Abstract
Objective
To investigate the efficacy and safety of brodalumab, a fully human anti-interleukin-17 receptor A monoclonal antibody, in patients with axial spondyloarthritis (axSpA).
Methods
In a multicentre, placebo-controlled phase 3 study (NCT02985983) conducted at 48 sites across Japan, Korea and Taiwan, patients with axSpA were randomised 1:1 to receive subcutaneous brodalumab 210 mg (n=80) or placebo (n=79) at baseline, weeks 1 and 2 and every 2 weeks thereafter, during the 16-week double-blind period. The primary endpoint was the proportion of patients with Assessment of SpondyloArthritis International Society (ASAS) 40 response at week 16. Secondary endpoints included the proportion of patients with ASAS 20 response and change in Ankylosing Spondylitis Disease Activity Score using C-reactive protein (ASDAS-CRP) at week 16 and safety.
Results
ASAS 40 response rate (n/N; 95% CI) was 43.8% (35/80; 32.7, 55.3) with brodalumab vs 24.1% (19/79; 15.1, 35.0) with placebo (rate difference, 19.7% (5.3, 34.1); p=0.018 by stratified Cochran-Mantel-Haenszel test). ASAS 20 response rate (n/N; 95% CI) was 67.5% (54/80; 56.1, 77.6) vs 41.8% (33/79; 30.8, 53.4) and least squares mean change (95% CI) from baseline (brodalumab, 2.660; placebo, 2.716) in ASDAS-CRP was –1.127 (–1.322, –0.931) with brodalumab vs –0.672 (–0.872, –0.473) with placebo at week 16. Treatment-emergent adverse events were reported in 44 (55%) and 45 (57%) patients in the brodalumab and placebo groups, respectively.
Conclusion
Brodalumab demonstrated a significant improvement at week 16 in patients with active axSpA. Safety of brodalumab was consistent with that reported in previous global/Japanese psoriasis studies.
Keywords: biological therapy, spondylitis, ankylosing, therapeutics, inflammation
Key messages.
What is already known about this subject?
Interleukin (IL)-17 cytokines play a pathophysiological role in axial spondyloarthritis (axSpA), and clinical trials have demonstrated the efficacy and safety of IL-17 inhibitors in the treatment of ankylosing spondylitis (AS) and non-radiographic axSpA.
Brodalumab is a novel IL-17 inhibitor that inhibits IL-17 by blocking IL-17 receptor A (IL-17RA).
What does this study add?
Brodalumab demonstrated a significantly higher Assessment of SpondyloArthritis International Society 40 response rate at 16 weeks vs placebo, with a rate difference of 19.7%, and was well tolerated in patients with axSpA.
The efficacy and safety of brodalumab was comparable with that previously demonstrated by other IL-17 inhibitors in patients with axSpA.
How might this impact on clinical practice or future developments?
Short-term results from this study indicate that brodalumab, a novel IL-17RA inhibitor, can be a potential therapeutic option for patients with axSpA.
Introduction
Axial spondyloarthritis (axSpA) is an inflammatory disease characterised by chronic (≥3 months) back pain with an age at onset of <45 years and both articular and extra-articular clinical manifestations.1–3 Based on the Assessment of SpondyloArthritis International Society (ASAS) 2009 classification criteria,1 axSpA was proposed as a single entity with two subtypes: ankylosing spondylitis (AS; radiographic axSpA) and non-radiographic axSpA (nr-axSpA).
The worldwide prevalence of SpA is 0.1%–1.4%,4 5 with an estimated pooled prevalence of 0.2% in South-East Asia.6 AS affects 9–30 individuals/10 000 general population.7 A 2018 epidemiological survey reported ~3200 AS cases in Japan.8 Overall, 54 857 (in 2010)9 and 27 419 (in 2015)10 AS cases were identified from health insurance databases in Taiwan and Korea, respectively. The prevalence of nr-axSpA remains unreported.11
Interleukin (IL)-17 cytokines play a pathophysiological role in axSpA.12 Clinical trials have demonstrated the efficacy and safety of IL-17 inhibitors in the treatment of AS13–23 and nr-axSpA.24 Inhibition of IL-17A and IL-17F prevents inflammation and pathological and new bone formation.25 26 Brodalumab, a fully human anti-IL-17 receptor A (IL-17RA) monoclonal antibody, inhibits the activity of several other cytokines (IL-17A/F, IL-17C and IL-17E), including IL-17A and IL-17F, thus demonstrating a broader inflammation-blocking activity than other selective IL-17 inhibitors.27 Brodalumab 210 mg is approved for the treatment of plaque psoriasis in North America, Canada, Europe,28 29 Japan30 and other Asian countries. This phase 3 study aimed to evaluate the efficacy and safety of brodalumab in patients with active axSpA, including those with nr-axSpA. Here, we report the interim analysis efficacy and safety results of brodalumab vs placebo from the 16-week double-blind randomised period.
Methods
Study design
This multicentre, randomised, placebo-controlled study, conducted at 48 sites across Japan (n=25), Korea (n=12) and Taiwan (n=11) from October 2016 to December 2019, comprised a 16-week double-blind period and a 52-week open-label extension period. An interim analysis was performed at week 16, following data cut-off, after all patients had completed their week 16 visit. Eligible patients were randomised 1:1 to receive subcutaneous brodalumab 210 mg (the dosage approved for psoriasis30) or placebo at baseline, weeks 1 and 2 and every 2 weeks thereafter. Use of analgesics, such as acetaminophen and tramadol, and temporary dose increase or initiation of non-steroidal anti-inflammatory drugs (NSAIDs) during disease flare-ups were permitted at the physicians’ discretion, except during 12 hours before a scheduled efficacy evaluation (excluding the screening test). NSAIDs were discontinued or their dose reduced upon flare-up resolution. Patients continuing conventional synthetic disease-modifying antirheumatic drugs (DMARDs) or oral corticosteroids started before study enrolment maintained a stable dose during the 16-week study period.
Randomisation and masking
Patients were randomised to treatment groups in the order of enrolment using an interactive web response system (IWRS) by dynamic allocation and stratified based on baseline C-reactive protein (CRP) level (≥/<upper limit of normal (ULN)), region (Japan/Korea/Taiwan), disease subpopulations (AS/nr-axSpA) and informed consent (IC) for pharmacokinetic (PK) additional sampling (yes/no). Parexel International Inc. created a master randomisation list and allocated the study drug using the IWRS according to a prior written procedure. Investigators, patients’ assessors, other study site personnel and the sponsor’s representatives remained blinded until week 16, and the randomisation list was unblinded at the end of the double-blind period after all the patients had completed their week 16 visit.
