Abstract
Background:
Whereas genetic susceptibility increases risk for major depressive disorder (MDD), non-genetic protective factors may mitigate this risk. In a large-scale prospective study of US Army soldiers, we examined whether trait resilience and/or unit cohesion could protect against the onset of MDD following combat deployment, even in soldiers at high polygenic risk.
Methods:
Data were analyzed from 3,079 soldiers of European ancestry assessed before and after their deployment to Afghanistan. Incident MDD was defined as no MDD episode at pre-deployment, followed by a MDD episode following deployment. Polygenic risk scores were constructed from a large-scale genome-wide association study of major depression. We first examined main effects of the MDD PRS and each protective factor on incident MDD. We then tested effects of each protective factor on incident MDD across strata of polygenic risk.
Results:
Polygenic risk showed a dose-response relationship to depression, such that soldiers at high polygenic risk had greatest odds for incident MDD. Both unit cohesion and trait resilience were prospectively associated with reduced risk for incident MDD. Notably, the protective effect of unit cohesion persisted even in soldiers at highest polygenic risk.
Conclusions:
Polygenic risk was associated with new-onset MDD in deployed soldiers. However, unit cohesion—an index of perceived support and morale—was protective against incident MDD even among those at highest genetic risk, and may represent a potent target for promoting resilience in vulnerable soldiers. Findings illustrate the value of combining genomic and environmental data in a prospective design to identify robust protective factors for mental health.
Keywords: Longitudinal, resilience, polygenic risk, depression, social support, genetics
Introduction
Exposure to stressful experiences is an important risk factor for major depressive disorder (MDD) (Kendler et al. 1999)—however, not all individuals exposed to stressful experiences develop MDD. This observation is of high relevance for the US Army, whose soldiers routinely encounter stressful events over the course of combat deployment (Adler et al. 2004) and show a correspondingly high burden of MDD following deployment (Wells et al. 2010; Shen et al. 2012; Bonde et al. 2016). Preventing MDD and its associated disability and comorbidities can improve individual/family wellbeing and troop readiness (Kline et al. 2010), and requires attention to risk and protective factors that influence depression.
The diathesis-stress model of depression (Hammen 2005) posits that some individuals have latent or pre-existing vulnerabilities, or diatheses, that are activated in the presence of stress to produce MDD. One such diathesis is genetic susceptibility, which has been found to substantially increase risk for MDD episodes in the presence of stressful life events (Kendler et al. 1995). Genetic susceptibility for a complex trait like MDD is thought to be polygenic—influenced by many common variants across the genome, each with relatively small effect sizes (Wray et al. 2014). This influence can be indexed by polygenic risk scores (PRS) that combine effects across common variants using results from a discovery genome-wide association study (GWAS). For MDD, a well-powered GWAS with 461,134 individuals (Wray & Sullivan 2017) has become available, and its derived PRS was recently validated in a diathesis-stress model for MDD in the context of life stressors (Colodro-Conde et al. 2017). However, to date, this PRS has not been prospectively validated in terms of new MDD onset following stress exposure.
While genetic susceptibility is a risk factor for depression, non-genetic protective factors may offset this risk, illuminating opportunities for prevention. Protective factors can be specific to the individual (intrinsic) or related to the individual’s environment (extrinsic) (Werner & Zigler 2000). One intrinsic factor that has been studied in Army populations is trait resilience, defined as perceived hardiness to stress and ability to cope adaptively with stressors (Connor & Davidson 2003). Unit cohesion—which includes emotional safety, bonding, and support between soldiers and with unit leaders—is an extrinsic factor that has also received substantial attention. Although these factors are well characterized for their protective effects on post-deployment mental health (Dolan & Adler 2006; Pietrzak et al. 2009; Wooten 2012; Zang et al. 2017), the extent to which they attenuate risk for MDD in the presence of genetic susceptibility has not been examined.
The Army Study of Risk and Resilience in Servicemembers (Army STARRS) has followed a large prospective sample of active duty soldiers with genomic data across one combat deployment cycle (Ursano et al. 2014). This provides a unique opportunity to test the effects of genetic susceptibility and candidate protective factors assessed shortly before deployment, in relation to development of MDD following deployment. Specifically, we examine whether two putative protective factors—trait resilience (intrinsic) and unit cohesion (extrinsic)—can reduce risk for incident post-deployment MDD even among soldiers at high polygenic risk for MDD.
Methods
Participants and procedures
The Pre/Post Deployment Study (PPDS) in Army STARRS is a multi-wave panel survey of US Army soldiers from three brigade combat teams that were deployed to Afghanistan in 2012. Soldiers from these brigade combat teams were eligible for this study if they provided written informed consent for participation. Soldiers completed baseline assessments within approximately six weeks before deployment, and follow-up assessments at three and nine months post-deployment. For this analysis, the sample was restricted to those with eligible survey responses and samples for genotyping. Procedures for Army STARRS and PPDS have been reported in detail elsewhere (Ursano et al. 2014; Stein et al. 2015), and were approved by the institutional review boards at the Uniformed Services University of the Health Sciences, Harvard University, University of Michigan, and University of California, San Diego.
Measures
Major depressive disorder (MDD).
MDD was ascertained at each assessment using items from the major depressive episode (MDE) scale of the WHO Composite International Diagnostic Interview-Screening Scales (CIDI-SC) (Kessler et al. 2013). Scale items assessed frequency of MDD symptoms (e.g., depressed mood, loss of interest) over the past 30 days, and were summed to yield overall symptom scores. Symptom scores were then dichotomized using receiver operating characteristic (ROC) curve analysis to determine clinical thresholds for past 30-day MDEs, as validated elsewhere (Kessler et al. 2013). Incident MDD was defined as no MDE at baseline, followed by a MDE at any point through nine months, while absence of incident MDD was defined as no MDE across both three and nine months (0=no incident depression, 1=incident depression). Soldiers who met criteria for an existing MDE at pre-deployment were excluded since MDD incidence could not be established. We included all remaining participants who had complete follow-up MDE data or otherwise met criteria for at least one MDE.
Trait resilience.
