Abstract
Globo H is a tumor-associated carbohydrate antigen (TACA), which serves as a valuable target for antitumor vaccine or cancer immunotherapies. However, most TACAs are T-cell-independent, and they cannot induce powerful immune response due to their poor immunogenicity. To address this problem, herein, several Globo H analogues with modification on the N-acyl group were prepared through a preactivation-based glycosylation strategy from the non-reducing end to the reducing end. These modified Globo H derivatives were then conjugated with carrier protein CRM197 to form glycoconjugates as anticancer vaccine candidates, which were used in combination with adjuvant glycolipid C34 for immunological studies. The immunological effects of these synthetic vaccine candidates were evaluated on Balb/c mice. The results showed that the fluorine-modified N-acyl Globo H conjugates can induce higher titers of IgG antibodies that can recognize the naturally occurring Globo H antigen on the surface of cancer cells and can eliminate cancer cells in the presence of a complement, indicating the potential of these synthetic glycoconjugates as anticancer vaccine candidates.
Fluorine-modified N-acyl Globo H conjugates induce higher titers of IgG antibodies that can recognize the native Globo H antigen on the surface of cancer cells and eliminate cancer cells, holding potential as anticancer vaccines.
Introduction
Cancer cells can often express some unique carbohydrate sequences on their surfaces because of the aberrant glycosyltransferase activity within these cells. These unusual glycans are known as tumor-associated carbohydrate antigens (TACAs).1,2 TACAs are important targets for the development of antitumor vaccines or cancer immunotherapies. Unfortunately, most TACAs are T-cell-independent antigens, and they are also expressed by normal tissues at a low level, and this leads to their poor immunogenicity.3 Strategies to enhance the immunogenicity of TACAs include conjugating TACAs with a suitable immunogenic carrier such as proteins,4,5 modification of TACAs through metabolic oligosaccharide engineering (MOE) which focuses on the modification of sialic acid,6,7 and introducing unnatural TACA analogues such as modification of carbohydrate antigen structures.8–10
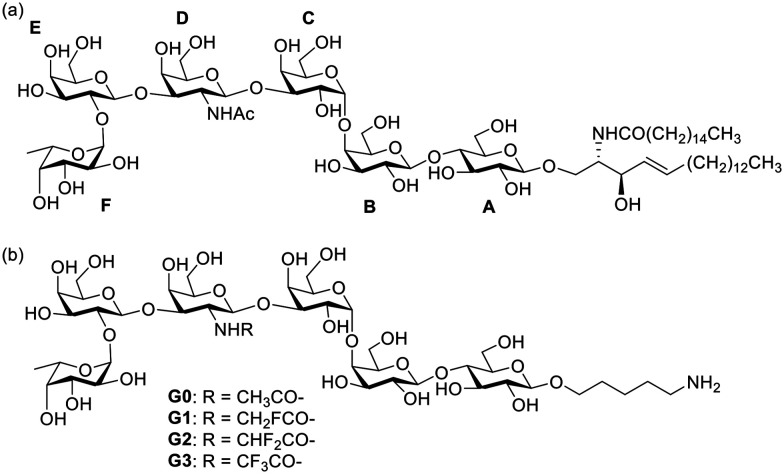
Globo H is a member of Globo series TACAs. It was first separated and characterized from human breast cancer cell line MCF-7 in 1983,11,12 and was later found to be overexpressed in many kinds of cancer cells in the form of glycosphingolipid.13Fig. 1a shows the natural Globo H antigen with the structure of α-l-Fuc-(1,2)-β-d-Gal (1,3)-β-d-GalNAc-(1,3)-α-d-Gal-(1,4)-β-d-Glc-(1,4)-β-Cer, where the fucose at the non-reducing end and the β-linkage at the CD junction play important roles in the recognition with its antibody.12,14 Globo H antigen has been conjugated with a protein carrier,15–17 monophosphoryl lipid A (MPLA),18 or polysaccharide A1 (PS A1)19 for the development of anticancer vaccines. Even though the Globo H-KLH vaccine has been approved for clinical trials,20,21 there are still limitations in their clinical application since this vaccine usually elicited higher titers of IgM antibody than those of IgG antibodies, implying that the T-cell-mediated immune response is weak, which is a major challenge in carbohydrate-based vaccine design.22
Fig. 1. (a) Natural Globo H antigen; (b) modified Globo H derivatives in this work.
