Abstract
Objective
This study aimed to investigate the role of long noncoding RNA (LncRNA) myocardial infarction-associated transcript (MIAT) in a heart failure (HF) model in vivo and in vitro by regulating the PI3K/Akt signaling pathway.
Methods
We established HF models in vivo and in vitro and evaluated the collagen content of these models and other factors.
Results
We found that when LncRNA MIAT was silenced, vascular endothelial growth factor, phosphorylated protein kinase B (Akt), and phosphorylated phosphoinositide 3-kinase (PI3K) mRNA and protein levels were significantly downregulated, which suggested that MIAT activated the PI3K/Akt signaling pathway. Akt and PI3K expression was not significantly changed. We also found that when LncRNA MIAT was silenced, collagen expression was significantly downregulated. This finding suggested that MIAT promoted myocardial fibrosis during the development of HF. The levels of inflammatory factors were also significantly reduced with silencing of LncRNA MIAT. This finding suggested that MIAT promoted the expression of inflammatory factors in myocardial fibrosis by activating the PI3K/Akt signaling pathway.
Conclusion
This study indicates that silencing LncRNA MIAT may improve myocardial fibrosis and alleviate HF through the PI3K/Akt signaling pathway, which may be helpful for patients with HF to obtain a better therapeutic effect.
Keywords: Long noncoding RNA, myocardial infarction-associated transcript, myocardial fibrosis, heart failure, phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), inflammation
Introduction
Heart failure (HF) affects more than 23 million people worldwide and causes a high rate of morbidity and mortality. 1 HF is an end-stage disease of various cardiovascular diseases and it has similar pathological characteristics. 2 At present, the focus of HF treatment is to prevent and reverse myocardial remodeling, and the treatment of myocardial fibrosis (MF) is the main research aim. Cardiac fibroblasts (CFs) are important cell groups responsible for extracellular matrix homeostasis. After myocardial infarction (MI), CFs transform into MFs and they play an important role in healing fibrosis.3,4 Therefore, research on MF is important in the field of HF treatment. Long noncoding RNA (LncRNA) is a new type of molecular regulatory factor in cardiac development and disease. LncRNA plays a part in shaping the structure and function of heart and defining the pathogenesis of cardiovascular disease. LncRNA plays an important role in cardiac remodeling, and MI-associated transcript (MIAT) is a powerful and dynamic modifier for cardiac remodeling. 5 In patients with acute MI, coronary heart disease, or angina pectoralis, the level of MIAT is significantly increased.6–8 A previous study showed that LncRNA MIAT induces cardiac fibrosis and LncRNA MIAT is a type of fibrotic factor, which is used to control cardiac fibrosis and regulate cardiac function in patients with MI. 8 Additionally, activation of the phosphoinositide 3-kinase/protein kinase B (PI3K/AKt) signaling pathway by LncRNA MIAT aggravates atherosclerosis (AS). Activation of the PI3K/Akt signaling pathway by LncRNA MIAT also promotes angiogenesis and the expression of inflammatory factors in mice. 9
The mechanism of LncRNA MIAT for improving HF by regulating MF has not been reported. Therefore, this study aimed to investigate this mechanism through the PI3K/Akt signaling pathway to relieve HF. Understanding this mechanism may help patients with HF achieve better treatment results.
Materials and methods
Human CF cultures and grouping
Human CFs (hCFs) were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in fibroblast medium (fetal bovine serum, Thermo Fisher Scientific; Waltham, MA, USA) containing 10% fetal bovine serum and 25% CO2 at 37°C. The medium was changed after 24 to 48 hours and passed once in 48 hours. Cultured hCFs were divided into the following four groups: negative control (NC) group, NC transfected with MIAT short interfering RNA (siRNA) (NC+si-MIAT) group, NC transfected with scramble siRNA (NC+Scramble) group, and NC induced by transforming growth factor (TGF)-β1 group. Cultured hCFs were treated with TGF-β1 (Sigma Aldrich, St Louis, MO, USA) at 20 ng/mL for 24 hours.
After transfection and induction at 24, 48, and 72 hours in the NC+Scramble, NC+si-MIAT, and TGF-β1 groups, the three groups were treated with TGF-β1 (20 ng/mL) for another 48 hours and then processed for downstream experiments. Cell proliferation activity was measured by the cholecystokinin-8 assay (Beyotime Institute of Biotechnology, Haimen, Jiangsu Province, China) in each group. Cell proliferation activity = (optical densityexperimental group – optical densityblank control group)/( optical densitycontrol group − optical densityblank control group).
