Abstract
A study was conducted to concentrate the betacyanin in red pitahaya extracts by removing the coexisting sugars by fermentation. Four lactic acid bacteria (Lactobacillus acidophilus, L. casei, L. rhamnosus and L. plantarum) and a yeast species (Saccharomyces cerevisiae) were screened to determine their efficiency to reduce sugar content in red pitahaya extracts for concentration of their betacyanin content. A reduction of sugar content (19.8–56.4%) and increase in the yield of betacyanins were observed in all extracts as compared to the control, which was not innoculated with any microorganisms after 1 day of fermentation. The lowest total sugar content (26.40 g/L) was observed in extracts fermented by S. cerevisiae. Extracts fermented by S. cerevisiae also showed greater numbers of microbial cells (10.75 log CFU/mL) and a lower pH value (3.54) compared to those (6.89–8.48 log CFU/mL and pH 4.64–5.42) of the Lactobacillus spp. after 1 day of fermentation. An optimization step using response surface methodology (RSM) was then conducted using S. cerevisiae. Temperature, time and agitation speed were found to have a significant effect on the total sugar content and BC of concentrated betacyanins from red pitahaya, while the yield of betacyanins was significantly influenced by temperature and agitation speed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-021-05116-2.
Keywords: Betalain, Hylocereus polyrhizus, Lactic acid bacteria, Response surface methodology, Red dragon fruit, Yeast
Introduction
Betalains are natural plant pigments that are currently gaining popularity as natural colorants in the food industry. Although betalains from red beet or beetroot are widely used as food colorant, betalains are not as well studied as compared to other natural plant pigments such as chlorophylls, carotenoids and anthocyanins (Choo 2018). However, betalains have potential applications in functional foods due to their pharmacological properties (Gengatharan et al. 2015). Red pitahaya (Hylocereus polyrhizus) which is native to Mexico, Central and South America is an edible fruit that is rich in betalains, a subgroup betacyanins which are purplish-red color water-soluble compounds. It is currently cultivated in Asia and Australia and known as red dragon fruit (Yong et al. 2018). Betacyanin extracts from red pitahaya showed good potential application as natural colorants in dairy products such as milk (Gengatharan et al. 2016), yogurt (Gengatharan et al. 2017) and ice cream (Gengatharan et al. 2020).
Betacyanin extracts contain sugars which may cause undesirable caramelization during thermal treatment in food processing. A step following the extraction is necessary to purify and concentrate the betacyanins. Although purification of betacyanins and betaxanthins using aqueous two-phase extraction (Chethana et al. 2007) was described previously, this method was found to be time consuming and may not be suitable for the use in the food industry. In addition, the use of techniques, such as membrane filtration and chromatography using Amberlite XAD 16 containing columns, to purify extracts with high sugar content is often limited by operational problems (Castellar et al. 2008). For this reason, the use of fermentation may be a useful alternative technique to eliminate undesirable sugars in betalain extracts. Pourrat et al. (1988) showed that the sugar molecules which are co-present with betalains were eliminated during the fermentation of red beetroot dye without the breakdown of the betanins (an abundant form of betacyanins). In addition, fermentation of heated beetroot extracts using Saccharomyces cerevisiae was found to eliminate more than 99% of sugar content (Koubaier et al. 2013). Sravan Kumar et al. (2015) reported a 72% decrease in total soluble solids which is an indication of sugar content upon performing fermentation of Basella rubra fruit juice water extract with Saccharomyces cerevisiae. A fermentation step may therefore be useful in reducing the total sugars and subsequently enabling the reproducible production of purified and concentrated betalains, specifically betacyanins, with minimal loss of pigments. Lactobacillus are catalase negative, Gram-positive microorganisms that produce mainly lactic acid as the major metabolic end product of carbohydrate fermentation (Holzapfel and Wood 2014). Many species of the genus Lactobacillus are known for their application in food fermentation and specific strains have been recognized as having probiotic properties.
The objective of this study was to investigate the fermentation of red pitahaya extract using lactic acid bacteria (Lactobacillus acidophilus, L. casei, L. rhamnosus and L. plantarum) and a yeast species (Saccharomyces cerevisiae) to reduce the sugar content and increase the betacyanin content. Subsequently, optimization of the fermentation process using the most suitable microorganism was performed using response surface methodology (RSM).
Materials and methods
Extraction of betacyanins from red pitahaya
The extraction of betacyanins from the pulps of red pitahaya with total soluble solid values of 20–22º Brix was performed by the method of Gengatharan et al. (2020) using distilled water at a fresh weight to solvent ratio (w/v) of 1:1. Commercially available pectinase enzyme (Pectinex SP-L) (Sigma-Aldrich, U.S.A.) with an enzyme activity of 10,292 PGU/mL at a concentration of 1.5% in water (w/v) was added to degrade the pectin and the mucilaginous compounds of the pulp. The pulp was homogenized for 2 h with occasional stirring before being filtered and centrifuged (Beckman Coulter, California, U.S.A.) at 15,000×g for 15 min. Supernatant containing the betacyanins was dried in a vacuum oven at 40 °C for 24 h and stored at 4 °C until further analysis. The dried extract contained 56 ± 5.65 g/100 g of total sugars.
