Abstract
Background: It is estimated that one in five people worldwide faces a diagnosis of a malignant neoplasm during their lifetime. Carvacrol and its isomer, thymol, are natural compounds that act against several diseases, including cancer. Thus, this systematic review aimed to examine and synthesize the knowledge on the antitumor effects of carvacrol and thymol.
Methods: A systematic literature search was carried out in the PubMed, Web of Science, Scopus and Lilacs databases in April 2020 (updated in March 2021) based on the PRISMA 2020 guidelines. The following combination of health descriptors, MeSH terms and their synonyms were used: carvacrol, thymol, antitumor, antineoplastic, anticancer, cytotoxicity, apoptosis, cell proliferation, in vitro and in vivo. To assess the risk of bias in in vivo studies, the SYRCLE Risk of Bias tool was used, and for in vitro studies, a modified version was used.
Results: A total of 1,170 records were identified, with 77 meeting the established criteria. The studies were published between 2003 and 2021, with 69 being in vitro and 10 in vivo. Forty-three used carvacrol, 19 thymol, and 15 studies tested both monoterpenes. It was attested that carvacrol and thymol induced apoptosis, cytotoxicity, cell cycle arrest, antimetastatic activity, and also displayed different antiproliferative effects and inhibition of signaling pathways (MAPKs and PI3K/AKT/mTOR).
Conclusions: Carvacrol and thymol exhibited antitumor and antiproliferative activity through several signaling pathways. In vitro, carvacrol appears to be more potent than thymol. However, further in vivo studies with robust methodology are required to define a standard and safe dose, determine their toxic or side effects, and clarify its exact mechanisms of action.
This systematic review was registered in the PROSPERO database (CRD42020176736) and the protocol is available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=176736.
Keywords: carvacrol, thymol, cancer, antitumor, anticancer
Introduction
It is estimated that one in five people worldwide faces the diagnosis of some malignant neoplasm during their lifetime, and the number of people with cancer is forecast to double by the year 2040 (World Health Organization, 2020). In fact, cancer is a major global public health problem, and it is one of the four main causes of premature death (before 70 years old) in most countries, resulting in 8.8 million deaths per year (National Cancer Institute, 2019). The antineoplastic agents available on the market have different mechanisms of action that impair cell proliferation and/or cause cell death, thereby increasing patient survival rate (Powell et al., 2014; Lee and Park, 2016). However, the toxicity and side effects of many treatments can worsen the quality of life of these individuals (Weingart et al., 2018; Hassen et al., 2019; Lu et al., 2019). Thus, despite being the subject of research for many years, cancer still remains a major concern and an important area of study in the search for a cure.
There is, therefore, an ongoing search for substances that can be used to develop more effective treatments, with less side effects, to use against cancer; one promising group of substances are natural products (NPs). There are many medicinal plants whose pharmacological properties have already been described and scientifically proven (Nelson, 1982; Mishra and Tiwari, 2011; Carqueijeiro et al., 2020). However, the enormous diversity of nature still holds many plant compounds without sufficient studies, particularly in the oncology area (Gordaliza, 2007; Asif, 2015). Historically, secondary plant metabolites have made important contributions to cancer therapy, such as, the vinca alkaloids (vinblastine and vincristine) and the paclitaxel terpene that was obtained from the Taxus brevifolia Nutt. species (Martino et al., 2018). More recently, other compounds, such as perillyl alcohol and limonene -monoterpenes found in aromatic plant species, have been widely studied due to their antitumor potential, and have been included in clinical phase studies (Shojaei et al., 2014; Arya and Saldanha, 2019).
In this context, carvacrol (5-isopropyl-2-methylphenol) and its thymol isomer (2-isopropyl-5-methylphenol), classified as natural multi-target compounds, deserve attention. Both are monoterpenoid phenols, the main components present in essential oils obtained from several plant species of the Lamiaceae and Verbenaceae families, such as oregano (Origanum vulgare L.), thyme (Thymus vulgaris L.) and “alecrim-da-chapada” (Lippia gracilis) (Santos et al., 2016; Salehi et al., 2018; Sharifi-Rad et al., 2018; Baj et al., 2020), which have already been reported to exhibit beneficial effects against many diseases (Silva et al., 2018), including cancer (Elbe et al., 2020; Pakdemirli et al., 2020). In addition, these compounds present anti-inflammatory (Li et al., 2018; Chamanara et al., 2019) and antioxidant (Arigesavan and Sudhandiran, 2015; Sheorain et al., 2019) activities that enable the reduction of inflammation and an increase in enzymatic and non-enzymatic antioxidants in the tumor environment (Gouveia et al., 2018). Hence, this systematic review aims to examine and synthesize knowledge about the antitumor and antiproliferative effect of carvacrol and thymol, as well as to report the main mechanisms of action already described for the two compounds against cancer. to provide guidance for future research.
Methods
Question and PICOS Strategy
The purpose of this systematic review was to answer the following question: Do carvacrol and thymol exhibit an anti-tumor effect on cancer cells (in vitro) or in animal models of cancer? The review followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021).
A PICOS strategy (patient or pathology, intervention, control, and other outcomes and type of study) was used based on: P: Animals with cancer or tumor cells; I: Treatment with carvacrol or thymol; C: No treatment, healthy cells or placebo (vehicle); O: Cytotoxic and antitumor effects, induction of apoptosis and inhibition of proliferation; S: Pre-clinical studies in vitro and in vivo.
Data Sources and Literature Search
The research was carried out in the databases PubMed, Web of Science, Scopus and Lilacs in April 2020 (updated in March 2021) using a combination of health descriptors, MeSH terms and their synonyms, such as antitumor, antineoplastic, anticancer, cytotoxicity, apoptosis, cell proliferation, in vitro, in vivo, carvacrol or 5-isopropyl-2-methylphenol and thymol or 5-methyl-2-propan-2-ylphenol (Supplementary Table S1, contains a complete list of these search terms).
Study Selection and Eligibility Criteria
Two independent reviewers (L.A.S. and L.T.S.P.) analyzed the research results and selected potentially relevant studies after reading their title and abstract, using the systematic review application, Rayyan (Ouzzani et al., 2016). We used the Kappa statistical test to measure the inter-rater reliability (Landis and Koch, 1977). Disagreements were resolved through a consensus between the reviewers, and the decision was supported by the assistance of a third reviewer when necessary (AGG). The following inclusion criteria were applied: Administration of carvacrol or pure thymol vs. placebo; in vitro studies of cancer cell lines, in vivo study of animals with cancer; cytotoxic effect, antitumor effect, inhibition of proliferation and apoptosis and experimental studies (in vitro and in vivo). The exclusion criteria were: experiments with derivatives of the carvacrol or thymol, association of the two compounds with other substances or in mixtures composing essential oils and extracts, animals with other diseases in addition to cancer, review articles, meta-analyses, abstracts, conference articles, editorials/letters and case reports. A manual search of the reference lists of all selected studies was also conducted, in order to identify additional primary studies for inclusion.
Data Extraction and Risk of Bias Assessment
We extracted the following data from the included articles: author, year, country, data about the monoterpene (source, obtention method), concentration and/or dose, type of animal or cell line, results (cytotoxicity, cell proliferation, apoptosis, cell cycle, histology), proposed mechanisms involved in the antitumor effect and conclusion. The authors of the included studies were contacted when necessary (whenever any data or article was not available).
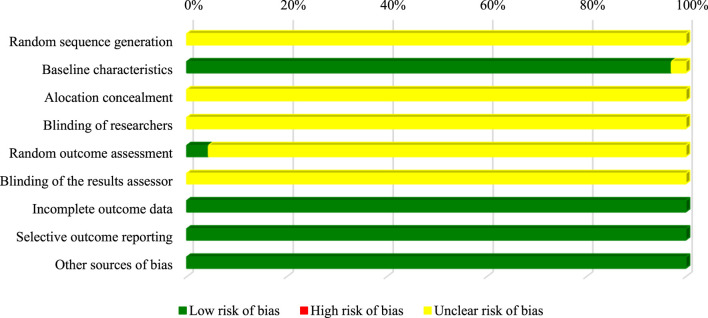
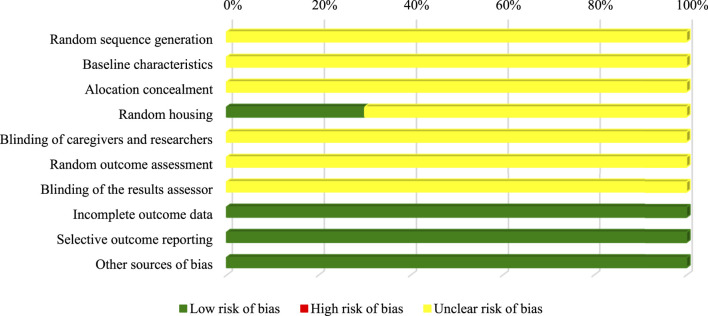
The SYRCLE Risk of Bias tool was used to assess the risk of bias of all in vivo experimental studies (Hooijmans et al., 2014). We analyzed the following ten domains: sequence generation, allocation concealment and random accommodation (selection bias), random accommodation and concealment (performance bias), random evaluation and concealment of results (detection bias), incomplete result data (bias of attrition), selective report of results (report bias) and other sources of bias, such as inappropriate influence from financiers. An adapted protocol of the SYRCLE Risk of Bias tool was used to evaluate the methodological quality of in vitro studies, as described by Chan et al. (2017). The methodological quality was classified as low, unclear or high, according to the established criteria (Hooijmans et al., 2014).
Statistical Analysis
IC50 values determined 24 h after the incubation of the studied cells with carvacrol or thymol were compiled, submitted to the standardization of the unit (μM) (Supplementary Table S2) and were expressed as mean ± standard error of the mean (SEM).
Results
Study Selection
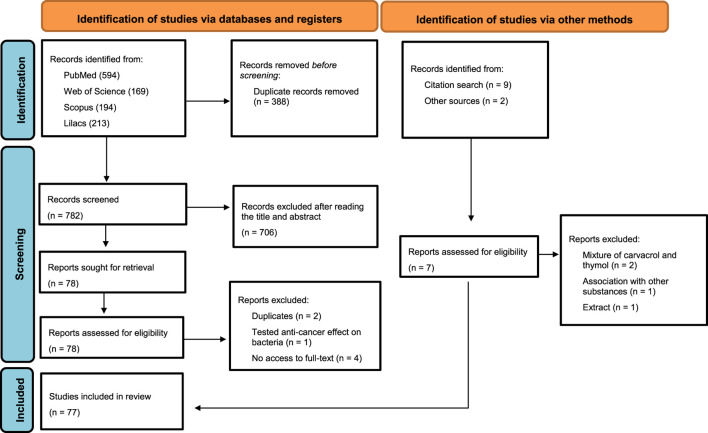
The initial search resulted in 1,170 records, of which 594, 169, 194, and 213 were found in PubMed, Web of Science, Scopus and Lilacs, respectively. Of these, 388 were excluded due to duplication. After screening the title and abstract, 706 reports were excluded, and 1 report was sought for retrieval, as it met the criteria after reading the full text, resulting in 77 studies. Of these, three were excluded after reading the full text (two for presenting the same results and one that tested the anti-cancer effect on bacteria) and four for not having access to the full text, resulting in 70 articles. In addition, 9 studies were identified after a manual search of the references and two studies obtained from other sources, but only seven studies were added (two studies were excluded for having a mixture between carvacrol and thymol, one for being associated with other substances, and one that studied the extract of a plant rich in thymol), finally resulting in 77 included studies (Figure 1). There was almost perfect (Landis and Koch, 1977) reliability/agreement (κ = 0.813) among the reviewers, after selecting the titles and abstracts.
FIGURE 1.
Flowchart of included studies.
Overview of Included Studies
The selected studies were carried out in different countries: India (n = 14), China (n = 14), Turkey (n = 13), the Republic of Korea (n = 5), Slovakia (n = 5), Iran (n = 5), Morocco (n = 2), Brazil (n = 2), Greece (n = 2), Iraq (n = 2), The United States of America (n = 2), Canada (n = 2), Egypt (n = 2), Croatia (n = 1), Lithuania (n = 1), Italy (n = 1), Spain (n = 1), The United Kingdom (n = 1), Peru (n = 1), Belgium (n = 1). Asia (51.9%) was the continent with the largest number of publications on the subject, followed by Europe (33.7%), the Americas (9.3%) and Africa (5.1%), with a greater trend of publications on this subject in the last three years (Supplementary Figure S1). Among the selected articles, 69 (89.6%) reported in vitro experiments and 10 (12.9%) were in vivo studies. Likewise, 43, 19, and 15 publications tested only carvacrol, thymol and both compounds, respectively. They were mostly obtained commercially, provided by Sigma Aldrich (n = 52), Fluka (n = 5), Aldrich Chemical (n = 2), Western Chemical (n = 1), Agolin SA (n = 1), Alfa Aesar (n = 1). In only five studies were the compounds isolated from essential oils. Remarkably, ten studies did not report the source of the tested compounds. A detailed description of the included studies is shown in Tables 1 and 2. A narrative summary of the results is presented below, divided into in vitro and in vivo studies.
TABLE 1.
Detailed description of the studies that used carvacrol, included in the systematic review.
| Model | Concentration/incubation time | Experimental methods for testing IC50 values | Results/targets | Conclusion | Authors (Year), Country | ||
|---|---|---|---|---|---|---|---|
| Increase | Decrease | IC50 | |||||
| Monoterpene carvacrol | |||||||
| In vitro studies | |||||||
| CO25 | 1–150 μg/mL | MTT assay | p21N−ras | Tumor growth | 60 μg/mL–24 h | Carvacrol has a cytotoxic effect and an antiproliferative effect | Zeytinoglu et al. (2003), Turkey |
| 24, 48, 72 h of incubation | DNA synthesis level | ||||||
| A549 | 100–1,000 μM | — | Apoptosis induction | Cell viability | — | Carvacrol may have an anticancer effect and be used as a drug substance to cure cancer | Koparal and Zeytinoglu (2003), Turkey |
| 24 h of incubation | Cell proliferation | ||||||
| HepG2 | 25–900 μmol | — | Cytotoxic effects | DNA damage level | — | HepG2 cells were slightly more sensitive to the effects | Horváthová et al. (2006), Slovakia |
| Caco-2 | 24 of incubation | ||||||
| Leiomyosarcoma | 10–4,000 μM | Trypan Blue | Antiproliferative effects | Cell growth | 90 μM–24 h | Carvacrol has anticarcinogenic, antiproliferative and antiplatelet properties | Karkabounas et al. (2006), Greece |
| 24 and 48 h of incubation | 67 μM–48 h | ||||||
| K-562 | 200–1,000 μM | Trypan blue exclusion | Cytotoxic effects | DNA damage level | 220 μM–24 h | Carvacrol has cytotoxic, antioxidant effects and has a protective action against DNA damage | Horvathova et al. (2007), Slovakia |
| 24 or 48 h of incubation | |||||||
| P-815 | 0.004–0.5% v/v | MTT assay | — | — | <0.004% v/v–48 h | Carvacrol is cytotoxic | Jaafari et al. (2007), Morocco |
| 48 h of incubation | |||||||
| HepG2 | 100–1,000 μM | Trypan blue exclusion | Cytotoxic effects | Cell proliferation | HepG2 - 350 μM–24 h | Carvacrol has antiproliferative and antioxidant effects | Slamenová et al. (2007), Slovakia |
| Caco-2 | 24 h of incubation | Caco-2 - 600 μM–24 h | |||||
| MDA-MB 231 | 20–100 μM | MTT assay | Apoptosis induction | Cell growth | 100 μM–48 h | Carvacrol can be a potent antitumor molecule against breast cancer metastatic cells | Arunasree, (2010), India |
| Caspase activation | S-phase cells | ||||||
| 24 or 48 h of incubation | Sub-stage G0/G1 | Mitochondrial membrane potential | |||||
| Cyt C | Bcl-2 | ||||||
| Bax | |||||||
| 5RP7 | 0.0002–0.1 mg/mL | MTT assay and Trypan Blue exclusion | Cytotoxic effects | — | 5RP7 - 0.04 mg/mL–24/48 h | Carvacrol promoted a cytotoxic effect, induced apoptosis and can be used in cancer therapy | Akalin and Incesu, (2011), Turkey |
| CO25 | 24 or 48 h of incubation | Apoptotic cells | CO25–0.1 mg/mL–24 h | ||||
| 0.05 mg/mL–48 h | |||||||
| SiHa | 25–500 μg/mL | MTT and LDH assay | Apoptosis induction | Cell proliferation | SiHa - 50 ± 3.89 mg/L | Carvacrol is a potent anticancer compound that exhibits cytotoxic effects and induces the inhibition of cell proliferation in both human cervical cancer cells | Mehdi et al. (2011), India |
| HeLa | 48 h of incubation | HeLa - 50 ± 5.95 mg/L | |||||
| HepG2 | 20–200 μg/mL | CellTiter-Blue® cell viability assay | Cytotoxic effects | Membrane damage | 53.09 μg/mL | Carvacrol exhibits antioxidant activity and anticancer effects on cells | Özkan and Erdogan (2011), Turkey |
| 24 h of incubation | Antiproliferative effects | Cell viability | |||||
| P-815 | 0.05–1.25 μM | MTT assay | Cytotoxic effects | Interruption of cell cycle progression in the S phase | P-815–0.067 μM | Carvacrol showed a cytotoxic effect in all strains tested | Jaafari et al. (2012), Morocco |
| CEM | CEM - 0.042 μM | ||||||
| K-562 | 48 h of incubation | K-562–0.067 μM | |||||
| MCF-7 | MCF-7 - 0.125 μM | ||||||
| MCF-7 gem | MCF-7 gem - 0.067 μM | ||||||
| DBTRG-05MG | 200–1,000 μM | — | Generation of ROS | Cell viability | — | Carvacrol was cytotoxic and induced cell death in human glioblastoma cells | Liang and Lu, (2012), China |
| 24 h of incubation | Caspase-3 | ||||||
| H1299 | 25–1800 μM | CellTiter-Blue® cell viability assay | MDA | Membrane and DNA damage | 380 μM–24 h | Carvacrol exhibited cytotoxic and antioxidant effects | Ozkan and Erdogan (2012), Turkey |
| 24 and 48 h of incubation | 8-OHdG | 244 μM–48 h | |||||
| B16-F10 | Not reported | Trypan blue assay and MTT assay | Cytotoxic effects | Cell viability | 550 μM | Carvacrol showed an antitumor effect with moderate cytotoxicity | Satooka and Kubo (2012), United States |
