Abstract
Non-small-cell lung cancer (NSCLC) causes significant mortality each year. After successful resection of disease stage IB (>4 cm) to IIIA (per AJCC 7), adjuvant platinum-based chemotherapy improves median overall survival and is the standard of care, but many patients still experience recurrence of disease. An adjuvant regimen with greater efficacy could substantially improve outcomes. Pembrolizumab, a programmed cell death-1 inhibitor, has become an important option in the treatment of metastatic NSCLC. ALCHEMIST is a clinical trial platform of the National Cancer Institute that includes biomarker analysis for resected NSCLC and supports therapeutic trials including A081801 (ACCIO), a three-arm study that will evaluate both concurrent chemotherapy plus pembrolizumab and sequential chemotherapy followed by pembrolizumab to standard of care adjuvant platinum-based chemotherapy.
Clinical trial registration: NCT04267848 (ClinicalTrials.gov)
Keywords: : adjuvant chemotherapy, lung adenocarcinoma, lung squamous cell carcinoma, NSCLC, pembrolizumab
Tweetable abstract
Non-small-cell lung cancer adjuvant platinum-based therapy is standard of care (SOC). Including pembrolizumab may improve efficacy. A081801 (ACCIO) is a three-arm study: SOC versus sequential chemotherapy–pembrolizumab versus concurrent chemotherapy + pembrolizumab.
Lung cancer overview
Lung cancer accounted for 1.76 million deaths worldwide in 2018 and claims more lives each year in the USA than breast, colon and prostate cancers combined [1,2]. Although surgically resectable non-small-cell lung cancer (NSCLC) is considered curable, many patients experience disease recurrence impacting survival [3]. Cisplatin-based adjuvant chemotherapy is the standard of care for stage IB (≥4 cm) – IIIA (per AJCC 7) NSCLC tumors, but the absolute benefit is marginal [3]. A systemic therapy regimen that further reduces recurrence rates after surgical resection of early stage NSCLC could significantly impact outcomes for a substantial number of patients.
Introduction to the trial
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial) is a National Cancer Institute, National Clinical Trial Network clinical trial platform offering biomarker analysis for high-risk resected NSCLC to support randomized trials of novel adjuvant therapies [4]. ALCHEMIST includes a screening trial (A151216, 5708 registered patients as of 5 October 2020) enrolling patients with completely resected clinical stage IB (≥4 cm)–IIIA (per AJCC 7) NSCLC. Tissue and blood are collected for biomarker testing including EGFR sequencing, ALK fluorescence in situ hybridization (FISH) and programmed death-ligand 1 (PD-L1) immunohistochemistry (IHC). Patients with EGFR mutations may enroll on a trial of adjuvant erlotinib versus observation (A081105, 384 randomized as of 5 October 2020) while those with ALK fusions may enroll on a trial of adjuvant crizotinib versus observation (E4512, 119 randomized as of 5 October 2020). A third trial offering adjuvant nivolumab versus observation regardless of PD-L1 status (EA5142, 935 randomized) completed enrollment on 19 October 2019. Each of these therapeutic trials enrolls patients after completion of adjuvant chemotherapy.
Pembrolizumab
Pembrolizumab is a humanized monoclonal antibody that binds to PD-1 on T cells, blocking the binding of PD-1 ligands (PD-L1 and PD-L2). Inhibition of the PD-1 pathway inhibits the downregulation of immune response generated by PD-1 receptor signaling [5]. This limits T-cell suppression allowing for an antitumor response [6]. Pembrolizumab is now one of the more commonly utilized first-line therapeutic options in the treatment of NSCLC either as a single agent or concurrent with chemotherapy [7–9].
