Abstract
Background
The European Society of Cardiology (ESC) published an updated stable chest pain guideline in 2019, recommending the use of an updated pre-test probability (PTP) risk score (RS) to assess the likelihood of coronary artery disease (CAD). We sought to compare the 2019 and 2013 PTPRS in a contemporary cohort of patients.
Methods
612 patients who were investigated with computed tomography coronary angiography (CTCA) for stable chest pain were included in a retrospective analysis.
Results
There were 255 patients with 2019 PTPRS 15–50% with a 9% yield of severe CAD on CTCA, compared with 402 patients and a 4% yield using the 2013 PTPRS (p = 0.01). 355 patients had a 2019 PTPRS of <15%, with 3% found to have severe CAD, compared with 67 patients and none with severe CAD using the 2013 PTPRS (p = 0.14). 336 of patients with 2019 PTPRS of <15% had a calcium score as part of the CTCA. 223 of these had a zero calcium score and only one had severe CAD. In comparison, 113 patients had a positive calcium score, and 10 (9%) had severe CAD (p < 0.001).
Discussion
The ESC 2019 PTPRS classifies more patients as at lower risk of CAD and hence reduces the risk overestimation associated with the 2013 PTPRS. However, in patients with a 2019 PTPRS of <15%, who would not be investigated, the use of the calcium score detected the majority of patients with significant CAD, who may benefit from secondary prevention and an associated mortality benefit as per the SCOT-Heart trial.
Keywords: Coronary artery disease, chronic ischaemic heart disease, calcium score, computed tomography coronary angiography, atherosclerosis, pre-test probability risk score, functional testing
Introduction
The European Society of Cardiology (ESC) 2019 guidelines 1 continue to recommend the use of pre-test probability risk scores (PTPRS) to guide decision making on whether to investigate patients suspected of having coronary artery disease (CAD), and the diagnostic modality with which they are investigated. Pre-test probability risk scores have been validated in predicting future coronary events. 2 The recommended diagnostic modalities are ischaemia assessment, with imaging stress tests for those with higher PTPRS, or anatomical testing, with computed tomography coronary angiography (CTCA) for those with lower PTPRS. The PTPRS is based on a triad of three simple parameters comprising age, gender, and typicality of chest pain. The previous ESC guideline in 2013 3 recommended the use of a PTPRS, based on data by the CAD consortium, 4 as an update to the Diamond and Forrester model. 5 The model published by the CAD consortium was based on a contemporary cohort of European patients with new chest pain, undergoing invasive coronary angiography (ICA), and was intended to improve the Diamond and Forrester model, which overestimated risk. However, the CAD consortium risk score was also found to overestimate likelihood of CAD. Hence, in the recent ESC 2019 guideline on stable chest pain, 1 the PTPRS was updated once more to reflect the current lower prevalence of CAD in contemporary European populations.
In comparison, the UK updated National Institute of Health and Care Excellence (NICE) guidelines 2016 6 removed PTPRS and simply recommended the use of CTCA, as the first line investigation for all patients presenting with stable chest pain.
We have previously established in a survey of UK Cardiologists that ESC stable chest pain guidelines continue to inform UK clinical practice. 7 We therefore wanted to assess the performance of the ESC 2019 PTPRS in comparison with the ESC 2013 PTPRS in a cohort of patients presenting with stable chest pain in whom CTCA was the initial diagnostic modality, as per the NICE guidelines, which is the departmental policy for patients referred with de-novo stable chest pain. We have previously retrospectively evaluated the performance of CTCA and the rates of downstream invasive coronary angiography on the same cohort. 8 We hypothesised the yield of significant coronary disease be superior with the revised 2019 PTPRS but may potentially underestimate risk, given the findings of Bing et al. 2 We therefore, also aimed to explore whether the additional use of the calcium score can contribute to the stratification of patients deemed to not require investigation, under the new ESC 2019 PTPRS.
Methods
The recruitment criteria have been previously described, 8 but in brief included patients aged >18 years and assessed in cardiology clinics. In accordance with NICE guidelines, CTCA is the local first line investigation for all patients presenting with new onset stable chest pain, unless there is a contraindication to CTCA. All such patients who underwent CTCA for new onset stable chest pain at our hospital between January 2017 and May 2018 were identified with a retrospective search of the radiology database. Patients with inconclusive CTCA were excluded from the analysis.