Because of the similar atom radius and lipophilicity of fluorine compared with those of a hydrogen atom, and the absence of fluorinated compounds in most organisms, fluorinated modifications of TACAs have been applied as a way to improve the immunogenicity in the development of anticancer vaccines.23,24 More importantly, fluorination of antigens has been shown to be a strategy to enhance the enzymatic stability of synthetic vaccines.24 Previously, our group conducted a series study of N-acyl modified STn antigens and discovered that fluorinated modifications on the STn can enhance the anti-STn IgG titers.25 Based on this previous work, we want to design and synthesize N-acyl modified Globo H derivatives (Fig. 1b) with the aim to improve its immune efficacy. Herein, we describe the design, synthesis, and immunological evaluation of these Globo H analogue glycoconjugates.
Results and discussion
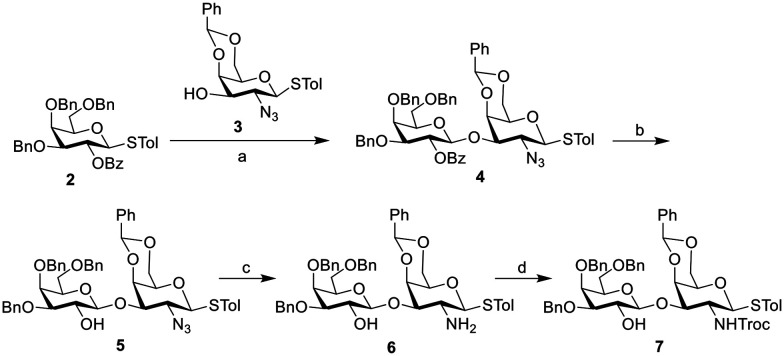
Because of the biological importance of the Globo H antigen in the development of anticancer vaccines, the preparation of the Globo H carbohydrate antigen has attracted considerable attention. Different strategies including chemical synthesis,26–31 and chemoenzymatic or enzymatic method17,32,33 have been developed for the construction of Globo H hexasaccharides. The strategy we used in this work mainly refers to the method reported by Huang's group31 with some modifications, where the Globo H hexasaccharide was retro-synthetically divided into four components: AB, C, DE, and F. The synthesis was carried out from the non-reducing end F to the reducing end AB through a preactivation-based strategy.34 Monosaccharide building blocks 1, 2, 3 and 9 were prepared according to the literature.31 The synthesis of DE disaccharide building block 7 is shown in Scheme 1. Glycosyl donor 2 was preactivated with Ph2SO/Tf2O followed by the addition of glycosyl acceptor 3 to afford disaccharide 4, which was subsequently converted to disaccharide building block 7 following a reported procedure.31
Scheme 1. Synthesis of building block 7. Reagents and conditions: a) Ph2SO, Tf2O, TTBP, DCM, preactivation of compound 2 at −72 °C for 15 min, then addition of compound 3, 85%; b) NaOMe, DCM/MeOH, 82%; c) 1,3-propanedithiol, Et3N, DCM/MeOH, 86%; d) TrocCl, NaHCO3, THF, 64%.
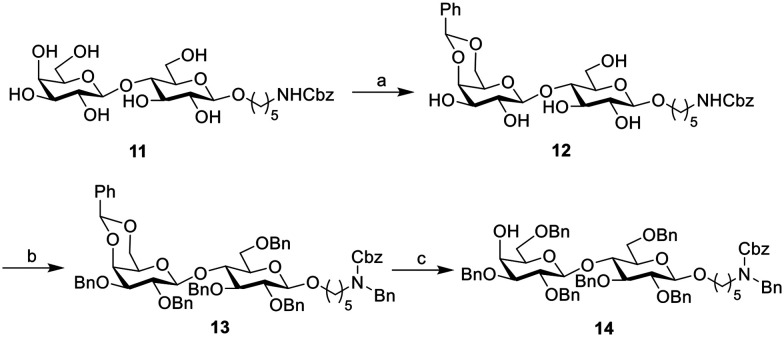
For the synthesis of lactose building block 14 (AB), compound 11 (ref. 35) bearing a five-carbon amino linker was reacted with benzaldehyde dimethyl acetal to protect the 4,6-hydroxyls on the galactose moiety, followed by the global benzyl protection to get compound 13, which was further converted to building block 14 as the last component in the assembly of the Globo H hexasaccharide (Scheme 2).
Scheme 2. Synthesis of lactose building block 14. Reagents and conditions: a) PhCH(OMe)2, CSA, DMF, 85%; b) BnBr, NaH, TBAI, DMF, 0 °C to r.t., 73%; c) NaBH3CN, HCl, THF, 0 °C to r.t, 82%.
