Abstract
HER2-targeted therapies are approved only for HER2-positive breast and gastric cancers. We assessed the safety/tolerability and activity of the novel HER2-targeted antibody–drug conjugate trastuzumab deruxtecan (T-DXd) in 60 patients with pretreated, HER2-expressing (IHC ≥ 1+), non-breast/non-gastric or HER2-mutant solid tumors from a phase I trial (NCT02564900). Most common (>50%) treatment-emergent adverse events (TEAE) were nausea, decreased appetite, and vomiting. Two drug-related TEAEs were associated with fatal outcomes. The confirmed objective response rate (ORR) was 28.3% (17/60). Median progression-free survival (PFS) was 7.2 [95% confidence interval (CI), 4.8–11.1] months. In HER2-mutant non–small cell lung cancer (NSCLC), ORR was 72.7% (8/11), and median PFS was 11.3 (95% CI, 8.1–14.3) months. Confirmed responses were observed in six tumor types, including HER2-expressing NSCLC, colorectal cancer, salivary gland cancer, biliary tract cancer, endometrial cancer, and HER2-mutant NSCLC and breast cancer. Results suggest T-DXd holds promise for HER2-expressing/mutant solid tumors.
SIGNIFICANCE: T-DXd demonstrated promising activity in a heterogeneous patient population with heavily pretreated HER2-expressing or HER2-mutant solid tumors, especially HER2-mutant NSCLC. The safety profile was generally acceptable. Interstitial lung disease can be severe and requires prompt monitoring and intervention. Further research of T-DXd is warranted to address these unmet medical needs.
INTRODUCTION
HER2-targeted therapies have greatly improved survival for patients with HER2-positive breast cancer in the adjuvant/neoadjuvant setting and in metastatic disease, as well as for HER2-positive metastatic gastric cancer (1–4). Dual blockade with trastuzumab and pertuzumab, anti-HER2 humanized mAbs, in combination with chemotherapy is the recommended first-line therapy for patients with HER2-positive metastatic breast cancer (5). Other HER2-targeted therapies such as the antibody–drug conjugate (ADC) trastuzumab emtansine (T-DM1) and the HER2 kinase inhibitor lapatinib are approved in later lines of therapy (5). For HER2-positive gastric cancer, a trastuzumab-based regimen is the standard of care for previously untreated metastatic disease (6).
HER2 overexpression and/or mutations are also observed in tumors other than breast and gastric cancers, such as non–small cell lung cancer (NSCLC) and colorectal cancer (7, 8). Despite the presence of HER2 overexpression and/or mutations in these solid tumors, there are no approved HER2-targeted therapies for these patients. HER2 overexpression (IHC 2+ or 3+) occurs in approximately 10% to 30% of NSCLCs, and HER2-activating mutations occur in approximately 1% to 3% of NSCLCs (7, 9, 10). In colorectal cancer, HER2 overexpression is reported in approximately 2% to 11% of tumors (8). HER2 overexpression/mutation is associated with poor prognosis in NSCLC adenocarcinomas, but its prognostic utility in colorectal cancer remains unclear (8, 11, 12).
Trastuzumab deruxtecan (T-DXd; DS-8201a) is a novel ADC with a humanized anti-HER2 antibody, a cleavable, peptide-based linker, and a potent topoisomerase I inhibitor payload (13). T-DXd was designed with the goal of delivering a potent cytotoxic payload to HER2-expressing tumor cells, while limiting off-target toxicity in normal cells. The novel peptide-based linker is stable in plasma and is thought to be selectively cleaved by lysosomal enzymes such as cathepsins that are upregulated in the tumor microenvironment (13). T-DXd has a drug-to-antibody ratio (DAR) of approximately 8 with homogeneous conjugation, which is 2-fold higher than T-DM1 (DAR 3–4), enabling efficient delivery of the payload to targeted cells (13, 14). The released payload is membrane-permeable, allowing it to exhibit a cytotoxic effect on neighboring tumor cells in close proximity to the targeted cell, regardless of their HER2 expression levels (i.e., a cytotoxic bystander effect; ref. 15). The high DAR and the cytotoxic bystander effect may be of particular importance in targeting tumors with heterogeneous HER2 expression, such as NSCLC and colorectal cancer (16, 17). In preclinical studies, T-DXd showed antitumor activity across a wide range of HER2-expressing tumor types (13, 15).
In August 2015, a dose-escalation and -expansion phase I trial was initiated to assess the safety/tolerability and efficacy of T-DXd in patients with advanced HER2-expressing or HER2-mutant solid tumors (18). During dose escalation, no dose-limiting toxicities were observed and the MTD for doses ranging from 0.8 to 8.0 mg/kg in patients with advanced breast cancer or gastric cancer was not reached (18). During the dose-expansion phase, T-DXd demonstrated promising antitumor activity with an acceptable safety profile in patients with HER2-positive breast cancer or gastric cancer treated with 5.4 or 6.4 mg/kg doses [investigator-assessed confirmed objective response rates (ORR) of 59.5% and 43.2%, respectively; refs. 19, 20]. In the HER2-expressing non-breast/non-gastric or HER2-mutant solid tumor cohort, the MTD during dose escalation was 6.4 mg/kg intravenously once every 3 weeks, and this dose was chosen for dose expansion in this cohort based on the principle of highest tolerated dose without dose-limiting toxicities (18). Here, we present the safety/tolerability and antitumor activity of T-DXd in patients with HER2-expressing non-breast/non-gastric or HER2-mutant solid tumors who received the 6.4 mg/kg dose of T-DXd.
RESULTS
Demographics and Baseline Characteristics
As of February 1, 2019, 60 patients with HER2-expressing non-breast/non-gastric and/or HER2-mutant solid tumors were enrolled (colorectal cancer, n = 20; NSCLC, n = 18; and other, n = 22); 59 patients received ≥1 dose of 6.4 mg/kg T-DXd (colorectal cancer, n = 20; NSCLC, n = 18; and other, n = 21). In this analysis, “other cancers” included 8 salivary gland tumors; 2 HER2-mutant breast cancers (1 HER2 IHC 2+ and 1 HER2 IHC missing per central laboratory assessment); 2 esophageal cancers; 2 endometrial cancers; 2 cases of Paget disease; 2 cases of biliary tract cancer; and 1 case each of pancreatic cancer, uterine cervix carcinoma, extraskeletal myxoid chondrosarcoma, and small-intestine adenocarcinoma. At the time of this analysis, 10 patients were still on treatment and 49 patients discontinued (Fig. 1). One patient discontinued prior to receiving the first dose of study drug due to the occurrence of an adverse event (AE). The most common reason for study discontinuation was progressive disease (PD) per RECIST version 1.1 (n = 34; 57.6%); 5 (8.5%) patients discontinued due to clinical progression, and 5 (8.5%) patients discontinued due to AEs. On the basis of Kaplan–Meier, the median [95% confidence interval (CI)] treatment duration was 6.3 (3.9–10.8) months; NSCLC, 10.6 (5.5–14.2) months; colorectal cancer, 3.0 (1.4–6.3) months; other, 6.3 (2.2–14.2) months.
Figure 1.
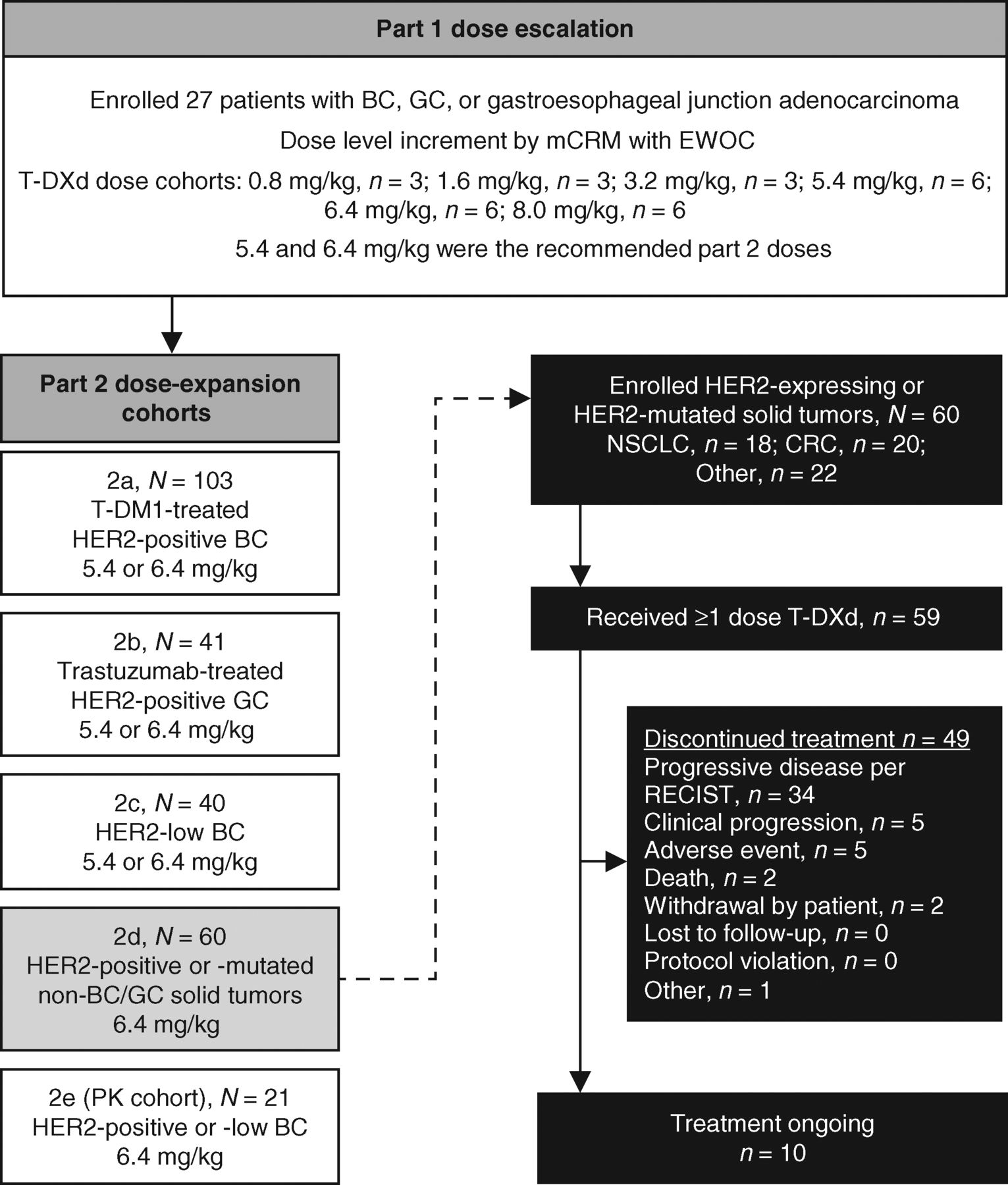
Study design and patient disposition. Data cutoff for this analysis was February 2019. BC, breast cancer; CRC, colorectal cancer; EWOC, escalation with overdose control; GC, gastric cancer; mCRM, modified continuous reassessment method; PK, pharmacokinetics.
