Abstract
Bracatinga (Mimosa scabrella Bentham) honeydew honey is a Brazilian dark honey in increasing international appreciation. In this sense, the knowledge of its composition and potential biological properties becomes indispensable. In the present study, the physicochemical characteristics, including mineral and phenolic composition, and the scavenging, reducing, and antimicrobial proprieties of bracatinga honeydew honey (bhh) from five different geographical locations, were investigated. Bhh proved to be a potential functional food due to its high content of minerals (up to 6395 mg kg−1) and phenolic compounds (up to 2393 µg 100 g−1) and high scavenging and reducing activities. High antimicrobial activity against four bacterial strains, with minimum inhibitory concentration values ranging from 10 to 60%, were also found. Additionally, through principal component analysis, partial discrimination of bhh was observed according to the geographical location, which favored the separation of samples from Lages, and mainly due to the presence of nectar in this honey, which was proposed for the samples from Bom Retiro.
Supplementary information
The online version of this article (10.1007/s13197-020-04937-x) contains supplementary material, which is available to authorized users.
Keywords: Mimosa scabrella Bentham, Phenolic compounds, Mineral, Identity and quality parameters, Antibacterial activity, Antioxidant activity
Introduction
Honey is a sweet product whose major compounds include sugars and water, and minor components that englobe phenolic compounds, minerals, organic acids, proteins, amino acids, enzymes, among others. The minor constituents play a crucial role in honey’s therapeutic properties and have been considered strongly dependent on many factors, including botanical and geographical origin, processing and storage conditions, and bees species (De-Melo et al. 2018; Bergamo et al. 2019a).
In recent years, honey’s interest increased and focused on its bioactive compounds and health benefits, including antioxidant, anti-inflammatory, wound-healing, and antimicrobial activities (Seraglio et al. 2019). Among these properties, special attention has been given to its antioxidant and antimicrobial potential. Its antioxidant activity is strongly related to the presence of compounds with reducing and/or scavenging proprieties, such as phenolic compounds and minerals. In contrast, its antimicrobial activity has been attributed to some specific characteristics, such as acidity, osmolality, generation of hydrogen peroxide, and phenolic compounds which are capable of inhibiting bacterial growth (Can et al. 2015; Lukasiewicz et al. 2015; Deng et al. 2018).
In this context, honeydew honeys (elaborated from secretions of living parts of plants or excretions of plant-sucking insects) have gained greater attention of consumers and the food industry than blossom honeys (produced from the nectar of flowers) (European Commission 2002). It is because honeydew honey commonly has lower glucose and fructose contents and higher levels of minerals, pH, electrical conductivity, proteins, phenolic compounds, and oligosaccharides than blossom honeys. Also, honeydew honey frequently has a darker color, intense flavor, and higher bioactive potential than blossom honeys (Can et al. 2015; De-Melo et al. 2018; Bergamo et al. 2018, 2019a; Seraglio et al. 2019).
Bhh is inserted in the European market and corresponds to a Brazilian dark honey produced by Apis mellifera bees from sugary excretions released by Tachardiella sp. or Stigmacoccus paranaensis plant-sucking insects which live in the bracatinga trees using its phloem as food (Bergamo et al. 2019a). Although European consumers appreciate this honeydew honey, few studies related to its physicochemical characteristics, bioactive composition, and potential health-promoting properties are still found. Despite the few studies, high levels of bioactive compounds and reducing and scavenging activities have been reported for bhh, suggesting promising biological proprieties (Seraglio et al. 2017; Bergamo et al. 2018, 2019a). Considering these promising biological potentials and the fact that, to the best of our knowledge, the antimicrobial activity of bhh has never been reported in the literature so far, the investigation of this propriety becomes relevant.
In this context, the present study aimed to investigate the physicochemical characteristics, the content of minerals and phenolic compounds, and the scavenging, reducing, and antimicrobial activities of bhh from five different geographical locations.
Materials and methods
Honeydew honey samples
Nineteen bracatinga (Mimosa scabrella Bentham) honeydew honeys were harvested in 2014, between February and June, by local professional beekeepers in five geographical locations of the Santa Catarina state, Brazil: Bocaina do Sul (n = 5; BS1, BS2, BS3, BS4, BS5); Urupema (n = 4; UP1, UP2, UP3, UP4); Lages (n = 4; LG1, LG2, LG3, LG4); Bom Retiro (n = 3; BR1, BR2, BR3); and Urubici (n = 3; UB1, UB2, UB3). Honeycombs were transported (5 ± 2 °C) until the laboratory, drained, homogenized, centrifuged, and frozen (− 20 ± 2 °C). Before analysis, samples were thawed and homogenized.
Reagents
All chemicals were of analytical grade, and ultrapure water was used (Milli-Q Simplicity® UV system, Millipore Corporation, Saverne, France). Ascorbic acid, potassium iodide, hydrochloric acid, sodium acetate, lactic acid, and sodium chloride were purchased from Vetec (Duque de Caxias, Brazil). Sodium hydroxide, sodium dodecyl sulfate (SDS), disodium tetraborate decahydrate (STB), acetic acid, and sodium carbonate were acquired from Merk (Rio de Janeiro, Brazil), and Mueller–Hinton broth (MHB) was obtained from Kasvi (Italy). HPLC-grade formic acid was acquired from J. T. Baker (Phillipsburg, NJ, USA), and HPLC-grade dimethyl sulfoxide, methanol, and acetonitrile were purchased from Merck (Darmstadt, Germany).
Folin–Ciocalteu reagent, 5-(hydroxymethyl)furfural (5-HMF), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), cetyltrimethylammonium bromide (CTAB), imidazole, caffeine, sorbic acid, soluble starch, ferric chloride, ferrous sulfate heptahydrate, phenolic compounds (apigenin, isorhamnetin, pinobanksin, ferulic acid, sinapic acid, p-aminobenzoic acid, p-coumaric acid, 4-methylumbelliferone, vanillic acid, rutin, naringin, (+)-catechin, sinapaldehyde, caffeic acid, chlorogenic acid, coniferaldehyde, syringaldehyde, chrysin, hesperidin, syringic acid, kaempferol, naringenin, (−)-epigallocatechin gallate, (−)-epicatechin, pinocembrin, galangin, salicylic acid, quercetin, gallic acid, benzoic acid, 3,4-dihydroxybenzoic acid, luteolin), minerals (K+, Ca2+, Na+, Mg2+, Mn2+, Ba2+), and carbohydrates (d-fructose, d-(+)-glucose monohydrate, sucrose) were obtained from Sigma-Aldrich (Saint Louis, MO, USA).
Physicochemical parameters
Moisture content (g 100 g−1) was determined according to AOAC method 969.38 (AOAC 2005) in a refractometer Abbe Tropenmodell I (Carl Zeiss Jena, Germany) at 20 °C.
Contents of fructose, glucose, and sucrose were determined according to the method previously validated proposed by Rizelio et al. (2012b) in a capillary electrophoresis system (Agilent Technologies, model 7100, Germany) equipped with a diode array detector (CE-DAD). Results were expressed in g 100 g−1.
Determination of pH and free acidity was performed according to AOAC method 962.19 (AOAC 2005). In honey solution (10 g of honey in 75 mL of ultrapure water), pH was determined using a digital pH meter MD-20 (Digimed, São Paulo, Brazil). This honey solution was titrated for free acidity until pH 8.5 with NaOH 0.05 mol L−1, and the result expressed in mEq kg−1.
