Abstract
Disseminated Intravascular Coagulation (DIC) commonly complicates sepsis and considerably worsens mortality. Recent studies suggested that anticoagulant therapies improved mortality only in specific sepsis populations, and key pathologies for selecting optimal targets needed to be identified. Anticoagulant activities were naturally altered with aging. This study aimed to evaluate age-related differences in efficacy of anticoagulant therapies in sepsis. This post hoc analysis of a nationwide multicenter cohort study was conducted in 42 intensive care units in Japan. Adult patients with septic DIC were divided into anticoagulant and control groups. Age-related changes in predicted mortality in both groups were compared using a logistic regression model including 2-way interaction terms. Patients were also stratified into 3 subsets based on age, and propensity score-adjusted Cox regression analyses were conducted to examine survival effect of anticoagulants in each subset. We included 1432 patients with septic DIC; 867 patients received anticoagulants and 565 received none. Age-related change in predicted mortality was significantly different between groups (P for interaction = 0.013), and the gap between groups was broad in the younger population. Similarly, in Cox regression analyses, anticoagulant therapies were associated with significantly lower mortality in the subsets of age ≤ 60 and 60-79 (hazard ratios = 0.461, 0.617, P = 0.007, 0.005, respectively), whereas there was no difference in survival between the groups in the subsets of age ≥ 80. The efficacy of anticoagulant therapies for septic DIC might be associated with patient age.
Keywords: anticoagulants, disseminated intravascular coagulation, sepsis
Introduction
Sepsis is a life-threatening condition defined as organ dysfunction caused by a dysregulated host response to infection. With a high short-term mortality rate of approximately 20%-30%, 1 sepsis affects millions of people and continues to be a major healthcare problem worldwide.
Sepsis invariably leads to blood coagulation disorders associated with the progression of multiple organ dysfunction syndrome and subsequent death. Therefore, anticoagulant therapies were historically expected to have survival benefit against sepsis. However, a meta-analysis, including 24 randomized controlled trials (RCTs) assessing efficacy of anticoagulant therapies against sepsis, showed that anticoagulant therapies did not improve mortality in the overall sepsis population but did improve mortality in patients with sepsis-induced disseminated intravascular coagulation (DIC). 2 Besides, a recent multicenter observational study reported that survival benefit of anticoagulant therapy was limited to patients with DIC. 3 This evidence suggested that anticoagulant therapies could achieve maximum survival efficacy when used in septic DIC. However, several RCTs targeting patients with sepsis-induced DIC found null effects of anticoagulant therapies against death, 4,5 indicating that DIC alone was not enough to select optimal targets for anticoagulant therapies. Thus, another key component for selecting optimal patients for anticoagulant therapies must be determined.
Several previous studies showed anticoagulant activities to be altered in accordance with aging, and the increased risk of death in an aged mice model with sepsis versus a young model was partly attributable to dysfunction of the coagulation system with aging. For example, Starr et al reported that aged mice with cecal ligation and puncture had decreased levels of activated protein C (APC) and significantly increased mortality compared with the young model. 6 Further, a human observational study reported dysfunction of the coagulant system in an elderly population to be associated with higher mortality compared to a younger population. 7
From these insights, we hypothesized that age-related dysfunction in the coagulant system would be associated with the efficacy of anticoagulant therapies. This study aimed to evaluate the difference in effects of anticoagulant therapies according to age using a Japanese nationwide multicenter registry database.
