Abstract
Histology-based skeletochronology is a widely used approach to determine the age of an individual, and is based on the assumption that temporal cessations or decelerations of bone growth lead to incremental growth marks (GM), reflecting annual cycles. We studied the reliability of histology-based skeletochronology in a variety of extant tetrapods by comparing two different approaches: petrographic ground sections versus stained microtomized sections. Each bone was cut into two corresponding halves at its growth centre in order to apply both approaches to one and the same sample. None of the samples unequivocally revealed the actual age of the specimens, but truly concerning is the fact that the majority of samples even led to conflicting age estimates between the two approaches. Although the microtomized sections tended to yield more GM and thus indicated an older age than the ground sections, the contrary also occurred. Such a pronounced ambiguity in skeletochronological data strongly challenges the value of the respective age determinations for both extant and extinct animals. We conclude that much more research on the fundamental methodological side of skeletochronology—especially regarding the general nature and microscopic recognition of GM—is required.
Keywords: age determination, bone histology, life history, skeletochronology
1. Introduction
Reliable age estimates provide the basis for reconstructing various life history traits such as longevity, age at maturity, growth rates and growth strategies [1–7], and can provide crucial demographic data needed for the successful management and conservation of endangered species [8–10]. Consequently, various approaches for age determinations have been developed. However, morphometric studies on, for example, size frequencies, testis lobation, lens weight or tooth wear can only provide rather rough estimates for the differentiation of adults and juveniles, or vague age classes [11–14]. Furthermore, such approaches not only require a defined frame of reference (individuals of known age), but frequently depend on soft tissues, and thus are inapplicable to fossils. Mark–release–recapture studies are often considered the gold standard, but span over numerous years, frequently pose great challenges concerning the accessibility of the sampling area and recapture likelihood, and are equally inapplicable to fossils.
Skeletochronology is a widely used alternative, based on the assumption that incremental growth marks (GM) are preserved in animal bones and teeth, and thus can serve the basis for absolute age determinations [15–17]. It is generally accepted that at least some temporal cessations or decelerations of growth, namely zones, annuli and lines of arrested growth (LAG), indeed indicate annual cycles [18,19]. The factors leading to and influencing the deposition of GM, however, are still inconclusively understood. They are invariably ascribed to unfavourable seasonal conditions that prohibited continued growth, which may explain their occurrence in ectotherms of temperate climates, but not their presence in endotherms or animals living in aseasonal climates [19,20]. Growth arrest may also be mediated by hormonal cues [21], and the expression of GM is governed by a genetically based internal rhythm synchronized and amplified by seasonal stressors [19,22].
In theory, no frame of reference is required for skeletochronology, and the utilization of mineralized hard tissues is furthermore applicable to fossils. Especially for extinct taxa, bone histology can offer valuable insights into certain otherwise inaccessible aspects of their physiology, and even allows—within certain limits—for taxonomic or individual allocations of specimens [21,23–26]. On closer examination, however, skeletochronology is not necessarily as simple as it appears at first, for reasons ranging from behavioural and environmental variables, differences in bone composition, growth and resorption, to the confident interpretation of GM (especially in the case of missing, additional, multiple and ambiguous marks) [19,27]. Considering that skeletochronology yields accurate age estimates for some species [28–30], but not for others—even closely related ones—or that accuracy drastically decreases with age [31–34], a frame of reference for each species (or even population) remains strongly advised [9,19,27]. However, this is rarely applied in practice and is impossible for fossils.
Another problem arises from the fact that substantially different approaches are used for skeletochronology: palaeontologists mainly use undecalcified petrographic ground sections (ranging in thickness from about 25–200 µm) examined with polarizing microscopes, whereas neontologists usually use decalcified microtomized and stained sections (ranging in thickness from 2 to 25 µm) examined under normal transmitted light. Considering the fundamental differences in the applied histological techniques, systematic evaluations of the comparability of different approaches are remarkably rare [35]. The aim of the present study was to address whether petrographic ground sections and microtomized sections yield the same age estimates, and how the number of GM corresponds to the known age of the individual studied. Or generally speaking, how reliable are histology-based skeletochronological age determinations really, and how meaningful is this approach for inferring life history traits in fossils?
