Abstract
Genetic predisposition to heavy drinking is a risk factor for alcohol misuse. We used selectively bred crossed high alcohol-preferring (cHAP) mice to study sex differences in alcohol drinking and its effect on glutamatergic activity in dorsolateral (DLS) and dorsomedial (DMS) striatum. We performed whole-cell patch-clamp recording in neurons from male and female cHAP mice with 5-week alcohol drinking history and alcohol-naïve controls. In DMS, alcohol-naïve males’ neurons displayed lower cell capacitance and higher membrane resistance than females’ neurons, both effects reversed by drinking. Conversely, in DLS neurons, drinking history increased capacitance only in males and changed membrane resistance only in females. Altered biophysical membrane properties were accompanied by disrupted glutamatergic transmission. Drinking history increased spontaneous excitatory postsynaptic current (sEPSC) amplitude in DMS and frequency in DLS female neurons, compared to alcohol-naïve females, without effect in males. Acute ethanol differentially impacted DMS and DLS neurons by sex and drinking history. In DMS, acute alcohol significantly increased sEPSC frequency only in neurons from alcohol-naïve females, an effect that disappeared after drinking history. In DLS, acute alcohol had opposing effects in males and females based on drinking history. Estrous cycle also impacted DMS and DLS neurons differently: sEPSC amplitudes were higher in DMS cells from drinking history than alcohol-naïve females, whereas estrous cycle, not drinking history, modified DLS firing rate. Our data show sex differences in cHAP ethanol consumption and neurophysiology, suggesting differential dysregulation of glutamatergic drive onto DMS and DLS after chronic ethanol consumption.
1. Introduction
Alcohol is the most widely misused drug in the United States. According to the National Survey on Drug Use and Health in 2019, 6.3% of American adults (18 and older) reported engagement in heavy alcohol use, and 5.6% of this age group had alcohol use disorder (AUD). AUD has been defined as a chronic, relapsing brain disease characterized by the impaired ability to stop or control the use of alcohol despite the adverse consequences (NIAAA, 2020).
The emergence and persistence of AUD result from long-lasting alcohol-induced changes in the brain. Alcohol produces a variety of neuroadaptations that contribute to the development of alcohol misuse. Chronic alcohol exposure not only affects excitatory and inhibitory transmission, but also alters the release of many neuromodulators, disrupting neuronal connectivity and synaptic function in multiple brain structures (Wilcox et al., 2014; Pleil et al., 2015; Ma et al., 2018; Gerchen et al., 2019; McGinnis et al., 2020; also see Abrahao et al., 2017). One brain region crucially involved in alcohol misuse is the striatum, the largest nucleus of the basal ganglia, which is comprised almost entirely of GABAergic medium-sized spiny neurons (MSNs), accounting for as much as 95% of the neuronal population (Smith and Bolam, 1990; Gerfen and Wilson, 1996). The striatum receives projections from virtually all areas of the cerebral cortex, activating MSNs through a topographically organized glutamatergic projection system (Lei et al., 2004; Reiner et al., 2010). Notably, the dorsal part of the striatum, originally considered a motor structure, performs important reward-related functions, including motivated behavior and substance misuse (Lovinger and Alvarez, 2017; Nonomura et al., 2018). Anatomically, the dorsal striatum is subdivided into dorsomedial striatum (DMS) and dorsolateral striatum (DLS), which play distinct roles in alcohol use. The DMS mainly engages in goal-directed behavior (Balleine and O’Doherty, 2010) and is hypothesized to contribute to alcohol seeking (Wang et al., 2015; Ma et al., 2018), whereas the DLS plays a key role in habit formation (Jog et al., 1999; Balleine and O’Doherty, 2010) and, therefore, might contribute to compulsive use of alcohol. Accordingly, prolonged alcohol self-administration in rodents produces habit-like responding with a gradual shift from DMS to DLS control (Corbit et al., 2012). Similarly, human studies have shown an overreliance on stimulus-response habit learning in alcohol-dependent patients as compared with healthy controls (Sjoerds et al., 2013). Habitual responses are inflexible, and it has been suggested that compulsive drive to seek drugs, including alcohol, is a form of adapted habit (Everitt and Robbins, 2005; Nelson and Killcross, 2006; Lüscher et al., 2020). Understanding how drugs of abuse alter neuronal function to promote compulsive seeking is a central goal of addiction research.
An important consideration is that the effects of misused drugs on neural circuits can be influenced by genetics, particularly in alcohol dependence. Genetic determinants of alcohol misuse have long been a focus of alcohol research, due to ample evidence for genetic contribution to AUD. For instance, a history of AUD in first-degree relatives confers greater risk to develop disordered drinking (Walters, 2002; Agrawal and Lynskey, 2008). Interestingly, the risk of offspring developing AUD significantly increases if the disorder is present in both parents (Mellentin et al., 2016; Yoon et al., 2013). Similarly, multigenerational family history of AUD is highly associated with more severe recurrence of alcohol misuse (Moss et al., 2007). Because genetic predisposition to drink alcohol is an important feature of AUD, preclinical rodent models based on genetic selection for alcohol preference are a meaningful tool to understand the bases of alcohol misuse.
Selective breeding involves screening offspring for high and low alcohol consumption or preference, with those at the extremes of the distribution selected for mating over multiple generations (see Bell et al., 2017). Genetic selection has successfully mimicked some aspects of AUD in rodents (Bell et al., 2017; Hansen and Spuhler, 1984; Li et al., 1987; Oberlin et al., 2011; Matson and Grahame, 2013). In mice, for example, multiple lines of low and high alcohol-preferring (LAP and HAP) mice have been selectively bred, all of which consistently self-administer pharmacologically relevant quantities of alcohol that correlate with high blood ethanol concentrations (BEC) (Oberlin et al., 2011; Matson and Grahame, 2013). Two HAP progenitor lines, HAP1 and HAP2, were reciprocally crossed to generate the crossed HAP (cHAP) line (Oberlin et al., 2011), yielding a useful phenotype for high alcohol consumption. The alcohol intakes reported in the cHAP line are among the highest known in murine models of alcoholism, exceeding alcohol consumption from both progenitor lines in four generations (Oberlin et al., 2011). In addition, cHAP mice have been shown to develop functional tolerance and to reach BECs from voluntary drinking comparable to those targeted in forced alcohol exposure paradigms, like alcohol vapor exposure, to produce alcohol dependence (Oberlin et al., 2011; Matson and Grahame, 2013; Lopez et al., 2017). Despite understanding the behavioral impact of genetic selection on alcohol-related behaviors, preexisting differences in neural function in these genetic lines of alcohol-preferring mice are poorly understood.
Recent evidence has shown dysregulated neurotransmission in alcohol-naïve HAP mice, exhibiting altered neuronal excitability in the striatum, particularly in DLS, when compared to LAP mice, suggesting basal differences in excitatory transmission (Fritz et al., 2019). Although cHAP mice have not been physiologically characterized, they display behavioral phenotypes suggestive of enhanced reliance on DLS function in the areas of habit-like behavior and drinking despite negative consequences. Even acute administration of alcohol is sufficient to promote the expression of habit-like behavior in cHAP mice (Houck and Grahame, 2018). Furthermore, they willingly drink alcohol containing the bitter tastant quinine, with alcohol experience increasing tolerance to very high levels of quinine, suggesting that cHAP mice have an innate aversion resistance (Houck et al., 2019). The underlying mechanisms driving this compulsive-like behavior in cHAP mice remain unknown.
Identifying aspects of neurophysiology resulting in vulnerabilities to compulsively consume alcohol may be key to finding new therapies to treat AUD. Given the propensity to develop habit-like behavior and compulsive-like drinking, we sought to investigate physiological properties of glutamatergic transmission in DMS and DLS neurons of cHAP mice and determine whether sex or alcohol drinking history produced different patterns of neuronal activity. We hypothesized that bath application of alcohol would acutely change spontaneous excitatory postsynaptic currents (sEPSCs) in alcohol-naïve cHAP mice, and that chronic alcohol drinking would alter neuronal function. Given that female cHAP mice consume more alcohol than their male counterparts (Oberlin et al., 2011), we also theorized that sex differences may exist in DMS or DLS function in cHAPs whether alcohol-naïve or with a chronic drinking history.
2. Materials and Methods
2.1. Subjects
Adult (9–13 weeks old) male and female cHAP mice, bred on site, were single housed and kept under controlled conditions of humidity and temperature, with water and food available ad libitum, and maintained on a 12-h reverse light/dark cycle. Details about the derivation and characterization of cHAP mice were published previously (Oberlin et al., 2011). The experimental procedures were carried out in accordance with the IUPUI School of Sciences Animal Care and Use Committee and adhered to guidelines set forth by the National Institutes of Health.
2.2. Two-Bottle Choice Alcohol self-administration
Forty-seven mice were randomly assigned to either the control group (0W; n=11 per sex) or the alcohol exposure group (5W; n=12 female, n=13 male), counterbalanced by sex and family. Mice from both groups were single housed in parallel and handled once a week for the cage change and for the recording of body weight, so that drinking fluid access (described below) was the only difference between the groups. Mice in the control group had a single water bottle available for the 5 weeks prior to recording. Mice in the alcohol exposure group had 5 weeks of free access to alcohol according to the two-bottle choice (TBC) paradigm: mice were provided continuous home cage access to two bottles, one containing ethanol (10% v/v; EtOH 190 proof, Decon Labs, Inc.) and one containing regular tap water. Each bottle was filled with 20–30 mL of drinking solution that was replenished every other day. At the start of the dark cycle (10:00 am), the consumption from each bottle was recorded three times a week for a total duration of five weeks. The position of the bottles was alternated every other day as a control for positional preference. The starting date of TBC was staggered so that the end of treatment aligned with the electrophysiological experiment day, as only one animal per day was euthanized for whole-cell patch-clamp recordings. Similarly, control mice were individually housed for the same duration prior to recording as alcohol-exposed mice.
2.3. Whole-cell patch-clamp electrophysiology
2.3.1. Brain slice preparation
Mice were deeply anesthetized with isoflurane (~20 s), decapitated, and brains were rapidly removed from the skull and immediately placed in oxygenated (95% O2/5% CO2) ice-cold cutting solution containing, in mM: 194 Sucrose, 30 NaCl, 4.5 KCl, 1.2 NaH2PO4, 26 NaHCO3, 1 MgCl2, 10 glucose (pH 7.4, ~310 mOsm). Coronal brain slices, 300 μm thick, were cut using a vibrating-blade microtome (Leica VT 1200S, Leica Biosystems, Wetzlar Germany). Slices were then placed in oxygenated artificial cerebrospinal fluid (aCSF) containing, in mM: 124 NaCl, 4.5 KCl, 1.2 NaH2PO4, 26 NaHCO3, 1 MgCl2, 10 glucose (pH 7.4, 300 mOsm), to rest at room temperature for at least 1 h prior to recording.