Trait resilience was self-reported by soldiers at baseline using a five-item scale derived from a larger pool of 17 items that were pilot-tested in earlier Army STARRS surveys and culled using exploratory factor analysis and item response theory analysis for administration in the PPDS. Information on the development and validation of this scale has been published elsewhere (Campbell-Sills et al. 2018). Participants reported on their abilities to “keep calm and think of the right thing to do in a crisis,” “manage stress,” or to “try new approaches if old ones don’t work” (all items described in Supplementary Materials S1A). Items were rated on a five-point Likert scale ranging from “poor” to “excellent,” and summed to yield continuous scores ranging from 0 and 20. Internal consistency was good (α=0.89). Scores were standardized to a mean of 0 and variance of 1 for analysis.
Unit cohesion.
Unit cohesion was assessed at baseline using a seven-item scale developed for this study and adapted from the Walter Reed Army Institute of Research (WRAIR) Military Cohesion Scales (Vaitkus 1994). Soldiers reported on perceived support and cohesion within their unit, including items such as “I can rely on members of my unit for help if I need it,” “I can open up and talk to my first line leaders if I need help,” and “My leaders take a personal interest in the well-being of all soldiers in my unit” (all items described in Supplementary Materials S1B). Items were rated on five-point Likert scale ranging from “strongly disagree” to “strongly agree,” and were summed to yield continuous scores ranging between 0 and 27. Internal consistency was high (α=0.89); a factor analysis confirmed that one single factor was sufficient to represent shared variability among these seven scale items. Scores were standardized to a mean of 0 and variance of 1 for analysis.
Combat stress exposure.
Within one month of return from deployment, soldiers also completed a 15-item measure of combat stress exposure—including engaging in combat patrol or other dangerous duties, and firing at and/or receiving enemy fire. These items (described in Supplementary Materials S2) were summed to reflect overall burden of combat stress exposure, as in previous research (Stein et al. 2015).
DNA processing
Detailed information about genotyping, imputation, quality control (QC), and population assignment in Army STARRs is available elsewhere (Stein et al. 2016). Briefly, DNA samples for each participant were genotyped using Illumina OmniExpress and Exome array with additional custom content. Initial quality control (QC) procedures were conducted to retain only (1) samples with genotype missingness < 0.02, no extreme autosomal heterozygosity, and no relatedness (if related pairs of individuals were identified, only one was kept); and (2) single-nucleotide polymorphisms (SNPs) with genotype missingness < 0.05 (before sample QC) and < 0.02 (after sample QC), minor allele frequency (MAF) > 0.05, and no violation of the Hardy-Weinberg equilibrium (p > 1×10−6).
Prior to imputation, SNPs were also removed if they were not present or had non-matching alleles in the 1000 Genomes Project reference panel (August 2012 phase 1 integrated release) (Auton et al. 2015) or had ambiguous alleles with MAF > 0.10. Following a two-step pre-phasing/imputation process (Howie et al. 2012), imputed SNPs were converted to “best guess” genotyped SNPs based on their imputation probability. Where no possible genotype met the threshold of 80% probability, information for that SNP was set as missing. SNPs were filtered again to retain missingness < 0.02 and imputation quality (INFO) score > 0.80, and duplicate SNPs were identified for exclusion in subsequent analyses.
Ancestry was inferred through principal component (PC) analyses as reported previously (Stein et al. 2016). Given that polygenic risk scores would be constructed using effect sizes obtained in samples of European ancestry (Wray & Sullivan 2017), only PPDS participants assigned to the European ancestry (EA) group were retained (N=4,900). By inspecting successive PC plots within the EA group for evidence of population structure, we determined only the first three principal components were likely relevant for inclusion as covariates in subsequent analyses.
Polygenic risk scoring
To construct the polygenic risk scores (PRS), we obtained summary statistics from the latest GWAS of major depression in 461,134 individuals (Wray & Sullivan 2017). For main analyses, we used the set of summary statistics without 23andMe data (N=173,005) that is now publicly available. After removal of ambiguous SNPs, we clumped the GWAS summary statistics using our EA genomic data to limit inclusion of highly correlated SNPs, using a r2 threshold of 0.25 and a 250kb window. These clumped summary statistics were used to compute PRS from our EA genomic data that included SNPs whose effects met the following p-value thresholds (pT) in decreasing order of stringency: 5×10−8, <0.0001, <0.001, 0.01, 0.05, 0.10, 0.50, 1.0. PRS were calculated as the total sum of risk alleles at each eligible SNP weighted by their estimated effect size (log odds ratio), divided by total number of SNPs included for scoring (Supplementary Table S2A).
Statistical analyses
First, we examined the MDD PRS at varying p-value thresholds in relation to incident MDD (Supplementary Materials Figure S3A). The PRS at the p-value threshold with largest Nagelkerke’s pseudo-R2 (pT=0.01) above and beyond a covariates-only model was selected for subsequent analyses (Ripke et al. 2013). This PRS was distributed across individuals and divided into three groups of polygenic risk (Supplementary Table S3B): low (quintile 1), intermediate (quintiles 2–4), and high (quintile 5) (Khera et al. 2016). Second, we used logistic regressions to examine the main effects of polygenic risk on incident MDD, using the low risk group as the reference group. Third, we used logistic regressions to examine the main effects of each protective factor on incident MDD. Fourth, we tested the effects of each protective factor (per standardized unit score) on incident MDD across polygenic risk groups, correcting for testing three separate models with a Bonferroni-corrected p-value of 0.017 (0.05/3). At each step, we adjusted for sex, age, and principal components to account for population stratification when polygenic scores were included. For any protective factor that showed protective effects for incident MDD in the context of polygenic risk, we conducted similar within-group analyses to test whether this factor would also show protective effects in the context of environmental risk (i.e., combat stress exposure). All analyses were conducted in R.
Results
Sample characteristics
Our sample consisted of PPDS participants of European ancestry who provided genome-wide data, excluding soldiers with an existing MDE at pre-deployment (N=310) and including all participants who had complete follow-up MDE data or otherwise met criteria for at least one MDE (resulting N=3,079). The sample was predominantly (96%) male and younger than 30 years old (mean=25.9, SD=5.8). At baseline, soldiers tended to report high trait resilience scores (mean=15.1, SD=4.0, max=20) and perceived their units as relatively cohesive (mean=19.7, SD=5.4, max=27). During deployment, soldiers reported experiencing an average of 4.1 major combat-related stressors (max=13.0, SD=2.8). Within nine months of returning from deployment, 13% (N=390) met criteria for incident MDD.
