OALA0101
SARS‐CoV‐2 immunity in COVID‐19 convalescent individuals living with HIV: bulk immune profiling and SARS‐CoV‐2‐specific humoral and cellular immune responses
ML Polo 1,2; A Czernikier1,2; D Gianone1,2; MB Vecchione2; Y Ghiglione1,2; S Balinotti3; G Turk1,4; Y Longueira1,2; N Laufer1,4; F Quiroga1,4; and BBEI working group
1CONICET, Instituto INBIRS, Buenos Aires, Argentina. 2Universidad de Buenos Aires, Facultad de Medicina, Buenos Aires, Argentina. 3BBEI Working Group, Buenos Aires, Argentina. 4Universidad de Buenos Aires, Facultad de Medicina, Departamento de Microbiología, Parasitología e Inmunología, Buenos Aires, Argentina
Background: SARS‐CoV‐2‐specific immune response features in PLWHA remain to be fully elucidated. The impact of HIV over the immune profile of lymphocyte populations in PLWHA recovered from COVID‐19, as well as the humoral and cellular response secondary to COVID‐19 were evaluated.
Methods: Samples from donors to the Argentinean Biobank of Infectious Diseases with COVID‐19 diagnosis: Twenty‐one PLWHA on ART and 21 HIV negative (HIVneg) were included. Plasma and PBMC were obtained. SARS‐CoV‐2‐specific IgG/IgM levels and IgG titres were determined by ELISA (COVIDAR test). Antibody neutralization capacity was evaluated against wild‐type SARS‐CoV‐2. IFN‐g‐secreting cells were detected by ELISPOT using SARS‐CoV‐2 Spike, RBD or Nucleocapsid protein (10 mg/mL,) or overlapping peptide pools spanning Spike or Nucleocapsid proteins (1mg/mL). The frequency and phenotype of bulk T, B and NK cells were assessed by flow cytometry.
Results: PLWHA median age was 47 (IQR: 39.5 to 54); LTCD4 = 513 cells/μL (IQR: 351 to 873). HIVneg median age was 41 (IQR: 35 to 57). All individuals presented mild/moderate COVID‐19. Mean time from symptoms onset to donation was 44 days (IQR: 29.5 to 55) for HIVneg and 62 (IQR: 35 to 93) for PLWHA. 75% of PLWHA and 85% of HIVneg had detectable SARS‐CoV‐2‐specific antibodies, with IgG levels not differing between groups. Among PLWHA, neutralization capacity correlated with IgG titres (r: 0.90, p < 0.001), LTCD4 count (r: 0.85, p: 0.001), LTCD8 count (r: 0.97, p < 0.001) and age (r: 0.63, p: 0.021). All donors, including those with undetectable antibody response, had SARS‐CoV2‐specific cellular immunity. While HIVneg displayed IFN‐g‐secreting cells in response to S protein, RBD and S peptide pools, PLWHA responses were detected in S protein and N peptide pool, although with decreased magnitude (both p < 0.01). Both groups displayed similar Treg (CD127‐CD25+CD4+T) frequency, similar effector/memory and T‐helper profile for LTCD4, and comparable exhaustion and memory profiles for LTCD8. No differences in NK, B or antibody‐secreting cell proportions were observed. PLWHA presented increased Tfh (CD4+CXCR5+T cells, p < 0.01) and CXCR1+Tfh (p < 0.05) cell frequency, enhanced expression of PD1+ on LTCD4 (p < 0.05), HLA‐DR on LTCD8 (p < 0.05) and higher expression of CD95 (p = 0.002), CD25 (p = 0.004), HLA‐DR (p < 0.0001), NKp46 (p = 0.035) and CD38/HLA‐DR (p = 0.002) on NK cells.
Conclusions: Although PLWHA showed an immune profile with enhanced activation and exhaustion, the severity of COVID‐19 was not exacerbated. Among PLWHA, SARS‐CoV‐2 infection could exert a significant humoral and cellular response, which could be associated with increased proportions of Tfh cells. The cellular response was lower compared to HIVneg individuals; nevertheless, a preserved LTCD4 count emerged as a key factor to achieve better antibody responses with higher neutralization capacity. These data reinforce the impact of ART not only in HIV control but in the capacity to control other infections.
OALA0102
SARS‐CoV‐2‐seronegative subjects target CTL epitopes in the SARS‐CoV‐2 nucleoprotein cross‐reactive to common cold coronaviruses
K Schmidt 1, K Nganou‐Makamdop2, M Tenbusch2, B El Kenz1,2, C Maier2, D Lapuente2, K Überla2, B Spriewald3, S Bergmann1, EG Harrer1, and T Harrer1
1Universitätsklinikum Erlangen and Friedrich‐Alexander‐University Erlangen‐Nürnberg, Department of Medicine 3, Erlangen, Germany. 2Universitätsklinikum Erlangen and Friedrich‐Alexander‐University Erlangen‐Nürnberg, Institute of Clinical and Molecular Virology, Erlangen, Germany. 3Universitätsklinikum Erlangen and Friedrich‐Alexander‐University Erlangen‐Nürnberg, Department of Medicine 5, Erlangen, Germany
Background: The beta‐coronavirus SARS‐CoV‐2 induces severe disease (COVID‐19) mainly in elderly persons with risk factors, whereas the majority of patients experience a mild course of infection. As the circulating common cold coronaviruses, OC43 and HKU1, share some homologous sequences with SARS‐CoV‐2, cross‐reactive T‐cell responses could influence the susceptibility to SARS‐CoV‐2 infection and the course of COVID‐19. To investigate the role of beta‐coronavirus cross‐reactive T cells, we analysed the T‐cell response against a 15 amino acid long peptide (DP15: DLSPRWYFYYLGTGP) from the SARS‐CoV‐2 nucleoprotein sequence with a high homology to the corresponding sequence (QLLPRWYFYYLGTGP) in OC43 and HKU1. As HIV‐1 infection is a potential risk factor for COVID‐19, we studied a cohort of HIV‐1‐infected patients on antiretroviral therapy.
Methods: PBMC from HIV‐1‐infected patients and from healthy controls were stimulated with peptide SCoV‐DP15. Outgrowing cells were tested for recognition of DP15 by g‐IFN‐ELISPOT assays and by flow cytometric assays. Epitopes were mapped using truncated peptides in ELISPOT assays. SARS‐CoV‐2 antibodies were measured by a flow cytometric antibody assay.
Results: Forty‐four out of 116 HIV‐1‐infected patients (37.9 %) and four out of 23 (17.4%) healthy donors showed a specific recognition of the SCoV‐DP15 peptide or of shorter peptides within DP15 by CD4+ T cells and/or by CD8+ T cells. All responders were SARS‐CoV‐2‐seronegative. We could define several new cross‐reactive HLA‐I‐restricted epitopes in the SARS‐CoV‐2 nucleoprotein. Epitope‐specific CD8+ T‐cell lines recognized corresponding epitopes within OC43 and HKU1 to a similar degree or even at lower peptide concentrations suggesting that they were induced by infection with OC43 or HKU1.
Conclusions: Our results confirm that SARS‐CoV‐2‐seronegative subjects can target SARS‐CoV‐2 not only by cross‐reactive CD4+ T cells but also by cross‐reactive CD8+ cytotoxic T cells (CTL). The delineation of cross‐reactive T‐cell epitopes contributes to an efficient epitope‐specific immunomonitoring of SARS‐CoV‐2‐specific T cells. Further prospective studies are needed to prove the protective role of cross‐reactive T cells and their restricting HLA alleles for control of SARS‐CoV‐2 infection. The frequent observation of SARS‐CoV‐2‐reactive T cells in HIV‐1‐infected subjects could be a reason that treated HIV‐1 infection does not seem to be a strong risk factor for the development of severe COVID‐19.
OALA0103
Viral hepatitis cascade of care among adults living with HIV in Asia‐Pacific
D Rupasinghe 1; JY Choi2; N Kumarasamy3; S Pujari4; PS Ly5; TP Merati6; MP Lee7; KV Nguyen8; S Kiertiburanakul9; CD Do10; A Avihingsanon11; J Ross12; and A Jiamsakul1
1The Kirby Institute, Sydney, Australia. 2Yonsei University College of Medicine, Division of Infectious Diseases, Department of Internal Medicine, Seoul, Korea, Republic of. 3CART CRS, Voluntary Health Services, Chennai, India. 4Institute of Infectious Diseases, Pune, India. 5National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia. 6Udayana University & Sanglah Hospital, Faculty of Medicine, Bali, Indonesia. 7Queen Elizabeth Hospital, Hong Kong, Hong Kong, SAR of China. 8National Hospital for Tropical Diseases, Hanoi, Vietnam. 9Ramathibodi Hospital, Mahidol University, Faculty of Medicine, Bangkok, Thailand. 10Bach Mai Hospital, Hanoi, Vietnam. 11HIV‐NAT/Thai Red Cross AIDS Research Centre and Tuberculosis Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. 12TREAT Asia, amfAR – The Foundation for AIDS Research, Bangkok, Thailand
Background: Data on viral hepatitis (VH) diagnosis, treatment and cure rates among PLHIV from the Asia‐Pacific region are limited. With targets set to eliminate VH as a global epidemic by 2030, this study aims to identify gaps in the hepatitis B virus (HBV) and hepatitis C virus (HCV) cascade of care (CoC) among PLHIV in the region.
Methods: PLHIV enrolled in a regional HIV observational cohort, on antiretroviral therapy (ART), and in follow‐up between 2010 and 2019 were included. Patients were considered as having VH co‐infection if they ever tested positive for HBV surface antigen (HBsAg) or anti‐HCV (HCVAb). The CoC included the proportion of patients with positive HBV or HCV, HBV/HCV serology testing, received therapy and subsequently reached HBV or HCV suppression.
Results: Of 22,340 patients included, most were male (64%) with a median age of 35 years (Interquartile range (IQR) 30 to 42) with heterosexual contact as the main mode of HIV exposure. Among those included, 39% (8612/22,340) had HBsAg screening tests with 8% (672/8612) testing positive. Of 672 HBsAg‐positive patients, 71% (474/672) initiated HBV treatment; 67% (318/474) had a subsequent HBV DNA test, with 18% (58/318) reaching HBV suppression (Figure 1a).
Screening for HCVAb was done on 37% (8231/22,340) of those included, of whom 9% (779/8231) tested positive. Of the 779 HCVAb‐positive participants, 68% (526/779) had a subsequent HCV RNA test, of whom 51% (267/526) tested positive. 65% (174/267) of those positive for HCV RNA initiated treatment. Of those treated, 40% (69/174) were tested for sustained virological response (SVR) and 90% (62/69) had confirmed SVR (Figure 1b).
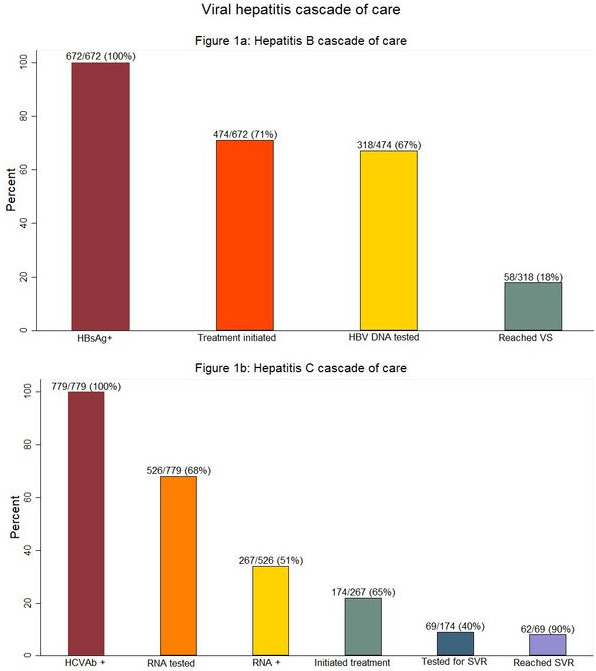
Abstract OALA0103‐Figure 1.
Conclusions: Our study identified low VH screening and low treatment monitoring with HBV DNA and HCV RNA testing. These findings suggest the need for improved access to affordable testing for screening and monitoring treatment response for VH through treatment programmes in the region.
OALA0201
The passenger hypothesis: HIV exploits CD4 T‐cell homeostasis to promote long‐term persistence of its reservoirs
D Reeves 1; C Bacchus‐Souffan2; M Fitch3; J Symons4; M Abdel‐Mohsen5; R Hoh2; H Ahn2; S Deeks2; S Lewin4; M Hellerstein3; JM McCune6; P Hunt2; and J Schiffer1
1Fred Hutchinson Cancer Research Center, Seattle, United States. 2University of California San Francisco, San Francisco, United States. 3University of California Berkley, Berkley, United States. 4The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia. 5The Wistar Institute, Philadelphia, United States. 6Bill and Melinda Gates Foundation, Seattle, United States
Background: The reservoir of latently infected CD4+ T cells ensures HIV persistence during suppressive antiretroviral therapy (ART). The proliferation of reservoir cells carries provirus along as a passenger and produces clonal HIV lineages that outlive single‐infected cells. We tested the hypothesis that differentiation between CD4+ T cell subsets helps sustain the reservoir.
Methods: We quantified HIV reservoir size and clonality longitudinally at 1 to 3 time points in 37 participants on ART in five resting CD4+ T cell subsets: naïve (TN), stem‐cell memory (TSCM), central memory (TCM), transitional memory (TTM) and effector memory (TEM). We tested 10 mathematical models including proliferation, death and differentiation mechanisms to select the most parsimonious model of the HIV reservoir in CD4+ T cell subsets. Deuterium labelling measurements were performed to impute cellular subset turnover rates into the model.
Results: Integrated HIV DNA was stable or decreased in each subset; median rates ranged from 0 (no clearance, TN) to a 42‐month half‐life (TEM). However, cellular turnover rates were substantially more rapid, ranging from 3 (TEM) to 30 (TN) month half‐lives, suggesting that infected cells are constantly being replaced via cellular proliferation. The best model followed linear differentiation from TN through to TEM. Estimated differentiation rates were on the order of but generally slower than turnover rates (TCM ‐> TEM was most rapid), suggesting many but not all proliferation events result in differentiation. Proliferation was the predominant mechanism of persistence in all subsets, with contributions of cellular longevity (especially TN) and differentiation (especially TEM). Depending on the participant and cell subset, model estimates revealed 102 to 104 new HIV‐infected cells were created per million CD4+ T cells in a typical year. Greater oligoclonal expansions in TCM (higher Gini index) were associated with more rapid clearance in the HIV reservoir (Spearman ρ = 0.4 to 0.7 for all subsets), suggesting TCM clones heavily influence total reservoir dynamics.
Conclusions: We show proliferation of HIV‐infected CD4+ T cells appears to be the predominant mechanism of reservoir persistence across T‐cell subsets. HIV proviruses, additionally, passage between subsets through cellular differentiation. Thus, reservoir reduction interventions should consider both proliferation and differentiation of T‐cell subsets.
OALA0202
Identifying host genetic determinants of HIV‐1 reservoir markers reveals PTDSS2 and IRF7 as potential modifying factors in HIV‐1 patients
W Trypsteen 1; Z Zhang2,3; M Blaauw4; JC dos Santos4; X Chu2,4,5,6; S Rutsaert1; L Vandekerckhove1; C‐J Xu4,5,6; MA Swertz2,3; Y Li2,4,5,6; and A van der Ven4
1Ghent University, HIV Cure Research Center – Department of Internal Medicine and Pediatrics, Gent, Belgium. 2University of Groningen, Department of Genetics, Groningen, Netherlands. 3University Medical Center Groningen, Genomics Coordination Center, Groningen, Netherlands. 4Radboud University Medical Center, Department of Internal Medicine and Radboud Center for Infectious Diseases, Nijmegen, Netherlands. 5Centre for Individualised Infection Medicine, Department of Computational Biology for Individualised Medicine, Hannover, Germany. 6Centre for Experimental and Clinical Infection Research, TWINCORE, Hannover, Germany
Background: Combination antiretroviral treatment (cART) cannot eradicate HIV‐1 from the body due to the establishment of persisting viral reservoirs which reinitiate new rounds of HIV‐1 replication after treatment interruption. These HIV‐1 reservoirs mainly comprise long‐lived resting memory CD4+ T cells and show high variability in size or activity among virally suppressed individuals. Therefore, the identification of host factors that contribute to this observed variation could open avenues for new HIV‐1 treatment strategies.
Methods: In this study, we conducted a genome‐wide quantity trait locus (QTL) analysis to probe functionally relevant genetic variants linked to levels of cell‐associated (CA)‐HIV‐1 DNA, CA‐HIV‐1 RNA and RNA:DNA ratio in CD4+T cells isolated from whole blood from a cohort of 207 (Caucasian) HIV‐1 patients under long‐term suppressive cART (median = 6.6 years). CA‐HIV‐1 DNA and CA‐HIV‐1 RNA levels were measured with corresponding droplet digital PCR assays and genotype information of 522,455 single‐nucleotide variants (SNV) was retrieved via the Infinium Global Screening array platform.
Results: The QTL mapping analysis involved an additive linear regression model with a correction for age, gender, CD4 nadir and HIV‐1 duration and identified one significant genetic association with CA‐HIV‐1 DNA (PTDSS2, p < 5 × 10−8), whereas four associations were found for RNA:DNA ratio (RNH1, IRF7, DEAF1 and RP11‐1149M10.2, p < 5 × 10−7). Next, we validated that the IRF7 SNV is significantly correlated with higher expression (qPCR) of the IRF7 gene in peripheral blood mononuclear cells (PBMC) from HIV‐1 patients and influences the IFN‐y production capacity of ex vivo stimulated PBMCs with TLR2/4/7 agonists, supporting its functional role in HIV‐1 infection.
Conclusions: The presented data suggest that the amount of CA‐HIV‐1 DNA and RNA:DNA ratio could be influenced by the PTDSS2 and IRF7 loci. Especially, the IRF7 SNV is functionally linked to higher expression levels of its gene product and modifies IFN‐y levels which contribute to the control of the relative HIV‐1 transcriptional activity and associated immunological burden. These observations provide novel knowledge on the molecular mechanisms involved in HIV‐1 reservoir establishment and/or maintenance and could indicate targets for future therapeutic strategies to lower HIV‐1 reservoir size or activity in patients.
OALA0203
Naïve CD4+ T cells form the bulk of the translation competent HIV‐1 reservoir in vertically infected children and adolescents
J Canape 1,2; H Dieumegard1,2; DG Rancy1; MA Diallo1,2; A Bitnun3,4; F Kakkar5,6,7; J Brophy8,9; L Samson8,9; MT Hawkes10; P Wender11; A Pagliuzza12; M Massanella12; N Chomont2,12; S Read3,4; A Le Campion2; H Soudeyns1,2,5,7; and EPIC4 Study Group
1Unité d’Immunopathologie Virale, Centre de Recherche du CHU Sainte‐Justine, Montréal, Canada. 2Département de Microbiologie, Infectiologie et Immunologie, Faculté de Médecine, Université de Montréal, Montréal, Canada. 3Hospital for Sick Children, Toronto, Canada. 4Department of Pediatrics, University of Toronto, Toronto, Canada. 5Centre d’Infectiologie Mère‐Enfant, CHU Sainte‐Justine, Montréal, Canada. 6Service des Maladies Infectieuses, CHU Sainte‐Justine, Montréal, Canada. 7Département de Pédiatrie, Faculté de Médecine, Université de Montréal, Montréal, Canada. 8Children’s Hospital of Eastern Ontario, Ottawa, Canada. 9Department of Pediatrics, University of Ottawa, Ottawa, Canada. 10Department of Pediatrics, University of Alberta, Edmonton, Canada. 11Department of Chemistry, Stanford University, Stanford, United States. 12Centre de Recherche du CHUM, Montréal, Canada
Background: Our inability to cure HIV/AIDS stems from the fact that HIV establishes and maintains cellular reservoirs where it shelters from the effects of combination antiretroviral therapy (cART) and host immunity. Whereas reservoir components are well known in adults, the composition and evolution of these reservoirs in vertically infected children are incompletely understood. Our objective was to examine the effects of the timing of cART initiation and achievement of sustained viral suppression (SVS) on the size and nature of the HIV reservoir in children and adolescents.
Methods: Using the HIV‐Flow method (Pardons et al., PLoS Pathog 15:e1007619, 2019), size and cell subset distribution of the translation competent viral reservoir were assessed in purified CD4+ T cells from vertically infected children and adolescents (n = 34) with and without SVS, who were enrolled in the EPIC4 study and stratified according to age (0 to 5, 5 to 10, 10 to 18 years).
Results: Differences in reservoir size between male and female participants or across age groups were not statistically significant (p = 0.5003, p = 0.9410). Naïve CD4+ T cells were the main contributor to the pool of p24‐producing cells in all age groups as compared to central memory (CM), effector memory (EM) and terminally differentiated (TD) (p = 0.001, p < 0.0001, p < 0.0001). The large representation of naive CD4+ cells in the total CD4+ T cells pool (approximately 68% to >80%) can explain this contribution. CM cells tended to carry higher frequencies of p24+ cells in adolescents compared to younger age groups but differences were not statistically significant (p>0.4442). A negative correlation was observed between the frequency of p24‐positive T cells and the cumulative proportion of life under SVS (r = −3588, p = 0.0403). Finally, the frequency of p24‐positive T cells was positively correlated with age at initiation of first cART (r = 0.4323, p = 0.0216).
Conclusions: Unlike HIV‐infected adults, the cellular reservoir harbouring translation competent HIV in vertically infected children and adolescents is mostly comprised of naïve CD4+ T cells, with a distribution profile progressively transitioning to that of adults. Importantly, the frequency of p24‐positive T cells was associated with adequacy of SVS and age of cART initiation. These results inform and reinforce evidence‐based guidance for the management of vertical HIV infection.
OAA0101
Defining an adipose tissue single cell atlas to understand metabolic disease in HIV
SS Bailin1; R Ram2; A Chopra2; R Gangula1; S Leary2; M Mashayekhi1; CL Gabriel1; BO Woodward1; M Lima1; L Hannah1; SA Kalams1; SA Mallal1; JR Koethe1; and CN Wanjalla 1
1Vanderbilt University Medical Center, Nashville, United States. 2Institute for Immunology and Infectious Diseases, Murdoch University, Perth, Australia
Background: Adipose tissue (AT) is a critical regulator of metabolic health and is emerging as important in HIV. Despite this, data on the complex cellular milieu and immune regulation are lacking. We sought to assess the AT microenvironment in persons with HIV (PWH).
Methods: We performed subcutaneous abdominal liposuction and isolated the stromal vascular fraction (SVF) from 16 HIV‐negative diabetics, 16 HIV‐positive non‐diabetics and 16 HIV‐positive diabetics on long‐term ART. Cells were stained with a panel of 5’ DNA‐sequence tagged antibodies (TotalSeq‐C) that represented standard lineages, activation and regulatory markers (45 antibodies). For the analysis, CellRanger (version 3.0.0) was used to demultiplex the raw sequencing data, extract filter and correct barcodes and unique molecular identifiers, remove cDNA PCR duplicates and align reads to the human transcriptome (GRCh38). The resulting BAM files and filtered count matrices were used in analyses. We assessed the AT cell types and their association of these subsets with the pre‐adipocytes (Spearman rank correlation).
Results: Agnostic to metabolic disease, PWH had lower proportions of pre‐adipocytes (median 20.4% in non‐diabetic and 36.4% in diabetic) compared with HIV‐negative diabetic participants (62.7%) (Figure 1). The proportion of CD8 T cells, monocytes and NK cells were significantly higher in PWH compared with HIV‐negative participants, irrespective of metabolic disease. Pre‐adipocyte and NK cells were inversely related in non‐diabetic PWH (r = −0.68, p = 0.005), diabetic PWH (r = −0.70, p = 0.004) and HIV‐negative diabetics (r = −0.51, p = 0.05). A similar trend was observed between CD8 T cells and pre‐adipocytes.
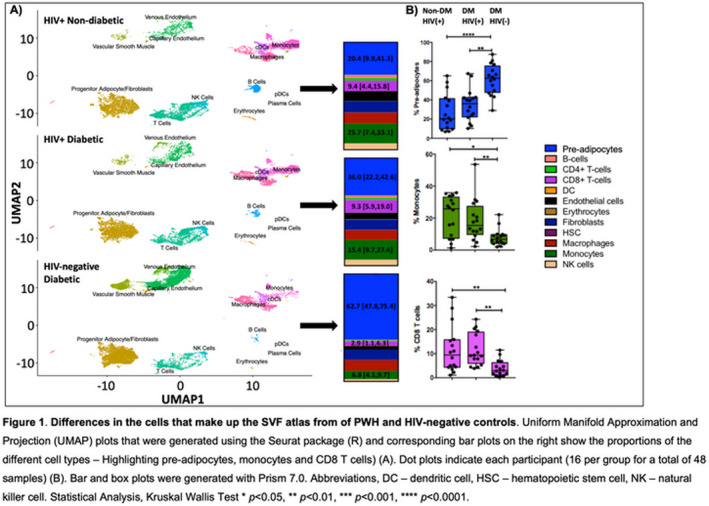
Abstract OAA0101‐Figure 1.
Conclusions: We have generated a detailed atlas of AT SVF by HIV and diabetes status and show that PWH have higher proportions of NK and T cells compared with diabetic HIV negative. We hypothesize that this may correlate with the HIV reservoir. Future studies will pair this data with measurements of the HIV reservoir quantification and ART drug levels to understand how AT contributes to viral persistence.
OAA0102
Mechanisms of residual immune activation in HIV‐1‐infected human lymphoid tissue ex vivo
L Margolis 1; V Mercurio1; W Fitzgerald1; C Vanpouille1; and I Molodsov2
1National Institutes of Health, National Institute of Child Health and Human Development, Bethesda, United States. 2N.F. Gamaleya Federal National Research Centre for Epidemiology and Microbiology, Moscow, Russian Federation
Background: HIV‐1 infection triggers immune activation, as reflected by the upregulation of various cytokines. This immune activation remains elevated despite efficient suppression of virus by antiretroviral therapy (ART) and leads to early age‐related diseases. Mechanisms of this residual immune activation remain unknown. Here, we addressed these mechanisms in HIV‐1‐infected human lymphoid tissues ex vivo subjected to ART.
Methods: Human lymphoid tissues ex vivo were infected with HIV‐1 and viral replication was suppressed by ART. Tissue immune activation was evaluated from measurements of 29 cytokines in the culture medium using multiplexed immunoassays.
Results: We investigated several potential causes of the residual immune activation, including:
a proinflammatory effect of ART drugs themselves;
an early HIV‐1–triggered “cytokine storm,” which could, in turn, trigger a sustained cytokine dysregulation;
herpesvirus reactivation;
HIV‐1 protein release; and
production of defective virions and extracellular vesicles (EVs).
Neither ART itself, nor simulated cytokine storms, nor exogenously added HIV‐1 proteins triggered a sustained cytokine upregulation. In contrast, defective (replicative‐incompetent) virions and EVs induced sustained cytokine upregulation, as did infectious virus. Tissue immune activation was accompanied by reactivation of CMV.
Conclusions: Immune activation in HIV‐1‐infected ex vivo human lymphoid tissue after HIV‐1 suppression is mediated by the EVs and/or defective viral particles.
OAA0103
The association of cardiovascular risk factors and disease in people living with HIV in the United Kingdom: a retrospective matched cohort study
T Gooden 1; M Gardner1; J Wang1; K Jolly1; DA Lane1,2; LA Benjamin3,4,5; HC Mwandumba6,7; V Kandoole6,8,9; S Manaseki‐Holland1; GYH Lip1,2; K Nirantharakumar1; and GN Thomas1
1University of Birmingham, Institute of Applied Health Research, Birmingham, United Kingdom. 2University of Liverpool, Liverpool Centre for Cardiovascular Science, Liverpool, United Kingdom. 3University College London, Laboratory of Molecular and Cell Biology, London, United Kingdom. 4University College London, Stroke Research Centre, London, United Kingdom. 5University of Liverpool, Institute of Infection, Veterinary and Ecological Sciences, Liverpool, United Kingdom. 6University of Malawi, Malawi Liverpool Wellcome Trust Clinical Research Programme, Blantyre, Malawi. 7Liverpool School of Tropical Medicine, Department of Clinical Sciences, Liverpool, United Kingdom. 8University of Malawi, College of Medicine, Blantyre, Malawi. 9University Hospitals Bristol and Western NHS Foundation Trust, Bristol Heart Institute, Bristol, United Kingdom
Background: Heightened risk of cardiovascular disease (CVD) and associated risk factors in people living with HIV (PLWH) have been reported in various settings; however, results are limited and differ geographically. We aimed to identify the association of CV risk factors and disease in PLWH compared to those without HIV in the United Kingdom.
Methods: A matched cohort was derived from The Health Improvement Network (THIN) database from January 2000 to January 2020. Adult (≥18 years) people with an HIV diagnosis (exposed) were eligible and matched for gender and age with up to four people without HIV (unexposed). Outcomes included CVD (stroke, myocardial infarction (MI), peripheral vascular disease (PVD), ischaemic heart disease (IHD) and heart failure (HF)), hypertension, diabetes, chronic kidney disease (CKD), lipid‐lowering drug use and all‐cause mortality. Cox proportional hazard regression models were used to compare the risk of each outcome between the exposed and unexposed groups.
Results: The cohort comprised 9233 exposed and 35721 unexposed individuals; 34% were females and the mean age was 41. Across all models, the exposed group was at a higher risk for CVD (HR 1.54, 95% CI 1.30, 1.83), specifically stroke (HR 1.49, 95% CI 1.11, 2.00), hypertension (HR 1.37, 95% CI 1.22, 1.55), lipid‐lowering drug use (HR 1.96, 95% CI 1.78, 2.16), CKD (HR 2.40, 95% CI 1.93, 2.98) and all‐cause mortality (HR 2.68, 95% CI 2.32, 3.10). CVD risk remained significant across sub‐groups of gender, age, smoking status and index year. Younger patients (≤40 years) had the highest risk of CVD (HR 2.01, 95% CI 1.29, 3.13) and all‐cause mortality (HR 6.09, 95% CI 4.36, 8.51). Females had double the risk for MI (HR 2.67, 95% CI 1.02, 6.95) and IHD (HR 2.34, 95% CI 1.17, 4.71), whereas males had a slightly increased risk for stroke (HR 1.55, 95% CI 1.11, 2.15) and IHD (HR 1.47, 95% 1.14, 1.91).
Conclusions: PLWH, particularly of younger age, are at a heightened risk for mortality, cardiovascular risk factors and disease. Therefore, screening for CV risk factors and disease in PLWH should be routine. Further research is needed to ascertain the drivers of these risks to inform prevention strategies.
OAA0104
Higher comorbidity and comedication burden in women and young people living with HIV
M Paudel1; G Prajapati2; E Buysman1; S Goswami2; J Mao2; K McNiff1; and P Kumar 3
1Optum, Eden Prairie, United States. 2Merck & Co, Inc., Kenilworth, United States. 3Georgetown University Medical Center, Washington, United States
Background: Advancements in antiretroviral therapies (ART) have led to longer life expectancies for people living with HIV (PLWH). An understanding of comorbidity and comedication prevalence in HIV sub‐populations is important for personalized care.
Methods: A retrospective study was conducted using an administrative claims database. Adults (≥18 years) with ≥1 pharmacy claim for an ART or HIV/AIDS diagnosis code in medical claims during 2018 (index date: earliest ART/HIV claim) were identified (PLWH). Adults without HIV (PLWoH) were matched 2:1 with PLWH on age, gender, race, region and insurance type. Continuous health plan enrolment of 12 months prior to (baseline), and 30 days after index date was required. Differences in baseline comorbidities and comedications between PLWH and PLWoH across age, gender and race were assessed using descriptive statistics.
Results: A total of 20,256 PLWH were matched to 40,512 PLWoH. The mean age was 52 years, 20% were women and 28% were Black. Multimorbidity (≥2 comorbidities) and polypharmacy (≥5 non‐ART drugs) prevalence was higher in PLWH than PLWoH, and increased with age, in women, and in Black populations, with the largest differences in prevalence observed in the 18 to 39 age group (Table 1). The prevalence of most comorbidities was higher in PLWH versus PLWoH in 18 to 39 age group, but differences varied in older groups. Comorbidities such as hypertension, cardiovascular disease (CVD), diabetes mellitus and chronic kidney disease (CKD) were more prevalent in women than men among PLWH, but differences between PLWH versus PLWoH by gender were inconsistent (Table 1). Neuropsychiatric conditions were more prevalent in PLWH than PLWoH in all strata (p < 0.05).
Abstract OAA0104‐Table 1
| PLWH | PLWoH | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 to 39 | 40 to 49 | 50 to 59 | 60 to 69 | 70+ | Women | Men | 18 to 39 | 40 to 49 | 50 to 59 | 60 to 69 | 70+ | Women | Men | |
| Multimorbidity | 24.1 | 38.9 | 53.0 | 69.0 | 80.0 | 59.4 | 48.5 | 11.1 | 28.6 | 46.7 | 60.2 | 75.4 | 52.9 | 39.3 |
| CVD | 3.2 | 5.5 | 11.4 | 19.5 | 32.9 | 14.3 | 12.0 | 1.2 | 4.6 | 10.9 | 19.2 | 32.0 | 12.6 | 11.3 |
| Hypertension | 8.2 | 20.5 | 35.3 | 52.0 | 68.3 | 44.6 | 31.2 | 4.5 | 18.0 | 35.2 | 50.0 | 66.7 | 38.8 | 30.5 |
| Diabetes mellitus | 2.3 | 7.6 | 13.7 | 23.1 | 30.4 | 21.0 | 12.1 | 1.6 | 8.6 | 17.8 | 25.3 | 31.5 | 19.5 | 14.9 |
| CKD | 3.5 | 7.0 | 12.7 | 20.9 | 33.7 | 18.0 | 12.5 | 1.3 | 5.1 | 9.7 | 14.1 | 22.5 | 11.0 | 9.0 |
| Neuropsychiatric | 18.8 | 22.8 | 26.9 | 29.6 | 28.5 | 29.0 | 24.4 | 10.2 | 16.8 | 21.4 | 19.6 | 20.9 | 25.6 | 16.1 |
| Polypharmacy | 56.6 | 70.5 | 79.5 | 87.6 | 92.4 | 81.5 | 75.0 | 31.0 | 50.3 | 65.8 | 78.6 | 86.5 | 74.9 | 57.3 |
All values presented are percents; Bold font indicates where p < 0.05 for differences between PLWH and PLWoH; Ages in years.
Conclusions: Comorbidity and polypharmacy burden were higher in PLWH than PLWoH with notable differences in specific comorbidities in younger age groups and women. An individualized approach to care including ART can minimize drug–drug interactions and adverse events and thereby improve patient outcomes.
OAA0201
STAT modulation as stratergy to improve NK cell cytotoxicity against HIV and cancer
AB Macedo 1; C Levinger1; A Santini1; B Nguyen2; K Crandal2; and A Bosque1
1The George Washington University, Microbiology, Immunology and Tropical Medicine, Washington DC, United States. 2The George Washington University, Computational Biology Institute, Milken Institute School of Public Health, Washington, United States
Background: NK cells are important effectors of the innate immune response to a variety of viral infections and malignant cells. HIV infection, even after initiation of antiretroviral therapy, results in significant defective NK cells function. Thus, strategies that improve NK cell activity are urgently needed. The janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway is critical for NK cell development, survival, proliferation and cytotoxic function. In this work, we tested whether the previously characterized HIV latency‐reversing agent (LRA) 3‐Hydroxy‐1,2,3‐benzotriazin‐4(3H)‐one (HODHBt), a modulator of STAT pathway activity could also enhance NK cell function.
Methods: NK cells from HIV‐negative donors were isolated from PBMCs and incubated in the presence of IL‐15, HODHBt or a combination of both. We performed RNASeq and a set of diverse assays to evaluate NK cell phenotype and cytotoxic function against HIV‐infected CD4T and cancer cells. We also evaluated the ability of HODHBt to improve cytokine‐induced memory‐like NK cell responses upon cytokine recall.
Results: We observed that NK cells treated with HODHBt plus IL‐15 increased their cytotoxic profile phenotype compared to those treated with IL‐15 alone. This was demonstrated by an increased expression of activation markers (CD25 and CD69), components of the cytotoxic cell granules (Granzyme A, Granzyme B, perforin, granulysin), death receptor ligands (APO2L/TRAIL and CD95L/FasL) and enhance pro‐inflammatory cytokine production (lFN‐g and CXCL‐10). Moreover, HODHBt enhanced the killing of different tumour cells and favoured the killing of HIV‐infected CD4T cells. Finally, HODHBt improved memory‐like NK cell responses upon cytokine recall.
Conclusions: Overall, our data suggest that enhancing the magnitude of JAK‐STAT signalling pathway with HODHBt may favour NK cell cytotoxicity phenotype and function, and this pathway could be explored for novel cell adoptive immunotherapeutic approaches using NK cells against HIV and associated malignancies.
OAA0202
Defining CTL immunotherapy candidates against replication‐competent and defective HIV
E Lee 1; B Horsburgh1; T Schlub2; J Milush3; R Hoh3; S Deeks3; and S Palmer1
1The Westmead Institute for Medical Research, The University of Sydney, Centre for Virus Research, Westmead, Australia. 2The University of Sydney, School of Public Health, Camperdown, Australia. 3University of California San Francisco, Department of Medicine, San Francisco, United States
Background: An effective HIV‐specific CD8+ T cell (CTL) response that targets cells expressing vulnerable regions of the genetically diverse proviruses will be required to control human immunodeficiency virus (HIV) during antiretroviral therapy (ART) interruption. To contribute to this effort, we defined immunogenic CTL epitopes within genetically intact and defective proviruses which are effective for multiple human leucocyte antigen class I (HLA‐I) alleles.
Methods: A repertoire of 8 to 14 mer peptides was generated from the gag, pol, vif, nef, vpr and env genomic regions extracted from 350 proviral sequences derived from six participants with known HLA‐I alleles. We then employed the Protein BLAST and NetMHClpan‐4.0 algorithms to select the peptides that are HIV‐specific and binders to participant HLA‐I alleles. Next, we applied protein network analysis to select the peptides derived from evolutionarily constrained regions that are crucial for the structural maintenance of HIV proteins. We also performed an interaction network analysis to delineate the peptides that can form a stable complex with both HLA‐I molecules and T‐cell receptor alpha/beta chains (TCRαβ).
Results: From the proviruses of long‐term‐treated individuals, we obtained a repertoire of 17.6 million peptides derived from gag, pol, vif, nef, vpr and env genomic regions. Only a fraction of these peptides (0.03%) were binders to the participant HLA‐I alleles. Of the six regions examined, vpr contained the highest density of HIV‐specific peptides that were binders to participant HLA‐I alleles. Of these, only four Vpr peptides (9 to 10 mer) can form a stable complex with TCRαβ and canonical forms of HLA‐I molecules. These four Vpr peptides were predicted to bind to multiple HLA‐I alleles/supertypes, including those associated with protection against HIV, with a global population coverage of 74%. Importantly, these peptides are identified from both genetically intact and defective proviruses suggesting CTL response to these peptides has the potential to target HIV‐infected cells containing diverse HIV genomes.
Conclusions: Employing our immunoinformatics analysis pipeline, we defined several peptides within topologically important regions of the Vpr protein. Future therapeutic vaccines and other immunotherapies should consider including these peptides as they are predicted to enhance CD8+ T‐cell response against HIV‐infected cells containing diverse proviruses.
OAA0203
Immunogens based on VLPs presenting epitopes of the HIV‐1 fusion peptide
A Rudometov; N Rudometova; T Esina; A Ilyichev; and L Karpenko
State Research Center of Virology and Biotechnology VECTOR, Koltsovo, Russian Federation
Background: The characterization of bnAbs epitopes allows the identification of vulnerable sites on the HIV‐1 Env, which are the basis for the development of HIV B‐cell immunogens. One of the relatively conserved targets of bnAbs is the fusion peptide (FP), which includes a linear epitope recognized by the antibody VRC34.01. One of the approaches to the development of immunogens is the construction of chimeric virus‐like particles exposing linear epitopes. A promising system for the presentation of foreign epitopes is HBcAg, which forms particles with a size of about 36 nm, consisting of 240 HBcAg monomers.
This study aims to develop an HBcAg‐based immunogen aimed at the induction of neutralizing antibodies to
FP HIV‐1.
Methods: Targets were represented by amino acid sequences corresponding to positions env 512 to 519 amino acid residues of the fusion peptide of HIV‐1 isolates – AIGLGAAF (subtype A6), VVGLGAVF (recombinant form CRF63_02A) and AVGIGAVF (consensus sequence). Furthermore, we designed and synthesized oligonucleotide duplexes encoding the selected epitopes and cloned them as part of the pET21‐HBcAg plasmid vector. After that, the plasmid constructs were used to transform E. coli BL21 cells. Recombinant proteins were purified using chromatography. The size and morphology of the obtained chimeric HBcAg particles were determined using electron microscopy. The antigenicity of chimeric particles was analysed by dot blot and ELISA using the neutralizing antibody VRC34.01.
Results: Three recombinant plasmids encoding HBcAg variants containing fragments of FP HIV‐1 in the region of the main antigenic determinant were obtained. Producer strains of HBcAg‐FP variants were obtained. Purified and soluble HBcAg‐FP preparations were obtained. It was found that the obtained proteins form particles of a characteristic spherical shape with a size of 40 to 50 nm. Moreover, it was shown that the antibody VRC34.01 interacts with FP in the HBcAg.
Conclusions: As a result of the work, HBcAg particles were obtained with FP HIV‐1 fragments on their surface. These particles are currently being tested for the ability to induce HIV‐neutralizing antibodies in laboratory animals.
The study was supported by the grant of the President of the Russian Federation MK‐583.2020.4.
OAA0204
Plasma IL‐21 associates with HIV‐1 Neutralizing potency of polyclonal IgG in the periphery
I Ngare; T Kuwata; G Barabona; and T Ueno
Kumamoto University, Kumamoto, Japan
Background: Although the development of HIV‐1 potent and broadly neutralizing antibodies (bNAbs) is strongly associated with viral load, host immune factors may play an additional role. IL‐21 contributes to antibody avidity and affinity maturation but it remains unclear whether IL‐21 associates with bNAb elicitation in HIV‐1 infection. We, therefore, investigated the correlates of IL‐21 in bNAb elicitation in patients infected with HIV‐1 non‐B subtypes.
Methods: A total of 417 HIV‐1‐infected treatment naïve and treated but failed individuals were recruited in Tanzania. For preliminary screening, neutralizing activity was assessed against subtype B, Tier 2 Envelope (strain JRFL), followed by secondary screening using a Global panel of 12 Envelopes spanning various HIV‐1 subtypes. Murine Leukaemia Virus Envelope was used as a specificity control and drug resistance mutations were inserted in pseudoviruses to prevent the effect of residual antiretroviral drugs in plasma. IgG fraction was purified from plasma as needed and used in neutralization assays. IgG neutralizing breadth was defined as the percentage of neutraliz ed Envelopes on the panel, whereas potency was the mean of IC50 values across the panel. Cytokines (such as IL‐21 and IL‐6) were quantified using Cytometric Bead Array.
Results: In a total of 417 plasma samples screened, 32 (7.7%) exhibited neutralizing potency against JRFL Envelope and therefore qualified for IgG purification. Among them, 3 (0.72%) could be Elite neutralizers since they exhibited neutralizing potency against ≥80% of the global Envelope panel. As expected, neutralizing breadth correlated with viral load within the neutralizers subset (p < 0.02), but not with sex or age. IL‐21 levels correlated with the Neutralizing potency of IgG fractions (p = 0.04) of the 32 neutralizers. Plasma IL‐6 and IL‐5 did not correlate with either IgG neutralizing potency or breadth.
Conclusions: Plasma IL‐21 level could be a surrogate marker for potent neutralizing antibodies in viraemic patients with HIV‐1 non‐B subtype infection.
OAA0301
Novel multiplex analyses reveal disparate natural killer cell signalling pathway activation during lentivirus infection
S Sugawara 1; B Hueber1; K Kroll1; C Manickam1; DR Ram1; S Jost1; and RK Reeves1,2
1Beth Israel Deaconess Medical Center, Brookline, United States. 2Ragon Institute of Mass General Hospital, MIT and Harvard, Cambridge, United States
Background: Natural killer (NK) cells are critical effector cells for modulating human immunodeficiency virus (HIV)‐1 and simian immunodeficiency virus (SIV) transmission and subsequent opportunistic disease. Unfortunately, NK cell responses are also often highly dysregulated in HIV‐1 and SIV infection, but the mechanisms remain unclear. Although perturbation of surface receptor expression and function of NK cells in infection has been widely reported, elucidation on the impact of downstream signalling events remains unclear. This is further complicated by the fact that most cell signalling assays can only assess at most three phosphorylation events at a time. In order to fill this knowledge gap, we decided to investigate the NK cell signalome in humans and macaques in greater detail during lentiviral infection.
Methods: We developed an NK cell multiplex signalling assay on the Luminex platform to assess the simultaneous phosphorylation (p) events of 10 major signalling molecules critical to NK cell function: p‐Syk, p‐lck, p‐LAT, p‐ZAP70, pJNK, p‐NFkB, p‐p70S6K, p‐Akt, p‐STAT3 and p‐STAT5. Analyses were performed on enriched human NK cells and NK cells from control and chronically SIVmac251‐infected rhesus macaques following stimulation by cross‐linking several classes of receptors including CD16, NKp46 (natural cytotoxicity receptor), NKG2D (NKG2 family receptor) and CD2 (co‐receptor).
Results: All stimulations tested activated immunoreceptor tyrosine‐based activating motif (ITAM)‐based signalling (Syk, lck, LAT, ZAP70), MAP kinase (JNK) and STAT5 pathways in human NK cells. As expected, CD16‐based activation was the most robust for all analytes, although CD2 stimulation induced additional STAT3 activation. Importantly, activation profiles regardless of stimulus were similar between uninfected human and macaque NK cells. Compared to controls, NK cell signalling in SIV‐infected animals was globally reduced in magnitude following CD16 stimulation, but signalling in response to CD2 stimulation was increased, specifically within the STAT5 pathway. These data were consistent with an upregulation of CD2 on NK cells during SIV infection.
Conclusions: We were able to establish a multiplex platform to evaluate complex cell signalling in NK cells, and demonstrated clear changes in CD16 versus CD2 signalling during SIV infection. The application of this technology will offer new insights into how HIV‐1 dysregulates the NK cell response and open up new avenues for immunotherapeutics.
OAA0302
HIV modifies the m6A and m5C epitranscriptomic landscape of the host cell
S Cristinelli 1; P Angelino2; A Janowczyk2; M Delorenzi3,2; and A Ciuffi1
1Lausanne University Hospital and University of Lausanne/Institute of Microbiology, Département Médecine de Laboratoire et Pathologie (DMLP), Lausanne, Switzerland. 2Bioinformatics Core Facility (BCF), SIB Swiss Institute of Bioinformatics, Lausanne, Switzerland. 3University of Lausanne, Translational Bioinformatics and Statistics Department of Oncology, Lausanne, Switzerland
Background: The study of RNA modifications, today known as epitranscriptomics, is of growing interest. The N6‐methyladenosine (m6A) and 5‐methylcytosine (m5C) RNA modifications are abundantly present on mRNA molecules, and impact RNA interactions with other proteins or molecules, thereby affecting cellular processes, such as RNA splicing, export, stability and translation. Recently, these epitranscriptomic marks were found to be present on HIV transcripts and affect viral replication. However, no study has been performed to date to investigate the impact of HIV replication on the transcript methylation level in the infected cell.
Methods: We used a productive HIV infection model to explore the landscape of m6A and m5C marks on the transcriptome of HIV‐infected cells. For this, the SupT1 T‐cell line was mock‐treated or infected with a high dose of VSV‐G pseudotyped HIVeGFP‐based vector to ensure approximately 80% infection efficiency. Cells were collected at 12, 24 and 36 hours post‐infection for mRNA extraction and FACS analysis. M6A RNA modifications were investigated by methylated RNA immunoprecipitation followed by sequencing (MeRIP‐Seq). M5C RNA modifications were investigated using a bisulphite conversion approach followed by sequencing (BS‐Seq). Untouched mRNAs were used as input controls. Libraries were prepared using TruSeq‐stranded mRNA protocols (Illumina) and sequenced on Illumina HiSeq2500.
Results: Our data suggest that HIV Infection impacted the methylation landscape of HIV‐infected cells, inducing mostly increased methylation of cellular transcripts upon infection. Indeed, differential methylation analysis identified 59 m6A hypermethylated and only 2 hypomethylated transcripts and 14 m5C hypermethylated transcripts and 7 hypomethylated ones. Furthermore, both m6A and m5C methylations were detected on viral transcripts and viral particle RNA genomes.
Conclusions: Our results provide a valuable resource for m6A and m5C transcripts in the non‐infected and HIV‐infected cells and highlight differentially methylated transcripts that may modulate HIV expression and thus HIV replication. Thus, epitranscriptomic analyses may uncover novel players in the HIV‐host interplay, thereby offering a novel array of opportunities to inhibit HIV replication.
OAA0303
GS‐9822, a preclinical LEDGIN, displays a block‐and‐lock phenotype in cell culture
A Bruggemans 1; G Vansant1; M Balakrishnan2; ML Mitchell2; R Cai2; F Christ1; and Z Debyser1
1KU Leuven, Molecular Virology and Gene Therapy, Leuven, Belgium. 2Gilead Sciences Inc., Foster City, United States
Background: The ability of HIV to integrate into the host genome and establish latent reservoirs is the main hurdle towards an HIV cure. LEDGINs are small‐molecule integrase that targets the binding pocket of LEDGF/p75, a cellular cofactor that substantially contributes to HIV integration site selection. They are potent antivirals that inhibit HIV integration and maturation. In addition, they retarget residual integrants away from transcription units towards a more repressive chromatin environment. A previous study also demonstrated that after CX14442 treatment, residually integrated proviruses are more latent and refractory to reactivation, supporting the use of LEDGINs in a functional cure strategy.
Methods: In this study, we compared GS‐9822, a potent, pre‐clinical lead compound, with the research compound CX14442 with respect to antiviral potency, integration site selection, latency and reactivation. Using AlphaScreen and multiple round HIV‐1 replication in MT‐4 cells we compared the activities of CX14442 and GS‐9822. In addition, integration sites after LEDGIN treatment were sequenced (Illumina Miseq) and the surrounding chromatin environments were compared using the INSPIRED platform. Using established double reporter viruses, we studied both latency and reactivation after treatment with either LEDGINs or the integrase inhibitor raltegravir as a control.
Results: GS‐9822, a pre‐clinical LEDGIN, is a potent antiviral with nanomolar activity against wild‐type HIV‐1. GS‐9822 inhibits the LEDGF/p75‐integrase interaction and reduces HIV‐1 integration. Much like CX14442, GS‐9822 was able to retarget integration of residual proviruses away from active genes and gene‐dense regions, resulting in a more repressive epigenetic landscape. Finally, when using a double reporter construct, CX14442 and GS‐9822 were shown to reduce HIV‐1 infectivity, increase immediate latency and decrease the reactivation potential of residual integrants. Remarkably, GS‐9822 induced these effects at 200‐300‐fold lower concentrations than CX14442.
Conclusions: The ability to retarget integration sites and induce a deep latent state (block‐and‐lock) is not specific for a single LEDGIN, CX14442, but a class‐effect related to the inhibition of the LEDGF/p75‐integrase interaction. Highly potent LEDGIN compounds that inhibit this interaction can induce these effects at doses achievable in the clinic, making LEDGINs an interesting candidate for functional HIV cure research.
OAA0304
Proteomic evidence of vesatolimod‐induced enhancement of “cross‐talk” between innate and adaptive immune cells in HIV controllers on ART
Y Cai 1; SG Deeks2; C Brinson3; M Ramgopal4; N Jones2; E DeJesus5; A Mills6; P Shalit7; B Moldt1; L Zhang1; E Vendrame1; DM Brainard1; D SenGupta1; O Podlaha1; and JJ Wallin1
1Gilead Sciences Inc., Foster City, United States. 2University of California, San Francisco, San Francisco, United States. 3Central Texas Clinical Research, Austin, United States. 4Midway Specialty Care Center, Fort Pierce, United States. 5Orlando Immunology Center, Orlando, United States. 6Southern California Men's Medical Group, Los Angeles, United States. 7Peter Shalit MD And Associates, Seattle, United States
Background: Vesatolimod (VES), an oral TLR7 agonist, induces interferon‐stimulated genes and circulating cytokines in a dose‐dependent manner in healthy volunteers and PWH on ART. In a Phase 1b trial of VES in HIV controllers, we observed modest but significant delay in viral rebound and decrease in viral set‐point, following ART interruption. We investigate mechanisms associated with these outcomes by assessing proteomic changes following VES.
Methods: We enrolled 25 HIV controllers (pre‐ART viral load 50 to 5000 copies/mL) on ART for ≥6 months. Seventeen participants received 10 biweekly doses of VES and 8 received placebo, followed by analytical treatment interruption. Immune cell activation after VES was evaluated using flow cytometry. Plasma samples were used for high‐throughput proteomic analysis with Proximity Extension Assay (PEA) technology. Data were analyzed with Ingenuity Pathway Analysis.
Results: Compared to placebo, VES cumulatively induced innate and adaptive immune cell activation. Geometric mean fluorescent intensity of CD40 on pDC, and frequency of CD69+CD56dim, CD69+CD56bright NK cells, and Ki67+CD4+ T cells were significantly increased 1‐day after VES dose‐10 (p = 0.0007, p = 0.0115, p = 0.0311, p = 0.0033). Frequency of activated monocytes (CD14+CD16+) and CD8+ T cells (CD38+CD8+) were increased by day‐3 after VES dose‐10 (p = 0.0056; p < 0.0001). Among 92 proteins evaluated by PEA, 21 proteins were significantly upregulated 1‐day after VES dose‐1 and 10 (p < 0.05). Pathway analysis of shifts revealed significant increase in immune responses following VES treatment, including pathways involved in T‐cell differentiation, recruitment, and migration (z‐score ≥ 1.96) and in crosstalk between dendritic cells and NK cells, NK‐cell signaling, Th1 pathways, and antiviral responses (z‐score ≥ 1.96; Figure 1).
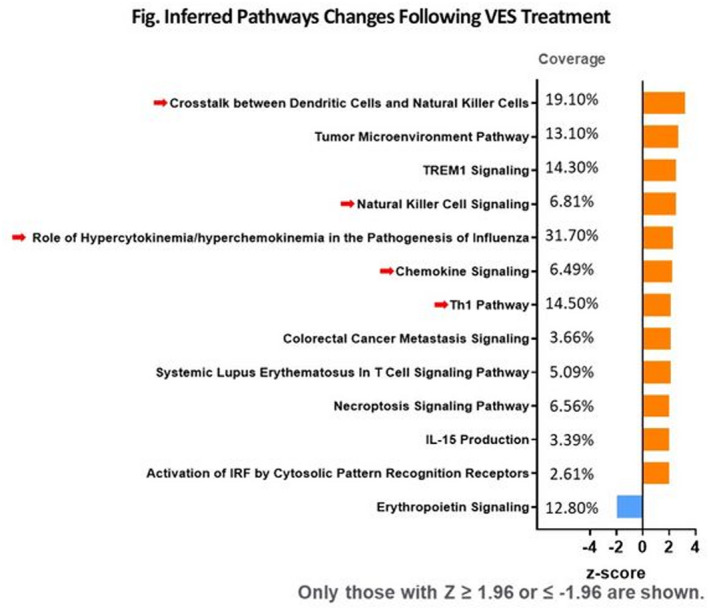
Abstract OAA0304‐Figure 1.
Conclusions: We utilized novel high‐throughput proteomic analysis approach to explore mechanisms associated with VES outcomes. An unbiased model revealed extensive shifts in immune function after administration of VES, with evidence of “cross‐talk” between innate and adaptive immune response. Findings support the hypothesis that achievement of post‐ART control requires combination of increased cellular immune responses coupled with balanced inflammatory response.
OAA0401
The circadian clock machinery regulates HIV transcription in CD4+ T cells
C‐D Ngassaki‐Yoka 1,2; D Chatterjee3,2; Y Zhang1,2; T Wiche Salinas3,2; L Raymond Marchand2; N Cermakian4; J‐P Routy5; L Solt6; and P Ancuta3,2
1Université de Montréal, Microbiologie, Infectiologie et Immunologie, Faculté de Médecine, Montréal, Canada. 2Centre de Recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM), Axe Immunopathologie, Montréal, Canada. 3Université de Montréal, Microbiologie, infectiologie et immunologie, faculté de médecine, Montréal, Canada. 4Douglas Mental Health University Institute, McGill University, Montréal, Canada. 5McGill University Health Centre: Glen Site, Research Institute, Montréal, Canada. 6The Scripps Research Institute, Department of Immunology and Microbiology, Jupiter, United States
Background: CD4+ T cells are key HIV‐1 infection targets and are highly enriched in viral reservoirs in people living with HIV (PLWH) receiving viral‐suppressive antiretroviral therapy (ART). Current antiretroviral drugs block different steps of the viral replication cycle but not the transcription, a process under the control of host‐cell transcription factors. Residual HIV transcription during ART is a major cause of chronic immune activation and non‐AIDS co‐morbidities. In previous studies, we demonstrated that the transcriptional signature associated with HIV permissiveness in Th17 cells includes the circadian clock components/regulators BMAL1 and REV‐ERBs. Of note, REV‐ERBs act as transcriptional repressors of BMAL1 (a transcriptional activator binding to E‐boxes in the HIV promoter) and RORC2 (the master regulator of Th17 polarization). Thus, we hypothesized that REV‐ERBs regulate both BMAL1‐mediated HIV replication and RORC2‐mediated effector functions in Th17 cells.
Methods: To test this hypothesis, we used the REV‐ERB agonists SR9009 and SR9011, reported to be efficient in decreasing Th17‐mediated autoimmune pathology in mice. Memory CD4+ T cells from uninfected individuals were stimulated with CD3/CD28 antibodies and exposed to HIV in vitro. A viral outgrowth assay (VOA) was performed with memory CD4+ T cells of ART‐treated PLWH activated via CD3/CD28 in the presence/absence of the REV‐ERB agonists. Lentiviral vectors were used to over‐express BMAL1 in primary CD4+ T cells. Cytokines and HIV‐p24 levels were measured by ELISA. HIV‐DNA integration was quantified by PCR.