2.3.2. Electrophysiological recordings
After the resting period, individual slices were transferred to a recording chamber fixed to the stage of an upright microscope and continuously perfused with oxygenated aCSF (2 mL/min) containing the GABAA receptor blocker bicuculine (10 μM, Tocris Bioscience, Bristol, UK) to isolate sEPSCs. Patch micropipettes (resistance 3–6 MΩ) were fabricated from borosilicate glass capillaries (1.5 mm outer diameter, 1.12 mm inner diameter) using a horizontal puller (P-1000, Sutter Instruments, Novato, CA). Recording pipettes were filled with a CsMeSO3-based internal solution containing, in mM: 120 CsMeSO3, 5 NaCl, 10 HEPES, 1.1 EGTA, 0.3 Na-GTP, 4 Mg-ATP (pH 7.4, 290 mOsm). Whole-cell recordings were obtained from DMS and DLS neurons with the voltage clamped at −60 mV. Between 1 and 3 neurons per region/animal were recorded. Approximate coronal sections and demarcated DMS and DLS regions from which neurons were selected are illustrated (Supplemental Figure 1). Cells were visualized using a Nikon Eclipse FN-1 microscope with a 40X water immersion objective and an ORCA-FLASH sCMOS camera (Hamamatsu) with differential interference contrast imaging. Recordings were obtained using a MultiClamp 700B amplifier and Digidata 1550B digitizer (Molecular Devices, Sunnyvale, CA). All recordings were filtered at 2 kHz and digitized at 10 kHz. Data were acquired using Clampex 11 software (Molecular Devices, Sunnyvale, CA). Neurons were selected based on visual examination of cell shape and size characteristic of MSNs. After patching cells, only neurons whose capacitance (>40 pF) and membrane resistance (<300 MΩ) were within the range specified for MSNs proceeded to sEPSC recording, whereas those with characteristic properties of interneurons were discarded. Basic membrane properties were determined with a depolarizing step voltage (5 mV) using the membrane test function integrated in the pClamp software. Neurons were allowed to equilibrate to the whole-cell configuration for at least 5 minutes prior to collecting sEPSC recordings. Access resistance ranged from 10–25 MΩ and was monitored throughout the experiment; recordings with changes in access resistance greater than 20% were not included in the data analysis. Following equilibration, sEPSC data were collected for at least 5 minutes to establish sEPSC baseline. To examine the acute effect of ethanol, a subset of cells was superfused with EtOH (50 mM). Data were analyzed over 2-minute bins, with the baseline bin selected just prior to EtOH application and the acute effects of ethanol analyzed beginning 6 to 8 minutes after the onset of ethanol application. We used an EtOH concentration previously reported for similar electrophysiological recordings in mouse slices (Wilcox et al., 2014). Data analysis for event detection and measurements of sEPSC amplitude, frequency, rise time and decay constant were performed by generating a customized command for event detection using the Template Search module in Clampfit 11 (Molecular Devices). We generated two categories of templates: one for fast rise time and another for slow rise time. To ensure a high degree of similarity of the events, we selected a match threshold value of 4 following manufacturers’ recommendations.
2.4. Blood Ethanol Concentrations
Single blood samples for BECs were collected on the last day of TBC. Trunk blood was sampled after decapitation around dark cycle onset; samples were centrifuged for 5 min at 5000 rpm to separate the plasma. Alcohol concentrations were measured using the Analox alcohol analyzer (AM1 Analox Instruments, Lunenburg, MA) as in (Linsenbardt and Boehm, 2013; Rangel-Barajas et al., 2020). Briefly, 5 μL plasma samples were loaded into a detection channel and the oxygen uptake of the analyte was estimated using oxidoreductase enzyme as a catalytic reagent (alcohol reagent kit; Analox Instruments, Lunenburg, MA). Calibration of the equipment with an ethanol standard analyte (100 mg/dL) was performed before samples were analyzed.
2.5. Estrous cycle phase determination
Single vaginal smears were collected at the time of brain collection to determine estrous cycle phase. After anesthesia, a cotton swab was moistened with sterile water and gently inserted into the vagina (~3–4 mm). The swab was slowly rotated to collect cell samples, and the samples were immediately transferred to slides and examined under a light microscope (Leica DMi1 Microsystems, Wetzlar Germany) to determine the stage of the estrous cycle. Three phases were used for classification based on cytology of the vaginal smear: a) diestrus: characterized by prominent leukocytes with a few epithelial and cornified cells; b) proestrus: identified by numerous round, nucleated cells, uniform in size; and c) estrus: mainly consisting of abundant anucleated cornified epithelial cells with irregular size and shape. Samples were obtained only at the end of alcohol self-administration to avoid interference with drinking behavior. Due to small sample size for proestrus, only estrus and diestrus were included in the examination of cycle effects on sEPSC parameters.
2.6. Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). Two-way analysis of variance (ANOVA) was used to determine significant treatment effects, with factors as listed below. Significant interactions in two-way ANOVAs were followed up with post hoc Sidak’s multiple comparisons test.
Behavior:
Weight-normalized alcohol intake was calculated as (grams alcohol consumed per day)/(mouse weight in kg), and average daily consumption was reported over five weeks. Preference ratios were calculated as (volume of ethanol solution consumed)/(total fluid intake volume). Intake and preference data were analyzed by two-way ANOVA, with the between-subjects factor sex and within-subjects factor day as the repeated measure. Correlational determinations were performed with linear regression analysis to determine the best fit and to obtain the coefficient of determination (r2).
Electrophysiology:
Parameters of spontaneous currents were averaged over 2-minute epochs to assess sEPSC amplitude, frequency, rise time and decay time. To examine acute ethanol effects, baseline measurements were performed in the 2 minutes immediately before ethanol application and ethanol’s effects measured at least 6 minutes after the onset of 50 mM EtOH application. Changes in sEPSC parameters following EtOH application were expressed as percent of baseline, calculated as (average following 50 mM EtOH application)/(average at baseline). All electrophysiological data were analyzed by two-way ANOVAs comparing the factors drinking history (0W and 5W) and sex (male and female). Acute ethanol effects were expressed as percent of baseline and individual treatment groups assessed by one-sample t-tests relative to 100%, indicating no change from baseline.
Estrous Cycle:
Distribution of mice in estrous cycle phases was analyzed by Chi-square (χ2) in conjunction with Fisher’s test to calculate the exact p-value. Electrophysiological data were analyzed by two-way ANOVAs with the factors estrous cycle and drinking history.
Analyses were performed using GraphPad Prism 8.0a Software (San Diego, CA). Significance for all statistical comparisons was set at p≤0.05.
3. Results
3.1. Voluntary oral alcohol intake and blood ethanol levels
To determine voluntary alcohol intake, cHAP mice were given free choice between alcohol (10% v/v) and water for five weeks (Figure 1). Analysis of estimated weight-normalized intake via two-way RM-ANOVA with sex and day as factors determined significant differences by sex [F(14,23) = 24.53, p<0.0001], day [F(14,322) = 9.56, p<0.0001], and their interaction [F(14,322) = 2.28, p=0.005]. Post hoc examination of the sex by day interaction indicated that females consumed significantly more alcohol than males beginning on day 18 of drinking and continuing for the rest of the treatment period (Sidak’s multiple comparisons test, p<0.01; Figure 1A). Estimated average weekly alcohol intakes confirmed that male and female drinking was similar in the first two weeks of access but diverged in weeks 3–5 (Supplemental Figure 2A–E). Despite the differences in weight-normalized alcohol intake, no sex differences were found in ethanol preference over five weeks of drinking history (Week 1: female, 87.16 ± 2.4%, male, 84.37 ± 2.7%; Week 5: female 98.85 ± 1.5%, male 95.47 ± 0.8%; F(1,23) = 1.04, p=0.31; Supplemental Figure 2F). To estimate the strength of association between alcohol intake and BECs, we performed correlational analyses using drinking data from the last day of access, immediately preceding the BEC measurement (Figure 1B and C). A strong and significant correlation between alcohol intake and BEC was detected for both groups (Female: r2=0.80, p<0.0001; Male: r2= 0.62, p=0.001).
Figure 1. Females consume more alcohol and display greater blood ethanol levels than males.
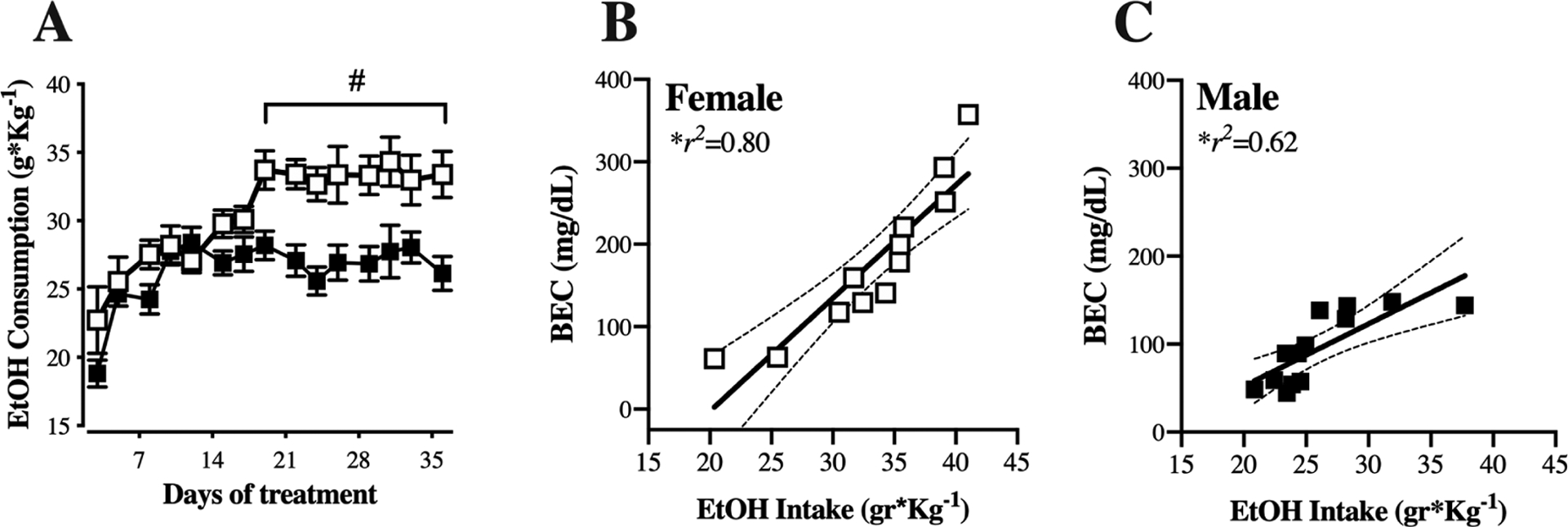
(A) Male (black symbols) and female (white symbols) cHAP mice were provided two-bottle choice access to 10% (v/v) alcohol and water in the home cage for 5 weeks. Intake was measured and bottle position alternated 3 times per week. Data are presented as mean ± SEM weight-normalized alcohol intake, calculated as (g alcohol consumed)/(body weight in kg), averaged across 2 or 3 days. (B-C) Correlation between weight-normalized alcohol intake on the day prior to euthanasia and the blood ethanol content (BEC) obtained at the time of euthanasia for females (B) and males (C). # p<0.01, female vs. male. n=12 female, n=13 male.