| 24 h of incubation | Relative melanogenesis | ||||||
| Relative melanin cell | |||||||
| HepG2 | 0.05–0.4 mmol/L | MTT assay | p-p38 | Cell viability | 0.4 mmol/L–24 h | Carvacrol caused inhibition of cell proliferation, inhibition of tumor cell growth and induction of apoptosis | Yin et al. (2012), China |
| 24 h of incubation | MAPK | p-ERK 1/2 | |||||
| Caspase-3 | Bcl-2 | ||||||
| OC2 | 200–1,000 μM | — | Generation of ROS | Cell viability | — | Carvacrol exhibited a cytotoxic effect and induced apoptosis in human oral cancer cells | Liang et al. (2013), China |
| 24 h of incubation | Caspase-3 | ||||||
| MCF-7 | 140–450 μM | MTT and LDH assay | Caspase-3, -6 and -9 | Cell viability | 244.7 ± 0.71μM–48 h | Carvacrol induces cytotoxicity and apoptosis in MCF-7 cells and may be a potential chemotherapeutic agent against cancer | Al-Fatlawi and Ahmad (2014), India |
| 24 and 48 of incubation | Bax | Bcl-2 | |||||
| p53 | |||||||
| N2a | 10–400 mg/L | — | TAC | — | — | Carvacrol has antioxidant and anticancer properties in N2a cells at concentrations of 200 and 400 mg/L | Aydın et al. (2014), Turkey |
| 24 h of incubation | TOS | ||||||
| Caco-2 | 100–2,500 μM | MTS assay | Apoptosis induction | Cell viability | 460 ± 3.6 μM–24 h | Carvacrol exhibited cytotoxic effects and induction of apoptosis | Llana-Ruiz-Cabello et al. (2014), Spain |
| 24 and 48 h of incubation | 343 ± 7.4 μM–48 h | ||||||
| HepG2 | 25–1,000 μM | Trypan Blue exclusion and MTT assay | Apoptosis induction | Cell growth | 425 μM–24 h | Carvacrol can be used as an anti-tumor molecule against cancer cells | Melusova et al. (2014), Slovakia |
| 24 h of incubation | SsDNA breaks | ||||||
| Oxidative DNA lesions | |||||||
| HepG2 | 100–600 μM | — | Cells in G1 phase | S-phase cells | — | Carvacrol caused induction of apoptosis and slowed cell division, resulting in cell death | Melušová et al. (2014), Slovakia |
| 24 h of incubation | |||||||
| U87 | 125–1,000 μM | MTT assay | Apoptosis induction | Cell viability | 561.3 μM–24 h | Carvacrol has therapeutic potential for the treatment of glioblastomas by inhibiting TRPM7 channels | Chen et al. (2015), Canada |
| 24, 48 or 72 h of incubation | Caspase-3 | Cell proliferation | |||||
| PI3K/Akt | |||||||
| MAPK | |||||||
| TRPM7 | |||||||
| MMP-2 | |||||||
| HCT116 | 100–900 μmol/L | MTT assay | Apoptosis induction | Cell growth | HCT116–544.4 μmol/L–48 h | Carvacrol can be a promising natural product in the management colon cancer | Fan et al. (2015), China |
| LoVo | 48 h of incubation | Cell migration and invasion | |||||
| Bcl-2 | |||||||
| Bax | MMP-2 and -9 | LoVo - 530.2 μmol/L–48 h | |||||
| Cyclin B1 | |||||||
| p-ERK | |||||||
| p-JNK | p-Akt | ||||||
| PI3K/Akt | |||||||
| Cell cycle stop in phase G2/M | |||||||
| AGS | 0.01–6 mg/mL | MTT assay | Cytotoxic effects | Cell viability | 30 μg/mL–48 h | Carvacrol exhibited a cytotoxic effect against gastric cancer cells | Maryam et al. (2015), Iran |
| 48 h of incubation | |||||||
| HL-60 | 10–200 μM | MTT assay | Apoptosis induction | Cell viability | HL-60–100 μM–24 h | Carvacrol effectively blocked the proliferation of cancer cells in vitro | Bhakkiyalakshmi et al. (2016), India |
| Jurkat | 24 h of incubation | Cytotoxic effects | MMP | Jurkat - 50 μM–24 h | |||
| Generation of ROS | Bcl-2 | ||||||
| Caspase-3 | |||||||
| Bax | |||||||
| Tca-8113 | 10–80 μM | — | Apoptosis induction | Cell proliferation | — | Carvacrol is a powerful new natural anti-cancer drug for human OSCC | Dai et al. (2016), China |
| SCC-25 | 24 and 48 h of incubation | S-Phase cells | |||||
| p21 | CCND1 | ||||||
| CDK4 | |||||||
| Bcl-2 | |||||||
| Bax | MMP-2 and -9 | ||||||
| COX-2 | |||||||
| A549 | 1–1,000 μM | SRB assay | Antiproliferative effects | — | A549–0.118 ± 0.0012 mΜ–72 h | Carvacrol exhibited antiproliferative and antioxidant effects. In addition, it exhibited more potent cytotoxicity against cells (A549). The cells (Hep3B) were more resistant to treatment and the cells (HepG2) were less sensitive | Fitsiou et al. (2016), Greece |
| HepG2 | 72 h of incubation | Cytotoxic effects | HepG2 - 0.344 ± 0.0035 mΜ–72 h | ||||
| Hep3B | Hep3B- 0.234 ± 0.017 mΜ–72 h | ||||||
| PC-3 | 250–750 μM | CCK-8 Kit | — | Cell viability | PC-3 - 498.3 ± 12.2 μM–24 h | Carvacrol treatment suppresses cell proliferation, migration and invasion, indicating that it has antiprostatic effects in vitro | Luo et al. (2016), China |
| DU 145 | 24, 48 and 72 h of incubation | Cell proliferation | DU 145–430.6 ± 21.9 μM–24 h | ||||
| Cell migration | |||||||
| Wound healing | |||||||
| MMP-2 | |||||||
| PI3K/Akt and MAPK | |||||||
| Cell invasion | |||||||
| TRPM7 | |||||||
| A549 | 0–250 μM | — | Cytotoxic effects | Cell viability | — | Carvacrol has cytotoxic activity | Coccimiglio et al. (2016), Canada |
| 24 h of incubation | |||||||
| U87 | 1–10,000 μM | MTT assay | Anticancer activity | — | U87–322 μM–24 h | Carvacrol exerted anticancer and antiproliferative activity with greater effect against the breast cancer cell line | Baranauskaite et al. (2017), Lithuania |
| MDA-MB 231 | 24 h of incubation | Antiproliferative activity | MDA-MB 231–199 μM–24 h | ||||
| Antioxidant activity | |||||||
| HepG2 | 0.01–0.25 μg/μL | MTT assay | — | Cell viability | 48 mg/L–24 h | Carvacrol has therapeutic potential in tumor cells without adverse effects in healthy cells | Elshafie et al. (2017), Italy |
| 24 h of incubation | Hepatocarcinoma cells | ||||||
| PC-3 | 100–800 μM | — | Cytotoxic effects | Cell viability | — | Carvacrol is cytotoxic | Horng et al. (2017), China |
| 24 h of incubation | |||||||
| DU 145 | 10–500 μM | MTT assay | Cytotoxic effects | Cell viability | 84.39 μM–24 h | Carvacrol has antiproliferative potential and can act as a chemopreventive agent in prostate cancer | Khan et al. (2017), India |
| 24 and 48 h of incubation | Apoptosis induction | Cell proliferation | 42.06 μM–48 h | ||||
| Caspase-3 | Mitochondrial membrane potential | ||||||
| Generation of ROS | Cell cycle stop | ||||||
| Cells in phase G0/G1 | Cells in S and G2/M phases | ||||||
| SiHa | 140–450 μM | MTT assay and LDH | Cytotoxic effects | Cell viability | SiHa - 424.22 μmol –24 h and 339.13 μmol–48 h | Carvacrol exhibited antiproliferative effects and may be a potential chemotherapeutic agent against cancer | Abbas and Al-Fatlawi (2018), Iraq |
| HepG2 | 24 and 48 h of incubation | Apoptosis induction | Bcl-2 | ||||
| Caspase-3, -6 and -9 | HepG2 - 576.52 μmol –24 h and 415.19 μmol –48 h | ||||||
| Bax | |||||||
| p53 | |||||||
| A375 | 3.906–1,000 μg/mL | MTT assay | Apoptosis induction | Cell viability | 40.41 ± 0.044 μg/mL–24 h | Carvacrol exhibits antiproliferative effects | Govindaraju and Arulselvi (2018), India |
| 24 of incubation | Sub-G1 phase | Cell growth | |||||
| Bcl-2 | |||||||
| Cell cycle stop | |||||||
| Cells in phase G0/G1 and G2/M | |||||||
| AGS | 10–600 µM | CellTiter-Glo Luminescent cell viability assay | Apoptotic effects | Cell viability | 82.57 ± 5.58 µM–24 h | Carvacrol has cytotoxic effects, apoptotic, genotoxic effects and dose-dependent ROS generators | Günes-Bayir et al. (2018), Turkey |
| 24 h of incubation | Necrosis | Bcl-2 | |||||
| Bax | |||||||
| Caspase-3 and -9 | |||||||
| Generation of ROS | GSH levels | ||||||
| Genotoxic effect | |||||||
| AGS | 10–600 µM | CellTiter-Glo Luminescent cell viability assay | Cytotoxic effects | Cell viability | 82.57 ± 5.5 μM–48 h | Carvacrol inhibited cell proliferation and induced cytotoxicity in cancer cells | Günes-Bayir et al. (2018), Turkey |
| 48 h of incubation | Apoptosis induction | Bcl-2 | |||||
| Bax | |||||||
| Caspase-3 e -9 | |||||||
| Generation of ROS | GSH levels | ||||||
| Genotoxic effect | |||||||
| MCF-7 | 10–200 μg/mL | MTT assay | — | Cell viability | MCF-7 - 46.5 μg/mL–24 h | Carvacrol has a cytotoxic effect and can cause inhibition of cell growth | Jamali et al. (2018), Iran |
| MDA-MB 231 | 24 h of incubation | MDA-MB 231–53 μg/mL– 24 h | |||||
| A549 | 30–300 μM | — | — | Cell viability | - | Carvacrol suppressed cell proliferation and migration and its inhibitory effect was attenuated in NSCLC cells with overexpression of AXL | Jung et al. (2018), Republic of Korea |
| H460 | 24 h of incubation | Cell proliferation | |||||
| AXL expression | |||||||
| Cell migration | |||||||
| JAR | 50–300 μM | — | Apoptosis induction | Cell proliferation | — | Carvacrol may be a possible new therapeutic agent or supplement for the control of human choriocarcinomas | Lim et al. (2019), Republic of Korea |
| JEG3 | 48 h of incubation | Sub-G1 phase | Cell viability | ||||
| Generation of ROS | PI3K/AKT | ||||||
| p-JNK | p-ERK1/2 | ||||||
| p-p38 | MMP | ||||||
| HeLa | 100–800 µM | XTT Reduction assay | Induction of cytotoxicity and apoptosis | Cyclin D1 | 556 ± 39 μM–24 h | Carvacrol can be used to treat cervical cancer, however, it should be avoided during cisplatin chemotherapy | Potočnjak et al. (2018), Croatia |
| 24 h of incubation | ERK1/2 | ||||||
| Caspase-9 | |||||||
| p21 | |||||||
| PC-3 | 100–800 μM | MTT assay | Cell death | Cell viability | 360 μM–48 h | Carvacrol inhibited the ability to invade and migrate PC3 cells and can be considered an anticancer agent | Heidarian and Keloushadi (2019), Iran |
| 48 h of incubation | Cell proliferation | ||||||
| Tumor cell invasion | |||||||
| IL-6 | |||||||
| p-STAT3 | |||||||
| p-ERK1/2 | |||||||
| p-AKT | |||||||
| PC-3 | 10–500 μM | MTT assay | Apoptosis induction | Cell viability | 46.71 μM–24 h | Carvacrol is a chemopreventive agent and has an antiproliferative effect on prostate cancer cells | Khan et al. (2019), India |
| Caspases -8 e -9 | Cell proliferation | ||||||
| Cell migration | |||||||
| 24 and 48 h of incubation | Generation of ROS | Cell cycle stop at G0/G1 | 39.81 μM–48 h | ||||
| Cells in S and G2/M phases Bcl-2 | |||||||
| Bax | Notch-1 | ||||||
| mRNA Jagged-1 | |||||||
| MCF-7 | 31.2–500 μg/mL | AlamarBlue® assay | Apoptosis induction | Cell proliferation | MCF-7 - 266.8 μg/mL–48 h | Carvacrol had the most cytotoxic effect among the other components studied | Tayarani-Najaran et al. (2019), Iran |
| PC-3 | 48 h of incubation | Cytotoxic effects | Cell viability | PC-3 - >500 μg/mL– 48 h | |||
| DU 145 | Bax | DU 145–21.11 μg/mL– 48 h | |||||
| PC-3 | 25–200 μg/mL | — | Cytotoxic effects | Cell viability | — | At the lowest concentration tested (25 μg/ml), carvacrol did not exhibit cytotoxicity to cancer cells | Trindade et al. (2019), Brazil |
| 24 and 48 h of incubation | |||||||
| HCT116 | 25–200 μM | xCELLigence Real-time cell Analysis | — | Cell proliferation | HCT116–92 μM–48 h | Carvacrol has an antiproliferative effect on both cell lines, but is more efficient against HT-29 compared to the HCT116 cell line | Pakdemirli et al. (2020), Turkey |
| HT-29 | 48 h of incubation | HT-29–42 μM–48 h | |||||
| MCF-7 | 25–250 μmol/L | MTT and LDH assay | Apoptosis induction | Cell viability | 200 μmol/L–24/48 h | Carvacrol can be used in a new approach for the treatment of breast cancer | Mari et al. (2020), India |
| Cells in phase G0/G1 | Cells in S and G2 phase | ||||||
| CDK4 and 6 | |||||||
| 24 and 48 of incubation | Cyclin D1 | ||||||
| Bax | Bcl-2 | ||||||
| PI3K/p-AKT | |||||||
| SKOV-3 | 100, 200, 400, 600 μM | MTT assay | Apoptosis induction | Cell viability | 322.50 µM–24 h | Carvacrol was cytotoxic to the ovarian cancer cell line | Elbe et al. (2020), Turkey |
| 24 and 48 h of incubation | 289.54 µM–48 h | ||||||
| Kelly | 12.5, 25, 50 µM | — | Antiproliferative effects | — | — | Carvacrol can be used to inhibit neuroblastoma cell proliferation | Kocal and Pakdemirli (2020), Turkey |
| SH-SY5Y | 24 h of incubation | ||||||
| BT-483 | 25–500 μM | — | Apoptosis induction | Cell viability | — | Carvacrol suppresses breast cancer cells by regulating the cell cycle and the TRPM7 pathway is one of the pharmacological mechanisms | Li et al. (2021), China |
| BT-474 | Cells in G1/G0 phase | S-phase and G2/M cells | |||||
| MCF-7 | 24 h of incubation | Cyclin C, D and E | Cell proliferation | ||||
| MDA-MB 231 | Cyclin A e B | ||||||
| MDA-MB 453 | CDK 4 | ||||||
| KG1 | 100, 200, 300, 400 μM | — | Cell death | Cell viability | — | KG1 cell lines were very sensitive to 300 µM carvacrol compared to the HL60 line, while the K562 line showed resistance after 48 h of treatment with 400 µM carvacrol | Bouhtit et al. (2021), Belgium |
| K-562 | 24 and 48 h of incubation | ||||||
| HL-60 | |||||||
| Model | Dose | Results/targets | Conclusion | Authors (Year), Country | |
|---|---|---|---|---|---|
| Increase | Decrease | ||||
| In vivo studies | |||||
| Chemical carcinogenesis induced by B [a]P in male wistar rats | 20 mL of carvacrol (ε = 976 mg/mL) mixed with 200mg of B [a]P | Anticarcinogenic effects | 30% tumor incidence | Carvacrol has anticarcinogenic, antiproliferative and antiplatelet properties | Karkabounas et al. (2006), Greece |
| Animal survival time | B [a]P carcinogenic potency | ||||
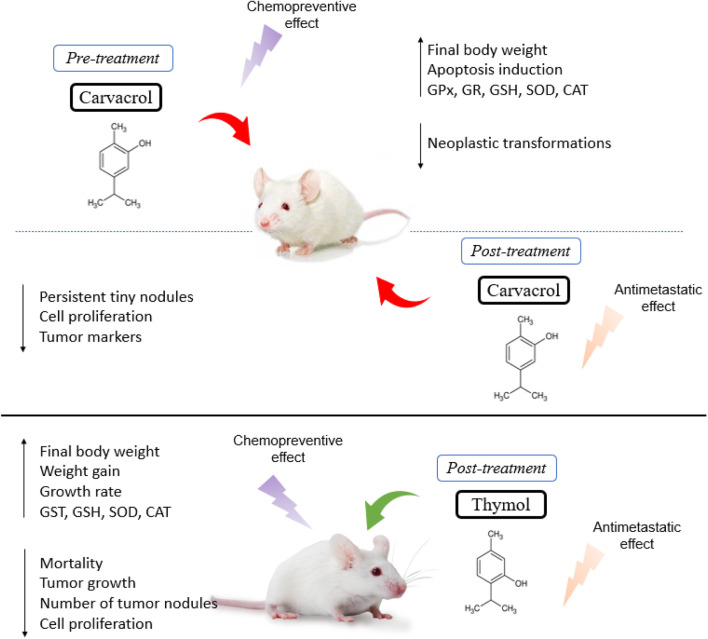
| Male wistar rats induced with hepatocarcinogenesis providing 0.01% DEN through drinking water for 16 weeks | Pre-treatment: (15 mg/kg b.wt) of carvacrol orally one week before DEN administration and up to 16 weeks | Final body weight | Pre-treatment: Number of nodules; neoplastic transformations; liver weight | Carvacrol has the ability to cause apoptosis in cancer cells and has a potent elimination of free radicals and antioxidant activities | Jayakumar et al. (2012), India |
| Post-treatment: (15 mg/kg b.wt) of carvacrol orally for 6 weeks after administration of DEN for 10 weeks | Chemopreventive effect apoptosis induction | Post-treatment: Persistent but tiny nodules; architecture; | |||
| GPx, GR, GSH, SOD, CAT | Likely to spread through intrahepatic veins | ||||
| AST, ALT, ALP, LDH, cGT | |||||
| Liver carcinogenesis chemically induced by NDEA in male wistar rats orally (dissolved in 0.9% normal saline), in a dose of 20 mg/kg body weight, five times a week, for 6 weeks | 15 mg/kg of carvacrol orally, five times a week for 15 weeks, after NDEA administration for 6 weeks | Antiproliferative effect | AFP | Carvacrol may have an antitumor effect through its antiangiogenic capacity, antiproliferative effect and apoptotic activity against tumor cells in vivo | Ahmed et al. (2013), Egypt |
| Apoptosis induction | VEGF | ||||
| Marked improvement in histological characteristic of liver tissue | AFU | ||||
| GGT | |||||
| Male wistar rats induced with hepatocarcinogenesis providing 0.01% DEN through drinking water for 16 weeks | Pre-treatment: (15 mg/kg b.wt) of carvacrol orally one week before DEN administration and up to 16 weeks | — | Cell proliferation | Carvacrol attenuates hepatocellular carcinoma by inhibiting cell proliferation and tumor metastases | Subramaniyan et al. (2014), India |
| Post-treatment: (15 mg/kg b.wt) of carvacrol orally for 6 weeks after administration of DEN for 10 weeks | Tumor markers | ||||
| Mast cell density | |||||
| PCNA | |||||
| MMP-2 and -9 | |||||
| AgNORs | |||||
| DMH-induced colon carcinogenesis in male Wistar rats who received subcutaneous injections of DMH (20 mg/kg b.wt) in the right thigh, once a week for the first 4 weeks of the experiment (four injections) | 20, 40 or 80 mg/kg every day from the day of the carcinogen treatment till the end of the 16th week | Weight gain | Incidence of tumors | Carvacrol has antiproliferative, anticarcinogenic and chemopreventive potential and its effects were better observed at a dose of 40 mg/kg b.wt | Sivaranjani et al. (2016), India |
| Growth rate | Growth of neoplastic polyps | ||||
| GPx, GR, GSH, SOD, CAT | |||||
| C57BL/6 mice induced with DEN hepatocellular carcinoma injected intraperitoneally at a dose of 100 mg/kg | — | Intrastromal and peritumor lymphocytes | Tumor growth | The direct regulation relationship between DAPK1 and PPP2R2A may be the biological mechanism of tumorigenesis and progression of hepatocellular carcinoma | Li et al. (2019), China |
| Intragastrically | DAPK1 | Tumor cells | |||
| 20 weeks | Mitotic phase | ||||
| PPP2R2A | |||||
| DMBA-induced breast cancer in female Holztman mice in a single administration of DMBA by oral gavage at a dose of 80 mg /kg body weight | 50, 100 and 200 mg/kg/day | Tumor latency | Number of tumors | Carvacrol had an antitumor effect on breast cancer in vivo and it is likely that this effect may be due to its antioxidant activity | Rojas-Armas et al. (2020), Peru |
| Oral gavage | 75% in the frequency of tumors | ||||
| 14 weeks | 67% in the incidence of tumors | ||||
| Average volume | |||||
TABLE 2.
Detailed description of the studies that used thymol, included in the systematic review.