2. Material and methods
Sampling comprised representatives of extant amphibians, reptiles, birds and mammals of known age. Samples were taken from existing specimens—no animals were killed specifically for this study. A complete list of specimens, ages, measurements and additional information is given in the electronic supplementary material (but see also table 1).
Table 1.
Morphometric data and GM counts for the specimens analysed. PC: GM counts based on petrographic ground sections; SC: GM counts based on stained microtomized sections. Length is given in mm for each bone, true age in years. The asterisk (*) indicates that these GM were heavily splitting.
| taxon | sample | humerus |
femur |
true age | ||||
|---|---|---|---|---|---|---|---|---|
| length | PC | SC | length | PC | SC | |||
| Lissamphibia | Bombina orientalis | 13 | 4 | 7 | 17 | 3 | 5 | 5 |
| Lepidosauria | Sphenodon punctatus | n.a. | n.a. | n.a. | 42 | 21 | 21 | n.a. |
| Lacerta viridis | 15 | 4 | 4 | 18 | 5 | 3 | 5 | |
| Pogona vitticeps | 37 | 6 | 12 | 40 | 7 | 11 | 16 | |
| Paroedura picta | 15 | 4 | 6 | 18 | 6 | 6 | 4–5 | |
| Testudines | Cuora amboinensis | 37 | 6 | 8 | 36 | 8 | 8 | ≥25 |
| Mammalia | Mus musculus | 13 | 2* | 2* | 16 | 1* | 1* | 1 |
| Dama dama | 215 | 6 | 6 | 176 | 5 | 7 | 15 | |
| Aves | Gallus domesticus | 80 | 0 | 0 | 88 | 0 | 1 | ≥7 |
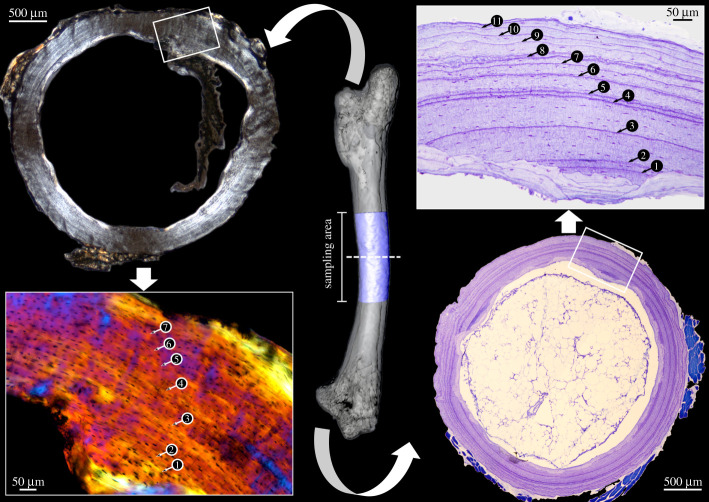
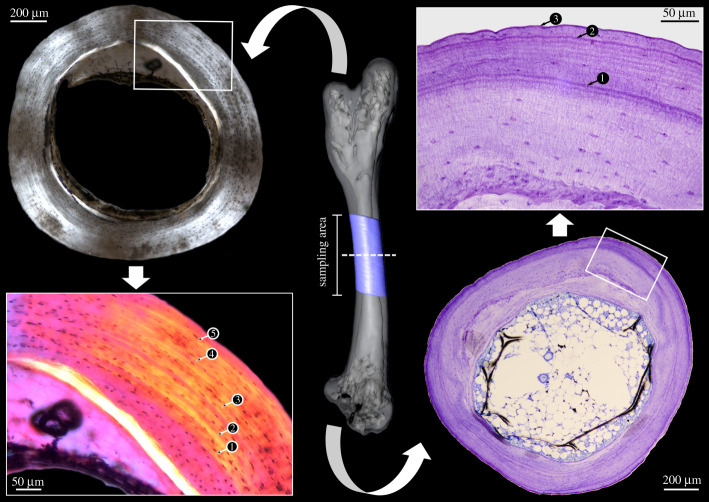
The right humeri and femora of all specimens were sampled (except the Sphenodon punctatus sample, for which only the isolated left femur was available). The isolated bones were µCT scanned (for details, see the electronic supplementary material) to determine the most suitable sampling area: their growth centre, yielding the most complete growth record, which was morphologically identified as the area with the smallest medullary cavity and thickest cortex in cross section. The bones were cut in the middle of the sampling area and the proximal halves were used for petrographic ground sections; the distal halves for conventional microtomized sections.