All study participants provided written voluntary IC. The study was designed and sponsored by Kyowa Kirin and is registered at ClinicalTrials.gov (NCT02985983) and conducted after consultation and in agreement with the Japanese regulatory authorities.
Patient and public involvement
Patients or the public were not involved in the design or conduct or reporting or dissemination plans of our research.
Patients
Eligible patients included adults (aged ≥18 years), diagnosed as having axSpA by rheumatologists, meeting the ASAS classification criteria for axSpA (online supplemental table S1).1 AS was diagnosed based on radiographic evidence of sacroiliitis grade ≥2 bilaterally or grade 3–4 unilaterally and ≥1 SpA feature (excluding Crohn’s disease) by the ASAS classification criteria for axSpA.1 In the absence of radiographic evidence for AS, either of the following criteria was required to be met for a diagnosis of nr-axSpA: presence of inflammatory lesions of the sacroiliac joint on magnetic resonance imaging (MRI) of SpA Research Consortium of Canada level ≥2 and ≥1 SpA feature by the ASAS classification criteria for axSpA (excluding Crohn’s disease) OR human leucocyte antigen (HLA)-B27 positivity and ≥2 SpA features by the ASAS classification criteria for axSpA (excluding Crohn’s disease), with one of these being elevated CRP >ULN (0.5 mg/dL) (online supplemental tables S1 and S2). Radiographic sacroiliitis and inflammatory lesions on MRI were read centrally by a single experienced reader. Other inclusion criteria were Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥4 (including a spinal pain score ≥4) and inadequate response to NSAID therapy of ≥3 months. Patients receiving prior therapy were included if the duration of conventional synthetic DMARDs was ≥3 months before study drug initiation, with a stable dose for ≥4 weeks, or the duration of oral corticosteroids was ≥4 weeks before study drug initiation. Patients with complete ankylosis of the spine; history of Crohn’s disease; history/evidence of suicidal ideation (severity: 4 or 5)/any suicidal behaviour based on the Columbia-Suicide Severity Rating Scale (C-SSRS), or severe depression based on the Patient Health Questionnaire-8 (PHQ-8); or previous biologic therapies, including >1 anti-tumour necrosis factor (anti-TNF) or any anti-TNF within 4–10 weeks of study start or anti-IL-17 or anti-IL-12/IL-23 within 6 months were excluded from the study (online supplemental table S1).
annrheumdis-2020-219406supp001.pdf (325KB, pdf)
Outcome measures
The primary endpoint was the proportion of patients with axSpA achieving an ASAS 40 response at week 16. Secondary endpoints included the proportion of patients with axSpA achieving ASAS 20, those with AS and nr-axSpA achieving ASAS 40 at week 16, change from baseline in Ankylosing Spondylitis Disease Activity Score (ASDAS) using CRP (ASDAS-CRP) at week 16 and ASDAS-CRP response rate by disease improvement and disease activity state. ASAS 20 response rate in the AS subpopulation was assessed post hoc. ASAS 40 response was defined as an improvement of ≥40% (≥20% for ASAS 20) and an absolute improvement of ≥2 units (≥1 unit for ASAS 20) (on a 10-unit scale) in ≥3 of the four main ASAS domains (ie, Patient Global Assessment (PGA) of axSpA, the average of total and nocturnal PGA of spinal pain, Bath Ankylosing Spondylitis Functional Index (BASFI) score and the mean of BASDAI Q5 and Q6) and no worsening (by ≥20% and ≥1 unit for ASAS 20) in the remaining domains.
Treatment-emergent adverse events (TEAEs) were summarised by system organ class (SOC) and preferred term (PT) using the Medical Dictionary for Regulatory Activities (V.19.1). TEAEs identified or considered as potential risks were assessed as ‘TEAEs of interest’ and labelled in the following six categories: neutrophil count decreased/serious infections/serious hypersensitivity (identified risks) and malignancy/inflammatory bowel disease/suicide or self-injury-related events (potential risks) (online supplemental tables S3 and S4).
Exploratory endpoints included BASFI, BASDAI, Bath Ankylosing Spondylitis Metrology Index (BASMI), AS Quality of Life Questionnaire (ASQoL), Short Form-36 (SF-36) Health Survey (version 2), enthesitis count, swollen joint count and PGA of spine pain and axSpA.
Subgroup analyses by prior anti-TNF therapy, HLA-B27 status and CRP level at screening were performed for ASAS 40 response rate at week 16.
Statistical analyses
At least 59 patients per treatment group were targeted for enrolment to achieve a power of 90% with a two-sided 5% significance level assuming an ASAS 40 response rate of 40.5% with brodalumab and 13.0% with placebo. The sample size was estimated based on the clinically significant difference in ASAS 40 response rate achieved in AS patients with secukinumab (~40%) and placebo (~12%) in the MEASURE 1 and MEASURE 2 studies15 and that in nr-axSpA patients with anti-TNF agents (43%) and placebo (15%) in five clinical studies and the expected number of enrolled nr-axSpA patients (maximum possible: 30 patients).4 This study was not powered to demonstrate treatment group differences by AS and nr-axSpA.
The full analysis set comprised all randomised patients associated with the assigned treatment excluding those who received no study drug or had no post-dosing efficacy data. The safety analysis set comprised all randomised patients associated with the assigned treatment excluding those who received no study drug.
Missing data at week 16 were imputed as no response for ASAS 20/40 and baseline-observation-carried-forward (BOCF) for ASDAS-CRP. ASAS 40 response rate at week 16 was compared between brodalumab and placebo using the Cochran-Mantel-Haenszel test adjusted with all stratification factors used for randomisation, except IC for PK additional sampling.
For each treatment group, ASAS 20/40 was summarised by visit, and the point estimates and 95% CIs of ASAS 20/40 were calculated. The point estimate and 95% CI of ASDAS-CRP and the change from baseline at week 16 were calculated for each treatment group using an analysis of covariance model adjusted with the baseline ASDAS-CRP and all stratification factors used for randomisation, except IC for PK additional sampling. Data were analysed using SAS V.9.4.