Establishment of the HF model in rats and grouping
Healthy male C57BL/6 rats (20–25 g) were fed under an ambient temperature of 25°C±2°C and a 12/12-hour of light/dark environment. All rats were used for subsequent experiments in accordance with the Laboratory Animal Management Regulations and Animal Ethical Requirements before modeling. The animal experimental protocol (approval no. 2019-013) was approved by the Ethics Committee of the Inner Mongolia People’s Hospital. Rats were sacrificed and anesthetized by intraperitoneal injection of pentobarbital sodium solution (1%) at a dose of 50 mg/kg. We reduced the number of rats that were used and reduced their suffering as much as possible.
The rats were divided into the four following groups: NC (NC group), HF model (HF group), HF model transfected with MIAT siRNA (HF+si-MIAT group), and HF model transfected with scramble siRNA (HF+Sham group). There were five rats in each group. All of the rats were designed and purchased from Invitrogen (Shanghai, China). The construct was inserted into the lentivirus vector plasmid PHy-LV-KD5.1 (Invitrogen, Shanghai, China). The recombinant lentiviral vector carrying siRNA MIAT was successfully constructed by homologous recombination.
The plasmid (70 μL, 108 TU/mL) carrying si-MIAT or siRNA was injected into the left ventricular chamber and was forced into the coronary arteries when the aortic artery was temporarily occluded for 15 s. Rats in the HF+Sham group were administered 70 μL of Dulbecco’s modified Eagle medium as controls.
Measurement of collagen content
The total collagen content was detected by the Sircol Collagen Assay kit (Biocolor Ltd., Northern Ireland, UK) as previously described. 10 The absorbance of the sample at 540 nm was determined by an enzyme-linked immunosorbent assay (ELISA). Using a linear calibration curve, the collagen content was converted into protein units and normalized to the total protein content of each sample.
Real-time reverse transcription polymerase chain reaction
RNA isolation from tissues and cells was performed with the Trizol standard protocol (Invitrogen Inc., Carlsbad, CA, USA), and quantified using the Nano-Drop 8000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). For each sample, RNA was converted to cDNA using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLVRT; Promega, Madison, WI, USA). The transcript levels were detected by SYBR Green I incorporation methods on the ABI 7500 fast Real Time PCR system (Applied Biosystems, Foster City, CA, USA). The sequences of primer pairs were synthesized by RiboBio (Guangzhou, China) and are listed in Table 1.
Table 1.
Primer sequences.
| Gene | Primer |
|---|---|
| VEGF | F: 5′-GCTTCGGCAGCACATATACTAAAAT-3′ R: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| PI3K | F: 5′-GAAAGCCGAGAACCTATTGCGAG-3′ R: 5′-GTTTGACTTCGCCATCTACCAC-3′ |
| Akt | F: 5′-CGCACCGGTATGACGACGTGGCTATTGTGAA-3′ R: 5′-CCGGAATTCTCAGGAAGTGCCGCTGG-3′ |
| TNF-α | F: 5′-CCGATGGGTTGTACCTTGTC-3′ R: 5′-GGGCTGGGTAGAGAATGGAT-3′ |
| IL-1β | F: 5′-TGTGATGAAAGACGGCACAC-3′ R: 5′-CTTCTTCTTTGGGTATTGTTTGG-3′ |
| IL-6 | F: 5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′ R: 5′-TCTGACCACAGTGAGGAATGTCCAC-3′ |
| Col I | F: 5′-GCTCCTCTTAGGGGCCACT-3′ R: 5′-CCACGTCTCACCATTGGGG-3′ |
| Col III | F: 5′-ACGTAGATGAATTGGGATGCAG-3′ R: 5′-GGGTTGGGGCAGTCTAGTG-3′ |
| GAPDH | F: 5′-AAGAAGGTGGTGAAGCAGGC-3′ R: 5′-TCCACCACCCTGTTGCTGTA-3′ |
F, forward; R, reverse; VEGF, vascular endothelial growth factor; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; TNF-α, tumor necrosis factor-α; IL, interleukin; Col, collagen; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
ELISA assay
Levels of interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, collagen (Col) I, and Col III were measured with commercial ELISA kits (Mlbio, Shanghai, China) according to the manufacturer’s protocol.
