Graphic abstract
In this study, the stability of 5-methyltetrahydrofolate (5-MTHF) in the model system and folate-enriched egg yolk and strategies for 5-MTHF stabilization were investigated. The oxygen, temperature and light affect the stability of 5-MTHF in the model system, among which oxygen is the main factor. In thermal pasteurization and spray-drying with normal air media, 5-MTHF is sensitive to oxidation, with the retention rate of blank group only reaching 74.96% ± 1.28%. The addition of vitamin C or vitamin E can protect 5-MTHF in egg yolk from degradation and the latter has a better protective effect. By adding 0.2% (w/v) vitamin E to egg yolk liquid, the retention rate of 5-MTHF during thermal pasteurization and spray-drying with normal air media were 94.16% ± 0.48% and 84.80% ± 0.82% respectively. Additionally, the spray-drying technique with inert gas media (N2) was also an effective method to improve the stability of the 5-MTHF in egg yolk. Our study explored the factors affecting the stability of 5-MTHF in both model systems and egg yolk liquid and provided effective strategies for the protection of 5-MTHF during the processing of egg.
Keywords: 5-methyltetrahydrofolate, Model system, Egg yolk, Degradation, Stabilization
Introduction
As an important water-soluble B vitamin, folate has a significant importance in the prevention of other diseases including cerebral vascular disease, cognitive impairments, some cancers and osteoporosis (Kawakita et al. 2018; Rachael et al. 2006; Wald et al. 2002). The word “folate” usually refers to the naturally occurring forms of folate, including tetrahydrofolate, 5-formyltetrahydrofolate and 5-methyltetrahydrofolate (5-MTHF). Among these, 5-MTHF can be directly absorbed by human body (Seyoum and Selhub 1998). Folic acid (FA) represents a synthetic and oxidized form of folate, and is usually found in fortification food and dietary supplements (Phillips et al. 2005). However, more and more evidence shows that consuming an excess of FA may mask vitamin B12 deficiency in early stage, cause the existence of unmetabolised FA in circulation, and increase the risk of certain cancer including prostate cancer and colon cancer (Sweeney et al. 2007). Considering concerns described above, an alternative way to achieve adequate and safe intake of folate should be considered. One approach is to develop novel functional foods based upon animal products rich in natural forms of folate through the addition of FA to basal diet of animals. Studies have shown that folate-enriched eggs can be produced by supplementing hens’ basal diet with FA. The reported highest egg folate content is as about 1.5–2 times as that of an ordinary egg with average size (Dickson et al. 2010; Hebert et al. 2005). Also, the folate in folate-enriched egg, mainly 5-MTHF (around 80%), is mostly located in egg yolk, and the minimal amount of unmetabolised FA accounts for less than 10% (Hoey et al. 2009).
Our previous research (not published yet) has also shown that folate-enriched eggs can be produced by adding FA and vitamin C to hens’ basal diet, with the highest 5-MTHF content reaching around 78 μg/egg (2.17-fold higher than an ordinary egg). However, the chemical structure of 5-MTHF (Fig. 1) differs from FA by being reduced in positions N (5), C (6) and C (7), and N (8), while hydrogen in position N (10) is substituted by a methyl group (Herbig et al. 2019). This makes 5-MTHF highly unstable and susceptible to oxidation, which brings a challenge when considering the further process of folate-enriched egg. Currently, both thermal pasteurization and spray-drying are widely used in egg processing and utilization to produce pasteurized egg yolk liquid and egg yolk powder, which are both useful egg products. 5-MTHF is susceptible to oxidation, and therefore it is stable in the absence of oxygen (Liu et al. 2012). Therefore, 5-MTHF of folate-enriched eggs will probably decrease in the thermal pasteurization and spray-drying as the oxidation may exist in these two processes. Lots of researches have shown that the vitamin C can decrease the degradation of 5-MTHF in model systems as it has antioxidant effect (Rozoy et al., 2012). Vitamin E also has a strong ability to serve as an antioxidant or radical scavenger, which is well described by many studies (Gottstein and Grosch 1990). Nitrogen-filled spray drying is widely used for dried dangerous chemical products. Luo et al. (2013) reported that increasing the nitrogen concentration decreased the loss of vitamin C and total phenol when using nitrogen-controlled heat pump drying for Hami melons, which is explained as the reduction of vitamin C and total phenol oxidation.
Fig. 1.
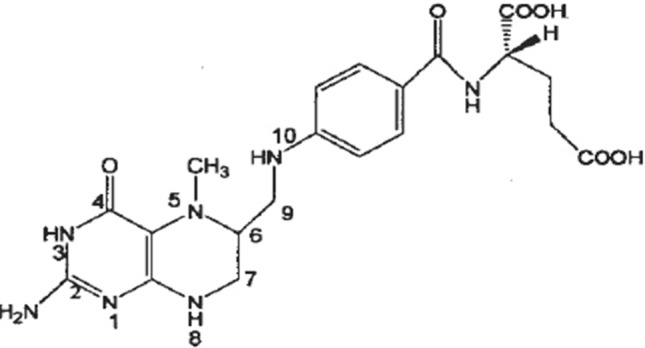
The chemical structural formula of 5-MTHF
To date, studies specially designed to investigate the degradation and protection of 5-MTHF in food systems are limited, especially in folate-enriched eggs. Also, little research has studied the application of nitrogen-filled spray drying in the food industry. Additionally, it is valuable for industrial producers to know more information about the strategies for 5-MTHF stabilization in the processing including thermal pasteurization and spray-drying. Therefore, the aim of this research, is to first identify the main factor for the degradation of 5-MTHF in the model system, then to study the effect of thermal pasteurization, spray-drying on 5-MTHF in folate-enriched egg yolk, and lastly to explore strategies for 5-MTHF stabilization using nitrogen-filled spray drying and antioxidants including vitamin C and vitamin E.
