Abstract
Objective:
To evaluate the potential of intracortical electrode array signals for brain-computer interfaces (BCIs) to restore lost speech, we measured the performance of decoders trained to discriminate a comprehensive basis set of 39 English phonemes and to synthesize speech sounds via a neural pattern matching method. We decoded neural correlates of spoken-out-loud words in the “hand knob” area of precentral gyrus, a step towards the eventual goal of decoding attempted speech from ventral speech areas in patients who are unable to speak.
Approach:
Neural and audio data were recorded while two BrainGate2 pilot clinical trial participants, each with two chronically-implanted 96-electrode arrays, spoke 420 different words that broadly sampled English phonemes. Phoneme onsets were identified from audio recordings, and their identities were then classified from neural features consisting of each electrode’s binned action potential counts or high-frequency local field potential power. Speech synthesis was performed using the ‘Brain-to-Speech’ pattern matching method. We also examined two potential confounds specific to decoding overt speech: acoustic contamination of neural signals and systematic differences in labeling different phonemes’ onset times.
Main results:
A linear decoder achieved up to 29.3% classification accuracy (chance = 6%) across 39 phonemes, while a recurrent neural network classifier achieved 33.9% accuracy. Parameter sweeps indicated that performance did not saturate when adding more electrodes or more training data, and that accuracy improved when utilizing time-varying structure in the data. Microphonic contamination and phoneme onset differences modestly increased decoding accuracy, but could be mitigated by acoustic artifact subtraction and using a neural speech onset marker, respectively. Speech synthesis achieved r = 0.523 correlation between true and reconstructed audio.
Significance:
The ability to decode speech using intracortical electrode array signals from a nontraditional speech area suggests that placing electrode arrays in ventral speech areas is a promising direction for speech BCIs.
Keywords: speech decoding, intracortical, BCI, precentral gyrus, motor cortex, human
1. Introduction
Neurological disorders such as stroke and amyotrophic lateral sclerosis (ALS) take away the ability to speak from millions worldwide (LaPointe and Stierwalt 2018). In the case of ALS, loss of speech is often considered the worst aspect of disease progression, necessitating augmentative communication within 1-2 years of symptom onset (Makkonen et al. 2018). Noninvasive assistive technologies such as sip-and-puff interfaces and eye tracking have helped re-establish communication, but suffer from low information transfer rates and limited expressivity (Koch Fager et al. 2019; Tai, Blain, and Chau 2008). In contrast, speech is amongst the most intuitive and fastest means of communication, with conversational speech conveying roughly 150 words per minute (Chang and Anumanchipalli 2020). Brain-computer interfaces (BCIs) are an emerging technology through which a person’s movement intentions are read out from their neural signals (Slutzky 2019; Tam et al. 2019). A high-performance speech BCI that could accurately identify what people with speech loss want to say would therefore substantially improve patients’ quality of life.
To gain direct access to the relevant neural signals, ongoing efforts to build speech BCIs (reviewed in (Rabbani, Milsap, and Crone 2019; Herff and Schultz 2016; Chakrabarti et al. 2015; Martin et al. 2018) have often used invasive electrocorticography (ECoG) measurements, in which electrode disks distributed across the cortical surface sample the aggregate activity of many tens of thousands of neurons under each recording site. This approach has been used in discrete classification of small speech building blocks such as phonemes, syllables, words, and sound snippets (Salari et al. 2018; Ramsey et al. 2018; Herff et al. 2015; Mugler et al. 2014; Martin et al. 2014; Kellis et al. 2010; Herff et al. 2019; Bouchard and Chang 2014). Recent work has also used neural networks to learn nonlinear mappings that transform neural signals into a variety of outputs such as acoustic speech features (Akbari et al. 2019; Angrick et al. 2019), including by way of intermediate articulatory kinematics (Anumanchipalli, Chartier, and Chang 2019), discrete targets such as phonemes or syllables (Livezey, Bouchard, and Chang 2019), and entire phrases and sentences (Moses et al. 2019; Makin, Moses, and Chang 2020).
While the most advanced speech BCIs to date are ECoG-based, other recording modalities are also being investigated. These include non-surgical methods such as electroencephalography (Nguyen, Karavas, and Artemiadis 2018) and magnetoencephalography (Dash, Ferrari, and Wang 2020), stereotactic electroencephalography to access field potentials from deeper brain structures (Herff, Krusienski, and Kubben 2020), and intracortical electrodes capable of recording neuronal spiking activity (Guenther et al. 2009; Brumberg et al. 2011). The intracortical approach, which can in principle sample from many distinct sources of information (individual neurons or small groups of neurons), is particularly promising for providing the sufficiently high neural bandwidth necessary to restore conversational speech. Intracortical BCIs that use multielectrode array signals to decode attempted arm and hand movements have demonstrated the highest performance to date for controlling computer cursors (Pandarinath et al. 2017), high degree-of-freedom robotic arms (Collinger et al. 2013), and the user’s own arm muscles (Ajiboye et al. 2017), with no prior user training (Brandman et al. 2018). In contrast, intracortical BCIs for decoding speech are far less mature. Several studies have examined speech production using small numbers of intracortical recording sites in lateral temporal lobe (Creutzfeldt, Ojemann, and Lettich 1989; Tankus, Fried, and Shoham 2012), ventral motor cortex (Guenther et al. 2009; Brumberg et al. 2011), orbitofrontal cortex (Tankus, Fried, and Shoham 2012), and subthalamic nucleus (Tankus and Fried 2019; Lipski et al. 2018). Ninety-six-electrode Utah arrays, of the type used in the aforementioned arm and hand BCIs, have been placed into superior temporal gyrus to decode heard speech in monkeys (Heelan et al. 2019) and both heard and spoken speech in a person (Chan et al. 2014). However, these previous studies did not use large numbers of electrodes to record from motor areas of cortex, which is the approach that has proved promising for restoring arm and hand function.
We are positioned to start to address this gap after having recently found speech-related activity in the dorsal “hand/knob” area of precentral gyrus (Stavisky et al. 2019; Willett et al. 2020), where a number of groups already place Utah arrays.These signals could be used to discriminate between a small number of syllables and words with high accuracy (Stavisky et al. 2018, 2019, 2020). In the present study, we build on this discovery to further evaluate the potential of using intracortical signals from two Utah arrays to decode among 39 phonemes, which could be used as a comprehensive basis set in a speech BCI, and to reconstruct speech sounds using the Brain-to-Speech pattern matching technique of (Herff et al. 2019). Importantly, we recorded from the dorsal precentral gyrus of two people with tetraplegia who already had arrays placed as part of their participation in the BrainGate2 clinical trial, which has the primary goal of evaluating the safety of these intracortical BCI devices. We recognize and wish to highlight that this cortical area, which has a well-established role in volitional arm and hand movements (Hochberg et al. 2006, 2012; Collinger et al. 2013; Wodlinger et al. 2015; Pandarinath et al. 2017; Ajiboye et al. 2017; Bouton et al. 2016; Downey, Weiss, et al. 2018; Rastogi et al. 2020) and to a lesser extent movements of other body parts (Stavisky et al. 2019; Willett et al. 2020), is likely sub-optimal for decoding speech. Nonetheless, this research context also presents a rare opportunity to evaluate the feasibility of decoding speech production from many simultaneously recorded intracortical signals, using ground truth data from people who can still speak, without additional risk. Our guiding motivation is that the results of this investigation should be viewed as a lower bound proof-of-concept on the intracortical performance that ought to be possible, and that higher performance will be possible with arrays specifically placed in ventral speech cortex where previous ECoG studies have shown strong speech production-related modulation (Kellis et al. 2010; Bouchard et al. 2013; Herff et al. 2019; Mugler et al. 2018; Chartier et al. 2018; Takai, Brown, and Liotti 2010).
We recorded neural signals as the participants spoke out loud a set of 420 different short words. This set included most of the same spoken words as previous ECoG studies (Mugler et al. 2014; Herff et al. 2019), which allowed us to make a well-matched comparison between decoding Utah array signals and these previous ECoG results. We first examined whether neural firing rates changed when the participant spoke just one or a handful of phonemes (“sparse tuning”), or across speaking many phonemes (“broad tuning”). This measurement is important for assessing the viability of intracortical speech BCIs, which in the near-term are likely to be restricted to hundreds (not thousands) of electrodes located within a relatively localized area of cortex. We found that activity recorded on these electrodes had broad tuning; this encoding makes it more likely that signals related to producing all the phonemes will be observable from a limited number of electrodes, and also has previously been shown to be more robust and decodable (Abbott and Dayan 1999). Next, we trained both conventional and deep learning classifiers to predict which of 39 phonemes was being spoken, using the available neural signals. We found that decoding performance was better using high-frequency local field potentials (HLFP) rather than threshold crossing spikes (i.e., action potentials from one or potentially several neurons near an electrode tip detected when the measured voltage drops below a set threshold), and was competitive with previous ECoG decoding performance (Mugler et al. 2014). Encouragingly, subsampling the data used for decoder training indicated that speech BCI performance is likely to improve as more training data and electrode recording sites become available. We also evaluated reconstructing continuous speech from these neural data by combining audio snippets corresponding to the closest matching neural window in a training data set, and again observed comparable performance to reported ECoG speech synthesis using the same technique (Herff et al. 2019).
While there are many advantages to using overt speaking data to establish proof-of-feasibility for a speech BCI, this widely used model (Suppes, Lu, and Han 1997; Kellis et al. 2010; Bouchard and Chang 2014; Pailla et al. 2016; Mugler et al. 2014; Herff et al. 2015; Ramsey et al. 2018; Salari et al. 2018; Angrick et al. 2019; Herff et al. 2019; Moses et al. 2019; Anumanchipalli, Chartier, and Chang 2019; Livezey, Bouchard, and Chang 2019; J. G. Makin, Moses, and Chang 2020) also introduces potential confounds. Here we investigated two potential limitations which have received little prior attention. The first is that using the recorded audio signal to detect voice onset may introduce systematic onset time biases across phonemes due to differences between when voice sounds become detectable versus when the speech articulators are being moved. This in turn can exaggerate neural differences between phonemes by artificially shifting what are actually condition-invariant neural signal components. The second confound, which was recently raised by Roussel and colleagues (Roussel et al. 2020), is that mechanical vibrations due to speaking might cause microphonic artifacts in the neural recordings. Our analyses suggest that while these confounds most likely do inflate speech decoding performance, their effects are not large. Furthermore, we introduce analysis methods that can be applied to neural data collected during overt speech to mitigate these confounds.
2. Methods
2.1. Participants
Research sessions were conducted with volunteer participants enrolled in the BrainGate2 pilot clinical trial (ClinicalTrials.gov Identifier: NCT00912041). The trial is approved by the U.S. Food and Drug Administration under an Investigational Device Exemption (Caution: Investigational device. Limited by Federal law to investigational use) and the Institutional Review Boards of Stanford University Medical Center (protocol #20804), Brown University (#0809992560), and Partners HealthCare / Massachusetts General Hospital (#2011P001036). The BrainGate2 trial’s purpose is to collect preliminary safety information and demonstrate feasibility that an intracortical BCI can be used by people with tetraplegia for communication and control of external devices; the present manuscript results from analysis and decoding of neural activity recorded during the participants’ engagement in research that is enabled by the clinical trial but does not report clinical trial outcomes.
Participant T5 is a right-handed man who was 65 years old at the time of the study. He was diagnosed with C4 AIS-C spinal cord injury eleven years prior to this study. T5 is able to speak and move his head, and has residual movement of his left bicep as well as trace movement in most muscle groups. Participant T11 is an ambidextrous man who was 35 years old at the time of the study. He was diagnosed with C4 AIS-B spinal cord injury eleven years prior to this study. T11 is able to speak and move his head. He has some residual movement in both arms. Both participants gave informed consent for this research and associated publications.
2.2. Many Words Task
The participants performed a simple visually prompted speaking task. On each trial they spoke one of 420 unique words that widely sample American English phonemes. The words used are an expanded set of the Modified Rhyme Test (House et al. 1963; Mines, Hanson, and Shoup 1978) and were previously used in several other studies (Mugler et al. 2018; Herff et al. 2019; Angrick et al. 2019). They include most, but not all, of the words used in Mugler and colleagues’ study (Mugler et al. 2014). These words were visually prompted (and subsequently spoken) one at a time. The participant was seated while fixating on a colored square centered on a computer screen in front of him. A trial started with an instruction period lasting 1.2 to 1.8 seconds, in which the central square was red, and white text above it instructed the word (e.g., ‘Prepare: “Word’, see Fig. 1B). The participant subsequently spoke the prompted word out loud following a go cue, which consisted of the square turning green, the text changing to “Go,” and an audible beep. The next trial began ~2.5 seconds later. Trials were presented in continuous blocks separated by short breaks, with the entire 420 word corpus divided across four blocks. Participant T5 performed three repetitions of each word (12 total blocks). Participant T11 performed 11 total blocks resulting in two to three repetitions of each word. Data from participant T5 were previously analyzed in (Stavisky et al. 2019), which examined the phonemic structure of trial-averaged, phoneme-aligned firing rates. The T11 data have not previously been reported.
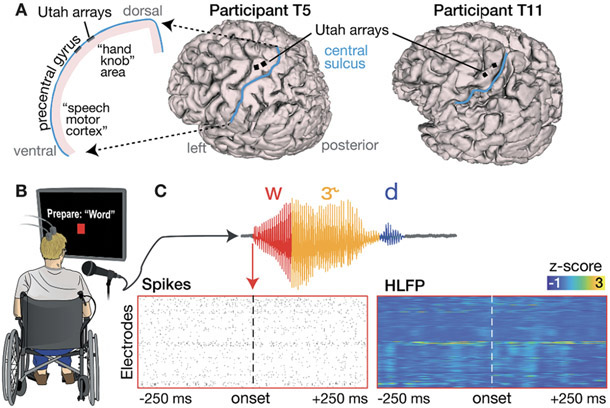
Figure 1.
