Abstract
Reward-seeking and relief from negative emotions are two central motivational drives underlying addictions. Impaired executive control over craving and negative emotions contributes to compulsive addictive behaviors. Neuroimaging evidence has implicated the prefrontal cortex (PFC) in regulating craving or emotions. This study aims at examining whether anodal transcranial direct current stimulation (tDCS) over a specific region of the PFC would enhance both regulation processes. Thirty-three men with internet gaming disorder received active (1.5 mA for 20 minutes) and sham tDCS over the right dorsolateral PFC (dlPFC) one week apart in a randomized order. During each stimulation session, participants regulated craving for gaming during a regulation of craving (ROC) task and negative emotions during an emotion regulation (ER) task using cognitive reappraisal. Subjective ratings of craving and negative emotions and skin conductance responses (SCRs) were recorded. For both craving and negative emotions, tDCS of the right dlPFC facilitated downregulation and upregulation: active relative to sham tDCS decreased ratings (ROC: 95% CI of difference −1.38 to −0.56, p < 0.001; ER: −1.65 to −0.70, p < 0.001) and/or SCRs (ROC: −1.99 to −0.41 μs, p = 0.004) for downregulation, and increased ratings (ROC: 0.24 to 0.82, p = 0.001; ER: 0.26 to 0.72, p < 0.001) for upregulation. These findings provide the first experimental evidence confirming that tDCS of the right dlPFC enhances both craving- and negative-emotion-regulation. This suggests a promising approach for concurrently enhancing executive control over two central motivational drives underlying addictions.
Keywords: Transcranial direct current stimulation, Craving regulation, Negative emotion regulation, Internet gaming disorder
1. Introduction
Theories of addictions propose that reward-seeking and relief from negative emotions are two important motivational drives underlying engagement in addictive behaviors (Baker et al., 2004; Nestler, 2005; Potenza, 2014). Impaired executive control over reward-regulation and negative-emotion-regulation contributes to compulsive addictive behaviors (Goldstein & Volkow, 2011; Volkow et al., 2016). Specifically, difficulties in curbing intense craving for addiction-related rewards (specific substances or activities) are associated with persistent addictive behaviors (Kober et al., 2010; Yang et al., 2017), which contribute to negative sequelae of addictions (e.g., aversive withdrawal symptoms and impairments in health and functioning). Difficulties in regulating negative emotions adaptively may also promote craving and engagement in addictive behaviors. Hypo-reactivity to long-term negative consequences of addictions may be one important factor that contributes to compulsive addictive behaviors (Yao et al., 2015; Yip et al., 2018). In other situations, hypersensitivity to withdrawal symptoms and negative events may induce or increase craving (Khosravani et al., 2017; Milivojevic & Sinha, 2018). To develop more effective treatments for addictions, it may be important for interventions to simultaneously target regulation of both craving and negative emotions. Prior intervention studies have suggested that treatments involving craving-regulation or negative-emotion-regulation training are effective in alleviating addictive behaviors (Azizi et al., 2010; Dolan et al., 2013), and that enhanced regulation of negative emotions by mindfulness meditation reduces drug use (Tang et al., 2016). Despite the important role of regulation tendencies in addictions and their treatment, few studies have investigated approaches to concurrently enhance regulation of craving and negative emotions.
Neuroimaging studies separately investigating these regulation processes indicate that craving-regulation and negative-emotion-regulation may share similar neural correlates, with cognitive-control networks involving prefrontal regions implicated in each regulation process. For instance, Kober et al (2010) instructed cigarette smokers to regulate craving induced by smoking-related cues using cognitive reappraisal. The results revealed that downregulation of craving was associated with increased activation of the dorsolateral prefrontal cortex (dlPFC) and decreased activation of the ventral striatum. Their findings suggest that regulation of craving may be exerted through dlPFC-related top-down regulation of activity in limbic regions. Meanwhile, other studies have found that downregulation and upregulation of negative emotions are associated with increased activation of prefrontal regions (Buhle et al., 2014; Goldin et al., 2008). Furthermore, individuals with addictions have shown deficits in regulation of craving and negative emotional states (Naqvi et al., 2015; Yip et al., 2018) and relative hypo-activation of prefrontal regions during these processes (Albein-Urios et al., 2014; Yip et al., 2018). Transcranial direct current stimulation (tDCS), a non-invasive technique that is proposed to increase activation of targeted cortical regions with anodal stimulation (Lefaucheur et al., 2017), permits us to investigate whether tDCS over a specific region of the PFC may enhance regulation of both craving and negative emotions.
We increased cortical activation in the right dlPFC via anodal tDCS in individuals with internet gaming disorder (IGD) and instructed participants to complete two well-validated tasks assessing regulation of craving (ROC) (Kober et al., 2010) and emotion regulation (ER) (Buhle et al., 2014) during the stimulation. Our rationale for choosing participants with IGD was twofold. First, IGD is a condition included in the DSM-5 (American Psychiatric Association, 2013) and gaming disorder has been approved by the WHO secretariat for inclusion in the ICD-11 (World Health Organization, 2018). The prevalence and severity of IGD may be particularly impactful in East Asian regions including China (Yao et al., 2017), making IGD worthy of further study in these jurisdictions. However, IGD is important worldwide. Individuals with IGD are frequently exposed to cues related to internet games in part due to the widespread availability and use of the internet, complicating efforts to curb cue-induced craving via avoidance. Second, as with substance addiction, considerable neuroimaging evidence has shown that IGD is characterized by impaired cognitive control involved in reward and negative-emotion processing (Yao et al., 2017; Yip et al., 2018), demonstrating similar neural alterations (i.e., relative hypo-activation of prefrontal regions) across addictions (Albein-Urios et al., 2014; Naqvi et al., 2015). Therefore, exploring effective approaches to enhance regulation of craving and negative emotions may be particularly relevant to public health efforts regarding IGD (Rumpf et al., 2018) and broadly relevant to those regarding individuals with addictions.