Samples for petrographic ground sections were placed in absolute ethanol for one week and air dried. Dry samples were embedded in epoxy resin (Araldite 2020, Huntsman), cut with a rock saw (Buehler Isomet 4000 linear precision), and ground on glass plates with silicon carbide powder (F400, F600, F800 grit size, Krantz) down to a thickness of 60–180 µm. The sections were cover-slipped using UV-glue (Verifix LV 740, Bohle).
Samples for conventional microtomized sections were fixed in 4% formaldehyde made fresh from paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) at 4°C, for 45 to 72 h, depending on sample size. Fixed samples were washed in 0.1 M PBS for 30 min. and stored in 70% ethanol until further processing. Samples were decalcified in 5% nitric acid, depending on size, for 1.5 to 50 h, and washed in 0.1 M PBS overnight. Samples were dehydrated in a graded series of ethanol and embedded in methacrylate (Technovit 7100, Heraeus-Kulzer). Samples were sectioned at 2–5 µm with an HM350 rotary microtome (Microm). Staining was done with 0.1% toluidine blue in 0.1% sodium tetraborate. Supplementary staining was done on selected consecutive sections with Ehrlich's haematoxylin, Weigert's haematoxylin + eosin, and cresyl violet. Imaging and GM counting was done with a Zeiss Axio Lab.A1, a Leica DM RE or Leica DM LP microscope (see electronic supplementary material for details).
3. Results and discussion
A general histological description for all specimens studied, together with a taxon-specific discussion, is provided in the electronic supplement. Except for Gallus domesticus (in which only the microtomized femoral section showed one GM), GM could be identified with both approaches in all specimens, a summary of which is given in table 1. However, visibility as well as GM counts generally varied greatly between the two approaches, and also between the respective skeletal elements.
The majority of samples yielded divergent counts when the two approaches were compared, with a larger number of GM recognizable in the microtomized sections as the more frequent case (figure 1). However, one sample also revealed a higher number of GM in the petrographic ground sections compared to the microtomized ones (figure 2). Only two microtomized samples agreed in GM counts for the humerus and femur, respectively, but none of the samples showed a consistent number of GM in the two bones sampled that would reflect their true known age. One may argue that the fact that most of our specimens derived from captivity had an effect on GM deposition and hence interfered with accurate age estimates. However, this circumstance cannot be held responsible for the observed differences between the two approaches, which were applied to one and the same bone sample.
Figure 1.
The right femur of Pogona vitticeps sectioned at its centre of growth. Note that the petrographic ground section (left) reveals a smaller number of GM (arrowheads) than the microtomized section (right). (Online version in colour.)
Figure 2.
The right femur of Lacerta viridis sectioned at its centre of growth. Note that the petrographic ground section (left) reveals a larger number of GM (arrowheads) than the microtomized section (right). (Online version in colour.)
Consistent GM counts between petrographic ground and microtomized sections were obtained only for a minority of samples. Three out of eight humeral samples and four out of nine femoral ones agreed in their GM counts for the same individual. Although identical in terms of the number of GM counted, only one of these seven ‘agreeing’ sample pairs revealed a trustworthy corresponding distribution of the GM along the cross section (Sphenodon punctatus; see electronic supplementary material). The other six sample pairs—based on the relative spacing between the individual GM—raise further doubts that the countable structures in fact correspond to each other (figure 3; electronic supplementary material).
Figure 3.