Neural data recorded during a word speaking task.
(A) Array placements on 3D reconstructions of each participant’s brain. The left side illustration highlights that we recorded neural correlates of overt speech in a dorsal cortical area that is distinct from the ventral areas where speech production is typically decoded. (B) Illustration of the visually-prompted word speaking task. (C) Example phoneme segmentation of a word from the recorded audio. Below we show threshold crossing spikes and high frequency LFP (HLFP) for a 500 ms window rates centered on voice onset for this utterance of /w/.
Phoneme classes with at least 30 repetitions in the T5 dataset were included in the phoneme-specific analyses (e.g., classification), resulting in a set of 39 different phonemes. One phoneme in the T11 dataset (/ɔI/) had fewer repetitions (26) but was nonetheless included for consistency across the two participants. Two phonemes which otherwise would have had too few repetitions for inclusion were consolidated with a very similar phoneme with more repetitions: /ɔ/ (14 T5 utterances, 14 T11 utterances) was grouped with /ʌ/ (115 T5 utterances, 96 T11 utterances), and /ɚ/ (18 T5 utterances, 18 T11 utterances) was grouped with /ɝ/ (30 T5 utterances, 29 T11 utterances).
In the phoneme classification analyses shown in Supplementary Figure 2, we sought to match, as best we could, a set of words for comparing phoneme decoding with that of a previous ECoG phoneme decoding study by Mugler and colleagues (Mugler et al. 2014). Our word set already had 256 of the 320 words of that study; these we included in our ‘matched’ data subset. To fill out the remaining 64 words, we picked similar words from the 190 additional words in our overall dataset. Our substituted words have the same total number of phonemes as the missing words in the Mugler and colleagues report (Mugler et al. 2014), and typically differed in only one or two phonemes. Examples of these substitutions are “bill” (bɪl) → “bell” (bεl), “late” (leɪt) → “loot” (lut), “mass” (mæs) → “mouse” (maʊs), “ray” (reɪ) → “reach” (ritʃ), “tip” (tɪp) → “type” (taɪp).
2.3. Intracortical electrode array recording
Participants T5 and T11 had two 96-electrode Utah arrays (Blackrock Microsystems Inc., Salt Lake City, USA) neurosurgically placed in the ‘hand knob’ area of their left dorsal motor cortex 29 months (T5) and 3 months (T11) prior to this study (Fig. 1A).
Neural signals were acquired with a NeuroPort™ system (Blackrock Microsystems Inc.), analog filtered from 0.3 Hz to 7,500 Hz, and digitized at 30,000 Hz at 16 bits/sample.
2.4. Neural signals
We common average referenced each electrode by subtracting the mean voltage across all other electrodes from its time series at each timepoint. Action potentials (spikes) were detected based on when voltage values became more negative than a threshold set at −3.5 × root mean square (RMS) of that electrode’s voltage. We did not spike sort these threshold crossing spikes (‘TCs’), which may capture action potentials from one or more neurons near the electrode tip, since here we were interested in decoding the neural state rather than characterizing the properties of putative single neurons (Ajiboye et al. 2017; Brandman et al. 2018; Collinger et al. 2013; Pandarinath et al. 2017; Trautmann et al. 2019; Even-Chen et al. 2018; Oby et al. 2016). The voltage time series were also bandpass-filtered using a 3rd order bandpass Butterworth causal filter from 125 to 5000 Hz to extract high-frequency local field potential (HLFP) signals, which have been previously shown to contain useful motor-related information (Pandarinath et al. 2017; Stavisky et al. 2018, 2019; Nason et al. 2020).
2.5. Audio recording
Audio recordings were made using a microphone (Shure SM-58), pre-amplified by ~60 dB (ART Tube MP Studio microphone pre-amplifier), and digitized at 30,000 Hz by the NeuroPort system via an analog input port.
Phoneme identities and onset times were manually labeled using the Praat software package (Boersma 2001). The spoken words contained between two and six phonemes with a majority being three phonemes long (Supplementary Fig. 1A). Supplementary Figure 1B,C shows the distribution of phoneme audio durations. Although the same word prompt set was used for both participants, participant T5 completed one additional block of the many words task, and both participants occasionally misspoke or chose to speak different words that are spelled the same way (e.g., “tear”). Thus, the exact utterance count (T5: 3,840, T11: 3,467) and distribution of spoken phonemes differed between participants.
2.6. Neural feature extraction and classification models
HLFP activity was clipped at 100 × median activity for each electrode to lessen the impact of rare, large-amplitude electrical noise artifacts. Furthermore, one trial from each of the participants’ datasets was removed due to a very large simultaneous noise artifact across all electrodes. HLFP and spike rasters were temporally smoothed using a moving average filter (20 milliseconds with 1 millisecond spacing), resulting in time series of binned TCs firing rates and HLFP power. Electrodes were then visually inspected and those with baseline firing rate nonstationarities or visible noise were excluded from the neural analyses; this resulted in 158 and 179 included electrodes for T5 and T11, respectively.
We trained (1) a multi-class logistic regression model and (2) a recurrent neural network (RNN) to predict phoneme identity from neural features using the Scikit-learn and Tensorflow (version 1.15.0) libraries respectively (Pedregosa et al. 2011; Abadi et al. 2016). Both models were trained in a cross-validated classification setup using multiple bins of activity per electrode as described below.
Except where otherwise mentioned, we extracted a 500 millisecond window of neural activity centered around the onset of each phoneme. This specific value, while somewhat arbitrary, was chosen to match a previous ECoG phoneme decoding study (Mugler et al. 2014) and exceeds the typical duration of the participants’ spoken phonemes (Supplementary Fig. 1). Figure 3E, J examines a wider range of neural activity analysis windows. For the logistic regression model, we averaged activity in this window within non-overlapping 50 millisecond bins (Mugler et al. 2014) and z-score normalized their magnitudes to account for different electrodes’ activity ranges. We then compressed the resulting feature set using principal components analysis (PCA, 75% variance retained). Z-score normalization mean and standard deviation (s.d.), and PCA coefficients were calculated from within each training fold and then applied to the test data.
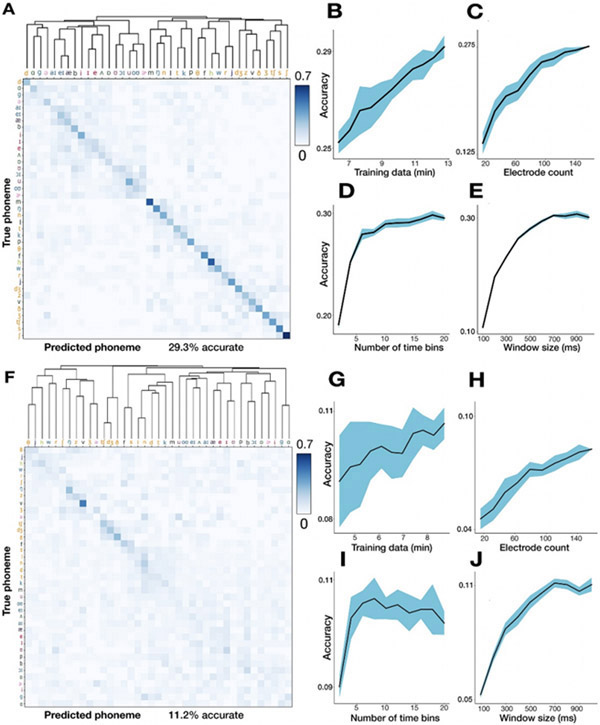
Figure 3.
Decoding 39 English phonemes and associated hyperparameter sweeps.
(A) T5 phoneme decoding confusion matrix, sorted by a hierarchical clustering dendrogram. Values are normalized so that each row adds up to 1. Overall accuracy was 29.3% using leave-one-out cross-validation. Note that the colorbar saturates at 0.7 (to better show the pattern of errors), not 1. Phoneme labels are colored based on their place of articulation group, which is examined further in Supplementary Figure 2. (B-C) Parameter sweeps for training set size (B) and number of electrodes (C). Shading denotes standard deviation across 10 repetitions of 10-fold cross-validation. (D) Creating finer-grained time bins from the overall 500 millisecond window improves performance. For example, twenty time bins (the rightmost point of this plot) means that each electrode contributes twenty bins each averaging HLFP across 25 ms to the overall neural feature vector. (E) Using a larger window (with 50 ms non-overlapping bins) increases performance until saturation around 600 ms. (F-J) same as above for T11 data.
For the RNN decoder analysis (see Section 2.8: Recurrent neural network decoder), we did not discard the 34 T5 electrodes as described above and instead used all 192 electrodes. Our reasoning was that even non-stationary or noisy electrodes could potentially be used by the RNN (Sussillo et al. 2016). In practice, however, this made little difference: the RNN decoder with the same electrode exclusions as the linear decoder performed at 32.4% accuracy, compared to 33.9% without excluding any electrodes.
2.7. Logistic regression for phoneme classification
The logistic regression model was trained using the Scikit-learn library (Pedregosa et al. 2011) in a leave-one-out cross-validation setup using default parameters except for the “lbfgs” optimization setting and L2 regularization value (λ = 100) to suppress overfitting. For multi-class classification, we used a one-vs-rest scheme where a different model is trained for each class to discriminate it against all others. For a given phoneme, model predictions are pooled and the highest positive class likelihood across models is taken as the decoder estimate.
We used 20-fold cross-validation for generating the phoneme classifications shown in Figs. 4, 5E, and 5F. We used leave-one-out cross-validation for the phoneme classifications shown in Figs. 3A, 3F, 5D and Supplementary Fig. 2. For all hyperparameter sweeps (Fig. 3), we used 10 repetitions of a 10-fold cross-validation procedure. Training data duration sweeps were performed by taking fractions of the overall phoneme data and then, for each such fraction, calculating the mean training data audio duration across training folds. This approach ensured that audio length estimates were not inflated by overlapping phoneme windows in the same training fold (e.g., if two consecutive phonemes are included), which would be the case if we had simply multiplied the number of phonemes used by our 500 millisecond window. The "number of electrodes" hyperparameter sweep was performed by randomly selecting a specific number of electrodes for both training and testing, and repeating this sampling 10 times for each number of electrodes.
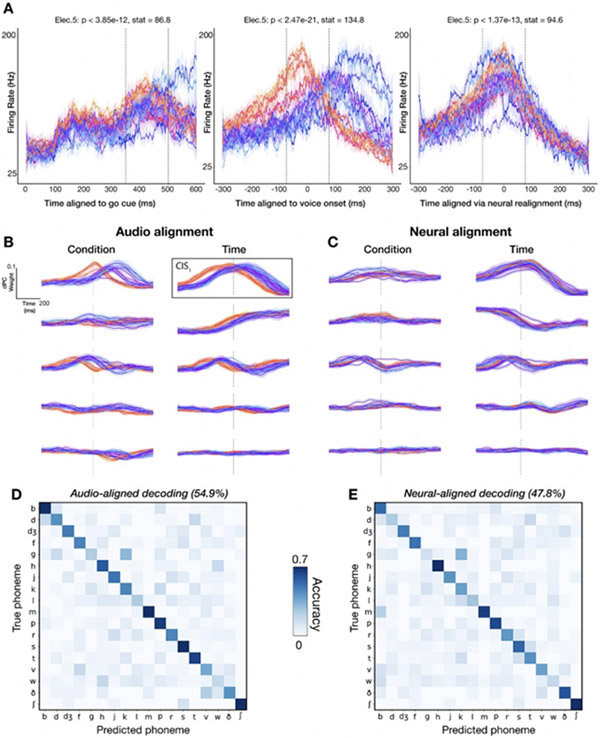
Figure 4:
Audio-based phoneme onset alignments cause spurious neural variance across phonemes.
(A) Firing rate (20 ms bins) of an example electrode across 18 phoneme classes is plotted for distinct alignment strategies (left to right: aligning the same utterances’ data to the go cue, voice onset, and the “neural onset” approach we introduce). Each trace is one phoneme, and shading denotes standard errors. Plosives are shaded with warm colors to illustrate how voice onset alignment systematically biases the alignment of certain phonemes.
(B-C) dPCs for phoneme-dependent and phoneme-independent factorizations of neural ensemble firing rates in a 1500 ms window. The top five dPC component projections (sorted by variance explained) are displayed for each marginalization for the audio and neural alignment approaches. (B) dPC projections aligned to voice onset (vertical dotted lines). Plosives (warm colors) have a similar temporal profile to other phonemes (cool colors) except for a temporal offset. This serves as a warning that voice onset alignment may artificially introduce differences between different phonemes’ trial-averaged activities. To compensate for this, we re-aligned data to a neural (rather than audio) anchor: each phoneme’s trial-averaged peak time of the largest condition-invariant component, outlined in black, was used to determine a “neural onset” for neural realignment. (C) Recomputed dPC projections using this CIS1-realigned neural data. Vertical dotted lines show estimated CIS1 peaks. (D) Decoder confusion matrix from predicting the first phoneme in each word using a 500 ms window centered on voice onset. (E) Confusion matrix when classifying the same phoneme utterances, but now using neurally realigned data.