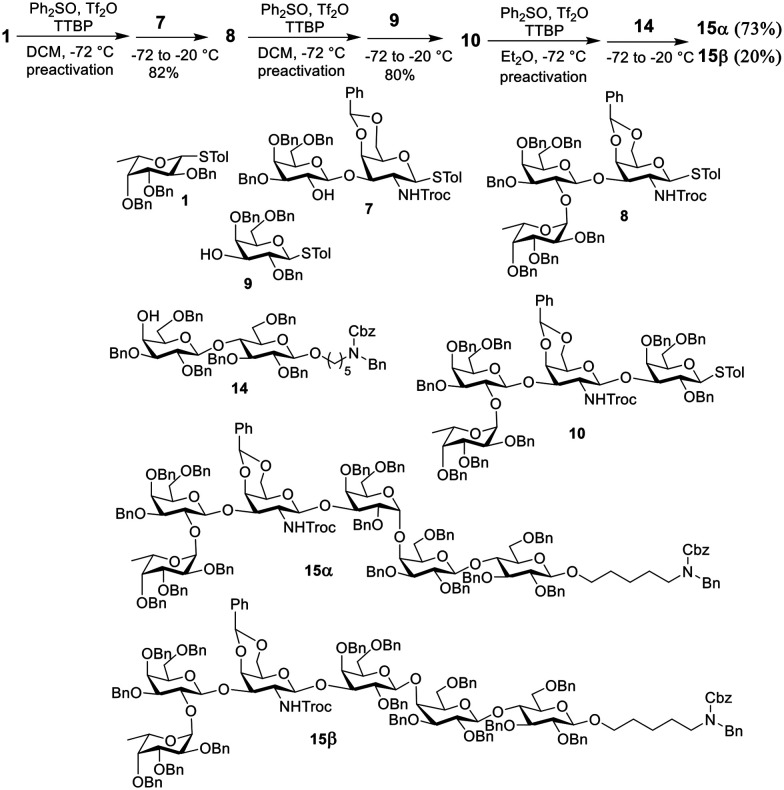
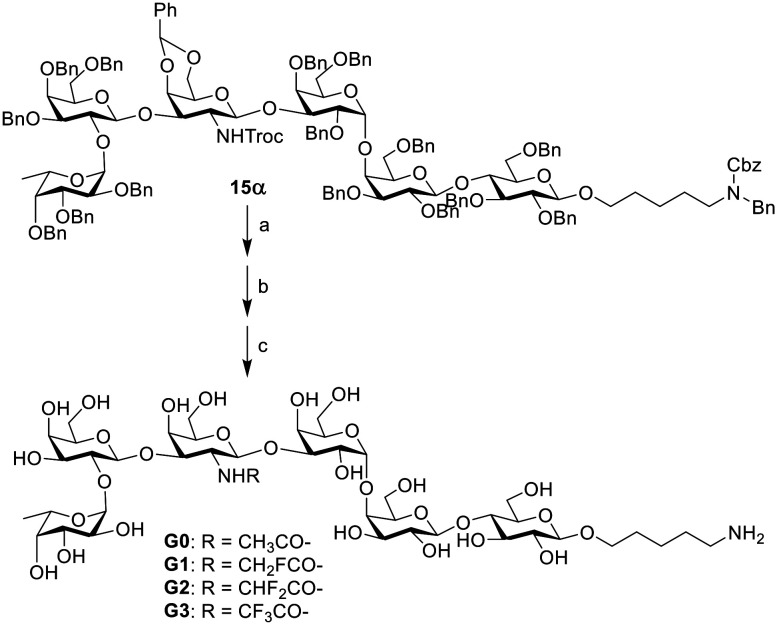
The preparation of the globally protected Globo H hexasaccharide is shown in Scheme 3. Starting from the non-reducing end, first, the preactivation of fucose building block 1 at −72 °C with Ph2SO/Tf2O in dichloromethane (DCM), which was followed by the addition of disaccharide acceptor 7, generated trisaccharide 8 in 82% isolated yield. The construction of tetrasaccharide 10 from trisaccharide 8 and galactose building block 9 was conducted in the same way by preactivation of 8 followed by the addition of 9. The last step was the α-glycosylation of compound 10 and lactose building block 14. The reaction was carried out, providing a coupled product, but the ratio of the desired product/byproduct (15α/15β) was only 1.2 when DCM was used as the solvent. To improve the yield of the α linkage product, when the solvent was switched to ether, the ratio of 15α/15β was increased to 3.7.