Results: CD3/CD28 triggering resulted in a significant down‐regulation of REV‐ERBα and REV‐ERBβ, and the up‐regulation on BMAL1 mRNA expression. The REV‐ERB agonists potently inhibited HIV replication in vitro and viral outgrowth in VOA. The antiviral effects coincided with decreased IL‐17A and IFN‐γ production. Single‐round infection with a VSV‐G‐pseudotyped HIV showed decreased HIV‐p24 expression/production but no differences in HIV‐DNA integration in presence of REV‐ERB agonists, indicative of an inhibitory effect post‐integration, likely during transcription. Finally, we confirmed that BMAL1 overexpression increases HIV replication.
Conclusions: These results provide a strong rationale for further evaluating the possibility to therapeutically target REV‐ERBs as a way to limit BMAL1‐dependent HIV transcription and subsequently diminish chronic immune activation and non‐AIDS co‐morbidities during ART.
OAA0402
RNA‐directed gene therapy protects CD4+ T cells during HIV challenge and delays virus rebound post‐ART in humanized mice
C Ahlenstiel 1; V Klemm1; S Ledger1; C Allison2; G Symonds3; M Pellegrini2; and A Kelleher1
1University of New South Wales, Kirby Institute, Sydney, Australia. 2University of Melbourne/Walter & Eliza Hall Institute, Melbourne, Australia. 3CSL Ltd, Australia, Sydney, Australia
Background: The HIV‐1 latent reservoir is a major barrier to developing an HIV cure. Gene therapy is a promising treatment, highlighted by the success of the Berlin and London patients. Using RNA‐directed gene‐modified stem cells to induce and enforce super‐latency, we aim to mimic natural virus latency in an HIV‐1 functional cure “block and lock” approach, combined with conventional CCR5 mRNA targeting. We have previously shown novel siRNAs induce potent HIV‐1 silencing in various cell lines in vitro and provide protection from virus challenge in a humanized mouse model of acute HIV‐1 infection. We now investigate their potential for gene therapy using shRNA‐transduced CD34+ haematopoietic stem cells in a humanized mouse model of chronic HIV‐1 infection with ART.
Methods: Human CD34+ stem cells were transduced using GFP‐labelled lentivirus expressing promoter‐targeted shRNA, shPromA or dual construct shPromA/shCCR5 or controls; mock‐ or empty(‐shRNA+loop)‐transduced, and transplanted into irradiated NSG mice. At 18 weeks of post‐engraftment mice were challenged with CCR5‐tropic HIV‐1JR‐FL. Mice were bled at weeks 3, 5, 7 and 10 post‐infection (p.i.), received ART for eight weeks, following which ART was interrupted. Virus rebound was measured for four weeks prior to/at sacrifice by flow cytometry analysis of CD4+ T cells/GFP expression, RT‐qPCR analysis of viral load and RNAscope in lymph nodes and spleen tissue.
Results: Transduction efficiencies ranged between 40% and 70%. At sacrifice transduced mice expressing shPromA or dual shPromA/shCCR5 showed up to 100% CD4+ GFP expression, with means of approximately 70%. This correlated with a stable CD4 T cell count in dual shPromA/shCCR5 transduced mice, over 40 weeks of challenge, ART and ART interruption, compared to mock and empty‐transduced mice, which were 1.5 and 2 logs lower respectively. Virus rebound was delayed seven days in dual‐transduced mice, which showed a 1 log decrease in viraemia at four weeks post‐ART interruption compared to controls. Quantification of RNAscope and immunostaining of lymph nodes and spleen will determine the level of virus silencing in tissue.
Conclusions: This study demonstrates RNA‐directed ex vivo gene therapy targeting shPromA/shCCR5 has the potential to protect against HIV‐1, following ART interruption in a humanized mouse model.
OAA0403
The balance of mucosal CD4 T cells prior to infection is associated with control of virus replication after therapeutic vaccination in SIV‐infected rhesus macaques
H Tunggal 1,2; P Munson1,2; M O'Connor1,2; D Bratt2; N Hajari2; S Wangari2; D May2; B Agricola2; J Smedley2; K Bagley3,4; and D Fuller1,2
1University of Washington, Microbiology, Seattle, United States. 2Washington National Primate Research Center, Seattle, United States. 3Profectus Biosciences, Baltimore, United States. 4Orlance, Inc., Seattle, United States
Background: A therapeutic vaccine that induces lasting control of HIV infection could eliminate the need for lifelong antiretroviral therapy (ART). However, barriers to an effective therapeutic vaccine include insufficient vaccine immunogenicity in the periphery and gut‐associated lymphoid tissue (GALT), and an incomplete understanding of what host parameters affect ART efficacy, vaccine immunogenicity and viral control. Here, we investigated a therapeutic SIV DNA vaccine and a novel combination of adjuvants and characterized immune parameters associated with viral control post‐ART.
Methods: Adult male rhesus macaques were infected with SIVΔB670 and initiated ART at six weeks of post‐infection (wpi). Beginning at 32wpi, animals received five therapeutic immunizations spaced four weeks apart. ART was suspended at 55wpi to evaluate efficacy. One group received a DNA vaccine (MAG) expressing SIV Gag, Pol, Env and Nef, with E. coli heat‐labile enterotoxin, LT, via Gene Gun (MAG+LT; N = 5). Another group received MAG and a genetic adjuvant combination expressing soluble CD80, soluble PD‐1, IL‐12, IL‐33, RALDH2 and the catalytic subunit of LT via intradermal electroporation (MAG+AC; N = 5). Controls received empty plasmid DNA via Gene Gun (Controls; N = 4). T‐cell responses and immunophenotyping in PBMC and GALT were determined by flow cytometry, whereas viraemia was measured by RT‐qPCR.
Results: Every animal exhibited robust acute viraemia (median 107 RNA copies/mL plasma), but ART did not fully suppress viral replication in all animals. Post‐ART, 3/5 MAG+AC animals controlled viraemia (median viral loads ≤103 RNA copies/mL plasma), compared to 1/5 MAG+LT and 1/4 control animals (controllers). Nine animals, among all groups, exhibited immediate viral rebound (median viral loads >103 RNA copies/mL, non‐controllers). Although was no significant difference between groups in protection from viral rebound, lower post‐ART viral burden correlated with increased ART responsiveness and polyfunctional SIV‐specific CD8+ T cells in mesenteric lymph nodes prior to and during ART interruption. Notably, improved responses to ART and control of viral rebound correlated with elevated frequencies of colonic CD4+ T cells and lower Th17/Treg ratios pre‐infection.
Conclusions: These results indicate that mucosal immunity prior to infection can influence ART efficacy and the outcome of immunotherapeutic vaccination, suggesting that therapies capable of modulating host mucosal immunity may be needed to achieve an HIV functional cure.
OAA0404
Early antiretroviral therapy favours post‐treatment SIV control, which is associated with enhanced CD8+ T‐cell antiviral activity against rebounding virus – the pVISCONTI study
C Passaes 1; D Desjardins2; V Avettand‐Fenoel3; A Chapel1; V Monceaux1; A Melard4; N Dimant2; F Perdomo‐Celis1; A David1; A Barrail‐Tran5; O Lambotte6; J Guedj7; N Dereuddre‐Bosquet2; M Muller‐Trutwin1; C Rouzioux8; R Le Grand2; A Saez‐Cirion1; and pVISCONTI Study Group
1Institut Pasteur, Unité HIV, Inflammation et Persistance, Paris, France. 2UMR 1184, Immunology of Viral, Auto‐Immune, Hematological and Bacterial Diseases (IMVA‐HB), IBFJ, CEA – Université Paris‐Saclay – INSERM, IDMIT Department, Fontenay‐aux‐Roses/Le Kremlin‐Bicêtre, France. 3Université de Paris , INSERM U 1016, CNRS 8104, APHP Hôpital Necker – Enfants Malades, Paris, France. 4Université de Paris, INSERM U 1016, CNRS 8104, Paris, France. 5Université Paris Saclay, Faculté de Pharmacie , AP‐HP, Hôpital Bicêtre, UMR 1184, Immunologie des Maladies Virales, Auto‐Immunes, Hématologiques et Bactériennes, Le Kremlin‐Bicêtre, France. 6APHP Université Paris‐Saclay, Hôpital Bicêtre, Inserm, CEA, Center for Immunology of Viral, Auto‐Immune, Hematological and Bacterial Diseases (IDMIT/IMVA‐HB), UMR 1184, Clinical Immunology Department, Le Kremlin Bicêtre, France. 7Université de Paris, IAME, INSERM, Paris, France. 8Université de Paris, CNRS 8104, APHP Hôpital Necker – Enfants Malades, Paris, France
Background: The VISCONTI study proposed that post‐treatment control (PTC) might be favoured by early antiretroviral treatment (cART) initiation. However, a formal demonstration has not been established and the underlying mechanisms leading to PTC remain elusive. We used a non‐human primate model to assess, in standardized conditions, the impact of early versus late cART initiation on immune responses and the outcome after analytical treatment interruption (ATI).
Methods: SIVmac251‐infected cynomolgus macaques (CyMs) remained untreated (n = 17) or initiated cART at primary (day 28 post‐infection [p.i.], n = 12) or at chronic (6 months p.i., n = 12) infection. cART was maintained for 24 months. The animals were then monitored for 12 months after ATI. Plasma viral loads (pVL), CD4+ T cells and CD8+ T‐cell responses (phenotype and viral inhibition assay) were analysed throughout the study.
Results: pVL levels were similar at cART initiation for both groups of CyMs receiving cART (D28 and M6). After ATI, all CyMs experienced viral rebound (>1000 copies/mL), except one animal in the D28 group. Viral rebound occurred earlier in the M6 group (17.5 days) when compared with D28 group (28 days) (p = 0.0009). Early treatment significantly impaired definitive loss of viral control (p = 0.012). Moreover, 82% of CyMs in the D28 group were defined as PTC (<400 copies/mL) at the end of the study, which was higher than in the M6 group (25%) or among non‐treated (12%) CyMs (p = 0.0003). The anti‐SIV activity of CD8+ T cells, as measured in the viral inhibition assay, was weak in all animals at primary SIV‐infection, but strongly increased after ATI, in particular in D28 CyMs (6.6x fold [3.5 to 9.8] post‐ATI vs. primary infection). The CD8‐antiviral activity that emerged following viral rebound was stronger in PTCs (p = 0.016) early after ATI and at the end of the study in blood and lymphoid tissues (spleen, peripheral and mesenteric lymph nodes). A negative correlation was found between the anti‐SIV activity of CD8+ T cells and cumulated pVLs post‐ATI (r = −0.41, p = 0.05).
Conclusions: Early cART initiation favoured PTC in SIVmac251‐infected CyMs. This was associated with the promotion of a robust secondary SIV‐specific CD8+ T‐cell response, which might contribute to efficiently counteract viral rebound after ATI in PTCs.
OAA0405
Evaluation of HIV‐1 reservoir size and broadly neutralizing antibody (bNAb) susceptibility in individuals who initiated ART during acute and chronic infection
B Moldt 1; H Günthard2; K Workowski3; S Little4; J Eron5; E Overton6; C Lehmann7; C Rokx8; M Kozal9; R Gandhi10; H Liu1; T Makadzange1; S Collins1; R Geleziunas1; and C Callebaut1
1Gilead Sciences, Inc, Foster City, United States. 2University Hospital Zurich, Zurich, Switzerland. 3Emory University, Atlanta, United States. 4University of California, San Diego, San Diego, United States. 5University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, United States. 6University of Alabama at Birmingham, Birmingham, United States. 7University Hospital of Cologne, Cologne, Germany. 8Erasmus University Medical Center, Rotterdam, Netherlands. 9Yale School of Medicine, New Haven, United States. 10Massachusetts General Hospital and Harvard Medical School, Boston, United States
Background: The persistence of the viral reservoir is the main barrier to curing HIV. Initiation of ART during primary HIV infection can limit the size and diversity of the viral reservoir. Characterization of the differences between individuals who initiate ART during primary and chronic infection will be critical for clinical trial design and HIV cure strategies.
Methods: A cross‐sectional, non‐interventional study was performed to characterize the viral reservoir in people living with HIV. Four cohorts were enrolled with participants that initiated ART during Fiebig I‐II, Fiebig III‐IV, early (≤3 months of infection) or chronic (≥6 months of infection) infection. Participants who underwent leukapheresis and viral reservoir in PBMCs were evaluated by the Intact Proviral DNA Assay (IPDA), the Total HIV DNA Assay (THDA) and the Quantitative Viral Outgrowth Assay (QVOA). Viral diversity and susceptibility to the bNAb elipovimab were determined by genotyping of the viral envelope gene.
Results: An increase in reservoir size was observed with increased time to ART initiation (Fiebig stages through chronic infection) when measured by IPDA and THDA, whereas no difference was observed by QVOA. Viral diversity was lower in participants initiating ART during acute infection than chronic infection, and acute ART‐treated individuals also showed higher susceptibility to elipovimab as 71% of cohort 1 participants, 78% of cohort 2 participants, 53% of cohort 3 participants and 44% of cohort 4 participants were sensitive.
Abstact OAA0405‐Table 1. Characteristic and HIV reservoir
|
Cohort 1 Fiebig I‐II (n = 16) |
Cohort .2 Fiebig III‐IV (n = 17) |
Cohort 3 Early infection (n = 14) |
Cohort 4 Chronic infection (n = 17) |
|
|---|---|---|---|---|
| Time on ART (years, median (Q1, Q3)) | 4.1 (1.3, 7.8) | 5.0 (3.0, 7.0) | 2.9 (2.0, 5.2) | 5.1 (2.9, 6.6) |
| Pre‐ART HIV‐1 RNA (log10 copies/mL, median (Q1, Q3)) | 5.95 (5.40, 6.73) | 5.88 (5.47, 6.69) | 5.43 (4.67, 6.33) | 4.44 (4.10, 4.75) |
| CD4+ cell count at screening (cells/μL, median (Q1, Q3)) | 784 (636, 1109) | 716 (675, 848) | 913 (860, 1134) | 824 (688, 968) |
| Intact HIV DNA, IPDA (copies/106 CD4+ cells, median (Q1, Q3)) | 28.86 (3.53, 58.09) | 28.63 (24.67, 125.90) | 82.28 (14.07, 206.10) | 57.72 (20.42, 185.00) |
| Cell‐associated HIV DNA, IPDA (copies/106 CD4+ cells, median (Q1, Q3)) | 163.30 (50.47, 319.00) | 79.92 (27.01, 465.40) | 308.50 (164.10, 641.30) | 359.50 (184.10, 1583.00) |
| Cell‐associated HIV DNA, THDA (copies/106 CD4+ cells, median (Q1, Q3)) | 32.40 (9.00, 249.15) | 37.12 (19.18, 129.08) | 50.77 (29.95, 304.60) | 138.27 (54.31, 499.81) |
| Replication competent HIV, QVOA (copies/106 CD4+ cells, median (Q1, Q3)) | 0.060 (0.014, 0.286) | 0.069 (0.014, 0.315) | 0.105 (0.014, 0.286) | 0.070 (0.014, 0.257) |
Conclusions: Early treated individuals had lower reservoir size, lower viral diversity and higher susceptibility to bNAbs (exemplified by elipovimab) supporting that individuals who initiate ART during Fiebig stages, and in particular during Fiebig I to IV, would be an attractive population for early proof of concept bNAb cure‐related trials. The IPDA provides both intact and total HIV DNA measurements and was able to differentiate between early and late cohorts and should therefore be given priority as a reservoir measurement in HIV cure trials.
OALB0301
CD4+ T‐cell count below 200 cells/mm3 is associated with worse COVID‐19 outcomes among people living with HIV regardless of virological suppression
DK Nomah 1,2; J Reyes‐Urueña1,3,4; Y Diaz1,4; S Moreno1,4; J Aceiton1,4; A Bruguera1,3,4; RM Vivanco‐Hidalgo5; JM Llibre6; P Domingo7; V Falcó8; A Imaz9; C Cortés10; L Force11; E Letang12; I Vilaró13; J Casabona1,2,3,4; JM Miro14; and The PISCIS Cohort Group
1Centre Estudis Epidemiològics sobre les Infeccions de Transmissió Sexual i Sida de Catalunya (CEEISCAT), Dept Salut, Generalitat de Catalunya, Badalona, Spain. 2Universitat Autònoma de Barcelona, Departament de Pediatria, d’Obstetrícia i Ginecologia i de Medicina Preventiva i de Salut Publica, Bellaterra, Spain. 3CIBER Epidemiologia y Salud Pública (CIBERESP), Barcelona, Spain. 4Institut d'Investigació Germans Trias i Pujol (IGTP), Barcelona, Spain. 5Agència de Qualitat i Avaluació Sanitàries de Catalunya, Barcelona, Spain. 6Hospital Universitari Germans Trias i Pujol, Badalona, Spain. 7Hospital de la Santa Creu i Sant Pau, Barcelona, Spain. 8Vall d'Hebron Research Institute (VHIR), Hospital de Vall d’Hebron, Barcelona, Spain. 9Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, Spain. 10Hospital Moises Broggi‐Consorci Sanitari Integral, L’Hospitalet de Llobregat, Spain. 11Hospital de Mataró, Mataró, Spain. 12Hospital del Mar, Barcelona, Spain. 13Hospital de Vic, Vic, Spain. 14Hospital Clínic‐Institut d’Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona, Barcelona, Spain
Background: Information about the relationship between HIV‐associated immune suppression and COVID‐19 outcomes is scarce. We aimed to characterize the epidemiological and clinical features, and impact of immunosuppression on COVID‐19‐related outcomes among persons living with HIV (PLWH).
Methods: PISCIS is a population‐based cohort of PLWH aged ≥16 years in care at 16 Catalan hospitals, which collects socio‐demographic and clinical data between 01/01/1998 and 15/12/2020. We linked PISCIS data with integrated healthcare, clinical and surveillance registries through the Public Data Analysis for Health Research and Innovation Program of Catalonia (PADRIS) to obtain COVID‐19 diagnosis‐related data and other comorbidities. Only patients with microbiologically confirmed SARS‐CoV‐2 infection (NAAT, antigen detection or antibodies) were included in the analysis. Factors associated with COVID‐19 diagnosis and severe outcomes were assessed using multivariate Cox regression models. The impact of immunosuppression on severe outcomes (hospital admission or death) was estimated in survival analysis.
Results: Of 13,264 PLWH in our cohort, 747 (5.63%) were diagnosed with COVID‐19. Among them, 616/747 (82.46%) were males and the median age was 44 years (IQR 37 to 53). One‐hundred and one (13.5%) were hospitalized, seven (0.92%) were admitted to the ICU and 11 (1.5%) died. Non‐Spanish origin (HR 1.6;95% CI: 1.3 to 1.9) and low socio‐economic status (HR 1.5;95% CI: 1.1 to 1.9) were associated with a higher odds of COVID‐19 diagnosis. Aged ≥75 years (HR 4.4;95% CI: 1.4 to 13.8), non‐Spanish origin (HR 2.1; 95% CI: 1.3 to 3.4), low socio‐economic status (HR 2.3;95% CI: 1.1 to 5.2) and comorbidities (metabolic, HR 4.8;95% CI:2.4 to 9.8; neuropsychiatric, HR 3.3;95% CI: 1.7 to 6.2; and cardiovascular, HR 7.7;95% CI: 3.5 to 17.0) were associated with higher odds of severe outcomes. The Kaplan–Meier estimator showed an increased risk of severe outcomes among patients with CD4 count <200 cells/mm3 (p < 0.001) and detectable viral load (p = 0.046). CD4 count <200 cells/mm3 remained associated with COVID‐19 severe outcomes even if the viral load was undetectable (p = 0.032).
Conclusions: COVID‐19 diagnosis was more common among migrants and PLWH with low socio‐economic status. Among PLWH with COVID‐19, those with CD4 count <200 cells/mm3, older age, non‐Spanish origin, low socio‐economic status, and metabolic, neuropsychiatric and cardiovascular comorbidities, had a higher risk of severe outcomes. Of note, CD4 count <200 cells/mm3 remained a risk factor for severe COVID‐19 outcomes despite virological suppression.
OALB0302
Long‐acting subcutaneous lenacapavir dosed every six months as part of a combination regimen in treatment‐naïve people with HIV: interim 16‐week results of a randomized, open‐label, phase 2 induction‐maintenance study (CALIBRATE)
SK Gupta 1; M Berhe2; G Crofoot3; J Sims4; P Benson5; M Ramgopal6; WE Sanchez7; P Ruane8; C McDonald9; A Scribner10; H Wang11; L VanderVeen11; H Dvory‐Sobol11; RH Hyland11; MS Rhee11; JM Baeten11; DM Brainard11; and E Koenig12
1Indiana University School of Medicine, Indianapolis, United States. 2North Texas Infectious Diseases Consultants, Dallas, United States. 3The Crofoot Research Center, Inc., Houton, United States. 4St. Hope Foundation, Bellaire, United States. 5Be Well Medical Center, Berkley, United States. 6Midway Specialty Care Center, Fort Pierce, United States. 7Floridian Clinical Research, Hialeah, United States. 8Ruane Clinical Research Group, Los Angeles, United States. 9Texas Centers for Infectious Disease Associates, Fort Worth, United States. 10Diagnostic Clinic of Longview Center for Clinical Research (DCOL), Longview, United States. 11Gilead Sciences Inc., Foster City, United States. 12Instituto Dominicano de Estudios Virológicos (IDEV), Santo Domingo, Dominican Republic
Background: Lenacapavir (LEN, GS‐6207), a potent first‐in‐class inhibitor of HIV‐1 capsid function, is in development as a long‐acting agent for treatment and prevention of HIV.
Methods: CALIBRATE is an ongoing, phase 2, randomized, open‐label, active‐controlled, induction‐maintenance study in treatment‐naïve people with HIV‐1 (TN‐PWH) with CD4+ cell count ≥200/µL. Participants were randomized (2:2:2:1) to treatment groups (TGs) A to D (Figure 1). TG‐A and B received subcutaneous (SC) LEN with oral daily emtricitabine/tenofovir alafenamide (F/TAF); at W28, those achieving HIV‐1 RNA (VL)<50 copies/mL switched F/TAF to oral daily TAF (TG1) or bictegravir (BIC) (TG2). TG‐C received oral daily LEN with F/TAF. TG‐D received oral daily B/F/TAF. The primary endpoint is VL < 50 copies/mL at W54 by FDA Snapshot. We report the pre‐specified W16 interim efficacy and safety analyses, for which there were no planned statistical comparisons.
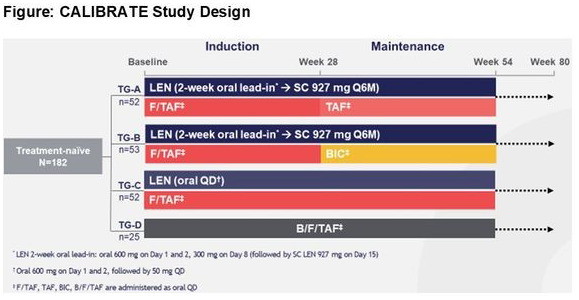
Abstract OALB0302‐Figure 1.
Results: A total of 182 participants (7% female, 54% Black) were randomized/dosed (n = 52, 53, 52, 25 in TG‐A to D). Median age was 29 years; 15% had VL>100,000 c/mL. At W16, 92% (48/52), 94% (50/53), 94% (49/52) and 100% (25/25) had VL < 50 copies/mL in TG‐A, B, C and D, respectively, by missing = failure, and 98% (48/49), 98% (50/51), 96% (49/51) and 100% (25/25) by missing = excluded. Four participants had VL>50 copies/mL: 3 with VL < 100 copies/mL (1 TG‐A, 2 TG‐C) and 1 with VL>5000 copies/mL (TG‐B). Resistance analysis is ongoing. No participant died, experienced a study drug‐related serious adverse event (AE), or discontinued study drug due to AE, and no Grade 3 or 4 AEs were considered study‐drug related. The most frequent AEs were injection site erythema, injection site pain (12% each), injection site swelling (11%) and headache (10%). All injection site reactions were mild or moderate.
Conclusions: LEN, given subcutaneously or orally, in combination with F/TAF led to high rates of viral suppression in TN‐PWH by W16. LEN was generally safe and well‐tolerated. Results support ongoing evaluation of LEN for treatment and prevention of HIV.
OALB0303
Switching to the 2‐drug regimen of dolutegravir/lamivudine (DTG/3TC) fixed‐dose combination (FDC) is non‐inferior to continuing a 3‐drug regimen through 24 weeks in a randomized clinical trial (SALSA)
JM Llibre 1; C Alves2; C‐Y Cheng3,4; O Osiyemi5; C Galera6; L Hocqueloux7; F Maggiolo8; O Degen9; E Blair10; B Wynne10; J Oyee11; M Underwood10; L Curtis11; G Bontempo10; and J van Wyk12
1Hospital Universitari Germans Trias i Pujol, Barcelona, Spain. 2Universidade Federal da Bahia, Salvador, Brazil. 3Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan, Taiwan, Province of China. 4School of Public Health, National Yang‐Ming University, Taipei, Taiwan, Province of China. 5Triple O Research Institute PA, West Palm Beach, United States. 6Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain. 7Centre Hospitalier Régional d’Orléans, Orléans, France. 8ASST Papa Giovanni XXIII, Bergamo, Italy. 9Universitätsklinikum Hamburg‐Eppendorf, Hamburg, Germany. 10ViiV Healthcare, Research Triangle Park, United States. 11GlaxoSmithKline, Uxbridge, United Kingdom. 12ViiV Healthcare, Brentford, United Kingdom
Background: Long‐term non‐inferior efficacy of the 2‐drug regimen (2DR) DTG/3TC compared with 3/4‐drug regimens (3/4DRs) has been demonstrated in treatment‐naive (DTG + TDF/FTC through 144 weeks) and treatment‐experienced individuals with HIV‐1 (TAF‐based regimens through 96 weeks), with a good safety profile and a high barrier to resistance. We evaluated the efficacy and safety of switching to DTG/3TC fixed‐dose combination (FDC) in adults with HIV‐1 on any current antiretroviral regimen (CAR).
Methods: SALSA is a randomized, controlled, open‐label study. Participants with HIV‐1 RNA <50 copies/mL for >6 months on a 3/4DR without prior virological failure or NRTI or DTG resistance‐associated mutations were randomized 1:1 (stratified by baseline third agent class) to switch to DTG/3TC or continue CAR for 52 weeks. The primary endpoint was the proportion of participants with plasma HIV‐1 RNA ≥50 copies/mL at Week 48 (FDA Snapshot algorithm, ITT‐E population). Planned Week 24 interim analysis assessed non‐inferiority with a 5% margin.
Results: Overall, 493 participants were randomized (59% white; 39% women; 39% aged >50 years; 50%/40%/10% on NNRTI/INSTI/PI at baseline). DTG/3TC was non‐inferior to continuing CAR at Week 24 using Snapshot virological failure, and results were consistent with the Snapshot virological success analysis (Table 1). No participants in either arm met confirmed virological withdrawal criteria; therefore, no resistance testing was done. Overall safety outcomes were comparable between the DTG/3TC and CAR groups for frequency of any AEs (60% vs. 60%), AEs leading to withdrawal (2% vs. <1%) and serious AEs (1% vs. 6%) respectively.
Abstract OALB0303‐Table 1. Week 24 study outcome by snapshot analysis
| n (%) | DTG/3TC (N = 246) | CAR (N = 247) | Adjusted difference (95% CI) |
|---|---|---|---|
| HIV‐1 RNA ≥50 copies/mL a | 0 | 1 (<1) | −0.4% (−1.2%, 0.4%) |
| HIV‐1 RNA <50 copies/mL (virological success) | 234 (95) | 237 (96) | −0.8% (−4.5%, 2.8%) |
| No virological data | 12 (5) | 9 (4) | – |
Estimates and confidence intervals were based on a stratified analysis using Cochran–Mantel–Haenszel weights adjusting for baseline third agent class.
Conclusions: In SALSA, switching to DTG/3TC was non‐inferior to continuing CAR in maintaining virological suppression at Week 24, with a safety profile consistent with the DTG and 3TC labels. Through 24 weeks, DTG/3TC 2DR offers a switch option with fewer antiretroviral drugs compared with traditional 3DRs, without increased risk of virological failure or resistance. The study is ongoing; the conference presentation will include lipid data and Week 48 results.
OALB0401
More frequent viral load testing, with point‐of‐care tests has no impact on viral suppression in postpartum HIV‐positive women in a randomized controlled trial in two clinics in Johannesburg, South Africa
L Fairlie 1; S Sawry1; S Pals2; G Sherman3,4; D Williamson2; A Chivafa1; B Ngeno2; L Berrie5; K Diallo5; M Hurlston Cox2; M Mogashoa5; S Modi2; and OPPTIM Study group
1Wits RHI, Johannesburg, South Africa. 2US Centers for Disease Control and Prevention (CDC), Atlanta, United States. 3Paediatric HIV Surveillance in the Centre for HIV and STI, National Institute for Communicable Diseases, Johannesburg, South Africa. 4University of the Witwatersrand, Department of Paediatrics and Child Health, Johannesburg, South Africa. 5Centers for Global Health, CDC‐South Africa, Division of Global HIV and Tuberculosis, Pretoria, South Africa
Background: Elevated maternal viral load (VL) increases HIV transmission risk for breastfeeding infants. We describe results from a non‐blinded randomized controlled trial in Johannesburg comparing three‐monthly point‐of‐care (POC) VL testing (arm 2), to six‐monthly standard‐of‐care (SOC) laboratory‐based VL testing (arm 1) in HIV‐positive post‐partum women on first‐line antiretroviral treatment. We evaluated differences in VL suppression rates per arm at six, twelve and eighteen months.
Methods: Mother‐child dyads were enrolled at the child’s 6/10/14‐week clinic visit. Women were randomized 1:1 to arm 1 or 2. For arm 2, trained nurse clinicians and field workers used Cepheid GeneXpert IV for POC VL testing.
We fit a generalized linear mixed model with VL suppression at enrolment, six, twelve and eighteen months as the outcome, indicator variables for time, study site, study arm and interaction variables (time x site, time x arm, site x arm, time x site x arm). The model included a random effect for study ID to account for correlation among multiple VL from the same woman over time. All interaction terms were non‐significant and were removed from the model. The final model tested for a difference by study arm, pooling across timepoints.
Results: At baseline, women in arms 1 and 2 were well‐balanced for socio‐economic status (Table 1). VL suppression rates were high throughout the study, with no difference at each time point between arms (p‐value 0.8937) after adjusting for baseline VL suppression; in arm 1 and 2, respectively, 94.0% and 88.6% at baseline, 96.2% and 91.2% at six months, 94.1% and 91.6% at twelve months and 94.1% and 94.3 % at eigteen months.
Abstract OALB0401‐Table 1: Baseline socio‐economic characteristics
|
Total n (%N) N = 405 |
Standard of care (SoC) arm (1) n (%N) N = 204 |
Point of care (POC) arm (2) n (%N) N = 201 |
P‐value* |
||
|---|---|---|---|---|---|
| Age in years, mean (standard deviation) | 30.3 (5.35) | 30.5 (5.5) | 30.1 (5.2) | .4463 | |
| Country of birth | South Africa | 188 (46.5) | 97 (47.5) | 91 (45.3) | .6462 |
| Other | 217 (53.5) | 107 (52.5) | 110 (54.7) | ||
| Married/cohabiting | 225 (55.8) | 113 (55.4) | 112 (55.7) | .9468 | |
| Secondary or higher education | 396 (98.3) | 201 (98.5) | 195 (97.0) | .3012 | |
| Unemployed | 221 (54.8) | 105 (51.5) | 116 (57.7) | .2072 | |
| Financial support | Receives any financial support | 352 (87.3) | 172 (84.3) | 180 (89.6) | .1181 |
| Financial support child’s father | 303 (75.2) | 150 (73.5) | 153 (76.1) | .5483 | |
| Receives a social grant | 67 (16.6) | 35 (17.2) | 32 (15.9) | .7378 | |
Comparison between SOC and POC arms.
Conclusions: In our study, there was no significant difference in VL suppression rates between six‐monthly SOC and three‐monthly POC VL testing in HIV‐positive postpartum women. VL suppression rates were high overall, indicating PMTCT programme success.
OALB0402
Obsession with suppression: comparison of virally suppressed and unsuppressed children and adolescents living with HIV (CALHIV) treated with dolutegravir regimens in Mbeya and Mwanza, Tanzania
J Bacha 1,2,3; B Mayalla3; M Chodota3; N Jiwa3; L Mwita3; and L Campbell1,2,3
1Baylor International Pediatric AIDS Initiative (BIPAI) at Texas Children’s Hospital, Baylor College of Medicine, Houston, United States. 2Baylor College of Medicine, Houston, United States. 3Baylor College of Medicine Children's Foundation – Tanzania, Mbeya, Tanzania, United Republic of
Background: Current guidelines recommend using dolutegravir (DTG) as a preferred ART regimen in eligible CALHIV. However, descriptions of CALHIV who remain virologically unsuppressed despite treatment with DTG remain unknown. We aimed to describe the cohort of CALHIV in care at the Baylor Tanzania HIV clinics who remain in treatment failure despite being on a DTG regimen.
Methods: A retrospective chart review was conducted to assess the clinical characteristics of CALHIV receiving DTG as part of their ART at the Baylor College of Medicine Children’s Foundation – Tanzania Centres of Excellence (COEs) in Mbeya and Mwanza, Tanzania between 1 March 2019 (when DTG became available) and 30 November 2020. HIV viral load (VL) suppression was defined as VL < 1000 copies/mL.
Results: A total of 1703 CALHIV received DTG, among which 1084 (63.7%) had a documented VL after being prescribed DTG and were included in the analysis. Among those with post‐DTG VL results, 7.6% (82/1084) remained virally unsuppressed despite their DTG regimen.
Compared to CALHIV virally suppressed on DTG (N = 1002, 94.4%), those unsuppressed on DTG had higher rates of malnutrition (3.6% vs. 0.7%, p < 0.01) and previous ART exposure (99% vs. 97%, p < 0.01), as well as lower rates of previous viral suppression (70% vs. 97%, p < 0.01) (Table 1). There were no differences among the group regarding sex, age, time on ART, history of TB disease, history of IPT use, or single versus multiple tab DTG regimens (pill burden).
Abstract OALB0402‐Table 1
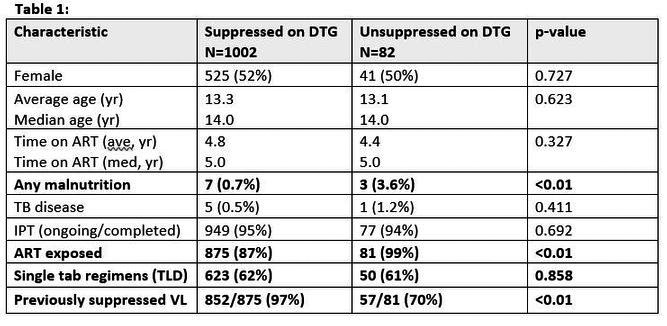
Conclusions: While DTG was highly effective in virally suppressing the majority of CALHIV, 7.6% remained unsuppressed. Unsuppressed patients were more likely to have prior ART exposure and prior lack of VL suppression, likely reflecting a subset of CALHIV with complex adherence challenges. The pill burden of DTG regimens did not appear to make a difference between groups. These unsuppressed CALHIV will require unique, patient‐centred support to improve their treatment success.
OALB0403
A rapid enzymatic assay for selective detection of HIV drugs that indicate long‐term and short‐term PrEP adherence
AO Olanrewaju; AH Gim; BP Sullivan; JD Posner; and PK Drain
University of Washington, Seattle, United States
Background: Tenofovir diphosphate (TFV‐DP) and emtricitabine triphosphate (FTC‐TP) are nucleotide analogue drugs used in PrEP that indicate long‐term (onr to three month) and short‐term (1‐week) medication adherence respectively. We recently developed the REverSe TRanscrIptase Chain Termination (RESTRICT) assay for rapid measurement of nucleotide analogues based on their inhibition of DNA synthesis by HIV reverse transcriptase (RT) enzyme and demonstrated proof of concept TFV‐DP measurement in clinical samples. Here we design RESTRICT assays for selective measurement of both TFV‐DP and FTC‐TP.
Methods: RESTRICT assays were completed by incubating RT, nucleotides, DNA templates and primers at 37˚C for 30 minutes followed by the addition of PicoGreen® dye to provide fluorescence output. We designed a guanosine‐rich DNA template for selective detection of FTC‐TP (cytidine analogue) by Watson‐Crick‐Franklin base pairing and excluded thymidine bases to prevent TFV‐DP (adenosine analogue) binding. Similarly, we designed a thymidine‐rich DNA template (excluding guanosine bases) for selective TFV‐DP detection. We spiked 1 µM of TFV‐DP and FTC‐TP into RESTRICT assays with each DNA template and measured endpoint fluorescence. We normalized fluorescence output using “no‐enzyme” negative controls and “no drug” positive controls.
Results: “No enzyme” controls produced no fluorescence since no DNA synthesis occurred, whereas “no drug” controls produced maximum fluorescence since there was inhibition of DNA synthesis (Figure 1). RESTRICT assays with guanosine‐rich DNA templates produced low fluorescence (4.0 ± 4.3%) with FTC‐TP and high fluorescence (86.2% ± 26.3%) with TFV‐DP indicating selective FTC‐TP detection without cross‐reactivity with TFV‐DP (p = 0.0060). Conversely, thymidine‐rich DNA templates produced high fluorescence (105.7% ± 3.1%) with FTC‐TP and low fluorescence (2.4% ± 1.5%) with TFV‐DP indicating selective TFV‐DP detection (p < 0.0001).
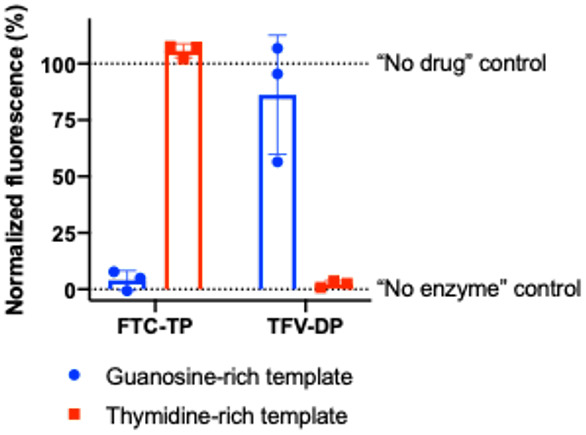
Abstract OALB0403‐Figure 1.
Conclusions: The RESTRICT assay enables rapid and selective detection of TFV‐DP and FTC‐TP. RESTRICT could help to monitor short‐term and long‐term PrEP adherence in near‐patient settings.
OAB0101
Robust SARS‐CoV‐2‐specific serological and functional T‐cell immunity in PLWHIV
L Donadeu1,2; J Tiraboschi 3,2; A Favà1,2; S Scévola3,2; D Podzamczer3,2; and O Bestard1,2
1Bellvitge University Hospital, Translational Nephrology and Transplantation Laboratory, L'Hospitalet de Llobregat, Spain. 2IDIBELL, L'Hospitalet de Llobregat, Spain. 3Bellvitge University Hospital, HIV Unit, Infectious Disease Service, L'Hospitalet de Llobregat, Spain
Background: While the description of protective humoral and T‐cell immune responses has been reported among immunocompetent (IC) individuals, its characterization among PLWHIV remains uncertain.
Methods: SARS‐CoV‐2‐specific serological and functional T‐cell immune responses against main immunogenic antigens were assessed in 11 HIV‐positive patients at three (T1) and six months (T2) following confirmed‐SARS‐CoV‐2‐infection, and compared to a cohort of 34 immunocompetent (IC) individuals developing mild (outpatient, n = 21) and severe (inpatient, n = 13) disease. Also, SARS‐CoV‐2 (Spike)‐specific memory B cells responses were investigated A healthy non‐infected group of 16 patients whose PBMC (peripheral blood mononuclear cells) had been bio‐banked before COVID‐19 pandemic (2018) were also analySed.
Results: Median (range) age was 51 (33 to 67); nadir and current T‐CD4 cell count was 219 cells/mL (28 to 600) and 633 cells/mL (284 to 1000) respectively. Only 5/11 patients needed hospital admission and one of them required ICU. Patients displayed similar IFN‐γ, IL2 and polyfunctional IFN‐γ/IL2 producing T‐cell frequencies than IC with mild symptoms at three and six months after infection. IC patients with more severe COVID‐19 infection exhibited the highest T‐cell immune responses (Figure 1). However, all (14/14) severe, 7/11 (63%) HIV and 3/18 (16.7%) mild IC patients showed IgG seropositivity at six months (p < 0.005) (Figure 2). Interestingly, a broad range of SARS‐CoV‐2 (Spike)‐specific memory B‐cell responses in the majority of HIV patients, despite the absence of SARS‐CoV‐2‐specific IgG antibodies at three (4/4) and six (2/4) months (Figure 3).
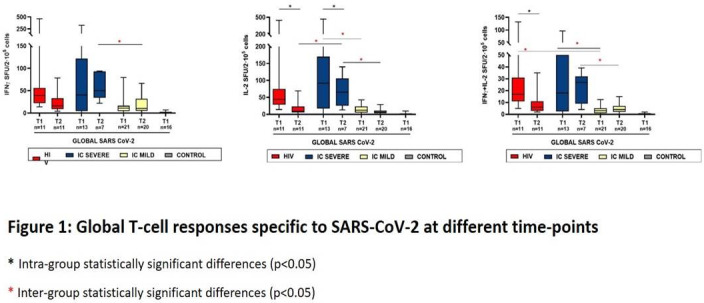
Abstract OAB0101‐Figure 1.
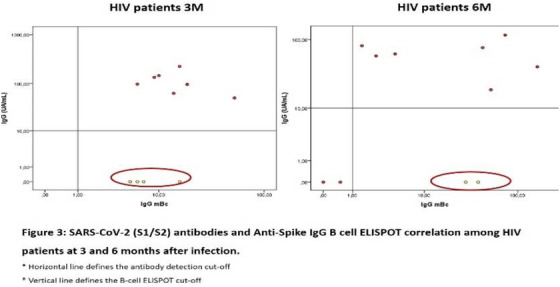
Abstract OAB0101‐Figure 2.
Conclusions: Our data suggest a comparable natural immunization among chronic HIV, similar to that of IC convalescent patients developing similar COVID‐19 disease severity. Notably, functional B‐ and T‐cell assessment may more reliably detect immunized patients with robust immune memory responses as compared to serological memory assessment during mid‐term convalescence.
OAB0102
Rising substance use linked to STI and HCV in Thai MSM after acute HIV infection
C Muccini 1,2; S Pinyakorn3,4; E Kroon2; C Sacdalan2; TA Crowell3,4; P Chan2; J Ananworanich5; R Paul6; S Vasan3,4; N Phanuphak2; and DJ Colby2,3,4
1Vita‐Salute San Raffaele University, Milan, Italy. 2SEARCH, Institute of HIV Research and Innovation, Bangkok, Thailand. 3U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, United States. 4Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, United States. 5Amsterdam Medical Centre, University of Amsterdam, Department of Global Health, Amsterdam, Netherlands. 6Missouri Institute of Mental Health, University of Missouri‐St. Louis, St. Louis, United States
Background: We report longitudinal trends in alcohol and recreational drug use and their associations with clinical outcomes in a Thai cohort of people living with HIV who are men who have sex with men (MSM).
Methods: From 2017 to 2019, participants in the RV254/SEARCH010 cohort of acute HIV infection completed a questionnaire every 24 weeks about drug, alcohol use and group sex. Positive use was defined as ≥1 self‐reports of substance use during a calendar year. Risky alcohol use was defined as AUDIT‐C score ≥4. Logistic regression with generalized estimating equations estimated odds ratios (ORs) and 95% confidence intervals (CIs) for factors associated with recreational drug and risky alcohol use.
Results: Among 604 participants with substance use data, the median age was 26 years and 93.5% were MSM. Alcohol consumption was reported in 83.3% and risky alcohol use in 38.1%. Recreational drug use was reported in 46.9%. From 2017 to 2019, rising trends were observed for risky alcohol use, any recreational drug use, poppers and methamphetamine injection (Table 1). New recruits to the cohort in 2017 to 2019 (n = 137) were more likely than those enrolled between 2009 and 2016 to report methamphetamine use (30% vs. 19%, p = 0.01) and injection of methamphetamine (20% vs. 3.9%, p < 0.01). Participants who used recreational drugs were more likely to have hepatitis C coinfection (OR 3.42, 95% CI 1.88 to 6.21), syphilis coinfection (OR 2.69, 1.75 to 4.13), gonorrhoea (OR 7.74, 5.04 to 11.89), chlamydia (OR 1.61, 1.12 to 2.31) and group sex (OR 7.74, 5.04 to 11.89). Methamphetamine injection was highly associated with group sex (OR 28.40, 10.99 to 73.41).
Abstract OAB0102‐Table 1. Proportion of participants reporting substance use by calendar year
| Substance | All (n = 604) | 2017 (n = 328) | 2018 (n = 548) | 2019 (n = 594) | p‐value test for trend |
|---|---|---|---|---|---|
| Alcohol | 503 (83.3) | 218 (66.5) | 412 (75.2) | 456 (76.8) | 0.001 |
| AUDIT‐C score ≥4 | 230 (38.1) | 49 (14.9) | 131 (23.9) | 192 (32.3) | <0.001 |
| Erectile dysfunction drugs | 172 (28.5) | 50 (15.2) | 119 (21.7) | 118 (19.9) | 0.170 |
| Recreational drug use (includes all below) | 283 (46.9) | 68 (20.7) | 191 (34.9) | 210 (35.4) | <0.001 |
| Poppers | 242 (40.1) | 69 (21.0) | 153 (27.9) | 173 (29.1) | 0.013 |
| Ecstasy | 33 (5.5) | 5 (1.5) | 24 (4.4) | 19 (3.2) | 0.312 |
| Oral amphetamines | 22 (3.6) | 2 (0.6) | 15 (2.7) | 13 (2.2) | 0.183 |
| Methamphetamine | 129 (21.4) | 33 (10.1) | 87 (15.9) | 85 (14.3) | 0.146 |
| Injection of methamphetamine | 46 (7.6) | 6 (1.8) | 24 (4.4) | 27 (4.5) | 0.061 |
Conclusions: Substance use has increased in Thai MSM living with HIV. Recreational drugs are strongly associated with the acquisition of sexually transmitted infections, including hepatitis C. Substance use screening at diagnosis and at every routine visit should be integrated into clinical practice for MSM with HIV in Thailand.
OAB0103
High HCV cure rates in C‐FREE, first community‐based study offering testing and treatment of viral hepatitis and HIV among people who use drugs and their partners in Thailand
T Wansom 1; A Thongmee1; S Chittmittrapap2; T Saraporn3; S Hasuwannakit3; S Waeuseng4; K Wanavanakorn4; N Chotirosniramit5; B Intharabut1; R Teuansiri1; K Kulprayong1; A Waesateh6; T Chumwangwapee1; S Pattarasuteewong7; S Pattarasuteewong7; T Pinyosinwat8; C Wongsuwon8; V Ngammee9; S Phueakchai10; S Lawpoolsri11; S Mills12; P Creac’H13; G Jourdain14; A Avihingsanon15; N Phanuphak16; and N Durier1
1Dreamlopments, Bangkok, Thailand. 2Chulalongkorn University, Bangkok, Thailand. 3Chana District Hospital, Chana, Thailand. 4SuNgaiKolok District Hospital, SuNgaiKolok, Thailand. 5Research Institute of Health Sciences, Chiang Mai University, Chiang Mai, Thailand. 6Dreamlopments, Chana, Thailand. 7Dreamlopments, Chiang Mai, Thailand. 8Raks Thai Foundation, Bangkok, Thailand. 9Ozone Foundation, Bangkok, Thailand. 10Thai Drug Users Network, Trang, Thailand. 11BIOPHICS, Mahidol University, Bangkok, Thailand. 12FHI 360, LINKAGES, Bangkok, Thailand. 13Global Fund to Fight AIDS, TB, and Malaria, Geneva, Switzerland. 14Institut de Recherche pour le Développement, Chiang Mai, Thailand. 15HIV‐NAT, Thai Red Cross AIDS Research Centre, Bangkok, Thailand. 16Institute of HIV Research and Innovation, Bangkok, Thailand
Background: Among people who inject drugs (PWID) in Thailand, HIV and hepatitis C (HCV) antibody prevalence is estimated at 25% and 70% respectively. C‐Free is a cohort study of community‐based testing and treatment of HCV, hepatitis B virus (HBV) and HIV for people who use drugs (PWUD), implemented at six drop‐in centres (DICs) offering harm reduction services in Thailand.
Methods: Individuals who currently/previously used drug(s), aged at least 18, were screened for HCV, HBV and HIV. Those with negative hepatitis B surface antigen (HBsAg) and antibody receive HBV vaccination. GeneXpert was utilized to measure HIV RNA, HCV RNA and HBV DNA on‐site, for those with reactive rapid tests. Those with confirmed HIV and HBV infection were referred to existing national programmes. Participants with HCV infection without evidence of decompensated cirrhosis, hepatocellular carcinoma or end‐stage renal disease, were offered a 12‐week course of sofosbuvir/velpatasvir. Those with negative HIV and/or HCV were offered repeat testing at three‐month intervals
Results: Between June 2019 and January 2021, 1118 participants enrolled, 949 (84.9%) were male, the median age was 43 years (range 18 to 73) and 841 (75.2%) reported a lifetime history of injecting drugs. HCV antibody was detected in 809 (72.4%), HIV antibody in 460 (41.1%) and HbsAg in 54 (4.8%). 72.6% of those with HIV were co‐infected with HCV. Among 809 with reactive HCV Ab, 667 (82.45%) had detectable HCV RNA, 226 (39.4%) reported actively injecting drugs, and 60 (9%) had evidence of cirrhosis using an AST to Platelet Ratio (APRI) of 2.0.
Of 652 participants who met HCV treatment eligibility criteria, 573 (87.9%) have started sofosbuvir/velpatasvir, 420 (73.3%) have completed treatment and 353 (61.6%) have reached the sustained virological response (SVR) timepoint. SVR was achieved by 326 participants; 92.4% (95% CI 89% to 95%) in the intent‐to‐treat analysis and 95.3% (95% CI 93% to 97%) of 342 in the per‐protocol analysis. No treatment‐related serious adverse events were observed.
Conclusions: Community‐based HCV treatment with sofosbuvir/velpatasvir for PWUD in Thailand, within harm reduction settings, is safe and highly effective. National programmes should urgently integrate community‐based HIV and HBV/HCV test and treat services as a standard of care for drug‐using populations to decrease morbidity and onward transmission of these infections.
OAB0104
Reaching HCV micro‐elimination in HIV/HCV co‐infected individuals in the Netherlands: exploring remaining barriers to HCV treatment
C Isfordink 1,2; C Smit3; A Boyd3,4; M de Regt5; B Rijnders6; R van Crevel7; R Ackens8; P Reiss1,3; J Arends2; M van der Valk1; and on behalf of the ATHENA observational cohort
1Amsterdam Institute for Infection and Immunity, Amsterdam University Medical Centers, Department of Internal Medicine, Division of Infectious Diseases, Amsterdam, Netherlands. 2UMC Utrecht, Department of Internal Medicine and Infectious Diseases, Utrecht, Netherlands. 3Stichting HIV Monitoring, Amsterdam, Netherlands. 4Public Health Service of Amsterdam, Department of Infectious Diseases, Research and Prevention, Amsterdam, Netherlands. 5Onze Lieve Vrouwe Gasthuis, Department of Internal Medicine and Infectious Diseases, Amsterdam, Netherlands. 6Erasmus MC, University Medical Center, Department of Internal Medicine, Section Infectious Diseases, Rotterdam, Netherlands. 7Radboud University Medical Center, Department of Internal Medicine and Radboud Center for Infectious Diseases, Nijmegen, Netherlands. 8Maastricht University Medical Center, Department of Internal Medicine, Division of Infectious Diseases, Maastricht, Netherlands
Background: With universal access to direct‐acting antivirals (DAA) since November 2015, the Netherlands is progressing towards micro‐elimination of hepatitis C virus (HCV) in people living with HIV (PLWH). However, some HCV‐viraemic PLWH have yet to receive DAA treatment. We described the barriers for DAA‐uptake in these individuals.
Methods: We included HCV‐viraemic individuals from a nationwide cohort of PLWH in the Netherlands with ≥1 visit during universal DAA‐access (database lock = 31 December 2019). Based on their last visit, these individuals were grouped as DAA‐treated or ‐untreated. We identified variables associated with being DAA‐untreated using multivariable logistic regression. In December 2020, physicians of DAA‐untreated PLWH were asked to complete an in‐depth questionnaire on barriers to DAA‐uptake and the risk of onward HCV transmission.
Results: Of the 25,196 PLWH ever screened for HCV, roughly 5% were HCV‐viraemic between 2003 and 2014, decreasing to 1.6% in 2016 and 0.7% in 2019. 983 PLWH were HCV‐viraemic during the universal DAA‐access era; 76/983 remained DAA‐untreated at the time of database lock. Being DAA‐untreated was associated with belonging to a key population other than men who have sex with men (OR = 10.6, 95% CI = 5.5 to 22.0), older age (OR/10 years = 1.6, 95% CI = 1.3 to 1.9), infrequent follow‐up (OR = 17.1, 95% CI = 8.3 to 36.6) and excessive alcohol use (OR = 1.7, 95% CI = 1.4 to 5.3). Of the 76 persons known to be DAA‐untreated at database lock, 41 were no longer in care (deceased, n = 23; lost to follow‐up, n = 12; moved abroad, n = 6), whereas six initiated DAA since database lock. The remaining 29 were still DAA‐untreated and in care in December 2020 (29/983, 3%), in whom the most common barriers to DAA‐uptake were patient‐related concerns (Table 1).
Abstract OAB0104‐Table 1. Physician‐reported barriers to DAA‐treatment and risk of onward transmission in HCV‐viraemic persons living with HIV in the Netherlands (n = 29)
| Barrier to DAA‐treatment uptakea | |
|---|---|
| Patient refusal | 9 (31%) |
| No liver fibrosis | 7 (24%) |
| Infrequent visit attendance | 6 (21%) |
| Severe comorbidity | 5 (17%) |
| Insufficient adherence expected | 3 (10%) |
| Physician‐reported risk of onward HCV transmissionb | 1 (3%) |
| Additional data collection form not returned | 9 (31%) |
Data obtained via a questionnaire by the treating physician (December 2020). DAA, direct‐acting antivirals; HCV, hepatitis C virus.
aFive most frequent barriers mentioned. Multiple barriers per individual are possible.
bRisk of onward sexual transmission and/or onward transmission through drug use.
Conclusions: The current prevalence of HCV‐viraemic PLWH in care is low in the Netherlands, coinciding with widespread DAA uptake since 2016. Patient refusal is the main barrier to DAA uptake in the remaining DAA‐untreated. Few of these individuals appear to engage in activities associated with the risk of onward HCV transmission.
OAB0105
Evaluation of the HCV cascade of care among people with HIV/hepatitis C co‐infection in New South Wales, Australia: a data linkage study
S Hosseini‐Hooshyar; M Alavi; M Martinello; G Matthews; and G Dore
The Kirby Institute, UNSW Sydney, Sydney, Australia
Background: Evaluating the hepatitis C virus (HCV) care cascade can provide a benchmark to track the effectiveness of interventions and identify service gaps at the population level. This study evaluated HCV RNA testing and treatment uptake pre‐ and post‐direct‐acting antiviral (DAA) availability and assessed factors associated with non‐treatment in the DAA era among people living with HIV/HCV co‐infection in NSW, Australia.
Methods: Records of individuals with HCV‐positive serology in NSW (1993 to 2017) were linked to HIV notification, perinatal data collection, hospitalization, births, deaths and marriages, opioid agonist therapy (OAT), incarceration and cancer registry datasets. These were then linked to national datasets of HCV RNA testing and HCV therapy dispensing from 2010. Factors associated with non‐treatment uptake in the DAA era were assessed using logistic regression.
Results: Among 988 people living with HIV with an HCV notification between 1993 and 2017 in NSW, 751 ever received RNA testing, and 419 ever initiated treatments. The proportion receiving HCV RNA testing remained stable from pre‐DAA era (2010 to 2015; 77% [260/336]) to post‐DAA era (2016 to 2018; 72% [89/123]). However, HCV treatment initiation dramatically increased from 7% (16/225) in the pre‐DAA era to 73% (194/267) in the post‐DAA era. Median time from HCV notification to RNA testing decreased from 13 weeks in 2010 to zero weeks in 2012 to 2017. Median time from HCV diagnosis to treatment decreased from 311 weeks in 2010 to less than five weeks in 2017. The unadjusted logistic regression model indicated no association between available demographic characteristics (i.e. year of birth, aboriginal ethnicity, country of birth, local health district of residence at the time of HCV) as well as drug and alcohol use (i.e. drug dependence and history of alcohol‐use disorder) and non‐treatment in DAA‐era.
Conclusions: A dramatic increase in HCV treatment uptake was seen in DAA era compared with pre‐DAA era among people living with HIV in Australia. These findings indicate that in settings of unrestricted DAA access, such as Australia, very high treatment uptake is possible in people living with HIV/HCV co‐infection that can lead to HCV elimination among this population.
OAB0201
Outcomes and incidence of TB among people living with HIV who received TB preventive therapy in Uganda
R Odeke 1; RN Ssebunya1; J Namulema1; I Kiconco1; E Sserunkuma1; R Tusiime1; A Mubiru2; AG Fitzmaurice2; LJ Nelson2; A Kekitiinwa1; and P Elyanu3
1Baylor College of Medicine Children’s Foundation, Uganda., Kampala, Uganda. 2Division of Global HIV/AIDS and TB (DGHT), US Centers for Disease Control and Prevention (CDC), Kampala, Uganda. 3Baylor College of Medicine Children’s Foundation, Uganda., Fort portal, Uganda
Background: TB preventive therapy (TPT) among people living with HIV (PLHIV) reduces the risk of developing TB disease. In Fort Portal region, data on TPT outcomes among PLHIV are scarce. We determined TPT outcomes and probability of TB occurrence among PLHIV who initiated TPT.