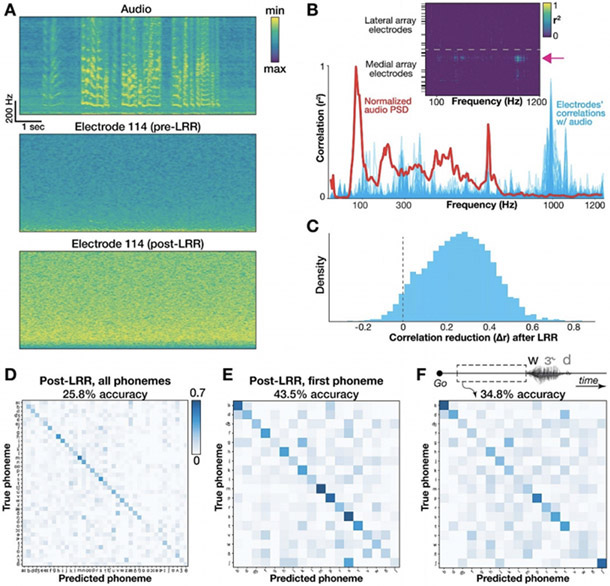
Fig. 5:
Quantifying and mitigating acoustic contamination of neural signals.
(A) Spectrograms for audio and neural data in the electrode and block exhibiting the strongest audio-neural correlations. Frequencies range from 5 to 1000 Hz. The bottom plot shows the same electrode after LRR “decontamination”. (B) Plot of the mean audio PSD (red) and all electrodes’ Pearson correlations (blue) from the same example block. Inset shows correlation coefficients of individual electrodes (rows) across frequencies (columns). Black horizontal ticks denote electrodes excluded from neural analyses. The pink arrow shows the example electrode from panel A. (C) Change in audio-neural correlations after LRR, pooled across all blocks, electrodes, and frequencies (restricted to electrodes with r2 > 0.1 originally). Values to the right of the dotted ‘0’ line indicate a reduction in correlation strength. The mean audio-neural correlation reduction was 0.26. (D) Full classifier confusion matrix after LRR (25.8% overall accuracy across 39 classes). (E) Confusion matrix for first phoneme decoding after applying LRR. As in D, the classifier used a 500 ms window centered on voice onset. (F) Confusion matrix showing decoding each word’s first phoneme using 500 ms leading up to voice onset to avoid possible audio contamination or neural activity related to auditory feedback.
To test for structure in the decoder’s misclassifications of T5’s neural data, we sorted our confusion matrix, where entry (i, j) corresponds to the percentage of times the ith phoneme in our decoding set is classified as the jth, by place of articulation (as in (Moses et al. 2019; Stavisky et al. 2019)) and measured the difference between within- and between-group confusions. We then built a null distribution of expected differences if there were no underlying structure correlated with place of articulation in these errors by generating random partitions of our phonemes (keeping the same group sizes) and re-measuring the test statistic 10,000 times from these permuted data.
2.8. Recurrent neural network decoder
A recurrent neural network (RNN) was trained to predict phoneme identities from 1000 ms neural data snippets from each electrode, each subdivided into fifty 20 ms bins. The RNN was built as a single layer of 512 bidirectional gated recurrent units (GRUs) (Cho et al. 2014) implemented with the cuDNN library (Nvidia Corp., Santa Clara, USA). When training, the input data at each 20 ms time step was the 192-dimensional HLFP feature vector, while the supervised target output was a 39-dimensional ones-hot vector where the element corresponding to the phoneme associated with this data snippet was set to 1, and all other phonemes were set to 0 (Supplementary Fig. 3A). The training cost function also included a L2 regularization term to penalize large network weights (λ = 1e-5). During testing, for each phoneme utterance a new 192×50 neural data matrix was input to the RNN, and a 39×50 output matrix was read out, which consisted of the RNN’s predicted likelihoods (logits) that the input data came from each of the 39 possible phonemes, for each bin. The most likely phoneme during the last bin was chosen as the RNN’s final prediction for that snippet (Supplementary Fig. 3B). The RNN was trained and evaluated across 10 folds of the data, such that each spoken phoneme utterance appeared in the test set once.
Given the relative paucity of available Many Words Task speaking data compared to the data corpus sizes in typical machine learning applications, two additional training data augmentation methods were used to regularize the RNN and prevent it from overfitting on the training data. These were adapted from our group’s recent work using RNNs to decode the neural correlates of handwriting (Willett et al. 2020) and conform to lessons learned from a previous iBCI RNN decoding study (Sussillo et al. 2016). First, white noise (s.d. = 0.1) was added to each time bin’s neural input feature vectors during training. Second, more slowly-varying artificial neural input feature drifts were added across the time bins of each snippet during training to make the RNN robust to potential nonstationarities in the neural data (Perge et al. 2013; Jarosiewicz et al. 2015; Downey et al. 2018). These drifts had two components: a constant offset vector applied separately to each utterance (s.d. = 0.6), and a random walk across bins (s.d. = 0.02).
The RNN was trained using the “Adam” gradient descent optimization (Kingma and Ba 2017) with a mini-batch size of 128 utterances, with no burn in steps, for 20,000 mini-batches. The learning rate decreased linearly from 0.01 (first mini-batch) to 0 (last mini-batch).
In order to directly and fairly compare this RNN approach to the logistic regression described in the previous section, we also trained and evaluated a logistic regression model using the exact same input data (including the same specific 10 cross-validation folds) as used for the RNN decoder evaluation.
2.9. Demixed Principal Components Analysis (dPCA)
We used dPCA (Kobak et al. 2016) to decompose population activity into components reflecting variance that can be attributed to phoneme classes and time (“phoneme-dependent”) or time only (phoneme-independent). Specifically, we took a 1500 ms window centered on voice onset and decomposed it into low-rank approximations that captured phoneme class-dependent and independent variance. Spike rasters were convolved with a symmetrical 25 ms s.d. Gaussian and then averaged within 10 ms, non-overlapping bins before applying dPCA.
To ensure that dPCs were not fitting to noise, we adopted the cross-validation procedure described in (Willett et al. 2020) to obtain 95% confidence intervals. We split our trials into ten separate folds to cross-validate dPC decompositions, thus avoiding overfitting to dataset noise. For each fold, all other folds are used to identify a dPCA decomposition. We then projected the held-out trials onto the identified dPCs. Geometrically, dPCA finds a linear subspace of a marginalized feature space where the axes correspond to a reconstruction error-minimizing subspace. Flipping the orientation of these vectors will preserve this subspace and hence also satisfy the optimization, but can confuse visualizations and comparisons across dPCA application instances. To avoid this issue and facilitate dPC comparisons across folds, we multiplied our components by −1 when this would result in a smaller angle between the kth principal components in a given marginalization.
We used the largest phoneme-independent neural component, which we refer to as the 1st component of the condition-invariant signal (CIS1) as in (Kaufman et al. 2016), to determine when phoneme production started from the neural data. This is in contrast to using acoustic data (Fig. 4). To do so, we trial averaged the neural activity for a given phoneme within each cross-validation fold and projected the resulting electrodes by time matrix onto the CIS1 neural dimension. This yields 10 CIS1 peak estimates (for 10 folds). The average CIS1 time course across these ten folds was used to determine each phoneme’s CIS1 peak (the time of maximum CIS1 value), which determined that phoneme’s neurally-derived onset. In practice, this approach is similar to simply aligning to the peak of each phoneme’s trial-averaged mean population firing rate (the Pearson correlation between CIS1 peaks and mean rate peaks was r = 0.669, p = 0.002), but is more principled since dPCA explicitly searches for a condition-invariant projection of the data.
2.10. Quantifying acoustic artifact and Linear Regression Reference (LRR) decontamination
To compare spectral content between recorded audio and electrodes (Fig. 5A,B), we convolved the voltage time series of each electrode and also the audio channel with a 200 ms Hamming window and then computed the power spectral density (PSD) in non-overlapping bins using a short-time Fourier transform (as in Roussel et al. 2020). We isolated ‘voicing epochs’ in which to compare audio and neural power time series by sub-selecting time points with summed audio power (across all frequencies) in the top ~10% of values across all audio data. For spectrogram visualizations in Fig. 5A, sliding time windows overlap by 90%.
The LRR procedure (Young et al. 2018) to remove putative acoustic contamination from each electrodes’ signal began with fitting linear regression models that predicted each electrode’s voltage (bandpass-filtered in the 125-5000 Hz range using a third-order Butterworth filter) at a given time sample from every other electrode’s voltage at that time sample. Regression weights were fit separately within each block in order to increase reliability if there were across-block changes in the acoustic contamination if, for example, the participant shifted posture. These linear models were fit using the scikit-learn library’s (Pedregosa et al. 2011) SGDRegressor class with default parameters save for regularization strength (alpha = 400), early stopping, and initial learning rate (eta0 = 0.0000004). Hyperparameter values were identified based upon manual tuning. Regressions were fit using voltage activity occurring during voicing epochs. These timepoints were identified by extracting the acoustic envelope using a Hilbert transform and then thresholding based upon a manually identified threshold.
2.11. Tuning fork control for microphonic pickup
We performed this control experiment during a separate research session with participant T5, twelve months after his primary Many Words Task session. The participant was seated in his chair, facing the microphone, and connected to the full recording system in the same way as during the primary data collection. The participant was asked to sit quietly, relax, and avoid moving while a researcher activated a tuning fork (by striking it with a mallet) and then held it in the air just in front of the participant’s head. The researcher then touched the tuning fork’s stem against T5’s head, then against each Blackrock cable pre-amplifier, and finally against each of the hanging cables itself. The participant reported that he could hear and/or feel the vibrations in all conditions but that they did not cause any discomfort.
We identified 7 second audio and neural snippets corresponding to when the tuning fork was activated and applied to the head/pre-amplifier/cable. Power spectrograms were calculated for these snippets’ raw voltage signals (sampled at 30,000 Hz with no additional filters applied except for the analog filters of the Blackrock Neural Signal Processor) using a short time Fourier transform (50 ms windows, 1 ms sliding window, 7.3 Hz resolution).
2.12. Speech synthesis via unit selection
Speech audio was reconstructed offline using the ‘Brain-to-Speech’ synthesis method recently developed by Herff and colleagues (Herff et al. 2019). To facilitate comparison with this previous ECoG study, we closely followed their methods and used the same set of spoken word prompts (although the exact set of utterances was not identical due to different trial counts and occasional missed trials or word substitutions). In overview, this unit selection-based approach (Hunt and Black 1996) consists of creating a training library of speech units (brief snippets of audio) with corresponding neural feature vectors. Then, for “new” neural data (here, held out test data), the closest matching neural data in the training library is identified. The audio snippet corresponding to this best matching neural data is selected for the speech reconstruction. This procedure advances step by step, selecting and concatenating together these best neurally-matching audio snippets until speech sound has been synthesized for the entire test epoch (here, one word and the silence immediately preceding and/or following it). The resulting performance is assessed by comparing the synthesized speech audio with the true spoken audio.
The T5 Many Words Task data were divided into ten folds for cross-validation, and the overall procedure was repeated such that each fold was used once for testing against a decoder trained on the remaining 90% of the data. An audio snippet unit was constructed at every 10 ms step of the recorded speech data. Each audio snippet was a 150 ms duration Hanning window, which facilitates smooth transitions when concatenating partially overlapping units.
For each audio snippet unit, a corresponding neural feature vector was generated as follows. HLFP power was calculated, clipped at 100 × median activity, and z-scored as described earlier. From each electrode, a 650 ms window (centered on the center of the audio snippet) of these neural data were divided into thirteen non-overlapping 50 ms bins and time-averaged within each bin. The choice of thirteen bins differs slightly from (Herff et al. 2019), who used eleven 50 ms bins (450 ms total window); we opted for 650 ms based on the phoneme decoding parameter sweeps in Fig. 3E. This yielded a 158 electrodes × 13 bins = 2054-dimensional neural vector for each audio snippet. Given the high dimensionality of these neural features relative to the amount of training data (~138,000 training samples per fold), we reduced the neural feature dimensionality (a form of regularization to reduce overfitting) using principal components analysis. As in (Herff et al. 2019), we kept as many principal components as necessary to explain at least 70% of the total neural variance, which for these data required 25 principal components. The training and test folds were constructed such that no test fold words were present in the training fold. Furthermore, PCA coefficients and neural feature means, medians, and standard deviations (for clipping and z-scoring) were computed only from the training fold, and then applied to the test fold.
For each 10 ms step of the test data, speech synthesis proceeded as follows. The 25-dimensional neural feature vector corresponding this this step, xt, was compared to all N training data neural features xi=1, …, N using cosine similarity, and then the closest matching training sample was selected:
The 150 ms audio snippet corresponding to training index i was then added to the synthesized speech, overlapping with 140 ms of the existing synthesized audio. This procedure was repeated for the entire test word. As in (Herff et al. 2019), we did not use any concatenation cost when selecting the best matching audio unit (i.e., we did not apply a statistical prior of what sounds are more or less likely to follow other sounds).
To quantify the quality of reconstruction, we used the preferred method of Herff and colleagues: decomposing the true and synthesized audio into time-frequency bins, and then measuring the correlation between the true and synthesized audio. Specifically, 50 ms windows of the audio (advanced by 40 ms with each step, resulting in 10 ms window overlaps) were transformed into the spectral domain onto the Mel scale (Stevens, Volkmann, and Newman 1937) using the Librosa package (McFee et al. 2019). Forty overlapping triangular Mel filters spanning 0 to 16 kHz were used, and the resulting power in each time-frequency bin was converted to decibels. This yielded forty vectors (one for each frequency bin) for each of the true and synthetic time-varying spectral power; we then calculated the Pearson correlation coefficient between each of these true and synthetic vectors. As in (Herff et al. 2019), the final reconstruction quality metrics reported are the mean across these forty individual frequency bin correlations.
To measure chance performance, this procedure was repeated 100 times but with the “best match” speech units randomly selected from the training data, rather than selecting the unit with the most similar neural feature vector.
