Abstract
As immunotherapies continue to emerge as a standard component of treatment for a variety of cancers, the imperative for testing these in combination with other standard cancer therapies grows. Radiation therapy may be a particularly well-suited partner for many immunotherapies. By modulating immune tolerance and functional immunogenicity at a targeted tumor site, radiation therapy may serve as a method of in situ tumor vaccination. In situ tumor vaccination is a therapeutic strategy that seeks to convert a patient’s own tumor into a nidus for enhanced presentation of tumor-specific antigens in a way that will stimulate and diversify an antitumor T cell response. The mechanisms whereby radiation may impact immunotherapy are diverse and include its capacity to simultaneously elicit local inflammation, temporary local depletion of suppressive lymphocyte lineages, enhanced tumor cell susceptibility to immune response, and immunogenic tumor cell death. Emerging data suggest that each of these mechanisms may display a distinct dose—response profile, making it challenging to maximize each of these effects using external beam radiation. Conversely, the highly heterogenous and conformal dose distribution achieved with brachytherapy may be optimal for enhancing the immunogenic capacity of radiation at a tumor site while minimizing off-target antagonistic effects on peripheral immune cells. Here, we review the immunogenic effects of radiation, summarize the clinical rationale and data supporting the use of radiation together with immunotherapies, and discuss the rationale and urgent need for further preclinical and clinical investigation specifically of brachytherapy in combination with immunotherapies. Harnessing these immunomodulatory effects of brachytherapy may offer solutions to overcome obstacles to the efficacy of immunotherapies in immunologically “cold” tumors while potentiating greater response in the context of immunologically “hot” tumors. © 2018 American Brachytherapy Society. Published by Elsevier Inc. All rights reserved.
Keywords: Immunotherapy, Brachytherapy, Checkpoint blockade, Radiation, In situ vaccine
Introduction
One of the hallmarks of tumorigenesis is the ability of cancer cells to suppress or circumvent the host immune system (1, 2). The mechanisms by which cancer cells evade both innate and adaptive immunity are complex and result, at least in part, from the selective effects of immunoediting (3). Advances in strategies to overcome tumor cell evasion of immune detection have led to a rapid pace of preclinical and clinical development in the field of cancer immunotherapy. Current immunotherapy treatments have shown clinical benefit in multiple malignancies including melanoma (4, 5), head and neck cancer (6), urothelial cancer (7), and lung cancer (8, 9). Nevertheless, durable tumor response remains elusive for the vast majority of patients with metastatic cancers, and immunotherapies are not typically effective in patients with immunologically “cold” tumors characterized by low levels of T cell infiltrate and/or few mutation-created neoantigens.
One potential approach to improving the response to cancer immunotherapies is to combine such treatments with radiotherapy. The mechanisms by which radiation may interact with the tumor immune microenvironment at a targeted site include (1) temporary local eradication of radiation-sensitive immune lineages including suppressor and effector lymphocytes, (2) local release of inflammatory cytokines and damage-associated molecular patterns resulting in local effects on endothelial cell expression of adhesion receptors, immune cell trafficking, and immune cell activation, (3) immunogenic tumor cell death and release of tumor-specific antigens, and (4) induction of phenotypic changes in the expression of immune susceptibility markers on tumor cells surviving radiation (10). Because of these effects, radiation may enhance dendritic cell maturation, antigen cross-presentation, and diversification of antitumor T cell responses (11, 12). As a result, radiation may elicit an “in situ tumor vaccination” effect—converting a patient’s own tumor into a nidus for presentation of tumor-specific antigens in a way that will stimulate and diversify an antitumor immune response (13).
Because most therapeutic radiation is delivered as external beam radiation therapy (EBRT), most approaches to combining radiation and immunotherapy have been studied using EBRT. Preclinical studies indicate that the immunogenic effects of radiation are sensitive to the timing, dose, field size, and fractionation of radiotherapy (14-16). Owing to its unmatched conformality and intratumoral dose heterogeneity, brachytherapy may confer meaningful advantages over EBRT when it comes to priming an in situ vaccine effect. Herein, we review current utilization of different types of cancer immunotherapies combined with radiation therapy, the potential advantages of a brachytherapy—immunotherapy tandem, and current preclinical and clinical studies evaluating this therapeutic combination.