Methods: We retrospectively pooled electronic medical records data of a cohort of PLHIV aged one year and older who received TPT (isoniazid) from August 2016 through June 2020 at Fort Portal Regional Referral Hospital, Kilembe Mines and Kabarole hospitals in Fort Portal region, Uganda. TPT outcomes included: completed TPT, defaulted, transferred out, died, stopped TPT or developed TB. TPT outcomes were measured six months after TPT initiation and were reported as frequencies. Clients were followed up for 24 months from the date of TPT initiation to estimate the risk of TB occurrence. TB diagnosis was based on either clinical diagnosis or bacteriological detection by GeneXpert MTB/Rif or sputum smear microscopy. We compared the incidence of TB among patients who completed TPT and those who defaulted.
Results: A total of 10,085 PLHIV on ART [65% female, mean age 39 years (SD = 12.7)] initiated TPT during the study period. Overall, 96% (9661/10,085) completed TPT, 0.4% (38/10,085) died, 0.13% (13/10,085) developed TB, 1.5% (152/10,085) defaulted, whereas 2% (219/10,085) transferred and 0.02% (2/10,085) stopped treatment due to side effects. In 114,993 person months of follow‐up, 0.1% (13/10,085) PLHIV developed TB, 69% (9/13) being male and above 35 years; 46% (6/13) developed TB within the first six months of TPT. During a mean of 11.4 months of follow‐up, the TB incidence was 0.011% (95% CI: 0.007 to 0.019) with those who completed TPT having a lower incidence rate (IR = 0.006%, 95% CI: 0.003 to 0.013) compared with those who had defaulted TPT (IR = 1.23%, 95% CI: 0.55 to 2.74). Thirteen percent (11/8243) of individuals who developed TB were on a dolutegravir‐based antiretroviral treatment regimen.
Conclusions: Although most of the PLHIV who initiated on TPT completed six months of treatment, a small proportion of TB disease and deaths still occurred. We recommend that clinicians emphasize screening for active TB before initiating TPT among PLHIV through clinical group sessions for challenging cases.
OAB0202
The clinical effects of durably low CD4 counts while virologically suppressed among ART‐initiating persons with HIV in Latin America
Y Caro‐Vega 1; PF Rebeiro2; BE Shepherd2; PF Belaunzarán‐Zamudio3; B Crabtree‐Ramírez1; C Cesar4; P Mendes Luz5; C Cortés6; D Padget7; E Gotuzzo8; CC Mc Gowan2; and J Sierra‐Madero1
1Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran, Departamento de Infectología, Mexico City, Mexico. 2Vanderbilt University, School of Medicine, Nashville, United States. 3National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, United States. 4Fundación Huesped, Buenos Aires, Argentina. 5Instituto Nacional de Infectologia Evandro Chagas, Rio de Janeiro, Brazil. 6Fundación Arriarán/Universidad de Chile, Santiago, Chile. 7Instituto Hondureño de Seguridad Social, Tegucigalpa, Honduras. 8Universidad Peruana Cayetano Heredia, Lima, Peru
Background: People with HIV (PWH) with insufficient immune responses after initiating antiretroviral therapy (ART) have higher risks of comorbidities and death. Cumulative time with low CD4+ counts (CD4), even with successful virological suppression (VS), has been associated with poor outcomes. In the Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a high proportion of deaths are attributed to late ART initiation, with approximately half of patients initiating ART with CD4 < 200 cells/µL. We estimated the effect of cumulative time with CD4 < 200 cells/µL on mortality and comorbidities among ART‐initiators with VS during 2000 to 2017.
Methods: We followed PWH in CCASAnet initiating ART with CD4 and HIV RNA measures from initial VS (HIV RNA < 200 copies/mL) until death or loss to follow‐up. Individuals were censored when HIV RNA was first ≥200 copies/mL. We fit Cox models to estimate the risk of death and/or AIDS‐ and non‐AIDS‐related severe comorbidities (SCM; including cancers and cardiovascular, liver and renal diseases) by time‐updated percentage of follow‐up time with CD4 < 200 cells/µL (%tCD4<200), adjusting for sex, age, HIV transmission route, education, calendar year, clinic site and in secondary analyses, time‐updated CD4 count.
Results: Among 9123 patients with VS contributing a median of 51.5 (IQR: 26.1, 91.5) months, 78% were men, median age was 34 years, 4668 (51%) started ART with CD4 < 200 cells/µL and median %tCD4<200 was 0% (IQR: 0, 15%). For those starting ART with CD4 < 200 cells/µL, median %tCD4<200 at 12 months was 36% (IQR: 0, 100%) and at 24 months was 18% (IQR: 0, 58%). A total of 283 (3%) deaths and 774 (8.4%) patients with SCM were identified. Comparing %tCD4<200 of 15% versus 0%, the adjusted relative hazard (aHR) of death was 1.27 (95% confidence interval [CI]: 1.20 to 1.34), of SCM was 1.13 (95% CI: 1.09 to 1.17), and of either was 1.15 (95% CI: 1.12 to 1.19). Estimates were similar when also adjusting for time‐updated CD4 count: aHR = 1.11, (95% CI: 1.05 to 1.18); aHR = 1.08, (95% CI: 1.04 to 1.12); and aHR = 1.10, (95% CI: 1.07 to 1.13) respectively.
Conclusions: Virologically suppressed PWH spending more time with CD4 < 200 cells/µL had an increased risk of death and severe comorbidities in this Latin American cohort. Vigilant screening for comorbidities is needed in these populations.
OAB0203
Correlates associated with mortality among HIV‐TB co‐infected patients in Mumbai, India
A Palkar1; S Acharya 1; and MS Setia2
1Mumbai Districts AIDS Control Society, Mumbai, India. 2Consultant Dermatologist and Epidemiologist, Mumbai, India
Background: Tuberculosis (TB) is the commonest opportunistic infection and cause of death in patients with human immunodeficiency virus (HIV) in developing countries. Successful TB treatment outcomes are lower among HIV‐infected compared with non‐infected patients. We studied the factors associated with mortality in patients co‐infected with HIV and TB in Mumbai, India.
Methods: We studied the association of demographic data (age, gender, migrant), clinical history (history of TB, type of TB), treatment history (ART and Anti TB Treatment [ATT], CD4 and weight at TB diagnosis) with mortality in 1571 co‐infected patients registered for care in 2018. Logistic regression models were used to estimate the odds ratios (OR) and 95% confidence intervals (CI) for multivariate analysis.
Results: Of 1571 co‐infected patients, 950 (60%) were men, 610 (39%) were women and 11 (1%) were male‐to‐female transgenders/Hijras. The mean age (standard deviation) was 39.1 (12.1) years. There was an equal proportion of pulmonary (790) and extra pulmonary TB (781). The median (interquartile range) CD4 count at the time of diagnosis was 226 (106, 386). About 859 (55%) patients were taking ART at the time of TB diagnosis and 712 (45%) patients were treatment naïve for both the diseases at the time of diagnosis. Nineteen percent of co‐infected patients (295) had died during the follow‐up period. Mortality was significantly associated with age (≥60 years) and CD4 count at the time of diagnosis (<200 CD4 cells). Higher weight at the time of diagnosis and individuals who were not from Mumbai were less likely to die. Detailed ORs and their CIs are presented in Table 1.
Abstract OAB0203‐Table 1
| Death | Odds ratio | [95% confidence Interval] | p>z | |
|---|---|---|---|---|
| 19 to 29 years | 2.16 | 0.84 | 5.52 | 0.109 |
| 30 to 59 years | 3.31 | 1.40 | 7.83 | 0.006 |
| ≥60 years | 4.57 | 1.57 | 13.31 | 0.005 |
| Treatment Naïve | 1.91 | 1.40 | 2.62 | 0.001 |
| Pulmonary TB | 0.91 | 0.66 | 1.24 | 0.535 |
| Weight | 0.96 | 0.95 | 0.98 | 0.001 |
| CD4 (201 to 500) | 1.00 | 0.58 | 1.75 | 0.992 |
| CD4 (<200) | 2.53 | 1.51 | 4.25 | 0.001 |
| Past history of TB | 1.20 | 0.88 | 1.65 | 0.242 |
Conclusions: Patient‐centric counselling and early diagnosis of both diseases can help improve TB treatment outcomes. Screening tests of urine LAM among patients with advanced HIV disease can help in early detection and management of TB, thereby reducing mortality.
OAB0204
Long‐term survival and predictors of mortality in HIV/TB patients in Uganda
I Lumu 1,2; J Musaazi2; I Handel1; F Parry1; and B Castelnuovo2
1University of Edinburgh, College of Medicine and Veterinary Medicine, Edinburgh, United Kingdom. 2Infectious Diseases Institute, Makerere University, Kampala, Uganda
Background: HIV/TB co‐infected patients may have reduced survival even after successful completion of TB treatment; however, the data on long‐term survival of these patients in sub‐Saharan Africa are limited. This five‐year retrospective cohort set out to determine the long‐term survival and predictors of mortality after completing TB treatment in antiretroviral therapy (ART) experienced HIV/TB co‐infected patients in Uganda.
Methods: This was a five‐year retrospective cohort analysis of all ART‐experienced HIV/TB co‐infected patients who completed TB treatment in a specialist HIV clinic between 2009 and 2014. The characteristics of patients were described using frequencies and medians. The survival and factors associated with all‐cause mortality were determined using Kaplan–Meier methods and Cox proportional hazard models respectively.
Results: 1128 patients completed TB treatment between 2009 and 2014, of which 573 (50. 8%) were males. The median age was 36 years (IQR: 31 to 43), the median BMI was 21.93 kgm2 (IQR: 20.05 to 24.22), the median CD4 cell count was 233 cells/μL (IQR: 138 to 365). The person time at risk during the five years of follow‐up was 4410.60 person‐years. 67 (5.9%) patients died during the study period and mortality occurred at 15.19 per 1000 person‐years (95% CI: 11.96‐ 19.30). The probability of death was 0.0251 (95% CI: 0.0173 to 0.0364), 0.0575 (95% CI: 0.0447 to 0.0738) and 0.0684 (95% CI: 0.0541 to 0.0862), at one, three and five years respectively. CD4 count <200 cells/μL, BMI < 18 kgm2 and TB relapse were associated with mortality: unadjusted Hazard Ratio (uHR)= 3.73, (95% CI: 2.01‐ 6.94, p < 0.001), uHR = 2.06 (95% CI: 1.21 to 3.49, p = 0.008) and uHR = 1.95, (95% CI: 1.11‐ 3.41, p = 0.020) respectively. However, in the multivariate analysis, only BMI < 18 kgm2 was associated with mortality, adjusted HR = 3.78, (95% CI: 1.93‐ 7.40, p < 0.001).
Conclusions: In this clinic, ART‐experienced HIV patients that were co‐infected with TB seemed to have a reasonably good five‐year survival probability (93.16%) after completing TB treatment. This could be attributed to the increased access to ART in Uganda. Notably, being malnourished is the single most important predictor of mortality in this cohort, and thus the continuous close monitoring of at‐risk patients could avert mortality.
OAB0205
Incidence and co‐factors of Mtb infection in first two years of life: observational follow‐up of a randomized controlled trial of INH to prevent primary Mtb infection among HIV‐exposed uninfected children
S LaCourse 1; J Escudero1; N Carimo1; B Richardson1; L Cranmer2; A Warr3; E Maleche‐Obimbo4; J Mecha5; D Matemo5; J Kinuthia5; T Hawn1; G John‐Stewart1; and infant TB Prevention Study (iTIPS)
1University of Washington, Seattle, United States. 2Emory University, Atlanta, United States. 3Baylor College of Medicine, Houston, United States. 4University of Nairobi, Nairobi, Kenya. 5Kenyatta National Hospital, Nairobi, Kenya
Background: In the infant TB Infection Prevention Study there was a trend for decreased TST‐positivity after 12‐month INH among Kenyan HIV‐exposed uninfected (HEU) infants. We present 24‐month observational follow‐up.
Methods: Infants age of six weeks without known TB exposure were randomized to 12‐month INH versus no INH. Mtb infection was measured at 12 months by interferon‐gamma release assay (IGRA, QFT‐Plus), with tuberculin skin test (TST, positive ≥10 mm) added six months after first study exit due to low accrued endpoints. Follow‐up was extended with repeat TST placed at 24 months. The observational outcome was cumulative Mtb infection by 24 months with any positive Mtb infection test considered “ever positive.” Correlates of Mtb infection were evaluated by generalized linear models and risk of conversions/reversions by multinomial regression.
Results: As previously reported, among 300 HEU infants enrolled (150/arm), 28/265 (11%) with 12‐month Mtb infection endpoints were positive, with a trend for lower 12‐month Mtb infection among infants randomized to INH (HR 0.53 [95% CI 0.24 to 1.14], p = 0.11]) driven by TST‐positivity (RR 0.48 [95% CI 0.22 to 1.05], p = 0.07). Among 228 children completing 24‐month follow‐up, 25 (13.3%) were TST positive.
Overall, 39/275 (14%) infants with Mtb infection outcome at 12 or 24 months were positive; cumulative Mtb infection incidence was 9.8/100 PY (INH 7.5 vs. no INH 9.8/100 PY, HR 0.75 [95% CI 0.40 to 1.42], p = 0.37] and associated with lack of flush toilet or running water (p < 0.001 and p = 0.02 respectively). Among 162 infants with TST at 12 and 24 months, 68% (17/25) with 12‐month TST‐positivity reverted; 5.4% (7/137) TST‐negative converted. While post‐trial TST conversions were similar among no INH and INH (2/83 [2.4%] vs. 5/79 [6.3%], RR 0.4 [95% CI 0.08 to 2.2], p = 0.30), children not receiving INH were more likely to have TST reversions (No INH [13/83, 15.7%] vs. INH [4/79, 5.1%], RR 3.4 [95% CI 1.03 to 10.8], p = 0.04).
Conclusions: In post‐RCT follow‐up, 24‐month cumulative Mtb infection incidence measured primarily by TST was high and associated with poorer household conditions in this cohort of HEU children. The trend for decreased TST‐positivity after 12 months of INH was not sustained. TST reversions occurred frequently; fewer reversions among INH recipients may reflect INH potential to delay the timing of primary infection.
OAB0301
Comparison of viral replication for the 2‐drug regimen (2DR) of dolutegravir/lamivudine (DTG/3TC) versus a 3/4‐drug tenofovir alafenamide‐based regimen (TBR) in the TANGO study through week 96
R Wang 1; J Wright2; N George2; M Ait‐Khaled3; T Lutz4; O Osiyemi5; M Gorgolas6; P Leone1; B Wynne1; J van Wyk3; and M Underwood1
1ViiV Healthcare, Research Triangle Park, United States. 2GlaxoSmithKline, Stockley Park, United Kingdom. 3ViiV Healthcare, Brentford, United Kingdom. 4Infektio Research, Frankfurt, Germany. 5Triple O Research Institute PA, West Palm Beach, United States. 6Jiménez Díaz Foundation University Hospital, Madrid, Spain
Background: TANGO demonstrated non‐inferior virological efficacy (HIV‐1 RNA ≥50 copies/mL, Snapshot) of switching to DTG/3TC versus continuing TBR in HIV‐1‐infected, virologically suppressed adults at 96 weeks. Abbott RealTime HIV‐1 assay measures viral load (VL) from 40 to 10,000,000 copies/mL, and provides qualitative target detected (TD) or target not detected (TND) outcomes for VL < 40 copies/mL. Clinical significance of low‐level VL < 50 copies/mL remains unclear. We assessed the proportion of participants with TD/TND and elevated VL through Week 96 (Wk96).
Methods: Proportions of participants with VL < 40 copies/mL and TND were analysed by visit (Snapshot) through Wk96. Participants’ TD/TND status over time, overall and by Baseline VL classifications, was assessed. The frequency of elevated VL categories including “blips” was determined.
Results: At Wk96, similar proportions of participants had TND with 2DR and 3DR (73% [271/369] vs. 69% [255/372] respectively; adjusted difference, 4.9%; 95% Cl, −1.7, 11.4; Snapshot). Across Baseline VL categories, proportions with TND at all visits through Wk96 were higher at 37% (137/369) with 2DR versus 31% (114/372) with 3DR (Table 1). Occurrence of elevated VL (Table 2) was low and similar across arms through Wk96, and most frequently observed VL rebounds were “blips.” Zero and three confirmed virological withdrawals were observed with 2DR and 3DR respectively.
Abstract OAB0301‐Table 1
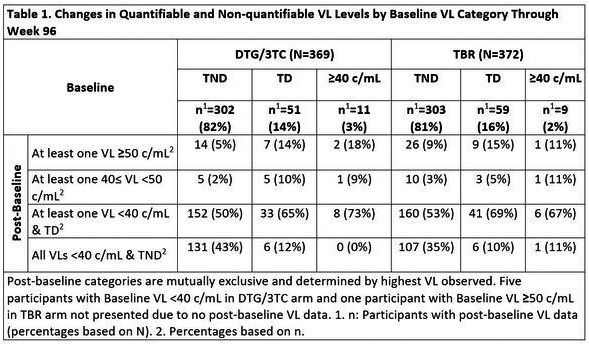
Abstract OAB0301‐Table 2
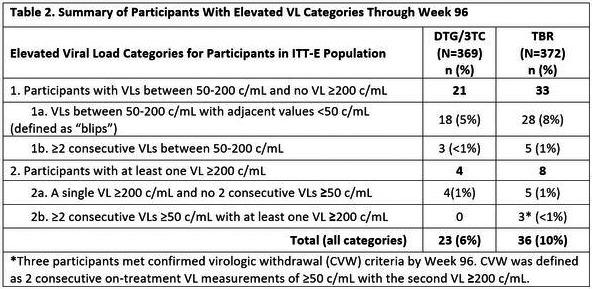
Conclusions: Similar proportions of participants had TND at all visits through Wk96 in both treatment arms. Regardless of Baseline VL, the incidence of intermittent viraemia was low and similar between arms. These “deep dive” virology findings further support the potency and durability of 2DR versus 3DR in maintaining viral suppression.
OAB0302
Week 124 results of the randomized, open‐label, Phase 3 FLAIR study evaluating long‐acting cabotegravir + rilpivirine for treatment in adults with HIV‐1 infection (ITT‐E population)
C Orkin 1; R D’Amico2; E Bernal Morell3; DHS Tan4; H Katner5; Y Singh6; H‐J Stellbrink7; E Belonosova8; R DeMoor9; S Griffith2; S Thiagarajah9; R Van Solingen‐Ristea10; SL Ford11; H Crauwels10; P Patel2; A Cutrell2; KY Smith2; K Vandermeulen10; M St Clair2; and WR Spreen2
1Queen Mary University, London, United Kingdom. 2ViiV Healthcare, Research Triangle Park, United States. 3Hospital General Universitario Reina Sofía, Murcia, Spain. 4Division of Infectious Diseases, St. Michael's Hospital, Toronto, Canada. 5Mercer Univer sity Medical School, Macon, United States. 6Desmond Tutu Health Foundation, Cape Town, South Africa. 7ICH Study Center, Hamburg, Germany. 8Orel Regional Center for AIDS, Orel, Russian Federation. 9GlaxoSmithKline, London, United Kingdom. 10Janssen Research & Development, Beerse, Belgium. 11GlaxoSmithKline, Research Triangle Park, United States
Background: Long‐acting (LA) intramuscular injections of cabotegravir (CAB) and rilpivirine (RPV) have been developed as an alternative to daily oral dosing for HIV‐1. The Phase 3 FLAIR study (NCT02938520) demonstrated non‐inferiority of switching virologically suppressed participants from daily oral dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) to monthly CAB+RPV LA through Week (W) 48 and W96. Results for participants who received CAB+RPV for 124 weeks are presented.
Methods: In the Maintenance Phase (W0–100), participants were randomized (1:1) to continue DTG/ABC/3TC or switch to monthly CAB+RPV LA after initially receiving a ≥4‐week oral lead‐in of CAB+RPV. At W100, participants receiving DTG/ABC/3TC could switch to CAB+RPV LA or withdraw. The primary endpoint was the proportion of participants with plasma HIV‐1 RNA ≥50 copies/mL at W48 (FDA Snapshot algorithm). W124 endpoints included the proportion of participants with HIV‐1 RNA ≥50 and <50 copies/mL, confirmed virological failure (CVF; two consecutive viral loads ≥200 copies/mL) and safety and tolerability.
Results: Overall, 283 participants received ≥1 dose of CAB+RPV; median (range) age was 34.0 (19 to 68) years, 22% were female (sex at birth) and 76% were white. At W124, 14 (4.9%) participants had HIV‐1 RNA ≥50 copies/mL, with 227 (80.2%) maintaining suppression (HIV‐1 RNA <50 copies/mL). Through W124, 5 (1.8%) participants met CVF, one additional participant since W96 (Table 1). Injection site reactions (ISRs) were the most common drug‐related adverse event (AE); most were Grade 1 or 2 (99.5%). The proportion of participants with ISRs decreased over time (W4: 72%; W48: 23%; W96: 19%; W124: 18%). Serious AEs and AEs leading to withdrawal occurred in 12% (one drug related) and 5% of participants, respectively, through 124 weeks.
Abstract OAB0302 Table 1
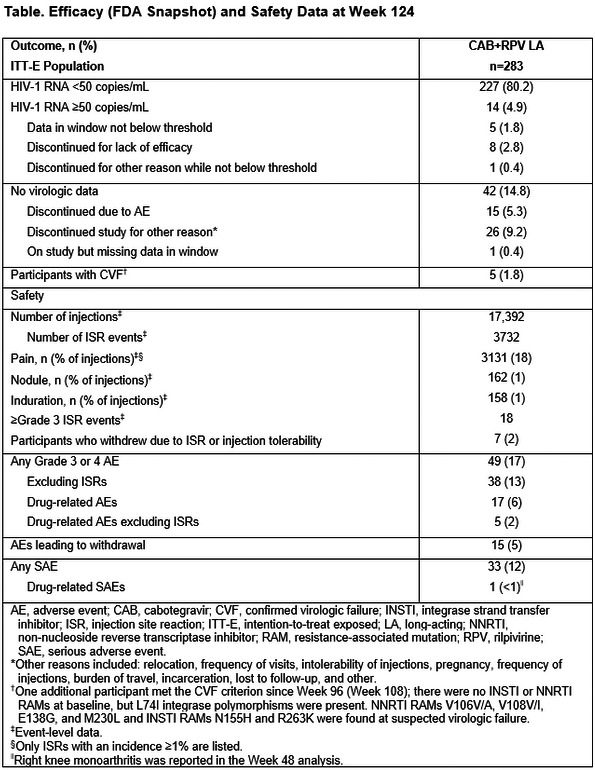
Conclusions: At W124, monthly CAB+RPV LA maintained virological suppression in most participants; the safety and tolerability profile was consistent with prior W48/W96 results. These results demonstrate the durability of CAB+RPV LA as a well‐tolerated, effective maintenance therapy.
OAB0303
Prevalence of baseline virological risk factors of increased virological failure to CAB+RPV among ARV‐naïve patients
C Charpentier 1; A Storto1; C Soulié2; VM Ferré1; M Wirden2; V Joly3; S Lambert‐Niclot4; L Morand‐Joubert4; R Landman3; K Lacombe5; C Katlama6; J Ghosn3; A‐G Marcelin2; V Calvez2; and D Descamps1
1Hôpital Bichat‐Claude Bernard, Service de Virologie, Paris, France. 2Hôpital Pitié‐Salpêtrière, Service de Virologie, Paris, France. 3Hôpital Bichat‐Claude Bernard, Service de Maladies Infectieuses et Tropicales, Paris, France. 4Hôpital Saint‐Antoine, Service de Virologie, Paris, France. 5Hôpital Saint‐Antoine, Service de Maladies Infectieusese et Tropicales, Paris, France. 6Hôpital Pitié‐Salpêtrière, Service de Maladies Infectieuses et Tropicales, Paris, France
Background: Multivariable baseline factor analysis across CAB+RPV phase 3 studies recently showed that HIV‐1 subtypes A6/A1, characterized by the L74I integrase (IN) polymorphism, and presence of RPV resistance‐associated mutations (RAM), as well as body mass index, were associated with an increased risk of virological failure of this dual‐therapy. The aim of this study was to describe the prevalence of CAB and RPV RAM among ARV‐naive patients depending on the subtype.
Methods: From 2010 to 2020, 4212 sequences from ARV‐naïve patients with both RT and IN available sequences were collected from three large Parisian Academic Hospitals genotypic databases. CAB and RPV RAM were defined according to the ANRS algorithm (www.hivfrenchresistance.org).
Results: Among 4212 sequences, 38.6% belonged to B subtype and the most prevalent non‐B subtype was CRF02_AG (32.4%). Subtype A represented 5.1% of the sequences within 85.5% was of subtype A6/A1 (n = 183/214). Overall, the presence of at least one CAB or RPV RAM was 16.2% and 14.3% respectively. The overall prevalence of L74I in IN and E138A in RT was 13.0% and 3.2%, respectively, and stable over the decade. The frequency of L74I was significantly higher in non‐B than in B subtypes (17.4% vs. 6.0%, p < 0.0001) with the highest prevalence observed in subtype A (49.5%). The frequency of E138A was significantly higher in non‐B than in B subtypes (3.8% vs. 2.2%, p = 0.0003) and was 7.9% in subtype A (n = 17/214). Sixteen patients (0.4%) displayed virus harbouring both E138A and L74I polymorphisms. Considering genotypic resistance interpretation, using ANRS algorithm, 0.74% (n = 31), 7.3% (n = 306) and 0.09% (n = 4) of sequences were resistant to cabotegravir, rilpivirine or both respectively. Thus, 183 sequences were subtype A6/A1 and 244 were interpreted as resistant to RPV (after excluding those of subtype A6/A1) leading to 427 (10.1%) of sequences combining both baseline virological risk factors of CAB+RPV dual‐therapy failure.
Conclusions: Among large sequences databases, when combining RPV RAMs and HIV‐1 subtype A6/A1 prevalence, 10.1% of ARV‐naive patients would not be eligible for CAB+RPV dual‐therapy. These data re‐emphasize the need of a pre‐therapeutic genotypic resistance test to detect polymorphisms, transmitted drug resistance and to define HIV‐1 subtype.
OAB0304
Islatravir safety analysis through week 96 from a Phase 2 trial in treatment naïve adults with HIV‐1 infection
D Cunningham 1; J‐M Molina2; Y Yazdanpanah3; AA Saud4; CC Anania5; SO Klopfer6; A Grandhi6; K Eves6; D Hepler6; C Hwang6; R Lahoulou7; and TA Correll6
1Pueblo Family Physicians Ltd, Phoenix, United States. 2Saint‐Louis Hospital and University of Paris, Department, of Infectious Disease, Paris, France. 3Bichat Hospital, Paris, France. 4University of Chile, Santiago, Chile. 5Hospital Hernan Henriquez Aravena of Temuco, Temuco, Chile. 6Merck & Co., Inc., Kenilworth, United States. 7MSD France, Puteaux, France
Background: Islatravir (ISL, MK‐8591) is a nucleoside reverse transcriptase translocation inhibitor (NRTTI) in development for the treatment and prevention of HIV‐1 infection. We previously showed that ISL + doravirine (DOR) was effective in maintaining viral suppression through week 96 and was well‐tolerated. Here we present a detailed safety analysis of those results.
Methods: In this Phase 2b trial, treatment‐naïve adults with HIV‐1 were randomized to receive ISL (0.25, 0.75, or 2.25mg) + DOR (100mg) and lamivudine (3TC, 300mg) QD, or a fixed‐dose combination of DOR (100mg), 3TC (300mg) and tenofovir disoproxil fumarate (300mg) (DOR/3TC/TDF) QD. In the ISL groups, participants who achieved HIV‐1 RNA <50 copies/mL at Week 20 or later, stopped 3TC at the next study visit. All participants receiving ISL were switched to ISL (0.75mg) between weeks 60 and 84. For the current analysis, we conducted a detailed review of adverse events (AEs) and examined these AEs for study periods at weeks 0 to 48, 48 to 96 and 0 to 96.
Results: A total of 121 participants received drug and were included in the analyses. Similar AE rates between treatment arms were observed across all arms of the trial for each time period. No dose‐dependent difference in the safety profile of ISL was observed. AEs were more frequent in the first 48 weeks of the trial, as compared to the second 48‐week period, for all treatment arms (see Table 1). During the 96 week‐period overall, diarrhoea, mostly mild and transient, was more frequently reported for DOR/3TC/TDF (19.4%) as compared to ISL groups (combined 7.9%), whereas headache was more common in ISL groups (combined 11.1%) as compared to the DOR/3TC/TDF group (6.5%).
Conclusions: ISL was well‐tolerated, regardless of dose, through 96 weeks of treatment, with most AEs reported as mild and transient. No participants had drug‐related AEs or discontinued the study due to a drug‐related AE between weeks 48 to 96.
Abstract OAB0304‐Table 1
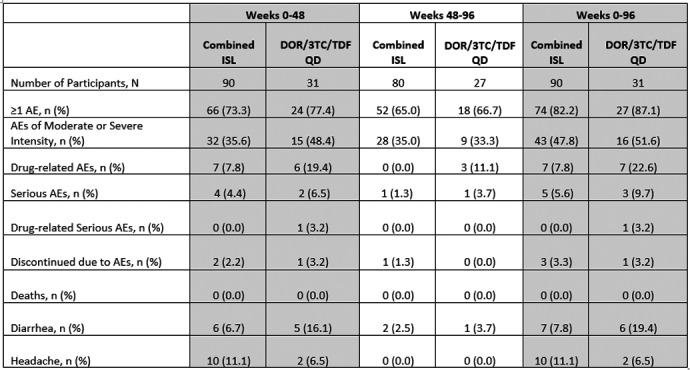
OAB0305
New generation of HYBRID CAR‐T cells efficiently kill HIV‐infected cells and neutralize cell‐free virus
Z Stylianidou; M Wejda; T Schynkel; S Gerlo; W Witkowski; and L Vandekerckhove
Ghent University, Department of Internal Medicine and Pediatrics, HIV Cure Research Center, Ghent, Belgium
Background: The current gold standard in HIV treatment fails to provide a definitive cure. Infected patients must adhere to a lifelong therapy burden, which on its own can impose side effects. High economic costs and patient stigmatization further contribute to the social impact of the HIV epidemic. Cell and gene therapies for HIV are increasingly gaining ground, especially over the last decade. However, most of them suffer from important practical limitations. Here we propose an innovative approach, Hybrid CAR, which combines the cytotoxic function mediated by Chimeric Antigen Receptor (CAR) with antiviral properties of broadly neutralizing antibodies (bNAbs) secreted from the same engineered cell. This will address both the cellular and viral components of HIV pathogenesis.
Methods: Primary CD8+ cells (derived from healthy donors) were lentivirally transduced to express anti‐HIV CAR and bNAb from the same bicistronic construct. CAR expression was validated by flow cytometry and RT‐qPCR, whereas the secreted antibody was detected by ELISA. Coculture of Hybrid CAR‐T cells with autologous, HIV‐infected CD4+ T cells was performed to evaluate the direct cytotoxic effects. Supernatants collected from Hybrid CAR T‐cell monocultures were used to evaluate neutralization potential of T cell secreted antibodies in a TZM‐bl assay.
Results: Hybrid CAR‐T cells demonstrated efficient killing of HIV‐infected, autologous CD4+ T cells with complete inhibition of viral replication. Secreted antibodies alone were able to reduce the infectivity of HIV, demonstrating dual, synergistic functionality of the Hybrid CAR.
Conclusions: These data provide proof of concept for the Hybrid CAR platform and show successful secretion of anti‐HIV antibodies from primary T cells, as well as retained killing function mediated by simultaneously expressed CAR. Ongoing experiments are being performed to provide evidence of antibody‐dependent cytotoxicity (ADCC) and antibody‐dependent phagocytosis (ADCP) exerted by the CAR‐T‐cell secreted bNAbs.
OAB0401
Is systematic Xpert MTB/RIF improved the detection of tuberculosis in HIV‐infected patients in a low tuberculosis incidence setting in West Africa?
DL Dahourou 1; MF Dogo2; Y Diallo3; KS Adjoh2; MT Traore3; K Agossou2; S Traore3; IT Traoré4,5; H Traore6; and CSC Merle6
1Centre National de la Recherche Scientifique et Technologique, Institut de Recherche en Sciences de la Santé, Ouagadougou, Burkina Faso. 2Programme National de Lutte contre la Tuberculose, Lomé, Togo. 3Cellule Sectorielle de Lutte contre le VIH/Sida, la Tuberculose et les Hépatites, Bamako, Mali. 4Institut National de la Santé Publique, Centre Muraz, Bobo‐Dioulasso, Burkina Faso. 5Université Nazi Boni, Institut National des Sciences de la Santé, Bobo‐Dioulasso, Burkina Faso. 6World Health Organisation, Special Programme for Research and Training in Tropical Disease, Geneva, Switzerland
Background: Tuberculosis is a leading cause of death in HIV patients, but its diagnosis is challenging. We assessed the performance of Xpert MTB/RIF (Xpert) in a low tuberculosis incidence setting in Lomé (Togo) and Bamako (Mali), regardless of the presence of tuberculosis presumptive symptoms.
Methods: We included all consenting HIV‐infected patient aged ≥15 years, not initiated on antiretroviral therapy (ART) or on ART ≤1 month. Participants were screened for tuberculosis with 4‐symptom screening (fever, cough, night sweats and weight loss) as recommended by World Health Organization (WHO), and with Xpert performed on a sputum sample. In addition, sputum was tested using mycobacterial culture. We compared the sensitivity and specificity of a WHO 4‐symptom screening strategy plus Xpert for those who reported any one of the symptoms, to Xpert for all HIV‐infected patients regardless of tuberculosis presumptive symptoms, with mycobacterial culture as a gold standard.
Results: Patients were recruited between January 1 and December 31, 2020. A total of 545 patients were enrolled, of whom 85% (467/545) who completed the diagnostic algorithm were included in this study (82% in Lomé; median age 37.4 years (interquartile range: 30.4 to 46.3); 65% female; WHO HIV stage I, 55%). Fever was reported in 15%, whereas cough, weight loss and night sweats were reported in 26%, 39% and 14% respectively. Overall, 46 (10%) tuberculosis cases were identified by Xpert or mycobacterial culture. Of these, four tuberculosis patients reported none of the four clinical symptoms. The rifampicin resistance was rare (2%, 1/46). Compare to mycobacterial culture, the WHO 4‐symptom screening strategy plus Xpert sensitivity was 53% (95% confidence interval [95% CI] 38% to 68%). While, the sensitivity of the systematic use of Xpert was 63% (95% CI 0.47 to 0.77). The specificity was 99% (95% CI 98% to 100%) for both strategies.
Conclusions: In this operational research supported and funded by WHO‐TDR, the prevalence of tuberculosis among HIV‐positive patients starting ART or on ART for less than one month was 10%. The use of the WHO 4‐symptom screening strategy plus Xpert will missed 9% of the tuberculosis cases. The systematic screening for tuberculosis using Xpert at ART initiation regardless of tuberculosis presumptive symptoms could improve its early diagnosis and treatment.
OAB0402
The point of care device needed for confirming active hepatitis C infection in the prison population
S Ubolyam 1; S Plakunmonthon1; J Sophonphan2; P Eiamyoung1; T Darodom1; A Mahanontharit1; S Gatechompol3; T Ueaphongsukkit4; and A Avihingsanon3
1HIVNAT, Laboratory, Bangkok, Thailand. 2HIVNAT, Statistician, Bangkok, Thailand. 3HIVNAT, Medical, Bangkok, Thailand. 4Chulalongkorn University, Faculty of Medicine, Bangkok, Thailand
Background: Hepatitis C virus (HCV) RNA screening is a critical step for HCV treatment and care. A rapid HCV RNA test or point‐of‐care (POC) test would be extremely helpful in diagnosing active HCV infection. GeneDrive is a novel POC HCV RNA assay, permits the identification of active HCV infection for all HCV genotype with a lower limit of detection of 1406 to 3203 IU/mL. We, therefore, validated a GeneDrive POC assay to detect HCV RNA in the high‐risk population with limited access to HCV RNA testing.
Methods: A pilot “Test and Treat HCV in prison” was conducted in a Central Prison in Bangkok, Thailand between 2019 and 2020. The Study addresses hepatitis C high‐risk population (prison group) and is to be tested on the GeneDrive HCV RNA assay. We validated the GeneDrive HCV assay of the pre‐treatment samples comparing results with those obtained from the Abbott RealTime HCV assay as a reference test with a limit of detection 12 IU/mL.
Results: A total of 158 samples from prisoners with positive anti HCV were included in this validation, 126 samples were HCV detection from Abbott RealTime HCV test (HCV RNA (log10) ranged1.8 to 7.4 IU/mL) and another 36 samples with HCV RNA undetectable (HCV RNA < 12 IU/mL). The GeneDrive HCV assay showed 100% sensitivity (95% CI 97% to 100%) and 97.2% specificity (95% CI 85.5% to 99.9%) to detect HCV. The sensitivity (100%) and specificity (97.2%) for the GeneDrive HCV RNA assay were the same as the gold standard test. Agreement between Abbott Real time PCR and GeneDrive is 99.4%. The performance of evaluation is shown in Table 1.
Abstract OAB0402‐Table 1. Performance of GeneDrive evaluation
| n/N | % (95% confidence interval) | |
|---|---|---|
| Sensitivity | 122/122 | 100 (97 to 100) |
| Specificity | 35/36 | 97.2 (85.5 to 99.9) |
| Positive predictive value | 122/123 | 99.2 (95.6 to 100) |
| Negative predictive value | 35/35 | 100 (90 to 100) |
| AUC | ‐ | 0.99 (0.96 to 1) |
Conclusions: GeneDrive, a novel POC HCV RNA assay yielded a high performance to confirm active HCV infection. To ending hepatitis C in resource‐limited settings (RLS), this portable POC device is a good alternative to facilitate “Test and treat HCV” in a real clinical setting in many RLS.
OAB0403
Plasma separation cards are suitable for HIV‐1 genotypic drug resistance testing
H Reddy1; YX Liao1; L Hans1,2; L Noble2; L Scott2; W Stevens1,2; and K Steegen 1,2
1National Health Laboratory Service, Department of Molecular Medicine and Haematology, Johannesburg, South Africa. 2University of the Witwatersrand, Faculty of Health Sciences, Johannesburg, South Africa
Background: Plasma specimens are considered the gold standard for HIV drug resistance testing. However, the collection, preparation, storage and transport of plasma remain a challenge in resource‐limited settings. To mitigate these challenges, Roche Molecular Diagnostics developed a sample collection device, the plasma separation card (PSC), to separate and stabilize plasma from whole blood using a proprietary membrane and stabilizing fleece. PSCs have been successfully used for HIV viral load testing. This study aimed to assess the feasibility of the PSC as an alternative collection matrix for HIV‐1 genotypic drug resistance testing.
Methods: The study was conducted at Charlotte Maxeke Johannesburg Academic Hospital from December 2019 to April 2020. Plasma, dried blood spot (DBS) and PSC samples were prepared from 35 routine specimens. Spotted cards were lysed using two‐hour and overnight lysis methods. Specimens from all collection matrices were extracted (Nuclisens EasyMag, Biomerieux) and sequenced using an in‐house developed Sanger‐based assay. Results were compared, using plasma as the gold standard, to determine amplification and sequencing success rates and drug resistance mutation concordance.
Results: The median plasma viral load was 4.4 log10 copies/mL (IQR: 3.68 to 4.99 log10 copies/mL). Amplification success rates for PSC lysed using the two‐hour or overnight lysis method (97%, n = 34 each), and DBS two‐hour lysis (97%, n = 34) or overnight lysis (86%, n = 30), were achieved. These were comparable to plasma samples (97%, n = 34). Sequencing success rates were higher using the two‐hour lysis method, 100% and 88% for PSC and DBS, respectively, compared to 91% and 82% using overnight lysis. A percentage similarity of >98% at drug resistance mutation sites was achieved for all specimens, except for one DBS 2h‐lysis sample and one DBS overnight lysis sample. Of the detected drug resistance mutation discordances between plasma and spotting samples, most had no or limited impact on the clinical management of patients and <1% resulted in clinically significant changes.
Conclusions: These findings indicate the PSC, lysed using a two‐hour method, is a suitable alternative to plasma for HIV‐1 drug resistance testing. Plasma Separation Cards may increase access to HIV drug resistance testing in settings experiencing challenges using plasma.
OAB0404
Evaluation of new high‐throughput platforms for the quantification of HIV‐1 RNA in plasma to support scale‐up of viral load testing in low‐ and middle‐income countries
G Zhang 1; L Hans2; S Jadczak1; K Sleeman1; L Makuwaza3; K Peloakgosi‐Shikwambani3; M Hurlston Cox1; H Alexander1; S Carmona2; and C Zeh1
1Centers for Disease Control and Prevention, Division of Global HIV & TB, Center for Global Health, Atlanta, United States. 2National Health Laboratory Service, and University of the Witwatersrand, Johannesburg, South Africa. 3Wits Health Consortium, Johannesburg, South Africa
Background: Roche announced that the conventional COBAS AmpliPrep/COBAS TaqMan (CAP/CTM) system will be phased out by early 2024 and replaced with new high‐throughput systems, such as the Roche cobas 4800 (c4800) and the cobas 6800/8800 (c6800/c8800). We sought to independently evaluate the analytical and clinical performance of the c4800 and the c6800/c8800 for HIV‐1 viral load testing using plasma for the World Health Organization Prequalification (WHO‐PQ) and for use in President’s Emergency Plan for AIDS Relief (PEPFAR)‐supported countries.
Methods: Analytical performance, including the limit of detection (LOD), precision, linear reportable range, subtype detection and cross‐contamination, were evaluated at the Centers for Disease Control and Prevention using the third WHO HIV‐1 RNA Standard and virus culture covering HIV‐1 subtypes A, B, C, D and CRF02‐AG. Remnant clinical specimens (n = 1349) were tested in South Africa to assess the assays’ accuracy and agreement with the reference assay on CAP/CTM. LOD was calculated using PROBIT analysis. Bland‐Altman and correlation analysis were used to analyse the bias and measurement agreement.
Results: LOD was estimated to be 20.6 copies/mL (95% confidence interval [CI]: 14.9 to 40.5) for the c4800 and 12.5 copies/mL (95% CI: 8.9 to 43.5) for the c6800/c8800. Within‐laboratory standard deviations were less than 0.2 log10 copies/mL on both platforms. A high degree of linearity was seen on all tested subtypes for both platforms with R2 values greater than 0.98. No cross‐contamination was observed for either platform. When compared to the reference assay, the c4800 had a demonstrated sensitivity of 96.8% (95% CI 93.8 to 98.4) and a specificity of 98.8% (93.6 to 99.8) in detecting virological failure (viral load ≥1000 copies/mL), whereas the c6800/c8800 had a sensitivity of 98.0% (95.3 to 99.1) and a specificity of 94.9% (89.9 to 97.5). The average bias between the new assay and the reference assay was less than 0.12 log10 copies/mL for both platforms.
Conclusions: The c4800 and c6800/c8800 demonstrate comparable accuracy and precision to the reference method. The improved chemistry of c6800/c8800 makes it more sensitive and supports use as the new reference standard for plasma viral load testing. These findings contributed to WHO‐PQ and PEPFAR approval for their use in scale‐up of viral load testing in low‐and middle‐income countries.
OAB0405
APOBEC editing in HIV DNA proviral vif and pol long‐reads issued from virologically suppressed patients included in the ANRS LAMIDOL trial
M Bertine1; A Storto1; V Joly2; S Lameiras3; C Burdet4; S Baulande3; R Landman2; F Raffi5; Y Yazdanpanah2; D Descamps1; C Charpentier 1; and LAMIDOL study group
1Hôpital Bichat‐Claude Bernard, Service de Virologie, Paris, France. 2Hôpital Bichat‐Claude Bernard, Service de Maladies Infectieuses et Tropicales, Paris, France. 3Institut Curie, Paris, France. 4Hôpital Bichat‐Claude Bernard, Service d'Épidémiologie Biostatistique et de Recherche Clinique, Paris, France. 5CHU de Nantes, Service de Maladies Infectieuses et Tropicales, Nantes, France
Background: In HIV‐1, hypermutated viruses induced by APOBEC3F/3G cytidine‐deaminase activity represent a part of defective viruses. Few data are available with long‐reads UDS technology regarding the linkage of APOBEC3F/3G editing. The objective of this study was to assess the proportion of APOBEC3F/3G defective viruses in PBMC from ARV‐treated patients with prolonged virological suppression.
Methods: UDS of vif (579pb) and pol (3012pb) regions was performed on HIV‐DNA from PBMC collected at baseline from virologically suppressed patients since a median of 4.5 years, switched to DTG+3TC in the ANRS167‐LAMIDOL trial. Long‐reads were obtained using PacBio‐Sequel system, alignments were performed with Geneious. In‐house Python programmes were designed to identify defective sequences and APOBEC‐related resistance mutations (APOMut). All hypermutated sequences and those containing at least one stop codon were considered defective.
Results: Among the 110 patients assessed, HIV vif and pol sequencing were available for 79 and 19 respectively. The median number of reads was 2200 (IQR = 1460 to 2931) and 5978 (IQR = 5357 to 8023) for vif and pol respectively. At least one proviral defective read was detected in 15 patients (19%, IC 95% = 11.0 to 29.4) in vif and 9 (47%, IC95 = 24.4 to 71.1) in pol. When present, the median percentage of reads with at least 1 stop codon was 36.2% (IQR = 28.7 to 100) and 4.2% (IQR = 1.8 to 37.1), in vif and pol respectively. Hypermutated reads were detected in proviruses of 3 (3.8%, IC95 = 0.8 to 10.7) and 4 (21.1%, IC95 = 6.1 to 45.6) patients, in vif and pol respectively. Eleven APOMut were detected in HIV‐DNA of five patients (26%): D30N, M46I and G73S in protease (all in a single patient); E138K (n = 1), M184I (n = 3) and M230I (n = 3) in RT; E138K (n = 1) in integrase. Eight of the 11 APOMut were present in minority proportions (range = 1.1% to 14.7%). Overall, APOMut and stop codons were present on the same reads in 98% of cases.
Conclusions: In these ART‐treated patients with prolonged virological suppression, 19% of vif and 47% of pol sequences harboured at least one defective provirus. Long‐read UDS showed that stop codons and APOMut were linked on the same read in almost all cases. In addition, we showed that virological suppression was maintained on DTG+3TC despite baseline minority APOMut in 26% of cases.
OAB0501
HIV immune reconstitution inflammatory response syndrome and the risk of adverse pregnancy‐foetal outcomes among ART naïve women aged 20 to 49 years in selected public hospitals, Nairobi, Kenya
J Muthuka 1; Y Kombe2; A Makokha3; and M Kiptoo1
1Kenya Medical Training College, Nairobi, Kenya. 2Kenya Medical Research Institute, Nairobi, Kenya. 3Jomo Kenyatta University, Nairobi, Kenya
Background: This study described the incidence of adverse pregnancy‐foetal outcomes (APFOs) in Kenyan HIV‐infected ART‐naive pregnant women and examined the relationship between maternal HIV‐immune reconstitution inflammatory response syndrome (IRIS) /related risk factors and APFOs. This prospective cohort study was carried out among 102 HIV‐IRIS‐exposed and 102 HIV‐IRIS non‐exposed pregnant women after initiating ART.
Methods: Both groups were enrolled from two hospitals in Nairobi County, Kenya in July 2019. Data were collected in a standard structured form, including maternal and demographic characteristics, HIV‐IRIS status, HIV‐IRIS related factors and their pregnancy outcomes. APFOs were assessed by maternal HIV‐IRIS status and HIV‐IRIS related factors using logistic regression analysis.
Results: The incidence of APFOs, over the entire period, in IRIS versus non‐IRIS, was 26.47% and 10.78% and the rates were 0.012 and 0.0045 per person’s week respectively. The RRs of APFOs were double‐fold among IRIS cases compared to non‐IRIS cases RR (2, 2.69 and 2) respectively. IRIS cases were three times more likely to experience an APFO compared to non‐IRIS cases [OR = 3; 95% CI: 1.4 to 6.4; p = 0.004]. At specific visit times, APFOs were associated with IRIS mostly at delivery (p = 0.006) as compared to other times; [OR = 2.1; 95% CI: 0.502 to 8.482; p = 0.16]; [OR = 2.5; 95% CI: 1.295 to 8.121; p = 0.006] and [OR = 2.4; 95% CI: 0.216 to 27.286; p = 0.71]. APFOs with higher frequencies at specific points among IRIS and non‐IRIS cases were; at the end of the second trimester; miscarriage, 3 (2.9%), 2 (2.0%), at delivery; LBW 11 (10.8%), 3 (2.9%) and within two weeks after delivery; newborn intensive care admission (newborn jaundice) 2 (2.0%), 1 (1.0%), respectively, all with p > 0.05 about HIV‐IRIS. LBW showed the highest incidence/significance relative to IRIS [OR = 3.8; 95% CI: 1.079, 14.754; p = 0.0019]. Multiple logistic regression for the entire follow‐up period dropped maternal HIV‐IRIS and revealed HIV‐RNA viral load at baseline of above 50 copies/mL [AOR = 2.7; 95% CI: 1.2 to 6.3; p = 0.017], closely, maternal placental syndrome (MPS) characterized by hypertensive events [AOR = 0.1; 95% CI: 0.0 to 1.0; p = 0.052] and mother’s general health during delivery [AOR = 4; 95% CI: 4.0: 1.8 to 9.1; p = 0.001] as independent predictors of APFOs.
Conclusions: Maternal HIV‐IRIS was associated with significantly increased risks of APFOs. Modifiable risk factors should be monitored and controlled in clinical practice more so towards delivery.
OAB0502
Factors associated with severity of Edinburgh Postnatal Depression Screen (EPDS) and optimal cut‐off of EPDS for diagnosis of depression and anxiety among postpartum HIV‐positive women in Lusaka, Zambia
MP Kasaro 1; B Blette2; R Paul3; S Meltzer‐Brody4; CE Schiller4; JM Ncheka3; B Spelke5; J Stringer6; and E Stringer5
1UNC Global Projects Zambia, Lusaka, Zambia. 2University of North Carolina, Gillings School of Global Public Health, Department of Biostatistics, Chapel Hill, United States. 3University of Zambia, School of Medicine, Department of Psychiatry, Lusaka, Zambia. 4University of North Carolina, School of Medicine, Department of Psychiatry, Chapel Hill, United States. 5University of North Carolina, School of Medicine, Department of Obstetrics and Gynecology, Chapel Hill, United States. 6University of North Carolina, School of Medicine, Department of Obstetrics and Gynecology, Chapel hill, United States
Background: Postnatal depression (PND) may affect adherence to HIV treatment and thus postnatal HIV transmission. The Edinburgh Postnatal Depression Screen (EPDS), widely used to screen for PND, has not been previously validated in HIV‐infected, perinatal women in LMICs. Additionally, factors associated with the severity of depression remain unknown.
Methods: As part of PND treatment study, we screened HIV‐infected women with the EPDS between 6 and 10 weeks postpartum. To identify women at the lower symptom threshold, those scoring ≥6 of 30 were referred for a Mini International Neuropsychiatric Interview (MINI). The optimal EPDS threshold for diagnosis of depression was determined using receiver operating characteristic (ROC) curve analysis. Multiple imputation was used to characterize those with EPDS < 6. Based on the literature, mild depression was defined as an EPDS 7‐13 and moderate to severe as an EPDS ≥14. Differences between groups and factors associated with severity of depression were calculated using Chi square and t‐tests.
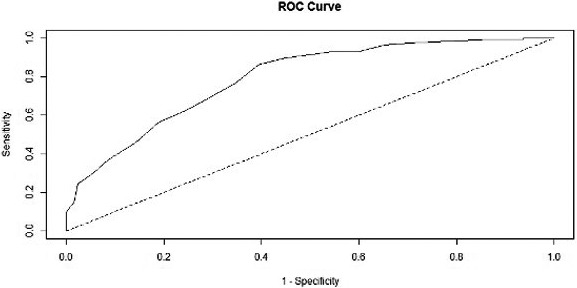
Abstract OAB0502‐Figure 1. Receiver Operating Characteristic (ROC) curve for optimal EPDS threshold defining depression
Results: 192/240 (80%) women screened had an EPDS ≥ 6, of whom 120 (63%) agreed to undergo MINI evaluation. Using the MINI diagnostic tool, an EPDS score of 10 performed best at discriminating depression with the sensitivity of 86% and specificity of 61%. The area under the curve was 0.79 after imputation. 59 MINI‐evaluated women (49%) scored ≥14, indicating moderate/severe depression. Factors associated with an EPDS ≤ 13 were living with partner (RR = =0.63; 95% CI 0.44, 0.90; p = 0.01), living in poverty (RR = 0.68; 95% CI 0.47, 0.98; p = 0.04) and having fewer life stressors (mean difference ‐1.0 (−1.8, −0.2) p = 0.01).
Conclusions: An EPDS ≥ 10 is the optimal threshold for determining depression in HIV‐infected postpartum women. Among women evaluated with a MINI, women with moderate to severe depression were more likely to not live with a partner and have a greater number of life stressors.
OAB0503
A longitudinal study on insulin resistance and metabolic syndrome in children with perinatal HIV infection and HIV exposed uninfected children in South Africa
C Davies 1; F Vaida2; S Browne2; B Ayele1; and S Innes1
1Stellenbosch University, Cape Town, South Africa. 2University of California, San Diego, United States
Background: HIV is associated with insulin resistance and Metabolic Syndrome, driven by HIV‐associated immune dysregulation and by antiretroviral therapy (ART). However, few longitudinal studies have been conducted in children living with perinatally acquired HIV (CLpHIV). We evaluated the trajectory of insulin resistance and Metabolic Syndrome in CLpHIV compared to children who are HIV‐unexposed and uninfected (CHUU), and children who are HIV‐exposed and uninfected (CHEU).
Methods: The study included children previously part of the Children with HIV Early antiRetroviral (CHER) trial and P1060 trial followed at Tygerberg Children’s Hospital in South Africa between 2014 and 2020, along with CHEU and CHUU from the same communities. The cohort comprised 485 children, with 141 CLpHIV, 169 CHEU and 175 CHUU, with a median age at baseline of nine years. The main outcome was the Homeostatic Model Assessment of Insulin Resistance (HOMA‐IR), and secondary outcomes included LDL cholesterol, triglyceride‐to‐HDL ratio, android fat mass and systolic blood pressure. We used a mixed effects model to model the progression of metabolic indicators over time in each HIV group. Directed Acyclic Graph analysis was used to identify covariates, whereafter the following were considered as confounders: gender, height, age group, Tanner puberty stage and ethnicity.
Results: Adjusted mean HOMA‐IR was 15% (95% CI: 2% to 29%) greater in CLpHIV than CHUU. Adjusted mean triglyceride‐to‐HDL ratio was 48%(95% CI: 35% to 62%) greater in CLpHIV than CHUU, and the adjusted mean LDL was 0.25 mmol/L greater in CLpHIV than CHUU (95% CI: 0.11 to 0.40). In all analyses, no significant difference was found between CHEU and CHUU.
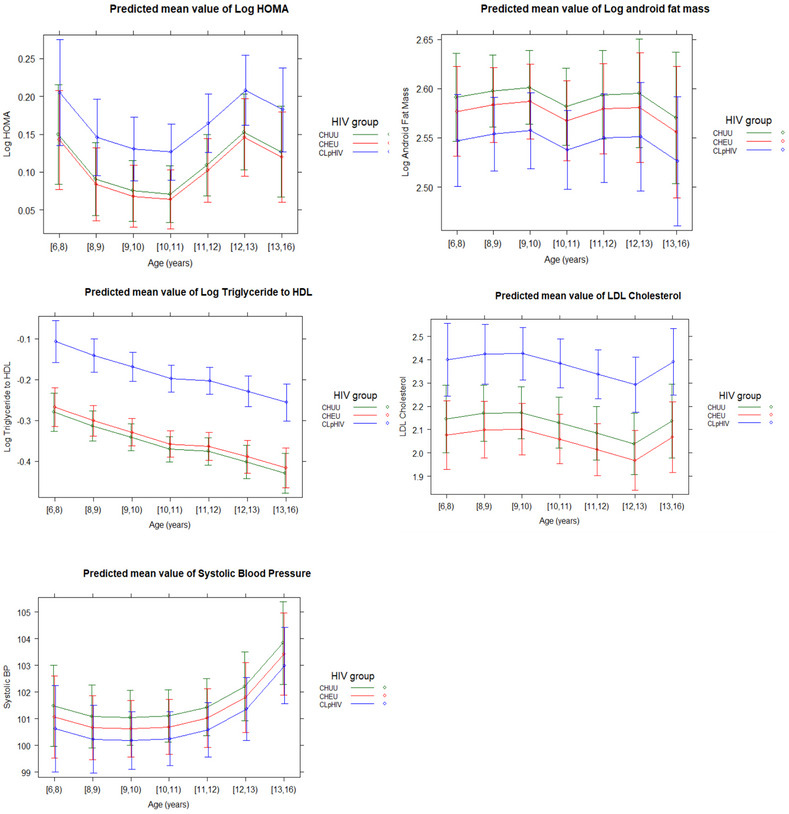
Abstract OAB0503‐Figure 1. Predicted values for each metabolic indicator over time, by HIV group
Conclusions: CLpHIV have persistently elevated insulin resistance, triglyceride‐to‐HDL ratio and LDL cholesterol into puberty, and therefore should be monitored carefully for subclinical cardiovascular disease and receive appropriate preventative interventions, as CLpHIV will have a lifelong exposure to HIV‐associated immune dysregulation and ART.
OAB0504
The road to success is paved with dolutegravir: Dolutegravir treatment success among in children and adolescents living with HIV (CALHIV) at the Baylor Tanzania Centres of Excellence
J Bacha 1,2,3; B Mayalla3; M Chodota3; N Jiwa3; L Mwita3; L Campbell1,2,3; and Baylor Tanzania DTG
1Baylor International Pediatric AIDS Initiative (BIPAI) at Texas Children’s Hospital, Baylor College of Medicine, Houston, United States. 2Baylor College of Medicine, Houston, United States. 3Baylor College of Medicine Children's Foundation – Tanzania, Mbeya, Tanzania, United Republic of
Background: Efficacy and safety data of novel antiretrovirals, such as dolutegravir (DTG), in children and adolescents, often lags behind adult data, and can lead to hesitation and slow uptake by HIV clinicians. Beginning in 2019, the Baylor Tanzania programme began an enthusiastic rollout of DTG among CALHIV. We describe outcomes and safety data of this DTG rollout among CALHIV enrolled at the Baylor Tanzania clinics in Mbeya and Mwanza, Tanzania.