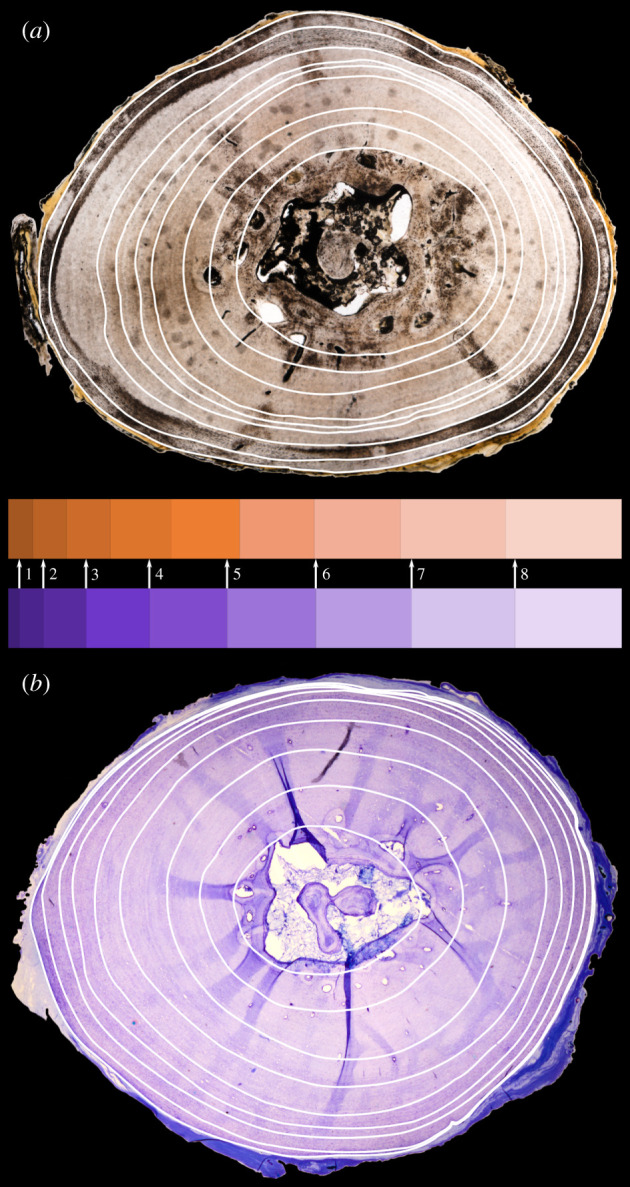
Comparison of the femoral ground section (a) and microtomized section (b) of Cuora amboinensis. Note that the relative spacing of the GM (indicated by the horizontal bar diagrams in the middle) does not show a trustworthy match between the two approaches (see arrows), albeit the numeric value of countable GM agrees in absolute terms. (Online version in colour.)
Our study presents the first data of a systematic comparison of the two primary approaches of GM identification and strongly suggests that there is a fundamental problem: different histological approaches (petrographic ground sections versus microtomized sections) can lead to substantially different age estimates. This is particularly concerning because these deviations can occur in both directions, namely petrographic ground sections can reveal a smaller number of GM than microtomized sections within the same bone (sampled immediately adjacent to each other), and vice versa. Even more concerning is that, also for those sample pairs in which the numbers of GM match, their distribution along the cross section does not correspond to each other, and thus raises doubts of whether the countable GM in the two approaches actually reflect the same structures. In other words: do the putative GM identifiable in ground sections correspond to the putative GM in stained microtomized sections at all? Or to put it even more provocatively: are either one true annual GM at all?
Bone undergoes constant changes, most importantly resorption and remodelling that can lead to the potential loss of GM [19]. Some of these transformations, such as endosteal resorption due to the expansion of the medullary cavity and/or region, can be accounted for by methods of back-calculation, but only if a sufficient growth series is available [20,28]. More difficult and usually not assessable are additional (non-annual) GM corresponding to physiological stresses, such as starvation, disease or abnormally harsh climatic conditions [36].