A081801 (ACCIO)
Background & rationale
Substantial benefit has been noted with concurrent chemotherapy plus checkpoint inhibitor in the metastatic setting [7,9,10]. To investigate the potential benefit of concurrent treatment with adjuvant chemotherapy and pembrolizumab after complete resection of stage IB (>4 cm) – IIIA (per AJCC 7) NSCLC, a new trial within the ALCHEMIST portfolio, activated as of 5 June 2020 enrolls patients prior to adjuvant chemotherapy. A three-arm design, including enrollment to a sequential arm of adjuvant chemotherapy followed by pembrolizumab will also allow for the potential to compare sequential to concurrent chemotherapy and pembrolizumab. Although multiple other studies have enrolled, or are currently enrolling, a sequential arm, this study is the first to enroll prior to any adjuvant chemotherapy. This difference in the time of enrollment has the potential to provide further perspective to outcomes in this population. Trials enrolling after chemotherapy may select out only those with the best functional status. At the same time, if one or more sequential immunotherapy studies mature and are reported, the trial will be modified to drop an arm and continue enrollment only to the relevant arms.
Quality of life
Understanding the impact of treatment and symptoms on patients’ daily lives is essential to balance efficacy versus safety of various treatments in clinical trials. In trials where there is a vast difference in outcomes between the study arms, the impact on quality of life (QoL) may not be as relevant, as it would be expected that the improved disease outcomes would naturally lead to better QoL. If the survival benefit detected is small but associated with substantial health related QoL (HRQOL) worsening, some patients or clinicians may feel it is not worth the tradeoff. Conversely, if a small survival benefit is detected with no decrement in HRQOL, then sentiments may be different. Hence, collecting HRQOL information in this setting is important for informing multiple stakeholders, including patients.
Patients with resected NSCLC tolerate four cycles of adjuvant chemotherapy well, and the additional side effects are outweighed by the improvement in overall survival (OS) [11]. Pembrolizumab has been well tolerated in metastatic NSCLC and either improved or maintained HRQOL compared with chemotherapy [12]. The present trial, ACCIO, assigns patients to approximately 1 year of pembrolizumab, either starting concurrently with platinum-based chemotherapy or sequentially. The cumulative adverse effects of extended therapy that may be accepted in the metastatic setting, may lead to decreased compliance and acceptance of therapy in the adjuvant setting.
Design
Study design