3.2. Basic membrane properties of neurons from DMS and DLS
Basic membrane properties were recorded at a holding potential of −60 mV (Table 1). We analyzed the mean membrane capacitance (Cm), mean membrane resistance (Rm), and mean decay time (τ) using two-way ANOVA with sex and drinking history as factors. In the DMS, we found significant main effects of sex and sex by drinking history interactions for both Cm and Rm (Cm, sex [F(1, 62)=4.04, p=0.048], interaction [F(1, 62)=4.89, p=0.030]; Rm, sex [F(1, 62)=5.53, p=0.021], interaction [F(1, 62)= 4.54, p=0.036]). Post hoc analyses showed a significantly lower capacitance in male neurons, as compared to female neurons, but a higher resistance in neurons from male mice, as compared to females, specifically within alcohol-naïve mice. Surprisingly, these sex differences were not evident after five weeks of drinking. In fact, the Rm was significantly reduced in neurons from males with a drinking history (from 171 ± 30 to 85.5 ± 9.5 MΩ; 0W and 5W, respectively, Sidak’s multiple comparisons test, p<0.05). Interestingly, membrane properties in the DLS were primarily affected by past alcohol consumption. There was a main effect of drinking history [F(1, 58)=4.03, p=0.049], likely carried by increased Cm in male mice after 5W drinking (from 88.9 ± 7.6 to 118.5 ± 9.2 pF). Changes in Rm in DLS also were observed after drinking history, with significant sex by drinking history interaction [F(1, 58)=4.55, p=0.037] due to a significantly higher Rm in female neurons after drinking history (from 91.9 ± 19.8 to 148.2 ± 22.7 MΩ, Sidak’s multiple comparisons test p<0.05) that was not observed in males. The mean τ was unchanged by sex or drinking history in either DMS or DLS (see Table 1).
Table 1.
Summary of basic membrane properties of neurons from DMS and DLS in cHAP mice
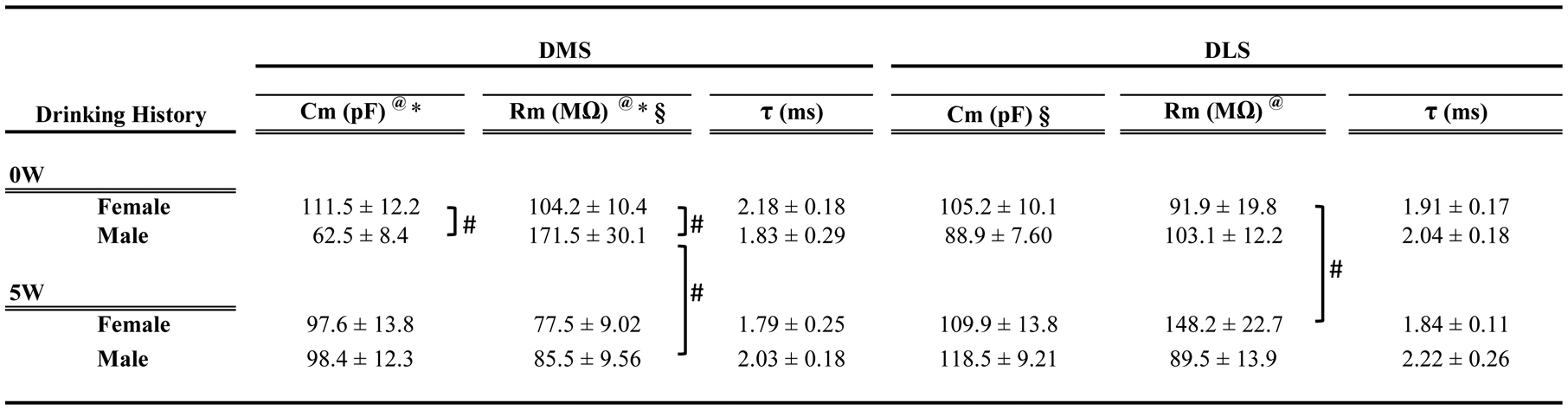
|
Data are expressed as meas ± SEM, n=13 to 19 cells per sex/group, from 8–10 mice per group. Cm, cell membrane capacitance; Rm, input resistance;
, time constant.
Symbols indicate statistical significance:
p<0.001, main effect by drinking history;
p<0.05 sex differences main effect;
p<0.05 interaction between the variables.
Post hoc Sidak’s multiple comparison p<0.05.
3.3. Increase in amplitude of sEPSC in neurons from female DMS after five weeks of drinking history
We next examined the impact of drinking history on spontaneous glutamatergic activity, particularly whether it differed by sex. Representative traces from female (Figure 2A) and male (Figure 2B) DMS neurons illustrate differential effects of past alcohol intake on neuronal activity by sex. The mean amplitude of sEPSCs from DMS neurons increased after five weeks of drinking history in female (from 13.35±0.67 to 16.81±0.64 pA) but not male mice (from 15.64±1.05 to 14.45±0.72 pA; Figure 2C). Two-way ANOVA with sex and drinking history as factors determined significant interaction between the variables, but not main effects (interaction [F(1,69)=8.81, p=0.004]; sex [F(1,69)=0.002, p= 0.96]; drinking history [F(1,69)=2.08, p=0.15]), and post hoc Sidak’s multiple comparisons test confirmed a significant difference between 0W and 5W alcohol in female (p=0.014) but not male neurons. Interestingly, there was no effect in sEPSC mean frequency or decay constant in any of the groups (Figure 2D and F), but sex differences were detected in the sEPSC rise time (sex [F(1,69)=6.92, p=0.010], Figure 2E) without effect of drinking history or interaction between the factors (drinking history [F(1,69)=0.02, p=0.88]; interaction [F(1,69)=1.7, p=0.19]).
Figure 2. Sex and alcohol history affect baseline properties of DMS neurons.
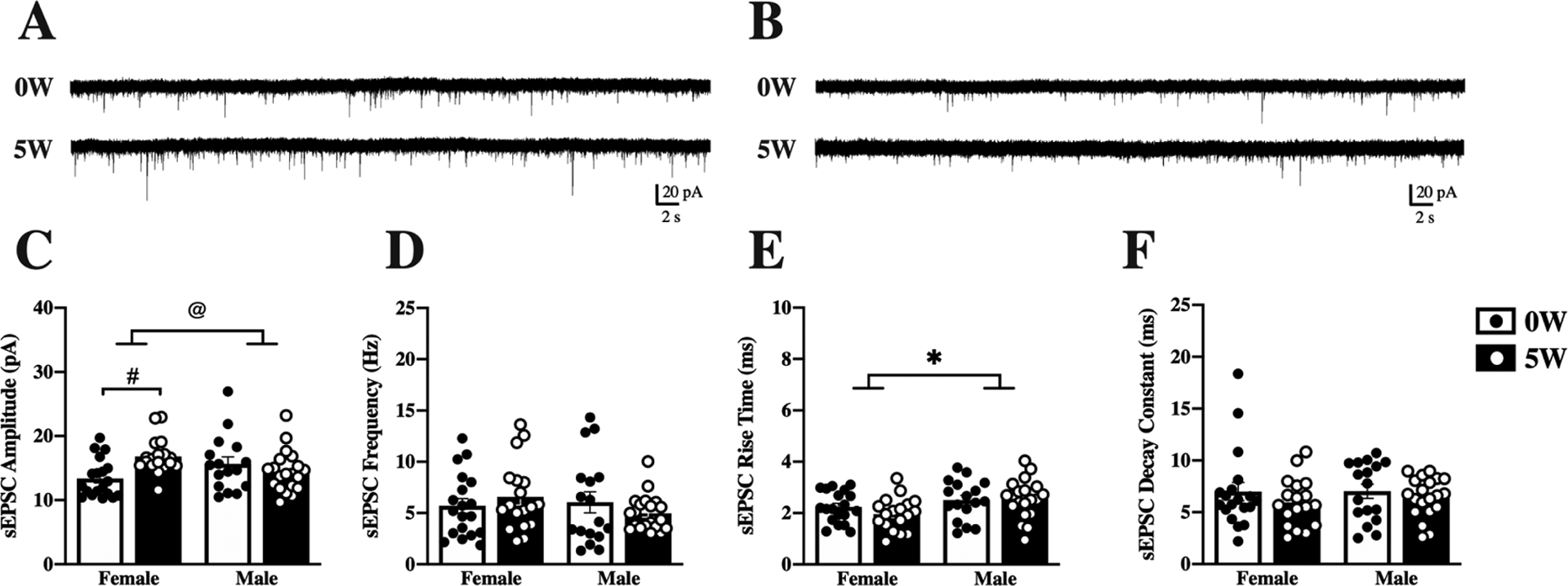
Mice were given access to 10% (v/v) alcohol and water in the home cage for 5 weeks (5W, filled bars & white dots) or water alone (0W, open bars & black dots) prior to euthanasia. Whole-cell spontaneous excitatory postsynaptic currents (sEPSCs) were recorded from dorsomedial striatal (DMS) neurons and properties of the currents measured and averaged across a 2-minute time window. Representative traces are shown for female (A) and male (B) neurons following 0W (top) and 5W (bottom) drinking history. (C) Amplitude of sEPSCs increased after 5W alcohol intake, compared to 0W, for females only. (D) Frequency of sEPSCs did not differ by sex or drinking history. (E) Rise time was higher for males than females, regardless of drinking history. (F) Sex and drinking history did not significantly impact decay time. Histograms represent mean ± SEM. @ p<0.05, sex by drinking history interaction, # p<0.05, 0W vs. 5W, * p<0.05 main effect of sex, as indicated. Number of cells: n=19, 0W female, n=18, 5W female, n=17, 0W male, n=20, 5W male; from 6–8 mice per group.
3.4. Altered sEPSC in DLS neurons after five weeks of drinking history
Alcohol drinking history differentially impacted DLS and DMS neurons, as illustrated by the representative traces from female (Figure 3A) and male (Figure 3B) neurons. In contrast to DMS, drinking history did not change sEPSC amplitude (Figure 3C), but instead altered frequency (Figure 3D) in DLS neurons. In females, the mean sEPSC frequency was significantly increased after five weeks of drinking (from 3.86±0.34 to 5.75±0.59 Hz), while male neurons were not affected (from 4.2±0.58 to 3.72±0.0.41 Hz). Analysis by two-way ANOVA confirmed a significant interaction between drinking history and sex [F(1,55)=5.62, p=0.02], and post hoc Sidak’s multiple comparisons confirmed this interaction was due to increased frequency in female (p=0.03) but not male (p=0.90) neurons. There was also a significant difference between the female 5W and male 5W groups (Sidak’s multiple comparisons test, p=0.03). Interestingly, other kinetic parameters of sEPSC were found to be different in the DLS as well. Sex differences were found in the mean rise time (sex main effect [F(1,55)=7.01, p=0.01], Figure 3E) and in the mean decay constant (sex main effect [F(1,55)=5.93, p=0.01], Figure 3F), without effects of drinking history or interactions between the factors.
Figure 3. Sex and alcohol history affect baseline properties of DLS neurons.
