Abstract
The present study is focused on the influence of amylose and amylopectin ratio on crystallinity, water barrier, mechanical, morphological and anti-fungal properties of starch-based bionanocomposite films. The different sources of starch containing various proportion of amylose and amylopectin (high amylose corn starch, 70:30; corn starch, 28:72; wheat starch, 25:75; and potato starch, 20:80) has been incorporated with chitosan (CH) and nanoclay (Na-MMT). Amylose and amylopectin ratio has regulated the orientation of molecular structure in the starch-based films. Experimental results have revealed that the prepared bionanocomposite films that of CS/CH/nanoclay has exhibited higher crystallinity and molecular miscibility among corn starch, with chitosan and nanoclay were confirmed by XRD. CS/CH/nanoclay has exhibited lowest water vapor permeability and highest tensile strength due to molecular space present in corn starch. Fourier transform infrared spectroscopy has confirmed the shift of amine peak to a higher wavenumber indicating a stronger hydrogen bond between starch and chitosan. Finally, the best bionanocomposite films were tested for food packaging applications. Low-density polyethylene has exhibited fungal growth on 5th day when packed with bread slices at 25 °C and 59% RH whereas CS/CH/nanoclay bionanocomposite film did not show the same for at least 20 days. CS/CH/nanoclay film could potentially be useful for active packaging in extending shelf life; maintaining its quality and safety of food products thus substituting synthetic plastic packaging materials.
Keywords: Starch, Amylose and amylopectin ratio, Barrier properties, Bionanocomposite films, Anti-fungal properties, Active packaging, Shelf life
Introduction
In the past few decades, one major contributor to the present scenario is conventional synthetic plastics as packaging materials major global environmental concern due to its decomposition period ranging from hundreds to sometimes thousands of years (Pagno et al. 2016). Biopolymer-based packaging material is a possible alternative to petrochemical-based plastics that overcomes the problem of biodegradability. Biodegradable polymer has made immense contribution to the development of food packaging industry (Nair et al. 2017). Consumers’ behaviour shows an increase in inclination toward bio based food packaging material as well as environmental friendly with prolonging shelf life during food preservation (Tan et al. 2015). The biodegradable polymer is gaining much attention in the application of food packaging that is a significant contributor to improving food safety, preserving food quality until consumption, minimizing chemical preservation, and reducing environmental impact (Ozcalik and Tihminlioglu 2013).
Starch is the most promising natural polymer used in food packaging because of its biocompatibility, film-forming ability and low cost (Sanyang et al. 2016). Starch based biodegradable films have a major drawback of strong hydrophilic behaviour, which imparts poor water barrier and lower mechanical properties comparative to petrochemical plastic films (Souza et al. 2012). Amylose is a linear homo-polymer chain of α-(1 → 4) linked glucose units. Amylopectin is a highly branched molecule with α-(1 → 6) glycosidic linkage at branch points as well as linear way with α-(1 → 4)-linked glucose units (Murphy 2000). Amylopectin contains a branching point that takes place in occurring every 24–30 glucose units and which leads the molecular structure and effects on biological and physical properties (Perez and Bertoft 2010). Different ratio of amylose: amylopectin signifies distinct molecular weight due to the nature of chain lengths, chain branching and structure characteristics in the starch-based products (Mua and Jackson 1997). Chitosan is a natural and second most abundant polymer material composed of D-glucosamine units linked by β-(1–4) bonds and N-acetyl-d-glucosamine. Chitosan is easily prepare by deacetylation of chitin that found in the core part of the exoskeleton of crustaceans, arthropods and cell walls of fungi. It is a biodegradable, bioactive, adequate water vapour barrier, low oxygen permeable and excellent film forming ability (Aguirre-Loredo et al. 2016; Jahit et al. 2016). Chitosan has unique property of food protection due to its inherent anti-bacterial and anti-fungal properties against spoilage microorganism and several pathogenic (Tan et al. 2015).
Recently the preparation of nanocomposites using montmorillonite (MMT) is a persuading method as filler material to improve the barrier, mechanical and thermal properties without affecting biodegradability of the food packaging industry (Siqueira et al. 2010; Alekseeva et al. 2017). MMT is a natural non-toxic nanoclaythat composed of aluminium silicates (clay minerals) layers classify as phyllosilicates, which is environmentally friendly and highly abundant at comparatively cheap rate (Ray and Bousmina 2005).
Furthermore, there are no study for different biodegradable films prepared from starch sources containing various proportion of amylose: amylopectin incorporated with chitosan and nanoclay based packaging film for food applications. The aim of this work is to study the influence of ratio of amylose-amylopectin of starches on functional properties (crystallinity, water barrier, mechanical, morphological and anti-fungal properties) of bionanocomposite eco-friendly food packaging.
Materials and methods
Materials
Corn starch (CS), wheat starch (WH) and potato starch (PS) have been supplied by Nacalai Tesque (Kyoto, Japan). The ratio of amylose and amylopectin is 28:72, 25:75, 20:80 and moisture content is 13.83%, 14.24%, 18.62% respectively. High amylose corn starch (HACS) in the range of amylose: amylopectin 70:30 and moisture content 14.18% has been obtained from Sanwa- Starch Corporation. (Nara, Japan). The Megazyme kit procedure has been used for determination amylose: amylopectin ratios in different sources of starch (Megazyme Ireland International, Ireland). Sodium MMT and chitosan from shrimp shells with < 75% deacetylation degree has been obtained from Sigma-Aldrich (St. Louis, MO, USA). Glycerol is used as plasticizer and glacial acetic acid is used as solvent, has been supplied from Nacalai Tesque (Kyoto, Japan).