Scheme 3. Synthesis of protected Globo H saccharide.
To synthesize the N-acyl modified derivatives, the trichloroethoxycarbonyl (Troc) group was deprotected to get the free amino functionality, which was further reacted with the corresponding acetic anhydride or fluorinated acetyl anhydride (fluorinated acetyl chloride for the monofluorinated derivative). Finally, global deprotection was conducted under catalytic hydrogenolysis conditions (H2/Pd(OH)2) to generate the target molecules G0–G3 (Scheme 4), which were purified by C18 reversed-phase column chromatography (H2O → H2O/MeOH, 8/1, v/v) to give the corresponding deprotected product. The final products were characterized by NMR (1H, 13C, HMBC) spectroscopy and HRMS analyses (see the ESI†). The 1H NMR data of the native compound G0 coincide with those reported in the literature.17 These globally deprotected products were further used to conjugate with protein to prepare glycoconjugates as vaccine candidates.
Scheme 4. Synthesis of Globo H derivatives. Reagents and conditions: a) 1 M NaOH, THF, 50 °C, overnight; b) anhydride of corresponding carboxylic acid, pyridine, DMAP; for compound G1, CH2FCOCl, pyridine, DMAP; c) H2, Pd(OH)2/C, THF/HOAc/H2O, 64% for compound G0, 47% for compound G1, 61% for compound G2, 66% for compound G3, over three steps.
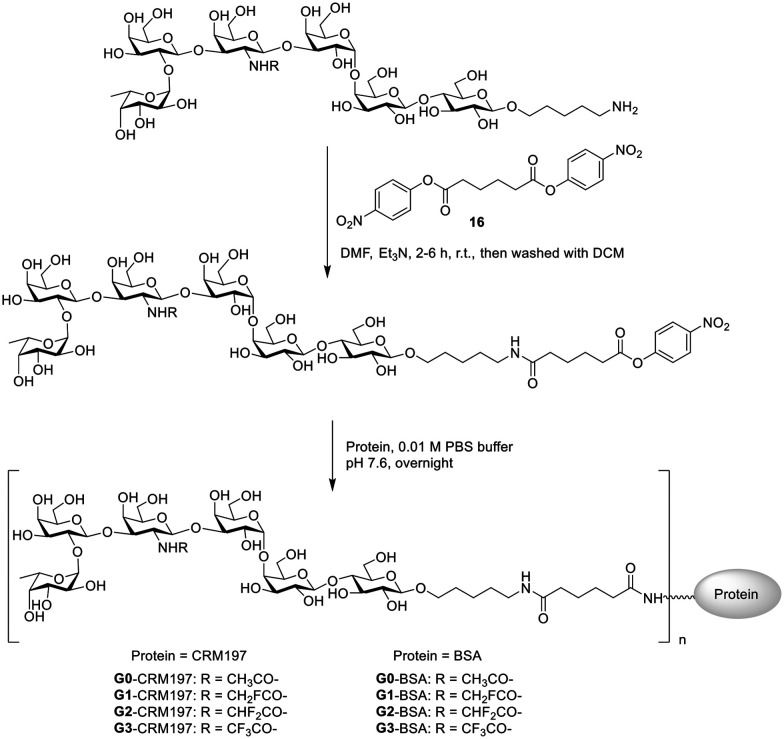
Carrier protein diphtheria toxoid cross-reactive material (CRM) 197 is a US FDA approved vaccine carrier protein. Compared with the Globo H-KLH+QS21 vaccine used in clinical phase III, the Globo H-CRM197+C34 vaccine has been shown to induce higher titers of IgG antibodies, which has been approved for clinical studies.16,36 Thus, we chose CRM197 as a carrier protein and glycolipid C34 as an adjuvant. With Globo H and its N-acyl modified derivatives in hand, the conjugation with protein was performed to prepare glycoconjugates. The glycoconjugates were prepared according to a reported procedure.37 As shown in Scheme 5, the Globo H analogues (G0–G3) were treated with an excess amount of linker 16 (ref. 38) to generate the corresponding ester intermediates. The reactions were monitored by TLC. These ester intermediates were subsequently incubated with proteins (CRM197 or BSA) in PBS buffer (pH 7.6). The resulting product was then purified through dialysis in PBS buffer to afford the corresponding glycoconjugate. The carbohydrate loading percentage was determined using the MALDI-TOF MS spectra (Table S1, ESI†) and the protein content was determined by the BCA assay.39
Scheme 5. Synthesis of Globo H-derived glycoconjugates.
