Abstract
Extracellular vesicles (EVs) are produced by diverse eukaryotic and prokaryotic cells. They have prominent roles in the modulation of cell-cell communication, inflammation versus immunomodulation, carcinogenic processes, cell proliferation and differentiation, and tissue regeneration. These acellular vesicles are more promising than cellular methods because of the lower risk of tumor formation, autoimmune responses and toxic effects compared with cell therapy. Moreover, the small size and lower complexity of these vesicles compared with cells have made their production and storage easier than cellular methods. Exosomes originated from mesenchymal stem cells has also been introduced as therapeutic option for a number of human diseases. The current review aims at summarization of the role of EVs in the regenerative medicine with a focus on their therapeutic impacts in liver fibrosis, lung disorders, osteoarthritis, colitis, myocardial injury, spinal cord injury and retinal injury.
Keywords: extracellular medicine, regenerative medicine, mesenchymal stem cells, biomarkers, expression
Introduction
Being released from diverse eukaryotic and prokaryotic cells, extracellular vesicles (EVs) have prominent roles in the modulation of cell-cell communication, inflammation versus immunomodulation, carcinogenic processes, cell proliferation and differentiation, and tissue regeneration (Soleymani-Goloujeh et al., 2018). Collectively, EV include an assorted cell-secreted assemblies enclosed by a bilayer phospholipid membrane containing various macromolecules such as proteins, lipids, and nucleic acids (Robbins and Morelli, 2014; Fuster-Matanzo et al., 2015). The interaction between EVs and target cells is accomplished via various routes including the interplay between transmembrane proteins on EVs and cellular surface receptors and induction of certain signaling pathways (Raposo and Stoorvogel, 2013). Alternatively, EVs can directly fuse with their target cells and release their constituents into the cytosol after endocytosis (Raposo and Stoorvogel, 2013). Being implicated in a wide range of pathophysiological processes, EVs can be used as biomarkers of diverse disorders and targets for the design of new cell-free therapeutic options (Fuster-Matanzo et al., 2015). Microvesicles and exosomes comprise two main categories of EVs with sizes about 100 nm–1 μm and 30–150 nm, respectively (Doyle and Wang, 2019).
Due to the heterogeneity of EVs and their sizes, isolation, identification and classification of EVs are challenging issues (Ramis, 2020). Yet, ample works are being conducted to enhance procedures for investigation of EVs. A new aqueous two-phase system-based method has been established for highly efficient isolation of EVs with high level of purity (Kırbas̨ O. K. et al., 2019). Another EV immunolabeling method has been introduced that can be incorporated into the currently used nanoparticle tracking analysis protocols to provide particle concentration, size scattering, and surface characteristics of EVs (Thane et al., 2019). Moreover, a luminescence-based assay has been developed that can obviously discriminate between EV uptake and EV binding to the surface of target cells (Toribio et al., 2019). Lastly, generation of an inducible CD9-GFP mouse has provided a method for EV labeling in a cell-type specific way and simultaneous analysis of EVs in vivo (Neckles et al., 2019). It is worth mentioning that the method used for isolation of EVs has clear impact on the integrity and purity of EVs.
Several studies have emphasized on the role of EVs in tissue engineering and regenerative medicine with the aim of reestablishment of an injured or abnormal-working tissue (De Jong et al., 2014). The current review aims at summarization of the role of EVs in the regenerative medicine with a focus on their therapeutic impacts in liver fibrosis, lung disorders, osteoarthritis, colitis, myocardial injury, spinal cord injury and retinal injury.
Liver Fibrosis
Mesenchymal stem cells (MSCs) has been introduced as therapeutic option for liver disease based on their ability to differentiate into hepatic cells and their aptitude in the reduction of inflammatory responses through secretion of certain anti-inflammatory cytokines (Lou et al., 2017a). MSC-derived exosomes are superior to MSCs regarding lower probability induction of tumors, rejection and toxicity (Lou et al., 2017a). Expression of miR-122 has been decreased in transactivated hepatic stellate cells (HSCs). Exosomes originated from adipose tissue-derived MSCs have been displayed to up-regulate miR-122. Up-regulation of miR-122 has suppressed the proliferation of LX2 cells through targeting P4HA1 gene. This miRNA has been shown to reduce collagen maturation and extracellular matrix synthesis (Li J. et al., 2013). MSC-derived substances might also be used in the treatment of fulminant hepatic failure (FHF). In a rat model of acute hepatic injury, systemic administration of MSC-conditioned medium has enhanced survival of animals, prevented the production of hepatic damage markers, decreased apoptosis rate of hepatic cells and increased the quantities of proliferating hepatic cells. Taken together, MSC-conditioned medium has direct anti-apoptotic and pro-mitotic impacts on hepatic cells and is a possible method for the management of FHF (Van Poll et al., 2008). Besides, MSC-conditioned medium (CM) has been revealed to influence apoptotic processes in cultured mouse primary hepatic cells following induction of hepatic injury using carbon tetrachloride (CCl4). In this study, bone marrow MSCs (BM-MSCs) have been used for generation of CM. Authors have demonstrated up-regulation of IL-6 in the CCl4-CM treated hepatocytes on the first day of culture. Moreover, levels of fibroblast-like-protein (FGL1) have been increased after 48 h, while annexin V positive hepatocytes have been decreased at day 3 post plating. These results have indicated the impact of this CM in attenuation of CCl4-induced apoptosis in liver cells via induction of FGL1 (Xagorari et al., 2013). Another study has assessed the impact of MSCs on the phenotype and activity of natural killer T (NKT) cells in a mouse model of hepatic injury induced by concanavalin A and α-galactosylceramide. In vitro culture of liver NKT cells with MSCs has resulted in production of lower quantities of TNF-α, IFN-γ and IL-4 proinflammatory cytokines while over-production of the anti-inflammatory cytokine IL-10 upon stimulation with α-galactosylceramide. MSCs have also deceased levels of apoptosis-inducing ligands on hepatic NKT cells and diminished levels of pro-apoptotic genes in the hepatic tissue. Notably, MSCs have decreased the cytotoxic effects of hepatic NKT cells against hepatocytes. These effects have been shown to be mediated by indoleamine 2,3-dioxygenase (IDO) and inducible nitric oxide synthase (iNOS). Moreover, human MSCs have also been shown to reduce release of proinflammatory cytokines in α-galactosylceramide-stimulated human peripheral blood mononuclear cells via a similar route and decrease their cytotoxic effects against hepatic cells (Gazdic et al., 2018). In addition, transplantation of human umbilical cord-MSCs (UC-MSCs) into acutely damaged and fibrotic liver have restored hepatic function and ameliorated liver fibrosis. Exosomes originated from these cells have decreased the surface fibrous capsules, lessened inflammatory responses in the hepatic tissue and collagen deposition in CCl4-associated fibrotic liver. Levels of collagen type I and III, TGF-β1 and phosphorylated Smad2 have also been decreased (Li T. et al., 2013). Table 1 reviews the results studies which reported the role of extracellular vesicles in the treatment of liver disorders.
TABLE 1.
Summary of studies which reported the role of extracellular vesicles in the treatment of liver disorders.