Historical identification of the immunogenic effects of radiation
In the 1950s, Mole et al. reported the capacity of radiation therapy to elicit a response at a distant nonradiated site. The authors described this as an abscopal effect, occurring “at a distance from the irradiated volume but within the same organism” (17). It was not until later that Ehlers et al. and Kingsley et al. used the term abscopal to more specifically describe spontaneous regression of nonradiated metastatic disease sites in case reports of patients with metastatic papillary adenoma (18) and metastatic melanoma (19). Several additional case reports have confirmed an abscopal anti-tumor response to radiation; however, it is clear that this effect is quite rare with radiation alone (20). Importantly, preclinical studies have demonstrated that the abscopal response to radiation is immune mediated (21).
Early preclinical studies eloquently demonstrated a cooperative interaction between radiation therapy and the host immune system with respect to tumor control. In 1979, Helen Stone, Lester Peters, and Luka Milas reported the radiation dose required for local tumor control in 50% of mice (TCD50) for a murine fibrosarcoma tumor in mice with one of three immunologic states: (1) immunocompetent, (2) immune compromised by whole body radiation ± surgical thymectomy, or (3) exposed to Corynebacterium parvum to stimulate the reticuloendothelial system (2). These investigators determined that immunosuppressed mice required a much higher TCD50 compared with immunocompetent mice (22). Immunocompromised mice also had higher incidence of lung metastases compared with immunocompetent in mice without local tumor recurrence. Conversely, they found that stimulation of the immune system with C. parvum reduced the TCD50. Demonstrating early insight into the concept of immunologically “hot” vs. “cold” tumors, they showed that this effect was less pronounced when a “weakly immunogenic tumor” was subject to similar analyses. Since the publication of this work, several groups have investigated the immune cell populations and molecular events that contribute to the interaction between radiation therapy response and host immunity. With the emergence of molecularly targeted immunotherapies, much of this focus has now shifted to evaluating the potential for cooperative interaction between these agents and radiation therapy.
Current immunotherapeutic treatment approaches with radiation
The concurrent emergence of a detailed mechanistic understanding of the immunogenic effects of radiation therapy and the prominent emergence of immunotherapy in clinical oncology has generated considerable interest in exploring the clinical potential for cooperative therapeutic interactions between these treatment modalities. This has given rise to the growing field of radio-immuno-oncology, which strives to elucidate immunologic effects of radiation therapy and evaluates the application of radiation therapy in combination with immunotherapy. Here, we briefly review prior preclinical and clinical studies that have evaluated the efficacy and safety of such combinations.
Cytokines
Cytokines are a broad category of naturally secreted autocrine, paracrine, or endocrine signaling peptides that are involved in the interaction of distinct immune cell lineages with each other and with their surrounding microenvironment. Early attempts to stimulate an anticancer immune response often sought to activate a patient’s immune system to better recognize and attack their cancer by systemically administering immune stimulatory cytokines. Clinical and preclinical studies by Rosenberg et al., demonstrated that high-dose systemic administration of the interleukin-2 (IL-2) cytokine could result in an effective antitumor immune response in patients with renal carcinoma or melanoma (23, 24). These landmark studies led to FDA approval of high-dose IL-2 as an immunotherapeutic treatment for these malignancies in 1992 and 1998, respectively (25, 26). Subsequently, the use of interferon alfa-2b was shown to confer a potential benefit in the adjuvant setting for patient with surgically resected, high-risk melanoma (27, 28). In pediatric patients with high-risk neuroblastoma, a combination cytokine therapy approach with IL-2, granulocyte—macrophage colony-stimulating factor (GM-CSF), isotretinoin, and the tumor-specific antidisialoganglioside antibody improved overall survival (29).
Early preclinical studies of radiation therapy in combination with cytokine treatments were reported by Cameron et al. These authors demonstrated that radiation therapy can synergize with and enhance the efficacy of high-dose IL-2 in a poorly immunogenic murine adenocarcinoma model (30). In addition to IL-2, other cytokines such as interleukins-3 and −12 (IL-3, IL-12), tumor necrosis factor-α, and others have been evaluated in preclinical studies together with radiation and demonstrated cooperative effects (31, 32). More recently, intratumoral delivery of cytokines has been shown to enhance the in situ vaccine effect of local EBRT in preclinical syngeneic tumor models (33).