Of note, we chose the right dlPFC as a target region for stimulation, rather than the left dlPFC or other prefrontal regions related to regulatory control (e.g., dorsomedial PFC and ventrolateral PFC), for two important reasons. First, tDCS of the right dlPFC has been particularly recommended for addiction interventions (Lefaucheur et al., 2017). Second, the right dlPFC may be particularly involved in negative emotional processing based on empirical evidence supporting the valence theory of hemisphere-lateralized activity of prefrontal regions (with negative and positive and emotions preferentially processed in the right and left hemispheres, respectively) (Leyman et al., 2009).
Based on the aforementioned neuroimaging evidence (Goldin et al., 2008; Kober et al., 2010), we hypothesized that increasing activation of the right dlPFC via tDCS would improve regulation of both craving and negative emotions. During performance of the ROC and ER tasks, participants were instructed to downregulate and upregulate craving induced by gaming images and negative emotions elicited by negative pictures using cognitive reappraisal, respectively. Subjective ratings of craving and negative feelings and skin conductance responses (SCRs) were recorded. We anticipated that tDCS of the right dlPFC would facilitate both downregulation and upregulation in both tasks: active relative to sham tDCS would decrease ratings for downregulation conditions, and increase ratings for upregulation conditions, respectively. Given that SCRs have been correlated with amygdala-frontal function (Feeser et al., 2014; Mangina & BeuzeronMangina, 1996; Williams et al., 2001) and may represent a sensitive physiological marker of craving-regulation and negative-emotion-regulation (Driscoll et al., 2009; Kim & Hamann, 2012), we hypothesized that SCR measures would operate in an analogous fashion as tCDS effects on subjective ratings.
2. Experimental procedures
2.1. Participants
We initially screened 364 young adults for eligibility via online advertisements targeting college students in Beijing, China. We calculated the desired sample size using G*Power 3.1.9.2 before conducting the study. The desired sample size was 30 when we assumed a moderate effect size (α = 0.05, power = 0.8, effect size = 0.25) (Cohen, 1988). Considering possible dropouts, we recruited 38 participants with IGD (aged 18–25 years). Given the higher prevalence of IGD in males (Meng et al., 2015) and gender-related differences related to craving (Dong et al., 2018 a,b), we included only male participants. Five participants discontinued due to time conflicts with the second visit, and the remaining 33 participants completed the whole study. Participants were diagnosed with IGD if they met ⩾ 5 items of DSM-5 proposed criteria for IGD (American Psychiatric Association, 2013), scored ⩾ 50 on a revised version of Young’s online internet addiction test (IAT) (Young, 2009), spent the majority of online time (more than 50%) on gaming, and played internet games ⩾ 20 hours/week for at least 1 year. Participants were excluded for substance dependence, psychiatric or neurological disorders, use of psychotropic medications, head trauma, the presence of metal in the head or face, or being left-handed. See detailed screening in the Supplemental Materials. Table 1 shows demographic and clinical characteristics of participants. The study was approved by a local research ethics committee at the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, and all participants provided written informed consent prior to participation, in accordance with the Declaration of Helsinki.
Table 1.
Demographic and Clinical Characteristics of Participants
| Mean ± SD or n (%) | |
|---|---|
| Age (years) | 21.21 ± 2.27 |
| Young’s online internet addiction test | 65.82 ± 10.53 |
| Years of internet gaming | 1.52 ± 0.75 |
| Weekly gaming time on the internet (hours) | 28.97 ± 9.98 |
| Alcohol user | 12(36%) |
| AUDIT score | 2.92 ± 1.68 |
| Tobacco smoker | 2(6%) |
| FTND score | 1.00 ± 0.00 |
| Beck depression inventory- II (BDI-II) score | 4.61 ± 5.62 |
| Beck anxiety inventory (BAI) score | 4.33 ± 4.62 |
Note: AUDIT, alcohol use disorder identification test; FTND, Fagerstöm test for nicotine dependence; SD, standard deviation. See detailed distribution of BDI-II and BAI scores in the Supplemental Materials.
2.2. Experimental Design
The study used a double-blind, within-subject, and sham-controlled design. Participants received active and sham tDCS sessions one week apart. Participants were randomly assigned to one of two groups in a 1:1 ratio to receive the two tDCS sessions either in active-sham or sham-active order. In each session, participants received 20 minutes of active or sham stimulation, during which they completed the ROC and ER tasks. Participants were instructed to regulate gaming-related craving and negative emotions using cognitive reappraisal during the ROC and ER tasks, respectively. Subjective ratings of craving and negative feelings and SCRs were recorded.
2.3. ROC and ER Tasks
The ROC task included gaming images from three internet games (League of Legends, King of Glory, and Playerunknown’s Battlegrounds) popular among young Chinese men. Participants were presented with 48 images from their most preferred game and were instructed to downregulate or upregulate craving induced by gaming images using cognitive reappraisal in the ROC task. In the ER task, participants were presented with 48 negative pictures taken from the International Affective Picture System (IAPS) and were instructed to downregulate or upregulate negative feelings elicited by negative pictures using cognitive reappraisal. Participants were trained to use cognitive reappraisal strategies for each task prior to the formal experiment. In the ROC task, participants were instructed to consider the long-term negative effects of excessive gaming in the downregulation condition (e.g., it harms physical health, psychosocial functioning, and academic performance) and to consider the immediate positive effects of internet gaming in the upregulation condition (e.g., it brings relaxation and excitement). In the ER task, in the downregulation condition, participants were instructed to imagine a better outcome of the situation than the one depicted, and to imagine a worse outcome in the upregulation condition. For example, for an image of a funeral, they might imagine that a very old man had died peacefully after enjoying a long and happy life in the downregulation condition, and that an innocent baby had died in a terrible car accident in the upregulation condition.