Methods
Study Design, Setting and Participants
This post hoc analysis of a nationwide multicenter retrospective cohort study (Japan Septic Disseminated Intravascular Coagulation; J-SEPTIC DIC study) was conducted in 42 intensive care units (ICUs) in Japan. 8 All adult patients diagnosed as having severe sepsis or septic shock by Sepsis-1 criteria during January 2011 through December 2013 were consecutively enrolled in the registry. Among the patients who were register into J-SEPTIC DIC study, we included the patients who fulfilled both the Sepsis-3 criteria and Japanese Association for Acute Medicine (JAAM) DIC criteria. Exclusion criteria included the use of warfarin/acetylsalicylic acid/thrombolytic therapies before study entry; the limitation of sustained life care or post-cardiopulmonary arrest resuscitation status; history of fulminant hepatitis, decompensated liver cirrhosis, or other serious liver disorder; treatment with any chemotherapy; history of hematologic malignant disease; other conditions increasing thrombosis risk at study entry; and patients with missing data for main analysis. We defined the day of study inclusion as “day 1.” In Japanese ICUs, patients with sepsis were typically treated according to the Surviving Sepsis Campaign Guidelines. 9
This study followed the principles of the Declaration of Helsinki. The study protocol was reviewed by the Ethics Committees of Osaka University Hospital, and the board waived the need for the registration because this study used an open and anonymous dataset.
Definition of Sepsis and DIC
We diagnosed “sepsis” according to the Sepsis-3 definition presented at the 45th Critical Care Congress of the Society of Critical Care Medicine in 2016. 10 In this new definition, sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock was also defined based on Sepsis-3 criteria as an elevated lactate level and sepsis-induced hypotension persisting despite adequate fluid resuscitation and requiring catecholamine infusion to improve hemodynamic status.
DIC was diagnosed at the time of inclusion based on JAAM DIC criteria. The JAAM DIC scoring system uses the SIRS score and global coagulation tests including platelet counts, prothrombin time, and FDP/D-dimer levels. 11 We also evaluated the ISTH overt DIC score proposed by the Scientific Subcommittee on DIC of the ISTH. 12 To calculate this score, FDP values were adopted as the fibrin-related marker and scored according to cut-off levels and ranges previously published by Gando et al. 13
Data Collection
Patients were followed until hospital discharge or death. A case report form was developed for the J-SEPTIC DIC registry, 14 and the following information was obtained: age, sex, illness severity scores on the day of ICU admission, source of ICU admission, preexisting comorbidities, primary source of infection, and therapeutic interventions such as mechanical ventilation, vasopressor treatment, immunoglobulin, low-dose steroid, renal replacement therapy, and low-dose heparin for prophylaxis against deep vein thrombosis (DVT). Illness severity was evaluated according to the Sequential Organ Failure Assessment (SOFA) score evaluated on days 1 and 4 and the Acute Physiology and Chronic Health Evaluation (APACHE) II score evaluated on day 1.
The primary outcome measure was all-cause in-hospital mortality. Secondary outcomes were severe bleeding events. In the current study, severe bleeding events were defined as any of the following conditions; 1) intracranial hemorrhage, 2) transfusion requirements related to bleeding, or 3) bleeding requiring surgical interventions.
Patient Categorization
Study patients were categorized into the anticoagulant group (those who underwent systemic administration at therapeutic doses of any anticoagulant agents such as antithrombin, recombinant human thrombomodulin, heparin/heparinoid or serine protease inhibitors) and the control group (who received no anticoagulant therapies for the purpose of DIC treatment). Patients receiving prophylactic administration of low-dose heparin/heparinoid for venous thromboembolism were included in both groups.
Statistical Analysis
To evaluate differences in efficacy and safety of anticoagulant therapies according to patient age, we conducted logistic regression analysis for primary and secondary outcomes including interactions terms between the treatment variable and patient age. The same logistic regression analyses were conducted to evaluate the survival benefit and risk of bleeding complication of several individual anticoagulant agents, namely, antithrombin and recombinant human thrombomodulin.
We also stratified the participants into 3 subsets based on their age: age ≤ 60, age 60-79, and age ≥ 80 years. We conducted multivariable Cox proportional hazard regression analyses to examine the effect of anticoagulant therapies on mortality in each age class separately. Besides, to evaluate the association of anticoagulant therapy and DIC recovery rate, logistic regression analysis was conducted in the 3 age classes. In this study, DIC recovery was defined as surviving with JAAM DIC score of less than 4 at day 7.
