Abstract
This study aimed to determine the correlation between serine hydroxymethyl transferase 1 (SHMT1) gene methylation and ischemic stroke. A total of 202 age- and sex-matched individuals were included. Quantitative methylation-specific polymerase chain reaction (qMSP-PCR) was used to analyze the DNA methylation level. The plasma homocysteine (Hcy) concentration was much higher in ischemic cases than in controls (p = 0.009), while the high-density lipoprotein (HDL) levels in stroke cases were considerably lower than in controls (p = 0.005). A significantly higher level of SHMT1 methylation was observed in the ischemic strokes (58.82 ± 17.83%) compared to that in the controls (42.59 ± 20.76%, p < 0.001). The SHMT1 methylation level was strongly correlated with HDL concentration in the healthy controls (r = 0.517, p < 0.001), while the high plasma level of Hcy showed strong association with SHMT1 methylation in ischemic strokes (r = 0.346, p < 0.001). Receiver operating characteristic (ROC) analysis of curve indicated that SHMT1 methylation has been an acceptable indicator for ischemic stroke in female patients [all sexes, area under the curve (AUC) = 0.71, p < 0.001; male patients AUC = 0.62, p = 0.032; and female patients AUC = 0.79, p < 0.001] and in all ages (AUC = 0.71, p < 0.001). In our samples, DNA methylation levels of the STHMI gene were significantly correlated with ischemic stroke in Han Chinese. STHMI hypermethylation was significantly associated with the high Hcy concentration in ischemic stroke and had value as a potential indicator for female ischemic stroke.
Keywords: Ischemic stroke, DNA methylation, serine hydroxymethyl transferase 1, homocysteine, high-density lipoprotein, sex
INTRODUCTION
Ischemic stroke is an acute cerebrovascular disease associated with extremely high mortality. It has become the leading cause of disease-related death in China [1]. However, the pathogenesis of ischemic stroke is not fully understood. Ageing, hypertension, hyperlipidemia [2], genetics [3], and lifestyle [4] are reported as the main risk factors for ischemic stroke [5]. Several studies have shown that genetic and epigenetic factors play key roles in the development of ischemic stroke. DNA methylation was previously suggested to be involved in stroke pathogenesis by affecting the expression of stroke-related genes [6]. For example, one study showed that the low level of long interspersed nucleotide element 1 gene methylation is associated with a high risk for ischemic stroke in men [7], while another suggested that matrix metalloproteinase-2 gene demethylation is associated with ischemic stroke in a sex and stroke subtype-specific manner [8].
The serine hydroxymethyl transferase 1 (SHMT1) gene plays an important role in folic acid metabolism. SHMT1 can promote the formation of 5-methyltetrahydrofolate during folate metabolism and the homocysteine (Hcy) metabolism pathway in the methionine cycle [9]. Hcy was shown to increase brain lesions after stroke [10] and has been suggested as a high risk factor for the onset of stroke [11]. SHMT1 variants may be involved in Hcy metabolism and, therefore, contribute to an increased risk of ischemic stroke [12]. The methylation of SHMT1 gene may affect the expression of SHMT1 [13], a protein that plays a key role in promoting the conversion of serine and tetrahydrofolate to glycine and 5,10-methylenetetrahydrofolate [14]. Moreover, SHMT1 expression was shown to be associated with phosphate-induced vascular smooth muscle cell calcification [15] and a SHMT1 variant was suggested as a risk factor in early-onset ischemic stroke [16]. In addition, the altered expression of SHMT1 could block folic acid metabolism and abnormal Hcy remethylation pathways. This causes excessive accumulation of Hcy, which could increase the risk of ischemic stroke [17]. A previous study showed that SHMT1 promoter hypermethylation was confirmed in both, patients with essential hypertension, and patients with hyperhomocysteinemia [13]. However, DNA methylation of this gene had not been examined in the context of ischemic stroke. Therefore, we hypothesized that DNA methylation of SHMT1 would be associated with ischemic stroke case–control status. The purpose of this study was to determine the correlation between SHMT1 gene methylation and ischemic stroke in a Chinese population. We also identified the effects of SHMT1 methylation on Hcy and circulating lipids in ischemic stroke patients.
