Abstract
A link between oxidative stress and hypertension has been firmly established in multiple animal models of hypertension, but remains elusive in humans. While initial studies focused on inactivation of nitric oxide by superoxide, our understanding of relevant reactive oxygen species (ROS: superoxide, hydrogen peroxide, peroxynitrite) and how they modify complex signaling pathways to promote hypertension has expanded significantly. In this review, we summarize recent advances in delineating the primary and secondary sources of ROS (NADPH oxidases (Nox), uncoupled endothelial nitric oxide synthase, endoplasmic reticulum and mitochondria), the post-translational oxidative modifications they induce on protein targets important for redox signaling, their interplay with endogenous antioxidant systems, and the role of inflammasome activation and endoplasmic reticular stress in the development of hypertension. We highlight how oxidative stress in different organ systems contributes to hypertension, describe new animal models that have clarified the importance of specific proteins and discuss clinical studies that shed light on how these processes and pathways are altered in human hypertension. Finally, we focus on the promise of redox proteomics and systems biology to help us fully understand the relationship between ROS and hypertension and their potential for designing and evaluating novel antihypertensive therapies.
Keywords: Reactive oxygen species, superoxide anion, hydrogen peroxide, Nox, redox signaling, antioxidants, biomarkers, endoplasmic reticulum stress
Subject Terms: Biomarkers, Hypertension, Oxidant Stress
INTRODUCTION
Multiple regulatory systems involving the heart, vessels, kidneys, brain and immune cells, underpin the pathophysiology of hypertension, and oxidative stress has been considered as a unifying factor linking these elements (1). The oxidative stress theory of disease is based on the premise that increased bioavailability of reactive oxygen species (ROS), comprising free radicals and non-free radicals, adversely affects cellular macromolecules, such as RNA, DNA, proteins, lipids and carbohydrates causing cell injury and death (2). As a protective mechanism, cellular antioxidant defense systems (superoxide dismutase (SOD), peroxidases, antioxidant vitamins) have evolved, which maintain oxidation-reduction (redox) status by preventing ROS accumulation. Oxidative stress was originally defined as ‘a disturbance in the prooxidant:antioxidant balance in favor of the former’, with the notion that free radicals are injurious species (3). However, following discoveries in the 1980s that i) ROS are critically involved in host:defense responses (4), ii) endothelial-derived nitric oxide (NO) is an endothelium-derived relaxing factor (EDRF) that controls vasorelaxation (5), iii) ROS generation is controlled by ‘professional oxidases’ [nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NADPH oxidases, or Noxes)] (6), and the more recent findings that levels of ROS are influenced by hormones and other factors (7–9), it became clear that cytosolic ROS are highly regulated active participants in diverse cellular processes contributing to normal signaling, cellular homeostasis and biological functions of living organisms (10,11). This led to a redefinition of ‘oxidative stress’ to incorporate the importance of redox signaling, which is now described as ‘an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage’ (12). This is the definition used in the present review.
The significance of oxidative stress in the context of hypertension relates to the fundamental role of ROS and redox signaling in molecular, cellular and systems processes that cause endothelial damage, vascular dysfunction, cardiovascular remodeling, renal dysfunction, sympathetic nervous system excitation, immune cell activation and systemic inflammation, important in the pathophysiology of hypertension (13–16). Redox signaling is the specific oxidation-reduction post-translational modification of downstream signaling molecules induced by free radicals, such as superoxide anion (O2−) and NO, and non-free radicals, such as hydrogen peroxide (H2O2) (17,18). Oxidative posttranslational modification (Ox-PTM) of proteins is a key molecular mechanism that controls proteins, which ultimately influence cellular biological responses (19). Normally, proteins are targets of reversible Ox-PTM, while in pathological conditions associated with oxidative stress, such as in hypertension, proteins undergo irreversible Ox-PTM leading to loss of protein function and consequent cell damage, tissue injury and target organ failure (20,21). Advances in oxidative proteomics have provided new insights on the role of redox biology in cardiovascular pathophysiology with recent studies focusing on cysteine (Cys) oxidation of proteins involved in cell proliferation, inflammation, contraction/relaxation, cytoskeletal organization and apoptosis (22,23).
Primary enzymatic sources of cardiovascular ROS are non-phagocytic Noxes, which are variably upregulated in hypertension (24–26). Emerging evidence indicates that in addition to Nox hyperactivation, nitric oxide synthase (NOS) uncoupling, endoplasmic reticulum (ER) stress and mitochondrial oxidative stress contribute to redox changes in hypertension (27–29). These processes are themselves redox-sensitive and may be considered as secondary mechanisms involved in oxidative stress in hypertension. Activation of eNOS typically generates NO, however in an oxidized milieu, NOS becomes uncoupled to generate vasoinjurious O2− instead of vasoprotective NO (30).
ER stress and the unfolded protein response (UPR) arise as a consequence of accumulation of misfolded proteins. These processes adversely affect cellular homeostasis, intracellular signaling and cell morphology leading to tissue injury (31). ER stress has been described as a novel regulator of cardiovascular disease since it plays a role in vascular cell phenotypic switching, dedifferentiation, calcification and apoptosis and contributes to endothelial dysfunction and vascular remodeling in hypertension and atherosclerosis (32,33). ROS-regulated ER stress may be an important process in hypertension-associated cardiovascular dysfunction and target organ damage (30,34). This is supported by studies showing that inhibition of ER stress protects against hypertension-induced vascular dysfunction (35), blunts development of hypertension in spontaneously hypertensive rats (SHR) (36,37), prevents cardiac impairment and hypertrophy in experimental models of hypertension and pulmonary arterial hypertension (38,39) and underlies increased angiotensin II (Ang II) signaling and sympathetic outflow (40,41).
Mitochondrial oxidative stress, which is associated with mitochondrial dysfunction and redox inactivation of mitochondrial deacetylase, Silent mating type information regulation 2 homolog 3 [Sirtuin 3 (SIRT3)], and the key mitochondrial antioxidant SOD2, may also contribute to the development of hypertension (42). These processes, which themselves cause oxidative stress, promote vascular dysfunction, cardiac fibrosis and blood pressure elevation in experimental models (43). Clinical studies support the potential role of mitochondrial oxidative stress because Sirt3 is downregulated in patients with essential hypertension (44). These findings have sparked interest in developing mitochondrial targeted interventions as vasoprotective and antihypertensive strategies (45). In particular scavenging of mitochondrial lipid peroxidation products (isolevuglandins) attenuates development of hypertension in Ang II-infused mice (46) and mitoQ, a mitochondrial-targeted SOD mimetic, ameliorates endothelial dysfunction, inhibits ROS generation and decreases blood pressure in models of hypertension (47). Similar favorable vascular responses have been demonstrated in humans treated with MitoQ, a mitochondrial antioxidant (48).
Extensive pre-clinical studies in experimental models demonstrate unambiguously that redox signaling is critically involved in cardiovascular pathophysiology and that oxidative stress is causally linked to the development of hypertension (49–51). However, this has not translated to the clinic where it has been difficult to demonstrate an etiological basis for ROS in blood pressure elevation. This may relate, in part, to a paucity of information on ROS biology in human tissue, sub-optimal methods to measure ROS in the clinical setting and inappropriately designed clinical trials to evaluate the antihypertensive effects of antioxidant therapies. Nonetheless, many clinical studies have demonstrated positive associations between systemic biomarkers of oxidative stress and blood pressure and antioxidant capacity has been shown to be reduced in hypertension (52–54).
The present review provides an up-to-date appraisal of the biology of ROS and oxidative stress in the pathogenesis of hypertension with a particular focus on emerging new concepts. Specifically we discuss i) primary (Noxes) and secondary mechanisms (NOS uncoupling, ER stress, mitochondrial stress) of ROS generation, ii) redox signaling, the ROS-regulated inflammasome and oxidative proteomics in hypertension, and iii) systems basis of oxidative stress in hypertension. Finally we provide some perspectives of the role of oxidative stress in human hypertension.
REACTIVE OXYGEN SPECIES OF RELEVANCE IN HYPERTENSION
ROS are produced as metabolic by-products of respiration, but also intentionally by enzymatic reactions, such as that catalyzed by Noxes. Over the last 30 years, their role as signaling molecules in regulating physiological functions has been well established. However, excess production of ROS or failure of antioxidant defenses in specific subcellular compartments or whole tissue results in pathological signaling as well as protein, lipid and DNA damage (24–26,55). Here we concentrate on those ROS that are intimately involved in the pathogenesis of hypertension: O2−, NO, peroxynitrite (OONO−) and H2O2 (56,57). Superoxide has a very short half-life (milliseconds at neutral pH) and can react rapidly with NO to form OONO− or is quickly converted to H2O2. While NO is vasoprotective and is an important vasodilator, OONO− contributes to tissue damage and H2O2 reacts with various cellular proteins to modify signaling pathways, including transcription factors, protein phosphatases, kinases, ion channels and small molecular weight G-proteins.
PRIMARY MECHANISMS OF ROS GENERATION IN HYPERTENSION
One of the most important enzymatic sources of O2− and H2O2 in the cardiovascular system is Nox, a family of transmembrane proteins that transfer electrons across membranes (24–26,58,59) (Figure 1). Noxes catalyze the reduction of O2 to produce O2− in an NADPH-dependent manner [NADPH + 2O2→NADP+ + H+ + 2O2−], which in turn dismutates to generate H2O2 (spontaneously or catalysed by SOD) (58). This cascade of reactions leads to generation of secondary ROS including the reaction of O2− with NO to form ONOO−, iron-catalysed Fenton reaction to produce hydroxyl radical (OH−), and peroxidase-catalysed generation of hypochlorous acid (HOCl). During activation, the electron donor NADPH provides two electrons that are transported across membranes to reduce O2 to O2−, with associated production of two protons that influence intracellular pH (60). Accordingly Noxes may have two functions, with the primary role the relay of electrons to generate ROS and secondly the production and transport of protons across membranes (58–60).
Figure 1. Sources of ROS in the cardiovascular system.
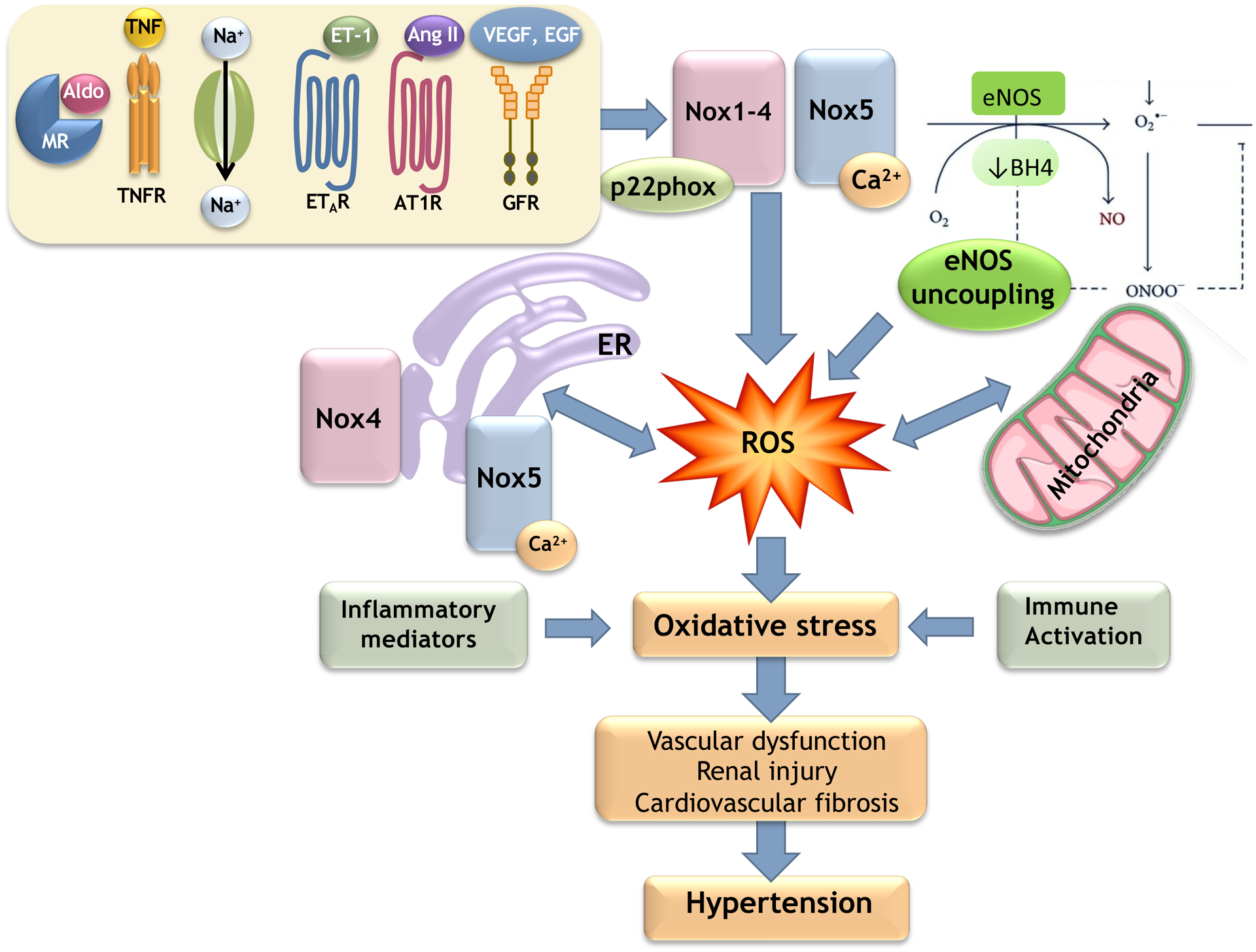
The major soure of ROS in the cardioavscular system is the Nox family. Nox1–4 are p22phox-dependent oxidases, whereas Nox5, a Ca2+-sensitive Nox, does not require p22phox for its activation. In the cardiovascular system, Noxes are regulated by pro-hypertensive and inflammatory factors including Ang II, ET-1, aldosterone, salt, growth factors (VEGF, EGF) and TNF. ROS are also generated by eNOS uncoupling, and mitochondrial and ER mechanisms, which are influenced by Nox/ROS. MR, mineralocorticoid receptor; AT1R, Ang II type 1 receptor; ETAR, ET-1 type A receptor; GFR, growth factor receptor; BH4, tetrahydrobiopterin.
