Abstract
Objective
To evaluate the efficacy of the Archimedes Navigation System (Broncus Medical, San Jose, CA, USA) for guidance during transbronchial cryobiopsy and the incidence of complications in patients with diffuse lung disease.
Methods
High-resolution computed tomography and transbronchial cryobiopsy were used to evaluate eight patients with diffuse lung disease. The Archimedes Navigation System was used before cryobiopsy to obtain the best path with which to avoid large vessels. Three to five cryobiopsy specimens were taken from each sampled segment.
Results
Preoperative planning using the Archimedes Navigation System was successfully performed on all eight patients. The probe-to-pleura distance was approximately 10 mm. No cases of pneumothorax occurred, one patient developed moderate bleeding, two developed minor bleeding, and five developed minimal bleeding that stopped spontaneously. A final diagnosis was obtained for seven patients, and ongoing follow-up was being conducted for the last patient at the time of this writing.
Conclusions
This is the first report of combining navigation technology with cryobiopsy to diagnose diffuse lung disease. The Archimedes Navigation System, which provides real-time guidance, is helpful in pre-cryobiopsy planning and diagnosis of diffuse lung disease. Moreover, this system can reduce the pneumothorax rate and bleeding risk by avoiding large vessels.
Keywords: Archimedes Navigation System, cryobiopsy, diffuse lung disease, complication, diagnosis, preoperative planning
Introduction
Diffuse lung diseases have complex and diverse etiologies and are diagnosed through the overall consideration of clinical, imaging, and histopathological manifestations. Surgical lung biopsy is the gold standard for obtaining histopathological information, but such biopsies are not often performed because of the high risk of surgery, high cost, and numerous complications.1–4 Cryobiopsy is being increasingly and widely used to obtain diagnostic information from patients with diffuse lung disease. Unlike the use of a conventional biopsy forceps, cryobiopsy facilitates the acquisition of a large tissue specimen and guarantees its structural integrity 5 ; therefore, this technology can markedly improve the diagnostic yield. 6 However, pneumothorax and bleeding are major complications. According to reports in the literature, the incidence rates of pneumothorax and moderate to severe blood loss range from 0% to 26% and from 0% to 78%, respectively, showing wide variation among different centers.5–8 The wide ranges of complication rates are related to several issues, including the procedural methods used, operator’s experience, freezing time, number of freezes, and evaluation techniques used immediately after the procedure (e.g., chest X-ray to confirm the presence of pneumothorax).5–8
Methods for reducing bleeding and pneumothorax associated with cryobiopsy have become a focus of research. A widely used technique for obtaining biopsy specimens at a distance of 1 to 2 cm from the pleura is the use of a cryoprobe under C-arm computed tomography (CT) guidance, which can maximize the acquisition of lesions near the pleura and reduce the occurrence of pneumothorax.9,10 However, C-arm CT-guided cryobiopsy cannot reduce the rate of bleeding complications. In addition, C-arm CT leads to prolonged radiation exposure in both operators and patients, further limiting its use. Small ultrasound probes have been used to detect the presence of major blood vessels in the areas intended for cryobiopsy, 11 but the use of small ultrasound probes is limited by their low resolution; such probes can only reveal large vessels within lesions associated with diffuse pulmonary disease.
Numerous bronchoscopic navigation technologies have been used for the diagnosis of lung disease. The main navigation technologies currently in use are virtual navigation, electromagnetic navigation, and the Archimedes Navigation System (Broncus Medical, San Jose, CA, USA). The Archimedes Navigation System, which is mainly applied for the diagnosis and treatment of peripheral lung nodules, not only has the functions of the other navigation systems but can also clearly show the pulmonary blood vessels. 12 It contains Doppler positioning capabilities, which can display different paths and demonstrate the best path to avoid blood vessels, thereby reducing bleeding complications. The Archimedes Navigation System not only has enhanced ability to detect blood flow in vessels near the region of interest but can also measure the distance from the region of interest to the pleura in real time. Therefore, we considered that a cryoprobe can be more accurately placed closer to the pleura using this device, making it possible to use cryobiopsy for the diagnosis of diffuse lung disease. To the best of our knowledge, no studies to date have examined the combination of navigation technology with cryobiopsy for the diagnosis of diffuse lung disease. In this preliminary study, we assessed the feasibility of a newly designed application involving use of the Archimedes Navigation System for preoperative planning and intraoperative-guided cryobiopsy for the diagnosis of diffuse lung disease. We also evaluated the safety and positive diagnostic rate of this technique.