| Cell origin | Type of secreted vesicle | Disease | Target cells or tissues | Molecular mechanism | Biological effect and therapeutic applications | References |
| CP-MSCs | Exosome | liver fibrosis | Hepatocytes | microRNA-125b | Increase liver Regeneration by inhibition of hedgehog (Hh) signal | Hyun et al., 2015 |
| βMSCs | Exosome | CLP | Hepatocytes | miR-146a | Diminish liver damage and decrease mortality | Song et al., 2017 |
| BM-MSCs | Conditioned medium | Acute liver failure | Th1 and Th17 cells | IL-10; CXCR3 and CCR5 | Decrease invasion in the injured liver | Van Poll et al., 2008 |
| HA-MSCs | EVs | Acute liver failure | Hepatocytes | lncRNA H19 | Increase hepatocytes proliferation and decrease mortality | Jin et al., 2018 |
| HA-MSCs | Exosome | Acute liver failure | Macrophages | miR-17 | Suppress the activation of NLRP3 inflammatory bodies | Liu et al., 2018 |
| UC-MSCs | Exosome | Liver fibrosis | Hepatic cells | TGF-β/Smad2 | Decrease collagen production | Li T. et al., 2013 |
| hUCMSCs | Exosome | Acute liver failure | Hepatocytes | miR-299-3p | Decrease inflammation through suppression of NLRP3-related pathways | Zhang et al., 2020 |
| MSC | Exosome | HBV | Macrophage | HBV-miR-3/SOCS5/STAT1 | Macrophage M1 polarization and IL-6 secretion | Zhao X. et al., 2020 |
| MSC | Exosome | HBV | Macrophage | HBV-infected hepatocyte exosomes/MyD88, TICAM-1, and MAVS | Enhance immune response in the host | Kouwaki et al., 2016 |
| BM-MSCs | Conditioned medium/Exosome | Acute liver failure | Hepatocytes | IDO-1/KYN; HGF; FLP1; IL-6/gp130; Bcl-xL; Cyclin D1 | Increase proliferation and suppress apoptosis | Xagorari et al., 2013; Gazdic et al., 2018; Milosavljevic et al., 2018 |
| hUCMSCs | Exosome | Liver failure | Hepatocytes | GPX1 | Decrease oxidative stress and apoptosis | Yan et al., 2017 |
| BM- MSCs | Exosome | Autoimmune hepatitis | Hepatocytes | miR-223 | ALT and AST levels were diminished and apoptosis was inhibited. | Chen et al., 2018 |
| BM-MSCs | Conditioned medium | Acute liver failure | Natural killer T cells | IDO-1/KYN | Decrease inflammatory Cytokines secretion and decrease cytotoxicity | Xagorari et al., 2013; Gazdic et al., 2018; Milosavljevic et al., 2018 |
| MSC | Exosome | NAFLD | Macrophage | miR122-5p/lysosome | M1 polarization | Zhao Z. et al., 2020 |
| MSC | Exosome | Hepatocellular carcinoma | Macrophage | lncRNA TUC339/Toll-like receptor signaling and FcgR-mediated phagocytosis | Decrease in pro-inflammatory cytokine secretion and enhance the phagocytosis | Li X. et al., 2018 |
| MSC | Exosome | Hepatocellular carcinoma | Macrophage | Exo-con/STAT3 | Enhance cytokine secretion in macrophages | Cheng et al., 2017 |
| BM-MSCs | Exosome | Acute liver failure/liver fibrosis | Leukocyte | IDO-1/KYN; TGF-; IL-10 | Suppressed activation of the inflammasome | Lou et al., 2017a; Milosavljevic et al., 2017 |
| MSC | Exosome | Alcoholic liver disease | Macrophage | miR-27A/CD206 on monocytes | M2 polarization | Saha et al., 2016 |
| MSC | Exosome | Alcoholic liver disease | Macrophage | CD40L/Caspase-3 | M1 polarization | Eguchi et al., 2016 |
| MSC | Exosome | Alcoholic liver disease | Monocytes | miR-122/HO-1 | Increase sensitivity of monocytes to LPS | Fairclough et al., 2014 |
| MSC | Exosome | Alcoholic liver disease | Kupffer cells | Mitochondrial double-stranded RNA/TLR3 in Kupffer cells | Increase in IL-1b and IL–17A levels | Lee et al., 2020 |
| MSC | Exosome | NAFLD | Macrophage | Hepatocyte-derived EV/DR5/Caspase/ROCK1 | Enhance macrophage pro-inflammatory | Hirsova et al., 2016 |
| MSC | Exosome | NAFLD | Monocytes | Lipotoxic EVs/ITGb1 | Increase monocyte adhesion and inflammatory response | Gallina et al., 2019 |
| MSC | Exosome | Hepatocellular carcinoma | Macrophage | miR-23a-3p/PTEN/AKT | Inhibition of T-cell function | Li T. et al., 2013 |
| MSC | Exosome | Hepatocellular carcinoma | Hepatocytes | miR-142-3p/RAC1 | supress hepatocellular carcinoma cell migration and invasion | Zhang et al., 2014 |
| UC- MSCs | EVs | Hepatitis | Liver cells | miR-let-7f, miR-145, miR-199a, miR-221 | Protect liver cells against HCV | Qian et al., 2016 |
| BM-MSCs | Exosome | Liver injury | – | Cationized pullulan | Anti-inflammatory effect | Tamura et al., 2017 |
| MenSCs | Exosome | Fulminant liver failure | Hepatocytes | ICAM-1, osteoprotegerin, angiogenin-2, | Decrease mortality and inhibits apoptosis | Yang et al., 2017a |
| HA-MSCs | Exosome | liver fibrosis | Hepatocytes | miR-122 | Decrease collagen deposition | Lou et al., 2017b |
| MSC | Exosome | HCV | Macrophage | Anti-HCV miRNA-29/TLR3-activated macrophages | Decrease HCV replication | Zhou et al., 2016 |
| MSC | Exosome | HCV | Monocytes | Exosome-packaged HCV/TLR7/8 | Differentiation of monocytes into macrophages | Saha et al., 2017 |
| MSC | Exosome | Alcoholic liver disease | Macrophage | miR-155/Hsp90 | Enhance in inflammatory macrophages | Babuta et al., 2019 |
| MSC | Exosome | NAFLD | Macrophage | mi R-192-5p/Rictor/Akt/FoxO1 | M1 polarization | Liu et al., 2020 |
BM-MSCs, bone marrow mesenchymal stem cells; UC-MSCs, umbilical cord mesenchymal stem cells; KYN, Kynurenine; CCR5, C-C chemokine receptor type 5; TGF-β, transforming growth factor beta; IDO-1, indoleamine 2,3 dioxygenase-1; HA-MSC, human adipose-derived mesenchymal stem cells; MenSC-Exos, human menstrual blood stem cell-derived exosomes; hUCMSC-Exos, Human umbilical cord mesenchymal stem cell-derived exosomes; CP-MSC, chorionic plate-derived mesenchymal stem cells; βMSC, MSC pre-treated with IL-1β; CLP, Puncture induced sepsis; NAFLD, Nonalcoholic fatty liver disease.
Lung Disorders
Exosomes originated from endothelial progenitor cells (EPCs) have been shown to preclude sepsis-associated endothelial dysfunction and lung damage partly because of the presence of miR-126 in these vesicles (Zhou et al., 2018). Moreover, intratracheal injection of EPC-derived exosomes has been shown to ameliorate lung damage following lipopolysaccharide-induced acute lung injury. This type of treatment has also decreased cell quantities, protein amounts, and cytokine levels in the bronchoalveolar lavage fluid, representing a decrease in permeability and inflammatory responses possibly through a miR-126-dependent mechanism. Similarly, up-regulation of miR-126-3p in human small airway epithelial cells has been shown to affect expression of PIK3R2, miRNA-126-5p has been shown to suppress expression of HMGB1 and VEGFα which are involved in the regulation of inflammatory responses and permeability, respectively. Notably, both miRNAs enhance the levels of tight junction proteins proposing a possible mechanism through which miR-126 alleviates LPS-induced lung damage (Zhou et al., 2019). Another study has demonstrated the efficacy of CM or EVs originated from BM-MSCs in amelioration of inflammation in an animal model of mixed Th2/Th17, neutrophil-associated allergic airway inflammation. Systemic injection of both CM and EVs isolated from human and murine MSCs, at the commencement of antigen challenge in formerly sensitized animal models has considerably amended the airway hyperreactivity, inflammatory reactions in lung, and the antigen-specific CD4 T-cell Th2 and Th17 phenotype (Cruz et al., 2015). Adipose tissue-derived MSCs and EVs have been shown to act in a different way on static lung elastance, regulatory T cells and CD3+CD4+ T cells of bronchoalveolar lavage fluid, and production of proinflammatory cytokines. Yet, their effects on reduction of eosinophils in lung tissue, content of collagen fibers in airways and lung parenchyma, production of TGF-β in lung tissue, and thymic CD3+CD4+ T cells have been similar (de Castro et al., 2017). Supplementary Table 1 gives a summary of studies which reported the role of EVs in the treatment of lung disorders.
Osteoarthritis
Mao et al. have demonstrated elevated exosomal levels of miR-92a-3p in the chondrogenic exosomes of MSCs despite its low levels in the osteoarthritis chondrocyte-originated exosomes. Notably, MSC-miR-92a-3p exosomes have stimulated cartilage proliferation and increased expressions of matrix genes in an MSC model of chondrogenesis and in primary human chondrocytes, respectively. miR-92a-3p has been shown to suppress expression of WNT5A in both models. Moreover, MSC-miR-92a-3p exosomes have inhibited cartilage destruction in the mouse model of osteoarthritis (Mao et al., 2018). Zhu et al. have shown the effects of exosomes originated from synovial membrane MSCs as well as exosomes of MSCs derived from iPSCs in the attenuation of osteoarthritis in an animal model of this disorder. Yet, the latter exosomes have had a greater therapeutic impact. Both types of exosomes have also enhanced chondrocyte migration and proliferation with those secreted by MSCs derived from iPSCs being superior to the other (Zhu et al., 2017). Supplementary Table 2 gives a summary of studies which reported the role of EVs in the treatment of osteoarthritis.
Colitis
Microvesicles containing miR-200b have been shown to amend the abnormal morphology of TGF-β1-treated intestinal epithelial cells and recover the 2,4,6-trinitrobenzene sulfonic acid-induced fibrosis in the colon possibly through inhibition of epithelial-mesenchymal transition (EMT) and mitigation of fibrosis. These effects have been accompanied by over-expression of E-Cad, and down-regulation of vimentin, α-SMA, ZBE1, and ZEB2 (Yang et al., 2017b). Mao et al. have appraised the impact of human UC-MSCs-derived exosomes in an animal model of dextran sulfate sodium- induced inflammatory bowel disease (IBD). These exosomes have been shown to relieve IBD course through enhancing IL-10 level while decreasing TNF-α, IL-1β, IL-6, iNOS, and IL-7 levels. Besides, treatment with these exosomes has led to reduction of macrophage infiltration into the colon (Mao et al., 2017). BM-MSC-derived EVs have also had beneficial effects in an animal model of 2,4,6-trinitrobenzene sulfonic acid-induced colitis when injected intravenously. These effects are possibly mediated through down-regulation of NF-κBp65, TNF-α, iNOS, and COX-2 in damaged colon. Moreover, these vesicles have remarkably decreased IL-1β and increased IL-10 levels. In addition, BM-MSC-derived EVs have been shown to modulate the anti-oxidant/oxidant equilibrium, and moderate apoptotic pathways (Yang et al., 2015). Supplementary Table 3 gives a summary of studies which reported the role of EVs in the treatment of colitis.