Statistical analysis
All statistical analyses were performed using IBM SPSS 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad Prism Software Inc., San Diego, CA, USA). Differences between two groups and multiple groups were analyzed using the t-test and one-way analysis of variance, respectively. A p value <0.05 was considered as statistically significant.
Results
Silencing of MIAT inhibits TGF-β1-induced collagen production and proliferation in hCFs
To determine the role of MIAT in CFs, the effects of LncRNA MIAT knockdown on collagen production in hCFs were studied. Cultured hCFs were transfected with MIAT siRNA or scramble siRNA for 48 hours, and TGF-β1 was used to induce hCFs. In the NC+si-MIAT group, cell proliferation activity was significantly lower compared with that in the TGF-β1 group (p<0.05, Figure 1a). This finding suggested that MIAT enhanced TGF-β1-induced proliferation of hCFs. LncRNA MIAT knockdown significantly inhibited TGF-β1-induced upregulation of Col I and Col III mRNA (Figure 1i, j) and protein (Figure 1k, l) levels (all p<0.05). MIAT mRNA levels in the NC+Scramble and NC+si-MIAT groups were significantly higher than those in the NC group (both p<0.05, Figure 1p).
Figure 1.
(a) Cell proliferation activity as measured by the CCK-8 assay in each group. (b) Measurement of collagen content in each group. (c–p) Expression levels of mRNA and protein for various factors in vitro with measurement by real-time reverse transcription polymerase chain reaction and ELISA assays.
*p<0.05 versus the NC group; **p<0.01 versus the NC group.
#p<0.05 versus the NC+Scramble group; ##p<0.01 versus the NC+Scramble group.
ϕp<0.05 versus the NC+si-MIAT group; ϕϕp<0.01 versus the NC+si-MIAT group.
NC, negative control; siMIAT, short interfering RNA myocardial infarction-associated transcript; TGF-ß, transforming growth factor-ß; CCK-8, cholecystokinin-8; VEGF, vascular endothelial growth factor; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; IL, interleukin; TNF-α, tumor necrosis factor-α; Col, collagen; ELISA, enzyme-linked immunosorbent assay; MIAT, myocardial infarction-associated transcript.
MIAT activates the PI3K/Akt signaling pathway
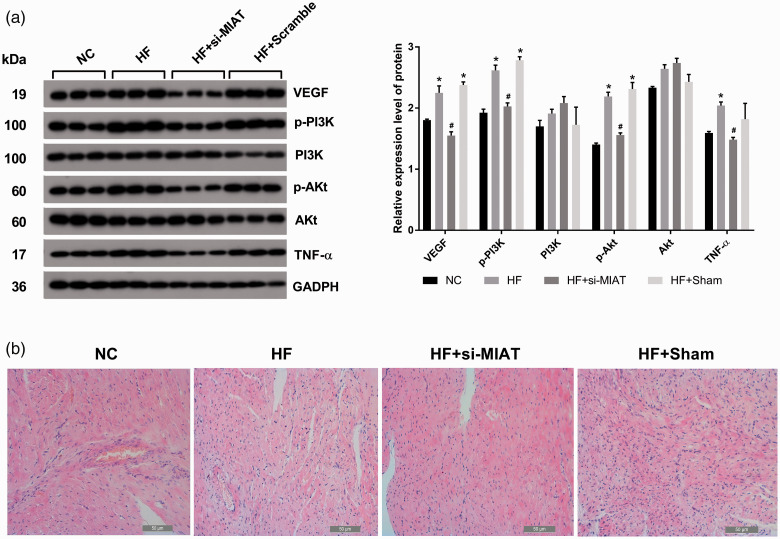
The activation state of the PI3K/Akt signaling pathway was determined by real-time reverse transcription-polymerase chain reaction (RT-PCR) and western blotting in in vitro and in vivo experiments. In in vitro experiments, PI3K mRNA levels (Figure 1d) and Akt mRNA levels (Figure 1e) in the NC+si-MIAT group were not significantly different compared with those in the NC+Scramble group. However, vascular endothelial growth factor (VEGF) mRNA levels were significantly lower in the NC+si-MIAT group than in the NC+Scramble group (Figure 1c). We also found the same results at the protein expression level for VEGF, PI3K, and Akt (Figure 2). Protein expression levels of phosphorylated (p)-PI3K and p-Akt were significantly lower in the NC+si-MIAT group than in the NC+Scramble group (both p<0.05, Figure 2). In in vivo experiments, we found that VEGF (Figure 3a), PIK3 (Figure 3b), and Akt (Figure 3c) mRNA expression levels in the HF and HF+Sham groups were significantly upregulated compared with the NC group (all p<0.05). VEGF (p<0.05, Figure 3a) and Akt (p<0.05, Figure 3c), but not PIK3, mRNA expression levels were significantly downregulated in the HF+si-MIAT group compared with the HF group. We found the same results at the protein expression level (Figure 4a). These results suggested that MIAT activated the PI3K/Akt signaling pathway.