MATERIALS AND METHODS
Study participants
The study was approved by the Ethics Committee of the Ningbo First Hospital (2014-002 and 2017-R028) and written informed consent was given by all participants. The study group included 101 patients with ischemic stroke (51 males and 50 females, mean age 61.07 ± 11.56 years) and 101 age- and sex- matched healthy volunteers (51 males and 50 females, mean age 62.49± 8.93 years). All the individuals were recruited from the stroke center of Ningbo First Hospital between September 2013 and December 2019. The diagnoses of ischemic stroke patients were confirmed based on international standardized definitions, and magnetic resonance imaging (MRI) and cranial computed tomography (CT) scan findings. The control group individuals were recruited from the health center and those with serious liver disease, kidney disease, or any cerebral vascular diseases were excluded from the study.
Biochemical measurements
General information for the individuals, including sex, age, hypertension (self-reported a history of antihypertensive drug use), diabetes (self-reported positive history of type 1 or type 2), drinking history (self-reported positive history of drinking, more than 50 ml drinks/week), smoking history (self-reported positive history of smoking), and body mass index (BMI), were obtained. A total of 5 mL fasting venous blood was collected from the volunteers on the first morning of cerebral infarction and used for the detection of biochemical indicators; the remaining blood was used for DNA extraction. The plasma levels of Hcy were measured by cycling enzymatic method; triglycerides (TGs), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were determined by the enzymatic method; and apolipoprotein A (ApoA), apolipoprotein B (ApoB), and apolipoprotein E (ApoE) were estimated through the transmission turbidimetric method. All the biochemical measurements were tested using an automatic biochemical analyzer (AU2700; Olympus, Japan).
DNA methylation data collection
Blood DNA was extracted using magnetic bead isolation method and performed on the Lab-Aid 820 Nucleic Acid Extractor (Xiamen Zhishan Biological Technology Co., Ltd., China). DNA methylation was measured using quantitative methylation-specific polymerase chain reaction (qMSP-PCR) assay and performed on the LightCycler 480 (Roche Diagnostics, Mannheim, Germany). ACTB was selected as the internal reference gene to standardize the amount of target DNA, and completely methylated DNA was used as a positive control [18]. A fragment (GRCh37/hg19, chr17: 18266824–18266911) located in the SHMT1 CpG island was selected to determine the level of DNA methylation. The qMSP primers and PCR reaction conditions for SHMT1 (forward primer: 5’-cgagtttaggaaggtgtatt-3’, reverse primer: 5’-ccatacttaactacgctctc-3’) and ACTB (forward primer: 5’-ccagtttaggaaggtgtatt-3’, reverse primer: 5’-ccatacttaactacgctctc-3’) were the same as those presented in a previous study [13]. The percentage of methylated reference (PMR) was calculated by the 2−ΔΔCt method to represent gene methylation (methylation percentage ranged from 0% to 100%). [19], in which ΔΔCt = sample DNA (Cttarget gene – Ct ACTB) − fully methylated DNA (Ct target gene – Ct ACTB) [20].
Statistical analysis
The data were expressed as mean ± standard deviation (SD) or number and analyzed by the t-test or Pearson Chi-square. The association between SHMT1 methylation and clinical data was assessed using the Spearman’s (Hcy) or Pearson’s (other factors normally distributed) correlation test and multivariate binary logistic regression analysis after adjustment for age, sex, BMI, Hcy, TG, TC, HDL, and LDL. In stratified analysis, ischemic stroke potential modifiers, such as sex (male or female) and age (<60 or ≥60 years), were assessed. The receiver operating characteristic (ROC) curve test was used to evaluate the sensitivity of SHMT1 methylation for ischemic stroke diagnosis. Data were analyzed using SPSS V20.0 (Armonk, NY, USA) and figures plotted using GraphPad Prism V8.0 (La Jolla, CA, USA). Statistical significance was considered at p < 0.05.
RESULTS
The clinical characteristics of all participants are shown in Table 1. The BMI was significantly different between the two groups (cases vs. controls: 24.45 ± 3.11 kg/m2 vs. 23.43 ± 3.10 kg/m2, respectively, p = 0.020). The plasma Hcy concentration was much higher in ischemic cases (18.48 ± 10.29 μmol/L) than in controls (15.27 ± 6.35 μmol/L, p = 0.009), while the HDL levels in stroke cases (1.05 ± 0.31 mmol/L) were considerably lower than in controls (1.16 ± 0.24 mmol/L, p = 0.005). In contrast, LDL concentration was higher in ischemic cases (2.91 ± 0.78 mmol/L) than in controls (2.61 ± 0.84 mmol/L, p = 0.011).