3. Results
3.1. Single electrode modulation when producing speech
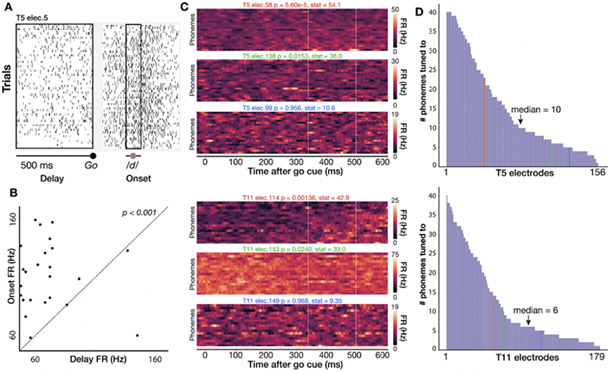
We first assessed single electrode phoneme tuning properties by measuring firing rate changes between the instructed delay period (when participants first read a short word) and around speech onset (when they spoke it out loud in response to a go cue). For each electrode, we examined a 500 millisecond window of delay period activity just prior to the go cue and a 100 millisecond window centered on each phoneme’s voice onset time. We then calculated average firing rates within each window (Fig. 2A) and tested the significance of FR changes across these two time windows (permutation sign-test, 1000 permutations; uncorrected) for all phoneme classes (Fig 2B, D). To maintain statistical independence of samples, if the same phoneme class occurred multiple times within a trial, we randomly selected only one occurence. Nearly all electrodes showed firing rate modulations to speaking at least one phoneme (156/158 electrodes for T5, 179/179 for T11). A sizable minority of electrodes showed tuning to at least half of all phoneme classes (40/158 electrodes for T5, 41/179 for T11, Fig. 2E-F). Across electrodes and phonemes, significant firing rate changes largely reflected increasing firing rates around voice onset (78% and 86% of significant changes for T5 and T11 respectively). Thus, a dominant feature of these data was broadly tuned firing rate increase after the go cue, consistent with our previous intracortical multielectrode recordings (Stavisky et al. 2019) and prior ECoG recordings in ventral sensorimotor cortex (Bouchard et al. 2013; Mugler et al. 2018).
Figure 2. Individual electrodes show broad tuning across phonemes.
(A) Spike rasters for a single T5 electrode across all instances of /d/ in the full dataset of spoken words. Black boxes show a 500 ms delay period analysis window before the go cue and a 100 ms analysis window centered around voice onset). (B) Scatter plot of firing rates during the delay and onset epochs for the electrode shown in A; each point is one trial. Firing rates are significantly higher around voice onset (two-sided permutation sign test; p<0.001). (C) Three example T5 electrodes (top) and T11 electrodes (bottom) chosen to exemplify high, low, and not significant selectivity between speaking different phones (Kruskal-Wallis across single trial firing rates from 350 to 500 ms after go cue, marked by vertical lines).. The phonemes were sorted by firing rate for each participant’s high tuning example electrode and then kept in the same order for the other two electrodes. (D) Distribution of number of phonemes to which T5’s (top) and T11’s (bottom) electrodes are tuned (i.e., having a significant firing rate difference between delay and onset epochs), sorted from broadest tuning to most narrow tuning. In general, electrodes show a broad tuning profile. Vertical colored lines indicate the corresponding color’s electrode in panel C.
These data did not contain strong preparatory activity or phoneme discriminability during the instructed delay period. Therefore, in the rest of this study we focus on decoding the activity immediately preceding and during overt speaking. Speech epoch firing rates (averaged across electrodes and a 1 s window centered on each phoneme utterance’s onset) were 18.1 ± 35.0 Hz for T5 (mean ± s.d.) and 10.6 ± 27.6 Hz for T11.
3.2. Decoding English phonemes using neural population activity
We next sought to assess the utility of these signals for a speech BCI by decoding (offline) the identity of individual phoneme utterances from the neural activity across all functioning electrodes. We trained logistic regression classifiers with neural data and phoneme labels across all utterances (~14 total minutes data) and assessed cross-validated classification accuracy across 39 phonemes. Our first major observation was that although TC-spikes contained substantial information about phoneme identity (14.8% accuracy for T5, 7.9% accuracy for T11, p < 0.002 compared to chance), classification was much more accurate when decoding high-frequency local field potential power (HLFP, 125-5000 Hz): 29.29% and 11.22% for T5 and T11 respectively (Fig. 3A,F). This is consistent with our previous study decoding a small number of syllables spoken by T5 (Stavisky et al. 2018) and previous arm and hand decoding/BCI studies (Stark and Abeles 2007; Pandarinath et al. 2017; Bouton et al. 2016; Zhang et al. 2018; Nason et al. 2020) employing Utah arrays. The superior performance of HLFP decoding may reflect this neural feature more robustly capturing spiking activity from more neurons in the local vicinity of each electrode (Asher et al. 2007; Waldert, Lemon, and Kraskov 2013; Buzsáki, Anastassiou, and Koch 2012; Nason et al. 2020). However, our result does not imply that there is universally more information in the spike band power than in the action potentials, and especially not in the case where isolatable single neuron activit(ies) would be observed on every electrode. Rather, the ensemble HLFP across electrodes may contain more decodable information than the ensemble spikes in a scenario where many electrodes do not record isolatable single units or even large amplitude multiunit threshold crossing spikes (which will often be the case for chronic electrode arrays, given today’s technology). Furthermore, combining the activities of several neurons by decoding TC-spikes (an information-destroying aggregation that will also occur in the HLFP signal), has been shown in practice to not substantially reduce the accuracy of decoding movements from motor cortex (Chestek et al. 2011; Christie et al. 2015; Trautmann et al. 2019). Since HLFP decoding outperformed TC-spikes decoding in these data, for the remaining analyses we will focus on this more informative neural feature.
To better understand how phoneme decoding varied as a function of the quantity and temporal granularity of the input intracortical data, we measured performance as a function of increasing training dataset size, electrode count, and the number of time bins within an overall 500 millisecond window. Increasing accuracy as a function of both more training data (Fig. 3B,G) and more electrodes used (Fig. 3C,H) did not show performance saturation. Importantly, this indicates that a path to improve intracortical speech BCI performance would be to collect more data and implant more electrodes. Taking advantage of time-varying structure by dividing each utterance’s neural window into more time bins substantially increased performance (Fig. 3D,I). This observation is in accordance with prior ECoG decoding work (Ramsey et al. 2018). Unlike T5’s almost monotonic improvement with more fine-grained time bins, T11’s classification performance saturated at 10 bins (each 100 ms long) and then declined. This trend likely follows from the lower signal-to-noise ratio (SNR) (and lower overall firing rates) in T11’s recordings, in which case short time bins will exacerbate noise in the inputs and reduce decoding accuracy.
Using a total time window of approximately 600 milliseconds maximized performance; this agrees with a similar analysis by Mugler and colleagues (Mugler et al. 2014). This optimal decoding window is substantially longer than the typical phoneme duration (Supplementary Fig. 1) and likely reflects that the motor cortical correlates of producing each phoneme are longer than that phoneme’s audible duration (Tourville and Guenther 2011; Hickok 2012). However, this increasing classification accuracy when using longer time windows should be interpreted with caution for at least two reasons. First, given the limited dictionary size in this study (420 words), increasing the decoding window may allow the classifier to exploit neural correlates of adjacent phonemes in a way that is not representative of what could be expected during open vocabulary decoding. Second, overly long time bins may introduce problematic delays if used in a closed-loop speech BCI (e.g., by delaying when decoded sounds/words are synthesized/typed) (Rabbani, Milsap, and Crone 2019). While the user might be able to compensate with slower speech, this would reduce the maximum potential communication rate; thus, the choice of online decoding window duration will likely reflect a speed versus accuracy trade-off.
Although phoneme prediction was above chance in both participants, classification accuracy was much higher in T5 than in T11. This result is consistent with ongoing efforts to characterize these T11 signals’ relationship to a variety of actualized and attempted movements (including attempted arm and hand movements), where preliminary findings indicate that T11’s arrays modulate substantially during movements, but exhibit less specificity than the T5 recording. This could reflect differences either in array placement or in modulation specific to this area of cortex in this participant. Given this lack of specificity, we believe that T5's signals more closely approximate those that would be available in ventral areas of motor cortex that would be recorded from in future work, specifically centered on building a speech prosthesis. Thus, subsequent analyses exploring phoneme decoding in more depth are restricted to the more informative T5 dataset.
To more directly compare the information content in T5’s intracortical recordings to a prior ECoG study reporting what is (to our knowledge) the highest accuracy in decoding phonemes within stand-alone short words (Mugler et al. 2014), we attempted a closely matched comparison to that study. This was facilitated by our having deliberately used a similar set of prompted words to that study: 232 of our words were also used by Mugler and colleagues (Mugler et al. 2014), and we picked replacements for the missing 80 words by choosing similar words from our (larger) word set (see 2.2: Many words task). For this analysis we also restricted our trials to match the trial count of the participant with the highest performance in Mugler and colleagues (Mugler et al. 2014) and used an equivalent 600 millisecond window centered on voice onset (Supplementary Fig. 2). Decoding vowels and consonants separately as in (Mugler et al. 2014), the T5 dataset accuracy was 36.8% across 24 consonant classes (vs. 36.1% in Mugler et al.’s best participant) and 27.7% across 16 vowels classes (vs. 23.9%).
In the previous analyses we used logistic regression decoding in the interest of pragmatic considerations (compute time for parameter sweeps) and facilitating comparison to previous work (Mugler et al. 2014). However, we recognize that additional performance may be gained by employing modern and powerful machine learning techniques, which may be particularly well-suited for decoding the dynamic neural activity underlying speech (Livezey, Bouchard, and Chang 2019; Angrick et al. 2019; Anumanchipalli, Chartier, and Chang 2019). To that end, we also decoded T5's phonemes dataset using a recurrent neural network with data augmentation (see 2.8 Recurrent neural network decoder). However, this resulted in only a modest performance improvement (33.4% accuracy versus 29.6% for a logistic regression with matched input data; Supplementary Figure 3).
3.3. A neural realignment strategy to correct for systematic voice onset biases between phonemes
We next turn to the first of two potential confounds by which studying audible speech may artifactually inflate the accuracy of decoding the corresponding neural data. Recall that, as per standard practice, we defined the start of each phoneme utterance based on the audio recording. This approach makes an important assumption: insofar as detectable speech sound lags the start of the underlying process of speech production, this lag is consistent across different phonemes. However, this assumption is unlikely to hold under most models of what motor cortex encodes. Specifically, if neural activity reflects articulatory movements (Lotte et al. 2015; Chartier et al. 2018; Mugler et al. 2018; Stavisky et al. 2019), then there may be across-phoneme differences between when articulatory movements start versus when the phoneme is clearly audible.
Figure 4A presents empirical evidence of this problem by showing different time alignments to generate an example electrode’s firing rates as T5 spoke different phonemes. In the left-most panel, neural data are aligned to the task go cue; note that these analyses only include the first phoneme of each word to facilitate aligning to the go cue or to the neural correlate of speech initiation, as described below. This electrode’s firing rate increased shortly after the go cue and was largely similar across phonemes, consistent with the presence of a large condition-invariant signal, ‘CIS’ (Kaufman et al. 2016), upon speech initiation (Stavisky et al. 2019). In addition to this general activity increase, there was also phoneme-specific information as indicated by significant firing rate differences between phonemes in the epoch from 350 ms to 500 ms after the go cue. These data also suggest that there were not large systematic differences in the reaction time when speaking words that start with different phonemes. In stark contrast, the Fig. 4A center panel shows the same trials aligned to audio-derived voice onset time. The firing rate traces for plosives (/p/, /b/, /k/, /g/, /t/, and /d/) are shown with warm colors to better highlight a systematic onset timing bias. These phonemes’ firing rates follow a similar time-course to the other phonemes’, except for a time offset, which leads to much greater differences across phonemes in an analysis epoch of the same duration (150 ms) now centered on voice onset. These plosive phonemes involve a temporary constriction of airflow (which produces little sound), followed by a rapid (and very audible) release of air, thereby increasing the latency between when phoneme production starts (e.g., movement of the lips) and when the voice onset is detected. This example vividly illustrates that a consequence of using audio data to mark the start of each phoneme utterance introduces systematic timing biases that appear as spuriously accentuated differences across phonemes.
As an alternative to audio-derived voice onset, we attempted to detect phoneme onsets directly from the neural data. We used the largest variance phoneme condition-invariant signal of the neural population signal, the ‘CIS1’, as a speech activity onset indicator. This linear readout was previously found to be time-locked to attempted motor activity (Kaufman et al. 2016), including during speaking (Stavisky et al. 2019). Since the CIS1’s temporal profile should be largely invariant across speaking different phonemes, we can perform a “neural realignment” by aligning CIS1 peaks across conditions. To do so, we used the demixed PCA (Kobak et al. 2016) dimensionality reduction technique to decompose and summarize the neural ensemble activity into a smaller number of condition-invariant and condition-specific components. Figure 4B shows ten of these population modes. The time-courses of these components across phonemes appear very similar except for temporal offsets, providing a population-level corroboration of the previous individual electrode example. Once again, the early peaking traces correspond to plosives, suggesting that audio-based timing cues are systematically biased across different phoneme classes and that aligning to voice onset introduced spurious variance into our trial-averaged neural activity.
We then used each phoneme class’ CIS1 peak to identify the offset from audio voice onset. This allowed us to realign the corresponding neural data to this ‘neural onset’. For instance, /b/ had an offset of −20 milliseconds; thus, for all trials with words that start with /b/, we shifted our neural data 20 milliseconds forward in time. Performing dPCA again on these realigned data (Fig. 4C), both the condition-invariant and the condition-specific components were more similar across phoneme classes. The right-most plot of Fig. 4A shows the same example electrode, now realigned using this CIS1-derived neural onset time. Here the time-courses of each phoneme appear more similar than when aligning to audio-derived voice onset, but there were still significant differences between phonemes.