Results
Patients
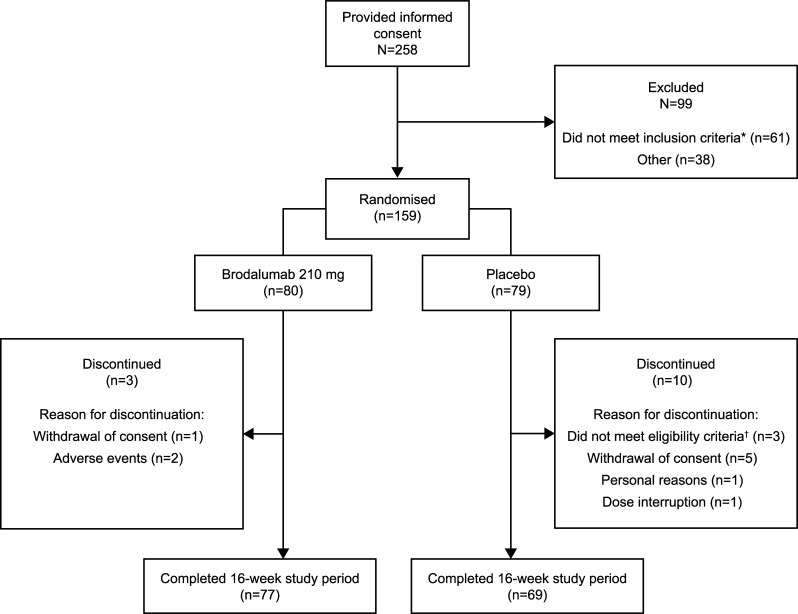
Overall, 159 eligible patients with axSpA were randomised 1:1 to receive brodalumab 210 mg (n=80) or placebo (n=79; figure 1). Of these, three in the brodalumab group and ten in the placebo group discontinued (figure 1), and 77 and 69, respectively, completed the 16-week period. No patient met the exclusion criteria based on the C-SSRS rating and/or PHQ-8 score.
Figure 1.
Patient disposition. *Did not meet the study criteria for AS or nr-axSpA, as judged by the central image readers. †Failed to meet the inclusion criteria or met one of the exclusion criteria. AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; nr-axSpA, non-radiographic axSpA.
The study population comprised mainly men (brodalumab: 66 (82.5%); placebo: 61 (77.2%)) and patients with AS (brodalumab: 63 (78.8%); placebo: 62 (78.5%)). The mean age was 36.6 years and 38.3 years in the brodalumab and placebo groups, respectively. Approximately 20% of patients in each treatment group had received prior anti-TNF therapy (table 1).
Table 1.
Patient demographics and other baseline characteristics (full analysis set)
| Characteristic | Brodalumab 210 mg N=80 |
Placebo N=79 |
| Sex, male—n (%) | 66 (82.5) | 61 (77.2) |
| Mean age (SD)—years | 36.6 (11.4) | 38.3 (10.8) |
| Mean BMI (SD)—kg/m2 | 25.1 (4.2) | 25.4 (4.1) |
| Disease subpopulations— n (%) | ||
| AS | 63 (78.8) | 62 (78.5) |
| nr-axSpA | 17 (21.3) | 16 (20.3) |
| Missing* | 0 | 1 (1.3) |
| Disease duration of axSpA†—n | 75 | 75 |
| Mean (SD)—years | 7.1 (7.7) | 6.5 (6.5) |
| Range—years | 0.1–33.9 | 0.1–26.8 |
| Region—n (%) | ||
| Japan | 15 (18.8) | 15 (19.0) |
| Korea | 22 (27.5) | 22 (27.8) |
| Taiwan | 43 (53.8) | 42 (53.2) |
| Spondyloarthritis features—n (%) | ||
| Inflammatory back pain | 79 (98.8) | 79 (100.0) |
| Arthritis | 25 (31.3) | 35 (44.3) |
| Enthesitis (heel) | 18 (22.5) | 19 (24.1) |
| Uveitis | 16 (20.0) | 11 (13.9) |
| Dactylitis | 2 (2.5) | 4 (5.1) |
| Psoriasis | 6 (7.5) | 5 (6.3) |
| Good response to NSAIDs | 25 (31.3) | 31 (39.2) |
| Family history of spondyloarthritis | 21 (26.3) | 31 (39.2) |
| HLA-B27 positive | 68 (85.0) | 65 (82.3) |
| Elevated CRP‡ | 49 (61.3) | 46 (58.2) |
| Prior anti-TNF therapy—n (%) | 16 (20.0) | 17 (21.5) |
| Adalimumab | 4 (5.0) | 4 (5.1) |
| Certolizumab pegol | 4 (5.0) | 3 (3.8) |
| Etanercept | 6 (7.5) | 5 (6.3) |
| Golimumab | 0 | 2 (2.5) |
| Infliximab | 2 (2.5) | 3 (3.8) |
| Prior anti-IL-12/23 therapy—n (%) | 0 | 0 |
| CRP level at screening, mean (SD)—mg/dL | 1.22 (1.45) | 1.15 (1.19) |
| ASDAS-CRP score, mean (SD) | 2.660 (0.615) | 2.716 (0.652) |
| BASFI score, mean (SD) | 3.7 (2.3) | 3.5 (2.5) |
| BASDAI score, mean (SD) | 6.2 (1.4) | 6.4 (1.6) |
| BASMI score, mean (SD) | 2.3 (1.7) | 2.9 (1.9) |
*The patient was initially randomised to the nr-axSpA subgroup at enrolment based on HLA-B-positive results observed on flow cytometry analysis at a local laboratory. However, based on HLA-B-negative results identified later using PCR-SBT at the central lab, the patient was excluded from the nr-axSpA subpopulation.
†Time from diagnosis to study enrolment.
‡CRP above ULN (0.5 mg/dL).
AS, ankylosing spondylitis; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMI, body mass index; CRP, C-reactive protein; HLA, human leucocyte antigen; IL, interleukin; nr-axSpA, non-radiographic axSpA; NSAID, non-steroidal anti-inflammatory drug; PCR-SBT, PCR-sequenced based typing; TNF, tumour necrosis factor; ULN, upper limit of normal.