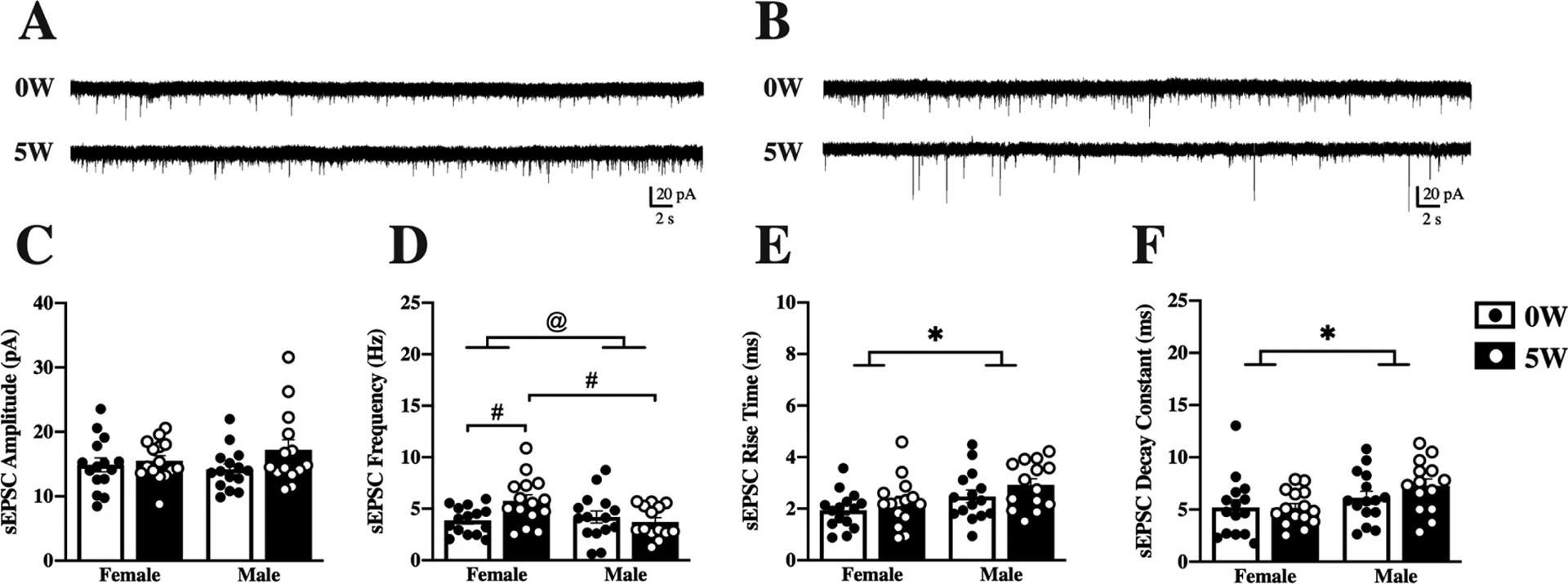
Mice were given access to 10% (v/v) alcohol and water in the home cage for 5 weeks (5W, filled bars & white dots) or water alone (0W, open bars & black dots) prior to euthanasia. Whole-cell spontaneous excitatory postsynaptic currents (sEPSCs) were recorded from dorsolateral striatal (DLS) neurons and properties of the currents measured and averaged across a 2-minute time window. Representative traces are shown for female (A) and male (B) neurons following 0W (top) and 5W (bottom) drinking history. (C) Amplitude of sEPSCs did not differ by sex or drinking history. (D) Frequency of sEPSCs was increased after drinking in females, and frequencies were higher in 5W female than 5W male neurons. (E) Rise time was higher for males than females, regardless of drinking history. (F) Decay time was higher in male than in female neurons, regardless of drinking history. Histograms represent mean ± SEM. @ p<0.05, sex by drinking history interaction, # p<0.05 between groups, as indicated; * p<0.05 main effect of sex, as indicated. Number of cells: n=15, 0W female, n=15, 5W female, n=15, 0W male, n=14, 5W male; from 6 to 8 mice per group.
3.5. Ethanol acutely alters spontaneous activity of DMS and DLS neurons differently by sex and drinking history
Next, we investigated the effect of acute EtOH treatment on neuronal activity, and whether this differed by sex or by alcohol drinking history. For these experiments, we perfused EtOH (50 mM) onto slices after collecting baseline sEPSC measurements and examined the change in sEPSC after EtOH application, expressed as percent of baseline (see Methods). Like baseline measures, acute ethanol effects differed in DMS (Figure 4) and DLS (Figure 5) neurons. For DMS neurons (representative traces, Figure 4A, B), two-way ANOVA found a significant interaction for amplitude [F(1,32)=6.9 , p=0.01; Figure 4C], with no main effects by sex or drinking history [sex [F(1,32)=0.077 p= 0.78]; drinking history [F(1,32)=1.86, p=0.18]. Despite the significant interaction, post hoc comparisons did not yield significant effects, although there was a trend in males (0W vs 5W, female: p =0.59, male p=0.057). No significant changes of amplitude from baseline were detected by one-sample t-test after EtOH application for any of the groups. Unlike amplitude, sEPSC frequency analysis by two-way ANOVA found a main effect of sex [F(1,32)=5.06, p=0.03] that did not interact with drinking history. One-sample t-tests determined a significant increase in frequency after EtOH application only in alcohol-naïve females ([t9=3.19, p=0.01]; Figure 4D), which likely contributed to sex differences found by two-way ANOVA. No additional interactions or main effects were detected for kinetic parameters of sEPSC (Figure 4E–F). Paired analysis of the non-normalized data for DMS sEPSC, including demonstration of individual data points, can be found in the supplementary material (Supplemental Figure 3).
Figure 4. Acute effects of alcohol on DMS neurons differ by sex and drinking history.
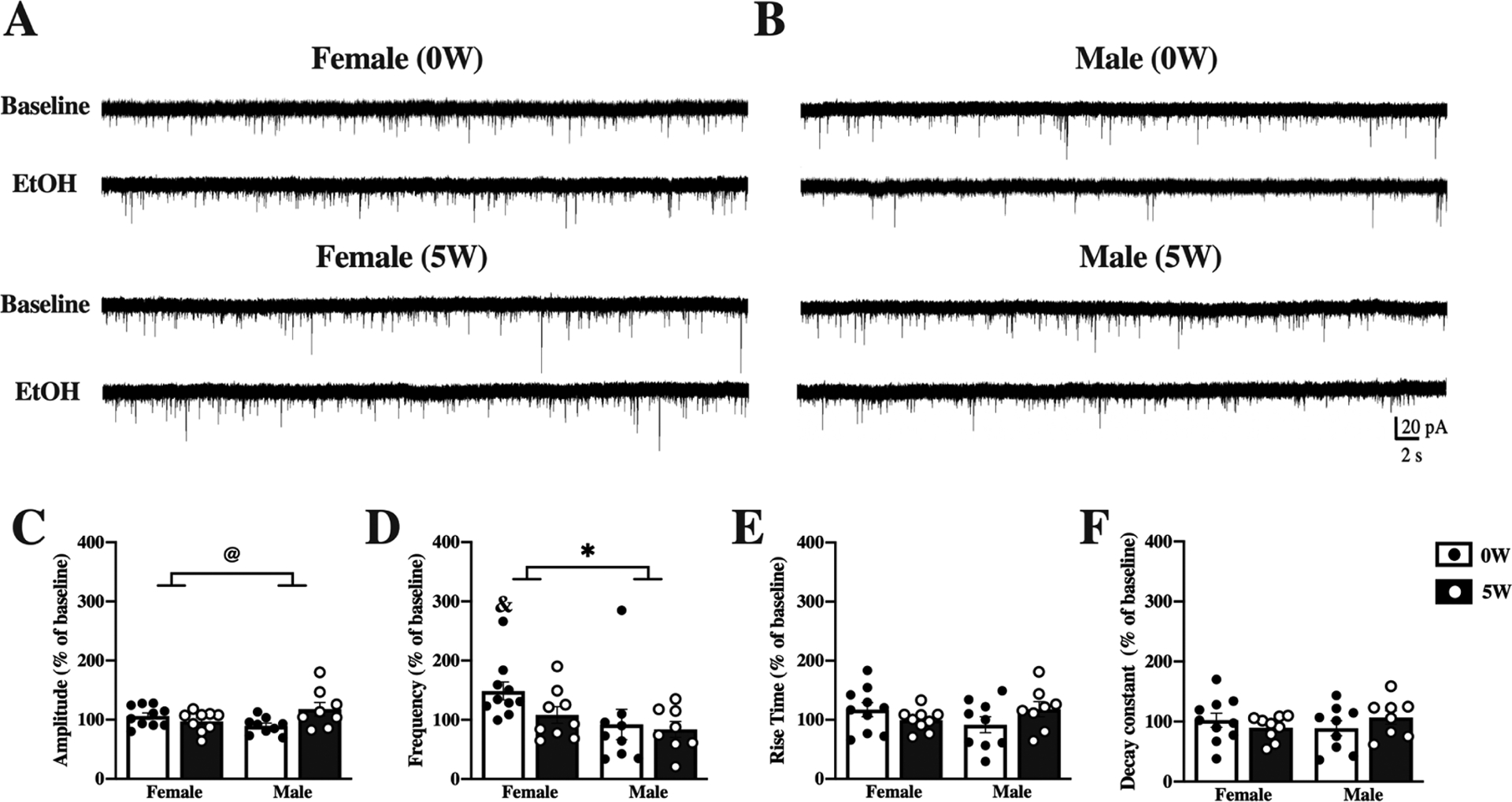
Mice were given access to 10% (v/v) alcohol and water in the home cage for 5 weeks (5W, filled bars & white dots) or water alone (0W, open bars & black dots) prior to euthanasia. Whole-cell spontaneous excitatory postsynaptic currents (sEPSCs) were recorded from dorsomedial striatal (DMS) neurons and properties of the currents measured and averaged across a 2-minute time window before (baseline) and after application of 50 mM ethanol (EtOH) to the slices. Representative traces at baseline and after EtOH treatment are shown for neurons from female (A) and male (B) mice with 0W (top) and 5W (bottom) drinking history. (C) Acute alcohol treatment slightly increased sEPSC amplitude in 5W males, compared to 0W, but slightly decreased amplitude in 5W females, compared to 0W. (D) Acute alcohol changed sEPSC frequency more in females than in males, significantly increasing frequency only in 0W females. Acute alcohol application did not alter rise time (E) or decay time (F). Histograms represent mean ± SEM baseline-normalized values, calculated as (sEPSC parameter after 50 mM EtOH application)/(sEPSC parameter at baseline). @ p<0.05, sex by drinking history interaction, * p<0.05, main effect of sex; & p<0.05 vs. 100% (one-sample t-test). Number of cells: n=10, 0W female, n=9, 5W female, n=9, 0W male n=8, 5W male; from 4 to 6 mice per group.
Figure 5. Acute effects of alcohol on DLS neurons differ by sex and drinking history.