The notion that Noxes are key players in hypertension was first suggested over 40 years ago in clinical studies when it was shown that antihypertensive drugs mediate effects through coenzyme Q10-NADPH oxidase (61) and that f-Met-Leu-Phe-stimulated NADPH-dependent O2− production in neutrophils of patients with essential hypertension is almost four-fold higher compared to that in neutrophils of normotensive individuals (62). Using a reverse translational approach, these clinical observations were tested in experimental models and in the 1990s a pivotal role for NADH/NADPH oxidase-generated ROS was demonstrated in Ang II-dependent hypertension (63,64). Since then it has been unambiguously demonstrated that Nox-derived ROS are involved in blood pressure elevation in all commonly studied models of hypertension (24–26,63–67). On the other hand, it has been more challenging to show a causal role for Nox/ROS in human hypertension (68,69)
The Nox family
The Nox family comprises seven isoforms: Nox1–5, duox1/duox2, with each isoform possessing a catalytic core (electron-transporting element), a transmembrane domain and NADPH oxidase subunits (p22phox, p47phox/Nox-activator 1 (NOXA1), p67phox/Nox-organizer 1 (NOXO1), p40phox). Nox1–4 require p22phox and different subunits for their activation, while Nox5 activation is independent of p22phox and NADPH oxidase subunits (70). All Noxes have six conserved predicted transmembrane alpha-helices with heme binding regions and a flavoprotein homology domain on the intracellular C-terminus containing binding sites for FAD and NADPH (70,71). Noxes are regulated by vasoactive agents, growth factors and physical stimuli as well as signaling molecules and localize in subcellular compartments (71–76). Nox1, Nox2, Nox4 and Nox5, which are expressed in cells of the heart, vessels, kidney and brain, play an important role in cardiovascular and cerebrovascular pathophysiology and have been linked to oxidative stress in hypertension (74–76). Many comprehensive reviews have described the role of Nox1, Nox2 and Nox4 in hypertension (77,78) and accordingly here we provide only a brief overview of these. On the other hand Nox5, the most recently characterized Nox, will be discussed in detail as it is emerging as an important mediator of vascular oxidative stress in human hypertension.
p22phox-dependent Noxes (Nox1–4) in the cardiovascular system
Nox1, the first Nox homologue to be discovered, was originally called Mox1 for ‘mitogenic oxidase’ because of its role in cell proliferation and mitogenesis (79). In addition to p22phox, it requires NOXO1 (p67 isoform), NOXA1 (p47phox isoform) and Rac1 for its full activation. It is an important driver of cardiovascular inflammation and fibrosis and inhibitors of Nox1 and Nox1/4 (GKT136901, GKT137831) prevent cardiovascular remodeling in hypertension (80,81).
Nox2 is typically expressed in neutrophils and macrophages, but also in cells of the heart, vessels, kidneys and brain (82). Activated Nox2 associates with membrane-bound p22phox, cytosolic subunits p47phox, p67phox and p40phox, and the small G proteins Rac1/2 (83). In phagocytic cells, Nox2 produces high levels of O2− in bursts, crucial in host defense responses, while in vascular and non-phagocytic cells O2− is produced at low levels in a sustained and controlled manner (84).
Nox3 is expressed almost exclusively in the inner ear (85), with little convincing evidence that it is functionally present in the cardiovascular system. However a few studies demonstrated that Nox3 is expressed in endothelial and cardiac cells (86,87).
Nox4 exists as a heterodimer linked to p22phox and does not require cytosolic subunits for its activation. Unlike other Noxes, Nox4 produces O2− and H2O2 and is constitutively active (88). Nox4 activity is directly linked to its abundance, cell localization, intracellular compartmentalization and association with proteins including regulatory protein polymerase delta–interacting protein 2 (poldip2) and tyrosine kinase substrate 5 (Tks5) (89,90). Nox4 is abundant in the kidney and tumour cells and has been considered to be an oncoprotein (91). It is also expressed in other cell types, including cardiomyocytes, endothelial and vascular smooth muscle cells (VSMC), osteoclasts, epithelial cells and hemopoietic stem cells. Nox4 associates with focal adhesions (92,93), is important in cell migration and localizes in the nucleus, mitochondria and ER. In the endothelium, Nox4-derived H2O2 has been described as an EDRF involved in vasodilation and in the stressed heart it is cardioprotective (94). Studies in Nox4 knockout and Nox4 over-expressing mice have shown that Nox4 is both cardiovascular protective and injurious (95,96), depending on the experimental model. Suggestions have also been made that Nox4 associates with Nox5, although mechanisms for this are elusive and the pathophysiological significance is unclear (97).
p22phox-independent Noxes in the cardiovascular system – importance of Nox5
Nox5 shares ≈ 50% homology with Nox2 and is unique in that it does not require p22phox or cytosolic NADPH oxidase subunits for its activation (98). Duox1/2 are also p22phox-independent isoforms and are involved in thyroid hormone synthesis (99). Duox1/2 do not appear to be important in cardiovascular pathophysiology although duox-mediated H2O2 generation has been described in zebrafish heart (100).
Nox5 is the most recently characterised Nox and currently is the only Nox to be crystalized (99,101). Nox5 has some distinguishing features: i) the rodent genome lacks Nox5 yet it is present in lower forms and mammals, ii) Nox5 generates O2− from a single gene product, iii) it has a unique N-terminal extension possesing Ca2+-binding helix-loop-helix structure domains, iv) Nox5 activation is regulated by changes in intracellular free Ca2+ concentration ([Ca2+]i) and undergoes conformational changes, and v) Nox5 is regulated by post-translational modifications, including phosphorylation, oxidation, S-nitrosylation and SUMOylation, but unlike other Noxes is not glycosylated and has been described as a ‘bona fide nonglycoprotein’ (102–104).
Six Nox5 isoforms have been identified [Nox5-α, -β, -γ, -δ, -ε, -ζ] (61). Nox5α, Nox5β and Nox5γ are functionally active and generate ROS (99). Nox5α and Nox5β are the primary isoforms in human cells and are negatively regulated by Nox5ε, which inhibits Nox5-mediated O2− generation (105). Noxδ, Nox5ε and Noxζ do not produce appreciable amounts of ROS and their functional significance remains unclear. Epigenetic factors including, eg. histone deacetylase 2 (HDAC2), cause upregulation of the Nox5 gene promotor activity in VSMCs (105).
Activation of Nox5 involves Ca2+-binding to the EF hand on the N-terminal region (102–104). The [Ca2+]i concentration needed for Nox5 activation is high, and thus additional systems involving regulatory proteins have evolved that increase sensitivity to Ca2+ thereby facilitating ROS generation at lower [Ca2+]i. Accordingly, Nox5 can be activated directly by Ca2+ or indirectly by interacting with other proteins and kinases (106–110). Nox5 regulatory proteins include PKC, calmodulin, caveolin-1, c-Abl1, c-Src and chaperone molecules (Hsp90, Hsp70). These proteins differentially influence Nox5 and may stabilise the enzyme. For example PKC and calmodulin increase Nox5 sensitivity to Ca2+ promoting activation, while interaction with caveolin-1 promotes Nox5 inactivation. Pro-inflammatory transcription factors (NF-κB, AP-1, STAT1/STAT3) have also been shown to regulate Nox5 in human aortic smooth muscle cells.
While Nox1–4 are primarily associated with the cell membrane, Nox5 localizes in intracellular compartments mainly the perinuclear area and ER (99,104,109,111). This is functionally relevant because the ER is a Ca2+-rich depot and the site of protein synthesis and post-translational modification. Within cells, Nox5 traffics from intracellular compartments to the cell membrane bringing it into close proximity to regulatory proteins such as PKC and c-Src that influence its activation and downstream signaling (109–113).
Nox5 is ubiquitously expressed in human tissues and is a major ROS-generating enzyme in cells of the cardiovascular system (113,114). Of the vascular isoforms, it is the key Nox responsible for O2− production in human vessels. It is activated by vasoactive agents (Ang II, endothelin-1 (ET-1)), growth factors (PDGF, EGF) and pro-inflammatory mediators (TGFβ, cytokines) (113–117) and inhibits Nox5-induced signaling (118).
Nox5 is involved in many physiological processes including sperm motility, sperm-oocyte fusion, cell proliferation and cytokine production (113,119). In the vascular system, we identified a key role for Nox5 in redox-sensitive vascular contraction through a feedforward system where Ca2+-regulated Nox5 promotes redox-mediated increases in [Ca2+]i that triggers contraction. Increased activation of endothelial cell Nox5 causes cell proliferation and formation of capillary-like structures, important in atherosclerosis and angiogenesis (120). In the heart Nox5 plays a role in the regulation of Ca2+-activated K+ channels involved in coronary artery contraction (121).
Multiple Nox isoforms are involved in hypertension
The role of Nox-derived ROS in the development of hypertension has been demonstrated primarily in transgenic mouse models where Nox isoforms or NADPH oxidase subunits have been deleted or overexpressed (122) (Table). A direct causal role for Noxes in the development of Ang II-induced hypertension has been demonstrated in mice lacking Nox1, Nox2, Nox4, p47phox or p22phox (122–124). Nox1-deficient mice have reduced vascular O2− production and blunted pressor responses, whereas in Nox1 overexpressing mice, Ang II-mediated vascular hypertrophy and blood pressure elevation are exaggerated (123,124). However, in a model of chronic Ang II-dependent hypertension (human renin-expressing mice) development of hypertension was independent of Nox1 and Nox2 (125,126). In Ang II-infused mice treated with siRNA targeted to renal p22phox, renal Nox activity was blunted, ROS formation was reduced, and blood pressure elevation was prevented (127). Recent studies have shown that fibroblast Nox2 contributes to vascular remodeling and hypertension through a paracrine effect (128). It should be highlighted that in these Nox knockout or transgenic studies, baseline cardiovascular phenotypes of mice are unexpectedly normal and it is only when challenged, eg. Ang II or salt, that mice exhibit vascular and blood pressure changes.
Table.
Effects of increased activation/expression of Nox isoforms and mitochondria in the pathophysiology of hypertension
| Source | Consequence |
|---|---|
| Nox1 | ↓endothelial relaxation |
| ↑vascular remodeling | |
| ↑fibrosis | |
| ↑blood pressure | |
| Nox2 | ↑inflammation |
| ↑blood pressure | |
| ↑neuronal excitability | |
| ↑T cell proliferation | |
| Nox4 | ↑endothelial relaxation |
| ↓vascular remodeling | |
| ↓or no △ blood pressure oncoprotein renal dysfunction | |
| Nox5 (SMC) | ↑vasoconstriction |
| ↓endothelial relaxation no change in blood pressure in young mice hypertension with aging stroke accelerated vascular remodeling vascular calcification | |
| Nox5 (EC) | ↓endothelial relaxation |
| ↑systolic hypertension hypertensive retinopathy | |
| Mitochondria | ↓endothelial relaxation |
| ↑blood pressure |
There is a paucity of information on Nox3 in hypertension, likley because this isoform is not typically expressed in the cardiovascular system. However a few studies suggest a role for Nox3 in hypertension (129–132). In two models of experimental hypertension, brain oxidative stress has been linked to Nox3 upregulation. In the cerebrum of stroke-prone SHR (SHRSP), mRNA and protein expression of Nox3 and ROS production were increased compared with Wistar Kyoto (WKY) rats (129). In the phenol renal injury model of hypertension, oxidative stress-induced activation of the sympathetic nervous system is associated with increased Nox3 expression and oxidative stress in the brain (130). In humans, data from large gene expression studies identified Nox3 as a predictor of future hypertension (131,132). In a case-control study in a Chinese population, pulmonary hypertension was associated with rs6557421 variant in Nox3 (131). In West Africans, a genome-wide search for regions linked to renal dysfunction identified Nox3 (6q25.1-q26) as a potential candidate gene implicated in hypertensive and diabetic nephropathy (132).
Nox4 seems to be especially important in salt-sensitive hypertension. It contributes to renal oxidative stress, kidney injury, hypercontractile vascular responses and blood pressure elevation in Dahl-salt-sensitive rats, DOCA-salt rats, aldosterone-salt rats and salt-fed mice with reduced renal mass (133–136). In these models Nox4-derived H2O2 is a major upstream regulator of mammalian target of rapamycin complex 1 (mTORC1), which contributes to salt-induced renal injury and hypertension (133). Underlying mechanisms may involve Nox4-dependent maladaptive upregulation of ENaC-regulated sodium reabsorption in the distal nephron. The importance of Nox4 in salt sensitive hypertension is supported by studies in Nox4-knockout Dahl salt-sensitive rats where renal injury and blood pressure elevation were blunted through processes that normalize renal mitochondrial bioenergetics and decreased mitochondrial ROS production (134).
Treatment of hypertensive mice with agents that inhibit Nox1, Nox2 and Nox4, such as apocynin, diphenylene iodinium, gp91ds-tat, and GKT137831, improved vascular function, normalized blood pressure and improved hypertensive cardiac remodelling, supporting the link between Nox activation, oxidative stress and hypertension (136).
Because rodents lack Nox5 it has been challenging to study Nox5 in traditional mouse or rat models of hypertension. To address this, Nox5 knock-in mice have been generated. Mice expressing human Nox5 in a podocyte-specific manner exhibited podocyte dysfunction, albuminuria and hypertension, processes that were exacerbated when mice were made diabetic with streptozotocin (137). Nox5-induced renal inflammation involves induction of cytokine expression and upregulation of toll-like receptors (TLRs), which causes a feed-forward system where TLR activation enhances Nox5-induced generation of ROS and consequent oxidative stress and renal injury (138). Expression of human Nox5 in mice in a VSMC-specific manner causes renal oxidative stress, glomerulosclerosis, mesangial expansion, renal inflammation and fibrosis, processes that accelerate progression of renal disease in diabetes (139). Vascular Nox5 has also been associated with hypertensive diabetic retinopathy (140). In oxygen-induced retinopathy in mice expressing human Nox5 in endothelial cells, retinal vascular permeability and neovascularization, and expression of angiogenic and inflammatory factors, were increased compared with wild-type littermates (140). The relationship between vascular Nox5 and blood pressure seems to be an age-dependent phenomenon, because blood pressure was not elevated in 16–20 week old mice expressing human Nox5 in VSMCs, while it was significanly elevated in 30–35 week old mice (141, unpublished data). This age-related phenomenon has also been described in mice expressing human Nox5 in an endothelial cell-specific manner (142). Aged, but not young, endothelial Nox5 knock-in mice developed systolic hypertension with endothelial dysfunction due to uncoupled NOS uncoupling (142). Increased blood pressure in endothelial Nox5 mice is associated with target organ damage and stroke (142) and vascular Nox5 expression is increased in CADASIL, a small vessel disease associated with premature vascular dementia (143).