The downregulation and upregulation conditions were manipulated block-wise in both tasks. Each block (Figure 1) started with a color cue for 2 seconds indicating the regulation condition (i.e., the blue screen for downregulation and the green screen for upregulation for half the participants, and vice versa for the other half). Afterwards, participants were presented with four pictures (gaming images in the ROC task or negative pictures in the ER task) for 8 seconds each followed by a 1-s fixation, and were asked to view and reappraise these pictures in accordance with the regulation condition of the current block. The background of each presentation was the same color with the cue of the current block. After each picture, a nine-point Likert scale (from 1 = ‘not at all’ to 9 = ‘very much’) was presented for 4 seconds, during which participants rated how much they craved playing the internet game in the ROC task and how negative they felt in the ER task. Overall, the ROC task consisted of four types of conditions (blocks): craving-downregulation blocks with active tDCS, craving-downregulation blocks with sham tDCS, craving-upregulation blocks with active tDCS and craving-upregulation blocks with sham tDCS. Analogously, the ER task consisted of four types of conditions: negative-emotion-downregulation blocks with active tDCS, negative-emotion-downregulation blocks with sham tDCS, negative-emotion-upregulation blocks with active tDCS and negative-emotion-upregulation blocks with sham tDCS. Ratings of craving and negative emotions were averaged in each experimental condition for each participant, respectively.
Figure 1.
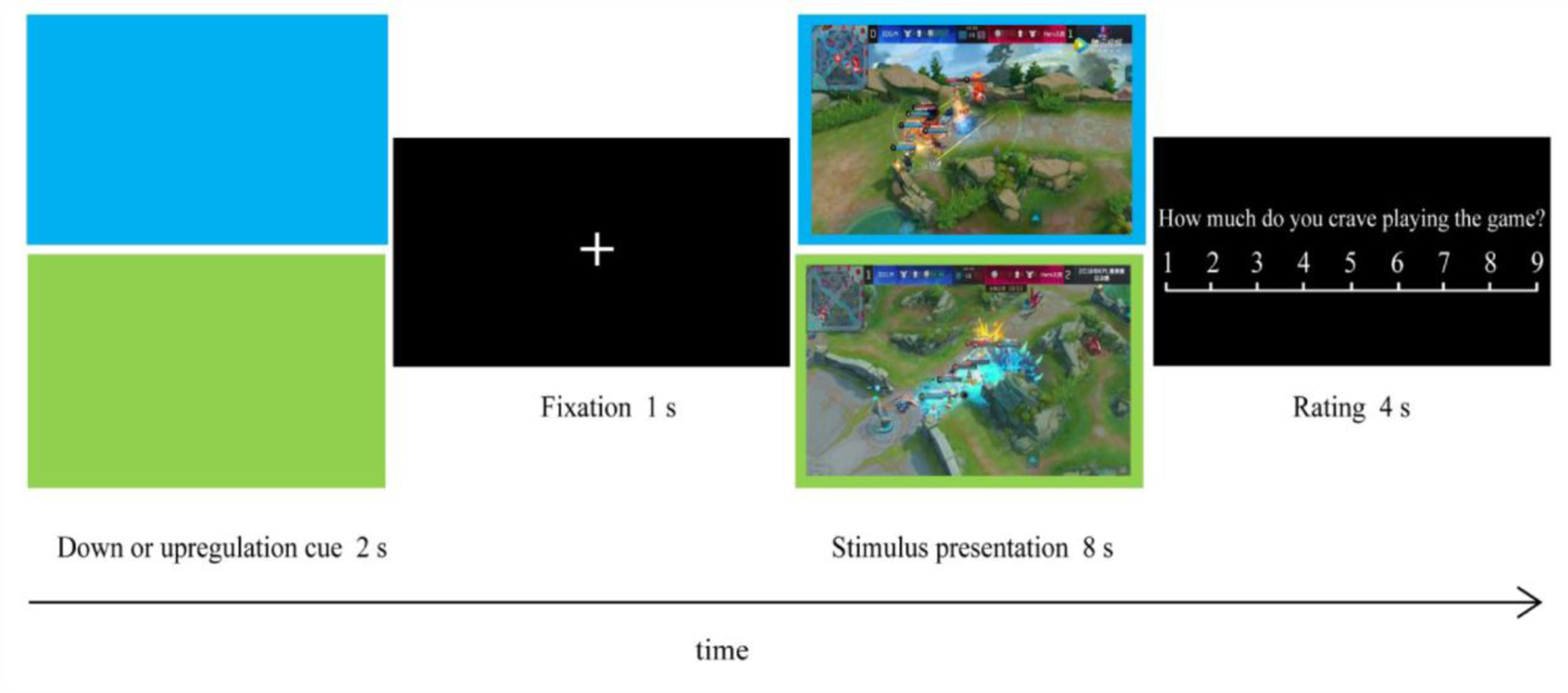
Structure for the ROC and ER blocks. Each block began with a down- or upregulation cue which was followed by four pictures (gaming pictures in the ROC task or negative pictures in the ER task). Participants were instructed to view and reappraise pictures in accordance with the regulation condition of the current block. After each picture, participants rated how much they craved playing internet games in the ROC blocks and rated how negative they felt in the ER blocks.