ACCIO (NCT04267848) is a randomized three-arm Phase III trial comparing the disease-free survival (DFS) and OS as dual primary end points between the standard of care adjuvant chemotherapy followed by observation (arm A), sequential adjuvant chemotherapy followed by pembrolizumab for 1 year (arm B) and combination adjuvant chemotherapy plus pembrolizumab followed by pembrolizumab (arm C). Both experimental arms (B & C) include the same duration of pembrolizumab (about 1 year). Individuals will be randomized based upon stratification factors including: nonsquamous versus squamous, PD-L1 expression (≥50 vs <50%), smoking status (never vs former/current) and pathological stage (IB and II vs IIIA). See Figure 1.
Figure 1. . Schema of the ACCIO trial including three arms.
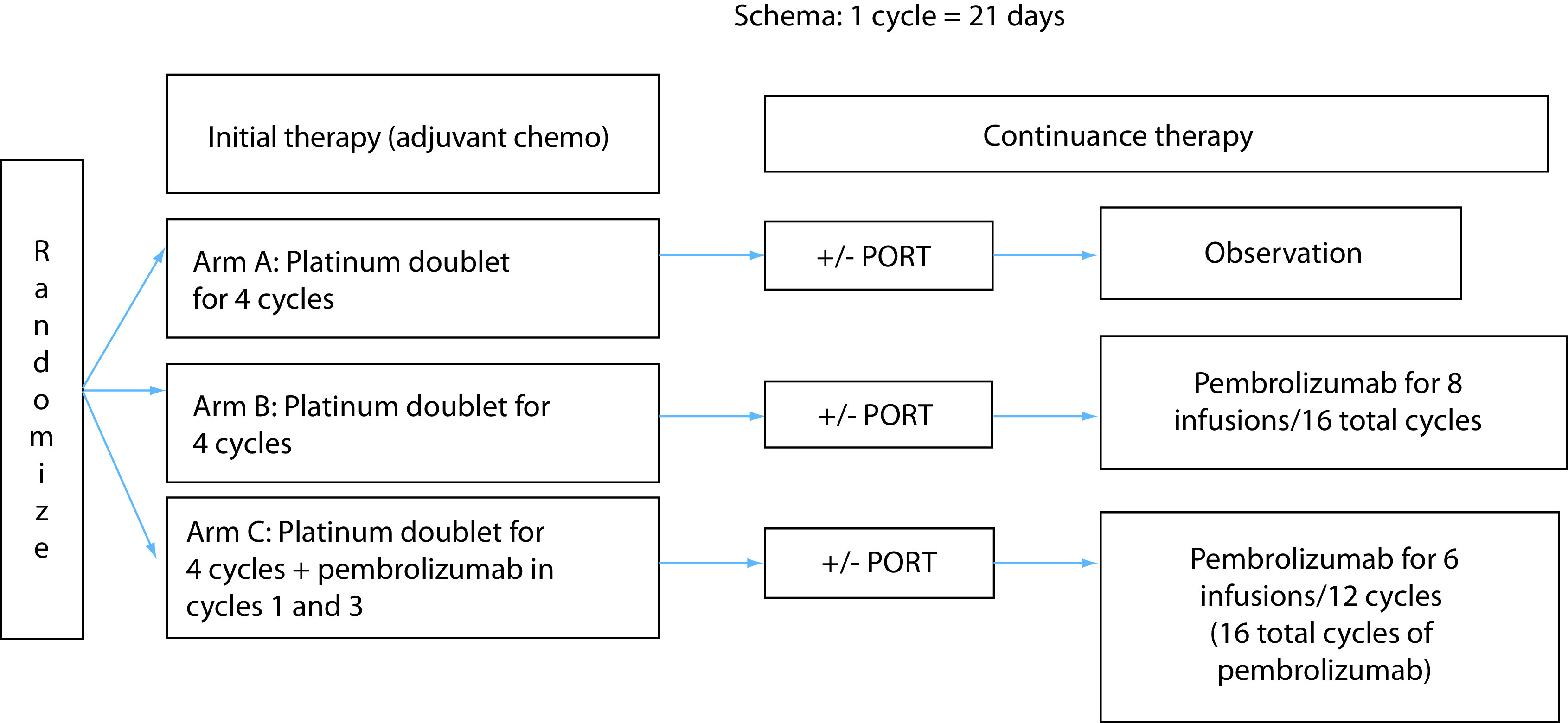
Pembrolizumab dosing is every 6 weeks. Sequential and concurrent arms each include about 1 year of pembrolizumab.
PORT: Postoperative radiation therapy.
Prior studies have shown that the median DFS is generally around 42 months in this patient population treated with standard of care adjuvant chemotherapy [3,13]. Reported median OS depends significantly on the stages enrolled. ANITA trial included stage IB–IIIA and reported median OS of 65.7 months (39% stage III, 24% stage II and 36% stage IB) [14]. IALT reported a 5-year OS of 44.5% with similar population of stage inclusion (40% stage III, 24% stage II and 36% stage I) [15]. Studies such as E1505 had fewer stage III patients (29%) and a predictably higher median OS (about 84 months).
Based on the AJCC clinical staging information for patients enrolled in the ALCHEMIST screening trial at the time of protocol development (12% stage IB, 51% stage II and 37% stage IIIA), a projected median DFS and OS of 42 and 66 months, respectively, for the standard of care arm (arm A) were considered reasonable. We hypothesize that the addition of pembrolizumab (in both the combination and sequential experimental arms, arms B and C) to the standard of care (arm A) will improve the median DFS to around 63 months and the median OS to around 99 months. We think this is achievable based on data in stage IV NSCLC [7]. We will also compare the adverse events, rate of drug discontinuation due to adverse events, and DFS and OS by different patient subgroups of interest (defined by PD-L1 expression, tumor mutational burden status, etc.).
Enrollment to checkpoint inhibitor clinical trials in the adjuvant setting has occurred after completing chemotherapy. ACCIO is the first trial to enroll to an adjuvant regimen that includes a checkpoint inhibitor prior to any adjuvant treatment, which may provide a more reliable comparison to adjuvant chemotherapy alone. The trial aims to complete enrollment within a timeframe that is prior to significant data reporting of other accruing trials, but the three-arm design also allows for dropping an arm that becomes less relevant if ongoing studies report data that are definitive.