Materials and methods
Study design and participants
This clinical prospective single-arm intervention study was performed to evaluate the application value of the Archimedes Navigation System for cryobiopsy. Patients with diffuse lung disease were enrolled from 5 May 2018 to 5 December 2019. The inclusion criteria were age of 18 to 75 years, diffuse bilateral lung disease of unknown cause, inability of high-resolution CT (HRCT) to identify the cause of the disease, interstitial pneumonia of unknown cause, understanding and agreement with the purpose of the study by both patients and their families, and patient provision of written informed consent for examination. The exclusion criteria were inability to tolerate tracheal endoscopy under general anesthesia, long-term use of anticoagulants (warfarin or aspirin that could not be stopped), abnormal platelet function or low platelet count (<100 × 109), history of massive hemorrhage under bronchoscopy, severe cardiac insufficiency, very low diffusion capacity of the lungs, and pregnancy or lactation.
General anesthesia was applied to all patients, and cryobiopsy was performed with the use of a rigid bronchoscope or a laryngeal mask airway for patient ventilation. Consultation with the Pathology Department was required to evaluate the pathological biopsy specimens. Data from patients who could not be clearly diagnosed were reviewed by a multidisciplinary clinical/pathological/imaging team. During and after surgery, information on complications and management was recorded.
This study was ethically conducted in accordance with the World Medical Association Declaration of Helsinki. Approval for this study was obtained from the ethics committee of Henan Provincial People’s Hospital (People’s Hospital of Zhengzhou University) (ethics approval no. 2019-88). Written informed consent to participate in this clinical research was provided by each patient.
Preoperative planning
HRCT was performed with a slice thickness of 0.625 mm. All patients’ HRCT data were collected and sent to a GEAW4.4 workstation with 64 slices, then imported to the Archimedes Navigation System for preoperative planning. There were five regions of interest per patient; these regions of interest were also the areas of lung cryobiopsy. First, we identified and marked each region of interest, in which the probe-to-pleura distance was measured as approximately 20 mm and the target region was located away from the vascular area (Figure 1). The Archimedes Navigation System allowed us to study the distribution of blood vessels with different diameters (1, 5, and 10 mm) in the region of interest, which could be used to plan the cryobiopsy path. When the diameter of vessels was smaller than 10 mm, the region of interest could be the target of lung cryobiopsy (Figure 2). The optimal planning path was then selected, and the distance from the tip of the bronchoscope to the midpoint of the region of interest was measured. The preoperative planning data of a representative patient are shown in Figure 3.
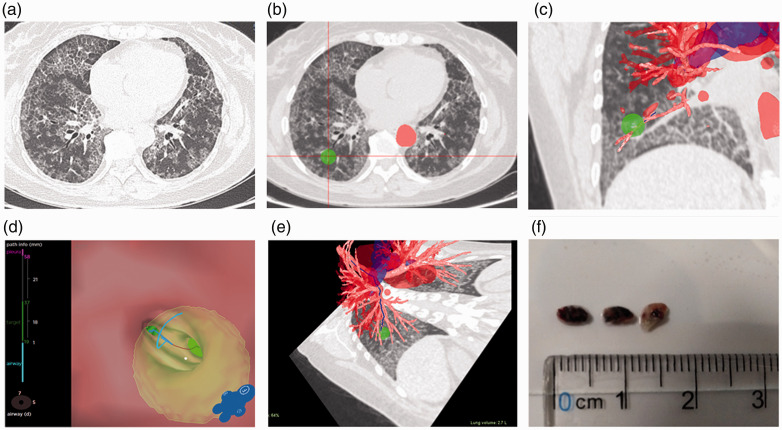
Figure 1.
Different images of the region of interest. (a) Computed tomography images for cryobiopsy show diffuse pavers in both lungs of Patient 6. (b) The region of interest is marked. (c) Coronal view of the region of interest. (d) Distance from the bronchial orifice to the region of interest is measured. (e) Path of endotracheal endoscope. (f) Three lung tissue specimens successfully extracted under the Archimedes Navigation System.