| Model | Concentration/Incubation time | Experimental methods for testing IC50 values | Results/targets | Conclusion | Authors (Year), Country | ||
|---|---|---|---|---|---|---|---|
| Increase | Decrease | IC50 | |||||
| Monoterpene thymol | |||||||
| In vitro studies | |||||||
| HepG2 | 150–900 μmol | – | Cytotoxic effects | DNA damage level | – | HepG2 cells were slightly more sensitive to the effects | Horváthová et al. (2006), Slovakia |
| Caco-2 | 24 of incubation | ||||||
| K-562 | 200, 400, 600, 800, 1,000 μM | Trypan blue exclusion | Cytotoxic effects | DNA damage level | 500 μM–24 h | Thymol has cytotoxic, antioxidant effects and has a protective action against DNA damage | Horvathova et al. (2007), Slovakia |
| 24 or 48 h of incubation | |||||||
| P-815 | 0.004–0.5% v/v | MTT assay | Cytotoxic effects | – | 0.015% v/v–48 h | Thymol is cytotoxic | Jaafari et al. (2007), Morocco |
| 48 h of incubation | |||||||
| HepG2 | 150–1,000 μM | Trypan blue exclusion | Cytotoxic effects | Cell proliferation | HepG2 - 400 μM–24 h | Thymol has antiproliferative and protective effects | Slamenová et al. (2007), Slovakia |
| Resistance to harmful DNA effects (antioxidant properties) | |||||||
| Caco-2 | 24 of incubation | Caco-2 - 700 μM–24 h | |||||
| HeLa | 15, 30.5, 61, 122, 244 ng/mL | – | Cytotoxic effects | Cell survival | – | Thymol has strong antitumor activity against the HeLa cell line | Abed, (2011), Iraq |
| Hep | 72 h of incubation | ||||||
| MG63 | 100, 200, 400, 600 μmol/L | – | Cytotoxic effects | Cell viability | – | Thymol showed antitumor activity in MG63 cells, moreover, its apoptotic effect is related to the pronounced antioxidant activity | Chang et al. (2011), China |
| 24 h of incubation | Apoptosis induction | ||||||
| Generation of ROS | |||||||
| HL-60 | 5, 25, 50, 75, 100 μM | – | Cytotoxic effects | Cell viability | – | Apoptosis induced by thymol in HL-60 cells involves the dependent and independent pathways of caspase | Deb et al. (2011), India |
| 24 h of incubation | Apoptosis induction | Cells in phases G0/G1, S and G2/M | |||||
| Cells in sub phase G0/G1 generation of ROS | Cell cycle stop in phase G0/G1 Bcl-2 | ||||||
| Caspase-9, -8 and -3 | |||||||
| DBTRG-05MG | 200, 300, 400, 500, 600, 800 μM | – | Cytotoxic effects | Cell viability | – | Thymol induces cell death in human glioblastoma cells | Hsu et al. (2011), China |
| Apoptosis induction and necrosis | |||||||
| 24 h of incubation | |||||||
| HepG2 | 20–200 μg/mL | CellTiter-Blue® cell viability assay | Cytotoxic effects | Membrane damage | 60.01 μg/mL–24 h | Thymol exhibits antioxidant activities and anti-cancer effects on cells | Özkan and Erdogan, (2011), Turkey |
| Antiproliferative effects | |||||||
| 24 h of incubation | |||||||
| P-815 | 0.05–1.25 μM | MTT assay | Cytotoxic effects | Cell cycle stop in phase G0/G1 | P-815–0.15 μM–48 h | Thymol showed relevant cytotoxic effects in all tested strains | Jaafari et al. (2012), Morocco |
| CEM | 48 h of incubation | CEM - 0.31 μM–48 h | |||||
| K-562 | K-562–0.44 μM–48 h | ||||||
| MCF-7 | MCF-7 - 0.48 μM–48 h | ||||||
| MCF-7gem | MCF-7gem - | ||||||
| H1299 | 10–2,000 μM | CellTiter-Blue® cell viability assay | Cytotoxic effects | Membrane and DNA damage | 497 μM–24 h | Thymol exhibited a cytotoxic and antioxidant effect | Ozkan and Erdogan, (2012), Turkey |
| 24 and 48 of incubation | MDA | 266 μM–48 h | |||||
| 8-OHdG | |||||||
| B16-F10 | 75, 150, 300, 600, 1,200 μM | Trypan blue and MTT assay | Cytotoxic effects | Cell viability | 400 μM | Thymol showed antitumor effect with moderate cytotoxicity | Satooka and Kubo, (2012), United States |
| 24 h of incubation | Generation of ROS | ||||||
| Density of melanoma cells | |||||||
| HepG2 | 1.56–50 μg/mL | Trypan blue assay | Cytotoxicity only for B16-F10 cells | – | HepG2 - > 25 μg/mL | Thymol showed cytotoxicity to B16-F10 cells | Ferraz et al. (2013), Brazil |
| K-562 | 72 h of incubation | Apoptosis induction in HepG2 cells | K-562–72 h | ||||
| B16-F10 | Induction of caspase-3-dependent apoptotic cell death in HepG cells | B16-F10–18.23 μg/mL–72 h | |||||
| PC-3 | 10, 30.50, 70, 100 μg/mL | MTT assay | Cytotoxic effects | Cell viability | PC-3 - 18 μg/mL–48 h | Thymol exhibited cytotoxicity and induced apoptosis | Pathania et al. (2013), India |
| MDA-MB 231 | Apoptosis induction | Cell proliferation | MDA-MB 231–15 μg/mL–48 h | ||||
| A549 | 48 h of incubation | DNA fraction sub G0 | PI3K/AKT/mTOR | A549–52 μg/mL–48 h | |||
| MCF-7 | TNF-R1 | MCF-7 - 10 μg/mL–48 h | |||||
| HL-60 | Bax | Bcl-2 | HL-60–45 μg/mL–48 h | ||||
| Caspase-8 and 9 | |||||||
| Caco-2 | 100–2,500 μM | – | – | – | – | The cells exposed to thymol remained unchanged and did not produce any cytotoxic, apoptotic or necrotic effects at any of the tested concentrations | Llana-Ruiz-Cabello et al. (2014), Spain |
| 24 and 48 h of incubation | |||||||
| A549 | 1–1.000 μM | SRB assay | Cytotoxic effects | – | A549–0.187 ± 0.061 mΜ–72 h | Thymol exhibited more effective cytotoxicity against cells (Hep3B), while cells (A549) were less sensitive to treatment and cells (HepG2) were more resistant | Fitsiou et al. (2016), Greece |
| HepG2 | 72 h of incubation | Antiproliferative effects | HepG2 - 0.390 ± 0.01 mΜ–72 h | ||||
| Hep3B | Hep3B- 0.181 ± 0.016 mΜ–72 h | ||||||
| AGS | 100, 200, 400 μM | – | Cytotoxic effects | Cell viability | – | Thymol has potent anticancer effects on gastric cancer cells | Kang et al. (2016), Republic of Korea |
| Apoptosis induction | |||||||
| Sub-G1 phase | Cell growth | ||||||
| 6, 12, 24 h of incubation | Generation of ROS | ||||||
| Bax | MMP | ||||||
| Caspase-8, -7 and -9 | |||||||
| C6 | 0.1, 0.3, 1, 3, 10, | – | – | Cell viability | – | Thymol is a potential candidate for the treatment of malignant gliomas | Lee et al. (2016), Republic of Korea |
| 30, 100, 200 µM | Cell migration | ||||||
| 24 h of incubation | p-ERK1/2 | ||||||
| MMP-2 and -9 | |||||||
| A549 | 0–250 μM | – | Cytotoxic effects | Cell viability | – | Thymol has cytotoxic and antioxidant activity and its cytotoxic effect was greater than that of carvacrol | Coccimiglio et al. (2016), Canada |
| 24 h of incubation | |||||||
| HCT-116 | 100, 150, 200 μg/mL | – | Cytotoxic effects | Cell proliferation | – | Thymol can be used as a potent drug against colon cancer due to its lower toxicity | Chauhan et al. (2018), Republic of Korea |
| 24 h of incubation | Apoptosis induction | Clonogenic potential | |||||
| Generation of ROS | |||||||
| Caspase-3 | |||||||
| p-JNK | |||||||
| Cyt C | |||||||
| HepG2 | 0.06, 0.11, 0.22, 0.45, 0.90 μg/μL | MTT assay | – | Cell viability | 289 mg/L–24 h | Thymol has therapeutic potential in tumor cells without adverse effects on healthy cells | Elshafie et al. (2017), Italy |
| 24 h of incubation | Hepatocarcinoma cells | ||||||
| T24 | 25, 50, 100, 150 μM | MTT assay | Cytotoxic effects | Cell viability | T24–90.1 ± 7.6 μM–24 h | Thymol can be used as a promising anticancer agent against bladder cancer | Li et al. (2017), China |
| SW780 | 24 h of incubation or 100 μM – | Apoptosis induction | Cell cycle stop in phase G2/M | SW780–108.6 ± 11.3 μM–24 h | |||
| p21 | Cyclin A and B1 | ||||||
| J82 | 6, 12, 24, 36 h of incubation | Caspase-3 and -9 | CDK2 | J82–130.5 ± 10.8 μM–24 h | |||
| p-JNK | |||||||
| p-p38 | |||||||
| MAPK | PI3K/Akt | ||||||
| Generation of ROS | |||||||
| PC-3 | 100, 300, 500, 700, 900 μM | – | Cytotoxic effects | Cell viability | – | Thymol was cytotoxic to PC-3 cells | Yeh et al. (2017), China |
| 24 h of incubation | Induction of cell death | ||||||
| Cal7 | 200–800 µM | Cell Titer 96 ® Aqueous non-Radioactive cell Proliferation assay | Cytotoxic effects | Cell viability | 350 μM–500 μM | Thymol had cytotoxic, antiproliferative and antitumor effects | De La Chapa et al. (2018), United States |
| SCC4 | 48 h of incubation | ||||||
| SCC9 | |||||||
| HeLa | |||||||
| H460 | |||||||
| MDA-231 | |||||||
| PC-3 | |||||||
| AGS | 10, 20, 30, 50, 100, 200, 400, 600 µM | CellTiter-Glo Luminescent cell viability assay | Apoptotic effects | Cell viability | 75.63 ± 4.01 µM–24 h | Thymol has cytotoxic, apoptotic, genotoxic and dose-dependent ROS-generating effects | Günes-Bayir et al. (2018), Turkey |
| 24 h of incubation | Necrosis | Bcl-2 | |||||
| Bax | |||||||
| Caspase-3 and -9 | |||||||
| Generation of ROS | GSH levels | ||||||
| Genotoxic effect | |||||||
| MCF-7 | 10, 15, 30, 50, 80, 100, 200 μg/mL | MTT assay | Cytotoxic effects | Bcl-2 | MDA-MB 231–56 μg/mL–24 h | Thymol has antiproliferative effects | Jamali et al. (2018), Iran |
| MDA-MB 231 | 24 h of incubation | Antiproliferative effect | Interruption of cell cycle progression in the S phase | MCF-7 - 47 μg/mL–24 h | |||
| Apoptosis induction | |||||||
| Caspase-3 | |||||||
| Bax | |||||||
| Generation of ROS | |||||||
| Sub-G1 phase | |||||||
| MCF-7 | 5, 10, 20, 30, 40, 50, 75, 100 g/mL | MTT assay | Cytotoxic effects | Number of cancer cells | 54 μg/mL - 48 h | Thymol can induce the process of apoptosis in MCF-7 and, therefore, can be considered an anticancer agent | Seresht et al. (2019), Iran |
| 48 and 72 h of incubation | p53 | Cell cycle arrest induction | 62 μg/mL - 72 h | ||||
| p21 | |||||||
| HT-29 | 62.5, 125, 250, 500, 750, 1,000 ppm | Trypan Blue exclusion assay | Cytotoxic effects | – | 152.1 ± 18.0 ppm–24 h | Thymol induces cytotoxicity and provides genoprotective effects | Thapa et al. (2019), United Kingdom |
| 24 h of incubation | Genoprotective effects | ||||||
| MDA-MB 231 | 100, 200, 400, 600, 800 µM | MTT assay | Cytotoxic effects | – | MDA-MB 231–208.36 μM–72 h; | Thymol has apoptotic and antiproliferative properties and can serve as a potential therapeutic agent | Elbe et al. (2020), Turkey |
| PC-3 | 24, 48 and 72 h of incubation | Antiproliferative effect | PC-3 - 711 μM–24 h, 601 μM–48 h and 552 μM–72 h; | ||||
| DU 145 | Apoptosis induction | DU 145–799 μM–24 h, 721 μM–48 h and 448 μM–72 h | |||||
| KLN 205 | KLN 205–421 μM–48 h and 229.68 μM–72 h | ||||||
| SKOV-3 | 100, 200, 400, 600 μM | MTT assay | Apoptosis induction | Cell viability | 316.08 μM–24 h | Thymol was cytotoxic to the ovarian cancer cell line and it was more potent than carvacrol | Elbe et al. (2020), Turkey |
| 24 and 48 h of incubation | 258.38 μM–48 h | ||||||
| HCT116 | 10, 20, 40, 80, 120 μg/mL | CCK-8 Kit | Apoptosis induction | Proliferative capacity | LoVo - 41.46 μg/mL - 48 h | Thymol treatment reduced the proliferative capacity of cells and suppressed cell migration and invasion | Zeng et al. (2020), China |
| HCT116–46.74 μg/mL - 48 h | |||||||
| LoVo | 24, 48 and 72 h of incubation | Bax | Cell migration and invasion | ||||
| Caspase-3 and PARP | Cell cycle stop | ||||||
| Cells in phase G0/G1 | Bcl-2 | ||||||
| Cells in S and G2/M phases | |||||||
| AGS | 0–600 μM | CellTiter-Glo Luminescent cell viability assay | Cytotoxic effects | Cell viability | 75.63 ± 4.01 μM–24 h | Thymol has cytotoxic and antioxidant effects in gastric adenocarcinoma | Günes-Bayir et al. (2020), Turkey |
| 24 h of incubation | Generation of ROS | GSH levels | |||||
| Apoptosis induction | |||||||
| Bax | Bcl-2 | ||||||
| Caspase-3 and -9 | |||||||
| DNA damage | |||||||
| A549 | 25–200 μg/mL | MTT assay | Antiproliferative effect | Cell viability | 745 μM–24 h | Thymol can act as a safe and potent therapeutic agent to treat non-small cell lung cancer | Balan et al. (2021), India |
| 12 and 24 h of incubation | Apoptosis induction | MMP | |||||
| DNA damage | Bcl-2 | ||||||
| Generation of ROS | |||||||
| Caspase-3 and -9 | |||||||
| Bax | SOD | ||||||
| Cells in phase G0/G1 | |||||||
| TBARBS | |||||||
| CARBONIL | |||||||
| KG1 | 25, 50, 100 μM | – | Cell death | Cell viability | – | KG1 cells treated with 50 µM thymol were more sensitive compared to the other two lines. At 100 μM, thymol induced complete cell death of KG1 and HL60 cells, while about 50% of K562 cells resisted cell death after 48 h of treatmentl | Bouhtit et al. (2021), Belgium |
| K-562 | 24 and 48 h of incubation | ||||||
| HL-60 | |||||||
| Model | Concentration | Results/Targets | Conclusion | Authors (Year), country | |
|---|---|---|---|---|---|
| Increase | Decrease | ||||
| In vivo studies | |||||
| Female athymic nude rats were injected subcutaneously in the right flank with 3 × 106 Cal27 or HeLa cells in 0.1 mL of sterile PBS | 4.3 mM thymol (32 μg diluted in 50 μl sterile saline with a final concentration of 0.25% DMSO) | Apoptotic cells | Tumor volume reduction | Thymol had cytotoxic, antiproliferative and antitumor effects | De La Chapa et al. (2018), United States |
| Proliferative cells | |||||
| Xenograft model: BALB/c male nude mice were injected subcutaneously with HCT116 cells (1 × 107 cells in 0.2 mL of PBS) on the back | Xenograft model and lung metastasis model: Intraperitoneal injection for 30 days with thymol at 75 mg/kg 1x on alternate days or thymol at 150 mg/kg 1x on alternate days | Necrotic lesions | Tumor growth and metastasis | Thymol inhibits the growth and metastasis of colorectal cancer in vivo by suppressing Wnt/β-catenin signaling and the EMT program | Zeng et al. (2020), China |
| Average number of tumor nodules on the surface of the lungs | |||||
| Bax | Ki-67 expression level | ||||
| Lung metastasis model: HCT116 cells (1 × 106) were intravenously injected into the tail vein of each mouse | Cell proliferation | ||||
| Bcl-2 | |||||
| Wnt/β-catenin signaling pathway | |||||
| Caderina-E | Vimentina | ||||
| Cyclin D1 | |||||
| C-myc | |||||
| Survivin | |||||
| Male Wistar rats injected with DMH (40 mg/kg intraperitoneally, twice a week) for 16 consecutive weeks | 20 mg/kg/day, orally, for 16 weeks | Final body weight | Mortality | Thymol administration had promising preclinical protective efficacy by promoting inhibition of oxidative stress, inflammation and induction of apoptosis | Hassan et al. (2021), Egypt |
| Weight gain | Incidence of ACF | ||||
| Growth rate | Serum CEA levels | ||||
| NRF2 | Serum levels of CA19-9 | ||||
| Caspase-3 | |||||
| TNF-α | |||||
| GST, GSH, SOD, CAT | NF-κB | ||||
| IL-6 | |||||
| Tissue content of MDA (colon lipid peroxidation) | |||||
Abbreviations: 5RP7, Mouse embryonic fibroblast with transformation of H-ras oncogenes; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; A375, Melanoma (skin) cancer cell line; A549, Lung Carcinoma Cell Line; ACF, Aberrant crypt foci; AFP, Alpha-fetoprotein serum; AFU, Alpha l-fucosidase; AgNORs, Proteins Associated with the Argyrophilic Nucleolar Organizing Region; AGS, Human gastric carcinoma cell line; ALP, Alkaline Phosphatase; ALT, Alanine transaminase; AST, Aspartate transaminase; AXL, Tyrosine Kinase Receptor; B[a]P, 3.4 benzopurene; B16-F10, Mouse melanoma cells; BT-474, Breast ductal carcinoma; BT-483, Breast ductal carcinoma; C6, Glioma cell line; Caco-2, Cell line derived from human colon carcinoma; CA 19–9, Tumor markers carbohydrate antigen 19–9; Cal27, Cell line of the squamous cell carcinoma of the tongue; CAT, Catalase; CEA, Carcinoembryonic antigen; CCK-8, Cell Counting Kit-8; CCND1, Gene encoding the cyclin D1 protein; CDK4 or 6, Cyclin-dependent kinases; cGT, Glutamyl transpeptidase Range; CyT C, Cytochrome C; c-Myc, Proto-oncogene; CO25, Mouse muscle cell line; COX-2, Cyclooxygenase; DAPK1, Protein kinase 1 associated with death; DBTRG-05MG, Human Glioblastoma Cells; DEN, Diethylnitrosamine; DMH, 1,2-dimethylhydrazine; DMBA, 7,12-dimethylbenz[a]anthracene; DMSO, Dimethylsulfoxide; DNA, Deoxyribonucleic acid; DU 145, Human Prostate Cancer Cell Line; EC50, Half of the maximum effective concentration; EMF, Acute T Lymphoblastoid Leukemia; EMT, Epithelial-mesenchymal transition; ERK 1/2, Kinase 1/2 regulated by extracellular signal; ERO, Reactive Oxygen Species; GGT, Gamma-Glutamyltransferase; GPx, Glutathione Peroxidase; GR, Glutathione reductase; GSH, Reduced Glutathione; H1299, Parental and Drug Resistant Human Lung Cancer Cell Line; H460, Non-small cell lung cancer cell line; HCT116, Colorectal adenocarcinoma cell line; HeLa, Human Cervical Cancer Cell Line; Hep, Human Laryngeal Squamous Cell Carcinoma; Hep3Β, Human Hepatocellular Carcinoma Cell Line; HepG2, Human Hepatocellular Carcinoma Cell Line; HL-60, Human Acute Promyelocytic Leukemia Cell Line; HT-29, Colorectal adenocarcinoma cell line; IC50, Half of the maximum inhibitory concentration; IL-6, Interleukin-6; J82, Bladder Cancer Cell Line; Jagged-1, Jagged Canonical Notch Ligand 1; JAR, Human Choriocarcinoma Cell Line; JEG3, Human Choriocarcinoma Cell Line; Jurkat, Lymphocytes derived from T-cell lymphoma; KG1 and K-562, Human Myelogenous Leukemia Cell Line; Kelly, Neuroblastoma cell line; Ki-67, Antigen, biomarker; KLN 205, Non-small cell lung cancer; LDH, Lactate dehydrogenase; LoVo, Colorectal Adenocarcinoma Cell Line; MAPK, Protein kinase activated by mitogen; MTT, Methyl Tetrazolium Test; MTS, Tetrazolium salt reduction; MCF-7, Human breast cancer cell line; MCF-7gem, Gemcitabine-resistant human breast adenocarcinoma; MDA, Malondialdehyde; MDA-MB 231, Human metastatic breast adenocarcinoma cell line; MDA-MB 453, Human metastatic breast adenocarcinoma cell line; MDPK, Myotonic dystrophy protein kinase; MG63, Human Osteosarcoma Cell Line; MMP, Potential of the mitochondrial membrane; MMP-2 or 9, Metalloproteinase-2 or 9 of the matrix; N2a, Rat neuroblastoma cell line; NDEA, N-nitrosodiethylamine; Notch-1, Signaling path; NSCLC, Non-small cell lung cancer; OC2, Human oral cancer cells; OSCC, Human oral squamous cell carcinoma; p21, WAF1 encoding gene; p38, Mitogen-activated protein kinases; p53, tumor protein; P-815, Murine Mastocytoma Cell Line; p-AKT, Phospho-protein kinase B; PBS, Sterile phosphate buffered saline; PC-3, Human Prostate Cancer Cell Line; PCNA, Proliferating Cell Nuclear Antigen; PI3K/AKT/mTOR, Phosphoinositide-3-kinase/Akt/mammalian target; PI3K/Akt, Phosphoinositide-3-kinase-Akt; p-JNK, Fosto-c-Jun N-terminal kinase; p-p38, Phospho-p38; PPP2R2A, Serine/threonine-protein phosphatase 2A; p-STAT3, Phospho-signal transducer and transcription activator; SRB, Sulforhodamine B; SCC-25, Human squamous cell carcinoma cell line; SCC4 and SCC9, Human oral squamous cell carcinoma cell line; SH-SY5Y, Neuroblastoma cell line; SiHa, Human Cervical Cancer Cell Line; SKOV-3, Ovarian cancer cell line; SOD, Superoxide dismutase; SW780, Bladder cancer cell line; T24, Bladder Cancer Cell Line; TAC, Total antioxidant capacity; TBARS, Thiobarbituric Acid Reactive Substances; TCA-8113, Human tongue squamous cell carcinoma cell line; TNF-α, Tumor Necrosis Factor-Alpha; TNFR1, Tumor necrosis factor 1 receptor; TOS, Total oxidant status; TRPM7, Subfamily M of the cation channel of the potential transient receptor Member 7; U87, Human glioblastoma cell line; VEGF, Vascular endothelial growth factor; XXT, 2.3‐bis(2‐methoxy‐4‐nitro‐5‐sulfophenyl)‐2H‐tetrazolium-5-carboxanilide inner salt.
Description of In Vitro Studies with Carvacrol and Thymol
Carcinomas
Carvacrol (500 and 1,000 μM) was able to inhibit the viability and proliferation of lung cancer cells (A549 cell line), in addition to inducing early apoptotic characteristics (Koparal and Zeytinoglu, 2003) and reducing the viability of the A549, H460 (Jung et al., 2018) and H1299 cells lines, the latter being resistant to epirubicin (Ozkan and Erdogan, 2012). These effects occurred mainly through the inhibition of tyrosine kinase receptor (AXL) expression and an increase in malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine levels (8-OHdG) (Ozkan and Erdogan, 2012; Jung et al., 2018).
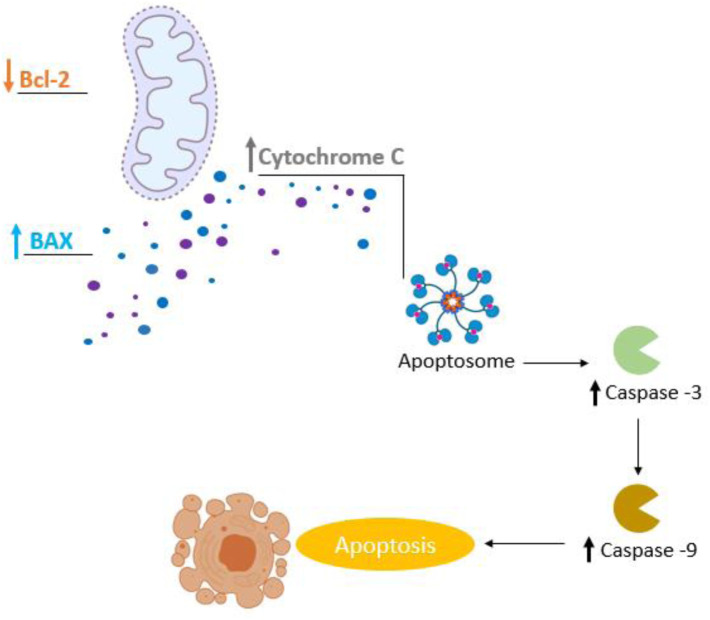
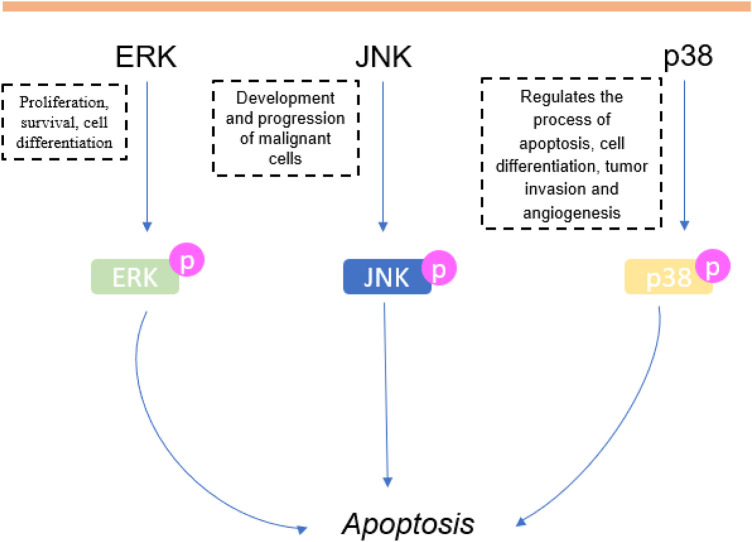
In relation to hepatocarcinomas (HepG2 cell line), carvacrol exhibited anticancer effects, provoking cell death and antiproliferative effects in a concentration-dependent manner (Özkan and Erdogan, 2011; Melusova et al., 2014). The inhibition of cell proliferation and apoptosis induction occurred via the mitochondria-mediated pathway, accompanied by caspase-3 activation and Bcl-2 inhibition (Yin et al., 2012). The via extracellular signal-regulated kinases (ERK) protein, and mitogen-activated protein kinases (p38) apoptotic pathways may also be involved (Yin et al., 2012). Similarly, Melušová et al. (2014) demonstrated a marked apoptotic effect of carvacrol at a concentration of 650 μM after 24 h of incubation, and an accumulation of cells in the G1 phase, together with a reduction of cells in the S phase, slowing cell cycle/mitosis and provoking cell death.