Diastase activity was determined quantitatively (in Shade units) according to AOAC method 920.180 (AOAC 2005) using a UV–visible spectrophotometer (Spectro Vision SB 1810-60 S, Beijing, China).
5-HMF content was determined in a CE-DAD system according to the method previously validated proposed by Rizelio et al. (2012a). Results were expressed in mg kg−1.
Electrical conductivity was determined in a conductivity meter Tec-4MP model (Tecnal, São Paulo, Brazil) in 20% (w/v; honey:water) honey solution at 25 °C (Bogdanov et al. 1999). Results were expressed in mS cm−1.
Chromatic analysis (L*, a*, b*) was performed in a colorimeter (Chroma Meter CR-400, Konica Minolta, Tokyo, Japan) in honeys without dilution (Bergamo et al. 2019b).
Minerals
Contents of K+, Na+, Ca2+, Mg2+, and Mn2+ were determined in a CE-DAD system according to the method proposed by Seraglio et al. (2017). This method was analytically validated for bhh in terms of system suitability, linearity, matrix effect, precision, accuracy, limit of detection, and limit of quantification (Online Resource 1). Results were expressed in mg kg−1.
Phenolic compounds
Phenolic compounds were determined according to the method previously validated proposed by Seraglio et al. (2016) in a liquid chromatography system (Agilent Technologies, model 1290, Wilmington, DE, USA) coupled to a hybrid quadrupole linear ion trap mass spectrometer QTRAP® 5500 (AB Sciex, Foster City, CA, USA) and an electrospray ionization source. Results were expressed in μg 100 g−1.
Scavenging and reducing activities in vitro
For the determination of scavenging and reducing activities, samples (0.5 ± 0.01 g) were diluted with ultrapure water, according to Bergamo et al. (2019a).
DPPH scavenging activity was determined according to Kim et al. (2002). Scavenging ability was calculated as inhibition (%) = [1 − (absorbance samplet=30 min/absorbance DPPH solutiont=0 min) × 100] and the results presented as mg ascorbic acid equivalents (AAE) 100 g−1.
Total reducing capacity was assessed by the Folin–Ciocalteu method (Singleton and Rossi 1965). Results were presented as mg gallic acid equivalent (GAE) 100 g−1.
Ferric reducing antioxidant power (FRAP) was assessed by the method proposed by Bertoncelj et al. (2007). Results were expressed as μmol Fe2+ 100 g−1.
Antimicrobial activity in vitro
Two Gram-positive (Staphylococcus aureus ATCC 25923 and Listeria monocytogenes ATCC 19111) and two Gram-negative (Escherichia coli ATCC 8739 and Salmonella Typhimurium ATCC 14028) bacterial strains were selected for antimicrobial assay.
Minimum inhibitory concentration (MIC) of honey samples was determined according to Ghramh et al. (2018), with some modifications. In sterilized test tubes, each honey sample was diluted with MHB to give the final concentrations of 80, 60, 40, 10, and 5% (w/v) in the final volume of 1 mL. An aliquot of 10 µL of bacterial suspension adjusted to 108 colony-forming units (CFU)/mL by dilution with MHB was inoculated in the tubes and then incubated at 35 ± 1 °C for 24 h. MIC was defined as the lowest concentration of honey inhibiting the bacterial growth and was determined by visual inspection (turbidity) and sediment formation after centrifugation.
Statistical analysis
Assays were performed in three independent experiments (n = 3), and the data presented as mean ± standard deviation. Shapiro–Wilk test was employed to evaluate the data normality, and the Kruskal–Wallis non-parametric test was used to multiple comparisons between the sample groups. The principal component analysis was used to explain and interpret the interdependence of data. Differences were considered statistically significant at the 5% level (p < 0.05). Statistica software (Statsoft Inc., Tulsa, OK, USA) was used to perform the statistical analyses.
Results and discussion
Physicochemical parameters
Moisture and sugars are important indicators of honey maturity, and their contents in bhh are shown in Table 1 (and Online Resource 2).
Table 1.
Moisture, carbohydrates quantified, and other physicochemical parameters in bracatinga (Mimosa scabrella Bentham) honeydew honey from different geographical locations
| Parameter | Content | Region | ||||
|---|---|---|---|---|---|---|
| Bocaina do Sul (n = 5) | Urupema (n = 4) | Lages (n = 4) | Bom Retiro (n = 3) | Urubici (n = 3) | ||
| Moisture (g 100 g−1) | Minimum | 16.4 | 16.0 | 15.0 | 16.6 | 15.7 |
| Maximum | 19.2 | 18.6 | 16.9 | 18.9 | 18.1 | |
| Mean ± SD | 17.7 ± 1.22a | 17.4 ± 1.32a | 15.9 ± 0.88a | 17.6 ± 1.17a | 16.8 ± 1.21a | |
| Fructose (g 100 g−1) | Minimum | 35.1 | 33.1 | 37.1 | 35.1 | 36.6 |
| Maximum | 46.2 | 44.8 | 52.2 | 38.7 | 40.0 | |
| Mean ± SD | 38.3 ± 4.52a | 37.9 ± 4.95a | 45.8 ± 6.31a | 36.7 ± 1.84a | 38.3 ± 1.70a | |
| Glucose (g 100 g−1) | Minimum | 24.6 | 24.2 | 25.3 | 25.9 | 23.7 |
| Maximum | 31.7 | 32.2 | 30.7 | 29.6 | 27.3 | |
| Mean ± SD | 26.9 ± 2.81a | 28.0 ± 3.28a | 28.4 ± 2.39a | 27.4 ± 1.97a | 26.0 ± 2.02a | |
| F + G (g 100 g−1) | Minimum | 77.9 | 57.4 | 62.4 | 61.0 | 60.3 |
| Maximum | 59.7 | 77.0 | 82.1 | 68.2 | 67.3 | |
| Mean ± SD | 65.3 ± 7.27a | 66.0 ± 8.16a | 74.2 ± 8.50a | 64.0 ± 3.75a | 64.3 ± 3.62a | |
| F/G ratio | Minimum | 1.37 | 1.32 | 1.47 | 1.31 | 1.41 |
| Maximum | 1.48 | 1.39 | 1.75 | 1.36 | 1.54 | |
| Mean ± SD | 1.42 ± 0.05a,b,c | 1.35 ± 0.03b,c | 1.61 ± 0.13a | 1.34 ± 0.03b,c | 1.47 ± 0.07a,b,c | |
| G/M ratio | Minimum | 1.32 | 1.30 | 1.54 | 1.49 | 1.43 |
| Maximum | 1.72 | 1.74 | 1.94 | 1.60 | 1.74 | |
| Mean ± SD | 1.53 ± 0.17a | 1.61 ± 0.21a | 1.79 ± 0.17a | 1.55 ± 0.06a | 1.55 ± 0.16a | |
| Free acidity (mEq kg−1) | Minimum | 56.3 | 60.0 | 50.4 | 57.5 | 52.5 |
| Maximum | 61.9 | 70.1 | 63.8 | 60.7 | 55.8 | |
| Mean ± SD | 58.1 ± 2.33a,b | 64.7 ± 4.14a | 54.5 ± 6.31a,b | 58.7 ± 1.74a,b | 54.6 ± 1.80b | |
| pH | Minimum | 4.70 | 4.27 | 4.48 | 4.02 | 4.62 |
| Maximum | 5.25 | 4.83 | 4.94 | 4.26 | 4.96 | |
| Mean ± SD | 4.94 ± 0.22a | 4.56 ± 0.22a,b | 4.78 ± 0.21a,b | 4.14 ± 0.12b | 4.78 ± 0.17a,b | |
| 5-HMF (mg kg−1) | Minimum | < LOD | < LOD | < LOD | < LOD | < LOD |