Table 1 shows the baseline demographics. The median time from initial diagnosis to the first dose of T-DXd was 28.8 [interquartile range (IQR), 15.9–44.0] months. Overall, 51.7% (31/60) of patients were female. At baseline, all patients had visceral disease. The median number of prior anticancer regimens was 3.0 (IQR, 2–5); 33.3% (20/60) of patients received ≥5 prior regimens. Among the 18 patients with NSCLC, 27.8% (5/18) had received a prior HER2-targeted regimen, 22.2% (4/18) had received a prior EGFR inhibitor, and 5.6% (1/18) had received a prior anaplastic lymphoma kinase inhibitor. Among the 20 patients with colorectal cancer, no patients had received a prior HER2-targeted therapy, 60.0% (12/20) had received a prior EGFR inhibitor, 80.0% (16/20) had received a prior VEGF inhibitor, and 90% (18/20) had received prior irinotecan. A total of 19 patients had a HER2 mutation; the specific mutations are listed in Supplementary Table S1.
Table 1.
Patient demographics and baseline characteristics (enrolled analysis set)
| Characteristic | NSCLC | CRC | Other cancers | Total (part 2d) |
|---|---|---|---|---|
| n = 18 | n = 20 | n = 22a | N = 60 | |
| Age, median (range), years | 58.0 (23–83) | 59.5 (35–75) | 58.0 (44–76) | 58.0 (23–83) |
| Sex | ||||
| Male | 5 (27.8) | 11 (55.0) | 13 (59.1) | 29 (48.3) |
| Female | 13 (72.2) | 9 (45.0) | 9 (40.9) | 31 (51.7) |
| Country | ||||
| Japan | 8 (44.4) | 17 (85.0) | 13 (59.1) | 38 (63.3) |
| United States | 10 (55.6) | 3 (15.0) | 9 (40.9) | 22 (36.7) |
| ECOG performance status | ||||
| 0 | 4 (22.2) | 13 (65.0) | 12 (54.5) | 29 (48.3) |
| 1 | 14 (77.8) | 7 (35.0) | 9 (40.9) | 30 (50.0) |
| Missing | 0 | 0 | 1 (4.5) | 1 (1.7) |
| Time from initial diagnosis, median (range), monthsb | 22.5 (0.1–111.9) | 27.4 (5.6–91.3) | 33.6 (2.0–95.2) | 28.8 (0.1–111.9) |
| Prior anticancer regimens, median (range) | 4.0 (1–10) | 4.5 (1–7) | 2.0 (0–7) | 3.0 (0–10) |
| ≥5 prior anticancer regimens | 5 (27.8) | 10 (50.0) | 5 (21.7) | 20 (33.3) |
| Chemotherapy | 18 (100) | 20 (100) | 18 (81.8) | 56 (93.3) |
| Immunotherapy | 13 (72.2) | 1 (5.0) | 2 (9.1) | 16 (26.7) |
| HER2-targeted therapy | 5 (27.8) | 0 | 12 (54.5) | 17 (28.3) |
| Hormone therapy | 0 | 1 (5.0) | 2 (9.1) | 3 (5.0) |
| VEGF inhibitors | 8 (44.4) | 16 (80.0) | 4 (18.2) | 28 (46.7) |
| EGFR inhibitor | 4 (22.2) | 12 (60.0) | 1 (4.5) | 17 (28.3) |
| ALK inhibitor | 1 (5.6) | 0 | 0 | 1 (1.7) |
| Previous cancer surgery | 6 (33.3) | 16 (80.0) | 18 (81.8) | 40 (66.7) |
| Previous radiotherapy | 11 (61.1) | 5 (25.0) | 15 (68.2) | 31 (51.7) |
| HER2 expression (IHC): local assessment | ||||
| 3+ | 2 (11.1) | 5 (25.0) | 10 (45.5) | 17 (28.3) |
| 2+ | 4 (22.2) | 5 (25.0) | 1 (4.5) | 10 (16.7) |
| ISH+ | 1 (5.6) | 3 (15.0) | 0 | 4 (6.7) |
| ISH− or NE | 1 (5.6) | 1 (5.0) | 0 | 2 (3.3) |
| ISH missing | 2 (11.1) | 1 (5.0) | 1 (4.5) | 4 (6.7) |
| 1+ | 0 | 1 (5.0) | 1 (4.5) | 2 (3.3) |
| 0 | 1 (5.6) | 0 | 0 | 1 (1.7) |
| NE | 0 | 0 | 1 (4.5) | 1 (1.7) |
| Not examined | 11 (61.1) | 9 (45.0) | 9 (40.9) | 29 (48.3) |
| HER2 expression (IHC): central laboratory assessment | ||||
| 3+ | 2 (11.1) | 9 (45.0) | 11 (50.0) | 22 (36.7) |
| 2+ | 1 (5.6) | 2 (10.0) | 6 (27.3) | 9 (15.0) |
| ISH+ | 0 | 1 (5.0) | 3 (13.6) | 4 (6.7) |
| ISH− or NE | 0 | 0 | 1 (4.5) | 1 (1.7) |
| ISH missing | 1 (5.6) | 1 (5.0) | 2 (9.1) | 4 (6.7) |
| 1+ | 8 (44.4) | 2 (10.0) | 1 (4.5) | 11 (18.3) |
| 0 | 5 (27.8) | 7 (35.0) | 0 | 12 (20.0) |
| NE | 0 | 0 | 0 | 0 |
| Not examined | 0 | 0 | 0 | 0 |
| HER2 mutation | 11 (61.1) | 6 (30.0) | 2 (9.1)c | 19 (31.7) |
| Kinase domain mutations | 8 (44.4) | 5 (25.0) | 2 (9.1) | 15 (25.0) |
| Transmembrane domain mutations | 2 (11.1) | 1 (5.0) | 0 (0.0) | 3 (5.0) |
| Extracellular domain mutations | 1 (5.6) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| RAS mutation | — | 7 (35.0) | — | — |
| KRAS mutation | — | 5 (25.0) | — | — |
| NRAS mutation | — | 2 (10.0) | — | — |
NOTE: All values are n (%), unless otherwise specified. Data cutoff for this analysis was February 1, 2019. The enrolled analysis set includes all patients with HER2-expressing non-breast/non-gastric solid tumors or HER2-mutant solid tumors who provided informed consent and were enrolled in the dose escalation or expansion. Percentage was calculated using the number of patients in the column heading as the denominator. Baseline was defined as the last nonmissing value taken before the first dose of study drug.
Abbreviations: ALK, anaplastic lymphoma kinase; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group.
Other cancers group includes eight salivary gland tumors; two HER2-mutant breast cancers (one HER2 IHC 2+ and one HER2 IHC missing per central laboratory assessment); two esophageal cancers; two endometrial cancers; two cases of Paget disease; two cases of biliary tract cancer; and one case each of pancreatic cancer, uterine cervix carcinoma, extraskeletal myxoid chondrosarcoma, and small-intestine adenocarcinoma.
Time from initial diagnosis was calculated as (date of registration − date of initial diagnosis + 1) × 12/365.25.
Both subjects were patients with breast cancer.
Overall, 36.7% (22/60) of patients were HER2 IHC 3+, 15.0% (9/60) were IHC 2+, 18.3% (11/60) were IHC 1+, and 20.0% (12/60) were IHC 0 as assessed by a central laboratory (Table 1). Among patients with NSCLC evaluated for HER2 expression/amplification by central laboratory assessment, 11.1% (2/18) were HER2 IHC 3+, 5.6% (1/18) were IHC 2+, 44.4% (8/16) were IHC 1+, and 27.8% (5/18) were IHC 0. HER2 mutations were reported in 61.1% (11/18) of patients with NSCLC; among them 9.1% (1/11) were HER2 IHC 2+, 36.4% (4/11) were HER2 IHC 1+, 36.4% (4/11) were IHC 0, and 18.2% (2/11) were not evaluated/missing for HER2 IHC. The most common HER2 mutations among patients with NSCLC were exon 20 insertions [44.4% (8/18)]. Among patients with colorectal cancer examined or evaluated for HER2 expression/amplification, 45.0% (9/20) were HER2 IHC 3+, 10.0% (2/20) were IHC2+, 10.0% (2/20) were IHC 1+, and 35.0% (7/20) were IHC 0. RAS gene (KRAS and NRAS) mutations were observed in 35.0% (7/20) of patients with colorectal cancer.
