Abstract
We investigated whether dogs (Canis familiaris) distinguish between human true (TB) and false beliefs (FB). In three experiments with a pre-registered change of location task, dogs (n = 260) could retrieve food from one of two opaque buckets after witnessing a misleading suggestion by a human informant (the ‘communicator’) who held either a TB or a FB about the location of food. Dogs in both the TB and FB group witnessed the initial hiding of food, its subsequent displacement by a second experimenter, and finally, the misleading suggestion to the empty bucket by the communicator. On average, dogs chose the suggested container significantly more often in the FB group than in the TB group and hence were sensitive to the experimental manipulation. Terriers were the only group of breeds that behaved like human infants and apes tested in previous studies with a similar paradigm, by following the communicator's suggestion more often in the TB than in the FB group. We discuss the results in terms of processing of goals and beliefs. Overall, we provide evidence that pet dogs distinguish between TB and FB scenarios, suggesting that the mechanisms underlying sensitivity to others' beliefs have not evolved uniquely in the primate lineage.
Keywords: theory of mind, dogs, false belief, breed differences, canine cognition
1. Background
Complex social life has been proposed as one of the main driving forces in the evolution of higher cognitive abilities in humans and non-human animals [1,2]. The capacity to recognize that other agents might have mental states different from one's own and that these mental states will usually guide the agents' behaviour can be of considerable advantage when navigating a complex social environment [3,4]. Such a meta-representational capacity has been assumed to lie at the heart of human communication, cooperation and culture [5,6].
A key marker and methodological approach to the study of such mind-reading abilities is the attribution of false beliefs (FB) to others. FB attribution has been regarded for decades as a distinctly human skill, and even as something that makes humans distinct from other animals. Inspired by the seminal article that launched comparative research on ‘theory of mind’ [5] and by Dennett's commentary [7], Wimmer & Perner [8] invented a now classic verbal FB task (later also called the ‘Sally-Anne’ task; [9]). In this, children viewed a scenario in which one person (Maxi) placed a piece of chocolate in a cupboard and then left the room. While Maxi was absent, his mother would transfer the piece of chocolate to another cupboard. FB processing was reflected by the children indicating that Maxi, after returning into the room, expected the chocolate to be in the first cupboard, which was contrary to the state of reality. Only children from the age of about four onwards could pass this test.
For many years thereafter, it was reasoned that non-human animals lacked FB understanding. For instance, while children around 5 years of age succeeded in a competitive FB task, chimpanzees (Pan troglodytes) and bonobos (Pan paniscus) failed [10]. Children, unlike apes, avoided the container that a human competitor tried to reach when the latter had a FB about the location of a hidden reward. However, apes' looking behaviour in this study—they looked more often at the unchosen container in FB trials than in true belief (TB) control trials—raised the possibility for an implicit FB understanding. Implicit FB tests developed for human infants [11–13] showed that chimpanzees, bonobos and orang-utans (Pongo abelii), and more recently even monkeys (Macaca fuscata), anticipated the location where an actor would search for an object based on the actor's FB about the object's location [14–16]. In another study using an explicit task, apes distinguished between TB and FB in their helping behaviour [17]. Thus, evidence has been accumulating that non-human primates are able to distinguish false from true beliefs, and may possess a theory of mind (but see [18]). However, it remains an open question whether the primates in these studies fully represented the actor's beliefs (the mentalist account) or relied on a sophisticated rule learned during their lives that agents tend to search for things where they last saw them (the behaviour rule account [6,18,19]). Even the looking behaviour and reaction times of human infants and adults tested on implicit TB tasks can in some cases be explained in terms of low-level, domain general processes, including retroactive interference and distraction [20,21], although recent findings with apes [22,23] and human infants [24] did not find any support for the submentalizing account of belief-based action prediction.
Little is known about FB understanding in other animals [6]. Among non-primates, dogs (Canis familiaris) constitute a particularly interesting case, as their social environment has been shared with humans for at least 14 000 years [25]. For this reason, dogs have been considered as a model species for the comparative investigation of socio-cognitive abilities [26–28]. Many studies have focused on dogs' understanding of human gestures, emotions and communication (for reviews, see [29–32]). In particular, dogs have shown to be sensitive to human attentional states [33,34] and they also seem to understand that human actions are goal-directed [35]. A study by Virányi et al. [36] found that dogs, similarly to human children, were more likely to indicate the location of a hidden toy to a human helper when this person was absent during the hiding relative to when she was present. Since then, the literature has seen accumulating empirical evidence for dogs’ visual perspective-taking abilities [37–39], including evidence for geometrical gaze following [40]. But the question remains whether, in addition to their behaviour-reading competences, dogs are also sensitive to some mental or psychological states of humans, apart from visual perspectives different from their own.
With the present, pre-registered study, we aimed at assessing whether pet dogs distinguish by active choice between the TB and FB of a human informant according to Wimmer & Perner's change of location paradigm [8]. We adapted for dogs interactive tasks that had been previously employed with human infants [41] and apes [17,42]. We chose to embed the FB task in a (mostly) cooperative situation because previous studies suggest that dogs excel at reading human communicative intentions and might interpret some repeated hiding-finding interactions as a social game with the experimenter (e.g. [43,44]).
In our test, dogs’ own witnessing of the events conflicted with the suggestion they received from an experimenter (the communicator) about the location of food. Food was visibly transferred from container A to container B either in the presence (true belief condition) or in the absence (FB condition) of the communicator. In both test conditions, dogs witnessed the initial hiding and the subsequent displacement of food but the communicator highlighted with multi-modal signals (including gaze alternation and talking [45]; figure 1) container A, that had been baited at the beginning of the trial, but was empty after the food displacement.
Figure 1.