Several early-phase clinical trials explored the combination of cytokine with radiation therapy. A Phase I/II trial by Seung et al. demonstrated that hypofractionated radiation (20 Gy × 1—3 fractions to a single site of disease in patients with metastatic RCC or melanoma) in conjunction with high-dose systemic IL-2 (started 3 days after radiation) could be delivered safely and resulted in a 66% objective response rate (34). More recently, Golden et al. combined EBRT (35 Gy in 10 fx) with subcutaneous delivery of GM-CSF in patients with metastatic solid tumors and observed abscopal antitumor responses in 27% of patients (35). A number of ongoing early-phase clinical trials currently are investigating combinations of radiation and cytokines, some with a particular focus on intratumoral delivery approaches or modified cytokine formulations that may exhibit greater tumor selectivity and reduced toxicity (Table 1).
Table 1.
Clinical trials investigating the use of radiation and immunotherapies
| Category | Examples of immune Rx | Disease site | Phase | Number of present studies |
Number involving brachytherapy |
|---|---|---|---|---|---|
| Checkpoint inhibitors | Anti-CTLA-4 | Cervix, melanoma, head and neck, pancreas, liver, lung | I/II/III | 19 | 1* *dual checkpoint blockade |
| PD-1/PD-L-1 | Esophageal, NSCLC, malignant glioma, melanoma (brain metastases), invasive bladder, oligometastatic breast, head and neck, pancreas, gastric, colorectal, follicular lymphoma | I/II/III | 93 | 1 | |
| Cytokines | IL-2, IFN, GM-CSF, and TGF-beta blockade | Metastatic breast, NSCLC, glioblastoma, follicular lymphoma, and pancreas | II | 9 | 0 |
| Cell therapy | CAR T cells (Anti BCMA, CD19, CD-30, TAI-meso, EGFRvIII, mesothelin, CD22) | B-cell lymphomas, pancreas, glioblastoma, follicular lymphoma, pancreas | I/II | 27 | 1 |
| Vaccines/oncolytic viruses | AdV-tk, sipluleucel-T, G207, ADV/HSV-tk, oncolytic adenovirus Ad5-yCD/mutTKSR39rep-hIL12 and Ad5-yCD/mutTKSR39rep-AD | Prostate, pancreas, malignant supratentorial neoplasms, NSCLC, triple-negative breast, prostate, glioma, ovarian, NSCLC, sarcoma, glioblastoma, neuroblastoma, | I/II/III | 35 | 0 |
| Other targeted immune Rx | L19-IL-2, IL-2, IFN-alpha, OX40 antibody, CDX-301, GITR, and TLR-4 agonist | Solid tumor, melanoma, renal cell carcinoma, NSCLC, breast, sarcoma, cutaneous T cell and recurrent lymphoma | I/II | 29 | 0 |
Tumor vaccines and oncolytic viruses
Tumor vaccines are a class of treatments that seek to activate a patient’s immune system to specifically recognize and attack cancer cells. Various vaccine approaches including dendritic cell vaccines, viral vaccines, nucleic acid vaccines, whole tumor cell vaccines, and protein/peptide vaccines have been developed and are in various stages of preclinical and clinical testing. Currently two cancer vaccines are FDA approved, sipuleucel-T (Provenge) and talimogene laherparepvec (T-VEC, or Imlygic). Sipuleucel-T is a dendritic cell-based tumor vaccine that is prepared by collecting peripheral blood mononuclear cells, including antigen-presenting cells, from patients with metastatic prostate cancer and activating these cells to prostatic acid phosphatase, a prostate-specific antigen, which is fused to GM-CSF to stimulate immune cell proliferation. These activated cells are subsequently injected back into the patients and have been shown to improve overall survival by 4.1 months over placebo with a 21% relative reduction in the risk of death (36). T-VEC is a herpes simplex type I oncolytic virus that has been modified to selectively lyse cancer cells, while inserting a gene encoding for GM-CSF to recruit and activate antigen presenting cells. T-VEC received FDA approval in 2015 after a Phase III trial demonstrated a significantly improved durable response rate (response lasting ≥ 6 months) as well as overall objective response compared with GM-CSF in patients with unresectable metastatic melanoma (37). In addition to these approved treatments, tumor vaccine approaches using nucleic acid vaccines, whole tumor cell vaccines, and protein/peptide vaccines are currently under investigation in varying phase clinical trials.