Microorganisms and culture conditions
Saccharomyces cerevisiae (ATCC 204508), Lactobacillus plantarum (ATCC 8014), Lactobacillus rhamnosus (ATCC 7469), Lactobacillus casei (ATCC 334) obtained from American Type Culture Collection, U.S.A. and Lactobacillus acidophilus LA-5 from Chr. Hansen, Denmark were used as starter cultures for the fermentation of betacyanins from red pitahaya. The cultures were maintained as glycerol stocks at − 20 °C. The S. cerevisiae and Lactobacillus spp. were activated and sub-cultured twice at 36 °C in nutrient broth and de Man, Rogosa & Sharpe (MRS) broth for 24 h or 48 h, respectively under anaerobic condition. The cells were collected by centrifugation at 5000×g for 10 min at 4 °C and were then suspended in betacyanin extract.
Fermentation of betacyanins
The betacyanins from the pulp of red pitahaya were dissolved in distilled water and standardized to an absorbance value of 0.70 ± 0.02 at a wavelength of 538 nm. This was followed by filtration using a 0.22 μm membrane filter and this sample (50 mL) was inoculated with 3% (v/v) bacterial or yeast suspension that was adjusted to 0.10 absorbance at 600 nm, which is equivalent to about 1 × 108 colony-forming unit (CFU) mL−1 in a 250 mL Erlenmeyer flask at 36 °C for 5 days and incubated in a shaking incubator at an agitation speed of 100 rpm. All samples were dried in a vacuum oven at 40 °C until constant weight was achieved. In this study, the vacuum-dried extracts are described as betacyanins. The betacyanins was analyzed from day-0 to day-5 of fermentation for total sugar content, betacyanin content, yield, pH and microbial numbers.
Total sugar content
Total sugar content was determined according to the phenol–sulphuric acid method (Dubois et al. 1956). Twenty mL of sample was centrifuged at 5000×g for 10 min at 4 °C. Two mL of the supernatant was pipetted into a test tube, and 0.05 mL of 80% phenol was added followed by 5 mL of concentrated sulfuric acid. The tubes were then carefully vortexed. The tubes were allowed to stand for 10 min and was placed in a water bath at 25 °C for 10 min. The tubes were vortexed before absorbance of the developed yellow orange colour was measured at 490 nm using a Lambda-25 UV–Vis spectrophotometer (Perkin Elmer, USA). The total sugar content was expressed as g/L.
Betacyanin content (BC)
The BC was determined according to the method of Stintzing et al. (2003). Absorbance was carried out using a Lambda 25 UV–Vis spectrophotometer (Perkin Elmer, U.S.A) at a wavelength of 538 nm. The betacyanin content (BC) was calculated as below.
where A538 = the absorbance at 538 nm (λmax), L (path length) = 1.0 cm, DF = dilution factor, V = extract volume (mL), W = fresh weight of extracting material (g), E = mean molar absorptivity of betanin which equals to 6.5 × 104 L mol−1 cm−1 in water and MW = 550.
High performance liquid chromatography (HPLC) analysis of betacyanins
HPLC analysis of betacyanins was carried out according to the method of Gengatharan et al. (2016) using a HPLC system (PerkinElmer, Waltham, U.S.A.) with a UV/VIS detector and a LiChroCart Purospher Star RP-18 (250 mm × 4.6 mm, 5 µm) column (Merck, Darmstadt, Germany). Isocratic elution of a mixture of 900 mL of 5 mL/L aqueous trifluoroacetic acid and 100 mL acetonitrile for 30 min at a flowrate of 1.0 mL/min at 25 °C was carried out. The injection volume was 20 µL and detection was at 538 nm.
Liquid chromatography-mass spectrometry (LC–MS) analysis of betacyanins
The identification of betacyanins was performed according to the method of Gengatharan et al. (2016) using a FX 15 ultra-high pressure liquid chromatography system (PerkinElmer, Waltham, USA) with a Zorbax C18 (150 mm × 4.6 mm, 5 µm) column (Agilent Technologies, Santa Clara, U.S.A.). A gradient elution from 100 mL/L to 900 mL/L of 0.1 mL/L formic acid in acetonitrile for 15 min with a flow rate of 500 mL/min was used. An AB Sciex 3200Q Trap Liquid Chromatography Tandem Mass LC/MS/MS Mass Spectrometer (AB Sciex Instruments, Foster City, USA) equipped with an electrospray source and operating in a positive ionization mode was used. The spray voltage was 5 kV and the temperature of the heated capillary was set to 400ºC. Data processing was carried out using a XCALIBUR software program.
Yield
The yield of the vacuum-dried betacyanins obtained from the fermentation study was calculated based on the formula below:
Enumeration of microorganisms
Colony forming units (CFU) were enumerated in plates after allowing S. cerevisiae and Lactobacillus spp. to incubate at 36 °C in nutrient agar and MRS agar for 24 h or 48 h, respectively, under anaerobic condition.
Optimization of fermentation of betacyanin extracts from red pitahaya using Saccharomyces cerevisiae
Design of experiments
Response surface methodology (RSM) was used to optimise and study the influence of fermentation parameters (independent variables) such as temperature, time, inoculum size and agitation speed on the yield, (BC) and total sugar content of betacyanins from red pitahaya. A Box-Behnken design using Minitab ® Release 14.1 software was used to establish the experiment designs with three factors and three levels. A design consisting of 27 experimental runs was used for the optimization of fermentation (Supplementary Table 1). A total of 27 samples of betacyanin preparations from red pitahaya were prepared at a standardized absorbance of 0.70 ± 0.02 at a wavelength of 537 nm and were fermented using S. cerevisiae. The inoculated betacyanin samples from red pitahaya were placed in a shaking incubator at the selected agitation speed, time and temperature. After fermentation, the samples were centrifuged at 5000×g for 10 min and the supernatant were collected in a clean weighed beaker. The betacyanin content was determined immediately prior to drying in vacuum at 40 °C for 24 h.