3.4. Classification is weakly affected by audio alignment-based phoneme onset timing bias
In the previous section we identified a phoneme timing bias from audio-derived onset labeling, and also demonstrated a method to correct for this bias (at least for the first phoneme of a word) using neurally-derived onset labeling. This allows us to now quantify how much phoneme onset biases can affect decoding audio-labeled phonemes by enabling a classifier to take advantage of spurious neural variance across phoneme classes.
To measure the impact of this confound, we trained separate decoders to classify the first phoneme of T5’s spoken words using 1) the original neural data windows, which were aligned to voice onset as in Fig. 3, and 2) the realigned neural data windows obtained using CIS1 peak alignment. For both procedures, we used the same preprocessing steps as before (see 3.2). Decoding each word’s first phoneme from neural data time windows centered on neurally-derived phoneme onsets yielded 47.8% accuracy across 18 classes (Fig. 4E), as compared to 54.9% using windows centered on audio-derived phoneme onsets (Fig. 4D), a relative decrease of 12.9%. This indicates that that audio-derived phoneme onset biases inflate overt speech decoding accuracies, but only modestly so.
3.5. Classification is weakly affected by microphonic artifact and is possible before voice onset
The second potential confounds stemming from studying neural correlates of audible speech is that speaking could mechanically jostle components of the electrophysiology recording system, which would interact with ambient electromagnetic fields to generate small electrical currents that affect the final voltage measurement (i.e., microphonic pickup). This could be a larger or smaller effect, or no effect at all, depending on electromagnetic shielding (e.g., of cables), ambient electromagnetic power (e.g., other powered devices nearby, including lighting) and how recording components are stabilized (e.g., cables immobilized or not). There is also the possibility that there is an acoustic effect, but that it arises from some other mechanism that is not understood. To identify if audio contamination was potentially impacting classification performance, we implemented four different analyses.
We first looked for frequency-specific correlations between audio and neural signals, as in (Roussel et al. 2020). We computed spectrograms for simultaneous speech audio (microphone) recordings and each electrode’s activity (Fig. 5A). This yielded a set of time series containing frequency-specific signal power from 5 to 1200 Hz. We isolated time snippets with audible speech (see 2.9: Quantifying acoustic artifact and Linear Regression Reference (LRR) decontamination) and correlated these electrode and audio power spectral density (PSD) time series at each frequency. If microphonic pickup had heavily contaminated our recordings, then the most prominent audio frequencies would most likely induce oscillations in the electrode signal at the corresponding frequency and manifest as stronger correlations with the associated “neural” signal power at those frequencies. Plotting the mean PSD across speech timepoints alongside our electrodes’ correlations for an example block, we found strong electrode-audio correlations across a range of frequencies (Fig. 5B) with a max correlation r = 0.953 across all 192 electrodes × 240 frequencies = 46,080 comparisons, and a median r = −0.002. This implies that our neural recordings contain microphonic contamination in at least some frequency bin(s) on some electrode(s). However, inspecting the example electrode which had the strongest overall audio-neural correlation (Fig. 5A), we still see little contamination visible to the eye. This contrasts with the striking obvious contamination in some of the recordings analyzed in (Roussel et al. 2020). The Fig. 5B inset summarizes the correlation with audio across all electrodes, and reveals a small set of electrodes with higher audio-neural correlations, all on the same (medial) array.
Second, to more directly test whether mechanical vibrations in the speech range could, in principle, affect our recordings, we performed a positive control where we intentionally applied a mechano-acoustic stimulus to the recording setup. During a separate research session with participant T5, we held an activated 340 Hz or 480 Hz tuning fork either in the air in front of the (fully connectorized) participant (Supplementary Fig. 4A), or pressed the activated fork’s stem to his head near the connector pedestals (Supplementary Fig. 4B), directly to the pre-amplifiers (Supplementary Fig. 4C), or directly to the connector cables (Supplementary Fig. 4D). We reasoned that since a tuning fork applies the majority of its mechanical energy into the recording apparatus at a specific frequency (unlike speech), and is unlikely to evoke biological neural oscillations in a motor cortical area at that same specific frequency, then a sharp increase in recorded signal power at the fork’s vibration frequency would clearly indicate the presence of an artifact. We did indeed observe such an artifact on some medial array electrodes when the tuning fork was applied to the pre-amplifier and cable, the two conditions in which there was putatively the most mechanical energy being transferred into the recording apparatus. This result demonstrates that our measurement and analysis methods do have the sensitivity to detect acoustic contamination, at least at these frequencies and amplitudes. We do not purport to equate this artificial tuning fork stimulation to the acousto-mechanical interaction between the participant speaking and the recording system; rather, this result, which is consistent with (Roussel et al. 2020), merely shows that there exist conditions under which artifactual acoustic contamination is possible. This lends support to the aforementioned suspicion that some of the speech-correlated neural activity was due to acoustic contamination.
Third, as a conservative decoding analysis that would avoid acoustic contamination, we extracted a 500 millisecond neural window just prior to each word but before speech onset. Since this neural activity precedes audible audio signals, it cannot be polluted by microphonic pickup. This analysis also serves as a control that excludes the potential contribution to decoding by neural processing of auditory feedback in this motor cortical area. Decoding these pre-voice neural windows for each word’s first phoneme yielded a cross-validated (20 fold) classification performance of 34.8% across 18 classes (Fig. 5F), well above chance performance (p < 0.002; permutation test, 500 permutations). For comparison, using a 500 ms neural window centered on the phoneme’s voice onset (which we expect to better capture phoneme production-specific neural activity, in addition to potential acoustic contamination and auditory feedback) yielded an accuracy of 54.9% (Fig. 4D).
Fourth, we implemented a denoising procedure (Young et al. 2018)linear regression reference or ‘LRR’, (Young et al. 2018) which aims to mitigate acoustic contamination of the neural signals. LRR works by subtracting out predictable signals shared across many electrodes (which is what we would expect from microphonic pickup) from each electrode’s activity. A linear regression model estimates each electrode’s activity using instantaneous activity from all other electrodes; this prediction is then subtracted from that electrode’s original time series to yield the denoised estimate. This approach was first applied in (Young et al. 2018) to remove electrical artifacts induced during intracortical microstimulation. Here we applied the method to putative acoustic contamination by fitting regression models to bandpass-filtered (125-5000 Hz to match our HLFP neural feature) voltage signals. Figure 5A shows an example electrode’s activity before and after LRR, while Supplementary Fig. 4E demonstrates that this technique successfully removes much (but not all) of the artifact introduced in the tuning fork positive control. Following LRR, we observed a substantial reduction in audio-neural correlations (Fig. 5C), indicating that the procedure was mitigating acoustic contamination. At the same time, there was only a small reduction in cross-validated accuracy decoding all phonemes (reduction from 29.3% to 25.8%, Fig. 5D), indicating that the procedure was not eliminating much phoneme-specific information. However, we note that the LRR technique does not guarantee that all acoustic contamination is eliminated (which would cause us to under-estimate the contribution of microphonic pickup to decoding accuracy), and it also can subtract out genuine neural signal that is shared across electrodes (which would cause us to over-estimate the contribution). Thus, these results should be viewed as just one piece of evidence towards estimating the effects of acoustic contamination in overt speech decoding studies.
In summary, our results support the concern raised by (Roussel et al. 2020) that microphonic artifacts are present in intracortical and ECoG recordings during overt speech. These audio-driven signals may well have somewhat increased the phoneme classification accuracies in this study. However, our further analyses described in this section bracket this potential artifactual contribution as small and, critically, show that we can decode phonemes even before audible voicing (Fig. 5F). This agrees with our prior findings that neural activity in this area modulates in response to unvoiced orofacial movements (Stavisky et al. 2019).
3.6. Classifier confusions correlate with place of articulation groupings
We now return to the observation that when sorting our decoder confusion matrices based on the results of agglomerative hierarchical clustering (Sokal & Michener 1958), the resulting dendrograms suggest divisions across phonemes relating to their phonemic groupings (Fig. 3A; e.g., broad vowel-consonant divisions). A permutation test (see 2.7: Logistic regression for phoneme classification) revealed significantly higher confusion within place of articulation groups than between groups (Supplementary Fig. 5A-B; p < 0.005), indicating that there was latent structure in our classifier errors putatively related to how similar the motoric demands of producing different phonemes were (Lotte et al. 2015; Mugler et al., 2018; Chartier et al. 2018; Stavisky et al. 2019). However, we identified in sections 3.3 and 3.4 that these classifier errors were somewhat affected by systematic across-phoneme differences in labeled voice onsets. This raises the concern that the pattern of decoding errors could be related to similarities in phonemes’ latencies between speech production initiation and voice onset, rather than place of articulation per se. We therefore repeated the place of articulation grouping analysis, but now applied to classifier errors from predicting the first phoneme of each word using neural alignment (as described for Fig. 4). We again observed higher confusion within place of articulation groups (Supplementary Fig. 5C-D; p < 0.005), suggesting that this error structure was not just an artifact of voice onset bias.
3.7. Speech synthesis using the ‘Brain-to-Speech’ unit selection method
In this work we have primarily evaluated the feasibility of using intracortical signals to decode discrete phonemes, which could be strung together by a speech BCI to generate audio or text. However, another approach for implementing a speech BCI is to directly synthesize speech sounds (Anumanchipalli, Chartier, and Chang 2019; Angrick et al. 2019; Herff et al. 2019). To more comprehensively test the suitability of intracortical array signals for a proof-of-concept speech BCI, we also applied a recently-published speech synthesis technique, termed ‘Brain-to-Speech’ by Herff and colleagues (Herff et al. 2019), to our data. This pattern matching approach compares each sliding window of neural data to a library of training data consisting of neural and corresponding audio data. At each time step, Brain-to-Speech selects the snippet of audio data corresponding to the best matching neural window; these snippets are then smoothly concatenated to generate a synthesized audio output. This method has a number of attractive attributes, including being fast and data-efficient to train; potentially reproducing the user’s own voice, including intonation and prosody, if ground truth speech training data is available; and being very fast to compute (i.e, amenable to real-time use). Typically, such unit selection techniques (Hunt and Black 1996) select the next unit (here, an audio snippet) based both on a “target cost”, i.e., how well the input data (neural features) match each unit in the training library, and a “concatenation cost” that uses a statistical model to evaluate how likely each unit would be given the previously selected units. However, as in (Herff et al. 2019), here we did not use a concatenation cost in order to more directly test the information content of the neural data alone, without the additional benefits of statistical priors which would vary depending on the specific speech BCI application.
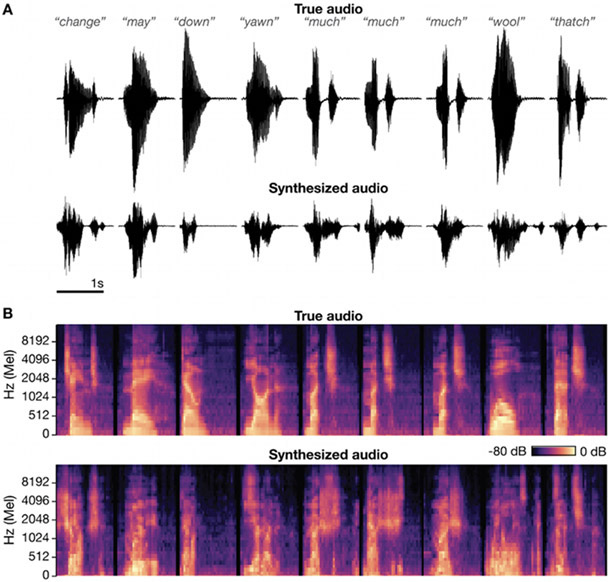
Figure 6 shows the result of applying this speech synthesis technique (offline) to the T5 dataset. As in (Herff et al. 2019), good examples were chosen to showcase that in some cases the reconstructed audio is borderline intelligibile. We quantified the reconstruction accuracy by decomposing the synthesized speech into time and frequency bins (40 logarithmically-spaced Mel-scale bins that approximate the human perception of speech) and calculating the correlation coefficient between the true and synthesized audio’s time-varying representation within each frequency bin. Averaging across all the data and all forty bins, the mean correlation was 0.523. Chance performance ranged from 0.019 to 0.038, with a mean of 0.027. For comparison, using the same performance metric, Herff and colleagues report an accuracy of r = 0.574 for the best participant in their ECoG study, and r = 0.246 averaged across all six participants. This indicates that intracortical signals are also competitive with ECoG for this speech synthesis approach. Supplementary Audio 1 contains the true and synthesized audio for 47 good example words (the first 9 of which are shown in Figure 6). Supplementary Audio 2 presents another 47 randomly chosen example words which demonstrate that most synthesis trials were not intelligible.
Fig. 6:
Speech synthesis using ‘brain-to-speech’ unit selection.
(A) Audio waveforms for the actual words spoken by participant T5 (top) and the synthesized audio reconstructed from neural data (bottom). (B) Corresponding acoustic spectrograms. The correlation coefficient between true and synthesized audio (averaged across all 40 Mel frequency bins) for these 9 good examples was 0.696.
4. Discussion
The present results indicate that decoding speech from intracortical arrays is a promising future direction for developing BCIs to restore lost speech. The phoneme classification accuracy and speech synthesis results demonstrated in the participant with more informative signals (T5) is similar to work performed with ECoG arrays that spanned a wide swath of speech-related cortical areas (Mugler et al. 2014; Livezey, Bouchard, and Chang 2019; Herff et al. 2019). This is despite the limited spatial coverage of the present study’s Utah arrays (10 x 10 grid of electrodes spanning 4.0 mm x 4.0 mm; array backplane is 4.2 mm x 4.2 mm) and their sub-optimal placement outside traditional speech areas. This accuracy also exceeds a prior intracortical phoneme decoding study which used fewer electrodes and reported 21% accuracy across 38 phonemes (Brumberg et al. 2011). However, we note the lower accuracy in the second participant (T11), and the overall gap between either participant’s decoder performance and the close to 100% accuracy one would aspire to for a speech BCI that approaches a healthy speaker’s capabilities. Thus, while we find these results encouraging, we do not believe that these dorsal precentral gyrus signals from two Utah arrays are sufficient to support fluent open-vocabulary communication using a speech BCI; rather, we view these results as a proof-of-concept demonstration that the intracortical approach is competitive with ECoG, and as motivation for future work using more electrodes placed in ventral speech areas.