Material and methods
Materials
Folate-enriched eggs were provided by Rongda Poultry Co., Ltd (Xuancheng, China). The 5-MTHF (≥ 98%) standard was obtained from Sigma Chemical Co, Ltd (Switzerland). Sodium ascorbate (≥ 98%) was obtained from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). Bis–Tris Propane (99%), vitamin E (≥ 99%) and vitamin C (≥ 99%) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Stability of 5-MTHF in the model system
Thermal treatment
The samples were immersed in a water bath (85 or 100 °C) for 15 min. Once the thermal treatments were finished, the samples were taken out and immediately cooled in an ice bath (0–4 °C) for 30 min to minimize further degradation of 5-MTHF before HPLC assay. The blank was defined as the same concentration of non-heat treatment sample in each experiment.
Sample preparation
5-MTHF was stored at -40 °C. Prior to sample preparation, phosphate buffer solution (0.1 M, pH 7) was prepared using Milli-Q water and kept at 25 °C till further use. For each experiment, samples of 5-MTHF were freshly dissolved in phosphate buffer solutions. Three experiments were conducted. In experiment one, the effect of light on 5-MTHF stability in the model system was studied and samples were divided into two groups and treated with heating process at 85 °C for 15 min. All samples were flushed with nitrogen for 10 min at room temperature. In one group, the preparation of 5-MTHF (40 mg/mL) was conducted under usual light, and then samples were put in transparent glass vials for further thermal treatment under usual light. In the other group, the procedures of sample preparation and all treatments were carried out under subdued light, and the samples were put in dark brown glass vials.
In experiment two, the effect of temperature on 5-MTHF in the model system was identified and samples were divided into two groups and went through thermal treatment at temperature of 85 and 100 °C respectively for 15 min. All procedures were performed under subdued light. All samples were immediately flushed with nitrogen for 10 min at room temperature before thermal treatment and then were put in dark brown glass vials and capped with precaution to avoid the contact with air.
In experiment three, the influence of oxygen on 5-MTHF in the model system was explored and samples were divided into two groups treated with heating process at 85 °C for 15 min. One group of 5-MTHF solution was flushed with nitrogen for 10 min before receiving thermal treatment while the other group was flushed with nothing. All samples were prepared under subdued light and also put in dark brown glass vitals. All procedures during the experiment were carried out under subdued light.
HPLC analysis for 5-MTHF
The concentration of the 5-MTHF in the model system was identified by HPLC. HPLC analysis was conducted on a LC-20AT liquid chromatograph (Shimadzu, Japan) equipped with an UV/vis detector and LabSolutions software. 20 μL sample was injected onto a reverse phase Atlantis C-18 column (3 μm, 150 mm × 4.6 mm i.d.) of Waters (Milford, USA) mounted in a thermostatic column compartment at 30 °C. The gradient elution conditions were as follows: 0.6 ml/min flow rate; solvent A, phosphate buffer (0.05 M, pH 3.5); solvent B, acetonitrile; elution was performed with linear gradients from 10 to 18% B in 10 min, from 18 to 10% in 1 min, followed by re-equilibration of the column. Chromatograms were recorded at 290 nm.
Stability of 5-MTHF in the processing of folate-enriched egg yolk and strategies for its stabilization
Effect of vitamin C or vitamin E on the stability of 5-MTHF of egg yolk liquid in thermal pasteurization
Thermal pasteurization of egg yolk was performed previously with some modifications (Wilkin and Winter 2006). 20 mL egg yolk liquids were pasteurized at 65 °C for 3.5 min, with and without vitamin C (0, 0.05%, 0.10%, 0.15%, 0.20% w/v) or vitamin E (0, 0.05%, 0.10%, 0.15%, 0.20% w/v). After thermal pasteurization, all samples were immediately placed in cold water and then lyophilized for further analysis. The freeze-drying was performed on SCIENTZ-10 N of Ningbo Scientz Biotechnology Co., Ltd (Ningbo, Zhejiang, China). The drying conditions were as follows: − 60 °C for cold trap temperature, 48 h for drying time. The vacuum degree was lower than 20 Pa.