Preparation of starch chitosan-clay of bionanocomposites
Chitosan powder (1 g) in 1% (v/v) acetic acid solution has been prepared. Starch (5 g) is mixed in 100 mL deionized water and the suspension has been agitated under constant magnetic stirring (700 rpm) at room temperature for 60 min followed by heating until complete gelatinization. The two solutions are obtained above have been mixed under constant agitation (700 rpm). Glycerol has been added as 40% (w/w) in solution under constant magnetic stirring. Clay nanoparticles (0.01 g) have been suspended into distilled water (50 mL) using ultrasonic mixing for 5 h (Ultrasonic cleaner, China). About 5 mL of this nanocaly have been mixed starch–chitosan films solutions and stirred for 15 min. Further, 10 mL of starch–chitosan films solutions has been poured into Petri dishes (12 cm in diameter). Film is formed by drying at 55 °C for 3 h of conventional oven subsequently kept at room temperature for about 24 h. All the prepared films have been conditioned at saturated sodium chloride solution (NaCl—75% RH) for 72 h prior to further testing. The film has been prepared using solution-casting method. The samples have been represented as HACS/CH/nanoclay CS/CH/nanoclay, WH/CH/nanoclay, and PS/CH/nanoclay and control sample as CH/nanoclay. The procedure for preparation of CH/nanoclay film is same as that of other films with the absence of starch solution.
Thickness measurement
Digital micrometre (Mitutyo, Japan) has been used to determine the thickness of the films to the nearest 0.001 mm. The measurements have been taken at three random positions of the film and average has been calculated.
X-ray diffraction (XRD)
X-ray diffraction pattern of the bionanocomposite films for crystallinity have been obtained using X-ray diffractometer (Bruker D8 Advance, Karlsruhe, Germany). The diffraction spectra have been recorded over the 2θ range of 5–50°, using Cu Kα radiation (20 mA, 40 kV) with a scan rate of 2 s per step and step size of 0.02°. Finally, the degree of crystallinity has been calculated by dividing area of crystalline region with total area under curve (Kumar et al. 2020).
Water barrier properties
Film moisture content
The moisture content (MC) of prepared sample films have been determined gravimetrically by drying the films (40 × 15 mm) in the oven at 105 °C for 24 h (Rhim et al. 2013).
Film solubility
The film solubility (FS) has been carried out according to Farahnaky et al. (2013) with minor modification. The films have been cut into sizes of 40 × 15 mm and is heated at 105 °C for 24 h the determination of initial dry weight. The dried sample films have been immersed in 50 mL of distilled water and is incubated at 25 °C using an incubator shaker (24 h, 100 rpm). Then, sample is taken out and is dried at 105 °C for 24 h in an oven to obtain the final dry weight of films. The film solubility of the sample is calculated as follows:
Water vapour permeability (WVP)
The water vapor permeability (WVP) has been estimated by gravimetric method according to the JIS Z0208 standard method known as the “cup method’’ (JIS 1976). Films have been prepared and is sealed over the circular opening of 0.00287 m2 of a permeation cell, and is stored at 25 °C in a desiccator. The cell has been completely filled with calcium chloride anhydrous (CaCl2—0% RH), and the system has been placed in a desiccator containing saturated sodium chloride solution (NaCl—75% RH). A plot of weight gain of the permeation cell versus time has been used to determine the water vapor transport. The water vapor transmission rate (WVTR) has been calculated from slope (g/s) divided by the transfer area (m2). The weight of cups has been examined at 12 h interval for 2 days. WVP (g.m−1 s−1 Pa−1) is calculated as follows:
where ∆P is the difference in partial water vapor pressure between two sides of the film specimens and L is the film thickness (m).
Mechanical properties
Tensile strength (TS) and elongation at break (E) of the films has been calculated according to ASTM D882-91 standard method (ASTM 1996) using universal testing machine (EZ-L, Shimadzu, Japan). All films have been cut into (80 × 5 mm) rectangular shapes and measured. The initial grip separation has been set at 50 mm and 5 mm/min crosshead speed has been set to determine tensile strength and elongation at break.
Dynamic mechanical thermal analysis (DMTA)
The small deformation analysis of bionanocomposite films has been performed in a dynamic mechanical thermal analysis (DMTA) using Q800 DMA (TA Instruments, USA). The films sample has been tested in clamp tension and for cooling sysem using liquid nitrogen. Specimen of dimensions 50 × 10 × 0.06 mm have been placed in clamp with initial grip lengh of 20 mm. The testing parameters have frequency = 1 Hz; strain = 0.05%; heating rate = 5 °C/min and temperature range = − 75 to 150 °C. Dynamic mechanical analysis has been used to calculated glass transition (Tg) value in the linear viscoelastic regime. The storage modulus (E′) and loss tangent (tan δ = ΔE′/E′′) have been recorded as a function of temperature from selected heating or cooling rate and with constant frequency.
Fourier transforms infrared spectroscopy (FTIR)
FTIR-ATR spectra of films has been recorded using FTIR spectrometer (Spectrum 100, Perkin-Elmer USA). Spectra has been collected in within the range of 4000–600 cm−1. For each spectrum, 25 consecutive has been scanned at 4 cm−1 resolutions for the sample. The background spectrum has been scanned under the same parameter before each sample scanning (Xu et al. 2005).
Morphological and anti-fungal properties of bionanocomposite film
The morphological structures of prepared films has been performed in a scanning electron microscopy (SEM) (S-4800, Hitachi, Japan). Sample morphology has been analysed at an accelerating potential of 15 kV. Micrographs has been taken at a magnification of 800 × to investigate the morphological characteristics. The film samples has been attached to SEM stub using double-sided carbon tape and coated with osmium (Frank et al. 2018).
The anti-fungal activity of films has been evaluated by direct contact with bread samples containing 40% moisture content according to procedure described by Tan et al. (2015). Bread pieces has been supplied from the local confectionery store. Comparing the anti-fungal activity of bionanocomposite films with conventional polymeric packaging materials with the same size of bread samples has also been packed with low-density polyethylene (LDPE). All packed bread samples have been stored at 25 °C and 59% RH for 20 days.
Statistical analysis
Analysis of variance (ANOVA) has been performed on a completely randomized experimental design using SPSS 20.0 (SPSS Inc., Chicago) and the mean has been compared with Tukey Test (confidence p < 0.05).
Results and discussion
X-ray diffraction
In order to study the role of amylose-amylopectin in the polymer matrix, performed XRD analyse of CH/nanoclay, PS/CH/nanoclay, WH/CH/nanoclay, CS/CH/nanoclay and HACS/CH/nanoclay bionanocomposite films are presented in Fig. 1.
Fig. 1.