Efficacy
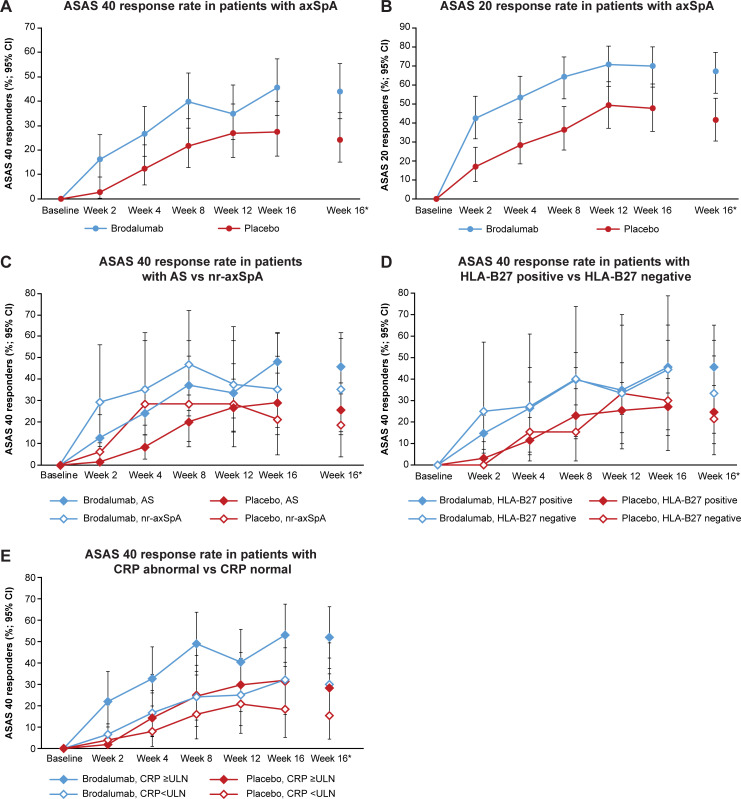
The primary endpoint, ASAS 40 response at week 16 (95% CI; non-responder imputation (NRI)), was achieved in 35 patients with axSpA (43.8%; 32.7, 55.3) in the brodalumab group (rate difference, 19.7% (5.3, 34.1); p=0.018) vs 19 patients (24.1%; 15.1, 35.0) in the placebo group (figure 2A and online supplemental table S5). The ASAS 20 response rate (95% CI; NRI) was higher with brodalumab (67.5%; 56.1, 77.6) vs placebo (41.8%; 30.8, 53.4) among patients with axSpA (figure 2B). Among subpopulations with AS and nr-axSpA (figure 2C and online supplemental table S5), the ASAS 40 response rate (95% CI; NRI) at week 16 was higher with brodalumab (AS: 46.0%; 33.4, 59.1, nr-axSpA: 35.3%; 14.2, 61.7) vs placebo (AS: 25.8%; 15.5, 38.5, nr-axSpA: 18.8%; 4.0, 45.6). In the AS subpopulation, the ASAS 20 response rate (n/N; 95% CI; NRI) at week 16 was 69.8% (44/63; 57.0, 80.8) with brodalumab vs 41.9% (26/62; 29.5, 55.2) with placebo. ASAS 40 response rate in the nr-axSpA subpopulation by MRI status is shown in online supplemental table S6.
Figure 2.
Response rates (full analysis set). (A) ASAS 40 response rate in patients with axSpA. (B) ASAS 20 response rate in patients with axSpA. (C) ASAS 40 response rate in patients with AS vs nr-axSpA. (D) ASAS 40 response rate in patients with HLA-B27 positive vs HLA-B27 negative. (E) ASAS 40 response rate in patients with CRP abnormal vs CRP normal. *Data are presented in online supplemental table S5. AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis International Society; axSpA, axial spondyloarthritis; CRP, C-reactive protein; HLA, human leucocyte antigen; nr-axSpA, non-radiographic axSpA; ULN, upper limit of normal.
Among anti-TNF-naïve patients, the ASAS 40 response rate was 45.3% (29/64) with brodalumab vs 25.8% (16/62) with placebo compared with 37.5% (6/16) vs 17.6% (3/17), respectively, for anti-TNF-experienced patients at week 16 (data not shown). The ASAS 40 response rate at week 16 was higher in patients with HLA-B27 positivity than in those with HLA-B27 negativity (brodalumab, 45.6% (31/68) vs 33.3% (4/12); placebo, 24.6% (16/65) vs 21.4% (3/14); figure 2D and online supplemental table S5) and in those with CRP level ≥ULN vs CRP level <ULN (brodalumab, 52.0% (26/50) vs 30.0% (9/30); placebo, 28.3% (15/53) vs 15.4% (4/26)) (figure 2E and online supplemental table S5).
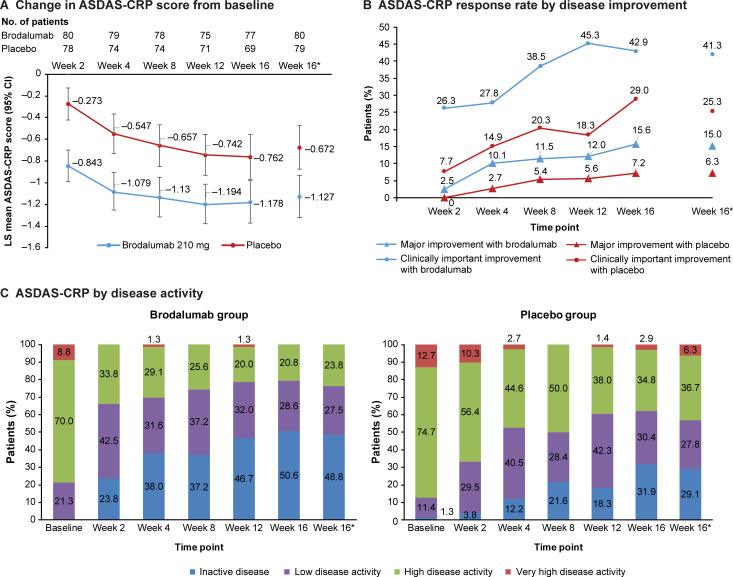
The least squares mean (LSM) (95% CI; BOCF) change from baseline in ASDAS-CRP was –1.127 (–1.322, –0.931) with brodalumab vs –0.672 (–0.872, –0.473) with placebo at week 16 (figure 3A), with an LSM difference (95% CI) in the change from baseline in ASDAS-CRP between the two groups of –0.454 (–0.689, –0.219). The proportion of patients with major and clinically important improvement at week 16 was 15.0% (12/80) and 41.3% (33/80), respectively, with brodalumab vs 6.3% (5/79) and 25.3% (20/79), respectively, with placebo (figure 3B). At week 16, 48.8% (39/80) of patients had inactive disease and 27.5% (22/80) had low disease activity in the brodalumab group (figure 3C). Improvements from baseline in BASFI, BASDAI, BASMI, enthesitis count, swollen joint count and PGA of spinal pain and axSpA at week 16 were slightly higher with brodalumab than with placebo (online supplemental table S7).