Colorectal cancer (Caco-2 cell line) also exhibited reduced cell viability and a significant increase of early apoptotic cells after carvacrol incubation (115 μM) (Llana-Ruiz-Cabello et al., 2014). There was also inhibition of HCT116, LoVo and HT-29 cells proliferation (Fan et al., 2015; Pakdemirli et al., 2020). Carvacrol also promoted a decrease in Bcl-2, metalloproteinase-2 and -9 (MMP-2 and MMP-9), p-ERK, p-Akt, cyclin B1 levels and an increase in p-JNK, Bax levels, resulting in cell cycle arrest at the G2/M phase (Fan et al., 2015).
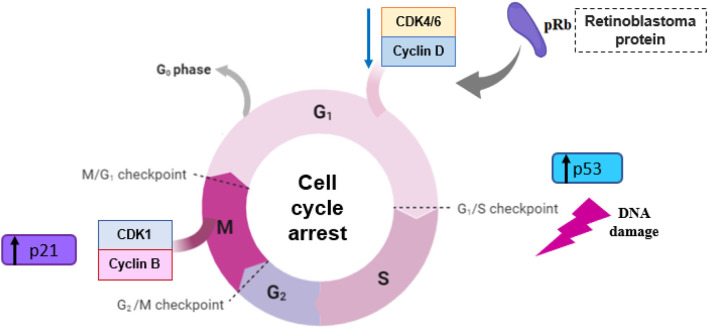
In respect of breast cancer, treatment with carvacrol decreases MDA-MB231 (Jamali et al., 2018; Li et al., 2021) and MCF-7 cells line viability (Al-Fatlawi and Ahmad, 2014; Jamali et al., 2018; Tayarani-Najaran et al., 2019; Li et al., 2021). At 200 μM, the MDA-MB-231 cell line was the most sensitive and MCF-7 was the least sensitive, indicating that the effectiveness of carvacrol may vary according to the types of breast cancer cell. In addition, the TRPM7 pathway is one of the suggested pharmacological mechanisms of action (Li et al., 2021). Carvacrol was more cytotoxic compared to thymol (Jamali et al., 2018), α-thujone, 4-terpineol, 1,8-cineol, bornyl acetate and camphor (Tayarani-Najaran et al., 2019). Tayarani-Najaran et al. (2019) also reported an apoptotic effect marked by an increased level of Bax protein, and cleaved both poly [ADP-ribose] polymerase 1 (PARP-1) and caspase-3. The antiproliferative activity of carvacrol was 1.2 times higher against MDA-MB231 cells compared to U87 cells (Baranauskaite et al., 2017). MDA-MB 231 cell proliferation slowed after treatment with carvacrol, accompanied by apoptosis induction with increased levels of Bax, decreased mitochondrial membrane potential, cytochrome C release, caspase activation, PARP cleavage, increased sub-phase G0/G1 of the cell cycle and a reduced number of cells in the S phase (Arunasree, 2010). The viability of MCF-7 cells was reduced after carvacrol treatment (200 μmol/L), with a significant increase in the number of early and late apoptotic cells, accompanied by a negative regulation of Bcl2 and positive regulation of Bax protein. An accumulation of cells in the G0/G1 phase was observed, along with a reduction of cells in the S and G2 phases, mainly through the reduced expressions of CDK4, CDK6, retinoblastoma protein (pRB), cyclin D and phosphoinositide-3-kinase-Akt (PI3K/p-AKT) (Mari et al., 2020).
It was also observed that the administration of carvacrol provoked cytotoxic and apoptotic effects on HeLa and SiHa cervical cell lines (Mehdi et al., 2011). In fact, Potočnjak et al. (2018) demonstrated that the cytotoxicity exhibited by carvacrol against HeLa cells occurred through the suppression of the cell cycle and induction of apoptosis, the latter accompanied by an increase in caspase-9, PARP cleavage, and activation of ERK, increasing the expression of phospho-ERK1/2. In SiHa cells, the reduction in viability and apoptosis induction occurred through p53 activation and Bax, caspase-3, -6, -9 expression, along, with negative regulation of Bcl-2 gene (Abbas and Al-Fatlawi, 2018). Furthermore, another study demonstrated that carvacrol and thymol were cytotoxic against ovarian cancer (SKOV-3 cell line) exhibiting apoptotic and antiproliferative properties (Elbe et al., 2020).
Carvacrol also induced cytotoxicity and apoptosis (via caspase-3 and reactive oxygen species—ROS) of human oral squamous cell carcinoma (OC2 cell line) in a concentration-dependent manner (Liang et al., 2013). In tongue cancer (Tca-8113, SCC-25 cell lines), Dai et al. (2016) reported that carvacrol effectively inhibited cell proliferation through the negative regulation of CCND1 and CDK4 expression, and the positive regulation of p21 expression, resulting in a significant decrease of cells in the S phase, in addition to inhibiting the migration and invasion abilities of Tca-8113 cells via phospho-focal adhesion kinase (p-FAK), p-catenin, ZEB1 and MMP-2 and -9 reduction. Apoptosis was marked by a reduction of anti-apoptotic Bcl-2 proteins expression and an increase of proapoptotic Bax proteins levels (Dai et al., 2016).
In a prostate cancer cell line (DU 145), carvacrol showed a significant reduction of cell viability and proliferation in a concentration and time dependent manner, marked by a cell cycle arrest, resulting in the accumulation of cells in the G0/G1 phase, and apoptosis, related to the increased activity of caspase-3, production of ROS and loss of mitochondrial membrane potential (Khan et al., 2017). PC-3 cells also exhibited cytotoxicity and decreased cell viability in a concentration dependent manner after carvacrol treatment (Horng et al., 2017). A blockade of TRPM7 channels, reduced expression of MMP-2 and F-actin, was also observed, together with the inhibition of PI3K/Akt and MAPK (Mitogen-activated protein kinases) signaling pathways was also observed (Luo et al., 2016). Similarly, Heidarian and Keloushadi (2019) reported that this monoterpene acts through the negative regulation of pERK1/2, pSTAT3 and pAKT expression, suggesting that inhibition of interleukin-6 (IL-6) signaling pathways can be a promising target for prostate cancer treatment. The induction of PC-3 cells apoptosis was mostly through the intrinsic pathway, associated with the production of ROS and mediated by the increase expression of caspase-3, -8 and -9 and Bcl-2/Bax. There was also a G0/G1 phase arrest of cell cycle, together with a considerable decrease of cells in the S and G2/M phase (Khan et al., 2019). Similarly, Tayarani-Najaran et al. (2019) demonstrated decreased cell viability in a concentration dependent manner and also marked apoptosis (mitochondrial pathway), accompanied by cleavage of PARP-1 and caspase-3 and an increased Bax protein level.
Günes-Bayir et al. (2018), Günes-Bayir et al. (2018), and Maryam et al. (2015) reported cytotoxic effects of carvacrol on gastric cancer (AGS cell line) significantly reducing cell viability in a manner dependent on concentration. There was also an induction of apoptosis with a reduction of Bcl-2 protein levels, and an increase in Bax, caspase-3 and -9 protein levels, besides the production of ROS. In their most recent study, Günes-Bayir et al. (2020) also identified the cytotoxic effects of thymol on AGS cell viability, in addition to inducing apoptosis, by increasing ROS, Bax, Caspase-3, -9 levels and reducing Bcl-2 and GSH levels.
Regarding human choriocarcinoma (JAR and JEG3 cell lines), carvacrol was able to inhibit proliferation and induce cell cycle arrest. The results showed that carvacrol reduced cell proliferation and provoked apoptosis mediated by mitochondrial membrane potential depolarization, increased mitochondrial calcium and activation of Bax and Cytochrome C expression. In addition, treatment with this monoterpene promoted an accumulation of cells in the sub-G1 phase, indicating that changes in intracellular calcium and ROS generation are related to the antiproliferative effects observed. Additionally, there was a marked phosphorylation of ERK1/2 and also inhibition of the PI3K/AKT signaling pathway, indicating that carvacrol regulates signaling pathways by inhibiting MAPK and PI3K (Lim et al., 2019).
In murine B16-F10 and A375 melanoma cell lines, carvacrol reduced cell viability and induced cytotoxicity (Satooka and Kubo, 2012; Ferraz et al., 2013; Govindaraju and Arulselvi, 2018). The antiproliferative effect was confirmed by Govindaraju and Arulselvi (2018), who reported marked cell cycle arrest, attested by the accumulation of G1 phase cells, a reduction in the number of G2/M cells and apoptosis through the mitochondria-mediated pathway and PARP cleavage/activation, together with a reduced expression of the anti-apoptotic protein Bcl-2.
Similarly, the administration of thymol to lung cancer cells promoted a reduction in cell viability in the A549, H460 and H1299 cell lines (Pathania et al., 2013; Coccimiglio et al., 2016; De La Chapa et al., 2018; Balan et al., 2021). Likewise, the cytotoxic effect of thymol on A549 cells was higher than carvacrol cytotoxicity (Coccimiglio et al., 2016). Thymol also promoted cytotoxicity and apoptosis of KLN 205 cells with an IC50 of 421 and 229.68 μM in 48 and 72 h, respectively (Elbe et al., 2020). In liver carcinoma cells (HepG2), thymol exhibited antioxidant activity at lower (<IC50 = 60.01 μg/mL) concentrations and antitumor effects (apoptosis and inhibition of cell proliferation) at higher concentrations (>IC50 = 60.01 μg/mL) (Özkan and Erdogan, 2011). Elshafie et al. (2017) reported HepG2 cell death, decreased cell viability and a selective action of thymol against these tumor cells.
Thymol also showed concentration-dependent cytotoxic effects and reduced the proliferation of Caco-2 cells (Horváthová et al., 2006). In contrast, Llana-Ruiz-Cabello et al. (2014) reported that Caco-2 cells exposed to thymol did not exhibit any cytotoxic, apoptotic or necrotic effects in any of the tested concentrations. HCT-116 and HT-29 cells, after thymol administration, displayed a cell number reduction, cell apoptosis by disrupting mitochondrial membrane potential and ROS production (Chauhan et al., 2018; Thapa et al., 2019). These effects may have been caused by the positive regulation of the caspase-3, PARP-1, p-JNK and Cytochrome C expression (Chauhan et al., 2018).
Breast cancer cells (MDA-MB 231 cell line) also exhibited a reduction in cell viability after thymol treatment (Pathania et al., 2013; De La Chapa et al., 2018; Elbe et al., 2020). The inhibition of cell proliferation and apoptosis on MDA-MB231 and MCF-7 cell lines occurred via the mitochondrial pathway and induction of oxidative damage to DNA through Bax/Bcl-2 modulation, decreased levels of procaspase-8, -9, -3, increased levels of cleaved caspase-3 and ROS, and also cell cycle arrest at S-phase (Jamali et al., 2018). According to the results found by Seresht et al. (2019), thymol produced cytotoxic effects and reduced the number of MCF-7 cells, suggesting that this monoterpene induces cell cycle arrest, probably due to p21 overexpression. Thymol also promoted a marked antitumor effect on cervical cancer (HeLa cell line), through cytotoxic effects on the concentration of 30.5 ng/mL (Abed, 2011). De La Chapa et al. (2018) also reported decreased viability of HeLa cells and induction of apoptosis by PARP cleavage, suggesting that the anticancer effect of thymol is caused by mitochondrial dysfunction and subsequent apoptosis.
The administration of thymol to bladder cancer (T24, SW780, J82 cell lines) provoked inhibition of cell proliferation and decreased the cell viability in a concentration and time dependent manner, along with marked cell cycle arrest in the G2/M phase and induction of apoptosis through the intrinsic pathway, together with the activation of caspase-3 and -9, JNK and p38, release of cytochrome C, negative regulation of Bcl-2 family proteins and production of ROS. In addition, a considerable decrease in the expression of cyclin A, B1 and CDK2, as well as an increase in the expression of p21 were observed after treatment with thymol, suggesting that its antitumor effect occurs by inhibiting the PI3K/Akt signaling pathway, via MAPKs, and generation of ROS (Li et al., 2017). In human laryngeal squamous cell carcinomas (Hep), thymol showed a pronounced reduction of cell proliferation and also apoptosis, at a concentration of 30.5 ng/mL. According to De La Chapa et al. (2018) thymol exhibited cytotoxicity and decreased cell viability in a concentration dependent manner on Cal27, SCC4 and SCC9 cell lines. However, this cytotoxicity was reversed by the N-acetyl-cysteine (NAC) antioxidant addition, providing evidence that the anticancer mechanism of action of thymol involves mitochondrial dysfunction, and generation of ROS, culminating in apoptosis (De La Chapa et al., 2018). Thymol also caused a decrease in cell viability of prostate cancer (PC-3 cell line), and provoked cytotoxic effects (Pathania et al., 2013; Yeh et al., 2017; De La Chapa et al., 2018). PC-3 cells demonstrated greater sensitivity to treatment with thymol compared to DU145 cells. In addition, the induction of apoptosis in both cell lines occurred in a concentration-dependent manner (Elbe et al., 2020). Similarly, thymol suppressed the viability of melanoma (B16-F10 cell line), also in a concentration-dependent manner, by reducing the cell number and provoking cytotoxic effects. These effects seem to be related to the oxidative damage observed after the increase of ROS levels (Satooka and Kubo, 2012).
Central Nervous System Cancers
Human glioblastoma cells (DBTRG-05MG) showed reduced viability in a concentration-dependent manner when treated with carvacrol (200–600 μM), induced apoptosis and necrosis by ROS production and caspase-3 activity (Liang and Lu, 2012). In a rat neuroblastoma (N2a cell line), treatment with carvacrol (200–400 mg/L) exhibited cytotoxic and antiproliferative effects, along with antioxidant activity (Aydın et al., 2014). Kelly and SH-SY5Y neuroblastoma cells also exhibited a reduced proliferation rate after exposure to carvacrol (Kocal and Pakdemirli, 2020). In glioblastoma (cell line U87), carvacrol induced apoptosis by increasing the levels of caspase-3 cleavage, moreover, its antitumor mechanism of action seems to be related to the inhibition of PI3K/Akt signaling pathways, activation of mitogen/protein kinase by extracellular signals (via MAPK/ERK) and decreased levels of MMP-2 protein (Chen et al., 2015).
Regarding thymol, treatment at concentrations of 100 and 200 µM induced a significant reduction in cell viability and inhibited the migration of glioma cells (C6 cell line) through phosphorylation of PKCα and ERK1/2, that resulted in decreased expression of MMP-9 and MMP-2 (Lee et al., 2016). In addition, in DBTRG-05MG cells, thymol exhibited a cytotoxic effect in a concentration-dependent manner, by reducing cell viability and inducing apoptosis. The 400–600 μM range of concentrations promoted cell necrosis and the 800 μM concentration killed all cultivated cells (Hsu et al., 2011).
Sarcomas
Treatment with carvacrol in leiomyosarcoma cells exhibited antiproliferative effects in a concentration dependent manner and also inhibition of cell growth (Karkabounas et al., 2006). In addition, carvacrol showed a greater cytotoxicity compared to thymol against murine mast cell cells (P-815 cell line) (Jaafari et al., 2007; Jaafari et al., 2012), with accumulation of cells in the S phase (Jaafari et al., 2012).
In relation to thymol, there were concentration-dependent cytotoxic effects and interruption of the cell cycle progression in the G0/G1 phase in P-815 cells (Jaafari et al., 2012). In human osteosarcoma cells (MG63 cell line), thymol reduced cell viability, induced cytotoxic effects and apoptosis, which occurred in a concentration-dependent manner. Additionally, there was an increase in the production of ROS and cell death (Chang et al., 2011).
Leukemias
Carvacrol showed cytotoxic effects against human myeloid leukemia cells (K-562 cell line) (Horvathova et al., 2007; Jaafari et al., 2012) and against T-cell acute lymphoblastic leukemia (CEM cell line) (Jaafari et al., 2012). Carvacrol was more cytotoxic than thymol, inducing accumulation of cells in the S phase (Jaafari et al., 2012). It was shown that carvacrol produced cytotoxic effects and reduced cell viability in human acute promyelocytic leukemia (HL-60 cell line) and lymphocytes derived from T-cell lymphoma (Jurkat cell line). Treatment with carvacrol (100 μM) showed early and late apoptotic cells accompanied by a reduction of mitochondrial membrane potential levels, suggesting that apoptosis was mediated by the mitochondrial pathway, with a significant increase of Bax pro-apoptotic proteins, decreased expression of the anti-apoptotic proteins Bcl2 and an increased caspase-3 protein level (Bhakkiyalakshmi et al., 2016).
Analyzing the effects of thymol on K-562 and CEM cells, Jaafari et al. (2012) revealed that the latter was more sensitive to thymol effects, resulting in the accumulation of cells in the G0/G1 phase. In addition, treatment with thymol also reduced HL-60 cell viability, exhibiting cytotoxicity with concentrations above 50 μM (Deb et al., 2011). Cell cycle arrest was observed in the G0/G1 phase, with decreased Bcl-2 protein levels and interruption of mitochondrial homeostasis; increased ROS production, mitochondrial production of H2O2, and Bax protein levels; and activation of caspase-8, -9, -3 and PARP (Deb et al., 2011). Thus, inhibition of the PI3K/Akt/mTOR signaling pathway may be a possible mechanism involved behind the effects of thymol on HL-60 cells (Pathania et al., 2013).
It was observed by Bouhtit et al. (2021) that at a concentration of 300 µM of carvacrol the KG1 cell lines were more sensitive compared to the HL60 cell line, and at 400 µM the K-562 cell line showed resistance after 48 h of treatment. Regarding thymol (50 µM), the KG1 cell line was also more sensitive when compared to the other two and at the 100 µM dose, thymol was able to induce complete cell death in the KG1 and HL60 cell lines (Bouhtit et al., 2021).
Transformed Cell Lines
When using mouse myoblast cells (CO25 cell line) transformed with human N-RAS oncogene, Zeytinoglu et al. (2003) showed that the concentrations of 1, 5, and 10 μg/mL of carvacrol provoked cytotoxic effects. The same effects were also observed for 5RP7 and CO25 cells transformed by H-RAS and N-RAS oncogenes, respectively, as well as apoptotic morphological changes in both cell lines. However, the fragmentation of internucleosomal DNA and the initial apoptotic determinants were observed only in the cell line 5RP7 cell line. In addition, H-RAS-transformed 5RP7 cells were more sensitive to carvacrol than N-RAS-transformed CO25 cells (Akalin and Incesu, 2011).
Based on these data, we compiled the IC50 (μM) values determined 24 h after the incubation of the studied cells with carvacrol or thymol. It was possible to verify that, in general, carvacrol (336.7 ± 35.0, n = 21) is more potent than thymol (527.1 ± 146.6, n = 11), with difference between means of 103.8 (±106.9). The lowest IC50 values for carvacrol were against prostate carcinoma (PC-3 IC50 = 46.71 μM, Khan et al., 2019; DU 145 IC50 = 84.39 μM, Khan et al., 2017) and gastric carcinoma (AGS IC50 = 82.57 μM, Günes-Bayir et al., 2018), whereas thymol appears to be more selective for gastric cancer carcinoma (AGS, IC50 = 75.63 μM, Günes-Bayir et al., 2018) as seen in Supplementary Table S2.
Description of In Vivo Studies with Carvacrol and Thymol
Anticarcinogenic effects were observed after treatment with carvacrol in Wistar rats, depicted by a reduction in the incidence of tumors, increased survival rate, and a reduced carcinogenic potency of the substance in inducing malignant tumors (Karkabounas et al., 2006). The pre- and post-treatment with carvacrol in animals with liver cancer induced by diethylnitrosamine (DEN) revealed a decrease in the number of nodules, a final body weight increase and a reduction in liver weight. In fact, carvacrol pre-treatment caused the disappearance of most tumoral foci and nodules, characterized by few neoplastic cells, suggesting a chemopreventive effect. In contrast, post-treatment with carvacrol demonstrated the presence of small persistent nodules, loss of cellular architecture and a lower tendency to spread through the intrahepatic veins. Moreover, carvacrol was able to increase the levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione (GSH), along with a reduction of lipid peroxides and the enzymes AST, ALT, ALP, LDH and γGT in the serum (Jayakumar et al., 2012).
Similarly, Subramaniyan et al. (2014) also evaluated the effect of carvacrol pre- and post-treatment on a DEN-induced hepatocarcinogenesis rat model and observed a stability in tumor marker levels, a reduced mast cell density and inhibition of cell proliferation. Furthermore, supplementation with carvacrol significantly restored the activities of liver microsomal xenobiotic metabolizing enzymes to normal, with a reduced expression of proliferative nuclear cell antigen (PCNA), MMP-2 and -9, and thereby prevented the local spread of carcinogenic cells, showing an antimetastatic effect (Subramaniyan et al., 2014). Hence, in a rat model of hepatocellular carcinoma induced by diethylnitrosamine (DEN), carvacrol treatment promoted DNA fragmentation indicating its potential as an apoptotic agent. In addition, carvacrol showed a reduction in serum levels of alpha-fetoprotein (AFP), alpha l-fucosidase (AFU), vascular endothelial growth factor (VEGF) and decreased expression of the gamma glutamyl transferase (GGT) gene (Ahmed et al., 2013).
Carvacrol supplementation significantly improved the weight gain and growth rate of animals with colon cancer induced by 1,2-dimethylhydrazine (DMH), exhibiting a lower incidence of tumors and pre-neoplastic lesions, along with a reduction in oxidative stress damage (higher levels of GSH, GPx, GR, SOD and CAT), suggesting that carvacrol presents chemopreventive effects (Sivaranjani et al., 2016).
Li et al. (2019) showed that tumor growth in mice with DEN-induced hepatocarcinoma and treated with carvacrol was limited, revealing tumor cell reduction, rare mitotic figures, normal arrangement of cells, few microvessels, a central necrotic area on tumor tissue and a reduction of intrastromal and peritumor lymphocytes. Likewise, there was an increased expression of the death-associated protein kinase 1 (DAPK1) and decreased expression of serine/threonine-protein phosphatase 2A (PPP2R2A) in tumor tissues (Li et al., 2019). More recently, Rojas-Armas et al. (2020) showed a better effect of carvacrol at a dose of 100 mg/kg/day compared to the other doses tested (50 and 200 mg/kg/day) in female Holztman rats with breast cancer induced by 7,12-dimethylbenzanthracene (DMBA), showing a reduction of 4 (of 16) tumors, in addition to a 75% reduction in the frequency of tumors, a 67% reduction in incidence, an increase in tumor latency and a reduction in the average tumor volume and cumulative tumor volume (Rojas-Armas et al., 2020).