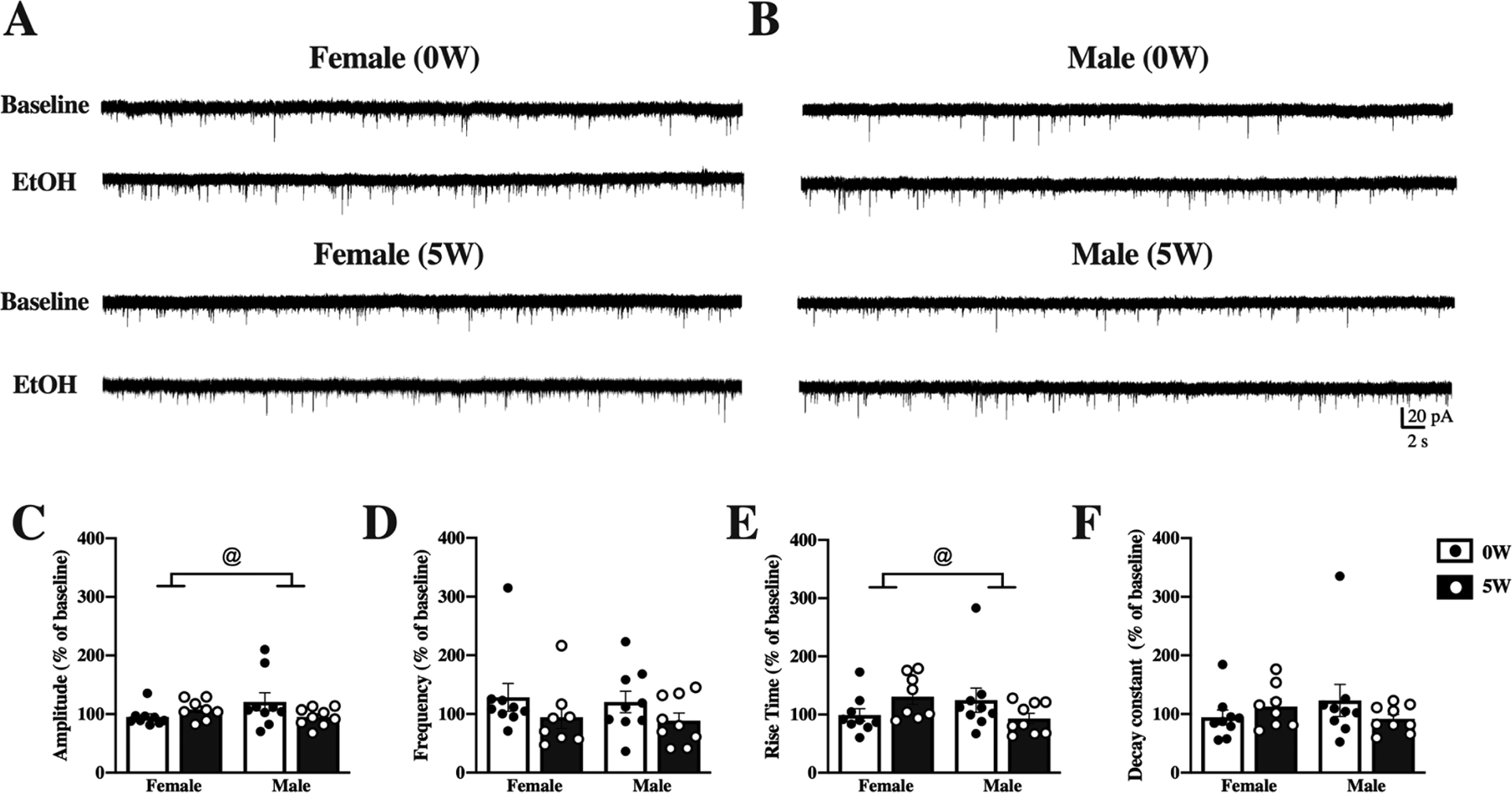
Mice were given access to 10% (v/v) alcohol and water in the home cage for 5 weeks (5W, filled bars & white dots) or water alone (0W, open bars & black dots) prior to euthanasia. Whole-cell spontaneous excitatory postsynaptic currents (sEPSCs) were recorded from dorsolateral striatal (DLS) neurons and properties of the currents measured and averaged across a 2-minute time window before (baseline) and after application of 50 mM ethanol (EtOH) to the slices. Representative traces at baseline and after EtOH treatment are shown for neurons from female (A) and male (B) mice with 0W (top) and 5W (bottom) drinking history. (C) Acute EtOH changed sEPSC amplitude differently by sex and drinking history. (D) Frequency of sEPSCs did not differ by sex or drinking history. (E) Acute EtOH increased rise times in 5W but not 0W females and 0W but not 5W males. (F) Decay constants did not differ by sex or drinking history. Histograms represent mean ± SEM baseline-normalized values, calculated as (sEPSC parameter after 50 mM EtOH application)/(sEPSC parameter at baseline). @ p<0.05, sex by drinking history interaction. Number of cells: n=9, 0W female, n=8, 5W female, n=8, 0W male, n=9 D, 5W male; from 4 to 6 mice per group.
Acute EtOH application differently affected DLS neurons (representative traces, Figure 5A–B), as compared to DMS neurons. Two-way ANOVA detected an interaction between sex and drinking history in response to acute EtOH application for sEPSC amplitude ([F(1,31)=4.22, p=0.048]; Figure 5C), likely due to opposite directions of change in acute EtOH effects in neurons from drinking history males and females, compared to same-sex alcohol-naïve controls, although no post hocs or one-sample t-test were significant. Distinctly from DMS, sEPSC frequency was not significantly changed after acute EtOH application, regardless of sex or drinking history, as determined by two-way ANOVA (Figure 5D). Analysis of DLS sEPSC kinetic properties by two-way ANOVA showed a significant interaction between sex and drinking history ([F(1,31)=4.8, p=0.03]; Figure 5E), an effect that was likely driven by drinking history in female mice (5W), as the acute effect of EtOH showed a trend to increase the rise time [t7= 2.3, p=0.054]. On the other hand, decay constant analysis did not yield interactions or main effects in the two-way ANOVA (Figure 5F), nor were changes from baseline found for any group when analyzed by one-sample t-test. Paired analysis of the non-normalized data for DLS sEPSC, including presentation of individual cells’ data points, can be found in the supplementary material (Supplemental Figure 4).
3.6. Estrous cycle and changes in mean amplitude of sEPSC from DMS neurons
Previous studies differ in reporting the impact of estrous cycle on alcohol intake, with some indicating cycle does not affect alcohol intake (Moore and Lynch, 2015; Priddy et al., 2017; Satta et al., 2018) and others demonstrating cycle-related changes in alcohol self-administration (Roberts et al. 1998). The impact of estrous cycle at the time of euthanasia can affect neurons’ acute responses to ethanol application (Logrip, et al., 2017), although this also can vary (Kirson et al. 2018). Females’ reduced sensitivity to sedative effects of alcohol, relative to males, are pronounced in proestrus and diestrus, an effect that was associated with reduced sIPSC frequency (Cha et al., 2006). Thus, we examined whether estrous cycle impacted sEPSC parameters in female mice during estrus and diestrus (Figure 6). The distribution of mice in estrus and diestrus at euthanasia significantly differed between alcohol naïve and 5W drinking history females. Alcohol-naïve female mice were mostly allocated in estrus (81%), with only 9% of the mice in diestrus, whereas in the drinking history group, mice were mostly allocated in diestrus (50%), with 20% in estrus (χ2=8.09, df=19, p=0.017). While estrous cycle was not associated with differences in alcohol intake (p=0.91) or BEC (p=0.80) attained prior to euthanasia, differences in sEPSC parameters were observed when data were stratified by cycle. DMS neurons had significantly higher mean sEPSC amplitudes in the drinking history group than cells from the alcohol-naïve mice, regardless of cycle (drinking history [F(1,32)=15.93, p=0.004]; Figure 6A), while no differences were found in mean sEPSC frequency in DMS (Figure 6B). Other sEPSC kinetic parameters in DMS were examined and no significant differences were found (data not shown). In DLS cells, however, no significant differences were observed in amplitude (Figure 6C), but there was a significant main effect of estrous cycle on sEPSC frequency ([F(1,26)=4.39, p=0.046]; Figure 6D). The impact of estrous cycle on acute alcohol effects could not be determined since the subset of neurons from the baseline analyses that were tested for acute EtOH effects were imbalanced across estrous cycle phases by group and thus insufficient to produce reliable ANOVA analyses.
Figure 6. Estrous cycle effects on spontaneous excitatory postsynaptic potentials.
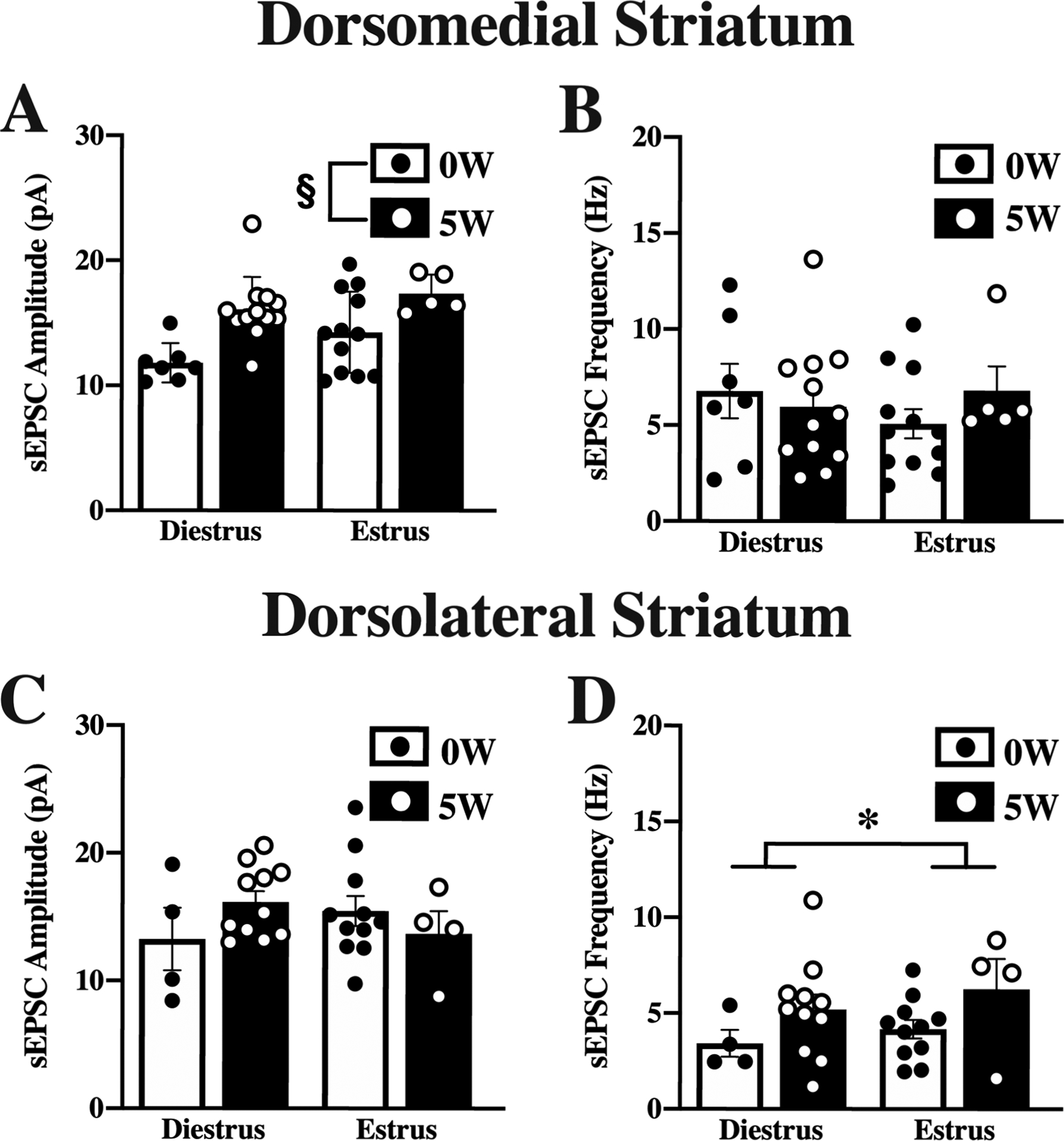
Mice were given access to 10% (v/v) alcohol and water in the home cage for 5 weeks (5W, filled bars & white dots) or water alone (0W, open bars & black dots) prior to euthanasia. Estrous cycle was determined at euthanasia. Whole-cell spontaneous excitatory postsynaptic currents (sEPSCs) were recorded from dorsomedial striatal (DMS; A,B) and dorsolateral striatal (DLS; C,D) neurons and properties of the currents measured and averaged across a 2-minute time window. In DMS, amplitude (A) but not frequency (B) differed by drinking history, with higher amplitude currents after 5W EtOH, independent of cycle. In DLS, amplitude (C) did not differ by drinking history or estrous cycle, but frequency (D) was higher in estrus than in diestrus. Histograms represent mean ± SEM. § p<0.05, main effect of drinking history, * p<0.05, main effect of cycle, as indicated. Number of cells from DMS: 0W; diestrus, n=7; estrus, n=12, 5W; diestrus, n=12; estrus, n=5. Number of cells from DLS: 0W; diestrus, n=4; estrus, n=11, 5W; diestrus, n=11, estrus, n=4. Number of animals, 6 to 8 mice per group.