While radiation therapy is not included as part of the currently approved cancer vaccine or oncolytic virus approaches, several preclinical studies have shown a synergistic effect of combining radiation with cancer vaccine strategies. For example, in preclinical studies utilizing the syngeneic CT26 murine colorectal tumor line, intratumoral injection of dendritic cells into an irradiated tumor in conjunction with low-dose cyclophosphamide (to deplete immune suppressive regulatory T cells [Tregs]) resulted in improved survival and increased response rates in both the radiated tumor as well as sites of distant nonradiated disease (38). Recognizing such potential, a number of clinical studies are now testing the cooperative interaction between radiation therapy and tumor vaccine or oncolytic virus regimens, including multiple studies evaluating combination of radiation with the FDA approved vaccines, T-VEC or sipuleucel-T (Table 1).
T cell checkpoint inhibitors
T cell checkpoint inhibitors are a class of antibodies that modulate tumor tolerance among T cells by recognizing and blocking specific inhibitory receptors on the surface of T cells and thereby enhancing T cell activation. These checkpoint receptors normally function as co-regulatory molecules and can inhibit T cell activation after antigen recognition by the T cell receptor. Such mechanisms, normally involved in maintenance of self-tolerance, can be co-opted by tumor cells to elicit immunologic exhaustion—thereby obviating tumor eradication in the face of an existing antitumor immune response. Monoclonal antibodies (mAbs) targeting checkpoint receptors such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) or its ligand PD-L1 may overcome immune exhaustion. Clinical studies demonstrate that anti-CTLA-4 mAb (ipilimumab) improves survival in patients with metastatic melanoma (39, 40) and treatment with anti-PD-1 or anti-PD-L1 mAbs results in improved survival for patients with various types of metastatic cancer, including melanoma, non—small cell lung cancer, and head and neck cancer (6, 41, 42). A frequent observation from clinical studies of T cell checkpoint inhibitors is that a small subgroup of patients who respond to treatment experience complete and durable regression of disease [1—2% complete response rate among melanoma patients treated with combined anti-CTLA-4 and anti-PD-1 (4, 42)]. This raises the possibility that augmenting response rates to checkpoint blockade may result in a dramatic impact on survival of patients even with advanced stage malignancy.
Preclinical studies demonstrate the potential for EBRT to augment the response to checkpoint blockade. In a 4T1 triple-negative breast cancer murine model, radiation therapy was shown to sensitize to anti-CTLA-4 therapy with significant improvement in survival and inhibition of distant metastases compared with treatment with anti-CTLA-4 alone (43). This synergistic effect of radiation and anti-CTLA-4 therapy was also shown in a syngeneic lung cancer model, Lewis lung carcinoma, where combination treatment significantly extended both tumor growth delay and survival (44). While anti-CTLA-4 treatment in conjunction with radiation has shown promising response rates in preclinical studies, resistance to this treatment approach and escape via T cell exhaustion can occur. A study by Twyman Saint Victor et al. demonstrated initial tumor regression after EBRT and anti-CTLA-4 in a preclinical B16 melanoma model; however, resistance to this therapy eventually developed due to upregulation of PD-L1. This resistance was reversible with anti-PD-L1 therapy, and in the setting of dual checkpoint blockade, these authors demonstrated a role for radiation therapy in increasing the diversity of the antitumor T cell response, consistent with in situ vaccine effect leading to enhanced tumor antigen recognition (12). In separate preclinical studies, the combination anti-CTLA-4 and anti-PD-1 treatment with radiation was also shown to significantly improve tumor growth delay, reduce lung metastases, and improve survival in a preclinical osteosarcoma model (45). These preclinical data indicate that dual checkpoint blockade in combination with radiation may be effective in generating an antitumor immune response against immunologically “cold” tumors. Several case reports and early phase clinical trials suggest safety and potential therapeutic benefit for the combination of radiation and checkpoint blockade. However, definitive evidence of clinical benefit is lacking for such combinations and results are pending from multiple ongoing studies that are testing these approaches in a variety of clinical contexts (Table 1).
Rationale for testing brachytherapy approaches in combination with immunotherapy
Nearly all preclinical and clinical studies evaluating radiation therapy in combination with immunotherapies have used EBRT. This reflects the predominant role of EBRT in the radiation oncology clinic and the relative ease of delivering EBRT in preclinical tumor models with available equipment. Yet there are potential advantages to brachytherapy over EBRT as a mode of delivering radiation for the purpose of tumor immunomodulation.