| Maximum | < LOD | < LOD | < LOD | < LOD | < LOD | |
| Mean ± SD | – | – | – | – | – | |
| Diastase (Schade units) | Minimum | 21.1 | 23.2 | 25.7 | 20.9 | 15.5 |
| Maximum | 41.4 | 37.9 | 40.4 | 28.1 | 25.6 | |
| Mean ± SD | 28.3 ± 8.62a | 30.1 ± 6.05a | 31.4 ± 6.56a | 23.8 ± 3.78a | 21.6 ± 5.37a | |
| Electrical conductivity (mS cm−1) | Minimum | 1.48 | 1.29 | 1.34 | 0.79 | 1.29 |
| Maximum | 1.74 | 1.47 | 1.71 | 1.02 | 1.41 | |
| Mean ± SD | 1.59 ± 0.10a | 1.39 ± 0.07a,b | 1.53 ± 0.17a,b | 0.90 ± 0.12b | 1.36 ± 0.06a,b | |
| Color—L* | Minimum | 22.2 | 25.9 | 24.9 | 30.6 | 27.6 |
| Maximum | 33.1 | 29.3 | 29.3 | 33.4 | 28.7 | |
| Mean ± SD | 26.1 ± 4.76a | 28.4 ± 1.64a | 26.4 ± 1.98a | 32.4 ± 1.54a | 28.0 ± 0.64a | |
| Color—a* | Minimum | 11.6 | 17.3 | 15.5 | 14.6 | 17.7 |
| Maximum | 17.1 | 19.6 | 19.6 | 17.1 | 18.4 | |
| Mean ± SD | 14.3 ± 2.27b | 18.8 ± 1.00a | 17.5 ± 1.68a,b | 15.7 ± 1.27a,b | 18.1 ± 0.35a,b | |
| Color—b* | Minimum | 10.3 | 16.3 | 14.0 | 24.3 | 19.5 |
| Maximum | 29.6 | 23.1 | 22.4 | 29.6 | 21.6 | |
| Mean ± SD | 17.2 ± 8.38a | 21.0 ± 3.17a | 17.4 ± 3.57a | 27.5 ± 2.82a | 20.9 ± 1.21a | |
SD standard deviation, F fructose, G glucose, M moisture, 5-HMF 5-hydroxymethylfurfural, LOD limit of detection (for 5-HMF: 3.37 mg kg−1); L* lightness, a* intensity of green (negative values) and red colors (positive values), b* intensity of blue (negative values) and yellow colors (positive values); (−) not applicable
Different superscript letters in the same row represent statistical (p < 0.05) difference by Kruskal–Wallis test
In all samples, the content of moisture (up to 19.2 ± 0.01 g 100 g−1) was below the maximum limit of 20 g 100 g−1 (European Commission 2002), indicating adequate climatic and processing conditions and minimization of possible fermentative processes in these samples (Karabagias et al. 2020).
Reducing sugars fructose and glucose are the main carbohydrates found in honeydew honey (Seraglio et al. 2019). Fructose and glucose contents ranged from 33.1 ± 1.98 to 52.2 ± 0.53 g 100 g−1 and 23.7 ± 2.50 to 32.2 ± 0.14 g 100 g−1, respectively. Their sum (fructose + glucose) was higher than 57 g 100 g−1, following the minimum limit of 45 g 100 g−1 established for honeydew honey (European Commission 2002). Premature harvesting is a factor that can result in high concentrations of sucrose (Seraglio et al. 2019). In this sense, a maximum limit of 5 g 100 g−1 is established for this sugar (European Commission 2002). In all samples, sucrose was below the limit of detection (0.44 g 100 g−1). Therefore, considering the results found for moisture and sugars, it can be suggested that bhh presented adequate maturation.
Low content of glucose favors the slow crystallization of honey. In this sense, fructose/glucose (F/G) and glucose/moisture (G/M) ratios are good estimators of honey crystallization (De-Melo et al. 2018). According to Table 1, all samples presented values lower than 2.0 for G/M ratio and higher than 1.3 for F/G ratio, suggesting slow crystallization of bhh. These results are following the previous report for this honey (Bergamo et al. 2019a).
Free acidity, pH, diastase activity, 5-HMF content, and electrical conductivity are important parameters indicators of deterioration or purity of honeys, and their values in bhh are shown in Table 1 (and Online Resource 2).
Considering the maximum limit of 50 mEq kg−1 established for free acidity (European Commission 2002), all samples were in disagreement with the regulations, presenting values ranging from 50.4 ± 1.68 to 70.1 ± 2.15 mEq kg−1. The high free acidity of honey is frequently related to fermentation processes, resulting mainly in acetic acid formation (Seraglio et al. 2019; Karabagias et al. 2020). However, high values of free acidity (up to 102.2 mEq kg−1) were already reported for honeydew honeys, including bhh (Terrab et al. 2003; Bergamo et al. 2019a; Karabagias et al. 2020). Therefore, high free acidity has been considering a typical characteristic of bhh and not indicative of honey deterioration (Bergamo et al. 2019a).
Although regulatory agencies do not require pH determination, this parameter is extensively related to honey’s microbiological stability (Seraglio et al. 2019). Honey pH seems dependent on factors such as bee species and saccharin source (nectar and exudate). It is observed that pH values commonly range from 3.6 to 4.6 in blossom honeys and from 4.5 to 6.5 in honeydew honeys. It is interesting to note that the honey pH is not directly related to the honey acidity (De-Melo et al. 2018). This fact is also observed in bhh, in which were found high free acidity values (> 50 mEq kg−1) but not very low pH values (4.02 ± 0.06–5.25 ± 0.02). This characteristic may be related to the presence of compounds with buffer capacity, such as salts and minerals (De-Melo et al. 2018). The pH values found in this study agree with those data reported in honeydew honeys from distinct botanical and geographical origins (Halouzka et al. 2016; Karabagias et al. 2020).
Diastase activity of honey is dependent on many factors such as the type of saccharin source and its abundance, age and physiological stage of the bee, bee species, season period, and temperature (De-Melo et al. 2018; Seraglio et al. 2019). In this sense, the diastase activity and the content of 5-HMF are parameters widely evaluated in honeys since they are considered indicators of prolonged storage and/or heating. In these conditions, a decrease in the diastase activity and an increase in the content of 5-HMF are expected (Seraglio et al. 2019; Karabagias et al. 2020). Diastase activity of samples ranged from 15.5 ± 0.28 to 41.4 ± 1.40 Schade units, following the minimum limit of 8 Schade units (European Commission 2002). The content of 5-HMF was below the limit of detection (3.37 mg kg−1), also in agreement with the maximum limit of 40 mg kg−1 (European Commission 2002). Therefore, these results indicate that bhh samples were fresh and not submitted to prolonged storage and/or heating.