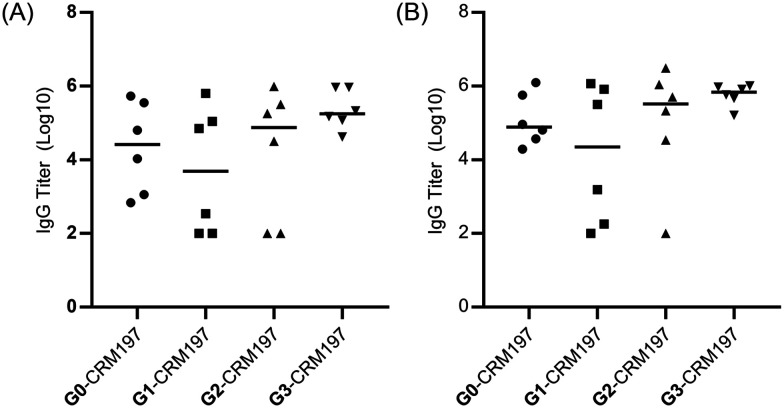
The immunogenicity of these Globo H-derived glycoconjugates was evaluated on Balb/c mice. Groups of six female mice were vaccinated intramuscularly four times at biweekly intervals with Globo H analogue-CRM197 (2 μg of Globo H analogue) and glycolipid adjuvant C34 (2 μg). The capabilities of the induced antibodies to recognize the native Globo H antigen and the modified Globo H antigens were examined by measuring the antibody titers of the pooled sera of each group or the serum antibody of each mouse using plates coated with the unmodified G0-BSA or the modified carbohydrate antigen-BSA glycoconjugates, respectively. The IgG antibody titers against the native Globo H antigen after the 3rd and 4th vaccinations are shown in Fig. 2. Compared with the antibody level of the unmodified glycoconjugate (G0-CRM197), the modified glycoconjugates showed enhanced antibody titers; in particular for the glycoconjugates G2-CRM197 and G3-CRM197, clear enhancement of antibody titers was observed. Also, the modified Globo H antigen-CRM197 conjugates elicited enhanced antibody titers against their corresponding modified Globo H antigen compared with that of the unmodified glycoconjugate (G0-CRM197) (Fig. S1, ESI†). Furthermore, the subclasses of IgG antibodies were identified, in which the reactivity of pooled sera IgG subclasses with G0-BSA was tested by ELISA. The results indicated that the IgG1 antibody was the predominant subtype (Table S2, ESI†), implying that T cell response was induced. The generation of IgG1 antibodies is the evidence of a type 2 T-helper (Th2) response.40,41
Fig. 2. Serum antibody titers against G0-BSA of individual mouse vaccinated with G0-CRM197, G1-CRM197, G2-CRM197 and G3-CRM197 after the (A) 3rd and (B) 4th vaccinations. Each data point represents the average titers of two detections of an individual mouse, and the black line in each series represents the median serum titer of six mice in each group. The data of IgG titers were plotted in the form of log 10.
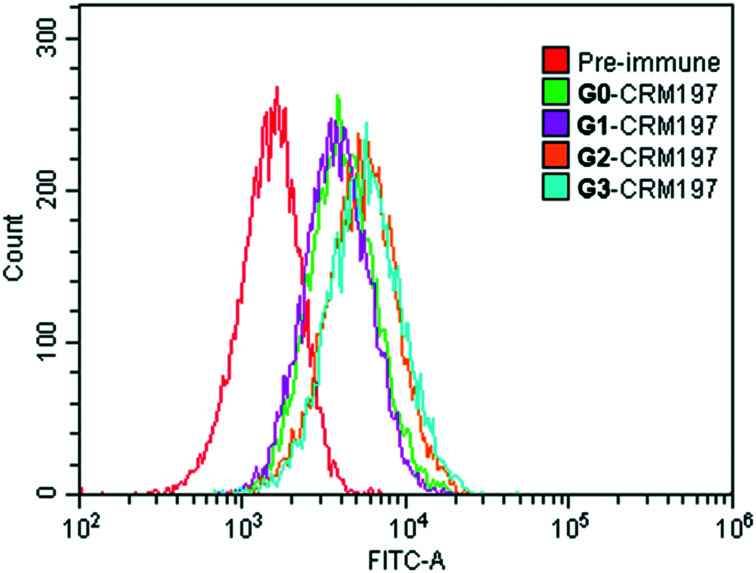
To investigate the capabilities of mouse sera induced by G0-CRM197, G1-CRM197, G2-CRM197 and G3-CRM197 to recognize the native Globo H antigen on cancer cells, the binding affinity of the immune serum antibodies for Globo H-positive MCF-7 human breast cancer cells was analyzed by flow cytometry. As shown in Fig. 3, the antisera elicited by the unmodified G0-CRM197 or the modified conjugates (G1-CRM197, G2-CRM197, G3-CRM197) can react well with the Globo H-positive MCF-7 cancer cells and the antisera induced by G2-CRM197 and G3-CRM197 bind better to the cells than those by G0-CRM197, which is consistent with the trend of the serum titer, indicating that the induced antibodies are specific to the Globo H antigen on the cell surface.