Effect of vitamin C or vitamin E on the stability of 5-MTHF of egg yolk liquid in spray-drying with normal air media
200 mL egg yolk liquids containing vitamin C (0, 0.05%, 0.10%, 0.15%, 0.20% w/v) or vitamin E (0, 0.05%, 0.10%, 0.15%, 0.20% w/v) were spray-dried for around 30 min. Spray-drying was conducted on SP-1500 of Shunyi Instrument Co., Ltd (Shanghai, China) whose evaporation capacity is 1500 mL/h with a pressure atomizer. According to previous studies, the drying conditions were set as follows: 180/70 °C for inlet/outlet air temperature, 18 m/s for the inlet air media flow rate, 25 °C for the feed temperature. The feed flow rate is 400 mL/h. The original concentration of 5-MTHF in egg yolk was determined as the 5-MTHF in freeze-dried egg yolk powder from the same batch egg.
Spray-drying with inert gas media (N2)
Spray-drying with inert gas media (N2) was conducted on BG-20 of Pingfeng Machine Technology Co., Ltd (Wuxi, Jiangsu, China) whose evaporation capacity is 20 kg/h with a pressure atomizer. The drying conditions were as the same as the common spray-drying described above: 180/70 °C for inlet/outlet air temperature, 18 m/s for the inlet gas media flow rate, 25 °C for the feed temperature. The feed flow rate is 1500 mL/h. The control group was identified as the lyophilized egg yolk powder from the same batch egg.
Assay of 5-MTHF in egg yolk
The extraction of 5-MTHF was performed as described previously with some modification (House et al. 2002). In short, egg was weighed, and yolk was displaced and weighed. Then, the yolk was lyophilized or spray-dried, and egg yolk powder was placed in -40 °C with recorded weight. Then, 5-MTHF in egg yolk powder was extracted with the trizma buffer (12.1 g/L trizma; 20 g/L sodium ascorbate; pH 7.8) and determined by UPLC-MS. The UPLC-MS was performed on a Waters ACQUITY UPLC ® system (Milford, USA) equipped with an electrospray ion source (ESI) and a triple quadrupole mass spectrometer (TOD). The analysis was carried out using an ACQUITY UPLC® HSS T3 column (1.8 μm, 2.1 × 100 mm). Methanol (solvent A) and water/formic acid (100:0.1 v/v) (solvent B) served as mobile phases. Oven temperature was set to 30 °C and sample size was 3 μL. The gradient elution conditions were as follows: flow rate 0.3 mL/min, 0–0.5 min of 98% A, 0.5–10 min of 60% A, and 10 -10.5 min of 98% A. Mass spectrometry operating conditions were as follows: capillary voltage 3,000 V, cone voltage 30 V, desolvation temperature 350 °C, cone gas 50 L/h and source temperature 120 °C. The product ion scans were performed for the precursor [M + H] + m/z 313.5.
Statistically analysis
A one-way analysis of variance (ANOVA) was used to evaluate the significant differences between mean values by SPSS (Version 24, IBM, America). When P ≤ 0.05, the differences between means were considered as significant by Duncan's-multiple range test. Results were displayed by mean values with their standard errors.
Results and discussions
Stability of 5-MTHF in the model system
The effect of light on 5-MTHF stability in the model system
In the first instance, the stability of 5-MTHF in a phosphate buffer solution (0.1 M, pH 7) heated at 85 °C for 15 min was investigated. The effect of light, temperature and oxygen on the retention rate of 5-MTHF in the model system was shown in Fig. 2. As is shown in Fig. 2, the retention rate of 5-MTHF under light condition was 58.45% ± 1.16% while the retention rate of 5-MTHF under dark condition was 72.86% ± 2.15%. Previous studies showed that folic acid was sensitive to sunlight, visible light and ultraviolet light (Nguyen et al. 2003). Akhtar et al. (2003) have postulated a mechanism of the degradation of folic acid in aqueous solution. They concluded that folic acid is motivated by UV light and then oxidized, that is, dehydrogenated in position C (9) and N (10). This cleavage of the bond between the C (9) and N (10) was also identified to occur in thermal degradation of folates. Since the C (9)–N (10) bond also exists in 5-MTHF, the degradation of 5-MTHF caused by light in our studies is probably attributed to the same cleavage of the bond.
Fig. 2.
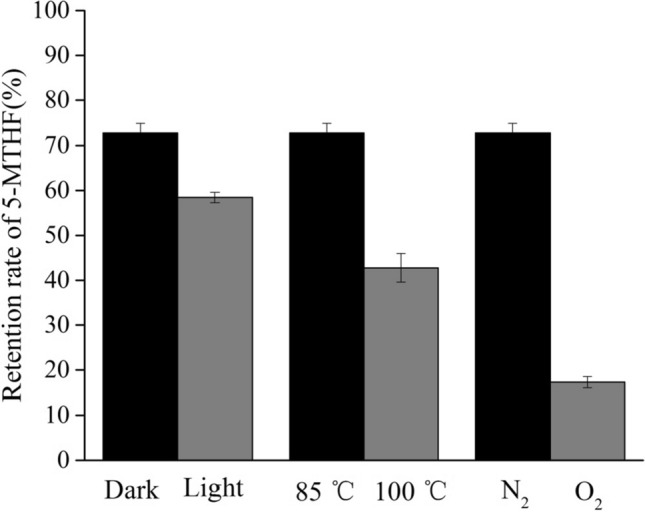
Stability of 5-MTHF in the model system
The effect of temperature on 5-MTHF stability in the model system
According to Fig. 2, the retention rate of 5-MTHF heated at 85 °C for 15 min under dark condition was 72.86% ± 2.15% while the retention rate of 5-MTHF heated at 100 °C for 15 min under dark condition was 42.76% ± 3.19%. According to literature, folates are also sensitive to heat. Nguyen et al. (2003) studied the stability of folic acid and 5-MTHF degradation during heat treatment in combination with high hydrostatic pressure. They suggested that the degradation of both folic acid and 5-MTHF followed first-order reaction kinetics. Studies on the reaction kinetics of folate degradation have also been carried out in some food systems, especially fruits and vegetables. Delchier et al. (2014b) studied the thermal degradation of total folate and 5-MTHF in spinach and green beans, with a conclusion of the first-order kinetic model. In agreement with their conclusion, the rise of temperature in our model system also resulted in an increase in the loss of 5-MTHF. Among light, temperature and oxygen, the oxygen has the most evident effect on the degradation of 5-MTHF in our study.