All CTCA were performed with a 64 slice LightSpeed VCT XTe GE scanner (GE Healthcare), using a commercially available protocol (SnapShot Pulse, GE healthcare) and the following scanning parameters: slice acquisition 64 x 0.625 mm, SFOV Cardiac, Z-axis detector coverage 40 mm, gantry rotation time of 350 ms. For adapted tube voltage; 100kv was used for small patients and 120kv for all other patients. Prospective gating was used as the standard acquisition protocol. A prospectively gated calcium score scan (gantry rotation time of 350 ms, 120kv and 150 mA) is undertaken as part of the CTCA protocol. Calcium score was performed in all patients above the age of 40. Patients were beta-blocked aiming to achieve a heart rate of <60bpm. CTCA were reported by a Cardiologist or a Radiologist.
The severity of coronary stenoses was classified, based on visual assessment as; severe > 70% or >50% in the left main stem (LMS), Moderate if >50–70% stenosis and mild if 30–50% stenosis. Calcium scores were performed as part of the CTCA acquisition protocol with the Agatston method.
The PTP risk scores based on age, gender and typicality of chest pain were calculated retrospectively from the clinical information provided. If the typicality of symptoms was not stated on the clinical referral, it was sought from the electronic patient record. In patients below the age of 30, the risk score was deemed indeterminate as this is the lower age cut off, and excluded from analysis. Both ESC risk scores classify those with PTPRS >85% as high likelihood of CAD where investigations are not needed for the diagnosis of CAD and PTPRS <15% as low likelihood of CAD where the recommendation is for no or deferred investigation. In the ESC 2019 guideline a PTPRS of 15–85% is classified as intermediate likelihood of CAD where investigation is recommended. 1 Imaging stress tests are recommended for those with intermediate to high likelihood, and CTCA for those with low to intermediate likelihood of CAD. The ESC 2013 guideline had subdivided this risk group as low to intermediate as 15–50% and as intermediate to high PTPRS as 50–85%. 3
In this analysis, descriptive statistics such as mean ± standard deviation and range are presented for continuous variables. Categorical data are presented as groups’ percentages and comparisons between groups were made using the Chi-squared or Fisher’s exact test, as appropriate. A P-value of <0.05 was deemed statistically significant. Statistical analyses were performed using Stata IC/15 (StataCorp, College Station, TX, US).
Results
A total of 749 patients were referred for CTCA between 01 January 2017 and 31 May 2018. A total of 97 patients did not attend for CTCA. Therefore, 652 patients underwent CTCA between 01 January 2017 and 31 May 2018, of which 309 were male and 303 were female, with an mean age of 56 years ±11 years (Table 1). 36 CTCA studies were inconclusive, and 4 patients had indeterminate PTPRS due to an age of <30. A total of 612 patients were analysed.
Table 1.
Patient demographics and typicality of chest pain used to calculate ESC PTPRS.
| Total number of patients | 612 |
|---|---|
| Male (%) | 309 (50) |
| Female (%) | 303 (50) |
| Mean Age | 56 ± 11 years, 24-90 |
| Typical chest pain (%) | 80 (13) |
| Atypical chest pain (%) | 503 (82) |
| Non-anginal chest pain (%) | 29 (5) |
Data expressed as mean ± standard deviation, range.
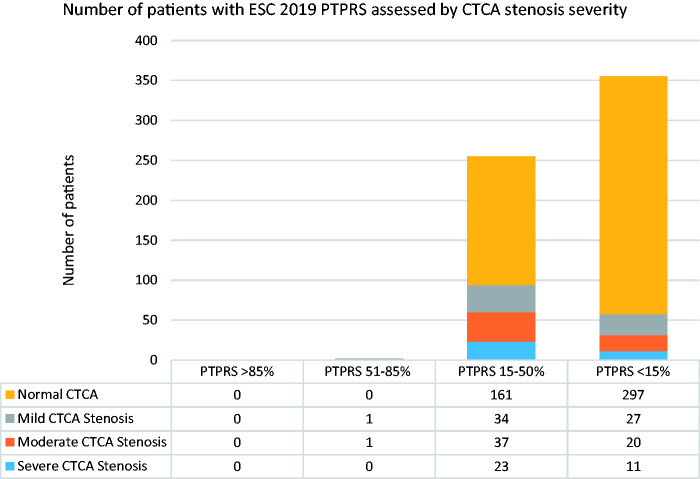
2019 ESC PTPRS (Figure 1)
Figure 1.