Highly conformal dose distribution
EBRT typically must pass through considerable volumes of normal tissue to reach targeted tumor volumes. Highly radiosensitive lymphoid immune cells (e.g., naive T cells—LD50 ~ 2 Gy) may be depleted in these volumes of normal tissue that receive low-dose radiation, leading to long-term lymphopenia in some cases (46). Such effects may limit the efficacy of immune stimulatory agents in conjunction with EBRT. To limit these off-target effects, many current clinical trials use stereotactic body radiation therapy, hypofractionation, image guidance, motion management, and constraints limiting dose to pertinent lymphoid tissues (e.g., draining lymphatics, blood pool, spleen, bone marrow), indeed with some evidence, this approach does reduce the negative effect on circulating immune components (47). In contrast to EBRT, radiation delivered using brachytherapy, particularly in the inverse planning and adaptive era, achieves unmatched dose conformality. Therefore, compared with other radiotherapy modalities, brachytherapy may be optimally suited for sparing normal tissue and pertinent lymphoid organs from the low doses of radiation that may eradicate sensitive immune cell lineages.
Dose heterogeneity
The heterogeneity of radiation dose delivered to the targeted tumor using brachytherapy, with high dose delivered close to the source and increasingly lower dose delivered with distance from the source, may facilitate optimal engagement of multiple immunogenic mechanisms that each have distinct dose—response profiles (Fig. 1). Specifically, in regions closest to the brachytherapy source, increasing dose of radiation should lead to greater tumor cell death, as predicted by the linear quadratic formulation and other models of tumor response to radiation therapy (48). This region should exhibit maximal immunogenic tumor cell death and release of tumor-specific antigens (43).
Fig. 1.

Schematic representation of dose heterogeneity of brachytherapy delivery and its subsequent impact on the tumor microenvironment.
Outside the highest dose regions, high—intermediate dose per fraction (8—12 Gy) may optimally induce cytoplasmic release of dsDNA and cGAS/STING/interferon-γ-driven phenotypic changes in the expression of immune susceptibility markers on tumor cells that survive radiation (15, 44). Higher radiation dose per fraction may undo this effect by inducing tumor cell expression of the Trex1 exonuclease, whereas lower dose per fraction may not maximally activate this effect.
Moderate dose per fraction (2—5 Gy), may potentiate the release of immune stimulatory cytokines, leading to enhanced tumor infiltration by immune cells. Prior studies suggest dose-dependent and dose rate—dependent effects of radiation on the release of the T cell stimulatory cytokine IL-12 and the inhibitory cytokine IL-10 from macrophages, with this effect peaking between 2 and 5 Gy and enhanced with lower dose rate (49). Similar effects on the TGF-β cytokine appear to play a role in modulating expression of the endothelial adhesion molecules ICAM-1 and VCAM, and this may enhance immune cell infiltration of tumors after radiotherapy (50).
Finally, low dose per fraction (1—2 Gy) may enable temporary local depletion of suppressive (and effector) tumor infiltrating lymphocyte populations through direct cytotoxic effects on these highly radiation-sensitive cell populations. T lymphocytes are among the most radiosensitive mammalian cell types and exhibit limited capacity for damage repair. Doses of 0.5, 2, and 3 Gy trigger apoptosis in 10%, 50%, and 90% of naïve T lymphocytes within 2—8 h of radiation exposure, respectively (51). This is in contrast to most tumor cells, which commonly die by mitotic catastrophe at a more delayed interval after radiation (52). Activated T effector cells and suppressive Tregs may be slightly less sensitive to radiation compared with naïve T cells (53, 54); yet both these lineages are sensitive to radiation, relative to most tumor cells, with 2 Gy of splenic radiation reported to reduce such T cell populations by 67% (55). Because T cells constantly circulate, this temporary local depletion may not result in a lasting immunologic effect, as seen with large-field EBRT, but rather may create a window of opportunity during which the tumor immune microenvironment may be favorably reconstituted in the absence of Tregs and potentially other suppressive lineages.
Intratumoral applicator placement
The immune system is complex and layered with potentially synergistic targets for immunotherapeutic applications. However, systemic combinations of immunotherapies have resulted in increased risk for systemic toxicities. One approach to limit this systemic toxicity while capitalizing on the potential for synergistic immunotherapy combinations is to deliver immunotherapies directly into a tumor via intratumoral injection. Because the adaptive immune system already is designed to generate systemic responses, these therapies may be particularly well suited to local delivery in a single tumor where high local concentrations can be achieved at doses that are relatively low compared with those needed to achieve efficacy with systemic delivery. An increasing number of preclinical studies have demonstrated systemic antitumor immune response after intratumoral injection of immunotherapies (33, 56) and the FDA approval of T-VEC for intratumoral injection has demonstrated clinical feasibility of this approach. Because brachytherapy radiation requires the insertion of a radiation source into the tumor tissue, this mode of radiation may be particularly amenable for combined modality approaches that require injection of immune modulating agents directly into tumor tissue. The potential to use of a single treatment catheter for these approaches would portend clinical advantages to both the treatment team and the patient.