The mere visibility of GM can be problematic as well and it usually seems to be neglected that the visibility might also greatly depend on the method of sample preparation [35,37]. With regard to the staining of microtomized sections alone, some authors claim that there is no difference [38], whereas others claim better results with one stain or the other [39,40]. We indeed noticed considerable differences in the distinctness of the GM among our samples. This was true for the toluidine blue staining alone, but was even more pronounced when different stains were compared (see electronic supplementary material). Another controversy derives from sample thickness. Ground sections that are too thin readily lose optical contrast [37], whereas a reduction of thickness in microtomized sections aids GM recognition, particularly in delineating peripheral ones [34].
The accurate recognition of GM that do not represent annual cycles, but instead mark important life history events such as metamorphosis in amphibians, hatching in lizards or reproductive phases in adults, and possibly weaning in mammals [4,19,41–43], is a general problem in skeletochronology that may lead to an overestimation of age. Equally problematic are multiple or splitting GM—as in our mouse or green lizard samples—which may be the result of differential bone growth on one side of the bone that should be counted as just one mark [42]. Multiple GM, however, can also indicate multiple cessations in growth per annum (e.g. hibernation in winter and aestivation in summer) and then have to be counted as one annual GM (e.g. [44]).
We conclude that a number of fundamental questions concerning skeletochronology are still awaiting an answer, which are at the very least: what actually constitutes the morphological structure that we see as a GM, and in how far does its extent of mineralization, or chemical composition, differ from that of the remaining bone (in extant and fossil bone)? What structural components or which molecules in GM are specifically targeted by the different stains? What is the influence of section thickness (in ground sections and microtomized sections) on the visibility of GM? Is there a difference between ground sections of extant bones or those from fossils, which due to their taphonomic history are often ‘naturally stained’ and also differ in mineral composition and degree of mineralization? And last but not least: how is it possible that the same bones prepared with the two different approaches lead to considerably different age estimates, including cases where the thicker ground sections reveal more GM than the thinner microtomized sections?
Bone histology undoubtedly remains a valuable tool for understanding certain aspects of population structure and life history traits in modern and extinct taxa. It of course cannot be ignored that there are some cases where skeletochronology provided very accurate age estimates (e.g. [30]). However, the reasons why it only occasionally works are completely unknown and in general far too much work is done without a reliable, systematic analysis of the methodological aspects involved. Our study once again highlights that there are numerous pitfalls in histology-based skeletochronology, especially regarding the absolute numeric aspects. The histological structure of the bone can provide, for example, insights into the state of maturity or the general growth rate. Determining the absolute age, on the other hand, unequivocally requires a well-supported frame of reference that indicates that a given species actually deposits annual GM at all. The required reliable information, however, might not always be available for taxa about which only very little is known and is simply inaccessible for extinct species. Concomitant with this latter circumstance is that the vast majority of studies that actually took external control data into account are based on microtomized sections only (but see [45]). For all of the numerous previous skeletochronological studies, it is impossible to (a) add such a frame of reference a posteriori, and (b) to conduct a comparison of the two approaches, given that this already needs to be considered at the stage of tissue sampling. Unless it can be shown that the growth dynamics of extinct taxa differ considerably from those of extant ones (resulting in much more unambiguous GM), this may, unfortunately, limit the usefulness of skeletochronology for fossil taxa, restricting its applicability to the rather general aspects of their life history mentioned above. Before the wide usage of skeletochronology in applied studies continues, particularly for extant taxa, we urge that much more research should be conducted on its fundamental biological and methodological aspects—especially in terms of understanding the general nature and microscopic recognition of GM.
Despite its appeal, the theoretical framework of histology-based skeletochronology does not seem to hold up in reality. The simplicity of the idea of ubiquitous cyclic GM deposition makes their analysis easy and a widely employed technique used by such diverse groups of biologists as physiologists, ecologists as well as those involved in conservational questions, and of course palaeontologists. However, this is usually done more as a means to an end rather than with the intent to actually understand the morphological dynamics that occur within the bone. The continued use of skeletochronology without prior clarification of the true identity of the structures one counts as putative GM is highly discouraged due to the high degree of ambiguity shown by our study, and eventually understanding these dynamics is required first.