Myocardial Injury
Mesenchymal stem cell-derived exosomes have been shown reduce the size of infarct in a mice model of myocardial ischemia/reperfusion injury thus being implicated in the tissue repair (Lai et al., 2010). MSCs have also been demonstrated to suppress myocardial cell apoptosis and enhance regenerative process in the endothelial cell microvasculature through production of exosomes. SDF1 has been identified as the effective exosome ingredient which has protective effects on cardiac function and suppresses myocardial injury (Gong et al., 2019). Akt-containing exosomes have improved cardiac function in an animal model of acute myocardial infarction. These vesicles could accelerate proliferation and migration of endothelial cells and construction of tube-like configurations and blood vessels in vitro and in vivo, respectively. These effects have been mediated through up-regulation of PDGF-D (Ma et al., 2017). Supplementary Table 4 provides a summary of studies which reported the role of EVs in the treatment of cardiac disorders.
Spinal Cord Injury
Bone marrow-MSC-derived EVs have been reported to decrease brain cell death, increased survival of neurons and improved regenerative processes and motor function. In addition, these vesicles has attenuated blood-spinal cord barrier and reduced pericyte coverage in the animal models of spinal cord injury. Exosomes have been shown to decrease pericyte migration through inhibition of NF-κB p65 signaling and reduction of the permeability of the blood-spinal cord barrier (Lu et al., 2019). miR-133b has been identified as an important ingredient of MSC-derived exosomes. Administration of miR-133b-containing exosomes has enhanced the recovery of hindlimb function in an animal model of spinal cord injury. Moreover, these exosomes have decreased lesion size, protected neurons, and stimulated regenerative processes of axons RhoA has been identified as a direct target of miR-133b. miR-133b-containing exosomes could activate neuron survival pathways such as ERK1/2, STAT3, and CREB (Li D. et al., 2018). Systemic injection of MSCs-derived exosomes has also been shown to reduce lesion dimension and enhance functional recovery after induction of spinal cord injury in animal models. These exosomes have also decreased cell apoptosis and inflammatory responses in the damaged spinal cord as evidenced by reduction of expressions of pro-apoptotic protein and TNF-α and IL-1β proinflammatory cytokines while increased levels of BCL2 and IL-10. MSCs-derived exosomes have also increased angiogenic processes (Huang et al., 2017). Supplementary Table 5 has shown summary of studies which reported the role of extracellular vesicles in the treatment of spinal cord injury.
Other Disorders
The beneficial effects of EVs in the treatment of several other disorders such as renal fibrosis, stroke, neurodegenerative disorders and retinal injury have been assessed in independent studies. For instance, experiments in an animal model of middle cerebral artery occlusion have shown the impact of MSCs in enhancement of miR-133b expression in the ipsilateral hemisphere. In vitro, expression of this miRNA has been increased in MSCs and in MSC-derived exosomes after exposure to ipsilateral ischemic tissue extracts. Expression of miR-133b has also been augmented in primary cultured neurons and astrocytes exposed with the exosome-enriched materials produced by these MSCs. The results of this study indicates communication between MSCs and brain parenchymal cells and the impact of such interplay on regulation of neurite outgrowth through exosome-mediated transfer of miR-133b to neural cells (Xin et al., 2012). The beneficial effects of BM-MSC-derived EVs have also been assessed in Alzheimer’s disease. Cui et al. have shown improvement of some neurological abnormalities in an animal model of this disorder following administration of MSC-derived exosomes. Administration of normoxic MSCs-derived exosomes has amended cognition and memory deficits, decreased plaque deposition and brain Aβ amounts. These effects are associated with down-regulation of TNF-α and IL-1β and up-regulation of IL-4 and IL-10. Exosomes from hypoxia-preconditioned MSCs have exerted superior effects in learning and memory abilities and plaque deposition and Aβ amounts (Cui et al., 2018). Another experiment in an animal model of glaucoma induced by chronic ocular hypertension has shown neuroprotective effect of BM-MSC-derived EVs and reduction of the quantity of degenerating axons in the optic nerve (Mead et al., 2018). In addition, BM-MSCs have been shown to extend the survival of allogenic renal transplant in animal models. Mechanistically, these cells increase miR-146a levels in dendritic cells of the treated animals. Similarly, BM-MSC-derived microvesicles enhance miR-146a levels in both immature and mature dendritic cells in vitro, while decreasing IL-12 levels in mature dendritic cells. Therefore, BM-MSCs-originated microvesicles enhance outcome of allogenic renal transplantation via suppression of dendritic cell maturity by miR-146a (Wu et al., 2017). Supplementary Table 6 summarizes the results of studies which reported the role of EVs in the treatment of various disorders.
Discussion
Extracellular vesicles are beneficial tools of delivery of biomolecules in the field of regenerative medicine. These acellular vesicles are more promising than cellular methods because of the lower risk of tumor formation, autoimmune responses and toxic effects compared with cell therapy (Katsuda et al., 2013a). Moreover, the small size and lower complexity of these vesicles compared with cells have make their production and storage easier than cellular methods (Katsuda et al., 2013a).
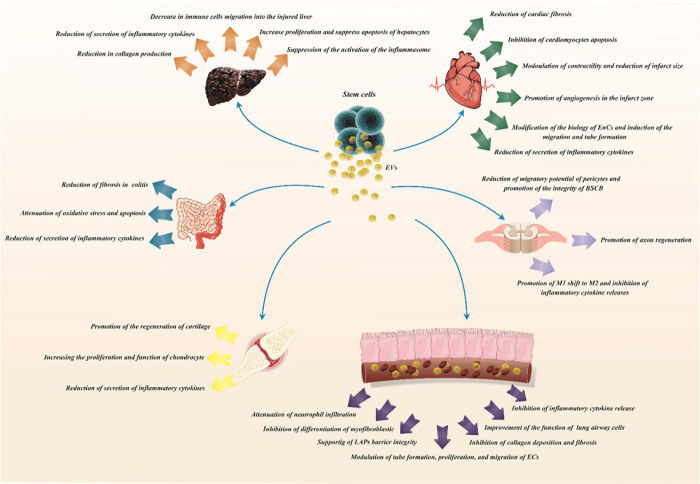
Mesenchymal stem cells have been suggested as the most favorable source for cell-based therapy due to their multi-lineage differentiation capacity and immuno-modulatory features (Harrell et al., 2019). As MSCs have therapeutic application in the prevention of parenchymal cell defects and enhancement of tissue regeneration in animal models of myocardial injury, renal failure, stroke and other disorders, the effects of MSC-derived EVs in the treatment of these disorders have been assessed reporting promising results. Figure 1 illustrates role of these particles in regeneration of different tissues.
FIGURE 1.
Role of MSC-derived extracellular vesicles in the regeneration of different tissues.
Proliferation, survival, apoptosis and senescence of MSCs might be affected by EVs. Endothelial cell-derived exosomes have been found to induce angiogenesis through suppression of cell senescence. Moreover, transfer of miR-214 by these vesicles has decreased expression of ATM gene in recipient cells, reducing their senescence (van Balkom et al., 2013). Further evidence for modulation of apoptosis by EVs has come from studies that revealed the presence of anti-apoptotic miRNAs in exosomes originated from human cardiac progenitor cells as well as bone marrow MSCs (Reis et al., 2012; Barile et al., 2014).
Conditioned medium or exosomes originated from MSCS can prevent liver injury through different mechanisms such as modulation of immune responses, induction of immune tolerance via affecting IDO and iNOS levels and changing expression of a number of miRNAs. In animal models of acute lung injury, administration of EPC-derived EVs has ameliorated tissue damage particularly through their cargo miR-126. Besides, a growing experience demonstrates beneficial effects MSC-based cell therapies in animal models of asthma suggesting a novel strategy for treatment of severe refractory asthma (Cruz et al., 2015).
The underlying mechanism of beneficial effects of MSC-derived EVs in the regeneration of tissues and inhibition of tissue damage has been verified in a number of studies through assessment of the cargo of EVs. However, the synergic effects of EV ingredients should not be ignored as these acellular particles contain several agents which might affect cellular processes via different routes. Moreover, EVs have several target cells in the microenvironment; therefore can affect the function of various cells such as endothelial cells, epithelial cells and different immune cells. The cell-specific functions of EVs should be also assessed in order to design the most appropriate therapeutic modalities.
The long half-life of exosomes and their ability in penetrating cell membranes and targeting specific kinds of cells have potentiated these vesicles as candidates for therapeutic applications. Moreover, the fact that exosomes are not perceived by immune system as foreign bodies makes them more appropriate for the these applications (Lai et al., 2013).
The efficacy of EVs originated from adipose tissue-MSCs in the amelioration of clinical and pathological features in animal models of disorders has indicated the vast source of finding MSCs and their related biomaterials, thus improving the applicability of these modalities in several settings. Exosomes secreted by iMSC might also have appropriate therapeutic impact in certain conditions due to their inexhaustible potential. Besides, microvesicles can be used for transferring certain cargo from genetically modified stem cells to target cells. Due to stability of exosomes in the circulation, systemic administration of these vesicles is an efficient method for transferring their cargo to target cells.