Figure 3.
ASDAS-CRP (full analysis set). (A) Change in ASDAS-CRP score from baseline at each efficacy timepoint over 16 weeks. *Baseline-observation-carried-forward ASAS response at week 16. (B) ASDAS-CRP response rate by disease improvement. Disease improvement was defined as clinically important and major for change from baseline in the ASDAS-CRP score of ≥1.1 and ≥2.0, respectively. (C) ASDAS-CRP response rate by disease activity state in the brodalumab (left) and placebo (right) groups. Disease activity state was defined by ASDAS-CRP score as follows: score <1.3, inactive disease; 1.3 to <2.1, low disease activity; 2.1 to <3.5, high disease activity; and ≥3.5, very high disease activity. ASAS, Assessment of SpondyloArthritis International Society; ASDAS-CRP, Ankylosing Spondylitis Disease Activity Score using C-reactive protein; LS, least squares.
Safety
Over 16 weeks, TEAEs were reported in 44 (55%) patients in the brodalumab group and 45 (57%) patients in the placebo group (table 2). No deaths were reported. The most commonly reported TEAEs by SOC in the brodalumab group were infections and infestations (n=18, 22.5%; placebo: n=15, 19.0%), gastrointestinal disorders (n=13, 16.3%; placebo: n=7, 8.9%) and investigations (n=7, 8.8%; placebo: n=1, 1.3%).
Table 2.
Safety summary (safety analysis set)
| TEAE—n (%) | Brodalumab 210 mg N=80 |
Placebo N=79 |
| Any TEAE | 44 (55.0) | 45 (57.0) |
| Deaths | 0 | 0 |
| Other serious TEAEs* | 4 (5.0) | 1 (1.3) |
| Other significant TEAEs† | 4 (5.0) | 3 (3.8) |
| Any drug-related TEAE | 26 (32.5) | 20 (25.3) |
| Deaths | 0 | 0 |
| Other serious TEAEs* | 1 (1.3) | 0 |
| Other significant TEAEs† | 2 (2.5) | 3 (3.8) |
| TEAEs of interest‡—n (%) | ||
| Any TEAE | 7 (8.8) | 2 (2.5) |
| Inflammatory bowel disease§ | 4 (5.0) | 2 (2.5) |
| Mouth ulceration | 3 (3.8) | 1 (1.3) |
| Stomatitis | 1 (1.3) | 1 (1.3) |
| Neutrophil count decreased | 1 (1.3) | 0 |
| Leucopenia | 1 (1.3) | 0 |
| Serious infections | 2 (2.5) | 0 |
| Herpes zoster oticus | 1 (1.3) | 0 |
| External ear cellulitis | 1 (1.3) | 0 |
| Suicide/self-injury-related events | 0 | 0 |
| Serious hypersensitivity | 0 | 0 |
| Malignancy | 0 | 0 |
*Any serious TEAE other than death.
†Any non-serious TEAE leading to withdrawal of the study drug or dose suspension.
‡TEAEs identified or considered as potential risks were assessed as ‘TEAEs of interest’ and labelled in the following six categories: neutrophil count decreased/serious infections/serious hypersensitivity (identified risks) and malignancy/inflammatory bowel disease/suicide or self-injury-related events (potential risks) (online supplemental tables S3 and S4).
§Ulcerative colitis or Crohn’s disease was not reported in any patient.
TEAE, treatment-emergent adverse event.
The most commonly reported TEAEs by PT in the brodalumab group were nasopharyngitis (10.0% (n=8); placebo: 11.4% (n=9)) and alanine aminotransferase and aspartate aminotransferase increased (5% (n=4) each; placebo: 1.3% (n=1) each) (table 3). Ulcerative colitis, Crohn’s disease, candidiasis or uveitis were not reported in any patient. Of the seven (8.8%) patients in the brodalumab group reporting TEAEs of interest, three had mouth ulceration and one each had stomatitis, herpes zoster oticus, external ear cellulitis and leucopenia. Other serious TEAEs were reported in four (5.0%) patients in the brodalumab group, all of which resolved, and included malocclusion/ankle fracture/external ear cellulitis (all drug unrelated) and herpes zoster oticus (relationship with drug unknown) in one patient each. In the placebo group, one serious TEAE of back pain was reported. No patient reported suicidal ideation or suicidal behaviour.
Table 3.
TEAEs by SOC and PT
| TEAEs (any TEAE ≥2.5% in any treatment group)—n (%) | Brodalumab 210 mg N=80 |
Placebo N=79 |
| Infections and infestations | 18 (22.5) | 15 (19.0) |
| Nasopharyngitis | 8 (10.0) | 9 (11.4) |
| Rhinitis | 3 (3.8) | 1 (1.3) |
| Upper respiratory tract infection | 2 (2.5) | 5 (6.3) |
| Gastrointestinal disorders | 13 (16.3) | 7 (8.9) |
| Diarrhoea | 3 (3.8) | 1 (1.3) |
| Mouth ulceration | 3 (3.8) | 1 (1.3) |
| Abdominal pain upper | 2 (2.5) | 0 |
| Vomiting | 2 (2.5) | 0 |
| Investigations | 7 (8.8) | 1 (1.3) |
| Alanine aminotransferase increased | 4 (5.0) | 1 (1.3) |
| Aspartate aminotransferase increased | 4 (5.0) | 1 (1.3) |
| Liver function test abnormal | 2 (2.5) | 0 |
| Musculoskeletal and connective tissue disorders | 6 (7.5) | 6 (7.6) |
| Back pain | 0 | 2 (2.5) |
| Injury, poisoning and procedural complications | 5 (6.3) | 6 (7.6) |
| Ligament sprain | 1 (1.3) | 2 (2.5) |
| Metabolism and nutrition disorders | 4 (5.0) | 2 (2.5) |
| Nervous system disorders | 3 (3.8) | 2 (2.5) |
| Skin and subcutaneous tissue disorders | 3 (3.8) | 5 (6.3) |
| Pruritus | 2 (2.5) | 1 (1.3) |
| General disorders and administration site conditions | 2 (2.5) | 6 (7.6) |
| Fatigue | 1 (1.3) | 2 (2.5) |
| Respiratory, thoracic and mediastinal disorders | 2 (2.5) | 3 (3.8) |
| Vascular disorders | 2 (2.5) | 2 (2.5) |
| Hypertension | 2 (2.5) | 2 (2.5) |
PT, preferred term; SOC, system organ class; TEAE, treatment-emergent adverse event.