Methods: Retrospective chart review was conducted to describe outcomes and safety data of CALHIV who received DTG as part of their ART at the Baylor College of Medicine Children’s Foundation – Tanzania Centres of Excellence (COEs) in Mbeya and Mwanza, Tanzania between 1 March 2019 (when DTG became available) and 30 November 2020. HIV viral load (VL) suppression was defined as VL < 1000 copies/mL.
Results: A total of 1703 CALHIV received DTG, representing 62.4% (1703/2727) of all CALHIV on ART and 78.1% (1703/2180) of CALHIV eligible for DTG by weight (>20kg) at the COE. TLD was used in 57.0% (970/1703), followed by 39.2% (667/1703) on ABC‐3TC‐DTG and 3.9% (66/170‐3) on AZT‐3TC‐DTG. Among the DTG cohort, 13.6% (231/1703) were new ART initiations, 63.2% (1077/1703) were shifted from a NNRTI regimen and 23.2% (395/1703) were shifted from a PI regimen.
Outcomes revealed no severe drug toxicity and no discontinuations of DTG, with 98.3% (1674/1703) remaining active in COE care and 1.7% (29/1703) transferred out. Multi‐month prescriptions were used in 73.6% (1254/1703) of DTG patients. At the end of the study period, 92.4% (1002/1084) of patients on DTG with documented VL were suppressed, compared to 86.4% (1257/1455) of those with VLs prior to DTG. Among those with pre‐ and post‐DTG VLs (n = 908), 85.6% (149/174) of previously unsuppressed became suppressed, and 94.6% (694/734) of previously suppressed remained suppressed.
Conclusions: DTG was well tolerated and highly effective in our clinically diverse cohort of CALHIV, and its use resulted in viral suppression for many previously unsuppressed CALHIV. These results encourage widespread use of DTG among eligible CALHIV, especially those who remain unsuppressed on their current regimens.
OAB0505
Neuropsychiatric manifestations and sleep disturbances in children and adolescents randomized to dolutegravir‐based ART versus standard‐of‐care in the ODYSSEY trial
A Turkova 1; A Kekitiinwa2; E White1; V Mumbiro3; E Khauda4; A Liberty5; E Dobbels6; GM Ahimbisibwe7; T Moloantoa5; L Atwine8; S Kanjanavanit9; NR Mosia10; T Puthanakit11; T Smit12; R Kobbe13; C Fortuny14; E Chidziva3; RC Kyambadde7; D Bbuye2; T Sarfati1; A Coelho15; Y Saïdi15; A Lugemwa8; N Klein12; M Bwakura‐ Dangarembizi3; C Kityo4; M Cotton6; C Giaquinto16; P Rojo17; DM Gibb1; D Ford1; and the ODYSSEY trial team
1MRC Clinical Trials Unit at UCL, Institute of Clinical Trials & Methodology, London, United Kingdom. 2Baylor College of Medicine, Kampala, Uganda. 3University of Zimbabwe, Harare, Zimbabwe. 4Joint Clinical Research Centre (JCRC), Kampala, Uganda. 5Perinatal HIV Research Unit, University of the Witwarsrand, Johannesburg, South Africa. 6Family Center for Research with Ubuntu, Department of Paediatrics & Child Health, Stellenbosch University, Tygerberg, South Africa. 7Makerere University‐Johns Hopkins University (MU‐JHU) Research Collaboration, Kampala, Uganda. 8Joint Clinical Research Centre (JCRC), Mbarara, Uganda. 9Nakornping Hospital, Chiang Mai Province, Thailand. 10Enhancing Care Foundation: King Edward VIII Hospital, Durban, South Africa. 11HIVNAT, Thai Red Cross AIDS Research Center, Bangkok, Thailand. 12Africa Health Research Institute (AHRI), Kwazulu‐Natal, South Africa. 13University Medical Center Hamburg‐Eppendorf, Hamburg, Germany. 14Hospital Sant Joan de Déu, Barcelona, Spain. 15INSERM/ANRS SC10‐US19, Essais Thérapeutiques et Maladies Infectieuses, Villejuif, France. 16University of Padova, Department of Women and Child Health, Padova, Italy. 17Hospital 12 de Octubre, Universidad Complutense, Department of Pediatrics, Madrid, Spain
Background: Dolutegravir is associated with neuropsychiatric adverse events (NPAEs) in adults. We present first randomized data in children and adolescents.
Methods: ODYSSEY is an open‐label, multi‐centre, randomized trial, comparing efficacy and safety of dolutegravir‐based ART (DTG) with the standard of care (SOC) in children initiating first‐ or second‐line therapy. We compared NPAEs, including serious adverse events (SAEs), grade ≥3 events, ART‐modifying events and suicidality‐related events and patient/carer mood‐and‐sleep questionnaire responses in DTG versus SOC.
Results: A total of 707 children ≥14 kg were randomized (sub‐Saharan Africa 88%, Thailand 9%, Europe 4%); 311 children started first‐line (92% efavirenz‐based in SOC); 396 second‐line (98% PI‐based). Median (IQR) age was 12.2 (9.1, 14.9); 362 (51%) were male; median follow‐up 142 (124,159) weeks.
There were 31 NPAEs (in 23 children): 18 (15) in DTG versus 13 (8) in SOC (Table 1). Median (IQR) age and time from enrolment at first event were 15.9 (10.4, 17.5) years and 72 (47,124) weeks respectively. Most NPAEs (23) were in children starting first‐line; and most (22) occurred in males. Ten participants (5 DTG;5 SOC) had 13 SAEs: 7 DTG (3 epilepsy/convulsions, 1 headache/hypertension, 1 depression, 1 parasuicide, 1 psychosis) versus 6 SOC (3 epilepsy/convulsions, 1 dizziness, 2 parasuicide). Twelve children (8 DTG;4 SOC) experienced 15 suicidality events: 10 suicidality ideation (6 DTG;4 SOC) and 5 parasuicide (2 DTG;3 SOC). ART‐modifying NPAE(s) included three DTG (2 depression, 1 psychosis) and two SOC (1 parasuicide, 1 dizziness).
A small number of participants/carers reported symptoms of self‐harm (8 DTG;1 SOC, p = 0.04), "life was not worth living"(17 DTG;5 SOC, p = 0.009) or suicidal thoughts (13 DTG;0 SOC, p < 0.001) in mood‐and‐sleep questionnaires; the reported symptoms were transient and did not lead to treatment change. There were no differences between treatment groups in low mood/feeling sad, problems concentrating, feeling worried or feeling angry/aggressive, time to fall asleep, nightmares/vivid dreams or sleep quality.
Conclusions: Numbers of NPAEs and reported neuropsychiatric symptoms were low. More participants reported neuropsychiatric symptoms in the DTG arm versus SOC, however, this difference should be interpreted with caution in an open‐label trial.
Abstract OAB0505‐Table 1. Summary of neuropsychiatric adverse events in ODYSSEY
| DTG, N = 350 | SOC, N = 357 | Total, N = 707 | p‐value | ||||
|---|---|---|---|---|---|---|---|
|
All neuropsychiatric adverse events, N [N participants] |
18 | [15] | 13 | [8] | 31 | [23] | 0.125* |
| Neurological adverse events | 6 | [6] | 6 | [5] | 12 | [11] | 0.736* |
| Psychiatric adverse events | 12 | [10] | 7 | [4] | 19 | [14] | 0.112* |
| Serious adverse events | 7 | [5] | 6 | [5] | 13 | [10] | |
| Grade ≥3 adverse events | 12 | [9] | 8 | [7] | 20 | [16] | |
| ART‐modifying events | 3 | [3] | 2 | [2] | 5 | [5] | |
| Hazard Ratio for time to first NPAE§ (95% CI) | 1.87 (0.79, 4.41) | 1 (ref) | 0.154 | ||||
NPAE, neuropsychiatric adverse events.
*Comparing number of participants with at least 1 event.
§Adjusted for ODYSSEY A and B.
OALC0501
Outcomes of participants switching from F/TDF to F/TAF for PrEP: week 48 results from the DISCOVER open label phase
C Spinner 1; A Avery2; JA Flamm3; G Crofoot4; C Brinson5; G Kronborg6; E DeJesus7; C Carter8; A Kintu8; Y Shao8; R Ebrahimi8; M Das8; JM Baeten8; and A Clarke9
1Technical University of Munich, School of Medicine, Munich, Germany. 2MetroHealth Medical Center, Cleveland, United States. 3Kaiser Permanente Medical Group, Sacramento, United States. 4The Crofoot Research Center, Inc., Houton, United States. 5Central Texas Clinical Research, Austin, United States. 6Hvidovre Hospital, Hvidovre, Denmark. 7Orlando Immunology Center, Orlando, United States. 8Gilead Sciences Inc., Foster City, United States. 9Sexual Health and Clinical Trials, Royal Sussex County Hospital, Brighton, United Kingdom
Background: DISCOVER is an ongoing, multinational, double‐blind, randomized controlled trial of F/TAF versus F/TDF for PrEP which demonstrated non‐inferior efficacy and improved bone mineral density (BMD) and renal safety biomarkers at week (W) 48 and 96 of the blinded phase. Here we report the W48 outcomes of the open‐label (OL) phase, where participants initially randomized to F/TDF switched to F/TAF.
Methods: After W96 of the blinded phase, participants could opt to receive F/TAF in the OL phase. We evaluated BMD, glomerular function (eGFR), biomarkers of proximal tubular injury (PTI; β2M/Cr, RBP/Cr), lipids and weight at OLW48 in participants who switched from F/TDF to F/TAF (switch group) and those remaining on F/TAF (stay group).
Results: The F/TAF OL phase included 2128 switch groups and 2080 stay group participants. Participants in the switch and stay groups had 2076 and 2075 person‐years of F/TAF exposure in the OL phase respectively. There were two HIV infections in the switch group and three in the stay group (Table 1). Hip and Spine BMD increased in the switch group. Switch group participants had lower eGFR at OL onset, which increased by OLW48. Both groups had improvements in PTI markers, which was greater in the switch group. LDL and HDL cholesterol increased in the switch group, with minimal change in total cholesterol:HDL ratio; fasting glucose change was small and not significantly different between groups. Weight gain was seen in both groups and was greater in the switch group.
Abstract OALC0501‐Table 1
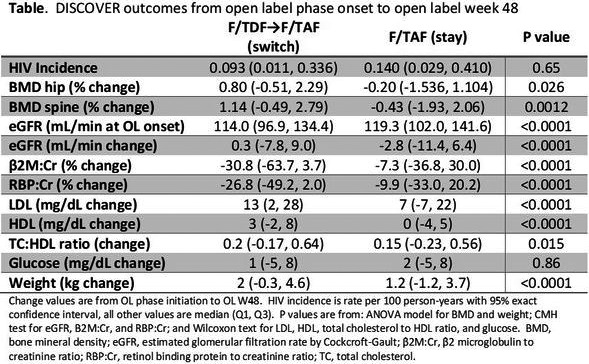
Conclusions: Participants switching from F/TDF to F/TAF in the OL phase of DISCOVER had a low HIV incidence rate, increased BMD and improved renal biomarkers. LDL, HDL and weight increased in these participants, consistent with a removal of TDF’s weight and lipid suppressive effects. These data support F/TAF as a safe and effective switch option for people currently taking F/TDF for PrEP.
OALC0502
Preferences for implementing long‐acting injectable pre‐exposure prophylaxis among cisgender men who have sex with men in the United States
SW Beckham 1; T Sanchez2; M Zlotorzynska2; M Rai2; P Sullivan2; S Hyman3; V Vannappagari4,5; S Sarkar4; A Reinhart4; K Rawlings4; and JF Bridges3
1Johns Hopkins Bloomberg School of Public Health, Health, Behavior and Soci ety, Baltimore, United States. 2Emory University Rollin School of Public Health, Epidemiology, Atlanta, United States. 3Ohio State University, College of Medicine, Columbus, United States. 4ViiV Healthcare, Research Triangle Park, United States. 5University of North Carolina at Chapel Hill, Chapel Hill, United States
Background: Long‐acting injectable HIV pre‐exposure prophylaxis (LAI‐PrEP) is efficacious and may overcome some challenges of daily oral PrEP. LAI‐PrEP has great potential to reduce HIV incidence in high‐risk populations, including men who have sex with men (MSM). Information about potential user preferences can be used to improve uptake, adherence and persistence and ultimately reduce HIV incidence. We sought to understand the preferences for implementation and perceived potential barriers of LAI‐PrEP among MSM.
Methods: We recruited participants online through the 2019 American Men’s Internet Survey. Eligible participants were HIV negative, sexually active MSM aged ≥15 and living in the United States. We designed and analysed a discrete‐choice experiment to identify preferred implementation profiles of LAI‐PrEP among the respondents. Attributes included perceived side effects, injection frequency, out‐of‐pocket cost, service location and negative judgement. We used mixed logit regression to calculate preference weights then relative importance by dividing the difference between the maximum and minimum preference weights within each attribute by the sum of the differences across all attributes, and multiplying by 100.
Results: N = 2241 participants responded. Perceived side effects were the most important potential barrier to LAI‐PrEP (52% of the total relative importance), followed by out‐of‐pocket cost of up to $100 (30%). Injection frequency comprised only 11% of the relative importance, with quarterly and semiannual injections slightly preferred over every two months. Perceived negative judgement from others (PrEP stigma) was relatively unimportant compared to other attributes (5%). Service location was the least important attribute (2%); participants only slightly preferred a private doctor’s office over a sexual health clinic and a pharmacy. See Figure.
Conclusions: This analysis provides insight into potential barriers to implementation of LAI‐PrEP among MSM in the United States. A LAI‐PrEP product with perceived severe side effects is likely to be a significant barrier to uptake, though potential users would probably tolerate mild‐to‐moderate side effects. Minimizing out‐of‐pocket costs is likely to increase uptake of LAI‐PrEP and is important to equitable access to populations most at risk. Reduction of injection frequency could marginally increase likelihood of PrEP utilization. Service location and potential negative judgement are unlikely to be barriers to uptake in this population.
OALC0503
Understanding participant experiences and preferences in an injectable PrEP trial: a qualitative sub‐study of barriers, facilitators and preferences for PrEP use among MSM and TGW
C Psaros 1; RJ Landovitz2; W Rice3,4; CF Kelley5,4; T Oyedele6,7; L Coelho8; N Phanuphak9; Y Singh10; K Middelkoop10; S Griffith11; M McCauley11; G Goodman1,12; J Rooney13; A Rinehart14; N Van de Velde15; J Clark16; V Go17; J Sugarman18; S Fields19; C Gordon20; A Adeyeye21; B Grinsztejn8; SA Safren22; and HPTN 083‐02 Study Team
1Massachusetts General Hospital, Department of Psychiatry, Boston, United States. 2UCLA Center for Clinical AIDS Research & Education, Division of Infectious Diseases, Los Angeles, United States. 3Rollins School of Public Health, Emory University, Department of Behavioral, Social and Health Education Sciences, Atlanta, United States. 4The Hope Clinic of the Emory Vaccine Center, Decatur, United States. 5Emory University School of Medicine, Division of Infectious Diseases, Atlanta, United States. 6Ruth M. Rothstein CORE Center, Adolescent & Young Adult Research, Chicago, United States. 7Ruth M. Rothstein CORE Center and Stroger Hospital of Cook County, Division of Infectious Diseases, Chicago, United States. 8Instituto Nacional de Infectologia Evandro Chagas, Fiocruz, Rio de Janeiro, Brazil. 9Thai Red Cross AIDS Research Center, Bangkok, Thailand. 10University of Cape Town, The Desmond Tutu HIV Centre, Institute of Infectious Disease and Molecular Medicine and Department of Medicine, Cape Town, South Africa. 11FHI 360, HIV Prevention Trials Network, Durham, United States. 12The Fenway Institute, Fenway Health, Boston, United States. 13Gilead Sciences, Foster City, United States. 14ViiV Healthcare, Durham, United States. 15ViiV Healthcare, Brentford, United Kingdom. 16UCLA David Geffen School of Medicine, Department of Medicine, Division of Infectious Diseases, Los Angeles, United States. 17University of North Carolina at Chapel Hill Gillings School of Global Public Health, Department of Health Behavior, Chapel Hill, United States. 18Berman Institute of Bioethics, School of Medicine, and Bloomberg School of Public Health, Johns Hopkins University, Baltimore, United States. 19Penn State College of Nursing, University Park, United States. 20National Institute of Mental Health, National Institutes of Health, HIV Prevention and Care Continuum, Co‐Morbidities, and Translational Research Branch, Rockville, United States. 21National Institute of Allergy and Infectious Diseases, National Institutes of Health, Division of AIDS, Rockville, United States. 22University of Miami, Department of Psychology, Coral Gables, United States
Background: HPTN083, a randomized, double‐blind international clinical trial of long‐acting injectable cabotegravir (CAB‐LA) versus daily oral emtricitabine/tenofovir disoproxil fumarate (TDF/FTC) for HIV prevention among cisgender men and transgender women who have sex with men (MSM/TGW), demonstrated a 66% reduction in HIV incidence in participants randomized to CAB‐LA versus TDF/FTC. Participants’ experiences prior to unblinding in May 2020 provide initial insights into preferences and best practices for implementing injectable PrEP.
Methods: Participants enrolled in HPTN083 were purposively sampled for individual qualitative interviews (n = 35) during the injection phase from three study sites (two U.S., one international), and categorized as adherent (n = 24), non‐adherent (n = 10) or early discontinuers (n = 1). Data were organized using NVivo (version 12) and analysed using content analysis.
Results: Reasons for enrolling in HPTN083 and using PrEP included a preference for using medication for HIV prevention versus for HIV treatment, that study participation was believed to be a means to enhance health via education and access to services, and a sense of contributing to community via research participation. Interviewees contrasted experiences with study staff and research sites with available clinical care, and emphasized increased scheduling flexibility, frequent and thorough communication, and the open, affirming environment of research sites (e.g. compassion, encouragement, less stigma). Injection experiences were positive overall with respect to ease of use; some described early anxiety around injections and shared perceptions about the study product (e.g. that efficacy would wane over time and/or before the next scheduled injection) and strategies for managing injection site discomfort. Facilitators of injection visit adherence generally centred around motivational factors (e.g. preservation of health, desire to contribute to research), use of reminder strategies, social support and clinic factors (e.g. flexibility). Barriers included structural factors (e.g. financial constraints, distance to clinic, homelessness) and competing demands (e.g. work schedules).
Conclusions: MSM/TGW viewed their participation in an injectable PrEP trial as a positive experience and a means by which to enhance wellbeing. Site/clinic flexibility and an open and affirming clinic environment were key facilitators to adherence. To support injection adherence over time, interventions that target structural barriers and flexible means of injection delivery may be most effective.
OALC0601
No impact of tenofovir/emtricitabine in estradiol exposure among transwomen on oral PrEP: results from the 12‐week drug–drug interaction PrEParadas substudy
V Cattani 1; E Jalil1; T Torres1; S Cardoso1; L Eksterman1; C Castro1; L Monteiro1; P Anderson2; E Wilson3; B Grinsztejn1; V Veloso1; and R Estrela1
1Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil. 2University of Colorado, Denver, United States. 3University of California, San Francisco, United States
Background: An important HIV prevention barrier for transwomen (TGW) is the concern that oral PrEP containing tenofovir (TDF) and emtricitabine (FTC) negatively affects the efficacy of feminizing hormone therapy (FHT). We aimed to assess the impact of PrEP on FHT among transwomen.
Methods: We performed a drug–drug interaction substudy among TGW enrolled in a TDF/FTC daily oral PrEP and using FHT (estradiol valerate plus spironolactone) in a demonstration study for TGW and PrEP in Rio de Janeiro, Brazil (NCT03220152). Participants had a first pharmacokinetic (PK) assessment and initiated PrEP 15 days after FHT initiation (W0), followed by a second PK evaluation 12 weeks later (W12). Blood samples were collected prior and after the directly observed therapy (0.5, 1, 2, 4, 6, 8 and 24 hours). Estradiol and spironolactone PK parameters were estimated by non‐compartmental analysis (Pharsight WinNonlin v. 7.6, Certara) and compared using Wilcoxon rank‐sum test (alpha = 0.05). We assessed PrEP adherence with DBS levels.
Results: Among 24 transwomen that completed the study, median age was 26.0 years (23.0 to 34.5) and body mass index was 22.7 kg/m2 (20.6 to 26.0). Condomless anal sex in the last six months was reported by 92%. At W12, 6, 13 and 5 participants had DBS levels consistent with 7+ doses/week, 4 to 6 doses/week, <2 doses/week. Estradiol exposure did not differ between W0 and W12: AUC/D 596.0 (387.0 to 757.0) at W0 and 511.0 (367.0 to 707.0) h*pg/mL/mg at W12 (p = 0.056), Cmax 36.0 (24.0 to 48.0) at W0 and 28.3 (22.0 to 43.0) pg/mL at W12 (p = 0.095). Although spironolactone AUC/D was significantly lower at W12 compared to W0 (2.8 [1.5 to 3.5] and 3.0 [2.1 to 5.1] h*ng/mL/mg, respectively, p = 0.008), its Cmax did not differ between the two assessments (W0: 0.9 [0.7 to 1.3], W12: 0.8 [0.7 to 1.2] ng/mL, p = 0.96).
Conclusions: Our results reassure that oral PrEP and FHT may be used concomitantly. This adds to limited data on the potential impact of oral PrEP on FHT among transwomen.
OALC0602
Hormone levels among transgender women and transgender men in a transgender‐led, integrated, gender‐affirming care and sexual health service at Tangerine Clinic in Bangkok, Thailand: a real‐world analysis
A Hiransuthikul 1,2; R Janamnuaysook1,3; P Getwongsa1; J Peelay1; N Teeratakulpisarn1; M Avery4; S Mills4; R Ramautarsing1; and N Phanuphak1,3
1Institute of HIV Research and Innovation (IHRI), Bangkok, Thailand. 2Chulalongkorn University, Department of Preventive and Social Medicine, Faculty of Medicine, Bangkok, Thailand. 3Center of Excellence in Transgender Health, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. 4FHI 360 and LINKAGES, Bangkok, Thailand
Background: Gender‐affirming hormone therapy (GAHT) is used among many transgender individuals who would like to achieve physical changes – feminizing hormone therapy (FHT) for transgender women and masculinizing hormone therapy (MHT) for transgender men. Without proper monitoring, GAHT can lead to suboptimal effect or put users at risk for adverse events. We aimed to determine hormone levels among transgender women and transgender men who were using GAHT.
Methods: Transgender individuals who were using GAHT at the entry to Tangerine Clinic and tested for hormone levels (blood estradiol [E2] and total testosterone [TT] levels for transgender women; TT for transgender men) between 2015 and 2020 were included. Hormone target levels were E2 of 100 to 200 pg/mL and TT of <50 ng/dL for transgender women; TT of 400 to 700 ng/dL for transgender men. Baseline and available hormone levels during the 12‐month follow‐up period were assessed to determine changes.
Results: A total of 1534 transgender women were included: 2.5% underwent orchiectomy, 70.8% used single‐hormone regimen and 5% were HIV positive. Median E2 and TT levels at baseline were 29 (14.3 to 45.3) pg/mL and 298.5 (22 to 646) ng/dL respectively. A total of 524 (32.2%) had any hormones within target levels: 28 (1.8%), both; 11 (0.7%), only E2 and 485 (31.6%), only TT. HIV status was not associated with the outcome of hormone target levels. Among 302 transgender women who came to follow‐up visit(s), 165 (54.6%) achieved or maintained either hormone within target levels. A total of 200 transgender men were included: none had gender‐affirming surgery and all were HIV negative. Median (IQR) TT levels were 45.5 (32.5 to 531.6) ng/dL, and 141 (70.5%), 26 (13.0%), 33 (16.5%) had suboptimal, optimal and supraphysiological TT levels respectively. Median haematocrit was significantly higher among those with optimal or supraphysiological TT levels compared to those with suboptimal TT levels (46.7 vs. 41.0% and 47.0 vs. 41.0 %, respectively, p < 0.001 in both comparisons), and seven had erythrocytosis. Median high‐density lipoprotein cholesterol (HDL) levels were significantly higher among transgender men with suboptimal TT levels compared to those with supraphysiological levels (61 vs. 50.5 mg/dL, p = 0.02). Among 152 transgender men who came to follow‐up visit(s), 51 (33.6%) achieved or maintained optimal TT levels.
Conclusions: One‐third of transgender women who were using FHT had any hormones within target levels and 13% of transgender men who were using MHT had optimal TT levels in this real‐world analysis. At follow‐up visits, there was an increase in the proportion with optimal hormone levels for both transgender groups, emphasizing a positive effect of supervised GAHT in a transgender‐competent healthcare facility.
OALC0603
Preference for long‐acting injectable pre‐exposure prophylaxis among transgender women clients of the Tangerine Clinic in Bangkok, Thailand
L Himma 1,2; S Siri2; R Janamnuaysook1,3; N Aimyoug2; C Munsawaengsub2; J Kongkapan1; J Rueannak1; P Srimanus1; M Avery4; S Mills4; R Ramautarsing1; and N Phanuphak1,3
1Institute of HIV Research and Innovation (IHRI), Bangkok, Thailand. 2Faculty of Public Health, Mahidol University, Bangkok, Thailand. 3Center of Excellence in Transgender Health (CETH), Chulalongkorn University, Bangkok, Thailand. 4Family Health International 360, Bangkok, Thailand
Background: Long‐acting injectable (LAI) cabotegravir demonstrated superior efficacy to oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) when used as pre‐exposure prophylaxis (PrEP) among men who have sex with men and transgender women (TGW). Alternative PrEP options are crucial for TGW as low uptake and retention have been seen in oral PrEP programmes. We explored the preference of LAI PrEP among Thai TGW clients who had ever used oral PrEP.
Methods: Tangerine Clinic is a trans‐led, integrated gender‐affirming care and sexual health clinic in Bangkok, Thailand. Between August and December 2020, we conducted a cross‐sectional study, recruiting consecutive HIV‐negative TGW clients at Tangerine who reported ever using oral PrEP. Participants completed a self‐administered questionnaire on demographics and risk behaviours, oral PrEP adherence, perceived PrEP benefits and preference for LAI PrEP. Logistic regression analysis was conducted to identify factors associated with LAI PrEP preference.
Results: Of 173 TGW who completed the survey, 94.2% were currently undergoing feminizing hormone therapy (FHT) and 24.2% had undergone gender‐affirming surgery. In the past six months, 86.1% had practiced receptive anal sex, 18.4% receptive neovaginal sex, 26.0% insertive anal sex and 67.2% reported inconsistent condom use. Sexually transmitted infections (STIs) were diagnosed among 30.7% of respondents during the past six months. The median age was 27 (IQR 24 to 29) years. Of all TGW, 76.3% were current PrEP users with a mean of 6.2 PrEP pills (SD = 0.8, min = 4 max = 7) taken per week, whereas 23.7% TGW had already discontinued PrEP. 123 of 166 (74.1%) who responded indicated a preference for LAI PrEP; 74.0% among current PrEP users and 74.3% among those who discontinued PrEP. Perceived benefits of PrEP (aOR 4.4, 95% CI 1.2 to 16.9, p = 0.021) and recent STI diagnosis (aOR 3.8, 95% CI 1.2 to 12.1, p = 0.020) were associated with preference for LAI PrEP.
Conclusions: Around three‐quarters of Thai TGW who had used oral PrEP reported a preference for LAI PrEP. Perceived benefit of PrEP and awareness of risk through STI diagnosis increased LAI PrEP preference. Future studies exploring LAI PrEP acceptability among TGW who have never used PrEP, potential interactions between FHT and LAI PrEP and alternative injection sites for TGW with buttock implants, are urgently needed.
OAC0101
Global adoption of guidelines on and use of oral pre‐exposure prophylaxis (PrEP): current situation and future projections
R Schaefer 1; H‐MA Schmidt1,2; G Ravasi3; A Mozalevskis4; BB Rewari5; F Lule6; K Yeboue7; A Brink8; N Mangadan Konath5; M Sharma5; N Seguy4; J Hermez9; AS Alaama9; N Ishikawa8; B Dongmo Nguimfack1; D Low‐Beer1; R Baggaley1; and S Dalal1
1World Health Organization, Global HIV, Hepatitis and STIs Programmes, Geneva, Switzerland. 2UNAIDS, Asia and the Pacific, Bangkok, Thailand. 3Pan‐American Health Organization, Washington, DC, United States. 4World Health Organization Regional Office for Europe, Copenhagen, Denmark. 5World Health Organization Regional Office for South‐East Asia, New Delhi, India. 6World Health Organization Regional Office for Africa, Brazzaville, Congo. 7World Health Organization Regional Office for Africa, Inter‐Country Support Team, Ouagadougou, Burkina Faso. 8World Health Organization Regional Office for the Western Pacific, Manila, Philippines. 9World Health Organization Regional Office for the Eastern Mediterranean, Cairo, Egypt
Background: With the 2020 global target of three million oral pre‐exposure prophylaxis (PrEP) users set during the UN General Assembly in 2016 at an end, we assessed global trends in the adoption of World Health Organization (WHO) PrEP recommendations into national guidelines and numbers of PrEP users, and estimated future trajectories of PrEP users.
Methods: Data obtained through the Global AIDS Monitoring (GAM) and WHO regional offices were collated to report numbers of PrEP users and WHO PrEP recommendations adoption by the country for 2016 to 2019. To forecast PrEP user numbers until 2023, model countries were selected in each region. PrEP use growth rates observed in these model countries were applied to countries in corresponding regions under different scenarios, including a COVID‐19 disruption scenario with static global PrEP use in 2020.
Results: In 2019, there were 630,000 PrEP users across 76 countries (41% in Americas region and 36% in African region), a 70% increase from 2018. 124 countries had adopted the WHO PrEP recommendations in national guidelines: 35 countries in 2018 and 24 in 2019. Without COVID‐19 disruptions, 1.0 to 1.1 million global PrEP users by the end of 2020 and 2.4 to 5.2 million by 2023 were projected (see Figure 1).
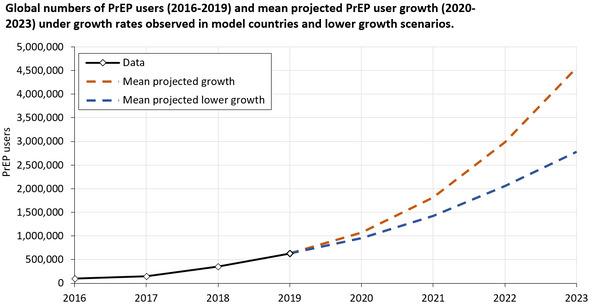
Abstract OAC0101‐Figure 1.
Conclusions: Widespread adoption of WHO PrEP recommendations coincided with a global increase in PrEP use. While the 2020 global PrEP target will be missed, we estimated future growth in PrEP use. In many countries, PrEP user numbers are small relative to numbers of new HIV infections and PrEP recommendations are not implemented at scale, limiting the current impact of PrEP on reducing HIV incidence. New PrEP products could expand the PrEP user base and, together with greater oral PrEP use through simplified delivery, PrEP could make a significant contribution to ending AIDS by 2030.
OAC0102
Early predictors of seroconversion among enrolees in a PrEP programme in Brazil, Mexico and Peru – the IMPREP Demonstration study
CF Caceres 1; KA Konda1; R Moreira2; I Leite2; M Cunha2; B Hoagland2; JV Guanira1; H Vermandere3; H Vega4; B Grinsztejn2; C Pimenta5; V Veloso2; and ImPrEP Study Group
1Universidad Peruana Cayetano Heredia, Center for Interdisciplinary Studies in Sexuality, AIDS and Society, Lima, Peru. 2Oswaldo Cruz Foundation/Fiocruz, Evandro Chagas National Institute of Infectious Diseases, Rio de Janeiro, Brazil. 3National Institute of Public Health, Cuernavaca, Mexico. 4Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, Mexico City, Mexico. 5Ministry of Health of Brazil, Brasilia, Brazil
Background: ImPrEP, a PrEP Demonstration Project for men who have sex with men (MSM) and transwomen in public health facilities in Brazil, Mexico and Peru, started enrolling mid‐2018 and as of December 2020 included 3842, 3005 and 2265 participants respectively. Since PrEP effectiveness requires personal commitment, PrEP programmes should offer additional support to people more likely to seroconvert. In this analysis, we assessed seroconversion in the cohort and modelled its early predictors.
Methods: ImPrEP enrolled consenting, HIV‐negative MSM and transwomen 18+ y.o. reporting recent (within six months): condomless anal sex; HIV positive/unknown status sex partner; STI diagnosis or signs/symptoms; or transactional sex. Enrolled participants received 30‐days of PrEP and returned at one‐month for a safety visit; subsequent prescriptions and visits were quarterly, all visits included HIV testing and behavioural assessments. Adherence was measured using the medication possession ratio (MPR): the number of pills prescribed divided by the days between visits. Anyone who tested HIV‐positive post‐enrolment was withdrawn from the cohort and linked to HIV care. Cox regression was used to identify early predictors of seroconversion, including baseline socio‐demographics and behaviours, plus MPR at the one‐month visit; the multivariate Cox model included all variables with p < 0.1, controlling by gender and country.
Results: Seroconversions by country, population and age group are shown in Table 1 below. In the multivariate Cox model, the risk of seroconversion was associated with being 18 to 24 y.o. (aHR 4.8, 95% CI 2.3 to 10.1), condomless receptive anal sex (aHR 2.0, 95% CI 1.1 to 3.6), MPR of 0.53 to 1 (aHR 2.8, 95% CI 1.5 to 4.9); MPR < 0.53 (aHR 3.7, 95% CI 1.8 to 7.6) and being from Peru (aHR 4.0, 95% CI 2.0 to 7.9), controlling for gender, transactional sex and initial intention to use PrEP.
Abstract OAC0102‐Table 1. Seroconversions by country, population and age group
| Country |
MSM 18 to 24 n [rate (95% CI)] |
TW 18 to 24 n [rate (95% CI)] |
MSM 25+ n [rate (95% CI)] |
TW 25+ n [rate (95% CI)] |
Total n [rate (95% CI)] |
|---|---|---|---|---|---|
| Brazil | 10 [0.87 (0.42 to 1.61)] | 1 [1.94 (0.05 to 10.81)] | 4 [0.1 (0.03 to 0.26)] | 0 [0.00 (0.00 to 2.82)] | 15 [0.28 [0.16 to 0.47)] |
| Mexico | 4 [1.16 (0.32 to 2.97)] | 0 [0.00 (0.00 to 49.92)] | 7 [0.4 (0.16 to 0.82)] | 0 [0.00 (0.00 to 18.01)] | 11 [0.52 (0.26 to 0.92)] |
| Peru | 22 [4.05 (2.54 to 6.13)] | 4 [9.47 (2.58 to 24.24)] | 17 [1.71 (0.99 to 2.73)] | 3 [1.71 (0.35 to 5.00)] | 46 [2.62 (1.92 to 3.49)] |
| Total | 36 [1.77 (1.24 to 2.45)] | 5 [4.94 (1.60 to 11.53)] | 28 [0.42 (0.28 to 0.6)] | 3 [0.92 (0.19 to 2.68)] | 72 [0.79 (0.62 to 0.99)] |
Conclusions: The risk for seroconversion in ImPrEP was associated with younger age (18 to 24), being from Peru, reporting condomless receptive anal sex, and early signs of non‐adherence (low MPR). Strategies are needed to support enrolees with these criteria to remain adherent and prevent seroconversion.
OAC0103
Comparing adherence to HIV Pre‐Exposure Prophylaxis (PrEP) among new, male PrEP users initiating F/TAF versus F/TDF
N Sacks1; B Healey1; L Rusie 2; M Pyra2; D Mezzio3; and J Gruber3
1Precision HEOR, Boston, United States. 2Howard Brown Health, Chicago, United States. 3Gilead Science, Foster City, United States
Background: Pre‐exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate or tenofovir alafenamide (F/TDF or F/TAF) is effective at preventing HIV when used consistently. There are limited real‐world data that compare adherence and seroconversion rates for F/TAF and F/TDF over concurrent timeframes. This study compared adherence measures and HIV incidence in new PrEP users initiating either F/TAF or F/TDF using a real‐world database.
Methods: This retrospective longitudinal analysis used PurpleLab, a nationally representative medical and pharmacy claims database (all U.S. payer types). Eligible users were (recorded as) male adults (≥18 years) with no history of PrEP (variable baseline from 10/1/2015), initiating F/TAF or F/TDF 10/1/2019 to 1/31/2020 (index date) with ≥1 medical claim 30 days prior (for improved linkage to clinical records). Users with evidence of HIV/HepB treatment or ≥1 HIV/HepB diagnosis (−12 months to +30 days of index date) were excluded. Included users were followed for ≥240 days. Outcomes included: proportion of days covered (PDC), adherence (PDC ≥ 0.8) and seroconversion (≥1 claim with HIV diagnosis). Differences in outcomes were assessed using Chi‐square and T‐tests. Multivariable logistic regression estimated the effect of PrEP regimen on adherence, controlling for age group, geography and type of insurance.
Results: A total of 1113 F/TAF and 1961 F/TDF users met study criteria. Adherence dropped over time for both regimens; F/TAF users had significantly higher mean PDC and adherence (PDC ≥ 0.8) at all time points (Table 1). In multivariable analyses, F/TAF users had 1.67 higher odds of adherence (PDC ≥ 0.8) relative to F/TDF (180 days; p < 0.0001); adherence odds also increased with older age, private insurance. Differences between seroconversion rates (F/TAF: 1.24/100 person‐years; F/TDF: 1.80/100 person‐years) were not significant (p>0.05).
Abstract OAC0103‐Table 1. Adherence measures over time, by PrEP regimen
| Regimen |
Mean PDC (180 d) |
Mean PDC (210 d) |
Mean PDC (240 d) |
Adherence (180d) |
Adherence (210d) |
Adherence (240d) |
|---|---|---|---|---|---|---|
| F/TAF (n = 1113) | 0.65** | 0.62** | 0.58** | 47%** | 44%** | 36%** |
| F/TDF (n = 1961) | 0.57 | 0.53 | 0.50 | 34% | 31% | 23% |
PDC, proportion of days covered; Adherence, proportion of users with PDC ≥0.8; d, days of follow‐up.
**Differences significant at p < 0.0001 comparing F/TAF to F/TDF over follow‐up period.
Conclusions: These results are the first to compare adherence to F/TDF and F/TAF over concurrent timeframes, using real‐world data. F/TAF users had higher levels of adherence, compared to F/TDF users over the same period. Overall seroconversion rates were low for both F/TDF and F/TAF; the study did not have sufficient power to detect differences between the two cohorts.
OAC0104
Variation in preferences for long‐acting injectable PrEP among US men who have sex with men: a latent class analysis
SW Beckham 1; S Hyman2; T Sanchez3; M Zlotorzynska3; M Rai3; P Sullivan3; V Vannappagari4,5; S Sarkar4; A Rinehart4; K Rawlings4; and JF Bridges2
1Johns Hopkins Bloomberg School of Public Health, Health, Behavior and Society, Baltimore, United States. 2Ohio State University, College of Medicine, Columbus, United States. 3Emory University Rollin School of Public Health, Epidemiology, Atlanta, United States. 4ViiV Healthcare, Research Triangle Park, United States. 5University of North Carolina‐Chapel Hill, Chapel Hill, United States
Background: Cabotegravir long‐acting injectable HIV pre‐exposure prophylaxis (LAI‐PrEP) is shown to be safe and efficacious. Understanding variations in potential user preferences for LAI‐PrEP may be useful to inform segmented implementation strategies, and subsequently improve uptake and thus community‐level effectiveness.
Methods: HIV negative, sexually active men who have sex with men (MSM) aged ≥15 living in the United States were recruited online for the 2019 American Men’s Internet Survey. They completed a discrete‐choice experiment with nine paired profiles with hypothetical LAI‐PrEP attributes: out‐of‐pocket cost, perceived side effects, injection frequency, perceived stigma and service location. Latent class analysis was used to segment respondents into groups based on their preferences for the hypothetical attributes presented and relative importance of preference weights and willingness‐to‐pay were calculated. Finally, associations with group membership based on sociodemographic characteristics and sexual risk behaviour were tested using logistic regression.
Results: Two latent classes emerged from 2206 respondents. “Value‐conscious” respondents (30%) exhibited strong dislike for higher out‐of‐pocket cost. The cost was 2.5 times more important than frequency and perceived side effects. “Outcomes‐conscious” respondents (70%) exhibited a strong dislike for perceived severe side effects. Their dislike for severe side effects was 3.2 times more important than cost. Neither group ascribed importance to service location nor stigma. Value‐conscious respondents were significantly younger (mean [IQR]: 29.1 [21 to 33] vs. 31.3 [22 to 37] years, chi‐2 p < 0.000), more likely to be racial/ethnic minorities, less educated, live in rural areas, and have public or no insurance. Outcome‐conscious respondents were marginally more likely to have ever used oral PrEP and were significantly less likely to have had condomless anal sex with an HIV‐serodiscordant partner in the past year or to have only casual partners. Willingness‐to‐pay analysis demonstrated that the outcomes‐conscious class would pay $354 to avoid side effects (vs. $32 in the value‐conscious class).
Conclusions: Perceived side effects emerged as an important barrier for the uptake of hypothetical LAI‐PrEP for a large proportion of potential MSM users. Minimizing out‐of‐pocket costs is likely to increase uptake, especially among younger racial minority groups, and is important to equitable access. Tailored communication strategies are recommended for the two different groups of potential LAI‐PrEP users.
OAC0105
Estimated long‐acting PrEP effectiveness in the HPTN 084 cohort using a model‐based HIV incidence in the absence of PrEP
M Moore 1; DJ Donnell1; M‐C Boily2; KM Mitchell1; S Delany‐Moretlwe3; M Hosseinipour4; JP Hughes1; B Hanscom1; and D Dimitrov1
1Fred Hutchinson Cancer Research Center, Vaccine and Infectious Disease, Seattle, United States. 2Imperial College London, Infectious Disease Epidemiology, London, United Kingdom. 3University of Witwatersrand, Wits RHI, Johannesburg, South Africa. 4University of North Carolina School of Medicine, Chapel Hill, United States
Background: HPTN 084 is a randomized double‐blind controlled‐superiority study assessing the safety and efficacy of long‐acting injectable cabotegravir for pre‐exposure prophylaxis (CAB‐LA) for preventing HIV in African women aged 18 to 45 years. Daily oral tenofovir disoproxil fumarate and emtricitabine (TDF/FTC) was an active comparator; there was no placebo control. We estimate the incidence in a hypothetical placebo control arm and project the effectiveness of CAB‐LA compared to placebo.
Methods: Our model‐based counterfactual predicts HIV risk in cohorts of sub‐Saharan Africa (SSA) women based on individual VOICE risk scores and HIV incidence, prevalence and viral load suppression among adult males in the communities of each trial site. HIV risk is used to predict cumulative HIV incidence over one year of follow‐up. This model was calibrated to data from the VOICE trial and previously validated by comparing predicted HIV incidence to that observed in HPTN 035, FEM‐PrEP, ASPIRE and ECHO.
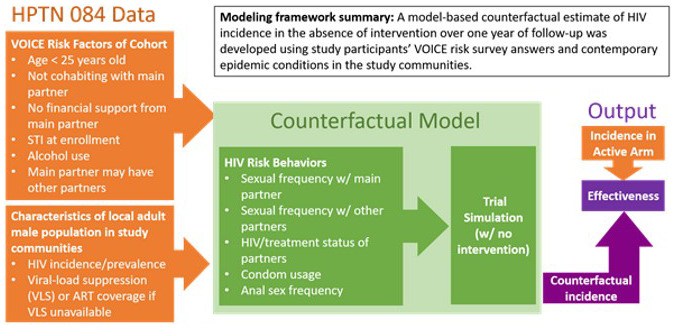
Abstract OAC0105‐Figure 1.
Results: Overall, we project a counterfactual placebo incidence of 2.2% (95% cred. int. 1.7% ‐ 2.8%) in the HPTN 084 study cohort compared to 0.2% (95% conf. int. 0.06% to 0.52%) in the CAB‐LA arm and 1.86% (95% conf. int. 1.3 to 2.57) in the TDF/FTC arm, suggesting an effectiveness against HIV acquisition of 91% (95% cred. int. 76% to 97%) and 15% (95% cred. int. ‐26%‐44%), respectively, compared to placebo.
Conclusions: For ethical reasons effectiveness of new HIV prevention products, such as CAB‐LA, must be compared to an approved PrEP product such as TDF/FTC, whose effectiveness depends on adherence. Counterfactual estimates of incidence allow for the comparison of such products against a hypothetical placebo control. This model‐based counterfactual, using contemporary epidemic data and participant risk factors, provides additional assurance that CAB‐LA reduced HIV acquisition risk by 90% among women in SSA. This effectiveness estimate can be further refined and validated with additional counterfactuals using other data and methodologies.
OAC0201
SARS CoV‐2 seroprevalence among HIV‐negative participants using tenofovir/emtricitabine‐based PrEP in 2020 – a sub‐study of PREVENIR‐ANRS and SAPRIS‐Sero
C Delaugerre 1; L Assoumou2; S Maylin3; M Minier3; A Gabassi3; M Genin2; L Beniguel2; J Ghosn4; X de lamballerie5; M El Mouhebb2; D Costagliola2; F Carrat2; J‐M Molina6; and PREVENIR study Group
1Hôpital Saint Louis, Université de Paris, Virologie, Paris, France. 2Institut Pierre Louis d'Epidémiologie et de Santé Publique, Sorbonne Université, Paris, France. 3Hôpital Saint Louis, Virologie, Paris, France. 4Hôpital Bichat, Université de Paris, Maladies Infectieuses, Paris, France. 5Unité des Virus Emergents, INSERM, Marseille, France. 6Hôpital Saint Louis – Université de Paris, Maladies Infectieuses, Paris, France
Background: Tenofovir (TDF) has shown activity on the SARS‐CoV‐2 RdR polymerase in vitro and in ferrets. There is controversy regarding the potential benefit of TDF to reduce SARS‐CoV‐2 infection or COVID‐19‐related morbidity. Our objective was to compare the seroprevalence rates of SARS CoV‐2 IgG among male participants using TDF/FTC‐based PrEP and matched controls.
Methods: Male participants from the PREVENIR study conducted in Ile de France, who are receiving on demand or daily PrEP and with an available sample between May and October 2020 were included in this study. The Sapris‐Sero study was a sub‐study of the national Sapris cohort, with several wave of sampling from May 2020 after the end of the March‐May lockdown. Male participants of the SAPRIS‐Sero study living in Ile de France were matched to each PREVENIR participant on age (±5 years), socio‐occupational category and date of sampling (±1 month). Odds ratio (OR) of the comparison between the two studies was calculated using stratified logistic regression. SARS‐CoV2 IgG anti S was measured using the Abbott SARS‐CoV‐2 IgG II Quant antibody test in PREVENIR and the Euroimmuns Anti‐SARS‐CoV‐2 ELISA IgG test in SAPRIS‐Sero.
Results: In PREVENIR, 844 participants with a median (IQR) age of 38 (31 to 45) years were matched to 844 participants of SAPRIS‐Sero cohort, aged of 41 (35 to 48) years. Matching was possible on the three variables for 729 participants. PrEP was on demand in 420 (49.8%) and daily in 424 (50.2%) individuals. For PREVENIR and SAPRIS‐Sero cohorts, SARS CoV‐2 IgG was negative in 753 (89.2%) and 738 (87.4%) subjects, low positive/undetermined in 4 (0.5%) and 28 (3.3%) and positive in 87 (10.3%) and 78 (9.2%) respectively. Considering low positive /undetermined as negative, OR was estimated as 1.13 (0.82 to 1.53). Sensitivity analyses (restricted to full matched samples and/or considering undetermined as positive) led to similar results.
Conclusions: Prevalence of SARS‐CoV‐2 IgG was similar in PrEP users and in a matched cohort in the Paris region after the COVID lockdown suggesting that TDF/FTC has no role in reducing SARS‐CoV‐2 acquisition.
OAC0202
Demand creation and HIV self‐testing delivery during COVID‐19 contingency measures of physical distancing among adolescents’ key population enrolled in PrEP in Brazil
L Magno 1,2; D Medeiros3; F Soares2; C Jefferson2; FM Duarte2; A Grangeiro4; and I Dourado2
1Universidade do Estado da Bahia, Departamento de Ciências da Vida, Salvador, Brazil. 2Universidade Federal da Bahia, Instituto de Saúde Coletiva, Salvador, Brazil. 3Universidade Federal da Bahia, Instituto Multidisciplinar em Saúde, Vitória da Conquista, Brazil. 4Universidade de São Paulo, Departamento de Medicina Preventiva, São Paulo, Brazil
Background: HIV self‐testing (HIVST) helps to prevent disruptions in the HIV testing services during COVID‐19 pandemic especially among adolescent’s key population (AKP) of men who have sex with men and transgender women. We aimed to analyse HIVST distribution to AKP in PrEP1519 cohort as part of the COVID‐19 contingency plan.
Description: Data are from the first PrEP demonstration cohort study among AKP aged 15 to 19 years old ongoing in three large Brazilian cities (PrEP1519). During physical distancing measures (PDM), started in March 2019 in Brazil, demand creation via social media was intensified and an HIVST delivery strategy was adopted in PrEP1519 clinics. A package with an HIVST and guidance was made available for AKP to be pick‐up at the clinics or by delivery services/mail to their preferred addresses. Video guidance was developed and publicized in PrEP1519 Instagram and YouTube. This analysis reports the findings comparing the pre‐pandemic (March 2019‐March 15th 2020) and pandemic (March 16th‐December 2020) periods. Bivariate analysis and chi‐square test were used to test differences in the HIVST distribution.
Lessons learned: In total, 1597 HIVST were delivered, 39.8% and 60.2% during the pre‐pandemic and pandemic periods respectively. The number of requests increased 86% during the pandemic, and 16% of these were delivered to home addresses. Among AKP who requested an HIVST before March 15th 2020, 27.1% reported private use, 24.0% gave to a sexual partner and 48.9% gave to a friend. COVID‐19 pandemic changed these patterns: 78.7% reported private use, 8.9% gave to a sexual partner and 12.3% gave to a friend (p < 0.001). Before the pandemic, most AKP used an HIVST because of condomless sex (34.0%) or as a checkup (34.0%). During PDM, condomless sex was reported by 49.6% as the main reason for self‐testing.
Conclusions/Next steps: HIVST requests among Brazilians AKP in PrEP1519 cohort increased significantly. Demand creation via social media made it possible to reach more AKP and will be sustained even after PDM restrictions are lifted. These experiences offer important lessons for other middle‐income countries, as well as other countries scaling up PrEP.
OAC0203
Real‐world utilization of F/TDF and F/TAF for HIV Pre‐Exposure Prophylaxis during the COVID‐19 pandemic in the United States, December 2019 – June 2020
L Tao; C Carter; M Das; V Shvachko; and D Magnuson
Gilead Sciences, Inc, Foster City, United States
Background: Extensive impact of the COVID‐19 pandemic on healthcare delivery has been reported, including impacts on the use of daily F/TDF and F/TAF for HIV pre‐exposure prophylaxis (PrEP). Using a real‐world claims database, we evaluated the utilization of PrEP in the United States (US) from December 2019 to June 2020.
Methods: HIV‐1‐negative individuals in the US who used F/TDF and F/TAF for PrEP between December 1, 2019 and June 31, 2020 were identified from a prescription claims database. We conducted a retrospective descriptive trend analysis.
Results: Over 46,000 individuals initiated F/TAF (median age 36 years, interquartile range, [IQR] 29−48) and 29,000 initiated F/TDF for PrEP (median age 31 years, IQR 25−40) between December 2019 and June 2020. Progressive drops in PrEP initiation were observed in February, March and April 2020, coinciding with the start of COVID‐19 spread in the United States and the resulting restrictions (Figure 1A). The overall number of PrEP users showed only a slight decrease after March 2020, mostly attributable to the attenuated increasing trend of F/TAF users after April (Figure 1B). PrEP initiation started to increase again after April, with more new PrEP prescriptions coming from family physicians and nurse practitioners/physician assistants and less from infectious disease physicians. Decreases in new PrEP use were seen in multiple geographic areas and across race/ethnicity groups, with decreases most pronounced in white individuals living in the Southern US states (Figure 1C).
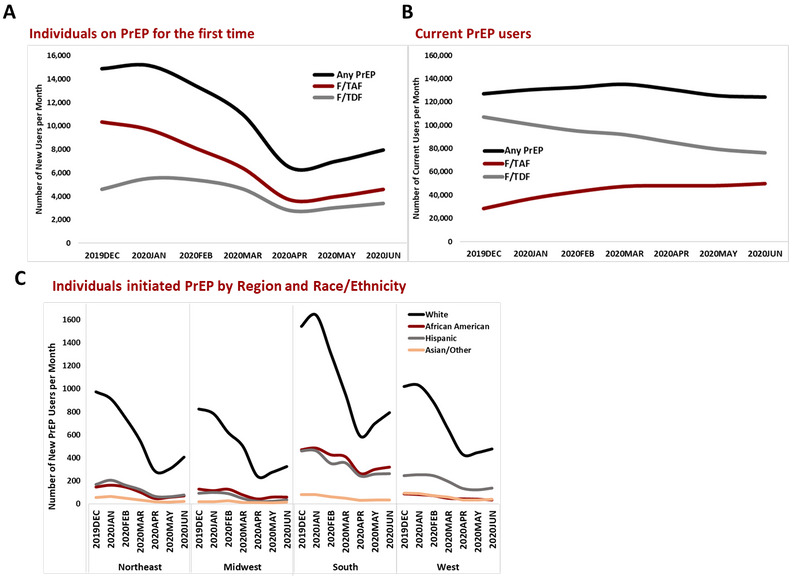
Abstract OAC0203‐Figure 1.
Conclusions: This real‐world analysis demonstrates how prescription claims data can be utilized to track PrEP use amidst the significant impact of the COVID‐19 pandemic. Our findings suggest that COVID‐19 has had a substantial impact on PrEP initiations, while having a lesser impact on overall use. These findings suggest that targeted efforts will be needed to provide PrEP to new users during and after the pandemic.
OAC0204
Going online to ensure uninterrupted HIV services during COVID‐19 in Nepal
K Bam 1; B Eveslage2; M Cassell3; A Sharma1; YR Sapkota1; PK Thakur2; RP Khanal1; and B Shrestha1
1EpiC Nepal/FHI 360, Kathmandu, Nepal. 2EpiC/FHI 360, Washington, DC, United States. 3EpiC/FHI 360, Hanoi, Vietnam
Background: In Nepal, the COVID‐19 pandemic placed substantial pressure on HIV programmes to adapt to new physical distancing policies and restrictions in movement that limited people from accessing HIV services in person. Building upon five years of online HIV service delivery, the PEPFAR‐ and USAID‐supported LINKAGES and EpiC programmes in Nepal amplified the government and community partners’ online efforts to ensure safe and sustained HIV service access during the COVID‐19 pandemic.
Description: From March to October 2020, EpiC Nepal supported a dramatic shift toward virtual client support and online HIV service access. New devices and mobile data packages were procured for outreach workers and community‐based supporters to maintain contact with key populations and people living with HIV (PLHIV). Virtual and online support was also facilitated by the development and dissemination of new key messages on HIV and COVID‐19 prevention, care and treatment. EpiC upgraded the project’s online reservation application, available at www.merosathi.net, with additional services available for booking and a new programme‐facing case management system. Several popular social media influencers were mobilized as “virtual peer champions” to promote the benefits of HIV treatment and care, including through the newly available virtual or online channels.
Lessons learned: From March to October 2020, 40,230 individuals received information on HIV and COVID‐19 prevention virtually – a rate five times higher than in July 2019 to February 2020 period. A total of 11,744 PLHIV were reached virtually for treatment adherence support, monitoring and education, which was not done before the COVID‐19 pandemic; 906 individuals tested for HIV and 141 were diagnosed with HIV from online approaches. Among all the individuals (906) who were linked through online engagement to off‐line HIV testing services, 16 percent received positive results – a rate three times higher than in the July 2019 to February 2020 period, when 612 individuals were tested and 31 individuals were diagnosed with HIV.
Conclusions/Next steps: Our findings suggest that online approaches provided continued service access for key populations and PLHIV. Institutionalizing virtual solutions in Nepal helped safeguard gains made in the HIV response from the ongoing COVID‐19‐related service disruptions and other future threats. Our experience helped facilitate the formal adoption of online interventions in Nepal.
OAC0205
Uptake of oral pre‐exposure prophylaxis for HIV infection among men who have sex with men and transgender: lessons learned during the SARS‐CoV‐2 pandemic from the first PrEP project in Myanmar
NN Tun 1,2; T Gils3; L Lynen3; M Min2; MY Aung1; F Smithuis4,5; K Gustafson6; and E Florence3
1Medical Action Myanmar, HIV, Yangon, Myanmar. 2Myanmar Oxford Clinical Research Unit, HIV, Yangon, Myanmar. 3Institute of Tropical Medicine, Clinical Science, Antwerp, Belgium. 4Medical Action Myanmar, director, Yangon, Myanmar. 5Myanmar Oxford Clinical Research Unit, Country director, Yangon, Myanmar. 6USAID Burma, HIV, Yangon, Myanmar
Background: HIV is concentrated among key populations in Myanmar. The first HIV daily pre‐exposure prophylaxis (PrEP) programme was initiated for men who have sex with men (MSM) and transgender (TG) by the medical organization Medical Action Myanmar (MAM). Event‐driven PrEP was not yet sanctioned. We assessed the uptake of daily PrEP and HIV‐seroconversion. A lockdown following increasing SARS‐CoV‐2‐cases required programmatic changes. We draw lessons learned from early findings.
Description: MAM estimated to enrol 200 HIV‐negative MSM/TG on daily PrEP in six months in Yangon. Community‐based peer educators (CBPE) raised awareness and provided counselling. Enrolled PrEP‐users should visit the clinic after one and three months and three‐monthly thereafter for HIV/STI screening. Enrolment started July 31, 2020 and SARS‐CoV‐2‐related stay‐at‐home measures were imposed on September 1. MAM swiftly adapted its strategy; CBPEs received support to use social media platforms and smartphones with a PrEP‐appointment application. A PrEP‐promotion webpage was launched. COVID precaution measures were implemented at the clinic. Counselling and history taking were done by phone and face‐to‐face appointments for drug re‐supplies and HIV/STI‐testing were made flexible.