As in prior ECoG studies (Mugler et al. 2014; Moses et al. 2019), we found that classifier errors exhibited articulatory structure (Supplementary Fig. 5). Phonemes spoken with similar place of articulation were more likely to be confused with one another compared to phonemes with different places of articulation. This suggests that the underlying neural signals relate to the movement of articulator muscles, broadly in line with ECoG studies (Mugler et al. 2014; Lotte et al. 2015; Mugler et al. 2018; Chartier et al. 2018; Moses et al. 2019) and earlier work looking at spiking activity in this dorsal ‘hand knob’ area (Stavisky et al. 2019). Assuming that our signals reflect articulator movements underlying speech, then the observed classification accuracy improvement from using multiple time bins likely reflects the increased ability to discriminate the time-varying movements underlying speech production (Ramsey et al. 2018; Chartier et al. 2018; Mugler et al. 2014). Future decoding approaches that seek to specifically assess and optimize performance with an eye toward eventual speech BCI applications may benefit from classifier cost functions and performance metrics tailored to speech perception, rather than treating all phoneme mistakes the same. For example, if the final BCI output is sound synthesis, then metrics based upon distortions of auditory spectral features (Anumanchipalli, Chartier, and Chang 2019) may be more informative: misclassifying similar sounding phonemes (e.g. /p/ and /b/) would be less damaging to overall speech comprehension compared to mistakes across groupings (e.g. /p/ and /r/).
We observed better performance classifying consonants versus vowels (Supplementary Fig. 2), which was also seen in earlier ECoG studies (Mugler et al. 2014; Livezey, Bouchard, and Chang 2019; Brumberg et al. 2011; Ramsey et al. 2018), although the factors explaining this phenomenon may differ for different recording modalities. In our case, lower vowel decoding accuracy might arise from the dataset statistics: most of the words our participants spoke were three phonemes long with consonant-vowel-consonant structure, resulting in more consonant training data. A second possibility is that the 500 millisecond decoding window used is short compared to the audio durations of vowels (Supplementary Fig. 1C, 319 ms on average for T5); the resulting neural features may therefore cover more of the relevant information for short duration consonants (173 ms on average for T5). Future work might consider a hierarchical classifier that first tries to decode vowel versus consonant, and then uses different window sizes accordingly. A third possibility is that the underlying articulator movements involved in vowel production are less well sampled by these recordings (Brumberg et al. 2011). Definitively testing the latter hypothesis will require future recordings of simultaneous neural and speech articulator activity.
The improved decoding performance when using high-frequency LFP power (as opposed to threshold crossing spikes) suggests that local voltage fluctuations can support phoneme classification without a need for spike detection. Indeed, prior decoding work across a more limited set of syllables found no accuracy difference between HLFP or spike features (Stavisky et al. 2018). The superior performance of HLFP in the present study’s more demanding phoneme classification task is consistent with studies showing that such spiking band power signals capture more task-related information from local neuronal activity than threshold crossings, including on electrodes that do not record sufficiently high SNR action potentials for spike detection (Stark and Abeles 2007; Nason et al. 2020). While this suggests that broadband LFP features are sufficient for high-performance decoding, we caution that microelectrode field potentials, which are largely dominated by local action potentials (Nason et al. 2020), reflect different underlying physiological sources than ECoG surface electrode recordings. In addition to being located on the surface of the brain (instead of ~1.5 mm below the surface), ECoG electrodes are much larger (~1 mm2 disks, versus ~0.001 mm2 Utah array electrode tips) and ECoG studies analyze the high gamma LFP power in a range of ~65 to 250 Hz (Bouchard et al. 2013; Chartier et al. 2018; Cheung et al. 2016; Dichter et al. 2018; Martin et al. 2014; Mugler et al. 2014; Ramsey et al. 2018) (versus this study’s 125 to 5,000 Hz). Importantly, performance here was dependent on sampling a sufficiently high number of electrodes from the two arrays (Fig. 2C). Corroborating this finding, individual electrodes had broad tuning profiles across phonemes, with 25% (T5) and 23% (T11) of all electrodes showing significant tuning to at least half of the 39 phonemes.
Leveraging a more computationally expressive machine learning approach for phoneme classification, we obtained a modest performance improvement using an RNN (33.9% accuracy, compared to 29.6% using logistic regression). Figure 3B,G indicates that performance had not saturated when increasing the number of electrodes or training data, and even more data should further improve classification accuracy, especially for deep learning methods (Livezey, Bouchard, and Chang 2019). Nonetheless, these results indicate that in the present data-impoverished context, deep learning provides only a small improvement compared to a simpler linear decoder. This echoes a pair of recent studies which synthesized speech from a shared dataset using either neural feature angle comparison (low complexity; (Herff et al. 2019) or convolutional neural networks (high complexity and only a small improvement; (Angrick et al. 2019). Furthermore, it seems unlikely that arrays in the hand knob area would support a very high performance speech BCI, even with more electrodes or training data. Future efforts will benefit from access to ventral speech cortex as well as higher electrode counts that record from a larger neural population.
In this study we also characterized and addressed two confounds that may be endemic to decoding overt speech. The first has not, to the best of our knowledge, been specifically examined before: labeling speech start times based on voice onset can artificially boost decoding performance by introducing systematic timing differences across phoneme classes between the onset labels and the true time when neural activity rapidly changes during speech production. This timing bias can be seen at the level of individual electrodes (Fig. 4A) and at the neural population level (Fig. 4B), and is consistent with the neural activity largely reflecting underlying articulatory movements (Bouchard et al. 2013; Lotte et al. 2015; Chartier et al. 2018; Mugler et al. 2018), rather than when these movements cause detectable sounds. A systematic discrepancy between voice and neural onset times was noted in a previous study (Jiang et al. 2016), which also proposed decoders that exploit relative neural onset differences between phoneme-specific and phoneme-independent neural components. It has also been previously noted (Mugler et al. 2014) that phoneme decoding accuracy is sensitive to the precision of onset timing labeling. Here, we realigned phonemes’ analysis windows to an onset time derived from the neural population signals themselves to account for this experimental confound and demonstrated that high performance classification was still possible (47.8% accuracy across 18 classes, Fig. 4E). We anticipate that this neural realignment strategy will also work in other recordings that contain strong condition-invariant responses time-locked to the speaking task. Because we observed an appreciable difference between decoder performance with and without this neural realignment (a 12.9% reduction in accuracy when classifying the first phoneme of each word), we caution that future speech BCI work based on voice onset labels should explicitly take across-labels timing biases into account.
We additionally found evidence of a second confound: acoustic contamination in our electrode signals (Roussel et al. 2020). Strong correlations between audio and “neural” activity occurred at specific frequencies, particularly in a cluster of electrodes on our medial array. However, unlike the characteristic correlation peaks around the fundamental frequency (and harmonics) of the more contaminated datasets in (Roussel et al. 2020), our correlations exhibited a less consistent relationship with T5’s audio PSD. This discrepancy may arise from the relatively short snippets of speech we had access to (as opposed to continuous speech), or they may reflect differences in the mechanics of acoustic contamination between specific setups. Further supporting our findings, we observed strong vibrations in the relevant frequency bands of some electrodes when applying a tuning fork to both our participant’s pre-amplifier and connector cable, consistent with the bench-top tests in (Roussel et al. 2020). To account for this contamination, we implemented two different approaches: 1) classification of each word’s first phoneme using pre-voice onset activity, and 2) the novel use of a decontamination procedure previously deployed for electrical stimulation artifact reduction (Young et al. 2018). By decoding phonemes using only pre-voicing neural activity (Fig. 5D, 34.8% accuracy across 18 classes), we showed that our performance is not solely a product of acoustic contamination. By mitigating correlations through a re-referencing approach, we found that we could still decode phonemes with relatively high accuracy (25.8% across 39 classes, although see 4.1). Thus, our conclusions are similar to that of (Roussel et al. 2020): microphonic pickup is a (manageable) nuisance for overt speech decoding studies, and that in a robust recording setup the majority of the measured voltage signals are biological and not artifactual.
4.1. Limitations
In this study we classified neural activity snippets aligned to phoneme onset, as determined from the audio recording of spoken speech. A clinical speech BCI will need to identify speech elements (such as phonemes) from unlabeled, continuous speaking data. Our speech synthesis results are one step in this direction, but free-running phoneme decoding from intracortical neural activity is a key next step for future work. Recent work decoding continuous speech from ECoG recordings indicates that this is feasible (Herff et al. 2015; Anumanchipalli, Chartier, and Chang 2019), and we anticipate that similar methods can be applied to intracortical recordings. While this presents a greater challenge, on the other hand, natural language processing methods may provide substantial help in the continuous regime (Li and Negoita 2018). In particular, leveraging the statistical structure of language may enable “denoising” of a neural decoder’s initial outputs (Moses et al. 2019; Willett et al. 2020). No such corrections were employed in this study, where we instead sought to understand ‘raw neural performance’ before applying any language model (the effectiveness of which will depend on the specifics of the speech BCI application, e.g., open vocabulary versus a more constrained vocabulary).
Our neural realignment procedure allowed us to disentangle the contribution of true (neural) variance across phonemes from that of spurious variance driven by systematic onset timing biases. While the results indicate relatively unimpaired performance following realignment (47.8% post-alignment vs 54.9%, 18 distinct classes), this check was restricted to classifying the first phoneme due to unreliable neural timing signals for subsequent phonemes. It thus remains an open challenge to quantify and mitigate the effect of voice onset biases for the later phonemes of a word.
Our recordings show evidence of acoustic contamination, which most likely improves classification performance. While our LRR procedure was able to mitigate this contamination, some acoustic contamination was probably still present. Future directions could examine a related artifact mitigation method (O’Shea and Shenoy 2018), with which we anticipate similar results. The present correlation analysis also assumes that the process of sound wave conversion into voltage signal fluctuations preserves frequencies, as opposed to shifting them around. While our tuning fork experiment suggests that this is approximately true (Supplementary Fig. 5 - although this is not necessarily the same mechanism underlying speech-induced acoustic contamination), we note that Roussel and colleagues observed a slight shift in correlation peaks for a participant with intracortical arrays relative to their voicing fundamental frequency.
Additionally, it is possible that neural signals relating to auditory feedback, as opposed to speech articulation, may drive some of the classification performance in this study. However, we were able to decode words’ first phonemes without an audible voicing activity and, in prior work (Stavisky et al. 2019), we also found strong tuning to unvoiced orofacial movements. Together, these findings suggest minimal feedback-driven signals compared to articulatory information. Future studies are needed to definitively disentangle these two processes two during attempted speech decoding.
These confounds complicate direct comparisons to previous work, as their extents may vary between different studies’ recording setups. Given the different corrections that can be applied (auditory feedback restriction, microphonic pickup controls, neural realignment, etc.) and combinations thereof, we therefore caution that the phoneme decoding and speech synthesis comparisons made here should not be viewed as exact, matched performance tests but rather ballpark estimates. With these points in mind, the demonstrated performance is at least comparable to prior studies (Brumberg et al. 2011; Mugler et al. 2014; Livezey, Bouchard, and Chang 2019) and supports further intracortical speech decoding work.
5. Conclusions
Taken together, our results indicate that the limited spatial coverage of current intracortical electrode arrays is more than offset by the high speech-related information provided by intracortical recordings. Our offline decode results suggest a lower bound on intracortically-driven speech BCI performance, since these arrays in a “hand/arm” area of precentral gyrus were likely suboptimally placed for speech decoding, and the participants did not receive online feedback that they could use to beneficially adjust their neural activity. This study de-risks and motivates future work in which arrays are implanted into ventral speech cortex in participants who cannot speak.
Supplementary Material
Acknowledgements
We thank participants T5, T11 and their caregivers for their generously volunteered time and effort as part of our BrainGate2 pilot clinical trial. We also thank Professor Mark Slutsky for providing the many words list; our Stanford NPTL and NPSL group for helpful discussions; Beverly Davis, Erika Siauciunas, and Nancy Lam for administrative support; and Dr. Darrel Deo (Stanford, NPTL group) for providing RNN decoder code documentation. Some of the computing for this project was performed on the Stanford High Performance Computing Cluster (Sherlock cluster). We thank Stanford University and the Stanford Research Computing Center for providing expert IT support for this computational resource where our primary compute node is housed.
This work was supported by an NSF Graduate Research Fellowship DGE-1656518 and Regina Casper Stanford Graduate Fellowship (G.H.W.); the A. P. Giannini Foundation, Wu Tsai Neurosciences Institute Interdisciplinary Scholars Fellowship, and Burroughs Wellcome Fund Career Award at the Scientific Interface (S.D.S.); Howard Hughes Medical Institute (F.R.W., D.T.A); US NIH NIDCD R01DC014034, NINDS R01NS066311, NIDCD R01DC009899, NICHDNCMRR N01HD53403, N01HD10018, Department of Veterans Affairs Rehabilitation Research and Development Service B6453R, MGH Deane Institute for Integrated Research on Atrial Fibrillation and Stroke (L.R.H.); NIDCD R01-DC014034, NIDCD U01-DC017844, NINDS UH2-NS095548, NINDS UO1-NS098968, Larry and Pamela Garlick, Samuel and Betsy Reeves, Wu Tsai Neurosciences Institute at Stanford (J.M.H and K.V.S); NIBIB R01-EB028171 (S.D.); Simons Foundation Collaboration on the Global Brain 543045 and Howard Hughes Medical Institute Investigator (K.V.S).