Statistical analysis
All experiments were conducted in three independent replicates (n = 3). Data were analysed by one way analysis of variance (ANOVA) using a SPSS software (IBM, U.S.A.). The Tukey test was used to determine the statistical significance at p < 0.05.
Results and discussion
Screening of concentration of betacyanins from red pitahaya fermentations
The screening study was conducted to investigate the suitability of lactic acid bacteria and a yeast to ferment and concentrate the betacyanins from the pulps of red pitahaya. The microorganism which concentrated betacyanins with the lowest total sugar content and highest yield and betacyanin content were subjected to a subsequent optimisation study.
Total sugar content, pH and microbial numbers
Measurement of total sugar content enabled the determination of consumption of sugars present in the betacyanins from red pitahaya by the selected microorganism used for fermentation. Similarly, the ability to readily utilize the sugars present in the betacyanins from red pitahaya can be evaluated by determining the pH and microbial numbers.
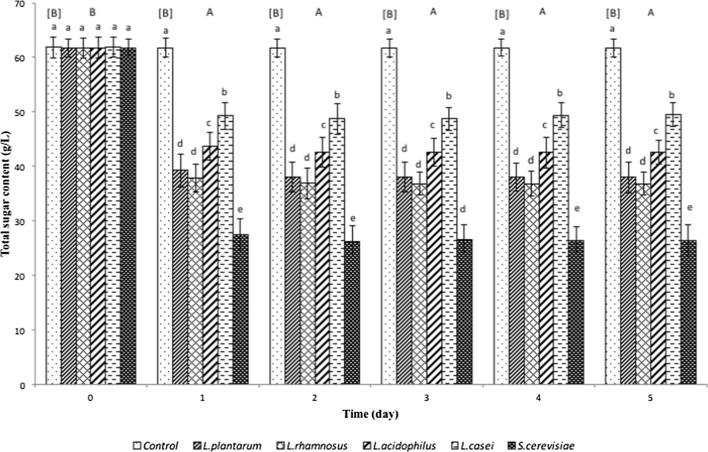
A significant reduction (p < 0.05) in total sugar content was observed on day-1 of fermentation in all fermented samples except for the control which was not inoculated with any microorganism (Fig. 1). This is because sugars are consumed during fermentation due to the microbial growth (Costa et al. 2013). However, no significant reduction in total sugar content was observed from day-1 onwards across all fermented samples (Fig. 1) indicating that the consumption of sugar by the selected microorganisms ceased within one day (24 h) of fermentation. This is also supported by a lack of decrease in pH values from day-1 of fermentation up to day-5 of fermentation (Table 1). The findings in this study are similar to that of Castellar et al. (2008) in which S. cerevisiae achieved 95% sugar reduction in Opuntia stricta fruits at 35 °C within 24 h. Sravan Kumar et al. (2015) reported a reduction between 42–72% in total solids content which was an indication of sugar content of Basella rubra fruits using three strains of S. cerevisiae after 3 h and 6 h of fermentation. Lin et al. (2021) demonstrated that inoculation ratio, concentration and sequence of S. cerevisiae can have effect on the sugar utilization of the yeast during pineapple wine fermentation.
Fig. 1.
Total sugar content of betacyanins from red pitahaya fermented by L. plantarum, L. rhamnosus, L. acidophilus, L. casei or S. cerevisiae. Results are presented as means ± standard deviations (n = 3). Superscript uppercase letters in square bracket indicate significant difference (p < 0.05) between fermentation days for controls while superscript uppercase letters without square brackets indicate significant difference (p < 0.05) between fermentation days for microorganisms. Superscript lowercase letters indicate significant difference (p < 0.05) between microorganisms within a particular day of fermentation
Table 1.
Microbial numbers and pH of Lactobacillus spp. and S. cerevisiae during fermentation of betacyanins from red pitahaya
| Time (day) | L. plantarum | L. rhamnosus | L. acidophilus | L. casei | S. cerevisiae | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| log CFU/mL | pH | log CFU/mL | pH | log CFU/mL | pH | log CFU/mL | pH | log CFU/mL | pH | |
| 0 | 5.23 ± 1.72a | 6.63 ± 0.08a | 5.51 ± 2.42a | 6.58 ± 0.04a | 4.82 ± 1.86a | 6. 61 ± 0.04a | 5.12 ± 0.88a | 6.60 ± 0.08a | 5.17 ± 2.35a | 6.59 ± 0.05a |
| 1 | 8.48 ± 1.45b | 4.64 ± 0.09c | 8.97 ± 1.88b | 4.58 ± 0.08c | 7.45 ± 2.11b | 5.34 ± 0.09b | 6.89 ± 1.05b | 5.42 ± 0.03b | 10.75 ± 1.62b | 3.54 ± 0.11b |
| 2 | 8.06 ± 2.41b | 4.64 ± 0.08c | 8.47 ± 2.36b | 4.57 ± 0.07c | 7.98 ± 1.65b | 5.32 ± 0.10b | 6.26 ± 2.03b | 5.41 ± 0.05b | 9.78 ± 1.87b | 3.57 ± 0.11b |
| 3 | 6.83 ± 0.78ba | 4.67 ± 0.06c | 7.34 ± 2.11b | 4.61 ± 0.06c | 6.09 ± 2.32b | 5.33 ± 0.06b | 5.16 ± 2.42b | 5.40 ± 0.04b | 8.89 ± 2.51b | 3.60 ± 0.12b |
| 4 | 5.65 ± 2.12a | 4.74 ± 0.07bc | 6.77 ± 1.38ba | 4.66 ± 0.06bc | 4.86 ± 1.41a | 5.36 ± 0.06b | 4.59 ± 1.37ba | 5.43 ± 0.05b | 7.65 ± 1.56ba | 3.62 ± 0.13b |