Breakdown of patients by ESC 2019 pre-test probability risk score (PTPRS) and the severity of coronary stenoses assessed by CT coronary angiography.
Using the ESC 2019 PTPRS there were no patients with PTPRS >85%. 2 patients had PTPRS 50–85%; one of these had moderate stenosis and one mild stenosis on CTCA.
There were 255 patients with PTPRS 15–50%; 23 (9%) of these had severe stenosis and 37 (15%) moderate stenosis, 34 (13%) mild stenosis, and 161 with normal CTCA.
A further 355 patients had PTPRS <15%; 11 (3%) had severe stenosis and 20 (6%) moderate stenosis. A further 27 (8%) patients had mild CTCA stenosis and 297 with normal CTCA. Hence, a total of 58 (16%) patients with a PTPRS <15% had some degree of CAD (Figure 1).
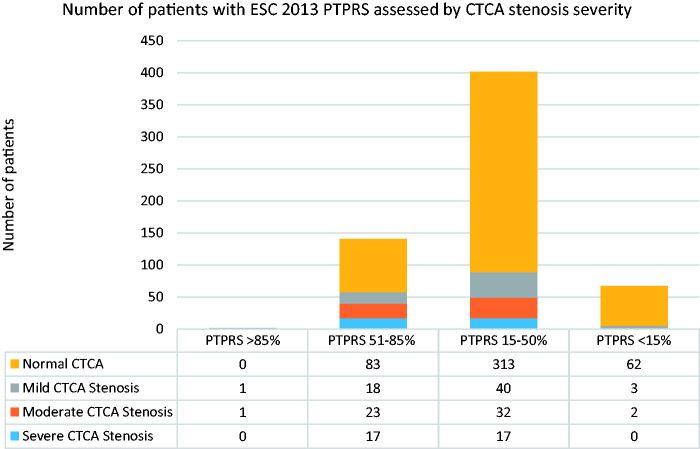
2013 ESC PTPRS (Figure 2)
Figure 2.
Breakdown of patients by ESC 2013 pre-test probability risk score (PTPRS) and the severity of coronary stenoses assessed by CT coronary angiography.
In comparison, ESC 2013 PTPRS identified two patients with PTPRS >85%; one had a moderate stenosis and one had a mild stenosis on CTCA.
There were 141 patients with PTPRS 50–85%; 17 (12%) of these were found to have a severe stenosis and 23 (16%) moderate stenosis, 18 (13%) mild stenosis, and 83 with normal CTCA.
A further 402 patients had a PTPRS 15–50%; 17 (4%) had a severe stenosis and 32 (8%) had a moderate stenosis, 40 (10%) mild stenosis, and 313 with normal CTCA. The yield of severe CTCA disease in patients classified PTPRS 15–50% was higher using the ESC 2019 guideline when compared to the 2013 guideline (9% versus 4%, respectively; p = 0.01).
Lastly, 67 patients had a PTPRS <15%; no patients had severe stenosis, 2 were found to have a moderate stenosis, 3 patients had mild stenosis, and 62 with normal CTCA. Thus, a total of 5 (7%) patients with a PTPRS <15% had some degree of CAD (Figure 2). In this risk group, there was insufficient evidence to support differences in the yield of severe CTCA disease between the 2019 and 2013 guidelines (3% versus 0%, respectively; p = 0.14).
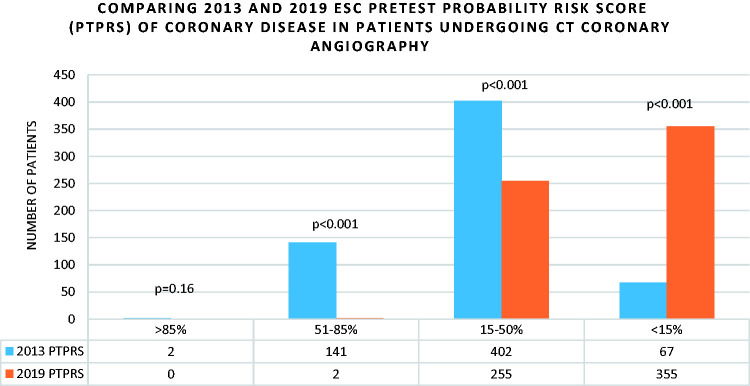
There were significant differences in the proportion of patients classified as PTPRS <15%, 15–50%, and 50–85% between the 2019 and 2013 guidelines (p < 0.001). The only exception was with PTPRS >85% wherein there were only 2 patients using the 2013 score (p = 0.16) (Figure 3).