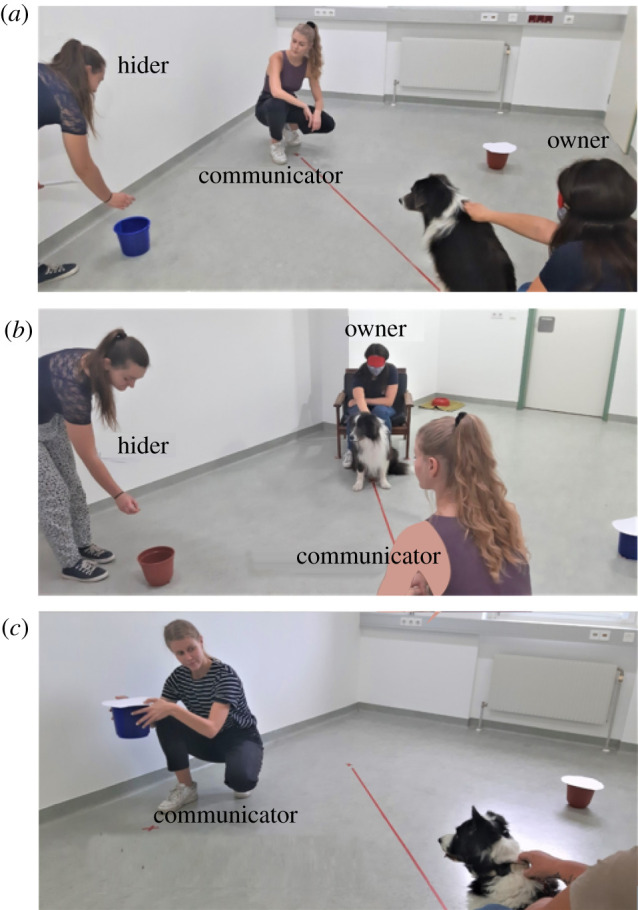
Dog and communicator faced each other and had an unrestricted view of the hider's actions (pictures a and b). The blindfolded owners sat on the armchair while holding their dog (pictures a and b). Three different video cameras recorded the scene. The communicator (during test trials of all conditions) used a different door than the hider to leave and re-enter the room. The communicator's door is partially visible in picture a, on the right. The hider always used the door visible in picture b to enter and exit the room. Picture a shows the hider hiding food in the first baited bucket (bucket A), while dog and communicator watched. Picture b shows the hider hiding the food in the second and final bucket (bucket B). Picture c shows the communicator suggesting bucket A. (Online version in colour.)
The rationale for testing dogs with the communicator's misleading information in both false and true belief conditions was the following. If dogs would only choose according to their knowledge of the final location of food, they would ignore the communicator's cue and choose bucket B equally in the two groups. So the first question we aimed to answer was whether dogs would react differently in TB and FB groups in response to the same misleading suggestion. Before data collection, and as pre-registered, we hypothesized that if dogs would choose in some cases bucket A following the communicator's suggestion, they would have more reason to do so in the true than in the FB condition according to the FB logic (see also [17,41], on which we based our pre-registered hypotheses). In essence, this logic goes as follows: in the true belief condition, the communicator suggests for the first time the empty container despite having herself witnessed the hiding of food in its final location. Dogs might interpret this novel, ‘misleading’ cue as driven by the intention to show something new about the empty bucket A. Hence, we expected that at least some of the dogs in the true belief group might have wanted to explore what else the communicator intended to show them. By contrast, if dogs attributed a FB to the communicator, they should have been less inclined to follow her suggestion to the empty container in the FB group, given that they had privileged knowledge about the food location compared to the communicator.
We conducted three consecutive experiments. After the main experiment, in which we compared dogs' performance between the TB and FB scenario, we ran two control experiments following-up and bolstering the conclusions from the first one. In the first of these two, we tested an additional group of dogs on a control version of the true belief scenario to assess whether retroactive interference (the disruption of memory of a previously encoded event when this is followed by a second salient event [46,47]) might have explained dogs’ behaviour in experiment 1. Finally, in the third experiment, we followed up the unexpected breed differences emerged in the first experiment. We explored breed group differences focusing on the functional groups proposed by the FCI (Fédération Cynologique Internationale, World Canine Organization). These are based on dogs' main behavioural characteristics and reflect the human selective efforts to create individuals well-suited to different activities (e.g. sheepdogs, retrievers, companion dogs, scent hounds, etc.).
2. Material and methods
The experimental design, hypotheses, procedure and data analysis for experiment 1 were pre-registered prior to data collection at the Open Science Framework (https://osf.io/g75km).
(a) . Subjects
The final sample for experiment 1 consisted of 120 pure-bred dogs (69 females and 51 males) belonging to 36 different breeds and classifiable under eight different FCI groups. The age range went from five months to 14 years; the mean age was 5.31 years. The sample was counterbalanced for age, sex, first baited bucket, communicator's identity and breed across groups. To determine the sample size, we had conducted a power simulation before registering our study plans (the details are reported in the pre-registration: https://osf.io/g75km; results in the electronic supplementary material).
The final sample for experiment 2 consisted of 60 pure-bred dogs (34 females) belonging to 21 different breeds and classifiable under the same eight FCI groups as the dogs in experiment 1. For the control condition, we matched as much as possible the number of subjects within each FCI group with the sample of dogs in the true belief group of experiment 1. Subjects' age range went from five months to 10 years (mean age: 4.43 years).
In experiment 3, we tested only border collies (n = 40, 25 females) and terriers belonging to 14 different breeds (n = 40, 22 females). The border collies’ age range went from five months to 12 years (mean age: 4.47 years). The terriers' age range went from six months to 12 years (mean age: 4.20 years). Both samples were balanced for age, sex, first baited box and (for terriers only) breed between conditions. A number of additional dogs participated but were not included in the analyses because they did not meet the pre-registered inclusion criteria, they were not pure-bred, or due to an experimenter mistake (see electronic supplementary material for more details).
Each dog participated in only one of the three experiments within this study (see electronic supplementary material, datasets S1–S3 for more information on the demographics and individual performance).
(b) . Set-up
All experiments were conducted in the same 6.05 × 3.33 m room of the Clever Dog Lab, Vienna. The room was accessible through two different doors (figure 1; electronic supplementary material, figure S1). The performance of dogs in all trials (familiarization and test phase) was recorded with a camera system consisting of three cameras. For experiment 1, the set-up was composed of two 15 cm high opaque plastic buckets of different colours (one blue, the other brown), positioned at 1.50 m from each other and equidistant to the dog (1.76 m, figure 1; electronic supplementary material, figure S1). The buckets were covered with an opaque, over-sized paper lid. Dogs could push the lid off with snout or paw. Before each session, the inside of both buckets was dusted with the same type of food that was used in the experiment.
In experiments 2 and 3, two new experimenters acted as hiders. The set-up was identical to experiment 1 (although in some cases, we used shorter buckets to test shorter breeds; see supplementary material for details). Across all experiments, all dogs were tested with communicators and hiders unfamiliar to them.
Water was available ad libitum.