Are polygenic risk and protective factors associated with incident MDD?
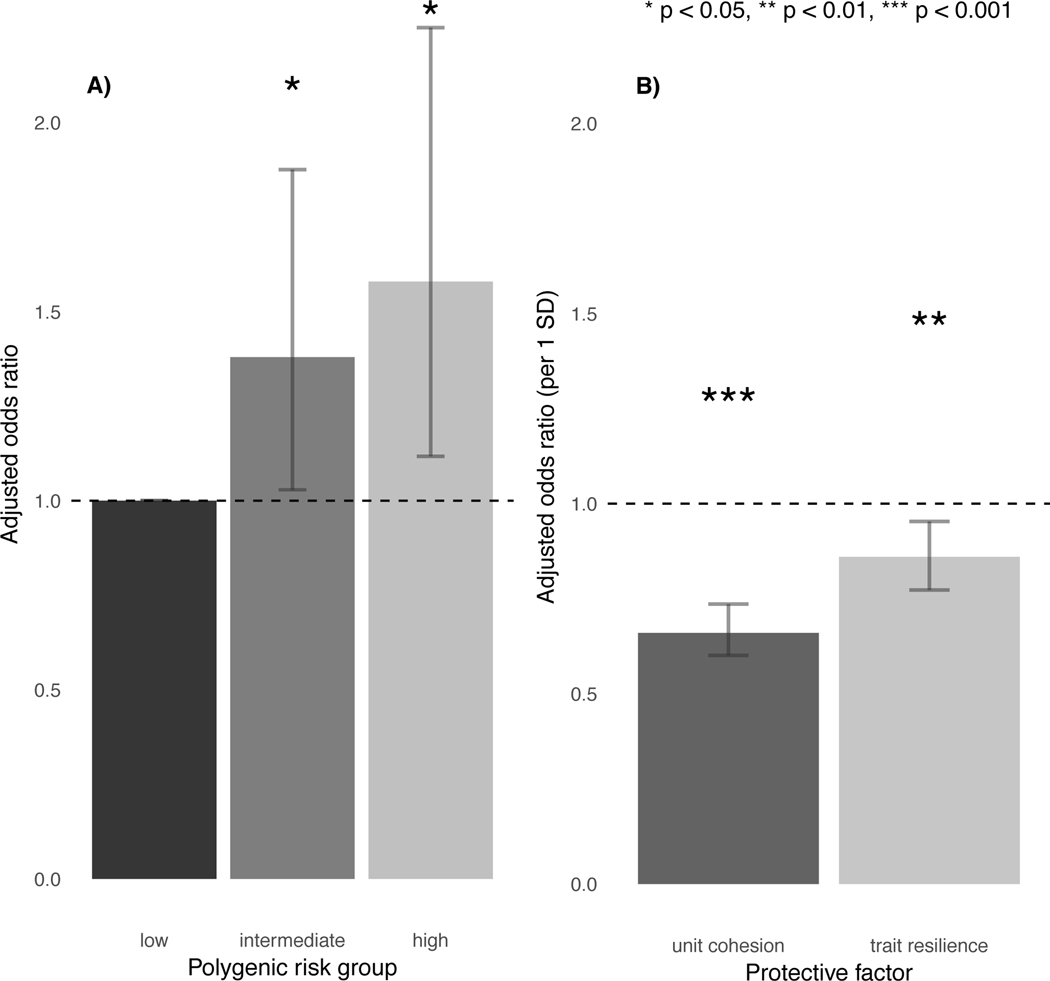
Polygenic risk showed a dose-response relationship with incident MDD. Compared to soldiers at low polygenic risk, odds for incident MDD were highest in soldiers at high polygenic risk (adjusted odds ratio [aOR]=1.58, 95% profile confidence interval [CI] = 1.12–2.25, p=.01) and more modestly increased in those at intermediate polygenic risk (aOR=1.38, 95% CI = 1.03–1.88, p=.04) (Figure 1a; full model results in Supplementary Table S3B).
Figure 1.
Main effects of A) polygenic risk and B) protective factors on incident MDD.
Soldiers who reported stronger unit cohesion at baseline had reduced odds for incident MDD (aOR=.67, 95% CI = 0.60–0.74, p=3.5×10−15), as did those who reported higher trait resilience (aOR=0.86, 95% CI = 0.77–0.95, p=3.2×10−3 (Figure 1b). Of note, unit cohesion and trait resilience were not significantly correlated with polygenic risk (r=−0.02, p=.30; r=−0.01, p=0.68, respectively), suggesting that the polygenic risk score for MDD was relatively specific to depression versus other traits in this sample.
Across the spectrum of polygenic risk, what are the effects of trait resilience and unit cohesion on incident MDD?
Across all strata of polygenic risk, soldiers who reported stronger unit cohesion had significantly lower odds for incident MDD (low: aOR=0.64, 95% CI = 0.50–0.83, p=5.4×10−4; intermediate: aOR=0.65, 95% CI = 0.57–0.74, p=6.4×10−11; high: aOR=0.75, 95% CI = 0.60–0.93, p=8.8×10−3), after Bonferroni correction. Notably, even among those at highest polygenic risk, unit cohesion was associated with reduced incidence of post-deployment MDD (Figure 2a). Trait resilience showed similar but only nominally significant effects for incident MDD across strata of polygenic risk (low: aOR=0.81, 95% CI = 0.62–1.05, p=.11; intermediate: aOR=0.90, 95% CI = 0.79–1.03, p=.13; high: aOR=0.80, 95% CI = 0.65–0.99, p=.04), after Bonferroni correction (Figure 2b). Follow-up regressions confirmed independent and non-interactive effects of polygenic risk—both categorically and continuously defined—as well as unit cohesion, but not trait resilience, on incident MDD (Supplementary Tables S3C, S3D, S3E, S3F).
Figure 2.