The retrospective design of this study caused baseline imbalances between the anticoagulant and control groups; therefore, all regression models were adjusted using propensity scores. The propensity score for the likelihood of undergoing anticoagulant therapies that patients actually received was calculated using multivariable logistic regression analysis including sex, pre-existing comorbidities, disease severity scores, primary source of infection, causal microorganisms, and other therapeutic interventions as covariates. The detailed combinations of the variables are described in Supplementary Table S1.
Descriptive statistics were calculated as medians (interquartile range) or proportions (numbers), as appropriate. Univariate differences between groups were assessed with the Mann-Whitney U test or chi-square test, as appropriate. Missing values were not imputed in any of the regression models.
All statistical inferences were performed with a 2-sided P at 5% significance level. Because of the underpowered nature of the interaction analysis, we used a 2-sided significance level of 20% with statistical inferences for the interaction analyses. 15 All statistical analyses were conducted using STATA Data Analysis and Statistical Software version 15.0 (StataCorp, College Station, TX).
Results
Study Participants
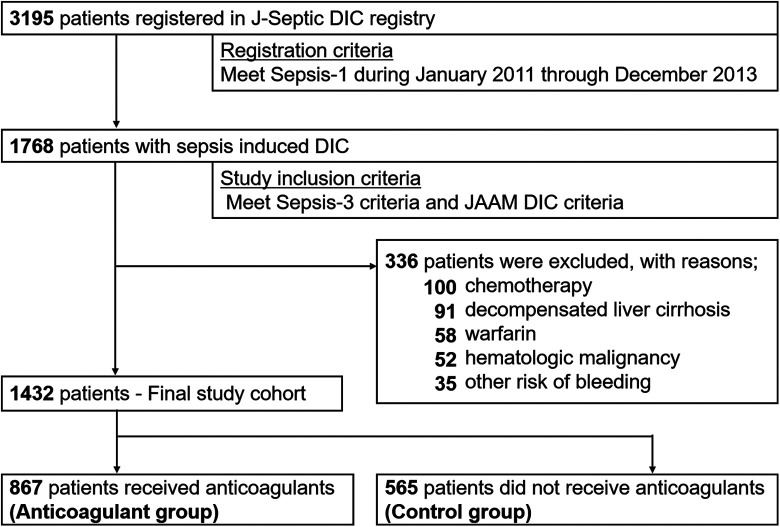
The patient flow diagram is shown in Figure 1. We included 1784 consecutive patients with sepsis-induced DIC. After excluding 336 patients meeting the exclusion criteria, the final study cohort comprised 1432 patients, of whom 867 (61.2%) received anticoagulant therapies (anticoagulant group) and 565 (38.8%) received no anticoagulant therapies for DIC treatment (control group). In anticoagulant group, 67.7% of patients received antithrombin concentrate, 61.9% received recombinant human thrombomodulin, 24.7% received serine protease inhibitors and 10.5% received Heparin/heparinoids.
Figure 1.
Patient flow diagram. J-Septic DIC, Japan Septic Disseminated Intravascular Coagulation; SCCM/ACCP, Society of Critical Care Medicine/American College of Chest Physicians; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation.
Baseline characteristics, illness severity scores at ICU admission and several outcomes are shown in Table 1. Although sex distribution was similar between the groups, the anticoagulant group was slightly but significantly younger. Severity of illness as indicated by SOFA and DIC scores was significantly higher in the anticoagulant group. DIC scores at day 3 and day 7 were still higher in the anticoagulant group. As well, the percentage of several therapeutic interventions other than anticoagulants, such as mechanical ventilation, vasopressor treatment, immunoglobulin, low-dose steroid, and renal replacement therapy, were significantly higher in the anticoagulant group. However, the percentage of low-dose heparin for prophylaxis against DVT was significantly higher in the control group. Both the length of ICU and hospital were longer in the anticoagulant group, however, there were no significant difference in ICU mortality and hospital mortality between the 2 groups.