TABLE 1.
The clinical characteristics for all participants
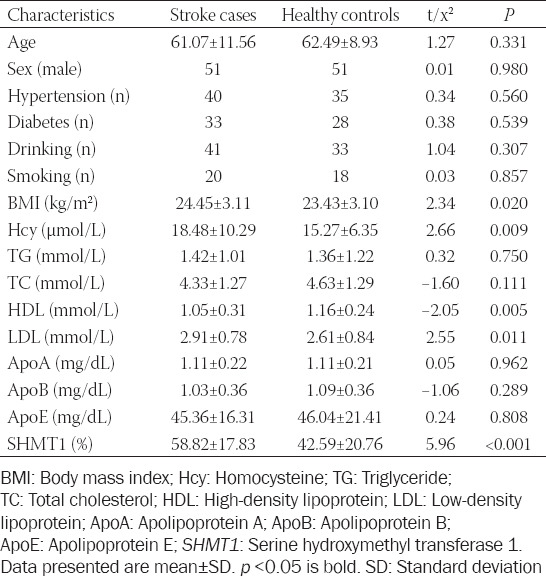
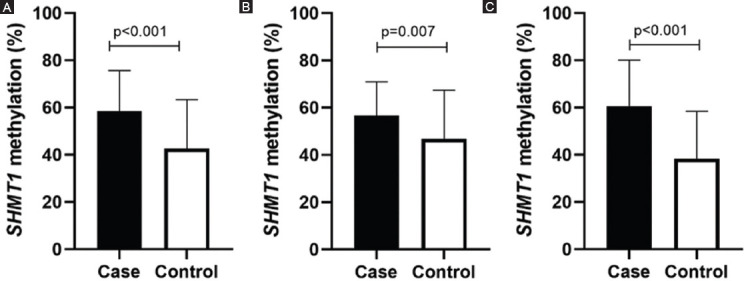
As shown in Figure 1, the SHMT1 methylation levels were significantly higher in the stroke patients (58.82 ± 17.83%) than in the controls (42.59 ± 20.76%, p < 0.001). Subgroup analysis by sex demonstrated that SHMT1 methylation levels in both male (56.59 ± 14.35%) and female (60.59 ± 19.51%) cases were higher than those in controls (46.82 ± 20.67%, p = 0.007 and 38.28 ± 20.16%, p < 0.001, respectively). Similarly, subgroup analysis by age indicated significantly higher levels of SHMT1 methylation in ischemic stroke patients than in controls in both the evaluated age groups (age ≤60 years, ischemic stroke vs. controls: 59.46 ± 17.94% vs. 43.49 ± 20.63%, p < 0.001; age > 60 years, ischemic stroke vs. controls: 57.73 ± 16.47% vs. 41.90 ± 21.02%, p < 0.001, Figure 2).
FIGURE 1.
Comparison of serine hydroxymethyl transferase 1 methylation (SHMT1) levels between cases and controls in male and female. (A) All groups, ischemic stroke versus controls: 58.82 ± 17.83 versus 42.59 ± 20.76, p < 0.001. (B) Male groups, ischemic stroke versus controls: 56.59 ± 14.35 versus 46.82 ± 20.67, p = 0.007. (C) Female groups, ischemic stroke versus controls: 60.59 ± 19.51 versus 38.28 ± 20.16, p < 0.001.
FIGURE 2.
Comparison of serine hydroxymethyl transferase 1 methylation (SHMT1) level in different ages. (A) Age ≤ 60 years, ischemic stroke versus controls: 59.46 ± 17.94 versus 43.49 ± 20.63, p < 0.001. (B) Age > 60 years, ischemic stroke versus controls: 57.73 ± 16.47 versus 41.90 ± 21.02, p < 0.001.