Supplementary Material
Acknowledgements
We wish to acknowledge Olaf Dülfer for assistance with the laboratory work. We furthermore wish to express our gratitude to Joachim Degen (LIMES), Morris Flecks (ZFMK), Iris Gockel-Böhner and Ute Müller (INRES), as well as Georg Gassner and Silke Schweiger (NHMW) for the provision of sample material. P. Martin Sander is thanked for critical discussions.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material [46].
Authors' contributions
P.J.S.: formal analysis, investigation, methodology, visualization, writing-original draft; N.K.: conceptualization, formal analysis, methodology, writing-review and editing; M.L.: conceptualization, formal analysis, methodology, project administration, supervision, writing-original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interest.
Funding
We received no funding for this article.
References
- 1.Cogălniceanu D, Miaud C. 2004. Variation in life history traits in Bombina bombina from the lower Danube floodplain. Amphibia-Reptilia 25, 115-119. ( 10.1163/156853804322992896) [DOI] [Google Scholar]
- 2.Sander PM, Klein N. 2005. Developmental plasticity in the life history of a prosauropod dinosaur. Science 310, 1800-1802. ( 10.1126/science.1120125) [DOI] [PubMed] [Google Scholar]
- 3.Lee AH, Werning S. 2008. Sexual maturity in growing dinosaurs does not fit reptilian growth models. Proc. Natl Acad. Sci. USA 105, 582-587. ( 10.1073/pnas.0708903105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hugi J, Sánchez-Villagra MR. 2012. Life history and skeletal adaptations in the Galapagos marine iguana (Amblyrhynchus cristatus) as reconstructed with bone histological data: a comparative study of iguanines. J. Herpetol. 46, 312-324. ( 10.1670/11-071) [DOI] [Google Scholar]
- 5.Griebeler EM, Klein N, Sander PM. 2013. Aging, maturation and growth of sauropodomorph dinosaurs as deduced from growth curves using long bone histological data: an assessment of methodological constraints and solutions. PLoS ONE 8, e67012. ( 10.1371/journal.pone.0067012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botha-Brink J, Codron D, Huttenlocker AK, Angielczyk KD, Ruta M. 2016. Breeding young as a survival strategy during Earth's greatest mass extinction. Sci. Rep. 6, 24053. ( 10.1038/srep24053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griebeler EM, Klein N. 2019. Life-history strategies indicate live-bearing in Nothosaurus (Sauropterygia). Palaeontology 62, 697-713. ( 10.1111/pala.12425) [DOI] [Google Scholar]
- 8.Castanet J, Newman DG, Girons HS. 1988. Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand. Herpetologica 44, 25-37. [Google Scholar]
- 9.de Buffrénil V, Castanet J. 2000. Age estimation by skeletochronology in the Nile monitor (Varanus niloticus), a highly exploited species. J. Herpetol. 34, 414-424. ( 10.2307/1565365) [DOI] [Google Scholar]
- 10.Marín-Moratalla N, Jordana X, Köhler M. 2013. Bone histology as an approach to providing data on certain key life history traits in mammals: implications for conservation biology. Mamm. Biol. 78, 422-429. ( 10.1016/j.mambio.2013.07.079) [DOI] [Google Scholar]
- 11.Tanaka S. 1962. A method of analysing a polymodal frequency distribution and its application to the length distribution of the porgy, Taius tumifrons (T. and S.). J. Fish. Res. Board. Can. 19, 1143-1159. ( 10.1139/f62-075) [DOI] [Google Scholar]
- 12.Humphrey RR. 1922. The multiple testis in urodeles. Biol. Bull. 43, 45-67. ( 10.2307/1536690) [DOI] [Google Scholar]