Table 1.
Baseline Characteristics, Illness Severity, and Outcomes in Patients With and Without Anticoagulant Therapy.a
| Anticoagulant group, n = 863 | Control group, n = 565 | P value | |
|---|---|---|---|
| Age, years | 72 (62-80) | 73 (64-82) | 0.013 |
| Sex, male | 482 (55.6%) | 316 (55.9%) | 0.901 |
| Illness severity | |||
| APACHE II score | 24 (18-29) | 23 (17-30) | 0.419 |
| SOFA score | 11 (8-14) | 9 (7-13) | <0.001 |
| JAAM DIC score | |||
| Day 1 | 6 (5-7) | 5 (4-6) | <0.001 |
| Day 3 | 5 (4-7) | 4 (2-5) | <0.001 |
| Day 7 | 3 (1-5) | 1 (0-4) | <0.001 |
| ISTH overt-DIC score | |||
| Day 1 | 4 (4-5) | 4 (3-5) | <0.001 |
| Day 3 | 4 (3-6) | 3 (1-4) | <0.001 |
| Day 7 | 2 (1-4) | 1 (0-3) | <0.001 |
| Preexisting comorbidities | |||
| Immunocompromised | 93 (10.7%) | 59 (10.4%) | 0.865 |
| Chronic kidney disease | 61 (7%) | 60 (10.6%) | 0.017 |
| Chronic heart failure | 51 (5.9%) | 19 (3.4%) | 0.031 |
| Chronic respiratory disorder | 28 (3.2%) | 22 (3.9%) | 0.503 |
| Liver insufficiency | 10 (1.2%) | 6 (1.1%) | 0.872 |
| Site of infection | 0.351 | ||
| Abdomen | 306 (35.3%) | 193 (34.2%) | |
| Lung | 157 (18.1%) | 116 (20.5%) | |
| Urinary tract | 183 (21.1%) | 115 (20.4%) | |
| Bone/soft tissue | 105 (12.1%) | 52 (9.2%) | |
| Cardiovascular | 34 (3.9%) | 23 (4.1%) | |
| Other/unknown | 82 (9.5%) | 66 (11.7%) | |
| Therapeutic interventions | |||
| Mechanical ventilation | 674 (77.7%) | 409 (72.4%) | 0.002 |
| Vasopressor treatment | 763 (88%) | 449 (79.5%) | <0.001 |
| Immunoglobulin | 418 (48.2%) | 79 (14%) | <0.001 |
| Low-dose steroid | 291 (33.6%) | 106 (18.8%) | <0.001 |
| Renal replacement therapy | 340 (39.2%) | 121 (21.4%) | <0.001 |
| Low-dose heparin | 90 (10.4%) | 94 (16.6%) | 0.001 |
| Interventions for source control | 410 (47.3%) | 218 (38.6%) | 0.001 |
| Outcomes | |||
| ICU length (days) | 9 (5-15) | 5 (3-11) | <0.001 |
| Hospital length (days) | 30 (16-60) | 26 (11-53) | <0.001 |
| ICU mortality | 197 (22.7%) | 135 (23.9%) | 0.608 |
| In-hospital mortality | 299 (34.5%) | 203 (35.9%) | 0.576 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; JAAM, Japanese Association for Acute Medicine; ISTH, International Society of Thrombosis and Hemostasis; DIC, disseminated intravascular coagulation.
a Data are presented as the median (first and third quartiles) for continuous variables and number (%) for categorical variables. Differences between groups were assessed using the Mann-Whitney U test or Chi-square test.