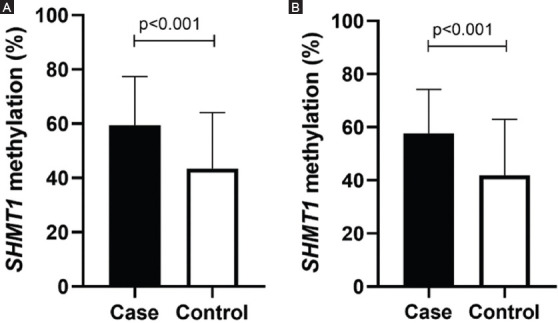
Next, we explored the relationship between SHMT1 methylation and biochemical indicators. As shown in Table 2, significant correlations were found between SHMT1 methylation and HDL concentration in controls (r = 0.517, p < 0.001), but not in ischemic cases (r = 0.153, p = 0.127, Pearson’s test). In contrast, the plasma level of Hcy showed strong association with SHMT1 methylation in ischemic stroke patients (r = 0.346, p < 0.001), but not in controls (r = 0.037, p = 0.715, Spearman’s test). In multivariate analysis, the levels of SHMT1 methylation (p < 0.001, OR [95% CI] = 1.07 [1.04-1.09]) and HDL (p < 0.001, OR [95% CI] = 0.02 [0.004-0.13], Table 3) were strongly related to ischemic stroke. The distributions of BMI, Hcy, HDL, and LDL are shown in Figure 3. SHMT1 methylation was also associated with Hcy in stroke cases (stroke cases, p = 0.034, controls, p = 0.950; total, p = 0.011) and HDL in controls (stroke cases, p = 0.146, controls, p < 0.0001, total, p = 0.001) using regression analysis adjusted for age, sex, BMI, Hcy, HDL, and LDL. BMI (stroke cases, p = 0.821, controls, p = 0.937; total, p = 0.673) and LDL (stroke cases, p = 0.603, controls, p = 0.646; total, p = 0.221) showed no association with SHMT1 methylation though regression analysis.
TABLE 2.
Correlation analysis between SHMT1 methylation and relative factors
TABLE 3.
Multivariable logistic regression analysis of association between SHMT1 methylation and the risk of ischemic stroke.
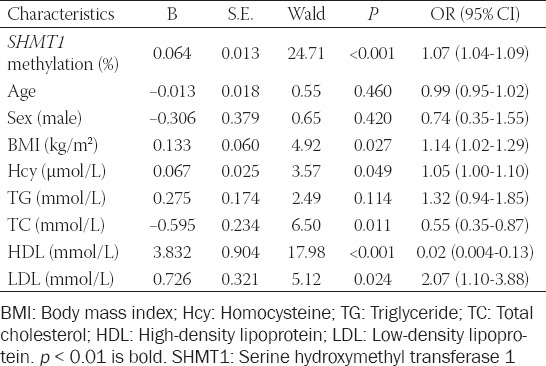
FIGURE 3.
The relationships between serine hydroxymethyl transferase 1 (SHMT1) methylation and homocysteine (Hcy), high-density lipoprotein (HDL), BMI, and low-density lipoprotein (LDL) in different groups. (A, B) SHMT1 methylation was associated with Hcy in stroke cases (stroke cases, p= 0.034, controls, p = 0.950; total, p = 0.011, (A) and HDL in controls (stroke cases, p = 0.146, controls, p < 0.0001, total, p = 0.001, (B) using regression analysis. (C, D) BMI (stroke cases, p= 0.821, controls, p = 0.937; total, p = 0.673, (C) and LDL (stroke cases, p = 0.603, controls, p = 0.646; total, p = 0.221, (D) showed no association with SHMT1 methylation though regression analysis.
As shown in Figure 4, the ROC analysis of curve showed that SHMT1 methylation had acceptable diagnostic value for ischemic stroke regardless of sex (area under the curve [AUC] = 0.71, p < 0.001; male patients, AUC = 0.62, p = 0.032; and female patients, AUC = 0.79, p < 0.001). The age subgroup analysis suggested similar results in all ages (age ≤ 60 years, AUC = 0.71, p < 0.001; age > 60 years, AUC = 0.71, p < 0.001).
FIGURE 4.