(c) . Procedure
All experiments took place during a single session of approximately 20 min. They consisted of three familiarization phases and a subsequent test of one trial only. The familiarization aimed at introducing the dog to the set-up, the experimenters and to the possibility of retrieving food from one of the buckets. We also used the familiarization as a screening to assess whether each dog was attentive enough to be tested (see electronic supplementary material for more details). By the end of the familiarization, dogs were accustomed to a series of events. One experimenter (the hider) always hid food in one container first (container A) and in half of the trials she additionally transferred the food to a second container (B) before leaving the room (figure 1a,b). The other experimenter (the communicator) always followed the hider's movements very carefully and, once the hiding event was completed, she suggested to the dogs where to look for food, based on what she had witnessed. This means that during the familiarization, the communicator always suggested to the dog the correct location of food. The communicative cue used to suggest consisted of approaching the container, crouching down close to it, picking it up, alternating gaze between it and the dog and uttering the sentence ‘look, this is good, this is very good’ (figure 1c). The identical cue was also used in the test phase.
The test phase consisted of one trial, showing either a true belief scenario (for dogs in the TB group) or a FB scenario (FB group). In the FB condition, the communicator, after witnessing the baiting of container A, walked outside of the room and closed the door behind her. At this point, the hider transferred the food from container A to container B in full view of the dog and left the room using the door behind the owner (figure 1). Upon returning, the (mistaken) communicator crouched down in the initial, neutral position and waited 2 s, looking to the floor motionlessly. Then she went to container A (the one which had been baited first) and performed the ostensive multi-modal cueing.
Also in the TB condition, the communicator left the room after the hiding of bucket A. However, while she was absent (for the same duration as in the FB condition), the hider quietly crouched down close to container A and waited for the communicator to re-enter the room. Only when the communicator had come back, and crouched down in her initial, neutral position, the hider removed the food from container A and visibly (for both dog and communicator) transferred it to container B. Apart from this difference, the sequence of events and especially the cueing to the dog was identical to the FB condition.
In the control true belief (CTB) condition, the hider hid food in container A, immediately displaced it to container B in the presence of the communicator and then left the room. Only at this point, also the communicator left the room, closed the door behind her, and re-entered immediately. She then proceeded as in the other conditions (pause and suggestion of bucket A).
Both during the familiarization and during the test, every time food was displaced, dogs could directly see as well as smell the bait being removed from container A and placed in container B. We only used visible displacements given that there is no clear evidence that dogs understand invisible displacements [48]. In all three test conditions, after hiding the reward in bucket B, the hider sneakily removed it from there and brought it with her outside of the room. Neither of the containers therefore contained a food reward when the dogs made their choice in the test trial, to avoid that dogs made their choice upon sniffing the piece of food in container B. Dogs did not seem to notice the removal of the food as they all still made a choice.
The dogs participating in experiments 1 and 3 were presented either with the FB or TB event. Those participating in experiment 2 were presented only with the CTB event.
(d) . Scoring and analyses
From the video recordings of the test trials, we scored dogs' choice and latency to choose. For the choice response, we scored whether dogs touched first (with their nose or one of the front paws) container A, as suggested by the communicator, or container B, where dogs saw food being hidden last. Following the reasoning described above, our pre-registered hypothesis was to find more dogs in the true belief group than in the FB group choosing the suggested container A. Additionally, we measured the time from the moment in which the dog was released by the owner until the first touch with nose or front paw on the chosen container (see electronic supplementary material).
For experiments 1 and 2, we analysed the bucket-A choices using two binomial GLMMs. We included the predictor variables group (FB/TB/CTB), age (in years), sex and ID of first baited box. We also included breed as random intercept and the random slopes of sex, condition, age and first baited box within breed. For experiment 3, we fitted separate binomial GLMs to the border collie and terrier choice data. We included the same predictor variables as in experiments 1 and 2 (see electronic supplementary material for more details concerning the analysis).
3. Results
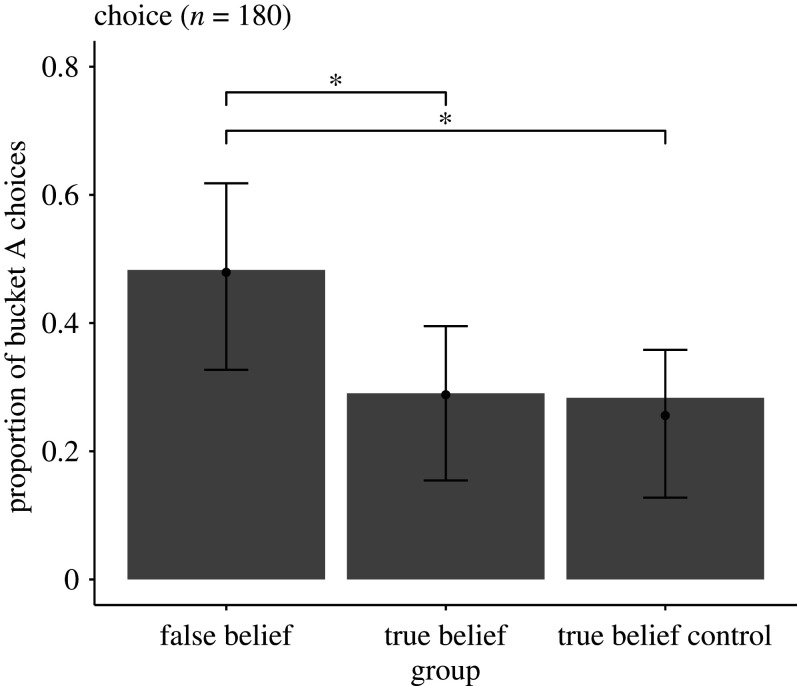
Across the two groups (TB and FB) of experiment 1, more dogs followed their own visual experience of where the food had been hidden rather than the communicator's suggestion (percentage of dogs that chose the suggested but empty container A: 38.5%). This percentage resulted from 48% of dogs in the FB group and 29% of dogs in the TB group choosing container A. This pattern of findings was opposite to our pre-registered predictions. A GLMM confirmed that dogs in the FB group were indeed significantly more likely to choose container A than dogs in the TB group (χ2 = 4.83, d.f. = 1, p = 0.028; electronic supplementary material, table S1; figure 2). Concerning model stability (see electronic supplementary material), the effect of experimental group was stable even when excluding one breed at a time (original estimate: 0.930, min: 0.791, max: 1.257).
Figure 2.
Comparison between false and true belief groups from experiment 1 (left and middle column) and control true belief group from experiment 2 (right column). The plot shows on the y-axis the proportion of dogs that chose the suggested (empty) bucket A. The experimental group is shown on the x-axis. Black lines with asterisks show statistically significant differences between false belief group and both true belief groups (p < 0.05). Black dots indicate fitted values and error bars 95% confidence intervals. Dogs reacted in the same way across true belief groups and in a different way between false belief and true belief groups.