A challenge in the field of application of MSCs in the regenerative medicine has arisen from the observed different effects of some MSC-derived EVs and MSCs on molecular targets, biomolecules and tissue construction which necessitate precise assessment of the pathways targeted by each modality.
Taken together, EVs have emerged as potential vehicles for amelioration of damaged tissues and improvement of tissue organization. However, the molecular mechanisms of EVs-induced changes in tissues should be appraised further. Moreover, the majority of studies have been conducted in experimental models. Therefore, applicability of these techniques in medical practice must be more comprehensively assessed. Besides, understanding the cargo trafficking pathways of EVs is necessary to control the cargo of EVs and avoid unspecific effects. Lack of knowledge in these fields has limited application of EVs in treatment of human disorders. Finally, lack of segregation of the therapeutic effects of “cells” versus “cell-derived EVs” is a limitation of a number of studies in this field.
Author Contributions
MT and SG-F wrote the draft and revised it. AB, MO, and VN designed the tables, figures, and collected the data. All authors approved submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.653296/full#supplementary-material
References
- Adamiak M., Cheng G., Bobis-Wozowicz S., Zhao L., Kedracka-Krok S., Samanta A., et al. (2018). Induced pluripotent stem cell (iPSC)–derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circulat. Res. 122 296–309. 10.1161/circresaha.117.311769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo I. M., Abreu S. C., Maron-Gutierrez T., Cruz F., Fujisaki L., Carreira H., et al. (2010). Bone marrow-derived mononuclear cell therapy in experimental pulmonary and extrapulmonary acute lung injury. Crit. Care Med. 38 1733–1741. 10.1097/ccm.0b013e3181e796d2 [DOI] [PubMed] [Google Scholar]
- Babuta M., Furi I., Bala S., Bukong T. N., Lowe P., Catalano D., et al. (2019). Dysregulated autophagy and lysosome function are linked to exosome production by micro-RNA 155 in alcoholic liver disease. Hepatology 70 2123–2141. 10.1002/hep.30766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Shao H., Wang H., Zhang Z., Su C., Dong L., et al. (2017). Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Scientific Rep. 7 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira E., Oliveira H., Silva J. D., Menna-Barreto R. F., Takyia C. M., Suk J. S., et al. (2018). Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis. Respir. Res. 19 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee E. R., Laflamme M. A., Papayannopoulou T., Kahn M., Murry C. E., Henderson W. R. (2012). Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One. 7:e33165. 10.1371/journal.pone.0033165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile L., Cervio E., Lionetti V., Milano G., Ciullo A., Biemmi V., et al. (2018). Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Circul. Res. 114 992–1005. 10.1093/cvr/cvy055 [DOI] [PubMed] [Google Scholar]
- Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L. M., et al. (2014). Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovas. Res. 103 530–541. 10.1093/cvr/cvu167 [DOI] [PubMed] [Google Scholar]
- Bruno S., Tapparo M., Collino F., Chiabotto G., Deregibus M. C., Soares Lindoso R., et al. (2017). Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Engin. Part A. 23 1262–1273. 10.1089/ten.tea.2017.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.-P., He X.-Y., Xu H.-T., Zou Z., Shi X.-Y. (2012). Autologous transplantation of peripheral blood-derived circulating endothelial progenitor cells attenuates endotoxin-induced acute lung injury in rabbits by direct endothelial repair and indirect immunomodulation. Anesthesiology 116 1278–1287. 10.1097/aln.0b013e3182567f84 [DOI] [PubMed] [Google Scholar]
- Chen C., Wang D., Moshaverinia A., Liu D., Kou X., Yu W., et al. (2017). Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 27 559–577. 10.1038/cr.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Wang S., Xiang H., Liu J., Zhang Y., Zhou S., et al. (2019). Microvesicles derived from human Wharton’s Jelly mesenchymal stem cells ameliorate acute lung injury partly mediated by hepatocyte growth factor. Int. J. Biochem. Cell Biol. 112 114–122. 10.1016/j.biocel.2019.05.010 [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Chang Y.-W., Tan K. P., Shen Y.-S., Wang Y.-H., Chang C.-H. (2018). Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PLoS One. 13:e0205563. 10.1371/journal.pone.0205563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Liu J., Liu Q., Liu Y., Fan L., Wang F., et al. (2017). Exosomes from melatonin treated hepatocellularcarcinoma cells alter the immunosupression status through STAT3 pathway in macrophages. Int. J. Biol. Sci. 13:723. 10.7150/ijbs.19642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Zhang G., Zhang L., Hu Y., Zhang K., Sun X., et al. (2018). Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell. Mol. Med. 22 261–276. 10.1111/jcmm.13316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. J., Kim M.-H., Jeon J., Kim O. Y., Choi Y., Seo J., et al. (2015). Active immunization with extracellular vesicles derived from Staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS One. 10:e0136021. 10.1371/journal.pone.0136021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson-Thomas V. J., Caterson B., Kao W. W. Y. (2013). Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells 31 2116–2126. 10.1002/stem.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F. F., Borg Z. D., Goodwin M., Sokocevic D., Wagner D. E., Coffey A., et al. (2015). Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl. Med. 4 1302–1316. 10.5966/sctm.2014-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G. H., Wu J., Mou F. F., Xie W. H., Wang F. B., Wang Q. L., et al. (2018). Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 32 654–668. 10.1096/fj.201700600r [DOI] [PubMed] [Google Scholar]
- Cui X., He Z., Liang Z., Chen Z., Wang H., Zhang J. (2017). Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-catenin signaling pathway. J. Cardiovas. Pharmacol. 70:225. 10.1097/fjc.0000000000000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro L. L., Xisto D. G., Kitoko J. Z., Cruz F. F., Olsen P. C., Redondo P. A. G., et al. (2017). Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 8 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Couto G., Gallet R., Cambier L., Jaghatspanyan E., Makkar N., Dawkins J. F., et al. (2017). Exosomal microRNA transfer into macrophages mediates cellular postconditioning. Circulation 136 200–214. 10.1161/circulationaha.116.024590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong O. G., Van Balkom B. W. M., Schiffelers R. M., Bouten C. V. C., Verhaar M. C. (2014). Extracellular vesicles: potential roles in regenerative medicine. Front. Immunol. 5:608. 10.3389/fimmu.2014.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Ge Z., Song Y., Wang H., Liu X., Zhang D. (2019). Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int. J. Biochem. Cell Biol. 114:105564. 10.1016/j.biocel.2019.105564 [DOI] [PubMed] [Google Scholar]
- Deng Y., Chen D., Gao F., Lv H., Zhang G., Sun X., et al. (2019). Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J. Biol. Engin. 13 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh P.-U. C., Paudel D., Brochu H., Popowski K. D., Gracieux M. C., Cores J., et al. (2020). Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 11 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. A., Kumar N., Noor M., Angelos M. G., Khan M., Chen C.-A., et al. (2018). Extracellular vesicles released by human induced-pluripotent stem cell-derived cardiomyocytes promote angiogenesis. Front. Physiol. 9:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L. M., Wang M. Z. (2019). Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 8:727. 10.3390/cells8070727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Zhang K., Zhang S., Wang R., Nie Y., Tao H., et al. (2017). Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 133 70–81. 10.1016/j.biomaterials.2017.04.030 [DOI] [PubMed] [Google Scholar]
- Du Y.-M., Zhuansun Y.-X., Chen R., Lin L., Lin Y., Li J.-G. (2018). Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 363 114–120. 10.1016/j.yexcr.2017.12.021 [DOI] [PubMed] [Google Scholar]
- Eguchi A., Du Jeu X. D. M., Johnson C. D., Nektaria A., Feldstein A. E. (2016). Liver Bid suppression for treatment of fibrosis associated with non-alcoholic steatohepatitis. J. Hepatol. 64 699–707. 10.1016/j.jhep.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Harane N., Kervadec A., Bellamy V., Pidial L., Neametalla H. J., Perier M.-C., et al. (2018). Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur. Heart J. 39 1835–1847. 10.1093/eurheartj/ehy012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili M., Niazi V., Pourfathollah A. A., Hosseini M. K. M., Nakhlestani M., Golzadeh K., et al. (2019). The impact of parathyroid hormone treated mesenchymal stem cells on ex-vivo expansion of cord blood hematopoietic stem cells. Gene Rep. 17:100490. 10.1016/j.genrep.2019.100490 [DOI] [Google Scholar]