Safety Outcomes
As of February 1, 2019, a total of 59 subjects received one dose of T-DXd and were included in the safety analysis. The median treatment duration overall was 6.3 months (range, 0.7–29.0 months). For patients with NSCLC, median treatment duration was 10.3 (range, 0.7–14.3) months, whereas for patients with colorectal cancer, median treatment duration was 3.0 (range, 0.7–18.5) months. The frequency of all-grade treatment-emergent adverse events (TEAE) was similar among the different tumor types in this analysis. All patients experienced at least 1 TEAE. The most common all-grade TEAEs were gastrointestinal and hematologic (Table 2; Supplementary Table S2). In total, 62.7% (37/59) of patients experienced grade ≥3 TEAEs (regardless of causality), of which the most common (≥5%) were anemia (15/59; 25.4%), decreased neutrophil count (12/59; 20.3%), decreased white blood cell count (11/59; 18.6%), decreased platelet count (9/59; 15.3%), decreased appetite (4/59; 6.8%), increased aspartate aminotransferase (3/59; 5.1%), febrile neutropenia (3/59; 5.1%), and hyponatremia (3/59; 5.1%; Table 2). Overall, 30.5% (18/59) of patients experienced serious TEAEs [regardless of causality; NSCLC, 11.1% (2/18); colorectal cancer, 45.0% (9/20); and other, 33.3% (7/21)]. In total, 8.5% (5/59) discontinued treatment due to TEAEs, most frequently (≥1%) due to pneumonitis (2/59; 3.4%) and interstitial lung disease (ILD; 1/59; 1.7%). Dose reduction associated with TEAEs occurred in 23.7% (14/59) of patients, most commonly (≥5%) due to platelet count decrease (6/59; 10.2%), anemia (4/59; 6.8%), and fatigue (3/59; 5.1%). Dose interruption associated with TEAEs occurred in 37.3% (22/59) of patients, most commonly (≥5%) due to decreased neutrophil count (10/59; 16.9%), anemia (6/59; 10.2%), decreased white blood cell count (3/59; 5.1%), and nasopharyngitis (3/59; 5.1%). Five patients experienced an AE associated with a fatal outcome, of which 2 were reported as drug-related by the investigator. Of the 2 patients who experienced an AE associated with a fatal outcome, one of them experienced an AE of respiratory failure summarized in detail below. The other patient was a 58-year-old male with colorectal cancer who experienced events of abnormal hepatic function, disseminated intravascular coagulation, and febrile neutropenia that were associated with a fatal outcome. This patient had a history of hepatic dysfunction and metastases at baseline. These events occurred 64 days after the first dose of T-DXd, and the patient died 14 days after receiving the last dose of the study drug.
Table 2.
Severity of TEAEs (safety analysis set)
| Worst NCI-CTCAE grade | Part 2d total (N = 59) | ||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Hematologic (>10% total) | |||||
| Anemiaa | 1 (1.7) | 7 (11.9) | 14 (23.7) | 1 (1.7) | 0 |
| Platelet count decreasedb | 9 (15.3) | 4 (6.8) | 6 (10.2) | 3 (5.1) | 0 |
| Neutrophil count decreasedc | 0 | 9 (15.3) | 8 (13.6) | 4 (6.8) | 0 |
| White blood cell count decreasedd | 1 (1.7) | 5 (8.5) | 8 (13.6) | 3 (5.1) | 0 |
| Gastrointestinal (>10% total) | |||||
| Nausea | 26 (44.1) | 16 (27.1) | 2 (3.4) | 0 | 0 |
| Vomiting | 24 (40.7) | 6 (10.2) | 1 (1.7) | 0 | 0 |
| Diarrhea | 12 (20.3) | 7 (11.9) | 0 | 0 | 0 |
| Constipation | 12 (20.3) | 3 (5.1) | 1 (1.7) | 0 | 0 |
| Stomatitise | 7 (11.9) | 2 (3.4) | 1 (1.7) | 0 | 0 |
| Abdominal painf | 4 (6.8) | 3 (5.1) | 1 (1.7) | 0 | 0 |
| Nervous system disorders (>10% total) | |||||
| Dysgeusia | 8 (13.6) | 1 (1.7) | 0 | 0 | 0 |
| Dizziness | 7 (11.9) | 1 (1.7) | 0 | 0 | 0 |
| Other (>10% total) | |||||
| Decreased appetite | 15 (25.4) | 16 (27.1) | 4 (6.8) | 0 | 0 |
| Fatigue | 11 (18.6) | 9 (15.3) | 1 (1.7) | 0 | 0 |
| Alopecia | 13 (22.0) | 7 (11.9) | 0 | 0 | 0 |
| Malaise | 13 (22.0) | 0 | 0 | 0 | 0 |
| Weight decreased | 3 (5.1) | 6 (10.2) | 2 (3.4) | 0 | 0 |
| Pyrexia | 5 (8.5) | 4 (6.8) | 0 | 0 | 0 |
| Hypokalemia | 8 (13.6) | 0 | 1 (1.7) | 0 | 0 |
| Epistaxis | 8 (13.6) | 0 | 0 | 0 | 0 |
| Hiccups | 6 (10.2) | 1 (1.7) | 0 | 0 | 0 |
| Dehydration | 2 (3.4) | 5 (8.5) | 0 | 0 | 0 |
| Blood alkaline phosphatase increased | 6 (10.2) | 1 (1.7) | 0 | 0 | 0 |
| Cough | 3 (5.1) | 3 (5.1) | 0 | 0 | 0 |
| Nasopharyngitis | 5 (8.5) | 1 (1.7) | 0 | 0 | 0 |
| Dry skin | 6 (10.2) | 0 | 0 | 0 | 0 |
| Peripheral edema | 4 (6.8) | 2 (3.4) | 0 | 0 | 0 |
| AEs of interest | |||||
| AST increased | 7 (11.9) | 2 (3.4) | 3 (5.1) | 0 | 0 |
| ALT decreased | 5 (8.5) | 1 (1.7) | 2 (3.4) | 0 | 0 |
| Ejection fraction decreased | 1 (1.7) | 4 (6.8) | 0 | 0 | 0 |
| Electrocardiogram QT prolonged | 0 | 1 (1.7) | 0 | 0 | 0 |
| Interstitial lung diseaseg | 1 (1.7) | 0 | 0 | 0 | 0 |
| Pneumonitisg | 3 (5.1) | 1 (1.7) | 1 (1.7) | 0 | 0 |
| Organizing pneumoniag | 0 | 0 | 0 | 0 | 1 (1.7)h |
| Blood bilirubin increased | 0 | 0 | 0 | 0 | 0 |
| Infusion-related reactions | 0 | 0 | 0 | 0 | 0 |
NOTE: Data are presented as n (%). Data cutoff for this analysis was February 1, 2019. Safety analysis set included all patients who received ≥1 dose of T-DXd. Although patients may experience more than one event per system organ class and preferred term, each patient is counted once for the worst CTCAE grade. One patient may be counted toward ≥2 preferred terms in the same system organ class category. System Organ Class was coded with Medical Dictionary for Regulatory Activities (MedDRA) version 20.1.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events.
Anemia includes hemoglobin decrease, red blood cell count decrease, and anemia.
Platelet count decrease includes platelet count decrease and thrombocytopenia.
Neutrophil count decrease includes neutrophil count decrease and neutropenia.
White blood cell count decrease includes leukopenia and white blood cell count decrease.
Stomatitis includes stomatitis, aphthous stomatitis, mouth ulceration, oral mucosa erosion, and oral mucosal blistering.
Abdominal pain includes abdominal discomfort, abdominal pain, abdominal pain lower, and abdominal pain upper.
As assessed by the investigator before independent adjudication.
Drug-related ILD as determined by the independent ILD adjudication committee; includes one grade 5 case of respiratory failure adjudicated as drug related.
In this study, 7 patients (4 NSCLC, 2 colorectal cancer, and 1 other cancer) experienced ILD or pneumonitis as reported by the investigator. There was 1 case of ILD (grade 1), 1 case of respiratory failure (grade 5), and 5 cases of pneumonitis (3 grade 1, 1 grade 2, and 1 grade 3). All 7 cases were sent for adjudication and 5 were adjudicated as drug-related ILD (2 grade 1 pneumonitis, 1 grade 2 pneumonitis, 1 grade 3 pneumonitis, and 1 grade 5 respiratory failure). The remaining 2 cases (1 grade 1 ILD and 1 grade 1 pneumonitis) were adjudicated as not ILD. No history of prior chest/lung radiotherapy was reported in these patients; 3 were from Japan and 2 were from the United States. In patients with NSCLC who experienced ILD, 3 of 4 cases were adjudicated as ILD related to the study drug, and the remaining case was adjudicated as not an ILD. Among the 3 patients with NSCLC with drug-related ILD, 1 patient experienced an AE of respiratory failure which was associated with a fatal outcome. This patient was a 48-year-old male with NSCLC who developed grade 3 respiratory failure 16 days after the first dose of T-DXd, which further worsened, and he died because of respiratory failure 26 days after receiving the first infusion. This patient had a history of ongoing dyspnea and pneumonectomy at baseline. The AE of respiratory failure associated with a fatal outcome was adjudicated as ILD and related to the study drug. The adjudicated onset date was determined to be before the patient started receiving the study drug.