Thus, dogs proved to be sensitive to the experimental manipulation; but they did so by showing the opposite pattern to what we had predicted based on findings in human infants and non-human primates. One explanation for their unexpected choice pattern might have resided in retroactive interference. Indeed, one could have hypothesized that dogs in the FB group were distracted by the communicator re-entering the room after the final hiding of food in bucket B. In the TB condition, this did not happen because the communicator was already inside the room during the displacement. Thus, retroactive interference might explain why dogs in the FB group were more inclined to follow the communicator's misleading suggestion.
To rule this hypothesis out, in experiment 2, we tested a new group of dogs (n = 60) on a CTB scenario and we compared their choices to those of the dogs in experiment 1 using a second binomial GLMM. In CTB condition, the possibly distracting events (the communicator leaving and re-entering the room) happened after the final hiding of food in bucket B, as in the FB condition. Therefore, based on the retroactive interference hypothesis, dogs should have chosen bucket A at similar rates in the FB and in the CTB group. If, however, dogs' choices were influenced by the communicator's belief, they should have chosen bucket A at similar rates in the TB and in the CTB group.
Dogs’ performance varied significantly across the three groups (GLMM: χ2 = 7.07, d.f. = 2, p = 0.029). In particular, dogs in the CTB group chose bucket A at similar rates (percentage: 28%) as in the TB group of experiment 1 (percentage: 29%; TBC–TB comparison: z = 0.36, p = 0.721). When comparing the CTB group to the FB group of experiment 1, we found once more that dogs in the FB group were significantly more likely to choose bucket A (percentage: 48%; comparison CTB–FB: z = 2.39, p = 0.017). Hence, retroactive interference does not seem a plausible explanation for dogs' differential reactions in the main experiment.
Additionally, younger dogs were significantly more likely to choose the suggested bucket A than older dogs irrespective of condition (χ2 = 8.16, d.f. = 1, p = 0.004).
Based on the visual inspection of dogs’ choices in experiment 1 (electronic supplementary material, figure S2), afterwards further supported by an exploratory analysis (electronic supplementary material, figure S8), the effect of experimental group (TB/FB) seemed to be completely reversed in one group (3: terriers), although no reliable conclusion could be drawn at that stage, given the small sample of terriers tested in experiment 1 (n = 10). To test the hypothesis that performance in this task might be influenced by the cooperativeness or independence of the breeds, we ran a follow-up experiment (experiment 3) in which we tested a larger sample of terriers and border collies.
Fifty-five per cent of the border collies in the FB group chose the empty container A, while 30% did so in the TB group. For the terriers, 20% of the dogs FB group chose the empty container A, while 50% did so in the TB group. A GLM confirmed that the two FCI groups reacted differently to the TB/FB manipulation (FCI × group interaction: χ2 = 6.70, d.f. = 1, p = 0.010; see electronic supplementary material, table S6). For the border collies, the TB/FB groups did not differ significantly (χ2 = 2.54, d.f. = 1, p = 0.111; see electronic supplementary material, table S7). The terriers in the TB group, however, were significantly more likely to choose container A than the terriers in the FB group (χ2 = 4.74, d.f. = 1, p = 0.029; see electronic supplementary material, table S8). Hence, the effects of experiment 1 were replicated for both border collies and terriers (electronic supplementary material, figure S2 and S6).
4. Discussion
This study aimed at investigating whether dogs would spontaneously behave in a different way in response to a misleading suggestion from a human informant with a TB or a FB and this is indeed what we found in experiment 1. The combined results of experiments 1 and 2 suggest that retroactive interference is not a likely explanation for the behaviour of dogs in this task. Finally, the results of experiment 3 show that performance in this task is subject to breed (group) differences.
Both conditions of experiment 1 (TB and FB) were characterized by very similar behavioural cues: the hider always moved food from container A to container B; the communicator always left and re-entered the room after the same amount of time, and afterwards always suggested the empty container A. Hence, the only difference between the TB and FB condition in experiment 1 was the moment in which the communicator re-entered the room: before (TB) or after (FB) the food displacement by the hider. From this difference, it could be inferred whether the communicator could or could not see the displacement of food and hence whether she was left with a FB (that food was still in bucket A), or whether her belief (that food was in bucket B) was updated and veridical.
In the absence of any previous training, dogs in the two groups of experiment 1 (TB, FB) behaved differently in response to the same misleading suggestion. More dogs in the FB than in the TB group approached and touched the suggested (empty) container. Given that the two scenarios differed in the timing of the communicator re-entering the room, we introduced a third condition to rule out the possible influence of the moment of re-entry. Indeed, one could have argued that dogs in the FB group of experiment 1 might have been distracted by the salient event of the communicator re-entering the room after the final hiding of food and hence were more inclined to trust the human signal due to retroactive interference (as already proposed for FB studies with human infants and adults [20,49]).
The events in the CTB condition had the potential to elicit the same retroactive interference (if not more) as those in the FB scenario. Indeed, dogs in the CTB witnessed the communicator both leaving and re-entering the room after the final hiding of food. The combined results of experiments 1 and 2, however, show that dogs in the two true belief scenarios reacted in the same way despite the difference in the order of the events. Therefore, dogs' responses did not depend on subtle details of the sequence of events in the experimental procedure. In line with findings that dogs showed an ability to judge human informants on the basis of what these have seen or not [40], our findings thus add further evidence that dogs possess the ability to differentiate between human knowledge states.
Prior to running the experiments, we had predicted that more dogs from the true belief group should have followed the communicator's cue. Surprisingly, instead, more dogs followed the communicator's misleading suggestion when the latter was absent during the food displacement (FB group). Thus, their behaviour was opposite to that of Buttelmann et al.'s human infants and apes [17,41], and to our own pre-registered hypothesis. A possible explanation for this behavioural pattern might reside in the way the communicator's intention was interpreted. In our experiment, the communicator first (during the familiarization trials) proved to be a reliable helper for the dogs and then during the test suggested for the first time the wrong container. In Buttelmann et al.'s studies [17,41], the participants were the ones asked to help the experimenter retrieve a hidden object, which was of no value for the participants themselves. In this kind of helping paradigm, the experimenter's goal was not to communicate to participants the location of the hidden object, therefore it is unlikely that participants viewed the experimenter as untrustworthy. In our task, however, it is possible that dogs in the true belief group interpreted the communicator's misleading behaviour as deceitful, or driven by another (unknown) intention, and therefore more dogs in this group (TB) than in the FB group ignored her suggestion and chose bucket B.