Primary & secondary objectives
The primary objective of the study is to compare the DFS and OS as dual primary end points between standard of care adjuvant chemotherapy (arm A) and combination chemotherapy and pembrolizumab (arm C), and standard of care adjuvant chemotherapy (arm A) versus sequential chemotherapy followed by pembrolizumab (arm B) in patients with resected (R0) stage IB (>4 cm)–IIIA NSCLC (7th edition). The three-arm design allows for a secondary objective to compare the DFS and OS as dual primary end points between arms B and C in the defined enrollment population. DFS is defined as the time from randomization to the first of either disease recurrence or death from any cause. OS is defined as the time from randomization to death from any cause.
The study includes QoL objectives to compare patient-reported QOL 1 year after randomization as assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) between patients randomized to receive adjuvant chemotherapy followed by pembrolizumab (arm B), and those randomized to receive adjuvant chemotherapy + observation (arm A) as well as compare combination adjuvant chemotherapy plus pembrolizumab (arm C) to adjuvant chemotherapy + observation (arm A). Comparison of patient-reported QOL as assessed by the EORTC QLQ-C30 between patients randomized to receive adjuvant chemotherapy + pembrolizumab concomitantly (arm C) and those randomized to receive adjuvant chemotherapy + observation or adjuvant chemotherapy followed by pembrolizumab (arms A and B combined) is also planned at completion of chemotherapy. The last QOL objective is to present longitudinal trajectories by arm of patient-reported dyspnea and coughing as assessed by the Lung Cancer Modular Supplement (EORTC QLQ-LC13).
Key eligibility criteria
Enrollment to A081801 (ACCIO) follows enrollment to A151216, which includes individuals that have undergone an R0 resection of NSCLC (any histologic subtype) 7th edition staging IB (≥4 cm) – IIIA. Mediastinal adenopathy that is incidentally detected by surgical specimen is eligible, but any known mediastinal adenopathy should not be treated with up front resection nor enrolled in this trial. Eligible functional status includes Eastern Cooperative Oncology Group (ECOG) Performance Status 0–1 after recovery from surgical resection. As part of A151216, tumor samples are tested centrally for EGFR, ALK and PD-L1, and when positive for EGFR or ALK, may enroll to A081105 and E4512, respectively. EGFR and ALK must be negative for enrollment of patients with nonsquamous tumors to ACCIO. To enable more rapid enrollment, local testing is accepted for ACCIO, but central testing on A151216 will also be performed. All patients must be eligible for standard of care adjuvant platinum-doublet chemotherapy and treatment with checkpoint inhibitor. Enrollment must occur 30–77 days post surgery. Standard laboratory values are required. PD-L1 testing must be completed prior to enrollment as this is a stratification factor, and the following assays are considered acceptable: DAKO 22c3, DAKO 28–8, E1L3N or SP-263. Patients must be age ≥18 years, have no ‘currently active’ second malignancy that is progressing or required treatment within the last 3 years, excluding specified low-risk cancers that have undergone potentially curative therapy. No known history of HBV or HCV is allowed.
Planned sample size & study period
The statistical analysis (further described later) includes 400 evaluable patients per arm for a total of 1200 evaluable patients. To account for possible dropouts/nonevaluable patients, we plan to overaccrue by 5%, which will include 421 per arm and a total of 1263 patients randomized. Based on enrollment within the ALCHEMIST studies, we expect accrual of at least 20 patients per month, which would result in full accrual around 5 years.
Dose & schedule of therapy
Arm A, the control arm, includes four cycles of standard of care adjuvant chemotherapy followed by observation. The acceptable chemotherapy regimens are the same across all arms and include: cisplatin + pemetrexed, carboplatin + pemetrexed, cisplatin + gemcitabine and carboplatin + paclitaxel. All regimens are Q3 week cycles. Arm B, sequential arm, includes four cycles of adjuvant chemotherapy followed by pembrolizumab for a total of about 1 year. Arm C, combination arm, includes four cycles of chemotherapy and concurrent pembrolizumab, followed by pembrolizumab. Arms B and C each include the same duration of pembrolizumab treatment of about 1 year. Pembrolizumab infusions are Q6 weeks for up to eight doses.