Preclinical studies combining immunotherapy and brachytherapy
Relatively few preclinical studies have tested the cooperative interaction between brachytherapy and immunotherapies. Indirect neutralization of Tregs with cyclophosphamide- and myeloid-derived suppressor cells with the phosphodiesterase inhibitor sildenafil, respectively, were found to synergize with radium (Ra)-224—loaded wires implanted into weakly immunogenic breast cancer xenografts (5). This combination, plus administration of the immunoadjuvant, CpG, resulted in the greatest reduction in metastatic tumor volume and number of lung metastases compared with any of the other combinations. The authors concluded that even tumors felt to be weakly immunogenic could be better controlled by combining brachytherapy and immunostimulatory measures. In a subsequent article, Hodge et al. used a CEA + mouse model and CEA-directed vaccine alone or in combination with an iodine-125 (I-125) seed implanted into the primary flank tumor and quantified lung metastases. While neither I-125 alone nor vaccine had any effect on the development of lung metastases, the combined therapy significantly reduced the burden of metastases in the lungs, demonstrating an abscopal effect (4).
In the only preclinical report of its kind, Rodriguez-Ruiz et al. used mouse models of colorectal cancer to measure the abscopal effect of brachytherapy (6). They aimed to explicitly determine if brachytherapy (8 Gy × 3 fractions delivered with an iridium-192 [Ir-192] source such that 90% of the CTV [D90] received the prescription dose) alone or combined with mAbs against PD-1 and/or CD137 induced an abscopal effect in a contralateral implanted tumor. Neither of the mAbs alone or in combination achieved primary or contralateral tumor control. Brachytherapy delivered to the primary tumor resulted in excellent local control independent of the addition of anti-PD-1 or anti-CD137 antibodies. Only the combination of brachytherapy and both immunostimulatory mAbs resulted in a measurable antitumor response at the nonradiated tumor, with control of this site in four of six mice in this group alone. This triplet therapy alone also extended survival, albeit for only two of six of the mice. These findings represent promising preclinical evidence to support the combination of brachytherapy and multiagent immune checkpoint inhibition.
While these studies demonstrate that brachytherapy approaches are capable of inducing antitumor immune response and enhancing efficacy of diverse immunotherapies, preclinical comparison of brachytherapy to EBRT (either standard fractionated or hypofractionated ablative regimens) are entirely lacking. This may be related to the limited number of investigators with experience and equipment necessary for testing brachytherapy in preclinical models. The dearth of brachytherapy utilization in combination with immunotherapies also likely reflects the relatively recent recognition of the potentially critical importance of radiation dose, dose per fraction, and dose rate in obtaining an optimal immune response. Going forward, using models such as that designed by Rodriguez-Ruiz et al. in future efforts to determine the optimal combinations of radiation and immunotherapy are critical to ensure proper application of brachytherapy in this setting.