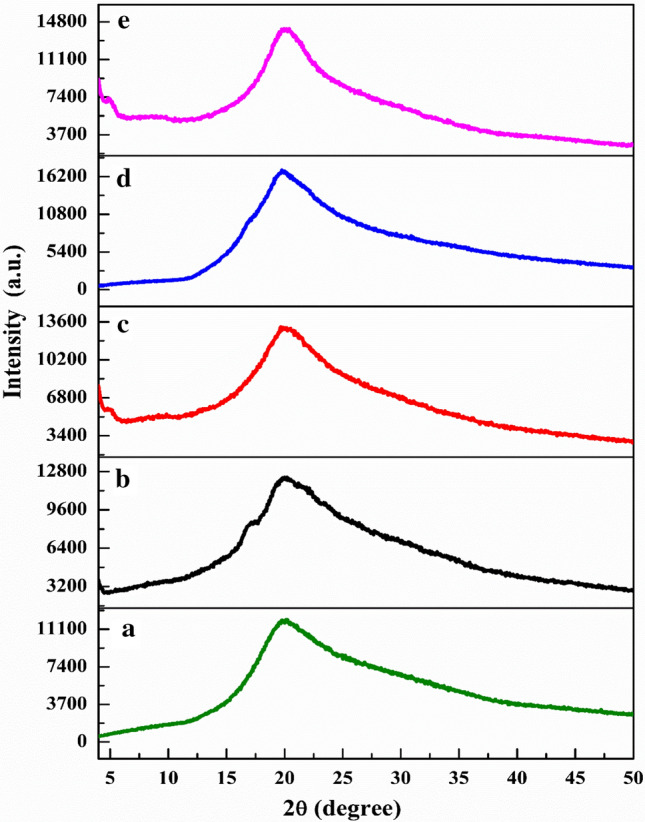
XRD pattern of different amylose–amylopectin ratios of starch-CH-nanoclayfilms: a CH/nanoclay(control), b PS/CH/nanoclay, c WH/CH/nanoclay, d CS/CH/nanoclayand, e HACS/CH/nanoclay
XRD diffraction patterns of native PS and HASC exhibit B-type which show main peaks around 2θ = 17°, 22° and 23° (Manek et al. 2012; Gernat et al. 1993). Whereas the diffraction patterns of native WH and CS shows A-type whose main peak is at 15°, 17°, 18° and 23° (Sun et al. 2014; Yu et al. 2016). The chitosan film shows diffraction peaks at around 8.08°, 11.6°, 17.84° and 22.38° (Ren et al. 2017). The peaks have been found in the diffraction spectra of starch-based films are not seen in case of bionanocomposite films which may be associated with the destruction of crystalline granules of starch during the process of gelatinization. Peak broadening and decreases in intensity can also be seen in the above case (Zhang et al. 2007).
Figure 1a When starch is completely solubilized with chitosan and reinforced by nanoclay, it depicts a film with three film-forming components (starch–chitosan-nanoclay) which show a main diffraction peak at (2θ = 20°) (Fig. 1b–d).
The shifting in diffraction peak is probably due to change in the chain orientation caused by an interaction of hydrogen-bond between chitosan and starch molecules (Bangyekan et al. 2006).
The broad peak intensity and percentage crystallinity increases like as CS/CH/nanoclay (33.79%) > HACS/CH/nanoclay (32.72%) > WH/CH/nanoclay (32.05) > PS/CH/nanoclay (30.03) > CH//nanoclay (26.26%) (Fig. 1a–e). This crystallinity range is found also by Feng et al. (2012) and Rindlav-Westling et al. (2002) for chitosan and different starch based films. These results have revealed that nanoclay (Na-MMT) inserts with chitosan or either starch polymer chain as well as both through silicate layers also thus developing starch/CH/nanoclay bionanocomposite film. The intercalation is affected by the ratio of amylose-amylopectin and OH group connected between nanoclay and polymer chains. The peak of Na-MMT around 7.21° (2θ) is not observed for all the bionanocomposite films which confirms proper intercalation of host polymer chain between nanoclay stacks. The intensity of the crystalline peak and percentage crystallinity in case of CS/CH/nanoclay is higher due to its chemical structure and compatibility, hydrogen bonding between CS and CH reveals a strong interaction of polymer matrix within nanoclay network.
Water barrier properties
The water barrier properties of the films consist of moisture content (MC), Film solubility (FS) and water vapor permeability (WVP) are shown in Fig. 2a–c.
Fig. 2.