De La Chapa et al. (2018), after treating female athymic nude mice injected with tongue squamous cell carcinoma (Cal27 cell line) and cervical cancer (HeLa cell line), reported a significant inhibition of tumor growth and volume, besides a significant reduction in the number of proliferative cells, with thymol increasing the quantity of apoptotic cells. In a later study, Zeng et al. (2020) established two in vivo models to investigate the effect of thymol on cancer progression. For the colorectal cancer model, HCT116 xenograft was injected (i.p.) into BALB/c mice, which after 7–10 days (when the tumors grew to approximately 100 mm3) were treated with thymol (75 or 150 mg/kg every other day). A significant reduction in cancer growth, a greater number of necrotic lesions and a lower level of Ki-67 expression were observed, which reflects cell proliferation. As for the lung metastasis model, HCT116 cells were injected into the tail vein of each mouse and then received treatment with thymol (75 or 150 mg/kg every other day). After 6 weeks, they found that the average number of tumor nodules on the lung surface of the two treatment groups was significantly lower, revealing an anti-metastatic effect, probably due to the inhibition of the Wnt/β-catenin signaling pathway (Zeng et al., 2020).
It was revealed in the study by Hassan et al. (2021) that the administration of thymol (20 mg/kg/day, p. o.) in male Wistar rats provided promising protective activity against colon cancer by significantly reducing elevated serum levels of colon-related tumor markers, carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA), as well as the apoptotic marker, caspase-3 compared to the colon cancer group. In addition, it promoted the reduction of oxidative stress by increasing the enzymatic antioxidants SOD, CAT, GSH and GST, inhibiting inflammation by decreasing TNF-α, NF-κB and IL-6 (Hassan et al., 2021). Figure 2 shows a summary of the main effects observed in the in vivo studies.
FIGURE 2.
Summary of main effects observed in in vivo studies.
Risk of Bias and Quality of Included Studies
Almost all studies did not present enough data to allow the judgment of the domains related to the generation of the random sequence, concealment of the allocation, blinding of the researchers and evaluators of the results. Only 4.3% (n = 3) of the studies recorded reported randomness when taking photos of selected areas regarding apoptotic activity assays. However, 97% of the studies applied the same conditions (temperature and incubation time, and purity, stability) for in vitro assays, ensuring a low risk of bias (Figure 3).
FIGURE 3.
Assessment of in vitro studies risk of bias.
Similarly, most of the SYRCLE tool domains for animal studies (random sequence generation; baseline characteristics; allocation concealment; blinding of caregivers and researchers; random evaluation of results; blinding of the results evaluator) were classified as uncertain due to the lack of information in the articles. In contrast, 30% (n = 3) record random allocation of the animals in the study (Figure 4).
FIGURE 4.
Assessment of in vivo studies risk of bias.
Discussion
Through the scientific evidence compiled in this systematic review, it was possible to verify the preventive and therapeutic effects of carvacrol and thymol in cancer in addition to the antimetastatic activity that these compounds were able to exert due to their cytotoxic and antiproliferative effects. Most studies have demonstrated the effect of these compounds on carcinomas, as they are one of the most common types of cancer (Neville, 2011).
Medicinal plants and their bioactive compounds have been an important source of recent drug discoveries (Van Wyk and Wink, 2018). Our review identified a considerable number of reports (77 studies) published in the last two decades, with an increasing trend over recent years. In fact, phytochemicals have great pharmaceutical significance due to their diverse structures (with more than 100,000 being described so far) and their pharmacological properties (Srivastav et al., 2020), and have already made an important contribution to cancer treatments (Ashraf, 2020). Our review also showed that carvacrol and its thymol isomer are capable of restraining growth and combating different tumor strains in vitro.
In Asia, the continent with the largest number of publications on the subject, the use of traditional, popular medicine continues to grow (De Boer and Cotingting, 2014) due to the low costs, easy access, the frequent reduced side effects and toxicity, and their better biodegradable properties (Soković et al., 2013). This is often reinforced by difficulties in accessing health services and obtaining essential medicines (WHO, 2007-2017; Ozawa et al., 2019). Moreover, India and China have, historically, made important contributions to knowledge about medicinal plants, being responsible for some of the most ancient reports about this issue, that were written approximately 5,000 and 4,000 years ago, respectively (Wiart, 2007; Kelly, 2009).
Neoplasia can be described as a disease of unchecked cell division, and its progression is related to abnormal activity of cell cycle regulators. The cell cycle consists of four discrete phases in which the cell increases in size and cellular content is duplicated (gap 1 or G1 phase), DNA is replicated (synthesis, or S phase), it prepares to divide (gap 2, or G2 phase), and then divides, creating two identical daughter cells (mitosis, or M phase). As a cell moves through each phase, stimulated by growth and transcription factors, it passes through several checkpoints, which ensure that mitosis occurs only when the cellular genome has been precisely replicated, avoiding mutations and generation of transformed cells (Hamilton and Infante, 2016; Ingham and Schwartz, 2017). The cell cycle is controlled mainly by cyclin-dependent kinases (CDKs) (Ingham and Schwartz, 2017) CDK4/6 are the kinases responsible for the inactivation/phosphorylation of retinoblastoma protein, at the G1/S phase transition checkpoint. In this review, it was noted that three in vitro studies (4.4%) reported that carvacrol decreased CDK4 protein expression in human tongue squamous cell carcinoma (Tca-8113 cells) (Dai et al., 2016) and in breast cancer (MCF-7 cells) (Mari et al., 2020; Li et al., 2021) and only one (1.4%) reported a decrease in CDK6 in MCF-7 cells (Mari et al., 2020). CDK inhibitor drugs are being used in some cancer types (Mari et al., 2020), such as acute myeloid leukemia (Lee and Zeidner, 2019) and breast cancer (Pernas et al., 2018). In addition, cell cycle arrest, reported in 14.7% (n = 10) of in vitro studies also represents a promising target of cancer treatment, since natural compounds can act as modulators, interrupting the cell cycle and, therefore, killing cancer cells (Bailon-Moscoso et al., 2017). The uncontrolled proliferation of cancer cells occurs due to their ability to prevent programmed cell death (apoptosis) (Olsson and Zhivotovsky, 2011; Dabrowska et al., 2016; Kim and Kim, 2018), that is responsible for eliminating aberrant proliferating cells or those with DNA damage/mutations (Dabrowska et al., 2016) (Figure 5). In this review, 55% (n = 38) of the studies reported induction of apoptosis after treatment with carvacrol or thymol in their in vitro studies and only 40% (n = 4) in in vivo studies.
FIGURE 5.
Cell cycle involvement in cancer treatment with carvacrol and thymol.
The tumor suppressor TP53 gene, also called “the guardian of the genome”, is activated in response to stress signals (DNA damage) and can interrupt DNA replication and cell division through cell cycle arrest (G1/S checkpoint), in order to restore genetic integrity, thereby preventing genetically transformed cell proliferation (Harris and Levine, 2005; Belyi et al., 2010; Georgakilas et al., 2017). When repair is not possible, p53 causes programmed cell death, interacting with the Bcl-2 family of proteins, triggering genes involved in apoptosis, such as Bax (Gottlieb and Oren, 1998; Song et al., 2014). In addition, evidence indicates that when there is a deficiency of p53, the p21 gene can act as an oncogenic factor, causing the cell cycle to be interrupted (Georgakilas et al., 2017). Treatment with carvacrol increased p53 expression in breast cancer (Al-Fatlawi and Ahmad, 2014), cervical and liver cancer cells (Abbas and Al-Fatlawi, 2018). Meanwhile, administration of thymol increased p21expression in bladder cancer cells (Li et al., 2017) and increased expression of both p53 and p21 in breast cancer cells (Seresht et al., 2019). In fact, TP53 genetic alterations are commonly observed in clinical tumor samples, since most mutations lead to function loss, in which cells can escape the destruction and repair process, resulting in a malignant transformation through favorable natural selection (Olsson and Zhivotovsky, 2011).
During cancer, overexpression of anti-apoptotic genes and under expression of pro-apoptotic genes can lead to the failure of the programmed cell death mechanism (Olsson and Zhivotovsky, 2011; Zhang et al., 2000). The expression of proapoptotic proteins, such as Bax (Li et al., 2017; Basu and Haldar, 1998), is an important mechanism of tumor regression (Backus et al., 2002). It triggers apoptosis by forming pores within the outer mitochondrial membrane, releasing cytochrome C that activates proteases such as caspase-9 and -3, that dismantle and destroy the cell (apoptosis) (Olsson and Zhivotovsky, 2011). Our results showed that 24.6% of in vitro studies (n = 17) and 10% of in vivo studies showed an increase in Bax levels after treatment with carvacrol or thymol. Additionally, 26% (n = 18) of in vitro studies reported negative regulation of the anti-apoptotic protein Bcl-2 (Swanton et al., 1999; Kroemer et al., 1998). Caspases are central apoptosis regulators and executors, and thus attractive targets for the development of therapeutic strategies for cancer treatment (Ghavami et al., 2009; Hensley et al., 2013; Fiandalo and Kyprianou, 2012; Shalini et al., 2015). Hence, positive expression of caspases increases tumor sensitization to treatment (Hensley et al., 2013) (Figure 6). In this review, 33.3% (n = 23) of in vitro studies reported an increase of caspase activity, more specifically caspase-3 (Deb et al., 2011; Liang and Lu, 2012; Yin et al., 2012; Ferraz et al., 2013; Liang et al., 2013; Al-Fatlawi and Ahmad, 2014; Chen et al., 2015; Bhakkiyalakshmi et al., 2016; Khan et al., 2017; Li et al., 2017; Abbas and Al-Fatlawi, 2018; Chauhan et al., 2018; Günes-Bayir et al., 2018; Günes-Bayir et al., 2018; Tayarani-Najaran et al., 2019), -6 (Al-Fatlawi and Ahmad, 2014; Abbas and Al-Fatlawi, 2018), -7 (Kang et al., 2016), -8 (Deb et al., 2011; Pathania et al., 2013; Kang et al., 2016; Khan et al., 2019) and -9 (Deb et al., 2011; Pathania et al., 2013; Al-Fatlawi and Ahmad, 2014; Kang et al., 2016; Li et al., 2017; Abbas and Al-Fatlawi, 2018; Günes-Bayir et al., 2018; Günes-Bayir et al., 2018; Potočnjak et al., 2018; Khan et al., 2019). It is noteworthy that the positive regulation of caspase-3 propagates and amplifies the apoptosis signal, and its loss of expression promotes tumorigenesis (Fiandalo and Kyprianou, 2012). Shalini et al. (2015) reported that high caspase-3 expression caused apoptosis of tumor cells, and is significantly associated with better prognosis in patients with non-small cell lung cancer (Yoo et al., 2004) and hepatocellular carcinomas (Huang et al., 2010). However, there is very little knowledge about the role of caspases-6 and -7 during cancer (Ghavami et al., 2009). In this review, caspase-6 and -7 expression was reported in only three studies. Overexpression of caspase-8 has been reported in prostate cancer cells treated with carvacrol (Khan et al., 2019) and in studies that tested thymol on promyelocytic leukemia (Deb et al., 2011; Pathania et al., 2013) and gastric cancer (Kang et al., 2016). The literature reveals that the activation of caspase-8 plays an important role in the initiation phase of apoptosis (Soung et al., 2005), as well as in suppressing oncogenic transformation, confirmed by an increased susceptibility to spontaneous mutations in its absence (Olsson and Zhivotovsky, 2011). Krelin et al. (2018) reported that caspase-8 deficient cells exhibit resistance to death, facilitating tumorigenic transformation (Krelin et al., 2008). The expression of caspase-9 was the second most reported in this review, due to its crucial role in apoptosis initiation among various types of cancer (Kim et al., 2015). Previous studies have reported that caspase-9 regulates the apoptosis process of cancer cells through interactions with signaling molecules (Bou-Hanna et al., 2015; Thakor et al., 2017). Natural compounds can regulate caspase-9 expression, and, therefore, favor apoptosis in cancer (Kim et al., 2015). Pre-treatment with caspase inhibitors caused a significant reduction in cytotoxicity and attenuation of apoptosis induced by carvacrol, observed in prostate cancer cells (Khan et al., 2019).
FIGURE 6.
Involvement of apoptosis in carvacrol and thymol treatment in cancer.
Another very important cell cycle regulator is MAPK. It controls cell growth, proliferation, differentiation and apoptosis, and represents one of the main signaling pathways involved in extracellular signals transduction (Binétruy et al., 2007; Kim and Choi, 2015; Li et al., 2018). In particular, JNK, ERK and p38 are the main proteins of MAPK pathways when approaching cancer. ERK is generally associated with cell proliferation, while JNK and p38 are closely related to the cell death process (Wagner and Nebreda, 2009). JNK is proven to be related to the development and progression of malignant cells (Wu et al., 2019). In this review, 5.7% (n = 4) of the studies that tested carvacrol or thymol showed that they were able to increase in vitro JNK phosphorylation in colon cancer (Fan et al., 2015; Chauhan et al., 2018), choriocarcinoma (Lim et al., 2019) and bladder cancer (Li et al., 2017). Studies showed that phosphorylation/activation of p38, MAPK and JNK contributes to cancer cell apoptosis (Liu et al., 2014; Wang et al., 2014) and that the p38 regulates apoptosis process, cycle growth progression and cell differentiation (Zarubin and Han, 2005; Krens et al., 2006). P38 can also directly affect tumor invasion and angiogenesis (Wagner and Nebreda, 2009). Herein, 4.3% (n = 3) of the studies in vitro induced phosphorylation of p38 in hepatocarcinoma cells (Yin et al., 2012), cancer bladder cells (Li et al., 2017) and choriocarcinoma cell (Lim et al., 2019). In fact, Li et al., (2017) suggested that the activation of JNK and p38 were pivotal to the cytotoxicity exhibited by thymol against cancer cells. In addition, the level of phosphorylated ERK decreased in hepatocarcinoma, colon cancer, choriocarcinoma and prostate cancer cells, after treatment with carvacrol, and p-ERK1/2 levels decreased after thymol treatment in glioma cells (Yin et al., 2012; Fan et al., 2015; Heidarian and Keloushadi, 2019; Lim et al., 2019). This corroborated the findings of Wang et al. (2018) who reported that patients with tumors with low p-ERK (activated form) had a higher survival rate (Low and Zhang, 2016) (Figure 7).
FIGURE 7.
Involvement of the MAPK pathway in carvacrol and thymol treatment in cancer.
The PI3K/AKT/mTOR signaling pathway is also studied in cancer since it regulates cell proliferation, growth, metabolism and motility (Song et al., 2019; O'Donnell et al., 2018). Its inhibition induces a pronounced anticancer activity (Li et al., 2016). In addition, some inhibitors of this signaling pathway have already been approved by the Food and Drug Administration for cancer treatment (Alzahrani, 2019). PI3k belongs to a family of lipid kinases, involved in extracellular signals transduction and cell growth promotion (Wullschleger et al., 2006; Alzahrani, 2019). AKT, also known as protein kinase B (PKB), is an oncogenic protein that regulates cell survival, proliferation, growth, apoptosis and glycogen metabolism (Alzahrani, 2019; Song et al., 2019). Excessive activation of mTOR (mammalian target of rapamycin), a serine/threonine kinase, is associated with the activation of hypoxia inducible factor (HIF) that regulates angiogenesis and tumor growth (Semenza, 2003). Its inhibition was identified by 2.8% (n = 2) of in vitro studies using thymol in human promyelocytic leukemia cell lines (Pathania et al., 2013) and bladder cancer cells (Li et al., 2017), and by 7.2% (n = 5) of in vitro studies that used carvacrol in colon cancer (Fan et al., 2015), glioblastoma (Chen et al., 2015), prostate cancer (Luo et al., 2016), choriocarcinoma (Lim et al., 2019) and breast cancer cells (Mari et al., 2020).
The mechanism of action reported in in vivo studies involves slightly more complex processes. Starting with Ahmed et al. (2013), the only authors of this review who reported in in vivo models a reduction in serum levels of AFP, AFU, VEGF and reduced GGT gene expression after treatment with carvacrol. The literature reveals that AFP, besides being one of the most useful biomarkers for the detection of hepatocellular carcinoma, also serves to monitor the response to anticancer therapy (high levels indicate tumor progression) (Bei and Mizejewski, 2011; Wong et al., 2015; Wang and Wang, 2018). On the other hand, AFU indicates clinical prognosis of several malignant tumors and helps to diagnose primary hepatocarcinoma (Giardina et al., 1992), colorectal cancer (Ayude et al., 2000), ovarian cancer (Abdel-Aleem et al., 1996), and was recently identified as an effective new biomarker for squamous cell carcinoma of the early esophagus (Yu et al., 2019). In addition, high levels of GGT influence proliferation and apoptosis and contribute to tumor progression (Koss and Greengard, 1982; Pompella et al., 2006; Zhang et al., 2006) serving as a biomarker in various types of cancer (Paolicchi et al., 1996; Whitfield, 2001; Pompella et al., 2006). The increased expression of VEGF during cancer, along with other pro-angiogenic factors, is responsible for new vascularization, representing a strategic point in the treatment (Collins and Hurwitz, 2005; Riaz et al., 2015; Siveen et al., 2017). The decrease in serum levels of these biomarkers in animals treated with carvacrol may prove to be an important antitumor characteristic that deserves attention and further studies to elucidate this mechanism (Ahmed et al., 2013). Together with VEGF, other enzymes collaborate for neoplastic invasion, such as mitochondrial membrane potential. They are matrix degradation enzymes (such as MMP), responsible for the degradation of the main constituents of basement membrane and extracellular matrix, facilitating the invasion of tumor cells and favoring the spread of cancer cells and metastases (Nagase et al., 2006; Shuman Moss et al., 2012; Jabłońska-Trypuć et al., 2016). High MMP-2 and -9 levels were associated with esophageal carcinomas (Koyama et al., 1990) and breast (Alrehaili et al., 2020), oral (Lin et al., 2004), bladder (Fouad et al., 2019), skin (Fundyler et al., 2004), larynx (Liu et al., 2005) cancer. In this systematic review, only one in vivo study (10%) reported a decrease in the levels of MMP-2 and MMP-9 in liver cancer after treatment with carvacrol (Subramaniyan et al., 2014), the majority 8.6% (n = 6) were in vitro studies. It is important to note that carvacrol prevented metastasis in vivo, demonstrated by tumors less likely to spread through intrahepatic veins (Jayakumar et al., 2012) and preventing local spread of cancer cells by suppressing the expression of MMP-2 and MMP-9 proteins (Subramaniyan et al., 2014), and thymol decreased the number of lung metastatic lesions in vivo, suppressing the Wnt/β-catenin signaling pathway (Zeng et al., 2020). However, there is almost nothing about this topic documented in the literature, and further studies are required to address this outcome.
Considering reactive oxygen species (ROS), these are a group of molecules that contain reduced forms of oxygen with short life and that are more energetically reactive than molecular oxygen (Srinivas et al., 2019). The generation of ROS inside the cell contributes to the antitumor process in order to induce DNA damage and slow the progression of the cell cycle, preventing cells with DNA damage (cancer cells) from continuing with cell division (Allawzi et al., 2019; Srinivas et al., 2019). In vitro, carvacrol and thymol increased the generation of reactive oxygen species in 24.63% (n = 17) of the studies, a fact that is also observed in chemotherapeutics such as doxorubicin (Conklin, 2004), cisplatin (Marullo et al., 2013) and bleomycin (Allawzi et al., 2019), which also increase ROS levels. Regarding in vivo studies, the pretreatment with carvacrol in colon cancer (Sivaranjani et al., 2016) and hepatocellular carcinoma (Jayakumar et al., 2012) increased the levels of enzymatic antioxidants such as GPx, SOD, CAT, GR, and GSH, revealing a chemopreventive effect of carvacrol and prevention of cell proliferation (Jayakumar et al., 2012; Sivaranjani et al., 2016). Similarly, thymol had a promising protective efficacy, revealing a chemopreventive effect against colon cancer observed by the increase in GST, GSH, SOD and CAT levels, in addition to inhibiting oxidative stress (Hassan et al., 2021). However, these results should be interpreted with caution, as low levels of ROS can be beneficial in preventing the development of cancer cells, since they can promote cancer (Prasad et al., 2017). In this sense, anticancer therapies can follow two paths; using compounds that prevent the formation of ROS, and thereby preventing carcinogenesis; or using compounds that have as their action mechanism the increase of ROS, promoting oxidative stress within the tumor (de Sá Junior et al., 2017). Further studies are needed to clarify this issue.
Post-treatment with carvacrol also promoted an increase in the expression of the DAPK1 gene and a decrease in the enzyme PPP2R2A (Li et al., 2019). DAPK1 is a serine/threonine kinase, a tumor suppressor protein that promotes apoptosis (Agodi et al., 2015; Zhai et al., 2019), while PPP2R2A, a regulatory subunit of protein phosphatase 2A (PP2A), controls the pathway of AKT signaling associated with tumor growth (Wang et al., 2016; Zeng et al., 2016). In fact, both may be involved in a possible antitumor mechanism of carvacrol, but it is still not very clear, requiring further studies (Li et al., 2019).
Through in vitro studies, we found that carvacrol proves to be more potent than thymol, and appears to have a greater cytotoxic effect for some cell lines, such as carcinomas (prostate and stomach) (Khan et al., 2017; Günes-Bayir et al., 2018; Khan et al., 2019). Thymol seems to act more against gastric cancer carcinoma (AGS, IC50 = 75.63 μM, Günes-Bayir et al., 2018). In fact, two recent studies published by Sisto et al. (2020) and Sisto et al. (2021) demonstrated the potential of these monoterpenes in the control of gastric carcinoma by reducing the viability of AGC cells.
In in vivo studies, on the other hand, the effect of carvacrol has been predominantly studied against hepatocarcinoma, with few studies for breast and colon cancer. In a way, it can be seen that the studies in this area do not seem to evolve toward a specific target, with low complementarity of the screenings carried out in vitro for the studies developed with experimental animals. This is a great barrier advances in this area of knowledge and helps to explain the discrepancy in the number of studies published in vitro (n = 69) and in vivo (n = 10) over the last two decades, as well as the absence of clinical trials. Thus, it is necessary to consider the studies already carried out for these compounds before conducting new primary studies on this topic, in order to evolve the research stages to the next level of drug development: in vivo studies and clinical trials.