Clinical trials combining brachytherapy and immunotherapy
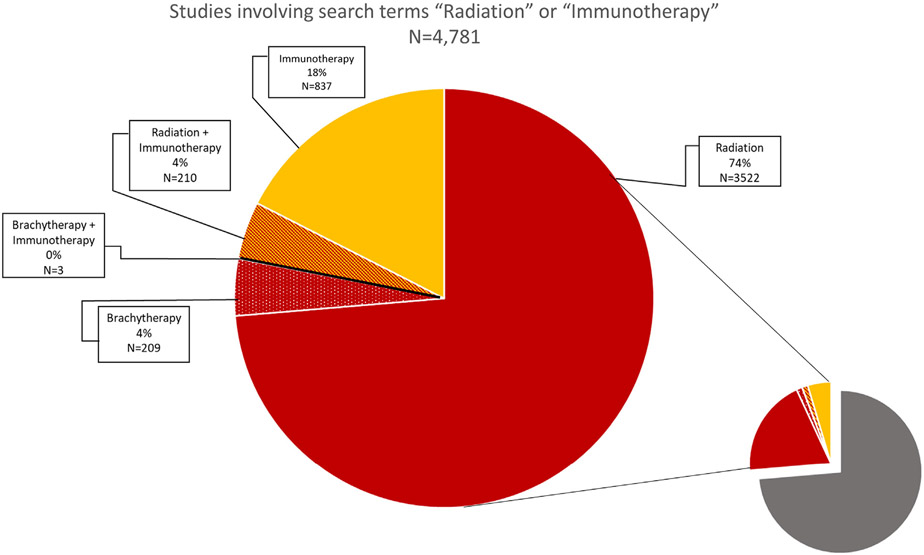
At the time of this review, over 1,000 clinical trials are identified on www.clinicaltrials.gov under the search terms “immunotherapy and cancer” (Fig. 2). Of these, only three trials involve a combination of brachytherapy and immunotherapy. Two of the three studies utilize 90Y microspherebased treatment of liver metastases in combination with either dual checkpoint blockade or a chimeric antigen receptor modified T cell targeting the CEA antigen (NCT02913417, NCT02416466). The final brachytherapy—immunotherapy combination study, which is actively enrolling patients, is testing the safety and efficacy of adding anti-PD-1 checkpoint blockade to standard chemoradiotherapy in patients with advanced cervical cancer. The study will compare anti-PD-1 mAb administered concurrently with brachytherapy/chemotherapy vs. administration of anti-PD-1 after the conclusion of chemoradiotherapy (NCT02635360). This study will determine whether patients receiving standard chemoradiotherapy may derive benefit from the addition of anti-PD-1 therapy; however, the study does not specifically evaluate for a cooperative interaction between brachytherapy and immune checkpoint blockade. Similarly, in extending this review of clinical trials to include those previously conducted only five studies were found to include the search terms “cancer”, “brachytherapy”, and “immunotherapy”, and on closer analysis, none of these tested for a cooperative effect of brachytherapy in potentiating response to an immunotherapy.
Fig. 2.
Graphical representation of current clinical trials involving cancer therapies. A Boolean search of open, interventional studies conducted on clinicaltrials.gov (April 27, 2018) with the search term “cancer” yielded 18,362 results. Of these results, 3,947 involved radiation in some form, and 212 of these studies utilized brachytherapy. Even further broken down, only three studies were found with the search terms “brachytherapy + immunotherapy”. This figure is quite small in comparison with the 212 studies found involving “radiation + immunotherapy”. Studies combining brachytherapy and immunotherapy represent 1.4% of all brachytherapy trials, while studies combining other forms of radiation and immunotherapy represent 5.4% of all radiation studies. In total, ongoing studies involving the larger umbrella terms of “radiation” and “immunotherapies” either in combination or separately comprise approximately 26% of all current ongoing interventional cancer trials.
Conclusion and future directions
Multiple studies of clinically relevant murine tumor models indicate that the most specific and immunogenic tumor antigens recognized by T cells are “private antigens” derived from random, patient-specific, nondriver protein mutations in tumor cells (57, 58). To capitalize on the strength of these antigens, it may be beneficial to utilize an in situ tumor vaccination strategy in combination with other immunotherapeutic approaches. Because of the mechanisms reviewed here, brachytherapy may be an ideal approach to achieving in situ tumor vaccination. Preclinical studies testing such an approach are limited in number but results to date have been encouraging. No reported preclinical studies compare EBRT, brachytherapy, or other local treatment approaches for their relative capacity to elicit in situ vaccination. Likewise, no clinical studies have reported on the safety or efficacy of brachytherapy in combination with immunotherapy. Planning such studies is not without challenges and it remains unknown what may be the optimal dose, dose rate, and fractionation of brachytherapy for any given immunotherapy as well as what will be the appropriate timing of immunotherapy administration relative to brachytherapy. Yet such challenges should not deter or delay what we see as a clear and immediate need for preclinical and early phase clinical studies evaluating combined modality approaches to in situ vaccination using brachytherapy and immunotherapy.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- [1].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100: 57–70. [DOI] [PubMed] [Google Scholar]
- [2].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- [3].Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991–998. [DOI] [PubMed] [Google Scholar]
- [4].Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wolchok JD, Chiarion-Sileni V, Rutkowski P, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bellmunt J, de Wit DJ, Vaughn Y, et al. Pembrolizumab as secondline Therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- [9].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Demaria S, Bhardwaj N, McBride WH, et al. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys 2005;63:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sharabi AB, Nirschl CJ, Kochel CM. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 2015;3:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 2010;28:4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Young KH, Baird JR, Savage T, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016;11:e0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234–241. [DOI] [PubMed] [Google Scholar]