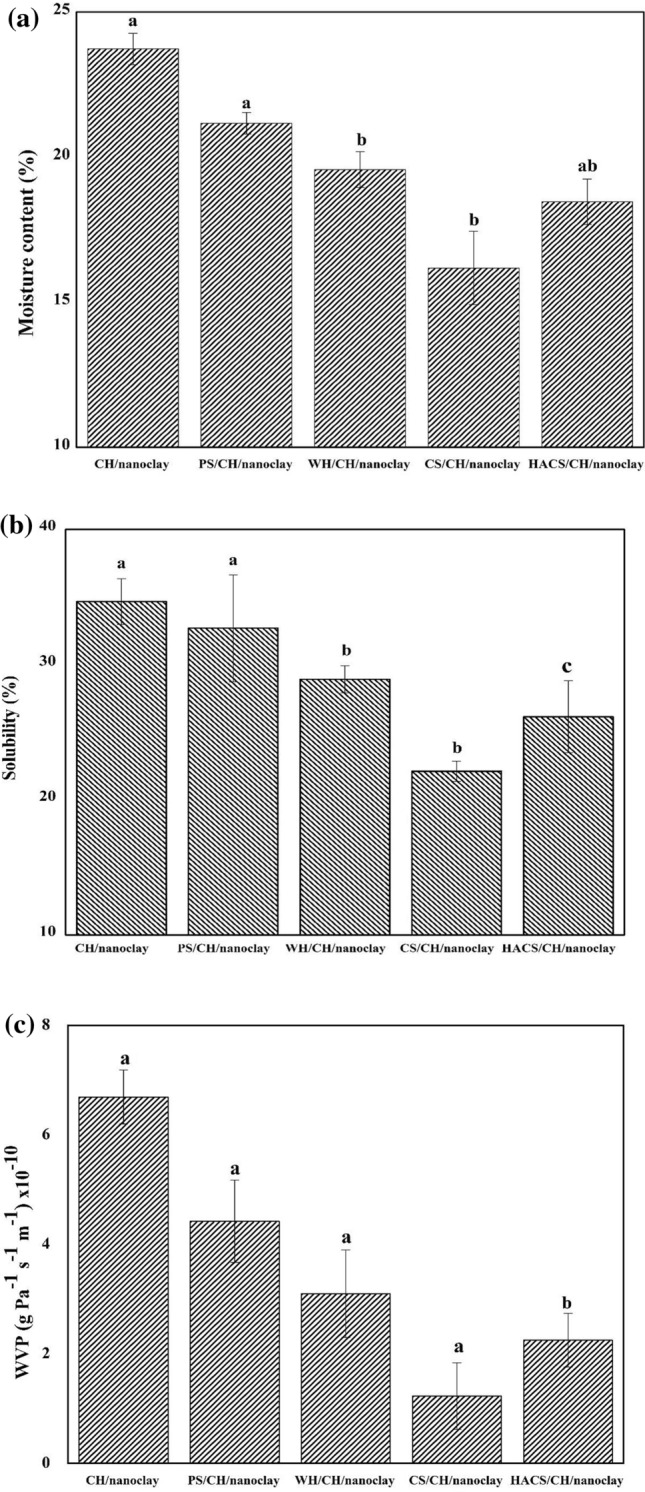
a Influence of amylose-amylopectin ratio on moisture content of starch based bionanocomposite films. b Influence of amylose-amylopectin ratio on film solubility of starch based bionanocomposite films. c Influence of amylose-amylopectin ratio on water vapor permeability of starch based bionanocomposite films. Means with different value letters represent significantly difference value p < 0.05 using Tukey Test
Moisture content of starch-based films does not depend only on relative humidity of environment but also on nature and chemical structure such as ratio of amylose-amylopectin content, size of branching point, which further guides the molecular architecture of starch granule (Mua and Jackson 1997; Bertoft and Blennow 2016). The existence of unbound –OH groups in case of CH/nanoclay (23.73%), PS/CH/nanoclay (21.17%) and WH/CH/nanoclay (19.57%) increase the interaction with water molecule. CS/CH/nanoclay films (16.18%) exhibit lesser hydrophilicity might be due to 28% amylose content that modifies its chemical structure and molecular space resulting in reduction of free water absorption site thus decreasing the percentage of moisture content. This may be associated with a strong hydrogen bonding group (–NH2) of CH and hydroxyl group (–OH) of CS. Though HACS/CH/nanoclay (18.46%) has the highest amylose content its MC is higher than CS/CH/nanoclay that may be due to the drastic reduction in amylopectin content. Thus, an increase in the amylose content to an optimum value results in very stable molecular orientation forming dense and stronger films enhancing the ability of its linear chains to interact with hydrogen bonds. The difference in moisture content is observed due to their difference in chemical structures and hygroscopic nature (Kurt and Kahyaoglu 2014). The results shown in XRD spectrum and moisture content together confirm the lower hydrophilic property of CS/CH/nanoclay. A similar result has also reported by Nair et al. 2017.
FS is one of the important parameters that determine the product reliability, shelf life and moisture resistance in food packaging (Nair et al. 2017). PS/CH/nanoclay had the higher film solubility (32.70%) as shown in Fig. 2b. Because of moisture content present crosslinking of starch and water resulting hydrophilic nature, lesser degree of interaction between starch and CH thus forming swollen film structure. This increases the solubility of the film that was in accordance with Kurt and Kahyaoglu 2014. While CS/CH/nanoclay showed a lower solubility (22.12%) in water due to lower water sensitivity and strong intermolecular interaction between polymers that resulted from the improved cohesiveness of the biopolymer matrix. These result in lower swelling of the films that maintain a stronger product integrity (Fig. 2b) in consistence with MC (Fig. 2a) and higher degree of crystallinity (Fig. 1d).
WVP of biopolymer films is yet another important property to considered for food packaging applications. Food spoilage is more when there is large transfer of moisture between the food and atmosphere. Normally, water penetration passes via amorphous regions of films (Mali et al. 2006). This WVP depends on the film structure, plasticizer, temperature and relative humidity of the surrounding atmosphere. Moreover, WVP plays an essential property for determine the shelf life of a packaged product. The results exhibit that WVP was in the direction CH/nanoclay > PS/CH/nanoclay > WH/CH/nanoclay > CS/CH/nanoclay (Fig. 2c). Low WVP of CS/CH/nanoclay (1.23 × 10−10 g Pa−1 s−1 m−1) showed to 28% amylose content due to which there is a higher intermolecular hydrogen interaction between CS and CH, thus reducing the molecular space for permeation of water molecule into the network. In case of HASC/CH/nanoclay (2.25 × 10–10 g Pa−1 s−1 m−1) film, 70% amylose ratio increases results in increase of the free volume of the film matrix that showed higher hydrophilicity. This leads to higher diffusivity and increased WVP. WVP obtained is in accordance with the results of higher degree of crystallinity (Fig. 1d) and MC (Fig. 2a).
Mechanical properties
Mechanical properties resulted from the tensile strength (TS) and elongation at break (EB) determine the properties such as film’s strength, barrier property and stretchable that in turn decides the application of packaging material such as handling, shipping and preserving the packed foods during storage. Figure 3a has shown TS on the influence of amylose-amylopectin ratios of starches incorporated with CH and nanoclay.
Fig. 3.