Each task contained 6 downregulation blocks and 6 upregulation blocks in total. The blocks were presented in a pseudorandomized order with a 10-s interblock interval. In the ROC task, the gaming images for downregulation and upregulation conditions were well-matched based on self-reported craving levels and arousal from a separate sample of 30 internet-game players in a pilot test (ps > 0.1). In the ER task, the negative pictures for downregulation and upregulation conditions were well-matched according to the valence and the arousal of the IAPS normed ratings (ps > 0.1).
2.4. tDCS protocol
Direct current was delivered through a pair of electrodes (5 × 7 cm2) connected to a DC-stimulator MC. The anode electrode was positioned on F4 (EEG 10–20 system). The cathode was placed on the left superior region of the trapezius muscle near the base of the participant’s neck (Clarke et al., 2014). Based on guidelines for tDCS protocols (Lefaucheur et al., 2017), a direct current of 1.5 mA was delivered for 20 minutes with a 30-second ramp up/down time during active stimulation. The sham tDCS only included 30-second ramp up and 30-second ramp down time. An assistant experimenter operated the DC-stimulator, so that the experimenter and participant were blind to the stimulation condition.
2.5. Recording and analysis of SCRs
SCRs were measured continuously during the ROC and ER tasks using an MP160 biosignal amplifier. SCR data were analyzed using the AcqKnowledge Software. The peak-to-base index was used as the indicator of SCRs (Nava et al., 2016). We calculated the difference between the maximal value recorded in the time window of 51 seconds after the onset of the first image within each block and an average value of 0.5 second pre-stimulus. We discarded SCR data from 1 participant owing to a technical problem with the recording.
2.6. tDCS-related measures
Ten potential side effects of tDCS (headache, scalp pain, neck pain, tingling, itching, burning, flushing skin, drowsiness, difficulty concentrating, and acute mood changes) were assessed using a four-point Likert scale (from 1 = ‘not at all’ to 4 = ‘very much’) after each active or sham stimulation. After completing the two stimulations, participants were asked, “Did you feel any difference between the two stimulations”. If the answer was “yes”, participants were asked to further describe the differences in detail.
2.7. Statistical analysis
Subjective ratings of craving and negative emotions as well as SCR measures for each task were analyzed using repeated measures analyses of variance (ANOVAs) with the following within-participant factors: regulation condition (downregulation vs. upregulation) and stimulation (active vs. sham). To control for the potential influence of emotional states on outcomes, BDI-II and BAI scores were added as covariates. For all ANOVAs, the significance level was set to alpha = 0.05, and ANOVAs were supplemented by paired two-tailed t-tests where appropriate. Effect sizes were calculated as partial eta squared.
3. Results
Below are results from 33 participants who completed the two tDCS sessions. Overall, the results revealed significant regulation × stimulation interactions. And we did not observe any significant interaction effects with BAI or BDI-II scores. These results indicate that emotional states measured through the BDI-II and BAI may have little impact on major experimental effects in the current study.
We also analyzed data from all 38 participants (with 5 participants who only received the first stimulation; see detailed statistical results in Supplemental Materials). The results exhibited the same significant pattern as these using the 33 participants, suggesting a robustness of the findings.
3.1. Effects of tDCS on craving ratings and SCRs during the ROC task
Analyses of craving ratings revealed a significant regulation × stimulation interaction [F(1, 30) = 21.76, p < 0.001, η2p = 0.42; Figure 2A]. Further post-hoc t-tests showed that for the downregulation condition active relative to sham tDCS decreased craving [t(32) = −4.84, mean: 2.35 vs. 3.32, 95% confidence interval (CI) of difference: −1.38 to −0.56, p < 0.001], and for the upregulation condition active relative to sham tDCS increased craving [t(32) = 3.68, mean: 7.53 vs. 7.00, 95% CI of difference: 0.24 to 0.82, p = 0.001]. To show tDCS effects on bidirectional regulation directly, we further calculated craving differences between upregulation and downregulation for each stimulation condition and compared such differences between active and sham conditions (active(upregulation - downregulation) vs. sham(upregulation - downregulation)). A paired t-test revealed that active relative to sham stimulation enlarged craving difference [t(32) = 6.90, mean: 5.18 vs. 3.68, 95% CI of difference: 1.06 to 1.94, p < 0.001], indicating enhanced bidirectional regulation.
Figure 2.