- [18].Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol 1973;46:220–222. [DOI] [PubMed] [Google Scholar]
- [19].Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol 1975;48:863–866. [DOI] [PubMed] [Google Scholar]
- [20].Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25–37. [DOI] [PubMed] [Google Scholar]
- [21].Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862–870. [DOI] [PubMed] [Google Scholar]
- [22].Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst 1979;63:1229–1235. [PubMed] [Google Scholar]
- [23].Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985;313:1485–1492. [DOI] [PubMed] [Google Scholar]
- [24].Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg 1989;210:474–484. discussion 484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105–2116. [DOI] [PubMed] [Google Scholar]
- [26].Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994;271:907–913. [PubMed] [Google Scholar]
- [27].Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol 2001;19:2370–2380. [DOI] [PubMed] [Google Scholar]
- [28].Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern cooperative oncology group trial EST 1684. J Clin Oncol 1996;14:7–17. [DOI] [PubMed] [Google Scholar]
- [29].Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med 1990; 171:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chiang CS, Hong JH, Wu YC, et al. Combining radiation therapy with interleukin-3 gene immunotherapy. Cancer Gene Ther 2000; 7:1172–1178. [DOI] [PubMed] [Google Scholar]
- [32].Hallahan DE, Beckett MA, Kufe D, et al. The interaction between recombinant human tumor necrosis factor and radiation in 13 human tumor cell lines. Int J Radiat Oncol Biol Phys 1990;19:69–74. [DOI] [PubMed] [Google Scholar]
- [33].Morris ZS, Guy EI, Francis DM, et al. In Situ tumor vaccination by combining local radiation and tumor-specific antibody or immunocytokine treatments. Cancer Res 2016;76:3929–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2–tumor and immunological responses. Sci Transl Med 2012;4:137ra74. [DOI] [PubMed] [Google Scholar]
- [35].Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795–803. [DOI] [PubMed] [Google Scholar]
- [36].Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–422. [DOI] [PubMed] [Google Scholar]
- [37].Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780–2788. [DOI] [PubMed] [Google Scholar]
- [38].Son CH, Shin DY, Kim SD, et al. Improvement of antitumor effect of intratumoral injection of immature dendritic cells into irradiated tumor by cyclophosphamide in mouse colon cancer model. J Immunother 2012;35:607–614. [DOI] [PubMed] [Google Scholar]
- [39].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- [41].Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- [42].Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PubMed] [Google Scholar]
- [43].Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005;11:728–734. [PubMed] [Google Scholar]
- [44].Yoshimoto Y, Suzuki Y, Mimura K, et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS One 2014;9:e92572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Takahashi Y, Yasui T, Tamari K, et al. Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS One 2017;12:e0189697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer 1977;39:737–743. [DOI] [PubMed] [Google Scholar]
- [47].Crocenzi T, Cottam B, Newell P, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer 2016; 4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fowler JF. 21 years of biologically effective dose. Br J Radiol 2010; 83:554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liu SZ. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlinearity Biol Toxicol Med 2003;1:71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rodriguez-Ruiz ME, Garasa S, Rodriguez I, et al. Intercellular adhesion Molecule-1 and vascular cell adhesion molecule are Induced by ionizing Radiation on lymphatic endothelium. Int J Radiat Oncol Biol Phys 2017;97:389–400. [DOI] [PubMed] [Google Scholar]
- [51].Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 1990;123:224–227. [PubMed] [Google Scholar]
- [52].Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol 2010;31:363–372. [DOI] [PubMed] [Google Scholar]
- [53].Liu R, Xiong S, Zhang L, et al. Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol 2010;7:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu S, Sun X, Luo J, et al. Effects of radiation on T regulatory cells in normal states and cancer: mechanisms and clinical implications. Am J Cancer Res 2015;5:3276–3285. [PMC free article] [PubMed] [Google Scholar]
- [55].Balogh A, Persa E, Bogdandi EN, et al. The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells. Inflamm Res 2013;62:201–212. [DOI] [PubMed] [Google Scholar]
- [56].Sagiv-Barfi I, Czerwinski DK, Levy S, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med 2018; 10(426):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].O’Sullivan T, Saddawi-Konefka R, Vermi W, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med 2012;209:1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Matsushita H, Vesey MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012;482:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]