Antitumor Activity Outcomes
As of February 1, 2019, the median (range) duration of follow-up was 7.8 (0.1–28.6) months for the overall cohort [NSCLC, 11.0 (0.9–21.9); colorectal cancer, 3.1 (0.8–18.9); other, 9.5 (0.1–28.6)]. The confirmed ORR based on independent central review in this heterogeneous population of patients with HER2-expressing non-breast/non-gastric and/or HER2-mutant solid tumors treated with T-DXd at the 6.4 mg/kg dose was 28.3% (17/60). The ORR based on investigator assessment was 36.7% (22/60; Table 3). Of the 60 patients, 51 were evaluable for tumor shrinkage. The median duration of response (DoR) was 11.5 [95% CI, 7.0–not estimable (NE)] months and the time to response (TTR) was 1.4 (95% CI, 1.4–2.9) months. Overall, 63.3% (38/60) of patients had a progression-free survival (PFS) event, and the median PFS was 7.2 (95% CI, 4.8–11.1) months; the median overall survival (OS) was 23.4 (95% CI, 15.6–NE) months (Fig. 2). Patients with NSCLC and other cancers showed better PFS than patients with colorectal cancer (Fig. 2). Tumor shrinkage by independent central review assessment was observed in 80.4% (41/51) of patients, of whom 92.7% (38/41) showed tumor shrinkage at the first assessment 6 weeks post-baseline (Fig. 3). Patients with HER2-mutant NSCLC had more pronounced tumor shrinkage than patients with NSCLC without documented mutations, regardless of IHC/ISH status. Among patients with colorectal cancer, the largest antitumor response was observed in those patients with HER2 IHC 3+. In the other cancers group, patients with HER2-mutant breast cancer and those with HER2-expressing or HER2-mutant endometrial cancer, salivary duct carcinoma, gallbladder cancer, and cholangiocarcinoma had the most pronounced tumor shrinkage (Fig. 3). Of the 19 patients with HER2 mutations, the most common confirmed response was partial response [PR; 9 (47.4%); Supplementary Table S1].
Table 3.
Clinical activity outcomes (enrolled analysis set)
| NSCLC | CRC | Other cancers | Total (part 2d) | |
|---|---|---|---|---|
| n = 18 | n = 20 | n = 22 | N = 60 | |
| Treatment duration, months, median (range)a | 10.3 (0.7–14.3) | 3.0 (0.7–18.5) | 6.3 (0.7–29.0)b | 6.3 (0.7–29.0)c |
| Duration of follow-up, months, median (range) | 11.0 (0.9–21.9) | 3.1 (0.8–18.9) | 9.5 (0.1–28.6) | 7.8 (0.1–28.6) |
| Investigator-assessed outcomes | ||||
| Best overall response with confirmation of CR/PR | ||||
| CR | 0 | 0 | 1 (4.5) | 1 (1.7) |
| PR | 10 (55.6) | 3 (15.0) | 8 (36.4) | 21 (35.0) |
| SD | 4 (22.2) | 13 (65.0) | 9 (40.9) | 26 (43.3) |
| PD | 3 (16.7) | 3 (15.0) | 3 (13.6) | 9 (15.0) |
| Not evaluable | 1 (5.6) | 1 (5.0) | 1 (4.5) | 3 (5.0) |
| ORR with confirmation of CR/PR, n/N (%) | 10/18 (55.6) | 3/20 (15.0) | 9/22 (40.9) | 22/60 (36.7) |
| 95% CI | (30.8–78.5) | (3.2–37.9) | (20.7–63.6) | (24.6–50.1) |
| DCR with confirmation of CR/PR/SD, n/N (%)d | 14/18 (77.8) | 16/20 (80.0) | 18/22 (81.8) | 48/60 (80.0) |
| 95% CI | (52.4–96.3) | (56.3–94.3) | (59.7–94.8) | (67.7–89.2) |
| TTR with confirmation, monthse | ||||
| n/N | 10/18 | 3/20 | 9/22 | 22/60 |
| Median (95% CI) | 2.1 (1.2–2.8) | 3.0 (1.4–8.1) | 1.6 (1.2–3.0) | 1.7 (1.4–2.9) |
| Min, max | 1.2, 5.4 | 1.4, 8.1 | 1.2, 5.7 | 1.2, 8.1 |
| Duration of response with confirmation, monthsf | ||||
| n/N | 10/18 | 3/20 | 9/22 | 22/60 |
| Median (95% CI) | 9.9 (6.9–11.5) | 7.4 (2.8–17.5) | 12.9 (3.0–NE) | 9.9 (9.0–17.5) |
| Min, max | 5.3+, 11.5 | 2.8, 17.5 | 3.0, 26.3+ | 2.8, 26.3+ |
| PFS, months | ||||
| Median (95% CI) | 11.3 (5.5–14.1) | 4.1 (2.1–5.9) | 11.1 (5.4–20.5) | 8.3 (5.4–11.8) |
| Assessment by independent central review | ||||
| Best overall response with confirmation of CR/PR | ||||
| CR | 0 | 0 | 1 (4.5) | 1 (1.7) |
| PR | 10 (55.6) | 1 (5.0) | 5 (22.7) | 16 (26.7) |
| SD | 5 (27.8) | 15 (75.0) | 12 (54.5) | 32 (53.3) |
| PD | 2 (11.1) | 2 (10.0) | 3 (13.6) | 7 (11.7) |
| Not evaluable | 1 (5.6) | 2 (10.0) | 1 (4.5) | 4 (6.7) |
| ORR with confirmation of CR/PR, n/N (%) | 10/18 (55.6) | 1/20 (5.0) | 6/22 (27.3) | 17/60 (28.3) |
| 95% CI | (30.8–78.5) | (0.1–24.9) | (10.7–50.2) | (17.5–41.4) |
| DCR with confirmation of CR/PR/SD, n/N (%)d | 15/18 (83.3) | 16/20 (80.0) | 18/22 (81.8) | 49/60 (81.7) |
| 95% CI | (58.6–96.4) | (56.3–94.3) | (59.7–94.8) | (69.6–90.5) |
| TTR with confirmation, monthse | ||||
| n/N | 10/18 | 1/20 | 6/22 | 17/60 |
| Median (95% CI) | 1.4 (1.2–2.8) | 3.0 (NE) | 1.6 (1.4–3.0) | 1.4 (1.4–2.9) |
| Min, max | 1.2, 5.8 | 3.0, 3.0 | 1.4, 3.0 | 1.2, 5.8 |
| Duration of response with confirmation, monthsf | ||||
| n/N | 10/18 | 1/20 | 6/22 | 17/60 |
| Median (95% CI) | 10.7 (6.9–11.5) | 13.4 (NE) | NR (3.0–NE) | 11.5 (7.0–NE) |
| Min, max | 3.3+, 11.5 | 13.4, 13.4 | 3.0, 21.1+ | 3.0, 21.1+ |
| PFS, months | ||||
| Median (95% CI) | 11.3 (7.2–14.3) | 4.0 (2.7–5.6) | 11.0 (2.8–NE) | 7.2 (4.8–11.1) |
| OS, months | ||||
| Median (95% CI) | NR (17.3–NE) | 15.6 (4.8–NE) | 23.4 (9.6–NE) | 23.4 (15.6–NE) |
| Min, max | 0.9, 21.9+ | 1.0+, 25.9+ | 0.1+, 28.6+ | 0.1+, 28.6+ |
NOTE: All values are n (%), unless otherwise specified. Data cutoff for this analysis was February 2019. The enrolled analysis set includes all patients with HER2-expressing non-breast/non-gastric solid tumors or HER2-mutant solid tumors who provided informed consent and were enrolled in the dose escalation or expansion. Min and max include the censored observations; using “+” after value indicates censoring.
Abbreviations: CR, complete response; CRC, colorectal cancer; DCR, disease control rate; max, maximum; min, minimum; NE, not estimable; NR, not reached; PR, partial response; SD, stable disease.
Safety analysis set.
n = 21.
n = 59.
DCR is calculated as the proportion of patients demonstrating CR, PR, or SD for a minimum of 5 weeks from date of first dose.
TTR was measured from the date of registration to the date at which criteria for CR or PR are first met.
Duration of response was measured from the time at which CR or PR criteria are first met, until the first date of objectively documented PD or death due to any cause.
Figure 2.