Currently postoperative radiation therapy (PORT) is allowed per investigator discretion and should be completed after initial therapy (adjuvant chemotherapy) and prior to initiation (or continuation) of pembrolizumab in the ‘continuance therapy’ timeframe of the protocol. Although controversial, in particular in light of the recent LUNG ART results presented recently, PORT is considered for pathologically involved N2 nodes in the surgical specimen. Although PORT may sometimes be considered for positive margin as well, any patients with postoperative positive margins are not eligible for enrollment to ACCIO.
Efficacy end points
ACCIO includes dual primary end points in the primary objective comparing both DFS and OS between standard of care adjuvant chemotherapy and combination adjuvant chemotherapy and pembrolizumab, and between standard of care adjuvant chemotherapy and sequential adjuvant chemotherapy followed by pembrolizumab in surgically resected NSCLC. Secondary objectives include a comparison of the DFS and OS of the sequential and combination arms. Secondary end points also include adverse events and discontinuation rates of each experimental arm compared with the control arm, a comparison of DFS and OS between the sequential and/or combination arms versus standard of care chemotherapy alone by PD-L1 expression (tumor proportion score ≥50% vs tumor proportion score <50%), and a comparison of DFS and OS between the sequential and/or combination arms versus standard of care chemotherapy alone in patients that received at least two cycles of initial adjuvant chemotherapy.
QoL measures
The EORTC QLQ-C30, is a well-validated scale that assesses the overall QoL. It is composed of six functional scales (physical, role, emotional, cognitive, social and global health status/QoL) and nine symptom scales/items (fatigue, nausea and vomiting, pain, dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial impact). The EORTC-QLQ-LC13 is a 13-item lung cancer-specific questionnaire that is added to the EORTC QLQ-C30 to obtain information about symptoms that are prevalent in lung cancer patients such as dyspnea, cough and hemoptysis [16]. These measures will be collected at four time points during the trial: baseline (after registration prior to first treatment), completion of chemotherapy, 1-year post randomization (+/− 4 weeks) and 2 years post randomization (+/− 4 weeks).
Statistical analyses
The primary goal is to compare each of the experimental arms to chemotherapy alone (arm A), where the alternative hypothesis is that the pembrolizumab arms will each demonstrate improved DFS compared with the control arm. 400 evaluable patients will be accrued to each arm of the study using a 1:1:1 randomization scheme (1200 evaluable total) to provide 90% power to detect an improvement in the median DFS from 42 to 63 months (hazard ratio [HR] = 0.67), assuming a one-sided significance level of 0.005. The primary analyses will be two individual comparisons (each experimental arm vs control) using a one-sided log-rank test. These analyses will take place after an approximate 63-month accrual period and after 363 total DFS events have occurred across both arms combined for each analysis (after a minimum follow-up of 30 months in all evaluable patients) or 5 years after accrual completion (whichever comes first).
The testing of the two pairwise comparisons (each of the experimental arms to the chemotherapy alone arm) will be done in a stepwise fashion. As the study design includes dual primary end points, a similar analysis will be performed with OS. With 400 evaluable patients per arm, there is 90% power to detect an improvement in the median OS from 66 to 99 months (HR = 0.67) assuming a one-sided significance level of 0.02. The primary analyses will be a comparison of each of the two experimental arms to the chemotherapy alone arm using a one-sided log-rank test for each analysis. These analyses will take place after an approximate 63-month accrual period and after 271 total OS events have occurred across both arms combined for each analysis (approximately after 30 months of minimum follow-up in all evaluable patients) or 8 years after accrual completion, whichever comes first. The testing of the two pairwise comparisons (each of the experimental arms to the chemotherapy alone arm) will be done in the same manner as the DFS analysis, each with a one-sided alpha of 0.02.