The effect of oxygen on 5-MTHF stability in the model system
As is shown in Fig. 2, the retention rate of 5-MTHF in nitrogen flushed aqueous solution heated at 85 °C under dark condition for 15 min was 72.86% ± 2.15% while the retention rate of 5-MTHF in aqueous solution under air heated at 85 °C for 15 min under dark condition was only 17.29% ± 1.24%. According to literature, the degradation of folates is universally assumed to be an oxidation process (Herbig et al. 2019). Moreover, compared with folic acid, 5-MTHF is highly susceptible to oxidation. In anaerobic condition, 5-MTHF is almost stable. Chen and Cooper (1979) were the first to study the thermal destruction of 5-MTHF in aqueous solution, under air or with nitrogen and ascorbate. They observed that the stability of 5-MTHF was increased in the presence of ascorbate and nitrogen atmosphere. Mnkeni and Beveridge (1983) also reported a decrease of 5-MTHF degradation when the initial oxygen concentration fell from 8 to 5.3 mg/L (by flushing nitrogen). Barrett and Luan (1989) also observed a faster degradation of 5-MTHF in the presence of oxygen in the model system (0.1 M, phosphate buffer, pH 7) comparing to the statistics obtained under oxygen-free system. Generally speaking, flushing nitrogen in the system can significantly protect the 5-MTHF from oxidation, which is also observed in our studies on the stability of 5-MTHF in the model system.
Stability of 5-MTHF in the processing of folate-enriched egg yolk
The effect of vitamin C on 5-MTHF stability in the thermal pasteurization of folate-enriched egg yolk
Thermal pasteurization is widely used in the production of egg yolk liquid or egg yolk powder as it can effectively destroy most of the bacteria existent and prolong the shelf life, especially at household refrigerator temperature. According to previous literatures, folate in egg yolk of the ordinary eggs was fairly shown to be unstable with folate retention estimated to be around 70% by various cooking methods, including boiling, frying, poaching and scrambling (Han et al. 2005). Of perhaps greater note, our previous experiment on the stability of 5-MTHF in the model system identified that thermal treatment would cause the degradation of 5-MTHF which was attributed to the exposure to the heat, oxygen and light. Thus, the stability of 5-MTHF and strategies for 5-MTHF stabilization in the thermal pasteurization of folate-enriched egg yolk were investigated in current study.
The effect of vitamin C on the stability of 5-MTHF in folate-enriched egg yolk liquid during thermal pasteurization was shown in Fig. 3. Similar with the observation in the model system, 5-MTHF in egg yolk liquid degraded a lot without the addition of vitamin C. In the blank group, the retention rate of 5-MTHF was only 74.96% ± 1.28%. Additionally, increasing the vitamin C concentration enhanced the 5-MTHF stability and the highest retention rate of 5-MTHF was 87.76% ± 1.44% with the addition of 0.2% vitamin C (w/v). This observation is in line with previous studies on the protection of vitamin C for the stability of folates in both model and food systems during thermal treatment. Oey et al. (2006) concluded that vitamin C increased the stability of folate during processing and the concentration of vitamin C must be at least two times as high as the molar concentration of the initial oxygen content in model systems. Delchier et al. (2014a) also reported the relationship between the concentration of oxygen and the loss of 5-MTHF in model systems. Therefore, as a water-soluble antioxidant, vitamin C can probably protect 5-MTHF in model systems from oxidation. As for the protective effect of vitamin C in model systems, Rozoy et al. (2012) confirmed that 5-MTHF could be highly preserved by the addition of an equal molar concentration of vitamin C. With regard to the protective effect of vitamin C in food systems, Herbig et al. (2019) reported that by adding vitamin C, the stability of 5-MTHF in apple and carrot purées could be increased, and the addition of 500 mg/kg vitamin C could make 5-MTHF stable. The addition of 500 mg/kg vitamin C is equivalent to 0.2% vitamin C in our study. Consistent with this, our study also indicated that 5-MTHF in folate-enriched egg yolk liquid was almost stable by adding 0.2% vitamin C during thermal pasteurization (65 °C, 3.5 min). Furthermore, Liu et al. (2012) studied the importance of antioxidant protection of 5-MTHF from thermal oxidation by using two food systems with antioxidant capacities, namely skim milk and soy milk, and found that food components with antioxidant activity could enhance the stability of 5-MTHF. Therefore, due to the oxidation resistance of vitamin C, the addition of vitamin C has an effective role in the protection of 5-MTHF in folate-enriched egg yolk during thermal pasteurization.