Cardiac hypertrophy, a consequence of hypertension and a cause of heart failure, was also associated with Nox5 upregulation (144). In cardiac tissue from patients undergoing heart transplant for cardiomyopathy and heart failure Nox5 expression was increased (144). In cardiac-specific Nox5 transgenic mice, left ventricular hypertrophy, interstitial fibrosis, and contractile dysfunction induced by pressure overload or Ang II, were exaggerated compared with wildtype counterparts (144). These effects were associated with oxidative stress and activation of redox-dependent MAPK (mitogen-activated protein kinase) and procontractile Ca2+ signaling pathways. Nox5 has also been shown to be important in VSMC phenotypic switching relevant to vascular calcification and aneurysm formation (145,146). With the emerging evidence that Nox5 may be an attractive therapeutic target in human cardiovascular disease, interest in developing selective Nox5 inhibitors is growing and a number of promising agents have been identified including melittin, gedunin and ML090 (147–149).
SECONDARY SOURCES AND MECHANISMS OF ROS GENERATION IN HYPERTENSION
It is well established that Noxes are the primary source for ROS in the cardiovascular system and a key driver for oxidative stress in hypertension. However, growing evidence indicates that secondary sources are also important, including NOS uncoupling, ER stress and mitochondrial dysfunction (Figure 1), processes that are influenced by Nox-derived ROS and that themselves contribute to Nox regulation and redox signaling in a compartmentalized fashion.
Nitric oxide synthase uncoupling
NO, the key EDRF, mediates vascular effects through soluble guanyl cyclase (sGC)-induced activation of cyclic guanosine monophosphate (cGMP) and phosphorylation of protein kinase A (150). NO also signals through cGMP-independent pathways by altering protein structure and function through S-nitrosylation (151). Production of NO is regulated by activation of NOS, of which there are three isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) (150). In the cardiovascular system, eNOS and nNOS are constitutively active in a Ca2+/calmodulin-dependent manner, whereas iNOS is activated by stress independently of Ca2+. In physiological conditions, in the presence of various cofactors including NADPH and tetrahydrobiopterin (BH4), NOS catalyzes L-arginine to produce L-citrulline plus NO (150). However in oxidative stress conditions, NOS removes an electron from NADPH donating it to O2 to generate O2− rather than NO (30). This process, termed NOS uncoupling, involves S-glutathionylation and phosphorylation of eNOS and BH4 oxidation (150,151).
An important trigger for NOS uncoupling is BH4 depletion through ROS-mediated processes. Superoxide directly oxidizes BH4 to BH2, destabilizing NOS leading to its uncoupling (150). All NOS isoforms are regulated by BH4 and hence all can undergo ‘uncoupling’ in stressed conditions. A key factor determining eNOS-derived NO versus O2− involves tryptophan 447 within the BH4-binding domain of eNOS (152). When this is mutated, BH4:eNOS interaction is impaired, leading to preferential O2− generation. Mechanistic studies in transgenic mice with endothelial-targeted overexpression of GTP cyclohydrolase (rate-limiting enzyme in BH4 synthesis) crossed with mice overexpressing eNOS, demonstrated that uncoupling is an independent and direct effect of a stoichiometric discordance between the enzyme and BH4 (152). Altering BH4 levels not only perturbs NOS-derived NO production, but it influences posttranslational protein modification by S-nitrosation, especially impacting proteins of the ubiquitin-proteasome system (151,152). Hence BH4 and eNOS act as a signaling node influencing many downstream pathways.
The overriding factor promoting NOS uncoupling is oxidative stress leading to decreased NO production, increased O2− generation and formation of unstable and reactive ONOO, which induces BH4 oxidation and nitration of prostacyclin synthase. This feedforward system where Nox-mediated oxidative stress begets oxidative stress through NOS uncoupling has been demonstrated in SHR, SHRSP, Ang II-induced hypertension and DOCA-salt rats and in patients with hypertension (153,154). However, not all hypertension models involve eNOS uncoupling, because sepiapterin, a BH4 precursor, had no blood pressure lowering effect in glucocorticoid-mediated hypertension (155).
Reversing eNOS uncoupling is vasoprotective
Normalizing NOS activity by increasing BH4 levels and eNOS transcription enhancing agents (4-fluoro-N-indan-2-yl-benzamide (AVE9488) and 2,2-difluoro-benzo[1,3]dioxole-5-carboxylic acid indan-2-ylamide (AVE3085)) has been suggested as a potential therapeutic strategy in cardiovascular disease (156). BH4 treatment normalized endothelial function in rodent hypertension and diabetes and improved endothelial function in patients with hypertension and atherosclerosis (156). High dose oral BH4 improved endothelial NO bioavailability and had a significant antihypertensive effect in patients with resistant hypertension (156). BH4 may have protective effects beyond preventing NOS uncoupling. In experimental hypertrophic cardiac disease, BH4 suppressed inflammatory responses and myocardial macrophage infiltration without impacting NOS activity (157). In patients with chronic kidney disease, BH4 ameliorated the exaggerated blood pressure response to exercise by decreasing sympathetic activity (156). Not all studies have demonstrated protective effects of BH4. In patients with established coronary artery disease and endothelial dysfunction, oral BH4 had no effect on vascular redox state or endothelial function and failed to decrease blood pressure in some patients with hypertension (158).
Nox:NOS interplay
While eNOS uncoupling may be an important source of O2− in cardiovascular disease, this is likely a secondary mechanism because the process is driven a priori by oxidative stress, which in hypertension is due mainly to Nox activation. The interplay between these systems may involve cross-talk between Noxes and NOS. For example p47phox and NOXO1-dependent activation of Nox1, but not other Noxes, mediates uncoupling of vascular eNOS in diabetes, effects that are ameliorated by Nox1 downregulation (159). A similar relationship between Nox1 and uncoupled eNOS was demonstrated in mice overexpressing Nox1 in VSMCs, where impaired endothelium-dependent relaxation was attributed to oxidative stress and eNOS uncoupling (160). In aortic aneursyms, Nox4 upregulation is linked to NOS uncoupling (161). Further adding to the complexity, there is evidence that mitochondria play a role in Nox-regulated eNOS uncoupling highlighting tight associations between ROS-generating systems, NOS and redox regulatory pathways in cardiovascular disease (162).
Endoplasmic reticulum, ROS generation and oxidative stress
The ER is increasingly being recognized as an important player in redox pathophysiology in the cardiovascular system (163–165). This is supported by: i) ER enzymes such as ER oxidoreductin (Ero1) and its thiol redox partner protein disulfide isomerase (PDI) are involved in ROS generation, ii) the ER is the site of p22phox and Nox synthesis, maturation and post-translational modification (glycosylation, phosphorylation, oxidation), iii) Nox interacts with the ER chaperone PDI, promoting Nox activity, iv) some Noxes, especially Nox4, are active in the ER, v) the ER communicates with mitochondria through mitochondria-associated ER membranes enabling Ca2+ and ROS exchange between compartments, especially important for Nox5, which is redox-and Ca2+-sensitive, and vi) the ER is critically involved in redox protein folding and stress responses (163–167). Moreover the ER provides a functional structure comprising molecular machinery for compartmentalized ROS production and redox signaling.
Protein folding in the ER is a tightly regulated redox-sensitive process. In stressed conditions the influx of unfolded proteins into the ER exceeds its capacity to fold them (ER stress) resulting in activation of ER stress signaling pathways, called the unfolded protein response (UPR) (168). The UPR comprises three major axes including, inositol-requiring protein 1 (IRE1), activating transcription factor-6 (ATF6) and PRKR-Like ER Kinase (PERK), which upon sensing ER stress, signal to downstream molecules. Activation of the UPR causes protein accumulation and misfolding leading to apoptosis, phenotypic switching, de-differentiation and trans-differentiation, processes that underlie cardiovascular remodeling and injury in hypertension (169,170). Noxes, ROS and oxidative stress are integrally associated with ER stress/UPR (171,172). Oxidative stress can directly trigger UPR, through oxidation of ER stress signaling molecules. In addition, ROS are generated during the UPR primarily through Nox4 activation and possibly Nox2. This likely involves ER chaperones, eg. PDI, which connects Noxes to ER-mediated functions.
Interplay between ER stress and ROS in hypertension
Interactions between ER stress, UPR and oxidative stress are implicated in the pathophysiology of hypertension and associated target organ damage (Figure 2). In Ang II-induced hypertension, the UPR proteins, binding immunoglobulin protein (BiP) and C/EBP homologous protein (CHOP), are upregulated in the aorta, mesenteric arteries and heart, as well as in the circumventricular subfornical organ of the brain (173–175). Treatment with ER stress inhibitors 4-PBA and TUDCA reduced blood pressure, decreased cardiovascular UPR activity and restored endothelial function in Ang II-induced hypertension and in SHR (176). In DOCA-salt hypertension, ER stress inhibition ameliorated hypertension-induced cardiac dysfunction by improving ER stress-associated calcium mishandling, apoptosis, inflammation and fibrosis (177). Pro-hypertensive effects of ER stress involve inactivation of redox-regulated SERCA2 and impaired sodium homeostasis, as demonstrated in transgenic mice expressing irreversibly oxidized SERCA (SERCA2 C674S) (178). ER stress has been suggested as a common factor in the synergistic effects of hypertension and diabetes in cardiovascular injury and target organ damage (179). In human hypertension, markers of ER stress and gene expression of ATF6, IRE1 and PERK are increased (180).
Figure 2. Interplay between oxidative stress and ER stress in hypertension.
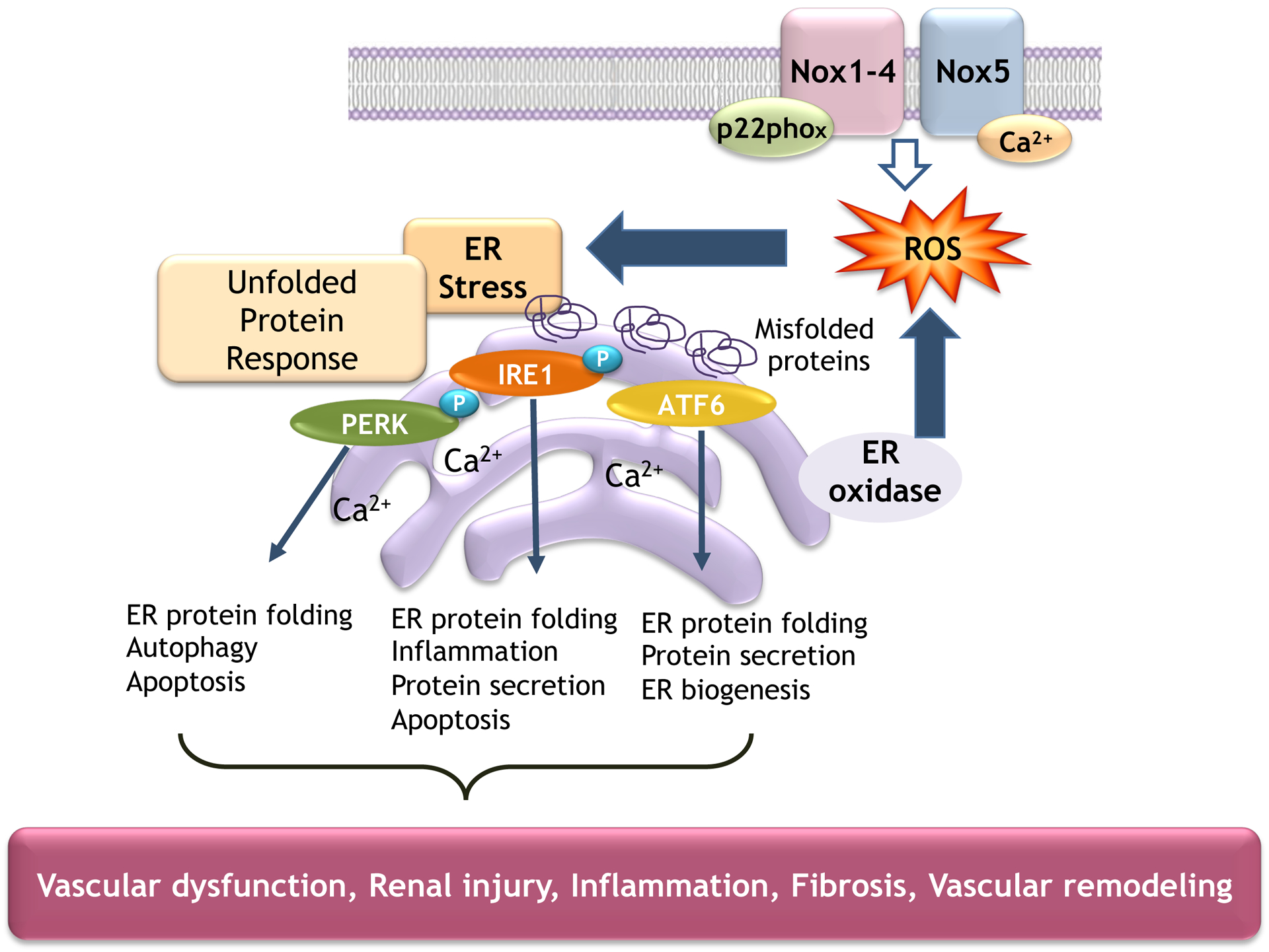
Increased Nox-induced ROS generation promotes activation of ER stress signaling pathways and the unfolded protein response that influence vascular function in hypertension.
Mitochondrial dysfunction and oxidative stress
Mitochondria are multifunctional organelles involved primarily in ATP synthesis via the TCA cycle and the electron transport chain (ETC), which comprises five multienzyme complexes (I, II, III, IV, V). The transfer of electrons across the ETC is coupled with the transport of protons, establishing the electrochemical gradient that generates ATP, which regulates K+ channels, that coordinate organelle performance and cellular energy requirements (181). Normally this is an efficient process with minimal electron leak. However in pathological conditions mitochondrial function is impaired with increased electron leak leading to O2− production (182,183). Within the mitochondria, SOD dismutates O2− to H2O2, which crosses the mitochondrial membrane to the cytosol by diffusion or via mitochondrial pores and voltage-dependent anion channels (182,183).
Mitochondrial oxidative stress and hypertension
The contribution of mitochondrial O2−/H2O2 to cellular ROS status is minor in physiological conditions but in pathological conditions, including hypertension, mitochondrial impairment contributes to cellular oxidative stress, a process itself that promotes mitochondrial dysfunction (184,185). In hypertension, cytosolic Nox-derived ROS traverses the mitochondrial membrane promoting electron leak and mitochondrial oxidative stress (185,186), processes that also involve NO because L-NAME prevents Ang II-stimulated mitochondrial O2− formation. These findings indicate important cross-talk between Noxes and mitochondrial oxidases through ROS/NO and suggest that the mitochondria may function as a central amplification system further contributing to mitochondrial and global oxidative stress in hypertension (187). Of the Noxes Nox2 is especially important in this (188).