Lessons learned: Of 695 eligible MSM/TG 243 (37%) were enrolled, 224 (92%) MSM, and 19 (8%) TG. The median age was 23 years (interquartile range: 20 to 28). Among 452 eligible MSM/TG who refused PrEP, 373 (83%) did so because they did not want to use daily PrEP, especially because social interaction was limited during the lockdown. In total, 487 phone consultations and 213 face‐to‐face consultations were done. Face‐to‐face client‐staff contact time was reduced from 45 to 15 minutes. Among enrolled PrEP‐clients, two (1%) had HIV‐seroconversion. One tested indeterminate at month 1 and positive when the test was repeated. The other person stopped taking PrEP after three months and tested positive on return (month 5).
Conclusions/Next steps: The number of MSM and TG who inquired about PrEP was very high despite the SARS‐CoV‐2 lockdown. The use of social media and the webpage might have facilitated this. The number of MSM/TG enrolled was substantial but most eligible MSM/TSG refused PrEP because of the daily pill burden as sexual contact was infrequent. Event‐driven PrEP could fill this important unmet need. Qualitative data could help understand barriers.
OAC0206
Combination HIV/HCV/HBV/STIs prevention among MSM and use of mobile applications/social networks at the COVID‐19 conditions in Ukraine
A Chernyshev 1; A Bogoslavets2; and R Marchenko3
1Alliance.Global, Public Organization, Advocacy & External Communication Department, Kyiv, Ukraine. 2Alliance.Global, Public Organization, Program Department, Kyiv, Ukraine. 3Alliance.Global, Public Organization, Program Coordinator, Kyiv, Ukraine
Background: The COVID‐19 pandemic and restrictions on displacement have made it more difficult to provide HIV testing and PrEP services to MSM in large Ukrainian cities. The purpose of the intervention is to maintain the effectiveness of the provision of these services during COVID‐19 and to implement new and innovative interventions in this regard, making the services more mobile.
Description: The methodology consists of conducting two national campaigns aimed at MSM recruiting in three largest cities of Ukraine, for conducting HIV/STI/HCV testing, as well as to attract to the PrEP programme, through targeted advertising on gay dating applications, search engines and in social networks for directing to web resources https://gettest.com.ua and https://prep.com.ua, for passing of testing and/or to receive equal counselling on PrEP by appointment, at Alliance.Global’s testing points. All MSM who have been positive result of HIV test, have been provided a social support to receive ART and be involved to the PLHIV/MSM support programme. For HIV‐negative MSM, we offer to become a member of the free PrEP programme and provide of bonuses.
Lessons learned: Thanks to advertising on two web resources, during September‐December 2020, 109,797 users visited the GetTest website, of which 841 MSM registered for HIV/HCV/STI testing and 80% of them were tested (the number of HIV‐positive results was approximately 4%). A total of 3244 MSM have learned about the PrEP programme during this period, approximately 300 new MSM have been attracted to the PrEP programme, in particular through bonuses and innovations such as taxi delivery, etc. (the coverage of the PrEP programme in three cities in 2020 amounted about 1300 MSM).
Conclusions/Next steps: Thanks to the introduction of two advertising campaigns on the Internet, in the conditions of lockdown and COVID‐19 pandemic, as well as such innovative interventions as delivery of clients by taxi to receive PrEP in a medical institution, receiving the free premium accounts in the mobile gay application Hornet, compliance with sanitary norms (free masks, disinfectants, etc.) and mandatory pre‐registration (to avoid queues), we were able to successfully provide services, and increase the intensity of testing and staging to the PrEP for MSM compared to the first half of 2020.
OAC0301
Outcomes of the Pride Plus Project for MSM/TW in Peru: a combination HIV intervention across the cascade of HIV prevention and care
KA Konda 1,2; CF Caceres2; A Maiorana3; DF Leon4; A Silva‐Santisteban2; GM Calvo2; E Lugo2; W Hamasaki2; L Pollack3; and S Kegeles3
1University of California, Los Angeles, Infectious Diseases, Los Angeles, United States. 2Universidad Peruana Cayetano Heredia, CIISSS, Lima, Peru. 3University of California, San Francisco, San Francisco, United States. 4Universidad Peruana Cayetano Heredia, CIISSS, lima, Peru
Background: Comprehensive HIV prevention and care strategies focused on MSM and transwomen (TW) in Peru remain elusive. We conducted a trial of a combination prevention strategy including establishing a community centre led by MSM/TW for MSM/TW, small group training for MSM/TW, healthcare provider (HCP) training and navigation for MSM/TW living with HIV.
Methods: In 2017 a cohort of MSM/TW was recruited in a southern neighbourhood of Lima to assess intervention outcomes along the HIV prevention and care cascade. One baseline and three yearly follow‐up assessments were planned (including anal swab NAAT testing for chlamydia/gonorrhoea), but a strict lockdown in response to the epidemic of COVID‐19 precluded follow‐up 3. From June 2017 to July 2020 a community centre for MSM/TW was active with trainings and social activities. HCP trainings were conducted in local hospitals and navigators supported MSM/TW living with HIV. To assess the intervention effect, the baseline and two‐year follow‐up assessments were compared using two‐sided Fisher’s exact tests; modelling is currently underway.
Results: The community centre successfully engaged MSM/TW leading to an ongoing leadership core group of 25 individuals, small group sessions with 120 MSM and 76 TW, and weekly social and educational activities. Among the assessment cohort, HIV testing increased from 51% at baseline to 65% at the second follow‐up, (p = 0.005). Engagement in HIV care increased among cohort participants living with HIV with 46% reporting an undetectable viral load and being ART‐adherent at baseline, compared to 59% at the final follow‐up (p = 0.010). However sexual behaviour was not modified (all p >0.05) and asymptomatic STIs increased (p = 0.023).
Abstract OAC0301‐Table 1. Outcome measures among a community‐based cohort of MSM and transwomen before and after a combination HIV prevention and care intervention
| Outcome | Baseline | Final follow‐up | p‐value |
|---|---|---|---|
| HIV Tested past 6 months (non‐positives at assessment) | 51.0% | 65.3% | 0.005 |
| Undetectable & ART‐adherent (HIV positives) | 45.5% | 59.0% | 0.010 |
| Success (above two outcomes combined) | 49.7% | 59.8% | 0.045 |
| Positive for Chlamydia/Gonorrhoea from rectal swabs | 17.2% | 27.4% | 0.023 |
| Unprotected anal intercourse | 37.6% | 29.9% | 0.114 |
| Condomless anal int. (total sample) b | 49.0% | 47.0% | 0.765 |
Condomless intercourse with participant mitigation (e.g. serosorting, strategic positioning, ART) is counted as a “NO”.
Any condomless act, regardless of circumstance is counted as a “YES”.
Conclusions: The Pride Plus Project yielded significant gains in HIV testing for HIV negatives and engagement in HIV care among MSM/TW living with HIV, the combined outcome of interest for this study. However, sexual behaviour remained unchanged and asymptomatic STIs increased. Further analysis of these results is ongoing.
OAC0302
HIV pattern and cascade of care among incarcerated people in Iran: Findings of three consecutive national bio‐behavioural surveillance surveys
A Shahesmaeili 1; M Shokouhi1,2,3; A Mirzazadeh1,4; S Amirzadeh5; Z Abdolahinia1; F Tavakoli1; S Hosseini Hooshyar1,6; N Ghalekhani1; R Khajehkazemi1; N Fahimfar7; M Karamouzian1,8; and H Sharifi1
1HIV/STI Surveillance Research Center, and WHO Collaborating Center for HIV Surveillance, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran, Islamic Republic of. 2Schulich School of Medicine & Dentistry, The University of Western Ontario, Department of Epidemiology & Biostatistics, London, Canada. 3Division of Social and Behavioural Health Sciences, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada. 4University of California San Francisco, Department of Epidemiology and Biostatistics, San Francisco, United States. 5Social Determinants of Health Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran, Islamic Republic of. 6The Kirby Institute, UNSW Sydney, Sydney, Australia. 7Osteoporosis Research Center, Endocrinology & Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran, Islamic Republic of. 8School of Population and Public Health, Faculty of Medicine, University of British Columbia, Vancouver, Canada
Background: In Iran, HIV outbreak was first noticed among incarcerated population in the mid‐1990s, who mostly were infected through the use of shared needles for drug injection. We evaluated the prevalence and pattern of HIV and status of HIV care cascade among incarcerated people in Iran from 2010 to 2017.
Methods: Data were obtained from three consecutive national bio‐behavioural surveillance surveys in 2010 (N = 4536), 2013 (N = 5490) and 2017 (N = 5785) through a multistage cluster sampling in Iran. HIV was tested using the ELISA method in the two first rounds and a rapid test in the third round. Information on demographic characteristics, risky behaviours and HIV testing and treatment was collected using face‐to‐face interviews. The viral suppression status was evaluated just among incarcerated people living with HIV who consented to be tested (N = 20).
Results: The overall prevalence of HIV was decreasing. The prevalence was estimated at 2.1% (95% CI: 1.2%, 3.6%) in 2010, 1.7% (95% CI: 1.3%, 2.1%) in 2013 and 0.8% (95% CI: 0.6, 1.1) in 2017 (trend p < 0.001). Among incarcerated people with a history of injection drug use, the HIV prevalence was estimated at 8.1% (95% CI: 4.6%, 13.7%) in 2010, 6.3 (95% CI: 4.8, 8.3) in 2013 and 3.9% (95% CI: 2.7, 5.7) in 2017. Based on the 2017 data, 32 out of 50 HIV‐positive incarcerated people (64%) were aware of their HIV status. Overall, nine out of 20 cases (45%) who tested for viral suppression and knew their HIV status were currently on ART of whom 44 % (n = 4) reached the viral suppression load.
Conclusions: Despite the decreasing pattern of HIV among Iranian incarcerated people, engagement in treatment and virologic suppression is low. More efforts are needed for incarcerated people living with HIV to be linked in and retain HIV care and treatment programmes.
OAC0303
HIV risk behaviours among retail pharmacy clients seeking sexual and reproductive health services in Kenya
M Asewe 1; V Omollo1; P Mogere2; K Oware1; J Odoyo1; Z Kwena1; JM Baeten3; K Ngure2; EA Bukusi1; and KF Ortblad4
1Kenya Medical Research Institute, Center for Microbiology Research, Kisumu, Kenya. 2Kenya Medical Research Institute, Center for Clinical Research, Thika, Kenya. 3Gilead Sciences, Foster, United States. 4University of Washington, Global Health, Seattle, United States
Background: The delivery of pre‐exposure prophylaxis (PrEP) for HIV prevention at retail pharmacies in Kenya may help overcome barriers (e.g. long wait times, stigma) to clinic‐delivered PrEP and reach individuals that do not regularly seek clinic‐based services. To understand the potential for this novel model of PrEP delivery, we evaluated HIV risk behaviour among pharmacy clients seeking sexual and reproductive health (SRH) services in Kenya.
Methods: At four retail pharmacies in Kisumu and Thika, Kenya, willing clients seeking SRH services (e.g. family planning) were screened for PrEP eligibility as part of a pharmacy‐based PrEP delivery pilot. To help determine eligibility, we used Kenya’s PrEP Rapid Assessment Screening Tool (RAST) routinely used in public HIV comprehensive care clinics. In the RAST, clients report their HIV status and that of their sexual partner(s) as well as a number of behaviours associated with HIV risk, including condom use, engagement in transactional sex and post‐exposure prophylaxis (PEP) use. We reported findings using descriptive statistics.
Results: From November 2020 to February 2021, 227 pharmacy clients completed the RAST to determine PrEP eligibility. Many clients sought contraceptive services (e.g. oral or emergency contraception) (29%) or PrEP (22%). Other services sought included pregnancy testing (8%), sexual performance‐enhancing drugs (8%) or HIV self‐testing (5%). The majority (80%) of clients reported some behaviour associated with HIV acquisition risk. Over half of clients (55%) reported inconsistent condom use, more than half (51%) reported not knowing the HIV status of their sexual partner(s), and almost a third (28%) reported multiple sex partners. Less commonly reported HIV associated risk behaviours included: sex under the influence of alcohol (11%), recurrent PEP use (4%), an STI in the past six months (4%), transactional sex (4%) and sex with partner(s) living with HIV (4%).
Conclusions: The prevalence of behaviours associated with HIV risk was high among clients accessing SRH services at retail pharmacies in Kenya. These findings suggest that the delivery of PrEP at retail pharmacies has great potential to expand the reach of PrEP to populations at HIV risk in Kenya and similar settings.
OAC0304
Key population lay providers can successfully link men who have sex with men clients to care after diagnosing sexually transmitted infections in community‐based organizations in Thailand
N Thammajaruk 1; S Chumseng1; O Nampaisan1; N Photisan1; J Peelay1; P Srivachiraroj1; S Promthong1; A Phunkron2; T Sangpasert3; S Sangtong4; P Brutrat5; M Avery6; S Mills6; P Phanuphak1; N Phanuphak1; and RA Ramautarsing1
1Institute of HIV Research and Innovation (IHRI), Bangkok, Thailand. 2Service Workers In Group Foundation, Bangkok, Thailand. 3Rainbow Sky Association of Thailand, Bangkok, Thailand. 4Mplus Foundation, Chiang Mai, Thailand. 5Service Workers In Group Foundation, Pattaya, Chonburi, Thailand. 6FHI 360, Bangkok, Thailand
Background: Through the key‐population‐led health services (KPLHS) model, trained key population (KP) lay providers deliver comprehensive HIV‐related services in community‐based organizations (CBOs) in Thailand. To increase access to services for sexually transmitted infections (STIs), we integrated point‐of‐care (POC) GeneXpert testing for chlamydia (CT) and gonorrhoea (NG) into KPLHS in four CBOs in Thailand. Here we assess rates of successful links to treatment after diagnosis.
Methods: POC STI testing was integrated into KPLHS in August 2019. Trained KP lay providers collected and tested single participant pooled urine, pharyngeal and rectal samples for CT/NG using the GeneXpert assay at the CBOs, and plasma was tested with rapid treponemal test and RPR test for syphilis serology. When an STI was detected, the client was assisted to treatment services by CBO care and support staff to healthcare facilities with which referral routes (including the acceptance of test results from the CBOs, thereby eliminating the need for repeat testing) were previously established. Successful linkage was assessed by follow‐up phone call, and the number of days between diagnosis and treatment was calculated.
Results: Between August 2019 and July 2020, 1008 participants (875 MSM [86.8%] and 133 TGW [13.2%]) were recruited. Among MSM, CT/NG/syphilis events were detected in 240/1029 (23.3%)/174/953 (18.3%)/98/961 (10.2%), of whom 206/239 (86.2%)/139/172 (80.8%)/82/98 (83.7%) successfully received treatment. Median (interquartile range‐IQR) days between diagnosis and treatment was 4 (1 to 10)/6 (2 to 15)/4 (2 to 7). Among TGW, CT/NG/syphilis events were detected in 33/155 (21.3%)/18/138 (13%)/16/140 (11.4%), of whom 22/33 (66.7%)/12/18 (66.7%)/7/16 (43.8%) successfully received treatment. Median (interquartile range‐IQR) days between diagnosis and treatment was 4 (1.5 to 11)/4 (3 to 5)/4 (2 to 6).
Conclusions: Integration of CT/NG testing into KPLHS to increase access to STI services among KPs resulted in significant STI diagnoses, with high rates of successful linkage to treatment services among MSM. Rates among TGW were lower, indicating tailored strategies to link this population to care are urgently needed. Despite the acceptance of CBO test results by referral facilities, time from diagnosis until treatment completion was long. Time to treatment should be further optimized by exploring methods to expedite treatment, such as fast‐track referral options or the integration of treatment services at CBOs to facilitate same‐day treatment.
OAC0305
Acceptability and satisfaction of self‐collection for chlamydia and gonorrhoea testing among transgender women in the Tangerine Clinic, Thailand: shifting toward the new normal
A Hiransuthikul 1,2; R Janamnuaysook1,3; L Himmad1; C Taya1; N Teeratakulpisarn1; N Thammajaruk1; M Avery4; S Mills4; R Ramautarsing1; and N Phanuphak1,3
1Institute of HIV Research and Innovation (IHRI), Bangkok, Thailand. 2Chulalongkorn University, Department of Preventive and Social Medicine, Faculty of Medicine, Bangkok, Thailand. 3Center of Excellence in Transgender Health, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. 4FHI 360 and LINKAGES, Bangkok, Thailand
Background: Provider‐collected swabs are an unappealing procedure for many transgender women due and may have led to suboptimal rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) testing. Self‐collection for CT and NG testing is recommended for men who have sex with men and cisgender women, but the information is lacking for transgender women. We aimed to determine the acceptability and satisfaction of self‐collection for Thai transgender women.
Methods: Thai transgender women who attended the Tangerine Clinic – a transgender‐led, integrated, gender‐affirming care and sexual health service in Bangkok – between May and July 2020 and had condomless sexual intercourse within the past six months were offered to collect urine and perform self‐swabs of pharyngeal, rectal, and if applicable, neovaginal compartments for pooled nucleic acid amplification testing for CT and NG (Abbott Real Time CT/NG, Abbott Molecular Inc., Illinois, USA). Participants were given a diagram of self‐collection instructions. Self‐administered questionnaires were used to assess satisfaction. The prevalence of CT and NG infections among those who accepted self‐collection was compared to our historical cohort consisting of 764 transgender women who underwent provider‐collected samples between 2015 and 2017.
Results: A total of 224 transgender women were offered self‐collection, and 143 (63.8%) accepted. All had pharyngeal, rectal and urethral samples collected. Of 28 who had undergone gender‐affirmative surgery, all accepted neovaginal self‐swab. Acceptance increased from 43.9% in May to 82.7% in July 2020. All transgender women who accepted self‐collection were able to perform it without assistance, and 82.8% were highly satisfied with the method. None reported dissatisfaction. No invalid results were reported from these samples. The pooled prevalence of CT and NG infections among transgender women performing self‐collection was 23.1% and 17.5% respectively; comparable to our historical cohort (22.9% CT infection, 14.3% NG infection).
Conclusions: Thai transgender women had high acceptability and satisfaction of self‐collection for CT and NG testing. Our results support the implementation of self‐collection services to maintain and/or increase sexually transmitted infection testing uptake, particularly during the COVID‐19 pandemic where physical distancing is the new normal. A larger study is warranted to determine and confirm the CT and NG test performance between self‐collection and provider collection.
OAC0401
Gonorrhoea and chlamydia prevalence and associated characteristics among transgender women in five U.S. cities, NHBS, 2019
J Chapin‐Bardales 1; M Johnson Jones1; T Dorji1; RD Kirkcaldy1; K Bernstein1; MR Morris1; E Nash1; K Lee1; E Olansky2; A Rivera3; D Trujillo4; A Martin5; WT Robinson6,7; J Todd8; C Wejnert1; and for the NHBS Study Group
1U.S. Centers for Disease Control and Prevention, Atlanta, United States. 2ICF International, Atlanta, United States. 3New York City Department of Health and Mental Hygiene, New York City, United States. 4San Francisco Department of Public Health, San Francisco, United States. 5Public Health—Seattle & King County, Seattle, United States. 6Louisiana Office of Public Health, New Orleans, United States. 7Louisiana State University Health Sciences Center School of Public Health, New Orleans, United States. 8Georgia Department of Public Health, Atlanta, United States
Background: Studies of sexually transmitted infections (STI) apart from HIV are lacking among transgender women in the United States. This paucity of information has limited guidance on testing and prevention of STIs for transgender women.
Methods: In 2019, National HIV Behavioral Surveillance recruited transgender women via respondent‐driven sampling in select U.S. cities. Eligibility included being ≥18 years old, assigned male at birth or intersex, and identifying as a transgender woman or a woman. Participants completed a survey, HIV testing, and in five cities (Atlanta, New Orleans, New York City, San Francisco, Seattle) gonorrhoea and chlamydia testing using self‐collected pharyngeal swabs, rectal swabs and urine. We report frequencies of gonorrhoea and chlamydia infections. Adjusted prevalence ratios and 95% confidence intervals were obtained using Poisson regression models with robust standard errors accounting for recruitment chain and adjusting for city and network size.
Results: Of 847 eligible participants, 824 (97.3%) consented and provided at least 1 STI specimen. Overall, 6.6% of participants had a positive test result for gonorrhoea, 8.0% for chlamydia and 12.5% for either gonorrhoea or chlamydia, at any of the anatomic sites. Rectal STI prevalence was highest at 9.8%, followed by pharyngeal at 4.3% and urogenital at 0.7%. Having either gonorrhoea or chlamydia at any of the anatomic sites was associated with younger age; being Hispanic, Asian, or Native Hawaiian/Pacific Islander race/ethnicity; and living in Atlanta or Seattle.
Conclusions: About one in eight transgender women had either gonorrhoea or chlamydia at 1 or more anatomic sites. Rectal STI prevalence was highest, signalling the importance of collecting rectal specimens as part of comprehensive STI testing for transgender women. Reaching young transgender women and transgender women of colour with culturally appropriate testing, prevention and treatment efforts will be key to reducing STI burden.
Abstract OAC0401‐Table 1
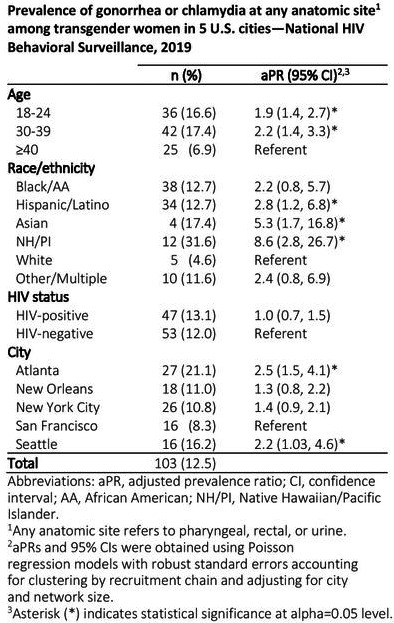
OAC0402
STI incidence among participants in the HIV Pre‐Exposure Prophylaxis (PrEP) impact trial in England
C Chiavenna 1; HD Mitchell1; R Osman1; E Mason1; MP Hibbert1; Q Enayat1; A Cartier2; S Jaffer2; V Diamente2; R Golombek2; C Estcourt3; C Sabin4; J Saunders1,4; A Charlett1; A Sullivan2,1; and Impact study group
1Public Health England, National Infection Service, London, United Kingdom. 2Chelsea and Westminster NHS Trust, London, United Kingdom. 3Glasgow Caledonian University, Glasgow, United Kingdom. 4University College London, London, United Kingdom
Background: Pre‐exposure prophylaxis (PrEP) is effective at reducing risk of HIV acquisition. However, there are concerns that widespread PrEP‐use may lead to changes in sexual behaviour that increase the transmission of sexually transmitted infections (STIs). We describe STI incidence among participants enrolled in a non‐interventional, non‐randomized trial of PrEP implementation at sexual health clinics (SHCs) across England.
Methods: Participants were enrolled between 13/10/2017 and 12/07/2020. Demographic, clinical and prescribing data were collected via electronic case report forms and routine STI surveillance. For this analysis, data were included up to 29/02/2020. We compared the incidence of chlamydia, gonorrhoea and syphilis across subgroups using univariate and multivariate zero‐inflated negative binomial regression models, adjusted for individual follow‐up time and testing frequency.
Results: This analysis included 18358 participants who had at least one post‐enrolment visit; the median follow‐up was 11.9 months [IQR 4.7 to 20.9].
19419 STIs were diagnosed among 8712 participants. Multiple infections were observed in 4580 (25.4%) participants. The mean incidence of any STI during follow‐up was 101.2 (95% CI 99.9 to 102.7) per 100 person‐years: 43.6 (95% CI 42.6– 44.5) for chlamydia, 50.5 (95% CI 49.5 to 51.5) for gonorrhoea and 7.2 (95% CI 6.8 to 7.6) for syphilis.
STI incidence was highest among 16 to 24 year olds (IRRs 0.93, 95% CI 0.87 to 0.98 for 25 to 34 yo, 0.79, 95% CI 0.74 to 0.84 for 35 to 44 yo, 0.66, 95% CI 0.61 to 0.71 for 45 to 54 yo and 0.59, 95% CI 0.53 to 0.66 for 55+yo) and in London (IRR 0.86, 95% CI 0.79 to 0.94 for Midlands and East, 0.84, 95% CI 0.78 to 0.90 for North and 0.80, 95% CI 0.74 to 0.89 for South). An STI diagnosis in the year before enrolment (IRR 1.51, 95% CI 1.44 to 1.58) and being born outside the United Kingdom (IRR 1.22, 95% CI 1.16 to 1.29 for Europe and 1.15, 95% CI 1.09 to 1.22 for elsewhere) were associated with increased STI incidence, while being on an event‐based PrEP instead of daily was associated with lower incidence (IRR 0.91, 95% CI 0.86 to 0.96). The lLower number of tests and STI diagnosis before enrolment, shorter follow‐up, and region of residence successfully predicted the chance of having zero STI diagnoses.
Conclusions: There are considerable differences in STI incidence among PrEP users, even after accounting for different attendance and testing frequency. Efforts in prevention should be focussed on the youngest living in London with the history of STIs.
OAC0403
The association of exposure to DREAMS combination HIV prevention on sexually acquiring or transmitting HIV amongst adolescent girls and young women living in rural South Africa: a cohort study
M Shahmanesh 1,2; N Mthiyane1; K Baisley3,1; T Zuma1,4; N Okesola1; J Dreyer1; C Herbst1; T Smit1; S Danaviah1; N McGrath5,1; G Harling2,1; L Sherr2; S Floyd3; I Birdthistle3; J Seeley1,3; and N Chimbindi1
1Africa Health Research Institute, Durban, South Africa. 2University College London/Institute for Global Health, London, United Kingdom. 3London School of Hygiene and Tropical Medicine, London, United Kingdom. 4University of KwaZulu‐Natal, Durban, South Africa. 5University of Southampton, Southampton, United Kingdom
Background: We investigate how the risk of sexually acquiring or transmitting HIV in adolescent girls and young women (AGYW) changed following the real‐world implementation of DREAMS (Determined, Resilient, Empowered, AIDS free, Mentored and Safe) combination HIV prevention.
Methods: We recruited a randomly selected population‐based cohort of AGYW aged 13 to 22 years (at baseline) living in rural KwaZulu‐Natal whom we interviewed annually (2017 to 2019). We measured exposure to DREAMS as self‐reported receipt of an invitation to participate and/or participation in DREAMS activities. HIV status was ascertained through blood tests on Dried Blood Spot. We used multivariable regression to assess the association between exposure to DREAMS and risk of acquiring HIV (incident HIV) or having transmissible HIV (being HIV positive with a detectable HIV viral load of ≥50 copies per millilitre) on the last available DBS. We adjusted for socio‐demographic, sexual relationship and migration.
Results: A total of 2184 (86.4%) of those eligible agreed to participate and 2016 (92.3%) participants provided data for at least one follow‐up time‐point. 1030 (54%) were exposed to DREAMS; HIV incidence was 2.2/100 person‐years (95% Confidence Interval [CI]: 1.66 to 2.86). There was no evidence that HIV incidence was lower in those exposed to DREAMS: adjusted rate ratio (aRR) 0.83 (95% CI: 0.46 to 1.52). HIV viral load was detectable for 169 (8.9%) respondents one to two years following enrolment; there was no evidence this was lower in those exposed to DREAMS with an adjusted risk difference, compared to those not exposed to DREAMS, of 0.99% [95% CI: −1.52 to 3.82]. Participants who lived in peri‐urban/urban setting were more likely to have incident HIV and transmissible HIV. Detectable HIV viral load was also associated with older age and ever having sex. Findings did not differ substantively by respondent age group.
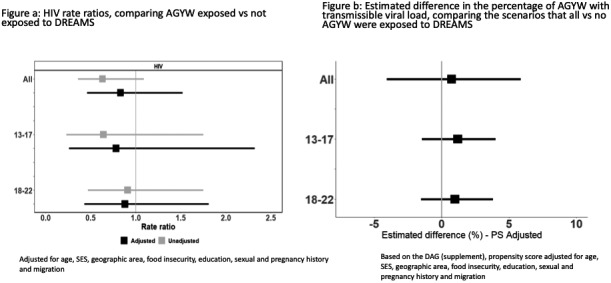
Abstract OAC0403‐Figure 1.
Conclusions: DREAMS exposure was not associated with reductions in risk of sexually acquiring or transmitting HIV among a representative cohort of AGYW in rural South Africa.
OAC0404
HPV increases HIV risk in African women: advancing the argument for HPV immunization
G Liu 1; N Mugo2; E Brown3; N Mgodi4; Z Chirenje4; J Marrazzo5; R Winer6; L Mansoor7; T Palanee‐Phillips8; S Siva9; L Naidoo9; N Jeenarain9; Z Gaffoor9; G Nair10; P Selepe11; C Nakabiito12; B Mkhize13; B Gati Mirembe12; M Taljaard11; J Baeten6; J Balkus1; F Hladik6; C Celum6; and R Barnabas6
1University of Washington, Epidemiology, Seattle, United States. 2Kenya Medical Research Institute, Nairobi, Kenya. 3University of Washington, Biostatistics, Seattle, United States. 4University of Zimbabwe, Harare, Zimbabwe. 5University of Alabama, Birmingham, United States. 6University of Washington, Seattle, United States. 7Centre for the AIDS Programme of Research in South Africa, Durban, South Africa. 8Wits Reproductive Health and HIV Institute, Johannesburg, South Africa. 9South Africa Medical Research Council, Durban, South Africa. 10Desmond Tutu HIV Centre, Cape Town, South Africa. 11The Aurum Institute, Klerksdorp, South Africa. 12Makerere University‐John Hopkins University Research Collaboration, Kampala, Uganda. 13University of Witwatersrand, Johannesburg, South Africa
Background: Adolescent girls and young women (AGYW) account for 25% of incident HIV infections in sub‐Saharan Africa. Human papillomavirus (HPV) infection is common among AGYW, but its role in HIV acquisition is uncertain. We evaluated the relationship between HPV and HIV acquisition using data from MTN‐003, a clinical trial of chemoprophylaxis for HIV among cisgender women in sub‐Saharan Africa.
Methods: Using a nested case‐control design, we matched 138 women who acquired HIV (cases) to 412 HIV‐negative controls. Cervical or vaginal swabs collected at one time‐point within six months before HIV seroconversion in cases were tested for 37 HPV types using a Luminex‐based liquid bead microarray – 14 of which are high‐risk carcinogenic types and 23 low‐risk types. We estimated the association between HPV and HIV using conditional logistic regressions, controlling for confounders including age, time‐varying sexual behaviours, vaginal infections and other sexually transmitted infections.
Results: Mean age in the study was 24 years (±4 years). Any, high‐risk and low‐risk HPV was detected in 84%, 74% and 66% of cases, and 65%, 55% and 48% of controls. Infection with ≥2 HPV types was common in cases (67%) and controls (49%). A high proportion (60% of cases and 42% of controls) had >1 type covered by the 9‐valent HPV vaccine, and 36% of cases and 22% of controls had >1 type covered by the quadrivalent vaccine. HIV risk increased 2.7‐fold with any HPV (adjusted odds ratio [aOR] 2.7, 95% confidence interval [CI] 1.5 to 5.1) or a high‐risk HPV infection (aOR 2.7, 95% CI 1.5 to 4.8) and 1.9‐fold with a low‐risk HPV infection (95% CI 1.1 to 3.0). Each additional HPV type detected was associated with a 20% increase in HIV risk (aOR 1.2, 95% CI 1.1 to 1.3). HIV acquisition was also associated with HPV types covered by the 9‐valent (aOR 2.2, 95% CI 1.3 to 3.6) and quadrivalent vaccines (aOR 1.8, 95% CI 1.1 to 3.0).
Conclusions: HPV infection was associated with HIV acquisition among AGYW living in high HIV burden settings. Infection with a 9‐valent HPV vaccine‐targeted type is prevalent in this population. In addition to preventing HPV‐associated cancers, increasing HPV vaccination coverage may reduce new HIV infections in sub‐Saharan Africa.
OAC0405
Development of a multiplex assay for use in multi‐analyte screening and surveillance
E Yufenyuy; S Vedapuri; G Cooley; D Danavall; S Mayur; M Kodani; C Chen; Y Tun; Y Fakile; D Martin; S Kamili; K Karem; and B Parekh
Centers for Disease Control and Prevention, Atlanta, United States
Background: Diagnostic assays that can simultaneously determine the presence of infection with multiple pathogens are key for diagnosis and surveillance. Single pathogen diagnostic tests for HIV, HSV, hepatitis viruses, syphilis and rubella are routinely used in disease surveillance in industrialized countries, but world‐wide applications are limited due to high cost, lack of expertise and poor laboratory infrastructure. We developed a multi‐analyte, pathogen detection assay for screening and sero‐surveillance using the Luminex MAGPIXTM platform that is simple, high‐throughput, cost‐effective, highly reproducible and helpful in monitoring multiple diseases.
Methods: The Luminex bead‐based 11‐Plex immunoassay for the detection of HIV‐1, HIV‐2, syphilis, hepatitis B, hepatitis C, HSV‐1, HSV‐2 and rubella was accomplished by coupling beads with specific antigens to detect IgG antibodies in plasma or serum samples. Each coupled antigen was systematically optimized and the performance of the monoplex and the multiplex (11‐Plex) were evaluated using a panel of well‐characterized specimens that contained a combination of antibodies to HIV‐1 (positive n = 70, negative n = 347), HIV‐2 (positive n = 5, negative n = 412), syphilis (positive n = 54, negative n = 363), hepatitis C (positive n = 67, negative n = 348), hepatitis B (positive n = 78, negative n = 337), HSV‐1 (positive n = 283, negative n = 134), HSV‐2 (positive n = 211, negative n = 206) and rubella (positive n = 391, negative n = 26).
Results: Both the monoplex and multiplex assay formats showed a overall sensitivity of 92.2% (95% CI, 90.2 to 94.0) and specificity of 98.1% (95% CI, 97.6 to 98.7) when compared to the reference data. The sensitivities and specificities of disease‐specific biomarker detection ranged from 68.7% to 100%, and from 95.6% to 100% respectively (Table 1). The results showed the 11‐Plex had an overall agreement of 96.7% (95% CI, 96.7 to 97.3) with reference tests and a corresponding kappa coefficient of 0.91 (95% CI, 0.90 to 0.93).
Conclusions: The 11‐Plex bead‐based surveillance tool is robust and allows for simultaneous detection of antibodies to multiple antigens in a high throughput format. This assay has the potential to simplify disease surveillance by providing an alternative to expensive, highly specialized individual tests. Additional clinical evaluation using maternal plasma or serum specimens is needed for the 11‐Plex to help curb mother‐to‐child transmission of multiple infections.
OAC0501
The cost and intermediary cost‐effectiveness of oral HIV self‐test kit distribution across eleven distribution models in South Africa
L Sande 1,2; K Matsimela3; C Mostert4; M d’Elbée1; M Majam5; J Phiri5; V Zishiri5; C Madondo6,7; S Khama6; K Hatzold8; C Johnson;9; T Chidarikire10; F Terris‐Prestholt1,11; and G Meyer‐Rath3
1London School of Hygiene and Tropical Medicine, Global Health & Development, London, United Kingdom. 2Malawi‐Liverpool‐Wellcome Trust Clinical Research Programme, Blantyre, Malawi. 3Health Economics and Epidemiology Research Office (HE2RO), Department of Internal Medicine, Johannesburg, South Africa. 4Wits Reproductive Health & HIV Institute, Johannesburg, South Africa. 5Ezintsha, Johannesburg, South Africa. 6Society for Family Health, Johannesburg, South Africa. 7The South African National AIDS Council, Johannesburg, South Africa. 8Population Services International, Cape Town, South Africa. 9World Health Organization, Geneva, Switzerland. 10National Department of Health, Pretoria, South Africa. 11Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland
Background: South Africa has made progress in reaching the population aged 15 to 64 with HIV testing, but testing gaps remain among key populations and men. HIV self‐testing (HIVST) can help fill these gaps by bringing testing closer. We conducted an economic evaluation of 11 innovative HIVST distribution approaches implemented across urban and rural settings in South Africa between 2018 and 2019 under the Self‐Testing AfRica Initiative.
Methods: We analysed the cost and outcomes along the care cascade from self‐testing to initiation of antiretroviral treatment (ART) across the country (Table 1). We conducted an ingredients‐based cost analysis from the provider’s perspective, combining bottom‐up and top‐down approaches. Cost analysis was limited to a 12‐months implementation period for all except two models with shorter implementation periods (transport hub and third‐party workplace models). We categorized cost items as capital versus recurrent. Capital costs were annualized over a two years lifespan to reflect the project duration and discounted using a 3% discount rate.
Abstract OAC0501‐Table 1
| Distribution setting | Model | Distribution approach | Target population |
|---|---|---|---|
| Facility | Horizontal primary healthcare (Antenatal care)/Horizontal primary healthcare (Index)/Vertical primary healthcare | Pregnant women received kits for their current sexual partner(s)/HIV‐positive clients attending PHC clinic received kits for their sexual partner(s)/On‐site HIV screening for clients attending PHC clinic for a wide array of services | Men & partners of HIV‐positive people/General population |
| Community distribution | Fixed point/Flexible community | Distribution at pre‐selected locations within communities, especially where men tend to congregate/Door‐to‐door distribution of kits | Men/Men and young people |
| Mobile integration/Workplace | Integrating HIVST to community‐based mobile HIV testing/Distributing kits at male‐dominated sector workplaces | ||
| Transport hub | Distributing kits in densely populated taxi ranks and train stations with high foot traffic | General population | |
| Key populations/Sex workers | Distributing kits to sex workers and truck drivers/Sex workers received kits for peers | Key populations/Sex workers |
Results: Slightly over a million kits were distributed; 49% through the flexible community model and the least kits (1%) through the mobile integration and PHC models. The self‐test positivity rate varied between 4% in the workplace model and 23% in the horizontal PHC model, with most models reporting a 5% positivity rate. The average cost per kit distributed ranged from $4.87 in the sex worker model to $18.07 in the mobile integration models. Facility models exhibited higher unit costs than community models. The average cost per reactive HIVST ranged from $28 in the sex worker model to $414 in the mobile integration model. The cost per confirmed positive result was between $66 in the sex worker model and $1229 in vertical PHC. Finally, the cost per ART initiation was between $116 in the sex worker model and $1278 in vertical PHC.
Conclusions: HIVST distribution cost varied widely across models, with the sex worker, transport hub and workplace models being the most efficient and least costly distribution approaches.
OAC0502
A successful launch of the first HIV self‐testing pharmacy‐based service demonstration project, Bangkok, Thailand
P Visavakum 1; N Pattarapayoon2; C Manopaiboon1; T Puangmalee1; C Kittinunvorakoon1; S Kohreanudom2; O Suksiripanich1; S Northbrook1; and H Ruksakom2
1U.S. Center for Disease Control and Prevention, Division of Global HIV and TB, Nonthaburi, Thailand. 2Ministry of Public Health Thailand, Department of Disease Control, Nonthaburi, Thailand
Background: The Thai Ministry of Public Health (MoPH) considers HIV self‐testing (HIVST) a complementary approach to increase access to and uptake of HIV testing especially among men who have sex with men (MSM) and transgender women (TGW), who historically have low access to HIV testing. We present results of the first HIVST pharmacy‐delivered model to assess the acceptance of unassisted HIVST among these populations in Bangkok.
Methods: Oral HIVST kits were available through 36 retail Boots pharmacies located in Bangkok. Participants who were directed to the project website during August and December 2020 and who met the inclusion criteria (i.e. Thai MSM/TGW ≥18 years of age, who lived/worked in Bangkok, had a smartphone with Internet connection, and had never been diagnosed with HIV) were invited to register online. All eligible participants provided written informed consent online, completed baseline and follow‐up questionnaires, picked up the free HIV test (OraQuick®) at the pharmacy, and performed the test unassisted. Participants testing HIV positive were encouraged to confirm test results at designated health facilities. Hotline was provided for additional counselling if needed. Questionnaire data including demographics, behavioural and HIV treatment information were collected electronically.
Results: Of 1511 MSM/TGW who consented, 826 (55%%) were eligible. Of these, 776 (94%) were MSM, 239 (31%) were 18 to 25 years old, 576 (70%) completed ≥bachelor’s degree, 653 (79%) were employed, over 70% had self‐perceived HIV risk and 343 (42%) had never tested for HIV. As of January 2021, 440 (53%) picked up the test kit at the pharmacy, 274 (62%) performed the test. Of these, 15 (5%) reported reactive test results and 205 (75%) reported that the test kit was easy to use. Few (n = 41) participants called the hotline for additional counselling.
Conclusions: This is the first project in Thailand that demonstrates the use of unassisted HIVST delivered via pharmacies. Based on our findings, HIVST is an effective way to reach MSM/TGW who have never tested for HIV. Those who reported the use of HIVST kits expressed high acceptability, suggesting HIVST as an acceptable and complementary strategy to HIV case‐finding strategy for these populations. More in‐depth analysis on linking HIV‐positive clients to confirmatory testing is underway.
OAC0503
User assessment of HIV self‐testing (HIVST) in Brazil: an acceptable tool with great potential for reaching key populations and maximize positivity yield
M Villares; A Bigolin; PC Gaspar; JBA Neto; R Ganassin; and GFM Pereira
Ministry of Health of Brazil, Department of Diseases of Chronic Condition and Sexually Transmitted Infections, Brasilia, Brazil
Background: Innovative strategies are paramount in a country that has a huge social disparity and an HIV epidemic concentrated in key populations (KP), who commonly experience barriers to access health services (HS). Aiming to reach the estimated 99.000 (11%) undiagnosed PLHIV, in 2018 Brazilian MoH started in 2018 a free HIVST distribution, focused on KP and young people. The aim of this study was to evaluate HIVST Brazilian users’ experience to guide public health policies.
Methods: From December 2018 to January 2021, we conducted a cross‐sectional study using an online self‐administered structured questionnaire to assess user acceptance and experience using HIVST. The study collected socio‐demographics, sexuality, risk behaviour and previous testing data from HIV self‐testers.
Users were encouraged to answer the post‐test anonymous questionnaire by using the QR code printed in the informative folder delivered with the HIVST.
Results: We obtained 813 answers. 363 (45%) of respondents were MSM and 50% were between 18 and 29 years old. 45% reported unprotected anal sex in the last six months and 32% of users were first‐time testers (46% in the 18 to 24 age group). Half of respondents (50%) realized the HIVST alone and 30% with a friend, partner or family member, 12% were assisted by an NGO member and 8% by a healthcare professional.
3% bought the test in pharmacies, 41% got it in a HS, 40% in outreach strategies and 13% received the test from a friend.
Regarding the testing experience, 91% of users found it easy to do, 98% would do an HIVST again and 99% would recommend it to a friend. The main advantages of HIVST pointed out were privacy (72%) and personal empowerment (51%).
Positive results were related by 28 (3.4%) people (Brazilian prevalence is 0.5%). Among the positive, 79% reported having already sought a HS to confirm the diagnosis.
Conclusions: HIVST is high acceptable, easy to perform and can maximize positivity yield. People prefer to self‐test alone or with someone they trust. Most people seek confirmation after a positive result. HIVST were mostly obtained outside HS, successfully reached first time testers, young people and KP.
OAC0504
Feasibility of HIV self‐testing among female sex workers in Iran: the SELFii study
R Khajehkazemi 1; L Taj2; T Amiri2; S Sindarreh3; M Nasirian3; S Hosseini‐Hooshyar4; A Esmaeili5; W McFarland6; M Mohraz2; and A Mirzazadeh6,1
1HIV/STI Surveillance Research Center, and WHO Collaborating Center for HIV Surveillance, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran, Islamic Republic of. 2Iranian Research Center for HIV/AIDS, Iranian Institute for Reduction of High‐Risk Behaviors, Tehran University of Medical Sciences, Tehran, Iran, Islamic Republic of. 3Epidemiology and Biostatistics Department, Health School, Isfahan University of Medical Sciences, Isfahan, Iran, Islamic Republic of. 4The Kirby Institute, UNSW Sydney, Sydney, Australia. 5U.S. Department of Veterans Affairs, Palo Alto, United States. 6Department of Epidemiology and Biostatistics, Institute for Global Health Sciences, University of California, San Francisco, United States
Background: The HIV epidemic in Iran may be shifting from injection to sexual transmission, presenting challenges to reach new key populations at high risk. Considering severe stigma, discrimination and criminalization, female sex workers’ (FSW) in Iran face barriers to HIV testing at health facilities. New testing strategies such as HIV self‐testing (HIVST) present a possible solution. We assessed the feasibility of HIVST among FSW in two cities of Iran.
Methods: Through peer‐referral sampling from 3/2019 to 8/2020, 492 FSW (aged 18 years or older who sold sex in the last month) in Tehran and Isfahan were invited by 18 peer‐educators to use HIVST. We collected data on their experiences in using HIVST, test outcomes and feasibility of reporting results through peer‐educators.
Results: Of 492 FSW participants, 54% were age 30 to 49 years, 44% used a condom at last sex and 60% had never tested for HIV. The most common places where FSW used the HIVST were at home (43%), followed by hotspots for sex work or houses that temporarily served as brothels (20%), and public places (18%). Most FSW were assisted by a peer‐educator to do the HIVST (76%). Two FSW (0.4%) self‐reported a positive HIVST result to a peer‐educator; both were referred and received confirmatory testing by the health system. At follow‐up, 33% of FSW reported using a condom at last sex; 32% reported no sexual contact after the HIVST. Half (50%) of FSW reported high stress to learn if they are positive before testing; 17% reported stress after the HIVST. Three‐fourths (75%) reported the HIVST was easy to use; 83% will recommend it to other FSW. A majority of FSW (54%) were willing to pay up to $2 USD for the HIVST.
Conclusions: Our study found that HIVST can be distributed by peer‐educators to FSW, with high acceptability for use at sex‐work venues or at home. Most accepted the assistance of peers to perform the test and record results. The issue of stress reported by some FSW needs further exploration to address this potential barrier. We found no indication that the self‐test results increased condomless sex.
OAC0505
Reaching the "first 95": a cross‐country analysis of HIV self‐testing in 177,572 people in nine countries in sub‐Saharan Africa
E van Empel 1,2; RA De Vlieg1,2; ME Marcus3; G Harling1,4,5,6,7; K Kahn7,8; TW Bärnighausen6,9,10; AT Choko11; L Montana12; and J Manne‐Goehler13
1Harvard University, Harvard Center for Population and Development Studies, Cambridge, United States. 2Maastricht University, Maastricht, Netherlands. 3University of Göttingen, Department of Economics and Centre for Modern Indian Studies, Göttingen, Germany. 4University College London, London, United Kingdom. 5Harvard T.H. Chan School of Public Health, Department of Epidemiology, Boston, United States. 6Africa Health Research Institute, KwaZulu‐Natal, South Africa. 7University of the Witwatersrand, MRC/Wits Agincourt Unit, Rural Public Health and Health Transitions Research Unit, Johannesburg, South Africa. 8INDEPTH Network, East Legon, Ghana. 9Heidelberg University, Heidelberg Institute of Global Health (HIGH), Heidelberg, Germany. 10Harvard T.H. Chan School of Public Health, Department of Global Health and Population, Boston, United States. 11Malawi – Liverpool Wellcome Trust Clinical Research Programme, Blantyre, Malawi. 12The Demographic and Health Surveys (DHS) Program, Cambridge, United States. 13Massachusetts General Hospital, Division of Infectious Diseases, Boston, United States
Background: HIV self‐testing (HIVST) offers a promising approach to increase diagnosis of HIV and advance progress towards the UNAIDS 95‐95‐95 targets. We aimed to understand patterns of awareness and utilization of HIVST in nine sub‐Saharan African (SSA) countries, with a goal to identify populations to target in disseminating this technology.
Methods: We pooled individual‐level population‐based data from nine Demographic and Health Surveys (DHS) in SSA from 2015 to 2019 (Burundi, Cameroon, Guinea, Malawi, Senegal, Sierra Leone, South Africa, Zambia, Zimbabwe). The primary outcomes of interest were awareness and utilization of HIVST. We then used logistic regression analyses with survey fixed effects to explore the relationship between sociodemographic characteristics and both (1) awareness and (2) utilization of HIVST. All models were adjusted for sex, age, rural/urban residence, education, wealth, marital status. We accounted for complex survey design.
Results: The total study sample included 177,572 people (66.0% women, mean age 29 ± 10 years), among whom 86.6% (95%‐CI 86.4 to 86.7) had never heard of HIVST, 11.7% (95%‐CI 11.6 to 11.9) had heard of HIVST but never tested and 1.7% (95%‐CI 1.6 to 1.8) had tested with HIVST. In adjusted models, women were less likely to be aware of HIVST (OR 0.75 95%‐CI 0.71 to 0.79), but more likely to have ever used HIVST (OR 1.17 95%‐CI 1.03 to 1.32) compared to men. Moreover, rural residents were less likely to be aware of or use HIVST; in addition, there were significant gradients in both wealth and education, with those who were least educated and poorest also least likely to have heard of or used HIVST (Table 1).
Abstract OAC0505‐Table 1. Multivariable logistic regression analysis
| Awareness of HIVST | Use of HIVST | |||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Rural a | 0.81 (0.75 to 0.88) | <0.001** | 0.74 (0.62 to 0.89) | 0.001* |
| Primary education b | 1.03 (0.96 to 1.11) | <0.001** | 0.79 (0.65 to 0.97) | <0.001** |
| Secondary education | 1.81 (1.68 to 1.95) | 1.64 (1.36 to 1.98) | ||
| Higher education | 4.89 (4.45 to 5.37) | 4.20 (3.43 to 5.16) | ||
| Poorer c | 1.26 (1.16 to 1.37) | <0.001** | 1.28 (1.04 to 1.59) | <0.001** |
| Middle |
1.45 (1.32 to 1.58) |
1.22 (0.96 to 1.55) |
||
| Richer | 1.70 (1.54 to 1.88) | 1.48 (1.17 to 1.86) | ||
| Richest | 2.36 (2.12 to 2.62) | 1.66 (1.31 to 2.11) | ||
HIVST, HIV self‐testing. Reference categories: a) urban, b) no education, c) poorest. Additionally, adjusted for sex, age and marital status.
*p < .05. **p < .001.
Conclusions: Overall awareness of HIVST was modest and uptake to date is low. Marginalized groups were least likely to have heard of or used HIVST. Efforts to scale‐up HIVST in these settings should aim to reach rural, less educated and lower income populations.
OALD0701
HIV service delivery to key populations in the time of COVID‐19: experiences from India
R Pollard 1; U Gopinath2; YA Reddy3; BR Kumar2; P Mugundu3; CK Vasudevan2; AK Srikrishnan2; A Singh3; AM McFall4; KH Mayer5,6; SH Mehta4; and SS Solomon1
1Johns Hopkins University School of Medicine, Baltimore, United States. 2YR Gaitonde Centre for AIDS Research and Education, Chennai, India. 3Johns Hopkins University School of Medicine, New Delhi, India. 4Johns Hopkins University School of Public Health, Baltimore, United States. 5The Fenway Institute, Boston, United States. 6Harvard Medical School, Boston, United States
Background: In March 2020, the Government of India revised HIV service delivery policies in response to COVID‐19 to include community distribution and multi‐month dispensation (MMD) of ART for stable and unstable PLHIV. There are limited data on the impact of COVID‐19‐associated disruptions and novel service strategies on HIV service access among key populations in low‐ and middle‐income countries.
Methods: Between November and December 2020, we conducted focus groups with purposively sampled men who have sex with men (MSM), female sex workers (FSW) and transgender women (TGW) in Telangana and Maharashtra, Indian states with high HIV burdens. Seven focus groups were conducted; five by phone and two in‐person with safety precautions. Discussion topics included service access experiences, medication adherence and preferences to ensure service continuation. Inductive coding identified themes across topics.
Results: Forty‐four individuals participated in focus groups (13 MSM; 16 FSW; 15 TGW) aged 20 to 49 years. Twenty‐four participants self‐identified as living with HIV. HIV‐negative participants reported challenges to get HIV tests at hospitals due to lockdown travel restrictions and fear of contracting COVID‐19. Some accessed HIV testing using transportation arranged by community‐based organizations. Most PLHIV reported uninterrupted ART refills; however, some reported lapses in ART adherence and delayed viral load testing. Participants receiving MMD shared consistent appreciation for the service as it saved time, money and reduced exposure to COVID‐19 and stigmatizing environments. PLHIV expressed gratitude for home deliveries which enabled access to ART, yet discouraged continuing home‐based services due to the risk of a confidentiality breach to family/neighbours. Most suggested community dispensation points. Other themes included loss of livelihood and requests for economic support across groups, and concerns about telemedicine as a service option from FSW and TGW related to limited smartphone access.
Conclusions: COVID‐19 had a greater impact on access to testing services (HIV testing, viral load) compared to treatment services. High acceptance of MMD and community‐based services support the need for differentiated service delivery models to overcome COVID‐19 disruptions. Varied preferences across key populations related to new service mechanisms and calls to address the impact of COVID‐19 on livelihood options underscore the importance of tailoring HIV care to community needs.
OALD0702
VIBRA trial – Village‐based refill of ART following home‐based same‐day ART initiation: a cluster‐randomized clinical trial
A Amstutz 1,2,3; TI Lejone4; L Khesa5; M Kopo4; M Kao5; J Muhairwe6; M Bresser1,2; T Klimkait7; M Battegay2,3; TR Glass2,1; and ND Labhardt1,2,3
1Swiss Tropical and Public Health Institute, Department of Medicine, Clinical Research Unit, Basel, Switzerland. 2University of Basel, Basel, Switzerland. 3University Hospital Basel, Division of Infectious Diseases and Hospital Epidemiology, Basel, Switzerland. 4SolidarMed, Partnerships for Health, Butha‐Buthe, Lesotho. 5SolidarMed, Partnerships for Health, Mokhotlong, Lesotho. 6SolidarMed, Partnerships for Health, Maseru, Lesotho. 7University of Basel, Department of Biomedicine, Molecular Virology, Basel, Switzerland
Background: Community‐based based antiretroviral treatment (ART) delivery is an important component of differentiated service delivery models in sub‐Sahara Africa. However, community‐based delivery systematically excludes new patients during their first six or 12 months on ART. The pragmatic VIBRA (Village‐based refill of ART) cluster‐randomized trial in rural Lesotho compared the option of ART refill by lay village health workers (VHW) versus clinic‐based refill after home‐based same‐day ART initiation during a door‐to‐door HIV testing campaign.
Methods: In village‐clusters randomized to the intervention, individuals found HIV positive were offered ART refill by VHWs after same‐day ART initiation. The trained VHWs dispensed drug supply for one to three months, scheduled a first clinic‐based follow‐up visit for blood draw at six months, and were supervised by a district ART nurse and the corresponding health facility. In control village‐clusters, participants were referred to the clinic for ART refill and follow‐up. The primary outcome was viral suppression <20 copies/mL. Secondary endpoints comprised three‐month linkage and 12‐month engagement in care, among others. Analyses were by intention‐to‐treat. Trial registration: NCT03630549.
Results: From August 15th, 2018, until May 28th, 2019, 139 individuals from 130 households in 60 clusters in control, and 118 individuals from 108 households in 57 clusters from intervention arm were enrolled. The majority were female (150 [58%]), with a median age of 36 years (interquartile range [IQR] 30 to 48), 200 [78%] were newly diagnosed. In the intervention arm, 48/118 (41%) opted for ART refill by the VHW, the remaining for clinic‐based refill. At 12 months, 64/139 (46%) and 46/118 (39%) participants in the control and intervention arm, respectively, achieved viral suppression below 20 copies/mL (adjusted absolute difference −0.07 [95% confidence interval −0.20 to 0.06]; p = 0.256). Linkage to care at three months did not differ between control versus intervention arm (65% vs. 68%; p = 0.630). 98/139 (71%) participants in control and 71/118 (60%) in intervention were active in care at 12 months (−0.12 [−0.23 to 0.003]; p = 0.058). Zero deaths occurred in control and seven deaths in intervention arm.
Conclusions: The offer of village‐based ART refill following home‐based ART initiation was not able to increase linkage to care, engagement in care and viral suppression compared to standard clinic‐based refill.
OALD0703
The Community HIV Epidemic Control Model: a community‐based intervention to achieve 90‐90‐90 via comprehensive HIV differentiated service delivery in rural communities in Zambia
C Claassen 1,2; I Kafunda2; L Mwango3; S Shiyando2; N Kancheya4; K Mweembo4; A Mwila4; L Hachaambwa1,3; and R Sheneberger1,2
1University of Maryland School of Medicine, Center for International Health, Education, and Biosecurity, Baltimore, United States. 2Maryland Global Initiatives Corporation Zambia, Lusaka, Zambia. 3Ciheb Zambia, Lusaka, Zambia. 4U.S. Centers for Disease Control and Prevention, Lusaka, Zambia
Background: Novel HIV differentiated service delivery models are needed to help Zambia achieve 90‐90‐90 epidemic control. University of Maryland Baltimore (UMB) designed and implemented the Community HIV Epidemic Control (CHEC) model to support HIV testing services (HTS), linkage to antiretroviral therapy (ART) and support viral load suppression (VLS).
Description: UMB implemented the CHEC model under the Stop Mother to Child HIV Transmission (SMACHT) project from 2015 to 2020 (Figure 1). Via health facilities, community health workers (CHW) were recruited and trained in HTS, and psychosocial and adherence counselling. CHWs conducted community HTS, escorted clients to initiate ART and delivered ART to patients who were stable‐on‐care (SOC), defined as on ART for >12 months with a suppressed viral load and willing to receive ART at home.