Electrical conductivity (EC) is one of the most important parameters for discrimination between honeydew honeys and blossom honeys. This parameter is strongly influenced by the mineral content (De-Melo et al. 2018). Therefore, considering that blossom honeys commonly present mineral content lower than honeydew honeys, it is expected that blossom honeys present EC values below 0.8 mS cm−1 and honeydew honeys and their mixtures showed EC values above 0.8 mS cm−1 (European Commission 2002). Considering that the blend of honeydew honeys with blossom decreases EC values, this parameter can contribute with information on the purity of honeydew honeys (Deng et al. 2018; Bergamo et al. 2019a). As shown in Table 1, all samples, except sample BR2, followed the regulatory limit (≥ 0.8 mS cm−1). However, considering that EC values ranging from 1.07 to 1.78 mS cm−1 were previously reported for bhh and that some dark blossom honeys such as Eucalyptus sp. honey present EC values above 0.8 mS cm−1 (Bergamo et al. 2019a), blossom honey may be present in samples BR1, BR2, and BR3.
Color has a significant impact on honeys’ acceptance and commercial value (Bergamo et al. 2019b). The color parameter L*, a*, and b* of bhh were evaluated (Table 1 and Online Resource 2). Low values of L* (lightness) and red and yellow shades (indicated by positive values of color parameter a* and b*, respectively) mean dark samples. The same tendency was observed for Brazilian and Slovenian honeydew honeys (Bertoncelj et al. 2007; Bergamo et al. 2019b). Dark tones of honeydew honeys are mainly related to the high content of phenolic compounds and minerals, but Maillard reaction products and other color compounds can also contribute (Brudzynski and Kim 2011). Considering that 5-HMF was not detected in the samples, it is possible to assume that minerals and phenolic compounds are probably the most important contributors to this honey’s color.
Considering these physicochemical parameters, significant differences were found for F/G ratio, free acidity, pH, electrical conductivity, and the color parameter a*. Samples from Lages showed the highest mean value of F/G ratio than the samples from Bom Retiro and Urupema. Samples from Bom Retiro also presented the lowest mean value of pH, and electrical conductivity than the samples from Bocaina do Sul. However, the samples from Urupema showed the highest mean values of free acidity, and the color parameter a* compared to the samples from Urubici and Bocaina do Sul, respectively. In this sense, the geographical origin appears to influence specific parameters according to each location. However, the influence of nectar (blossom honeys) on these samples’ composition cannot be neglected.
Minerals
Minerals are an important class of compounds found in honeydew honey. Minerals are involved in biological activities and the botanical and geographical authentication of honeys, since their contents are highly dependent on the soil composition and plant’s absorption capacity (Deng et al. 2018; Bergamo et al. 2018; Seraglio et al. 2019).
In this study, five minerals were investigated in bhh (Table 2 and Online Resource 2). The dominant mineral found in the samples was K+ (up to 6332 ± 124.7 mg kg−1), representing ≥ 95% of the total content of investigated minerals. Magnesium was the second main mineral, followed by Ca2+ and Na+ in all samples, except for LG2, BR1, BR2, and BR3, in which Ca2+ was the second main mineral, followed by Mg2+ and Na+. Manganese was below the limit of detection in all samples (0.37 mg kg−1). The major minerals reported for bhh were K+, followed by Mg2+, Ca2+, and Na+, whereas in blossom honeys were K+, followed by Ca2+, Mg2+, Na+, and, in some samples, Mn2+ (Bergamo et al. 2018). Therefore, the results found for minerals in this study corroborate data observed for EC, reinforcing the hypothesis that the samples BR1, BR2, and BR3 have a percentage of blossom honey in their composition. Also, mineral content was mostly similar to those reported for honeydew honeys from Spanish and New Zealand (Vanhanen et al. 2011; Escuredo et al. 2013).
Table 2.
Content (mg kg−1) of minerals quantified in bracatinga (Mimosa scabrella Bentham) honeydew honey from different geographical locations
| Compound | Content | Region | ||||
|---|---|---|---|---|---|---|
| Bocaina do Sul (n = 5) | Urupema (n = 4) | Lages (n = 4) | Bom Retiro (n = 3) | Urubici (n = 3) | ||
| K+ | Minimum | 5159 | 3789 | 4632 | 3529 | 4564 |
| Maximum | 6332 | 5253 | 6048 | 2323 | 6072 | |
| Mean ± SD | 5723 ± 456.2a | 4597 ± 614.8a,b | 5153 ± 617.3a,b | 3085 ± 662.5b | 5295 ± 755.0a,b | |
| Na+ | Minimum | 2.421 | 8.670 | 4.691 | 8.710 | 2.894 |
| Maximum | 8.442 | 13.31 | 8.782 | 9.870 | 8.133 | |
| Mean ± SD | 4.801 ± 2.310b | 11.01 ± 2.080a | 7.110 ± 1.742a,b | 9.202 ± 0.602a,b | 5.370 ± 2.632a,b | |
| Ca2+ | Minimum | 4.792 | 19.33 | 41.57 | 97.93 | 6.241 |
| Maximum | 34.75 | 38.05 | 124.7 | 139.6 | 29.74 | |
| Mean ± SD | 19.59 ± 12.66a | 26.01 ± 8.621a | 64.07 ± 40.49a | 112.3 ± 23.63a | 15.17 ± 12.73a | |
| Mg2+ | Minimum | 50.90 | 40.38 | 49.55 | 56.42 | 43.33 |
| Maximum | 55.44 | 49.10 | 80.66 | 61.21 | 55.78 | |
| Mean ± SD | 53.53 ± 1.661a,b | 46.47 ± 4.144b | 63.02 ± 13.31a | 59.19 ± 2.481a | 50.81 ± 6.591a,b | |
| Sum | Minimum | 5253 | 3866 | 4846 | 2533 | 4642 |
| Maximum | 6395 | 5352 | 6163 | 3699 | 6140 | |
| Mean ± SD | 5801 ± 445.8a | 4681 ± 623.4a,b | 5287 ± 593.7a,b | 3265 ± 638.1b | 5366 ± 750.2a,b | |
SD standard deviation
Different superscript letters in the same row represent statistical (p < 0.05) difference by Kruskal–Wallis test
Considering these data, a possible influence of geographical origin but also of nectar source (blossom honey) in the mineral content of bhh can be proposed, since samples from Bocaina do Sul showed the highest mean content of K+ and sum of minerals than to the samples from Bom Retiro, but the lowest mean content of Na+ compared to the samples from Urupema. For Mg2+, samples from Lages and Bom Retiro showed the highest mean content compared to the samples from Urupema.
Phenolic compounds
Phenolic compounds are another important class of compounds found in honeydew honey, mainly due to their bioactive and authenticity potential (Can et al. 2015; Lukasiewicz et al. 2015; Seraglio et al. 2019). Thirty-two phenolic compounds belonging to the classes of flavonoids, coumarins, lignin-derived aldehydes, and phenolic acids were investigated in bhh (Table 3 and Online Resource 2).
Table 3.