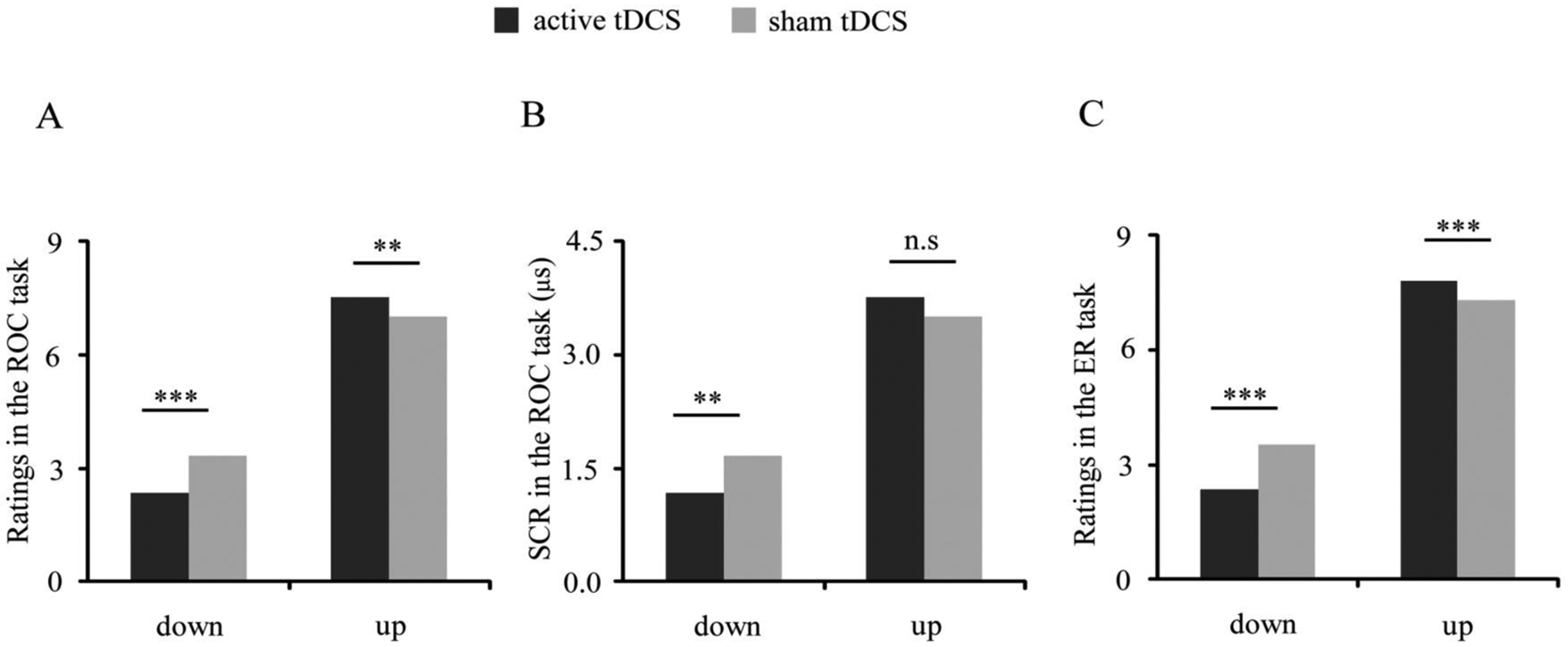
tDCS effects on regulation of craving and negative emotions. (A) For downregulation, decreased craving was observed during active vs. sham stimulation; for upregulation, increased craving was observed during active vs. sham stimulation. (B) For downregulation, decreased SCRs were observed during active vs. sham stimulation during performance of the ROC task. (C) For downregulation, decreased negative emotional responses were observed during active vs. sham stimulation; for upregulation, increased negative emotional responses were observed during active vs. sham stimulation. (*p < 0.05, **p < 0.01, and ***p < 0.001; n.s, not significant)
Analyses of SCRs also revealed a significant regulation × stimulation interaction [F(1, 29) = 5.91, p = 0.02, η2p = 0.17; Figure 2B]. Further post-hoc t-tests showed that for the downregulation condition, SCRs were lower during active vs. sham stimulation [t(31) = −3.08, mean: 2.63 vs. 3.83 μs, 95% CI of difference: −1.99 to −0.41 μs, p = 0.004], but that for the upregulation condition no significant difference was observed [t(31) = −0.24, p > 0.1]. Moreover, a paired t-test revealed that active relative to sham stimulation enlarged SCR difference between upregulation and downregulation [t(31) = 2.45, mean: 0.62 vs. −0.46, 95% CI of difference: 0.18 to 1.99 μs, p = 0.02], consistent with the effect of tDCS on self-reported craving.
3.2. Effects of tDCS on ratings of negative emotions and SCRs during the ER task
Analysis of negative emotions during the ER task revealed a similar pattern as that observed for craving during the ROC task. We observed a significant regulation × stimulation interaction [F(1, 30) = 12.97, p = 0.001, η2p = 0.30; Figure 2C]. Further post-hoc t-tests showed that for the downregulation condition active relative to sham tDCS decreased negative emotions [t(32) = −5.02, mean: 2.36 vs. 3.53, 95% CI of difference: −1.65 to −0.70, p < 0.001], and for the upregulation condition active relative to sham tDCS increased negative emotions [t(32) = 4.39, mean: 7.80 vs. 7.31, 95% CI of difference: 0.26 to 0.72, p < 0.001]. Moreover, a paired t-test revealed that active relative to sham stimulation enlarged difference of negative emotions between upregulation and downregulation [t(32) = 5.81, mean: 5.44 vs. 3.78, 95% CI of difference: 1.08 to 2.25, p < 0.001]. Analysis of SCRs did not reveal significant results. See Figure S1 regarding tDCS effects on regulation of craving and negative emotions in each participant in Supplemental Materials.
3.3. tDCS adverse effects and blinding
tDCS was well tolerated among all the subjects. Statistically, average ratings of tDCS adverse effects were 1.18 (SD = 0.29) in the active session and 1.18 (SD = 0.23) in the sham session, indicating that there were few or no clinically significant adverse effects. Paired t-tests revealed that adverse effects in the active condition were not significantly different from those in the sham (p > 0.1). Furthermore, participants were asked whether they had felt differences between the two stimulations after completing the study. Twenty-six participants reported no subjective differences, and 7 participants felt that one stimulation (active tDCS) generated slightly more itching than the other (sham tDCS). One sample binomial test revealed that the percentage (i.e., 21%) of participants who felt differences between active and sham tDCS was not statistically significantly different from chance (p > 0.1). Moreover, we analyzed results for tDCS effects on the regulation of craving and negative emotions using the remaining 26 participants (with the 7 participants reporting differences between the sham and active tDCS removed; see detailed statistical results in Supplemental Materials). The results exhibited the same significant pattern as those using all 33 participants). These data supported the efficacy of the blinding.