As for security, some studies have suggested that normal cells tolerate exposure to carvacrol (Koparal and Zeytinoglu, 2003; Yin et al., 2012; Khan et al., 2017; Lim et al., 2019) and thymol (Deb et al., 2011; Ferraz et al., 2013; Chauhan et al., 2018; Balan et al., 2021) in different concentration ranges well. However, some studies have demonstrated the action of these compounds, in a negative way, in some normal cells, such as human fibroblast cells (WS-1; IC50 of 138.1 ± 8.7 μM of carvacrol (Günes-Bayir et al., 2018); breast epithelial cells (fR2; IC50 of 86 μg/mL of thymol; (Pathania et al., 2013), normal lymphocyte (PBMC; IC50 > 25 μg/ml of thymol; (Ferraz et al., 2013); rat embryonic fibroblasts (mild toxicity of 20.94% after thymol 5, 30.5, 61, 122, 244 ng/mL; (Abed, 2011). Moreover, it has been shown that at concentrations of 0.5% (v/v) carvacrol and thymol exhibited a proliferative effect on normal human PBMC cells (Jaafari et al., 2007), and a lower concentration (10 μM) of carvacrol also caused a statistically significant proliferation of WS-1 cells (Günes-Bayir et al., 2018). In their most recent study, Günes-Bayir et al. (2020) reported that high doses of thymol can act on cancerous and healthy cells, while low doses seem to protect healthy cells but harm cancer cells. Moreover, genotoxic effects have been shown to be exhibited by carvacrol (Günes-Bayir et al., 2018) and thymol (Günes-Bayir et al., 2018), and it was also shown that a high concentration of carvacrol (460 µM) exhibited mutagenic and genotoxic effects causing DNA damage (Llana-Ruiz-Cabello et al., 2015). In vivo, Suntres et al. (2015) reported that the average lethal dose of carvacrol after intravenous administration in dogs was 310 mg/kg, for rats it was 810 mg/kg when administered orally and 80 and 73 mg/kg when injected intravenously or intraperitoneally, respectively. In mice, the lethal dose was 110–233.3 mg/kg, after inducing ataxia and drowsiness (Suntres et al., 2015). However, a phase I randomized clinical trial conducted in healthy subjects treated with carvacrol (1 or 2 mg/kg daily, p.o., during one month) revealed that this monoterpene did not lead to clinically significant changes, and did not cause any adverse effects, showing clinical safety and tolerability for this agent (Ghorani et al., 2021). Thus, the dose-response relationship must be considered in more detail, so that further studies in more advanced stages are developed to assess the antitumor effects of these compounds.
The methodological quality of the studies included revealed many items classified as “unclear” or “uncertain” indicating that the report—and presumably the experimental design—of these studies can be improved, especially with regard to the generation of the random sequence, the concealment of allocation and the blinding of researchers and evaluators, important factors in respect of a reliable result. Therefore, future studies need to improve their methodological quality, particularly in respect of risk of bias, in order to produce more reliable results. Even after almost two decades of research in this field, there has been no progress in research in humans, possibly due limitations of preclinical studies and the lack of knowledge about pharmacokinetics and toxicity of carvacrol and thymol. These factors limit the introduction of these compounds as a therapeutic option, and highlight the new for innovative studies to be undertaken.
Seven studies used a positive control to compare the antitumor effect of carvacrol (Jaafari et al., 2007; Maryam et al., 2015; Tayarani-Najaran et al., 2019) and/or thymol (Deb et al., 2011; Ferraz et al., 2013; Kang et al., 2016; Zeng et al., 2020) in their studies. In vitro, methotrexate and vincristine (0.5 µg/100 µL) (Jaafari et al., 2007), 5-fluorouracil (5-FU) (2.6 μg/μM) (Maryam et al., 2015), doxorubicin (0.5, 5, 10, and 20 μg/mL) (Tayarani-Najaran et al., 2019) (1.0 μg/mL) (Ferraz et al., 2013) and (2 μM) (Kang et al., 2016), camptothecin (5 μM) (Deb et al., 2011) and in vivo, doxorubicin (2 mg/kg) (Zeng et al., 2020), were the main substances used to compare the potential therapeutic effect of these monoterpenes. We emphasize here the importance of using a standard substance as a positive control as a parameter for comparing the effect of new candidates for cancer treatment.
In summary, we observed that more in vivo studies, particularly in respect of thymol, are needed. Further studies are required to help unravel and define the mechanisms of action of the two compounds against cancer cells, as well as studies that further explore their chemopreventive and anti-metastatic effects. It is of note that several studies suggested that signaling pathways (PI3K/AKT/mTOR and MAPKs) may be the main mechanism of action of these monoterpenoids, but more advanced studies are needed to elucidate this issue. There is also still a wide variation in the doses used, requiring the establishment of a consensus in respect of defining potentially effective and safe doses. In addition, further studies are also needed to clarify the effects of these two compounds on normal/healthy cells in vitro, and in appropriate in vivo models.
Conclusion
The knowledge obtained through the reviewed studies provides strong evidence of the antitumor and antiproliferative activity promoted by carvacrol and thymol. However, there are still gaps regarding the standard or ideal dose, the exact mechanism of action and safety of the two compounds, revealing challenges for future studies. In addition, it was found through in vitro studies that carvacrol seems more potent than thymol, and was shown to have a greater cytotoxic effect for some cell lines. Moreover, further animal studies should be encouraged, as they have as yet made limited progress, and most of the current results are based on in vitro studies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Conception and design: AG, DT, and LS. Data acquisition: LS and LP. Analysis and interpretation of data: LS and LP. Elaboration of the manuscript: LS. Review of intellectual content: MS, AG, and DT. Final approval of the completed manuscript: AG and DT.
Funding
Financial support was provided by the Fundação de Apoio à Pesquisa e Inovação Tecnolόgica do Estado de Sergipe (FAPITEC/SE), the Conselho Nacional de Desenvolvimento Científico e Tecnolόgico (CNPq/Brazil), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) and Federal University of Sergipe.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.702487/full#supplementary-material
References
- Abbas A., Al-Fatlawi A. (2018). Anti-proliferative and Pro-apoptotic Activity of Carvacrol on Human Cancer Cells. Int. J. Pharm. Res. 10 (2), 174–180. 10.3892/or.2012.1877 [DOI] [Google Scholar]
- Abdel-Aleem H., Ahmed A., Sabra A. M., Zakhari M., Soliman M., Hamed H. (1996). Serum Alphal-Fucosidase Enzyme Activity in Ovarian and Other Female Genital Tract Tumors. Int. J. Gynecol. Obstet. 55 (3), 273–279. 10.1016/s0020-7292(96)02770-1 [DOI] [PubMed] [Google Scholar]
- Abed R. (2011). Cytotoxic, Cytogenetics and Immunomodulatory Effects of Thymol from Thymus Vulgaris on Cancer and Normal Cell Lines In Vitro and In Vivo . Al-mustansiriyah J. Sci. 22 (5), 41–53. 10.3403/30303631 [DOI] [Google Scholar]
- Agodi A., Barchitta M., Quattrocchi A., Maugeri A., Vinciguerra M. (2015). DAPK1 Promoter Methylation and Cervical Cancer Risk: A Systematic Review and a Meta-Analysis. PLoS ONE 10 (8), e0135078. 10.1371/journal.pone.0135078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H., Shousha W., El-Mezayen H., Ismaiel N., Mahmoud N. (2013). In Vivo antitumor Potential of Carvacrol against Hepatocellular Carcinoma in Rat Model. World J. Pharm. Pharm. Sci. 2 (5), 2367–2396. 10.3748/wjg.v8.i3.464 [DOI] [Google Scholar]
- Akalin G., Incesu Z. (2011). The Effect of Carvacrol on Apoptosis of H-RAS and N-RAS Transformed Cell Lines. Turk J. Pharm. Sci. 8 (2), 105–116. 10.1007/978-1-4757-1235-3_13 [DOI] [Google Scholar]
- Al-Fatlawi A., Ahmad A. (2014). Cytotoxicity and Pro-apoptotic Activity of Carvacrol on Human Breast Cancer Cell Line MCF-7. World J. Pharm. Sci. 2 (10), 1218–1223. 10.3923/pjbs.2021.646.655 [DOI] [Google Scholar]
- Allawzi A., Elajaili H., Redente E. F., Nozik-Grayck E. (2019). Oxidative Toxicology of Bleomycin: Role of the Extracellular Redox Environment. Curr. Opin. Toxicol. 13 (13), 68–73. 10.1016/j.cotox.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrehaili A. A., Gharib A. F., Karam R. A., Alhakami R. A., El Sawy W. H., Abd Elrahman T. M. (2020). Clinical Significance of Plasma MMP‐2 and MMP‐9 Levels as Biomarkers for Tumor Expression in Breast Cancer Patients in Egypt. Mol. Biol. Rep. 47 (2), 1153–1160. 10.1007/s11033-019-05216-5 [DOI] [PubMed] [Google Scholar]
- Alzahrani A. S. (2019). PI3K/Akt/mTOR Inhibitors in Cancer: At the Bench and Bedside. Semin. Cancer Biol. 59, 125–132. 10.1016/j.semcancer.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Arigesavan K., Sudhandiran G. (2015). Carvacrol Exhibits Anti-oxidant and Anti-inflammatory Effects against 1, 2-dimethyl Hydrazine Plus Dextran Sodium Sulfate Induced Inflammation Associated Carcinogenicity in the colon of Fischer 344 Rats. Biochem. Biophysical Res. Commun. 461 (2), 314–320. 10.1016/j.bbrc.2015.04.030 [DOI] [PubMed] [Google Scholar]
- Arunasree K. M. (2010). Anti-proliferative Effects of Carvacrol on a Human Metastatic Breast Cancer Cell Line, MDA-MB 231. Phytomedicine 17 (8–9), 581–588. 10.1016/j.phymed.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Arya R., Saldanha S. N. (2019). “Dietary Phytochemicals, Epigenetics, and Colon Cancer Chemoprevention,” in Epigenetics of Cancer Prevention. Editors Bishayee A., Bhatia D. (Cambridge: Academic Press; ), 205–229. 10.1016/B978-0-12-812494-9.00010-X [DOI] [Google Scholar]
- Ashraf M. A. (2020). Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature's Bounty. Biomed. Res. Int. 2020, e8602879. 10.1155/2020/8602879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif M. (2015). Role of Fruit and Vegetable in the Treatment of Cancer. Curr. Sci. Perspect. 1 (1), 33–40. 10.1533/9781845694296.4.272 [DOI] [Google Scholar]
- Aydın E., Türkez H., Keleş M. S. (2014). The Effect of Carvacrol on Healthy Neurons and N2a Cancer Cells: Some Biochemical, Anticancerogenicity and Genotoxicity Studies. Cytotechnology 66 (1), 149–157. 10.1007/s10616-013-9547-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayude D., Fernández-Rodríguez J., Rodríguez-Berrocal F. J., Martínez-Zorzano V. S., De Carlos A., Gil E., et al. (2000). Value of the Serum Alpha-L-Fucosidase Activity in the Diagnosis of Colorectal Cancer. Oncology 59 (4), 310–316. 10.1159/000012188 [DOI] [PubMed] [Google Scholar]
- Backus H. H. J., Van Groeningen C. J., Vos W., Dukers D. F., Bloemena E., Wouters D., et al. (2002). Differential Expression of Cell Cycle and Apoptosis Related Proteins in Colorectal Mucosa, Primary colon Tumours, and Liver Metastases. J. Clin. Pathol. 55 (3), 206–211. 10.1136/jcp.55.3.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailon-Moscoso N., Cevallos-Solorzano G., Romero-Benavides J., Ramirez Orellana M. (2017). Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genomics 18 (2), 106–131. 10.2174/1389202917666160808125645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj T., Biernasiuk A., Wróbel R., Malm A. (2020). Chemical Composition and In Vitro Activity of Origanum Vulgare L., Satureja Hortensis L., Thymus Serpyllum L. And Thymus Vulgaris L. Essential Oils towards Oral Isolates of Candida Albicans and Candida Glabrata. Open Chem. 18 (1), 108–118. 10.1515/chem-2020-0011 [DOI] [Google Scholar]
- Balan D. J., Rajavel T., Das M., Sathya S., Jeyakumar M., Devi K. P. (2021). Thymol Induces Mitochondrial Pathway-Mediated Apoptosis via ROS Generation, Macromolecular Damage and SOD Diminution in A549 Cells. Pharmacol. Rep. 73 (1), 240–254. 10.1007/s43440-020-00171-6 [DOI] [PubMed] [Google Scholar]
- Baranauskaite J., Kubiliene A., Marksa M., Petrikaite V., Vitkevičius K., Baranauskas A., et al. (2017). The Influence of Different Oregano Species on the Antioxidant Activity Determined Using HPLC Postcolumn DPPH Method and Anticancer Activity of Carvacrol and Rosmarinic Acid. Biomed. Res. Int. 2017, 1681392. 10.1155/2017/1681392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Haldar S. (1998). The Relationship between BcI2, Bax and P53: Consequences for Cell Cycle Progression and Cell Death. Mol. Hum. Reprod. 4 (12), 1099–1109. 10.1093/molehr/4.12.1099 [DOI] [PubMed] [Google Scholar]
- Bei R., Mizejewski G. J. (2011). Alpha Fetoprotein Is More Than a Hepatocellular Cancer Biomarker: from Spontaneous Immune Response in Cancer Patients to the Development of an AFP-Based Cancer Vaccine. Curr. Mol. Med. 11 (7), 564–581. 10.2174/156652411800615162 [DOI] [PubMed] [Google Scholar]
- Belyi V. A., Ak P., Markert E., Wang H., Hu W., Puzio-Kuter A., et al. (2010). The Origins and Evolution of the P53 Family of Genes. Cold Spring Harbor Perspect. Biol. 2 (6), a001198. 10.1101/cshperspect.a001198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E., Suganya N., Sireesh D., Krishnamurthi K., Saravana Devi S., Rajaguru P., et al. (2016). Carvacrol Induces Mitochondria-Mediated Apoptosis in HL-60 Promyelocytic and Jurkat T Lymphoma Cells. Eur. J. Pharmacol. 772, 92–98. 10.1016/j.ejphar.2015.12.046 [DOI] [PubMed] [Google Scholar]
- Binétruy B., Heasley L., Bost F., Caron L., Aouadi M. (2007). Concise Review: Regulation of Embryonic Stem Cell Lineage Commitment by Mitogen-Activated Protein Kinases. Stem Cells 25 (5), 1090–1095. 10.1634/stemcells.2006-0612 [DOI] [PubMed] [Google Scholar]
- Bou-Hanna C., Jarry A., Lode L., Schmitz I., Schulze-Osthoff K., Kury S., et al. (2015). Acute Cytotoxicity of MIRA-1/NSC19630, a Mutant P53-Reactivating Small Molecule, against Human normal and Cancer Cells via a Caspase-9-dependent Apoptosis. Cancer Lett. 359 (2), 211–217. 10.1016/j.canlet.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Bouhtit F., Najar M., Moussa Agha D., Melki R., Najimi M., Sadki K., et al. (2021). New Anti-leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways. Molecules 26 (2), 410. 10.3390/molecules26020410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carqueijeiro I., Langley C., Grzech D., Koudounas K., Papon N., O’Connor S. E., et al. (2020). Beyond the Semi-synthetic Artemisinin: Metabolic Engineering of Plant-Derived Anti-cancer Drugs. Curr. Opin. Biotechnol. 65, 17–24. 10.1016/j.copbio.2019.11.017 [DOI] [PubMed] [Google Scholar]
- Chamanara M., Abdollahi A., Rezayat S. M., Ghazi-Khansari M., Dehpour A., Nassireslami E., et al. (2019). Thymol Reduces Acetic Acid-Induced Inflammatory Response through Inhibition of NF-kB Signaling Pathway in Rat colon Tissue. Inflammopharmacol 27 (6), 1275–1283. 10.1007/s10787-019-00583-8 [DOI] [PubMed] [Google Scholar]
- Chan H., Ho J., Liu X., Zhang L., Wong S. H., Chan M., et al. (2017). Potential and Use of Bacterial Small RNAs to Combat Drug Resistance: a Systematic Review. Infect. Drug Resist. 10, 521–532. 10.2147/IDR.S148444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-T., Hsu S.-S., Chou C.-T., Cheng J.-S., Wang J.-L., Lin K.-L., et al. (2011). Effect of Thymol on Ca2+ Homeostasis and Viability in MG63 Human Osteosarcoma Cells. Pharmacology 88 (3–4), 201–212. 10.1159/000331864 [DOI] [PubMed] [Google Scholar]
- Chauhan A. K., Bahuguna A., Paul S., Kang S. C. (2018). Thymol Elicits HCT-116 Colorectal Carcinoma Cell Death through Induction of Oxidative Stress. Acamc 17 (14), 1942–1950. 10.2174/1871520617666170327121228 [DOI] [PubMed] [Google Scholar]
- Chen W.-L., Barszczyk A., Turlova E., Deurloo M., Liu B., Yang B. B., et al. (2015). Inhibition of TRPM7 by Carvacrol Suppresses Glioblastoma Cell Proliferation, Migration and Invasion. Oncotarget 6 (18), 16321–16340. 10.18632/oncotarget.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccimiglio J., Alipour M., Jiang Z.-H., Gottardo C., Suntres Z. (2016). Antioxidant, Antibacterial, and Cytotoxic Activities of the EthanolicOriganum vulgare Extract and its Major Constituents. Oxidative Med. Cell. longevity 2016, 1404505. 10.1155/2016/1404505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T. S., Hurwitz H. I. (2005). Targeting Vascular Endothelial Growth Factor and Angiogenesis for the Treatment of Colorectal Cancer. Semin. Oncol. 32 (1), 61–68. 10.1053/j.seminoncol.2004.09.026 [DOI] [PubMed] [Google Scholar]
- Conklin K. A. (2004). Chemotherapy-associated Oxidative Stress: Impact on Chemotherapeutic Effectiveness. Integr. Cancer Ther. 3 (4), 294–300. 10.1177/1534735404270335 [DOI] [PubMed] [Google Scholar]
- Dabrowska C., Li M., Fan Y. (2016). Apoptotic Caspases in Promoting Cancer: Implications from Their Roles in Development and Tissue Homeostasis. Adv. Exp. Med. Biol. 930, 89–112. 10.1007/978-3-319-39406-0_4 [DOI] [PubMed] [Google Scholar]
- Dai W., Sun C., Huang S., Zhou Q. (2016). Carvacrol Suppresses Proliferation and Invasion in Human Oral Squamous Cell Carcinoma. Onco Targets Ther. 9, 2297–2304. 10.2147/OTT.S98875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer H. J., Cotingting C. (2014). Medicinal Plants for Women's Healthcare in Southeast Asia: A Meta-Analysis of Their Traditional Use, Chemical Constituents, and Pharmacology. J. Ethnopharmacology 151 (2), 747–767. 10.1016/j.jep.2013.11.030 [DOI] [PubMed] [Google Scholar]
- De La Chapa J. J., Singha P. K., Lee D. R., Gonzales C. B. (2018). Thymol Inhibits Oral Squamous Cell Carcinoma Growth via Mitochondria-Mediated Apoptosis. J. Oral Pathol. Med. 47 (7), 674–682. 10.1111/jop.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sá Junior P. L., Câmara D. A. D., Porcacchia A. S., Fonseca P. M. M., Jorge S. D., Araldi R. P., et al. (2017). The Roles of ROS in Cancer Heterogeneity and Therapy. Oxidative Med. Cell. longevity 2017, 2467940. 10.1155/2017/2467940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb D. D., Parimala G., Saravana Devi S., Chakraborty T. (2011). Effect of Thymol on Peripheral Blood Mononuclear Cell PBMC and Acute Promyelotic Cancer Cell Line HL-60. Chemico-Biological Interactions 193 (1), 97–106. 10.1016/j.cbi.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Elbe H., Yigitturk G., Cavusoglu T., Baygar T., Ozgul Onal M., Ozturk F. (2020). Comparison of Ultrastructural Changes and the Anticarcinogenic Effects of Thymol and Carvacrol on Ovarian Cancer Cells: Which Is More Effective? Ultrastructural Pathol. 44 (2), 193–202. 10.1080/01913123.2020.1740366 [DOI] [PubMed] [Google Scholar]
- Elbe H., Yigitturk G., Cavusoglu T., Uyanikgil Y., Ozturk F. (2020). Apoptotic Effects of Thymol, a Novel Monoterpene Phenol, on Different Types of Cancer. Bratisl Lek Listy 121 (2), 122–128. 10.4149/BLL_2020_016 [DOI] [PubMed] [Google Scholar]
- Elshafie H., Armentano M., Carmosino M., Bufo S., De Feo V., Camele I. (2017). Cytotoxic Activity of Origanum Vulgare L. On Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of its Biological Activity. Molecules 22 (9), 1435. 10.3390/molecules22091435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K., Li X., Cao Y., Qi H., Li L., Zhang Q., et al. (2015). Carvacrol Inhibits Proliferation and Induces Apoptosis in Human colon Cancer Cells. Anticancer Drugs 26 (8), 813–823. 10.1097/CAD.0000000000000263 [DOI] [PubMed] [Google Scholar]
- Ferraz R. P. C., Bomfim D. S., Carvalho N. C., Soares M. B. P., da Silva T. B., Machado W. J., et al. (2013). Cytotoxic Effect of Leaf Essential Oil of Lippia Gracilis Schauer (Verbenaceae). Phytomedicine 20 (7), 615–621. 10.1016/j.phymed.2013.01.015 [DOI] [PubMed] [Google Scholar]