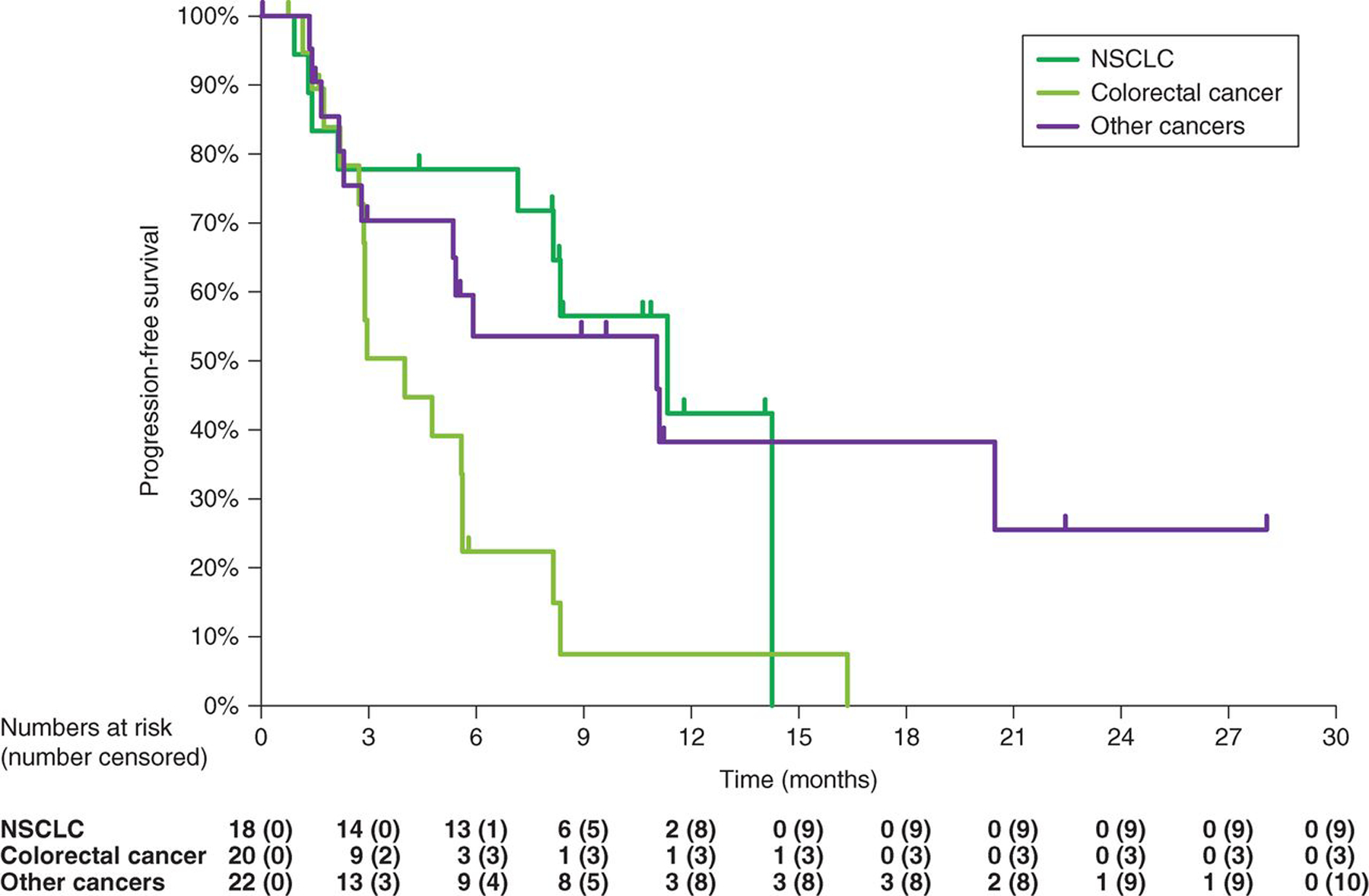
Kaplan–Meier estimate for PFS for T-DXd (6.4 mg/kg) in patients with HER2-expresssing non-breast, non-gastric cancers or HER2-mutant advanced solid tumors. Data cutoff for this analysis was February 2019.
Figure 3.
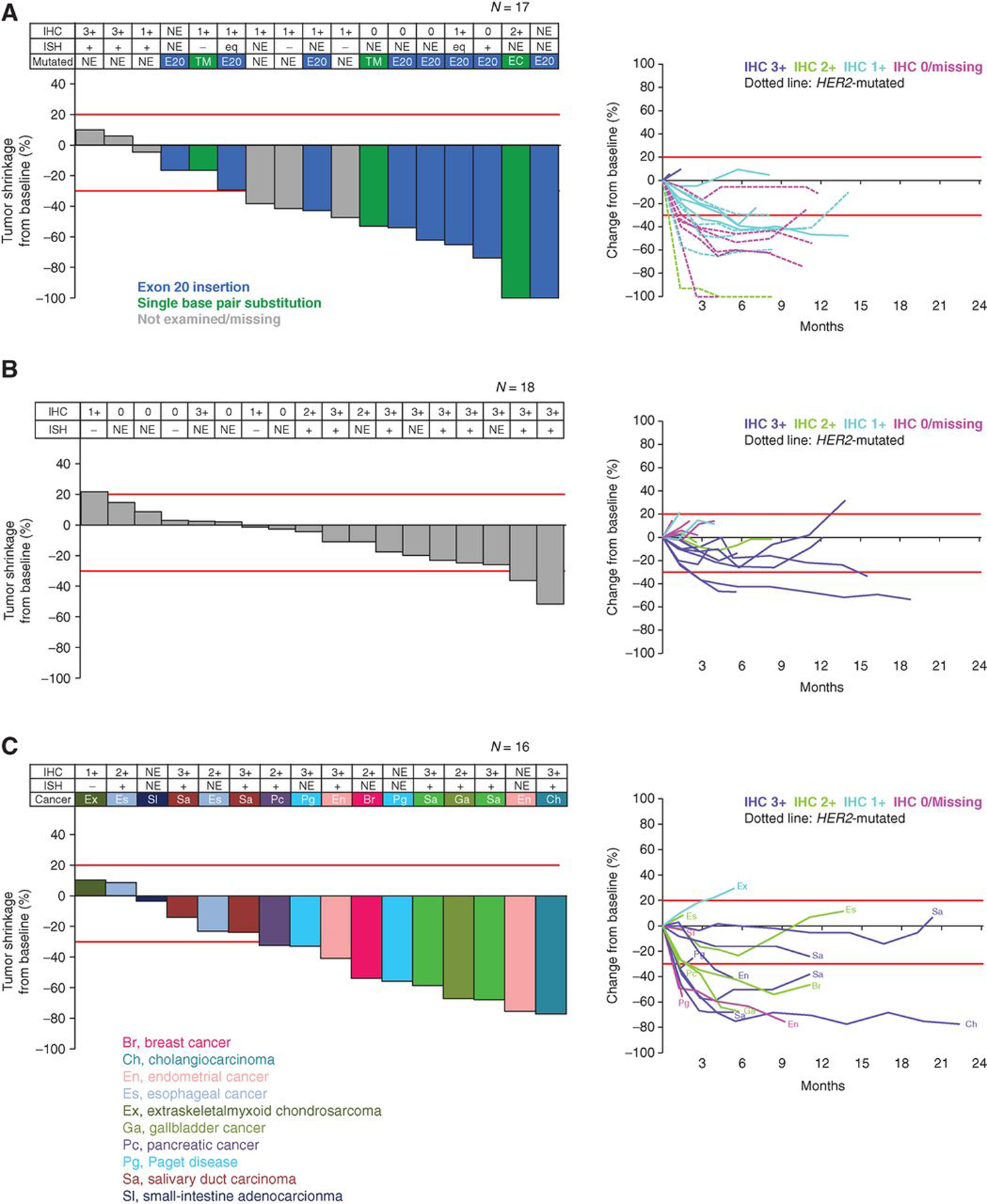
Best percentage change in tumor size and tumor shrinkage over time (based on central laboratory assessment) after T-DXd in patients with NSCLC (A), colorectal cancer (B), or other cancers (C). HER2 expression and mutation values shown are based on central laboratory assessment. HER2 expression and mutation status at enrollment was determined via local assessment. Of the 60 patients, 51 were evaluable for tumor shrinkage. Eight patients did not have baseline measurable disease and 1 patient did not have a post-baseline tumor assessment. Data cutoff for this analysis was February 2019. Red lines denote 20% increase or 30% reduction in tumor size, respectively. Br, breast cancer; Ch, cholangiocarcinoma; E20, exon 20 insertion; En, endometrial cancer; Es, esophageal cancer; Ex, extraskeletal myxoid chondrosarcoma; Ga, gallbladder cancer; NE, not evaluated; Pc, pancreatic cancer; Pg, Paget disease; Sa, salivary duct carcinoma; SI, small-intestine adenocarcinoma; TM, single base pair substitution at transmembrane domain.
In the HER2-expressing or HER2-mutant NSCLC subgroup, 55.6% (10/18) of patients had a confirmed objective response, with a median DoR of 10.7 (95% CI, 6.9–11.5) months and a TTR of 1.4 (95% CI, 1.2–2.8) months. A total of 50.0% (9/18) of patients with NSCLC had a PFS event, and the median PFS was 11.3 (95% CI, 7.2–14.3) months (Fig. 2). Among the subset of patients with NSCLC with HER2-mutant disease, the confirmed ORR was 72.7% (8/11), with a median DoR of 9.9 (95% CI, 6.9–11.5) months. The disease control rate (DCR) was 90.9% (10/11) and the median PFS was 11.3 (95% CI, 8.1–14.3) months (Table 4).
Table 4.
Clinical activity outcomes in HER2 -mutant NSCLC (enrolled analysis set)
| Outcome | HER2-mutant NSCLC |
|---|---|
| n = 11 | |
| Duration of follow-up, median (range), monthsa | 11.5 (5.1–14.6) |
| Investigator-assessed outcomes | |
| Best overall response with confirmation of CR/PR | |
| CR | 0 |
| PR | 8 (72.7) |
| SD | 2 (18.2) |
| PD | 1 (9.1) |
| NE | 0 |
| Confirmed ORR | 8/11 (72.7) |
| 95% CI | (39.0–94.0) |
| Confirmed DCRb | 10/11 (90.9) |
| 95% CI | (58.7–99.8) |
| TTR, monthsc | |
| n/N | 8/11 |
| Median (95% CI) | 1.4 (1.2–2.8) |
| Min, max | 1.2, 5.4 |
| Duration of response, monthsd | |
| n/N | 8/11 |
| Median (95% CI) | 9.9 (6.9–11.5) |
| Min, max | 5.3+, 11.5 |
| PFS, months | |
| Median (95% CI) | 11.3 (5.5–14.3) |
| Min, max | 2.8, 14.3 |
| Assessment by independent central review | |
| Best overall response with confirmation of CR/PR | |
| CR | 0 |
| PR | 8 (72.7) |
| SD | 2 (18.2) |
| PD | 1 (9.1) |
| NE | 0 |
| Confirmed ORR | 8/11 (72.7) |
| 95% CI | (39.0–94.0) |
| Confirmed DCRb | 10/11 (90.9) |
| 95% CI | (58.7–99.8) |
| TTR, monthsc | |
| n/N | 8/11 |
| Median (95% CI) | 1.4 (1.2–1.4) |
| Min, max | 1.2, 2.8 |
| Duration of response, monthsd | |
| n/N | 8/11 |
| Median (95% CI) | 9.9 (6.9–11.5) |
| Min, max | 3.3+, 11.5 |
| PFS, months | |
| Median (95% CI) | 11.3 (8.1–14.3) |
| Min, max | 1.3, 14.3 |
| OS, months | |
| Median (95% CI) | 17.3 (17.3–NE) |
| Min, max | 8.4, 20.9+ |
NOTE: All values are n (%), unless otherwise specified. Data cutoff for this analysis was February 1, 2019. Dashes indicate value not reached. Min and max include the censored observations; using “+” after value indicates censoring. Abbreviations: max, maximum; min, minimum; NE, not evaluable; ORR, objective response rate.