Overall study design has one-sided alpha = 0.025 with 90% power to evaluate the first pairwise comparison of interest (A vs C) for the dual primary end points of DFS and OS. If combination treatment is not found to be significantly different than control for DFS or OS, the control treatment will be recommended. If combination treatment is found to be significantly better for OS or DFS as compared with control, the next pairwise test will compare sequential treatment versus control (arm B vs A), with an overall one-sided alpha = 0.025 and 90% power for the dual primary end points of DFS and OS. If sequential treatment is not found to be significantly different than control for OS or DFS, the combination treatment will be recommended. If sequential treatment found significantly better than control for OS or DFS, the preplanned secondary comparisons for comparing sequential versus combination therapy will be undertaken.
We plan to conduct a secondary comparison of combination (arm C) versus sequential (arm B) strategies. We recognize that during enrollment, there may be data reported from other sequential studies that could guide a statistical plan to compare sequential to combination therapy. This could guide an amendment to adequately design a comparison of these arms by an independent statistician. Currently there are no data to guide this analysis, but we estimate the following for now: with 400 evaluable patients per arm based on an intent-to-treat analysis, alpha = 0.05 combined (OS = 0.04; DFS = 0.01), power = 80% will enable us to detect an HR = 0.75 (comparing combination to sequential therapy). Minimum follow-up of at least 4 years will be needed (325 events needed for OS and 491 events needed for DFS).
All randomized patients with outcome data will be considered evaluable for the coprimary end point based on the intent-to-treat principle. All randomized patients who withdraw consent prior to receiving treatment will be excluded completely from all summaries and analyses. A secondary sensitivity analysis will assess the patients who complete at least two cycles of chemotherapy. OS and DFS will be estimated using the Kaplan–Meier method, where the stratified log-rank test will be used to compare the distributions across the treatment arms.
Pembrolizumab has been well tolerated, including in combination with chemotherapy. For example, in KEYNOTE-189 (carboplatin and pemetrexed +/− pembrolizumab for nonsquamous NSCLC), there were 67.2 versus 65.8% grade 3+ adverse events in the pembrolizumab and control arms, respectively, reflecting the expected toxicities of chemotherapy [7]. In this adjuvant study, the protocol specifies overview by a data-safety monitoring board, and accrual will be temporarily suspended if at any time adverse events in the concurrent and/or sequential arms are substantially higher than the control arm particularly related to grade 4+ nonhematologic events. An early stopping rule is included for futility of DFS at 50 and 75% of the needed events.
Conclusion
NSCLC causes significantly more fatalities than any other cancer. Advances in treatment of resected early stage NSCLC beyond platinum-based chemotherapy could significantly impact many lives. The ALCHEMIST platform has successfully collected many samples from patients that have undergone resection of stage IB (≥4 cm)–IIIA (per AJCC 7) NSCLC and enrolled to three adjuvant systemic therapy protocols. The latest ALCHEMIST trial, A081801 (ACCIO), will evaluate the integration of immune checkpoint inhibitors and adjuvant systemic therapy in resected NSCLC. Patients are being randomized to standard of care adjuvant platinum-based chemotherapy alone versus sequential chemotherapy followed by pembrolizumab versus concurrent chemotherapy plus pembrolizumab. By building off the ongoing ALCHEMIST platform, we hope to facilitate rapid enrollment to ACCIO across the participating National Clinical Trial Network sites.
Executive summary.
Lung cancer overview
Lung cancer accounted for 1.76 million deaths worldwide. Unfortunately, many individuals that undergo curative intent treatment experience recurrence that often leads to mortality from lung cancer.
Pembrolizumab is a humanized monoclonal antibody that binds to programmed cell death-1 on T cells, inhibiting downregulation of immune response. This limited suppression of T cells allows for an antitumor response. Pembrolizumab is one of the more commonly utilized first-line therapeutic options in the treatment of non-small-cell lung cancer (NSCLC).