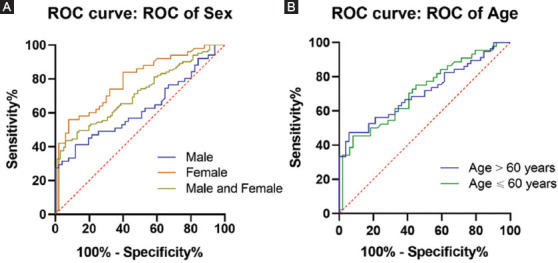
ROC curves of serine hydroxymethyl transferase 1 gene DNA methylation in ischemic strokes. ROC: Receiver operating characteristic, AUC, area under the curve. (A) In the male and female patients, AUC = 0.71, p < 0.001; male patients, AUC = 0.62, p = 0.032; and female patients, AUC = 0.79, p < 0.001. (B) In the group of age ≤ 60 years, AUC = 0.71, p < 0.001; age > 60 years, AUC = 0.71, p < 0.001.
DISCUSSION
In the present study, we explored the association between SHMT1 methylation and ischemic stroke. Our results showed that the methylation levels of SHMT1 were much higher in ischemic stroke patients than in healthy controls. STHMI hypermethylation was significantly associated with plasma Hcy concentration in ischemic stroke patients. In addition, ROC analysis suggested that SHMT1 methylation may be a useful predictor for female ischemic stroke.
Hcy was a sulfur-containing amino acid produced by the demethylation of methionine [21]. The previous studies had demonstrated that plasma Hcy was an independent risk factor for stroke [22,23]. Hcy could increase the risk of developing ischemic stroke and lacunar infarction [23]. Plasma Hcy concentration in the acute phase was suggested to associate with ischemic stroke mortality in patients [24]. SHMT1 was an important supplier of carbon unit in the process of folate metabolism [25]. The SHMT1 hypermethylation could reduce the expression of SHMT1 [13] causing folic acid metabolism and Hcy remethylation pathways to be blocked [26]. Subsequently, excessive accumulation of Hcy caused to hyperhomocysteinemia [27], which led to stroke. Our results showed that the plasma Hcy concentration was much higher in ischemic stroke patients and was significantly associated with SHMT1 methylation. These associations were consistent with the above inference.
Genetic factors and environmental factors are known to participate in the pathological process of ischemic stroke development, with several studies suggesting that patients with a high BMI may have a higher risk for ischemic stroke [28] due to higher blood lipid levels [29]. In fact, serum levels of HDL and LDL are significantly associated with increased risk of ischemic stroke [30] and people with lower concentration of LDL have a lower risk of subsequent stroke [31]. The current study showed that HDL concentration was associated with the ischemic stroke severity, and the level of HDL was much higher in severe stroke compared with mild stroke [32]. HDL has been suggested as a new target for stroke treatment, which might impact the care of stroke patients [33]. Other studies showed that the high level of HDL cholesterol was showed to associate with a decreased risk of ischemic stroke [34]. Low level of HDL cholesterol was associated with increased risk of ischemic stroke [35]. In this study, the results showed that BMI and LDL levels were significantly higher in ischemic stroke patients. Moreover, the HDL concentration was lower in the ischemic stroke patients and the SHMT1 methylation levels were associated with HDL concentration in controls. The previous studies showed that altered Hcy metabolism can have an effect on HDL levels [36], which may partly explain this association in the controls.
Age and sex are well known risk factors for ischemic stroke [37]. With age, the risk of stroke in the elderly increased significantly, and other conditions related to aging might aggravate clinical and functional consequences [38]. Margaret et al. showed that older age (age >60 years) of stroke onset is associated with greater disability [39]. Stroke has shown to affect females more than males because of their physical characteristics and living habits [40]. Females have also shown to have a much higher prevalence of stroke after age 45 [41], which largely due to a sharp increase risk in older postmenopausal female [42]. The previous studies suggested that there were sex differences in methylation of ischemic stroke-related genes [43]. Such as, the DNA methylation level of long interspersed nucleotide element 1 gene was associated with a higher risk for ischemic stroke in male, but not in female patients [7]. Our results showed that SHMT1 methylation levels were associated with ischemic stroke in both sexes, regardless of age. Moreover, the ROC analysis showed that SHMT1 methylation had better diagnostic value in female patients.