Footnotes
Declaration of interests
The MGH Translational Research Center has a clinical research support agreement with Neuralink, Paradromics, and Synchron, for which L.R.H. provides consultative input. JMH is a consultant for Neuralink Corp and Proteus Biomedical, and serves on the Medical Advisory Board of Enspire DBS. KVS consults for Neuralink Corp. and CTRL-Labs Inc. (part of Facebook Reality Labs) and is on the scientific advisory boards of MIND-X Inc., Inscopix Inc., and Heal Inc. All other authors have no competing interests.
References
- Abadi Martín, Barham Paul, Chen Jianmin, Chen Zhifeng, Davis Andy, Dean Jeffrey, Devin Matthieu, et al. 2016. “Tensorflow: A System for Large-Scale Machine Learning.” In 12th ${USENIX} Symposium on Operating Systems Design and Implementation ({OSDI}$ 16), 265–83. [Google Scholar]
- Abbott LF, and Dayan P. 1999. “The Effect of Correlated Variability on the Accuracy of a Population Code.” Neural Computation 11 (1): 91–101. [DOI] [PubMed] [Google Scholar]
- Ajiboye A. Bolu, Willett Francis R., Young Daniel R., Memberg William D., Murphy Brian A., Miller Jonathan P., Walter Benjamin L., et al. 2017. “Restoration of Reaching and Grasping Movements through Brain-Controlled Muscle Stimulation in a Person with Tetraplegia: A Proof-of-Concept Demonstration.” The Lancet 389 (10081): 1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari Hassan, Khalighinejad Bahar, Herrero Jose L., Mehta Ashesh D., and Mesgarani Nima. 2019. “Towards Reconstructing Intelligible Speech from the Human Auditory Cortex.” Scientific Reports 9 (1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrick Miguel, Herff Christian, Mugler Emily, Tate Matthew C., Slutzky Marc W., Krusienski Dean J., and Schultz Tanja. 2019. “Speech Synthesis from ECoG Using Densely Connected 3D Convolutional Neural Networks.” Journal of Neural Engineering 16 (3): 036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumanchipalli Gopala K., Chartier Josh, and Chang Edward F.. 2019. “Speech Synthesis from Neural Decoding of Spoken Sentences.” Nature 568 (7753): 493–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher Itay, Stark Eran, Abeles Moshe, and Prut Yifat. 2007. “Comparison of Direction and Object Selectivity of Local Field Potentials and Single Units in Macaque Posterior Parietal Cortex during Prehension.” Journal of Neurophysiology 97 (5): 3684–95. [DOI] [PubMed] [Google Scholar]
- Boersma Paul. 2001. “Praat, a System for Doing Phonetics by Computer.” Glot International. [Google Scholar]
- Bouchard Kristofer E., and Chang Edward F.. 2014. “Neural Decoding of Spoken Vowels from Human Sensory-Motor Cortex with High-Density Electrocorticography.” Conference Proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference; 2014: 6782–85. [DOI] [PubMed] [Google Scholar]
- Bouchard Kristofer E., Mesgarani Nima, Johnson Keith, and Chang Edward F.. 2013. “Functional Organization of Human Sensorimotor Cortex for Speech Articulation.” Nature 495 (7441): 327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton Chad E., Shaikhouni Ammar, Annetta Nicholas V., Bockbrader Marcia A., Friedenberg David A., Nielson Dylan M., Sharma Gaurav, et al. 2016. “Restoring Cortical Control of Functional Movement in a Human with Quadriplegia.” Nature 533 (7602): 247–50. [DOI] [PubMed] [Google Scholar]
- Brandman David M., Hosman Tommy, Saab Jad, Burkhart Michael C., Shanahan Benjamin E., Ciancibello John G., Sarma Anish A., et al. 2018. “Rapid Calibration of an Intracortical Brain–computer Interface for People with Tetraplegia.” Journal of Neural Engineering 15 (2): 026007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg Jonathan S., Wright E. Joe, Andreasen Dinal S., Guenther Frank H., and Kennedy Philip R.. 2011. “Classification of Intended Phoneme Production from Chronic Intracortical Microelectrode Recordings in Speech Motor Cortex.” Frontiers in Neuroscience 5. 10.3389/fnins.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki György, Anastassiou Costas A., and Koch Christof. 2012. “The Origin of Extracellular Fields and Currents — EEG, ECoG, LFP and Spikes.” Nature Reviews. Neuroscience 13 (6): 407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti Shreya, Sandberg Hilary M., Brumberg Jonathan S., and Krusienski Dean J.. 2015. “Progress in Speech Decoding from the Electrocorticogram.” Biomedical Engineering Letters 5 (1): 10–21. [Google Scholar]
- Chan Alexander M., Dykstra Andrew R., Jayaram Vinay, Leonard Matthew K., Travis Katherine E., Gygi Brian, Baker Janet M., et al. 2014. “Speech-Specific Tuning of Neurons in Human Superior Temporal Gyrus.” Cerebral Cortex 24 (10): 2679–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Edward F., and Anumanchipalli Gopala K.. 2020. “Toward a Speech Neuroprosthesis.” JAMA: The Journal of the American Medical Association 323 (5): 413–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier Josh, Anumanchipalli Gopala K., Johnson Keith, and Chang Edward F.. 2018. “Encoding of Articulatory Kinematic Trajectories in Human Speech Sensorimotor Cortex.” Neuron 98 (5): 1042–54.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestek Cynthia A., Gilja Vikash, Nuyujukian Paul, Foster Justin D., Fan Joline M., Kaufman Matthew T., Churchland Mark M., et al. 2011. “Long-Term Stability of Neural Prosthetic Control Signals from Silicon Cortical Arrays in Rhesus Macaque Motor Cortex.” Journal of Neural Engineering 8 (4): 045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Connie, Hamilton Liberty S., Johnson Keith, and Chang Edward F.. 2016. “Correction: The Auditory Representation of Speech Sounds in Human Motor Cortex.” eLife 5 (April). 10.7554/eLife.17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Kyunghyun, Bart van Merrienboer Caglar Gulcehre, Bahdanau Dzmitry, Bougares Fethi, Schwenk Holger, and Bengio Yoshua. 2014. “Learning Phrase Representations Using RNN Encoder-Decoder for Statistical Machine Translation.” arXiv [cs.CL]. arXiv http://arxiv.org/abs/1406.1078. [Google Scholar]
- Christie Breanne P., Tat Derek M., Irwin Zachary T., Gilja Vikash, Nuyujukian Paul, Foster Justin D., Ryu Stephen I., Shenoy Krishna V., Thompson David E., and Chestek Cynthia A.. 2015. “Comparison of Spike Sorting and Thresholding of Voltage Waveforms for Intracortical Brain–machine Interface Performance.” Journal of Neural Engineering. 10.1088/1741-2560/12/1/016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinger Jennifer L., Wodlinger Brian, Downey John E., Wang Wei, Tyler-Kabara Elizabeth C., Weber Douglas J., McMorland Angus J. C., Velliste Meel, Boninger Michael L., and Schwartz Andrew B.. 2013. “High-Performance Neuroprosthetic Control by an Individual with Tetraplegia.” The Lancet 381 (9866): 557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, and Lettich E. 1989. “Neuronal Activity in the Human Lateral Temporal Lobe.” Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale 77 (3): 451–75. [DOI] [PubMed] [Google Scholar]
- Dash Debadatta, Ferrari Paul, and Wang Jun. 2020. “Decoding Imagined and Spoken Phrases From Non-Invasive Neural (MEG) Signals.” Frontiers in Neuroscience 14 (April): 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter Benjamin K., Breshears Jonathan D., Leonard Matthew K., and Chang Edward F.. 2018. “The Control of Vocal Pitch in Human Laryngeal Motor Cortex.” Cell 174 (1): 21–31.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey John E., Schwed Nathaniel, Chase Steven M., Schwartz Andrew B., and Collinger Jennifer L.. 2018. “Intracortical Recording Stability in Human Brain–computer Interface Users.” Journal of Neural Engineering. 10.1088/1741-2552/aab7a0. [DOI] [PubMed] [Google Scholar]
- Downey John E., Weiss Jeffrey M., Flesher Sharlene N., Thumser Zachary C., Marasco Paul D., Boninger Michael L., Gaunt Robert A., and Collinger Jennifer L.. 2018. “Implicit Grasp Force Representation in Human Motor Cortical Recordings.” Frontiers in Neuroscience 12 (October): 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Chen N, Stavisky SD, Pandarinath C, Nuyujukian P, Blabe CH, Hochberg LR, Henderson JM, and Shenoy KV. 2018. “Feasibility of Automatic Error Detect-and-Undo System in Human Intracortical Brain–Computer Interfaces.” IEEE Transactions on Biomedical Engineering 65 (8): 1771–84. [DOI] [PubMed] [Google Scholar]
- Guenther Frank H., Brumberg Jonathan S., Wright E. Joseph, Nieto-Castanon Alfonso, Tourville Jason A., Panko Mikhail, Law Robert, et al. 2009. “A Wireless Brain-Machine Interface for Real-Time Speech Synthesis.” PloS One 4 (12): e8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heelan Christopher, Lee Jihun, O’Shea Ronan, Lynch Laurie, Brandman David M., Truccolo Wilson, and Nurmikko Arto V.. 2019. “Decoding Speech from Spike-Based Neural Population Recordings in Secondary Auditory Cortex of Non-Human Primates.” Communications Biology 2 (1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herff Christian, Diener Lorenz, Angrick Miguel, Mugler Emily, Tate Matthew C., Goldrick Matthew A., Krusienski Dean J., Slutzky Marc W., and Schultz Tanja. 2019. “Generating Natural, Intelligible Speech From Brain Activity in Motor, Premotor, and Inferior Frontal Cortices.” Frontiers in Neuroscience 13. 10.3389/fnins.2019.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herff Christian, Heger D, De Pesters A, and Telaar D. 2015. “Brain-to-Text: Decoding Spoken Phrases from Phone Representations in the Brain.” Frontiers in. https://www.frontiersin.org/articles/10.3389/fnins.2015.00217/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herff Christian, Krusienski Dean J., and Kubben Pieter. 2020. “The Potential of Stereotactic-EEG for Brain-Computer Interfaces: Current Progress and Future Directions.” Frontiers in Neuroscience 14 (February): 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herff Christian, and Schultz Tanja. 2016. “Automatic Speech Recognition from Neural Signals: A Focused Review.” Frontiers in Neuroscience 10 (September): 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok Gregory. February/2012. “Computational Neuroanatomy of Speech Production.” Nature Reviews. Neuroscience 13 (2): 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Leigh R., Bacher Daniel, Jarosiewicz Beata, Masse Nicolas Y., Simeral John D., Vogel Joern, Haddadin Sami, et al. 2012. “Reach and Grasp by People with Tetraplegia Using a Neurally Controlled Robotic Arm.” Nature 485 (7398): 372–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Leigh R., Serruya Mijail D., Friehs Gerhard M., Mukand Jon A., Saleh Maryam, Caplan Abraham H., Branner Almut, Chen David, Penn Richard D., and Donoghue John P.. 2006. “Neuronal Ensemble Control of Prosthetic Devices by a Human with Tetraplegia.” Nature 442 (7099): 164–71. [DOI] [PubMed] [Google Scholar]
- House Arthur S., Williams Carl, Hecker Michael H. L., and Kryter Karl D.. 1963. “Psychoacoustic Speech Test: A Modified Rhyme Test.” PsycEXTRA Dataset. 10.1037/e414082004-001. [DOI] [PubMed] [Google Scholar]
- Hunt AJ, and Black AW. 1996. “Unit Selection in a Concatenative Speech Synthesis System Using a Large Speech Database.” In 1996 IEEE International Conference on Acoustics, Speech, and Signal Processing Conference Proceedings, 1:373–76 vol. 1. [Google Scholar]
- Jarosiewicz Beata, Sarma Anish A., Bacher Daniel, Masse Nicolas Y., Simeral John D., Sorice Brittany, Oakley Erin M., et al. 2015. “Virtual Typing by People with Tetraplegia Using a Self-Calibrating Intracortical Brain-Computer Interface.” Science Translational Medicine 7 (313): 313ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Werner, Pailla Tejaswy, Dichter Benjamin, Chang Edward F., and Gilja Vikash. 2016. “Decoding Speech Using the Timing of Neural Signal Modulation.” Conference Proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 2016 (August): 1532–35. [DOI] [PubMed] [Google Scholar]
- Kaufman Matthew T., Seely Jeffrey S., Sussillo David, Ryu Stephen I., Shenoy Krishna V., and Churchland Mark M.. 2016. “The Largest Response Component in the Motor Cortex Reflects Movement Timing but Not Movement Type.” eNeuro 3 (4). 10.1523/ENEURO.0085-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis Spencer, Miller Kai, Thomson Kyle, Brown Richard, House Paul, and Greger Bradley. 2010. “Decoding Spoken Words Using Local Field Potentials Recorded from the Cortical Surface.” Journal of Neural Engineering 7 (5): 056007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma Diederik P., and Ba Jimmy. 2017. “Adam: A Method for Stochastic Optimization.” arXiv:1412.6980 [cs], January. http://arxiv.org/abs/1412.6980. [Google Scholar]
- Kobak Dmitry, Brendel Wieland, Constantinidis Christos, Feierstein Claudia E., Kepecs Adam, Mainen Zachary F., Qi Xue-Lian, Romo Ranulfo, Uchida Naoshige, and Machens Christian K.. 2016. “Demixed Principal Component Analysis of Neural Population Data.” Edited by van Rossum Mark C. W.. eLife 5 (April): e10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fager Koch, Susan Melanie Fried-Oken, Jakobs Tom, and Beukelman David R.. 2019. “New and Emerging Access Technologies for Adults with Complex Communication Needs and Severe Motor Impairments: State of the Science.” Augmentative and Alternative Communication 35 (1): 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe Leonard L., and Stierwalt Julie. 2018. “Aphasia and Related Neurogenic Language Disorders.” 10.1055/b-005-148885. [DOI] [Google Scholar]