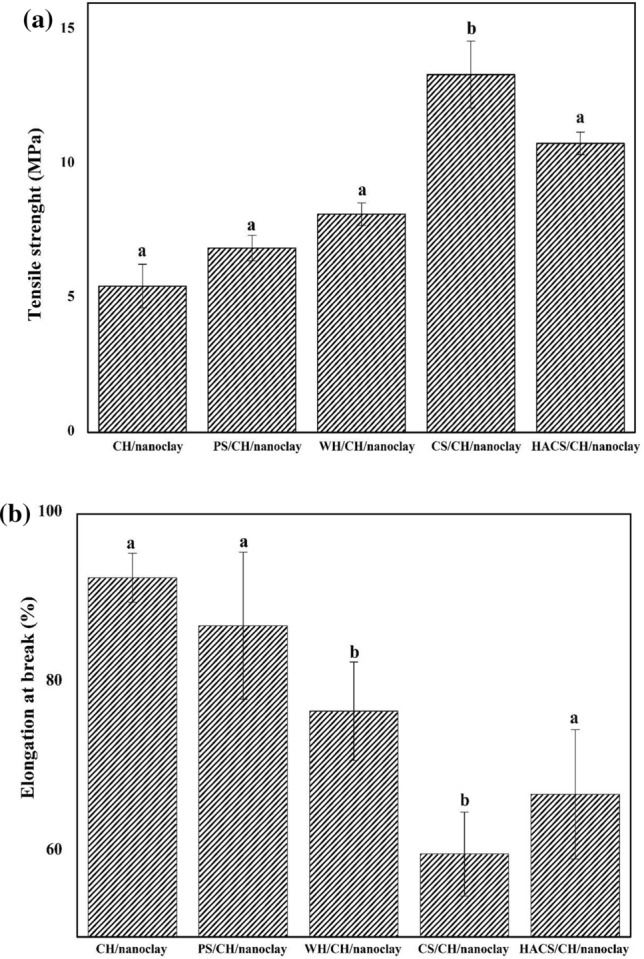
a Influence of amylose-amylopectin ratio on tensile strength of starch based bionanocomposite films. b Influence of amylose-amylopectin ratio on elongation at break of starch based bionanocomposite films. Means with different value letters represent significantly difference value p < 0.05 using Tukey Test
TS shows the capacity to accept load or maximum force applied on bionanocomposite before breaking and it depends on the chemical structure of starch matrix binder with CH and the reinforcement nanoclay in composite materials. Tensile values determine both stretch ability and strength that is a significant requirement for food-packing material (Arvanitoyannis et al. 1998). Mechanical properties are related with moisture content and glycerol concentration (plasticizing agent) within hydrophilic material film (Aguirre-Loredo et al. 2016). TS of control film (CH/nanoclay) has been found to be 5.42% due to the hydrophilic nature of film. The results has confirmed that PS/CH/nanoclay with 20% amylose has an average TS of 6.86 MPa, WH/CH/nanoclay film containing 25% amylose shows close to 8.13 MPa, CS/CH/ nanoclay film containing 28% amylose is near 13.33 MPa and the TS of HACS/CS/ nanoclay with 70% amylose is 10.76 MPa. The amino (NH2) functional groups in the backbone of chitosan are protonated and converted into NH3+ in the acetic acid solution. Nanoclay is a potential filler that enhances the ordered crystallinity structures of starch/CH molecules. CS/CH/nanoclay (28% amylose) shows the highest tensile strength which might be due to a highly linear structure formed during gelatinization consequence in formation of a stronger intermolecular hydrogen bonding between NH3+ in the backbone of chitosan and OH− of the starch. This result contributes in a denser packing of polymer network compared to PS/CH/nanoclay and WH/CH/nanoclay. Despite having 70%, amylose content in HACS/CH/nanoclay has lower TS as compared to CS/CH/nanoclay. The reason attributed to this is the drastic increase in amylose content that lead to the weakening of intra-molecular attraction between the polymer chains due to more exposure of unbound free-OH groups. These results support that CS/CH/nanoclay has a higher degree of crystallinity as shown in XRD (Fig. 1) and lower MC (Fig. 2a).
Elongation at the break (EB) estimates both extensibility and flexibility in packaging films. Figure 3b has presented the influence of amylose-amylopectin ratios on EB for starches incorporated with chitosan and reinforce with nanoclay. The average EB values of the biodegradable blend film behaved inversely to the tensile strength value decreasing in the order 92.46% (CH/nanoclay) 86.81% (PS/CH/nanoclay) > 79.69% (WH/CH/nanoclay) > 66.87% (HACS/CH/nanoclay) > 59.82% (CS/CH/nanoclay). It is interesting to note that on increasing amylose content (from 20 to 28%), EB as well as moisture content decreased which was probably due to –OH groups of amylose bound with NH3+ of chitosan with formation of strong intermolecular hydrogen bonding interactions in the presence of nanoclay (Na-MMT). While in case of higher moisture content, the effect of reinforcement is weak which is due to interaction of water with polymer matrix. Aforementioned, the increase in amylose improves the film strength along with decrease in its flexibility. In case of 70% amylose content (HACS/CH/ nanoclay) which shows more flexibility and chain mobility of polymers than CS/CH/nanoclay, the behaviour could be explained by the presence of high moisture content and exposure to free –OH groups of starch that interact with water leading to decrease in the rigidity of the polymer network. According to the result of high TS and low EB, CS/CH/nanoclay with 28% amylose content is optimum with its high crystallinity (Fig. 1d) and less WVP properties (Fig. 2c).
Dynamic mechanical thermal properties
The thermo-mechanical properties determine storage modulus and tan delta of starch-CH reinforced with nanoclay bionanocomposite films are presented in Fig. 4a, b.
Fig. 4.

a Influence of amylose-amylopectin ratio on storage modulus of starch based bionanocomposite films. b Influence of amylose-amylopectin ratio on tan delta of starch based bionanocomposite films
The storage modulus of CS/CH/nanoclay film is found to be higher than PS/CH/nanoclay, WH/CH/nanoclay, HACS/CH/nanoclay and CH/nanoclay films as presented in Fig. 4a. The stiffness of the composite film measure from storage modules. The stiffness of CS/CH/nanoclay is higher due to a strong inter-chain bond between CS and CH existing with nanoclay. This implies that stiffness of the polymer material in CS/CH/nanoclay is high as it restrains the chain mobility leading to a higher value of storage modulus of the films.