4. Discussion
Familial incidence of alcohol use disorder is well documented (Walters, 2002; Agrawal and Lynskey, 2008). In preclinical research, alcohol-preferring rodents have shown some hallmarks of human AUD; such models have been extremely useful and have led to important findings on motivational aspects and neural substrates mediating alcohol misuse. For this study, we used cHAP mice as a preclinical model of a family history of heavy drinking. Through selective breeding, cHAP mice were derived using two high alcohol-preferring lines as progenitors. It is important to note that the alcohol intakes reported for these mice are among the highest in rodent lines (Oberlin et al., 2011; Matson et al., 2014), surpassing both progenitor lines in four generations (see Oberlin et al., 2011). Here, we found interesting sex differences in drinking patterns that generated neurophysiological alterations in putative MSNs from the dorsal striatum.
4.1. Sex Differences in voluntary alcohol self-administration in cHAP mice
In many animal models, female rodents drink more than males (Oberlin et al., 2011; Garcia-Burgos et al., 2010; also see Priddy et al., 2017), yet only a small percentage of preclinical studies have included female rodents to understand this sex difference. Here we evaluated alcohol-drinking patterns over five weeks in female and male cHAP mice. In agreement with previous studies (Oberlin et al., 2011; Matson and Grahame, 2013; Matson et al., 2014), we found extremely high alcohol consumption under 24-h free-choice access to TBC. Interestingly, after two weeks, female mice consumed significantly higher amounts of alcohol than males. Sex differences were reported previously in cHAP mice, with higher mean alcohol intakes in female mice, but only after two or three weeks of drinking history (Matson et al., 2014). It is noteworthy that in our study, both females and males showed very similar patterns of intake escalation in the first 16 days of TBC, but female mice continued to escalate, while males did not. Matson and colleagues (2014) reported that escalation in cHAP mice was accompanied by the development of behavioral tolerance. Thus, sex differences in tolerance might account for the divergent patterns of escalation of alcohol consumption found here.
Accordingly, higher alcohol intakes correlated with greater BECs. Previously, it was reported that cHAP mice reached BEC over 250 mg/dL with free-choice alcohol consumption (Matson and Grahame, 2013). Consistently, we found a positive and significant correlation between BECs and EtOH intake in both female and male mice. While these BECs demonstrate pharmacologically relevant alcohol intake in both sexes, the source of the higher BECs where intake was similar between the sexes is unknown. One possibility may be sex differences in alcohol metabolism, as one study showed slower alcohol metabolism in females vs. males when the alcohol dose was delivered intragastrically, but not intraperitoneally (Desroches et al., 1995), though it should be noted that BECs did not differ by sex in the progenitor HAP1 mice after 30-min scheduled alcohol consumption (Grahame and Grose, 2003). It is noteworthy that we collected blood samples only once at euthanasia, to avoid stress effects from multiple sample collection, and that our correlation with intake spans a 24-h drinking period. This may represent a limitation for interpretation of this predictive analysis, since amounts of drinking in the interval immediately prior to BEC sampling could, at random, differ by sex, increasing the strength of association with BECs in females vs. males. Although the study of specific patterns of intake and their correlations with BECs in cHAP mice was not the aim of the present work, it would be interesting to explore in future studies. Nonetheless, the fast acquisition of alcohol drinking and substantial intake histories in both male and female cHAP mice support the need to elucidate basal and alcohol drinking modulation of neurophysiological properties in the DMS and DLS, brain regions important for promoting drinking as a goal-directed behavior and as a habitual response, respectively.
4.2. Changes of basic membrane properties of neurons from DMS and DLS
Passive membrane properties of a neuron refer to inherent membrane characteristics of conduction that can affect electrical signaling. For instance, the membrane capacitance, or Cm, can affect the time course of electrical signals as they travel across the cell membrane area, while the resistive membrane properties, or Rm, can affect the efficiency of conduction of electrical signals (Hille, 2001). Therefore, we analyzed these parameters in putative MSNs from both regions of dorsal striatum, DMS and DLS, in alcohol-naïve mice and after five weeks of drinking.
We found a significantly lower Cm in alcohol-naïve male mice as compared to females in DMS neurons. Unexpectedly, this effect was no longer observed after five weeks of drinking. Sex differences in capacitance of DMS neurons in alcohol-naïve mice were surprising. As capacitance reflects aspects of cell membrane area, this could be indicative of morphological differences prior to alcohol exposure, such as somatic size and/or dendritic arborization. In contrast to DMS, neurons from DLS displayed an enhanced Cm after chronic alcohol exposure, again only in male mice. Interestingly, it has been reported that chronic alcohol exposure increased the number of dendrites in MSNs from the nucleus accumbens of alcohol-preferring male rats (Zhou et al., 2007). Conversely, unaltered morphology and spine density of neurons from DMS and DLS was reported after chronic alcohol exposure in C57BL/6J mice (Wilcox et al., 2014). Hence, further studies are needed to determine if baseline sex differences in capacitance and consequent reversion by chronic alcohol history are associated with an altered neuronal morphology inherent to the cHAP line.
Intrinsic neuronal properties like resistance are maintained by a combination of factors, including cell size, number of channels, channel subunit composition, and neurotransmitter transporters, in such a way that specific conductances can affect the input resistance of a neuron (Marder and Goaillard, 2006). Here, we found sex differences in resistive properties of the membrane. The Rm in DMS neurons in alcohol-naïve male mice was higher than females. This might reflect a lower number of conductive membrane channels or be due to changes in the structural integrity of neurons, like lower membrane surface area. Consistent with a possible change in morphology, the Cm was lower in alcohol-naïve male mice, as discussed above. Importantly, the Rm was significantly reduced after five weeks of drinking history. In the striatum, inwardly rectifying potassium conductances highly contribute to reduction of input resistance (Nisembaum and Wilson, 1995). Thus, adjustment of number and/or properties of potassium channels after chronic alcohol exposure in male mice might play a role in this effect. In DLS, on the other hand, no sex differences were observed in alcohol-naïve mice, but females displayed a higher Rm after five weeks of drinking history, without changes in Cm. This suggests that altered resistance could be associated with changes in the activity and/or distribution of conductive channels after chronic alcohol exposure, rather than morphological changes. Although changes in biophysical membrane properties are expected to influence synaptic integration, observed sex differences in the capacitance and resistance of neurons from alcohol-naïve mice were not coupled with significant differences in sEPSC amplitude or kinetics. This disconnect might result from differential activation of dopamine or acetylcholine receptors by sex, both of which can alter membrane resistance (Pineda et al., 1995; Pacheco-Cano et al., 1996), or from co-occurring differences in other factors (e.g. ion channel expression, glutamate receptor subunit composition or transporter expression) that might oppose the observed divergence in membrane capacitance. Future studies to directly address differential expression of conductive channels and their contribution to observed electrophysiological properties in cHAP mice are important to provide further insight into the role these channels play in striatal MSN function. Nonetheless, these findings indicate that in the striatum of cHAP mice, there are baseline sex differences in biophysical membrane properties that are region specific. Furthermore, chronic alcohol exposure differentially alters basic membrane properties in male and female DLS.
4.3. Altered glutamatergic inputs to DMS and DLS after alcohol drinking history
MSNs in the striatum receive glutamatergic input from widespread areas of the cortex and subthalamus (Lei et al., 2004; Reiner et al., 2010; Mathai and Smith, 2011). At rest, striatal MSNs have a negative holding potential that keeps these neurons in a so-called “down” state, a feature that makes MSNs fire sparsely and depend on coordinated excitatory synaptic input to initiate spiking (Wilson and Kawaguchi, 1996). Neuroadaptive changes in glutamatergic transmission in the dorsal striatum have been implicated in addictive processes, including the development of alcohol drinking (Wang et al., 2007; Wang et al., 2010; Cheng et al., 2017; Gremel and Lovinger, 2017). Thus, to study if high drinking patterns observed in female cHAP mice were associated with altered excitatory synaptic transmission, we analyzed the effect of chronic alcohol consumption by cHAP mice on multiple parameters of excitatory synaptic transmission onto DMS and DLS, compared to alcohol-naïve cHAP mice.
In DMS, five-week drinking history significantly increased the mean amplitude of sEPSC only in female mice, without changing the frequency, suggesting that chronic alcohol exposure altered neural signals at the postsynaptic level. The lack of effect of drinking on male current amplitude contrasts with previous data following 7–9 weeks of low-intensity drinking in Wistar Han rats, where alcohol experience increased the amplitude of evoked field potentials, relative to alcohol-naïve neurons (Lagstrom et al., 2019). Whether these differences arise from strain, alcohol consumption patterns or recording type (field vs. whole cell) remains to be determined. Interestingly, there was also a main effect of sex in the kinetics of glutamatergic responses, as male mice had a higher sEPSC mean rise time than females, regardless of drinking history. This effect suggests sex differences in the postsynaptic action of glutamate. The lack of differences in frequency suggests that chronic alcohol exposure did not change glutamate release in cHAP DMS, but rather altered the postsynaptic integration of glutamate-gated currents. Similarly, after six weeks of binge-like alcohol drinking in C57BL/6J male mice, no changes in sEPSC mean frequency were observed in DMS (Wilcox et al., 2014). In contrast, recent studies found elevated sEPSC frequency in DMS from alcohol-naïve HAP mice when compared to LAP mice (Fritz et al., 2019). Augmented EPSC frequency also was found in primates, especially in the anterior portion of the caudate nucleus (which is similar to DMS in rodents), accompanied by increased amplitude of inhibitory postsynaptic currents (IPSC) and kinetic parameters such as rise time and decay constant (Cuzon Carlson et al., 2018). Potentiated inhibitory GABAergic frequency and amplitude have been reported as well in a specific cell population in the DMS (Chen et al., 2017). Future studies are needed to explore if imbalances of GABA and glutamate occur in cHAP mice after drinking history and whether sex is a mitigating factor.