Fig. 3.
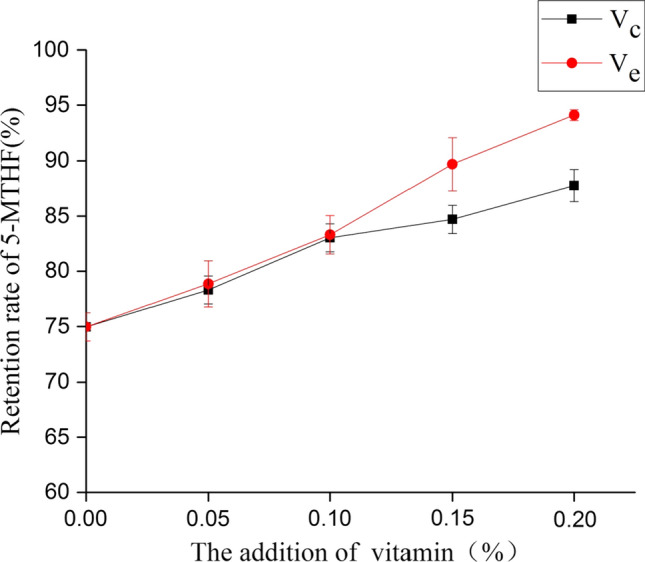
The effect of vitamin C and vitamin E on the stability of 5-MTHF of folate-enriched egg yolk liquid in thermal pasteurization
The effect of vitamin E on 5-MTHF stability in the thermal pasteurization of folate-enriched egg yolk
As mentioned above, the addition of vitamin C could protect 5-MTHF in folate-enriched egg yolk liquid from degradation during thermal pasteurization and increasing the concentration of vitamin C could also improve 5-MTHF stability. As is shown in Fig. 3, similar results and trends were observed for the addition of vitamin E. This could also be attributed to the role of vitamin E as a potent antioxidant (Dror and Allen 2011). Gottstein and Grosch (1990) reported that vitamin E could protect cooking oil from oxidation due to its antioxidant ability. Moreover, according to previous study, vitamin E also has a protective effect for immune system (Zhu et al. 2006). However, compared with vitamin C, vitamin E had a better protective effect for 5-MTHF in folate-enriched egg yolk. The highest retention rate of 5-MTHF stood at 94.16% ± 0.48% with the addition of 0.2% (w/v) vitamin E. This discrepancy could probably be explained by two reasons. Firstly, vitamin E and vitamin C have different chemical properties. Vitamin E used in this study is a kind of lipophilic antioxidant while vitamin C is a kind of water-soluble antioxidant (Yuan et al. 2008). Additionally, Naderi et al. (2017) identified that 5-MTHF was located in the egg yolk granule. Egg yolk granule is composed of low density lipoprotein (LDL), high-density lipoprotein (HDL) and phosvitin, which are all hydrophobic. Thus, compared with vitamin C, vitamin E may have a better access to 5-MTHF in egg yolk in order to protect it from oxidation. Secondly, vitamin C and vitamin E are also different in the stability in solutions with certain pH. A previous study has reported that vitamin C is more stable in the solution under lower pH condition. (Du et al. 2006). On the contrary, vitamin E has an excellent thermal stability (Xu et al. 2014). As the pH of egg yolk is around 6.3, the stability of vitamin E is better than that of vitamin C in egg yolk liquid during thermal pasteurization.
Stability of 5-MTHF of folate-enriched egg yolk in the spray-drying with normal air media
Spray drying is one of the most common and cheapest ways to produce egg yolk powder from egg yolk liquid which is generally used in food industry. As mentioned before, oxidation is the main reason for the degradation of 5-MTHF in both the model system and thermal pasteurization of egg yolk liquid. Therefore, high temperature and consistent air circulation in the process of spray-drying may also result in a remarkable loss of 5-MTHF in folate-enriched egg yolk. Additionally, according to previous studies, the spray-drying resulted in an evident loss of unstable vitamins in milk powder production, including vitamin E (loss rate 69.0%), vitamin A (loss rate 10.7%), vitamin B2 (loss rate 10.2%) and vitamin K1 (loss rate 2.51%) (Chen et al. 2008). As for the spray-dried egg powder, most studies concentrated on microbiological quality, the physical and functional properties while few studies focused on the stability of nutrients (Lechevalier et al. 2017). Furthermore, Caboni et al. (2005) reported the formation of cholesterol oxides occurred in the spray drying of egg yolks. Therefore, it’s necessary to investigate the stability of 5-MTHF and strategies for 5-MTHF stabilization in the spray-drying of folate-enriched egg yolk.