Previous studies have shown that dogs do not follow human misleading pointing gestures blindly (although sometimes they find them difficult to ignore [50]); instead, they can adjust their behaviour flexibly depending on the trustworthiness of the informant [51] and can discriminate between helpful and uncooperative experimenters [52]. Along this line of argument, it seems plausible to assume that dogs in both groups remembered the final location of food (bucket B). However, the communicator's misleading suggestion in the TB scenario might have appeared as deceitful if dogs attributed a true belief to the communicator. Whereas the same misleading suggestion (of bucket A) might have appeared as a mistake ‘in good will’ in the FB scenario if dogs understood that the communicator lacked the relevant knowledge (ignorance) or that she believed food was actually in container A (FB). This might explain why more dogs in the FB than in the TB group followed the misleading suggestion. Indeed, previous research indicates that dogs readily conform to a familiar and unfamiliar human's influence in object-choice tasks even when there is no apparent need to do so and, crucially, even when conforming leads to a suboptimal outcome for the dog [45,53–57]. In particular, Prato-Previde et al. [53] found that younger dogs were more easily misled by a human's influence than older ones, similar to the age effect revealed when pooling experiments 1 and 2 in the current study. We decided to test dogs from five months of age because Barnard et al. [55] had shown that the tendency to conform to human misleading suggestions is present in puppies already at 4 months. Similarly to what happened in our setting, Topál et al. [44] found that dogs in a hiding-finding game kept searching for a toy in previous hiding locations they knew to be empty. The authors suggest that such a ‘rule-following’ behaviour might minimize social conflicts and enhance social cohesion with humans [26,58]. In our task, the communicator with a FB might have been perceived as a mistaken informant who was still playing the game by the same rules as in the familiarization. Instead, the communicator with a true belief (suddenly switching to uncooperativeness) might have been perceived as less trustworthy or violating the rules of the game and this might explain why her suggestion was ignored more often.
Although we did not predict nor pre-register substantial breed effects, we had decided to test only pure-bred dogs in order to be able to explore possible differences. This resulted in the finding that the behaviour of terriers deviated from the one of most other breed groups (electronic supplementary material, figure S2 and S6). In particular, already in experiment 1, we observed a difference in the choice pattern of terriers (FCI group 3), on the one hand, and other breed groups, such as FCI group 2 (in our sample, Schnauzers, Molossoids and Swiss Mountain and Cattledogs), FCI group 7 (pointing dogs) and FCI group 8 (in our sample, retrievers) on the other hand. Unlike other breeds, more terriers from the TB group than from the FB group chose the empty container.
To confirm this unpredicted result, we tested new cohorts of terriers and border collies in experiment 3. We replicated our initial findings: while border collies behaved in accordance with the majority of breeds in experiment 1 (although we did not find a statistically significant difference between conditions; for comparison, the performance of the border collies in experiment 1 is shown in electronic supplementary material, figure S2) terriers exhibited the opposite behavioural pattern. The response pattern of the latter FCI group matches our initial prediction and is consistent with human infants' and great apes’ performance in a similar task [17,41].
We can only speculate about the reasons for the observed differences between FCI groups. The breed differences that have been reported in a scientific context mainly concern specific temperament traits [59,60], behaviours [61–63] and interspecific communicative abilities (e.g. tendency to look at a human's face and to follow pointing gestures [64–66]). Based on the working history of some breeds, Gácsi et al. [64] classified dogs into two main categories: cooperative and independent workers. Accordingly, dogs in the first category have been selected for cooperating while keeping continuous visual contact with their human partner, whereas the latter have been selected for working without any human visual contact. The authors found that cooperative workers (e.g. shepherds and gundogs) were more willing than independent ones (e.g. terriers, hounds, greyhounds and sledge dogs) to follow human distal, temporary pointing gestures. However, only limited attention has been devoted to the empirical investigation of dog breed differences in cognition [67]. An interesting exception is the study by Heberlein et al. [68], who found that independent workers and family dogs, when forbidden to eat food, were more skilled at taking their owner's perspective than cooperative workers. Based on the results of experiment 1, we decided to compare dogs considered as cooperative workers (here: border collies) to independent workers (here: terriers) in experiment 3. Terriers were chosen because they had shown the initially hypothesized response pattern in experiment 1; border collies because they have been extensively tested in studies on social cognition in our and other laboratories [69–71].
In the current study, breed differences might be indicative of different interpretations of the intentions behind human communicative signals. Indeed, terriers were not only more independent of the communicators' signal irrespective of condition, but they also reacted in the opposite way to the scenarios compared to pointing dogs, retrievers, molossoids and border collies (electronic supplementary material, figure S2 and S6). In particular, it is possible that many of the ‘cooperative workers’ in our study interpreted the communicators' cue in the TB scenario as deceitful while many terriers interpreted it as motivated by the intention to show something new. Marshall-Pescini et al. [45] suggested that the intentions behind human actions might play an important role in causing dogs’ social bias (i.e. the tendency to make counterproductive choices under the influence of human signalling). From our findings, it seems possible that artificial selection made cooperative workers more skilled at detecting human deception relative to independent workers. Indeed, it has been suggested that one of the necessary conditions for the emergence of reciprocal altruism is that the cooperating animals need to be able to recognize cheaters [72]. However, to test this hypothesis, future research is needed to target specifically the reaction of a larger sample of other ‘independent workers' (e.g. sled dogs, hounds and greyhounds) to this task.
The evolutionary origin of dogs' ability to distinguish between TB and FB of humans remains an open question. Future studies should examine how dogs and wolves (Canis lupus) compare in the current paradigm. If dogs’ increased attention to human mental states results from the process of domestication, wolves are not likely to perform similarly to dogs. Additionally, future research should clarify based on broader phylogenetic comparisons (e.g. comparing dogs with other domesticated species or primate species) whether identical or only superficially similar mechanisms have evolved across species and taxa.
In conclusion, our study provides the first experimental evidence that dogs distinguish between a TB and a FB condition in a change-of-location task. Although in both conditions the communicator suggested the empty container, different numbers of dogs in the two groups followed this cue. For most dog breeds, this response pattern was in contrast to those found in human infants and great apes—with the notable exception of the terrier breed group. This raises the possibility that pet dogs attribute to human informants, in the absence of any training, not only different knowledge states, but also different intentions and beliefs. Distraction [20] is very unlikely to account for this finding. Indeed, not only the good performance in the familiarization phase but also the fact that the majority of dogs in experiment 1 (61.5%) and in the CTB group (72%) followed their own knowledge proved that the dogs were sufficiently attentive to find food hidden and displaced.