- Fiandalo M. V., Kyprianou N. (2012). Caspase Control: Protagonists of Cancer Cell Apoptosis. Exp. Oncol. 34 (3), 165–175. [PMC free article] [PubMed] [Google Scholar]
- Fitsiou E., Anestopoulos I., Chlichlia K., Galanis A., Kourkoutas I., Panayiotidis M. I., et al. (2016). Antioxidant and Antiproliferative Properties of the Essential Oils of Satureja Thymbra and Satureja Parnassica and Their Major Constituents. Anticancer Res. 36 (11), 5757–5764. 10.21873/anticanres.11159 [DOI] [PubMed] [Google Scholar]
- Fouad H., Salem H., Ellakwa D. E. S., Abdel‐Hamid M. (2019). MMP‐2 and MMP‐9 as Prognostic Markers for the Early Detection of Urinary Bladder Cancer. J. Biochem. Mol. Toxicol. 33 (4), e22275. 10.1002/jbt.22275 [DOI] [PubMed] [Google Scholar]
- Fundyler O., Khanna M., Smoller B. R. (2004). Metalloproteinase-2 Expression Correlates with Aggressiveness of Cutaneous Squamous Cell Carcinomas. Mod. Pathol. 17 (5), 496–502. 10.1038/modpathol.3800066 [DOI] [PubMed] [Google Scholar]
- Georgakilas A. G., Martin O. A., Bonner W. M. (2017). p21: A Two-Faced Genome Guardian. Trends Mol. Med. 23 (4), 310–319. 10.1016/j.molmed.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Ghavami S., Hashemi M., Ande S. R., Yeganeh B., Xiao W., Eshraghi M., et al. (2009). Apoptosis and Cancer: Mutations within Caspase Genes. J. Med. Genet. 46 (8), 497–510. 10.1136/jmg.2009.066944 [DOI] [PubMed] [Google Scholar]
- Ghorani V., Alavinezhad A., Rajabi O., Mohammadpour A. H., Boskabady M. H. (2021). Safety and Tolerability of Carvacrol in Healthy Subjects: a Phase I Clinical Study. Drug Chem. Toxicol. 44 (2), 177–189. 10.1080/01480545.2018.1538233 [DOI] [PubMed] [Google Scholar]
- Giardina M. G., Matarazzo M., Varriale A., Morante R., Napoli A., Martino R. (1992). Serum Alpha-L-Fucosidase. A Useful Marker in the Diagnosis of Hepatocellular Carcinoma. Cancer 70 (5), 1044–1048. [DOI] [PubMed] [Google Scholar]
- Gordaliza M. (2007). Natural Products as Leads to Anticancer Drugs. Clin. Transl Oncol. 9 (12), 767–776. 10.1007/s12094-007-0138-9 [DOI] [PubMed] [Google Scholar]
- Gottlieb T. M., Oren M. (1998). p53 and Apoptosis. Semin. Cancer Biol. 8 (5), 359–368. 10.1006/scbi.1998.0098 [DOI] [PubMed] [Google Scholar]
- Gouveia D. N., Costa J. S., Oliveira M. A., Rabelo T. K., Silva A. M. d. O. e., Carvalho A. A., et al. (2018). α-Terpineol Reduces Cancer Pain via Modulation of Oxidative Stress and Inhibition of iNOS. Biomed. Pharmacother. 105, 652–661. 10.1016/j.biopha.2018.06.027 [DOI] [PubMed] [Google Scholar]
- Govindaraju S., Arulselvi P. I. (2018). Characterization ofColeus Aromaticusessential Oil and its Major Constituent Carvacrol Forin Vitroantidiabetic and Antiproliferative Activities. J. Herbs, Spices Med. Plants 24 (1), 37–51. 10.1080/10496475.2017.1369483 [DOI] [Google Scholar]
- Günes-Bayir A., Kocyigit A., Güler E. M., Bilgin M. G., Ergün İ. S., Dadak A. (2018). Effects of Carvacrol on Human Fibroblast (WS-1) and Gastric Adenocarcinoma (AGS) Cells In Vitro and on Wistar Rats In Vivo . Mol. Cel Biochem. 448 (5), 237–249. 10.1007/s11010-018-3329-5 [DOI] [PubMed] [Google Scholar]
- Günes-Bayir A., Kocyigit A., Guler E. M., Dadak A. (2020). In Vitro Hormetic Effect Investigation of Thymol on Human Fibroblast and Gastric Adenocarcinoma Cells. Molecules 25 (14), 3270. 10.3390/molecules25143270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günes-Bayir A., Kocyigit A., Güler E. M. (2018). In Vitro effects of Two Major Phenolic Compounds from the Family Lamiaceae Plants on the Human Gastric Carcinoma Cells. Toxicol. Ind. Health 34 (8), 525–539. 10.1177/0748233718761698 [DOI] [PubMed] [Google Scholar]
- Hamilton E., Infante J. R. (2016). Targeting CDK4/6 in Patients with Cancer. Cancer Treat. Rev. 45, 129–138. 10.1016/j.ctrv.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Harris S. L., Levine A. J. (2005). The P53 Pathway: Positive and Negative Feedback Loops. Oncogene 24 (17), 2899–2908. 10.1038/sj.onc.1208615 [DOI] [PubMed] [Google Scholar]
- Hassan H. F. H., Mansour A. M., Salama S. A., El-Sayed E.-S. M. (2021). The Chemopreventive Effect of Thymol against Dimethylhydrazine And/or High Fat Diet-Induced colon Cancer in Rats: Relevance to NF-Κb. Life Sci. 274, 119335. 10.1016/j.lfs.2021.119335 [DOI] [PubMed] [Google Scholar]
- Hassen A. M., Taye G., Gizaw M., Hussien F. M. (2019). Quality of Life and Associated Factors Among Patients with Breast Cancer under Chemotherapy at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. PLoS ONE 14 (9), e0222629. 10.1371/journal.pone.0222629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidarian E., Keloushadi M. (2019). Antiproliferative and Anti-invasion Effects of Carvacrol on PC3 Human Prostate Cancer Cells through Reducing pSTAT3, pAKT, and pERK1/2 Signaling Proteins. Int. J. Prev. Med. 10, 156. 10.4103/ijpvm.IJPVM_292_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley P., Mishra M., Kyprianou N. (2013). Targeting Caspases in Cancer Therapeutics. Biol. Chem. 394 (7), 831–843. 10.1515/hsz-2013-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans C. R., Rovers M. M., De Vries R. B., Leenaars M., Ritskes-Hoitinga M., Langendam M. W. (2014). SYRCLE's Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 14, 43. 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng C.-T., Chou C., Sun T., Liang W., Kuo C., Wang J., et al. (2017). Effect of Carvacrol on Ca^(2+) Movement and Viability in PC3 Human Prostate Cancer Cells. Chin. J. Physiol. 60 (5), 275–283. 10.4077/cjp.2017.bag506 [DOI] [PubMed] [Google Scholar]
- Horvathova E., Turcaniova V., Slamenova D. (2007). Comparative Study of DNA-Damaging and DNA-Protective Effects of Selected Components of Essential Plant Oils in Human Leukemic Cells K562. Neoplasma 54 (6), 478–483. [PubMed] [Google Scholar]
- Horváthová E., Sramková M., Lábaj J., Slamenová D. (2006). Study of Cytotoxic, Genotoxic and DNA-Protective Effects of Selected Plant Essential Oils on Human Cells Cultured In Vitro . Neuro Endocrinol. Lett. 27 (Suppl. 2), 44–47. 10.4172/2167-0412.1000170 [DOI] [PubMed] [Google Scholar]
- Hsu S.-S., Lin K.-L., Chou C.-T., Chiang A.-J., Liang W.-Z., Chang H.-T., et al. (2011). Effect of Thymol on Ca2+ Homeostasis and Viability in Human Glioblastoma Cells. Eur. J. Pharmacol. 670 (1), 85–91. 10.1016/j.ejphar.2011.08.017 [DOI] [PubMed] [Google Scholar]
- Huang H., Zhang X.-F., Zhou H.-J., Xue Y.-H., Dong Q.-Z., Ye Q.-H., et al. (2010). Expression and Prognostic Significance of Osteopontin and Caspase-3 in Hepatocellular Carcinoma Patients after Curative Resection. Cancer Sci. 101 (5), 1314–1319. 10.1111/j.1349-7006.2010.01524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham M., Schwartz G. K. (2017). Cell-Cycle Therapeutics Come of Age. J. Clin. Oncol. 35 (25), 2949–2959. 10.1200/JCO.2016.69.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafari A., Mouse H. A., Rakib E. M., M’barek L. A., Tilaoui M., Benbakhta C., et al. (2007). Chemical Composition and Antitumor Activity of Different Wild Varieties of Moroccan Thyme. Rev. Bras. Farmacogn. 17 (4), 477–491. 10.1590/S0102-695X2007000400002 [DOI] [Google Scholar]
- Jaafari A., Tilaoui M., Mouse H. A., M'bark L. A., Aboufatima R., Chait A., et al. (2012). Comparative Study of the Antitumor Effect of Natural Monoterpenes: Relationship to Cell Cycle Analysis. Rev. Bras. Farmacogn. 22 (3), 534–540. 10.1590/S0102-695X2012005000021 [DOI] [Google Scholar]
- Jabłońska-Trypuć A., Matejczyk M., Rosochacki S. (2016). Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzyme Inhib. Med. Chem. 31 (Suppl. 1), 177–183. 10.3109/14756366.2016.1161620 [DOI] [PubMed] [Google Scholar]
- Jamali T., Kavoosi G., Safavi M., Ardestani S. (2018). In-vitro Evaluation of Apoptotic Effect of OEO and Thymol in 2D and 3D Cell Cultures and the Study of Their Interaction Mode with DNA. Sci. Rep. 8 (1), 15787. 10.1038/s41598-018-34055-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar S., Madankumar A., Asokkumar S., Raghunandhakumar S., Gokula dhas K., Kamaraj S., et al. (2012). Potential Preventive Effect of Carvacrol against Diethylnitrosamine-Induced Hepatocellular Carcinoma in Rats. Mol. Cel Biochem. 360 (1–2), 51–60. 10.1007/s11010-011-1043-7 [DOI] [PubMed] [Google Scholar]
- Jung C. Y., Kim S. Y., Lee C. (2018). Carvacrol Targets AXL to Inhibit Cell Proliferation and Migration in Non-small Cell Lung Cancer Cells. Anticancer Res. 38 (1), 279–286. 10.21873/anticanres.12219 [DOI] [PubMed] [Google Scholar]
- Kang S.-H., Kim Y.-S., Kim E.-K., Hwang J.-W., Jeong J.-H., Dong X., et al. (2016). Anticancer Effect of Thymol on AGS Human Gastric Carcinoma Cells. J. Microbiol. Biotechnol. 26 (1), 28–37. 10.4014/jmb.1506.06073 [DOI] [PubMed] [Google Scholar]
- Karkabounas S., Kostoula O. K., Daskalou T., Veltsistas P., Karamouzis M., Zelovitis I., et al. (2006). Anticarcinogenic and Antiplatelet Effects of Carvacrol. Exp. Oncol. 28 (2), 121–125. 10.1080/01913123.2020.1740366 [DOI] [PubMed] [Google Scholar]
- Kelly K. (2009). The History of Medicine. The Middle Ages. Medicine: Stonehenge, 500–1450. [Google Scholar]
- Khan F., Khan I., Farooqui A., Ansari I. A. (2017). Carvacrol Induces Reactive Oxygen Species (ROS)-mediated Apoptosis along with Cell Cycle Arrest at G0/G1 in Human Prostate Cancer Cells. Nutr. Cancer 69 (7), 1075–1087. 10.1080/01635581.2017.1359321 [DOI] [PubMed] [Google Scholar]
- Khan F., Singh V. K., Saeed M., Kausar M. A., Ansari I. A. (2019). Carvacrol Induced Program Cell Death and Cell Cycle Arrest in Androgen-independent Human Prostate Cancer Cells via Inhibition of Notch Signaling. Anticancer Agents Med. Chem. 19 (13), 1588–1608. 10.2174/1871520619666190731152942 [DOI] [PubMed] [Google Scholar]
- Kim B., Srivastava S. K., Kim S.-H. (2015). Caspase-9 as a Therapeutic Target for Treating Cancer. Expert Opin. Ther. Targets 19 (1), 113–127. 10.1517/14728222.2014.961425 [DOI] [PubMed] [Google Scholar]
- Kim C., Kim B. (2018). Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 10 (8), 1021. 10.3390/nu10081021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. K., Choi E.-J. (2015). Compromised MAPK Signaling in Human Diseases: an Update. Arch. Toxicol. 89 (6), 867–882. 10.1007/s00204-015-1472-2 [DOI] [PubMed] [Google Scholar]
- Kocal G., Pakdemirli A. (2020). Antiproliferative Effects of Carvacrol on Neuroblastoma Cells. J. Dr Behcet Uz Child. Hosp. 10 (1), 61–64. 10.5222/buchd.2020.59251 [DOI] [Google Scholar]
- Koparal A. T., Zeytinoglu M. (2003). Effects of Carvacrol on a Human Non-small Cell Lung Cancer (NSCLC) Cell Line, A549. Cytotechnology 43 (1–3), 149–154. 10.1023/b:cyto.0000039917.60348.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss B., Greengard O. (1982). Effect of Neoplasms on the Content and Activity of Alkaline Phosphatase and Gamma-Glutamyl Transpeptidase in Uninvolved Host Tissues. Cancer Res. 42 (6), 2146–2158. [PubMed] [Google Scholar]
- Koyama H., Iwata H., Kuwabara Y., Iwase H., Kobayashi S., Fujii Y. (1990). Gelatinolytic Activity of Matrix Metalloproteinase-2 and -9 in Oesophageal Carcinoma; a Study Using In Situ Zymography. Eur. J. Cancer 36 (16), 2164–2170. 10.1016/s0959-8049(00)00297-5 [DOI] [PubMed] [Google Scholar]
- Krelin Y., Zhang L., Kang T.-B., Appel E., Kovalenko A., Wallach D. (2008). Caspase-8 Deficiency Facilitates Cellular Transformation In Vitro . Cell Death Differ. 15 (9), 1350–1355. 10.1038/cdd.2008.88 [DOI] [PubMed] [Google Scholar]
- Krens S. F. G., Spaink H. P., Snaar-Jagalska B. E. (2006). Functions of the MAPK Family in Vertebrate-Development. FEBS Lett. 580 (21), 4984–4990. 10.1016/j.febslet.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Kroemer G., Dallaporta B., Resche-Rigon M. (1998). The Mitochondrial Death/life Regulator in Apoptosis and Necrosis. Annu. Rev. Physiol. 60, 619–642. 10.1146/annurev.physiol.60.1.619 [DOI] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. (1977). The Measurement of Observer Agreement for Categorical Data. Biometrics 33 (1), 159–174. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- Lee D. J., Zeidner J. F. (2019). Cyclin-dependent Kinase (CDK) 9 and 4/6 Inhibitors in Acute Myeloid Leukemia (AML): a Promising Therapeutic Approach. Expert Opin. Investig. Drugs 28 (11), 989–1001. 10.1080/13543784.2019.1678583 [DOI] [PubMed] [Google Scholar]
- Lee H. S., Park S. W. (2016). Systemic Chemotherapy in Advanced Pancreatic Cancer. Gut Liver 10 (3), 340–347. 10.5009/gnl15465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. P., Kim J.-E., Park W.-H., Hong H. (2016). Regulation of C6 Glioma Cell Migration by Thymol. Oncol. Lett. 11 (4), 2619–2624. 10.3892/ol.2016.4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Yang M., Liao A., Zeng B., Liu D., Yao Y., et al. (2018). Linc00483 as ceRNA Regulates Proliferation and Apoptosis through Activating MAPKs in Gastric Cancer. J. Cel Mol Med. 22 (8), 3875–3886. 10.1111/jcmm.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Hu J., Wu S., Wang L., Cao X., Zhang X., et al. (2016). Auranofin-mediated Inhibition of PI3K/AKT/mTOR axis and Anticancer Activity in Non-small Cell Lung Cancer Cells. Oncotarget 7 (3), 3548–3558. 10.18632/oncotarget.6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., He L., Wu Y., Zhang Y. (2021). Carvacrol Affects Breast Cancer Cells through TRPM7 Mediated Cell Cycle Regulation. Life Sci. 266, 118894. 10.1016/j.lfs.2020.118894 [DOI] [PubMed] [Google Scholar]
- Li S., Zhao H., Bao L. (2019). Preliminary Study on the Mechanism of Carvacrol Regulating Hepatocellular Carcinoma Based on Network Pharmacology. Lett. Drug Des. Discov. 16 (11), 1286–1295. 10.2174/1570180816666190516105906 [DOI] [Google Scholar]
- Li Y., Wen J.-m., Du C.-j., Hu S.-m., Chen J.-x., Zhang S.-g., et al. (2017). Thymol Inhibits Bladder Cancer Cell Proliferation via Inducing Cell Cycle Arrest and Apoptosis. Biochem. Biophysical Res. Commun. 491 (2), 530–536. 10.1016/j.bbrc.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Li Y., Xu J. z., Gu C. x., Liu G. l., Tian K. (2018). Carvacrol Suppresses Inflammatory Responses in Rheumatoid Arthritis Fibroblast‐like Synoviocytes. J. Cel Biochem. 120 (5), 8169–8176. 10.1002/jcb.28098 [DOI] [PubMed] [Google Scholar]
- Liang W.-Z., Chou C.-T., Lu T., Chi C.-C., Tseng L.-L., Pan C.-C., et al. (2013). The Mechanism of Carvacrol-Evoked [Ca2+]i Rises and Non-ca2+-triggered Cell Death in OC2 Human Oral Cancer Cells. Toxicology 303, 152–161. 10.1016/j.tox.2012.10.026 [DOI] [PubMed] [Google Scholar]
- Liang W. Z., Lu C. H. (2012). Carvacrol-induced [Ca2+]i Rise and Apoptosis in Human Glioblastoma Cells. Life Sci. 90 (17–18), 703–711. 10.1016/j.lfs.2012.03.027 [DOI] [PubMed] [Google Scholar]
- Lim W., Ham J., Bazer F. W., Song G. (2019). Carvacrol Induces Mitochondria-Mediated Apoptosis via Disruption of Calcium Homeostasis in Human Choriocarcinoma Cells. J. Cel Physiol. 234 (2), 1803–1815. 10.1002/jcp.27054 [DOI] [PubMed] [Google Scholar]
- Lin S.-C., Lo S.-S., Liu C.-J., Chung M.-Y., Huang J.-W., Chang K.-W. (2004). Functional Genotype in Matrix Metalloproteinases-2 Promoter Is a Risk Factor for Oral Carcinogenesis. J. Oral Pathol. Med. 33 (7), 405–409. 10.1111/j.1600-0714.2004.00231.x [DOI] [PubMed] [Google Scholar]
- Liu J., Wu N., Ma L.-N., Zhong J.-T., Liu G., Zheng L.-H., et al. (2014). p38 MAPK Signaling Mediates Mitochondrial Apoptosis in Cancer Cells Induced by Oleanolic Acid. Asian Pac. J. Cancer Prev. 15 (11), 4519–4525. 10.7314/apjcp.2014.15.11.4519 [DOI] [PubMed] [Google Scholar]
- Liu W.-W., Zeng Z.-Y., Wu Q.-L., Hou J.-H., Chen Y.-Y. (2005). Overexpression of MMP-2 in Laryngeal Squamous Cell Carcinoma: a Potential Indicator for Poor Prognosis. Otolaryngol. Head Neck Surg. 132 (3), 395–400. 10.1016/j.otohns.2004.09.050 [DOI] [PubMed] [Google Scholar]
- Llana-Ruiz-Cabello M., Gutiérrez-Praena D., Pichardo S., Moreno F. J., Bermúdez J. M., Aucejo S., et al. (2014). Cytotoxicity and Morphological Effects Induced by Carvacrol and Thymol on the Human Cell Line Caco-2. Food Chem. Toxicol. 64, 281–290. 10.1016/j.fct.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Llana-Ruiz-Cabello M., Gutiérrez-Praena D., Puerto M., Pichardo S., Jos Á., Cameán A. M. (2015). In Vitro pro-oxidant/antioxidant Role of Carvacrol, Thymol and Their Mixture in the Intestinal Caco-2 Cell Line. Toxicol. Vitro 29 (4), 647–656. 10.1016/j.tiv.2015.02.006 [DOI] [PubMed] [Google Scholar]
- Low H. B., Zhang Y. (2016). Regulatory Roles of MAPK Phosphatases in Cancer. Immune Netw. 16 (2), 85–98. 10.4110/in.2016.16.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. C., Tsay S. L., Chang S. Y., Chen C. M., Liu C. Y. (2019). Daily Activity, Mood, and Quality of Life in Colorectal Cancer Patients with Chemotherapy‐induced Peripheral Neuropathy: A Mediation Effect Analysis. Cancer Med. 8 (3), 963–971. 10.1002/cam4.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Wu J.-Y., Lu M.-H., Shi Z., Na N., Di J.-M. (2016). Carvacrol Alleviates Prostate Cancer Cell Proliferation, Migration, and Invasion through Regulation of PI3K/Akt and MAPK Signaling Pathways. Oxidative Med. Cell Longevity 2016, 1–11. 10.1155/2016/1469693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari A., Mani G., Nagabhishek S. N., Balaraman G., Subramanian N., Mirza F. B., et al. (2020). Carvacrol Promotes Cell Cycle Arrest and Apoptosis through PI3K/AKT Signaling Pathway in MCF-7 Breast Cancer Cells. Chin. J. Integr. Med. 12, 133. 10.1007/s11655-020-3193-5 [DOI] [PubMed] [Google Scholar]
- Martino E., Casamassima G., Castiglione S., Cellupica E., Pantalone S., Papagni F., et al. (2018). Vinca Alkaloids and Analogues as Anti-cancer Agents: Looking Back, Peering Ahead. Bioorg. Med. Chem. Lett. 28 (17), 2816–2826. 10.1016/j.bmcl.2018.06.044 [DOI] [PubMed] [Google Scholar]
- Marullo R., Werner E., Degtyareva N., Moore B., Altavilla G., Ramalingam S. S., et al. (2013). Cisplatin Induces a Mitochondrial-ROS Response that Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PloS one 8 (11), e81162. 10.1371/journal.pone.0081162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryam K., Shakeri S., Kiani K. (2015). Preparation and In Vitro Investigation of Antigastric Cancer Activities of Carvacrol‐loaded Human Serum Albumin Nanoparticles. IET nanobiotechnol. 9 (5), 294–299. 10.1049/iet-nbt.2014.0040 [DOI] [PubMed] [Google Scholar]
- Mehdi S., Ahmad A., Irshad M., Manzoor N., Rizvi M. (2011). Cytotoxic Effect of Carvacrol on Human Cervical Cancer Cells. Biol. Med. 3 (2), 307–312. 10.26226/morressier.5770e29ad462b80290b4ba57 [DOI] [Google Scholar]
- Melušová M., Jantová S., Horváthová E. (2014). Carvacrol and Rosemary Oil at Higher Concentrations Induce Apoptosis in Human Hepatoma HepG2 Cells. Interdiscip. Toxicol. 7 (4), 189–194. 10.2478/intox-2014-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melusova M., Slamenova D., Kozics K., Jantova S., Horvathova E. (2014). Carvacrol and Rosemary Essential Oil Manifest Cytotoxic, DNA-Protective and Pro-apoptotic Effect Having No Effect on DNA Repair. Neoplasma 61 (6), 690–699. 10.4149/neo_2014_084 [DOI] [PubMed] [Google Scholar]
- Mishra B. B., Tiwari V. K. (2011). Natural Products: an Evolving Role in Future Drug Discovery. Eur. J. Med. Chem. 46 (10), 4769–4807. 10.1016/j.ejmech.2011.07.057 [DOI] [PubMed] [Google Scholar]
- Nagase H., Visse R., Murphy G. (2006). Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 69 (3), 562–573. 10.1016/j.cardiores.2005.12.002 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (2019). National Cancer Institute. Available at: https://www.inca.gov.br/estimativa/introducao (Acesseced November 13, 2020).