4. Discussion
By employing tDCS with a sham-controlled, within-subject, and double-blind design, the current study is the first empirical evidence demonstrating a common role for the right dlPFC in enhancing cognitive regulation of both craving and negative emotions among individuals with an addiction broadly and IGD specifically. In the ROC and ER tasks, increasing cortical activation in the right dlPFC via tDCS facilitated both downregulation and upregulation: active relative to sham tDCS decreased ratings and/or SCRs for downregulation conditions, and increased ratings for upregulation conditions, respectively. These findings reveal common neural substrates underlying craving/reward-regulation and negative-emotion-regulation. More importantly, these findings suggest a promising approach concurrently enhancing executive control over the two central motivational drives underlying addictions. The findings suggest tDCS may help individuals with IGD resist cravings and negative-reinforcement-related engagement in gaming, although this possibility warrants direct examination in future studies. Furthermore, the findings provide a strong rationale for neural mechanisms underlying effective addiction interventions.
The present study builds on and extends prior work in at least three ways. First, extending prior neuroimaging work that has implicated prefrontal regions in regulating craving or emotions (Buhle et al., 2014; Kober et al., 2010), the current study shows that stimulation of this region enhances regulation and suggests a possible causal influence of dlPFC on regulation efficacy. Individuals with addictions have shown deficits in regulation of craving and negative emotional states (Naqvi et al., 2015; Yip et al., 2018). The current study suggests a promising treatment approach that may alleviate both deficits.
Second, considerable prior work has shown that tDCS of dlPFC reduces craving or addictive behaviors (Boggio et al., 2008; Falcone et al., 2016; Lee et al., 2018), but cognitive mechanisms underlying such alterations have been unclear. The present study focuses on alterations in regulation during tDCS, revealing that tDCS of dlPFC enhances regulation of craving. Overall, the enhanced regulation of craving in the current study fits with previous findings showing that tDCS of the dlPFC altered functional connectivity between dlPFC and limbic brain regions (one pathway underlying cognitive regulation of craving) when heavy smokers were instructed to view cigarette-related cues after stimulation (Yang et al., 2017). The current findings preliminarily suggest that enhanced executive control over motivation and emotion may be one cognitive mechanism underlying tDCS effects on individuals with addictions. As such, the current study is an important addition to understanding the effects of multiple-session tDCS treatment in addictions.
Third, prior research of people with addictions has focused on inhibition of negative emotions (Albein-Urios et al., 2014; Yip et al., 2018). The current study focuses on enhancing bidirectional regulation over negative emotions. Specifically, active relative to sham tDCS facilitated both downregulation and upregulation over negative emotions. These findings suggest that cortical stimulation of the right dlPFC contributes to the enhancement of bidirectional regulatory control over negative emotions. The findings are in line with previous neuroimaging data showing that downregulation and upregulation of negative emotions are both accompanied with increased activation in prefrontal regions among healthy individuals (Buhle et al., 2014; Goldin et al., 2008). These findings are also consistent with the hypothesis that the prefrontal region is involved in the inhibition and selection of various responses (Feeser et al., 2014; Ochsner et al., 2004). Given that hyper- and hypo-reactivity to negative emotions may both relate to engagement in addictive behaviors in different situations (Khosravani et al., 2017; Yao et al., 2015), targeting enhancement of bidirectional regulation, as the current study did, relative to solely reducing negative emotions, suggests an approach that may improve efficacy in treatment development efforts.
Intriguingly, we observed effects of active vs. sham tDCS on both ratings and SCRs for the ROC task, but only effects on ratings for the ER task. This differs from previous research in healthy individuals showing that tDCS of the right dlPFC facilitates regulation of negative emotions as indexed by both self-reported ratings and SCRs (Feeser et al., 2014). Taken together with prior data indicating that changes in SCRs are associated with amygdala-frontal circuitry function (Mangina & BeuzeronMangina, 1996; Williams et al., 2001), and that individuals with IGD exhibit blunted prefrontal and amygdala responses to negative images (Yip et al., 2018), the current findings suggest that blunted neural processing of negative emotions may be a characteristic of IGD, as is the case for other addictions (Wilcox et al., 2016).
With respect to improving the efficacies of interventions, the current study has translational implications for clinical contexts in several ways. First, prior work has shown that treatments involving training in craving-regulation or negative-emotion-regulation can reduce addictive behaviors (Azizi et al., 2010; Dolan et al., 2013), and that enhanced regulation of negative emotions via mindfulness meditation can decrease drug use (Tang et al., 2016). By suggesting an approach that enhances regulation over these craving and negative emotions, the current findings may contribute to the development of related and more efficacious treatments for addictions. Second, given that craving, difficulties in regulating negative emotions, and addictions may develop in a complex and interacting fashion (Skinner & Aubin, 2010; Volkow et al., 2016), the current intervention targets both craving and negative emotions, and such interventions may have greater efficacies than those targeting either factor separately. Third, the confirmation of tDCS effects on regulation suggests enhanced executive control over motivation and emotion may be a critical cognitive mechanism underlying tDCS effects on addiction. The findings may provide a foundation for identifying predictors of tDCS effects and developing individualized treatments. Fourth, the direct confirmation of a common role for the right dlPFC in craving-regulation and negative-emotion-regulation here suggests that functioning and functional alterations of the right dlPFC should be considered in treatment development efforts for IGD (and perhaps other addictions) as a target or potential link to outcomes.