Discussion
In patients with AS and nr-axSpA, the efficacy and safety of selective IL-17 A and/or F blockade were demonstrated previously.13–24 However, this is the first study in patients with axSpA comprising subpopulations of both disease subtypes, AS and nr-axSpA, which reports the efficacy and safety of brodalumab, an IL-17RA monoclonal antibody that inhibits multiple IL-17 cytokines.27 Over 16 weeks, the ASAS 40 response rate in patients with axSpA consistently improved with brodalumab and was significantly higher vs placebo at week 16.
ASAS 40/20 responses were observed as early as week 2 among patients treated with brodalumab; at week 16, 43.8%/67.5% of patients demonstrated ASAS 40/20 response. The ASAS 40 response rate with brodalumab at week 16 was comparable in the subpopulations with AS (46.0%) and nr-axSpA (35.3%). Disease activity (as assessed by the ASDAS-CRP score) started improving as early as week 2 and consistently improved over 16 weeks. At week 16, 76.3% of patients achieved inactive disease or low disease activity, and 41.3% of patients had major and/or clinically important improvement with brodalumab treatment. The incidence of TEAEs was similar between the placebo and brodalumab groups, with no new safety signals identified with brodalumab treatment over 16 weeks. Importantly, no TEAEs related to suicidal ideation or behaviour were reported, as cautioned in the brodalumab label.31
The ASAS 40/20 response rates observed in this study with brodalumab in patients with AS (46.0%/69.8%) were comparable with those achieved at week 16 with secukinumab in previous studies in Asian (43.5%/69.6%)17 and Japanese (46.7%/70.0%)19 populations with active AS. The improvement in ASDAS-CRP was also similar to that previously reported with secukinumab in the Asian population.17 Although the study populations were similar, comparison between studies should be undertaken with caution because the efficacy may be impacted by other patient characteristics, such as CRP levels and HLA-B27 positivity. ASAS 40/20 response rates of 38.8%/59.5% and 35.9%/61.5% at week 16 with secukinumab with and without a loading dose, respectively,18 and 30%–46%/41%–72% at week 12 with 16–320 mg doses of bimekizumab have been reported previously in global populations.20
In patients with nr-axSpA, the ASAS 40 response rate achieved at week 16 in the current study (35.3%) was comparable to that achieved in the global COAST-X study (35%–40%) at week 16 with ixekizumab, an IL-17A inhibitor.24
The global phase 3 studies, COAST-V in biological DMARD–naïve21 and COAST-W in anti-TNF-experienced patients13 with AS, reported ASAS 40 response rates at 16 weeks of about 50% and 25%–30%, respectively, with two dose regimens of ixekizumab. Subgroup analyses in our study demonstrated comparable ASAS 40 response rates in anti-TNF-naïve (45.3%) and anti-TNF-experienced (37.5%) patients with brodalumab. The results from the global phase 3 study of secukinumab also suggested that prior anti-TNF use may not impact response with IL-17 inhibitors.18 High CRP levels and HLA-B27 positivity were previously reported as good predictors of response in patients with SpA/AS treated with anti-TNF agents.32–34 In this study as well, HLA-B27 positivity (vs negativity) and CRP levels ≥ULN (vs <ULN) were associated with higher ASAS 40 response rates in brodalumab-treated patients.
Improvement in BASDAI at week 16 (mean, –2.9) was comparable to that (LSM, –2.6) previously reported with secukinumab in the Asian population.17 Improvement in other measures, including patient-reported outcomes (PROs), in the present study was negligible vs placebo and lower for ASQoL and SF-36. A similar high placebo effect on subjective PROs was reported with secukinumab at 2 years in a global population18 and was attributed to the growing awareness about the efficacy of this drug. However, the relatively long disease duration (7.1 years) of the brodalumab group could also be a reason, where causes other than inflammation (such as non-physiological stress, chronic muscle imbalance) potentially contributed more to disease symptoms, leading to a disconnect between improvement in objective and subjective signs, as reported previously.35 The safety profile of brodalumab in the current study was similar to that reported in previous global and Japanese studies of psoriasis.36 37
Study limitations include limited generalisability of results since the study population was specific to Japan, Korea and Taiwan, where Kyowa Kirin has development/marketing rights; inability to assess statistical significance between disease subpopulations due to the small sample size of the nr-axSpA population; and unavailability of MRI outcomes until 16 weeks. Further, the number of patients with AS exceeded the planned sample size of 90 because too many patients were screened due to the concern of not being able to reach the targeted sample size, arising from a high rate of screening failures initially. Moreover, the current results demonstrate the short-term efficacy and safety of brodalumab treatment in axSpA. The 52-week (weeks 16–68) results from the open-label extension of this study will further confirm the long-term efficacy and safety of brodalumab.
In conclusion, this study demonstrated that brodalumab is a potential therapeutic option for patients with axSpA.
annrheumdis-2020-219406supp002.pdf (1.1MB, pdf)
Acknowledgments
Masayuki Takanuma of Kyowa Kirin analysed the data. Medical writing support was provided by Dr. Deepali Garg, MBBS, PGDHA, of Cactus Life Sciences (part of Cactus Communications) and was funded by Kyowa Kirin. These results were presented, in part, at the European Congress of Rheumatology (EULAR 2019, 12–15 June) in Madrid, Spain. http://scientific.sparx-ip.net/archiveeular/?view=1&c=a&searchfor=brodalumab&item=2019OP0234.
Footnotes
Handling editor: Josef S Smolen
Correction notice: This article has been corrected since it published Online First. The first author's affiliations have been updated.