- Faccini J., Ruidavets J.-B., Cordelier P., Martins F., Maoret J.-J., Bongard V., et al. (2017). Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Scient. Rep. 7:42916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough D., Brown J., Carlish B., Crisafulli B., Keay I. (2014). Breathing life into fisheries stock assessments with citizen science. Scient. Rep. 4 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S., Xu C., Zhang Y., Xue C., Yang C., Bi H., et al. (2016). Umbilical cord-derived mesenchymal stem cell-derived exosomal micrornas suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl. Med. 5 1425–1439. 10.5966/sctm.2015-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Fullwood M. J. (2016). Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom. Proteom. Bioinform. 14 42–54. 10.1016/j.gpb.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Huang W., Wani M., Yu X., Ashraf M. (2014). Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 9:e88685. 10.1371/journal.pone.0088685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster-Matanzo A., Gessler F., Leonardi T., Iraci N., Pluchino S. (2015). Acellular approaches for regenerative medicine: on the verge of clinical trials with extracellular membrane vesicles? Stem Cell Res. Ther. 6:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet R., Dawkins J., Valle J., Simsolo E., De Couto G., Middleton R., et al. (2017). Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina P., Gallo O., Nicoletti C., Romanelli R. G. A. (2019). hydrodynamic hypothesis for the pathogenesis of glymphatic system impairment in hepatic encephalopathy. J. Hepatol. 71 228–229. 10.1016/j.jhep.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Gangadaran P., Rajendran R. L., Lee H. W., Kalimuthu S., Hong C. M., Jeong S. Y., et al. (2017). Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control. Rel. 264 112–126. 10.1016/j.jconrel.2017.08.022 [DOI] [PubMed] [Google Scholar]
- Gao J., Wang S., Wang Z. (2017). High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 135 62–73. 10.1016/j.biomaterials.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Sun J., Dong C., Zhao M., Hu Y., Jin F. (2020). Extracellular vesicles derived from adipose mesenchymal stem cells alleviate PM2. 5-Induced lung injury and pulmonary fibrosis. Med. Sci. Monitor 26 e922782–e922781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdic M., Simovic Markovic B., Vucicevic L., Nikolic T., Djonov V., Arsenijevic N., et al. (2018). Mesenchymal stem cells protect from acute liver injury by attenuating hepatotoxicity of liver natural killer T cells in an inducible nitric oxide synthase-and indoleamine 2, 3-dioxygenase-dependent manner. J. Tissue Engin. Regener. Med. 12 e1173–e1185. [DOI] [PubMed] [Google Scholar]
- Ghafouri-Fard S., Niazi V., Taheri M. (2020a). Role of miRNAs and lncRNAs in hematopoietic stem cell differentiation. Non Coding RNA Res. 6 8–14. 10.1016/j.ncrna.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri-Fard S., Niazi V., Taheri M. (2020b). Role of miRNAs in conveying message of stem cells via extracellular vesicles. Exp. Mol. Pathol. 117:104569. 10.1016/j.yexmp.2020.104569 [DOI] [PubMed] [Google Scholar]
- Ghafouri-Fard S., Niazi V., Taheri M. (2021a). Contribution of extracellular vesicles in normal hematopoiesis and hematological malignancies. Heliyon 7”e06030. 10.1016/j.heliyon.2021.e06030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri-Fard S., Niazi V., Taheri M., Basiri A. (2021b). Effect of Small Molecule on ex vivo Expansion of Cord Blood Hematopoietic Stem Cells: A Concise Review. Front. Cell Devel. Biol. 9:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri-Fard S., Glassy M. C., Abak A., Hussen B. M., Niazi V., Taheri M. (2021c). The interaction between miRNAs/lncRNAs and Notch pathway in human disorders. Biomed. Pharmacother. 138:111496. 10.1016/j.biopha.2021.111496 [DOI] [PubMed] [Google Scholar]
- Gong M., Yu B., Wang J., Wang Y., Liu M., Paul C., et al. (2017). Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8:45200. 10.18632/oncotarget.16778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X. H., Liu H., Wang S. J., Liang S. W., Wang G. G. (2019). Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J. Cell. Physiol. 234 13878–13893. 10.1002/jcp.28070 [DOI] [PubMed] [Google Scholar]
- Gonzalez-King H., García N. A., Ontoria-Oviedo I., Ciria M., Montero J. A., Sepúlveda P. (2017). Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 35 1747–1759. 10.1002/stem.2618 [DOI] [PubMed] [Google Scholar]
- Gray W. D., French K. M., Ghosh-Choudhary S., Maxwell J. T., Brown M. E., Platt M. O., et al. (2015). Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circul. Res. 116 255–263. 10.1161/circresaha.116.304360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Gudapati V., Monsel A., Park J. H., Hu S., Kato H., et al. (2019). Mesenchymal stem cell–derived extracellular vesicles decrease lung injury in mice. J. Immunol. 203 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Zhu Y.-G., Monsel A., Gennai S., Lee T., Xu F. (2015). Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Transl. Med. 4 832–840. 10.5966/sctm.2015-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell C. R., Fellabaum C., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. (2019). Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 8:467. 10.3390/cells8050467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting M. T., Srivastava A. K., Zhaorigetu S., Bair H., Prabhakara K. S., Toledano Furman N. E., et al. (2018). Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 36 79–90. 10.1002/stem.2730 [DOI] [PubMed] [Google Scholar]
- He X., Dong Z., Cao Y., Wang H., Liu S., Liao L., et al. (2019). MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019:7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzenecker A., Seidl M., Kosovac K., Herfarth H., Kellermeier S., Obermeier F., et al. (2012). Downregulation of the ubiquitin-proteasome system in normal colonic macrophages and reinduction in inflammatory bowel disease. Digestion 86 34–47. 10.1159/000336353 [DOI] [PubMed] [Google Scholar]
- Hirsova P., Ibrahim S. H., Krishnan A., Verma V. K., Bronk S. F., Werneburg N. W., et al. (2016). Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 150 956–967. 10.1053/j.gastro.2015.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Park J., Liu A., Lee J., Zhang X., Hao Q., et al. (2018). Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl. Med. 7 615–624. 10.1002/sctm.17-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.-H., Yin X.-M., Xu Y., Xu C.-C., Lin X., Ye F.-B., et al. (2017). Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J. Neurotr. 34 3388–3396. 10.1089/neu.2017.5063 [DOI] [PubMed] [Google Scholar]
- Huang P., Wang L., Li Q., Tian X., Xu J., Xu J., et al. (2020). Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovas. Res. 116 353–367. 10.1093/cvr/cvz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Wang L., Li Q., Xu J., Xu J., Xiong Y., et al. (2019). Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res. Ther. 10 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Qin C., Wang J., Hu Y., Zheng G., Qiu G., et al. (2019). Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging 11:7996. 10.18632/aging.102314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J., Wang S., Kim J., Kim G. J., Jung Y. (2015). MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Scient. Rep. 5 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Wang J., Li H., Gao S., Shi R., Yang D., et al. (2018). Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine 34 231–242. 10.1016/j.ebiom.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger-Messerli M. S., Oppliger B., Spinelli M., Thomi G., Di Salvo I., Schneider P., et al. (2018). Extracellular vesicles derived from Wharton’s jelly mesenchymal stem cells prevent and resolve programmed cell death mediated by perinatal hypoxia-ischemia in neuronal cells. Cell Transpl. 27 168–180. 10.1177/0963689717738256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C., Shen Y., Ma G., Liu Y., Cai J., Kim I.-M. (2018). Transplantation of cardiac mesenchymal stem cell-derived exosomes promotes repair in ischemic myocardium. J. Cardiovas. Transl. Res. 11 420–428. 10.1007/s12265-018-9822-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Z., Ma J., Wang C., Yu J., Qiao Y., Hei F. (2017). Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation 40 486–496. 10.1007/s10753-016-0494-0 [DOI] [PubMed] [Google Scholar]
- Katakowski M., Buller B., Zheng X., Lu Y., Rogers T., Osobamiro O., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 335 201–204. 10.1016/j.canlet.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda T., Kosaka N., Takeshita F., Ochiya T. (2013a). The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 13 1637–1653. [DOI] [PubMed] [Google Scholar]
- Katsuda T., Tsuchiya R., Kosaka N., Yoshioka Y., Takagaki K., Oki K., et al. (2013b). Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Scientific Rep. 3:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kervadec A., Bellamy V., El Harane N., Arakélian L., Vanneaux V., Cacciapuoti I., et al. (2016). Cardiovascular progenitor–derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J. Heart Lung Transpl. 35 795–807. 10.1016/j.healun.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Khan M., Nickoloff E., Abramova T., Johnson J., Verma S. K., Krishnamurthy P., et al. (2015). Embryonic stem cell–derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circul. Res. 117 52–64. 10.1161/circresaha.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri M., Richardson L. A., Meulia T. (2018). Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 9 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Joglekar M. V., Hardikar A. A., Phan T. H., Khanal D., Tharkar P., et al. (2019). Placenta stem/stromal cell–derived extracellular vesicles for potential use in lung repair. Proteomics 19:1800166. 10.1002/pmic.201800166 [DOI] [PubMed] [Google Scholar]