Several limitations should be discussed. First, although demonstrating robust immediate tDCS effects on craving-regulation and negative-emotion-regulation, the current study did not examine tDCS effects on other addiction-related symptoms (e.g., addictive behaviors) or long-term tDCS effects on regulation. However, as mentioned earlier, previous research has demonstrated that enhanced craving-regulation and negative-emotion-regulation contributes to decreased addictive behaviors (Azizi et al., 2010; Dolan et al., 2013; Tang et al., 2016). Hence, demonstration of tDCS effects on enhancing regulation likely has important clinical implications. Additionally, multiple-session tDCS has demonstrated efficacy in reducing craving and addictive behaviors (Boggio et al., 2008; Falcone et al., 2016; Lee et al., 2018). By revealing potential cognitive mechanisms underlying tDCS effects, the current study is an important addition to understanding behavioral effects of multiple-session tDCS treatment in addictions and may provide a foundation for future studies into identifying predictors of tDCS effects. Nevertheless, longitudinal studies involving multiple-session tDCS effects on comprehensive addiction-related symptoms are needed to examine potential relationships between regulation of emotional and motivational processes and addictive behaviors. Second, unlike studies simply focusing on ROC or ER performance, the current study did not include the “look” condition (only including downregulation and upregulation conditions), which led to the absence of comparisons between look and downregulation/upregulation. However, this study focused on examining whether tDCS would enhance regulation of craving and negative emotions. These effects were indexed by response differences between active and sham stimulation for the downregulation condition (i.e., active(downregulation) - sham(downregulation)), and for the upregulation condition (i.e., active(upregulation) - sham(upregulation)), respectively. Although the absence of a “look” condition ostensibly had little effect on the validity of the current findings, inclusion of such a condition may have helped to enrich the current findings. Third, the self-report ratings of craving and negative emotions may be subjective, which may be subject to experimental demand. However, given that participants received the same experimental instructions during both active and sham tDCS, the sham-controlled setting in the current study controls for effects relating to subjectivity of self-reports. Additionally, the combination of subjective feelings (i.e., self-report ratings) and objective physiological reactivity (i.e., SCRs) further supports the validity of the current findings. Fourth, the current findings are innovative among individuals with an addiction broadly and IGD in particular. Although deficits in craving-regulation and negative-emotion-regulation are commonly reported across addictions (Albein-Urios et al., 2014; Naqvi et al., 2015), the current findings remain to be generalized to other addictions.
Supplementary Material
Highlights.
Internet gaming disorder (IGD) has few empirically validated treatments.
2. tDCS of the right dlPFC enhanced craving- and negative-emotion-regulation in IGD.
3. Common substrates underlie reward- and negative-emotion-regulation in IGD.
4. tDCS may be a promising approach for treating IGD.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (grant 31871122 and grant 31700966), the Project of Humanities and Social Sciences from the Ministry of Education in China (grant 15YJA190010), and the Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning (CNLZD1802). Dr. Potenza’s involvement was supported by the Connecticut Department of Mental Health and Addiction Services, the Connecticut Council on Problem Gambling and the National Center for Responsible Gaming. Dr. Kober’s involvement was supported by National Institute of Drug Abuse grants R01 DA042911, R01 DA043690, and P50 DA09241. Dr. Yip’s involvement was supported by NIDA grants K01DA039299 and K12DA000167, the National Center on Addiction and Substance Abuse (CASA) and an Open Project grant from the State Key Laboratory of Cognitive Neuroscience and Learning. The funding agencies did not have input into the content of the manuscript and the views in the manuscript may not reflect those of the funding agencies. Dr. Potenza has consulted for and advised INSYS, Shire, RiverMend Health, Lakelight Therapeutics/Opiant and Jazz Pharmaceuticals; has received research support from the Mohegan Sun Casino and the National Center for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; and has consulted for law offices and gambling entities on issues related to impulse control or addictive disorders.
Role of the Funding source
Not applicable. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: clinicaltrials.gov Identifier: NCT03352973
Conflict of interest
The authors declare no conflict of interest.
Reference
- Albein-Urios N, Verdejo-Roman J, Asensio S, Soriano-Mas C, Martinez-Gonzalez JM, Verdejo-Garcia A, 2014. Re-appraisal of negative emotions in cocaine dependence: dysfunctional corticolimbic activation and connectivity. Addict Biol. 19, 415–426. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Azizi A, Borjali A, Golzari M, 2010. The effectiveness of emotion regulation training and cognitive therapy on the emotional and addictional problems of substance abusers. Iran J Psychiatry. 5, 60–65. [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC, 2004. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 111, 33–51. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F, 2008. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend. 92, 55–60. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN, 2014. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex. 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJF, Browning M, Hammond G, Notebaert L, MacLeod C, 2014. The Causal Role of the Dorsolateral Prefrontal Cortex in the Modification of Attentional Bias: Evidence from Transcranial Direct Current Stimulation. Biol Psychiatry. 76, 946–952. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences(2nd ed.) Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Dolan SL, Rohsenow DJ, Martin RA, & Monti PM, 2013. Urge-specific and lifestyle coping strategies of alcoholics: relationships of specific strategies to treatment outcome. Drug Alcohol Depend. 128, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GH, Wang ZL, Wang Y, Du X, Potenza MN, 2018. Gender-related differences in neural responses to gaming cues before and after gaming: implications for gender-specific vulnerabilities to Internet gaming disorder. Soc Cog Affect Neurosci. 