| 5 | 5.44 ± 1.25a | 4.79 ± 0.05b | 6.01 ± 1.67a | 4.73 ± 0.04b | 3.52 ± 1.13a | 5.38 ± 0.04b | 3.71 ± 1.48a | 5.46 ± 0.05b | 6.79 ± 1.84a | 3.70 ± 0.13b |
Results are presented as means ± standard deviations (n = 3). Superscript lowercase letters indicate significant difference (p < 0.05) between fermentation days within a microorganism
The consumption of sugar is related to the increase of total viable counts of the selected microorganisms used for fermentation of betacyanins from red pitahaya (Table 1) from day-0 to day-1. After day-4 or day-5 of fermentation, a decrease in microbial numbers was found for all strains (Table 1). Throughout the five days of fermentation, the lowest total sugar content of concentrated betacyanins was observed in betacyanins from red pitahaya fermented by S. cerevisiae followed by L. plantarum or L. rhamosus and L. acidophilus or L. casei (Fig. 1). This indicates that S. cerevisiae is a better choice of microorganism to concentrate betacyanins from red pitahaya as compared to Lactobacillus strains tested. This is also further supported by a greater microbial number and a greater reduction in pH (Table 1) of betacyanin preparations fermented by S. cerevisiae as compared to those of the Lactobacillus strains. The presence of high acid and low pH in fermentation medium as a result of fermentation may have caused the lactic acid bacteria to rapidly enter stationary phase (Siegumfeldt et al. 2000) and consequently lose fermentation ability. In contrast, S. cerevisiae was able to sustain their growth with a decrease in pH due to their acidophilic nature and are able to grow well in acidic condition (Narendranath and Power 2005).
Among the selected Lactobacillus strains used for fermentation of betacyanin preparations from red pitahaya, L. plantarum and L. rhamnosus were found to reduce higher amounts of sugars compared to those of L. acidophilus and L. casei. This may be because the metabolism of carbohydrate by Lactobacillus is strain and substrate dependent (Hou et al. 2000).
Betacyanin content
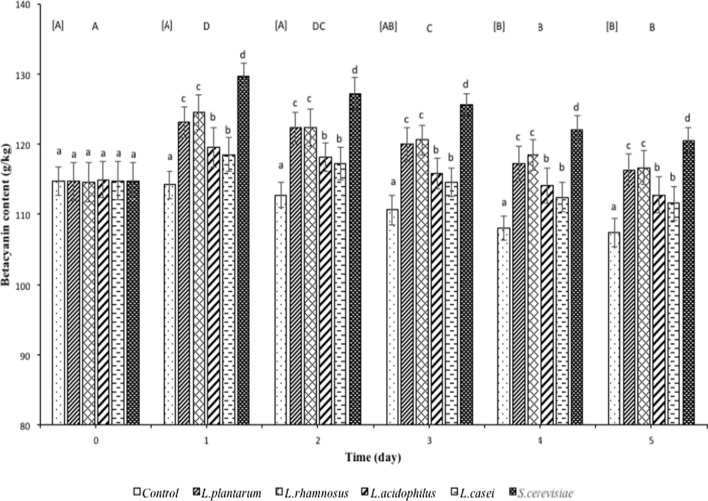
Betacyanin content (BC) is an important parameter to assess the pigment quality of the concentrated betacyanins from red pitahaya. Overall, it was found that fermentation significantly increased (p < 0.05) the BC of betacyanins from red pitahaya in all fermented samples compared to unfermented controls (Fig. 2) within the first day of fermentation. The highest BC was observed in betacyanins from red pitahaya fermented by S. cerevisiae followed by L. plantarum or L. rhamnosus and L. acidophilus or L. casei. This again can be attributed to the better ability of S. cerevisiae to ferment as discussed previously. The sugar content showed an inverse relationship to the betacyanin content with a greater decrease in sugar content attributable to a greater pigment content. Koubaier et al. (2013) found that yeast fermentation increased the betacyanin and betaxanthin content in heated red beetroot extracts. Castellar et al. (2008) found that fermentation of Opuntia stricta juice using S. cerevisiae var. bayanus increased the colour recovery rate by 85% due to the increase in pigment concentration.
Fig. 2.
Betacyanin content of betacyanins from red pitahaya fermented by L. plantarum, L. rhamnosus, L. acidophilus, L. casei or S. cerevisiae. Results are presented as means ± standard deviations (n = 3). Superscript uppercase letters in square bracket indicate significant difference (p < 0.05) between fermentation days for controls while superscript uppercase letters without square brackets indicate significant difference (p < 0.05) between fermentation days for microorganisms. Superscript lowercase letters indicate significant difference (p < 0.05) between microorganisms within a particular day of fermentation
There is a pattern of decreasing BC in fermented betacyanins preparation from day-2 of fermentation onwards. This may be due to the lowered pH of the fermented betacyanins (Table 1) which may have induced reactions such as hydrolysis or dehydrogenation which occurs under low pH conditions (pH 3 to pH 5) (Herbach et al. 2006a). This is in accordance with the study by Gengatharan et al. (2017) whereby betacyanin content of red pitahaya significantly decreased from pH 5 to pH 4 and pH 3.