Figure 2.
Silencing MIAT inhibits the PI3K/Akt signaling pathway in vitro.
**p<0.01 versus the NC group; ##p<0.01 versus the NC+Scramble group.
ϕϕp<0.01 versus the NC+si-MIAT group.
NC, negative control; siMIAT, short interfering RNA myocardial infarction-associated transcript; TGF-ß1, transforming growth factor-ß1; VEGF, vascular endothelial growth factor; p-PI3K, phosphorylated phosphoinositide 3-kinase; PI3K, phosphoinositide 3-kinase; p-Akt, phosphorylated protein kinase B; Akt, protein kinase B; TNF-α, tumor necrosis factor-α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 3.
Expression levels of mRNA and protein for various factors in vivo with measurement by real-time reverse transcription polymerase chain reaction and ELISA assays.
*p<0.05 versus the NC group; **p<0.01 versus the NC group.
#p<0.05 versus the HF group; ##p<0.01 versus the HF group.
NC, negative control; HF, heart failure; siMIAT, short interfering RNA myocardial infarction-associated transcript; VEGF, vascular endothelial growth factor; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; IL, interleukin; TNF-α, tumor necrosis factor-α; Col, collagen; ELISA, enzyme-linked immunosorbent assay; MIAT, myocardial infarction-associated transcript.
Figure 4.
(a) Silencing MIAT inhibits the PI3K/Akt signaling pathway (protein expression). (b) Hematoxylin and eosin staining in in vivo experiments.
*p<0.05 versus the NC group; #p<0.05 versus the HF group.
NC, negative control; HF, heart failure; siMIAT, short interfering RNA myocardial infarction-associated transcript; VEGF, vascular endothelial growth factor; p-PI3K, phosphorylated phosphoinositide 3-kinase; PI3K, phosphoinositide 3-kinase; p-Akt, phosphorylated protein kinase B; Akt, protein kinase B; TNF-α, tumor necrosis factor-α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
MIAT regulates MF through the PI3K/Akt signaling pathway
In in vivo experiments, we found that Col I (Figure 3g) and Col II (Figure 3h) mRNA expression levels were significantly upregulated in the HF and HF+Sham groups compared with the NC group (all p<0.05). Col I (Figure 3i) and Col II (Figure 3j) protein expression levels were significantly downregulated in the HF+si-MIAT group compared with the HF group. These results suggested that MIAT promoted MF in rats with HF by activating the PI3K/Akt signaling pathway.
MIAT promotes inflammatory factor expression by activating the PI3K/Akt signaling pathway
In in vitro experiments, IL-1β, IL-6, and TNF-α mRNA (Figure 1f–h) and protein (Figure 1m–o) expression levels were significantly downregulated in the NC+si-MIAT group compared with the NC+Scramble group (all p<0.05). In in vivo experiments, IL-1β, IL-6, and TNF-α mRNA (Figure 3d–f) and protein (Figure 3k, l, n) expression levels were significantly higher in the HF and HF+Sham groups compared with the NC group (all p<0.05). There were no significant differences in IL-1β, IL-6, or TNF-α expression between the NC and HF+si-MIAT groups. These results suggested that MIAT promoted inflammatory factor expression in MF by activating the PI3K/Akt signaling pathway.
Discussion
Cardiomyocyte apoptosis leads to blocked arterial flow to the heart, damage to cardiac muscle, and eventually heart failure and sudden cardiac death. 11 LncRNA is a cell response molecule of bioactive polyphenols that is used in the treatment of many diseases. LncRNA MIAT is expressed in the heart and fetal brain tissue. 12 HF is a common complication of MI, leading to high mortality worldwide.13–15 Recent studies have shown that upregulation of LncRNA MIAT aggravates the development of AS 16 and ischemic stroke, 17 and the mechanism of developing AS is related to the activation of the PI3K/Akt signaling pathway. Activation of the PI3K/Akt signaling pathway can reduce the level of reactive oxygen species and lipid deposition, thus inhibiting plaque formation and reversing the progression of AS. 18 The role of MIAT in HF through the PI3K/Akt signaling pathway has not been reported. Therefore, there is an urgent need to identify effective therapeutic targets to reduce the incidence of HF. In the present study, we examined the role of LncRNA MIAT in an HF model through regulation of the PI3K/Akt signaling pathway. Our study suggested that silencing MIAT reduced the occurrence of HF by activating the PI3K/Akt signaling pathway.