Figure 4b. has shown that the tan delta curve as a function of temperature. Glass transition temperature (Tg) depend upon presence of water molecule in the polymer which have significant role in the molecular structure that show hydrophilic nature as well as arrangement of inter-and intra-molecular forces in the polymer matrix. The value of Tg increases with polymer–polymer interaction while the value decreases with an increase in moisture content because of polymer-water-polymer interactions resulting in more flexible polymeric chains (Aguirre-Loredo et al. 2016). The tan delta is sensitive to the molecular motions and its peak is related to the glass transition temperature. The curve of thermoplastic starch (TPS) has revealed two thermal transitions (Tg) because of their two distinct parts namely starch-poor part (lower) and starch-rich part (upper). The upper part is generally regarded as glass transition temperature (Tg) of TPS materials. The upper transition for CH/nanoclay, PS/CH/nanoclay, WH/CH/nanoclay, CS/CH/nanoclay and HACS/CH/nanoclay is 36.5 °C, 68.3 °C, 78.9 °C, 88.2 °C and 83.5 °C respectively in the direction (CH/nanoclay < PS/CH/nanoclay < WH/CH/nanoclay < HACS/CH/nanoclay < CS/CH/nanoclay). In case of CS/CH/nanoclay reinforced with nanoclay particles that attribute to highest Tg because of the restricted rotational motion in polymer matrix leads to decrease in distance between adjacent chains of polymer thereby reducing the free volume thus, larger crystal domain with stronger strength is formed. On the other hand, lower Tg of PS/CH/nanoclay, WH/CH/nanoclay and HACS/CH/nanoclay than CS/CH/nanoclay is due to their higher free volume resulting in higher molecular mobility. This is in conformity with the result obtained in case of MC as shown in Fig. 2a.
Fourier transforms infrared spectroscopy
ATR-FTIR spectroscopy is a very powerful tool for examining the interaction of the three components of starch, chitosan and nanoclay in composite films is shown in Fig. 5. The broad peak in between 3600–3000 cm−1 is the stretching vibration of O–H and –NH due to the presence of chitosan (CH) film. The broad band observed around 3350–3365 cm−1 was associated with free intermolecular O–H stretching present in the starch granules and to the absorption of water during the gelatinization of the starch–chitosan– nanoclay mixture used for film preparation (Nagar et al. 2020). There is peak in the region at 2919 cm−1 attributed to the C–H stretching. The two characteristic peaks have been observed at 1640 cm−1 and 1580 cm−1 in pure chitosan film are due to the presence of a carbonyl group, C=O stretching vibrations (amide I) and NH bending (amide II) of pure chitosan film, respectively (Fig. 5a). The peak located at 1408 and 1340 cm−1 is attributed to C–H in plane bending. Characteristic peaks are appearing in region 1040–1066 cm−1 stretching vibration of C–O–C of glucosidic bonds (Chai and Isa 2013; Pinotti et al. 2007; Raucci et al. 2015). The Si–O and Al–OH stretching vibrations in CH/nanoclay were is found at 1112 cm−1, 911 cm−1 and 680 cm−1, respectively (Fig. 5a).
Fig. 5.
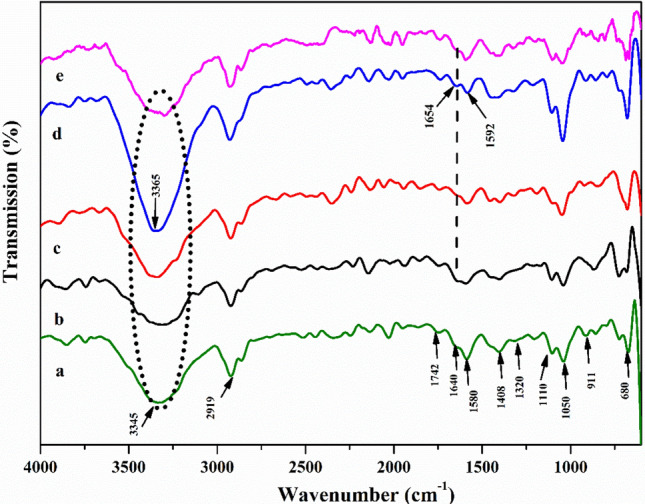
FTIR pattern of different amylose-amylopectin ratio of starch based bionanocomposite films a CH/nanoclay, b PS/CH/nanoclay, c WH/CH/nanoclay, d CS/CH/nanoclayand, e HACS/CH/nanoclay
It is possible that starch mixed CH-nanoclay in composite films, chitosan (CH) containing functional groups of –OH, –NH2, and C=O can be interacted with starch functional group –OH and are reinforced with silicate (Si–O–Si) and aluminium hydroxide (Al–OH) deformed with aluminates present on the surface of nanoclay bounded through intermolecular hydrogen bonding. The broad peak has been displaced from 3345 cm−1 to 3365 cm−1 and 3350 cm−1 showing to a higher wavelength that attributed the formation of intermolecular hydrogen bonding with chitosan. The peak for CS/CH/nanoclay and HACS/CH/nanoclay with high intensity and increased peak area improved its compatibility (Fig. 5d, e). However, –OH peak has been moved to a lower wavenumber for PS/CH/nanoclay and WH/CH/nanoclay indicating weaker intermolecular interactions between starch and CH films (Fig. 5b, c). In the spectrum of CS/CH/nanoclay bionanocomposite films, main peak related to the amino group N–H bending peak (amide I) has been shifted from 1580 to 1592 cm−1 toward higher wavenumber. The small peak shifted from 1640 cm−1 to 1654 cm−1 is associated with C=O stretching (amide II) with high intensity for CS/CH/nanoclay among all other bionanocomposite films, suggesting strong intermolecular hydrogen bonding between –OH functional group of corn starch (CS) and C=O, –NH2 functional group of chitosan (CH) (Fig. 5d). The broad area and peak intensity has been increased in CS/CH/nanoclay related to aluminate and silicate layers of Na-MMT (1110 cm−1, 911 cm−1 and 680 cm−1) compared with PS/CH/nanoclay, WH/CH/nanoclay and HACS/CH/nanoclay as presented in Fig. 5b–e. This is because stronger intermolecular hydrogen bonding networks between CH and starch polymer chains as it results in higher intercalation with nanoclay. This is confirmed with intensity of crystalline peak increased in XRD pattern of CS/CH/nanoclay (Fig. 1). Similar hydrogen bonding has been reported by Ji et al. (2017) between hydroxyl group of corn starch and amino group of chitosan in bionanocomposite films.