Fig. 3. Serological IgG analysis results of MCF-7 human breast cancer cells after the 4th vaccination by flow cytometry.
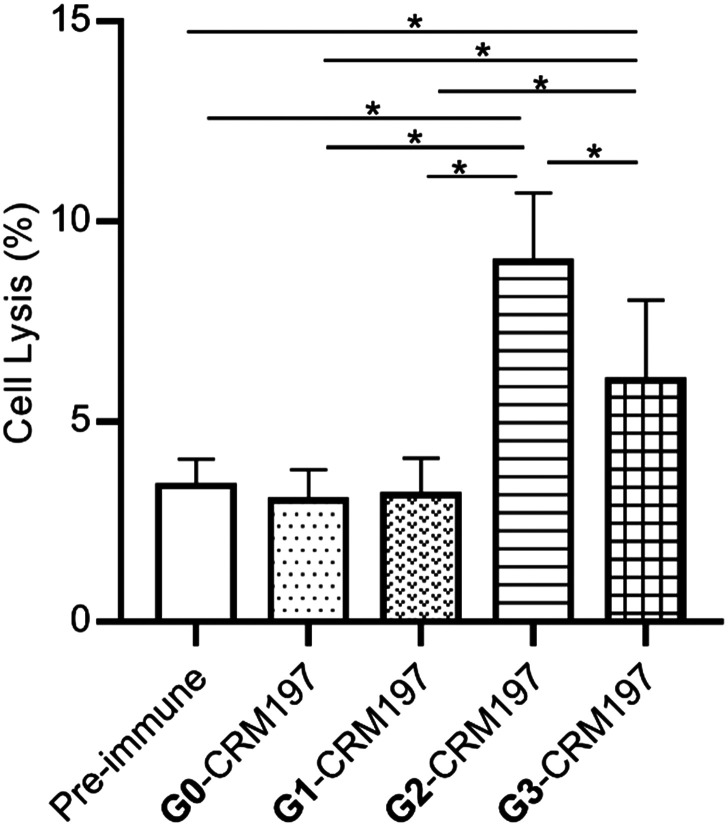
Complement-dependent cytotoxicity (CDC) was then investigated to determine whether the modified Globo H-CRM197-provoked immune response is useful for cancer immunotherapy. As shown in Fig. 4, the antisera obtained from vaccination with G2-CRM97 and G3-CRM197 can induce much stronger cancer cell cytotoxicity compared with those of the untreated mice (pre-immune mice), G0-CRM97 and G1-CRM197 immunized mice.
Fig. 4. Antibodies elicited by glycoconjugates mediate complement-dependent cytotoxicity (CDC) to eliminate Globo H-positive MCF-7 cancer cells. Cell lysis of pooled sera (1 : 10 dilution) after the 4th vaccination. Data are the mean ± SEM of three independent experiments. Cytotoxicity is shown with percent cell lysis determined by the lactate dehydrogenase (LDH) assay. Three parallel holes were arranged for each measurement. One-way ANOVA analysis was performed. Asterisks represent p < 0.05.
Overall, our results showed that fluorination of the Globo H antigen can enhance its immunogenicity. This may be attributed to the exogenetic properties of the fluorinated compounds and the increased enzymatic stability in vivo.23
Conclusions
In conclusion, different N-acyl modified Globo H derivatives and their carrier protein conjugates were designed and synthesized. The immunological properties of these glycoconjugates were evaluated using Balb/c mice. According to the results, among the three N-fluoroacetyl modified analogues, the difluorinated glycoconjugate G2-CRM197 and trifluorinated glycoconjugate G3-CRM197 induced obviously enhanced titers of IgG antibodies compared with the unmodified Globo H conjugate G0-CRM197, thus enhancing the immunogenicity. This study demonstrates that the N-acyl modification of Globo H holds potential for the development of anticancer vaccines.