Yield
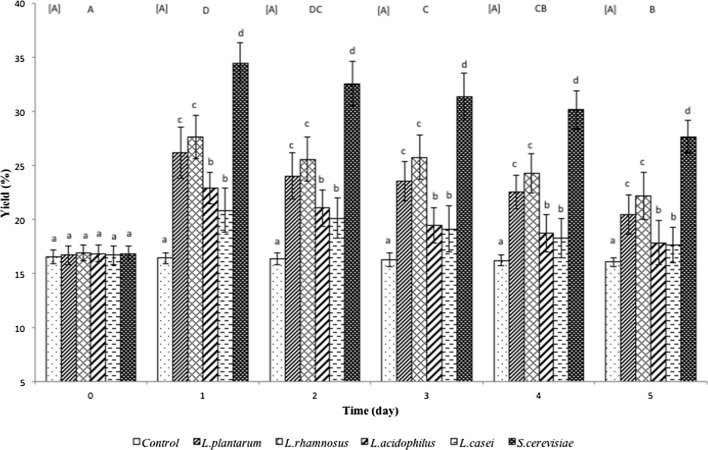
The yield of concentrated betacyanins from red pitahaya was evaluated to determine the betacyanin recovery efficiency from the fermentation process. Overall, it was found that fermentation significantly increased (p < 0.05) the yield of betacyanins from red pitahaya in all samples fermented by the selected Lactobacillus spp. and S. cerevisiae compared to unfermented controls (Fig. 3) throughout the fermentation. The highest yield was observed in betacyanins preparations from red pitahaya fermented by S. cerevisiae followed by L. plantarum or L. rhamnosus and L. acidophilus or L. casei. There was an approximately 50% increase in the yield of betacyanins. This again can be attributed to the better fermenting ability of S. cerevisiae as discussed previously.
Fig. 3.
Yield of betacyanins from red pitahaya fermented by L. plantarum, L. rhamnosus, L. acidophilus, L. casei or S. cerevisiae. Results are presented as means ± standard deviations (n = 3). Superscript uppercase letters in square bracket indicate significant difference (p < 0.05) between fermentation days for controls while superscript uppercase letters without square brackets indicate significant difference (p < 0.05) between fermentation days for microorganisms. Superscript lowercase letters indicate significant difference (p < 0.05) between microorganisms within a particular day of fermentation
There was a significant decrease in the yield of concentrated betacyanins in all fermented samples on day-3 of fermentation while the yield for the unfermented sample remained the same throughout the five days. This may be due to the decrease in pH (Table 1) or the production of alcohol by S. cerevisiae, or lactic acid by Lactobacillus spp., which may have initiated the degradation of betacyanins causing a reduction in the yield of betacyanins with fermentation time. A possible explanation for these observations may lie in the occurrence of hydrolytic cleavage of betacyanin in the presence of higher alcohol concentration or lower pH (Herbach et al. 2006b). Overall, S. cerevisiae was found to be the most effective microorganism to ferment and concentrate the betacyanins from the pulps of red pitahaya. For this reason, an optimisation of fermentation using S. cerevisiae was conducted.
Response surface methodology
The temperature range used in this study was from 24 to 36 °C and was selected based on the temperature range used for fermentation of Opuntia stricta juice by S. cerevisiae (Castellar et al. 2008). The time length of fermentation used in this study was 10–24 h due the outcome of the previous screening study that showed the cessation of fermentation within day-1 (24 h). The inoculum size and agitation speed range selected for this study was 1–5% and 100 rpm to 150 rpm, respectively. This was based on a preliminary study that was conducted to select the range of fermentation parameters to concentrate betacyanins from red pitahaya.
The suitability of the models was also assessed based on the significance of each factor (temperature, time, inoculum size and agitation speed) and their combinations to the selected response (total sugar content, betacyanin content, yield) which was obtained using an ANOVA analysis (Table 2). In addition, the multiple regression analysis of the data deduced the second-order polynomial equation for the studied responses (total sugar content, betacyanin content and yield) based on the fermentation factors that had significant effect (p < 0.05) on the studied responses. The three second-order polynomial equations are shown as follows.
Table 2.
Analysis of variance for the total sugar content, betacyanin content and yield of S. cerevisiae fermented betacyanins from red pitahaya
| P value | |||
|---|---|---|---|
| Total sugar content (g/L) | Betacyanin content (g/kg) | Yield (%) | |
| Temperature, X1 | 0.000 | 0.004 | 0.004 |
| Time, X2 | 0.000 | 0.000 | 0.320 |
| Inoculum size, X3 | 0.905 | 0.576 | 0.750 |
| Agitation speed, X4 | 0.000 | 0.000 | 0.000 |
| X1X2 | 0.512 | 0.526 | 0.634 |
| X1X3 | 0.176 | 0.687 | 0.566 |
| X1X4 | 0.038 | 0.115 | 0.725 |
| X2X3 | 0.804 | 0.920 | 0.763 |
| X2X4 | 0.125 | 0.216 | 0.019 |
| X3X4 | 0.710 | 0.240 | 0.980 |
| X12 | 0.048 | 0.198 | 0.004 |
| X22 | 0.041 | 0.001 | 0.001 |
| X32 | 0.703 | 0.849 | 0.067 |
| X42 | 0.000 | 0.000 | 0.000 |
| R2 | 0.982 | 0.993 | 0.927 |
| R2-adj | 0.961 | 0.986 | 0.842 |
Statistical parameters such as coefficient of determination (R2) and adjusted coefficient of determination (R2-adjusted) (Table 2) provided better measurements of the accuracy of the prediction models. The R2 is defined as the regression of sum of square proportion to the total sum of squares and illustrates the adequacy of a model. R2 ranges from 0 to 1. R2 values closer to 1 indicate more accuracy of a model (Yuan et al. 2008). The high R2-adjusted also determines if the model adequately fits the data (Badwaik et al. 2012; Yolmeh et al. 2014). The values of R2 and R2-adj (Table 2) of the three predicted polynomial models were close to 1 revealing that the full quadratic models were adequate and well fitted in terms of total sugar content, betacyanin content and yield, respectively.