Morphological and anti-fungal properties of bionanocomposite film
The microstructure of the starch–CH–nanoclaybionanocomposite films has been examined using SEM as shown in Fig. 6a. Results exhibit that all the bionanocomposite film showed a slight rough surface without any phase separation (Fig. 6a). These images has revealed that the introduction of nanoclay has enhanced the biopolymeric interaction in CS/CH/nanoclay than PS/CH/nanoclay,WH/CH/nanoclay,HACS/CH/nanoclay,CH/nanoclay. In micrograph of CS/CH reinforcement with Na–MMT films exhibit a homogenous surface due to its better miscibility as a result of interfacial adhesion between amylose of corn starch and CH which is due to extensive dispersion of nanoclay leading to strong intermolecular interactions in the polymer chain. Resulting CS/CH/nanoclay has shown to be relatively more smooth (plain without any pores or cracks), continuous, compact and maintain good structural integrity due to its better compatibility which is in accordance with XRD (Fig. 1 d) and FTIR pattern (Fig. 5d). While CH/nanoclay, PS/CH/nanoclay, WH/CH/nanoclay and HACS/CH/nanoclay impregranted with nanoclay bionanocomposite film in absense of large agglomerates and smooth surfaces are observed as a result of lesser chance interactions thus forming a weak interaction and adhesion on the interface with filler and matrix (Fig. 6a).
Fig. 6.
a SEM images of starch–CH–nanoclaybionanocomposite films, b comparison of bread quality (spoilage) sample packed in CS/CH/nanoclaybionanocomposite films with low density polyethylene films (control) on 1st day and 5th days
CS/CH/nanoclay film has been evaluated for anti-fungal activity by direct contact with bread slice sample. Uniform bread slice samples of size 2 × 2 cm has been sealed in direct contact with CS/CH/nanoclay bionanocomposite films. Sample packed in low-density polyethylene (LDPE) serve as the control. All of the samples has been stored at 25 °C at 59% RH. Comparative study of bread sample packed by CS/CH/nanoclay bionanocomposite films and low density polyethylene (LDPE) films on 1st day and 5th days is shown in Fig. 6b.
Because of impoved water barrier and better mechanical properties, the CS/CH/nanoclay bionanocomposite film has been considered for bread packaging. The LDPE film serve as control. The fungal growth has been observed on the 5th day in samples that are packed with LDPE films. But, in the case of samples packed with CS/CH/nanoclay films, the fungal growth has been inhibited until 20 days (Fig. 6b). Investigation shows that the presence of chitosan disrupts the membrane leading to the leak of cellular protein by binding its positively charged amino (–NH3+) group to negatively charged carboxylate (–COO–) group on the surface of microbial cell wall and retards its growth exhibiting anti-fungal activity our results also corroborate well with a previous study by Singh et al. (2015). Also, it may be due to that the CS/CH/nanoclay film structure improve water barrier properties and thus delayed the proliferation of fungal growth. Therefore, this bionanocomposite film can find potential applications in active food packaging to improve the quality of food during storage thereby extending the shelf life of products ensuring food safety.
Conclusion
The influence of different amylose-amylopectin ratios of starches on functional properties (crystallinity, water barrier, mechanical, morphological and anti-fungal properties) were comprehensively studied. The results obtained in the current study showed that CS (28:72) amylose-amylopectin ratio based films improved the higher TS, lower WVP and higher Tg. FTIR and XRD results confirmed that CS/CH/nanoclay films formed hydrogen bonds between corn starch and chitosan reinforced with nanoclay. These prepared films exhibited proper molecular miscibility, which raised in crystallinity peak intensity and showed heat-sealing properties. The prepared CS/CH/nanoclay bionanocomposite films exhibited anti-fungal activity in a stored bread sample at 25 °C, 59% RH for 20 days. These results showed that the prepared films could potential be exploited as bionanocomposite films for food packaging applications.
Acknowledgments
The author is grateful to Ministry of Human Resource Development, Govt. of India for providing financial support of this research. The author wish to thank Central Instrument Facility (CIF) and Department of Chemical Engineering, Indian Institute of Technology Guwahati and DST-FIST, Govt. of India for providing instruments facilities to carry out the research work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguirre-Loredo RY, Rodríguez-Hernández AI, Morales-Sánchez E, Gómez-Aldapa CA, Velazquez G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016;196:560–566. doi: 10.1016/j.foodchem.2015.09.065. [DOI] [PubMed] [Google Scholar]
- Alekseeva OV, Rodionova AN, Bagrovskaya NA, Agafonov AV, Noskov AV. Hydroxyethyl cellulose/bentonite/magnetite hybrid materials: structure, physicochemical properties, and anti-fungal activity. Cellulose. 2017;24:1825–1836. [Google Scholar]
- Arvanitoyannis I, Biliaderis CG, Ogawa H, Kawasaki N. Biodegradable films made from low-density polyethylene (LDPE), rice starch and potato starch for food packaging applications. Carbohydr Polym. 1998;36:89–104. [Google Scholar]
- ASTM (1996) Standard test methods for tensile properties of thin plastic sheeting, D882-91. Annual book of ASTM. American Society for Testing and Material, Philadelphia
- Bangyekan C, Aht-Ong D, Srikulkit K. Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr Polym. 2006;63:61–71. [Google Scholar]
- Bertoft E, Blennow A (2016) Structure of potato starch. In: Advances in potato chemistry and technology. Academic Press, pp 57–73
- Chai MN, Isa MI. The oleic acid composition effect on the carboxymethyl cellulose based biopolymer electrolyte. JCPT. 2013;3:1–4. [Google Scholar]
- Farahnaky A, Saberi B, Majzoobi M. Effect of glycerol on physical and mechanical properties of wheat starch edible films. J Texture Stud. 2013;44:176–186. [Google Scholar]
- Frank K, Garcia CV, Shin GH, Kim JT. Alginate biocomposite films incorporated with cinnamon essential oil nanoemulsions: physical, mechanical, and antibacterial properties. Int J Polym Sci. 2018;2018:1–8. [Google Scholar]
- Feng F, Liu Y, Zhao B, Hu K. Characterization of half N-acetylated chitosan powders and films. Procedia Eng. 2012;27:718–732. [Google Scholar]
- Gernat C, Radosta S, Anger H, Damaschun G. Crystalline parts of three different conformations detected in native and enzymatically degraded starches. Starch Stärke. 1993;45:309–314. [Google Scholar]
- Jahit IS, Nazmi NN, Isa MI, Sarbon NM. Preparation and physical properties of gelatin/CMC/chitosan composite films as affected by drying temperature. Int Food Res J. 2016;23:1068–1074. [Google Scholar]
- Japan Standards Association (1976) Testing method for determination of the water vapour transmission rate of moisture-proof packaging materials JIS Z 0208.