Mortality Change With Aging
We show the regression line for age-related change in predicted mortality in the anticoagulant and control groups as assessed by logistic regression analysis that included a 2-way interaction term in Figure 2. Although predicted mortality did not change with aging in the control group, it sharply increased mortality in the anticoagulant group in accordance with the increase of age, and the difference in age-related mortality change between groups was statistically significant (P for interaction = 0.013). In other words, predicted mortality between the anticoagulant and control groups tended to be largely different in the younger population. Almost same findings were observed in age-related change in predicted mortality in the antithrombin group and recombinant human thrombomodulin groups, both which were significantly different from those in the control group (Figure S1).
Figure 2.
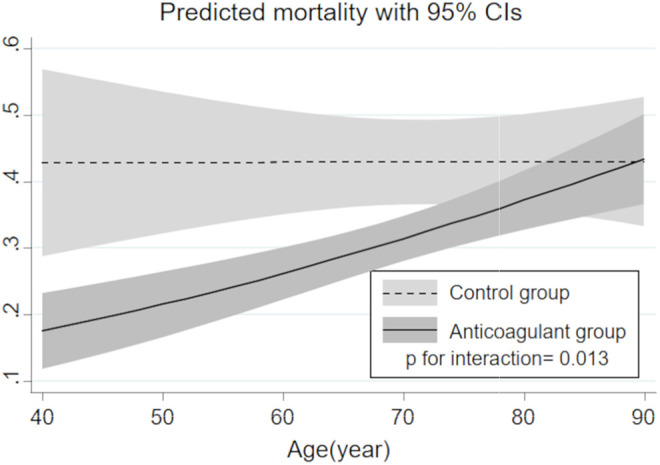
Regression line for age-related change in predicted mortality in each treatment group estimated by logistic regression model with a 2-way interaction term. CI indicates confidence interval.
Survival curves estimated by Cox proportional hazard regression in the 3 age classes are shown in Figure 3. Significant associations between anticoagulant therapies and lower in-hospital mortality were observed in the subsets of age ≤ 60 and age 60-79 (adjusted hazard ratio [HR] with 95% confidence interval [CI] = 0.461 [0.263-0.867] and 0.617 [0.441-0.863], P = 0.007 and 0.005, respectively). However, there was no significant difference in survival between the 2 groups in the subset of older patients (age ≥ 80) (0.934 [0.648-1.347], P = 0.717).
Figure 3.
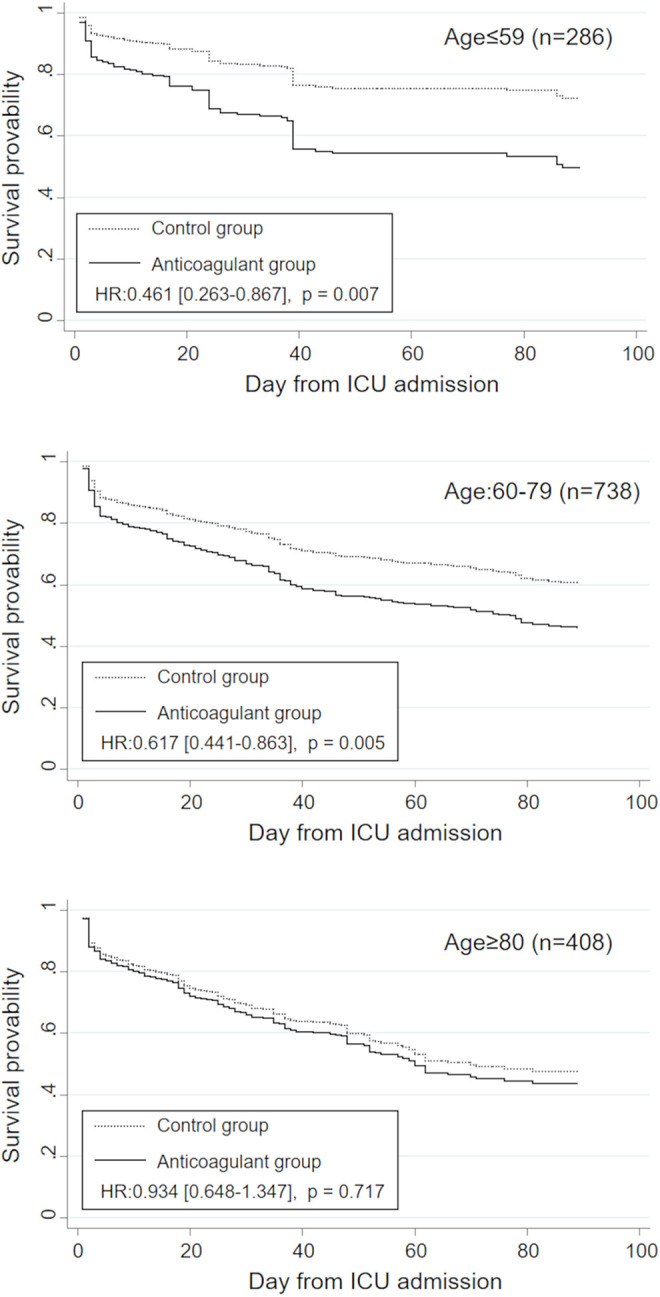
Adjusted estimated survival curves in the anticoagulant and control groups stratified by age class (age ≤ 60, 60-79, and ≥ 80 years). HR indicates hazard ratio.
Bleeding Complications