The Akt family is involved in a variety of cellular processes, including promoting cell survival, glucose metabolism, and cellular protein synthesis in cardiomyocytes. 19 Generally, Akt contains three isoforms of Akt1, Akt2, and Akt3. Short-term Akt1 activation promotes cardiac growth, 20 whereas long-term Akt1 activation induces pathological hypertrophy 21 because Akt1 overexpression leads to HF. 22 A previous study also showed that MIAT activated the PI3K/Akt signaling pathway. 9 Our study is consistent with this previous study because we found that p-PI3K and p-Akt expression was significantly decreased after silencing MIAT as shown by real-time RT-PCR and WB analysis. Interestingly, MIAT functions as an miR-150-5p sponge and affects the inhibitory effect of VEGF, which promotes the migration of inflammatory cells to the intima and induces pathological angiogenesis. 17 Our study showed that MIAT knockdown inhibited VEGF expression by real-time RT-PCR and WB analysis. TGF-β is the major fiber-derived growth factor in the MF process. 23 TGF-β signaling plays an important role in inflammation and immune regulation.24,25 TGF-β1/Smad2/3 signaling contributes to MF. 26 We found that MIAT silencing inhibited the production and proliferation of TGF-β1-induced MF as shown by collagen expression. MIAT promotes angiogenesis and expression of inflammatory factors (IL-1β, IL-6, and TNF-α) in mice with AS by activating the PI3K/Akt signaling pathway. 9 TNF-α is a major cytokine implicated in the progression of AS. 22 In this study, silencing MIAT suppressed inflammatory factor expression by activating the PI3K/Akt signaling pathway. We also found that MIAT knockdown significantly inhibited the proliferation of MF in the HF rat model. Additionally, MIAT knockdown attenuated Col I and Col III levels in the HF model. MIAT may have regulated CF through the PI3K/Akt signaling pathway. Taken together, our results suggest that MF is ameliorated by MIAT through the PI3K/Akt signaling pathway and this relieves HF.
There are some limitations to the present study. Conservation of the MIAT sequence is low between rats and humans, and LncRNAs are generally poorly conserved among species. 27 Therefore, the clinical significance of this study remains unclear. Furthermore, except for the PI3K/Akt pathway, we cannot exclude the involvement of other mechanisms in regulating LncRNA MIAT in HF. Another limitation of this study is that we cannot rule out involvement of other mechanisms of regulation of cardiac fibrosis by MIAT other than the PI3K/Akt pathway.
This study indicates that silencing MIAT ameliorates MF by the PI3K/Akt signaling pathway to relieve heart failure. However, further research is required to support our results. Our findings suggest that LncRNA MIAT is a molecular target for intervention in HF in humans, but further study is required to verify the potential mechanism for LncRNA MIAT in treating HF.
Footnotes
Author contributions: Conception and design of the research: Xingsheng Zhao and Yu Ren; acquisition of data: Hongkun Ren; analysis and interpretation of data: Huan Chen and Chun Ying; statistical analysis: Yun Wu and Xi Liu; drafting the manuscript: Yu Ren; obtaining funding and revision of manuscript for important intellectual content: Xingsheng Zhao.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by the Inner Mongolia Autonomous Region Science and Technology Innovation Guidance Project (KCBJ2018039) and the Inner Mongolia Autonomous Region Science and Technology Project (201702118).