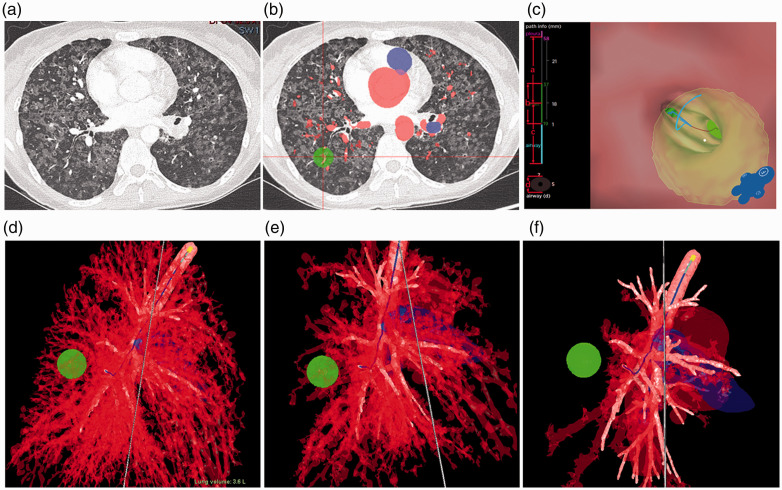
Figure 2.
Blood vessels in Patient 8 according to the Archimedes Navigation System planning. (a) Conventional computed tomography scan. (b) Enhanced computed tomography scan. (c) Pathological vascular changes in lungs. (d) 1-mm region showing close association with blood vessels. (e) 5-mm region where blood vessels are relatively sparse. (f) 10-mm region without obvious blood vessels.
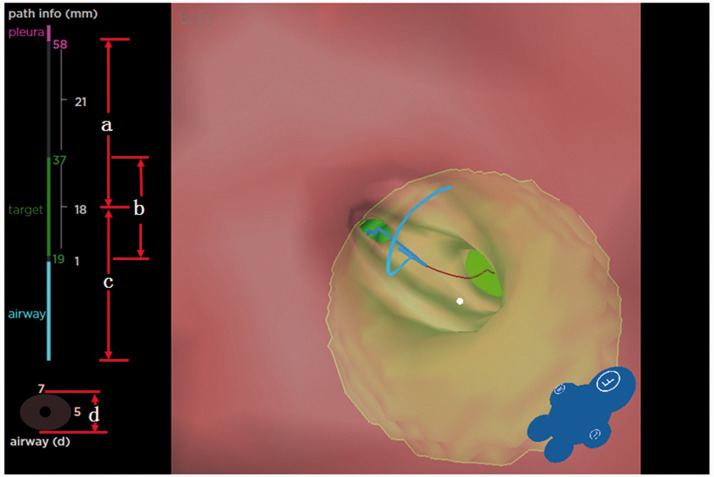
Figure 3.
Preoperative planning data for Patient 6. (a) Distance from region of interest to visceral pleura. (b) Diameter of region of interest. (c) Distance from region of interest to bronchial orifice. (d) Diameter of bronchus at the region of interest.
Procedure
Cryobiopsy was performed with a laryngeal mask airway in two patients and a rigid tracheoscope in the remaining patients based on their willingness. The positioning board was used to register the intraoperative bodies with the preoperative CT images. The patients underwent volume-controlled ventilation during the operation, and the volume corresponded to the resting-state tidal volume; thus, a consistent respiration amplitude was maintained during the CT examination, reducing the influence of respiration on the navigation system and ensuring accurate positioning. The bronchoscope (1TQ290; Olympus Medical Systems, Tokyo, Japan) was matched with the Archimedes Navigation System and then directed to the entrance of the bronchus in the region of interest. The distance from the bronchial orifice to the region of interest was measured as distance c (Figure 3). A 1.9-mm flexible cryoprobe (Erbe, Tübingen, Germany) was introduced to approximately distance c, but was extended from the tip of the bronchoscope. Cryobiopsy was performed (5- to 7-second freeze time) in the region of interest in the lung according to the preoperative planning. Three to five specimens were taken from each region and collected for histopathological examinations (unless uncontrollable bleeding or other serious complications occurred). Intraoperative hemorrhage was observed and record. A C arm (Ziehm 8000; Ziehm Imaging, Nuremberg, Germany) was used to check for pneumothorax during the operation.
Management of complications
Blood loss was categorized according to a modified classification system 13 as follows: grade 0, no blood loss or insignificant blood loss that resolved without intervention; grade 1, mild blood loss that was resolved by cold (2°C–4°C) 0.9% sodium chloride solution or epinephrine solution (1 mg/100 mL saline); grade 2, moderate blood loss requiring argon plasma coagulation (APC) or bronchial balloon blockage; and grade 3, severe blood loss leading to transfusion of blood products, vasopressor support, rescue surgery, or death. Grade 1, 2, and 3 bleeding required at least one type of hemostatic intervention.