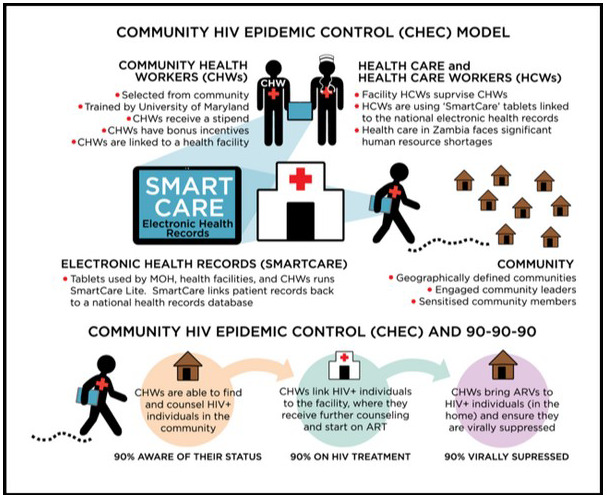
Abstract OALD0703‐Figure 1.
Lessons learned: In the first year of CHEC implementation, HTS increased from 21,051 in 2015 to 71,289 clients in 2016; 29% were tested in the community by CHWs (up from 0%).
From 2015 to 2020, SMACHT provided HTS to 1,379,387 clients, of whom 46,138 were identified as HIV positive for a positivity yield of 3.3%. Of these, 41,366 were linked to ART, 90% linkage overall.
A February 2017 sub‐study of all SOC patients found that of 1091 clients, 97% were virally suppressed with near 100% retention in care. By 2020, 66,841 clients on ART had received a VL test and 60,694 were suppressed, 91% viral load suppression.
Conclusions/Next steps: By task‐shifting HIV service delivery into the community, CHEC achieved 90% ART linkage and 91% VLS, with 97% VLS among SOC clients. Community‐based programmes can increase uptake of HTS and linkage to care. However, positivity yields may be low, necessitating targeted strategies such as index testing. In‐home delivery of ART to SOC patients supports adherence and results in high levels of VLS.
OALD0801
Using multi‐disease health screening campaigns to increase uptake of health and HIV testing services (HTS) in the Democratic Republic of the Congo (DRC)
S Muyano 1; C Tendo1; M Bazola1; S Wakimu1; M Ngoie1; D Kasongo Mukabi2; R Musao Kalemba2; P Kyansa2; P Kunda2; I Thior3; D Canagasabey3; P Kibwa1; J‐C Kiluba1; and L Mueller3
1PATH, Democratic Republic of the Congo Country Program, Lubumbashi, Congo, Democratic Republic of the. 2Kasumbalesa Referral Health Center, Kasumbalesa, Congo, Democratic Republic of the. 3PATH, HIV/TB Team, Washington, United States
Background: In 2019, only 56% of estimated HIV‐positive individuals in the DRC were diagnosed and enrolled on antiretroviral treatment (ART) indicating a need for new HTS strategies to reach undiagnosed PLHIV. PATH, through the USAID‐funded Integrated HIV/AIDS Project, piloted use of a multi‐disease screening campaign to improve uptake of HTS services.
Description: Under this campaign, health facilities offered free screenings during weekend and evening hours for hypertension, hyperglycaemia, sexually transmitted infections (STI), pneumonia, dermatitis and HIV. Community health volunteers disseminated information to raise awareness of the free consultations. At the consultation, clients completed an HIV risk assessment, then were screened for diseases based on eligibility and client preference; HIV testing was the last test. Clients who screened positive for any of the diseases were linked to care and treatment services, including same‐day ART initiation if HIV positive. PATH piloted this model at eight facilities in Haut‐Katanga, using descriptive and inferential statistics to analyse data from September 2020 through January 2021.
Lessons learned: A total of 2860 clients (57% male) participated in the screenings, with the highest representation among clients over 49 years (19%). Overall HIV prevalence was 12%, with higher prevalence among females (13%) than males (12%) and clients 25 years of age and older. 91% (192/210) of HIV‐positive individuals were initiated on ART. Overall STI prevalence was 22%, and the HIV/STI co‐infection rate was 14%, with higher co‐infection among females than males (19% vs. 10%; p < 0.05), and highest HIV/STI co‐infection prevalence among those in the 40 to 44 (26%) age band. More females than males had hypertension (11% vs. 8%; p < 0.05). Hyperglycaemia was detected among 7% of clients. These results highlight our campaign’s success in bringing individuals to facilities for multi‐disease screenings, including HIV. The high HIV/STI co‐infection rates reinforce the need to ensure provider‐initiated HTS at STI entry points.
Conclusions/Next steps: Our results highlight the promise of using multi‐disease screening campaigns to increase engagement in health services and improve HIV diagnosis among unreached PLHIV. We plan to continue testing this strategy, including investigating cost‐effectiveness, to further optimize the model for potential expansion across DRC in support of epidemic control.
OALD0802
Combined interventions to accelerate delivery on outcomes for young children affected by HIV in southern Africa
H Mebrahtu 1; S Skeen2; WE Rudgard3; S Du Toit2; K Haag1; KJ Roberts1; SL Gordon2; M Orkin3,4; L Cluver3,5; M Tomlinson2,6; and L Sherr1
1University College London, London, United Kingdom. 2Stellenbosch University, Cape Town, South Africa. 3University of Oxford, Oxford, United Kingdom. 4University of Witwatersrand, Johannesburg, South Africa. 5University of Cape Town, Cape Town, South Africa. 6Queens University, Belfast, United Kingdom
Background: Young children affected by HIV living in Africa face multiple vulnerabilities that hinder future success. The UNDP endorses “accelerator” interventions that drive success across multiple health and wellbeing outcomes as key for sustainable development. This study aimed to identify entry points for accelerator interventions relevant for children affected by HIV in southern Africa.
Methods: This study tracked child wellbeing outcomes among 989 children affected by HIV enrolled in community‐based organizations in South Africa and Malawi. Data from participating children (4 to 13 years) and their caregivers were collected at baseline and at 12‐ to 15‐month follow‐up. We investigated five hypothesized protective factors: food security, cash grant, positive parenting, living in a safe community and community acceptance; and 12 child outcomes related to the Sustainable Development Goals (SDGs): health status, nutrition, education, cognitive development and mental health. Protective factors were measured as consistent receipt at baseline and follow‐up and had to be positively associated with several child outcomes across three or more SDGs. Associations were evaluated using multivariate multivariable logistic regression controlling for baseline covariates. Adjusted probabilities of experiencing each SDG‐aligned outcome conditional on receipt of single, combined or all identified accelerators were also calculated.
Results: Three protective factors that had an impact on nine different child outcomes across three SDG‐aligned targets were identified. Household food security was positively associated with child education and cognitive development outcomes. Cash grant receipt was positively associated with nutrition and cognitive development outcomes. Living in a safe community was positively associated with all mental health outcomes. Experiencing a combination of two protective factors was associated with a higher adjusted probability of positive child outcomes. Moreover, experiencing all three protective factors was associated with the highest probability of positive child outcomes (+10.4 to 28.4% points). Substantial improvements were noted in child education outcomes.
Conclusions: The accelerator model of combining protective factors yielded greater improvements in child outcomes across different developmental domains than single provisions. Household food security, cash grant receipt and residence in a safe community may be key for the success of children affected by HIV. Promoting services to support all three of these factors would yield the greatest improvement in child outcomes in similar settings.
OALD0803
Reduction trends in AIDS indicators (incidence, hospitalization and mortality rates) associated with conditional cash transfer measures in Brazil: an ecological study
G Sampaio Morais 1; L Magno1,2; A Ferreira da Silva1; R Volmir Anderle1; LE Souza1; J Macinko3; I Dourado1; and D Rasella1
1Federal University of Bahia (UFBA), Institute of Collective Health (ISC), Salvador, Brazil. 2University of the State of Bahia, Life Science Department, Salvador, Brazil. 3University of California Los Angeles (UCLA), UCLA Fielding School of Public Health, Los Angeles, United States
Background: Brazil has long been recognized for its strong response to the HIV/AIDS epidemic. Although the epidemic is classified as stable at the national level, AIDS incidence, hospitalizations and mortality rates vary geographically. Brazil is also one of the most unequal countries in the world, and it has implemented in the last two decades one of the largest Conditional Cash Transfer (CCT) programmes, the Bolsa Familia Programme (BFP). BFP’s target populations are poor households earning between US$35 to 70 per person per month. It is important to note that the BFP has two conditionalities: the beneficiary families are obliged to keep the children in school and to be accompanied in health units. We aimed to evaluate the impact of BFP coverage on trends in the rates of AIDS incidence, hospitalizations and mortality in Brazil.
Methods: An ecological panel data study, with all 5507 Brazilian municipalities over the 2004 to 2012 period, was performed. We employed a fixed‐effects multivariate negative binomial model to estimate the association between BFP coverage – of the eligible poor population – classified as low (0% to 29%); intermediate (30% to 69%) and high (≥70%), and AIDS indicators, adjusting for all relevant covariates.
Results: At the national level, a BFP coverage of 70% or more of the poorest population, in municipalities with the highest AIDS incidence, was associated with a 10.3% (95% CI: 3.7 to 16.5) reduction in the incidence, with an even stronger effect among women (15%; 95% CI: 6.6 to 22.6) and children under 14 years old (38.7%; 95% CI: 20.9 to 52.5). Higher BFP coverage was also associated with a decline in AIDS‐related hospitalizations (26.4%; 95% CI: 18.0 to 34.0) and AIDS mortality rates (9.7%; 95% CI: 1.8 to 17.0).
Conclusions: This is the first study to evaluate the association between BFP coverage and trends in AIDS indicators in all Brazilian municipalities over a long period. BFP contributed to reduce the incidence, hospitalizations and mortality by AIDS in Brazil, which could be explained by both its money allowances and conditionalities. These results have important implications for countries with social protection measures such as conditional cash transfers. They are evidence of the impact of a policy that can be adapted to other low‐ and middle‐income countries with high socio‐economic inequalities.
OAD0101
Identifying implementation barriers and facilitators of an integrated PrEP and HIV service delivery model at public facilities in urban Uganda
D Thomas 1; K Ortblad1; T Muwonge2; S Namanda2; J Kibuuka2; M Nakitende2; F Nambi2; L Nakabugo2; A Mujugira2; and R Heffron1
1University of Washington, Global Health, Seattle, United States. 2Infectious Diseases Institute, Kampala, Uganda
Background: Practical HIV pre‐exposure prophylaxis (PrEP) delivery models for limited‐resource settings are critical for improving PrEP coverage and interrupting HIV transmission. This research uses technical assistance (TA) reports – a pragmatic data source – to understand implementation barriers and facilitators of an innovative PrEP delivery model that integrates PrEP and antiretroviral therapy (ART) delivery for HIV serodifferent couples in public health facilities in Kampala, Uganda (NCT03586128).
Methods: We used data from the Partners PrEP Programme (PPP); a stepped‐wedge cluster randomized trial that is testing an integrated model of oral PrEP and antiretroviral therapy (ART) delivery for HIV serodifferent couples at eight purposively sampled public health facilities in Kampala, Uganda. Technical advising teams, comprised of PPP staff, conducted monthly TA visits to implementing facilities to identify and address implementation challenges alongside facility staff. Findings were recorded in TA reports, which were completed using standardized forms, informed by the Consolidated Framework for Implementation Research (CFIR), to identify implementation barriers and facilitators. We used a content analysis approach to evaluate TA reports from January to December 2019 and assigned CFIR strength and valence ratings to understand the strength and magnitude of identified barriers and facilitators.
Results: Among the 39 reports from eight facilities (approximately 5 per facility), we identified 11 CFIR constructs. Key implementation facilitators included sensitizing and educating facility staff about PrEP (Knowledge and Beliefs about the Innovation); establishing formal and informal feedback and accountability mechanisms (Reflecting and Evaluating); and empowering facility staff to address implementation challenges (Self‐Efficacy). Key implementation barriers were related to ineffective recruitment and referral of eligible individuals from nearby facilities (Cosmopolitanism) as well as stockouts of laboratory reagents and testing supplies (Available Resources).
Conclusions: This analysis provides important context related to early implementation barriers and facilitators to inform scale‐up efforts for PrEP delivery within and beyond Uganda. Further, we found TA reports to be a pragmatic data source for assessing and documenting implementation challenges. Technical assistance reports provide a practical tool for assessing and addressing implementation challenges associated with expanded PrEP delivery. Future work will explore the identified key themes through in‐depth qualitative interviews with staff from implementing facilities.
OAD0102
Home‐based testing strategies for older adults in rural South Africa: a randomized controlled trial
ME Marcus1; N Mahlalela 2; D Drame3; FX Gomez‐Olive Casas2; J Rohr3; J Manne‐Goehler 4; S Tollman2; L Berkman3; K Kahn2; and T Bärnighausen5
1University of Goettingen, Goettingen, Germany. 2University of the Witwatersrand, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), Johannesburg, South Africa. 3Harvard University, Harvard Center for Population and Development Studies, Boston, United States. 4Harvard University, Department of Medicine, Boston, United States. 5Heidelberg Institute of Public Health, Faculty of Medicine, University of Heidelberg, Heidelberg, Germany
Background: Many older adults in rural South Africa still lack knowledge of their HIV status despite a high burden of HIV in this population. HIV self‐testing may offer a powerful approach to increase HIV testing in older populations. This study sought to establish the comparative effectiveness of three different home‐based HIV testing strategies for older adults in rural South Africa.
Methods: We randomized 3578 individuals in the “Health and Ageing in Africa: a Longitudinal Study of an INDEPTH Community in South Africa (HAALSI)” cohort study 1:1:1 to:
home‐based HIV rapid testing plus counselling;
home delivery of HIV self‐testing kits and
both home‐based HIV rapid testing plus counselling and home delivery of HIV self‐testing kits.
In a modified Poisson regression analysis yielding risk ratios, we estimate the treatment effects on our primary outcomes of
ever testing for HIV and
testing since trial enrolment.
Results: There was no significant difference in testing uptake or knowledge of HIV status across groups (see Table 1). However, respondents in the treatment arms containing self‐test kits were significantly more likely to test at home compared to the rapid testing only group, suggesting a preference for self‐testing in this population. We also found no adverse effects due to self‐test kits in any of our secondary outcomes, namely knowledge of HIV status, linkage to care for HIV and comorbidities, recent sexual partners, or HIV treatment uptake. Finally, being in either treatment arm with self‐testing significantly decreased depression scores by 0.5 to 0.6 points on the CESD‐20 scale.
Abstract OAD0102‐Table 1
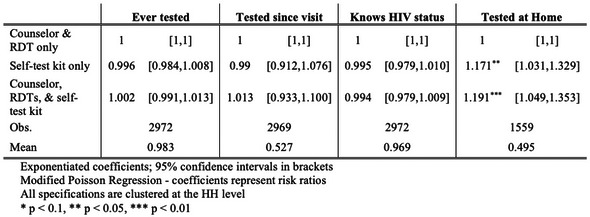
Conclusions: Our results indicate that HIV self‐testing is a safe and preferred home‐based testing option for older adults in rural South Africa, offering another promising policy tool in the effort to achieve the UNAIDS 90‐90‐90 targets.
OAD0103
Integrating Pre‐Exposure Prophylaxis Delivery in Decentralized Community HIV‐testing sites in rural KwaZulu Natal, South Africa
MK Kitenge 1,2; EC Casas3; P Isaakidis3; SJ Steele3; J Kunene4; and L Ohler1
1Médecins Sans Frontières (MSF), Eshowe, South Africa. 2Stellenbosch University, Department of Global Health, Division Epidemiology and Biostatistics, Cape Town, South Africa. 3Médecins Sans Frontières (MSF), Southern African Medical Unit, Cape Town, South Africa. 4Department of Health, King Cetshwayo District, KwaZulu‐Natal, South Africa
Background: Several demonstration projects have been conducted in urban‐based clinics across South Africa to explore pre‐exposure prophylaxis (PrEP) uptake in routine programmatic implementation conditions. However, retention in care (RIC) and adherence to integrating PrEP into decentralized community HIV‐ testing sites within a larger rural community remains unclear. We present preliminary results from a study evaluating PrEP delivery in rural community HIV testing sites under programmatic conditions.
Methods: This was a single‐arm study. From March 2019 to March 2020, daily oral PrEP was offered to HIV‐negative females aged 18 to 35 years, at four Médecins Sans Frontières (MSF)‐supported community HIV‐testing sites in Eshowe/Mbongolwane area, KwaZulu Natal, South Africa. Risk reduction counselling, adherence counselling, HIV‐testing, screening and treatment of sexually transmitted infections (STIs) were conducted at 3‐, 6‐ and 12‐month follow‐up visits. PrEP adherence was assessed measuring tenofovir diphosphate (TFV‐DP) blood concentrations at every visit. A threshold of ≥700 fmol/punch TFV‐DP concentration indicated adherence. Descriptive analysis of patient demographics, RIC and adherence were conducted.
Results: A total of 1564 participants were offered PrEP and 172 (11%) participants accepted PrEP initiation and enrolled in the study across sites. Participants’ mean age was 25 years (SD 5.6). 6.4% (11/172) participants tested positive for at least one STI at enrolment. Overall STI incidence was 0.15 cases per 100 person‐years (95% CI: 0.06 to 0.36).
Overall study retention at 12 months was 39.2%. Median discontinuation time was 145 days (IQR: 57 to 210) and PrEP was discontinued by 41 (24.0%) of the 172 participants at month three. Additionally, 17.5% (23/131), 29.6% (32/99),14.5% (11/67) of women discontinued PrEP at months 6, 9 and 12 respectively. The retention rate was higher in older participants (aged 25 to 35 years) than in younger participants (aged 18 to 24 years), 48.4% and 28.4% respectively. Adherence at three months was 57% (53/93) and 53.4% (31/58) at six months.
Conclusions: We observed relatively low PrEP uptake, retention and adherence. However, PrEP retention was higher among older women. This study underscores existing evidence that PrEP adherence remains a challenge. Strategies to address retention and adherence, including adherence support groups, peer‐mentors and cash incentives should be further explored.
OAD0104
From Internet to the health centre: WhatsApp as a tool to promote HIV testing among men who have sex with men recruited online
L Menacho Alvirio 1; and G Diaz Gervasi1
1Universidad Peruana Cayetano Heredia, Instituto de Medicina Tropical Alexander Von Humboldt, Lima, Peru
Background: HIV testing uptake remains low among MSM in Peru. As a result, many people are not aware of their status and cannot be linked to HIV care. The objective of this study was to assess the efficacy of a mobile app‐based intervention to increase HIV testing among MSM recruited online.
Methods: In this randomized controlled trial, Peruvian MSM, 18 years or older, HIV negative, without a recent HIV testing (last six months) were recruited online and randomly assigned to an intervention or control group. Participants were recruited online using Ads in Facebook and in two of the most visited local websites by gay men. After being recruited online, participants completed a short baseline survey. Later, a trained health worker used the mobile application WhatsApp to follow each participant for 12 weeks. They shared different topics of interest with an emphasis on HIV prevention in the second half of the period of follow‐up. Participants in control groups received standard of care. The main outcome was the number of participants who got an HIV test at one of the health centres of the research project.
Results: Participants were recruited between July and November 2015. A total of 400 participants were randomly assigned to the intervention group (n = 200) or the control group (n = 200). Eighty‐two participants (41%) in the intervention group and 17 (8.5%) in the control group went to the health centre to receive an HIV test (adjusted odds ratio: 7.64; 95% CI: 4.3 to 13.5).
Conclusions: Our combined strategy based on trained health workers using a mobile application for follow‐up, was efficacious to take MSM recruited online to a health centre for HIV testing. It is important to use mobile applications that are already widely used and accepted by the target population.
OAD0105
Barriers to expanding PrEP uptake among cisgender African American women in the South
C Psaros 1; G Goodman1; A Blyler1; C Ott2; J Wise2; K Kudroff2; L Elopre3; D Krakower4; and M‐C Kempf5
1Massachusetts General Hospital, Behavioral Medicine, Boston, United States. 2University of Alabama Birmingham, Birmingham, United States. 3University of Alabama Birmingham, Medical Education, Birmingham, United States. 4Beth Israel Deaconess Medical Center, Infectious Disease, Boston, United States. 5University of Alabama Birmingham, Nursing, Birmingham, United States
Background: Pre‐exposure prophylaxis (PrEP) is a potent biomedical tool for HIV prevention; however, PrEP is underutilized among African American (AA) women in the Deep South of the United States, despite an inequitable HIV burden. This study explored patient and provider perceptions, attitudes and preferences for PrEP service delivery, aiming to identify social, behavioural and cultural factors influencing uptake among AA women.
Methods: In‐depth, semi‐structured qualitative interviews were conducted among cisgender AA women at risk for HIV (based on sexual activity in the last six months), both with PrEP experience (N = 6) and without PrEP experience (N = 15), as well as providers (N = 20) from two federally qualified health centres (FQHCs) and HIV service agencies serving rural communities in Alabama. Data were coded in NVivo software (v.12), and analysed using content analysis.
Results: Overall attitudes and perceptions of PrEP among AA women and providers were positive; however, numerous barriers to widespread PrEP uptake were discussed. Barriers to effective patient–provider relationships reported by AA women included perceived discrimination (e.g. based on patients’ income, education, and/or race), a lack of provider empathy (e.g. feeling judged due to one’s health status or circumstances), a lack of shared medical decision making, general healthcare‐related anxiety, difficulty assessing one’s true HIV risk, and a desire for providers to view patients’ health more holistically. Both patients and providers desired an “under one roof” approach, whereby all PrEP‐related services are consolidated (e.g. prescribers, pharmacy, laboratories, educational materials), along with mechanisms for reducing PrEP‐related costs and improving access. Participants also discussed a need for normalization of PrEP use specifically among AA women, via increased and frequent visibility in public‐facing contexts (e.g. marketing campaigns, incorporation of PrEP information into standard medical appointment checklists), as well as increased PrEP training for providers.
Conclusions: These data identify key determinants that will influence PrEP uptake among cisgender AA women in the South receiving care at FQHCs and HIV service organizations. Individual, structural and system level barriers were identified that will inform adaptations of effective patient–provider communication interventions to be formally tested using implementation science frameworks (i.e. Exploration, Preparation, Implementation, Sustainment [EPIS] and Dynamic Adaptation Process [DAP] frameworks).
OAD0201
Improving health equity and ending the HIV epidemic in the United States: a distributional cost‐effectiveness analysis in six cities
AML Quan 1,2; C Mah2; E Krebs2,3; X Zang4; S Chen2; K Althoff5; W Armstrong6; CN Behrends7; J Dombrowski8,9; E Enns10; DJ Feaster11; K Gebo12; W Goedel4; M Golden8,9; BDL Marshall4; S Mehta12; A Pandya13; BR Schackman7; S Strathdee14; P Sullivan15; H Tookes16; B Nosyk2,3; and Localized HIV Modeling Study Group
1University of Toronto, Dalla Lana School of Public Health, Toronto, Canada. 2Simon Fraser University, Faculty of Health Sciences, Burnaby, Canada. 3BC Centre for Excellence in HIV/AIDS, Vancouver, Canada. 4Brown University, Department of Epidemiology, School of Public Health, Providence, United States. 5Johns Hopkins University, Department of Epidemiology, Bloomberg School of Public Health, Baltimore, United States. 6Emory University, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, United States. 7Cornell University, Department of Population Health Sciences, Weill Cornell Medical College;, New York, United States. 8University of Washington, Department of Medicine, Division of Allergy & Infectious Disease, Seattle, United States. 9Public Health – Seattle & King County, HIV/STD Program, Seattle, United States. 10University of Minnesota, Division of Health Policy and Management, Minneapolis, Canada. 11University of Miami, Department of Public Health Sciences, Leonard M. Miller School of Medicine, Miami, United States. 12Johns Hopkins University, Bloomberg School of Public Health, Baltimore, United States. 13Harvard University, T.H. Chan School of Public Health, Cambridge, United States. 14University of California San Diego, School of Medicine, San Diego, United States. 15Emory University, Department of Epidemiology, Atlanta, United States. 16University of Miami, Department of Medicine, Miller School of Medicine, Miami, United States
Background: In the United States, Black and Hispanic/Latinx individuals continue to be disproportionately impacted by HIV. Applying a distributional cost‐effectiveness framework, we estimated the distributional health impacts and cost‐effectiveness of two combination implementation approaches to determine the approach that best meets the objectives of improving population health and reducing racial/ethnic health disparities.
Methods: We adapted a dynamic HIV transmission model to characterize HIV microepidemics in six US cities: Atlanta, Baltimore, Los Angeles, Miami, New York and Seattle. We considered combinations of 16 evidence‐based interventions to diagnose, treat and prevent HIV transmission, implemented by race/ethnicity in proportion to 1) existing service levels (proportional services approach) and 2) the distribution of new diagnoses by race/ethnicity (between Black, Hispanic/Latinx and white/other individuals; equity approach). We estimated total costs, quality‐adjusted life‐years (QALYs), and incremental cost‐effectiveness ratios of strategies implemented from 2020 to 2030 (health‐care perspective; 20‐year time horizon; 3% annual discount rate). We identified city‐specific optimal strategies as the cost‐effective bundle that produced the highest health benefit. Using optimal strategies under each approach, we estimated three measures of health inequality (Between‐Group Variance, Index of Disparity, Theil Index), HIV incidence and incidence rate ratios.
Results: In all cities, optimal combination strategies under the equity approach generated more QALYs than those with proportional services, ranging from a 3.1% increase (95% CrI: 1.4% to 5.3%) in New York to more than double (101.9% [75.4% to 134.6%]) in Atlanta. Compared to proportional services, the equity approach delivered lower costs over 20 years in 4/6 cities, differences ranged from $74.0M ($6.9‐$164.6M) in Miami to $574.3M ($252.3 to 934.6M) in Atlanta. Incidence reductions in 2030 were greatest under the equity approach in all cities (up to 80.7% [71.0% to 86.1%] in Baltimore, Figure).
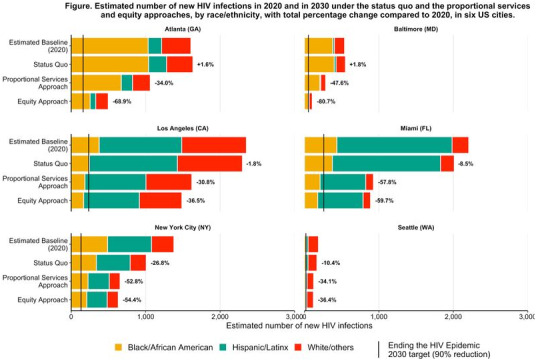
Abstract OAD0201‐Figure 1.
Conclusions: Equity‐focused HIV combination implementation strategies that reduce disparities for Black and Hispanic/Latinx individuals can significantly improve population health, reduce costs and drive progress toward Ending the HIV Epidemic.
OAD0202
Leveraging community ART dispensation through community health volunteers to enhance ART retention among the pastoralist PLHIVs of lower socio‐economic status in Kajiado: a case of Oltepesi Dispensary
P Nyaribo 1; T Wabwire1; A Bwire1; J Wanyonyi2; S Sarune2; and R Ndunge3
1African Development and Emergency Organization (ADEO), Kajiado County, Kenya. 2Kenya Red Cross Society (KRCS), Kajiado County, Kenya. 3Kajiado County Department of Health, Kajiado County, Kenya
Background: Oltepesi Dispensary is in Kajiado West, which has vast distances between villages and nearest healthcare facilities. Major barriers to retention are distance to healthcare facility and socio‐economic status. Funded by Global Fund‐Kenya Red Cross Society since 2018, ADEO supports PLHIVs at the dispensary using CHVs. CHVs are allocated a 15 to 20 PLHIV cohort for adherence support to improve adherence, reducing defaulter rate and ensure clients take charge of their health.
Description: Community ART dispensation strategy was adopted. CHVs pick ARVs from the facility, and deliver to PLHIV at home. It deviates from Community ART Groups where PLHIV pick drugs for their peers. It strengthened home visits where health talks are done involving adherence counselling, nutritional education and psychosocial support. The PLHIV’s inability to pick drugs from the facility due to distance barrier and transport cost prompted the intervention. CHVs voluntarily used their motorcycles in delivering drugs to PLHIVs and conducted enhanced adherence counselling. In 2018, the viral suppression rate was 20%. Aggressive community dispensation using CHVs during home visits increased it to 71% in 2019. In 2020, 53 clients were on care; 23 of 27 with a viral load result were suppressed (85%).
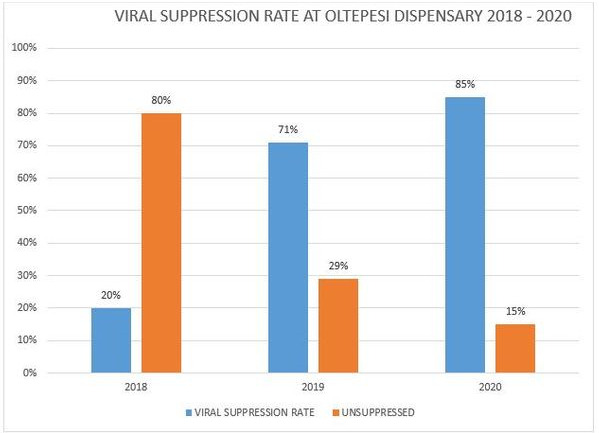
Abstract OAD0202‐Figure 1. Graph showing viral suppression rate at Oltepesi Dispensary since 2018 when ADEO started implementing the TCS programme.
Lessons learned: Evidence‐based interventions modelled on community ART dispensation are essential in reaching nomadic communities and clients where the distance to the nearest healthcare facility and between households is large. CHVs bridge the distance between PLHIVs and their care facilities. SCHMTs, CHAs and implementing partners must customize interventions for specific demographics.
Conclusions/Next steps: CHVs are vital in retaining PLHIVs on ART. Strengthening community ART dispensation in remote areas with limited infrastructure is essential in attaining the 90‐90‐90 objectives. HIV/AIDS programmes must consider integrating community dispensation using CHVs in areas where the distance to the healthcare facility is large.
OAD0203
Targeted virtual HIV‐sensitive case management of children and adolescents living with HIV amidst COVID‐19 in Zimbabwe: insights from Family AIDS Caring Trust (FACT) Orphaned and Vulnerable Children (OVC) Programme in Zimbabwe
D Chimedza 1; G Shumba1; J Tavengerwei1; B Mukome1; T Takaidza1; T Mugoni1; G Mavheneke1; C Chigaba1; E Nyakanda1; T Nyatsana1; R Makurumidze1; A Gwasira2; and S Chidiya3
1Family AIDS Caring Trust, Safeguarding and Sustainable Livelihoods, Mutare, Zimbabwe. 2Ministry of Health and Child Care, Community Health, Mutasa District, Zimbabwe. 3USAID, Health, Population and Nutrition, Harare, Zimbabwe
Background: With support from US President’s Emergency Plan for AIDS Relief, FACT Zimbabwe is implementing Children Tariro (CT) programme to mitigate the impacts of HIV/AIDS among Zimbabwe’s OVC, 0 to 17 years. CT contributes towards the achievement of UNAIDS’ 95‐95‐95 global goals by supporting the most HIV‐affected children and their families in six districts in Manicaland and Masvingo; two provinces in Zimbabwe to access HIV treatment, care and support and GBV prevention and clinical care.
Description: In Q1 October 2020, CT enrolled 5381 out of the targeted 6524 Children and Adolescents Living with HIV (CALHIV) from 238 public and faith‐based health facilities for HIV antiretroviral treatment (ART) adherence support. The remaining 1143 CALHIV on ART line‐listed for saturation follow‐up. CT partnered with the health facilities and community health workers (CHWs) to identify, track and support the targeted CALHIV and their families with ART adherence and psychosocial support, viral load testing and enhanced ART adherence counselling for those with high viral load. CT also referred to the Department of Social Development CALHIV with high viral load and their caregivers in desperate need for food consumption support. COVID‐19’s outbreak in Zimbabwe in March 2020 resulted in lockdown and mobility restrictions that affected access to the indicated support. CT provided partner health facilities and CHWs with airtime to continue implementing the interventions virtually through SMS, WhatsApp and phone calls.
Lessons learned: From the first to the second quarter 2020, we retained 6009 CALHIV on CT support. This represented a 10% increase in enrolment of CALHIV and indicated effective follow‐up of 628 of the 1143 CALHIV. In quarters three and four, our reach declined to 6002 and 5932 CALHIV respectively. These declines were because 59 CALHIV had aged out of CT support, 14 moved to non‐CT districts and 4 deceased from opportunistic infections. The data demonstrated uninterrupted access to CT support by targeted CALHIV and their families even after pivoting to virtual case management
Conclusions/Next steps: CT programme results highlight the efficacy of virtual case management for the most HIV‐affected CALHIV amidst COVID‐19.
OAD0204
Men missing from the HIV care continuum: a meta‐analysis and meta‐synthesis
M Nardell 1,2,3; O Adeoti4,5; C Peters6; A Tsai7,8,2; and I Katz9,10,2
1Brigham and Women's Hospital, Division of Global Health Equity, Department of Medicine, Boston, United States. 2Harvard Medical School, Boston, United States. 3Beth Israel Deaconess Medical Center, Department of Medicine, Boston, United States. 4Boston University School of Medicine, Boston, United States. 5Boston Medical Center, Boston, United States. 6University of Iowa, Department of Epidemiology, Iowa City, United States. 7Massachusetts General Hospital, Center for Global Health, Boston, United States. 8Massachusetts General Hospital, Mongan Institute, Boston, United States. 9Harvard Global Health Institute, Cambridge, United States. 10Brigham and Women's Hospital, Division of Women's Health, Department of Medicine, Boston, United States
Background: Men continue to fall off the HIV care continuum. Understanding where and how this happens is critical to achieving UNAIDS 95‐95‐95 goals. We sought to estimate the proportions of men meeting each of the 95‐95‐95 goals across studies in sub‐Saharan Africa and summarize qualitative evidence on factors influencing their care engagement.
Methods: We conducted a systematic review of peer‐reviewed literature in PubMed and Embase between 2014 and 2020. The meta‐analysis included studies in sub‐Saharan Africa involving men ≥15 years with data from 2009 onward and reporting on one or more of the 95‐95‐95 goals. We estimated pooled proportions using DerSimonion‐Laird random effects models. We quantified heterogeneity by country, setting (healthcare vs. community), outcome definition (e.g. the threshold for viral load suppression) and study quality. We used meta‐synthesis to summarize qualitative studies exploring barriers to men’s HIV care engagement in sub‐Saharan Africa and to develop a third‐order interpretation of the data.
Results: From 14,670 studies screened, 130 studies were included in the meta‐analysis. Forty‐seven studies reported data on knowledge of serostatus, 45 studies reported data on ART use and 75 studies reported data on viral suppression. We estimated the proportions of men meeting the 95‐95‐95 goals: knowledge of serostatus, 0.49 (95% CI, 0.41 to 0.58); being on ART, 0.57 (95% CI, 0.50 to 0.64) and achieving viral suppression, 0.79 (95% CI, 0.77 to 0.80). In studies including both men and women, compared with women, a lower proportion of men knew their serostatus (0.53 [95% CI, 0.44 to 0.63] vs. 0.66 [95% CI, 0.59 to 0.73], p = 0.04) or were virally suppressed (0.79 [95% CI, 0.77 to 0.80] vs. 0.81 [95% CI, 0.80 to 0.83], p = 0.01). Heterogeneity was high and partially explained by variation in the study population, study setting and outcome definition. The meta‐synthesis included 40 studies and identified three third‐order labels encompassing barriers to men’s care engagement: mistrust of the health system, poverty and perceived threats to heteronormative masculinity.
Conclusions: Men in sub‐Saharan Africa are falling behind especially in testing and treatment. Interventions that improve trust in the health system, provide affordable, convenient care and which fundamentally change masculine norms are needed to better engage men in HIV care.
OAD0205
Structural vulnerability and the impacts of the COVID‐19 pandemic on HIV risk behaviours and prevention needs among people who inject drugs
A Bazzi 1; C Buchholz2; and J Syvertsen3
1University of California, San Diego, Herbert Wertheim School of Public Health, La Jolla, United States. 2Boston University, School of Public Health, Boston, United States. 3University of California, Riverside, Department of Anthropology, Riverside, United States
Background: In many regions of the United States, continued high levels of opioid and polysubstance use have contributed to new HIV outbreaks among people who inject drugs (PWID). The unprecedented COVID‐19 pandemic, as well as measures undertaken to mitigate it, may have altered the HIV prevention needs of PWID. To inform HIV prevention services for this population, we explored PWID experiences in the COVID‐19 context.
Methods: From July to December 2020, we partnered with diverse syringe service programmes (SSPs) across Massachusetts to recruit individuals ≥18 years old reporting past‐month injection drug use. SSP staff introduced interested individuals to offsite study personnel using secure video‐conferencing on tablets in private indoor and outdoor spaces. Trained interviewers obtained verbal informed consent before administering brief quantitative surveys and in‐depth qualitative interviews via video. Thematic analysis identified common experiences related to HIV risks and prevention needs in the COVID‐19 context.
Results: Among 27 participants, the median age was 35 years (IQR: 30 to 43). Sixteen (59%) identified as male, 11 (41%) as female; 24 (89%) identified as white, 10 (37%) as Hispanic, 3 (11%) as Black. All 27 (100%) injected heroin/fentanyl and 22 (81%) also injected cocaine/crack (past month); additional drugs used recently included benzodiazepines (n = 12; 44%) and methamphetamine 9 (33%). Injection frequency was high, with 12 (44%) injecting ≥10 times daily. In the COVID‐19 context, most participants described having the “same routine,” with minimal changes to their injection behaviours or syringe access. However, participants discussed numerous structural challenges worsened by COVID‐19, including difficulty securing income (e.g. reduced ability to “hustle”) and reduced access to housing, healthcare, and addiction treatment. Participants also described stigmatizing experiences of being “presumed positive” for COVID‐19 within healthcare and social service settings.
Conclusions: Rather than drastically altering individuals’ injection behaviours, our findings illustrate how large‐scale public health emergencies like COVID‐19 may impact HIV vulnerability among PWID indirectly through changes in social and structural contexts. Despite initial SSP closures, expanded mobile outreach across this region helped PWID maintain access to sterile syringes. PWID narratives instead highlighted how COVID‐19 exacerbated structural vulnerability by destabilizing access to essential services while intensifying stigma through the conflation of addiction, homelessness and infectious diseases.
OAD0301
Gender‐based violence shadows COVID‐19: Increased sexual violence, HIV exposure and teen pregnancy among girls and women in Uganda
R Apondi 1; AC Awor1; LJ Nelson1; J Cheptoris2; F Ngabirano3; CD Egbulem1; S Alamo1; LA Mills1; and J Hegle4
1Division of Global Health HIV & TB, Center for Global Health, Division of Global Health HIV and TB, US Centers for Disease Control and Prevention, Kampala, Uganda. 2Ministry of Health, Kampala, Uganda. 3Ministry of Gender, Labor and Social Development, Kampala, Uganda. 4Division of Global Health HIV & TB, Center for Global Health, Division of Global Health HIV and TB, US Centers for Disease Control and Prevention, Atlanta, United States
Background: The COVID‐19 pandemic is associated with increased gender‐based violence (GBV) perpetration. However, the COVID‐19 response did not prioritize GBV services, negatively impacting post‐violence care ser vice access among girls/women in Uganda. We analysed routine programme data to correlate COVID‐19 restrictions with GBV violence reports, post‐exposure prophylaxis (PEP) uptake and teen pregnancy among Ugandan females.
Methods: Violence data from the Uganda Health Management System (HMIS) and Ministry of Gender Labor and Social Development reports (Uganda Child‐Helpline (UCHL)) were analysed. The analysis included reports involving females (all ages) from HMIS and females aged <18 years from UCHL. Two six‐month time periods were compared: October‐2019 to March‐2020 (pre‐COVID‐19 period) and April‐2020 to Septempter‐2020 (COVID‐19 period). From HMIS, selected outcome variables for sexual violence were post‐rape reports and PEP uptake; from UCHL selected outcomes were sexual violence (SV) reports and reported teen pregnancy. Frequency distributions to measure prevalence and chi‐square statistics were calculated to assess significant differences and computed odds of occurrence associated with time period.
Results: In pre‐COVID‐19 period, 17,702 females reported for post‐rape care and 3274 received PEP compared to 22,013 and 3348, respectively, during COVID‐19 period. This translates to a 24% increase in post‐rape reports and 18% reduction in PEP uptake between two periods. The odds of receiving PEP during COVID‐19 period were 0.79 times (95% CI 0.75 to 0.83) lower compared to pre‐COVID‐19 period. Over 50% of those who reported post‐rape care after the recommended 72‐hour intervention timeframe cited lockdown restrictions as the main reason for coming late.
In pre COVID‐19 period, 593 girls reported SV, and 73 reported teen pregnancy compared to 860 SV and 117 teen pregnancies in COVID‐19 period. The odds of reporting SV during COVID‐19 period was 1.30 times (95% CI: 1.12 to 1.51) higher compared to pre‐COVID‐19 period. There was a 17% increase in teen pregnancy between two periods, not statistically significant (OR 1.121, 95% CI, 0.82 to 1.53).
Conclusions: During Uganda’s COVID‐19 lockdown, sexual violence reports increased, increasing HIV exposure in national data, taking into consideration possible underestimated true GBV increase associated with COVID‐19 related disruptions. Investment in unhindered, flexible and adaptable GBV mitigation is important during pandemics.
OAD0302
Violence across the life course and opportunities for intervention design: findings from the Maisha Fiti study with female sex workers in Nairobi, Kenya
TS Beattie 1; R Kabuti2; P Shah1; A Beksinska1; E Nyakiri2; H Babu2; M Kungu2; TMFS Champions2; C Nyabuto2; M Okumu2; A Mahero2; P Ngurukiri2; Z Jama2; E Irungu2; W Adhiambo2; P Muthoga2; R Kaul3; H Weiss4; J Seeley1; and J Kimani2
1London School of Hygiene and Tropical Medicine, Global Health and Development, London, United Kingdom. 2Partners for Health and Development for Africa, Nairobi, Kenya. 3University of Toronto, Toronto, Canada. 4London School of Hygiene and Tropical Medicine, London, United Kingdom
Background: Violence against women and girls is associated with an increased risk of HIV acquisition, with effects both direct (e.g. rape) and indirect (e.g. from child maltreatment). We examined violence experiences among female sex workers (FSWs) and how this relates to HIV risk using a syndemics and life‐course perspective, in order to identify opportunities for interventions.
Methods: Maisha Fiti is a mixed‐methods longitudinal study with FSWs aged 18 to 45 years randomly selected from across Nairobi. Baseline behavioural‐biological surveys (n = 1003) were conducted June‐December 2019. Violence was assessed using the WHO Adverse Childhood Experiences (ACE) and Violence Against Women questionnaires. Harmful alcohol and substance use were assessed using the WHO ASSIST Tool. Descriptive statistics and multivariable logistic regression models were used to examine violence across the life‐course, and correlates of recent (past six months) sexual or physical violence experience.
Results: 1003 FSWs participated; HIV prevalence was 28.0%. Reports of adverse experiences in childhood were high: 41.4% were orphaned, 12.0% lived on the streets and 79.3% experienced physical or sexual violence. We found substantial overlap between violence in childhood, and subsequent partner and non‐partner violence in adulthood, with 72.2% reporting multiple types of violence. We also found high levels of recent violence (past six months), with 64.9% reporting physical or sexual violence, 30.7% police arrest, 2.4% gang rape and 2.8% rape in the past seven days. In adjusted analyses, recent sexual or physical violence (by any perpetrator) was associated with a high ACE score (AOR 5.3 (95% CI 3.3 to 8.7)), forced sexual debut (AOR 1.4 (1.0 to 2.0)) ever being married/co‐habiting (AOR 1.5 (1.0 to 2.1)), recent hunger (AOR 1.3 (1.0 to 1.8)), recent police arrest (AOR 2.3 (1.6, 3.2)), current harmful alcohol or substance use (AOR 1.6 (1.2 to 2.2)) and condomless last sex (AOR 1.4 (1.0 to 1.9)).
Conclusions: We report strong evidence of concurrent and sequential violence victimization across the life‐course by different perpetrators, and syndemics with harmful alcohol and substance use and HIV risk. Large‐scale holistic violence interventions are needed to reduce violence against women and girls in this setting. Interventions which focus on violence prevention during childhood and adolescence should help prevent future adverse trajectories, including HIV risk.
OAD0303
Sexual violence is longitudinally associated with reduced likelihood of viral suppression among transgender women living with HIV in Brazil
AM Leddy 1; J Sevelius1; G Saggese1; KC Bassichetto2; H Gilmore1; PGC de Carvalho2; LF Maschião2; MAdSM Veras2; and SA Lippman1
1University of California, San Francisco, Medicine, San Francisco, United States, 2Santa Casa School of Medical Sciences, São Paulo, Brazil
Background: Globally, transgender women (TGW) are disproportionately affected by HIV and gender‐based violence (GBV), defined as physical, sexual and emotional violence perpetrated against an individual based on their gender identity or expression. While a growing body of evidence demonstrates that GBV prevents engagement in HIV care and treatment among cis‐gender women, less research has examined this association among TGW. We conducted a longitudinal analysis to assess the impact of GBV on viral suppression among TGW living with HIV in Brazil.
Methods: A pilot trial of a peer navigation intervention to improve engagement in HIV care and treatment among TGW was carried out in São Paulo, Brazil between 2018 and 2019. TGW living with HIV were recruited and randomized into the intervention or control and participated in a baseline and nine‐month follow‐up survey. Surveys assessed self‐reported lifetime experiences of physical and sexual abuse. Laboratory confirmed viral suppression, defined as viral load less than 1000 copies/mL, was extracted from patient medical charts. We conducted an intent‐to‐treat analysis, whereby those with missing medical records data (n = 23) were assumed to not be virally suppressed. We used generalized linear model regressions with a Poisson distribution to estimate the relative risk (RR) for the association of physical and sexual violence at baseline with viral suppression at follow‐up, adjusting for baseline sociodemographic characteristics.
Results: A total of 113 TGW were enrolled. Retention was 70% at follow‐up. At baseline, the mean age was 33 years and 27% were living below the international poverty line. Over half of the participants (62%) reported lifetime physical violence and 45% reported lifetime sexual violence. At follow‐up, 32% had confirmed viral suppression. In adjusted models, lifetime physical violence was not significantly associated with viral suppression (RR: 0.69; 95% CI: 0.40, 1.20; p = 0.19). Lifetime sexual violence was significantly associated with a 58% reduction in viral suppression (RR: 0.42; 95% CI: 0.22, 0.81; p = 0.01).
Conclusions: Our findings are among the first to demonstrate that lifetime experiences of sexual violence are longitudinally associated with a reduced likelihood of viral suppression among TGW. Interventions seeking to improve antiretroviral therapy adherence should assess and address experiences of GBV among this population.
OAD0304
Do childhood and adolescence sexual violence experiences relate to HIV testing and PrEP uptake in young adulthood among MSM of colour living in an urban area of the United States?
M Mosley 1; L Wilton2; E Greene1; H Tieu3; M Paige1; V Nandi3; and V Frye1
1CUNY School of Medicine, Community Health and Social Medicine, New York, United States. 2SUNY Binghamton, Binghamton, United States. 3New York Blood Center, New York, United States
Background: Meta‐analyses have established that gay, bisexual and other men who have sex with men (MSM) who experienced childhood sexual abuse are significantly more likely to report HIV‐related outcomes, such as condomless anal intercourse, sex after alcohol and drug use and HIV acquisition. A recent study in the United States (US) on the impact of “adverse childhood experiences” (ACEs) on MSM sexual health found that ACEs were significantly associated with condomless anal intercourse. However, no significant association was reported between ACE exposure and HIV testing, the gateway to post‐/pre‐exposure HIV prophylaxis (PEP/PrEP), a cornerstone of the HIV prevention strategy in the US. Limited data are available on the association between childhood/young‐adulthood sexual violence experiences (SVEs) and PrEP use in later life among MSM of colour.
Methods: Using baseline data from TRUST, an NIH‐funded randomized and attention‐control trial of an HIV self‐testing intervention for young, Black/African‐American MSM, and standard uni‐ and bi‐variate descriptive statistics (Chi‐square and t‐tests), we assessed relations among SVEs on recent HIV testing in the past three months and recent PrEP use among 372 HIV‐negative MSM of colour. SVEs assessed perceptions of “first experience of anal sex” and asked participants to recall if it was “forced,” “coerced,” “non‐consensual” or “for survival.” We also assessed if they had a “sexual experience prior to age 18 that was forced, pressured or otherwise unwelcome.”
Results: Nearly a third of the sample (mean age = 24 years, SD = 5) experienced forced or pressured first sexual experiences; nearly two‐thirds reported a sexual experience prior to age 18 with a partner five or more years older and/or that was forced, coerced, or otherwise unwanted. In bivariate analyses, no associations were found between SVE and recent HIV testing or PrEP use among this sample of MSM of colour.
Conclusions: Although no associations were identified between childhood/adolescence SVEs and HIV testing and PrEP uptake in later life, provider knowledge of exposures is needed to deliver optimal, trauma‐informed sexual healthcare to MSM of colour. More research is needed to determine whether experiences of adulthood violence and/or an accumulation of childhood SVEs/ACEs over time relate to multiple HIV preventive behaviours among MSM of colour.
OAD0305
Using the Girl Group Leadership Model (GGLM) in improving economic strengthening to reduce new HIV incidences among adolescent girls and young women in Homabay County, Kenya
N ASIRA 1,2; and M MUIA3,2
1PATH/Afya Ziwani DREAMS Project, Homa Bay, Kenya. 2NOPE – Kenya, Nairobi, Kenya. 3PATH/Afya Ziwani DREAMS Project, Nairobi, Kenya
Background: Adolescent Girls and Young Women (AGYW) need leadership skills for personal development, economic empowerment and networking. Most AGYW lack opportunities to assume leadership in their health and life goals.
Description: The aim of GGLM IS to decrease the AGYW involvement with cross‐generational sex, multiple partners and transactional sex through economic empowerment. This model increased the networks and safety nets of the AGYW and championed gender‐based prevention of HIV. The GGLM incorporated leadership training in all facets of economic empowerment where the AGYW self‐lead themselves through economic empowerment processes. The GGLM involved a lot of girl groups whose membership was between 25 and 30 AGYW.
Lessons learned: GGLM created an opportunity for AGYW to gain social protection hence boosting their ability to reduce the risks of acquiring new HIV infections. Through GGLM, the AGYW have been able to start businesses and village savings and loaning groups. Half of the Girl groups created in the implementation period have since transacted more businesses than when they had not been exposed to this girl leadership model. The AGYW have been able to receive interventions such as entrepreneurship training, microenterprise start‐up support and facilitated access to employment and internships. GGLM as a best practice has enabled AGYW to rise into positions of leadership not only in their groups but also in the programmatic implementation as programme associates. Some groups have been registered with the ministry of Youth Gender and Social services to operate businesses across the country. These girl groups enabled AGYW to survive the COVID 19 pandemic period in the country. For example Superstars – a girl group in Homabay County who has adopted the GGLM started soap making business during the COVID 19 pandemic and made 10,000 Kenyan Shillings ($92) in loans to its members.
Conclusions/Next steps: Systematic strengthening of AGYW in Leadership gives them an edge to express themselves better, network and champion matters affecting them. Girl Group Leaders through a comprehensive model has enhanced retention and motivation for DREAMS beneficiaries in uptake of services. GGL model is an enabler for adaptation, resiliency and economic empowerment for vulnerable girls and young women towards sustainability.
OAD0401
Trends in HIV testing yield need to be interpreted within the context of changing testing patterns: analysis of individual‐level programme data from Zimbabwe’s national sex work programme, 2009 to 2019
HS Jones 1; S Musemburi2; L Chinyanganya3; A Takaruza3; ST Chabata3; P Matambanadzo3; B Rice1; JR Hargreaves1; B Hensen4; and FM Cowan3,5
1London School of Hygiene and Tropical Medicine, Public Health and Policy, London, United Kingdom. 2Ministry of Health and Child Care, AIDS and TB Prevention Department, Harare, Zimbabwe. 3Centre for Sexual Health and HIV/AIDS Research (CeSHHAR) Zimbabwe, Harare, Zimbabwe. 4London School of Hygiene and Tropical Medicine, Epidemiology and Population Health, London, United Kingdom. 5Liverpool School of Tropical Medicine, Global Health, Liverpool, United Kingdom
Background: Yield is used as a marker of HIV programme performance, but is increasing yield a valid indicator of success? Understanding the influence of HIV testing delivery and individual testing frequency could enhance our interpretation of this indicator and improve programme implementation. We analysed yield in the context of increased testing coverage among women accessing Zimbabwe’s national sex work programme (Sisters).
Methods: We analysed HIV test data and self‐report testing history among female sex workers attending Sisters clinics between 2009 and 2019. We defined yield as the proportion of tests delivered by the programme that were HIV positive. We used logistic regression to analyse yield over three time periods: 2009 to 2013, 2014 to 2016 and 2017 to 2019. We adjusted for confounding by demographic factors and included testing frequency in our model, defined as a woman having last tested within six months, to investigate its mediating role.
Results: During the 10‐year study period, 54,503 tests were recorded among 39,462 women, with increasing numbers of clinic sites and women reached and tested over time. While individual testing frequency increased, both within and outside Sisters, programme testing yield decreased (Figure 1). Between 2017 and 2019, yield was 9.6% (2608/27,024), compared to 47.9% (1934/4039) between 2009 and 2013 (aOR 6.1 95% CI 4.7 to 7.9) and 18.8% (4417/23,440) between 2014 and 2016 (aOR 2.2 95% CI 1.9 to 2.4). Including testing frequency in our model reduced odds ratios for yield between 2009 and 2013 (aOR 2.8 95% CI 2.1 to 3.6) and 2014 to 2016 (aOR 1.9 95% CI 1.7 to 2.1) compared to 2017 to 2019.
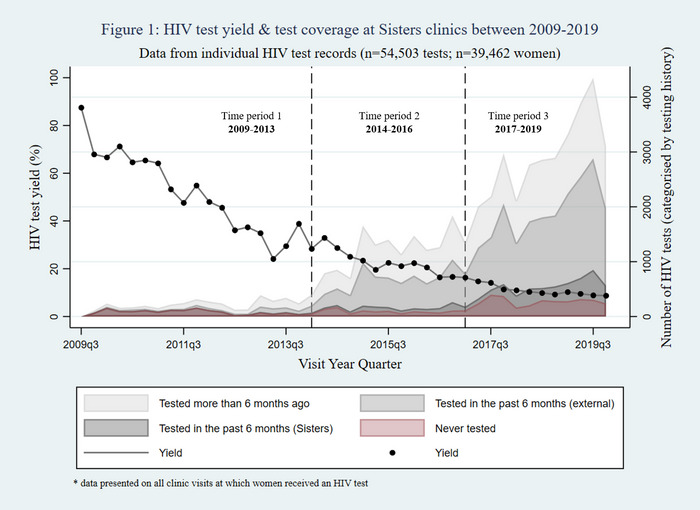
Abstract OAD0401‐Fgure 1.
Conclusions: Yield decreased among women testing through Sisters, with evidence this was mediated by more frequent testing. Earlier in the programme, HIV‐positive tests were likely longer standing undiagnosed infections, with more recent infections being picked up in later time periods. We recommend that, for yield to be a useful programme indicator, consideration needs to be given to the impact of changing testing patterns and what can be learned from this.
OAD0402
Acceptability and challenges of self‐collected rectal swab for sexually transmitted infections testing among men who have sex with men and transgender women in Kigali, Rwanda
JO Twahirwa Rwema 1; N Okonkwo2; MM Hamill3; E Uwizeye4; JD Makuza5; CE Lyons1; B Liestman1; J Nyombayire6; S Nsanzimana5; P Mugwaneza5; P Sullivan7; S Allen8; E Karita6; and S Baral1
1Johns Hopkins Bloomberg School of Public Health, Epidemiology, Baltimore, United States. 2Johns Hopkins School of Medicine, Baltimore, United States. 3Johns Hopkins School of Medicine, Infectious Diseases, Baltimore, United States. 4University of Maryland Baltimore County, Biotechnology, Baltimore, United States. 5Rwanda Biomedical Center, HIV, STI and OBBI Infections, Kigali, Rwanda. 6Project San Francisco, Kigali, Rwanda. 7Emory University, Epidemiology, Atlanta, United States. 8Emory University, Pathology and Laboratory Medicine, Atlanta, United States
Background: Rectal sexually transmitted infections (STI) are highly prevalent among men who have sex with men (MSM) and transgender women (TGW), but reticence to provider‐collected rectal swabs has been reported in sub‐Saharan Africa (SSA). Although self‐collection of rectal specimens is commonly used globally, there are limited data on its implementation across SSA. Here, we report experiences of self‐collecting rectal specimen for STI testing among MSM and TGW in Kigali.
Methods: From March‐August 2018, 738 MSM/TGW ≥18 years of age were recruited in a cross‐sectional study using respondent‐driven sampling in Kigali. Neisseria gonorrhoea (NG) and Chlamydia trachomatis (CT) were tested using Cepheid GeneXpert CT/NG platform on self‐collected rectal swabs. Likert scales were used to assess the difficulty and comfort with collecting the rectal swab. Multinomial logistic regression analyses were performed to characterize factors associated with difficulty in self‐collecting a rectal swab.
Results: Overall, 14% identified as TGW. The prevalence of CT was 9.1%(67) and NG was 8.8%(65) at any site. Overall, 27% and 6% of CT infections and 52% and 19% of NG infections were rectal and dual‐site respectively. In total, 78%(577) reported that collecting the rectal swab was easy/very easy, whereas 7%(52) and 15%(108) were neutral or found it difficult/very difficult respectively. The majority, 92%(679), were comfortable/very comfortable with the test and 98%(730) said they would repeat the test in the future. A total of 10%(76) of rectal swabs returned indeterminate results (66 invalid results and 10 errors).
In multivariable multinomial logistic regression adjusting for demographic characteristics, factors positively associated with difficulty collecting the rectal swab were discomfort with the test (adjusted relative risk ratio ((aRRR): 11.8 (95%: 6.5 to 21.3)) and a history of STI (aRRR: 1.8 (95%: 1.00 to 3.16). TGW were less likely to report difficulty performing the test compared to cisgender MSM (aRRR: 0.34 (95%: 0.13 to 0.89)).
Conclusions: Among MSM/TGW in Kigali, self‐collected rectal swabs were highly acceptable, easy to perform and comfortable. Patient education on self‐collection of rectal specimens should be introduced to reduce discomfort associated with this test, as it can support clinic‐ and community‐based STI testing. The high proportion of indeterminate results have significant cost implications, thus measures to ensure adequate sample collection and processing are necessary.