Content (µg 100 g−1) of individual phenolic compounds quantified in bracatinga (Mimosa scabrella Bentham) honeydew honey from different geographical locations
| Compound | Content | Region | ||||
|---|---|---|---|---|---|---|
| Bocaina do Sul (n = 5) | Urupema (n = 4) | Lages (n = 4) | Bom Retiro (n = 3) | Urubici (n = 3) | ||
| Flavonoids | ||||||
| Apigenin | Minimum | < LOQ | < LOD | < LOD | 2.920 | < LOQ |
| Maximum | 2.510 | < LOQ | 2.762 | 6.913 | 3.050 | |
| Mean ± SD | 0.502 ± 1.123a | – | 0.690 ± 1.382a | 4.670 ± 2.042a | 1.023 ± 1.760a | |
| Chrysin | Minimum | < LOQ | < LOD | < LOQ | < LOD | < LOD |
| Maximum | 6.592 | < LOD | 6.403 | < LOQ | < LOD | |
| Mean ± SD | 2.861 ± 2.860a | – | 2.590 ± 3.152a | – | – | |
| Galangin | Minimum | < LOD | < LOD | < LOD | < LOD | < LOD |
| Maximum | < LOQ | < LOD | 4.670 | < LOQ | < LOD | |
| Mean ± SD | – | – | 1.173 ± 2.344 | – | – | |
| Hesperidin | Minimum | 1.912 | < LOD | 15.57 | 8.360 | < LOD |
| Maximum | 16.35 | < LOD | 91.22 | 12.64 | 5.360 | |
| Mean ± SD | 5.702 ± 6.201a | – | 38.85 ± 35.20a | 10.56 ± 2.142a | 1.792 ± 3.091a | |
| Isorhamnetin | Minimum | 7.224 | 6.132 | 7.483 | 9.184 | 4.442 |
| Maximum | 12.55 | 15.89 | 13.29 | 11.36 | 10.92 | |
| Mean ± SD | 9.631 ± 2.290a | 11.72 ± 4.091a | 10.14 ± 2.521a | 9.960 ± 1.223a | 8.012 ± 3.291a | |
| Kaempferol | Minimum | 10.18 | 10.56 | 13.29 | 33.44 | 6.942 |
| Maximum | 22.15 | 32.21 | 26.56 | 35.81 | 31.51 | |
| Mean ± SD | 14.77 ± 4.632a | 20.15 ± 9.940a | 20.77 ± 6.202a | 34.44 ± 1.230a | 16.99 ± 12.88a | |
| Luteolin | Minimum | 3.323 | 2.964 | 3.953 | 10.94 | 4.313 |
| Maximum | 6.031 | 14.40 | 8.934 | 21.45 | 9.051 | |
| Mean ± SD | 4.500 ± 1.242a | 7.980 ± 5.242a | 5.684 ± 2.222a | 16.42 ± 5.272a | 6.842 ± 2.394a | |
| Naringenin | Minimum | 2.702 | 1.201 | 0.462 | 6.190 | 1.863 |
| Maximum | 8.393 | 4.400 | 2.502 | 15.67 | 6.441 | |
| Mean ± SD | 4.832 ± 2.141a,b | 2.994 ± 1.392a,b | 1.731 ± 0.924b | 10.66 ± 4.762a | 4.610 ± 2.433a,b | |
| Pinobanksin | Minimum | 2.960 | 1.592 | 1.853 | 7.041 | 2.301 |
| Maximum | 9.911 | 5.113 | 3.944 | 19.51 | 8.070 | |
| Mean ± SD | 5.770 ± 2.600a,b | 3.631 ± 1.533a,b | 2.663 ± 0.991b | 12.94 ± 6.263a | 5.702 ± 3.020a,b | |
| Pinocembrin | Minimum | < LOQ | < LOD | 0.971 | 0.841 | < LOD |
| Maximum | 7.400 | < LOD | 6.423 | 2.312 | < LOD | |
| Mean ± SD | 3.321 ± 2.914a | – | 3.163 ± 2.420a | 1.550 ± 0.742a | – | |
| Quercetin | Minimum | 17.16 | 14.01 | 24.07 | 45.02 | 6.832 |
| Maximum | 28.98 | 16.64 | 71.41 | 68.32 | 34.75 | |
| Mean ± SD | 21.02 ± 5.251a,b | 15.55 ± 1.232b | 41.83 ± 20.51a,b | 56.84 ± 11.66a | 24.87 ± 15.64a,b | |
| Rutin/unknown compound | Minimum | 7.281 | 6.513 | 21.68 | 29.30 | 1.941 |
| Maximum | 18.33 | 12.87 | 69.06 | 76.23 | 16.78 | |
| Mean ± SD | 12.09 ± 4.422a | 9.190 ± 2.660a | 39.89 ± 20.95a | 49.24 ± 24.25a | 10.27 ± 7.591a | |
| Lignin-derived aldehydes | ||||||
| Coniferaldehyde | Minimum | < LOD | 5.982 | < LOD | 8.531 | < LOQ |
| Maximum | < LOQ | 13.97 | < LOQ | 12.85 | < LOQ | |
| Mean ± SD | – | 10.31 ± 3.550a | – | 11.26 ± 2.380a | – | |
| Syringaldehyde | Minimum | < LOQ | < LOQ | < LOQ | 4.321 | < LOQ |
| Maximum | < LOQ | 5.453 | 6.554 | 7.061 | < LOQ | |
| Mean ± SD | – | 2.624 ± 3.033a | 2.623 ± 3.210a | 5.430 ± 1.452a | – | |
| Phenolic acids | ||||||
| Benzoic acid | Minimum | 46.69 | 66.41 | 632.2 | 605.9 | 125.4 |
| Maximum | 176.0 | 249.0 | 1520 | 1121 | 230.0 | |
| Mean ± SD | 116.4 ± 61.80b | 168.8 ± 82.45a,b | 1048 ± 458.4a | 886.2 ± 260.7a,b | 193.7 ± 59.23a,b | |
| Caffeic acid | Minimum | < LOQ | 18.19 | 19.98 | < LOQ | < LOQ |
| Maximum | 17.46 | 37.03 | 38.75 | < LOQ | 84.46 | |
| Mean ± SD | 5.612 ± 8.061a | 27.64 ± 7.910a | 27.93 ± 9.010a | – | 51.89 ± 45.42a | |
| Chlorogenic acid | Minimum | 5.211 | 19.24 | 23.39 | 66.21 | 8.980 |
| Maximum | 23.91 | 57.09 | 54.32 | 109.7 | 19.39 | |
| Mean ± SD | 12.55 ± 8.020b | 37.02 ± 15.63a,b | 36.97 ± 13.66a,b | 89.31 ± 21.88a | 15.30 ± 5.552a,b | |
| p-Coumaric acid | Minimum | 19.39 | 31.48 | 33.27 | 21.99 | 36.92 |
| Maximum | 29.38 | 48.81 | 41.29 | 31.24 | 85.72 | |
| Mean ± SD | 26.53 ± 4.161b | 41.50 ± 7.784a,b | 35.87 ± 3.763a,b | 25.77 ± 4.852a,b | 57.18 ± 25.43a | |
| 3,4-Dihydroxybenzoic acid | Minimum | 97.04 | 114.4 | 140.5 | 117.6 | 102.4 |
| Maximum | 129.1 | 164.9 | 228.4 | 135.0 | 156.4 | |
| Mean ± SD | 117.7 ± 13.61b | 136.2 ± 21.03a,b | 195.9 ± 38.31a | 124.2 ± 9.431a,b | 128.0 ± 27.10a,b | |
| Ferulic acid | Minimum | 7.644 | 15.94 | 13.97 | 43.93 | 10.21 |
| Maximum | 28.34 | 64.07 | 33.25 | 81.09 | 39.58 | |
| Mean ± SD | 16.16 ± 8.221b | 41.58 ± 22.43a,b | 25.01 ± 8.390a,b | 57.16 ± 20.76a | 29.50 ± 16.71a,b | |
| Gallic acid | Minimum | 35.82 | 28.44 | 33.43 | 16.02 | 30.57 |
| Maximum | 43.61 | 29.41 | 43.17 | 29.98 | 37.54 | |