Safety analysis set.
DCR is calculated as the proportion of patients demonstrating CR, PR, or SD for a minimum of 6 weeks (±1 week) from date of first dose.
TTR was measured from the date of registration to the date at which criteria for CR or PR are first met.
Duration of response was measured from the time at which CR or PR criteria are first met until the first date of objectively documented PD.
In the HER2-expressing or HER2-mutant colorectal cancer subgroup, 5.0% (1/20) of patients had a confirmed objective response. A total of 85.0% of patients with colorectal cancer (17/20) had a PFS event, and the median PFS was 4.0 (95% CI, 2.7–5.6) months (Fig. 2). Among 9 patients with HER2 IHC 3+ colorectal cancer, the confirmed ORR was 11.1% (1/9) and the confirmed DCR was 100% (9/9).
In addition to NSCLC and colorectal cancer, responses were also observed across a number of other tumor types, including HER2-expressing or HER2-amplified salivary gland cancer, biliary tract cancer, and endometrial cancer, and HER2-mutant nonamplified breast cancer (Fig. 3). In this group, the ORR was 6 of 22 (27.3%) and the median DoR was not reached (95% CI, 3.0–not reached). The median PFS was 11.0 months (95% CI, 2.8–NE; Table 3).
DISCUSSION
In this first-in-human clinical study investigating T-DXd for treatment of patients with advanced solid tumors, the 6.4 mg/kg dose demonstrated encouraging preliminary antitumor activity with an acceptable safety profile in patients with heavily pretreated, HER2-expressing and/or HER2-mutant solid tumors. In particular, T-DXd showed promising antitumor activity in patients with HER2-mutant NSCLC (confirmed ORR 72.7%; median PFS 11.3 months as assessed by an independent central review). The previously reported clinical activity in the HER2-positive breast cancer and HER2-positive gastric cancer cohorts of this phase I study included investigator-assessed confirmed ORR of 59.5% and 43.2%, respectively (19, 20). In addition, in the phase II DESTINY-BREAST01 trial, T-DXd 5.4 mg/kg demonstrated a confirmed ORR of 60.9% with a PFS of 16.9 months in patients with HER2-positive metastatic breast cancer previously treated with T-DM1 (21).
This study was performed in a heterogeneous population of HER2-expressing or HER2-mutant cancers, including NSCLC, colorectal cancer, salivary gland carcinoma, biliary tract cancer, endometrial cancer, small-intestinal adenocarcinoma, chondrosarcoma, and HER2-mutant breast cancer. Although sample sizes are too small to draw firm conclusions specific to a tumor type, T-DXd activity appears to be most pronounced in HER2-mutant NSCLC.
Beyond HER2-positive breast and gastric cancer, there is a need to develop new treatment options for patients with other HER2-expressing or HER2-mutant solid tumors, as conventional therapies (e.g., trifluridine/tipiracil and regorafenib in colorectal cancer, and pemetrexed, platinum, and taxanes in NSCLC) often show limited efficacy with a high frequency of AEs (22–25). HER2-expressing lung, bladder, and endometrial cancers are known to be associated with highly aggressive disease, and these patients carry a poor prognosis (26–28). Prior studies investigating trastuzumab and chemotherapy in patients with uterine and urothelial carcinomas demonstrated high response rates, but are limited by challenges in ascertaining the contribution of HER2-targeted therapy (29, 30). Compared with breast and gastric cancers, the rates of HER2 amplification are lower in NSCLC, colorectal cancer, and the other solid tumors examined in this study. However, mutations or amplification in HER2 are becoming more readily identified in these cancers in part due to the increasing use of next-generation sequencing (NGS; ref. 31).
The increasing use of NGS in lung cancers routinely identifies HER2 mutations, which are known oncogenic drivers in NSCLC (7). However, there are no HER2-targeted agents approved in patients with HER2-mutant NSCLC (32). HER2 mutations in advanced lung adenocarcinomas are associated with worse prognosis compared with patients with other oncogene-driven advanced lung adenocarcinomas (12). Analyses of mutations in the kinase and extracellular domains of HER2 found in lung and breast cancers indicate that these HER2 mutations have potential oncogenic activity and confer sensitivity to HER2-targeted therapies in vitro (33, 34).
There is limited clinical evidence to support the efficacy of HER2-targeted therapy in patients with HER2-mutant lung adenocarcinomas. The antitumor activity of trastuzumab in HER2-mutant lung adenocarcinomas has largely been demonstrated in case studies, and the evidence in support of the use of pan-HER family tyrosine kinase inhibitor (TKI), including afatinib, dacomitinib, and neratinib, is modest at best (35–40). Poziotinib and pyrotinib are two novel irreversible HER TKIs that showed promising activity in NSCLC with HER2 exon 20 insertions (41–43). Another HER2-targeted ADC, trastuzumab duocarmazine, has shown activity in a phase I study of HER2 low-expressing solid tumors, including endometrial and urothelial cancers (44). Although HER2-targeted ADCs have shown promising results, the number of patients studied is still quite limited (45). For instance, a recent phase II basket study reported an ORR of 44% and a median PFS of 5 months with T-DM1 in 18 heavily pretreated patients with HER2-mutant lung adenocarcinomas (45). Given this context, the ORR of >70% with T-DXd reported here in 11 patients with HER2-mutant NSCLC is particularly promising, with responders observed across HER2 mutation subtypes including exon 20 insertions and extracellular domain point mutations. However, these results need to be confirmed in larger studies.
In addition to NSCLC, the current results demonstrated preliminary activity for T-DXd in other HER2-expressing solid tumors, including colorectal cancer. There is limited data to support the efficacy of HER2-targeted therapies in HER2-expressing colorectal cancer. Here, we report an ORR of 5% with T-DXd in patients with colorectal cancer, of whom 35% had a KRAS/NRAS mutation. Among the patients with HER2 IHC 3+ colorectal cancer, the confirmed ORR was 11.1% (1/9) and the confirmed DCR was 100%. In this study, 45% of patients with colorectal cancer were HER2 IHC 1 or 0, which is disproportionately a low-expressing population compared with studies of trastuzumab in metastatic colorectal cancer (46, 47). In our study, the lower levels of HER2 expression, the advanced stage of treatment (50% had ≥5 prior regimens), and the increased genetic heterogeneity with concurrent RAS mutations for patients with colorectal cancer may have contributed to the low response rate observed. Furthermore, 90% of patients with colorectal cancer in this study progressed on prior irinotecan. Although topoisomerase I inhibition may be more targeted with T-DXd than with irinotecan, many of the patients with colorectal cancer had low HER2 expression, and the targeting provided by T-DXd may not be enough to overcome topoisomerase I resistance in these patients. It is important to note that the small sample size of patients with colorectal cancer precludes any conclusions on efficacy. Further studies in a HER2-overexpressing population of patients with colorectal cancer are warranted.
The observed antitumor response in the heterogeneous populations investigated in this study, particularly in patients with HER2-mutant NSCLC independent of HER2 IHC status, may relate to the unique mechanism of action of T-DXd. Unlike other anti-HER2–targeted antibody treatments that act through antibody-dependent cell-mediated cytotoxicity or inhibition of the signaling pathway, the HER2 antibody of T-DXd mainly functions to target HER2-expressing cells that then internalize the toxic payload. The high DAR and the bystander effect caused by the membrane permeability of the cytotoxic payload may contribute to enhanced targeting of HER2-expressing or HER2-mutant cancer cells (13, 15). It can be hypothesized that the activating HER2 mutations may lead to enhanced internalization by forming homodimers or heterodimers, which could increase the antitumor activity of ADCs (45, 48). This theory is supported by the enhanced response to trastuzumab that was first observed in patients with NSCLC with extracellular and transmembrane domain mutations (V659E or S310F) known to activate dimers (33, 49). The conformational change of the HER2 receptor caused by HER2 mutations may provide opportunities for newer anti-HER2 agents with high potency (45, 50).
Although not every patient had sufficient archival tissue for comprehensive genomic and proteomic testing, the biomarker analyses conducted in this trial have shed some light on the clinical development of HER2-targeted ADCs. First, HER2 mutations in NSCLC seem to be the most promising biomarker for predicting T-DXd activity, and responses occurred regardless of HER2 protein expression by IHC 0, 1, 2, or 3. Second, responses to T-DXd occurred in a variety of HER2-activating mutation subtypes in NSCLC, which is consistent with results from recent trials of T-DM1 (refs. 45, 51, 52; Supplementary Table S1). Third, HER2 amplification by ISH or NGS seems to predict responses across a variety of tumor types.