Based on the experience dogs made during the familiarization phase that the communicator's suggestion was trustworthy, the cueing of the empty container in the test has likely caused a conflicting information for the dogs. A possible account for the difference between FB and TB groups in dealing with this misleading suggestion by the human informant is in terms of mental state attribution. In the FB group, a decent number of dogs from cooperative breeds might have followed the wrong suggestion of the informant by attributing to her a FB and consequently a ‘justified’ mistake in good will. However, in the TB groups, a lower number of dogs followed the same suggestion because this appeared deceitful or at least unjustified based on the informant's epistemic state. By contrast, dogs from more independent breeds like terriers may have interpreted the TB informant's suggestion as invitation to explore the first hiding place further and therefore relatively more terriers of the TB group followed it. Of course, such mentalistic accounts in terms of how the situation appears from the communicator's perspective and what the intention of the communicator is when suggesting the wrong container, would need additional evidence from experiments with specific controls for other accounts (behavioural rules, ignorance, submentalizing, minimalist accounts, experiential record-keeping and awareness relations; see [6,18,20,49]). Until that, the possibility that dogs possess what seems at least an implicit FB understanding remains an exciting hypothesis.
Supplementary Material
Acknowledgements
The authors are grateful to all owners and dogs who participated in the study. We also wish to thank Roger Mundry for assistance with statistical analysis; Karin Bayer for administrative support; and several hiders and communicators for their help with data collection.
Ethics
The study was discussed and approved (ETK-030/02/2020) by the ethics and animal welfare committee of the University of Veterinary Medicine of Vienna in accordance with GSP guidelines and national legislation. Written consent to participate in the study was obtained by the dogs’ owners.
Data accessibility
All datasets, statistical tools and code used for the analyses and plots are available as electronic supplementary material [73,74].
Authors' contributions
L.L.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing-original draft, writing-review and editing; C.J.V.: conceptualization, data curation, formal analysis, methodology, software, supervision, validation, visualization, writing-review and editing; C.L.: conceptualization, funding acquisition, resources, supervision, validation, writing-review and editing; L.H.: conceptualization, funding acquisition, methodology, resources, supervision, validation, writing-original draft, writing-review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Vienna Science and Technology Fund (WWTF), the City of Vienna and Ithuba Capital AG through project CS18-012 and the Austrian Science Fund (FWF) through project W1262-B29.
References
- 1.Dunbar R, Dunbar RIM. 1998. Grooming, gossip, and the evolution of language. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Humphrey NK. 1976. The social function of intellect. In Growing points in ethology (ed. Hinde RA), pp. 303-317. Cambridge, MA: Cambridge University Press. [Google Scholar]
- 3.Heyes CM. 1998. Theory of mind in nonhuman primates. Behav. Brain Sci. 21, 101-114. ( 10.1017/S0140525X98000703) [DOI] [PubMed] [Google Scholar]
- 4.Lurz RW. 2011. Mindreading animals: the debate over what animals know about other minds. Cambridge, MA: The MIT Press. [Google Scholar]
- 5.Premack D, Woodruff G. 1978. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1, 515-526. ( 10.1017/S0140525X00076512) [DOI] [Google Scholar]
- 6.Krupenye C, Call J. 2019. Theory of mind in animals: current and future directions. Wiley Interdiscip. Rev. Cogn. Sci. 10, e1503. ( 10.1002/wcs.1503) [DOI] [PubMed] [Google Scholar]
- 7.Dennett DC. 1978. Beliefs about beliefs [P&W, SR&B]. Behav. Brain Sci. 1, 568-570. ( 10.1017/S0140525X00076664) [DOI] [Google Scholar]
- 8.Wimmer H, Perner J. 1983. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition 13, 103-128. ( 10.1016/0010-0277(83)90004-5) [DOI] [PubMed] [Google Scholar]
- 9.Baron-Cohen S, Leslie AM, Frith U. 1985. Does the autistic child have a ‘theory of mind’? Cognition 21, 37-46. ( 10.1016/0010-0277(85)90022-8) [DOI] [PubMed] [Google Scholar]
- 10.Krachun C, Carpenter M, Call J, Tomasello M. 2009. A competitive nonverbal false belief task for children and apes. Dev. Sci. 12, 521-535. ( 10.1111/j.1467-7687.2008.00793.x) [DOI] [PubMed] [Google Scholar]
- 11.Onishi KH, Baillargeon R. 2005. Do 15-month-old infants understand false beliefs? Science 308, 255-258. ( 10.1126/science.1107621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements WA, Perner J. 1994. Implicit understanding of belief. Cogn. Dev. 9, 377-395. ( 10.1016/0885-2014(94)90012-4) [DOI] [Google Scholar]
- 13.Southgate V, Senju A, Csibra G. 2007. Action anticipation through attribution of false belief by 2-year-olds. Psychol. Sci. 18, 587-592. ( 10.1111/j.1467-9280.2007.01944.x) [DOI] [PubMed] [Google Scholar]
- 14.Kano F, Krupenye C, Hirata S, Tomonaga M, Call J. 2019. Great apes use self-experience to anticipate an agent's action in a false-belief test. Proc. Natl Acad. Sci. USA 116, 20 904-20 909. ( 10.1073/pnas.1910095116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupenye C, Kano F, Hirata S, Call J, Tomasello M. 2016. Great apes anticipate that other individuals will act according to false beliefs. Science 354, 110-114. ( 10.1126/science.aaf8110) [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, et al. 2020. Macaques exhibit implicit gaze bias anticipating others' false-belief-driven actions via medial prefrontal cortex. Cell Rep. 30, 4433-4444. ( 10.1016/j.celrep.2020.03.013) [DOI] [PubMed] [Google Scholar]