- Nelson R. L. (1982). The Comparative Clinical Pharmacology and Pharmacokinetics of Vindesine, Vincristine, and Vinblastine in Human Patients with Cancer. Med. Pediatr. Oncol. 10 (2), 115–127. 10.1002/mpo.2950100202 [DOI] [PubMed] [Google Scholar]
- Neville B. (2011). Patologia Oral e Maxilofacial. Amsterdam: Elsevier Brasil, 3475. [Google Scholar]
- O'Donnell J. S., Massi D., Teng M. W. L., Mandala M. (2018). PI3K-AKT-mTOR Inhibition in Cancer Immunotherapy, Redux. Semin. Cancer Biol. 48, 91–103. 10.1016/j.semcancer.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Olsson M., Zhivotovsky B. (2011). Caspases and Cancer. Cel Death Differ 18 (9), 1441–1449. 10.1038/cdd.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. (2016). Rayyan-a Web and mobile App for Systematic Reviews. Syst. Rev. 5 (1), 1–10. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Shankar R., Leopold C., Orubu S. (2019). Access to Medicines through Health Systems in Low- and Middle-Income Countries. Health Policy Plan 34, iii1–iii3. 10.1093/heapol/czz119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkan A., Erdogan A. (2011). A Comparative Evaluation of Antioxidant and Anticancer Activity of Essential Oil from Origanum Onites (Lamiaceae) and its Two Major Phenolic Components. Turk J. Biol. 35 (2011), 735–742. 10.1016/j.toxlet.2010.03.459 [DOI] [Google Scholar]
- Ozkan A., Erdogan A. (2012). A Comparative Study of the Antioxidant/prooxidant Effects of Carvacrol and Thymol at Various Concentrations on Membrane and DNA of Parental and Drug Resistant H1299 Cells. Nat. Prod. Commun. 7 (12), 1557–1560. 10.1177/1934578x1200701201 [DOI] [PubMed] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. Plos Med. 18 (3), e1003583. 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakdemirli A., Karaca C., Sever T., Daşkin E., Leblebici A., Yiğitbaşi T., et al. (2020). Carvacrol Alters Soluble Factors in HCT-116 and HT-29 Cell Lines. Turk J. Med. Sci. 50 (1), 271–276. 10.3906/sag-1907-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicchi A., Pompella A., Tonarelli P., Gadducci A., Genazzani A. R., Zunino F., et al. (1996). Gamma-glutamyltranspeptidase Activity in Human Ovarian Carcinoma. Anticancer Res. 16 (5B), 3053–3058. [PubMed] [Google Scholar]
- Pathania A. S., Guru S. K., Verma M. K., Sharma C., Abdullah S. T., Malik F., et al. (2013). Disruption of the PI3K/AKT/mTOR Signaling cascade and Induction of Apoptosis in HL-60 Cells by an Essential Oil from Monarda Citriodora. Food Chem. Toxicol. 62, 246–254. 10.1016/j.fct.2013.08.037 [DOI] [PubMed] [Google Scholar]
- Pernas S., Tolaney S. M., Winer E. P., Goel S. (2018). CDK4/6 Inhibition in Breast Cancer: Current Practice and Future Directions. Ther. Adv. Med. Oncol. 10, 175883591878645. 10.1177/1758835918786451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompella A., De Tata V., Paolicchi A., Zunino F. (2006). Expression of γ-glutamyltransferase in Cancer Cells and its Significance in Drug Resistance. Biochem. Pharmacol. 71 (3), 231–238. 10.1016/j.bcp.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Potočnjak I., Gobin I., Domitrović R. (2018). Carvacrol Induces Cytotoxicity in Human Cervical Cancer Cells but Causes Cisplatin Resistance: Involvement of MEK-ERK Activation. Phytother Res. 32 (6), 1090–1097. 10.1002/ptr.6048 [DOI] [PubMed] [Google Scholar]
- Powell H. A., Tata L. J., Baldwin D. R., Potter V. A., Stanley R. A., Khakwani A., et al. (2014). Treatment Decisions and Survival for People with Small-Cell Lung Cancer. Br. J. Cancer 110 (4), 908–915. 10.1038/bjc.2013.812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Gupta S. C., Tyagi A. K. (2017). Reactive Oxygen Species (ROS) and Cancer: Role of Antioxidative Nutraceuticals. Cancer Lett. 387, 95–105. 10.1016/j.canlet.2016.03.042 [DOI] [PubMed] [Google Scholar]
- Riaz S. K., Iqbal Y., Malik M. F. A. (2015). Diagnostic and Therapeutic Implications of the Vascular Endothelial Growth Factor Family in Cancer. Asian Pac. J. Cancer Prev. 16 (5), 1677–1682. 10.7314/apjcp.2015.16.5.1677 [DOI] [PubMed] [Google Scholar]
- Rojas-Armas J. P., Arroyo-Acevedo J. L., Palomino-Pacheco M., Herrera-Calderón O., Ortiz-Sánchez J. M., Rojas-Armas A., et al. (2020). The Essential Oil of Cymbopogon Citratus Stapt and Carvacrol: An Approach of the Antitumor Effect on 7,12-Dimethylbenz-[α]-Anthracene (DMBA)-Induced Breast Cancer in Female Rats. Molecules 25 (14), 3284. 10.3390/molecules25143284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Mishra A. P., Shukla I., Sharifi-Rad M., Contreras M. d. M., Segura-Carretero A., et al. (2018). Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytotherapy Res. 32 (9), 1688–1706. 10.1002/ptr.6109 [DOI] [PubMed] [Google Scholar]
- Santos C. P. d., Pinto J. A. O., Santos C. A. d., Cruz E. M. O., Arrigoni-Blank M. d. F., Andrade T. M., et al. (2016). Harvest Time and Geographical Origin Affect the Essential Oil of Lippia Gracilis Schauer. Ind. Crops Prod. 79, 205–210. 10.1016/j.indcrop.2015.11.015 [DOI] [Google Scholar]
- Satooka H., Kubo I. (2012). Effects of Thymol on B16-F10 Melanoma Cells. J. Agric. Food Chem. 60 (10), 2746–2752. 10.1021/jf204525b [DOI] [PubMed] [Google Scholar]
- Semenza G. L. (2003). Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 3 (10), 721–732. 10.1038/nrc1187 [DOI] [PubMed] [Google Scholar]
- Seresht H. R., Albadry B. J., Al-mosawi A. K. M., Gholami O., Cheshomi H. (2019). The Cytotoxic Effects of Thymol as the Major Component of Trachyspermum Ammi on Breast Cancer (MCF-7) Cells. Pharm. Chem. J. 53 (2), 101–107. 10.1007/s11094-019-01961-w [DOI] [Google Scholar]
- Shalini S., Dorstyn L., Dawar S., Kumar S. (2015). Old, New and Emerging Functions of Caspases. Cel Death Differ 22 (4), 526–539. 10.1038/cdd.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M., Varoni E. M., Iriti M., Martorell M., Setzer W. N., Del Mar Contreras M., et al. (2018). Carvacrol and Human Health: A Comprehensive Review. Phytotherapy Res. 32 (9), 1675–1687. 10.1002/ptr.6103 [DOI] [PubMed] [Google Scholar]
- Sheorain J., Mehra M., Thakur R., Grewal S., Kumari S. (2019). In Vitro anti-inflammatory and Antioxidant Potential of Thymol Loaded Bipolymeric (Tragacanth Gum/chitosan) Nanocarrier. Int. J. Biol. Macromolecules 125, 1069–1074. 10.1016/j.ijbiomac.2018.12.095 [DOI] [PubMed] [Google Scholar]
- Shojaei S., Kiumarsi A., Moghadam A. R., Alizadeh J., Marzban H., Ghavami S. (2014). “Perillyl Alcohol (Monoterpene Alcohol), Limonene,” in The Enzymes Natural Products and Cancer Signaling: Isoprenoids, Polyphenols and Flavonoids. Editors Zahra Bathaie S., Tamanoi F. (Cambridge: Academic Press; ), 1–258. 10.1016/B978-0-12-802215-3.00002-1 [DOI] [PubMed] [Google Scholar]
- Shuman Moss L. A., Jensen-Taubman S., Stetler-Stevenson W. G. (2012). Matrix Metalloproteinases. Am. J. Pathol. 181 (6), 1895–1899. 10.1016/j.ajpath.2012.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E. R., de Carvalho F. O., Teixeira L. G. B., Santos N. G. L., Felipe F. A., Santana H. S. R., et al. (2018). Pharmacological Effects of Carvacrol in In Vitro Studies: A Review. Curr. Pharm. Des. 24 (29), 3454–3465. 10.2174/1381612824666181003123400 [DOI] [PubMed] [Google Scholar]
- Sisto F., Carradori S., Guglielmi P., Spano M., Secci D., Granese A., et al. (2021). Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line. Molecules 26 (7), 1829. 10.3390/molecules26071829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto F., Carradori S., Guglielmi P., Traversi C. B., Spano M., Sobolev A. P., et al. (2020). Synthesis and Biological Evaluation of Carvacrol-Based Derivatives as Dual Inhibitors of H. pylori Strains and AGS Cell Proliferation. Pharmaceuticals 13 (11), 405. 10.3390/ph13110405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaranjani A., Sivagami G., Nalini N. (2016). Chemopreventive Effect of Carvacrol on 1,2-dimethylhydrazine Induced Experimental colon Carcinogenesis. J. Cancer Res. Ther. 12 (2), 755–762. 10.4103/0973-1482.154925 [DOI] [PubMed] [Google Scholar]
- Siveen K. S., Prabhu K., Krishnankutty R., Kuttikrishnan S., Tsakou M., Alali F. Q., et al. (2017). Vascular Endothelial Growth Factor (VEGF) Signaling in Tumour Vascularization: Potential and Challenges. Curr. Vasc. Pharmacol. 15 (4), 339–351. 10.2174/1570161115666170105124038 [DOI] [PubMed] [Google Scholar]
- Slamenová D., Horváthová E., Sramková M., Marsálková L. (2007). DNA-protective Effects of Two Components of Essential Plant Oils Carvacrol and Thymol on Mammalian Cells Cultured In Vitro . Neoplasma 54 (2), 108–112. 10.7287/peerj.9626v0.1/reviews/1 [DOI] [PubMed] [Google Scholar]
- Soković M., Glamoclija J., Ciric A. (2013). Natural Products from Plants and Fungi as Fungicides. Fungicides—Showcases of Integrated Plant Disease. Management from Around the World. Chapter: Natural Products from Plants and Fungi as Fungicides. Berlin: Springer, 185–232. 10.5772/50277 [DOI] [Google Scholar]
- Song H.-Y., Deng X.-H., Yuan G.-Y., Hou X.-F., Zhu Z.-D., Zhou L., et al. (2014). Expression of Bcl-2 and P53 in Induction of Esophageal Cancer Cell Apoptosis by ECRG2 in Combination with Cisplatin. Asian Pac. J. Cancer Prev. 15 (3), 1397–1401. 10.7314/apjcp.2014.15.3.1397 [DOI] [PubMed] [Google Scholar]
- Song M., Bode A. M., Dong Z., Lee M.-H. (2019). AKT as a Therapeutic Target for Cancer. Cancer Res. 79 (6), 1019–1031. 10.1158/0008-5472.CAN-18-2738 [DOI] [PubMed] [Google Scholar]
- Soung Y. H., Lee J. W., Kim S. Y., Jang J., Park Y. G., Park W. S., et al. (2005). CASPASE-8 Gene Is Inactivated by Somatic Mutations in Gastric Carcinomas. Cancer Res. 65 (3), 815–821. 10.1038/sj.onc.1208244 [DOI] [PubMed] [Google Scholar]
- Srinivas U. S., Tan B. W. Q., Vellayappan B. A., Jeyasekharan A. D. (2019). ROS and the DNA Damage Response in Cancer. Redox Biol. 25, 101084. 10.1016/j.redox.2018.101084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastav V. K., Egbuna C., Tiwari M. (2020). Plant Secondary Metabolites as lead Compounds for the Production of Potent Drugs. Phytochemicals as Lead Comp. New Drug 14, 3–14. 10.1016/B978-0-12-817890-4.00001-9 [DOI] [Google Scholar]
- Subramaniyan J., Krishnan G., Balan R., Mgj D., Ramasamy E., Ramalingam S., et al. (2014). Carvacrol Modulates Instability of Xenobiotic Metabolizing Enzymes and Downregulates the Expressions of PCNA, MMP-2, and MMP-9 during Diethylnitrosamine-Induced Hepatocarcinogenesis in Rats. Mol. Cel Biochem. 395 (1), 65–76. 10.1007/s11010-014-2112-5 [DOI] [PubMed] [Google Scholar]
- Suntres Z. E., Coccimiglio J., Alipour M. (2015). The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 55 (3), 304–318. 10.1080/10408398.2011.653458 [DOI] [PubMed] [Google Scholar]
- Swanton E., Savory P., Cosulich S., Clarke P., Woodman P. (1999). Bcl-2 Regulates a Caspase-3/caspase-2 Apoptotic cascade in Cytosolic Extracts. Oncogene 18 (10), 1781–1787. 10.1038/sj.onc.1202490 [DOI] [PubMed] [Google Scholar]
- Tayarani-Najaran Z., Akaberi M., Hassanzadeh B., Shirazi N., Asili J., Al-Najjar H., et al. (2019). Analysis of the Essential Oils of Five Artemisia Species and Evaluation of Their Cytotoxic and Proapoptotic Effects. Mini Rev. Med. Chem. 19 (11), 902–912. 10.2174/1389557519666190311155021 [DOI] [PubMed] [Google Scholar]
- Thakor P., Subramanian R. B., Thakkar S. S., Ray A., Thakkar V. R. (2017). Phytol Induces ROS Mediated Apoptosis by Induction of Caspase 9 and 3 through Activation of TRAIL, FAS and TNF Receptors and Inhibits Tumor Progression Factor Glucose 6 Phosphate Dehydrogenase in Lung Carcinoma Cell Line (A549). Biomed. Pharmacother. 92, 491–500. 10.1016/j.biopha.2017.05.066 [DOI] [PubMed] [Google Scholar]
- Thapa D., Richardson A. J., Zweifel B., Wallace R. J., Gratz S. W. (2019). Genoprotective Effects of Essential Oil Compounds against Oxidative and Methylated DNA Damage in Human Colon Cancer Cells. J. Food Sci. 84 (7), 1979–1985. 10.1111/1750-3841.14665 [DOI] [PubMed] [Google Scholar]
- Trindade G. G. G., Thrivikraman G., Menezes P. P., França C. M., Lima B. S., Carvalho Y. M. B. G., et al. (2019). Carvacrol/β-cyclodextrin Inclusion Complex Inhibits Cell Proliferation and Migration of Prostate Cancer Cells. Food Chem. Toxicol. 125, 198–209. 10.1016/j.fct.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Van Wyk B., Wink M. (2018). Medicinal Plants of the World. Germany: CABI. University of Johannesburg, South Africa, Heidelberg University. [Google Scholar]
- Wagner E. F., Nebreda Á. R. (2009). Signal Integration by JNK and P38 MAPK Pathways in Cancer Development. Nat. Rev. Cancer 9 (8), 537–549. 10.1038/nrc2694 [DOI] [PubMed] [Google Scholar]
- Wang C., Jin H., Gao D., Lieftink C., Evers B., Jin G., et al. (2018). Phospho-ERK Is a Biomarker of Response to a Synthetic Lethal Drug Combination of Sorafenib and MEK Inhibition in Liver Cancer. J. Hepatol. 69 (5), 1057–1065. 10.1016/j.jhep.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Wang J., Su B., Zhu H., Chen C., Zhao G. (2016). Protective Effect of Geraniol Inhibits Inflammatory Response, Oxidative Stress and Apoptosis in Traumatic Injury of the Spinal Cord through Modulation of NF-Κb and P38 MAPK. Exp. Ther. Med. 12 (6), 3607–3613. 10.3892/etm.2016.385010.3892/etm.2016.3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang Q. (2018). Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity. Can. J. Gastroenterol. Hepatol. 2018, e9049252. 10.1155/2018/9049252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., He Q.-Y., Chiu J.-F. (2014). Dioscin Induced Activation of P38 MAPK and JNK via Mitochondrial Pathway in HL-60 Cell Line. Eur. J. Pharmacol. 735, 52–58. 10.1016/j.ejphar.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Weingart S., Zhang L., Sweeney M., Hassett M. (2018). Chemotherapy Medication Errors. Lancet Oncol. 19 (4), 191–199. 10.1016/S1470-2045(18)30094-9 [DOI] [PubMed] [Google Scholar]
- Whitfield J. B. (2001). Gamma Glutamyl Transferase. Crit. Rev. Clin. Lab. Sci. 38 (4), 263–355. 10.1080/20014091084227 [DOI] [PubMed] [Google Scholar]
- WHO (2007-2017). Ten Years in Public Health 2007-2017. World Health Organization; Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Available at: http://www.who.int/publications/10-year-review/en/ (Acesseced November 20, 2020).
- Wiart C. (2007). Ethnopharmacology of Medicinal Plants—Asia and the Pacific | Christophe Wiart | Springer. Available at: https://www.springer.com/gp/book/9781588297488 (Acesseced December 21, 2020).
- Wong R. J., Ahmed A., Gish R. G. (2015). Elevated Alpha-Fetoprotein. Clin. Liver Dis. 19 (2), 309–323. 10.1016/j.cld.2015.01.005 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020). Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Available at: https://www.who.int/publications/i/item/who-report-on-cancer-setting-priorities-investing-wisely-and-providing-care-for-all (Acesseced November 13, 2020).
- Wu Q., Wu W., Fu B., Shi L., Wang X., Kuca K. (2019). JNK Signaling in Cancer Cell Survival. Med. Res. Rev. 39 (6), 2082–2104. 10.1002/med.21574 [DOI] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M. N. (2006). TOR Signaling in Growth and Metabolism. Cell 124 (3), 471–484. 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Yeh J.-H., Chou C-T., Chen I-S., Lu T., Lin K-L., Yu C-C., et al. (2017). Effect of Thymol on Ca^(2+) Homeostasis and Viability in PC3 Human Prostate Cancer Cells. Chin. J. Physiol. 60 (1), 32–40. 10.4077/CJP.2017.BAF447 [DOI] [PubMed] [Google Scholar]
- Yin Q.-h., Yan F.-x., Zu X.-Y., Wu Y.-h., Wu X.-p., Liao M.-c., et al. (2012). Anti-proliferative and Pro-apoptotic Effect of Carvacrol on Human Hepatocellular Carcinoma Cell Line HepG-2. Cytotechnology 64 (1), 43–51. 10.1007/s10616-011-9389-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J. y., Kim C. H., Song S. H., Shim B. Y., Jeong Y. J., Ahn M. I., et al. (2004). Expression of Caspase-3 and C-Myc in Non-small Cell Lung Cancer. Cancer Res. Treat. 36 (5), 303–307. 10.4143/crt.2004.36.5.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zhang R., Yang T., Zhang M., Xi K., Lin Y., et al. (2019). Alpha-l-fucosidase: a Novel Serum Biomarker to Predict Prognosis in Early Stage Esophageal Squamous Cell Carcinoma. J. Thorac. Dis. 11 (9), 3980–3990. 10.21037/jtd.2019.08.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarubin T., Han J. (2005). Activation and Signaling of the P38 MAP Kinase Pathway. Cell Res. 15 (1), 11–18. 10.1038/sj.cr.7290257 [DOI] [PubMed] [Google Scholar]
- Zeng L. P., Hu Z. M., Li K., Xia K. (2016). miR‐222 Attenuates Cisplatin‐induced Cell Death by Targeting the PPP 2R2A/Akt/mTOR Axis in Bladder Cancer Cells. J. Cel. Mol. Med. 20 (3), 559–567. 10.1111/jcmm.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q., Che Y., Zhang Y., Chen M., Guo Q., Zhang W. (2020). Thymol Isolated from Thymus Vulgaris L. Inhibits Colorectal Cancer Cell Growth and Metastasis by Suppressing the Wnt/β-Catenin Pathway. Drug Des. Devel Ther. 14, 2535–2547. 10.2147/DDDT.S25421810.2147/dddt.s254218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeytinoglu H., Incesu Z., Baser K. H. C. (2003). Inhibition of DNA Synthesis by Carvacrol in Mouse Myoblast Cells Bearing a Human N-RAS Oncogene. Phytomedicine 10 (4), 292–299. 10.1078/094471103322004785 [DOI] [PubMed] [Google Scholar]
- Zhai C.-L., Tang G.-M., Qian G., Hu H.-L., Wang S.-J., Yin D., et al. (2019). MicroRNA-98 Attenuates Cardiac Ischemia-Reperfusion Injury through Inhibiting DAPK1 Expression. IUBMB Life 71 (2), 166–176. 10.1002/iub.1879 [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu H., Iles K. E., Liu R.-M., Postlethwait E. M., Laperche Y., et al. (2006). 4-Hydroxynonenal Induces Rat γ-Glutamyl Transpeptidase through Mitogen-Activated Protein Kinase-Mediated Electrophile Response Element/Nuclear Factor Erythroid 2-Related Factor 2 Signaling. Am. J. Respir. Cel Mol Biol 34 (2), 174–181. 10.1165/rcmb.2005-0280OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yu J., Park B., Kinzler K., Vogelstein B. (2000). Role of BAX in the Apoptotic Response to Anticancer Agents. Science 290 (5493), 989–992. 10.1126/science.290.5493.989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.