Collaborators: Kurisu Tada (Juntendo University Hospital, Japan); Mitsumasa Kishimoto (Kyorin University Hospital, Japan); Kou Katayama (Katayama Seikeigeka Rheumatism Clinic, Japan); Atsuo Taniguchi (Tokyo Women's Medical University Hospital, Japan); Yohei Seto (Tokyo Women's Medical University Yachiyo Medical Center, Japan); Mitsuhiro Morita (Fujita Health University Hospital, Japan); Kazuhiro Hatta (Tenri Hospital, Japan); Tetsuya Tomita (Osaka University Hospital, Japan); Nobuo Negoro (Osaka City University Hospital, Japan); Hitoshi Goto (Osaka City General Hospital, Japan); Shigeyoshi Tsuji (National Hospital Organization Osaka Minami Medical Center, Japan); Norikazu Murata (Yukioka Hospital, Japan); Kiyoshi Matsui (Hyogo College of Medicine, Japan); Masahiro Yamamura (Okayama Saiseikai General Hospital Outpatient Center, Japan); Hiroaki Dobashi (Kagawa University Hospital, Japan); Junichi Fukushi (Kyushu University Hospital, Japan); Satoshi Ikemura (Kyushu University Hospital, Japan); Akira Maeyama (Fukuoka University Hospital, Japan); Mitsuyo Kinjo (Okinawa Prefectural Chubu Hospital, Japan); Yukitaka Ueki (Sasebo Chuo Hospital, Japan); Eishi Uechi (Tomishiro Central Hospital, Japan); Tatsuya Atsumi (Hokkaido University Hospital, Japan); Hideto Kameda (Toho University Ohashi Medical Center, Japan); Yoshinori Taniguchi (Kochi Medical School Hospital, Japan); Sei Muraoka (Toho University Omori Medical Center, Japan); Masanobu Oishi (Chihaya Hospital, Japan); Seung Jae Hong (Kyung Hee University Hospital, Korea); Won Park (Inha University Hospital, Korea); Shin Seok Lee (Chonnam National University Hospital, Korea); Chang Hee Suh (Ajou University Hospital, Korea); Seong Wook Kang (Chungnam National University Hospital, Korea); Tae-Hwan Kim (Hanyang University Seoul Hospital, Korea); Jung Yoon Choe (Daegu Catholic University Medical Center, Korea); Ji Hyeon Ju (The Catholic University of Korea Seoul St. Mary’s Hospital, Korea); Jin Kyun Park (Seoul National University Hospital, Korea); Seung-Geun Lee (Pusan National University Hospital, Korea); Yun Jong Lee (Seoul National University Bundang Hospital, Korea); Sang-Heon Lee (Konkuk University Medical Center, Korea); Cheng Chung Wei (Chung Shan Medical University Hospital, Taiwan); Tien Tsai Cheng (Chang Gung Medical Foundation, Kaohsiung Chang Gung Memorial Hospital, Taiwan); Wen Chan Tsai (Kaohsiung Medical University Chung-Ho Memorial Hospital, Taiwan); Chung Ming Huang (China Medical University Hospital, Taiwan); Hsin Hua Chen (Taichung Veterans General Hospital, Taiwan); Der-Yuan Chen (Taichung Veterans General Hospital, Taiwan); Meng Yu Weng (National Cheng Kung University Hospital, Taiwan); Shue Fen Luo (Chang Gung Medical Foundation, LinKou Chang Gung Memorial Hospital, Taiwan); Kun Hung Chen (Cathay General Hospital, Taiwan); Ling Jung Yen (Kaohsiung Veterans General Hospital, Taiwan); Cheng Han Wu (National Taiwan University Hospital, Taiwan); Hsiang Cheng Chen (Tri-Service General Hospital, Taiwan).
Contributors: JC-CW, T-HK and MK contributed to the collection and interpretation of data and review of the manuscript. SK contributed to the study design, study oversight, data interpretation and review of the manuscript. NO and HJ contributed to the study design, data interpretation and review of the manuscript.
Funding: This study was funded by Kyowa Kirin.
Competing interests: JC-CW reports grants from Kyowa Kirin for the work under consideration for publication; grants from AbbVie, BMS, Celgene and UCB Pharma; personal fees from TSH Taiwan; and grants and personal fees from Janssen, Novartis and Pfizer, outside the submitted work. T-HK reports grants from Kyowa Kirin for the work under consideration for publication. MK reports personal fees from Kyowa Kirin for the work under consideration for publication and personal fees from AbbVie, Eli Lilly, Celgene, Pfizer, Gilead, Janssen, UCB Pharma, Eisai, Novartis, Tanabe-Mitsubishi, Ayumi and Astellas, outside the submitted work. NO is an employee of Kyowa Kirin. HJ is an employee of Kyowa Kirin Korea. SK reports personal fees from Kyowa Kirin for the work under consideration for publication; personal fees from AbbVie GK, Bristol-Myers Squibb, Eisai, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Teijin Pharma, Novartis Pharma K.K., Eli Lilly Japan K.K. and Asahikasei Pharma, outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
4827-006 study group:
Kurisu Tada, Mitsumasa Kishimoto, Kou Katayama, Atsuo Taniguchi, Yohei Seto, Mitsuhiro Morita, Kazuhiro Hatta, Tetsuya Tomita, Nobuo Negoro, Hitoshi Goto, Shigeyoshi Tsuji, Norikazu Murata, Kiyoshi Matsui, Masahiro Yamamura, Hiroaki Dobashi, Junichi Fukushi, Satoshi Ikemura, Akira Maeyama, Mitsuyo Kinjo, Yukitaka Ueki, Eishi Uechi, Tatsuya Atsumi, Hideto Kameda, Yoshinori Taniguchi, Sei Muraoka, Masanobu Oishi, Seung Jae Hong, Won Park, Shin Seok Lee, Chang Hee Suh, Seong Wook Kang, Tae-Hwan Kim, Jung Yoon Choe, Ji Hyeon Ju, Jin Kyun Park, Seung-Geun Lee, Yun Jong Lee, Sang-Heon Lee, Cheng Chung Wei, Tien Tsai Cheng, Wen Chan Tsai, Chung Ming Huang, Hsin Hua Chen, Der-Yuan Chen, Meng Yu Weng, Shue Fen Luo, Kun Hung Chen, Ling Jung Yen, Cheng Han Wu, and Hsiang Cheng Chen
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study protocol was approved by the institutional review board or independent ethics committee at each study site.
References
- 1. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 2. Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68 Suppl 2:ii1–44. 10.1136/ard.2008.104018 [DOI] [PubMed] [Google Scholar]
- 3. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. 10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 4. Corbett M, Soares M, Jhuti G, et al. Tumour necrosis factor-α inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess 2016;20:1–334. 10.3310/hta20090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. López-Medina C, Moltó A. Update on the epidemiology, risk factors, and disease outcomes of axial spondyloarthritis. Best Pract Res Clin Rheumatol 2018;32:241–53. 10.1016/j.berh.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 6. Stolwijk C, van Onna M, Boonen A, et al. Global prevalence of spondyloarthritis: a systematic review and meta-regression analysis. Arthritis Care Res 2016;68:1320–31. 10.1002/acr.22831 [DOI] [PubMed] [Google Scholar]
- 7. Wang R, Ward MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol 2018;30:137–43. 10.1097/BOR.0000000000000475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ankylosing spondylitis (designated intractable disease 271). Japan Intractable Diseases Information Center. Available: http://www.nanbyou.or.jp/entry/4847 [Accessed 11 Sep 2019].