- 17.Buttelmann D, Buttelmann F, Carpenter M, Call J, Tomasello M. 2017. Great apes distinguish true from false beliefs in an interactive helping task. PLoS ONE 12, e0173793. ( 10.1371/journal.pone.0173793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horschler DJ, MacLean EL, Santos LR. 2020. Do non-human primates really represent others’ beliefs? Trends Cogn. Sci. 24, 594-605. ( 10.1016/j.tics.2020.05.009) [DOI] [PubMed] [Google Scholar]
- 19.Perner J, Ruffman T. 2005. Infants' insight into the mind: how deep? Science 308, 214-216. ( 10.1126/science.1111656) [DOI] [PubMed] [Google Scholar]
- 20.Heyes C. 2014. Submentalizing: i am not really reading your mind. Perspect. Psychol. Sci. 9, 131-143. ( 10.1177/1745691613518076) [DOI] [PubMed] [Google Scholar]
- 21.Heyes C. 2014. False belief in infancy: a fresh look. Dev. Sci. 17, 647-659. ( 10.1111/desc.12148) [DOI] [PubMed] [Google Scholar]
- 22.Kano F, Krupenye C, Hirata S, Call J, Tomasello M. 2017. Submentalizing cannot explain belief-based action anticipation in apes. Trends Cogn. Sci. 21, 633-634. ( 10.1016/j.tics.2017.06.011) [DOI] [PubMed] [Google Scholar]
- 23.Krupenye C, Kano F, Hirata S, Call J, Tomasello M. 2017. A test of the submentalizing hypothesis: apes’ performance in a false belief task inanimate control. Commun. Integr. Biol. 10, e1343771. ( 10.1080/19420889.2017.1343771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surian L, Franchin L. 2020. On the domain specificity of the mechanisms underpinning spontaneous anticipatory looks in false-belief tasks. Dev. Sci. 23, e12955. [DOI] [PubMed] [Google Scholar]
- 25.Benecke N. 1987. Studies on early dog remains from Northern Europe. J. Archaeol. Sci. 14, 31-49. ( 10.1016/S0305-4403(87)80004-3) [DOI] [Google Scholar]
- 26.Topál J, et al. 2009. The dog as a model for understanding human social behavior. Adv. Study Behav. 39, 71-116. [Google Scholar]
- 27.Miklosi A. 2014. Dog behaviour, evolution, and cognition. Oxford, UK: OUP. [Google Scholar]
- 28.Hare B, Tomasello M. 2005. Human-like social skills in dogs? Trends Cogn. Sci. 9, 439-444. ( 10.1016/j.tics.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 29.Bensky MK, Gosling SD, Sinn DL. 2013. The world from a dog's point of view. Adv. Study Behav. 45, 209-406. [Google Scholar]
- 30.Huber L. 2016. How dogs perceive and understand us. Curr. Dir. Psychol. Sci. 25, 339-344. ( 10.1177/0963721416656329) [DOI] [Google Scholar]
- 31.Kaminski J, Marshall-Pescini S. 2014. The social dog: behavior and cognition. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 32.Wynne CD. 2016. What is special about dog cognition? Curr. Dir. Psychol. Sci. 25, 345-350. ( 10.1177/0963721416657540) [DOI] [Google Scholar]
- 33.Call J, Bräuer J, Kaminski J, Tomasello M. 2003. Domestic dogs (Canis familiaris) are sensitive to the attentional state of humans. J. Comp. Psychol. 117, 257. ( 10.1037/0735-7036.117.3.257) [DOI] [PubMed] [Google Scholar]
- 34.Schwab C, Huber L. 2006. Obey or not obey? Dogs (Canis familiaris) behave differently in response to attentional states of their owners. J. Comp. Psychol. 120, 169. ( 10.1037/0735-7036.120.3.169) [DOI] [PubMed] [Google Scholar]
- 35.Marshall-Pescini S, Ceretta M, Prato-Previde E. 2014. Do domestic dogs understand human actions as goal-directed? PLoS ONE 9, e106530. ( 10.1371/journal.pone.0106530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zs Virányi, Topál J, Miklósi Á, Csányi V. 2006. A nonverbal test of knowledge attribution: a comparative study on dogs and children. Anim. Cogn. 9, 13-26. ( 10.1007/s10071-005-0257-z) [DOI] [PubMed] [Google Scholar]
- 37.Kaminski J, Pitsch A, Tomasello M. 2013. Dogs steal in the dark. Anim. Cogn. 16, 385-394. ( 10.1007/s10071-012-0579-6) [DOI] [PubMed] [Google Scholar]
- 38.Maginnity ME, Grace RC. 2014. Visual perspective taking by dogs (Canis familiaris) in a Guesser–Knower task: evidence for a canine theory of mind? Anim. Cogn. 17, 1375-1392. ( 10.1007/s10071-014-0773-9) [DOI] [PubMed] [Google Scholar]
- 39.Kaminski J, Tomasello M, Call J, Bräuer J. 2009. Domestic dogs are sensitive to a human's perspective. Behaviour 146, 979-998. ( 10.1163/156853908X395530) [DOI] [Google Scholar]
- 40.Catala A, Mang B, Wallis L, Huber L. 2017. Dogs demonstrate perspective taking based on geometrical gaze following in a Guesser–Knower task. Anim. Cogn. 20, 581-589. ( 10.1007/s10071-017-1082-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buttelmann D, Carpenter M, Tomasello M. 2009. Eighteen-month-old infants show false belief understanding in an active helping paradigm. Cognition 112, 337-342. ( 10.1016/j.cognition.2009.05.006) [DOI] [PubMed] [Google Scholar]
- 42.Call J, Tomasello M. 1999. A nonverbal false belief task: the performance of children and great apes. Child Dev. 70, 381-395. ( 10.1111/1467-8624.00028) [DOI] [PubMed] [Google Scholar]
- 43.Topál J, Gergely G, Erdohegyi A, Csibra G, Miklosi A. 2009. Differential sensitivity to human communication in dogs, wolves, and human infants. Science 325, 1269-1272. ( 10.1126/science.1176960) [DOI] [PubMed] [Google Scholar]
- 44.Topál J, Kubinyi E, Gácsi M, Miklósi Á. 2005. Obeying social rules: a comparative study on dogs and humans. J. Cult. Evol. Psychol. 3, 223-243. ( 10.1556/JCEP.3.2005.3-4.1) [DOI] [Google Scholar]
- 45.Marshall-Pescini S, Passalacqua C, Miletto Petrazzini ME, Valsecchi P, Prato-Previde E. 2012. Do Dogs (Canis lupus familiaris) make counterproductive choices because they are sensitive to human ostensive cues?. PLoS ONE 7, e35437. ( 10.1371/journal.pone.0035437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baddeley AD. 1999. Essentials of human memory. Hove, UK: Psychology Press. [Google Scholar]
- 47.Pearce JM. 2013. Animal learning and cognition: an introduction. Hove, UK: Psychology Press. [Google Scholar]
- 48.Jaakkola K. 2014. Do animals understand invisible displacement? A critical review. J. Comp. Psychol. 128, 225. ( 10.1037/a0035675) [DOI] [PubMed] [Google Scholar]