Mitochondrial-targeted antioxidants
Antioxidants targeted to the mitochondria ameliorate mitochondrial dysfunction and reduce blood pressure in experimental hypertension. Inhibitors of cyclophilin D, which opens the mPTP, prevent development of hypertension in Ang II-infused mice (189). MitoQ, the mitochondrial-targeted antioxidant, attenuates hypertension-associated target organ damage and reduces blood pressure in SHRSP, salt-sensitive hypertension, pre-eclampsia and pulmonary hypertension (47,48). Clinical studies demonstrated that 6-week treatment with MitoQ improved endothelial function (brachial artery flow-mediated dilation) and reduced plasma biomarkers of oxidative stress in elderly individuals (48).
Mitochondrial dysfunction, SIRT3 and hypertension
Another mitochondrial system that is gaining attention is SIRT3, which is a key mitochondrial deacytelase that activates cyclophilin D and the mitochondrial anti-oxidant SOD2 (42). The antioxidative function of SIRT3 is attributed mainly to its deacytelation and activation of SOD2. In hypertension, vascular SIRT3 levels are reduced and SOD2 is hyperacetylated, leading to mitochondrial O2− accumulation and redox-sensitive vascular dysfunction (40–44). In SIRT3 knockout mice, Ang II- and DOCA-salt-induced hypertension, vascular oxidative stress, endothelial dysfunction and blood pressure elevation were exaggerated, responses that were normalized in hypertensive mice overexpressing SIRT3. The relationship between SIRT3 and blood pressure seems to be especially important in females, since SIRT3 knockout only in females was associated with cardiac dysfunction and hypertension, suggesting a sex-specific role for endothelial SIRT3 (190). In cell-based systems, Ang II-induced epithelial-mesenchymal transition was associated with decreased SIRT3 expression and oxidative- and mitochondrial stress (191), effects attenuated by SIRT3 overexpression (191). Decreased expression and activity of other SIRT isoforms, SIRT1 and SIRT6, which localize in the nucleus, have also been implicated in cardiovascular disease and hypertension (192,193).
Together these studies suggest that upregulating SIRTs may be an attractive strategy to prevent tissue fibrosis, hypertension and target organ damage. A machine-learning strategy supports this notion. Agents that activate SIRTs such as polyphenols (eg, resveratrol, quercetin) and modulators of acetylation (eg, curcumin, honokiol, oroxilyn A, quercetin, epigallocatechin-3-gallate, bakuchiol, tyrosol, and berberine) have been shown to reduce blood pressure and cardiovascular injury in experimental hypertension (194). However, not all studies have shown a protective effect of SIRT3 in hypertension. Sunitinib-induced hypertension and cardiotoxicity in mice were associated with increased SIRT3 levels in cardiovascular tissue (195). These cardiovascular toxicities were attenuated in SIRT3-knockout.
ROLE OF ANTIOXIDANT ENZYMES IN PERTURBED REDOX BALANCE IN HYPERTENSION
Redox homeostasis in cardiovascular pathophysiology is tightly regulated by antioxidant enzymes including SOD [cytoplasmic Cu/Zn SOD (SOD1), mitochondrial SOD (SOD2), extracellular SOD (SOD3)], glutathione peroxidase (GPX), catalase, and thioredoxin-peroxiredoxin (TRX-Prdx), and non-enzymatic antioxidants (α-tocopherol, β-carotene, bilirubin and uric acid) (196,197). SOD dismutates O2− into H2O2 while catalase catalyzes H2O2 into O2 and H2O. The peroxidases catalyze oxidation/reduction processes and promote reduction of oxidized proteins. A major transcription factor controlling cellular antioxidants is Nuclear factor erythroid 2-related factor 2 (Nrf2), the master regulator of antioxidant genes and hence of antioxidant status.
Altered antioxidant systems, including decreased expression and activity of antioxidant enzymes and reduced plasma levels of vitamins A/C/E, have been consistently demonstrated in experimental hypertension (198–200). Administration of exogenous SOD supports the notion that ROS play a pathophysiological role in hypertension. Membrane permeable SOD and SOD mimetics improve hypertension, endothelial dysfunction, cardiovascular remodeling, renal injury and oxidative stress in experimental models of hypertension (200–202). In transgenic mice deficient in antioxidant enzymes, eg. SOD1 and GSH synthase, blood pressure is elevated (203). Epigenetic silencing of SOD2 has been demonstrated in pulmonary hypertension (204). On the other hand, overexpression of antioxidants, catalase, SOD1 and SOD3, prevented blood pressure elevation and protected against kidney damage in Ang II-dependent hypertension and models of pulmonary hypertension (205). SOD knockout or overexpression do not influence blood pressure in normotensive animals, indicating that beneficial antioxidant effects occur in the context of oxidative stress and hypertension.
A common factor that may be responsible for dysfunctional antioxidant enzymes in hypertension is Nrf-2 (206,207). In two kidney-one clip (2K1C) hypertensive rats, nuclear accumulation of Nrf-2 and expression of Nrf2-regulated genes are reduced (206). Treatment with nitrites decreased oxidative stress, improved vascular function and reduced blood pressure by upregulating Nrf-2 and increasing antioxidant enzymatic activity. It has also been suggested that activation of the protective axis of the renin angiotensin system mediates effects by upregulating Nrf2, since ACE2 overexpression in mice was associated with increased Nrf-2-sensitive antioxidant genes (207).
Clinical studies have also demonstrated abnormal antioxidant status in patients with hypertension. Plasma levels of vitamin C are inversely related to blood pressure and antioxidant capacity in circulating cells is decreased in hypertension (208). In the Alpha-Tocopherol, Beta Carotene Cancer Prevention study, a 30-year prospective cohort analysis, higher baseline serum alpha tocopherol levels were associated with reduced cardiovascular disease risk and overall mortality (209). Moreover, an evaluation of dietary carotenoids and hypertension in the National Health and Nutrition Examination Survey 2007–2014 showed that higher intake of alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene and lutein was associated with lower risk of hypertension (210). Antioxidant vasoprotective effects seem to be amplified with aging as evidenced by the negative correlation between SOD and age in patients with hypertension (211).
Antioxidants in hypertension
Studies in experimental models of hypertension and pre-eclampsia consistently demonstrated amelioration of oxidative stress and blood pressure-lowering by antioxidant vitamins, SOD mimetics and resveratrol (212,213) Antioxidants also improved endothelial function, normalized vascular remodeling and reduced arterial stiffness, in part by decreasing Ox-PTM of proteins and restoring normal redox signaling in these models (212,213). A meta-analysis on the SOD-mimetic tempol clearly showed beneficial effects in experimental hypertension (214). However, these favourable responses have not translated to the clinic. Except for a few small studies, large antioxidant trials, such as the GISSI and HOPE trials, have been mostly negative (215,216). Reasons for this are multifactorial. To address some of these concerns, it has been suggested that compartment-specific agents including MitoQ or antioxidants that directly influence protein oxidation may have beneficial effects in patients (217).
Indirect antioxidants and Nrf2 agonists – new approaches to reduce oxidative stress
Most antioxidant therapies investigated clinically have direct effects by donating one or two electrons. A new concept suggests that agents may have indirect actions where agents augment endogenous antioxidant responses without being antioxidants themselves. Indirect antioxidants include the polyphenol resveratrol and Nrf-2 agonists (218,219). Resveratrol increases expression of antioxidant enzyes SOD, catalse and GPX, enhances Nrf-2-heme oxygenase-1 pathway, restores activity of eNOS and reduces expression of ROS-generating Nox4 (218,219). Hypertensive patients treated acutely with resveratrol showed improved vascular function, but without blood pressure-lowering effect (220). When added to standard anti-hypertensive therapy, resveratrol further reduced blood pressure in patients with hypertension (221). Based on these promising findings, randomized, crossover, double-blinded, placebo-controlled trials are underway to assess efficacy of resveratrol in controlling hypertension (222). Nrf2 activators, such as bardoxolone methyl and sulforaphane, are also indirect antioxidants and induce effects by enhancing expression of antioxidant and cytoprotective genes. Clinical efficacy of bardoxolone methyl and sulforaphane have been tested in many conditions including diabetes, kidney disease, cancer and pulmonary hypertension, with some favorable outcomes (222,223). Whether this is also evident in essential hypertension has yet to be demonstrated.
THE MOLECULAR BASIS OF OXIDATIVE STRESS IN HYPERTENSION
Molecular mechanisms whereby ROS contribute to hypertension are rooted in their effects on protein structure and function and associated redox signaling. ROS activate and inactivate transcription factors, membrane channels and metabolic enzymes, stabilize cytoskeletal proteins, and regulate Ca2+-sensitive and phosphorylation signaling pathways (224–226). Proteins are highly sensitive to the action of O2− and H2O2 and constitute ~70% of ROS targets. Specific ROS effects are mediated by covalent modification of redox-sensitive amino acids including histidine, tyrosine and cysteine. Oxidation and reduction of Cys-containing thiol proteins are amongst the key processes whereby ROS integrate into intracellular signaling pathways influencing cell function. Under normal conditions proteins are targets of reversible oxidative modifications, involved in signal transduction and cell homeostasis. In pathological conditions, as a result of oxidative stress, proteins undergo irreversible oxidation, which triggers protein degradation pathways and cell death (227). Dysregulated Ox-PTM of proteins is emerging as an important molecular process in cardiovascular disease (228).
ROS are highly reactive. Production of O2− in the vicinity of NO, or as a result of eNOS uncoupling, results in inactivation of NO and impairs endothelium-dependent relaxation (30). Peroxinitrite reacts with tyrosine to form 3-nitrotyrosine. Whether, and how, 3-nitrotyrosine affects protein function remains unclear. H2O2, on the other hand, reacts with deprotonated Cys groups to form sulfenic (R-SOH), sulfinic (R-SO2H) or sulfonic (R-SO3H) acids, as well as disulfides (RSSR) (229). While formation of sulfinic and sulfonic acids are reversible, sulfonic modifications are not (226,227). Sulphenic acids can also react with glutathione (GSH), resulting in S-glutathiolation. Both disulfides and s-glutathiolated residues can be reduced back to the thiol by thioredoxin/thioreductase or GSH/glutathione reductase. Alternatively, reactive Cys can interact with NO to generate S-nitrosothiols. By virtue of the fact that these reactions are fast, reversible and localized, all of these modifications can be classified as redox-sensitive intracellular signals that influence cell function (Figure 3).
Figure 3. Post translational oxidative modification of proteins.
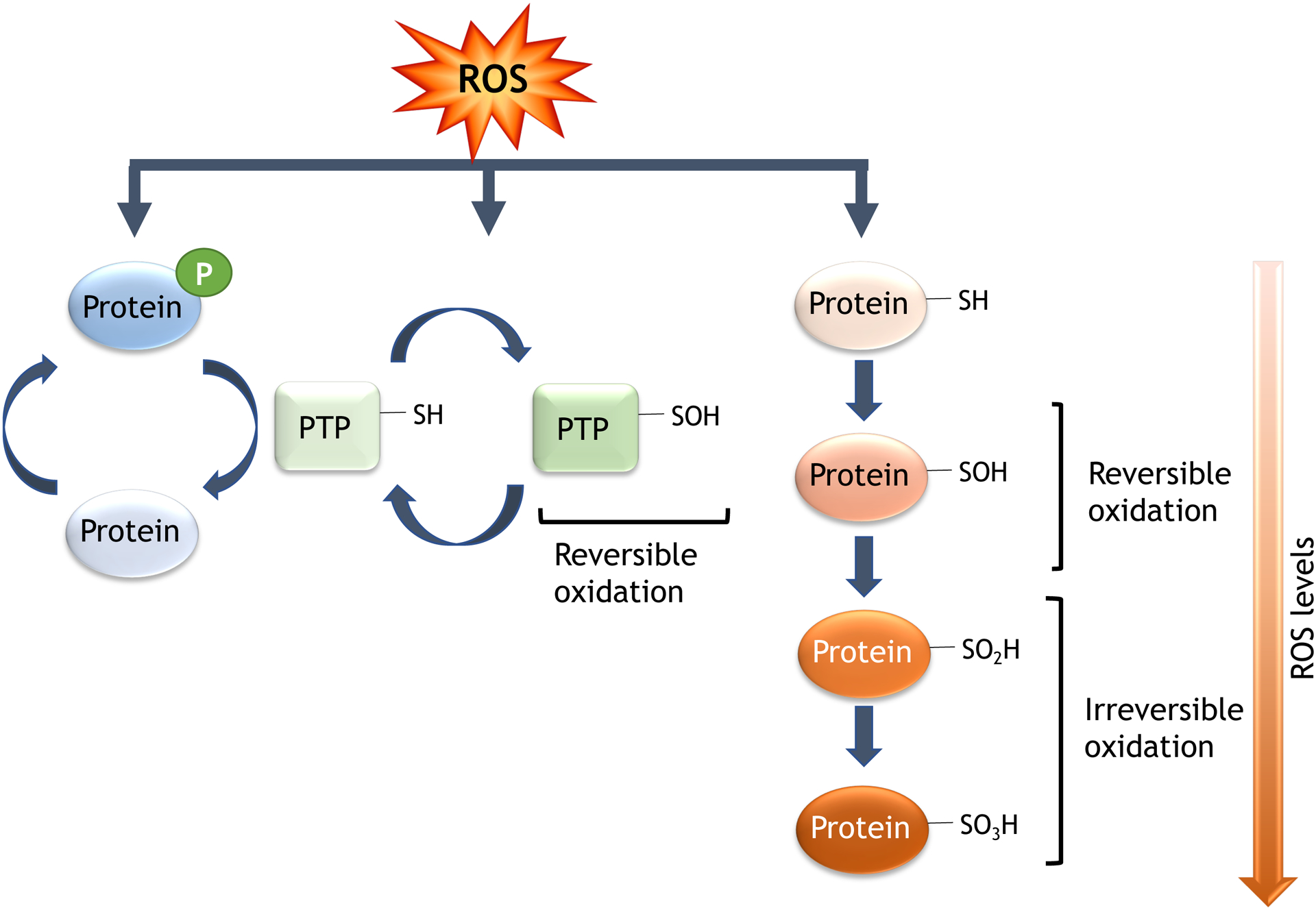
Main reactions leading to reversible and irreversible oxidation of proteins in cardiovascular cells.