The R2 value for total sugar content, betacyanin content and yield of fermented betacyanins (Table 2) indicate that 98.2%, 99.3% and 92.7% of the variation in the responses can be explained by the variation in the experimental factors. The remaining variation in the responses may be explained by other factors that are not investigated in this study. The R2 value for total sugar content, betacyanin content and yield of betacyanins was more than 0.9, which is considered as relatively high, indicating the high accuracy of the predicted models. The adjusted R2 for the studied prediction models were close to the R2 values with difference of less than 0.1. This again indicates the suitability of the selected range of factors and the high desirability of the predicted quadratic models. A possible factor which may have caused the remaining variation in the responses may be the amount of dissolved oxygen during fermentation.
Effects of fermentation parameters
Temperature strongly influences the growth rate of yeast cells during fermentation (Sener et al. 2007) which is important for sugar consumption of the fermentation media. In this study, temperature has a significant effect (p < 0.05) on the total sugar content of betacyanins from red pitahaya (Table 2). In addition to total sugar content, temperature plays a significant role in influencing the betacyanin content and yield of betacyanins (Table 2). Temperature was found to play an important role in drawing out betalains from its matrix constituent (Harivaindran et al. 2008) and extracting pectins from peach pomace (Faravash and Ashtiani 2008). The antioxidative potency of fermentation product from Ganoderma lucidum broth produced at a fermentation temperature of 30 °C was better than those produced at 18 °C and 24 °C (Chien et al. 2011). This indicates the importance of a suitable temperature to increase the betacyanin content and yield of concentrated betacyanins from red pitahaya.
In addition to temperature, time was found to have significant influence (p < 0.05) on the total sugar content and betacyanin content of betacyanins from red pitahaya (Table 2). Increasing fermentation time was found to significantly decrease the residual sugar content in Emir grape extracts which were used for wine production (Sener et al. 2007). Wang et al. (2015) reported an increase in total conversion rate of sugars to butyric acid in sweet sorghum juice with time suggesting influence of time in reducing the total sugar content.
Agitation speed was found to have significant effect (p < 0.05) on the total sugar content of concentrated betacyanins produced by fermentation from red pitahaya. Agitation speed influences the oxygen transfer rate in an exponential order (Huang et al. 2006). An increase in agitation speed increases the dissolved oxygen concentration which results in a quick start of growth (shortened lag time) and increased biomass which is favorable for sugar consumption (Rodmui et al. 2008). Aeration could also be beneficial to the growth and performance of microbial cells by improving the mass transfer characteristics with respect to substrate and product (Rodmui et al. 2008). Agitation also functions to mix the fermentation broth may play a role in stimulating the exponential growth phase of the microorganisms involved in the fermentation, which are responsible for the consumption of sugars in the fermentation media.
Agitation speed was also found to have significantly effect (p < 0.05) on the betacyanin content and yield of concentrated betacyanins produced by fermentation from red pitahaya (Table 2). Agitation ensures the uniform distribution of substrates in fermentation broths (Chhabra 2003) which may influence the betacyanin content of the betacyanins. Mohamad et al. (2013) reported that a higher agitation rate led to higher mass transfer coefficient which improved the convective mass transfer rate, thus facilitating the extraction process and leading to improvised extraction of phytochemicals. However, inoculum size did not show a significant effect on all three responses studied (Table 2).
A significant interaction effect (p < 0.05) was observed between agitation speed and temperature on the total sugar content of the concentrated betacyanins produced by fermentation from red pitahaya (Table 2). This is because agitation influences the oxygen transfer rate in an exponential order (Huang et al. 2006). Similarly, temperature influences growth rate of yeast cells during fermentation (Sener et al. 2007) which is important for sugar consumption during fermentation.
There were no significant effects on the interactions between fermentation parameters (temperature, time, inoculum size and agitation speed) on the betacyanin content of concentrated betacyanins produced by fermentation. In addition, there was a significant effect (p < 0.05) between time and agitation speed on the yield of concentrated produced by fermentation betacyanins (Table 2). This may be because time may influence the duration of interaction of dissolved oxygen with biomass in the fermentation media.
Optimization of fermentation parameters
The optimum fermentation conditions to concentrate the betacyanins from red pitahaya are: fermentation at a temperature of 36 °C for 16.5 h at an inoculum size of 2.7% and agitation speed of 107 rpm. This optimum fermentation step was able to reduce the total sugars by 83%. To verify the validity of the optimum conditions, experiments were performed thrice independently using the optimum fermentation parameters and the experimental results obtained were compared to the predicted results (Table 3).
Table 3.