Figure 3.
Comparing ESC 2013 and 2019 pre-test probability risk score in patients undergoing CT Coronary angiography.
Calcium scores
A total of 336 of 355 patients with PTPRS <15% based on ESC 2019 PTPRS also had a CT calcium score. 223 patients were found to have a calcium score of zero, and 113 patients had a Agatston score greater than zero, with a range between 1 and 930. Of patients with zero calcium score, only 1 (0.4%) patient had severe stenosis, 2 (0.9%) moderate stenoses and 5 (2%) mild stenosis. In contrast, in patients with positive calcium scores, 10 (9%) had severe stenosis, 17 (15%) moderate stenosis, and 21 (19%) mild stenosis (Table 2).
Table 2.
Comparing CTCA stenosis severity by calcium score.
| CTCA stenosis severity | Zero calcium score | >0 Calcium score | p Value |
|---|---|---|---|
| Severe | 1 (0.4%) | 10 (9%) | p < 0.001 |
| Moderate | 2 (0.9%) | 17 (15%) | p < 0.001 |
| Mild | 5 (2%) | 21 (19%) | p < 0.001 |
| Normal/minimal | 215 (96%) | 65 (58%) | p < 0.001 |
| Total number of patients | 223 | 113 |
Discussion
This study shows that, as expected, there is an overall significant reduction in risk classification with the ESC 2019 PTPRS compared with the ESC 2013 PTPRS (Figure 3). Both 2013 and 2019 PTPRS demonstrate our cohort to be a low-intermediate risk group for having significant CAD, given there were no patients with ESC 2019 PTPRS of >85%, and only 2 patients with a 2019 PTPRS 50–85% compared with 2 and 141 patients respectively under the 2013 PTPRS (Figure 3).
There were fewer patients classified with a PTPRS 15–50% in the 2019 score versus the 2013 score (255 and 402 patients respectively). The downward risk modification has led to an associated improvement in yield of severe CTCA disease (9% versus 4%, p = 0.01). However, in doing so, the ESC 2019 PTPRS incorrectly classified a small proportion of patients (3%) with significant CAD as low risk with PTPRS <15%, versus none with the 2013 PTPRS (0 = 0.14). The lack of statistical difference here may be explained by a discrepancy in the group sizes of 355 versus 67 patients respectively which a larger study sample may overcome, and the low incidence of severe stenoses, which would be expected in the lowest risk sub-group. Importantly, the calcium score detected the majority of patients with an ESC 2019 PTPRS <15% who had any degree of CAD (Table 2). Furthermore, previous evaluation of this cohort of patients, had identified a 73% yield of severe CAD on invasive coronary angiography following the identification of severe CTCA stenosis. 8
The authors of the 2019 ESC guideline acknowledge that they were trying to reduce the number of unnecessary investigations in patients who have low likelihood of having CAD for cost reasons and to avoid the potential harm from false positive tests. The purpose of the PTPRS is to help risk stratify the likelihood of having CAD. The ESC risk score in the 2013 guidelines was based on the Diamond and Forrester risk score from the 1970s and was updated by the CAD consortium 4 based on contemporary European populations, but still using the same simple clinical parameters of age, sex and typicality of chest pain. Specifically the prevalence of CAD for a given risk score was lower using the CAD consortium model than in the Diamond and Forrester original data. 5 However, several studies demonstrated that the CAD consortium model still overestimated the prevalence of CAD. 9 The ESC’s concern here was that this overestimation would compound the low diagnostic yield of the non-invasive tests. Hence the new PTPRS was designed to reduce this overestimation and to reduce the number of patients requiring investigations. The ESC guidelines do emphasise that the new PTPRS was based on patients from countries with low prevalence of CAD and acknowledge that the updated PTPRS could underestimate risk, but emphasise that those patients with PTPRS <15%, are at low risk with <1% annual mortality, and their investigation can be safely deferred. 10 Our data are consistent with this expectation. The ESC 2019 PTPRS underestimated the prevalence of CAD in patients with PTPRS <15% with 11 (3%) found to have severe stenosis, and 20 (6%) having moderate stenosis on CTCA. Conversely, although the ESC 2013 PTPRS overestimated the prevalence of CAD it did not miss anyone found to have severe coronary stenosis on CTCA. Importantly, 58 patients with an ESC 2019 PTPRS <15%, had some degree of CAD, and hence potentially would benefit from primary prevention which has been shown to reduce the risk of mortality and myocardial infarction based on the SCOT-Heart trial. 11 A subsequent post-hoc analysis of SCOT-Heart 2 assessing the performance of the ESC 2019 PTPRS showed it to be predictive of CAD and prognostic in patients with a PTPRS >15%. It did however tend to underestimate the presence of CAD which is similar to our experience.