Pneumothorax was confirmed by chest X-ray examination within 3 hours after the cryobiopsy procedure. Once pneumothorax had occurred, the patients were treated by insertion of a drainage tube; this was based on the volume of the pneumothorax or the patients’ signs and symptoms. Other procedure-related complications were observed during and after the procedure.
Results
Eight patients were included in this study. The patients comprised three men and five women with a median age of 56 years (range, 23–66 years). The patients’ clinical features and final diagnoses are shown in Table 1. One patient with a history of rhinitis was diagnosed with diffuse bronchiolitis based on the results of the histopathological examination of the cryobiopsy specimens and imaging examination. One patient with a history of arthritis was diagnosed with pulmonary fibrosis secondary to arthritis based on the results of the histopathological examination of the cryobiopsy specimens combined with the medical history and pathological results. The remaining patients had no specific underlying disease. Two patients underwent complete cryobiopsy under a laryngeal mask airway, and the others underwent cryobiopsy under rigid bronchoscopic ventilation. Four patients underwent cryobiopsies of the right anterior basal segment (RB8) and right lateral basal segment (RB9). One of these four patients was biopsied six times, with three attempts to acquire satisfactory specimens. Their intraoperative bleeding was moderate and stopped by APC. The remaining four patients underwent cryobiopsy of segment RB8, with minimal bleeding that stopped spontaneously.
Table 1.
Clinical features, complications, and diagnoses.
| Patient number |
Clinical features |
Complications |
Diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Medical history | Check ways | Target lobe | Biopsies (n) | Specimens (n) | Bleeding | Pneumothorax | Pathologically confirmed | MDT* | Final diagnosis achieved | |
| 1 | 66 | F | None | Laryngeal | RB8 | 4 | 3 | Grade 0 | No | – | DPB | Yes |
| 2 | 62 | M | Diabetes | Laryngeal | RB8/RB9 | 5 | 3 | Grade 1 | No | – | IPF | Yes |
| 3 | 58 | F | Arthritis | Rigid | RB6 | 5 | 3 | Grade 0 | No | – | Secondary interstitial (arthritis) | Yes |
| 4 | 48 | M | Rhinitis | Rigid | RB8/RB9 | 5 | 3 | Grade 2 | No | – | DPB | Yes |
| 5 | 23 | M | None | Rigid | RB8 | 4 | 3 | Grade 0 | No | – | Secondary interstitial | Yes |
| 6 | 54 | F | None | Rigid | RB8 | 4 | 3 | Grade 1 | No | PAP | Yes | |
| 7 | 62 | F | None | Rigid | RB8/RB9 | 6 | 3 | Grade 0 | No | – | No | |
| 8 | 28 | F | None | Rigid | RB8/RB9 | 6 | 3 | Grade 0 | No | Adenocarcinoma | Yes | |
F, female; M, male; RB8, right anterior basal segment; RB9, right anterior lateral segment; RB6, right superior segment; PAP, pulmonary alveolar proteinosis; MDT, multidisciplinary team; IPF, idiopathic pulmonary fibrosis; DPB, diffuse pantothenic bronchiolitis.
*Cases were discussed by a team consisting of a clinician, pathologist, and imaging physician.
The Archimedes Navigation System planning data are shown in Table 2. A planning path was selected based on the region of interest and its connection to a bronchus while avoiding peripheral vessels.
Table 2.
Archimedes Navigation planning path and data.
| Patient number | b (region of interest) (mm) | Region of interest communicating with bronchus | a (center of region of interest to pleura) (mm) | c (entrance of bronchus to center of region of interest) (mm) | d (diameter of bronchus) (mm) | C-arm |
|---|---|---|---|---|---|---|
| 1 | 15 | Yes | 26 | 35 | 7 | No |
| 2 | 16 | Yes | 22 | 29 | 8 | No |
| 3 | 17 | Yes | 20 | 36 | 7 | No |
| 4 | 15 | Yes | 23 | 34 | 6 | No |
| 5 | 18 | Yes | 22 | 27 | 6 | No |
| 6 | 15 | Yes | 20 | 19 | 5 | No |
| 7 | 14 | Yes | 19 | 28 | 6 | No |
| 8 | 15 | No | 25 | 39 | 7 | No |
Cryobiopsy guided by the Archimedes Navigation System was completed for all the study patients. The distance from the probe to the midpoint of the region of interest is shown as c in Figure 2. No death, acute pulmonary edema, continuous fever, or other serious complications occurred in any patient. Minimal bleeding (grade 0) occurred in five patients, and minor bleeding (grade 1) that stopped after the administration of a hemostatic agent occurred in two patients. A moderate amount of bleeding occurred in only one patient and stopped after APC treatment. None of the patients developed pneumothorax.