Experimental and predicted output values of the optimized fermentation process
| Experimental | Predicted | |
|---|---|---|
| Total sugar content (g/L) | 6.08 ± 0.96a | 5.67a |
| Betacyanin content (g/kg) | 122.83 ± 3.08a | 126.65a |
| Yield (%) | 46.17 ± 4.72a | 48.06a |
Different superscript lowercase letters within a row indicate significant difference at p < 0.05
The mean values obtained for the concentrated betacyanins from red pitahaya in terms of total sugar content, BC and yield through the confirmation experiments were within 90% to 97% of the predicted values (Table 3). The reliability of the prediction models was also further validated by statistical analysis (one-way ANOVA) and there was no significant difference (p < 0.05) between the observed and the predicted values of total sugar content, betacyanin content and yield of fermentation-assisted concentrated betacyanins from red pitahaya. This is expected as the adjusted R2 values for all three prediction models were close to 1. Therefore, the developed quadratic models are valid, well-suited and reliable.
Betacyanin composition of non-fermented and fermented betacyanins under the optimum conditions is shown in Table 4. Fermentation resulted in a significant decrease in the betanin and isobetanin contents but a significant increase in the phyllocactin and hylocerenin contents (Table 4). Temperatures above 50 °C can degrade betacyanins by isomerisation, dehydrogenation and decarboxylation (Herbach et al. 2004, 2006b). However, fermentation was conducted at 36 °C. The change in the betacyanin composition is most likely due to possible regeneration mechanism of betacyanins after breaking down during the fermentation. Herbach et al. (2006a) stated that betacyanins are able to regenerate as long as the basic building blocks such as cyclo-DOPA ring and betalamic acid are present to re-form other betacyanin chromophores. According to Castellar et al. (2003), phyllocactin contributes towards a better stability of betacyanins in red pitahaya.
Table 4.
Percentage of betacyanins from non-fermented and fermented red pitahaya extract under optimum conditions
| Betacyanin | Non-fermented | Fermented |
|---|---|---|
| Betanin | 31.75 ± 0.76b | 22.95 ± 1.02a |
| Isobetanin | 5.42 ± 0.27b | 3.26 ± 0.64a |
| Phyllocactin | 53.13 ± 1.01a | 60.47 ± 1.26b |
| Hylocerenin | 9.46 ± 0.57a | 13.21 ± 1.14b |
Results are presented as means ± standard deviations (n = 3). Superscript lowercase letters indicate significant difference (p < 0.05) between samples
Conclusion
This study demonstrated that fermentation is a simple and useful technique to reduce sugars in red pitahaya extract. Among the four strains of Lactobacillus sp. used, L. rhamnosus and L. plantarum reduced more total sugar content and thereby increased the betacyanin content from red pitahaya compared to L. casei and L. acidophilus. S. cerevisiae was found to be the most suitable microorganism to reduce sugars from red pitahaya extract compared to all strains of Lactobacillus sp. An optimization study (RSM) using S. cerevisiae showed that fermentation variables influenced the yield, betacyanin content and total sugar content of the betacyanins from red pitahaya. Optimization of fermentation parameters was therefore important to increase fermentation efficiency. Red pitahaya extract with higher betacyanin content and lower sugar content could potentially be used as a functional food ingredient that provide health benefits to the consumer but this requires further studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors' contributions
AG—original draft, writing, performed the analysis and data collection. GAD—supervision, editing and writing. WSC—conceptualization, project management, supervision, editing and writing.
Funding
(Information that explains whether and by whom the research was supported). This work was funded by School of Science, Monash University Malaysia.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declares that they have no conflict of interest statement.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Badwaik LS, Prasad K, Deka SC. Optimization of extraction conditions by response surface methodology for preparing partially defatted peanut. Int Food Res J. 2012;19:341–346. [Google Scholar]
- Castellar MR, Obόn JM, Alacid M, Fernández-López JA. Colour properties and stability of betacyanins from Opuntia fruits. J Agric Food Chem. 2003;51:2772–2776. doi: 10.1021/jf021045h. [DOI] [PubMed] [Google Scholar]
- Castellar MR, Obόn JM, Alacid M, Fernández-López JA. Fermentation of Opuntia stricta (Haw.) fruits for betalains concentration. J Agric Food Chem. 2008;56:4253–4257. doi: 10.1021/jf703699c. [DOI] [PubMed] [Google Scholar]
- Chethana S, Nayak CA, Raghavarao KSMS. Aqueous two phase extraction for purification and concentration of betalains. J Food Eng. 2007;81:679–687. [Google Scholar]
- Chhabra RP. Fluid-dynamics and heat transfer with non-Newtonian liquids in mechanically agitated vessels. Adv Heat Transf. 2003;37:77–178. [Google Scholar]
- Chien YL, Ho CT, Chiang BH, Hwang LS. Effect of fermentation time on antioxidative activities of Ganoderma lucidum broth using leguminous plants as part of the liquid fermentation medium. Food Chem. 2011;126:1586–1592. doi: 10.1016/j.foodchem.2010.12.024. [DOI] [PubMed] [Google Scholar]
- Choo WS. Betalains: Application in functional foods. In: Merillon J-M, Ramawat KG, editors. Bioactive molecules in food. Cham: Springer; 2018. pp. 1–28. [Google Scholar]