There are some limitations to our study that need to be considered. First, this was a candidate gene study, we had not studied how DNA methylation affected gene expression and other factors (such as cell-type heterogeneity), SHMT1 methylation and expression. These confounding factors should be studied in the future. Second, SHMT1 played an important role in the folic acid metabolism, so the function of other genes should be considered in future research. Third, the sample size was small and, therefore, we could not find any association between ischemic stroke and other clinical characteristics such as smoking, drinking, hypertension, diabetes, and blood lipids. Thus, future studies with larger samples that include multiple ethnic populations are needed to confirm our findings.
CONCLUSION
In our samples, DNA methylation levels of the STHMI gene were significantly correlated with ischemic stroke. STHMI hypermethylation was significantly associated with Hcy concentration in ischemic stroke and was shown to be a potential diagnostic tool for ischemic stroke in females. However, future studies to confirm these findings are required prior to potential clinical application of these results.
ACKNOWLEDGMENTS
This study was supported by grants from the Medicine and Health Science and Technology Projects of Zhejiang Province (2017KY610, 2018KY674) and the Ningbo Health Branding Subject Fund (PPXK2018-04).
Footnotes
Conflict of interests: The authors declare no conflict of interests.
REFERENCES
- 1.Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China:Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 2.Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74(1):54–66. doi: 10.1016/j.jacc.2019.03.524. https://doi.org/10.1016/j.jacc.2019.03.524. [DOI] [PubMed] [Google Scholar]
- 3.Meschia JF, Worrall BB, Rich SS. Genetic susceptibility to ischemic stroke. Nat Rev Neurol. 2011;7(7):369–78. doi: 10.1038/nrneurol.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutten-Jacobs LC, Larsson SC, Malik R, Rannikmae K, consortium M. International Stroke Genetics C. Genetic risk, incident stroke and the benefits of adhering to a healthy lifestyle:Cohort study of 306 473 UK biobank participants. BMJ. 2018;363:k4168. doi: 10.1136/bmj.k4168. https://doi.org/10.1136/bmj.k4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Wu H, Yue W, Dai Q, Liang H, Bian H, et al. Prevalence of stroke and vascular risk factors in China:A nationwide community-based study. Sci Rep. 2017;7(1):6402. doi: 10.1038/s41598-017-06691-1. https://doi.org/10.1038/s41598-017-06691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng GX, Xu N, Huang Q, Tan JY, Zhang Z, Li XF, et al. Association between promoter DNA methylation and gene expression in the pathogenesis of ischemic stroke. Aging (Albany NY) 2019;11(18):7663–77. doi: 10.18632/aging.102278. https://doi.org/10.18632/aging.102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin RT, Hsi E, Lin HF, Liao YC, Wang YS, Juo SH. LINE-1 methylation is associated with an increased risk of ischemic stroke in men. Curr Neurovasc Res. 2014;11(1):4–9. doi: 10.2174/1567202610666131202145530. [DOI] [PubMed] [Google Scholar]
- 8.Lin HF, Hsi E, Huang LC, Liao YC, Juo SH, Lin RT. Methylation in the matrix metalloproteinase-2 gene is associated with cerebral ischemic stroke. J Investig Med. 2017;65(4):794–99. doi: 10.1136/jim-2016-000277. https://doi.org/10.1136/jim-2016-000277. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane AJ, Perry CA, McEntee MF, Lin DM, Stover PJ. Shmt1 heterozygosity impairs folate-dependent thymidylate synthesis capacity and modifies risk of Apc(min)-mediated intestinal cancer risk. Cancer Res. 2011;71(6):2098–107. doi: 10.1158/0008-5472.CAN-10-1886. https://doi.org/10.1158/0008-5472.can-10-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jindal A, Rajagopal S, Winter L, Miller JW, Jacobsen DW, Brigman J, et al. Hyperhomocysteinemia leads to exacerbation of ischemic brain damage:Role of GluN2A NMDA receptors. Neurobiol Dis. 2019;127:287–302. doi: 10.1016/j.nbd.2019.03.012. https://doi.org/10.1016/j.nbd.