Redox signaling in hypertension
Oxidative modifications affect the structure and function of proteins, with multiple molecular and cellular consequences; for example, oxidation of protein tyrosine phosphatases reduces activity and oxidation of protein kinases such as Src or ASK-1 activates them (228–230) (Figure 4). Relevant to control of vessel tone, oxidation of protein kinase G activates it independently of cyclic GMP leading to vasorelaxation (229). Moreover, oxidant-based activation of MAP kinases, including ERK, JNK and p38MAPK, contributes to vascular and cardiac inflammation, proliferation, hypertrophy and migration (230) all of which are involved in cardiovascular remodeling and hypertension-induced end organ damage. Along with redox-sensitive activation of Src and Rho kinase, the MAP kinases can regulate contraction (231). Similarly, other tyrosine kinases, including PI3K/Akt and receptor tyrosine kinases (VEGFR, EGFR, PDGFR) can be modulated by ROS (232,233).
Figure 4. Schematic of redox-sensitive signaling pathways involved in hypertension.
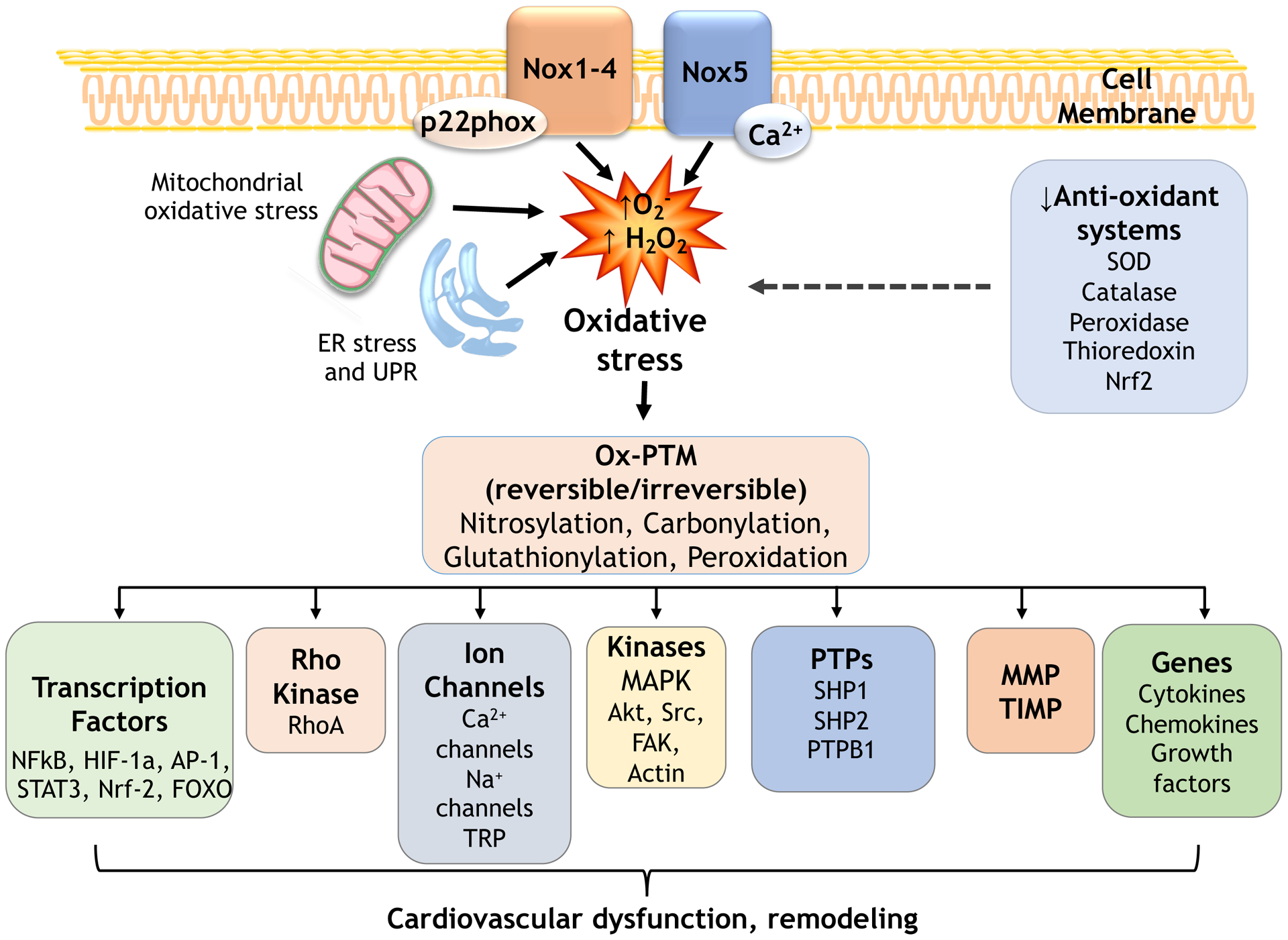
ROS influence many signaling molecules that regulate cardiovascular function including kinases, phosphatases, Ca2+ channels, transcription factors, genes. These processes involve oxidative post-translational modifications (Ox-PTM) of downstream redox-sensitive targets. Oxidative stress causes abnormal redox signaling leading to cardiovascular dysfunction and remodeling in hypertension. Decreased antioxidants contribute to oxidative stress. PTP, protein tyrosine phosphatases; MMP, matrix metalloproteinases; UPR, unfolded protein response.
Other proteins particularly susceptible to regulation by oxidative stress are cytoskeletal proteins, including actin (234). Actin oxidation not only influences its polymerization rate, but also its interaction with actin-binding proteins such as vinculin (235). Focal adhesion regulation by FAK as well as turnover are modulated by Nox4-derived H2O2 (234,236). Similarly, studies have shown that high ROS concentrations have adverse effectS on cardiac myofilament function (237). Perhaps more importantly, ROS activate transcription factors, most notably NFκB and HIF-1, which in turn regulate expression of proinflammatory chemokines and cytokines, leading to activation and recruitment of macrophages and immune cells, leading to cardiovascular inflammation and fibrosis, hallmarks of hypertension-induced end-organ damage (238). Other redox-sensitive transcription factors include STAT3 and AP-1, as well as Nrf-2/ Kelch-like ECH-associated protein 1 (KEAP1) (239). Nrf-2 is normally bound to and targeted for degradation by Keap1/Cullen-3, but when Cys residues on Keap1 are oxidatively modified, Nrf-2 is stabilized and translocates to the nucleus, inducing expression of antioxidant genes. Many of these pathways are protective, such that activation of Nrf-2 reduces oxidative stress and lowers blood pressure in SHR.
An important class of redox-sensitive signaling proteins in the cardiovascular system are Ca2+-binding proteins and channels (240), but the functional consequences are complex. Transient receptor potential (TRP) channels, store-operated Ca2+ channels, inositol trisphosphate receptors (IP3Rs) and voltage-gated Ca2+ channels are subject to regulation by ROS and many are directly regulated by cysteine oxidation (242). TRP channels, and in particular TRPM2 and TRPM7, are activated by ROS (241,242). In contrast, oxidation of ORAI1 channels by H2O2 inhibits activity, and STIM2, which senses endoplasmic reticulum calcium levels and gates ORAI channels, can also be oxidized, resulting in further inhibition of activity (243). H2O2 also downregulates IP3Rs, leading to reduced Ca2+ efflux in VSMCs (244). The overall effect of ROS on Ca2+ signaling thus depends on the interplay of multiple cascading targets.
Redox signaling and compartmentalization
Another important consideration for ROS-mediated signaling is subcellular compartmentalization. Because antioxidants are prevalent throughout the cell, O2− and H2O2 act upon signaling molecules in their immediate vicinity. For Nox1, that means lipid rafts and internalized vesicles (245) while for Nox4, signaling occurs mainly in the ER and potentially in the focal adhesions (246). Nox2 is found in neutrophil phagosomes where it is released extracellularly, but in other cell types, including endothelial cells, it is found in lamellipodial focal complexes, membrane ruffles and endosomes (247). Mitochondrial oxidants act upon ferrochelatase in the inner mitochondrial membrane to reduce heme and indirectly inhibit soluble guanylate cyclase (248). In addition, ROS-induced ROS release can amplify and spread signal generation, as is the case for Nox2-induced mitochondrial ROS production in mice treated with Ang II (249). Many redox-sensitive signaling proteins localize in cholesterol-rich microdomains, especially caveolae and lipid rafts, which facilitates ROS production with redox-sensitive targets in close proximity (250).
Redox signaling and the inflammasome in hypertension
The inflammasome, important in immune and inflammatory cells as well as VSMCs and endothelial cells, is increasingly being recognized to play a role in vascular inflammation in hypertension (251,252). Several inflammasomes have been described, but the nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome is best characterized (251). The NLRP3 inflammasome contains ASC (apoptosis-associated speck-like protein) adapter protein and procaspase-1. In the presence of immune activators, cell stresses and oxidative stress, the conformation of NLRP3 is altered giving rise to the NLRP3 inflammasome, which triggers cleavage of procaspase-1, generating caspase-1, which induces pyroptosis, (caspase-1-specific form of cell death leading to inflammation), and IL-1β and IL18 production and consequent induction of pro-inflammatory signaling pathways (251, 252). ROS have an important role in activation of the inflammasome by activating NFκB or by directly activating the inflammasome (253) (Figure 5). ROS act as second messengers and activate inflammasomes through Nox and redox-sensitive pathways involving ERK1/2, PI3K, thioredoxin and apolipoprotein C3 (ApoC3) (254). TRP cation channels that function as polymodal sensors and scaffolding proteins have also been implicated in inflammasome activation (255). Of significance, TRPM2, which we described as a point of cross-talk between redox and Ca2+ signaling in VSMCs (256), is an important link between ROS and the inflammasome (257). Because of its association with injurious signals as well as its dependency on ROS, activation of inflammasome is increasingly being considered as an important mechanism leading to chronic inflammatory processes in cardiovascular disease, including hypertension.
Figure 5. Oxidative stress and the inflammasome in hypertension.
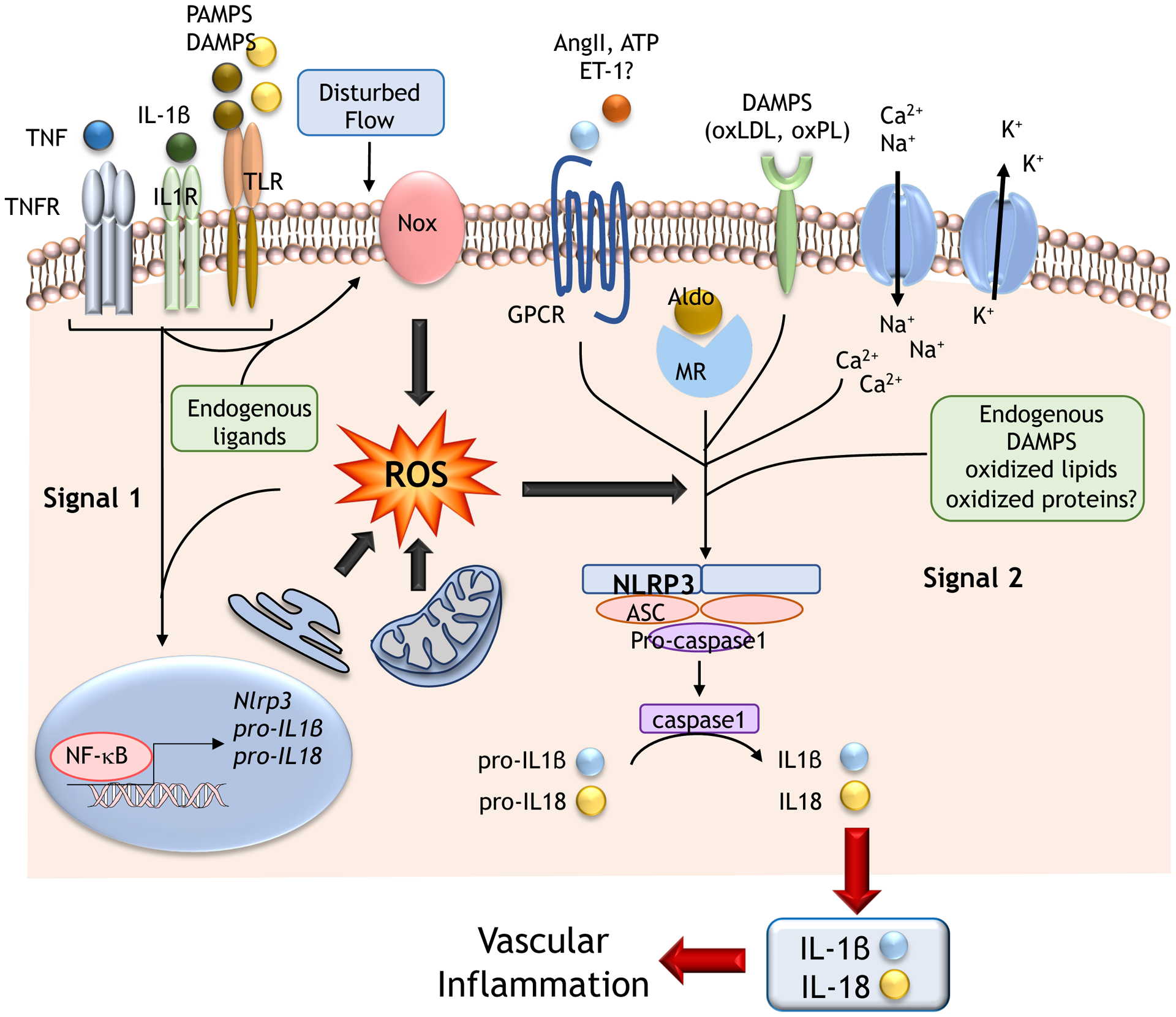
Prohypertensive factors induce activation of NLRP3 inflammasome through ROS. ROS influences signal 1 (cytokines, pathogen-associated molecular patterns (PAMPS), danger-associated molecular patterns (DAMPS)), leading to activation of NFkB and gene expression of components of the inflammasome. ROS also influence signal 2, by PAMPS, DAMPS, oxidized LDL (oxLDL), oxidized phospholipids (oxPL), ATP, Ang II, ET-1, aldosterone (aldo) and cations (K+, Ca2+, Na+). These processes lead to assembly of the inflammasome complex (NLRP3, ASC and pro-caspase 1), and consequent activation of caspase 1, cleavage of pro-IL-1β and pro-IL-18, and production of active forms of IL-1β and IL-18, which increase the inflammatory response, fibrosis and vascular remodelling in hypertension. MR, mineralocorticoid receptor; ASC - Apoptosis-Associated Speck-Like Protein Containing CARD; question mark (?) indicates possible effect.