The association between age-related change in predicted bleeding probabilities and anticoagulant use is shown in Figure 4. Changes in predicted bleeding probabilities in accordance with aging were only slight and not statistically different between groups (P for interaction = 0.776). Regardless of patient age, the anticoagulant group had a consistently higher risk of severe bleeding complications compared to the control group.
Figure 4.
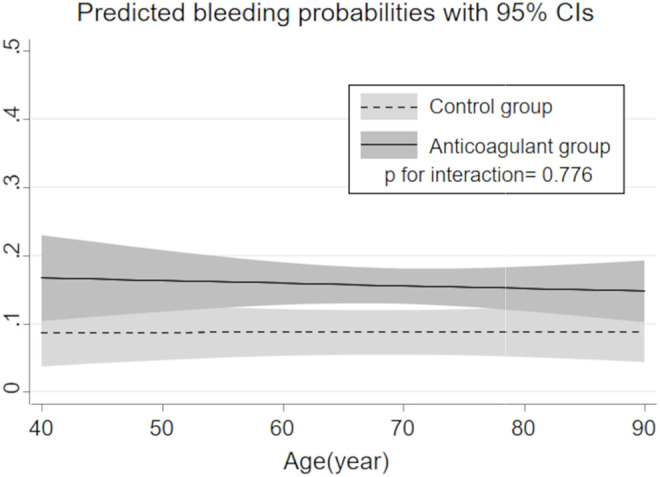
Regression line for age-related change in the risk of severe bleeding complication in each treatment group estimated by logistic regression model with a 2-way interaction term. CI indicates confidence interval.
DIC Recovery and Coagulation Biomarkers
The association of anticoagulant therapy and DIC recovery rate in the 3 age classes was evaluated by logistic regression analysis adjusted by propensity score (Figure S2). Anticoagulant therapy was tended to be associated with the increased rate of DIC recovery in all age classes but the differences were not statistically significant.
The time-related alteration in coagulation biomarkers in patients with and without anticoagulant therapy is summarized in Table S2. From day 1 to day 7, platelet counts and fibrinogen levels were constantly lower in the Anticoagulant group. In contrast, the levels of Fibrin/fibrinogen degradation product and D-dimer in the Anticoagulant group were sharply decreased with time, and became significantly lower compared to those in the control group after day 4.
Discussion
Summary of Findings
Initiating anticoagulant therapies without appropriate target selection should be avoided because of the increased risk of bleeding complications with no demonstrable survival benefit.
The present nationwide study evaluated the impact of aging on the effect of anticoagulant therapies and revealed that the survival benefit of anticoagulant therapies might differ based on age and might be maximized when used for relatively younger populations, and the risk of bleeding associated with anticoagulant therapies was constant regardless of patient age.