| Mean ± SD | 40.29 ± 3.282a | 29.10 ± 0.463b | 39.33 ± 4.261a,b | 22.22 ± 7.111b | 34.57 ± 3.603a,b | |
| Salicylic acid | Minimum | 80.35 | 75.71 | 186.5 | 308.3 | 111.0 |
| Maximum | 220.3 | 109.4 | 255.1 | 830.3 | 126.5 | |
| Mean ± SD | 119.5 ± 57.13b | 95.46 ± 15.82b | 227.7 ± 29.23a,b | 578.2 ± 261.4a | 120.3 ± 8.202a,b | |
| Syringic acid | Minimum | 18.75 | 14.91 | 43.16 | 30.32 | 31.80 |
| Maximum | 49.17 | 20.01 | 132.6 | 63.38 | 47.33 | |
| Mean ± SD | 26.43 ± 12.85a,b | 17.32 ± 2.214b | 78.91 ± 38.51a | 42.39 ± 18.24a,b | 41.19 ± 8.260a,b | |
| Sum | Minimum | 437.3 | 558.9 | 1421 | 1469 | 598.6 |
| Maximum | 823.2 | 822.4 | 2356 | 2393 | 873.8 | |
| Mean ± SD | 566.2 ± 155.1b | 678.7 ± 121.6a,b | 1887 ± 447.3a | 2049 ± 505.4a | 751.8 ± 140.2a,b | |
SD standard deviation, LOD limit of detection, LOQ limit of quantification; (−) not applicable
Different superscript letters in the same row represent statistical (p < 0.05) difference by Kruskal–Wallis test
LOD and LOQ, respectively, of: 0.20 and 0.80 µg L−1 for apigenin; 0.20 and 0.80 µg L−1 for chrysin; 0.20 and 1.60 µg L−1 for galangin; 0.20 and 0.40 µg L−1 for hesperidin; 0.20 and 0.40 µg L−1 for pinocembrin; 0.20 and 1.60 µg L−1 for coniferaldehyde; 0.40 and 1.60 µg L−1 for syringaldehyde; 1.60 and 3.20 µg L−1 for caffeic acid
For all samples, (+)-catechin, (−)-epicatechin, (−)-epigallocatechin gallate, naringin, 4-methylumbelliferone, sinapaldehyde, sinapic acid, and vanillic acid were below the limits of detection, and p-aminobenzoic acid was below the limit of quantification. Fifteen phenolics were quantified in all samples, and eight compounds (apigenin, chrysin, galangin, hesperidin, pinocembrin, coniferaldehyde, syringaldehyde, and caffeic acid) were quantified only in some of them. Phenolic acids were the main class of phenolics present in the samples, and benzoic acid (46.69 ± 1.962–1520 ± 36.44 µg 100 g−1), salicylic acid (75.71 ± 2.501–830.3 ± 9.771 µg 100 g−1), and 3,4-dihydroxybenzoic acid (97.04 ± 3.012–228.4 ± 22.73 µg 100 g−1) were the most abundant phenolics. These compounds were also some of the majority phenolic compounds found in bhh and honeydew honeys from different geographical and botanical origins, such as Quercus robur and Pinus brutia (Can et al. 2015; Seraglio et al. 2016). Similar contents for most of the investigated phenolics were also reported for honeydew honey from Turkey and Czech Republic (Silici et al. 2013; Halouzka et al. 2016).
For almost all investigated phenolics, significant differences were not observed between the five geographical locations, but they occurred in some cases. Samples from Bom Retiro presented the highest mean content of naringenin and pinobanksin than samples from Lages. Also, their quercetin content was higher than samples from Urupema; the same for their chlorogenic acid and ferulic acid contents in relation to the samples from Bocaina do Sul; and salicylic acid compared to the samples from Bocaina do Sul and Urupema. The highest mean content of benzoic and 3,4-dihydroxybenzoic acids was observed in the samples from Lages and p-coumaric acid in samples from Urubici compared to the samples of Bocaina do Sul. Samples from Bocaina do Sul only showed the highest mean content of gallic acid than Urupema and Bom Retiro samples. Consequently, samples from Lages and Bom Retiro showed the highest mean content related to the sum of phenolic compounds compared to samples from Bocaina do Sul. Therefore, these findings suggest that the geographic origin and the possible presence of nectar (blossom honey) also affect the profile and concentration of the samples’ phenolic compounds.
Scavenging and reducing activities in vitro
The possible antioxidant potential of honeys can be indicated by their scavenging and reducing activities in vitro. Figure 1 (and Online Resource 2) shows the scavenging capacity, investigated by DPPH method, and reducing ability, assessed by Folin–Ciocalteu and FRAP methods, of bhh.
Fig. 1.
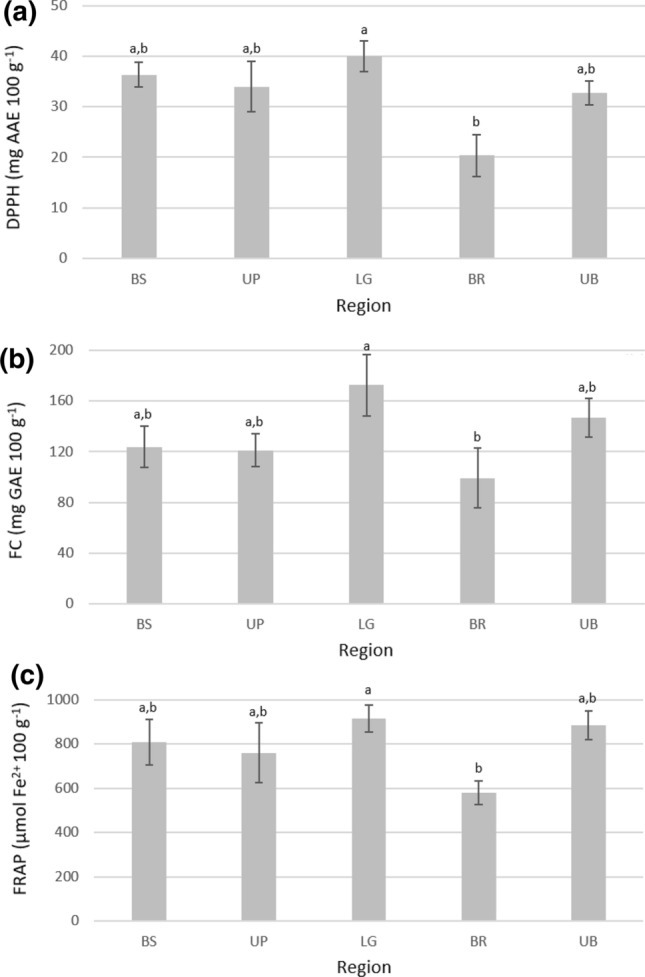
Results of a DPPH free radical scavenging activity, b Folin–Ciocalteu reducing capacity (FC), and c ferric reducing antioxidant power (FRAP) of bracatinga (Mimosa scabrella Bentham) honeydew honey from different geographical locations. BS Bocaina do Sul, UP Urupema, LG Lages, BR Bom Retiro, UB Urubici; DPPH 2,2-diphenyl-1-picrylhydrazyl, AAE ascorbic acid equivalent, GAE gallic acid equivalent; results expressed as mean ± standard deviation; Different superscript letters in the same column represent statistical (p < 0.05) difference by Kruskal–Wallis test
DPPH scavenging activity of samples ranged from 16.6 ± 0.52 to 43.4 ± 1.82 mg AAE 100 g−1. Similar values were found in bhh and in honeydew honeys from Burkina Fasan and Czech Republic (24.8–54.1 mg AAE 100 g−1) (Meda et al. 2005; Lachman et al. 2010; Seraglio et al. 2017; Bergamo et al. 2019a).