The ESC guideline advocates the use of modifiers to the PTPRS to incorporate cardiovascular risk factors, ECG changes, echocardiography, exercise ECG or the use of CT calcium score. There is extensive data demonstrating that patients with zero calcium score have a very low incidence of CAD and very low incidence of cardiovascular events.12,13 We demonstrated this in our data where only one patient with zero calcium score was found to have a severe coronary stenosis. Thus, the patients with PTPRS <15% who may otherwise have had no or deferred investigation under ESC 2019 PTPRS, could benefit from the use of the calcium score as a simple and low-cost additional risk classifier. The subgroup of patients with raised calcium score could go on to be investigated further with CTCA, or an imaging stress test. The use of CTCA following a positive calcium score in a tiered approach was shown to be clinically safe and cost effective in the CRESCENT trial. 14 Similarly, imaging stress tests have been used to investigate for ischaemia after a positive calcium score, particularly with calcium score >400. 15 Perhaps most importantly, these patients could benefit from commencing primary prevention.
Study limitations
This is a retrospective registry of patients undergoing CTCA at a single centre. A previous audit from our centre has demonstrated 9.4% of patients assessed at rapid access chest pain clinics are referred directly for ICA as they were deemed to be high risk, and a further 13.7% referred for DSE as they were deemed to be unsuitable for CTCA on 64-slice CT (predominantly patients with asthma, COPD and ventricular ectopy), which introduces a degree of selection bias. 16 Furthermore the diagnosis of CAD has been established non-invasively from CTCA, whereas most guidelines consider the diagnosis of significant CAD to be defined from invasive angiography as a > 70% stenosis or with invasive fractional flow reserve <0.8. 17 However, there is now extensive data from the CONFIRM registry which demonstrates that patients with “significant” coronary stenosis on CTCA have an adverse outcome, hence it should be reasonable to define the presence of CAD using CTCA. Furthermore, we have previously reported that 73% of the patients found to have severe stenosis on CTCA in this cohort were confirmed to have severe stenosis on invasive coronary angiography. Additionally, we acknowledge in adopting a retrospective approach, no assessment is made of potential modifiers such as a traditional risk factors for coronary disease, which may have influenced the choice of test had the score been used prospectively.
Conclusion
As per the ESC guideline authors’ intentions, the ESC 2019 PTPRS reclassifies patients with a lower risk score when compared with ESC 2013 PTPRS, thereby potentially reducing unnecessary investigation and the risk of false positive tests. Our results however suggest this is at the risk of missing a small percentage of patients with important CAD. Use of the simple, low cost, calcium score in patients with an ESC 2019 PTPRS <15% detected the majority of patients with significant CAD and may be utilised as a screening tool to identify patients with CAD in this group of patients. This may warrant a larger study to substantiate our findings.
Supplemental Material
Supplemental material, sj-jpg-1-cvd-10.1177_20480040211032789 for ESC 2019 guidelines on chronic coronary syndromes: could calcium scoring improve detection of coronary artery disease in patients with low risk score. Findings from a retrospective cohort of patients in a district general hospital by S Fyyaz, H Rasoul, C Miles, O Olabintan, S David, S Plein and K Alfakih in JRSM Cardiovascular Disease
Footnotes
Acknowledgements: We are grateful to Mr. Athanasios Katsigris (CT Superintendent Radiographer) and Ms. Edith Avorno (Cardiology Specialist Nurse for Rapid Access Chest Pain Clinic) for their help.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Formal ethics approval was not necessary due to the retrospective nature of the study and analysis of our own institutional data. The clinical audit was submitted and agreed with the local audit department
Guarantor: Saad Fyyaz and Khaled Alfakih accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Contributorship: SF and KA contributed to the design, compilation and authorship of the study. HR and OO contributed to data collection. SF and HR analysed the data. CM assisted with statistical analysis and critical revision. SD and KA co-reported all CTCA. SP contributed to critical revision of the manuscript.