Optimal Target of Anticoagulants
DIC is a key pathology in which anticoagulant therapies can achieve maximum efficacy in sepsis. 2,3,16 However, the presence of DIC itself is not enough to select the optimal sepsis patients for anticoagulant therapies. In fact, several RCTs targeting patients with septic DIC reported null effects of anticoagulant therapies to improve survival. 4,5 Thus, another pathophysiology associated with higher survival benefit of anticoagulant therapies must be identified.
The present study results clearly suggested that the survival effect of anticoagulant therapies was different based on patient age and might be higher in a relatively younger population, even among patients with septic DIC.
Furthermore, elderly patients with sepsis and septic shock typically have higher levels of inflammatory response, along with the enhanced activity of coagulation systems. 17,18 Overwhelming inflammatory response causes not only dysregulation of coagulation systems, but also the activation of platelets and enhanced platelet-monocyte aggregation. Therefore, an increased risk of death regardless of the use of anticoagulant agents, observed in elderly patients, might partly be attributable to the higher level of inflammation and the platelet-monocyte aggregation in elderly septic patients. 19 Besides, elderly patients who developed sepsis or septic shock typically had a number of pre-existing comorbidities that potentially affected the regulation of immune system and the acute inflammatory response. Thus, age-related increase in the rate of pre-existing comorbidities might be another explanation for the age-associated change in the efficacy of anticoagulants in septic DIC. 20
Although the present study, to our knowledge, is the first to evaluate the effect of patient age on survival benefit of anticoagulant therapies in septic patients, several previous studies showed that the effects of anticoagulant therapies widely vary by age class in other diseases. For example, previous clinical research showed that coagulation pathway dysfunction was related to aging in patients with nonvalvular atrial fibrillation (NVAF). Stroke or systemic embolic events increased more in the elderly patients treated with warfarin than in younger patients. 21 Age has a greater influence on the effectiveness of anticoagulant therapies, and thromboembolic risk increases in accordance with aging in patients with NVAF.
Based on these insights, another key component, other than DIC, for selecting patients for anticoagulants might be relatively younger age.
Mechanism for Heterogeneity in Survival Benefit
Age-related difference in the effects of anticoagulant therapies on mortality can be partly explained by the alteration of coagulation and anticoagulation systems with aging. Previous animal studies showed that anticoagulant system activity was associated with aging. For example, aged mouse sepsis models had higher mortality rates than young models due to suppressed APC level. 22 Another study reported that aged mice with sepsis developed severe coagulation disorders compared to those in young models due to dysfunction of the protein C activation pathway. 6 It can thus be assumed that the strategy of anticoagulant therapies for elderly patients, whose anticoagulant systems are naturally suppressed, needs to be distinguished from that for younger patients.
We also showed that predicted bleeding probabilities did not change in accordance with an increase in age in either group. We do not have a clear explanation for this constant risk of bleeding regardless of patient age, but one possible reason might relate to the balance between coagulation and bleeding. 23 Namely, fragile artery walls caused by atherosclerosis and hypertension could increase the risk of bleeding, which might be comparable to the decreased risk of bleeding caused by hyper-coagulation in the elderly.
Limitations
This analysis has several limitations. First, we assumed that all anticoagulant agents were the same for analysis purposes. Second, this is an observational study and hence suffers from potential selection and ascertainment bias. Third, the dose and duration of each anticoagulant therapies were not recorded in the J-SEPTIC DIC registry, and therefore, dose-related change in the effect of anticoagulant agents were not evaluated in this study. To compensate for these imbalances, we developed a propensity score approach that forces the analysts to explicitly focus on the potential biases.
Conclusion
Our analysis using the nationwide multicenter J-Septic study revealed that the efficacy of anticoagulant therapies for septic DIC might be associated with patient age. Future RCTs for anticoagulant therapies in sepsis should consider patient age as an inclusion criterion.