- Li Leon, and Negoita Serban. 2018. “Brain-to-Speech Decoding Will Require Linguistic and Pragmatic Data.” Journal of Neural Engineering 15 (6): 063001. [DOI] [PubMed] [Google Scholar]
- Lipski Witold J., Alhourani Ahmad, Pirnia Tara, Jones Peter W., Christina Dastolfo-Hromack Leah B. Helou, Crammond Donald J., et al. 2018. “Subthalamic Nucleus Neurons Differentially Encode Early and Late Aspects of Speech Production.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 38 (24): 5620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livezey Jesse A., Bouchard Kristofer E., and Chang Edward F.. 2019. “Deep Learning as a Tool for Neural Data Analysis: Speech Classification and Cross-Frequency Coupling in Human Sensorimotor Cortex.” Edited by Miller Kai. PLoS Computational Biology 15 (9): e1007091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotte Fabien, Brumberg Jonathan S., Brunner Peter, Gunduz Aysegul, Ritaccio Anthony L., Guan Cuntai, and Schalk Gerwin. 2015. “Electrocorticographic Representations of Segmental Features in Continuous Speech.” Frontiers in Human Neuroscience 9 (February). 10.3389/fnhum.2015.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin Joseph G., Moses David A., and Chang Edward F.. 2020. “Machine Translation of Cortical Activity to Text with an Encoder–decoder Framework.” Nature Neuroscience, March, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen Tanja, Ruottinen Hanna, Puhto Riitta, Helminen Mika, and Palmio Johanna. 2018. “Speech Deterioration in Amyotrophic Lateral Sclerosis (ALS) after Manifestation of Bulbar Symptoms.” International Journal of Language & Communication Disorders / Royal College of Speech & Language Therapists 53 (2): 385–92. [DOI] [PubMed] [Google Scholar]
- Martin Stephanie, Brunner Peter, Holdgraf Chris, Heinze Hans-Jochen, Crone Nathan E., Rieger Jochem, Schalk Gerwin, Knight Robert T., and Pasley Brian N.. 2014. “Decoding Spectrotemporal Features of Overt and Covert Speech from the Human Cortex.” Frontiers in Neuroengineering 7 (May): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Stephanie, Iturrate Iñaki, Millán José del R., Knight Robert T., and Pasley Brian N.. 2018. “Decoding Inner Speech Using Electrocorticography: Progress and Challenges Toward a Speech Prosthesis.” Frontiers in Neuroscience 12. 10.3389/fnins.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFee Brian, Lostanlen Vincent, McVicar Matt, Metsai Alexandros, Balke Stefan, Thomé C, Raffel Colin, et al. 2019. “Librosa/librosa: 0.7. 1.” Version 0. 7 1 [Google Scholar]
- Mines MA, Hanson BF, and Shoup JE. 1978. “Frequency of Occurrence of Phonemes in Conversational English.” Language and Speech 21 (3): 221–41. [DOI] [PubMed] [Google Scholar]
- Moses David A., Leonard Matthew K., Makin Joseph G., and Chang Edward F.. 2019. “Real-Time Decoding of Question-and-Answer Speech Dialogue Using Human Cortical Activity.” Nature Communications 10 (1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler Emily M., Patton James L., Flint Robert D., Wright Zachary A., Schuele Stephan U., Rosenow Joshua, Shih Jerry J., Krusienski Dean J., and Slutzky Marc W.. 2014. “Direct Classification of All American English Phonemes Using Signals from Functional Speech Motor Cortex.” Journal of Neural Engineering 11 (3): 035015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler Emily M., Tate Matthew C., Livescu Karen, Templer Jessica W., Goldrick Matthew A., and Slutzky Marc W.. 2018. “Differential Representation of Articulatory Gestures and Phonemes in Precentral and Inferior Frontal Gyri.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 38 (46): 9803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason SR, Vaskov AK, Willsey MS, Welle EJ, An H, Vu PP, Bullard AJ, et al. 2020. “Low-Power ‘spiking Band’ Feature Is Dominated by Local Single Units and Improves Brain-Machine Interface Performance.” Nature Biomedical Engineering. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Chuong H., Karavas George K., and Artemiadis Panagiotis. 2018. “Inferring Imagined Speech Using EEG Signals: A New Approach Using Riemannian Manifold Features.” Journal of Neural Engineering 15 (1): 016002. [DOI] [PubMed] [Google Scholar]
- Oby Emily R., Perel Sagi, Sadtler Patrick T., Ruff Douglas A., Mischel Jessica L., Montez David F., Cohen Marlene R., Batista Aaron P., and Chase Steven M.. 2016. “Extracellular Voltage Threshold Settings Can Be Tuned for Optimal Encoding of Movement and Stimulus Parameters.” Journal of Neural Engineering 13 (3): 036009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea Daniel J., and Shenoy Krishna V.. 2018. “ERAASR: An Algorithm for Removing Electrical Stimulation Artifacts from Multielectrode Array Recordings.” Journal of Neural Engineering 15 (2): 026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailla Tejaswy, Jiang Werner, Dichter Benjamin, Chang Edward F., and Gilja Vikash. 2016. “ECoG Data Analyses to Inform Closed-Loop BCI Experiments for Speech-Based Prosthetic Applications.” Conference Proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 2016 (August): 5713–16. [DOI] [PubMed] [Google Scholar]
- Pandarinath Chethan, Nuyujukian Paul, Blabe Christine H., Sorice Brittany L., Saab Jad, Willett Francis R., Hochberg Leigh R., Shenoy Krishna V., and Henderson Jaimie M.. 2017. “High Performance Communication by People with Paralysis Using an Intracortical Brain-Computer Interface.” Edited by Kastner Sabine. eLife 6 (February): e18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa Fabian, Varoquaux Gaël, Gramfort Alexandre, Michel Vincent, Thirion Bertrand, Grisel Olivier, Blondel Mathieu, et al. 2011. “Scikit-Learn: Machine Learning in Python.” The Journal of Machine Learning Research 12: 2825–30. [Google Scholar]
- Perge János A., Homer Mark L., Malik Wasim Q., Cash Sydney, Eskandar Emad, Friehs Gerhard, Donoghue John P., and Hochberg Leigh R.. 2013. “Intra-Day Signal Instabilities Affect Decoding Performance in an Intracortical Neural Interface System.” Journal of Neural Engineering 10 (3): 036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani Qinwan, Milsap Griffin, and Crone Nathan E.. 2019. “The Potential for a Speech Brain-Computer Interface Using Chronic Electrocorticography.” Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics 16 (1): 144–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Salari E, Aarnoutse EJ, Vansteensel MJ, Bleichner MG, and Freudenburg ZV. 2018. “Decoding Spoken Phonemes from Sensorimotor Cortex with High-Density ECoG Grids.” NeuroImage, New advances in encoding and decoding of brain signals, 180 (October): 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi Anisha, Vargas-Irwin Carlos E., Willett Francis R., Abreu Jessica, Crowder Douglas C., Murphy Brian A., Memberg William D., et al. 2020. “Neural Representation of Observed, Imagined, and Attempted Grasping Force in Motor Cortex of Individuals with Chronic Tetraplegia.” Scientific Reports 10 (1): 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel Philémon, Gaël Le Godais Florent Bocquelet, Palma Marie, Hongjie Jiang, Zhang Shaomin, Giraud Anne Lise, et al. 2020. “Observation and Assessment of Acoustic Contamination of Electrophysiological Brain Signals during Speech Production and Sound Perception.” Journal of Neural Engineering, August. 10.1088/1741-2552/abb25e. [DOI] [PubMed] [Google Scholar]
- Salari E, Freudenburg ZV, Vansteensel MJ, and Ramsey NF. 2018. “The Influence of Prior Pronunciations on Sensorimotor Cortex Activity Patterns during Vowel Production.” Journal of Neural Engineering 15 (6): 066025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutzky Marc W. 2019. “Brain-Machine Interfaces: Powerful Tools for Clinical Treatment and Neuroscientific Investigations.” The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry 25 (2): 139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark Eran, and Abeles Moshe. 2007. “Predicting Movement from Multiunit Activity.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 27 (31): 8387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavisky Sergey D., Rezaii Paymon, Willett Francis R., Hochberg Leigh R., Shenoy Krishna V., and Henderson Jaimie M.. 2018. “Decoding Speech from Intracortical Multielectrode Arrays in Dorsal ‘Arm/Hand Areas’ of Human Motor Cortex.” In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 93–97. [DOI] [PubMed] [Google Scholar]
- Stavisky Sergey D., Willett Francis R., Avansino Donald T., Hochberg Leigh R., Shenoy Krishna V., and Henderson Jaimie M.. 2020. “Speech-Related Dorsal Motor Cortex Activity Does Not Interfere with iBCI Cursor Control.” Journal of Neural Engineering 17 (1): 016049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavisky Sergey D., Willett Francis R., Wilson Guy H., Murphy Brian A., Rezaii Paymon, Avansino Donald T., Memberg William D., et al. 2019. “Neural Ensemble Dynamics in Dorsal Motor Cortex during Speech in People with Paralysis.” Edited by Makin Tamar R., Shinn-Cunningham Barbara G., Makin Tamar R., Gallego Juan Álvaro, and Scott Sophie K.. eLife 8 (December): e46015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SS, Volkmann J, and Newman EB. 1937. “A Scale for the Measurement of the Psychological Magnitude Pitch.” The Journal of the Acoustical Society of America 8 (3): 185–90. [Google Scholar]
- Suppes P, Lu ZL, and Han B. 1997. “Brain Wave Recognition of Words.” Proceedings of the National Academy of Sciences of the United States of America 94 (26): 14965–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussillo David, Stavisky Sergey D., Kao Jonathan C., Ryu Stephen I., and Shenoy Krishna V.. 2016. “Making Brain–machine Interfaces Robust to Future Neural Variability.” Nature Communications 7 (1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Kelly, Blain Stefanie, and Chau Tom. 2008. “A Review of Emerging Access Technologies for Individuals with Severe Motor Impairments.” Assistive Technology: The Official Journal of RESNA 20 (4): 204–19; quiz 220–21. [DOI] [PubMed] [Google Scholar]
- Takai Osamu, Brown Steven, and Liotti Mario. 2010. “Representation of the Speech Effectors in the Human Motor Cortex: Somatotopy or Overlap?” Brain and Language 113 (1): 39–44. [DOI] [PubMed] [Google Scholar]
- Tam Wing-Kin, Wu Tong, Zhao Qi, Keefer Edward, and Yang Zhi. 2019. “Human Motor Decoding from Neural Signals: A Review.” BMC Biomedical Engineering 1 (1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankus Ariel, and Fried Itzhak. 2019. “Degradation of Neuronal Encoding of Speech in the Subthalamic Nucleus in Parkinson’s Disease.” Neurosurgery 84 (2): 378–87. [DOI] [PubMed] [Google Scholar]
- Tankus Ariel, Fried Itzhak, and Shoham Shy. 2012. “Structured Neuronal Encoding and Decoding of Human Speech Features.” Nature Communications 3 (1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville Jason A., and Guenther Frank H.. August/2011. “The DIVA Model: A Neural Theory of Speech Acquisition and Production.” Language and Cognitive Processes 26 (7): 952–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann Eric M., Stavisky Sergey D., Lahiri Subhaneil, Ames Katherine C., Kaufman Matthew T., O’Shea Daniel J., Vyas Saurabh, et al. 2019. “Accurate Estimation of Neural Population Dynamics without Spike Sorting.” Neuron 103 (2): 292–308.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldert Stephan, Lemon Roger N., and Kraskov Alexander. 2013. “Influence of Spiking Activity on Cortical Local Field Potentials.” The Journal of Physiology 591 (21): 5291–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett Francis R., Avansino Donald, Hochberg Leigh, Henderson Jaimie, and Shenoy Krishna. 2020. “High-performance brain-to-text communication via imagined handwriting.” bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett Francis R., Deo Darrel R., Avansino Donald T., Rezaii Paymon, Hochberg Leigh R., Henderson Jaimie M., and Shenoy Krishna V.. 2020. “Hand Knob Area of Premotor Cortex Represents the Whole Body in a Compositional Way.” Cell, S0092867420302208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodlinger B, Downey JE, Tyler-Kabara EC, Schwartz AB, Boninger ML, and Collinger JL. 2015. “Ten-Dimensional Anthropomorphic Arm Control in a Human Brain- Machine Interface: Difficulties.” Solutions, and Limitations. J. Neural Eng 12 (016011): 20. [DOI] [PubMed] [Google Scholar]
- Young D, Willett F, Memberg WD, Murphy B, Walter B, Sweet J, Miller J, Hochberg LR, Kirsch RF, and Ajiboye AB. 2018. “Signal Processing Methods for Reducing Artifacts in Microelectrode Brain Recordings Caused by Functional Electrical Stimulation.” Journal of Neural Engineering 15 (2): 026014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Mingming, Schwemmer Michael A., Ting Jordyn E., Majstorovic Connor E., Friedenberg David A., Bockbrader Marcia A., Mysiw W. Jerry, et al. 2018. “Extracting Wavelet Based Neural Features from Human Intracortical Recordings for Neuroprosthetics Applications.” Bioelectronic Medicine 4 (July): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.