Seven patients received a final diagnosis. Lung adenocarcinoma was diagnosed in one patient and pulmonary alveolar proteinosis was diagnosed in one patient based on the histopathological examination. After a multidisciplinary discussion of the imaging, histopathology, and respiratory findings, diffuse bronchiolitis, secondary interstitial pneumonia, and idiopathic pulmonary fibrosis were diagnosed in two, two, and one patient, respectively. The diagnosis was unclear for one patient who refused to undergo a surgical biopsy. Ongoing follow-up of this patient was being conducted at the time of this writing.
Discussion
Surgical lung biopsy is the gold standard for obtaining histopathological information, 1 which can then be used to diagnose diffuse lung disease. However, lung biopsy has significant drawbacks such as the high risk of surgery, high cost, limited availability, and numerous complications.2–4 Hence, increasingly more studies are being conducted to reduce and avoid these drawbacks. First reported in 1977, 14 transbronchial lung cryobiopsy has emerged as a promising option for obtaining lung tissue. Compared with conventional biopsy, the cryobiopsy technique can obtain sufficiently large specimens and has significantly increased the diagnostic yield of airway lesions. 15 Many reports have documented the safety and feasibility of transbronchial lung cryobiopsy to diagnose respiratory disease. Shintani et al. 16 reported that transbronchial lung cryobiopsy and histology led to a diagnosis of acute fibrinous and organizing pneumonia in a 53-year-old Japanese woman. Troy et al. 17 investigated the diagnostic agreement between transbronchial lung cryobiopsy and surgical lung biopsy for interstitial lung disease and found that the yield of transbronchial lung cryobiopsy was higher than 70% when compared with the gold standard of surgical biopsy, showing high levels of agreement for both histopathological interpretation and multidisciplinary discussion. Different medical centers have used transbronchial lung cryobiopsy for the diagnosis of diffuse lung disease. However, the results of many studies also suggest that visualization of the position of the cryobiopsy probe under the C arm before cryobiopsy must be improved. 10
Most guided bronchoscopy systems, including virtual navigation, augmented fluoroscopy technology, and electromagnetic navigation systems, depend on preprocedural CT scans to create a virtual lung map. When changes in lung anatomy occur, the resulting “CT-to-body divergence” may cause inaccurate intraprocedural lesion localization and reduced diagnostic yield.18–20 In our study, a positioning board and volume-controlled ventilation were applied to reduce the influence of the divergence and respiration on the navigation system. Moreover, the distance between the area of interest and its bronchial orifice was measured by the Archimedes Navigation System after the region of interest had been located. During cryobiopsy, the distance that the cryoprobe needed to be moved to enter the orifice of the region of interest was consistent with the navigation measurement distance. This confirmed that the biopsy was performed in the region of interest and indicates that the Archimedes Navigation System can ensure accurate positioning. Based on its Doppler positioning capabilities, the Archimedes Navigation System can detect blood flow in vessels near the region of interest to provide an appropriate path that avoids bleeding and to allow the cryoprobe to be placed with increased accuracy closer to the pleura. In addition, the system can measure the distance from the region of interest to the pleura in real time. In this study, preoperative planning using the Archimedes Navigation System was successfully performed in all eight patients as expected, and the probe-to-pleura distance was approximately 20 mm. Our study confirms the feasibility of using the Archimedes Navigation System in pre-cryobiopsy planning. This is also the first report of combining navigation technology with cryobiopsy to diagnose diffuse lung disease.