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low HQ, Chen CP, Kasiman K, Thalamuthu A, Ng SS, Foo JN, et al. A comprehensive association analysis of homocysteine metabolic pathway genes in Singaporean Chinese with ischemic stroke. PLoS One. 2011;6(9):e24757. doi: 10.1371/journal.pone.0024757. https://doi.org/10.1371/journal.pone.0024757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G, Wang C, Ying X, Kong F, Ji H, Zhao J, et al. Serine hydroxymethyltransferase 1 promoter hypermethylation increases the risk of essential hypertension. J Clin Lab Anal. 2019;33(3):e22712. doi: 10.1002/jcla.22712. https://doi.org/10.1002/jcla.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16(10):650–62. doi: 10.1038/nrc.2016.81. https://doi.org/10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 15.Boehme B, Schelski N, Makridakis M, Henze L, Vlahou A, Lang F, et al. Role of cytosolic serine hydroxymethyl transferase 1 (SHMT1) in phosphate-induced vascular smooth muscle cell calcification. Kidney Blood Press Res. 2018;43(4):1212–21. doi: 10.1159/000492248. https://doi.org/10.1159/000492248. [DOI] [PubMed] [Google Scholar]
- 16.Giusti B, Saracini C, Bolli P, Magi A, Martinelli I, Peyvandi F, et al. Early-onset ischaemic stroke:Analysis of 58 polymorphisms in 17 genes involved in methionine metabolism. Thromb Haemost. 2010;104(2):231–42. doi: 10.1160/TH09-11-0748. https://doi.org/10.1160/th09-11-0748. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Dong Z, Cheng M, Zhao Y, Wang M, Sai N, et al. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J Neuroinflammation. 2017;14(1):187. doi: 10.1186/s12974-017-0963-x. https://doi.org/10.1186/s12974-017-0963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji H, Zhou C, Pan R, Han L, Chen W, Xu X, et al. APOE hypermethylation is significantly associated with coronary heart disease in males. Gene. 2019;689:84–9. doi: 10.1016/j.gene.2018.11.088. https://doi.org/10.1016/j.gene.2018.11.088. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Chen X, Zhou S, Lin Z, Yu X, Huang Y. DNA methylation of AHCY may increase the risk of ischemic stroke. Bosn J Basic Med Sci. 2020;20(4):471–6. doi: 10.17305/bjbms.2020.4535. https://doi.org/10.17305/bjbms.2020.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H, Wang T, Pan R, Yang Y, Li B, Zhou C, et al. Hypermethylated promoters of secreted frizzled-related protein genes are associated with colorectal cancer. Pathol Oncol Res. 2019;25:567–75. doi: 10.1007/s12253-018-0505-6. https://doi.org/10.1007/s12253-018-0505-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Wang X, He M, Qin X, Tang G, Huo Y, et al. Homocysteine and stroke risk:Modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke. 2017;48(5):1183–90. doi: 10.1161/STROKEAHA.116.015324. https://doi.org/10.1161/strokeaha.116.015324. [DOI] [PubMed] [Google Scholar]
- 22.Bostom AG, Rosenberg IH, Silbershatz H, Jacques PF, Selhub J, D'Agostino RB, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons:The Framingham study. Ann Intern Med. 1999;131(5):352–5. doi: 10.7326/0003-4819-131-5-199909070-00006. https://doi.org/10.7326/0003-4819-131-5-199909070-00006. [DOI] [PubMed] [Google Scholar]
- 23.Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109(22):2766–72. doi: 10.1161/01.CIR.0000131942.77635.2D. https://doi.org/10.1161/01.cir.0000131942.77635.2d. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z, Guan Y, Huo YR, Liu S, Zhang M, Lu H, et al. Elevated total homocysteine levels in acute ischemic stroke are associated with long-term mortality. Stroke. 2015;46(9):2419–25. doi: 10.1161/STROKEAHA.115.009136. https://doi.org/10.1161/strokeaha.115.009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducker GS, Ghergurovich JM, Mainolfi N, Suri V, Jeong SK, Li SH, et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2017;114(43):11404–9. doi: 10.1073/pnas.1706617114. https://doi.org/10.1073/pnas.1706617114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaudin AE, Abarinov EV, Noden DM, Perry CA, Chu S, Stabler SP, et al. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am J Clin Nutr. 2011;93(4):789–98. doi: 10.3945/ajcn.110.002766. https://doi.org/10.3945/ajcn.110.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hainsworth AH, Yeo NE, Weekman EM, Wilcock DM. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID) Biochim Biophys Acta. 2016;1862(5):1008–17. doi: 10.1016/j.bbadis.2015.11.015. https://doi.org/10.1016/j.bbadis.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazzano LA, Gu D, Whelton MR, Wu X, Chen CS, Duan X, et al. Body mass index and risk of stroke among Chinese men and women. Ann Neurol. 2010;67(1):11–20. doi: 10.1002/ana.21950. https://doi.org/10.1002/ana.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Yatsuya H, Iso H, Yamagishi K, Saito I, Kokubo Y, et al. Body mass index and risks of incident ischemic stroke subtypes:The Japan public health center-based prospective (JPHC) study. J Epidemiol. 2019;29(9):325–33. doi: 10.2188/jea.JE20170298. https://doi.org/10.2188/jea.je20170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68(8):556–62. doi: 10.1212/01.wnl.0000254472.41810.0d. https://doi.org/10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Bejot Y, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382(1):9. doi: 10.1056/NEJMoa1910355. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Zhang J, Luo Y. Impact of triglyceride playing on stroke severity correlated to bilirubin. Medicine (Baltimore) 2020;99(36):e21792. doi: 10.1097/MD.0000000000021792. https://doi.org/10.1097/md.0000000000021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reina SA, Llabre MM, Allison MA, Wilkins JT, Mendez AJ, Arnan MK, et al. HDL cholesterol and stroke risk:The multi-ethnic study of atherosclerosis. Atherosclerosis. 2015;243(1):314–9. doi: 10.1016/j.atherosclerosis.2015.09.031. https://doi.org/10.1016/j.atherosclerosis.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63(10):1868–75. doi: 10.1212/01.wnl.0000144282.42222.da. https://doi.org/10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Total and high-density lipoprotein cholesterol and stroke risk. Stroke. 2012;43(7):1768–74. doi: 10.1161/STROKEAHA.111.646778. https://doi.org/10.1161/strokeaha.111.646778. [DOI] [PubMed] [Google Scholar]
- 36.Namekata K, Enokido Y, Ishii I, Nagai Y, Harada T, Kimura H. Abnormal lipid metabolism in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. J Biol Chem. 2004;279(51):52961–9. doi: 10.1074/jbc.M406820200. https://doi.org/10.1074/jbc.m406820200. [DOI] [PubMed] [Google Scholar]
- 37.Andersen KK, Andersen ZJ, Olsen TS. Age- and gender-specific prevalence of cardiovascular risk factors in 40,102 patients with first-ever ischemic stroke:A Nationwide Danish Study. Stroke. 2010;41(12):2768–74. doi: 10.1161/STROKEAHA.110.595785. https://doi.org/10.1161/strokeaha.110.595785. [DOI] [PubMed] [Google Scholar]
- 38.Michael KM, Shaughnessy M. Stroke prevention and management in older adults. J Cardiovasc Nurs. 2006;21(5 Suppl 1):S21–6. doi: 10.1097/00005082-200609001-00006. [DOI] [PubMed] [Google Scholar]
- 39.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke:The Framingham study. J Stroke Cerebrovasc Dis. 2003;12(3):119–26. doi: 10.1016/S1052-3057(03)00042-9. https://doi.org/10.1016/s1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 40.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke:Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–26. doi: 10.1016/S1474-4422(08)70193-5. https://doi.org/10.1016/s1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han MK, et al. Gender differences in the age-stratified prevalence of risk factors in Korean ischemic stroke patients:A nationwide stroke registry-based cross-sectional study. Int J Stroke. 2014;9(6):759–65. doi: 10.1111/ijs.12146. https://doi.org/10.1111/ijs.12146. [DOI] [PubMed] [Google Scholar]
- 42.Howe MD, McCullough LD. Prevention and management of stroke in women. Expert Rev Cardiovasc Ther. 2015;13(4):403–15. doi: 10.1586/14779072.2015.1020300. [DOI] [PubMed] [Google Scholar]
- 43.Qin X, Li J, Wu T, Wu Y, Tang X, Gao P, et al. Overall and sex-specific associations between methylation of the ABCG1 and APOE genes and ischemic stroke or other atherosclerosis-related traits in a sibling study of Chinese population. Clin Epigenetics. 2019;11(1):189. doi: 10.1186/s13148-019-0784-0. https://doi.org/10.1186/s13148-019-0784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