As for the effect of the addition of vitamin C or vitamin E on the retention rate of 5-MTHF of folate-enriched eggs in the spray-drying with normal air media, the results were presented in Fig. 4. Consistent with the observation in the model system and thermal pasteurization, the addition of vitamin C or vitamin E could protect 5-MTHF from degradation. In the blank group, the retention rate of 5-MTHF was only 61.70% ± 0.27%, which was lower than that of thermal pasteurization. This could be attributed to the difference on the concentration of oxygen between the thermal pasteurization and the spray-drying. During the thermal pasteurization, the container was closed, thus, the concentration of oxygen is limited. In contrast, the oxygen was incessant during the spray-drying. With the same amount of vitamin C or vitamin E adding to the egg yolk liquid during the spray-drying, vitamin E had a better protection for the 5-MTHF. Therefore, the main reason could be concluded as the same as that in the thermal pasteurization, namely the chemical properties and the stability of vitamin C or vitamin E. Furthermore, the same observation was also attained in the processing of milk. Chen et al. (2008) reported that the loss of vitamin C was more than that of vitamin E during the thermal pasteurization and the spray-drying. This result also shows that vitamin E may be more stable than vitamin C.
Fig. 4.
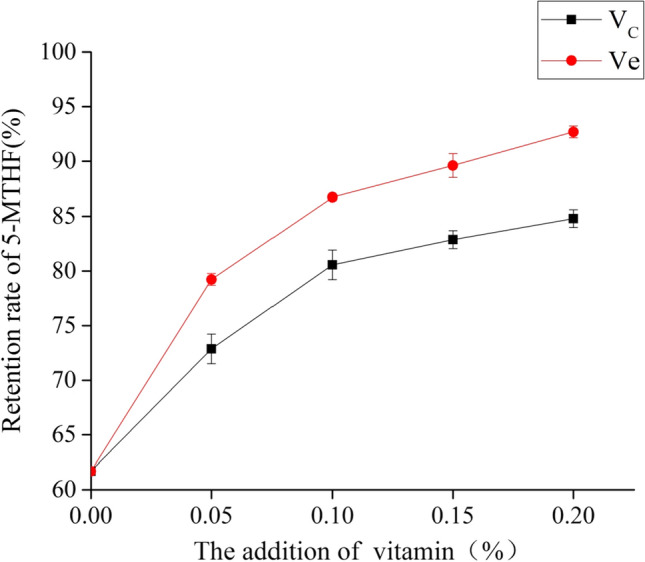
The effect of vitamin C and vitamin E on the stability of 5-MTHF of folate-enriched egg yolk in the spray-drying with normal air media
The effect of spray-drying with inert gas media (N2) on the stability of 5-MTHF of folate-enriched egg yolk
According to previous results in our study, the addition of vitamin C or vitamin E could protect the 5-MTHF in folate-enriched egg yolk from degradation in the spray-drying process due to their anti-oxidation effect. However, the highest addition of vitamin E still cannot completely protect 5-MTHF in folate-enriched egg yolk from degradation. The oxidation has proven critical in the degradation of 5-MTHF of folate-enriched egg yolk in previous experiments about the model system, thermal pasteurization and spray-drying with normal air media. Additionally, a new kind of spray-drying with nitrogen has already been used in the drying of easily oxidizable materials, inflammable and explosive chemical products, and flammable and explosive solvents, including petrol and ethyl alcohol (Qiang 2017). Therefore, the application of spray-drying with inert gas media (N2) was investigated in current study in order to completely protect the 5-MTHF of folate-enriched egg yolk from oxidation during the process of spray-drying. As is shown in Fig. 5, the 5-MTHF in folate-enriched egg yolk liquid almost remained stable during the spray drying with nitrogen while 5-MTHF decreased during spray drying with normal air media (loss rate 38.3% ± 0.27%). This observation was in accordance with that of the model system, thermal pasteurization and the common spray-drying. That is to say, oxidation is the main reason during the degradation of 5-MTHF in folate-enriched egg yolk and the spray-drying with inert gas media (N2) has huge potential in the drying for foods rich in 5-MTHF, especially the folate-enriched egg.
Fig. 5.
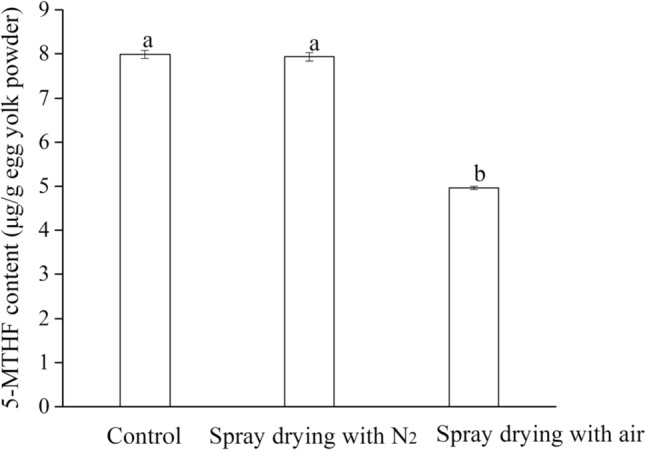
The effect of spray-drying with inert gas media (N2) on the stability of 5-MTHF of folate-enriched egg yolk a, b Mean values with different superscript letters were significantly different (P 0.05). Control group represents the lyophilized egg yolk powder
Conclusion
In the model system, it was found that oxygen, temperature and light affected the stability of 5-MTHF, among which oxygen was the main factor. In the thermal pasteurization of egg yolk liquid (65 °C, 3.5 min), the retention rate of 5-MTHF without any addition of antioxidant was only 74.96% ± 1.28%. The addition of vitamin C or vitamin E both can protect 5-MTHF in egg yolk from degradation with the highest retention rate reaching 94.16% ± 0.42% (The addition of vitamin E, 0.2%, m/v). In the spray-drying with normal air, the addition of vitamin C or vitamin E can also protect 5-MTHF from degradation due to their antioxidant properties. However, due to the incessant oxygen flow in the spray-drying with normal air media, the high concentration of antioxidants still cannot completely protect the 5-MTHF in egg yolk. Considering that the main factor for degradation is oxidation, a new kind of spray-drying with inert gas media (N2) was proposed for the spray-drying of egg yolk and the 5-MTHF was almost stable using the nitrogen-filled drying. Thus, our study not only identified the main factor, oxidation, during the processing of egg yolk, but also provided several ways to protect the 5-MTHF in egg yolk from degradation, including the addition of vitamin C or vitamin E and the use of spray-drying with inert gas media (N2).