To date, almost all studies on cryobiopsy have focused on safety.8,10,11,21,22 The main complications are bleeding and pneumothorax. The incidence of bleeding and pneumothorax, which was also studied in our research, varies among different centers. The Archimedes Navigation System was used to analyze the vessels and bronchi around the region of interest according to the HRCT imaging findings and planning path, allowing us to determine the distance from the tip of the cryoprobe to the region of interest before cryobiopsy. When blood vessels were sufficiently far from the region of interest, the cryobiopsy could be conducted while avoiding large blood vessels (Figure 2). In this way, the combination of the Archimedes Navigation System and transbronchial cryobiopsy may be superior to the use of a single technology. None of the eight patients developed pneumothorax, indicating that this method can reduce the rate of occurrence of pneumothorax and avoid operator exposure to imaging radiation under C-arm contrast-enhanced CT. Two (25.0%) patients developed grade 1 hemorrhage, one (12.5%) patient developed grade 2 hemorrhage, and the remaining patients (62.5%) developed grade 0 hemorrhage; these findings are consistent with previous reports.7,8,23 In addition, the complications that occur in patients without the application of navigation in our hospital are similar to the results of the present study; however, the former requires X-ray guidance, which leads to radiation exposure.
Diffuse lung diseases present major problems to clinical investigators. The diagnostic yield of conventional biopsy is low, and difficulties are encountered in most patients undergoing open-chest lung biopsy because of the risk of many complications and the long duration of hospitalization. Since the implementation of the cryobiopsy technique, many patients have agreed to undergo cryobiopsy to attain a definitive diagnosis. Patients’ acceptance of cryobiopsy is of great significance to the diagnosis of diffuse pulmonary disease.
In this study, the Archimedes Navigation System was selected to locate the region of interest preoperatively, and the region of interest was then directly cryobiopsied under navigation guidance. Of the eight patients, two (25.0%) patients’ diagnoses were clear on histopathology, five (62.5%) were clearly diagnosed after discussion by a multidisciplinary team, and one (12.5%) was not clearly diagnosed. This indicates that a team of physicians with specialties in different fields plays an important role in the diagnosis of diffuse pulmonary disease. The proportion of patients with a confirmed diagnosis (87.5% of 8 patients) was higher than that found in other studies8,23; we consider the following reasons for this discrepancy. First, all patients in the present study had bilateral lung disease (including nodules, voids, diffuse exudation, and fibrosis) rather than bilateral diffuse interstitial lung disease. Second, the study included a small number of patients; more patients are needed to confirm the feasibility of this method. In addition, the low complication rate in this study requires further validation. Third, no surgical lung biopsies were performed for further histological comparison, and the results discussed by the multidisciplinary team were unclear. We have herein proposed an experimental method for the diagnosis of diffuse lung disease by lung biopsy, verifying the feasibility of cryobiopsy guided by the Archimedes Navigation System.
In conclusion, the Archimedes Navigation System can be used to perform lung cryobiopsy while accurately locating the region of interest and avoiding the occurrence of pneumothorax. In addition, its Doppler function might reduce the rate of bleeding complications, resulting in a minimally invasive procedure that does not lead to increased risk and increased use of consumables. The Archimedes Navigation System can be used for diagnostic cryobiopsies in patients with diffuse lung disease because the operator and patient are not exposed to radiation from C-arm HRCT. However, more cases or randomized controlled studies are needed for further verification of the effectiveness and safety of the Archimedes Navigation System.
Acknowledgment
We wish to thank the staff of the Department of Respiratory and Critical Care Medicine and Department of Medical Equipment of Henan Provincial People’s Hospital, Zhengzhou University People's Hospital for their assistance.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the Medical Science and Technology Foundation of Henan Province (201602240, 2018020404) the Science and Technology Foundation of Henan Province (20161430) and the Health Science and Technology Talents Overseas Training Project of Henan Province (2016048).
Authors’ contributions: Guarantors of the integrity of the entire study: Xiaoju Zhang and Quncheng Zhang. Study conception and manuscript review: Xiaoju Zhang. Study design, data acquisition, data analysis, and statistical analysis: Quncheng Zhang. Definition of intellectual content, literature research, manuscript preparation, and manuscript editing: Huili Li. Clinical studies: Guannan Sun, Yong Qi, Dongjun Cheng, Zheng Wang, Weixia Xuan, and Yunxia An.