Treatment with T-DXd resulted in an acceptable safety profile that was observed to be consistent across other study cohorts (patients with HER2-positive breast cancer and gastric cancer), as described previously (19, 20). Our experience of pulmonary toxicity draws parallels with many EGFR-targeting agents, which have also been associated with an increased risk of drug-induced ILD, yet the specific mechanism remains unknown. Preclinical research directed by the TRISTAN consortium is under way to determine a general mechanism by which drug-induced ILD occurs (53). On the basis of a pooled analysis of ILD across multiple studies (multiple tumor types) of T-DXd, Japanese ethnicity and higher dose were associated with a higher risk factors for developing ILD (54). ILD is an important identified risk associated with T-DXd. ILD was managed per-protocol with dose reductions or discontinuation, corticosteroids, and supportive care (see Supplementary Data A). Close monitoring for signs and symptoms of ILD is recommended for early detection, and, once diagnosed, interruption of T-DXd and prompt intervention with steroids when appropriate may help reduce severity of this complication (20, 54). However, more research is needed to further characterize the risk.
There were several aspects of this study that could limit the generalizability of the results. This was a nonrandomized, phase I study with a heterogeneous patient population and a relatively limited sample size. Therefore, the results of this study should be viewed as exploratory, with results to be confirmed in ongoing, larger, tumor type–specific phase II studies, including in NSCLC and colorectal cancer. In addition, the biomarker selection was heterogeneous (HER2 IHC status and HER2 mutation status were locally assessed using archival samples). The full spectrum of concomitant mutations was not captured, and thus it was not possible to fully assess their impact on response to T-DXd.
In summary, T-DXd demonstrated promising antitumor activity with an acceptable safety profile in patients with heavily pretreated HER2-expressing or HER2-mutant solid tumors, especially in HER2-mutant NSCLC. If the results of this trial are replicated in subsequent trials, T-DXd may represent a promising option for patients with a variety of HER2-expressing or HER2-mutant cancers, for which there are no approved HER2-targeted therapies. Future directions include ongoing cancer type–specific phase II studies in HER2-overexpressing or HER2-mutant advanced NSCLC (NCT03505710), and HER2-expressing recurrent or metastatic colorectal cancer (NCT03384940). More clinical trials are needed to develop HER2-targeted agents for other solid tumors such as biliary tract, endometrial, and salivary gland cancers. The encouraging results of this clinical trial and the exploratory biomarker analyses conducted may serve to propel innovative trial designs and accelerate the clinical development of T-DXd and other HER2-targeted ADCs so that the substantial therapeutic benefits brought by HER2-targeted agents may be extended to patients with tumors other than breast or gastric cancer.
METHODS
Study Design and Participants
This was a first-in-human, phase I, nonrandomized, open-label, multiple-dose, two-part study conducted at six sites in Japan and eight in the United States (ClinicalTrials.gov NCT02564900; ClinicalTrials.jp 152978). This study complies with the ethical standards established by the Declaration of Helsinki, the International Council for Harmonisation, Good Clinical Practice consolidated Guideline E6, and applicable local regulations including those out-lined by FDA and Japanese Ministry of Health, Labor, and Welfare. Patients were required to provide written informed consent prior to study enrollment.
Detailed methods were reported previously (18–20). In brief, in the dose-escalation phase safety/tolerability and the MTD/recommended dose for expansion (RDE) were assessed in patients with advanced breast cancer or gastric cancer who had failed prior therapy in doses ranging from 0.8 to 8.0 mg/kg. In dose expansion, safety/tolerability and clinical activity of T-DXd at the RDE (5.4 or 6.4 mg/kg intravenous every 3 weeks) were assessed in five dose-expansion cohorts: post–T-DM1 HER2-positive (IHC 3+ or ISH+) breast cancer (part 2a); post-trastuzumab HER2-positive (IHC 3+ or IHC 2+/ISH+) gastric cancer (part 2b); HER2 low-expressing (IHC 1+ or 2+, ISH−) breast cancer (part 2c); other HER2-expressing (IHC ≥1+ or amplified by ISH or NGS) or HER2-mutant solid tumors (part 2d); and HER2-expressing (IHC 3+, 2+, 1+, and/or ISH+) advanced/unresectable or metastatic breast cancer for pharmacokinetic analysis (part 2e). The design for parts 1 and 2 of this study are shown in Fig. 1.
Study Population
This analysis included patients with HER2-expressing (IHC ≥1+ or amplified by ISH or NGS) or HER2-mutant (as determined by NGS or other analysis techniques as appropriate) advanced solid tumors (part 2d), including NSCLC, colorectal cancer, and other solid cancers. Patient HER2 status at enrollment was defined on the basis of local laboratory testing data. A retrospective analysis of HER2 IHC and ISH of archived formalin-fixed, paraffin-embedded tumor tissue sections was done after enrollment by a central laboratory (HercepTest, Dako). Key inclusion/exclusion criteria are presented in Supplementary Table S3.
Procedures
The study drug was provided by the sponsor (Daiichi Sankyo Inc.; Daiichi Sankyo Co. Ltd.) and was supplied in single-use glass vials. In patients with HER2-expressing non-breast and non-gastric or HER2-mutant solid tumors, the 6.4 mg/kg dose of T-DXd was administered once every 3 weeks until withdrawal of patient consent, PD, death, or loss to follow-up. Dosage interruptions of up to 4 weeks from the planned study drug administration date were permitted.
Tumor assessments and response to treatment measures have been described previously (18). In brief, assessments (CT or MRI) were performed every 6 weeks for the first 24 weeks, and every 12 weeks thereafter. RECIST version 1.1 was used by investigators, and by an independent central imaging facility to evaluate tumor response. TEAEs were recorded at every visit and graded according to the Common Terminology Criteria for Adverse Events version 4.03. Cardiac evaluations (echocardiography or multigated acquisition scanning) were performed at screening, before infusion on cycles 2 and 3, and thereafter at every other cycle until treatment termination. Reported cases of ILD (including pneumonitis and organizing pneumonia and additional preferred terms agreed to with the ILD adjudication committee including respiratory failure and acute respiratory failure) were reviewed by an independent adjudication committee.
Outcomes
Efficacy endpoints include ORR [rate of complete response (CR) plus rate of PR], DoR (first time CR or PR criteria were met to the first date of objectively documented PD or any cause of death), TTR (measured from registration to the time when criteria for CR or PR were first met), DCR [the sum of CR, PR, and stable disease (SD; neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD) per RECIST v1.1 for at least 5 weeks from the date of the first dose], percent change in a target lesion, duration of SD, PFS (time from the date of registration to the first objective documentation of radiographic PD or death due to any cause, whichever was earlier), and OS (time from registration to death from any cause).
Safety endpoints include TEAEs and TEAEs of special interest (ILD, ejection fraction decrease, electrocardiogram QT prolongation, and infusion-related reactions). An independent ILD adjudication committee was responsible for providing ongoing review of all potential ILD cases throughout the study (including cases of pneumonitis and organizing pneumonia). Cases were sent for adjudication from the trigger event based on 42 preferred terms (all from the ILD Standardized Med-DRA Query) and two additional preferred terms of respiratory failure and acute respiratory failure (44 preferred terms total) to identify cases eligible for adjudication. Additional data collected for cases brought to adjudication included an in-depth relevant medical history (smoking history, chronic obstructive pulmonary disease, and other chronic lung conditions), diagnostic evaluation, treatment, and outcome.
Statistical Analysis
The dose-escalation phase did not include formal statistical assessments for sample size. Cohort 2d had a planned enrollment of 60 patients, providing at least 80% power to exclude an ORR ≤15% at the 5% type I error (one-sided), when the true ORR is 30% based on a binomial distribution using SAS version 9.2.
The enrolled analysis set includes all patients with HER2-expressing non-breast/non-gastric solid tumors or HER2-mutant solid tumors who provided informed consent and were enrolled in the dose escalation or expansion. The response-evaluable set includes all patients with HER2-expressing non-breast/non-gastric solid tumors or HER2-mutant solid tumors who received at least one dose of T-DXd, and baseline measurable tumors assessed by independent central review. The safety analysis set includes all HER2-expressing non-breast/non-gastric solid-tumor patients or all HER2-mutant solid-tumor patients who received at least one dose of T-DXd.
Point estimates and exact binomial 95% CIs are provided for ORR and DCR. Descriptive summaries using the Kaplan–Meier method are provided with CIs based on the Brookmeyer–Crowley method for time to event variables (PFS, TTR, and DoR). Descriptive statistics are used to summarize demographic and safety.
Supplementary Material
Acknowledgments
This study was funded by Daiichi Sankyo Co., Ltd. B.T. Li is supported by the NIH/NCI Cancer Center Support Grant/Core Grant (P30 CA008748) at Memorial Sloan Kettering Cancer Center.