Several lines of evidence point to a possible overreliance on DLS neural activity after chronic alcohol exposure in both humans and animal models (Sjoerds et al., 2013; Corbit et al., 2012). Indeed, in cHAP mice, a single acute administration of ethanol is sufficient to evoke habit-like behavior (Houck and Grahame, 2018), suggesting an enhanced reliance on DLS function. We found that after five weeks of drinking, there was an increase in presynaptic glutamate release, based on the significantly higher sEPSC frequency in DLS neurons from female mice. Although no changes in amplitude were found, there were other sex differences in the sEPSC kinetic parameters rise time and decay constant. In agreement with our findings, higher frequency of sEPSC was found in DLS from alcohol-naïve HAP mice, as compared to LAPs, specifically in line 2 (Fritz et al., 2019), which is one of the progenitor lines used to generate the cHAP line (Oberlin et al., 2011). Long-term drinking history in primates also induced an increased firing rate in the caudoventral portion of the putamen, which is similar to the DLS in rodents (Cuzon Carlson et al., 2011). In contrast, after chronic alcohol consumption, no changes were observed in sEPSC frequency in DLS from C57BL/6J mice, but rather changes in sIPSC were reported (Wilcox et al., 2014), while the amplitude of evoked field potentials in DLS from Wistar Han rats was decreased (Lagstrom et al., 2019). Elevated sIPSC frequency and amplitude also were reported in HAP compared to LAP mice (Fritz et al., 2019). Interestingly, GABAergic interneurons from DLS may play a key role in these effects. It was recently reported that ablation of fast-spiking interneurons significantly reduced compulsive-like alcohol consumption and sIPSC amplitude (Patton et al., 2021). Our data and the recent evidence of increased excitability in MSNs from DLS in alcohol-naïve HAP mice (Fritz et al., 2019) suggest that genetic mechanisms might underlie altered glutamatergic transmission in cHAP DLS, an effect that may be derived from the HAP progenitor line, thereby increasing the risk of compulsive-like alcohol consumption in cHAP mice. Importantly, changes in sEPSC parameters were not associated with BECs at euthanasia or final ethanol intake measurements, as no significant correlations were found (Supplemental Table 1). It should be noted that the current studies, as well as previous comparisons of HAP and LAP mice, do not distinguish between neuronal subtypes in the striatum, particularly with respect to dopamine receptor expression. In MSNs expressing dopamine D1 receptors, chronic intermittent alcohol exposure was shown to strengthen glutamatergic transmission and enhance excitability (Wang et al., 2015; Renteria et al., 2017; Cheng et al., 2017), whereas increased GABAergic transmission was observed mainly in MSNs containing D2 dopamine receptors (Cheng et al., 2017). Thus, cell type-specific effects of chronic alcohol exposure in cHAP mice are an important venue of future investigation.
4.4. Acute effects of alcohol on glutamatergic transmission are altered by drinking history
Acute response to alcohol is directly related to development of disordered drinking, as males with low initial response to alcohol have shown heightened risk for developing AUD, a phenotype more commonly seen in those with a family history of alcohol misuse (Schuckit, 1994). Additionally, a central feature of escalating alcohol intake is the development of tolerance to alcohol’s effects (Abrahao et al., 2017). However, neuronal responses to acute alcohol application in cHAPs, as well as the impact of chronic alcohol intake on acute responses to alcohol at the neuronal level, were not known. To test cHAP striatal neurons’ response to acute alcohol exposure, we perfused 50 mM EtOH onto slices from alcohol-naïve and 5-week alcohol-experienced mice. In DMS, we found a significant sex by drinking history interaction for sEPSC amplitude, indicating that the impact of past drinking on alcohol’s acute effects differed in males and females. However, this interaction did not yield significant post hoc comparisons, nor did one-sample t-tests identify any group as significantly different from baseline after alcohol treatment, although for males, we did observe a trend for a difference in acute EtOH effects based on drinking history. The lack of significant change in amplitude after alcohol treatment may indicate an overall blunted alcohol response in the cHAP line, relative to rodents without a family history of high alcohol preference, which have shown decreased field potential amplitude following alcohol superfusion, regardless of drinking history (Lagstrom et al., 2019). While the literature does not provide context for our observations in cHAP females, the impact of acute alcohol in male DMS neurons has varied. The suppression of postsynaptic responses by acute EtOH is supported by studies showing concentration-dependent reduction in sEPSC amplitude in Sprague-Dawley rat dorsal striatum (Choi et al. 2006), noting that responses to 50 mM EtOH were highly variable and significant ethanol effects were only observed after 100 mM EtOH treatment. Our data also showed a very modest, not significant reduction in sEPSC amplitude after 50 mM EtOH application in male neurons, with a subset of cells increasing rather than decreasing amplitude (Supplemental Figure 3E). Thus, one explanation of our findings would be an insufficiently high acute alcohol concentration to overcome variability in individual response. However, other studies support the use of 50 mM EtOH as sufficient to reduce glutamatergic current amplitude, particularly when isolating the NMDA component of the response following local stimulation. When measured in whole-cell configuration, ethanol significantly reduced the amplitude of pharmacologically isolated NMDA currents (Yin et al., 2007; Wang et al., 2010), but either reduced (Lagstrom et al., 2019) or had no effect (Yin et al., 2007) on the amplitude of extracellularly recorded field potentials. It remains to be determined whether postsynaptic differences exist in cHAP neurons even prior to alcohol drinking that would blunt sensitivity to acute alcohol exposure; this is an important future direction of study.
Contrary to the equivocal findings for amplitude, acute EtOH increased event frequency in alcohol-naïve female mice, indicative of potentiated presynaptic glutamate release, but this effect was absent in females with an alcohol drinking history and in all males. The lack of alcohol-induced change in frequency in alcohol-naïve males aligns with Choi and colleagues (2006), who observed no acute EtOH-induced change in paired-pulse ratio, another indicator of presynaptic release, in dorsal striatal MSNs. Few studies have examined the effects of acute EtOH on alcohol-naïve females, so determining whether acute EtOH consistently increases sEPSC frequency in female DMS MSNs, or specifically in cHAP females, is an important future investigation.
In DLS, on the other hand, sEPSC parameters were bidirectionally affected by drinking history, such that both amplitude and rise time were higher in neurons from female cHAPs but lower in neurons from male mice with 5-week drinking history, as compared to neurons from same-sex alcohol-naïve mice. Although within-sex comparisons did not yield significant effects of drinking history, the data indicate there may be differences in how male and female neurons adapt to drinking history. Unlike DMS, no change was observed in sEPSC frequency for DLS neurons, suggesting no change in glutamate release at DLS synapses following acute alcohol superfusion, regardless of alcohol history or sex. This finding contrasts with the observation that 3 days of alcohol injections significantly decreased glutamate release, resulting in an increased paired-pulse ratio, in male Sprague-Dawley rats (Abburi et al., 2016). How 3 days of alcohol injections, which produced locomotor tolerance in males, relate to 5 weeks of drinking, marked by escalation of intake indicative of building tolerance, in their ability to alter synaptic function remains to be determined. A critical future direction lies in identification of the molecular adaptations producing the observed physiological changes in acute alcohol responses after drinking, particularly how these adaptations differ by sex, resulting in the divergent impacts on DMS and DLS sEPSCs observed in male and female cHAP mice.
4.5. Estrous cycle effects on glutamatergic activity
The role of circulating hormones, which differ across the estrous cycle in females, to modulate synaptic responsivity remains understudied, although estradiol has been shown to modulate excitatory synaptic efficacy in both male and female neurons, albeit via different mechanisms (Jain et al., 2019). Some studies have suggested that neural changes across the estrous cycle alter the effectiveness of alcohol. For instance, IPSC frequency was lower in hippocampal neurons from females than from males, and female rats showed less sensitivity to the sedative effects of ethanol than males, particularly during proestrus and diestrus, but not estrus and metestrus phases (Cha et al., 2006). Indeed, the subunit composition of GABAA receptors significantly changed during diestrus (Lovick et al., 2005), suggesting one possible source for differential sedation across the estrous cycle. Subunit composition of GABAA receptors has been shown to regulate binge drinking and some behavioral effects of ethanol withdrawal (Melon et al., 2019). Estrous cycle effects on excitatory synaptic physiology have been demonstrated in the central nucleus of the amygdala (Logrip et al., 2017; Kirson et al., 2018), but the impact of cycle on dorsal striatal physiology remains unknown. Thus, we sought to assess if estrous cycle impacted any changes observed in sEPSC in cHAP females. We found that drinking history may alter estrous phase distribution of female mice, as mice were more likely to be in diestrus and proestrus phases than in estrus after 5 weeks’ drinking. This may be due to disruption of normal cyclicity by chronic alcohol, which has previously been demonstrated (Sanchis et al., 1985). Conversely, most alcohol-naïve mice were in estrus on the day of recording. For DMS neurons, although sEPSC amplitude was generally higher after 5-week drinking, the magnitude of increase was more striking during diestrus. We also observed a main effect of estrous cycle on DLS sEPSC frequency, suggesting greater glutamate release in estrus, as compared to diestrus. No baseline differences were observed for DMS frequency, DLS amplitude, or kinetic parameters of sEPSCs in either striatal subdivision. Although estrous cycle does not seem to alter alcohol intake in free-cycling rodents (Priddy et al., 2017; Roberts et al., 1998), hormone levels associated with different phases of the estrous cycle might modify synaptic organization. To the best of our knowledge, the effect of estrous cycle on excitatory transmission in dorsal striatal MSNs is unknown. In NAc core, however, sex differences were found in mEPSC frequency during proestrus and estrus but not diestrus, and lower amplitude observed in females during diestrus and estrus, as compared to mEPSC from male rats (Proaño et al., 2018). Interestingly, the authors also demonstrated increased intrinsic excitability of neurons during diestrus but decreased excitability during estrus and proestrus. Importantly, these sex differences disappeared after gonadectomy, indicating that sex and cycle differences in ventral striatal neural activity might be caused by sex-specific hormone expression. One important consideration is that sex differences in excitatory synapse morphology may account for the differences we observed, as previous studies found that female rats have more glutamatergic input onto the distal dendrites of MSNs in NAc (Forlano and Woolley, 2010). An important limitation to consider in the interpretation of our data is imbalance in sample sizes when data were stratified by estrous phase, resulting in several groups with low cell numbers (i.e., DLS 0W-diestrus and 5W-estrus). Although the recordings came from different subjects, low sample size remains a caveat. Thus, additional studies are needed to specifically investigate the contribution of estrous cycle and hormone levels to sEPSC activity in the dorsal striatum of cHAP female mice. Nonetheless, these results emphasize the importance of considering biological variables inherent to female mice.