Activation of NLRP3 inflammasome has been shown in Ang II-mediated and DOCA/salt hypertension, and in preeclampsia, pulmonary hypertension, and hypertensive nephropathy (252,258,259). NLRP3 inflammasome activation contributes to Ang II-induced VSMC phenotypic transformation, cell proliferation, vascular remodeling and hypertension (260). In Ang II-infused NLRP3−/− mice, development of hypertension, vascular remodeling and VSMC phenotypic switching (contractile to proliferative) were blunted compared with wildtype mice (261). The NLRP3 inflammasome is also involved in Ang II-induced podocyte injury, mitochondrial dysfunction, renal dysfunction and cardiac hypertrophy (262). In Ang II-infused mice, hypertension-associated cardiac remodeling, which involves the serum- and glucocorticoid-inducible kinase (SGK1), was ameliorated by EMD638683, a highly selective SGK1 inhibitor, by suppressing NLRP3 expression, caspase-1 activation and IL-1β secretion (263). Benefical effects of EMD638683 on cardiac fibrosis were abolished by supplementation with exogenous IL-1β and the NLRP3 inflammasome inhibitor MCC950, indicating that EMD638683 reduces Ang II-induced cardiac inflammation and fibrosis by inhibiting the NLRP3 inflammasome/IL-1β secretion axis. This inflammasome process may be important in aldosterone-induced redox-mediated cardiac fibrosis and hypertension because aldosterone signals through SGK1 (264).
The thiol proteome in hypertension
Advances in LC-MS and biotin-switch methodologies have enabled proteome-wide assessments of Ox-PTMs formation in cardiovascular tissue (22,265). We demonstrated increased protein sulfenylation and hyperoxidation of protein tyrosine phosphatases and peroxiredoxins in vessels from experimental models of hypertension and showed that protein hyperoxidation is associated with oxidative and ER stress through upregulation of plasmalemmal-Nox1 and ER-Nox4 (21–23). Using a redox proteomics approach, protein targets of carbonylation in genetically obese hypertensive rats have been described. These rats have increased carbonylation of proteins associated with lipid metabolism, redox regulation and protein chaperone activity and decreased carbonylation of catalase, processes that promote metabolic syndrome and increased cardiovascular risk (266). Increased carbonylation has also been demonstrated in salt-sensitive hypertension in Dahl-salt-sensitive rats (267). In SHR kidney, total free thiol content was significantly lower than that of control rats, indicating increased oxidation of sulfhydryls (268). This was attributed, in part, to reduced thioredoxin reductase activity. Similar profiles have been described in human hypertension, where plasma levels of protein carbonyls and advanced protein oxidation products were increased with associated decreased levels of total thiols (269). Plasma protein carbonyl may be a biomarker of oxidative stress, while also playing a role in the pathogenesis of hypertension (270).
One of the most abundantly oxidized vascular proteins of relevance in hypertension is actin, critically involved in cytsoskeletal organization and cell contraction (234,235). Exposure of VSMC to H2O2 causes cytsoskeletal rearrangement, F-actin fragmentation, impaired polymerization and phenotypic changes, processes causing vascular dysfunction and arterial remodeling in hypertension (271). In conditions associated with severe oxidative stress, such as end stage heart failure and hypertension-associated target organ damage, carbonylation (irreversible form of Ox-PTM) of actin was associated with loss of cell viability and contractile dysfunction (271).
S-nitrosothiols in the cardiovascular system regulate multiple processes that are mainly protective, including NOS activity and vasodilation, cardiomyocyte Ca2+ homeostasis and myocardial contraction, ATP production and mitochondrial bioenergetics and inhibition of platelet aggregation and hemostasis. In hypertension S-nitrosylation of various proteins has been implicated in vascular dysfunction (272). For example, S-nitrosylation of eNOS (Cys101) promotes eNOS uncoupling and ROS production, while S-Nitrosylation of cyclooxygenase-2 (COX-2), Ca2+ channels and angiotensinogen (Cys18, Cys138) influence vascular tone (273). In heart failure, Cys294 S-nitrosylation and S-glutathionylation of ATP synthase are negatively correlated with ATP hydrolysis, indicating that Ox-PTMs cause inactivation of enzymatic activity and reduced ATP production and myocardial injury (274).
The number of identified proteins that undergo Ox-PTM in cardiovascular disease and hypertension continues to grow (21–23). In addition to those above, other proteins of interest include ryanodine receptor 2 (RyR2), Keap/Nrf-2, bone morphogenic proteins (BMP), NFATc4/ERK/AKT, Trx1, histone deacytelase 4 (HDAC4) and TRPM2 (275–277). We demonstrated in human VSMCs, that under basal conditions 92% of proteins were similarly oxidized in cells from normotensive and hypertensive patients, while ~ 3% were hyperoxidized and ~ 5% showed reduced oxidation in the hypertensive group (278). Some of the potentially interesting candidate vascular proteins that were highly oxidized included β-actin, annexin A1, galectin-1, FK506 binding protein polymerase I and transcript release factor (PTRF, −1.92) and vimentin (278). Of significance, many of these proteins play an important role in cytoskeletal organization and contraction.
While there have been enormous technological developments in proteomics, the field of redox proteomics in human physiology and disease mechanisms is still immature. However, this is an emerging field in cardiovascular research and new discoveries defining the oxidative proteome in hypertension will help delineate which proteins undergo Ox-PTM, how these underpin redox mechanisms of disease and whether they are useful biomarkers to predict and track development of hypertension.
OXIDATIVE STRESS AND ORGAN SYSTEMS IN HYPERTENSION
Oxidative stress is a unifying mechanism of tissue injury in multiple systems and organs that play a role in the pathophysiology of hypertension (Figure 6). Moreover, hypertension-associated target organ damage is a consequence of oxidative stress.
Figure 6. Systems biology, oxidative stress and hypertension.
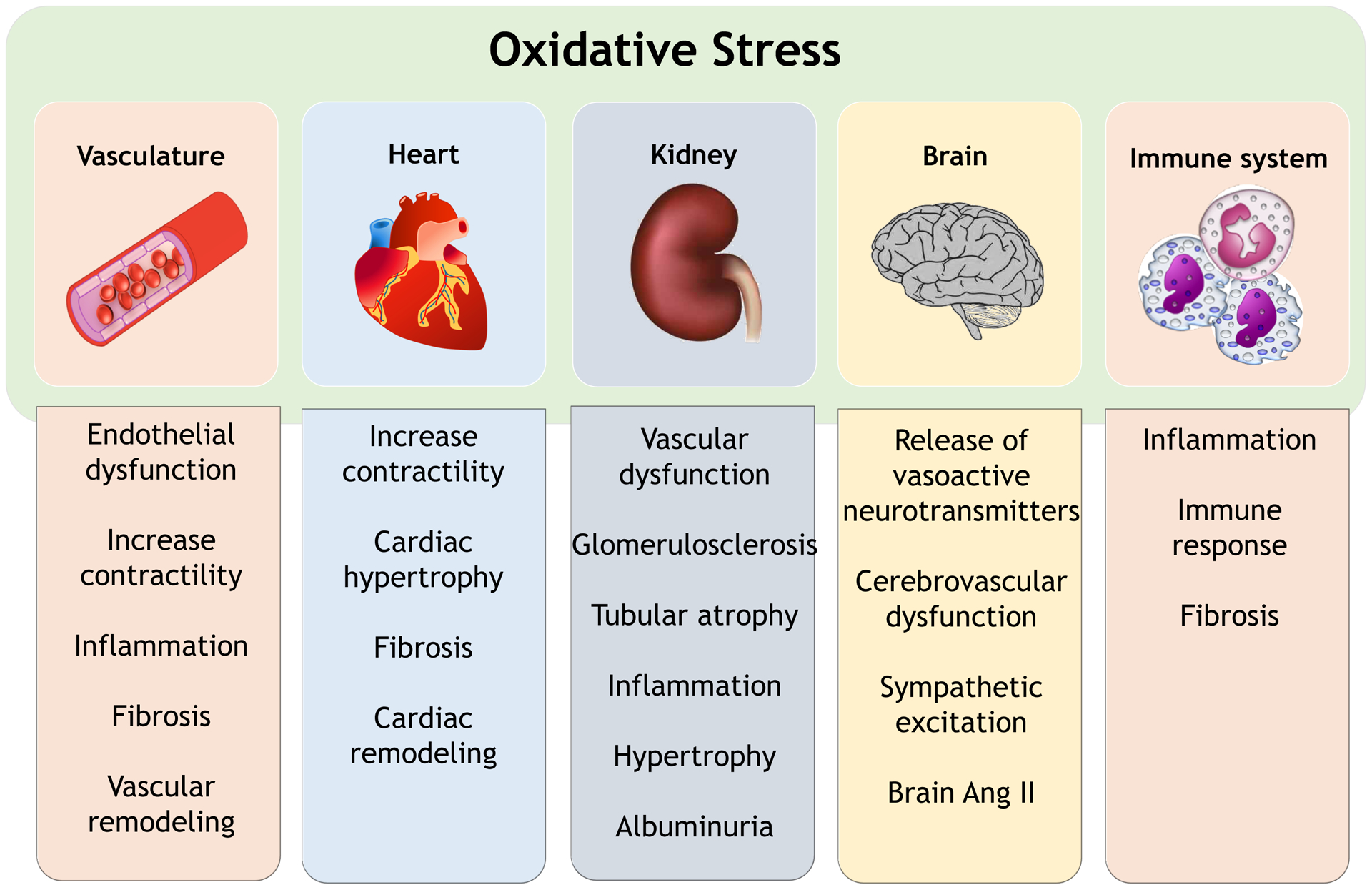
Functional effects of oxidative stress in regulatory systems and organs in the pathophysiology of hypertension.
The vascular system
Vasculopathy, consisting of impaired endothelium-dependent relaxation, increased arterial stiffness, enhanced contractility, inflammation, vascular calcification and remodeling is both a cause and consequence of hypertension (279,280). These processes that occur with aging, are accelerated in hypertension (280). Oxidative stress and redox-sensitive signaling contribute to these dysfunctions, as has been demonstrated both in vitro and in vivo (279–281). Many pro-hypertensive factors instigate and amplify ROS production in endothelial cells, VSMCs, adventitia, and perivascular adipose tissue, leading to vascular injury.
Ang II was the first vasoactive agent shown to induce the production of ROS in vascular cells in vitro and in aortas of hypertensive mice (282). Vessels from Ang II-infused mice and resistance arteries from hypertensive patients show increased vascular expression of Nox1 and Nox2 and increased ROS production, which in turn activate complex signaling pathways including MAP kinases, Src, Rho kinase and Ca2+ channels (282,283). These processes cause vascular hyperreactivity, endothelial dysfunction, vascular remodeling, influx of inflammatory cells into the vessel wall, increased wall stiffness and fibrosis. In hypertensive animals, genetic deletion of Noxes, pharmacological Nox inhibitors, ROS scavengers or antioxidants inhibit vascular remodeling, reduce inflammation and normalise endothelial function with associated blunting in blood pressure elevation (284).
Other ROS-dependent contributors to hypertension include aldosterone, ET-1, growth factors, immune factors and salt (285,286). Aldosterone, through mineralocorticoid receptors, signals via genomic and non-genomic pathways to increase ROS in VSMC and endothelial cells by activating Nox1 and Nox4 respectively. Inhibition of mineralcorticoid receptors attenuates Ang II-induced ROS production in the vasculature, while AT1 receptor signaling is required for aldosterone-induced oxidative stress, suggesting interplay between these two pro-hypertensive systems (285). Mice overexpressing endothelial ET-1 are hypertensive and exhibit oxidative stress, with associated increase in Nox1 and Nox2 expression (287). Ang II and ET-1, which signal through G-protein coupled receptors, transactivate growth factor receptors such as PDGF, EGF and IGF-1, which amplifies redox-dependent vascular processes including medial hypertrophy, vascular remodeling, inflammation and fibrosis (288).
Another tissue source of ROS, besides the endothelium and vascular media, that may influence vascular function in hypertension is perivascular adipose tissue. Adipose tissue possesses functionally active Nox4, which generates ROS, important in adipocyte function and adipokine production (289). Perivascular adipose tissue influences vascular tone through adipocyte-derived vasoactive factors and ROS, processes that are amplified in hypertension, obesity and diabetes mellitus.
The renal system
The kidneys play a critical role in the pathophysiology of hypertension through multiple processes that involve ROS (290,291). Increased generation of ROS in the kidney can trigger blood pressure elevation and accelerates development of hypertension in experimental models. The major source of renal ROS is Nox4, although other Noxes also play a role. Increased O2− causes vascular and tubular dusfunction, while H2O2 seems to be important in regulating pressure natriuresis by decreasing preglomerular vascular reactivity and increasing medullary blood flow (291). In vivo studies of single nephron function and in vitro studies with the double-perfused juxtaglomerular apparatus preparation have shown extensive interaction between O2− and NO in macula densa to regulate afferent arteriolar tone mediated by the tubuloglomerular feedback response (291). Oxidative stress in the kidney also promotes renin release, activates renal afferent nerves, causes dysfunction of glomerular cells and disrupts Na+ and water homeostasis, processes that contribute to the development of hypertension (290,291). Moreover production of O2− in specific nephron segments increases reactivity to Ang II. Recent evidence suggests that renal dopamine receptors (D1-D5R) regulate the redox state by decreasing ROS generation and that dopamine receptor dysfunction promotes oxidative stress and hypertension (292). The impact of renal oxidative stress on hypertension occurs early in life because oxidative stress plays a pathogenic role in developmental programming of kidney disease (293). Dampening oxidative stress in the kidney using ROS scavengers, antioxidants and Nox inhibitors lead to improved renal function and ameliorates blood pressure elevation in experimental hypertension (294). The involvement of renal oxidative stress in hypertension is complex and has been extensively reviewed (290–293).
The central nervous system
Oxidative stress in the brain plays an important role in the development of hypertension. Nox-induced ROS production in the nucleus tractus solitarii (NTS) and rostral ventrolateral medulla (RVLM) causes sympathoexcitation in hypertensive rats, through mechanisms that involve NO and pro-inflammatory processes (296). Redox signaling in the subfornical organ (SFO), an important forebrain circumventricular organ, is critical for sympathetic activation, driving the elevation in blood pressure in Ang II-infused mice (297). This was demonstrated in mice in which p22phox was deleted in the SFO, where Ang II failed to elicit a hypertensive response. Mechanisms through which the SFO mediates ROS-related blood pressure effects in the brain involve activation of the PVN causing increased plasma vasopressin, upregulation of ET-1 in cerebral small arteries and activation of ETA and AT1 receptors (297,298).
The brain has an active renin angiotensin system involved in the development of hypertension through oxidative mechanisms. Acute administration of Ang II to the paraventricular nucleus (PVN) caused an increase in blood pressure (299) and intracerebroventricular infusion of Ang II leads to an increase in Nox subunit expression and O2− production in the in the RVLM (300). Moreover, chronic Ang II infusion leads to elevated ROS in the subfornical organ, and administration of SOD selectively to this organ inhibits the associated increase in blood pressure (301). Additional work has shown that, similar to the vasculature, Nox-derived ROS induce dysfunction of mitochondrial electron transport, which in turn contributes to neurogenic hypertension.