OAD0403
Impact of a brief community health worker‐administered index case testing screening tool on paediatric HIV case identification: early results from Malawi
KR Simon 1,2; BE O'Brien1,2; S Masiano2; E Wetzel1,2; E Kavuta2; A Kabwinja2; CM Cox1,2; S Ahmed1,2; and MH Kim1,2
1Baylor College of Medicine International Pediatric AIDS Initiative, Houston, United States. 2Baylor College of Medicine Children's Foundation, Lilongwe, Malawi
Background: Nearly 100% of pregnant/lactating women living with HIV (WLHIV) in Malawi are on ART, yet only 68% of children living with HIV (CLHIV) are. This treatment gap between mothers and children signifies a missed opportunity to identify CLHIV. Index Case Testing (ICT) is a WHO‐endorsed model for identifying CLHIV. A contributor to suboptimal ICT implementation is the lack of methods to systematically track HIV status of children of WLHIV. We evaluated the impact of a screening tool on WLHIV screened for ICT, paediatric HIV testing and CLHIV identified.
Methods: The brief (<5 minutes) ICT screening tool assesses HIV testing status of children of WLHIV at ART clinic. Data captured include number of children 0 to 19 years, children’s names, ages and HIV status. Completed tools are attached to mother’s ART record for review at subsequent visits. WLHIV attending clinics in 118 health facilities in Malawi were screened from 1 October to 31 December 2020. De‐identified programme data from ICT registers were used to determine WLHIV screened, children tested and CLHIV identified. Results were compared to paediatric testing and case identification over the same period in 2019. A single sample t‐test was used to test differences in the mean number of women screened. Paired t‐tests were used to test differences in the mean number of children tested and CLHIV identified.
Results: The total number of women screened, children tested and CLHIV identified increased during ICT tool implementation (Table 1). The mean number of WLHIV screened weekly was 1411 in 2020 compared to 950 in 2019 (p = 0.042). The mean number of children tested weekly was 319 in 2020 compared to 192 in 2019 (p = 0.018). In 2020, the mean number CLHIV identified weekly was 10 compared to six in 2019 (p = 0.059). In both periods, approximately 3% of children tested HIV positive.
Abstract OAD0403‐Table 1.
| Outcome | Oct to Dec 2019 (without ICT tool) | Oct to Dec 2020 (with ICT tool) | Change |
|---|---|---|---|
| Total women screened | 12,350 | 18,342 | +49% |
| Total paediatric clients tested | 2500 | 4075 | +63% |
| Total paediatric clients tested HIV+ | 78 | 123 | +58% |
Conclusions: Systematic documentation of children’s ICT status using a brief ICT screening tool is a useful approach to identify untested children of WLHIV. Further examination of characteristics of WLHIV with untested children may inform programmatic interventions to identify CLHIV.
OAD0404
An evaluation of family index testing amongst biological children of people living with HIV in Nigeria
N Torbunde 1; T Jasper2; D Yahaya1; A Datti3; C David‐Onuoha4; G Haruna3; I Bakzak5; J Chineke5; M Chikeremma4; M Dimas5; M Yashimankut6; R Nwagwu4; T Hosle6; Y Adetunji6; and N Sam‐Agudu1
1Institute of Human Virology Nigeria, Pediatric and Adolescent Unit, Prevention, Care and Treatment Department, Abuja, Nigeria. 2Institute of Human Virology Nigeria, Pediatric Quality Improvement Unit, Abuja, Nigeria. 3Institute of Human Virology Nigeria, Katsina State Office, Prevention, Care and Treatment Department, Katsina, Nigeria. 4Institute of Human Virology Nigeria, Rivers State Office, Prevention, Care and Treatment Department, Port Harcourt, Nigeria. 5Institute of Human Virology Nigeria, Nasarawa State Office, Prevention, Care and Treatment Department, Lafia, Nigeria. 6Institute of Human Virology Nigeria, FCT Regional Office, Prevention, Care and Treatment Department, Abuja, Nigeria
Background: HIV case‐finding among children is a significant challenge in Nigeria. Of the estimated 150, 000 children aged 0 to 14 years and 110, 000 adolescents aged 10 to 19 years living with HIV in Nigeria, only 36% and 40%, respectively, are on treatment.
PEPFAR recommends family index testing (FIT) as a targeted strategy for improving case finding in children. FIT entails HIV testing for all biological children of people living with HIV (PLHIV).
The Pediatric Program at Institute of Human Virology Nigeria (IHVN) conducted a programme evaluation to determine the proportion of biological children of PLHIV in its treatment network not yet tested for HIV.
Methods: The evaluation was conducted between September and October 2020 at eight randomly selected high‐burden sites across four states – Rivers, FCT, Nasarawa and Katsina. A representative sample of adult PLHIV ≥ 18 years old with living biological children aged 0 to 19 years were randomly selected per site. Participants were interviewed via phone calls; those with untested children were invited to either bring their child(ren) to the facility for HIV testing, or given the option of home‐based testing.
Results: In total, 803 eligible adult PLHIV were interviewed (67% female). Of the 1, 732 children and adolescents elicited, 63% (1083) had a known HIV status, with 6% (67) identified as “known HIV positive”.
Age at HIV diagnosis could be remembered for only 82% (55/67) of known positives, and indicated that 62%, 27%, 9% and 2% were diagnosed at 0 to 4, 5 to 9, 10 to 14 and 15 to 19 years of age respectively.
Ninety‐two percent (597/649) of children with unknown HIV status were tested, with a yield of 1.7% (10/597) HIV positive.
Of children newly identified positive, 30%, 0%, 30% and 40% were identified from the 0‐ to 4‐, 5‐ to 9‐, 10‐ to 14‐ and 15‐ to 19‐year age bands respectively.
Conclusions: Significant progress has been made in scaling up FIT in Nigeria, however, a third of biological children of PLHIV in our evaluation had unknown HIV status. Intensified and coordinated efforts across Adult, PMTCT, Vulnerable Children and other community‐based programmes are needed to reach and test eligible children. Children in all age bands should remain prioritized for FIT.
OAD0405
Increasing efficiency in HIV testing services for prison inmates through the use of risk assessment: experience from EpiC Nigeria
C Oraelosi 1; P Ikani1; C Akolo2; I Iyortim3; M Oladele1; and J Achanya1
1Meeting Targets and Maintaining Epidemic Control (EpiC) Project, Abuja, Nigeria. 2FHI 360, Meeting Targets and Maintaining Epidemic Control (EpiC) Project, Washington DC, United States. 3United States Agency for International Development, Abuja, Nigeria
Background: Correctional facility inmates are a distinct key population (KP) group reached by the Key Population Investment Fund (KPIF) programme, which is implemented by the FHI 360‐led EpiC project in Nigeria with support from USAID and PEPFAR. The HIV positivity rate among prison inmates is about 2.8%, which is lower than that of other KP groups. The project team deployed a specially designed risk assessment tool (RAT) to help increase the efficiency of HIV testing services (HTS) among this population in Niger State, Nigeria.
Description: As part of routine programme implementation, the team assessed the outcome of deploying the RAT for HTS in 6 prisons. The RAT is a questionnaire administered by trained HTS providers to assess recent risky behaviours, such as having anal or vaginal sex without using a condom, engaging in transactional sex and sharing sharp objects. Only clients assessed to be at high risk of HIV were offered HTS. A retrospective comparative analysis was conducted using data collected 10 weeks before and after the deployment of the RAT.
Lessons learned: During the 10 weeks (February to April 2020) prior to RAT deployment, 5 (0.78%) of the 643 inmates who were offered HTS tested HIV positive (a rate similar to the HIV prevalence among the age 15 to 64 general population in Niger State). Within 10 weeks (April to June 2020), testing volume was reduced to 250 inmates, but 27 of those individuals (10.8%) tested positive. The monthly HIV positivity rate increased from 0% to 1% to 4% to 27% with the use of the RAT (Figure 1)
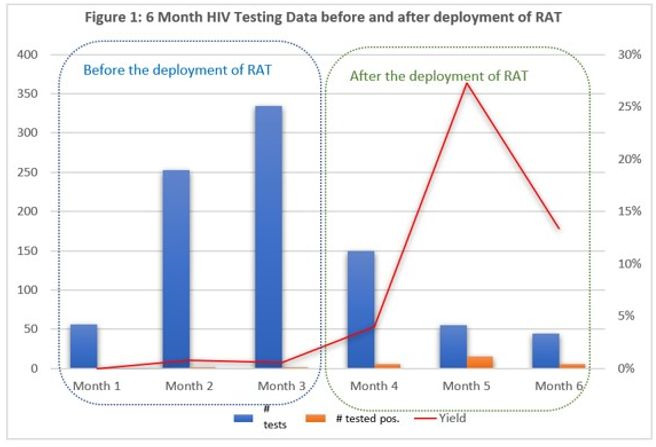
Abstract OAD0405‐Figure 1.
Conclusions/Next steps: The deployment of the RAT among inmates helped reduce HIV testing volume, increased testing efficiency and resulted in increases in case detection. Plans are underway to scale up the tool’s use across all EPiC‐supported correctional facilities in Nigeria.
OAD0406
“It is a process” – a qualitative evaluation of provider acceptability of HIV assisted partner services in western Kenya: experiences, challenges and facilitators
W Liu 1; B Wamuti2; M Owuor3; H Lagat3; E Kariithi3; C O’Bong’o3; M Mugambi4; M Sharma2; R Bosire5; S Masyuko2,4; D Katz2; C Farquhar2,6,7; and B Weiner2
1University of Washington, School of Nursing, Seattle, United States. 2University of Washington, Department of Global Health, Seattle, United States. 3PATH‐Kenya, Kisumu, Kenya. 4National AIDS and STI Control Programme, Kenya Ministry of Health, Nairobi, Kenya. 5Kenya Medical Research Institute, Nairobi, Kenya. 6University of Washington, Department of Medicine, Seattle, United States. 7University of Washington, Department of Epidemiology, Seattle, United States
Background: Assisted partner service (APS) is effective for increasing HIV testing services (HTS) uptake among sexual partners of people diagnosed with HIV with rare social harm. The acceptability of APS to HTS providers is important for the quality and effectiveness of APS delivery. Within an ongoing implementation science study of APS in western Kenya, we qualitatively evaluated the provider acceptability of APS.
Methods: From May to June 2020, we conducted virtual, semi‐structured in‐depth interviews with 14 HTS providers recruited from 8 of 31 study health facilities in Homa Bay and Kisumu counties. Participants were selected using criteria‐based purposive sampling to maximize variation on patient volume (assessed by the number of index clients tested for HIV) and APS performance (assessed by sexual partners’ elicitation and enrolment). Interviews inquired providers’ experiences providing APS including challenges and facilitators and the impact of contextual factors. Data were analysed using an inductive approach.
Results: Overall, HTS providers found APS acceptable. It was consistently reported that doing APS was a continuous process rather than a one‐day job, which required rapport development and persistent follow‐ups. Benefits of APS including efficiency in HIV case finding, expanded testing coverage in men and increased HIV status awareness and linkage to care motivated the providers. Advantages of provider referral were identified such as independent contact with partners on behalf of index clients and efficiency in partner tracing. Challenges of providing APS involved protecting clients’ confidentiality, difficulty obtaining partners’ accurate contact information, logistic barriers of tracing and clients’ refusal due to fear of being judged for multiple sexual partners, fear of breach of confidentiality and HIV stigma. Building rapport with clients, communicating with patience and non‐judgemental attitude and assuring confidentiality were examples of facilitators. Working in rural areas and bigger facilities, training, supportive supervision and community awareness of APS promoted APS delivery while low salaries, lack of equipment and high workload undermined it.
Conclusions: HTS providers found APS acceptable. Taking APS as a process was the key to success. Future scale‐up of APS could consider encouraging provider referral instead of the other APS methods to improve efficiency and reduce potential harm to clients.
OAD0501
Quality improvement collaborative approach to improving viral load suppression in the 15‐ to 24‐year age group in four regions of Namibia, 2018 to 2020
T Shikesho 1; A Basenero2; J Neidel3; L Bikinesi1; SY Hong4; H Ashivudhi4; and G Mutandi4
1Ministry of Health and Social Services, HIV Care and Treatment Subdivision, Windhoek, Namibia. 2Ministry of Health and Social Services, Quality Assurance, Windhoek, Namibia. 3University of California San Francisco, Quality Assurance, Windhoek, Namibia. 4Centers for Disease Control and Prevention, Clinical Services, Windhoek, Namibia
Background: Namibia’s antiretroviral therapy (ART) programme has been successful in achieving a high level of viral load suppression (VLS) of 91% (NAMPHIA, 2017). Sub‐populations and geographic areas need approaches to reach optimal VLS. In August 2018, VLS in four high‐burden HIV regions was 92% (n = 2527) overall, but only 44% (n = 407) among 15 to 24 years of age. We aimed to improve VLS in the 15 to 24 age group using a quality improvement collaborative (QIC) approach; a methodology that accelerates improvement where a performance gap is identified.
Description: Twenty‐five ART healthcare facilities in Kavango, Omusati, Oshana and Oshikoto regions that provide care to almost 66,400 people living with HIV (PLHIV) were selected to participate. A team of three healthcare workers (ART nurse, data clerk and medical officer) per facility attended the inaugural QIC learning session in July 2018. Regional HIV mentors were trained as quality improvement (QI) coaches, whereas the national QI coaches provided overall coordination and data management. Each facility set up a QI team and identified specific ideas to test using the model for improvement (Plan, Do, Study Act cycles). Facilities compiled and submitted monthly reports to the national level using an Excel template. VLS was defined as being active on ART with a viral load <1000 copies/mL.
Lessons learned: The key outcome was VLS in the 15‐ to 24‐year age group improved from a baseline of 44% (n = 407) in August 2018 to 74% by December 2020 (n = 719). Change ideas that were successfully implemented in the 25 participating sites to improve adherence to ART, patient tracking and management included the use of high VL registers (100% of the sites), enhanced adherence counselling (80%), multidisciplinary team management (72%), direct observation therapy (52%), use of pillboxes (32%), initiating and strengthening teen clubs (64%) and timely switch to working regimen (100%).
Conclusions/Next steps: A QIC model applied with a dedicated team of healthcare workers and QI coaches led to the improvement in VLS in the 15‐ to 24‐year age group. Facility level teamwork and QI learning sessions were critical to the success of the initiative. The QIC model may be used in other settings to optimize treatment outcomes for other indicators.
OAD0502
Applying machine learning and natural language processing of qualitative client‐reported data to design client‐centred interventions for maximal impact
AG Fitzmaurice 1; D Bogere1; BW Kafeero1; T Nsubuga‐Nyombi1; H Kadama2; J Ssendiwala3; and LJ Nelson1
1US Centers for Disease Control and Prevention (CDC), Division of Global HIV and TB (DGHT), Kampala, Uganda. 2Ministry of Health, Kampala, Uganda. 3Makerere University School of Public Health, Monitoring and Evaluation Technical Support Program (MakSPH‐METS), Kampala, Uganda
Background: As countries approach HIV epidemic control, client‐centred interventions are necessary to address persistent barriers to HIV prevention, care and treatment. We describe a deep‐learning model to rapidly analyse qualitative client‐reported data to design client‐centred interventions.
Methods: We purposively asked each client from 104 facilities in 32 districts why they missed appointments (N = 2267) or had unsuppressed viral loads (N = 2196); we classified open‐ended responses into eight intervention categories (Table 1). We used R (v4.0.2; quanteda package) for natural language processing (e.g. stemming “facility”/”facilities” to “facil”) and generated a matrix with scaled frequencies of stemmed words/phrases for each open‐ended response. We randomly subsetted data into training (60%) and test (40%) datasets, trained the model 30 times (neuralnet package; backpropagation through eight hidden neural network layers; 105 maximum steps) and validated the model by comparing a priori and model classifications on the test dataset.
Results: The model achieved 96% accuracy (1817 classifications consistent with 1889 a priori classifications). Model and a priori analyses revealed the same conclusions, validating the model for use in various contexts. For example model and a priori analyses concluded that disproportionately more males than females need interventions for traveling. The model prioritized addressing distance/mobility issues (for 22.2% of clients) and post‐missed‐appointment contact (18.5%) for this population, consistent with a priori analysis (22.1%, 19.3% respectively). The model was robust for smaller samples (e.g. 91 0‐ to 14 year‐olds constituting 4.8% of test dataset, prioritizing pre‐appointment reminder (26.4% of clients in model; 27.5% a priori), contact (17.6%; 17.6%), distance/mobility (16.5%; 19.8%)).
Conclusions: While these findings might improve outcomes in other facilities and countries, the major impact is this novel model’s ability to rapidly analyse other datasets to identify emerging and persistent population‐specific challenges needing intervention (e.g. by age, sex, facility, district). This model can facilitate client‐centred healthcare necessary to maintain HIV epidemic control.
Abstract OAD0502‐Table 1.
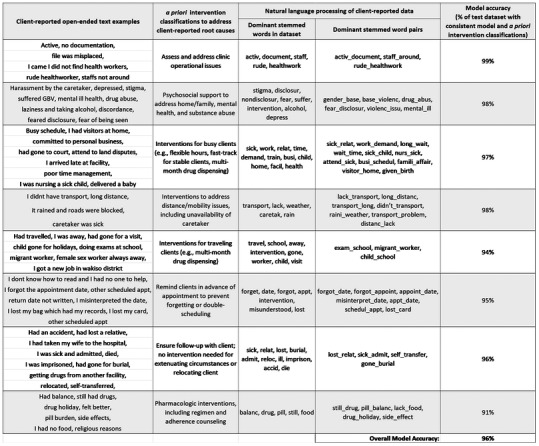
OAD0503
#SaferNowPH: a COVID‐adapted, key population‐responsive, community‐led integrated marketing communications campaign on HIV combination prevention in the Philippines
JO Corciega; R Domingo; E Bagasol Jr; CD Rozul; JM Maynes; JD Ruanto; and R Pagtakhan
LoveYourself Inc., Mandaluyong City, Philippines
Background: The COVID pandemic calls for an intensified promotion of innovative approaches in delivering HIV prevention information and services to minimize key populations’ (KP) vulnerability to HIV, given their reduced access to sexual health services, and to take advantage of their increased online time‐use.
Description: Informed by an online survey, FGDs and KIIs with 1000+ combined KP respondents, the community‐led National HIV Prevention Month (NHPM), themed #SaferNowPH, was launched August 2020 to promote four HIV prevention methods: condoms & lube, PrEP, PEP and treatment‐as‐prevention. The campaign hosted online mobilization activities (webinars, photography & free PrEP contests, a PLHIV dating show, an LGBT‐ & PLHIV‐themed film festival, and a game show featuring local advocates and influencers) and disseminated posters and videos fronted by health professionals with KP‐targeted messaging. Online content reached 236,000+ individuals. Over 45 localized online, in‐facility and community activation events were held nationwide through partner community‐based organizations, with support from Global Fund and other institutional/corporate partners. Combination prevention IEC materials and campaign‐branded kits were distributed for HIV & COVID protection. An interactive chatbot was also introduced to provide KPs recommended prevention method/s based on their responses to behaviour questions, and subsequently direct them to nearby HIV service providers.
Lessons learned: No single prevention approach can stop the HIV epidemic. The NHPM & #SaferNowPH campaign allowed for focused, sustained awareness activities on HIV combination prevention, while giving due emphasis on each prevention method. It illustrated how innovation and differentiation are essential, not only in HIV service delivery, but also in education, demand generation and the use of new media. Tapping influencers was effective at amplifying campaign reach while engaging community partners from campaign development to evaluation was integral in ensuring their ownership and commitment throughout campaign implementation. These combined reach helped increase partner co‐financing.
Conclusions/Next steps: The campaign successfully modelled KP‐targeted communications and the combination prevention approach to HIV education provided in the Philippine HIV Strategic Plan. Government endorsement is anticipated to sustain the NHPM annually. Community engagement is crucial to adapt key messages to varying KP and geographic needs, and to more effectively inform, generate demand from and link KP to prevention services.
OAD0504
Structured support groups improve PrEP uptake among female sex workers in Nairobi: a case study of BHESP
S Mwangi; C Miloyo; J Gacheru; D Kwala; L Opiyo; and P Mwangi
Bar Hostess Empowerment and Support Program (BHESP), Programs, Nairobi, Kenya
Background: Bar Hostess Empowerment & Support Program (BHESP), is a female sex worker led organization that advocate for the human rights and facilitates access to health services for sex workers (FSW) in Kenya. BHESP implements health and advocacy programmes targeting the same population.
To increase uptake of sexual reproductive health services BHESP is running Drop‐in Centers (Clinics) that are friendly to FSW. BHESP implemented oral Pre‐ Exposure Prophylaxis (PrEP) roll out targeting FSW at substantial risk of HIV infection in Nairobi. From October 2018, 6900 FSW were enrolled on oralPrEP and at the end of September 2019, only 1250 (18%) were continuing with PrEP which is an 82% self‐discontinuation.
Methods: BHESP administered Exit interviews to FSWs who discontinued PrEP use to establish the reasons. It was found that lack of knowledge, myths, misconception and stigma contributed to 60% of PrEP discontinuation among FSWs between 2018 and 2019.
To address this gap, BHESP introduced well‐structured PrEP support groups targeting newly enrolled and those that have missed their pill appointments. Support groups were deliberately planned to coincide with the PrEP refill/appointment days.
The objectives of the support group were to provide a peer support environment in which FSW on PrEP can come together and share experiences safely and openly. It also aimed to increase knowledge and awareness of transmission, prevention of HIV and STIs, PrEP adherence and to reduce stigma and feelings of isolation and discrimination among support group members.
Results: From October 2019 to September 2020, BHESP PrEP continuation for the enrolled FSW increased to 60% from 22% (3540/2120) in the previous year of implementation. After conducting exit interviews, FSWs who discontinued the use of PrEP reported to be as a result of reduced risk to HIV. Cases of missed appointments had also significantly reduced within the same period.
Conclusions: PrEP support groups for newly enrolled on PrEP and those missing refill appointments strengthened adherence and retention in the regimen as well as to increase knowledge and awareness on PrEP use. If PrEP support groups are well planned and structured, they can improve the uptake and adherence of oral PrEP among FSWs.
OAD0505
Optimizing antiretroviral treatment and viral suppression for adolescents and young people living with HIV by implementing Operation Triple Zero (OTZ) in four states in Nigeria
F Emerenini 1; R Fayorsey1; O Fadare1; E Okwor2; I Umahi1; S Orisayomi1; R Opara1; C Momah2; and E Atuma2
1ICAP at Columbia University, New York, United States. 2Jhpiego, Baltimore, United States
Background: In Nigeria, adolescents (10 to 19 years) account for about 23% of the total population, and 7% of people living with HIV (PLHIV); treatment outcomes for adolescents and youth living with HIV (AYPLHIV) are quite low. RISE‐Nigeria commenced Operation Triple Zero (OTZ) in 33 facilities across four states in February 2020 to improve treatment outcomes. This analysis reviews the effect of OTZ on treatment outcomes among AYPLHIV six months after implementation.
Description: The OTZ model focuses on health system modifications, adolescent‐centredness and involvement in health, and education of caregivers and health workers. Case managers were identified and trained on non‐judgemental approaches to counselling and optimal antiretroviral therapy (ART) for AYPLHIV; Clinic settings modified with adolescent‐friendly themes; all services integrated and systems for peer‐to‐peer adherence support strengthened; Extended and weekend clinic hours established, with an appointment system for age bands 10 to 14, 15 to 19 and 20 to 24 years; Viraemia clinics established for the virally unsuppressed AYPLHIV; Case‐based learning introduced for capacity building of health workers; Talent nurturing and skill development incorporated into AYPLHIV club meetings; and HIV status disclosure support offered to caregivers with opportunity for caregivers interaction during OTZ meetings facilitating peer‐to‐peer learning.
Lessons learned: After six months, AYPLHIV enrolment into OTZ increased from 615/3306 (18.6%) to 3595/4304 (83.5%); p < 0.001. Optimal regimen utilization pre‐intervention was 284/765 (37.1%%), 285/760 (37.5%) and 709/1526 (46.5%) pre‐intervention, and increased to 807/819 (98.5%), 985/991 (99.4%) and 2478/2484 (99.8%); p < 0.001 post‐intervention for age bands 10 to 14, 15 to 19 and 20 to 24 years respectively. Viral load coverage (VLC) was 255/765 (33.3%), 230/761 (30.2%), 492/1772 (27.7%) pre‐intervention and increased to 740/819 (90.1%), 806/991 (81.3%) and 1794/2484 (72.2%); p < 0.001 in respective age bands post‐intervention. Viral suppression (VS) rate increased from 390/586 (66.6%), 286/552 (51.8%) and 1155/1690 (68.3%) pre‐intervention to 611/749 (81.6%), 700/844 (82.9%) and 2030/2384 (85.2%); p < 0.001 in respective age bands post‐intervention. Overall VS was higher 2487/2935 (84.7%) among OTZ enrollees compared to non‐enrollees 852/1040 (81.9%); p = 0.03.
Conclusions/Next steps: OTZ implementation improved the use of optimal ARV regimen, VLC and VS among AYPLHIV. These results validate the use of integrated, asset‐based strategies to improve HIV treatment outcomes among AYPLHIV.
OAD0601
Fast and friendly is key to keeping men on HIV treatment! Results from a discrete choice experiment to understand men’s preferences in Johannesburg, South Africa for HIV treatment services
S Pascoe 1,2; C Govathson1,2; S Mngadi Ncube1,2; N Ngcobo1,2; M Nardell3,4; I Katz3,4,5,6; and L Long1,2,7
1Health Economics and Epidemiology Research Office (HE2RO), Johannesburg, South Africa. 2University of the Witwatersrand, Department of Internal Medicine, School of Clinical Medicine, Johannesburg, South Africa. 3Department of Medicine, Brigham and Women’s Hospital, Boston, MA, United States. 4Harvard Medical School, Boston, United States. 5Massachusetts General Hospital Center for Global Health, Boston,MA, United States. 6Havard Global Health Institute, Cambridge, MA, United States. 7Boston University School of Public Health, Department of Global Health, Boston, United States
Background: Men have been shown to be less likely to engage in care across the HIV care cascade, in particular retention in care and treatment. Understanding men’s preferences for HIV treatment models would provide data to optimize service delivery to improve retention and outcomes. A discrete choice experiment (DCE) was conducted to explore HIV treatment preferences of men in Johannesburg (South Africa).
Methods: We conducted a DCE in late 2020 with adult men (≥18 years) recruited from six community sites (e.g. hostel, homeless shelter, taxi rank) to ensure representation from men with varied HIV treatment experience. Each participant completed a DCE with nine choice sets focusing on preferences for eight HIV treatment attributes. Using conditional logistic regression, the strength of preference for each attribute level was estimated.
Results: 150 respondents completed the DCE (median age 35 years; 12% HIV+). Participants indicated a strong preference (OR = 2.55; 95% CI: 2.16 to 3.01) that providers are friendly, welcoming and non‐judgemental. There was a preference for consultations not to occur in groups (OR = 0.69; 95% CI: 0.58 to 0.82), not to be scheduled for dates early in the month (OR = 0.77; 95% CI: 0.61 to 0.98); and not to dispense three (OR = 0.77; 95CI: 0.60 to 0.98) or six months (OR = 0.77, 95% CI: 0.60 to 0.96) of drug at a time. There was a preference for services to be free (OR = 1.66; 95% CI: 1.29 to 2.13), but no clear preference for location of services, alternative drug formulation (pill vs. injection) or waiting time.
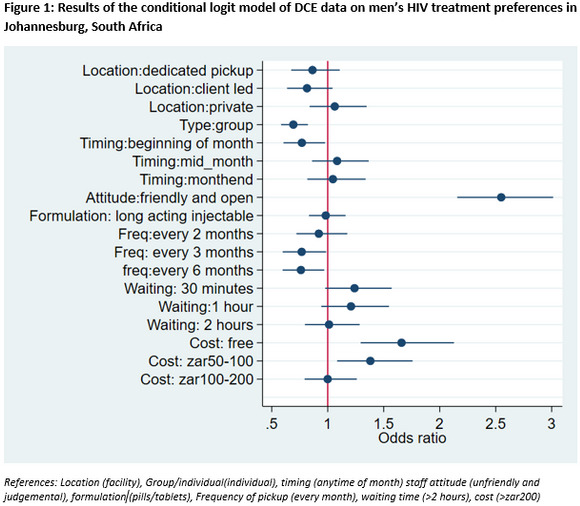
Abstract OAD0601‐Figure 1.
Conclusions: As has been seen with other populations provider attitude is a critical service delivery attribute for men accessing treatment. The preference for individual consultations and relatively frequent drug pickups suggests that typical models of differentiated care, which leverage peer group meetings and long dispensing intervals, may not be appropriate for retaining these men in care. Current service delivery models should consider these factors when designing interventions to retain men in care and treatment.
OAD0602
“I wish to continue receiving the reminder Short Messaging Service”: a mixed methods study on acceptability of digital adherence tools among adults living with HIV on antiretroviral treatment, Tanzania
K Ngowi 1,2; F Pima1; BT Mmbaga1,3; RE Aarnoutse4; P Reiss5; PT Nieuwkerk2; MA Sprangers2; and M Sumari‐de Boer1
1Kilimanjaro Clinical Research Institute, Research, Moshi, Tanzania, United Republic of. 2Amsterdam UMC, Location AMC, University of Amsterdam, Medical Psychology, Amsterdam, Netherlands. 3Kilimanjaro Christian Medical Center, Research, Moshi, Tanzania, United Republic of. 4Radboudumc, Radboud Institute for Health Sciences, Department of Pharmacy, Nijmegen, Netherlands. 5Amsterdam UMC, Location AMC, University of Amsterdam, Global Health and Amsterdam Institute for Global Health and Development, Amsterdam, Netherlands
Background: The rapid increase in the use of mobile phones across sub‐Saharan Africa over the past years have opened the door to the use of Digital adherence tools (DATs) to promote adherence to antiretroviral treatment (ART) for HIV. However, their effectiveness and acceptability in limited resource settings have been challenging. In this study, we examine the acceptability of DATs to improve adherence to ART.
Methods: This study was part of a three‐arm randomized controlled trial (REMIND) which investigated the effect of two different DAT’s: SMS text messages (SMS) or real‐time medication monitoring (RTMM) on treatment adherence; compared to standard of care. Exit interviews and in‐depth interviews were conducted at 48 weeks of follow‐up, to collect data on their experiences (successes, challenges and barriers) and behaviours regarding the implementation of the interventions. Translated transcripts, memos and field notes were imported to NVivo software version 12. We used a thematic framework analysis which drew from Sekhon’s theoretical framework of acceptability (TFA), which comprises of seven constructs (affective attitude, perceived burden, perceived effectiveness, ethicality, self‐efficacy, intervention coherence and opportunity costs).
Results: Of the 166 participants enrolled, 143 (86%) were interviewed (68 in the SMS arm and 75 in the RTMM arm). Participants were highly satisfied (98%) with the DAT system and the majority of them reported it motivated them to take their medication (99%). The majority of participants reported they were confident in their ability to comply with the intervention and understood how the intervention worked (97%). Very few reported negatively about the devices (carrying the device), with only 6% reporting that they did not feel comfortable and 8% had ethical concerns with the SMS content A few participants reported challenges with their connectivity/network and that the visits were too time‐consuming. A few participants reported that they incurred extra cost for the sake of the study.
Conclusions: Overall, the acceptability of these DATs was high. However, several factors may hamper their acceptability including the content and number of SMS, carrying the devices and the network availability.
OAD0603
Preferences for care engagement among people with HIV experiencing homelessness or unstable housing: a discrete choice experiment
E Imbert 1; MD Hickey1; JD Rosario1; M Conte2; AD Kerkoff1; A Clemenzi‐Allen3; E Lynch1; J Kelley1; J Oskarsson1; ED Riley1; DV Havlir1; and M Gandhi1
1University of California San Francisco, Division of HIV, Infectious Diseases, and Global Medicine, San Francisco, United States. 2Zucker School of Medicine at Hofstra/Northwell, Hempstead, United States. 3San Francisco Department of Public Health, San Francisco, United States
Background: In San Francisco, 39% of people with HIV (PWH) experiencing homelessness or unstable housing (HUH) were virally suppressed, compared to 75% overall. We conducted a discrete choice experiment (DCE) to evaluate preferences for strategies to improve care engagement for PWH‐HUH.
Methods: From July to November 2020, we enrolled PWH‐HUH at Ward 86 in our drop‐in, incentivized care programme (“POP‐UP”) and PWH‐HUH in traditional primary care who had an unsuppressed viral load (≥200 copies/mL) in the prior year. The DCE included five service attributes: single versus team of providers; incentives for clinic visit ($0, $10, $20); clinic location (Ward 86 only or additional site); drop‐in versus scheduled visits; in‐person only versus optional telehealth visits and patient navigator assistance with visits. We estimated relative utilities using mixed‐effects logistic regression and conducted latent class analysis to evaluate preference heterogeneity.
Results: We enrolled 115 participants (59 POP‐UP, 56 traditional care); 78% cisgender men, 54% used methamphetamines daily, 40% lived outdoors. Overall, strongest preferences were for same provider (β = 0.94, 95% CI 0.48 to 1.41), incentives (β = 0.56 per $5; 95% CI 0.47 to 0.66) and drop‐in visits (β = 0.47, 95% CI 0.12 to 0.82; Figure 1). Latent class analysis revealed two distinct groups: 78 (68%) preferred a flexible care model including an additional clinic location (β = 0.55, 95% CI 0.25 to 0.84), navigator assistance (β = 0.61, 95% CI 0.24 to 0.99), drop‐in visits (β = 0.61, 95% CI 0.17 to 1.06) and incentives (β = 0.77, 95% CI 0.62 to 0.92); 37 (32%) preferred continuity with the same provider (β = 3.12, 95% CI 2.26 to 3.98).
Conclusions: We identified heterogeneous care preferences among PWH‐HUH via a unique DCE analysis, with one‐third of respondents preferring provider continuity and two‐thirds preferring a more flexible care model. All respondents preferred incentives. There was no preference for telehealth, even when facilitated by a navigator. These findings highlight the importance of in‐person incentivized care for PWH‐HUH with the option to choose between provider continuity and flexibility.
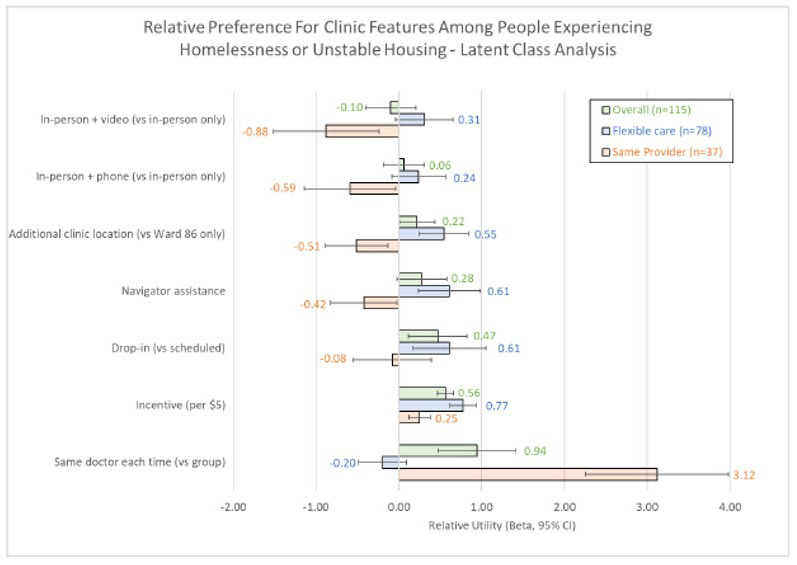
Abstract OAD0603‐Figure 1.
OAD0604
Patient and provider perspectives on a novel, low‐threshold PrEP programme for people who inject drugs and experience homelessness
A Bazzi 1; K Biello2; S Vahey3; M Harris3; and J Brody3
1University of California, San Diego, Herbert Wertheim School of Public Health and Human Longevity Science, La Jolla, United States. 2Brown University School of Public Health, Providence, United States. 3Boston Health Care for the Homeless Program, Boston, United States
Background: New HIV clusters continue to be identified among people who inject drugs (PWID) and experience homelessness in the Northeastern U.S. and other regions. Antiretroviral pre‐exposure prophylaxis (PrEP) is efficacious and recommended for HIV prevention; however, uptake remains low in this marginalized population. We explored patient and provider experiences with a novel, low‐threshold programme designed to support PrEP uptake, adherence and persistence among PWID experiencing homelessness.
Methods: Boston Health Care for the Homeless Program (BHCHP) implemented PrEP services for PWID experiencing homelessness in October 2018. From March to December, 2020, we conducted qualitative interviews with current and former adult PrEP programme patients and providers (e.g. BHCHP clinicians, patient navigators, outreach workers; and collaborators from affiliated organizations). Semi‐structured interviews were conducted in private outdoor areas using secure video‐conferencing on tablets. Rapid thematic analysis identified common patient and provider experiences with BHCHP’s PrEP programme.
Results: Among 21 PrEP programme participants, the median age was 35.5 years (IQR: 31 to 37.5), 15 (71%) identified as male, 6 (29%) as female. Thirteen (62%) identified as white, 4 (19%) Hispanic and 4 (19%) Black. Sixteen (76%) were currently taking PrEP. All participants reported past‐month heroin/fentanyl and polysubstance use (commonly methamphetamine [n = 19], cocaine/crack [n = 18], non‐prescribed benzodiazepines [n = 17] and gabapentin [n = 13]). Injection frequency was high: 12 reported injecting 4 to 9 times daily; 4 injected ≥10 times daily. Eleven providers had a median of six years working with PWID (IQR: 4.5 to 13). Programme participants and providers expressed concerns about ongoing HIV transmission, linking it to polysubstance use and sexual behaviours (including transactional sex work and sex with HIV‐positive partners). They described same‐day and short‐term prescribing (7‐day prescriptions), on‐site medication storage and coordination with other local service agencies as particularly helpful elements of the PrEP programme. Participants also discussed the approachability and persistent street presence of PrEP nurses and programme staff in facilitating their PrEP uptake and ongoing adherence and retention.
Conclusions: Our findings illustrate how innovative, culturally competent strategies can help engage and retain PWID experiencing homelessness in low‐threshold, outreach‐based PrEP services. Specific elements of this programme could be considered in a range of community‐based settings, including syringe service programmes and shelter to mitigate future outbreaks.
OAD0605
A people‐centred approach to develop intervention packages for HIV partner notification: facilitators and barriers under a socio‐ecological framework
X Yan 1; Y Xu1; W Tang1; J Tucker2; and W Miller2
1UNC Project‐China, Guangzhou, China. 2UNC Project‐China, London, United Kingdom
Background: The rate of HIV infections among MSM in China has been on the rise in recent years despite public health efforts to reach key populations for HIV prevention. The limited acceptability and usage of partner notification services (PS) would be one reason. People‐centred approach such as crowdsourcing, which collects ideas from the public to solve a certain problem, may be promising for developing more effective intervention packages in promoting PS.
Methods: This study used mixed methods to develop PS intervention strategies and analyse emerging themes of facilitators and barriers for PS. First, we used a community‐based participatory approach to organize a crowdsourcing contest that solicited innovative works for promoting PS among MSM in China. Second, descriptive analysis was used to examine the demographic characteristics of the participants and the features of the eligible entries. Finally, we conducted a content analysis using inductive and deductive coding methods under a socio‐ecological framework, to identify facilitators and barriers of PS.
Results: In total, 77 people from 31 cities submitted a total of 92 submissions, of which 53 remained eligible. Among participants with eligible entries, 60% were male, more than half identified as homosexual or bisexual, and 11% disclosed as living with HIV. Content analysis identified novel strategies to facilitate PS, including differentiation of care and stepwise notification. In addition, people‐centred principles were highlighted, as emerged themes from the submissions emphasized the index education and self‐empowerment, and the necessity to provide safe and supportive disclosure services.
Conclusions: The contest engaged a diverse population of participants to contribute to the development of people‐centred PS for MSM living with HIV in China. Differentiation of care and stepwise notification could be valuable for the nest‐step design of a more comprehensive, integrated intervention package. The emphasis on people‐centred PS is also insightful for HIV‐related policy design in China.
OAD0701
Between empathy and anger: healthcare workers’ perspectives on patient disengagement from antiretroviral treatment in Khayelitsha, Cape Town
T Nhemachena 1; T Cassidy2,3; C Spath1; C Keene2; N Zokufa2; KD Arendse2; L Wagner4; EV Heyden4; K Whitehouse2; and A Swartz1
1University of Cape Town, School of Public Health and Family Medicine, Division of Social and Behavioural Sciences, Cape Town, South Africa. 2Medécins Sans Frontières, Belgium Trust, Khayelitsha Project, Cape Town, South Africa. 3University of Cape Town, School of Public Health and Family Medicine., Cape Town, South Africa. 4Western Cape, Department of Health, Cape Town, South Africa
Background: The individual and public health benefits of antiretroviral treatment (ART) are undermined by suboptimal or poor engagement with care. Healthcare workers (HCW) attitudes and punitive treatment of patients have been repeatedly linked to poor engagement, but little is known about their perspectives or understanding of disengagement.
Methods: We used qualitative methodology to explore HCWs’ perspectives on ART disengagement, in Khayelitsha, an HIV‐prevalent, peri‐urban area in South Africa. HCWs were purposively recruited and included doctors, nurses, counsellors, social workers, data clerks, security guards and allied health professionals. Thirty semi‐structured in‐depth interviews were conducted. HCWs were asked to give examples of patients who interrupt treatment and how they feel when dealing with a patient who is returning to care. Transcripts were analysed using an inductive thematic analysis approach.
Results: Findings from this study show that staff had contradictory feelings towards disengaged patients, experiencing both empathy and anger. Most staff were knowledgeable about the complexities of disengagement and highlighted potential barriers to sustaining adherence to ART, including mental health challenges and non‐disclosure to family and partners. Empathy for patients who interrupted treatment was frequently reported when discussing potential barriers to engagement. However, many also expressed feelings of anger and frustration towards these patients, partly because of the increased workload from additional medical and psychosocial support needs of patients. Some staff, particularly those taking chronic medication, perceived that patients who disengage from ART do not take adequate responsibility for their health.
Conclusions: Punitive HCW behaviour and negative attitudes can drive poor engagement and act as a barrier to re‐engagement, undermining patients’ willingness and ability to sustain lifelong engagement with ART services. While the extent of negative attitudes towards patients identified is highly concerning, the understanding of the challenges that patients face and the empathy HCWs express represents an important opportunity for service improvement. We propose implementing measures to promote non‐judgemental patient‐centred care and contribute to reducing incidences of disengagement. For example a dedicated support mechanism for staff would help to reduce feelings of resentment or overburdened with work for patients requiring intense psychosocial and medical support.
OAD0702
The impact of the COVID‐19 pandemic on provision of HIV care: perspectives of HIV‐dedicated healthcare workers in East Africa
H Byakwaga 1; Z Kwena2; F Nalugoda3; M Aaloke4; M Assenzio5; C Kasozi6; W Muyindike7; K Ngonyani8; M Scanlon9; E Geng4; K Wools‐Kaloustian9; and J Martin5
1Mbarara University of Science and Technology, Mbarara, Uganda. 2Family AIDS Care and Education Services (FACES), Kisumu, Kenya. 3Rakai Health Sciences Program, Kalisizo, Uganda. 4Washington University, St. Louis, United States. 5University of California, San Francisco, United States. 6Masaka Regional Referral Hospital, Masaka, Uganda. 7ISS Clinic, Mbarara Regional Referral Hospital, Mbarara, Uganda. 8Tumbi Regional Referral Hospital, Kibaha, Tanzania, United Republic of. 9Indiana University, Indianapolis, United States
Background: The COVID‐19 pandemic presents unprecedented challenges. In resource‐limited settings, one concern is to what extent the pandemic has negatively affected HIV care, leading to setbacks in the remarkable progress of the past decade. Reports from frontline healthcare workers (HCWs) provide among the earliest opportunities to understand the impact of the pandemic on HIV care.
Methods: We surveyed HCWs providing care to people living with HIV at seven primary care facilities in Uganda and Kenya (affiliated with East Africa International Epidemiology Databases to Evaluate AIDS (IeDEA)) and who were part of an ongoing longitudinal study of care providers. HCWs completed an online self‐administered questionnaire regarding care delivery during April, May and June 2020.
Results: All 184 HCWs approached agreed to participate. Among these, 66% were women; the median age was 33 years; and 5% were doctors, 16% clinical officers, 33% nurses, 5% pharmacists/pharmacy technicians, 18% social workers/counsellors, 1% nutrition assistants and 22% non‐formally trained assistants (e.g. peer mentors). More than 50% of HCWs reported cessation, reduction, or delays in a variety of routine functions at their clinics (Table 1). In response to these challenges, 76% of HCWs reported an increase in communication with patients via phone or text, 86% reported a larger than usual supply of antiretroviral therapy (ART) being given to patients and 79% reported initiation or increased delivery of ART to patients in community settings.
Absract OAD0702‐Table 1. HIV care providers’ report of activities during COVID‐19 pandemic
| Activities at HIV clinic | Stopped | Reduced or delayed | Not affected | Not sure |
|---|---|---|---|---|
| New patient intake | 3% | 56% | 39% | 2% |
| ART initiation in new patients | 2% | 35% | 59% | 3% |
| Follow‐up visits at the clinic | 3% | 74% | 20% | 3% |
| Evaluation of patients by clinicians during follow‐up | 7% | 58% | 32% | 3% |
| ART adherence counselling at the clinic | 5% | 49% | 44% | 2% |
| Preventative screening for co‐morbidities/coinfections | 11% | 47% | 40% | 2% |
| HIV viral load testing | 1% | 38% | 59% | 2% |
| Home visits | 49% | 39% | 9% | 3% |
Conclusions: Among a representative sample of HIV care providers in East Africa, there were ample reports of HIV care disruptions as a result of the pandemic. There were, however, many attempts at rapid solutions to these disruptions, and dissemination of best practices might benefit other clinics. Our findings motivate formal investigation of the ultimate relevant outcomes – HIV‐related morbidity and mortality. The work also highlights elements of requisite preparedness for the next pandemic.
OAD0703
Evaluating the integration of telehealth in same‐day antiretroviral initiation service during COVID‐19 in Bangkok, Thailand
S Amatavete 1; S Lujintanon1; N Teeratakulpisarn1; S Thitipatarakorn11; P Seekaew2; C Hanaree1; J Sripanjakun1; C Prabjuntuek1; N Kulsinsub1; J Peelay1; P Phanuphak1; N Phanuphak1; and R Ramautarsing1
1Institute of HIV Research and Innovation, Bangkok, Thailand. 2Columbia University Mailman School of Public Health, Department of Epidemiology, New York, United States
Background: Same‐day antiretroviral therapy (SDART) initiation has been implemented since 2017 at the Thai Red Cross Anonymous Clinic (TRCAC), an HIV testing centre in Bangkok, Thailand. Clients who are willing and clinically eligible start ART on the day of HIV diagnosis. In response to the COVID‐19 pandemic, a lockdown was announced in Thailand in March 2020, limiting access to healthcare facilities. Telehealth for SDART follow‐up was established at TRCAC to minimize clinic visits. We present an evaluation of its implementation.
Methods: Pre‐COVID (until February 2020), clients who initiated SDART received a two‐week drug supply and returned to the clinic after two weeks for clinical evaluation and referral to long‐term care facilities. If no adverse events (AEs) were established, an eight‐week supply was provided while referral was arranged. During COVID‐19 lockdown (March–May 2020), a four‐week ART supply was provided, and the option of a video call for clinical consultation and physical examination instead of a clinical visit at two weeks was given. Clients with severe AEs were required to return to TRCAC; those without received another six‐week supply by courier to bridge the transition to long‐term facilities. A subset of clients was interviewed to assess experiences and preferences.
Results: During the lockdown, 238 clients were diagnosed with HIV at TRCAC, 183 (76.9%) were eligible for SDART, 176 (96.2%) accepted and 160 (90.9%) enrolled. Of 159 (99.4%) follow‐up visits completed, 52 (34.4%) occurred virtually – all with clients who did not have AEs prompting a clinic visit. Seven clients were interviewed; all experienced telehealth as positive and found it convenient and time saving. Due to the success of telehealth with ART delivery, it was continued. Post‐COVID (June–August 2020), 406 clients were diagnosed with HIV, 319 (78.6%) were eligible for SDART, 297 (93.1%) accepted and 283 (95.3%) enrolled. Of 232 (99.2%) follow‐up visits completed, 83 (38.8%) occurred virtually.
Conclusions: Telehealth follow‐up with ART delivery for SDART clients is a feasible and safe option for providing differentiated ART initiation services at TRCARC, leading to its continuation beyond COVID‐19. Therefore, telehealth in hospitals and for purposes beyond ART initiation should be explored.
OAD0704
Successful implementation of telemedicine and pharmacy enhanced HIV services as response to COVID‐19 quarantine among health insured patients in Argentina
P RodrÍguez‐Iantorno 1; D Cecchini1; R Mauas1; E Bottaro1; F Rombini1; J Ballivian1; H Visciglio1; Y Sarnagiotto2; and I Cassetti1
1Helios Salud, Buenos Aires, Argentina. 2Helios Pharma, Buenos Aires, Argentina
Background: Due to the spread of SARS‐CoV‐2 in Argentina, authorities implemented quarantine and community containment measures for 234 days during 2020, which may have hindered HIV care continuum. Our institution is the main ambulatory HIV care centre for health‐insured patients in Argentina, with 10500 patients in active follow‐up, mostly from Buenos Aires city (and surrounding areas) with a countrywide network. Since years, the institution achieved UNAIDS objectives of 90% ART coverage and 90% virological suppression. In order to minimize impact of quarantine in medical follow‐up, ART pharmacy withdrawals and virological suppression, telemedicine (E‐visits) and pharmacy enhanced services were implemented since April 2020 as contingency plan in pandemic lockout context.
Description: Telemedicine was based on linkage between the institutional electronic medical record and WhatsApp through a specific application, allowing patient–physician video call through mobile devices. After each E‐visit, a satisfaction survey (Likert type scale 1=”bad” to 5=”excellent”) was submitted to the patient. Pharmacy enhanced services consisted of pharmacy delivery for patients in vulnerable situation (from our main pharmacy to either patient´s home or next‐door pharmacy) and bimonthly withdrawals. To evaluate the impact of these services, we analysed number of medical visits, ART coverage, pharmacy withdrawals and virological suppression (viral load <200 copies/mL) in our population in 2020 versus 2019 (non‐pandemic year).
Lessons learned: During 2019, 34843 medical visits were done (no E‐visits). ART coverage, pharmacy withdrawals and virological suppression were 97.5%, 95.9% and 97% respectively. During 2020, 32400 medical visits were done, being 10355 (32%) E‐visits. Median patient satisfaction was 5 points (IQR: 5 to 5). ART coverage, pharmacy withdrawals and virological suppression were 98.7%, 98.1% and 94%, respectively, showing the success of these contingency measures in preserving patient follow‐up and ART coverage, adherence and suppression >90%.
Conclusions/Next steps: Telemedicine and pharmacy enhanced services were successful interventions in pandemic context for preserving institutional standards according to UNAIDS targets. Due to high patient satisfaction, telemedicine should be implemented as standard‐of‐care in our population.
OAD0705
CUSTOMIZE: overall results from a hybrid III implementation‐effectiveness study examining implementation of cabotegravir and rilpivirine long‐acting injectable for HIV treatment in US healthcare settings; final patient and provider data
M Czarnogorski 1; C Garris1; R D'Amico1; J Flamm2; G Sinclair3; M Wohlfeiler4; L Mena5; M Dalessandro6; C McHorney7; S Mansukhani8; W Williams9; D Merrill1; and W Spreen1
1ViiV Healthcare, Research Triangle Park, United States. 2Kaiser Permanente, Sacramento, United States. 3AIDS Arms, Inc., Dallas, United States. 4AIDS Healthcare Foundation, Miami Beach, United States. 5University of Mississippi Medical Center, Jackson, United States. 6ViiV Healthcare, Collegeville, United States. 7Evidera, Bethesda, United States. 8Evidera, Waltham, United States. 9GlaxoSmithKline, Collegeville, United States
Background: CUSTOMIZE examined implementation strategies for provider‐administered long‐acting (LA) injectable ART in diverse US healthcare settings. Findings from staff and patients after 12 months of cabotegravir+rilpivirine (CAB+RPV) LA implementation are reported.
Methods: Twenty‐four staff [physicians, injectors, administrators (n = 8 each)] from eight clinics completed surveys and interviews at baseline, interim (M4) and Month 12 (M12, n = 23). All patients received monthly CAB+RPV LA and completed surveys (n = 09 and 102), a subset completed interviews at baseline and M12 (n = 34 and 31) respectively. Interviews were recorded, transcribed and analysed using ATLAS.ti (v8.1).
Results: Staff found CAB+RPV LA acceptable, appropriate and feasible to implement across clinic types (Table 1). At baseline, providers’ (80.8%) top concern was patient ability to maintain monthly appointments; at M12, only 51.3% had this concern. Most providers (78.3%) felt optimal implementation was achieved within one to three months. Providers reported three key successful implementation strategies with patient adherence: good communication about target dosing window, appointment reminder systems and designated staff accountable for appointment tracking. Top strategies for successful clinic implementation were good staff communication, teamwork and web‐based treatment planner. Qualitative insights varied by clinic type. Federally Qualified Health Centers’ initial concerns on leadership support were mitigated by clinical data and implementation ease. University‐based clinics’ initial concern about patients keeping appointments was mitigated through tracking and patient‐friendly reminder systems. Private practices were initially concerned about injection visit frequency and length; by M12, visit length was short and increased touchpoints benefited patient–provider relationships. Some clinics noted patients’ enthusiasm and monthly compliance were key to success. Sustainability and scalability tactics included increasing/training new staff and effective injection schedule management. By M12, 74% of patients reported nothing interfered with their ability to receive CAB+RPV LA; 94% preferred LA over oral dosing.
Abstract OAD0705‐Table 1. Staff participant acceptability, appropriateness and feasibility by clinic type at month 12
|
Federally Qualified Health Centres (FQHCs) (n = 6) Agreed/Completely Agreed % (mean scale score) |
University Clinics (n = 6) Agreed/Completely Agreed % (mean scale score) |
Private Practice (n = 5) Agreed/Completely Agreed % (mean scale score) |
AIDS Healthcare Foundation (AHF) (n = 3) Agreed/Completely Agreed % (mean scale score) |
Health Management Organization (HMO) (n = 3) Agreed/Completely Agreed % (mean scale score) |
Total (n = 23) Agreed/Completely Agreed % (mean scale score) |
|
|---|---|---|---|---|---|---|
| Acceptability of Intervention Measure (AIM) | 100% (4.29) | 100% (4.75) | 100% (4.40) | 100% (5.00) | 66.7% (3.67) | 95.6% (4.45) |
| Intervention Appropriateness Measure (IAM) | 100% (4.33) | 100% (4.83) | 100% (4.60) | 100% (5.00) | 100% (4.33) | 100% (4.61) |
| Feasibility of Intervention Measure (FIM) | 100% (4.38) | 100% (4.75) | 80% (4.40) | 91.6% (4.58) | 100% (4.00) | 94.5% (4.46) |
AIM, IAM and FIM are each 4 item measures scored 1 to 5; 1 = completely disagree and 5 = completely agree. Mean Scores and Mean proportion who agreed (4) or completely agreed (5) to each of 4 statements for each measure are reported.
Conclusions: In CUSTOMIZE, LA ART was successfully implemented across a range of US healthcare settings. Barriers were mitigated with minor process adjustments. Patients reported few barriers to monthly appointments and most preferred CAB+RPV LA over daily oral therapy.
OAD0706
Patterns of patient–provider communications in public HIV clinics in Zambia: a latent class analysis using RIAS
N Mukamba 1; C Mwamba1; AS Namwase2; M Foloko1; K Lumbo1; H Nyirenda1; DL Roter3; A Sharma1; S Simbeza1; K Sikombe1; LK Beres4; J Pry1,5; K Christopoulos6; CB Holmes7,8; EH Geng2; I Sikazwe1; C Bolton‐Moore1; and A Mody2
1Centre for Infectious Disease Research in Zambia, Research, Lusaka, Zambia. 2Washington University School of Medicine in St. Louis, Division of Infectious Diseases, St. Louis, United States. 3Johns Hopkins Bloomberg School of Public Health, Baltimore, Health, Behavior and Society, Baltimore, United States. 4Johns Hopkins Bloomberg School of Public Health, Baltimore, International Health, Baltimore, United States. 5University of California at Davis, Epidemiology, Davis, United States. 6University of California at San Francisco, Department of Medicine, San Francisco, United States. 7Johns Hopkins University School of Medicine, Division of Infectious Diseases, Baltimore, United States. 8Georgetown University Medical Center, Centre for Global Health and Quality, Washington, United States
Background: Poor patient–provider communication is a clinic‐based barrier to long‐term adherence and retention in care among HIV patients. Objective assessments of this key metric of patient experience are lacking in sub‐Saharan Africa. We used the Roter Interaction Analysis System (RIAS) to quantitatively parse and assess distinctive patterns of patient–provider communication at public health HIV clinics in Zambia.
Methods: We enrolled adults at 24 Ministry of Health facilities providing antiretroviral therapy (ART) in Lusaka province, supported by the Centre for Infectious Disease Research in Zambia (CIDRZ). Clinic visits were audio‐recorded and coded by RIAS trained research staff who demonstrated high levels of intercoder reliability (Pearson correlation 0.8). We performed latent class analysis to identify visit interactions falling within distinctive patterns of communication.
Results: Among 120 patient–provider pairs (patients: 63% female; providers: 45% female, 84% physicians, 16% nurses), three distinct profiles of patient–provider communication were identified (Figure 1): (1) “Patient‐Centred Interactions” (21.2% of interactions) characterized by relatively high levels of patient‐centred communications, including shared decision making, discussion of psychosocial concerns and barriers to care and positive use of discretionary power; (2) “Positive Medical Interactions” (63.6%) characterized by a predominantly biomedical focus with providers both asking and providing information and patients responding to the provider with informative and positive statements and (3) “Negative Medical Interactions” (15.2%) similarly characterized by a biomedical focus but predominated by medical provider questions with relatively little information exchange between patients and providers, as well as patient expression of more negative than positive statements.