Supplemental Material
Supplemental Material, sj-pdf-1-cat-10.1177_10760296211033030 for Age Is Associated With the Efficacy of Anticoagulant Therapies Against Sepsis-Induced Disseminated Intravascular Coagulation by Kyosuke Takahashi, Yutaka Umemura, Kazuma Yamakawa, Hiroshi Ogura and Takeshi Shimazu in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
We thank all the institutions cooperating with the study and the J-SEPTIC group members.
Authors’ Note: The study protocol was reviewed by the Ethics Committees of Osaka university hospital, and the board waived the need for the registration because this study used the open and anonymous dataset.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article:
ORCID iD: Kyosuke Takahashi  https://orcid.org/0000-0001-9051-6518
https://orcid.org/0000-0001-9051-6518
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. [DOI] [PubMed] [Google Scholar]
- 2. Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2015;14(3):518–530. [DOI] [PubMed] [Google Scholar]
- 3. Yamakawa K, Umemura Y, Hayakawa M, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(1):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17(6):R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vincent JL, Ramesh MK, Ernest D, et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41(9):2069–2079. [DOI] [PubMed] [Google Scholar]
- 6. Starr ME, Takahashi H, Okamura D, et al. Increased coagulation and suppressed generation of activated protein C in aged mice during intra-abdominal sepsis. Am J Physiol Heart Circ Physiol. 2015;308(2):H83–H91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–187. [DOI] [PubMed] [Google Scholar]
- 8. Hayakawa M, Yamakawa K, Saito K, et al. Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study group. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost. 2016;115(6):1157–1166. [DOI] [PubMed] [Google Scholar]
- 9. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup. surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. [DOI] [PubMed] [Google Scholar]
- 10. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. [DOI] [PubMed] [Google Scholar]
- 12. Taylor FB, Jr, Toh CH, Hoots WK, et al. Towards a definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 13. Gando S, Saitoh D, Ogura H, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–150. [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa M, Yamakawa K, Saito S, et al. Nationwide registry of sepsis patients in Japan focused on disseminated intravascular coagulation 2011-2013. Sci Data. 2018;5:180243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19(3):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kienast J, Juers M, Wiedermann CJ, et al. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4(1):90–97. [DOI] [PubMed] [Google Scholar]
- 17. Cohen HJ, Harris T, Piper CF. Coagulation and activation of inflammatory pathways in the development of function decline and mortality in the elderly. Am J Med. 2003;114(3):180–187. [DOI] [PubMed] [Google Scholar]
- 18. Ginde AA, Blatchford PJ, Trzeciak S, et al. Age-related differences in biomarkers of acute inflammation during hospitalization for sepsis. Shock. 2014;42(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rondina MT, Carlisle M, Fraughton T, et al. Platelet-monocyte aggregate formation and mortality risk in older patients with severe sepsis and septic shock. J Gerontol Ser A Biol Sci Med Sci. 2014;70(2):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hotchkiss R.S., Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato ET, Giugliano RP, Ruff CT, et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF—TIMI 48 trial. J Am Heart Assoc. 2016;5(5):e003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Starr ME, Ueda J, Takahashi H, et al. Age-dependent vulnerability to endotoxemia is associated with reduction of anticoagulant factors activated protein C and thrombomodulin. Blood. 2010;115(23):4886–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamamoto K, Takeshita K, Saito H. Plasminogen activator inhibitor-1 in aging. Semin Thromb Hemost. 2014;40(6):652–659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cat-10.1177_10760296211033030 for Age Is Associated With the Efficacy of Anticoagulant Therapies Against Sepsis-Induced Disseminated Intravascular Coagulation by Kyosuke Takahashi, Yutaka Umemura, Kazuma Yamakawa, Hiroshi Ogura and Takeshi Shimazu in Clinical and Applied Thrombosis/Hemostasis