Disclosure of Potential Conflicts of Interest
J. Tsurutani reports receiving commercial research grants from Eisai, Kyowa Kirin, Daiichi Sankyo, Eli Lilly, Nihon Kayaku, Chugai, Pfizer, and MSD, has received speakers bureau honoraria from Eisai, Kyowa Kirin, Taiho, Chugai, Eli Lilly, Novartis, Daiichi Sankyo, and Nihon Kayaku, and has cosultant/advisory board relationships with Daiichi Sankyo, Eli Lilly, and Asahi Kasei. H. Iwata is a consultant at Chugai, Pfizer, Novartis, Daiichi Sankyo, AstraZeneca, Lilly, Kyowa Hakko Kirin, AbbVie, and Odonate, and has received speakers bureau honoraria from Chugai/Roche, Daiichi Sankyo, AstraZeneca, Lilly, Pfizer, Novartis, Taiho, and Eisai. I. Krop is a consultant at Daiichi Sankyo, AstraZeneca, Genentech Roche, Context Therapeutics, Macrogenics, Bristol-Myers Squibb, Seattle Genetics, and Taiho Oncology, has received speaking fees from Celltrion, and has received other remuneration from Merck and Novartis. P.A. Janne is a consultant at AstraZeneca, Boehringer Ingelheim, Roche/Genentech, Pfizer, ACEA Biosciences, Eli Lilly, Araxes Biosciences, SFJ Pharmaceuticals, Daiichi Sankyo, Novartis, Sanofi Oncology, and Takeda Oncology, is an SAB member at Biocartis, Ignyta, LOXO Oncology, Voronoi, and Mirati Therapeutics, reports receiving commercial research grants from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, PUMA, Eli Lilly, Revolution Medicines, Takeda Oncology, and Astellas Pharmaceuticals, has ownership interest (including patents) in Lab Corp, LOXO Oncology, and Gatekeeper Pharmaceuticals. S. Takahashi reports receiving commercial research grants from Daiichi Sankyo, Sanofi, Lilly, Ono Pharmaceutical, Pfizer, Astellas Pharma, Eisai, Bayer, Taiho, MSD, Novartis, Chugai Pharma, AstraZeneca, and Bristol-Myers Squibb, and has received speakers bureau honoraria from Daiichi Sankyo, Sanofi, Ono Pharmaceutical, Nihonkayaku, Eisai, Bayer, Taiho Pharmaceutical, MSD, Novartis, Chugai Pharma, AstraZeneca, and Bristol-Myers Squibb. H. Park reports receiving a commercial research grant from Daiichi Sankyo. K. Saito is a manager at Daiichi Sankyo. M. Sugihara is a manager at Daiichi-Sankyo Co., Ltd. T. Jikoh is a global deputy team leader for DS-8201a for Daiichi Sankyo, Inc. G. Gallant is senior vice president, global head, oncology development, at Daiichi Sankyo. B.T. Li is a consultant/advisor at Biosceptre International, Guardant Health, Hengrui Therapeutics, Mersana, Thermo Fisher Scientific, and Roche/Genentech, reports receiving commercial research grants from AstraZeneca, BioMed Valley Discoveries, MORE Health, Daiichi Sankyo, GRAIL, Guardant Health, Hengrui Therapeutics, Illumina, Amgen, Lilly, and Roche/Genentech, and has ownership interest in institutional patents at Memorial Sloan Kettering Cancer Center (US62/685,05, US62/514,661). No potential conflicts of interest were disclosed by the other authors.
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
REFERENCES
- 1.Krop IE, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689–99. [DOI] [PubMed] [Google Scholar]
- 2.Larionov AA. Current therapies for human epidermal growth factor receptor 2-positive metastatic breast cancer patients. Front Oncol 2018;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practical Guidelines in Oncology. Breast cancer, version 2.2019. 2019.
- 6.National Comprehensive Cancer Network. NCCN Clinical Practical Guidelines in Oncology. Gastric cancer, version 2.2019. 2019.
- 7.Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997–2003. [DOI] [PubMed] [Google Scholar]
- 8.Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol 2018; 29:1108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinmoller P, Gross C, Beyser K, Schmidtgen C, Maass G, Pedrocchi M, et al. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res 2003;9:5238–43. [PubMed] [Google Scholar]
- 10.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005;65:1642–6. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Shao X, Gao W, Bai J, Wang R, Huang P, et al. The role of human epidermal growth factor receptor 2 as a prognostic factor in lung cancer: a meta-analysis of published data. J Thorac Oncol 2010; 5:1922–32. [DOI] [PubMed] [Google Scholar]
- 12.Pillai RN, Behera M, Berry LD, Rossi MR, Kris MG, Johnson BE, et al. HER2 mutations in lung adenocarcinomas: a report from the Lung Cancer Mutation Consortium. Cancer 2017;123:4099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakada T, Masuda T, Naito H, Yoshida M, Ashida S, Morita K, et al. Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg Med Chem Lett 2016;26:1542–5. [DOI] [PubMed] [Google Scholar]
- 14.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280–90. [DOI] [PubMed] [Google Scholar]
- 15.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016;107: 1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grob TJ, Kannengiesser I, Tsourlakis MC, Atanackovic D, Koenig AM, Vashist YK, et al. Heterogeneity of ERBB2 amplification in adenocarcinoma, squamous cell carcinoma and large cell undifferentiated carcinoma of the lung. Mod Pathol 2012;25:1566–73. [DOI] [PubMed] [Google Scholar]
- 17.Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, et al. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol 2010;41:1577–85. [DOI] [PubMed] [Google Scholar]
- 18.Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 2017;18: 1512–22. [DOI] [PubMed] [Google Scholar]
- 19.Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol 2019;20:827–36. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol 2019;20: 816–26. [DOI] [PubMed] [Google Scholar]
- 21.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roed Skarderud M, Polk A, Kjeldgaard Vistisen K, Larsen FO, Nielsen DL. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a systematic review. Cancer Treat Rev 2018;62:61–73. [DOI] [PubMed] [Google Scholar]
- 23.Peeters M, Cervantes A, Moreno Vera S, Taieb J. Trifluridine/tipiracil: an emerging strategy for the management of gastrointestinal cancers. Future Oncol 2018;14:1629–45. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez C, Orlova M, Verzura MA, Minatta JN, Scibona P, Jauregui EG, et al. Severe toxicity in adult patients with lung cancer under treatment with pemetrexed: a prospective cohort study. J Chemother 2019;31:95–104. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Zhang H, Zhou C, Long X, Guan R, Yang N, et al. Real-world outcomes of various regimens of recombinant human endostatin combined with chemotherapy in non-driver gene mutation advanced non-small cell lung cancer. Cancer Med 2019;8:1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lonn U, Lonn S, Friberg S, Nilsson B, Silfversward C, Stenkvist B. Prognostic value of amplification of c-erb-B2 in bladder carcinoma. Clin Cancer Res 1995;1:1189–94. [PubMed] [Google Scholar]
- 27.Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol 2006;24:2376–85. [DOI] [PubMed] [Google Scholar]
- 28.Takenaka M, Hanagiri T, Shinohara S, Kuwata T, Chikaishi Y, Oka S, et al. The prognostic significance of HER2 overexpression in non-small cell lung cancer. Anticancer Res 2011;31:4631–6. [PubMed] [Google Scholar]
- 29.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol 2018;36:2044–51. [DOI] [PubMed] [Google Scholar]
- 30.Hussain MH, MacVicar GR, Petrylak DP, Dunn RL, Vaishampayan U, Lara PN Jr, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218–24. [DOI] [PubMed] [Google Scholar]
- 31.Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004;431:525–6. [DOI] [PubMed] [Google Scholar]
- 32.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:504–35. [DOI] [PubMed] [Google Scholar]
- 33.Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A 2012;109:14476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006;10:25–38. [DOI] [PubMed] [Google Scholar]
- 35.De Greve J, Teugels E, Geers C, Decoster L, Galdermans D, De Mey J, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 2012;76:123–7. [DOI] [PubMed] [Google Scholar]
- 36.Gatzemeier U, Groth G, Butts C, Van Zandwijk N, Shepherd F, Ardizzoni A, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004;15:19–27. [DOI] [PubMed] [Google Scholar]
- 37.Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018;554:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O’Connell J, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 2015;26:1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai WV, Lebas L, Barnes TA, Milia J, Ni A, Gautschi O, et al. Afatinib in patients with metastatic or recurrent HER2-mutant lung cancers: a retrospective international multicentre study. Eur J Cancer 2019; 109:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chuang JC, Stehr H, Liang Y, Das M, Huang J, Diehn M, et al. ERBB2-mutated metastatic non-small cell lung cancer: response and resistance to targeted therapies. J Thorac Oncol 2017;12:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robichaux JP, Elamin YY, Vijayan RSK, Nilsson MB, Hu L, He J, et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 2019;36:444–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Jiang T, Qin Z, Jiang J, Wang Q, Yang S, et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 2019;30:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019;20:1124–35. [DOI] [PubMed] [Google Scholar]
- 45.Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Adotrastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol 2018;36:2532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2019;20:518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:738–46. [DOI] [PubMed] [Google Scholar]
- 48.Pahuja KB, Nguyen TT, Jaiswal BS, Prabhash K, Thaker TM, Senger K, et al. Actionable activating oncogenic ERBB2/HER2 transmembrane and juxtamembrane domain mutations. Cancer Cell 2018;34:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serra V, Vivancos A, Puente XS, Felip E, Silberschmidt D, Caratu G, et al. Clinical response to a lapatinib-based therapy for a Li-Fraumeni syndrome patient with a novel HER2V659E mutation. Cancer Discov 2013;3:1238–44. [DOI] [PubMed] [Google Scholar]
- 50.Heymach J, Negrao M, Robichaux J, Carter B, Patel A, Altan M, et al. OA02.06 a phase II trial of poziotinib in EGFR and HER2 exon 20 mutant non-small cell lung cancer (NSCLC). J Thorac Oncol 2018;13:S323–S4. [Google Scholar]
- 51.Hotta K, Aoe K, Kozuki T, Ohashi K, Ninomiya K, Ichihara E, et al. A phase II study of trastuzumab emtansine in HER2-positive non-small cell lung cancer. J Thorac Oncol 2018;13:273–9. [DOI] [PubMed] [Google Scholar]
- 52.Peters S, Stahel R, Bubendorf L, Bonomi P, Villegas A, Kowalski DM, et al. Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non-small cell lung cancer: efficacy, safety, and biomarkers. Clin Cancer Res 2019;25:64–72. [DOI] [PubMed] [Google Scholar]
- 53.Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med 2018;7:pii: E356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell C, Camidge D, Gemma A, Kusumoto M, Baba T, Kuwano K, et al. Characterization, monitoring and management of interstitial lung disease in patients with metastatic breast cancer: Analysis of data available from multiple studies of DS-8201a, a HER2-targeted antibody drug conjugate with a topoisomerase I inhibitor payload. [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4–8; San Antonio, TX. Philadelphia (PA): AACR; 2019;79(4 Suppl):Abstract nr P6-17-06. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