Less is known about the redox targets in the brain that regulate the hypertensive response. In general, Ang II signaling in neurons increases Ca2+, activates Noxes, stimulates mitochondrial ROS production, and regulates voltage-gated K+ channels (302,303). In particular, O2− mediates Ang II-induced influx of extracellular Ca2+ and inhibition of the outward K+ current leading to increased neuronal excitability (303). Calcium/calmodulin-dependent protein kinase II (CaMKII) regulates these processes. Adenoviral-mediated overexpression of CaMKII in the subfornical organ enhanced Ang II-induced inhibition of the voltage-gated K+ current and increased the pressor response to intracerebroventricular injection of AngII. In addition, evidence shows that targeting c-Src in the PVN inhibits NFκB, pro-inflammatory cytokine production, and oxidative stress induced during salt-sensitive hypertension (304). Ang II administration to the RVLM of rats made hypertensive by exposure to repeated foot shocks and noise resulted in an increase in SAPK/JNK activity, which could be blocked with apocynin and contributed to elevation of blood pressure (305). Oxidative stress caused by intracerebroventricular infusion of Ang II can be attenuated by overexpression of ACE2, which in turn upregulates Nrf-2 (306).
The immune system
Inflammation has long been associated with hypertension, ranging from early studies reporting that immunosuppression lowers blood pressure following renal infarction to current work focused on the role of the innate and adaptive immune system in hypertension and searching for initiating signals. In 2007, Harrison’s group showed that mice lacking both T and B cells due to deletion of the recombination-activating gene 1 (RAG) had a reduced hypertensive response to angiotensin II or deoxycorticosterone acetate (DOCA) plus salt. Adoptive transfer of T cells restored the blood pressure response (307). Similar findings were made in RAG1-deficient Dahl salt-sensitive rats and in SCID mice, which are deficient in lymphocytes (308,309). Patients with hypertension tend to have an increased number of circulating “immunosenescent” proinflammatory CD8+ T cells, which generate excessive IFN-γ and TNF-α compared to CD8+ T cells from non-hypertensive individuals (310). CD4+ T cells also have a role in hypertension, particularly with regard to increased production of IL-17, which can raise blood pressure and impair endothelium-dependent dilatation (311). In addition, depletion of B cells using a CD20 antibody or using mice deficient in B-cell-activating factor receptor (which prevents B cell maturation) partially inhibits the blood pressure increase caused by angiotensin II infusion (312). Monocyte and macrophage depletion can also normalize the hypertensive response to angiotensin II (313).
Most relevant to the role of oxidative stress in hypertension are the findings that dendritic cells derived from monocytes of hypertensive mice generate excess ROS, which leads to formation of isolevuglandin adducts that act as neoantigens for T cells (314). Importantly, sodium uptake via an amiloride-sensitive channel in dendritic cells stimulates ROS by activating Nox2, resulting in activation of T cell proliferation and production of IL-17, both of which exacerbate hypertension (315). In addition, mice with CD4-targeted Nox2 deficiency had a reduced blood pressure increase following angiotensin II infusion that was accompanied by an increase in tissue-resident regulatory Tregs and a reduction in infiltrating effector T cells (316). In support of this finding, it has been shown that T-cell Nox2 is critical to the development of hypertension in Dahl salt-sensitive rats (317). It should also be noted that as T-cells become activated in hypertension, they secrete cytokines that act upon surrounding tissue and can increase ROS production by resident cells (317). In the case of the vasculature, cytokine-stimulated ROS production leads to inactivation of NO and impaired endothelium-dependent relaxation.
CLINICAL PERSPECTIVES
Despite the overwhelming pre-clinical findings that oxidative stress is causally linked to the development of hypertension, this has been more difficult to prove in humans. Nonetheless there is growing evidence that Nox activity, ROS generation and redox signaling are amplified in cardiovascular and renal tissue and VSMCs from resistance arteries from patients with hypertension, recapitulating some findings in experimental models (278,280,284,318,319). To further support a role for Nox/ROS in vascular regulation in humans, studies in patients with genetic Nox deficiency (chronic granulomatous disease) demonstrated higher flow-mediated dilation, lower intima-media thickness, reduced urinary isoprostanes and serum Nox2 activity, increased NO bioavailability, and higher serum nitrite/nitrate compared with controls, suggesting reduced vascular damage (320).
Genetic factors have been implicated in oxidative stress-related hypertension in humans. Normotensive individuals with a positive family history of hypertension have greater ROS production than blood pressure-matched subjects without a family history of hypertension (321,322). Polymorphisms in Nox subunits (CYBA (p22phox)) and Nox isoforms (Nox3) are associated with hypertension in different populations (131,132,323,324). More recently, findings from a large GWAS of almost half a million people identified Nox4 and Nox5 as new blood pressure-associated genes (325).
Beyond genetics, epigenetic factors influence development of hypertension, especially during critical windows in the early stages of development. Oxidative stress is an important epigenetic modulator since ROS regulate histone modifications (methylation, acetylation), DNA modifications expression of non-coding RNAs and ATP-dependent chromatin modelling (326). Numerous vascular targets for epigenetic programming have been identified in essential hypertension such as redox-sesnitive genes encoding for vasorelaxation/vasoconstriction, vasoactive peptides, inflammatory mediators and VSMC phenotypes. In addition redox-regulated DNA modification of genes involved in endocrine hypertension have been described including hydroxysteroid (11-beta) dehydrogenase 2 (HSD11B2), somatic angiotensin-converting enzyme (sACE), Na+/K+/2Cl− cotransporter 1 (NKCC1), angiotensinogen (AGT), and α-adducin (ADD1) gene (326,327). Also a number of miRNAs implicated in hypertension pathophysiology have been linked to oxidative stress, such as miR-200 family members that play a role in redox-dependent endothelial dysfunction, and miR-210 a key player in mitochondrial metabolism (326,328). Furthermore histone acetylation plays an important role in endothelial gene expression of eNOS through processes involving redox-regulated HDAC1 (326). Histone methylation and acetylation are reversible processes, accordingly interventions that reduce oxidative stress may be an attractive strategy to normalize epigentic changes involved in early programming of essential hypertension.
Most studies examining ROS in humans are based on associations between circulating or urine biomarkers of oxidative stress and blood pressure. Hypertensive patients exhibit higher levels of plasma H2O2, increased plasma and urine markers of oxidative stress such as thiobarbituric acid-reactive substances (TBARS), oxidized low-density lipoprotein (oxLDL), and 8 iso-prostane compared with normotensive individuals (329,330). Biomarkers of oxidative stress have also been demonstrated in patients with secondary hypertension (hyperaldosteronism) and preeclampsia (331,332). Because of the potential predictive value of biomarkers, there is growing interest in identifying more specific and direct indices of oxidative stress including oxidized proteins in serum, plasma, and urine. Patient studies have shown that plasma levels of reactive carbonyl species correlate with blood pressure and predict future hypertension, while serum levels of nitrosylated proteins are increased in patients with hypertension (333,334).
Markers of decreased antioxidant defense systems have also been demonstrated in human hypertension. Patients with essential hypertension exhibit reduced activity and decreased plasma levels of antioxidant enzymes, including SOD, glutathione peroxidase, and catalase (335–337). Ferric reducing antioxidant power (FRAP), an index of antioxidant status, and plasma levels of antioxidant vitamins A/C/E are lower in newly diagnosed untreated hypertensive patients compared with normotensive controls (336,337). In patients with white coat hypertension, serum protein carbonyl (indicating protein oxidation) was increased and endogenous antioxidant proteins (protein thiol, SOD, glutathione) were decreased compared with normotensive individuals, further supporting a relationship between low antioxidant capacity, increased oxidative stress, and hypertension (338). Population studies have demonstrated an inverse association between plasma vitamin C levels and blood pressure and a meta-analysis reported that vitamin C supplementation reduced systolic and diastolic blood pressure (339).
Considering the potential importance of oxidative stress in the pathophysiology of hypertension and the convincing experimental data, it is reasonable to imagine that reducing ROS bioavailability through antioxidants, ROS scavengers, and Nox inhibitors would have protective and blood pressure-lowering effects. Interest in developing therapies to target Nox/ROS is growing as highlighted in recent reviews (136,147–149). However, it should be emphasised that currently there are no clinically approved Nox/ROS inhibitors or antioxidants for use in the treatment of hypertension. Although further clinical research and drug development in the field are still needed, clinical utility of agents that reduce oxidative stress to manage hypertension and target organ damage is promising.
CONCLUSIONS
Evidence from animal studies and mechanistic studies in cultured cells have established a strong causative link between ROS, redox signaling, and hypertension. An important pathophysiological role for Noxes, uncoupled eNOS, mitochondrial ROS and ER stress in experiemnatl hypertension is clear. Common to these elements is oxidative stress. While antioxidant trials in humans have been largely negative, much remains to be learned about the timing, targeting, delivery and efficacy of novel agents that target ROS. Drugs that prevent production of ROS in specific subcellular compartments or drugs that activate endogenous antioxidant defenses show promise. However, to fully evaluate their potential as anti-hypertensives better biomarkers are needed to ensure that the expected targets are in fact inhibited and to assess the ability of these new therapies to interfere with hypertensive signaling while maintaining redox homeostasis. Redox proteomics offers a promising new approach to not only identify biomarkers to test the efficacy of antioxidant therapies, but also to provide mechanistic insights into the actual molecular targets of elevated oxidative stress. Additional delineation of the intersection between inflammasomes, ER stress, ROS, and mitochondrial function can be derived from systems biology and experimental medicine approaches in clinical samples or tissues from hypertensive animal models. These new technologies offer hope to finally resolve the paradox between the clearly established role of ROS in hypertension in animals and the current equivocal evidence in human hypertension.
ACKNOWLEDGEMENTS
KKG is supported by a grant from the NIH (HL152167). RMT is supported by grants from the British Heart Foundation (RE/18/6/34217, CH/4/29762). ACM is supported by a University of Glasgow Walton Fellowship.
ABBREVIATIONS
- Ang II
Angiotensin II
- ATF6
Activating transcription factor 6
- BiP
Binding-Immunoglobulin Protein (Endoplasmic Reticulum Chaperone)
- cGMP
Cyclic guanosine-3′,5′-monophosphate
- CHOP
CCAAT-enhancer-binding protein homologous protein
- EDRF
Endothelium-derived relaxing factor
- ER
Endoplasmic reticulum
- ET-1
Endothelin 1
- H2O2
Hydrogen Peroxide
- HDAC
Histone Deacetylase
- IRE-1
Inositol-requiring enzyme 1
- MAPK
mitogen-activated protein kinase
- mTORC1
mammalian target of rapamycin complex 1
- NO
Nitric Oxide
- NOS
Nitric oxide synthase
- NOX
NADPH oxidase
- O2
Anion superoxide
- Ox-PTM
Oxidative post-translational modification
- 4-PBA
4-phenylbutyric acid
- PERK
Protein kinase RNA-like endoplasmic reticulum kinase
- PKA
Protein kinase A
- PKG
Protein kinase G
- ROS
Reactive oxygen species
- SERCA2
Sarcoplasmic/endoplasmic reticulum calcium ATPase 2
- sGC
Soluble guanylyl cyclase
- SHR
Spontaneously Hypertensive Rats
- SHRSP
Spontaneously Hypertensive Stroke Prone Rats
- SIRT
Sirtuin
- SOD
Superoxide dismutase
- TUDCA
Taurine-conjugated ursodeoxycholic acid
- UPR
Unfolded protein response
Footnotes
CONFLICTS TO DECLARE
There are no conflicts to declare.