- 13.Teska WR, Pinder JE. III. 1986. Effects of nutrition on age determination using eye lens weights. Growth 50, 362-370. [PubMed] [Google Scholar]
- 14.Harris S, Cresswell WJ, Cheeseman CL. 1992. Age determination of badgers (Meles meles) from tooth wear: the need for a pragmatic approach. J. Zool. 228, 679-684. ( 10.1111/j.1469-7998.1992.tb04467.x) [DOI] [Google Scholar]
- 15.Peabody FE. 1961. Annual growth zones in living and fossil vertebrates. J. Morphol. 108, 11-62. ( 10.1002/jmor.1051080103) [DOI] [Google Scholar]
- 16.Klevezal GA, Kleinenberg SEE. 1969. Age determination of mammals from annual layers in teeth and bones (ed. Salkind J). Jerusalem: Israel Program for Scientific Translations. [Google Scholar]
- 17.Castanet J, Meunier FJ, Ricqlès AD. 1977. L'enregistrement de la croissance cyclique par le tissu osseux chez les vertébrés poïkilothermes: données comparatives et essai de synthèse. Bull. biol. Fr. Bel. 111, 183-202. [Google Scholar]
- 18.Francillon-Vieillot H, de Buffrénil V, Castanet J, Géraudie J, Meunier FJ, Sire JY, Zylberberg L, de Ricqlès A. 1990. Microstructure and mineralization of vertebrate skeletal tissues. In Skeletal biomineralization: patterns, processes and evolutionary trends, vol 1 (ed. Carter JG), pp. 471-530. New York, NY: Van Nostrand Reinhold. [Google Scholar]
- 19.Castanet J, Francillon-Vieillot H, Meunier FJ, de Ricqlès AJ. 1993. Bone and individual aging. In Bone, vol. 7: bone growth (ed. Hall BK), pp. 245-283. Boca Raton, FL: CRC Press. [Google Scholar]
- 20.Woodward HN, Padian K, Lee AH. 2013. Skeletochronology. In Bone histology of fossil tetrapods: advancing methods, analysis, and interpretation (eds Padian K, Lamm ET), pp. 187-207. Berkeley, CA: University of California Press. [Google Scholar]
- 21.Köhler M, Marín-Moratalla N, Jordana X, Aanes R. 2012. Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature 487, 358. ( 10.1038/nature11264) [DOI] [PubMed] [Google Scholar]
- 22.Castanet J. 2006. Time recording in bone microstructures of endothermic animals; functional relationships. C. R. Palevol. 5, 629-636. ( 10.1016/j.crpv.2005.10.006) [DOI] [Google Scholar]
- 23.Padian K, de Ricqlès AJ, Horner JR. 1995. Bone histology determines identification of a new fossil taxon of pterosaur (Reptilia: Archosauria). C. R. Acad. Sci., Serie II 320, 77-84. [Google Scholar]
- 24.Sander PM. 2000. Longbone histology of the Tendaguru sauropods: implications for growth and biology. Paleobiology 26, 466-488. () [DOI] [Google Scholar]
- 25.Horner JR, de Ricqlès AJ, Padian K. 2000. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: growth dynamics and physiology based on an ontogenetic series of skeletal elements. J. Vert. Paleontol. 20, 115-129. ( 10.1671/0272-4634(2000)020[0115:LBHOTH]2.0.CO;2) [DOI] [Google Scholar]
- 26.Scannella JB, Horner JR. 2011. ‘Nedoceratops’: an example of a transitional morphology. PLoS ONE 6, e28705. ( 10.1371/journal.pone.0028705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dammers K. 2006. Using osteohistology for ageing and sexing. In Recent Advances in Ageing and Sexing Animal Bones: Proceedings of the 9th Conference of the International Council of Archaeozoology), Durham, August 2002 (ed. Ruscillo D), pp. 9-39. Oxford, UK: Oxbow Books. [Google Scholar]
- 28.Hemelaar A. 1985. An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (L.) and its application to populations from different latitudes and altitudes. Amphibia-Reptilia 6, 323-341. ( 10.1163/156853885X00326) [DOI] [Google Scholar]
- 29.Trenham PC, Shaffer HB, Koenig WD, Stromberg MR. 2000. Life history and demographic variation in the California Tiger Salamander (Ambystoma californiense). Copeia 2000, 365-377. ( 10.1643/0045-8511(2000)000[0365:LHADVI]2.0.CO;2) [DOI] [Google Scholar]