Concerning the total reducing capacity, the values ranged from 84.5 ± 1.14 to 197 ± 3.66 mg GAE 100 g−1. Such results follow reports from bhh and honeydew honeys from Quercus robur, Pinus brutia, and Quercus ilex (61.4–187.0 mg GAE 100 g−1) (Can et al. 2015; Seraglio et al. 2017; Bergamo et al. 2019a; Karabagias et al. 2020).
Ferric reducing ability of samples ranged from 525 ± 11.5 to 1005 ± 10.4 µmol Fe2+ 100 g−1, similar values compared to those reported for bhh and Salix spp., Quercus robur, and Pinus brutia honeydew honeys (148–1260 µmol Fe2+ 100 g−1) (Tuberoso et al. 2011; Can et al. 2015; Seraglio et al. 2017; Bergamo et al. 2019a).
These results suggest high scavenging and reducing potential of these samples, especially compared to blossom honeys (Can et al. 2015; Bergamo et al. 2019a). Also, the geographical origin seems to have a low influence on these activities. However, the presence of nectar in the bhh, as proposed for samples BR1, BR2, and BR3, seems to decrease honey scavenging and reducing activities. This behavior was observed for the samples from Bom Retiro, in which the lowest mean value was verified compared to the samples from Lages, for all assays.
Antimicrobial activity in vitro
Antimicrobial properties of bhh against Gram-positive and Gram-negative bacterial strains were investigated for the first time. As shown in Table 4 (and Online Resource 2), bhh presented high activity against all bacterial strains tested. Except for samples BS1 and BS2, which demonstrated MIC values of 60% against the four bacterial strains studied, all other samples presented MIC values between 10 and 40%. The high antimicrobial activity showed by the samples from Urupema and Lages can be highlighted, in which MIC values between 10 and 20% were found. Particular attention should be paid to sample LG4, the only sample which presented MIC values of 10% for all bacterial strains. In general, all bacterial strains tested appear to be sensitive to bhh, being not possible to identify a strain more or less sensitive to this type of honey.
Table 4.
Minimum inhibitory concentration (MIC) of bracatinga (Mimosa scabrella Bentham) honeydew honey from different geographical locations
| Bacterium | MIC (% w/v honey) | Region | ||||
|---|---|---|---|---|---|---|
| Bocaina do Sul (n = 5) | Urupema (n = 4) | Lages (n = 4) | Bom Retiro (n = 3) | Urubici (n = 3) | ||
| Escherichia coli | Minimum | 20 | 10 | 10 | 20 | 20 |
| Maximum | 60 | 10 | 10 | 40 | 20 | |
| Staphylococcus aureus | Minimum | 20 | 10 | 10 | 20 | 20 |
| Maximum | 60 | 20 | 20 | 40 | 20 | |
| Listeria monocytogenes | Minimum | 20 | 20 | 10 | 40 | 40 |
| Maximum | 60 | 20 | 20 | 40 | 40 | |
| Salmonella Typhimurium | Minimum | 40 | 10 | 10 | 20 | 20 |
| Maximum | 60 | 20 | 20 | 40 | 40 | |
Antimicrobial properties of honey are related to a set of factors that includes high sugar osmolarity, enzymatic generation of hydrogen peroxide, acidity, and presence of other minor compounds, such as methylglyoxal, peptides, and phenolic compounds (Deng et al. 2018; Seraglio et al. 2019). In this sense, the high antimicrobial activity of bhh may be associated with its high values of acidity, minerals, and phenolic compounds. Besides, the variations in antimicrobial activity observed between samples from different geographic regions and between samples from the same geographic area, as verified for samples BS1 and BS2 compared to samples BS3, BS4, and BS5, may be associated with the presence in different concentrations of compounds with antimicrobial action not evaluated in this study such as hydrogen peroxide, methylglyoxal, and peptides. Although bhh is produced from sugary excretions released by plant-sucking insects fixed on bracatinga tree, conditions such as soil, the incidence of light, temperature, attack by insects or pests, altitude, among other factors, are different in each microlocal and can affect the tree and your phloem differently. This consequently can influence the composition of the sugary excretion used by the bees to produce bhh and thus the composition and properties of this honey.
Despite the few studies found in the literature, promising antimicrobial activity has been reported for honeydew honeys, with activity against some bacterial strains such as Micrococcus luteus, S. aureus, E. coli, Pseudomonas putida, Bacillus subtilis, Proteus myxofaciens, L. monocytogenes, and S. Typhimurium (Vorlová et al. 2005; Lukasiewicz et al. 2015). Considering that antimicrobial activity against E. coli and S. aureus strains are commonly investigated in honeydew honeys, this study contributes with additional information related to the antimicrobial potential of honeydew honeys against L. monocytogenes and S. typhimurium, two important foodborne pathogens widely associated with disease outbreaks in humans, reinforcing the potential of honeydew honeys as antimicrobial agents.
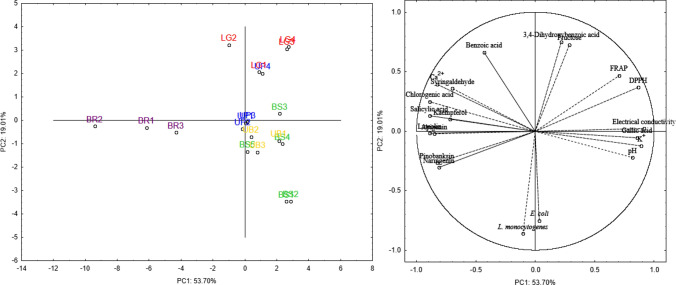
Principal component analysis
Principal component analysis (PCA) was carried out with data of physicochemical characteristics, minerals, phenolic compounds, scavenging, reducing, and antimicrobial activities of bhh to examine possible grouping of the samples according to their geographical location and which variables are influencing. Considering all the available data, a sum of the first five principal components (PCs) represented 75.41% of the data’s total variability, indicating a poor explanation of the data. Therefore, only the parameters with a correlation coefficient above 0.70 with PC1 and PC2 were selected, and a new PCA was generate aiming a better percentage of explanation of the data.
In this new analysis, PC1 (53.70%) and PC2 (19.01%) together explained 72.71% of the total data variance (Fig. 2). The dominant variables for PC1 were electrical conductivity (0.916), chlorogenic acid (− 0.892), salicylic acid (− 0.891), K+ (0.891), luteolin (− 0.889), DPPH (0.867), gallic acid (0.856), apigenin (− 0.849), pinobanksin (− 0.828), Ca2+ (− 0.826), pH (0.816), naringenin (− 0.810), kaempferol (− 0.717), FRAP (0.707), and syringaldehyde (− 0.699). For PC2, the dominant variables were antimicrobial activity against L. monocytogenes (− 0.862) and E. coli (− 0.753), 3,4-dyhydroxizenzoic acid (0.751), fructose (0.726), and benzoic acid (0.663).
Fig. 2.
Plot of principal component loadings for bracatinga (Mimosa scabrella Bentham) honeydew honeys from BS Bocaina do Sul, UP Urupema, LG Lages, BR Bom Retiro, and UB Urubici and the descriptors
In the projection of the score graph PC2 versus PC1 (Fig. 2), a partial grouping of the samples was observed. Samples from Bom Retiro were clearly separated from the other samples in PC1 mainly because of the low values of gallic acid, K+, FRAP, DPPH, pH, and electrical conductivity, and high levels of Ca2+ and some phenolics, such as pinobanksin, naringenin, and kaempferol. This result was expected since the samples from Bom Retiro presented expressive differences for many parameters compared to other samples. Also, this finding reinforces the probable presence of nectar (blossom honey) in enough quantity to affect these samples’ composition.