- 9. Hsieh M-Y, Kuo C-F. FRI0428 Epidemiology of ankylosing spondylitis in Taiwan: a nationwide population study. Ann Rheum Dis 2016;75:590–1. 10.1136/annrheumdis-2016-eular.5595 [DOI] [Google Scholar]
- 10. Park J-S, Hong J-Y, Park Y-S, et al. Trends in the prevalence and incidence of ankylosing spondylitis in South Korea, 2010-2015 and estimated differences according to income status. Sci Rep 2018;8:7694. 10.1038/s41598-018-25933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bohn R, Cooney M, Deodhar A, et al. Incidence and prevalence of axial spondyloarthritis: methodologic challenges and gaps in the literature. Clin Exp Rheumatol 2018;36:263–74. [PubMed] [Google Scholar]
- 12. Paine A, Ritchlin CT. Targeting the interleukin-23/17 axis in axial spondyloarthritis. Curr Opin Rheumatol 2016;28:359–67. 10.1097/BOR.0000000000000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol 2019;71:599–611. 10.1002/art.40753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther 2017;19:285. 10.1186/s13075-017-1490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- 16. Baraliakos X, Kivitz AJ, Deodhar AA, et al. Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the phase 3 MEASURE 1 trial. Clin Exp Rheumatol 2018;36:50–5. [PubMed] [Google Scholar]
- 17. Wei JC-C, Baeten D, Sieper J, et al. Efficacy and safety of secukinumab in Asian patients with active ankylosing spondylitis: 52-week pooled results from two phase 3 studies. Int J Rheum Dis 2017;20:589–96. 10.1111/1756-185X.13094 [DOI] [PubMed] [Google Scholar]
- 18. Kivitz AJ, Wagner U, Dokoupilova E, et al. Efficacy and safety of secukinumab 150 mg with and without loading regimen in ankylosing spondylitis: 104-week results from MEASURE 4 study. Rheumatol Ther 2018;5:447–62. 10.1007/s40744-018-0123-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kishimoto M, Taniguchi A, Fujishige A, et al. Efficacy and safety of secukinumab in Japanese patients with active ankylosing spondylitis: 24-week results from an open-label phase 3 study (MEASURE 2-J). Mod Rheumatol 2020;30:132–40. 10.1080/14397595.2018.1538004 [DOI] [PubMed] [Google Scholar]
- 20. van der Heijde D, Gensler LS, Deodhar A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 2020;79:595–604. 10.1136/annrheumdis-2020-216980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Heijde D, Wei JC-C, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018;392:2441–51. 10.1016/S0140-6736(18)31946-9 [DOI] [PubMed] [Google Scholar]
- 22. Baraliakos X, Braun J, Deodhar A, et al. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD Open 2019;5:e001005. 10.1136/rmdopen-2019-001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pavelka K, Kivitz AJ, Dokoupilova E, et al. Secukinumab 150/300 mg provides sustained improvements in the signs and symptoms of active ankylosing spondylitis: 3-year results from the phase 3 MEASURE 3 study. ACR Open Rheumatol 2020;2:119–27. 10.1002/acr2.11102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020;395:53–64. 10.1016/S0140-6736(19)32971-X [DOI] [PubMed] [Google Scholar]
- 25. van Tok MN, van Duivenvoorde LM, Kramer I, et al. Interleukin-17A inhibition diminishes inflammation and new bone formation in experimental spondyloarthritis. Arthritis Rheumatol 2019;71:612–25. 10.1002/art.40770 [DOI] [PubMed] [Google Scholar]
- 26. Shah M, Maroof A, Gikas P, et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 2020;6:e001306. 10.1136/rmdopen-2020-001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nirula A, Nilsen J, Klekotka P, et al. Effect of IL-17 receptor A blockade with brodalumab in inflammatory diseases. Rheumatology 2016;55:ii43–55. 10.1093/rheumatology/kew346 [DOI] [PubMed] [Google Scholar]
- 28. Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context 2019;8:212570 10.7573/dic.212570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. CADTH common drug review: CADTH Canadian Drug Expert Committee Recommendation (Final), 2018. Available: https://www.cadth.ca/sites/default/files/cdr/complete/SR0547_cdr_complete_Siliq_June_22_18.pdf [Accessed 25 Oct 2019].
- 30. New drugs approved in FY 2016. Pharmaceuticals and Medical Devices Agency, Japan, 2016. Available: https://www.pmda.go.jp/files/000229078.pdf [Accessed 12 Sep 2019].
- 31. SILIQ™ (brodalumab) injection, for subcutaneous use. Valeant Pharmaceuticals North America LLC, Bridgewater, NJ 08807, USA, 2017. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf [Accessed 17 Sep 2019].
- 32. Bisson-Vaivre A, Alcaix D, Zarnitsky C, et al. Efficacy of anti-TNF in patients with spondyloarthritis in absence of any imaging sign. Joint Bone Spine 2013;80:280–6. 10.1016/j.jbspin.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 33. Polo Y La Borda J, Campos J, Sanz J, et al. Predictive clinical-genetic model of long-term non-response to tumor necrosis factor-alpha inhibitor therapy in spondyloarthritis. Int J Rheum Dis 2019;22:1529–37. 10.1111/1756-185X.13607 [DOI] [PubMed] [Google Scholar]
- 34. Rudwaleit M, Claudepierre P, Wordsworth P, et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 2009;36:801–8. 10.3899/jrheum.081048 [DOI] [PubMed] [Google Scholar]
- 35. Weiß A, Song I-H, Haibel H, et al. Good correlation between changes in objective and subjective signs of inflammation in patients with short- but not long duration of axial spondyloarthritis treated with tumor necrosis factor-blockers. Arthritis Res Ther 2014;16:R35. 10.1186/ar4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci 2016;81:44–52. 10.1016/j.jdermsci.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 37. Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2016;175:273–86. 10.1111/bjd.14493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-219406supp001.pdf (325KB, pdf)
annrheumdis-2020-219406supp002.pdf (1.1MB, pdf)
Data Availability Statement
No data are available.