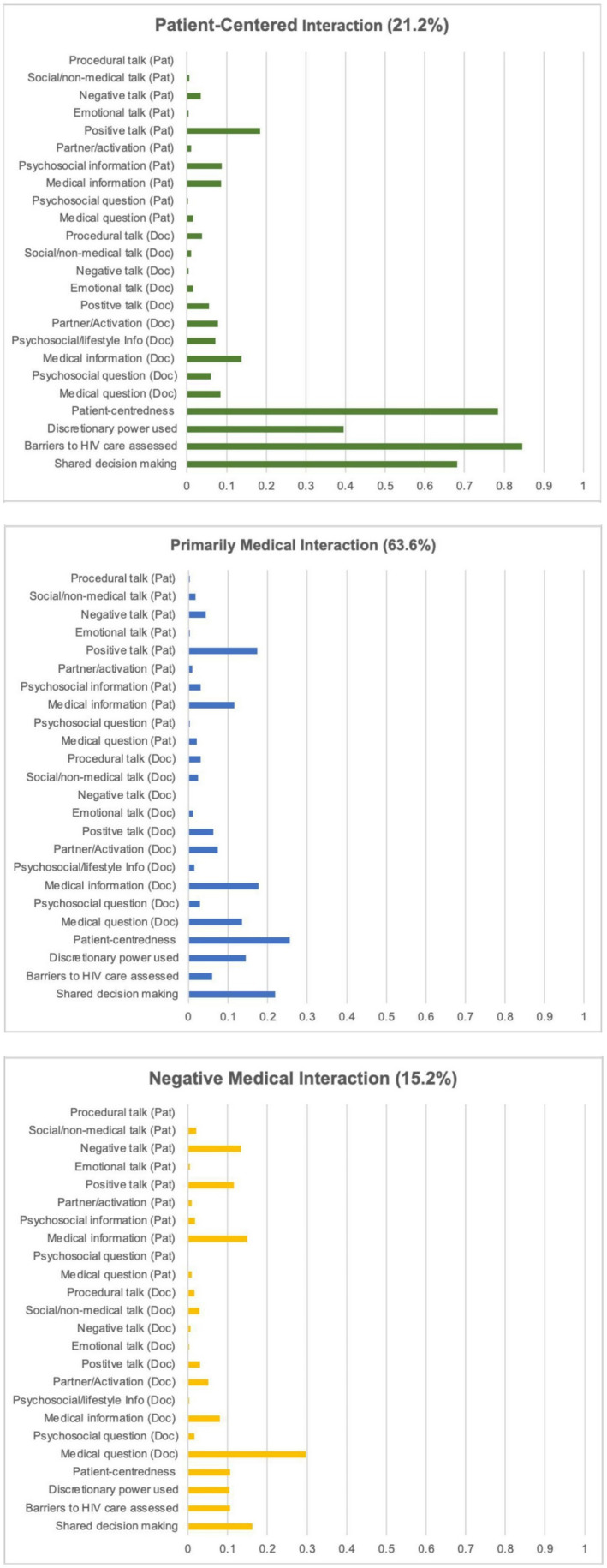
Abstract OAD0706‐Figure 1.
Conclusions: Patient–provider communication patterns primarily focused on medical aspects of living with HIV. Strengthening communication behaviours may be an important strategy for improving retention in HIV treatment programmes.
OALA01LB01
In‐vivo imaging using anti‐ENV probes in SIV infected monkeys: a reproducibility study
I Kim1; S Srinivasula1; P DeGrange2; B Long2; H Jang1; H Imamichi3; JA Carrasquillo3; C Lane3; and M Di Mascio 3
1Leidos Biomedical Research, Inc., Frederick, United States. 2Integrated Research Facility, Frederick, United States. 3National Institutes of Health, Bethesda, United States
Background: It has been reported that positron emission tomography (PET) with anti‐ENV monoclonal antibodies (mAbs) can be used to non‐invasively image SIV in tissues of chronically infected as well as combination antiretroviral treated (cART) and viral‐load suppressed animals. Recent clinical studies have attempted to reproduce these findings in cART‐treated and untreated HIV‐infected patients; however, conflicting observations have been reported. We attempted to reproduce the anti‐ENV imaging system in SIV‐infected RMs.
Methods: Two anti‐ENV probes‐ 7D3, and a mixture of ITS06.01 and ITS103.01 were radiolabeled for PET or single‐photon‐emission‐computed (SPECT) imaging, and administered intravenously (Table 1). Binding specificity was tested in‐vitro. Uninfected or SIV‐infected animals were imaged up to day 5 post‐radiotracer injection, followed by necropsy studies. Spleen and lymph node (LN) sections, and LN primary cells from two additional uninfected and two additional SIV‐infected RMs (PVL approximately 106 copies/ml) were used for ex‐vivo autoradiography and cell‐binding assays using 89Zr‐Df‐7D3 mAb. To test for presence of endogenous Abs in plasma that compete for the 7D3 binding site, plasma binding assay coupled with radio‐HPLC were run for RMs utilized in this study and 5 additional SIVmac239‐nef‐stop RMs during the first year of infection.
Results: Plasma binding assay revealed that competing endogenous Abs were absent at day 7 post infection (p.i.), inhibited 7D3 binding from 0% to 52% (mean 25%) at day 14 p.i., and fully abrogated binding in all RMs at year 1 p.i. Consistently, competing endogenous Abs were absent in the two animals imaged during pre‐acute SIV‐infection but fully abrogated binding in the chronically SIV‐infected animal. Autoradiography, binding assay of primary cells, and in‐vivo imaging up to five days post‐anti‐ENV probe injection did not detect differences in probe uptake between the uninfected and the SIV‐infected RMs.
Conclusions: In SIV pre‐acutely or chronically infected RMs, radiolabeled anti‐ENV mAb tracers did not detect ENV expression in‐vivo or ex‐vivo.
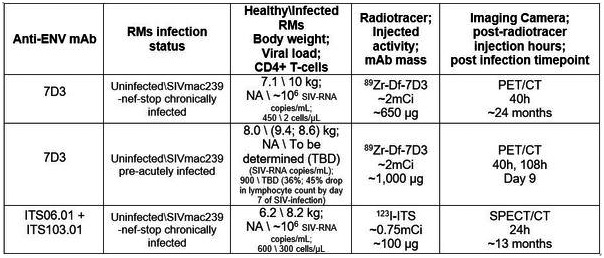
Abstract OALA01LB01‐Table 1.
OALA01LB02
Dual IL‐10 and PD‐1 blockade in SIVmac239 infected macaques promotes sustained virologic control in absence of ART
Z Strongin 1; SP Ribeiro2; TN Hoang1; K Nguyen1; J Harper1; JD Estes3; L Micci4; JD Lifson5; BJ Howell6; D Gorman7; DJ Hazuda6; M Paiardini1,8; and R‐P Sekaly2
1Emory University, Division of Microbiology and Immunology, Atlanta, United States. 2Emory University, Pathology Advanced Translational Research Unit, Atlanta, United States. 3Oregon Health & Science University, Division of Pathobiology and Immunology, Beaverton, United States. 4Merck & Co., Inc., Department of Discovery Oncology, Boston, United States. 5Frederick National Laboratory for Cancer Research, AIDS and Cancer Virus Program, Frederick, United States. 6Merck & Co., Inc., Department of Infectious Disease, Kenilworth, United States. 7Merck & Co., Inc., Department of Discovery Biologics, South San Francisco, United States. 8Emory University, Department of Pathology and Laboratory Medicine, Atlanta, United States
Background: IL‐10 production and PD‐1 expression are both elevated during chronic HIV/SIV infection under ART. Both are associated with virus persistence and T cell impaired effector function, which leads to virus rebound upon analytical treatment interruption (ATI). We hypothesized that IL‐10 and PD‐1 blockade will act synergistically to simultaneously reduce the SIV reservoir and boost T‐cell function, leading to improved control of viral rebound after ATI.
Methods: 28 rhesus macaques (RMs) were infected i.v. with SIVmac239, initiated ART (TDF/FTC/DTG) at day‐42 post‐infection, which was maintained for 14‐months prior to therapeutic intervention. RMs received rhesus aIL‐10 (#10), aIL‐10/aPD‐1 (#10), or vehicle (#8), with infusions every three‐weeks (Figure 1). Animals interrupted ART 12‐weeks after dose 1, and continued infusions for 14‐weeks after ATI.
Results: PD‐1 receptor occupancy was demonstrated in all aPD‐1 treated animals in blood and tissue throughout the intervention. Through 14‐weeks of ATI, 8/10 aIL‐10+aPD‐1 animals demonstrated viral suppression with plasma viremia (pVL) <100 copies/ml at least once after viral rebound, compared to 1/8 control and 3/10 aIL‐10 animals (Figure 2). Suppression was durable, with 6/10 aIL‐10+aPD‐1 animals exhibiting, in the first 14‐weeks post‐ATI, between four to twelve weeks of pVL < 400 copies/ml. Notably, aIL‐10+aPD‐1 treatment resulted in enhanced ability to control viremia at ATI as compared to the pre‐ART value, with 4‐log lower viral load, as compared to 1.4‐log lower in controls (p = 0.007).
Conclusions: These data demonstrate that combined anti‐IL‐10 and anti‐PD‐1 blockade can facilitate sustained virologic control in the absence of ART and interventions targeting these pathways represent a promising path towards HIV cure.
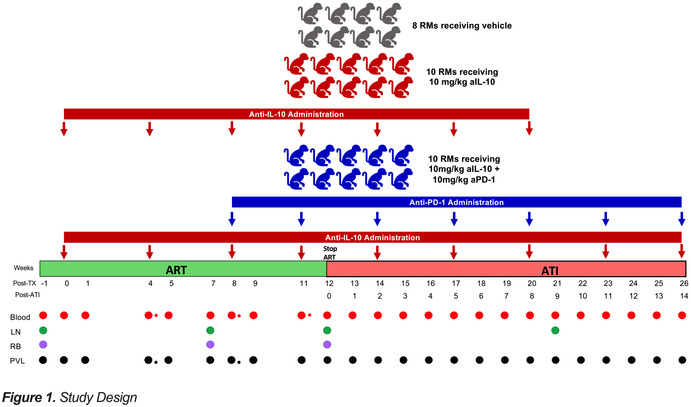
Abstract OALA01LB02‐Figure 1.
OALA01LB03
Combination therapy with the broadly neutralizing antibody VRC07‐523LS and the latency reversal agent Vorinostat fails to substantially reduce latent, resting CD4+ T cell infection or reduce low‐level viremia
DM Margolis 1; KS James1; M Tuyishime2; SD Falcinelli1; SB Joseph1; B Allard1; JL Kirchherr1; M Clohosey1; RJ Gorelick3; L Gama4; RA Koup4; JR Mascola4; M Floris‐Moore1; G Ferrari2; JJ Eron1; JD Kuruc1; NM Archin1; and CL Gay1
1University of North Carolina at Chapel Hill, UNC HIV Cure Center, Chapel Hill, United States. 2Duke University Medical Center, Department of Surgery, Durham, United States. 3Frederick National Laboratory for Cancer Research, Frederick, United States. 4Vaccine Research Center, NIAID, National Institutes of Health, Bethesda, United States
Background: Approaches to deplete persistent HIV infection are needed. We investigated the combined impact of the latency reversing agent vorinostat (VOR) and VRC‐07‐523‐LS, a broadly‐neutralizing HIV antibody with prolonged half‐life, on the HIV reservoir in HIV+ participants on stable antiretroviral therapy (ART).
Methods: Participants with HIV‐1 Infection on ART with a CD4 T cell count ≥350 cells/mm3 and viral suppression for ≥24 months received two cycles of intravenous VRC07‐523LS at 40 mg/kg followed by 10 oral doses of 400 mg VOR every 72 hours. Cycles were separated by at least one month. ART was maintained throughout the study. Change in low‐level HIV viremia, resting cell‐associated HIV RNA (rca‐RNA), Intact Proviral DNA assay (IPDA), and the frequency of resting CD4+ T‐cell infection (QVOA; quantitative viral outgrowth assay) was measured at baseline and after the treatment cycles.
Results: No serious treatment‐related adverse events were observed among eight participants. Following cycles of VRC07‐523LS and VOR, declines of IPDA or QVOA were seen, that did not reach statistical significance. Of note, we observed significant declines of rca‐RNA despite exposure to VOR in three participants although non‐significant depletions of IPDA and QVOA were observed. Viral isolates recovered from resting CD4 cell outgrowth assays did not acquire increased resistance to VRC‐07 during the study. Low‐level viremia (≤50 copies/ml) was absent or barely measurable in most participants. However, one participant maintained viremia of ca. 30 copies/ml throughout the study, despite the lack of evidence of VRC07 resistance.
Conclusions: VRC07‐523LS and VOR were safe and well‐tolerated. Downward trends in some parameters of HIV persistence were observed, but a definitive reduction in the HIV reservoir as measured by a 50% decrease in QVOA was not measured. The persistence of low‐level viremia in one participant raises the concern that Ab‐directed clearance may not be efficient enough to impact small populations of transiently productive infected cells. More efficacious antiviral immune interventions, likely paired with more effective latency reversal approaches that are now emerging, must be developed to clear persistent HIV infection.
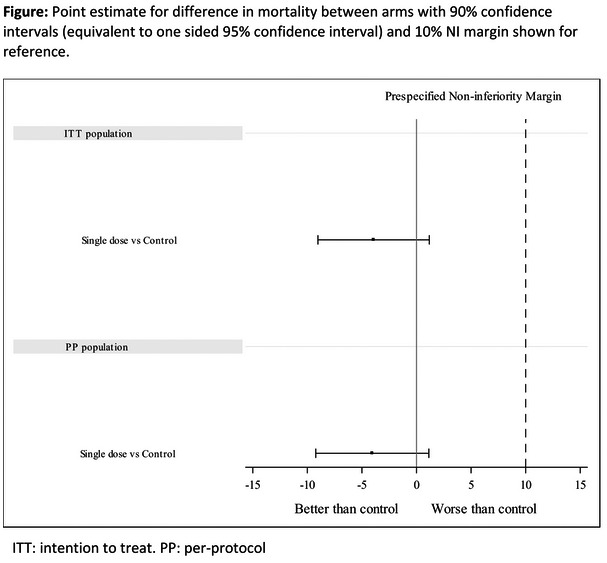
Abstract OALB01LB03‐Figure 1
OALB01LB01
Early termination of randomisation into TB‐PRACTECAL, a novel six months all‐oral regimen Drug Resistant TB study
B‐T Nyang'wa; I Motta; E Kazounis; and C Berry
Medecins Sans Frontieres, Manson Unit, London, United Kingdom
Background: Almost 500,000 people develop multidrug resistant tuberculosis (MDR‐TB) annually with a treatment success rate of around 60%. Treatment consists of up to 20 pills per day taken between nine and twenty‐four months. TB‐PRACTECAL (NCT02589782) is a multi‐arm multi‐stage, randomised controlled, open‐label phase II/III trial to evaluate the safety and efficacy of regimens containing bedaquiline, pretomanid and linezolid for the treatment of MDR‐TB. On 18 March, 2021, randomisation was terminated early following recommendations from the trial’s Data and Safety Monitoring Board (DSMB). We present the trial design, rationale for this decision and the planned next steps.
Description: Adults and children aged from 15 years were enrolled from Uzbekistan, Belarus and South Africa. An adaptive phase IIB/III design was chosen to accelerate the trial. Stage 1/phase IIB comprised of 3 investigational arms compared to locally approved standard of care (SoC). The best performing arm was selected for stage 2/PIII. In Stage 2, patients were randomised to either PRACTECAL‐1 arm (B‐Pa‐Lzd‐Mfx) or SoC. The primary outcome was patients with an unfavourable outcome (treatment failure, death, treatment discontinuation, recurrence, loss to follow‐up) at 72 weeks post‐randomisation. Target sample size was 201 per arm.
Lessons learned: Recruitment termination decision was based on 120 patients randomised to PRACTECAL‐1 arm and 120 to SoC. Around 25% of patients have HIV co‐infection. Interim analysis of the primary outcome showeda difference of at least three standard deviations favouring PRACTECAL‐1 compared to SoC. The difference was driven by a higher rate of treatment discontinuations in the SoC arm. There were five deaths in the SoC versus none in PRACTECAL‐1. Total patients randomised at randomisation termination was 552.
Conclusions/Next steps: The results of the interim analyses convinced the DSMB that equipoise between the two arms no longer exists. Accumulated data of all 552 patients will be analysed and submitted to answer PICO questions for the World Health Organization Rifampicin resistant TB guidelines development process. Results will be published by the end of the year. All patients will be followed up to at least 72 weeks. Given the positive findings, MSF is developing guidance and collaborations to scale up the regimen.
OALB01LB02
High rate of successful outcomes treating highly resistant TB in the ZeNix study of pretomanid, bedaquiline and alternative doses and durations of linezolid
F Conradie 1; D Everitt2; M Olugbosi3; G Wills4; S Fabiane4; J Timm2; and M Spigelman2
1University of Witwatersrand, Johannesburg, South Africa. 2TB Alliance, New York, United States. 3TB Alliance, Pretoria, South Africa. 4University of College London, London, United Kingdom
Background: In the Nix‐TB trial, a six‐month BPaL regimen, starting with 1200 mg linezolid daily, resulted in 89% durable cure at 24 months post therapy follow‐up, but a high rate of linezolid‐related adverse events. The subsequent ZeNix trial enrolled patients with highly resistant TB in South Africa, Russia, Georgia and Moldova and treated them for six months with bedaquiline (B), pretomanid (Pa) and varying doses and durations of linezolid (L), with follow up to the primary endpoint six months after completion of treatment.
Methods: Patients were treated for six months with bedaquiline (200 mg daily for eight weeks followed by 100 mg daily for 18 weeks), pretomanid (200 mg daily) and were equally randomized, dose‐blinded, to daily linezolid starting at 1200 mg for six months (1200L6M), 1200 mg for two months (1200L2M), 600 mg for six months (600L6M), or 600 mg for two months (600L2M). Clinical, laboratory and sputum liquid culture evaluations were performed at baseline, weekly for eight weeks and then every two to four weeks through the end of treatment, monthly for three months, and at the primary endpoint six months after completion of treatment.
Results: 181 participants with highly resistant TB were enrolled. A high success rate at the primary endpoint, similar to Nix‐TB, was observed: 93% in 1200L6M, 89% in 1200L2M, 91% in 600L6M and 84% in 600L2M. Patients in the 1200L6M arm had higher rates of adverse events of peripheral neuropathy and myelosuppression: 38% and 29% in 1200L6M, 24% and 15% in 1200L2M, 24% and 13% in 600L6M, and 13% and 16% in 600L2M, respectively. Four patients had optic neuropathy that reversed, all in the 1200L6M arm. More patients in the 1200L6M arm required linezolid dose modification (reduction, interruption, or discontinuation): 51% in 1200L6M, 28% in 1200L2M, 13% in 600L6M, and 13% in 600L2M.
Conclusions: The ZeNix trial confirms the high relapse‐free cure rate for the BPaL regimen in highly resistant TB and suggests that reduced doses and/or shorter durations of linezolid than 1200 mg for six months have similar efficacy and improved safety.
OALB01LB03
Single high‐dose liposomal amphotericin based regimen for treatment of HIV‐associated Cryptococcal Meningitis: results of the phase‐3 Ambition‐cm Randomised Trial
DS Lawrence 1,2; DB Meya3; E Kagimu3; J Kasibante3; E Mpoza3; M Rutakingirwa3; K Ssebambulidde3; L Tugume3; J Rhein4,3; DR Boulware4,3; H Mwandumba5,6; M Alufandika5; H Mzinganjira5; C Kanyama7; MC Hosseinipour7; C Chawinga7; G Meintjes8; C Schutz8; K Comins8; A Singh8; C Muzoora3; S Jjunju3; E Nuwagira3; M Mosepele2,9; T Leeme2; K Siamisang2; CE Ndhlovu10; A Hlupeni10; C Mutata10; E van Widenfelt6; W Hope11; O Lortholary12; DG Lalloo6; A Loyse13; T Chen6; SF Molloy13; T Boyer‐Chammard12; D Wang6; N Youssouf1,2; S Jaffar6; TS Harrison14,15,16; JN Jarvis1,2; and The Ambition Study Group1
1London School of Hygiene and Tropical Medicine. Department of Clinical Research. Faculty of Infectious and Tropical Diseases. London. United Kingdom. 2Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana. 3Makerere University, Infectious Diseases Institute, Kampala, Uganda. 4University of Minnesota, Department of Medicine, Minneapolis, United States. 5Malawi‐Liverpool‐Wellcome Trust Clinical Research Programme, Blantyre, Malawi. 6Liverpool School of Tropical Medicine, Department of Clinical Sciences and International Public Health, Liverpool, United Kingdom. 7Lilongwe Medical Relief Trust (UNC Project), Lilongwe, Malawi. 8University of Cape Town, Wellcome Centre for Infectious Diseases Research in Africa (CIDRI‐Africa), Institute of Infectious Disease and Molecular Medicine, Cape Town, South Africa. 9University of Botswana, Department of Internal Medicine, Gaborone, Botswana. 10University of Zimbabwe College of Health Sciences, Department of Medicine, Harare, Zimbabwe. 11University of Liverpool, Liverpool, United Kingdom. 12Institut Pasteur, Molecular Mycology Unit and National Reference Centre for Invasive Mycoses, Paris, France. 13St George's University of London, Institute for Infection and Immunity, London, United Kingdom. 14St George's University of London, Centre for Global Health, Institute of Infection and Immunity, London, United Kingdom. 15St George's University Hospitals NHS Foundation Trust, Clinical Academic Group in Infection and Immunity, London, United Kingdom. 16University of Exeter, MRC Centre for Medical Mycology, Exeter, United Kingdom
Background: Cryptococcal meningitis (CM) is a leading cause of HIV‐related mortality. Based on phase‐II study data showing that a single high‐dose of 10 mg/kg liposomal amphotericin‐B (AmBisome, Gilead Sciences Inc) was non‐inferior to 14 days of standard dosing in clearing Cryptococcus from the cerebrospinal fluid we performed a phase‐III randomised controlled non‐inferiority trial to examine the impact of a single high‐dose of AmBisome in averting all‐cause mortality from CM.
Methods: HIV‐positive adults with a first episode of CM in Botswana, Malawi, South Africa, Uganda and Zimbabwe were randomised to induction therapy of either (i) single, high‐dose AmBisome (10 mg/kg) given with 14 days of flucytosine 100 mg/kg/day and fluconazole 1200 mg/day (AmBisome) or (ii) 7 daily doses of amphotericin B deoxycholate (1 mg/kg) plus seven days of flucytosine 100 mg/kg/day, followed by seven days of fluconazole 1200 mg/day (control). All participants received consolidation therapy of fluconazole 800 mg/day for eight weeks. The primary endpoint was all‐cause mortality at 10 weeks with the trial powered to show non‐inferiority with a 10% margin.
Results: We randomised 844 participants from January 2018 to February 2021. 60.2% were men, with median age of 37 years, median CD4 of 27 cells/mm2, and 28.5% had abnormal mental status; 30 participants met early withdrawal exclusion criteria, leaving 814 in the intention‐to‐treat (ITT) population. None were lost to follow‐up. In the primary ITT analysis 10‐week mortality was 24.82% (101/407) in the AmBisome arm and 28.75% (117/407) in the control arm. The difference in mortality between the AmBisome arm and control arm was −3.93%, with the upper limit of the 1‐sided 95%CI for the difference being 1.17%, well below the pre‐specified 10% non‐inferiority margin. The single high‐dose AmBisome treatment was well tolerated.
Conclusions: Single high‐dose AmBisome on a backbone of flucytosine and fluconazole was non‐inferior to the current WHO recommended standard of care for HIV‐associated cryptococcal meningitis.
OALC01LB01
Adherence to the dapivirine vaginal ring and oral PrEP among adolescent girls and young women in Africa: interim results from the REACH study
G Nair 1; K Ngure2; D Szydlo3; E Brown4; C Akello5; T Palanee ‐ Phillips6; P Macdonald7; B Siziba8; S Hillier9; M Garcia10; S Johnson10; L Levy10; T McClure10; L Soto‐Torres11; and C Celum12
1Stellenbosch University, Centre for Medical Ethics and Law, Cape Town, South Africa. 2Jomo Kenyatta University of Agriculture and Technology, Department of Community Health, Nairobi, Kenya. 3Fred Hutchison Cancer Research Institute, Seattle, United States. 4Fred Hutchinson Cancer Research Center,, Vaccine and Infectious Disease Division, Seattle, United States. 5Makerere University – Johns Hopkins University Research Collaboration, Kampala,, Uganda. 6University of the Witwatersrand, Wits Reproductive Health and HIV Institute, Johannesburg, South Africa. 7University of Cape Town, Desmond Tutu HIV Centre, Cape Town, South Africa. 8University of Zimbabwe, Harare, Zimbabwe. 9University of Pittsburgh, Department of Obstetrics, Gynecology, and Reproductive Sciences, Pittsburgh, United States. 10FHI 360, North Carolina, United States. 11National Institute of Health, Department of Allergy and Infectious Diseases, Maryland, United States. 12University of Washington, Medicine, Seattle, United States
Background: Adolescent girls and young women (AGYW) account for most new HIV infections in sub‐Saharan Africa. WHO has endorsed oral PrEP and dapivirine vaginal ring (ring) for women at substantial risk of HIV infection. However, adherence to both products was lower among younger women in randomized placebo‐controlled trials. We assessed interim safety, adherence and acceptability of both products among AGYW between February 2019 and April 2021.
Methods: MTN‐034 (REACH) enrolled 16 to 21‐year‐old HIV‐uninfected, non‐pregnant AGYW from South Africa, Zimbabwe, and Uganda. In the first two study phases, AGYW were randomized to either monthly dapivirine ring or daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for six months, then switched to the second product for six months. Safety is assessed by ≥ grade 2 adverse events (AEs); adherence by residual drug levels in returned rings and plasma dried blood spots (DBS) for oral PrEP. In this analysis, dapivirine levels indicating release of ≥0.1071 mg/day (0.9 mg/28 day) were defined as moderate and ≥0.1426 mg/day (3.0 mg/28 day) as high adherence. DBS concentration of ≥700 fmol tenofovir diphosphate/punch was defined as moderate adherence (associated with 100% efficacy among men who have sex with men) and ≥1200 fmol/punch as high. Acceptability was measured by self‐report.
Results: 247 AGYW were enrolled with an average age of 18. Twenty six months into the study, retention to study visits is 94.4%. Approximately 35% of participants had at least 1 sexually transmitted infection at baseline (chlamydia: 28.7% [71/247]; gonorrhea 8.5% [21/247]). Most participants had at least moderate adherence to ring (77.8% [1064/1368]) and oral PrEP (58.6% [768/1310]). High adherence was observed in 50.2% of ring and 22.4% of oral PrEP users (687/1368 and 294/1310 of timepoints). AEs of ≥ grade 2 were experienced by 78% (187/241) of ring users and 77% (188/245) of oral PrEP users. Acceptability varied, with 88.5% (193/218) liking ring and 63.9% (140/219) liking oral PrEP. One HIV acquisition and 4 incident pregnancies were reported.
Conclusions: Adherence to oral PrEP and dapivirine ring was higher than previously observed among African AGYW, and both were well‐tolerated and highly acceptable. Dapivirine ring is a viable, promising new HIV prevention method, and adherence to both products can be achieved with support strategies.
OALC01LB02
High rates of drug resistance in individuals diagnosed with HIV in tenofovir disoproxil fumarate (TDF)‐based pre‐exposure prophylaxis rollout programs in Kenya, Zimbabwe, Eswatini and South Africa
UM Parikh 1; L Kudrick1; L Levy2; E Bosek3; B Chohan4,5; N Ndlovu6; I Mahaka6; A Hettema7; S Mullick8; K McCormick1; C Wallis9; L Wiesner10; P Anderson11; I Mukui12; S Masyuko13; M Mugambi13; O Mugurungi14; G Ncube14; M Dunbar15; NA Dlamini16; S Matse16; J Peterson2; J Baeten4,17; C Celum4; B Richardson4; D Castor18; K Torjesen2; JW Mellors1; and Global Evaluation of Microbicide Sensitivity (GEMS) Project
1University of Pittsburgh, Pittsburgh, United States. 2FHI360, Durham, United States. 3Consultant, Nairobi, Kenya. 4University of Washington, Seattle, United States. 5KEMRI, Nairobi, Kenya. 6Pangaea Zimbabwe AIDS Trust, Harare, Zimbabwe. 7Consultant, Mbabane, Eswatini. 8The Wits Reproductive Health and HIV Institute, Johannesburg, South Africa. 9BARC‐SA/Lancet Laboratories, Johannesburg, South Africa. 10University of Cape Town, Cape Town, South Africa. 11University of Colorado, Denver, United States. 12Ministry of Health, Nairobi, Kenya. 13National AIDS and STI Control Programme, Nairobi, Kenya. 14Ministry of Health and Child Care, Harare, Zimbabwe. 15Consultant, Oakland, United States. 16Ministry of Health, Mbabane, Eswatini. 17Gilead Sciences, Foster City, United States. 18Columbia University, New York, United States
Background: The ongoing rollout of oral TDF‐based PrEP has the potential to reduce HIV incidence in Sub‐Saharan Africa (SSA) but HIV drug resistance (HIVDR) in PrEP breakthrough infections could threaten treatment effectiveness, contribute to spread of resistance, and undermine efforts to control HIV. Accordingly, the Global Evaluation of Microbicide Sensitivity (GEMS) project was established to monitor HIVDR in PrEP rollout programs in SSA.
Methods: USAID/PEPFAR‐supported GEMS implemented resistance monitoring in PrEP users diagnosed with HIV while participating in national PrEP programs in Kenya, Zimbabwe and Eswatini, or rollout projects in South Africa. Blood samples were collected from consenting participants diagnosed with HIV on PrEP. Demographics and self‐reported adherence were collected via questionnaire. Tenofovir‐diphosphate (TFV‐DP) levels were measured by liquid chromatography–mass spectrometry. HIVDR mutations were detected by population genotyping and analyzed using Stanford HIVdb v9.0.
Results: Of 204 reported seroconversions on PrEP, 175 (86%) participants provided a sample, including 72 (41%) from South Africa, 58 (33%) from Kenya, 28 (16%) from Zimbabwe and 17 (10%) from Eswatini. These 175 participants had a median age of 24 years (range 16 to 67) and 74% were female. Key populations included HIV serodiscordant partnerships (21%), female sex workers (10%), men who have sex with men (9%), and transgender individuals (6%). 26% of infections occurred within 60 days of PrEP initiation. TFV‐DP was detectable (≥31.25 fmol/punch) in 63 of 86 (73%) samples, with 49 of those 63 (78%) self‐reporting good/fair adherence. 104 (59%) samples were successfully genotyped; insufficient HIV RNA (35% of all samples) was the predominant reason for no result. At least one major HIVDR mutation was detected in 47 (45%) samples, including 3TC/FTC‐associated M184IV (21%), TDF‐associated K65R (3%) and K70EN (3%). Transmitted NNRTI mutations unrelated to PrEP included K101E (1%), K103NS (13%), V106IM (5%), Y181C (2%), and G190A (7%).
Conclusions: The high frequency of HIV drug resistance in HIV‐infected individuals on PrEP (21% with M184IV; 3% with K65R) exceeds background levels of transmitted nucleoside/tide resistance in SSA (≤5%). Improved identification of acute infection before initiating PrEP, and HIVDR monitoring on PrEP is essential for PrEP rollout programs to preserve antiretroviral options for both treatment and prevention.
OALC01LB03
Safety and pharmacokinetics of oral islatravir once monthly for HIV pre‐exposure prophylaxis (PrEP): week 24 analysis of a phase 2a trial
S Hillier 1; L‐G Bekker2; SA Riddler3; CW Hendrix4; S Badal‐Faesen5; S Rasmussen6; H Schwartz7; P Macdonald2; J Lombaard8; Y Caraco9; A Peer10; M Patel11; B Evans11; B Homony11; K Nedrow11; V Teal11; P Hwang11; MN Robertson11; and RM Plank11
1Magee‐Women's Research Institute & Foundation, Pittsburgh, United States. 2Desmond Tutu HIV Centre, Cape Town, South Africa. 3University of Pittsburgh, Pittsburgh, United States. 4John Hopkins Hospital, Baltimore, United States. 5University of the Witwatersrand, Clinical HIV Research Unit, Johannesburg, South Africa. 6Celerion, Lincoln, United States. 7Research Centers of America, Hollywood, United States. 8Josha Research, Bloemfontein, South Africa. 9Hadassah Medical Center, Jerusalem, Israel. 10Rambam Health Care Campus, Haifa, Israel. 11Merck & Co., Inc., Kenilworth, United States
Background: Islatravir (ISL) is a nucleoside reverse transcriptase translocation inhibitor in development for prevention of HIV‐1. Phase 3 trials of oral ISL 60 mg once monthly (QM) are enrolling. We present unblinded safety and pharmacokinetic (PK) results through Week 24 of an ongoing phase 2a trial of monthly ISL for PrEP.
Methods: This randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter trial (NCT04003103) assesses the safety, tolerability, and PK of oral ISL in adults (age 18 to 65 years) at low‐risk for HIV‐1 acquisition. Participants were randomly assigned (2:2:1) to receive 6 QM doses of ISL 60 mg, ISL 120 mg, or matching placebo. ISL in plasma was measured in all participants; ISL‐triphosphate (ISL‐TP) in peripheral blood mononuclear cells (PBMCs) was measured in a subset. Safety assessments included adverse event (AE) reporting and laboratory results monitoring. Aggregate safety results through 05 April 2021 are reported here; unblinded safety results for all participants through Week 24 will be available for presentation.
Results: Of 242 participants randomized (median age 31 years, 67% female, 53% white, 42% Black or African American), 189 completed dosing, 15 discontinued study intervention, and 38 were ongoing as of 05 April 2021. AEs were reported by 60% of participants; the most common AEs were headache (9%), diarrhea (5%), and nausea (5%). AEs considered drug‐related by the investigator were reported in 15% of participants; all drug‐related AEs were mild or moderate (DAIDS grade 1 to 2). Two participants discontinued study drug due to drug‐related AEs (mild foreign body sensation in throat; moderate rash and pruritis). Two serious AEs (including one death) were reported; neither was considered drug‐related. Grade 3 to 4 laboratory values were uncommon (Table 1). ISL‐TP trough concentrations after both ISL 60 and 120 mg QM dosing remained above 0.05 pmol/106 PBMCs, the pre‐specified threshold for PrEP.
Conclusions: Oral ISL 60 and 120 mg QM were well‐tolerated over 24 weeks and achieved the pre‐specified PK threshold for HIV‐1 prevention.
Abstract OALC01LB03‐Table 1
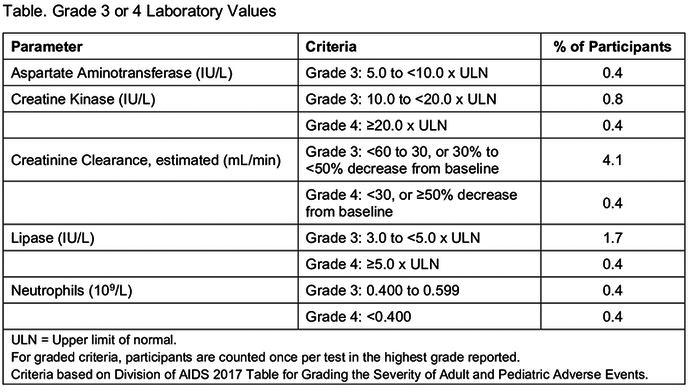
OALD01LB01
Factors associated with 12‐month retention after referral to a differentiated service delivery for HIV treatment model in Zambia
Y Jo1; S Rosen2,3; B Phiri 4; A Grimsrud5; M Mwansa6; H Shakwele4; P Haimbe4; MM Mwenechanya7; P Lumano Mulenga6; BE Nichols2,3,8
1Boston Medical Center, Section of Infectious Diseases, Department of Medicine, Boston, United States. 2Boston University School of Public Health, Department of Global Health, Boston, United States. 3University of the Witwatersrand, Health Economics and Epidemiology Research Office, Department of Internal Medicine, School of Clinical Medicine, Johannesburg, South Africa. 4Clinton Health Access Initiative, Lusaka, Zambia. 5International AIDS Society, Cape Town, South Africa. 6Ministry of Health, Lusaka, Zambia. 7The Centre for Infectious Disease Research in Zambia, Lusaka, Zambia. 8Amsterdam University Medical Center, Department of Medical Microbiology, Lusaka, Zambia
Background: Many countries across sub‐Saharan Africa are rapidly scaling up differentiated service delivery (DSD) for HIV treatment models to support person‐centered care. We assessed the associations between patient and facility characteristics and 12‐month retention after referral to DSD across DSD models in Zambia.
Abstract OALD01LB01‐Figure 1
Methods: A retrospective record review using electronic medical records was done including adults (≥15 years) who started DSD between October 2019 and March 2020. Retention was defined as in ART care on 31 December 2020. We categorized DSD models into six groups: multi‐month dispensing (MMD), fast‐track, group models, alternative pick‐up points, home delivery, and extended facility hours. Relative risk of loss to follow‐up (LTFU) was estimated by DSD model adjusted for age, gender, location (urban/rural), and care level stratified by antiretroviral therapy (ART) dispensing interval. Using linear regression, a facility‐level analysis assessed the association between mean percentage of patients LTFU per facility with location, care level, number of DSD models and percentage of patients receiving 4 to 6 MMD.
Results: Of 90,829 patients referred to DSD models, the majority (78.3%, n = 71,101) received 4 to 6 MMD. Among those receiving 4 to 6 MMD, those in fast‐track and group models had lower adjusted risk of LTFU after 12 months compared to those receiving only MMD (adjusted risk ratio (aRR) 0.63, 95% confidence interval (CI) 0.59 to 0.67; aRR 0.72, 95%CI 0.62 to 0.84, respectively) (Figure 1). Among those receiving 3MMD, there was no difference in LTFU by DSD model. At the facility level, the adjusted risk of LTFU increased with having multiple DSD models available at the facility compared to having just one DSD model (increased LTFU of +2%, 95% CI: 0% to 5%).
Conclusions: Twelve‐month retention varied by DSD model, MMD duration, and facility‐level characteristics in Zambia. Efforts are needed to support long‐term retention in DSD models and understand the interaction between specific models, health facility and patient level characteristics.
OALD01LB02
Community‐led quality improvement of HIV services using community scorecards in Vietnam
L Hoai Nguyen 1,2; V Nhat Thi Dang1,2; P Thi Do1,2; T Pollack1,2; N Tuyet Thi Vo1,2; M Tra Dang1,2; T Minh Dao3; H Huy Dam4; L The Vuong5; A Kim Ai Le6; D Weikum7; and A Nguyen8
1Beth Israel Deaconess Medical Center, Boston, United States. 2Partnership for Health Advancement in Vietnam (HAIVN), Hanoi, Vietnam. 3Community Advisory Board, Binh Duong, Vietnam. 4Community Advisory Board, Thai Nguyen, Vietnam. 5Binh Duong Center for Disease Control, Binh Duong, Vietnam. 6Thai Nguyen Center for Disease Control, Thai Nguyen, Vietnam. 7US Centers for Disease Control and Prevention, Division of Global HIV & TB, Atlanta, United States. 8US Centers for Disease Control and Prevention, Division of Global HIV & TB, Hanoi, Vietnam
Background: Meaningful community engagement decreases stigma and discrimination (S&D) and improves the quality of HIV services. Community scorecards (CSC) were introduced in two Vietnamese high‐burden provinces to reduce S&D, facilitate community engagement, and improve services.
Description: The CSC is a two‐way, participatory, community‐led quality improvement tool adapted for the Vietnamese context. Community representatives led CSC indicator development with inputs from health staff (HS). Communities and HS both rated 15 quantitative indicators (score, 1 [poorest quality] to 10 [best quality]) covering prevention, counseling and testing, care and treatment, facilities, policy and procedures, and overall satisfaction. Discussions also provided qualitative data for all indicators. Each CSC scoring meeting included 15 to 20 representatives from HS and the community. Quarterly CSC implementation began in January 2020 at seven sites; three successive rounds have been completed by April 2021 with advocacy action plans.
Abstract OALD01LB02‐Figure 1
Lessons learned: CSC substantially improved services, as exemplified by two sites (Figure 1). After CSC, clients reported increased access to free prevention commodities (e.g., condoms, needles) and informational materials. Facilities also designated individual counseling rooms to meet clients’ request for increased privacy. Finally, CSC promoted friendlier services. Facilities improved client spaces and comfort (e.g., restroom, waiting space furniture) and introduced new procedures to reduce waiting time and S&D. No incidents of S&D were reported after round 2.
Quantitative scores sometimes did not accurately reflect service quality. For example, at times clients gave high scores, HS gave themselves low scores, but qualitative discussions indicated intermediate quality. Clients and HS reported that CSC provided a platform to review service quality, understand service gaps, and build trusting relationships.
Conclusions/Next steps: CSC in Vietnam successfully facilitated meaningful partnerships between the HIV‐affected community and HS and led to improvements. Both groups shared decision‐making responsibility. Future efforts will include expansion of CSC to more sites across Vietnam and linking CSC findings with community‐led monitoring initiatives.
OALD01LB03
RAPID‐VL intervention improves viral load ordering, results turnaround time and viral suppression: a cluster randomized trial in HIV clinics in Uganda
V Jain 1; A Owaraganise2; D Black1; B Twinamatsiko2; M Ayebare2; B Wandera2; F Semitala2; C Twinamatsiko2; S Kabageni2; M Naluguza3; L Mills3; A Namale3; A Zee4; C Dawson Rose5; E Charlebois6,7; and M Kamya8,2
1University of California, San Francisco, Division of HIV, Infectious Diseases & Global Medicine, University of California, San Francisco, San Francisco, United States. 2Infectious Diseases Research Collaboration (IDRC), Kampala, Uganda. 3Centers for Disease Control and Prevention, Kampala, Uganda. 4Centers for Disease Control and Prevention, Atlanta, United States. 5University of California, San Francisco, Department of Nursing, San Francisco, United States. 6University of California, San Francisco, Division of HIV, Infectious Diseases & Global Medicine, San Francisco, United States. 7University of California, San Francisco, Center for AIDS Prevention Studies, San Francisco, United States. 8Makerere University, Department of Medicine, Kampala, Uganda
Background: HIV viral load (VL) monitoring is crucial for long‐term antiretroviral therapy (ART) success. However, challenges in Africa include suboptimal VL ordering by clinicians, delayed VL turnaround time, and suboptimal VL counseling/interpretation.
Methods: A cluster‐randomized controlled “pre‐post” trial was conducted in 20 PEPFAR‐supported HIV clinics (10 intervention/10 control) in southwestern Uganda. We enrolled four high‐risk patient groups (pregnant/breastfeeding women, children/adolescents (two to seventeen years), viremic patients, patients overdue for VL), and non‐high‐risk adults. Retrospective clinic data (2017 to 2018 “pre‐intervention”) on N = 1200 participants (60/clinic; 20 clinics) was obtained, and N = 1200 new participants enrolled prospectively (2018 to 2020 “post‐intervention”; N = 2400 total). The RAPID‐VL intervention included (1)‐a VL‐ordering flowsheet with quarterly performance feedback, (2)‐rapid near‐point‐of‐care VL testing (Cepheid GeneXpert) with same/next‐day telephone delivery of VL results to patients, and (3)‐clinician training on VL results counseling. Control clinics used standard‐of‐care VL ordering/testing/counseling per Uganda’s national program. Primary outcomes were (1)‐VL turnaround time (result delivery to patients) and (2)‐% of visits with guideline‐concordant VL ordering. Secondary outcome was VL suppression one‐year post‐intervention. Intervention effect was analyzed by cluster‐adjusted difference‐in‐difference estimation.
Results: Of 2400 participants, 66.4% were female, mean age 37 (range 18 to 88), and median ART duration 2.8 years. Pediatric participants were 50.9% female, mean age 9 (range 2 to 17), and median ART duration three years. Pre‐intervention VL turnaround time was not significantly different between intervention and control clinics (mean 73.4 days; p = 0.20 cluster‐adjusted).
Post‐intervention, turnaround time was significantly reduced in intervention vs. control clinics (median = 1 vs. 56 days). Intervention‐associated change in mean turnaround time, adjusting for temporal trends and clinic‐level clustering, was −67.3 days (p<0.0001). Significant reductions were seen within every patient subgroup. Pre‐intervention, VL ordering was not significantly different in intervention vs. control clinics (70.5% vs. 72.2%; p = 0.081). Post‐intervention, the intervention‐associated improvement in VL ordering was +10.4% (p = 0.01). One‐year viral suppression post‐intervention in measured participants was 83.1% in intervention clinics and 76.0% in control clinics (+7.1%, p = 0.0091).
Conclusions: In this large cluster RCT in Uganda, a multi‐component intervention with boosted clinician training and rapid near‐point‐of‐care VL testing: (1)‐significantly reduced VL turnaround time, (2)‐significantly improved guideline‐concordant VL ordering, and (3)‐significantly improved one‐year viral suppression. The RAPID‐VL intervention may strengthen VL operations within national ART programs.
OALX01LB01
Comparative functional analysis of HIV‐1 accessory proteins Nef and Vpu in African LTNPs and chronic progressors
G Umviligihozo 1; S W Jin1; FM Mwimanzi1; H Sudderuddin2; ZL Brumme1,2; and MA Brockman1,2
1Simon Fraser Univeristy, Faculty of Health Sciences, Burnaby, Canada. 2BC Centre for Excellence in HIV/AIDS, Vancouver, Canada
Background: HIV‐1 Nef and Vpu enhance viral pathogenicity through partially overlapping immune evasion functions, but few studies have assessed both proteins together in HIV‐infected individuals exhibiting slower disease progression. Here, we analyzed and compared the functions of Nef and Vpu sequences isolated from 38 HIV‐1 infected long term non‐progressors (LTNPs) from Rwanda with those of 24 Vpu and 92 Nef sequences isolated previously from chronically infected individuals (CI).
Methods: HIV RNA was extracted from plasma and nested RT‐PCR was used to amplify Nef and Vpu coding regions. Amplicons were cloned into an expression vector, which features dual promotors driving Nef/Vpu and GFP expression. Nef and Vpu clones were transfected by electroporation into an immortalized CD4+ T‐cell line (CEM). The ability of each Nef clone to down‐regulate CD4/HLA and each Vpu clone to down‐regulate CD4/Tetherin was quantified by flow cytometry and the resulting data normalized to that of negative (empty vector) and positive (Nef SF2 and Vpu NL4.3) controls.
Results: Normalized Vpu‐mediated downregulation activity among LTNPs (median [IQR]) was 0.97 [0.78 to 1.11] for CD4 and 0.93 [0.79 to 0.99] for Tetherin, while Nef‐mediated downregulation activity was 0.98 [0.90 to 1.0] for CD4 and 0.71 [0.47 to 0.74] for HLA. Vpu‐mediated CD4 downregulation activity and Nef‐mediated HLA downregulation functions were significantly lower in LTNPs compared to CI (p = 0.003 and p < 0.0001, respectively).
Conclusions: Our results show variable Nef and Vpu activity in LTNPs versus CI, suggesting a modest functional impairment in LTNPs that may contribute to a delayed clinical disease.
OALX01LB02
Efficacy and safety of long‐acting subcutaneous lenacapavir in phase 2/3 in heavily treatment‐experienced people with HIV: week 26 results (Capella study)
J‐M Molina 1; S Segal‐Maurer2; H‐J Stellbrink3; A Castagna4; M Berhe5; GJ Richmond6; PJ Ruane7; GI Sinclair8; K Siripassorn9; H Wang10; H Patel10; N Margot10; H Dvory‐Sobol10; RH Hyland10; MS Rhee10; J Baeten10; and E DeJesus11
1Hopital Saint Louis, Paris, France. 2New York Presbyterian Queens, New York, United States. 3ICH Study Center, Hamburg, Germany. 4IRCCS Ospedale San Raffaele, Milan, Italy. 5North Texas Infectious Diseases Consultants, Dallas, United States. 6Gary J Richmond, MD, PA, Fort Lauderdale, United States. 7Ruane Clinical Research Group, Los Angeles, United States. 8PrismHealth North Texas, Dallas, United States. 9Bamrasnaradura Infectious Diseases Institute, Nonthaburi, Thailand. 10Gilead Sciences Inc., Foster City, United States. 11Orlando Immunology Center, Orlando, United States
Background: Lenacapavir (LEN), a long‐acting first‐in‐class HIV capsid inhibitor with full activity against multidrug‐resistant mutants, is in clinical development. The ongoing Phase 2/3 Capella study in heavily treatment‐experienced (HTE) people with HIV (PWH) failing their current regimen with multidrug‐resistance achieved the primary endpoint demonstrating short term potent antiviral activity of LEN versus placebo during the 14‐day functional monotherapy period.
Methods: In the randomized cohort, participants were randomized (2:1) to add oral LEN or placebo to their failing regimen (600 mg on Day 1 [D] and 2 and 300 mg on D8). At D15, those on oral LEN received subcutaneous (SC) LEN 927 mg (Q6M); those on placebo started the oral lead‐in, followed by SC Q6M. All randomized participants initiated an investigator‐selected, optimized background regimen (OBR) at D15. In the non‐randomized cohort, participants started OBR concurrent with LEN (oral lead‐in à SC). We report the secondary endpoint of Week 26 (W26) efficacy in the randomized cohort, and additional available efficacy and safety from both cohorts.
Results: 72 participants enrolled: 36 in each cohort. Overall, 25% were female; 38% Black. Median age was 52 years; median CD4 count was 150 cells/µl; mean HIV‐1 RNA (VL) was 4.17 log10 c/ml. Resistance to ≥2 ARVs in each class was 99%(NRTIs), 97%(NNRTIs), 81%(PIs) and 69%(INSTIs). At W26 in the randomized cohort, 81%(29/36) had VL<50 copies/ml via FDA‐Snapshot algorithm. In participants with data through W26 from both cohorts, 79%(33/42) had VL<50 copies/ml via missing=failure. Median CD4 count increased by 82 cells/µl. Four randomized participants had emergent LEN resistance; 3 suppressed afterwards, one with OBR change and two without. Resistance analysis in non‐randomized participants is ongoing. There were no study drug‐related serious adverse events (AEs) or AEs leading to discontinuations. LEN‐related ISRs occurred in 56% (40/72) and were mostly mild or moderate (38/40). Most common ISRs (>20%) were swelling (26%) and erythema (24%); both resolved within days.
Conclusions: Subcutaneous LEN in combination with OBR led to sustained virologic suppression in 81% of HTE PWH at W26. LEN was safe and well tolerated. These results support the ongoing evaluation of LEN for treatment and prevention of HIV‐1 infection.
OALX01LB03
Initial results of recent HIV infection surveillance in Cambodia, 2020
PS Ly 1; K Soch2; V Ouk1; B Ngauv1; C Chea1; C Mom1; C Bora1; B Eng2; V Ly2; R Albalak2; C Yang3; S Ebrahim3; T Dobbs3; A Ernst4; S Welty4; S Stephens4; L Buback4; B Kiernan4; and A Suthar3
1National Centre for HIV Dermatology and STD (NCHADS), Phnom Penh, Cambodia. 2Centre for Disease Control and Prevention (CDC), National Institute of Public Health, Phnom Penh, Cambodia. 3Centre for Disease Control and Prevention (CDC), Atlanta, United States. 4University of California San Francisco (UCSF): Institute for Global Health Sciences (IGHS), San Francisco, United States
Background: Recent HIV infection surveillance can help to identify populations and geographies with active transmission. We compared risk factors among recent and long‐term infections from the initial ten months of recent HIV infection surveillance in Cambodia to help target prevention interventions and rapid treatment initiation among newly diagnosed individuals of recent HIV infection.
Methods: We used demographic and risk data collected in the National HIV Voluntary Counselling and Testing (VCT) data from March through December 2020. Clients aged ≥15 years and newly diagnosed as HIV‐positive were offered recency testing at 66 facilities in all 25 provinces of Cambodia. Blood specimens from consenting individuals were tested by the Asante HIV‐1 Rapid Test for Recent Infection (RTRI). We compared the distribution of clients with RTRI‐recent and long‐term infection by select characteristics using chi‐square tests in STATA16.
Results: Of 2464 newly diagnosed HIV‐positive VCT clients, 2080 (84%) consented to RTRI testing, and 161 (8%) were classified as RTRI‐recent infections. The percentage of clients with recent infection did not vary by sex (men, 76%; women, 24%; p = 0.629). Overall, there were statistically significant differences in some age groups, population groups, and provinces. Recent infections were significantly more frequent among clients aged 20 to 34 years (p < 0.001), entertainment workers (p = 0.001), and men who have sex with men (MSM) (p < 0.001) compared to those with long‐term infections. Similarly, recent infections were more frequent identified in Siem Reap (p = 0.010) and Phnom Penh (p = 0.038) provinces (Table 1).
Conclusions: Initial recent HIV surveillance data suggest that recent transmission in Cambodia may be driven by several age, population, and geographic groups different from those with long‐term infections. Continued surveillance may facilitate improved targeting of HIV prevention and treatment interventions.
Abstract OALX01LB03‐Table 1. Comparison of RTRI‐long term and RTRI‐recent by demographic and risk group characteristics
| Demographic characteristics | Percentage of RTRI‐Long‐term infection (n = 1919) | Percentage of RTRI‐recent infection (n = 161) | p‐value |
|---|---|---|---|
| <20 years | 6% (109) | 6% (9) | 0.962 |
| 20 to 34 years | 60% (1145) | 76% (123) | <0.001 |
| ≥35 years | 35% (664) | 18% (29) | <0.001 |
| Male | 75% (1433) | 76% (123) | 0.629 |
| Female | 25% (486) | 24% (38) | |
| Siem Reap province | 12% (234) | 19% (31) | 0.010 |
| Phnom Penh province | 42% (815) | 51% (82) | 0.037 |
| Other provinces | 26% (496) | 12% (20) | <0.001 |
| Risk group characteristics | |||
| Entertainment worker | 3% (54) | 7% (12) | 0.001 |
| Men who have sex with men | 41% (793) | 58% (94) | <0.001 |
| General population | 50% (967) | 32% (51) | <0.001 |
OALX01LB04
Impact of COVID‐19 on HIV treatment interruption in seven PEPFAR countries, April–June 2020
N Mehta 1; A Stewart1; K Fisher1; S Ghosh1; L Santos1; P Harvey2; M Li3; S Mazibuko3; S Hong4; G Mutandi4; C Nyagatare5; ML Umwangange5; E Yoboka6; J Kalamya7; S Agolory8; K Mweebo8; L Mulenga9; P Nyika10; R Weber10; C Mupanguri11; S Patel1; and J Aberle‐Grasse1
1Centers for Disease Control and Prevention, Atlanta, United States. 2Centers for Disease Control and Prevention, Gaborone, Botswana. 3Centers for Disease Control and Prevention, Mbabane, Eswatini. 4Centers for Disease Control and Prevention, Windhoek, Namibia. 5Centers for Disease Control and Prevention, Kigali, Rwanda. 6Ministry of Health, Kigali, Rwanda. 7Centers for Disease Control and Prevention, Kampala, Uganda. 8Centers for Disease Control and Prevention, Lusaka, Zambia. 9Ministry of Health, Lusaka, Zambia. 10Centers for Disease Control and Prevention, Harare, Zimbabwe. 11Ministry of Health, Harare, Zimbabwe
Background: Modeling estimates indicated that the COVID‐19 pandemic would impact access to treatment for people living with HIV due to national lockdowns, restricted mobility, and overwhelmed healthcare infrastructures. We evaluated seven U.S. President’s Emergency Plan for AIDS Relief (PEPFAR)‐supported countries to determine if the COVID‐19 pandemic caused interruption in HIV services.
Methods: We reviewed quarterly program data from Centers for Disease Control and Prevention HIV treatment sites among seven PEPFAR‐supported countries with mature treatment programs (>80% treatment coverage). Interruption in treatment (IIT) in the months with the most restrictive mitigation measures or lockdown (April to June 2020 [P2]) were compared with the three‐month periods before (January–March 2020 [P1]) and after (July–September 2020 [P3]). Narrative data were reviewed for context.
Results: During December 2019, 1,838,396 individuals were receiving antiretroviral therapy (ART) in the seven PEPFAR‐supported countries assessed. Overall, in the quarter before the lockdowns (P1 vs. P2), 23% more patients experienced IIT; in the quarter after the lockdowns (P3 vs. P2), 10% fewer patients experienced IIT (Table 1). Although results varied by country, the number of patients experiencing IIT after the lockdown was either less than that during the lockdown or remained lower than before the lockdown, with the exception of Botswana. Common themes from narratives showed that programs used alternate facility refills, multi‐month dispensation, community‐based ART refills, and social distancing and mitigation measures in clinics to adapt to the COVID‐19 pandemic.
Abstract OALX01LB04‐Table 1. Interruptions in treatment during the COVID‐19 pandemic
| Country | Patients experiencing an interruption in treatment (IIT) | Positive value indicates P1 had a greater IIT than P2* | Negative value indicates P2 had a greater IIT than P3* | ||
|---|---|---|---|---|---|
| January to March 2020 (P1) | April to June 2020 (P2 to lockdown) | July to September 2020 (P3) | % Change between P1 and P2: (P1 to P2)/P2 × 100 | % Change between P3 and P2: (P3 to P2)/P2 × 100 | |
| Total | 51,966 | 42,133 | 37,780 | 23 | −10 |
| Botswana | 221 | 1891 | 1113 | −88 | −41 |
| eSwatini | 298 | 181 | 186 | 65 | 3 |
| Namibia | 2276 | 1974 | 2047 | 15 | 4 |
| Rwanda | 548 | 534 | 525 | 3 | −2 |
| Uganda | 16,115 | 23,046 | 15,069 | −30 | −35 |
| Zambia | 25,968 | 9060 | 14,806 | 187 | 63 |
| Zimbabwe | 6540 | 5447 | 4034 | 20 | −26 |
Conclusions: During the initial COVID‐19 lockdowns, treatment interruptions did not increase across PEPFAR‐supported countries with high ART coverage. These findings suggest the rapid adoption of innovative strategies including policies around multi‐month dispensing and community ART access sustained HIV treatment during the initial months of the COVID‐19 pandemic. However, further research is warranted to understand the variation in IIT among these countries.