4.6. Summary
Due to very high voluntary alcohol intake, the cHAP mouse line presents a useful model for examining neuroadaptations associated with excessive alcohol intake. Here we characterize sex differences in cHAP DMS and DLS neurons, demonstrating how a history of significant alcohol intake differentially impacts glutamatergic transmission in male and female neurons and alters acute responses to alcohol application. The findings described herein lay the initial foundation for future investigation into the molecular bases of physiological differences in cHAP neurons. Such studies may reveal determinants of excessive alcohol intake, enumerating possible novel targets for medication development to treat excessive drinking in individuals with a family history of AUD.
Supplementary Material
Highlights:
Sex differences in voluntary alcohol self-administration
Altered glutamate transmission onto dorsal striatum induced by alcohol drinking history
Drinking history changed neurons’ responses to acute alcohol treatment
Estrous cycle affected glutamatergic transmission
Acknowledgments
The authors would like to thank Dr. Luis F. Lopez-Santiago and Dr. René Caballero-Florán for their insight and expertise on electrophysiological experiments and Shannon Roy and B.S. Manav Bharat Patel for their excellent technical support in the execution of the TBC procedure. We also thank Dr. Nicholas Grahame and the Animal Production Core of the Indiana Alcohol Research Center for provision of cHAP mice. This research was supported by a pilot grant from the Indiana Alcohol Research Center [P60 AA007611, PI, Dr. David Kareken]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Alcohol Abuse and Alcoholism. The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abburi C, Wolfman SL, Metz RA, Kamber R, McGehee DS, McDaid J, 2016. Tolerance to ethanol or nicotine results in increased ethanol self-administration and long-term depression in the dorsolateral striatum. eNeuro 3, ENEURO.0112–15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahao KP, Salinas AG, Lovinger DM, 2017. Alcohol and the brain: Neuronal molecular targets, synapses, and circuits. Neuron 96, 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, 2008. Are there genetic influences on addiction: Evidence from family, adoption and twin studies. Addiction 103, 1069–1081. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP, 2010. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, Rodd ZA, 2017. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 122, 201–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, Li Q, Wilson WA, Swartzwelder HS, 2006. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res 30, 113–118. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, Wang J, 2017. Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption. Biol Psychiatry 81, 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Kim KJ, Cho HS, Kim SY, Cho YJ, Hahn SJ, Sung KW, 2006. Acute inhibition of corticostriatal synaptic transmission in the rat dorsal striatum by ethanol. Alcohol 40, 95–101. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH, 2012. Habitual alcohol seeking: Time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry 72, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Grant KA, Lovinger DM, 2018. Synaptic adaptations to chronic ethanol intake in male rhesus monkey dorsal striatum depend on age of drinking onset. Neuropharmacology 131, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA, 2011. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36, 2513–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroches D, Orevillo C, Verina D, 1995. Sex- and strain-related differences in first-pass alcohol metabolism in mice. Alcohol 12, 221–226. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2005. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci 8, 1481–1489. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS 2010. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol. 15, 1330–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM, Munoz B, Atwood BK, 2019. Genetic selection for alcohol preference in mice alters dorsal striatum neurotransmission. Alcohol Clin Exp Res 43, 2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Burgos D, Manrique Zuluaga T, Gallo Torre M, Gonzalez Reyes F, 2010. Sex differences in the alcohol deprivation effect in rats. Psicothema 22, 887–892. [PubMed] [Google Scholar]
- Gerchen MF, Rentsch A, Kirsch M, Kiefer F, Kirsch P, 2019. Shifts in the functional topography of frontal cortex-striatum connectivity in alcohol use disorder. Addict Biol 24, 1245–1253. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ, 1996. The Basal Ganglia in: Swanson LW, Björklund A, Hökfelt T (Eds), Handbook of Chemical Neuroanatomy Elsevier B.V pp 371–468. [Google Scholar]
- Grahame NJ, Grose AM, 2003. Blood alcohol concentrations after scheduled access in high-alcohol-preferring mice. Alcohol 31, 99–104. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Lovinger DM, 2017. Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs. Genes Brain Behav 16, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Spuhler K, 1984. Development of the national institutes of health genetically heterogeneous rat stock. Alcohol Clin Exp Res 8, 477–479. [DOI] [PubMed] [Google Scholar]
- Hille B, 2001. Ion Channels of Excitable Membranes, third ed. Sinauer Associates, Inc., Massachusetts. [Google Scholar]
- Houck CA, Carron CR, Millie LA, Grahame NJ, 2019. Innate and acquired quinine-resistant alcohol, but not saccharin, drinking in crossed high-alcohol-preferring mice. Alcohol Clin Exp Res 43, 2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck CA, Grahame NJ, 2018. Acute drug effects on habitual and non-habitual responding in crossed high alcohol preferring mice. Psychopharmacology (Berl) 235, 2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Huang GZ, Woolley CS, 2019. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J Neurosci 39, 1552–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM, 1999. Building neural representations of habits. Science 286, 1745–1749. [DOI] [PubMed] [Google Scholar]
- Kirson D, Oleata CS, Parsons LH, Ciccocioppo R, Roberto M, 2018. CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msP and wistar rats. Addict Biol 23, 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagstrom O, Danielsson K, Soderpalm B, Ericson M, Adermark L, 2019. Voluntary ethanol intake produces subregion-specific neuroadaptations in striatal and cortical areas of wistar rats. Alcohol Clin Exp Res 43, 803–811. [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A, 2004. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci 24, 8289–8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM, 1987. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl 1, 91–96. [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL 2nd. 2013. Determining the heritability of ethanol-induced locomotor sensitization in mice using short-term behavioral selection. Psychopharmacology 230, 267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Oleata C, Roberto M, 2017. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology 114, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Miles MF, Williams RW, Becker HC, 2017. Variable effects of chronic intermittent ethanol exposure on ethanol drinking in a genetically diverse mouse cohort. Alcohol 58, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL, 2005. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in wistar rats. Neuroscience 131, 397–405. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Alvarez VA, 2017. Alcohol and basal ganglia circuitry: Animal models. Neuropharmacology 122, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Robbins TW, Everitt BJ, 2020. The transition to compulsion in addiction. Nat Rev Neurosci 21, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Cheng Y, Roltsch Hellard E, Wang X, Lu J, Gao X, Huang CCY, Wei XY, Ji JY, Wang J, 2018. Bidirectional and long-lasting control of alcohol-seeking behavior by corticostriatal LTP and LTD. Nat Neurosci 21, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM, 2006. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7, 563–574. [DOI] [PubMed] [Google Scholar]
- Mathai A, Smith Y, 2011. The corticostriatal and corticosubthalamic pathways: Two entries, one target. so what? Front Syst Neurosci 5, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ, 2013. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol 18, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Kasten CR, Boehm SL 2nd, Grahame NJ, 2014. Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res 38, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MM, Parrish BC, Chappell AM, Alexander NJ, McCool BA, 2020. Chronic ethanol differentially modulates glutamate release from dorsal and ventral prefrontal cortical inputs onto rat basolateral amygdala principal neurons. eNeuro 7, ENEURO.0132–19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellentin AI, Brink M, Andersen L, Erlangsen A, Stenager E, Bjerregaard LB, Christiansen E, 2016. The risk of offspring developing substance use disorders when exposed to one versus two parent(s) with alcohol use disorder: A nationwide, register-based cohort study. J Psychiatr Res 80, 52–58. [DOI] [PubMed] [Google Scholar]
- Melon LC, Nasman JT, John AS, Mbonu K, Maguire JL, 2019. Interneuronal delta-GABAA receptors regulate binge drinking and are necessary for the behavioral effects of early withdrawal. Neuropsychopharmacology 44, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CF, Lynch WJ, 2015. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav 132, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY, 2007. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend 91, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA-National Institute on Alcohol Abuse and Alcoholism, 2020, October. Alcohol Facts and Statistics Retrieved from https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics
- Nelson A, Killcross S, 2006. Amphetamine exposure enhances habit formation. J Neurosci 26, 3805–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ, 1995. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci 15, 4449–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura S, Nishizawa K, Sakai Y et al. , 2018. Monitoring and updating of action selection for goal-directed behavior through the striatal direct and indirect pathways. Neuron 99, 1302–1314.e5. [DOI] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N, 2011. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet 41, 288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Cano MT, Bargas J, Hernández-López S, Tapia D, Galarraga E., 1996. Inhibitory action of dopamine involves a subthreshold Cs(+)-sensitive conductance in neostriatal neurons. Exp Brain Res 110, 205–11. [DOI] [PubMed] [Google Scholar]
- Patton MS, Heckman M, Kim C, Mu C, Mathur BN, 2021. Compulsive alcohol consumption is regulated by dorsal striatum fast-spiking interneurons. Neuropsychopharmacology 46, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JC, Bargas J, Flores-Hernández J, Galarraga E., 1995. Muscarinic receptors modulate the afterhyperpolarizing potential in neostriatal neurons. Eur J Pharmacol 281, 271–7. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA et al. , 2015. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF, 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proaño SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J, 2018. Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol 120, 1356–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Barajas C, Coronel I, Zhang Y, Hernández M, Boehm II SL, 2020. Low-level developmental lead exposure does not predispose to adult alcohol self-administration but does increase the risk of relapsing to alcohol seeking in mice: Contrasting role of GLT1 and xCT brain expression. Neuropharmacology 15,181. [DOI] [PubMed] [Google Scholar]
- Reiner A, Hart NM, Lei W, Deng Y, 2010. Corticostriatal projection neurons - dichotomous types and dichotomous functions. Front Neuroanat 4, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Maier EY, Buske TR, Morrisett RA, 2017. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology 112, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF, 1998. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 22, 1564–1569. [PubMed] [Google Scholar]
- Satta R, Hilderbrand ER, Lasek AW, 2018. Ovarian hormones contribute to high levels of binge-like drinking by female mice. Alcohol Clin Exp Res 42, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, 1994. Alcohol sensitivity and dependence. Exs 71, 341–348. [DOI] [PubMed] [Google Scholar]
- Sanchis R, Esquifino A, Guerri C, 1985. Chronic ethanol intake modifies estrous cyclicity and alters prolactin and LH levels. Pharmacology Biochemistry & Behavior 23, 221–224. doi: 10.1016/0091-3057(85)90560-x. [DOI] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman AT, Penninx BW, Veltman DJ, 2013. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry 3, e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Bolam JP, 1990. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13, 259–265. [DOI] [PubMed] [Google Scholar]
- Walters GD, 2002. The heritability of alcohol abuse and dependence: A meta-analysis of behavior genetic research. Am J Drug Alcohol Abuse 28, 557–584. [DOI] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D., 2012. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci 32, 15124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, Ben Hamida S, Ron D, 2015. Alcohol elicits functional and structural plasticity selectively in dopamine D1 receptor-expressing neurons of the dorsomedial striatum. J Neurosci 35, 11634–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D., 2010. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci 30, 10187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA, 2014. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39, 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y, 1996. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16, 2397–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM, 2007. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci 25, 3226–3232. [DOI] [PubMed] [Google Scholar]
- Yoon G, Westermeyer J, Kuskowski MA, Nesheim L, 2013. Impact of the number of parents with alcohol use disorder on alcohol use disorder in offspring: A population-based study. J Clin Psychiatry 74, 795–801. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P, 2007. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res 1134, 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