A081801 (ACCIO)
Background & rationale: substantial outcome improvement has been demonstrated with concurrent pembrolizumab in the metastatic setting. This leads to the logical hypothesis that inclusion of pembrolizumab in the adjuvant setting will also improve outcomes. ACCIO also includes a health-related quality of life analysis with the recognition that cumulative adverse effects of extended therapy that may be accepted in the metastatic setting, may lead to decreased compliance and acceptance of therapy in the adjuvant setting.
Design: ACCIO is a three-arm study under the ALCHEMIST umbrella that will evaluate both concurrent chemotherapy plus pembrolizumab and sequential chemotherapy followed by pembrolizumab to standard of care adjuvant platinum-based chemotherapy. A dual end point design of disease free and overall survival (OS) is utilized, with a planned enrollment of 1200 evaluable patients.
Primary and secondary objectives: the primary objective of the study is to compare the disease-free survival (DFS) and OS as dual primary end points between the standard of care adjuvant chemotherapy (arm A) and combination chemotherapy and pembrolizumab (arm C), and standard of care adjuvant chemotherapy (arm A) versus sequential chemotherapy followed by pembrolizumab (arm B). The three-arm design allows for a secondary objective to compare the DFS and OS as dual primary end points between the sequential (arm B) and concurrent (arm C) arms. Secondary end points include patient-reported quality of life 1-year end points and analysis based upon programmed cell death-ligand 1 (PD-L1) expression of ≥50% versus <50%.
Key eligibility criteria: enrollment to ACCIO follows enrollment to A151216 including those with R0 resection of NSCLC (any histologic subtype) 7th edition staging IB (≥4 cm) – IIIA. Inclusion criteria specifies functional status of ECOG 0–1, EGFR and ALK negative, completed PD-L1 testing (all expression is eligible), no second malignancy within the past 3 years (excluding specified low-risk cancers that have undergone potentially curative treatment). Patients must be eligible for adjuvant chemotherapy and pembrolizumab along with other standard eligibility criteria.
Planned sample size and study period: statistical analysis include 400 evaluable patients per arm for a total of 1200 evaluable patients. Interim futility analyses are planned for DFS alone at 50 and 75% of the needed events.
Dose and schedule: all chemotherapy regimens include the established dosing schedule. Pembrolizumab dosing is 600 mg every 6 weeks for up to eight doses in both the sequential and concurrent arms.
Conclusion
NSCLC causes significantly more fatalities than any other cancer. Advances in the treatment of resected early stage NSCLC could significantly impact many lives. The ALCHEMIST platform has successfully enrolled many patients after surgical resection of NSCLC, and ACCIO is currently enrolling patients after R0 resection of NSCLC with stage IB (>4 cm)–IIIA that are eligible for adjuvant chemotherapy and pembrolizumab who have not already received any treatment other than resection. EGFR, ALK and PD-L1 results are required for enrollment. All NSCLC subtypes and PD-L1 expression are eligible.
Footnotes
Financial & competing interests disclosure
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233180, UG1CA233253, UG1CA233247, UG1CA233339, and UG1CA232760. https://acknowledgments.alliancefound.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JM Sands reports honoraria for consulting from AstraZeneca, Takeda, Boehringer Ingeheim, Medtronic, Jazz Pharmaceuticals, Pharma Mar, Blueprint Pharmaceuticals, Daiichi Sankyo, Eli Lilly, Loxo, Foundation Medicine, Guardant and Abbvie. GR Oxnard reports employment with Foundation Medicine and equity in Roche. R Govindan reports honoraria for advisory board from Genentech, Pfizer, Roche, Nektar, Bristol Myers Squibb, Partner Therapeutics, GlaxoSmithKline, Jounce and Achilles; and consulting from AstraZeneca, Amgen, Horizon Pharmaceuticals, GenePlus and Inivata. J Gray reports honoraria for consulting from AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, EMD Serono, Inivata, Merck and Novartis; and research support from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, GI Therapeutics, Merck, Novartis, Pfizer and Ludwig Institute of Cancer Research. JK Salama reports research support to institution by Abbvie; and support to institution of spouse by Bristol Myers Squibb, Genentech, Immunocore and Merck. L