Ethical statement
All experiments were carried out according to the International Association for the Study of Pain ethical guidelines (Zimmermann, 1983) and approved by the Institutional Animal Care and Use Committee of Peking University.
Author contributions
C. Z. conducted all the synthesis and compound characterization. X. Z. conducted all biological work. C. S. helped with some biological work. X. Y. designed and supervised the project.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21738001 and 81821004) and the National Key Research and Development Program of China (Grant No. 2018YFA0507602).
Electronic supplementary information (ESI) available: Compound characterization and supplementary data. See DOI: 10.1039/d1md00067e
Notes and references
- Hakomori S. I. Zhang Y. Chem. Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- A. B. T-cell Danishefsky S. J. Allen J. R. Angew. Chem., Int. Ed. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Mettu R. Chen C.-Y. Wu C.-Y. J. Biomed. Sci. 2020;27:9. doi: 10.1186/s12929-019-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir J. A. Abdel-Motal U. M. Jondal M. Wilson I. A. Immunity. 1999;10:51–61. doi: 10.1016/s1074-7613(00)80006-0. [DOI] [PubMed] [Google Scholar]
- Brocke C. Kunz H. Bioorg. Med. Chem. 2002;10:3085–3112. doi: 10.1016/s0968-0896(02)00135-9. [DOI] [PubMed] [Google Scholar]
- Pan Y. Chefalo P. Nagy N. Harding C. Guo Z. J. Med. Chem. 2005;48:875–883. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Guo Z. Bioconjugate Chem. 2006;17:1537–1544. doi: 10.1021/bc060103s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z. Huang X. J. Carbohydr. Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J. Roy R. Gamian A. J. Immunol. 1986;137:1708–1713. [PubMed] [Google Scholar]
- Liu C. C. Ye X. S. Glycoconjugate J. 2012;29:259–271. doi: 10.1007/s10719-012-9399-9. [DOI] [PubMed] [Google Scholar]
- Kannagis R. Leverys S. B. Ishigamis F. Hakomorisj S. Lynne H. Knowlesll B. B. Solterb D. J. Biol. Chem. 1983:8934–8942. [Google Scholar]
- Bremer E. G. Levery S. B. Sonnino S. Ghidoni R. Canevari S. Kannagi R. Hakomori S. J. Biol. Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- Livingston P. O. Semin. Cancer Biol. 1995;6:357–366. doi: 10.1016/1044-579x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Kim I. J. Park T. K. Hu S. Abrampah K. Zhang S. Livingston P. O. Danishefsky S. J. J. Org. Chem. 1995;60:7716–7717. [Google Scholar]
- Ragupathi G. Park T. K. Zhang S. Kim I. J. Graber L. Adluri S. Lloyd K. O. Danishefsky S. J. Livingston P. O. Angew. Chem., Int. Ed. Engl. 1997;36:125–128. [Google Scholar]
- Huang Y. L. Hung J. T. Cheung S. K. C. Lee H. Y. Chu K. C. Li S. T. Lin Y. C. Ren C. T. Cheng T. J. R. Hsu T. L. Yu A. L. Wu C. Y. Wong C. H. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2517–2522. doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. Y. Chen C. Y. Tsai T. I. Li S. T. Lin K. H. Cheng Y. Y. Ren C. T. Cheng T. J. R. Wu C. Y. Wong C. H. J. Am. Chem. Soc. 2014;136:16844–16853. doi: 10.1021/ja508040d. [DOI] [PubMed] [Google Scholar]