- Costa MGM, Fonteles TV, Jesus ALT, Rodrigues S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: process optimisation and product stability. Food Chem. 2013;139:261–266. doi: 10.1016/j.foodchem.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Faravash RS, Ashtiani FZ. The influence of acid volume, ethanol-to-extract ratio and acid washing time on the yield of pectic substances extraction from peach pomace. Food Hydrocoll. 2008;22:196–202. [Google Scholar]
- Gengatharan A, Dykes G, Choo WS. Betalains: natural plant pigments with potential application in functional foods. LWT Food Sci Technol. 2015;64:645–649. [Google Scholar]
- Gengatharan A, Dykes G, Choo WS. Stability of betacyanin from red pitahaya (Hylocereus polyrhizus) and its potential application as a natural colourant in milk. Int J Food Sci Technol. 2016;51:427–434. [Google Scholar]
- Gengatharan A, Dykes G, Choo WS. The effect of pH treatment and refrigerated storage on natural colourant preparations (betacyanins) from red pitahaya and their potential application in yoghurt. LWT Food Sci Technol. 2017;80:437–445. [Google Scholar]
- Gengatharan A, Dykes G, Choo WS. Betacyanins from Hylocereus polyrhizus: pectinase-assisted extraction and application as a natural food colourant in ice cream. J Food Sci Technol. 2020;58:1401–1410. doi: 10.1007/s13197-020-04651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harivaindran KV, Rebecca OPS, Chandran S. Study of optimal temperature, pH and stability of dragon fruit (Hylocereus polyrhizus) peel for use as potential natural colourant. Pak J Biol Sci. 2008;11:2259–2263. doi: 10.3923/pjbs.2008.2259.2263. [DOI] [PubMed] [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Impact of thermal treatment on colour and pigment pattern of red beet (Beta vulgaris L.) preparations. J Food Sci. 2004;69:C491–C498. [Google Scholar]
- Herbach KM, Rohe M, Stintzing FC, Carle R. Structural and chromatic stability of purple pitahaya (Hylocereus polyrhizus [Weber] Britton and Rose) betacyanins as affected by the juice matrix and selected additives. Food Res Int. 2006;39:667–677. [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Betalain stability and degradation-structural and chromatic aspects. J Food Sci. 2006;71:R41–R50. [Google Scholar]
- Holzapfel WH, Wood BJB. Introduction to the LAB. In: Holzapfel WH, Wood BJB, editors. Lactic acid bacteria—biodiversity and taxonomy. Chichester: Wiley; 2014. pp. 1–12. [Google Scholar]
- Hou JW, Yu RC, Chou CC. Changes in some components of soymilk during fermentation with bifidobacteria. Food Res Int. 2000;33:393–397. [Google Scholar]
- Huang WC, Chen SJ, Chen TL. The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation. Biochem Eng J. 2006;32:239–243. [Google Scholar]
- Koubaier HBH, Essaidi I, Snoussi A, Zgoulli S, Chaabouni M, Thonart P, Bouzouita N. Effect of Saccharomyces cerevisiae fermentation on the colorants of heated red beet root extracts. Afr J Biotechnol. 2013;12:728–734. [Google Scholar]
- Lin X, Jia Y, Li K, Hu X, Li C, Liu S. Effect of the inoculation strategies of selected Metschnikowia agaves and Saccharomyces cerevisiae on the volatile profile of pineapple wine in mixed fermentation. J Food Sci Technol. 2021 doi: 10.1007/s13197-021-05019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad M, Ali MW, Ripin AA. Effect of extraction process parameters on the yield of bioactive compounds from the roots of Eurycoma longifolia. Jurnal Teknologi. 2013;60:51–57. [Google Scholar]
- Narendranath NV, Power R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of Lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl Environ Microbiol. 2005;71:2239–2243. doi: 10.1128/AEM.71.5.2239-2243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourrat A, Lejeune B, Grand A, Pourrat H. Betalains assay of fermented red beet root extract by high performance liquid chromatography. J Food Sci. 1988;53:294–295. [Google Scholar]
- Rodmui A, Kongkiattikajorn J, Dandusitapun Y. Optimization of agitation conditions for maximum ethanol production by co-culture. Kasetsart J Nat Sci. 2008;42:285–293. [Google Scholar]
- Sener A, Canbaş A, Ünal MÜ. The effect of fermentation temperature on the growth kinetics of wine yeast species. Turk J Agric For. 2007;31:349–354. [Google Scholar]
- Siegumfeldt H, Rechinger KB, Jakobsen M. Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl Environ Microbiol. 2000;66:2330–2335. doi: 10.1128/aem.66.6.2330-2335.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sravan Kumar S, Manoj P, Giridhar P. A method for red-violet pigments extraction from fruits of Malabar spinach (Basella rubra) with enhanced antioxidant potential under fermentation. J Food Sci Technol. 2015;52:3037–3043. doi: 10.1007/s13197-014-1335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzing FC, Schieber A, Carle R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur Food Res Technol. 2003;216:303–311. [Google Scholar]
- Wang L, Ou MS, Nieves I, Shanmugam KT. Fermentation of sweet sorghum derived sugars to butyric acid at high titer and productivity by a moderate thermophile Clostridium thermobutyricum at 50°C. Bioresour Technol. 2015;198:533–539. doi: 10.1016/j.biortech.2015.09.062. [DOI] [PubMed] [Google Scholar]
- Yolmeh M, Najafi MBH, Farhoosh R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM) Food Chem. 2014;155:319–324. doi: 10.1016/j.foodchem.2014.01.059. [DOI] [PubMed] [Google Scholar]
- Yong YY, Dykes G, Lee SM, Choo WS. Effect of refrigerated storage on betacyanin composition, antibacterial activity of red pitahaya (Hylocereus polyrhizus) and cytotoxicity evaluation of betacyanin rich extract on normal human cell lines. LWT Food Sci Technol. 2018;91:491–497. [Google Scholar]
- Yuan Y, Gao Y, Mao L, Zhao J. Optimisation of conditions for the preparation of β carotene nanoemulsions using response surface methodology. Food Chem. 2008;107:1300–1306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.