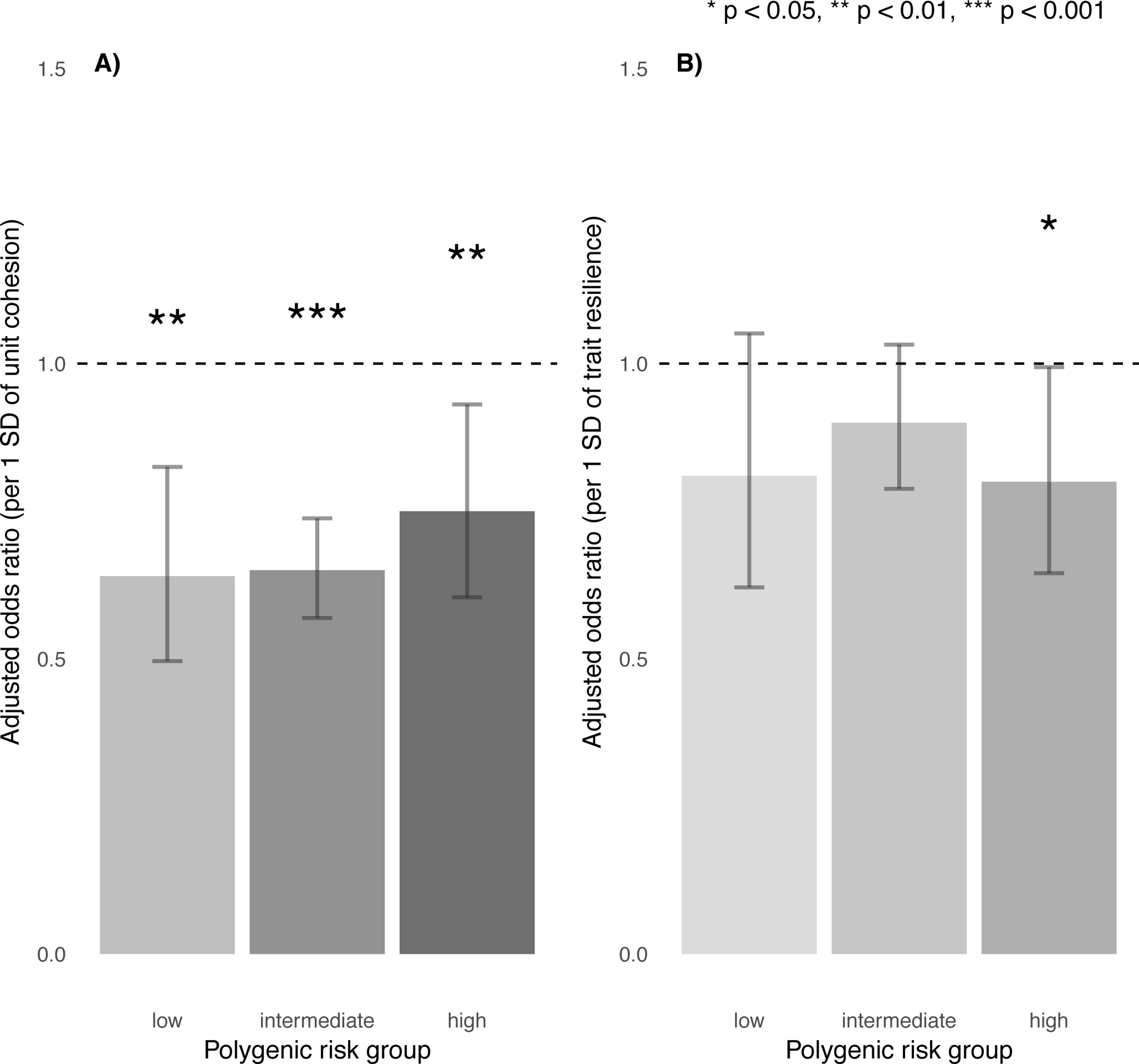
Effects of A) unit cohesion and B) trait resilience on incident MDD, stratified by polygenic risk.
Does unit cohesion also protect against MDD across different levels of environmental risk?
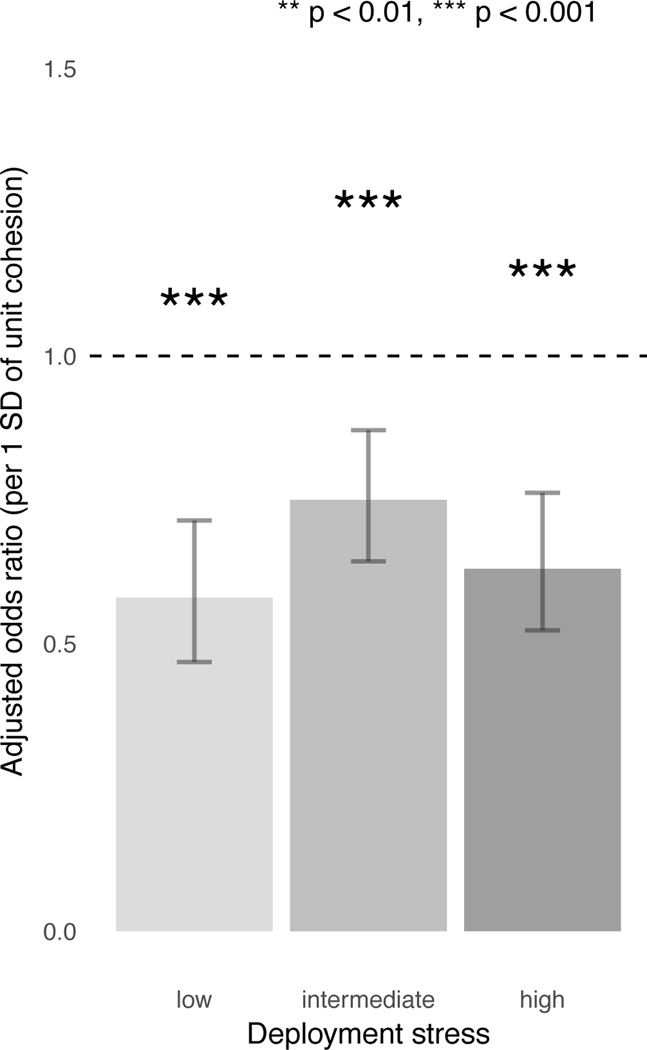
To further explore the protective effect of unit cohesion across different sources of risk, we stratified soldiers by combat stress exposure: low (quintile 1), intermediate (quintiles 2–4), and high (quintile 5). Combat stress exposure was itself a risk factor for incident MDD (aOR=1.11 per additional exposure, 95% CI = 1.07–1.15, p=3.1×10−8). Unit cohesion remained protective against incident MDD across all levels of combat stress exposure (low: aOR=0.58, 95% CI = 0.47–0.71, p=3.7×10−7; intermediate: aOR=0.75, 95% CI = 0.64–0.87, p=1.7×10−4; high: aOR=0.63, 95% CI = 0.52–0.76, p=1.7×10−6), even for those who reported high levels of combat stress (Figure 3). Follow-up regression confirmed independent and non-interactive effects of polygenic risk, unit cohesion, and combat stress exposure on incident MDD (Supplementary Table S3G).