- Kırbas̨ O. K., Bozkurt B. T., Asutay A. B., Mat B., Ozdemir B., Öztürkoğlu D., et al. (2019). Optimized Isolation of Extracellular Vesicles From Various Organic Sources Using Aqueous Two-Phase System. Scient. Rep. 9:19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwaki T., Fukushima Y., Daito T., Sanada T., Yamamoto N., Mifsud E. J., et al. (2016). Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Front. Immunol. 7:335. 10.3389/fimmu.2016.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R. C., Arslan F., Lee M. M., Sze N. S. K., Choo A., Chen T. S., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4 214–222. 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Lai R. C., Yeo R. W. Y., Tan K. H., Lim S. K. (2013). Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol. Adv. 31 543–551. 10.1016/j.biotechadv.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Lankford K. L., Arroyo E. J., Nazimek K., Bryniarski K., Askenase P. W., Kocsis J. D. (2018). Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 13:e0190358. 10.1371/journal.pone.0190358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Shim Y. R., Seo W., Kim M. H., Choi W. M., Kim H. H., et al. (2020). Mitochondrial Double-Stranded RNA in Exosome Promotes Interleukin-17 Production Through Toll-Like Receptor 3 in Alcohol-associated Liver Injury. Hepatology 72 609–625. 10.1002/hep.31041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhang P., Yao X., Li H., Shen H., Li X., et al. (2018). Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front. Neurosci. 12:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ghazwani M., Zhang Y., Lu J., Li J., Fan J., et al. (2013). miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 58 522–528. 10.1016/j.jhep.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Jin S., Zhang Y. (2015). Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int. J. Clin. Exp. Med. 8:3825. [PMC free article] [PubMed] [Google Scholar]
- Li L.-F., Liu Y.-Y., Yang C.-T., Chien Y., Twu N.-F., Wang M.-L., et al. (2013). Improvement of ventilator-induced lung injury by IPS cell-derived conditioned medium via inhibition of PI3K/Akt pathway and IP-10-dependent paracrine regulation. Biomaterials 34 78–91. 10.1016/j.biomaterials.2012.09.042 [DOI] [PubMed] [Google Scholar]
- Li Q.-C., Liang Y., Su Z.-B. (2019). Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 316 L1107–L1117. [DOI] [PubMed] [Google Scholar]
- Li T., Yan Y., Wang B., Qian H., Zhang X., Shen L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Devel. 22 845–854. 10.1089/scd.2012.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Lei Y., Wu M., Li N. (2018). Regulation of macrophage activation and polarization by HCC-derived exosomal lncRNA TUC339. Int. J. Mol. Sci. 19:2958. 10.3390/ijms19102958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zhang L., Wang S., Han Q., Zhao R. C. (2016). Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 129 2182–2189. 10.1242/jcs.170373 [DOI] [PubMed] [Google Scholar]
- Lima Correa B., El Harane N., Gomez I., Rachid Hocine H., Vilar J., Desgres M., et al. (2021). Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovas. Res. 117 292–307. 10.1093/cvr/cvaa028 [DOI] [PubMed] [Google Scholar]
- Liu J., Fan L., Yu H., Zhang J., He Y., Feng D., et al. (2019). Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology 70 241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-S., Du J., Cheng X., Zhang X.-Z., Li Y., Chen X.-L. (2019). Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J. Chin. Med. Assoc. 82 895–901. 10.1097/jcma.0000000000000189 [DOI] [PubMed] [Google Scholar]
- Liu L., Jin X., Hu C.-F., Li R., Shen C.-X. (2017). Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell. Physiol. Biochem. 43 52–68. 10.1159/000480317 [DOI] [PubMed] [Google Scholar]
- Liu X. L., Pan Q., Cao H. X., Xin F. Z., Zhao Z. H., Yang R. X., et al. (2020). Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology 72 454–469. 10.1002/hep.31050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lou G., Li A., Zhang T., Qi J., Ye D., et al. (2018). AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 36 140–150. 10.1016/j.ebiom.2018.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López E., Blázquez R., Marinaro F., Álvarez V., Blanco V., Báez C., et al. (2019). The intrapericardial delivery of extracellular vesicles from cardiosphere-derived cells stimulates M2 polarization during the acute phase of porcine myocardial infarction. Stem Cell Rev. Rep. 16:626. 10.1007/s12015-020-09962-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verrilli M. A., Caviedes A., Cabrera A., Sandoval S., Wyneken U., Khoury M. (2016). Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 320 129–139. 10.1016/j.neuroscience.2016.01.061 [DOI] [PubMed] [Google Scholar]
- Lou G., Chen Z., Zheng M., Liu Y. (2017a). Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 49:e346. 10.1038/emm.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou G., Yang Y., Liu F., Ye B., Chen Z., Zheng M., et al. (2017b). MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell. Mol. Med. 21 2963–2973. 10.1111/jcmm.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy H., Kuok D. I., Hui K. P., Choi M. H., Yuen W., Nicholls J. M., et al. (2019). Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A (H5N1) Virus–Associated Acute Lung Injury. J. Infect. Dis. 219 186–196. 10.1093/infdis/jiy478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Zhou Y., Zhang R., Wen L., Wu K., Li Y., et al. (2019). Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front. Neurosci. 13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther K. M., Haar L., McGuinness M., Wang Y., Lynch I. V. T. L., Phan A., et al. (2018). Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell. Cardiol. 119 125–137. 10.1016/j.yjmcc.2018.04.012 [DOI] [PubMed] [Google Scholar]
- Ma J., Zhao Y., Sun L., Sun X., Zhao X., Sun X., et al. (2017). Exosomes derived from AKt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl. Med. 6 51–59. 10.5966/sctm.2016-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Chen Y., Chen Y., Meng Q., Sun J., Shao L., et al. (2018). MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. 2018:3290372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M. N., Mazzoli-Rocha F., Casquilho N. V., Maron-Gutierrez T., Ortenzi V. H., Morales M. M., et al. (2018). Bone marrow-derived mononuclear cell therapy in papain-induced experimental pulmonary emphysema. Front. Physiol. 9:121. 10.3389/fphys.2018.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri N., Willis G. R., Fernandez-Gonzalez A., Reis M., Nassiri S., Mitsialis S. A., et al. (2019). Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI insight 4:e128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao F., Wu Y., Tang X., Kang J., Zhang B., Yan Y., et al. (2017). Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. BioMed Res. Int. 2017:5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Zhang Z., Hu S., Zhang Z., Chang Z., Huang Z., et al. (2018). Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 9 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao M., Wang S.-N., Lv X.-J., Wang Y., Xu J.-C. (2010). Intravenous delivery of bone marrow-derived endothelial progenitor cells improves survival and attenuates lipopolysaccharide-induced lung injury in rats. Shock 34 196–204. 10.1097/shk.0b013e3181d49457 [DOI] [PubMed] [Google Scholar]
- Maring J. A., Lodder K., Mol E., Verhage V., Wiesmeijer K. C., Dingenouts C. K., et al. (2019). Cardiac progenitor cell–derived extracellular vesicles reduce infarct size and associate with increased cardiovascular cell proliferation. J. Cardiovas. Transl. Res. 12 5–17. 10.1007/s12265-018-9842-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens A., Ordies S., Vanaudenaerde B. M., Verleden S. E., Vos R., Van Raemdonck D. E., et al. (2017). Immunoregulatory effects of multipotent adult progenitor cells in a porcine ex vivo lung perfusion model. Stem Cell Res. Ther. 8:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayourian J., Ceholski D. K., Gorski P. A., Mathiyalagan P., Murphy J. F., Salazar S. I., et al. (2018). Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circulat. Res. 122 933–944. 10.1161/circresaha.118.312420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B., Ahmed Z., Tomarev S. (2018). Mesenchymal Stem Cell–Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Ophthalmol. Visual Sci. 59 5473–5480. 10.1167/iovs.18-25310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B., Tomarev S. (2017). Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through mirna-dependent mechanisms. Stem Cells Transl. Med. 6 1273–1285. 10.1002/sctm.16-0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic N., Gazdic M., Simovic Markovic B., Arsenijevic A., Nurkovic J., Dolicanin Z., et al. (2018). Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells–an experimental study. Transpl. Int. 31 102–115. 10.1111/tri.13023 [DOI] [PubMed] [Google Scholar]
- Milosavljevic N., Gazdic M., Simovic Markovic B., Arsenijevic A., Nurkovic J., Dolicanin Z., et al. (2017). Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. 23 1040–1050. 10.1002/lt.24784 [DOI] [PubMed] [Google Scholar]
- Monsel A., Zhu Y.-G., Gennai S., Hao Q., Hu S., Rouby J.-J. (2015). Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 192 324–336. 10.1164/rccm.201410-1765oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. J., Jackson M. V., Cunningham E. K., Kissenpfennig A., McAuley D. F., O’Kane C. M., et al. (2017). Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 196 1275–1286. 10.1164/rccm.201701-0170oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Miyaki S., Ishitobi H., Matsuyama S., Nakasa T., Kamei N., et al. (2015). Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 589 1257–1265. 10.1016/j.febslet.2015.03.031 [DOI] [PubMed] [Google Scholar]
- Nandra K. K., Takahashi K., Collino M., Benetti E., Wong W. F., Goh F. Y., et al. (2012). Acute Treatment With Bone Marrow–Derived Mononuclear Cells Attenuates the Organ Injury/Dysfunction Induced by Hemorrhagic Shock in the Rat. Shock 37 592–598. 10.1097/shk.0b013e31824e4c0d [DOI] [PubMed] [Google Scholar]