- Zhou Z. Liao G. Mandal S. S. Suryawanshi S. Guo Z. Chem. Sci. 2015;6:7112–7121. doi: 10.1039/c5sc01402f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. Trabbic K. R. Shi M. Nishat S. Eradi P. Kleski K. A. Andreana P. R. Chem. Sci. 2020;11:13052–13059. doi: 10.1039/d0sc04595k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilewski T. Ragupathi G. Bhuta S. Williams L. J. Musselli C. Zhang X. F. Bencsath K. P. Panageas K. S. Chin J. Hudis C. A. Norton L. Houghton A. N. Livingston P. O. Danishefsky S. J. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-S. Yu A. L. Tseng L.-M. Chow L. W. C. Hou M.-F. Hurvitz S. A. Schwab R. B. Murray J. L. Chang H.-K. Chang H.-T. Chen S.-C. Kim S.-B. Hung J.-T. Ueng S.-H. Lee S.-H. Chen C.-C. Rugo H. S. J. Immunother. Cancer. 2020;8:e000342. doi: 10.1136/jitc-2019-000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun F. Toth I. Stephenson R. J. Adv. Drug Delivery Rev. 2020;165–166:117–126. doi: 10.1016/j.addr.2020.04.006. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Röder A. Kaiser A. Wagner S. Gaidzik N. Kowalczyk D. Westerlind U. Gerlitzki B. Schmitt E. Kunz H. Angew. Chem., Int. Ed. 2010;49:8498–8503. doi: 10.1002/anie.201003810. [DOI] [PubMed] [Google Scholar]
- Jiménez-Barbero J. Linclau B. Ardá A. Reichardt N. C. Sollogoub M. Unione L. Vincent S. P. Chem. Soc. Rev. 2020;49:3863–3888. doi: 10.1039/c9cs00099b. [DOI] [PubMed] [Google Scholar]
- Yang F. Zheng X. J. Huo C. X. Wang Y. Zhang Y. Ye X. S. ACS Chem. Biol. 2011;6:252–259. doi: 10.1021/cb100287q. [DOI] [PubMed] [Google Scholar]
- Bilodeau M. T. Park T. K. Hu S. Randolph J. T. Danishefsky S. J. Livingston P. O. Zhang S. Danishefsky S. J. J. Am. Chem. Soc. 1995;117:7840–7841. [Google Scholar]
- Park T. K. Kim I. J. Hu S. Bilodeau M. T. Randolph J. T. Kwon O. Danishefsky S. J. J. Am. Chem. Soc. 1996;118:11488–11500. [Google Scholar]
- Lassaletta J. M. Schmidt R. R. Liebigs Ann. 1996;1996:1417–1423. [Google Scholar]
- Burkhart F. Zhang Z. Wacowich-Sgarbi S. Wong C. H. Angew. Chem., Int. Ed. 2001;40:1274–1277. doi: 10.1002/1521-3773(20010401)40:7<1274::aid-anie1274>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Bosse F. Marcaurelle L. A. Seeberger P. H. J. Org. Chem. 2002;67:6659–6670. doi: 10.1021/jo025834+. [DOI] [PubMed] [Google Scholar]
- Wang Z. Zhou L. El-Boubbou K. Ye X. S. Huang X. J. Org. Chem. 2007;72:6409–6420. doi: 10.1021/jo070585g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D. M. Eguchi H. Yi W. Li L. Wang P. G. Xia C. Org. Lett. 2008;10:1009–1012. doi: 10.1021/ol703121h. [DOI] [PubMed] [Google Scholar]
- Wang Z. Gilbert M. Eguchi H. Yu H. Cheng J. Muthana S. Zhou L. Wang P. G. Chen X. Huang X. Adv. Synth. Catal. 2008;350:1717–1728. doi: 10.1002/adsc.200800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X. Ye X. S. Chin. J. Chem. 2021;39:531–542. [Google Scholar]
- Biswas G. Jeon O. Y. Lee W. S. Kim D. C. Kim K. T. Lee S. Chang S. Chung S. K. Chem. – Eur. J. 2008;14:9161–9168. doi: 10.1002/chem.200801160. [DOI] [PubMed] [Google Scholar]
- Danishefsky S. J. Shue Y. K. Chang M. N. Wong C. H. Acc. Chem. Res. 2015;48:643–652. doi: 10.1021/ar5004187. [DOI] [PubMed] [Google Scholar]
- Huo C. X. Zheng X. J. Xiao A. Liu C. C. Sun S. Lv Z. Ye X. S. Org. Biomol. Chem. 2015;13:3677–3690. doi: 10.1039/c4ob02424a. [DOI] [PubMed] [Google Scholar]
- Guo K. Chu C. C. Chkhaidze E. Katsarava R. J. Polym. Sci., Part A: Polym. Chem. 2005;43:1463–1477. [Google Scholar]
- Smith P. K. Krohn R. I. Hermanson G. T. Mallia A. K. Gartner F. H. Provenzano M. D. Fujimoto E. K. Goeke N. M. Olson B. J. Klenk D. C. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D. Holmes J. Paul W. E. Annu. Rev. Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Skwarczynski M. Zaman M. Urbani C. N. Lin I. C. Jia Z. Batzloff M. R. Good M. F. Monteiro M. J. Toth I. Angew. Chem., Int. Ed. 2010;49:5742–5745. doi: 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.