Figure 3.
Effects of unit cohesion on incident MDD across levels of combat stress exposure.
Discussion
Prior research has established that genetic vulnerability and stressful experiences increase risk for depression—however, two important areas have remained underexplored. First, an emphasis on risk factors has provided little information about the potential for protective factors to promote resilience in the face of genetic or environmental risks. Second, while we know that genetic vulnerability confers risk for depression, implications for actionable or clinically relevant opportunities for prevention are largely unknown. Here, we take advantage of a unique, large-scale, prospectively studied cohort of Army soldiers for whom both genomic data and exposure to a defined class of stressors is available, to answer questions about the nature of risk and protective factors for depression. We find that unit cohesion—an index of social support and morale—is prospectively associated with reduced risk for post-deployment depression, and that this protective effect persists even for soldiers with high genetic or environmental risk.
Our work makes a number of contributions to the literature. First, we demonstrate for the first time that polygenic risk is prospectively associated with new-onset depression following stress exposure. Drawing on large-scale GWAS of major depression (Wray & Sullivan 2017), we observe a dose-response relationship between polygenic risk and incident MDD following combat deployment, with a 52% increase in relative odds between soldiers in the top and bottom quintiles of polygenic risk. Such differences suggest that PRS meaningfully explained increased risk for depression in our sample. Although PRS have known limitations (Wray et al. 2013; Bogdan et al. 2018)—notably the fact that they still explain limited variance in psychiatric outcomes, in addition to being constrained to the scope and size of existing GWAS, which limit current predictive utility in clinical settings—they may prove informative in their ability to stratify risk for epidemiological investigation.
Second, we provide novel evidence that strong unit cohesion prior to deployment may offset psychiatric risk despite underlying genetic susceptibility. Protective effects in the presence of high polygenic risk have been shown in cardiology (Khera et al. 2016) and we now apply this framework in psychiatry. While previous research has identified unit cohesion as a protective factor for mental health following deployment, most studies have been cross-sectional (Pietrzak et al. 2009; Armistead-Jehle et al. 2011; Du Preez et al. 2012; Goldmann et al. 2012; Jones et al. 2012; Kanesarajah et al. 2016; Zang et al. 2017) and ours represents at least a four-fold increase in scale compared to existing prospective studies of unit cohesion and mental health (Polusny et al. 2011; Han et al. 2014), in addition to being the first to integrate genetic data.
Third, we corroborate prior evidence that unit cohesion is associated with reduced risk for incident MDD despite high levels of combat stress exposure (Armistead-Jehle et al. 2011; Polusny et al. 2011; Jones et al. 2012; Han et al. 2014; Kanesarajah et al. 2016) and extend this to show that pre-deployment unit cohesion, combat stress exposure, and genetic susceptibility additively, and to some extent orthogonally, influence risk for incident MDD. Together, this suggests that unit cohesion may be widely beneficial for soldiers despite genetic or environmental risk. Unit cohesion has been conceptualized as a multi-faceted construct (Siebold 1999), including horizontal cohesion (e.g., perceived support from fellow soldiers, sense of bonding and camaraderie between soldiers, trust and reliance on fellow soldiers) and vertical cohesion (e.g., respect and appreciation from unit leaders; clear communication with unit leaders) (Manning 1994). Our measure tapped into both aspects of unit cohesion, particularly respect and support between soldiers and with their leaders. Given inevitable stressors encountered during deployment, feeling comfortable seeking help and/or raising concerns may facilitate better coping than self-directed efforts to regulate stress (Berkman 2000). Moreover, strengthening such dimensions of unit cohesion is putatively actionable (Greden et al. 2010; Williams et al. 2016)—for example, by providing leadership skills training, facilitating regular team-based interactions between soldiers during training, and keeping units operationally intact across training and deployment—though interventions remain to be rigorously tested.
Our study has several limitations. First, our analyses focused on two protective factors as exemplars of intrinsic versus extrinsic pre-deployment features, and have not tested a comprehensive set of protective factors that could reduce risk for MDD. Notably, even with the inclusion of protective factors, polygenic risk, demographic factors, and combat exposure, and our models still explained a relatively small proportion of variance in risk for MDD (Supplementary Tables S3B-S3G), which highlights the complexity of predicting MDD even with robust variables and a relatively homogeneous population. Second, our construct of unit cohesion was measured at the individual level and may thus capture both extrinsic factors (e.g., quality of relationships, unit culture) as well as intrinsic factors that influence soldiers’ perceptions of unit cohesion (e.g., agreeableness, current distress). Future studies could use unit-level cohesion scores to further circumvent reporting bias, account for potential clustering within units, and better isolate unit cohesion as an exogenous risk factor. However, individual reports may better capture the soldier’s own social experience within the unit, which could be most relevant for psychiatric risk. Third, in order to establish incident MDD, we restricted our sample so that no participant met criteria for a 30-day depressive episode shortly before deployment. However, we did not exclude subthreshold symptoms; lifetime MDD in partial or full remission at the baseline assessment; and/or other comorbid psychopathology; and it is possible that such factors would have contributed to predicting post-deployment MDD. Despite combat exposure, rates of MDD in this Army sample were generally comparable to those observed in the population at large. This may be due to the fact that soldiers with greater mental health vulnerabilities may have not been deployed to combat or previously left Army service. We also chose to examine MDD as a form of clinically significant depression requiring targeted attention, rather than depressive symptoms across a continuum. Notably, we found similar results (not shown) when considering average post-deployment depressive symptoms—in that polygenic risk was not only associated with greater symptom severity, but unit cohesion was also linked to significantly decreased symptom severity even among soldiers at high polygenic risk. While this study focused on MDD given availability of a relevant polygenic score, future work could also examine other manifestations of psychopathology following deployment, including substance use and other stress-related disorders, as genome-wide discovery progresses. Fourth, our MDD polygenic risk score was based on a reduced GWAS meta-analytic sample for which results are publicly available, which may limit power to capture all polygenic influences on MDD. Finally, our sample was primarily male and was limited to individuals of European ancestry (to maximize the power of the PRS which was based on GWAS of European ancestry subjects). Thus, our results may not generalize beyond Army populations or to female or non-EA individuals.