ORCID iD: Xiaoju Zhang https://orcid.org/0000-0002-4189-0121
References
- 1.Kuse N, Inomata M, Awano N, et al. Management and utility of transbronchial lung cryobiopsy in Japan. Respir Investig 2019; 57: 245–251. [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Wu CT, Hsu HH, et al. Surgical lung biopsy for diffuse pulmonary disease: experience of 196 patients. J Thorac Cardiovasc Surg 2005; 129: 984–990. [DOI] [PubMed] [Google Scholar]
- 3.Cooley J, Balestra R, Aragaki-Nakahodo AA, et al. Safety of performing transbronchial lung cryobiopsy on hospitalized patients with interstitial lung disease. Respir Med 2018; 140: 71–76. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura H, Awano N, Inomata M, et al. Diagnostic utility of transbronchial lung cryobiopsy: two cases of anti-aminoacyl-tRNA synthetase syndrome with respiratory failure. Respir Investig 2019; 57: 399–403. [DOI] [PubMed] [Google Scholar]
- 5.Johannson KA, Marcoux VS, Ronksley PE, et al. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. A systematic review and metaanalysis. Ann Am Thorac Soc 2016; 13: 1828–1838. [DOI] [PubMed] [Google Scholar]
- 6.Ganganah O, Guo SL, Chiniah M, et al. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology 2016; 21: 834–841. [DOI] [PubMed] [Google Scholar]
- 7.Ravaglia C, Wells AU, Tomassetti S, et al. Transbronchial lung cryobiopsy in diffuse parenchymal lung disease: comparison between biopsy from 1 segment and biopsy from 2 segments - diagnostic yield and complications. Respiration 2017; 93: 285–292. [DOI] [PubMed] [Google Scholar]
- 8.Ussavarungsi K, Kern RM, Roden AC, et al. Transbronchial cryobiopsy in diffuse parenchymal lung disease: retrospective analysis of 74 cases. Chest 2017; 151: 400–408. [DOI] [PubMed] [Google Scholar]
- 9.Poletti V, Casoni GL, Gurioli C, et al. Lung cryobiopsies: a paradigm shift in diagnostic bronchoscopy? Respirology 2014; 19: 645–654. [DOI] [PubMed] [Google Scholar]
- 10.Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the Cryobiopsy Working Group on Safety and Utility and a call for standardization of the procedure. Respiration 2018; 95: 188–200. [DOI] [PubMed] [Google Scholar]
- 11.Viglietta L, Inchingolo R, Pavano C, et al. Ultrasonography for the diagnosis of pneumothorax after transbronchial lung cryobiopsy in diffuse parenchymal lung diseases. Respiration 2017; 94: 232–236. [DOI] [PubMed] [Google Scholar]
- 12.Herth FJ, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015; 70: 326–332. [DOI] [PubMed] [Google Scholar]
- 13.Schumann C, Hetzel M, Babiak AJ, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg 2010; 139: 997–1000. [DOI] [PubMed] [Google Scholar]
- 14.Rodgers BM, Rosenfeld M, Talbert JL. Endobronchial cryotherapy in the treatment of tracheal strictures. J Pediatr Surg 1977; 12: 443–449. [DOI] [PubMed] [Google Scholar]
- 15.Schumann C, Hetzel J, Babiak AJ, et al. Cryoprobe biopsy increases the diagnostic yield in endobronchial tumor lesions. J Thorac Cardiovasc Surg 2010; 140: 417–421. [DOI] [PubMed] [Google Scholar]
- 16.Shintani R, Oda T, Niwa T, et al. Transbronchial lung cryobiopsy in idiopathic acute fibrinous and organizing pneumonia. Respir Med Case Rep 2019; 28: 100888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troy KL, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020; 8: 171–181. [DOI] [PubMed] [Google Scholar]
- 18.Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis 2018; 10: 6950–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchett M. Comparison of pulmonary nodule location between preprocedural CT and intra-procedural cone-beam CT during guided bronchoscopy. J Thorac Oncol 2018; 13: S403. [Google Scholar]
- 20.Pritchett MA, Bhadra K, Calcutt M, et al. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis 2020; 12: 1595–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinfort DP, D'Agostino RD, Vrjlic I, et al. CT-Fluoroscopic guidance for performance of targeted transbronchial cryobiopsy: a preliminary report. Respiration 2018; 96: 472–479. [DOI] [PubMed] [Google Scholar]
- 22.Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016; 193: 745–752. [DOI] [PubMed] [Google Scholar]
- 23.Segmen F, Aktas Z, Ozturk A, et al. How many samples would be optimal for endobronchial cryobiopsy? Surg Endosc 2017; 31: 1219–1224. [DOI] [PubMed] [Google Scholar]