ORCID iD: S Fyyaz https://orcid.org/0000-0002-8630-9265
References
- 1.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S and Bax JJ. ESC Scientific Document Group, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC), European Heart Journal 2020; 41(3): 407–477. 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 2.Bing R, Singh T, Dweck MR, Mills NL, Williams MC, Adamson P D, Newby DE. Validation of European Society of Cardiology pre-test probabilities for obstructive coronary artery disease in suspected stable angina. Eur Heart J Qual Care Clin Outcomes. 2020 Oct 1; 6(4): 293–300. doi: 10.1093/ehjqcco/qcaa006. PMID: 31977010; PMCID: PMC7590886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003. [DOI] [PubMed] [Google Scholar]
- 4.Genders TSS, Steyerberg EW, Alkadhi H, et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. Epub ahead of print 2011. DOI:10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 5.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300: 1350–1358. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Chest pain of recent onset: assessment and diagnosis. NICE Guideline CG95 [2016 Update 2010]. London: National Institute for Health and Care Excellence. [PubMed]
- 7.Fyyaz S, Alfakih K, Papachristidis A, et al. Opinions on the expanding role of CTCA in patients with stable chest pain and beyond: a UK survey. Br J Cardiol 2018; 25:107–9. DOI:10.5837/bjc.2018.019 [Google Scholar]
- 8.Fyyaz S, Hudson J, Olabintan O, et al. Computed tomography coronary angiography: diagnostic yield and downstream testing. Clin Med (Lond) 2020; 20: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juarez-Orozco LE, Saraste A, Capodanno D, et al. Impact of a decreasing pre-test probability on the performance of diagnostic tests for coronary artery disease. Eur Heart J Cardiovasc. Epub ahead of print 2019. DOI:10.1093/ehjci/jez054. [DOI] [PubMed] [Google Scholar]
- 10.Foldyna B, Udelson JE, Karády J, et al. Pretest probability for patients with suspected obstructive coronary artery disease: re-evaluating Diamond-Forrester for the contemporary era and clinical implications: Insights from the PROMISE trial. Eur Heart J Cardiovasc Imaging. Epub ahead of print 2019. DOI:10.1093/ehjci/jey182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newby DE, Adamson PD, Berry C, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. Epub ahead of print 2018. DOI:10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 12.Zeb I, Budoff M. Coronary artery calcium screening: does it perform better than other cardiovascular risk stratification tools? Int J Mol Sci 2015. 16(3):6606-20. doi: 10.3390/ijms16036606. PMID: 25807266; PMCID: PMC4394551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rijlaarsdam-Hermsen D, Lo-Kioeng-Shioe MS, Kuijpers D, et al. Prognostic value of the coronary artery calcium score in suspected coronary artery disease: a study of 644 symptomatic patients. Neth Heart J 2020; 28: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubbers M, Dedic A, Coenen A, et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J 2016; 37: 1232–1243. [DOI] [PubMed] [Google Scholar]
- 15.Rijlaarsdam-Hermsen D, Lo-Kioeng-Shioe M, van Domburg RT, et al. Stress-only adenosine CMR improves diagnostic yield in stable symptomatic patients with coronary artery calcium. JACC Cardiovasc Imaging. Epub ahead of print 2020. DOI:10.1016/j.jcmg.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Denny S, Papachristidis A, Vaughan GF, et al. Moderated posters – new roles for cardiac imaging 2018: state of the art. 431 CT coronary angiography for all patients presenting with stable chest pain – UK NICE 2016 guidelines. Eur Hear J – Cardiovasc Imaging 2019; 20: i275–i283. [Google Scholar]
- 17.Reeh J, Therming CB, Heitmann M, et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J. Epub ahead of print 2019. DOI:10.1093/eurheartj/ehy806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-cvd-10.1177_20480040211032789 for ESC 2019 guidelines on chronic coronary syndromes: could calcium scoring improve detection of coronary artery disease in patients with low risk score. Findings from a retrospective cohort of patients in a district general hospital by S Fyyaz, H Rasoul, C Miles, O Olabintan, S David, S Plein and K Alfakih in JRSM Cardiovascular Disease