In conclusion, our findings support a role for both genetic and environmental factors in influencing psychiatric risk in soldiers across combat deployment. In this prospective inquiry, we showed that soldiers who experienced strong unit cohesion shortly before deployment were at reduced risk for incident MDD following deployment, regardless of their genetic susceptibility. This study illustrates the potential of protective factors to offset psychiatric risk following exposure to stressful events. Importantly, potentially actionable factors such as group cohesion and social support may protect against depression even among those most genetically susceptible, and represent promising targets for promoting resilience in at-risk populations.
Supplementary Material
Acknowledgments
Funding:
Army STARRS was sponsored by the Department of the Army and funded under cooperative agreement number U01MH087981 (2009–2015) with the National Institutes of Health, National Institute of Mental Health (NIH/NIMH). Subsequently, STARRS-LS was sponsored and funded by the Department of Defense (USUHS grant number HU0001-15-2-0004). The contents are solely the responsibility of the authors and do not necessarily represent the views of the Department of Health and Human Services, NIMH, the Department of the Army, or the Department of Defense. Dr. Choi was supported in part by a NIMH T32 Training Fellowship (T32MH017119). Dr. Smoller is a Tepper Family MGH Research Scholar and supported in part by the Demarest Lloyd, Jr, Foundation and NIH grant K24MH094614. The Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium was supported in part by NIH grant U01MH109528.
Conflicts of interest:
Dr. Stein has in the past three years been a consultant for Actelion, Aptinyx, Dart Neuroscience, Healthcare Management Technologies, Janssen, Neurocrine Biosciences, Oxeia Biopharmaceuticals, Pfizer, and Resilience Therapeutics. Dr. Stein owns founders shares and stock options in Resilience Therapeutics and has stock options in Oxeia Biopharmaceticals. Dr. Smoller is an unpaid member of the Scientific Advisory Board of Psy Therapeutics, Inc and of the Bipolar/Depression Research Community Advisory Panel of 23andMe. In the past three years, Dr. Kessler has been a consultant for Hoffman-La Roche, Inc., Johnson & Johnson Wellness and Prevention, and Sanofi-Aventis Groupe. Dr. Kessler has served on advisory boards for Mensante Corporation, Plus One Health Management, Lake Nona Institute, and U.S. Preventive Medicine. Dr. Kessler owns 25% share in DataStat, Inc. The remaining authors report nothing to disclose.
Footnotes
The STARRS Team:
Co-Principal Investigators: Robert J. Ursano, MD (Uniformed Services University of the Health Sciences) and Murray B. Stein, MD, MPH (University of California San Diego and VA San Diego Healthcare System)
Site Principal Investigators: Steven Heeringa, PhD (University of Michigan), James Wagner, PhD (University of Michigan) and Ronald C. Kessler, PhD (Harvard Medical School) Army liaison/consultant: Kenneth Cox, MD, MPH (USAPHC (Provisional))
Other team members: Pablo A. Aliaga, MS (Uniformed Services University of the Health Sciences); COL David M. Benedek, MD (Uniformed Services University of the Health Sciences); Susan Borja, PhD (NIMH); Tianxi Cai, ScD (Harvard School of Public Health); Laura Campbell-Sills, PhD (University of California San Diego); Carol S. Fullerton, PhD (Uniformed Services University of the Health Sciences); Nancy Gebler, MA (University of Michigan); Robert K. Gifford, PhD (Uniformed Services University of the Health Sciences); Paul E. Hurwitz, MPH (Uniformed Services University of the Health Sciences); Kevin Jensen, PhD (Yale University); Kristen Jepsen, PhD (University of California San Diego); Tzu-Cheg Kao, PhD (Uniformed Services University of the Health Sciences); Lisa Lewandowski-Romps, PhD (University of Michigan); Holly Herberman Mash, PhD (Uniformed Services University of the Health Sciences); James E. McCarroll, PhD, MPH (Uniformed Services University of the Health Sciences); Colter Mitchell, PhD (University of Michigan); James A. Naifeh, PhD (Uniformed Services University of the Health Sciences); Tsz Hin Hinz Ng, MPH (Uniformed Services University of the Health Sciences); Caroline Nievergelt, PhD (University of California San Diego); Nancy A. Sampson, BA (Harvard Medical School); CDR Patcho Santiago, MD, MPH (Uniformed Services University of the Health Sciences); Ronen Segman, MD (Hadassah University Hospital, Israel); Alan M. Zaslavsky, PhD (Harvard Medical School); and Lei Zhang, MD (Uniformed Services University of the Health Sciences).