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Author contributions
YY carried out the experiments, performed theoretical calculations and wrote the manuscript; LG, YS and CC revised the manuscript; SD and YL analyzed data; YY and JL designed experiments.
Funding
The authors would like to thank for the financial support from the National Key Research and Development Program of China (No. 2018YFD0400303) and the National Natural Science Foundation of China (No. 31671809 and 31801483).
Compliance with ethical standards
Conflicts of interest
The authors declare there is no conflicts of interest regarding the publication of this paper.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barrett DM, Luan DB. Effect of oxygen on thermal degradation of 5-methyl-5,6,7,8-tetrahydrofolic acid. J Food Sci. 1989;54:146–149. [Google Scholar]
- Caboni MF, Boselli E, Messia MC, Velazco V, Fratianni A, Panfili G, Marconi E. Effect of processing and storage on the chemical quality markers of spray-dried whole egg. Food Chem. 2005;92:293–303. [Google Scholar]
- Chen T-S, Cooper RG. Thermal destruction of folacin: effect of ascorbic acid, oxygen and temperature. J Food Sci. 1979;44:713–716. [Google Scholar]
- Chen WL, Sun KJ, He J. Study on stability of vitamins in milk powder production. J Dairy Sci Technol. 2008;31:66–83. [Google Scholar]
- Delchier N, Ringling C, Cuvelier M-E, Courtois F, Rychlik M, Renard CMGC. Thermal degradation of folates under varying oxygen conditions. Food Chem. 2014;165:85–91. doi: 10.1016/j.foodchem.2014.05.076. [DOI] [PubMed] [Google Scholar]
- Delchier N, Ringling C, Maingonnat J-F, Rychlik M, Renard CMGC. Mechanisms of folate losses during processing: diffusion versus heat degradation. Food Chem. 2014;157:439–447. doi: 10.1016/j.foodchem.2014.02.054. [DOI] [PubMed] [Google Scholar]
- Dickson TM, Tactacan GB, Hebert K, Guenter W, House JD. Optimization of folate deposition in eggs through dietary supplementation of folic acid over the entire production cycle of Hy-Line W36, Hy-Line W98, and CV20 laying hens. J Appl Poul Res. 2010;19:80–91. [Google Scholar]
- Dror DK, Allen LH. Vitamin E deficiency in developing countries. Food Nutr Bull. 2011;32:124–143. doi: 10.1177/156482651103200206. [DOI] [PubMed] [Google Scholar]
- Du FH, Tian LY, Wang J, Wang L. Studies on the stability of Vc in the sea buckthorn juice. Sci Technol Food Ind. 2006;27:81–83. [Google Scholar]
- Gottstein T, Grosch W. Model study of different antioxidant properties of α- and γ-tocopherol in fats. Fat Sci Technol. 1990;92:139–144. [Google Scholar]
- Han YH, Yon M, Hyun TH. Folate intake estimated with an updated database and its association to blood folate and homocysteine in Korean college students. Eur J Clin Nutr. 2005;59:246–254. doi: 10.1038/sj.ejcn.1602065. [DOI] [PubMed] [Google Scholar]
- Hebert K, House JD, Guenter W. Effect of dietary folic acid supplementation on egg folate content and the performance and folate status of two strains of laying hens. Poul Sci. 2005;84:1533–1538. doi: 10.1093/ps/84.10.1533. [DOI] [PubMed] [Google Scholar]
- Herbig AL, Delchier N, Striegel L, Rychlik M, Renard C. Stability of 5-methyltetrahydrofolate in fortified apple and carrot purees. LWT Food Sci Technol. 2019;107:158–163. [Google Scholar]
- Hoey L, Mcnulty H, Mccann EM, Mccracken KJ, Scott JM, Marc BB, Molloy AM, Graham C, Pentieva K. Laying hens can convert high doses of folic acid added to the feed into natural folates in eggs providing a novel source of food folate. Brit J Nutr. 2009;101:206–212. doi: 10.1017/S0007114508995647. [DOI] [PubMed] [Google Scholar]
- House JD, Braun K, Ballance DM, O’Connor CP, Guenter W. The enrichment of eggs with folic acid through supplementation of the laying hen diet. Poul Sci. 2002;9:1332–1337. doi: 10.1093/ps/81.9.1332. [DOI] [PubMed] [Google Scholar]
- Akhtar MJ, Khan MA, Ahmad I. Identification of photoproducts of folic acid and its degradation pathways in aqueous solution. J Pharmaceut Biomed. 2003;31:579–588. doi: 10.1016/s0731-7085(02)00724-0. [DOI] [PubMed] [Google Scholar]