Raez reports research support to institution from Bristol Myers Squibb, Genentech/Roche, Pfizer, Nanthealth, MSD, Merck Serono, Lilly Oncology, Boehringer Ingelheim, Syndax, Novartis, Heat Biologics, AstraZeneca, Exosomes DX, Liquid Genomics and Loxo Oncology. A Ganti reports honoraria for consulting from Genentech, AstraZeneca and Flagship Biosciences; honoraria for advisory board from Genentech, AstraZeneca, G1 Therapeutics and Jazz Pharmaceuticals; DSMC for Y-mAbs Therapeutics; and research support to institution from Merck, NEKTAR, Takeda, AstraZeneca and TAB Biosciences. J Bradley reports honoraria for consulting from Varian, Inc. and Genentech; and scientific advisory board from Mevion Medical systems, Inc. SS Ramalingam reports honoraria for consulting from Amgen, Bristol Myers Squibb, Genentech/Roche, Merck, AstraZeneca, Takeda, Eisai, Daiichi Sankyo, Sanofi, GlaxoSmithKline and Eli Lilly; and grants from Tesaro, Merck, AstraZeneca, Advaxis, Bristo Myers Squibb, Amgen, Takeda, Genmab and GlaxoSmithKline. TE Stinchcombe reports honoraria for consulting from Takeda, AstraZeneca, Genentech/Roche, Foundation Medicine, Pfizer, EMD Serono, Novartis, Daiichi Sankyo, Lilly, Medtronic, Puma Biotechnology, Janssen Oncology and Regeneron; and research funding to institution by Genentech/Roche, Blueprint Medicines, AstraZeneca, Takeda, Advaxis and Regeneron. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.International Agency for Research on Cancer. World Health Organization. Global Cancer Observatory: Cancer Today. Cancer fact sheet: all cancers. http://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 68(1), 7–30 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Pignon JP, Tribodet H, Scagliotti GV et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 26(21), 3552–3559 (2008). [DOI] [PubMed] [Google Scholar]; • Evaluates the benefit of adjuvant chemotherapy for non-small-cell lung cancer (NSCLC).
- 4.Govindan R, Mandrekar SJ, Gerber DE et al. ALCHEMIST trials: a golden opportunity to transform outcomes in early-stage non-small-cell lung cancer. Clin. Cancer Res. 21(24), 5439–5444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the ALCHEMIST platform.
- 5.Hamid O, Robert C, Daud A et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369(2), 134–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Ribas A, Wolchok JD et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a Phase I trial. Lancet 384(9948), 1109–1117 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Gandhi L, Rodriguez-Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378(22), 2078–2092 (2018). [DOI] [PubMed] [Google Scholar]; • KEYNOTE-189 was a landmark publication as it led to a change in the standard of care to include pembrolizumab with chemotherapy in first-line nonsquamous NSCLC.
- 8.Reck M, Rodriguez-Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375(19), 1823–1833 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379(21), 2040–2051 (2018). [DOI] [PubMed] [Google Scholar]; • KEYNOTE-407 was a landmark publication that led to the approval of pembrolizumab being included with chemotherapy in the first-line treatment of squamous cell NSCLC.
- 10.West H, McCleod M, Hussein M et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, Phase III trial. Lancet Oncol. 20(7), 924–937 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Bezjak A, Lee CW, Ding K et al. Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10. J. Clin. Oncol. 26(31), 5052–5059 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Rodriguez-Abreu D, Robinson AG et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label Phase III trial. Lancet Oncol. 18(12), 1600–1609 (2017). [DOI] [PubMed] [Google Scholar]; • This study demonstrated an improvement in health-related quality of life in patients with metastatic NSCLC treated with first-line pembrolizumab as compared to those treated with chemotherapy.
- 13.Wakelee HA, Dahlberg SE, Keller SM et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, Phase III trial. Lancet Oncol. 18(12), 1610–1623 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douillard JY, Rosell R, De Lena M et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 7(9), 719–727 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Arriagada R, Bergman B, Dunant A et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 350(4), 351–360 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur. J. Cancer 30A(5), 635–642 (1994). [DOI] [PubMed] [Google Scholar]