References
- Adler AB, McGurk D, Stetz MC, Bliese PD (2004). Military Occupational Stressors in Garrison, Training, and Deployed Environments. In Modeling Military Stressors: The WRAIR Occupational Stress Research Program. Toronto. [Google Scholar]
- Armistead-Jehle P, Johnston SL, Wade NG, Ecklund CJ (2011). Posttraumatic Stress in U.S. Marines: The Role of Unit Cohesion and Combat Exposure. Journal of Counseling & Development 89, 81–88. [Google Scholar]
- Auton A, Abecasis GR, Altshuler DM, Durbin RM, Abecasis GR, Bentley DR, Chakravarti A, Clark AG, Donnelly P, Eichler EE, Flicek P, Gabriel SB, Gibbs RA, Green ED, Hurles ME, Knoppers BM, Korbel JO, Lander ES, Lee C, Lehrach H, Mardis ER, Marth GT, McVean GA, Nickerson DA, Schmidt JP, Sherry ST, Wang J, Wilson RK, Gibbs RA, Boerwinkle E, Doddapaneni H, Han Y, Korchina V, Kovar C, Lee S, Muzny D, Reid JG, Zhu Y, Wang J, Chang Y, Feng Q, Fang X, Guo X, Jian M, Jiang H, Jin X, Lan T, Li G, Li J, Li Y, Liu S, Liu X, Lu Y, Ma X, Tang M, Wang B, Wang G, Wu H, Wu R, Xu X, Yin Y, Zhang D, Zhang W, Zhao J, Zhao M, Zheng X, Lander ES, Altshuler DM, Gabriel SB, Gupta N, Gharani N, Toji LH, Gerry NP, Resch AM, Flicek P, Barker J, Clarke L, Gil L, Hunt SE, Kelman G, Kulesha E, Leinonen R, McLaren WM, Radhakrishnan R, Roa A, Smirnov D, Smith RE, Streeter I, Thormann A, Toneva I, Vaughan B, Zheng-Bradley X, Bentley DR, Grocock R, Humphray S, James T, Kingsbury Z, Lehrach H, et al. (2015). A global reference for human genetic variation. Nature 526, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF (2000). Social Support, Social Networks, Social Cohesion and Health. Social Work in Health Care 31, 3–14. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Baranger DAA, Agrawal A (2018). Polygenic Risk Scores in Clinical Psychology: Bridging Genomic Risk to Individual Differences. Annual Review of Clinical Psychology 14, 119–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP, Utzon-Frank N, Bertelsen M, Borritz M, Eller NH, Nordentoft M, Olesen K, Rod NH, Rugulies R (2016). Risk of depressive disorder following disasters and military deployment: Systematic review with meta-analysis. British Journal of Psychiatry 208, 330–336. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Kessler RC, Ursano RJ, Sun X, Taylor CT, Heeringa SG, Nock MK, Sampson NA, Jain S, Stein MB (2018). Predictive validity and correlates of self-assessed resilience among U.S. Army soldiers. Depression and Anxiety 35, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colodro-Conde L, Couvy-Duchesne B, Zhu G, Coventry WL, Byrne EM, Gordon S, Wright MJ, Montgomery GW, Madden P a. F, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Ripke S, Eaves LJ, Heath AC, Wray NR, Medland SE, Martin NG (2017). A direct test of the diathesis-stress model for depression. Molecular Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Davidson JRT (2003). Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depression and Anxiety 18, 76–82. [DOI] [PubMed] [Google Scholar]
- Dolan CA, Adler AB (2006). Military hardiness as a buffer of psychological health on return from deployment. Military Medicine 171, 93–98. [DOI] [PubMed] [Google Scholar]
- Du Preez J, Sundin J, Wessely S, Fear NT (2012). Unit cohesion and mental health in the UK armed forces. Occupational Medicine (Oxford, England) 62, 47–53. [DOI] [PubMed] [Google Scholar]
- Goldmann E, Calabrese JR, Prescott MR, Tamburrino M, Liberzon I, Slembarski R, Shirley E, Fine T, Goto T, Wilson K, Ganocy S, Chan P, Serrano MB, Sizemore J, Galea S (2012). Potentially modifiable pre-, peri-, and postdeployment characteristics associated with deployment-related posttraumatic stress disorder among ohio army national guard soldiers. Annals of Epidemiology 22, 71–78. [DOI] [PubMed] [Google Scholar]
- Greden JF, Valenstein M, Spinner J, Blow A, Gorman LA, Dalack GW, Marcus S, Kees M (2010). Buddy-to-Buddy, a citizen soldier peer support program to counteract stigma, PTSD, depression, and suicide: Buddy-to-Buddy for citizen soldiers support. Annals of the New York Academy of Sciences 1208, 90–97. [DOI] [PubMed] [Google Scholar]
- Hammen C (2005). Stress and Depression. Annual Review of Clinical Psychology 1, 293–319. [DOI] [PubMed] [Google Scholar]
- Han SC, Castro F, Lee LO, Charney ME, Marx BP, Brailey K, Proctor SP, Vasterling JJ (2014). Military unit support, postdeployment social support, and PTSD symptoms among active duty and National Guard soldiers deployed to Iraq. Journal of Anxiety Disorders 28, 446–453. [DOI] [PubMed] [Google Scholar]
- Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR (2012). Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nature Genetics 44, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, Seddon R, Fear NT, McAllister P, Wessely S, Greenberg N (2012). Leadership, cohesion, morale, and the mental health of UK Armed Forces in Afghanistan. Psychiatry 75, 49–59. [DOI] [PubMed] [Google Scholar]
- Kanesarajah J, Waller M, Zheng WY, Dobson AJ (2016). Unit cohesion, traumatic exposure and mental health of military personnel. Occupational Medicine (Oxford, England) 66, 308–315. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA (1999). Causal Relationship Between Stressful Life Events and the Onset of Major Depression. American Journal of Psychiatry 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ (1995). Stressful life events, genetic liability, and onset of an episode of major depression in women. The American Journal of Psychiatry 152, 833–842. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Santiago PN, Colpe LJ, Dempsey CL, First MB, Heeringa SG, Stein MB, Fullerton CS, Gruber MJ, Naifeh JA, Nock MK, Sampson NA, Schoenbaum M, Zaslavsky AM, Ursano RJ (2013). Clinical reappraisal of the Composite International Diagnostic Interview Screening Scales (CIDI-SC) in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS): Clinical reappraisal of the CIDI-SC in Army STARRS. International Journal of Methods in Psychiatric Research 22, 303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, Fuster V, Boerwinkle E, Melander O, Orho-Melander M, Ridker PM, Kathiresan S (2016). Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. The New England Journal of Medicine 375, 2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A, Falca-Dodson M, Sussner B, Ciccone DS, Chandler H, Callahan L, Losonczy M (2010). Effects of Repeated Deployment to Iraq and Afghanistan on the Health of New Jersey Army National Guard Troops: Implications for Military Readiness. American Journal of Public Health 100, 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning F (1994). Morale and cohesion in military psychiatry. In Military psychiatry: Preparing in peace for war. vol (Part 1), pp1–8. [Google Scholar]
- Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM (2009). Psychological resilience and postdeployment social support protect against traumatic stress and depressive symptoms in soldiers returning from Operations Enduring Freedom and Iraqi Freedom. Depression and Anxiety 26, 745–751. [DOI] [PubMed] [Google Scholar]
- Polusny MA, Erbes CR, Murdoch M, Arbisi PA, Thuras P, Rath MB (2011). Prospective risk factors for new-onset post-traumatic stress disorder in National Guard soldiers deployed to Iraq. Psychological Medicine 41, 687–698. [DOI] [PubMed] [Google Scholar]
- Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, Bergen SE, Collins AL, Crowley JJ, Fromer M, Kim Y, Lee SH, Magnusson PKE, Sanchez N, Stahl EA, Williams S, Wray NR, Xia K, Bettella F, Borglum AD, Bulik-Sullivan BK, Cormican P, Craddock N, de Leeuw C, Durmishi N, Gill M, Golimbet V, Hamshere ML, Holmans P, Hougaard DM, Kendler KS, Lin K, Morris DW, Mors O, Mortensen PB, Neale BM, O’Neill FA, Owen MJ, Milovancevic MP, Posthuma D, Powell J, Richards AL, Riley BP, Ruderfer D, Rujescu D, Sigurdsson E, Silagadze T, Smit AB, Stefansson H, Steinberg S, Suvisaari J, Tosato S, Verhage M, Walters JT, Multicenter Genetic Studies of Schizophrenia Consortium, Levinson DF, Gejman PV, Kendler KS, Laurent C, Mowry BJ, O’Donovan MC, Owen MJ, Pulver AE, Riley BP, Schwab SG, Wildenauer DB, Dudbridge F, Holmans P, Shi J, Albus M, Alexander M, Campion D, Cohen D, Dikeos D, Duan J, Eichhammer P, Godard S, Hansen M, Lerer FB, Liang K-Y, Maier W, Mallet J, Nertney DA, Nestadt G, Norton N, O’Neill FA, Papadimitriou GN, Ribble R, Sanders AR, Silverman JM, Walsh D, Williams NM, Wormley B, Psychosis Endophenotypes International Consortium, Arranz MJ, Bakker S, Bender S, Bramon E, et al. (2013). Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nature Genetics 45, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y-C, Arkes J, Williams TV (2012). Effects of Iraq/Afghanistan Deployments on Major Depression and Substance Use Disorder: Analysis of Active Duty Personnel in the US Military. American Journal of Public Health 102, S80–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold GL (1999). The Evolution of the Measurement of Cohesion. Military Psychology 11, 5–26. [Google Scholar]
- Stein MB, Chen C-Y, Ursano RJ, Cai T, Gelernter J, Heeringa SG, Jain S, Jensen KP, Maihofer AX, Mitchell C, Nievergelt CM, Nock MK, Neale BM, Polimanti R, Ripke S, Sun X, Thomas ML, Wang Q, Ware EB, Borja S, Kessler RC, Smoller JW, Army Study to Assess Risk and Resilience in Servicemembers (STARRS) Collaborators (2016). Genome-wide Association Studies of Posttraumatic Stress Disorder in 2 Cohorts of US Army Soldiers. JAMA psychiatry 73, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Kessler RC, Heeringa SG, Jain S, Campbell-Sills L, Colpe LJ, Fullerton CS, Nock MK, Sampson NA, Schoenbaum M, Sun X, Thomas ML, Ursano RJ, On behalf of the Army STARRS collaborators (2015). Prospective Longitudinal Evaluation of the Effect of Deployment-Acquired Traumatic Brain Injury on Posttraumatic Stress and Related Disorders: Results From the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). American Journal of Psychiatry 172, 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursano RJ, Colpe LJ, Heeringa SG, Kessler RC, Schoenbaum M, Stein MB, Army STARRS collaborators (2014). The Army study to assess risk and resilience in servicemembers (Army STARRS). Psychiatry 77, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkus MA (1994). Unit Manning System: Human Dimensions Field Evaluation of the COHORT Company Replacement Model. In Report No. WRAIR/TR-94e0017. Walter Reed Army Institute of Research: Washington, DC. [Google Scholar]
- Wells TS, LeardMann CA, Fortuna SO, Smith B, Smith TC, Ryan MAK, Boyko EJ, Blazer D, for the Millennium Cohort Study Team (2010). A Prospective Study of Depression Following Combat Deployment in Support of the Wars in Iraq and Afghanistan. American Journal of Public Health 100, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner EE, Zigler EF (2000). Protective Factors and Individual Resilience. In Handbook of Early Childhood Intervention 2nd edn Eds Shonkoff JP & Meisels SJ, pp115–132. Cambridge University Press: Cambridge. [Google Scholar]
- Williams J, Brown JM, Bray RM, Anderson Goodell EM, Rae Olmsted K, Adler AB (2016). Unit Cohesion, Resilience, and Mental Health of Soldiers in Basic Combat Training. Military Psychology 28, 241–250. [Google Scholar]
- Wooten NR (2012). Deployment cycle stressors and post-traumatic stress symptoms in Army National Guard women: the mediating effect of resilience. Social Work in Health Care 51, 828–849. [DOI] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, Middeldorp CM (2014). Research review: Polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry, and Allied Disciplines 55, 1068–1087. [DOI] [PubMed] [Google Scholar]
- Wray NR, Sullivan PF (2017). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Yang J, Hayes BJ, Price AL, Goddard ME, Visscher PM (2013). Pitfalls of predicting complex traits from SNPs. Nature Reviews. Genetics 14, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Gallagher T, McLean CP, Tannahill HS, Yarvis JS, Foa EB, STRONG STAR Consortium (2017). The impact of social support, unit cohesion, and trait resilience on PTSD in treatment-seeking military personnel with PTSD: The role of posttraumatic cognitions. Journal of Psychiatric Research 86, 18–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.