- Kawakita D, Lee Y-CA, Gren LH, Buys SS, Vecchia LC, Hashibe M. The impact of folate intake on the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian cancer screening trial (PLCO) cohort. Bri J Cancer. 2018;118:299–306. doi: 10.1038/bjc.2017.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechevalier V, Guérin-Dubiard C, Anton M, Beaumal V, Briand ED, Gillard A, Gouar YL, Musikaphun N, Pasco M, Dupont D, Nau F. Effect of dry heat treatment of egg white powder on its functional, nutritional and allergenic properties. J Food Eng. 2017;195:40–51. [Google Scholar]
- Liu Y, Tomiuk S, Rozoy E, Simard S, Bazinet L, Green T, Kitts DD. Thermal oxidation studies on reduced folate, l-5-methyltetrahydrofolic acid (L-5-MTHF) and strategies for stabilization using food matrices. J Food Sci. 2012;77:236–243. doi: 10.1111/j.1750-3841.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- Luo L, Wang Q, Ren GY, Duan X, Liu YH, Zhu WX, Chu GY, Zhu M. Study on heat pump drying process of cantaloupe slice by filling nitrogen and lowering oxygen. Dry Technol Eq. 2013;11:50–57. [Google Scholar]
- Mnkeni AP, Beveridge T. Thermal destruction of 5-methyltetrahydrofolic acid in buffer and model food systems. J Food Sci. 1983;48:595–599. [Google Scholar]
- Naderi N, Doyen A, House JD, Pouliot Y. The use of high hydrostatic pressure to generate folate-enriched extracts from the granule fraction of hen’s egg yolk. Food Chem. 2017;232:253–262. doi: 10.1016/j.foodchem.2017.03.144. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Indrawati HM. Model studies on the stability of folic acid and 5-methyltetrahydrofolic acid degradation during thermal treatment in combination with high hydrostatic pressure. J Agri Food Chem. 2003;51:3352–3357. doi: 10.1021/jf026234e. [DOI] [PubMed] [Google Scholar]
- Oey I, Verlinde P, Hendrickx M, Loey AV. Temperature and pressure stability of l-ascorbic acid and/or [6s] 5-methyltetrahydrofolic acid: a kinetic study. Eur Food Res Technol. 2006;223:71–77. [Google Scholar]
- Phillips KM, Wunderlich KM, Holden JM, Exler J, Gebhardt SE, Haytowitz DB, Beecher GR, Doherty RF. Stability of 5-methyltetrahydrofolate in frozen fresh fruits and vegetables. Food Chem. 2005;92:587–595. [Google Scholar]
- Qiang L (2017) Non-oxygen type nitrogen drying machine. China: CN 107866138 A
- Rachael ZS-S, Shih-Chen C, Michael FL, Karen AJ, Christine J, Saundra SB, Robert NH, Regina GZ. Folate intake, alcohol use, and postmenopausal breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Clin Nutr. 2006;4:895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- Rozoy E, Simard S, Liu YZ, Kitts D, Lessard J, Bazinet L. The use of cyclic voltammetry to study the oxidation of L-5-methyltetrahydrofolate and its preservation by ascorbic acid. Food Chem. 2012;132:1429–1435. doi: 10.1016/j.foodchem.2011.11.132. [DOI] [PubMed] [Google Scholar]
- Seyoum E, Selhub J. Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. J Nutr. 1998;11:1956–1960. doi: 10.1093/jn/128.11.1956. [DOI] [PubMed] [Google Scholar]
- Sweeney MR, McPartlin J, Scott J. Folic acid fortification and public health: Report on threshold dosesabove which unmetabolised folic acid appear in serum. BMC Public Health. 2007;7:41–47. doi: 10.1186/1471-2458-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ Bri Med J. 2002;325:1–7. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin M, Winter AR. Pasteurization of egg yolk and white. Poul Sci. 2016;26:136–142. [Google Scholar]
- Xu Y, Zhong WS, Yuan NN, Duan WJ. Stability research of vitamin E. Zhejiang Chem Ind. 2014;45:22–24. [Google Scholar]
- Yuan MX, Huang XN, Han SY, Xi Y, Wong HB. Progress in natural vitamin E. J Biol. 2008;25:13–15. [Google Scholar]
- Zhu CL, Zhang WB, Sun P, Zhou QL, Jing XQ, Liu X, Hou WM. Effects of vitamin C and vitamin E combination on immunoregulation and antioxidation. Chin J Clin Rehabil. 2006;10:120–122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.