- Ji N, Qin Y, Xi T, Xiong L, Sun Q. Effect of chitosan on the antibacterial and physical properties of corn starch nanocomposite films. Starch Stärke. 2017;69:1600114. [Google Scholar]
- Kumar Y, Singh L, Sharanagat VS, Patel A, Kumar K. Effect of microwave treatment (low power and varying time) on potato starch: microstructure, thermo-functional, pasting and rheological properties. Int J Biol Macromol. 2020;155:27–35. doi: 10.1016/j.ijbiomac.2020.03.174. [DOI] [PubMed] [Google Scholar]
- Kurt A, Kahyaoglu T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr Polym. 2014;104:50–58. doi: 10.1016/j.carbpol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Mali S, Grossmann MV, García MA, Martino MN, Zaritzky NE. Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J Food Eng. 2006;75:453–460. [Google Scholar]
- Manek RV, Builders PF, Kolling WM, Emeje M, Kunle OO. Physicochemical and binder properties of starch obtained from Cyperus esculentus. AAPS Pharm Sci Tech. 2012;13:379–388. doi: 10.1208/s12249-012-9761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mua JP, Jackson DS. Relationships between functional attributes and molecular structures of amylose and amylopectin fractions from corn starch. J Agric Food Chem. 1997;45:3848–3854. [Google Scholar]
- Murphy P. Starch. In: Phillips GO, Williams PA, editors. Handb hydrocolloids. Cambridge, England: Woodhead Publishing Limited; 2000. pp. 41–65. [Google Scholar]
- Nagar M, Sharanagat VS, Kumar Y, Singh L. Development and characterization of elephant foot yam starch–hydrocolloids based edible packaging film: physical, optical, thermal and barrier properties. J Food Sci Technol. 2020;57(4):1331–1341. doi: 10.1007/s13197-019-04167-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SB, Alummoottil NJ, Moothandasserry SS. Chitosan-konjac glucomannan-cassava starch-nanosilver composite films with moisture resistant and antimicrobial properties for food-packaging applications. Starch Stärke. 2017;69:1600210. [Google Scholar]
- Ozcalik O, Tihminlioglu F. Barrier properties of corn zein nanocomposite coated polypropylene films for food packaging applications. J Food Eng. 2013;114:505–513. [Google Scholar]
- Pagno CH, de Farias YB, Costa TM, de Oliveira RA, Flôres SH. Synthesis of biodegradable films with antioxidant properties based on cassava starch containing bixin nanocapsules. J Food Sci Technol. 2016;53:3197–3205. doi: 10.1007/s13197-016-2294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez S, Bertoft E. The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch Stärke. 2010;62:389–420. [Google Scholar]
- Pinotti A, García MA, Martino MN, Zaritzky NE. Study on microstructure and physical properties of composite films based on chitosan and methylcellulose. Food Hydrocoll. 2007;21:66–72. [Google Scholar]
- Raucci MG, Alvarez-Perez MA, Demitri C, Giugliano D, De Benedictis V, Sannino A, Ambrosio L. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J Biomed Mater Res Part A. 2015;103:2045–2056. doi: 10.1002/jbm.a.35343. [DOI] [PubMed] [Google Scholar]
- Ray SS, Bousmina M. Biodegradable polymers and their layered silicate nanocomposites: in greening the 21st century materials world. Prog Mater Sci. 2005;50:962–1079. [Google Scholar]
- Ren L, Yan X, Zhou J, Tong J, Su X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int J Biol Macromol. 2017;105:1636–1643. doi: 10.1016/j.ijbiomac.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Rindlav-Westling Å, Stading M, Gatenholm P. Crystallinity and morphology in films of starch, amylose and amylopectin blends. Biomacromol. 2002;3(1):84–91. doi: 10.1021/bm010114i. [DOI] [PubMed] [Google Scholar]
- Rhim JW, Park HM, Ha CS. Bio-nanocomposites for food packaging applications. Prog Polym Sci. 2013;38:1629–1652. [Google Scholar]
- Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (arenga pinnata) starch for food packaging. J Food Sci Technol. 2016;53:326–336. doi: 10.1007/s13197-015-2009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Chatli M, Sahoo J. Development of chitosan based edible films: process optimization using response surface methodology. J Food Sci Technol. 2015;52:2530–2543. doi: 10.1007/s13197-014-1318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira G, Bras J, Dufresne A. Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers. 2010;2:728–765. [Google Scholar]
- Souza AC, Benze RF, Ferrão ES, Ditchfield C, Coelho AC, Tadini CC. Cassava starch biodegradable films: influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT Food Sci Technol. 2012;46:110–117. [Google Scholar]
- Sun Q, Xu Y, Xiong L. Effect of microwave-assisted dry heating with xanthan on normal and waxy corn starches. Int J Biol Macromol. 2014;68:86–91. doi: 10.1016/j.ijbiomac.2014.04.032. [DOI] [PubMed] [Google Scholar]
- Tan YM, Lim SH, Tay BY, Lee MW, Thian ES. Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater Res Bull. 2015;69:142–146. [Google Scholar]
- Xu YX, Kim KM, Hanna MA, Nag D. Chitosan-starch composite film: preparation and characterization. Ind Crops Prod. 2005;21:185–192. [Google Scholar]
- Yu K, Wang Y, Xu Y, Guo L, Du X. Correlation between wheat starch annealing conditions and retrogradation during storage. Czech J Food Sci. 2016;34:79–86. [Google Scholar]
- Zhang QX, Yu ZZ, Xie XL, Naito K, Kagawa Y. Preparation and crystalline morphology of biodegradable starch/clay nanocomposites. Polymer. 2007;48:7193–7200. [Google Scholar]