- 49.Butterfill SA, Apperly IA. 2013. How to construct a minimal theory of mind. Mind Lang. 28, 606-637. ( 10.1111/mila.12036) [DOI] [Google Scholar]
- 50.Petter M, Musolino E, Roberts WA, Cole M. 2009. Can dogs (Canis familiaris) detect human deception? Behav. Process. 82, 109-118. ( 10.1016/j.beproc.2009.07.002) [DOI] [PubMed] [Google Scholar]
- 51.Takaoka A, Maeda T, Hori Y, Fujita K. 2015. Do dogs follow behavioral cues from an unreliable human? Anim. Cogn. 18, 475-483. ( 10.1007/s10071-014-0816-2) [DOI] [PubMed] [Google Scholar]
- 52.Heberlein MT, Manser MB, Turner DC. 2017. Deceptive-like behaviour in dogs (Canis familiaris). Anim. Cogn. 20, 511-520. ( 10.1007/s10071-017-1078-6) [DOI] [PubMed] [Google Scholar]
- 53.Prato-Previde E, Marshall-Pescini S, Valsecchi P. 2008. Is your choice my choice? The owners' effect on pet dogs’(Canis lupus familiaris) performance in a food choice task. Anim. Cogn. 11, 167-174. ( 10.1007/s10071-007-0102-7) [DOI] [PubMed] [Google Scholar]
- 54.Marshall-Pescini S, Prato-Previde E, Valsecchi P. 2011. Are dogs (Canis familiaris) misled more by their owners than by strangers in a food choice task? Anim. Cogn. 14, 137-142. ( 10.1007/s10071-010-0340-y) [DOI] [PubMed] [Google Scholar]
- 55.Barnard S, Passalacqua C, Pelosi A, Valsecchi P, Prato-Previde E. 2019. Effects of breed group and development on dogs' willingness to follow a human misleading advice. Anim. Cogn. 22, 757-768. ( 10.1007/s10071-019-01272-3) [DOI] [PubMed] [Google Scholar]
- 56.Kupán K, Miklósi Á, Gergely G, Topál J. 2011. Why do dogs (Canis familiaris) select the empty container in an observational learning task? Anim. Cogn. 14, 259-268. ( 10.1007/s10071-010-0359-0) [DOI] [PubMed] [Google Scholar]
- 57.Szetei V, Miklósi Á, Topál J, Csányi V. 2003. When dogs seem to lose their nose: an investigation on the use of visual and olfactory cues in communicative context between dog and owner. Appl. Anim. Behav. Sci. 83, 141-152. ( 10.1016/S0168-1591(03)00114-X) [DOI] [Google Scholar]
- 58.Miklósi Á, Topál J. 2012. The evolution of canine cognition. In The Oxford handbook of comparative evolutionary psychology (eds TK, Shackelford, J Vonk), pp. 194-213. Oxford, UK: OUP. [Google Scholar]
- 59.Serpell JA, Hsu YA. 2005. Effects of breed, sex, and neuter status on trainability in dogs. Anthrozoös 18, 196-207. ( 10.2752/089279305785594135) [DOI] [Google Scholar]
- 60.Scott JP, Fuller JL. 2012. Genetics and the social behavior of the dog. Chicago, IL: University of Chicago Press. [Google Scholar]
- 61.Netto WJ, Planta DJU. 1997. Behavioural testing for aggression in the domestic dog. Appl. Anim. Behav. Sci. 52, 243-263. ( 10.1016/S0168-1591(96)01126-4) [DOI] [Google Scholar]
- 62.Christiansen FO, Bakken M, Braastad BO. 2001. Behavioural differences between three breed groups of hunting dogs confronted with domestic sheep. Appl. Anim. Behav. Sci. 72, 115-129. ( 10.1016/S0168-1591(00)00202-1) [DOI] [PubMed] [Google Scholar]
- 63.Kolm N, Temrin H, Miklósi Á, Kubinyi E, Garamszegi LZ. 2020. The link between selection for function and human-directed play behaviour in dogs. Biol. Lett. 16, 20200366. ( 10.1098/rsbl.2020.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gácsi M, McGreevy P, Kara E, Miklósi Á. 2009. Effects of selection for cooperation and attention in dogs. Behav. Brain Funct. 5, 31. ( 10.1186/1744-9081-5-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pongrácz P, Miklósi Á, Vida V, Csányi V. 2005. The pet dogs ability for learning from a human demonstrator in a detour task is independent from the breed and age. Appl. Anim. Behav. Sci. 90, 309-323. ( 10.1016/j.applanim.2004.08.004) [DOI] [Google Scholar]
- 66.Jakovcevic A, Elgier AM, Mustaca AE, Bentosela M. 2010. Breed differences in dogs’ (Canis familiaris) gaze to the human face. Behav. Process. 84, 602-607. ( 10.1016/j.beproc.2010.04.003) [DOI] [PubMed] [Google Scholar]
- 67.Gnanadesikan GE, Hare B, Snyder-Mackler N, MacLean EL. 2020. Estimating the heritability of cognitive traits across dog breeds reveals highly heritable inhibitory control and communication factors. Anim. Cogn. 23, 953-964. ( 10.1007/s10071-020-01400-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heberlein MTE, Turner DC, Manser MB. 2017. Dogs' (Canis familiaris) attention to human perception: influence of breed groups and life experiences. J. Comp. Psychol. 131, 19-29. ( 10.1037/com0000050) [DOI] [PubMed] [Google Scholar]
- 69.Chapagain D, Virányi Z, Wallis LJ, Huber L, Serra J, Range F. 2017. Aging of attentiveness in border collies and other pet dog breeds: the protective benefits of lifelong training. Front. Aging Neurosci. 9, 100. ( 10.3389/fnagi.2017.00100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Udell MAR, Ewald M, Dorey NR, Wynne CDL. 2014. Exploring breed differences in dogs (Canis familiaris): does exaggeration or inhibition of predatory response predict performance on human-guided tasks? Anim. Behav. 89, 99-105. ( 10.1016/j.anbehav.2013.12.012) [DOI] [Google Scholar]
- 71.Karl S, et al. 2020. Exploring the dog–human relationship by combining fMRI, eye-tracking and behavioural measures. Sci. Rep. 10, 1-15. ( 10.1038/s41598-020-79247-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35-57. ( 10.1086/406755) [DOI] [Google Scholar]
- 73.Lonardo L, Völter C, Lamm C, Huber L. 2021. Dogs follow human misleading suggestions more often when the informant has a false belief. Figshare. [DOI] [PMC free article] [PubMed]
- 74.Lonardo L, Völter C, Lamm C, Huber L. 2021. Data for: Dogs follow human misleading suggestions more often when the informant has a false belief. [cited 2021 Apr 1]. See https://github.com/lonardol/data_and_code_for_Lonardo_et_al.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lonardo L, Völter C, Lamm C, Huber L. 2021. Dogs follow human misleading suggestions more often when the informant has a false belief. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All datasets, statistical tools and code used for the analyses and plots are available as electronic supplementary material [73,74].