It was also observed that the geographical origin has a restricted influence on the composition of bhh. Besides the samples from Bom Retiro, only the samples from Lages were separated from the other samples, except for sample UP4, in the PC2, mainly due to the high contents of benzoic acid, 3,4-dihydroxybenzoic acid, and fructose, and low MIC values related to antimicrobial activity against E. coli and L. monocytogenes. These results indicate that the antimicrobial activity of bhh is highly influenced by the content of benzoic acid, 3,4-dihydroxybenzoic acid, and fructose.
Conclusion
In this study, physicochemical proprieties, minerals, phenolic compounds, and scavenging, reducing, and antimicrobial activities of bhh from distinct locations were investigated. Independent of the geographical location, all samples showed high acidity, high content of minerals and phenolic compounds, and high scavenging, reducing, and antimicrobial activities, indicating that bhh is a potential functional food. Although the samples share common characteristics, it was possible to verify partial discrimination of this honey type according to the geographical location and mainly due to the presence of nectar, factors that have been shown to affect the composition and biological activity of bhh.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. Authors also wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq), Fundação de Amparo à Pesquisa do Estado de Santa Catarina—Brasil (FAPESC), Agronomist Saulo Luis Poffo (Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina—EPAGRI), and the participating beekeepers from Santa Catarina state.
Authors contribution
M.S.A. coordinated the work and, together with S.K.T.S., designed all experiments, performed the antioxidant and minerals experiments, interpreted these data, wrote, edited, and revised the manuscript; G.O.R. and G.B. performed the physicochemical experiments, interpreted these data, and revised the manuscript; A.C.V. and H.D. performed the phenolic compounds experiments, interpreted these data, and revised the manuscript; M.M. performed the antimicrobial experiments, interpreted these data, and revised the manuscript; A.C.O.C., L.V.G., and R.F. supervised the work and revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Siluana Katia Tischer Seraglio: CNPq Scholarship—Brazil (160175/2019-4).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Siluana Katia Tischer Seraglio, Email: siluanaseraglio@hotmail.com.
Ana Carolina Oliveira Costa, Email: ana.costa@ufsc.br.
References
- AOAC . Official methods of analysis of AOAC International. 18. Gaithersburg: AOAC International; 2005. [Google Scholar]
- Bergamo G, Seraglio SKT, Gonzaga LV, et al. Mineral profile as a potential parameter for verifying the authenticity of bracatinga honeydew honeys. LWT. 2018;97:390–395. [Google Scholar]
- Bergamo G, Seraglio SKT, Gonzaga LV, et al. Physicochemical characteristics of bracatinga honeydew honey and blossom honey produced in the state of Santa Catarina: an approach to honey differentiation. Food Res Int. 2019;116:745–754. doi: 10.1016/j.foodres.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Bergamo G, Seraglio SKT, Gonzaga LV, et al. Differentiation of honeydew honeys and blossom honeys: a new model based on colour parameters. J Food Sci Technol. 2019;56:2771–2777. doi: 10.1007/s13197-019-03737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncelj J, Dobersek U, Jamnik M, Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105:822–828. [Google Scholar]
- Bogdanov S, Lüllmann C, Martin P, et al. Honey quality and international regulatory standards: review by the International Honey Commission. Bee World. 1999;80:61–69. [Google Scholar]
- Brudzynski K, Kim L. Storage-induced chemical changes in active components of honey de-regulate its antibacterial activity. Food Chem. 2011;126:1155–1163. [Google Scholar]
- Can Z, Yildiz O, Sahin H, et al. An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- De-Melo AAM, de Almeida-Muradian LB, Sancho MT, Pascual-Maté A. Composition and properties of Apis mellifera honey: a review. J Apic Res. 2018;57:5–37. [Google Scholar]
- Deng J, Liu R, Lu Q, et al. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018;252:243–249. doi: 10.1016/j.foodchem.2018.01.115. [DOI] [PubMed] [Google Scholar]
- Escuredo O, Míguez M, Fernández-González M, Seijo MC. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013;138:851–856. doi: 10.1016/j.foodchem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- European Commission Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off J Eur Communities. 2002;10:47–52. [Google Scholar]
- Ghramh HA, Khan KA, Alshehri AMA. Antibacterial potential of some Saudi honeys from Asir region against selected pathogenic bacteria. Saudi J Biol Sci. 2018;26:1278–1284. doi: 10.1016/j.sjbs.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halouzka R, Tarkowski P, Zeljković SĆ. Characterisation of phenolics and other quality parameters of different types of honey. Czech J Food Sci. 2016;34:244–253. [Google Scholar]
- Karabagias IK, Karabournioti S, Karabagias VK, Badeka AV. Palynological, physico-chemical and bioactivity parameters determination, of a less common Greek honeydew honey: “dryomelo”. Food Control. 2020;109:106940. [Google Scholar]
- Kim D-O, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Lachman J, Orsák M, Hejtmánková A, Kovářová E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT Food Sci Technol. 2010;43:52–58. [Google Scholar]
- Lukasiewicz M, Kowalski S, Makarewicz M. Antimicrobial and antioxidant activity of selected Polish herbhoneys. LWT Food Sci Technol. 2015;64:547–553. [Google Scholar]
- Meda A, Lamien CE, Romito M, et al. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- Rizelio VM, Gonzaga LV, da Silva Campelo Borges G, et al. Development of a fast MECK method for determination of 5-HMF in honey samples. Food Chem. 2012;133:1640–1645. [Google Scholar]
- Rizelio VM, Tenfen L, da Silveira R, et al. Development of a fast capillary electrophoresis method for determination of carbohydrates in honey samples. Talanta. 2012;93:62–66. doi: 10.1016/j.talanta.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Seraglio SKT, Valese AC, Daguer H, et al. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res Int. 2016;87:60–67. doi: 10.1016/j.foodres.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Seraglio SKT, Valese AC, Daguer H, et al. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res Int. 2017;99:670–678. doi: 10.1016/j.foodres.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Seraglio SKT, Silva B, Bergamo G, et al. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res Int. 2019;119:44–66. doi: 10.1016/j.foodres.2019.01.028. [DOI] [PubMed] [Google Scholar]
- Silici S, Sarioglu K, Karaman K. Determination of polyphenols of some turkish honeydew and nectar honeys using HPLC-DAD. J Liq Chromatogr Relat Technol. 2013;36:2330–2341. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Terrab A, González AG, Díez MJ, Heredia FJ. Characterisation of Moroccan unifloral honeys using multivariate analysis. Eur Food Res Technol. 2003;218:88–95. [Google Scholar]
- Tuberoso CIG, Jerkoví I, Bifulco E, Marijanović Z. Biodiversity of Salix spp. honeydew and nectar honeys determined by RP-HPLC and evaluation of their antioxidant capacity. Chem Biodivers. 2011;8:872–879. doi: 10.1002/cbdv.201000359. [DOI] [PubMed] [Google Scholar]
- Vanhanen LP, Emmertz A, Savage GP. Mineral analysis of mono-floral New Zealand honey. Food Chem. 2011;128:236–240. doi: 10.1016/j.foodchem.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Vorlová L, Karpíšková R, Chabinioková I, et al. The antimicrobial activity of honeys produced in the Czech Republic. Czech J Anim Sci. 2005;50:376–384. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.