- Neckles V. N., Morton M. C., Holmberg J. C., Sokolov A. M., Nottoli T., Liu D., et al. (2019). A transgenic inducible GFP extracellular-vesicle reporter (TIGER) mouse illuminates neonatal cortical astrocytes as a source of immunomodulatory extracellular vesicles. Scient. Rep. 9:3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi V., Parseh B., Ahani M., Karami F., Gilanchi S., Atarodi K., et al. (2020). Communication between stromal and hematopoietic stem cell by exosomes in normal and malignant bone marrow niche. Biomed. Pharmacother. 132:110854. 10.1016/j.biopha.2020.110854 [DOI] [PubMed] [Google Scholar]
- Ornellas D. S., Maron-Gutierrez T., Ornellas F. M., Cruz F. F., Oliveira G. P., Lucas I. H., et al. (2011). Early and late effects of bone marrow-derived mononuclear cell therapy on lung and distal organs in experimental sepsis. Respir. Physiol. Neurobiol. 178 304–314. 10.1016/j.resp.2011.06.029 [DOI] [PubMed] [Google Scholar]
- Pan J., Alimujiang M., Chen Q., Shi H., Luo X. (2019). Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction- induced myocardial damage via downregulation of early growth response factor 1. J. Cell. Biochem. 120 4433–4443. 10.1002/jcb.27731 [DOI] [PubMed] [Google Scholar]
- Park J., Kim S., Lim H., Liu A., Hu S., Lee J., et al. (2019). Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax 74 43–50. 10.1136/thoraxjnl-2018-211576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prota L. F. M., Lassance R. M., Maron-Gutierrez T., Castiglione R. C., Garcia C. S. B., Santana M. C. E., et al. (2010). Bone marrow mononuclear cell therapy led to alveolar-capillary membrane repair, improving lung mechanics in endotoxin-induced acute lung injury. Cell Transpl. 19 965–971. 10.3727/096368910x506845 [DOI] [PubMed] [Google Scholar]
- Qian X., Xu C., Fang S., Zhao P., Wang Y., Liu H., et al. (2016). Exosomal microRNAs derived from umbilical mesenchymal stem cells inhibit hepatitis C virus infection. Stem Cells Transl. Med. 5 1190–1203. 10.5966/sctm.2015-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramis J. M. (2020). Extracellular Vesicles in Cell Biology and Medicine. Scient. Rep. 10:8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200 373–383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi S., Molavi Z., Mirmotalebisohi S. A., Niknam Z., Sameni M., Niazi V., et al. (2021). Mesenchymal stem cells in the treatment of new coronavirus pandemic: a novel promising therapeutic approach. Tabriz Univ. Med. Sci. 10.1007/s12015-020-09973-w [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis L. A., Borges F. T., Simões M. J., Borges A. A., Sinigaglia-Coimbra R., Schor N. (2012). Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 7:e44092. 10.1371/journal.pone.0044092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. D., Morelli A. E. (2014). Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14 195–208. 10.1038/nri3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce S. G., Patel K. P., Mao W., Zhu D., Lim R., Samuel C. S. (2019). Serelaxin enhances the therapeutic effects of human amnion epithelial cell-derived exosomes in experimental models of lung disease. Br. J. Pharmacol. 176 2195–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B., Kodys K., Adejumo A., Szabo G. (2017). Circulating and exosome-packaged hepatitis C single-stranded RNA induce monocyte differentiation via TLR7/8 to polarized macrophages and fibrocytes. J. Immunol. 198 1974–1984. 10.4049/jimmunol.1600797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B., Momen-Heravi F., Kodys K., Szabo G. (2016). MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J. Biol. Chem. 291 149–159. 10.1074/jbc.m115.694133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah T., Qin S., Vashi M., Predescu D. N., Jeganathan N., Bardita C., et al. (2018). Alk5/Runx1 signaling mediated by extracellular vesicles promotes vascular repair in acute respiratory distress syndrome. Clin. Transl. Med. 7 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L., Zhang Y., Lan B., Wang J., Zhang Z., Zhang L., et al. (2017). MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Res. Int. 2017:4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Liu J., Zhang F., Wang Y., Qin Y., Zhou Z., et al. (2016). CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016:1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shentu T.-P., Huang T.-S., Cernelc-Kohan M., Chan J., Wong S. S., Espinoza C. R., et al. (2017). Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Scient. Rep. 7 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., Wang Y., Zhao R., Long X., Deng W., Wang Z. (2018). Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS One. 13:e0191616. 10.1371/journal.pone.0191616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yang Y., Guo Q., Gao Q., Ding Y., Wang H., et al. (2019). Exosomes derived from human umbilical cord mesenchymal stem cells promote fibroblast-to-myofibroblast differentiation in inflammatory environments and benefit cardioprotective effects. Stem Cells Devel. 28 799–811. 10.1089/scd.2018.0242 [DOI] [PubMed] [Google Scholar]
- Shigemoto-Kuroda T., Oh J. Y., Kim D.-K., Jeong H. J., Park S. Y., Lee H. J. (2017). MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 8 1214–1225. 10.1016/j.stemcr.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue S.-J., Rau R.-H., Shiue H.-S., Hung Y.-W., Li Z.-X., Yang K. D., et al. (2019). Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury–induced pain in rats. Pain 160 210–223. 10.1097/j.pain.0000000000001395 [DOI] [PubMed] [Google Scholar]
- Silva J. D., de Castro L. L., Braga C. L., Oliveira G. P., Trivelin S. A., Barbosa-Junior C. M., et al. (2019). Mesenchymal stromal cells are more effective than their extracellular vesicles at reducing lung injury regardless of acute respiratory distress syndrome etiology. Stem Cells Int. 2019:8262849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleymani-Goloujeh M., Saberi S., Shekari F. (2018). Extracellular vesicles in regenerative medicine, a brief review. Modern Med. Lab. J. 2 118–126. [Google Scholar]
- Song Y., Dou H., Li X., Zhao X., Li Y., Liu D., et al. (2017). Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells 35 1208–1221. 10.1002/stem.2564 [DOI] [PubMed] [Google Scholar]
- Su V. Y. F., Chiou S. H., Lin C. S., Chen W. C., Yu W. K., Chen Y. W., et al. (2017). Induced pluripotent stem cells reduce neutrophil chemotaxis via activating GRK2 in endotoxin-induced acute lung injury. Respirology 22 1156–1164. 10.1111/resp.13053 [DOI] [PubMed] [Google Scholar]
- Su V. Y. F., Yang K. Y., Chiou S. H., Chen N. J., Mo M. H., Lin C. S., et al. (2019). Induced Pluripotent Stem Cells Regulate Triggering Receptor Expressed on Myeloid Cell-1 Expression and the p38 Mitogen-Activated Protein Kinase Pathway in Endotoxin-Induced Acute Lung Injury. Stem Cells 37 631–639. 10.1002/stem.2980 [DOI] [PubMed] [Google Scholar]
- Sun L., Xu R., Sun X., Duan Y., Han Y., Zhao Y., et al. (2016). Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy 18 413–422. 10.1016/j.jcyt.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Sun L., Zhu M., Feng W., Lin Y., Yin J., Jin J., et al. (2019). Exosomal miRNA Let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxidat. Med. Cell. Long 2019:4506303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Shan A., Wei Z., Xu B. (2018). Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 503 2611–2618. 10.1016/j.bbrc.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Tamura R., Uemoto S., Tabata Y. (2017). Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 57 274–284. 10.1016/j.actbio.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Tan J. L., Lau S. N., Leaw B., Nguyen H. P., Salamonsen L. A., Saad M. I., et al. (2018). Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl. Med. 7 180–196. 10.1002/sctm.17-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. D., Shi L., Monsel A., Li X. Y., Zhu H. L., Zhu Y. G., et al. (2017). Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells 35 1849–1859. 10.1002/stem.2619 [DOI] [PubMed] [Google Scholar]
- Tao S.-C., Yuan T., Zhang Y.-L., Yin W.-J., Guo S.-C., Zhang C.-Q. (2017). Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7:180. 10.7150/thno.17133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapparo M., Bruno S., Collino F., Togliatto G., Deregibus M. C., Provero P., et al. (2019). Renal regenerative potential of extracellular vesicles derived from miRNA-engineered mesenchymal stromal cells. Int. J. Mol. Sci. 20:2381. 10.3390/ijms20102381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thane K. E., Davis A. M., Hoffman A. M. (2019). Improved methods for fluorescent labeling and detection of single extracellular vesicles using nanoparticle tracking analysis. Scient. Rep. 9 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ti D., Hao H., Tong C., Liu J., Dong L., Zheng J., et al. (2015). LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 13:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofiño-Vian M., Guillén M. I., del Caz M. D. P., Silvestre A., Alcaraz M. J. (2018). Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 47 11–25. 10.1159/000489739 [DOI] [PubMed] [Google Scholar]
- Toribio V., Morales S., López-Martín S., Cardeñes B., Cabañas C., Yáñez-Mó M. (2019). Development of a quantitative method to measure EV uptake. Scient. Rep. 9 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseliou E., Fouad J., Reich H., Slipczuk L., De Couto G., Aminzadeh M., et al. (2015). Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J. Am. College Cardiol. 66 599–611. 10.1016/j.jacc.2015.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Balkom B. W., De Jong O. G., Smits M., Brummelman J., den Ouden K., de Bree P. M., et al. (2013). Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 121 3997–4006. 10.1182/blood-2013-02-478925 [DOI] [PubMed] [Google Scholar]
- Van Poll D., Parekkadan B., Cho C. H., Berthiaume F., Nahmias Y., Tilles A. W., et al. (2008). Mesenchymal stem cell–derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 47 1634–1643. 10.1002/hep.22236 [DOI] [PubMed] [Google Scholar]