13(11), 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GH, Zheng H, Liu X, Wang Y, Du X, Potenza MN, 2018. Gender-related differences in cue-elicited cravings in Internet gaming disorder: The effects of deprivation. J Behav Addict. 7(4), 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D, Tranel D, Anderson SW, 2009. The effects of voluntary regulation of positive and negative emotion on psychophysiological responsiveness. International Journal of Psychophysiology. 72, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Bernardo L, Ashare RL, Hamilton R, Faseyitan O, McKee SA, Loughead J, Lerman C, 2016. Transcranial Direct Current Brain Stimulation Increases Ability to Resist Smoking. Brain Stimulation. 9, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeser M, Prehn K, Kazzer P, Mungee A, Bajbouj M, 2014. Transcranial Direct Current Stimulation Enhances Cognitive Control During Emotion Regulation. Brain Stimul. 7, 105–112. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ, 2008. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 63, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani V, Bastan FS, Ghorbani F, Kamali Z, 2017. Difficulties in emotion regulation mediate negative and positive affects and craving in alcoholic patients. Addictive Behaviors. 71, 75–81. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S, 2012. The effect of cognitive reappraisal on physiological reactivity and emotional memory. International Journal of Psychophysiology. 83, 348–356. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN, 2010. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America. 107, 14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Im. JJ, Oh JK, Choi EK, Yoon S, Bikson M, Song IU, Jeong H, Chung YA, 2018. Transcranial direct current stimulation for online gamers: A prospective single-arm feasibility study. Journal of Behavioral Addictions. 7(4), 1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, Ridder DD, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Paulus W, 2017. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 128, 56–92. [DOI] [PubMed] [Google Scholar]
- Leyman L, De Raedt R, M-A V, Baeken C, 2009. Influence of high-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex on the inhibition of emotional information in healthy volunteers. Psychological Medicine. 39, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Mangina CA, BeuzeronMangina JH, 1996. Direct electrical stimulation of specific human brain structures and bilateral electrodermal activity. International Journal of Psychophysiology. 22, 1–8. [DOI] [PubMed] [Google Scholar]
- Meng YJ, Deng W, Wang HY, Guo WJ, Li T, 2015. The prefrontal dysfunction in individuals with Internet gaming disorder: a meta-analysis of functional magnetic resonance imaging studies. Addict Biol. 20, 799–808. [DOI] [PubMed] [Google Scholar]
- Milivojevic V, Sinha R, 2018. Central and Peripheral Biomarkers of Stress Response for Addiction Risk and Relapse Vulnerability. Trends Mol Med. 24, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Ochsner KN, Kober H, Kuerbis A, Feng T, Wall M, Morgenstern J, 2015. Cognitive Regulation of Craving in Alcohol-Dependent and Social Drinkers. Alcoholism-Clinical and Experimental Research. 39, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava E, Romano D, Grassi M, Turati C, 2016. Skin conductance reveals the early development of the unconscious processing of emotions. Cortex. 84, 124–131. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, 2005. Is there a common molecular pathway for addiction? Nat Neurosci. 8, 1445–1449. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ, 2004. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Potenza MN, 2014. Biased behaviors: towards understanding vulnerability and resilience factors in addictions. Biol Psychiatry. 75, 94–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpf HJ, Achab S, Billieux J, Bowden-Jones H, Carragher N, Demetrovics Z, Poznyak V, 2018. Including gaming disorder in the ICD-11: The need to do so from a clinical and public health perspective. Journal of Behavioral Addictions. 7, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MD, Aubin HJ, 2010. Craving’s place in addiction theory: Contributions of the major models. Neuroscience and Biobehavioral Reviews. 34, 606–623. [DOI] [PubMed] [Google Scholar]
- Tang YY, Tang R, Posner MI, 2016. Mindfulness meditation improves emotion regulation and reduces drug abuse. Drug Alcohol Depend. 163, 13–18. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 374, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Pommy JM, Adinoff B, 2016. Neural Circuitry of Impaired Emotion Regulation in Substance Use Disorders. American Journal of Psychiatry. 173, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, Gordon E, 2001. Arousal dissociates amygdala and hippocampal fear responses: Evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 14, 1070–1079. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Criteria for Gaming Disorder in the Eleventh Edition of the International Classification of Diseases. https://icdwhoint/browse11/l-m/en#/http%3a%2f%2fidwhoint%2ficd%2fentity%2f1448597234. 2018; Accessed November 30, 2018.
- Yang LZ, Shi B, Li H, Zhang W, Liu Y, Wang HZ, , Lv W, Ji X, Hudak J, Zhou Y, Fallgatter AJ, Zhang XC, 2017. Electrical stimulation reduces smokers’ craving by modulating the coupling between dorsal lateral prefrontal cortex and parahippocampal gyrus. Social Cognitive and Affective Neuroscience. 12, 1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YW, Chen PR, Li S, Wang LJ, Zhang JT, Yip SW, Chen G, Deng LY, Liu QX, Fang XY, 2015. Decision-Making for Risky Gains and Losses among College Students with Internet Gaming Disorder. Plos One, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YW, Liu L, Ma SS, Shi XH, Zhou N, Zhang JT, Potenza MN, 2017. Functional and structural neural alterations in Internet gaming disorder: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 83, 313–324. [DOI] [PubMed] [Google Scholar]
- Yao YW, Potenza MN, Zhang JT, 2017. Internet Gaming Disorder Within the DSM-5 Framework and With an Eye Toward ICD-11. Am J Psychiatry. 174, 486–487. [DOI] [PubMed] [Google Scholar]
- Yip SW, Gross JJ, Chawla M, Ma SS, Shi XH, Liu L, Yao YW, Zhu L, Worhunsky PD, Zhang J, 2018. Is Neural Processing of Negative Stimuli Altered in Addiction Independent of Drug Effects? Findings From Drug-Naive Youth with Internet Gaming Disorder. Neuropsychopharmacology. 43, 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, 2009. Internet addiction test (IAT). http://www.netaddiction.com/resources/internet_addiction_test.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