- 30.Snover ML, Hohn AA. 2004. Validation and interpretation of annual skeletal marks in loggerhead (Caretta caretta) and Kemp's ridley (Lepidochelys kempii) sea turtles. Fish. Bull. 102, 682-692. [Google Scholar]
- 31.Lapeña M, Domínguez DP, Hernández F, de Lope Rebollo F, de la Fuente ÁJ. 1993. Two examples showing contradictory results by using skeletochronology in birds. Archaeofauna 2, 175-179. [Google Scholar]
- 32.Bjorndal KA, Bolten AB, Bennett RA, Jacobson ER, Wronski TJ, Valeski JJ, Eliazar PJ. 1998. Age and growth in sea turtles: limitations of skeletochronology for demographic studies. Copeia 1998, 23-30. ( 10.2307/1447698) [DOI] [Google Scholar]
- 33.Eden CJ, Whiteman HH, Duobinis-Gray L, Wissinger SA. 2007. Accuracy assessment of skeletochronology in the Arizona tiger salamander (Ambystoma tigrinum nebulosum). Copeia 2007, 471-477. ( 10.1643/0045-8511(2007)7[471:AAOSIT]2.0.CO;2) [DOI] [Google Scholar]
- 34.Sinsch U. 2015. Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol. J. 25, 5-13. [Google Scholar]
- 35.Goshe LR, Avens L, Bybee J, Hohn AA. 2009. An evaluation of histological techniques used in skeletochronological age estimation of sea turtles. Chelonian Conserv. Biol. 8, 217-222. ( 10.2744/CCB-0777.1) [DOI] [Google Scholar]
- 36.Peabody FE. 1958. A Kansas Drouth Recorded in Growth Zones of a Bullsnake. Copeia 1958, 91-94. ( 10.2307/1440547) [DOI] [Google Scholar]
- 37.Woodward HN, Horner JR, Farlow JO. 2014. Quantification of intraskeletal histovariability in Alligator mississippiensis and implications for vertebrate osteohistology. PeerJ 2, e422. ( 10.7717/peerj.422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinsch U, Leskovar C, Drobig A, König A, Grosse WR. 2007. Life-history traits in green toad (Bufo viridis) populations: indicators of habitat quality. Can. J. Zool. 85, 665-673. ( 10.1139/Z07-046) [DOI] [Google Scholar]
- 39.Klevezal G. 1996. Recording structures of mammals. Determination of age and reconstruction of life history. Rotterdam, The Netherlands: A. A. Balkema. [Google Scholar]
- 40.Rossell CR Jr, Sheehan JL. 1998. Comparison of histological staining procedures for skeletochronological studies. Herpetol. Rev. 29, 95. [Google Scholar]
- 41.Castanet J, Smirina E. 1990. Introduction to the skeletochronological method in amphibians and reptiles. Ann. Sci. Nat., Zool. Biol. Anim. 13, 191-196. [Google Scholar]
- 42.Smirina EM. 1994. Age determination and longevity in amphibians. Gerontology 40, 133-146. ( 10.1159/000213583) [DOI] [PubMed] [Google Scholar]
- 43.Castanet J, Croci S, Aujard F, Perret M, Cubo J, de Margerie E. 2004. Lines of arrested growth in bone and age estimation in a small primate: Microcebus murinus. J. Zool. 263, 31-39. ( 10.1017/S0952836904004844) [DOI] [Google Scholar]
- 44.Caetano MH, Castanet J, Francillon H. 1985. Détermination de l'âge de Triturus marmoratus marmoratus (Latreille 1800) du Parc National de Peneda Gerês (Portugal) par squelettochronologie. Amphibia-Reptilia. 6, 117-132. ( 10.1163/156853885X00010) [DOI] [Google Scholar]
- 45.Tucker AD. 1997. Validation of skeletochronology to determine age of freshwater crocodiles (Crocodylus johnstoni). Mar. Freshwater Res. 48, 343-351. ( 10.1071/MF96113) [DOI] [Google Scholar]
- 46.Schucht PJ, Klein N, Lambertz M. 2021. Data from: What's my age again? On the ambiguity of histology-based skeletochronology. Figshare. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material [46].