- Varkouhi A. K., Jerkic M., Ormesher L., Gagnon S., Goyal S., Rabani R., et al. (2019). Extracellular vesicles from interferon-γ–primed human umbilical cord mesenchymal stromal cells reduce Escherichia coli–induced acute lung injury in rats. Anesthesiology 130 778–790. 10.1097/aln.0000000000002655 [DOI] [PubMed] [Google Scholar]
- Wang B., Yao K., Huuskes B. M., Shen H.-H., Zhuang J., Godson C., et al. (2016). Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol. ther. 24 1290–1301. 10.1038/mt.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Morales J. E., Calame D. G., Alcorn J. L., Wetsel R. A. (2010). Transplantation of human embryonic stem cell–derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol. Ther. 18 625–634. 10.1038/mt.2009.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zheng R., Chen Q., Shao J., Yu J., Hu S. (2017). Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res. Ther. 8 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen S., Ma X., Cheng C., Xiao X., Chen J., et al. (2013). Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxidative Med. Cell. Long. 2013:572729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Huang R., Xu Q., Zheng G., Qiu G., Ge M., et al. (2020). Mesenchymal Stem Cell–Derived Extracellular Vesicles Alleviate Acute Lung Injury Via Transfer of miR-27a-3p. Crit. Care Med. 48 e599–e610. [DOI] [PubMed] [Google Scholar]
- Wang J., Jia H., Zhang B., Yin L., Mao F., Yu J., et al. (2018). HucMSC exosome-transported 14-3-3ζ prevents the injury of cisplatin to HK-2 cells by inducing autophagy in vitro. Cytotherapy 20 29–44. 10.1016/j.jcyt.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Wang K., Jiang Z., Webster K. A., Chen J., Hu H., Zhou Y., et al. (2017). Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl. Med. 6 209–222. 10.5966/sctm.2015-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Pei S., Han L., Guo B., Li Y., Duan R., et al. (2018). Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell. Physiol. Biochem. 50 1535–1559. 10.1159/000494652 [DOI] [PubMed] [Google Scholar]
- Wang N., Chen C., Yang D., Liao Q., Luo H., Wang X., et al. (2017). Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim. Biophys. Acta 1863 2085–2092. 10.1016/j.bbadis.2017.02.023 [DOI] [PubMed] [Google Scholar]
- Wang X. L., Zhao Y. Y., Sun L., Shi Y., Li Z. Q., Zhao X. D., et al. (2018). Exosomes derived from human umbilical cord mesenchymal stem cells improve myocardial repair via upregulation of Smad7. Int. J. Mol. Med. 41 3063–3072. [DOI] [PubMed] [Google Scholar]
- Wang X., Gu H., Qin D., Yang L., Huang W., Essandoh K., et al. (2015). Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Scient. Rep. 5:13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fu B., Sun X., Li D., Huang Q., Zhao W., et al. (2015). Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-β1-mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Res. Ther. 6:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Liu Z., Hu L., Gu W., Zhu L. (2018). Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp. Cell Res. 370 13–23. 10.1016/j.yexcr.2018.06.003 [DOI] [PubMed] [Google Scholar]
- Wu X.-Q., Yan T.-Z., Wang Z.-W., Wu X., Cao G.-H., Zhang C. (2017). BM-MSCs-derived microvesicles promote allogeneic kidney graft survival through enhancing micro-146a expression of dendritic cells. Immunol. Lett. 191 55–62. 10.1016/j.imlet.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Xagorari A., Siotou E., Yiangou M., Tsolaki E., Bougiouklis D., Sakkas L., et al. (2013). Protective effect of mesenchymal stem cell-conditioned medium on hepatic cell apoptosis after acute liver injury. Int. J. Clin. Exp. Pathol. 6:831. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., et al. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30 1556–1564. 10.1002/stem.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H., Li Y., Liu Z., Wang X., Shang X., Cui Y., et al. (2013). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31 2737–2746. 10.1002/stem.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Shao Y., Ye K., Qu Y., Memet O., He D., et al. (2019). Mesenchymal stem cell-derived exosomes attenuate phosgene-induced acute lung injury in rats. Inhalation Toxicol. 31 52–60. 10.1080/08958378.2019.1597220 [DOI] [PubMed] [Google Scholar]
- Xu R., Zhang F., Chai R., Zhou W., Hu M., Liu B., et al. (2019). Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J. Cell. Mol. Med. 23 7617–7631. 10.1111/jcmm.14635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Jiang W., Tan Y., Zou S., Zhang H., Mao F., et al. (2017). hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol. Ther. 25 465–479. 10.1016/j.ymthe.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X.-X., Fan H., Tang Q., Shou Z.-X., Zuo D.-M., et al. (2015). Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 10:e0140551. 10.1371/journal.pone.0140551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang X., Chen X., Wang L., Yang G. (2017a). Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Ther. Nucleic Acids 7 278–287. 10.1016/j.omtn.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhou C. Z., Zhu R., Fan H., Liu X. X., Duan X. Y., et al. (2017b). miR-200b-containing microvesicles attenuate experimental colitis associated intestinal fibrosis by inhibiting epithelial-mesenchymal transition. J. Gastroenterol. Hepatol. 32 1966–1974. 10.1111/jgh.13797 [DOI] [PubMed] [Google Scholar]
- Yang K.-Y., Shih H.-C., How C.-K., Chen C.-Y., Hsu H.-S., Yang C.-W., et al. (2011). IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest 140 1243–1253. 10.1378/chest.11-0539 [DOI] [PubMed] [Google Scholar]
- Yu B., Kim H. W., Gong M., Wang J., Millard R. W., Wang Y., et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 182 349–360. 10.1016/j.ijcard.2014.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Shao H., Su C., Jiang Y., Chen X., Bai L., et al. (2016). Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Scientific Rep. 6:34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Wang D., Wen X., Tang X., Qi D., He J., et al. (2020). Adipose-derived exosomes protect the pulmonary endothelial barrier in ventilator-induced lung injury by inhibiting the TRPV4/Ca2+ signaling pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 318 L723–L741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Guo F., Chang M., Zhou Z., Yi L., Gao C., et al. (2019a). Exosome-delivered syndecan-1 rescues acute lung injury via a FAK/p190RhoGAP/RhoA/ROCK/NF-κB signaling axis and glycocalyx enhancement. Exp. Cell Res. 384:111596. 10.1016/j.yexcr.2019.111596 [DOI] [PubMed] [Google Scholar]
- Zhang C., Shao K., Liu C., Li C., Yu B. (2019b). Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur. Rev. Med. Pharmacol. Sci. 23 6691–6699. [DOI] [PubMed] [Google Scholar]
- Zhang D., Lee H., Wang X., Rai A., Groot M., Jin Y. (2018). Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol. Ther. 26 2119–2130. 10.1016/j.ymthe.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Shan W.-F., Wu G.-Q., Xiong X.-X., Jin H.-Y., Zhu S.-M. (2014). Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J. Transl. Med. 12 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Chu W., Lai R., Lim S., Hui J., Toh W. (2016). Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartilage 24 2135–2140. 10.1016/j.joca.2016.06.022 [DOI] [PubMed] [Google Scholar]
- Zhang S., Chuah S. J., Lai R. C., Hui J. H. P., Lim S. K., Toh W. S. (2018). MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 156 16–27. 10.1016/j.biomaterials.2017.11.028 [DOI] [PubMed] [Google Scholar]
- Zhang S., Jiang L., Hu H., Wang H., Wang X., Jiang J., et al. (2020). Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 246:117401. 10.1016/j.lfs.2020.117401 [DOI] [PubMed] [Google Scholar]
- Zhao J., Li X., Hu J., Chen F., Qiao S., Sun X., et al. (2019). Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovas. Res. 115 1205–1216. 10.1093/cvr/cvz040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Sun L., Mu T., Yi J., Ma C., Xie H., et al. (2020). An HBV-encoded miRNA activates innate immunity to restrict HBV replication. J. Mol. Cell Biol. 12 263–276. 10.1093/jmcb/mjz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zhong L., Li P., He K., Qiu C., Zhao L., et al. (2020). Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Exp. Cell Res. 387:111738. 10.1016/j.yexcr.2019.111738 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Li P., Goodwin A. J., Cook J. A., Halushka P. V., Chang E., et al. (2018). Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol. Ther. 26 1375–1384. 10.1016/j.ymthe.2018.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li P., Goodwin A. J., Cook J. A., Halushka P. V., Chang E., et al. (2019). Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit. Care. 23:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang X., Sun L., Zhou L., Ma T. C., Song L., et al. (2016). Toll-like receptor 3-activated macrophages confer anti-HCV activity to hepatocytes through exosomes. FASEB J. 30 4132–4140. 10.1096/fj.201600696r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Lu K., Zhang N., Zhao Y., Ma Q., Shen J., et al. (2018). Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 46 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Huang X., Yu W., Chen H., Chen Y., Dai Y. (2018). Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50:e12871. 10.1111/and.12871 [DOI] [PubMed] [Google Scholar]
- Zhu L.-P., Tian T., Wang J.-Y., He J.-N., Chen T., Pan M., et al. (2018). Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 8:6163. 10.7150/thno.28021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. G., Feng X. M., Abbott J., Fang X. H., Hao Q., Monsel A., et al. (2014). Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 32 116–125. 10.1002/stem.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., et al. (2017). Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 8 1–11. 10.1155/2018/9601623 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.