ORCID iD: Xingsheng Zhao https://orcid.org/0000-0002-1346-6913
References
- 1.Poustchi F, Amani H, Ahmadian Z, et al. Combination Therapy of Killing Diseases by Injectable Hydrogels: From Concept to Medical Applications. Adv Healthc Mater 2021; 10: e2001571. [DOI] [PubMed] [Google Scholar]
- 2.Lu M, Qin Q, Yao J, et al. Induction of LOX by TGF-beta1/Smad/AP-1 signaling aggravates rat myocardial fibrosis and heart failure. IUBMB Life 2019; 71: 1729–1739. [DOI] [PubMed] [Google Scholar]
- 3.Zhou R, Gao J, Xiang C, et al. Salvianolic acid A attenuated myocardial infarction-induced apoptosis and inflammation by activating Trx. Naunyn Schmiedebergs Arch Pharmacol 2020; 393: 991–1002. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Wang B, Li N, et al. Salvia miltiorrhiza and Carthamus tinctorius Extract Prevents Cardiac Fibrosis and Dysfunction after Myocardial Infarction by Epigenetically Inhibiting Smad3 Expression. Evid Based Complement Alternat Med 2019; 2019: 6479136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen S, Jiang H, Bei Y, et al. Long Non-Coding RNAs in Cardiac Remodeling. Cell Physiol Biochem 2017; 41: 1830–1837. [DOI] [PubMed] [Google Scholar]
- 6.Wang XM, Li XM, Song N, et al. Long non-coding RNAs H19, MALAT1 and MIAT as potential novel biomarkers for diagnosis of acute myocardial infarction. Biomed Pharmacother 2019; 118: 109208. [DOI] [PubMed] [Google Scholar]
- 7.Toraih EA, El-Wazir A, Alghamdi SA, et al. Association of long non-coding RNA MIAT and MALAT1 expression profiles in peripheral blood of coronary artery disease patients with previous cardiac events. Genet Mol Biol 2019; 42: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu X, Du Y, Shu Y, et al. MIAT Is a Pro-fibrotic Long Non-coding RNA Governing Cardiac Fibrosis in Post-infarct Myocardium. Sci Rep 2017; 7: 42657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun G, Li Y, Ji Z. Up-regulation of MIAT aggravates the atherosclerotic damage in atherosclerosis mice through the activation of PI3K/Akt signaling pathway. Drug Deliv 2019; 26: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu W, Li C, Qu X, et al. Arsenic-induced interstitial myocardial fibrosis reveals a new insight into drug-induced long QT syndrome. Cardiovasc Res 2012; 96: 90–98. [DOI] [PubMed] [Google Scholar]
- 11.Xiao H, Zhang H, Wang D, et al. Impact of smoke-free legislation on acute myocardial infarction and stroke mortality: Tianjin, China, 2007-2015. Tob Control 2020; 29: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu XH, Yuan YX, Rao S, et al. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur Rev Med Pharmacol Sci 2016; 20: 3653–3660. [PubMed] [Google Scholar]
- 13.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017; 70: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128: E147–E239. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Li Y, Sheng C, et al. Tanshinone IIA inhibits apoptosis in the myocardium by inducing microRNA-152-3p expression and thereby downregulating PTEN. Am J Transl Res 2016; 8: 3124–3132. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu M, Li N, Luo P, et al. Peripheral Blood Leukocyte Expression of lncRNA MIAT and Its Diagnostic and Prognostic Value in Ischemic Stroke. J Stroke Cerebrovasc Dis 2018; 27: 326–337. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Sun G, Dong X, et al. Isorhamnetin Attenuates Atherosclerosis by Inhibiting Macrophage Apoptosis via PI3K/AKT Activation and HO-1 Induction. PLoS One 2015; 10: e0120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail 2011; 13: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiojima I, Yefremashvili M, Luo Z, et al. Akt Signaling Mediates Postnatal Heart Growth in Response to Insulin and Nutritional Status. J Biol Chem 2002; 277: 37670–37677. [DOI] [PubMed] [Google Scholar]
- 21.Kemi OJ, Ceci M, Wisloff U, et al. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol 2008; 214: 316–321. [DOI] [PubMed] [Google Scholar]
- 22.Shiojima I, Sato K, Izumiya Y, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 2005; 115: 2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen RR, Fan XH, Chen G, et al. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGFβ1/Smad2/3 signaling axis. Chem Biol Interact 2019; 302: 11–21. [DOI] [PubMed] [Google Scholar]
- 24.Chen WJ, Dijke PT. Immunoregulation by members of the TGFβ superfamily. Nat Rev Immunol 2016; 16: 723–740. [DOI] [PubMed] [Google Scholar]
- 25.Matsuki K, Hathaway CK, Chang AS, et al. Transforming growth factor beta1 and aldosterone. Curr Opin Nephrol Hypertens 24: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Shen W, Zhang JY, et al. Stevioside attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-kappaB/TGF-beta1/Smad signaling pathway. Food Funct 2019; 10: 1179–1190. [DOI] [PubMed] [Google Scholar]
- 27.Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009; 458: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]