REFERENCES
- 1.Touyz RM, Rios FJ, Alves-Lopes R, Neves KB, Camargo LL, Montezano AC. Oxidative Stress: A Unifying Paradigm in Hypertension. Can J Cardiol. 2020;36(5):659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghezzi P, Jaquet V, Marcucci F, Schmidt HHHW. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br J Pharmacol. 2017;174(12):1784–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sies H Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McPhail LC, Shirley PS, Clayton CC, Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985;75(5):1735–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith TM, Edwards DH, Lewis MJ, Newby AC, Henderson AH. The nature of endothelium-derived vascular relaxant factor. Nature. 1984;308(5960):645–7. [DOI] [PubMed] [Google Scholar]
- 6.Gabig TG. The NADPH-dependent O-.2-generating oxidase from human neutrophils J Biol Chem. 1983;258(10):6352–6356. [PubMed] [Google Scholar]
- 7.Welch WJ. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension. 2008;52(1):51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107(7):1053–8. [DOI] [PubMed] [Google Scholar]
- 9.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sies H Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–383. [DOI] [PubMed] [Google Scholar]
- 12.Lushchak VI, Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–175. [DOI] [PubMed] [Google Scholar]
- 13.Case AJ, Tian J, Zimmerman MC. Increased mitochondrial superoxide in the brain, but not periphery, sensitizes mice to angiotensin II-mediated hypertension. Redox Biol. 2017;11:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes RA, Neves KB, Tostes RC, Montezano AC, Touyz RM. Downregulation of Nuclear Factor Erythroid 2-Related Factor and Associated Antioxidant Genes Contributes to Redox-Sensitive Vascular Dysfunction in Hypertension. Hypertension. 2015;66(6):1240–50. [DOI] [PubMed] [Google Scholar]
- 15.Silva GC, Silva JF, Diniz TF, Lemos VS, Cortes SF. Endothelial dysfunction in DOCA-salt-hypertensive mice: role of neuronal nitric oxide synthase-derived hydrogen peroxide. Clin Sci (Lond). 2016;130(11):895–90. [DOI] [PubMed] [Google Scholar]
- 16.Guzik TJ, Touyz RM. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension. 2017;70(4):660–667. [DOI] [PubMed] [Google Scholar]
- 17.Krylatov AV, Maslov LN, Voronkov NS, Boshchenko AA, Popov SV, Gomez L, Wang H, Jaggi AS, Downey JM. Reactive Oxygen Species as Intracellular Signaling Molecules in the Cardiovascular System. Curr Cardiol Rev. 2018;14(4):290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtenberg D, Pinchuk I, Lichtenberg D. Oxidative stress, the term and the concept. Biochem Biophys Res Commun. 2015;461(3):441–444. [DOI] [PubMed] [Google Scholar]
- 19.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res. 2013;112(2):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomin T, Schittmayer M, Honeder S, Heininger C, Birner-Gruenberger R. Irreversible oxidative post-translational modifications in heart disease. Expert Rev Proteomics. 2019;16(8):681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba SP, Bhatnagar A. Role of thiols in oxidative stress. Curr Opin Toxicol. 2018;7:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Victorino VJ, Mencalha AL, Panis C. Post-translational modifications disclose a dual role for redox stress in cardiovascular pathophysiology. Life Sci. 2015;129:42–47. [DOI] [PubMed] [Google Scholar]
- 23.Mannaa A, Hanisch FG. Redox Proteomes in Human Physiology and Disease Mechanisms. J Proteome Res. 2020;19(1):1–17. [DOI] [PubMed] [Google Scholar]
- 24.Sedeek M, Hébert RL, Kennedy CR, Burns KD, Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens. 2009;18(2):122–127. [DOI] [PubMed] [Google Scholar]
- 25.Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A. eNOS uncoupling in cardiovascular diseases--the role of oxidative stress and inflammation. Curr Pharm Des. 2014;20(22):3579–3594 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Murugesan P, Huang K, Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol. 2020;17(3):170–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dromparis P, Paulin R, Stenson TH, Haromy A, Sutendra G, Michelakis ED. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation. 2013;127(1):115–125 [DOI] [PubMed] [Google Scholar]
- 28.Dikalov SI, Dikalova AE. Crosstalk Between Mitochondrial Hyperacetylation and Oxidative Stress in Vascular Dysfunction and Hypertension. Antioxid Redox Signal. 2019;31(10):710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daiber A, Xia N, Steven S, Oelze M, Hanf A, Kröller-Schön S, Münzel T, Li H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int J Mol Sci. 2019;20(1):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21(8):421–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanahan CM, Furmanik M. Endoplasmic Reticulum Stress in Arterial Smooth Muscle Cells: A Novel Regulator of Vascular Disease. Curr Cardiol Rev. 2017;13(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochoa CD, Wu RF, Terada LS. ROS signaling and ER stress in cardiovascular disease. Mol Aspects Med. 2018;63:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Ding Y, Ramprasath T, Zou MH. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid Redox Signal. 2021;34(9):750–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlisle RE, Werner KE, Yum V, Lu C, Tat V, Memon M, No Y, Ask K, Dickhout JG. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J Hypertens. 2016. August;34(8):1556–1569. [DOI] [PubMed] [Google Scholar]
- 36.Naiel S, Carlisle RE, Lu C, Tat V, Dickhout JG. Endoplasmic reticulum stress inhibition blunts the development of essential hypertension in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2019;316(5):H1214–H1223. [DOI] [PubMed] [Google Scholar]
- 37.Spitler KM, Matsumoto T, Webb RC. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2013;305(3):H344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bal NB, Han S, Kiremitci S, Sadi G, Uludag O, Demirel-Yilmaz E, Bal NB, et al. Hypertension-induced cardiac impairment is reversed by the inhibition of endoplasmic reticulum stress. J Pharm Pharmacol. 2019;71(12):1809–1821. [DOI] [PubMed] [Google Scholar]
- 39.Pan T, Zhang L, Miao K, Wang Y. A crucial role of endoplasmic reticulum stress in cellular responses during pulmonary arterial hypertension. Am J Transl Res. 2020. May 15;12(5):1481–1490. [PMC free article] [PubMed] [Google Scholar]
- 40.Wenceslau CF, McCarthy CG, Webb RC. To Be, or Nox to Be, Endoplasmic Reticulum Stress in Hypertension. Hypertension. 2018;72(1):59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young CN. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122(11):3960–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Flynn CR, Uzhachenko R, Fessel JP, Gamboa JL, Harrison DG, Dikalov SI. Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ Res 2017;121: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su H, Zeng H, Liu B, Chen JX. Sirtuin 3 is essential for hypertension‐induced cardiac fibrosis via mediating pericyte transition. J Cell Mol Med. 2020;24(14):8057–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, Billings FT 4th, Verdin E, Auwerx J, Harrison DG, Dikalov SI. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ Res. 2020;126(4):439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller FJ Jr. Hypertension and Mitochondrial Oxidative Stress Revisited: Sirtuin 3, the Improved “Antioxidant”. Circ Res. 2020;126(4):453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison DG, Dikalov S. Isolevuglandins Contribute to Vascular Oxidative Stress and Mitochondria-Targeted Scavenger of Isolevuglandins Reduces Mitochondrial Dysfunction and Hypertension., Hypertension. 2020;76(6):1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, Murphy MP, Dominiczak AF, Graham D, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54(2):322–328. [DOI] [PubMed] [Google Scholar]
- 48.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR, Rossman MJ, et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 2018;71(6):1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinheiro LC, Oliveira-Paula GH. Sources and Effects of Oxidative Stress in Hypertension. Curr Hypertens Rev. 2020;16(3):166–180 [DOI] [PubMed] [Google Scholar]
- 50.Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34(5):575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J, Asico LD, Beitelshees AL, Feranil JB, Wang X, Jones JE, Armando I, Cuevas SG, Schwartz GL, Gums JG, Chapman AB, Turner ST, Boerwinkle E, Cooper-DeHoff RM, Johnson JA, Felder RA, Weinman EJ, Zeng C, Jose PA, Villar VAM. Sorting nexin 1 loss results in increased oxidative stress and hypertension FASEB J. 2020;34(6):7941–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pawluk H, Pawluk R, Robaczewska J, Kędziora-Kornatowska K, Kędziora J, Pawluk H, et al. Biomarkers of antioxidant status and lipid peroxidation in elderly patients with hypertension. Redox Rep. 2017;22(6):542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang HY, Lee TH, Yang HY, Antioxidant enzymes as redox-based biomarkers: a brief review. BMB Rep. 2015;48(4):200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol. 2015;31(5):631–641. [DOI] [PubMed] [Google Scholar]
- 55.Wojtovich AP, Berry BJ, Galkin A. Redox Signaling Through Compartmentalization of Reactive Oxygen Species: Implications for Health and Disease. Antioxid Redox Signal. 2019;31(9):591–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A; American Heart Association Council on Basic Cardiovascular Sciences. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ Res. 2016;119(5):e39–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cifuentes ME, Pagano PJ. Targeting reactive oxygen species in hypertension. Curr Opin Nephrol Hypertens. 2006;15(2):179–186. [DOI] [PubMed] [Google Scholar]
- 58.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. [DOI] [PubMed] [Google Scholar]
- 59.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11(10):2607–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeCoursey TE, Cherny VV, Zhou W, Thomas LL. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci U S A. 2000;97:6885–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kishi H, Kishi T, Folkers K. Bioenergetics in clinical medicine. III. Inhibition of coenzyme Q10-enzymes by clinically used anti-hypertensive drugs. Res Commun Chem Pathol Pharmacol. 1975;12(3):533–540. [PubMed] [Google Scholar]
- 62.Pontremoli S, Salamino F, Sparatore B, De Tullio R, Patrone M, Tizianello A, Melloni E. Enhanced activation of the respiratory burst oxidase in neutrophils from hypertensive patients. Biochem Biophys Res Commun. 1989;158(3):966–972. [DOI] [PubMed] [Google Scholar]
- 63.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95(3):588–593. [DOI] [PubMed] [Google Scholar]
- 64.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97(8):1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reckelhoff JF, Romero DG, Yanes Cardozo LL. Sex, Oxidative Stress, and Hypertension: Insights From Animal Models. Physiology. 2019;34(3):178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manning RD Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand. 2003;179(3):243–250. [DOI] [PubMed] [Google Scholar]
- 67.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med. 2012;44:S2–16. [DOI] [PubMed] [Google Scholar]
- 68.Seifert R, Hilgenstock G, Fassbender M, Distler A. Regulation of the superoxide-forming NADPH oxidase of human neutrophils is not altered in essential hypertension. J Hypertens. 1991;9(2):147–153. [DOI] [PubMed] [Google Scholar]
- 69.Sagar S, Kallo IJ, Kaul N, Ganguly NK, Sharma BK. Oxygen free radicals in essential hypertension. Mol Cell Biochem. 1992;111(1–2):103–108. [DOI] [PubMed] [Google Scholar]
- 70.Gabig TG, Babior BM. The O2(−) -forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J Biol Chem. 1979;254(18):9070–9074. [PubMed] [Google Scholar]
- 71.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278(27):25234–25246. [DOI] [PubMed] [Google Scholar]
- 72.Brandes RP, Weissmann N, Schröder K. Nox family NADPH oxidases: Molecular mechanisms of activation Free Radic Biol Med. 2014;76:208–226. [DOI] [PubMed] [Google Scholar]
- 73.Jandeleit-Dahm K Endothelin in diabetes associated atherosclerosis: Opportunity “NOX”. Cardiovasc Res. 2021;cvab018. [DOI] [PubMed] [Google Scholar]
- 74.Guzik TJ, Griendling KK. NADPH oxidases: molecular understanding finally reaching the clinical level? Antioxid Redox Signal. 2009;11(10):2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schröder K NADPH oxidases in redox regulation of cell adhesion and migration. Antioxid Redox Signal. 2014;20(13):2043–2058. [DOI] [PubMed] [Google Scholar]
- 76.Brandes RP, Schröder K. Differential vascular functions of Nox family NADPH oxidases. Curr Opin Lipidol. 2008;19(5):513–518. [DOI] [PubMed] [Google Scholar]
- 77.Lassègue B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110(10):1364–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med. 2017;109:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401(6748):79–82. [DOI] [PubMed] [Google Scholar]
- 80.Buvelot H, Jaquet V, Krause KH. Mammalian NADPH Oxidases. Methods Mol Biol. 2019;1982:17–36. [DOI] [PubMed] [Google Scholar]
- 81.Teixeira G, Szyndralewiez C, Molango S, Carnesecchi S, Heitz F, Wiesel P, Wood JM. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br J Pharmacol. 2017;174(12):1647–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens. 2011;5(3):137–153. [DOI] [PubMed] [Google Scholar]
- 83.Schröder K, Weissmann N, Brandes RP. Organizers and activators: Cytosolic Nox proteins impacting on vascular function. Free Radic Biol Med. 2017;109:22–32. [DOI] [PubMed] [Google Scholar]
- 84.Trevelin SC, Shah AM, Lombardi G. Beyond bacterial killing: NADPH oxidase 2 is an immunomodulator Immunol Lett. 2020;221:39–48. [DOI] [PubMed] [Google Scholar]
- 85.Bánfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279(44):46065–46072. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Shan P, Srivastava A, Jiang G, Zhang X, Lee PJ. An Endothelial Hsp70-TLR4 Axis Limits Nox3 Expression and Protects Against Oxidant Injury in Lungs. Antioxid Redox Signal. 2016;24(17):991–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carnesecchi S, Carpentier JL, Foti M, Szanto. Insulin-induced vascular endothelial growth factor expression is mediated by the NADPH oxidase NOX3. Exp Cell Res. 2006;312(17):3413–3424. [DOI] [PubMed] [Google Scholar]
- 88.Pagano PJ, Cifuentes-Pagano E. The Enigmatic Vascular NOX: From Artifact to Double Agent of Change: Arthur C. Corcoran Memorial Lecture - 2019. Hypertension. 2021;77(2):275–283. [DOI] [PubMed] [Google Scholar]
- 89.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassègue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009;2(88):ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gray SP, Shah AM, Smyrnias I. NADPH oxidase 4 and its role in the cardiovascular system. Vasc Biol. 2019;1(1):H59–H66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Y, Ni T, Lin J, Zhang C, Zheng L, Luo M. Long non-coding RNA H19, a negative regulator of microRNA-148b-3p, participates in hypoxia stress in human hepatic sinusoidal endothelial cells via NOX4 and eNOS/NO signaling. Biochimie. 2019;163:128–136. [DOI] [PubMed] [Google Scholar]
- 93.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45(9):1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. [DOI] [PubMed] [Google Scholar]
- 95.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC and Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. [DOI] [PubMed] [Google Scholar]
- 96.Nosalski R, Mikolajczyk T, Siedlinski M, Saju B, Koziol J, Maffia P, Guzik TJ. Nox1/4 inhibition exacerbates age dependent perivascular inflammation and fibrosis in a model of spontaneous hypertension. Pharmacol Res. 2020:105235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond). 2011;120(4):131–141. [DOI] [PubMed] [Google Scholar]
- 98.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269(1–2):131–140. [DOI] [PubMed] [Google Scholar]
- 99.Bánfi B, Molnár G, Maturana A, Steger K, Hegedûs B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276(40):37594–37601. [DOI] [PubMed] [Google Scholar]
- 100.Weaver CJ, Leung YF, Suter DM. Expression dynamics of NADPH oxidases during early zebrafish development. J Comp Neurol. 2016;524(10):2130–2141. [DOI] [PubMed] [Google Scholar]
- 101.Magnani F, Nenci S, Millana Fananas E, Ceccon M, Romero E, Fraaije MW, Mattevi A. Crystal structures and atomic model of NADPH oxidase. Proc Natl Acad Sci. 2017;114(26):6764–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei CC, Hay E, Smith D, Lloyd L, Acharya G, Ngo R. Binding of Nox5’s EF-Hand domain to the peptides corresponding to the phosphorylatable region and regulatory inhibitory loop in its dehydrogenase domain. Biophys Chem. 2020;262:106379. [DOI] [PubMed] [Google Scholar]
- 103.Millana Fañanás E, Todesca S, Sicorello A, Masino L, Pompach P, Magnani F, Pastore A, Mattevi A. On the mechanism of calcium-dependent activation of NADPH oxidase 5 (NOX5). FEBS J. 2020;287(12):2486–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen F, Yin C, Dimitropoulou C, Fulton DJ. Cloning, Characteristics, and Functional Analysis of Rabbit NADPH Oxidase 5. Front Physiol. 2016;7:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Görlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42(4):446–459. [DOI] [PubMed] [Google Scholar]
- 106.Pandey D, Fulton DJ. Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am J Physiol Heart Circ Physiol. 2011;300(4):H1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282(9):6494–6507. [DOI] [PubMed] [Google Scholar]
- 108.Pandey D, Chen F, Patel A, Wang CY, Dimitropoulou C, Patel VS, Rudic RD, Stepp DW, Fulton DJ. SUMO1 negatively regulates reactive oxygen species production from NADPH oxidases. Arterioscler Thromb Vasc Biol. 2011;31(7):1634–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen F, Barman S, Yu Y, Haigh S, Wang Y, Black SM, Rafikov R, Dou H, Bagi Z, Han W, Su Y, Fulton DJ. Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med. 2014;73:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen F, Haigh S, Yu Y, Benson T, Wang Y, Li X, Dou H, Bagi Z, Verin AD, Stepp DW, Csanyi G, Chadli A, Weintraub NL, Smith SM, Fulton DJ. Nox5 stability and superoxide production is regulated by C-terminal binding of Hsp90 and CO-chaperones. Free Radic Biol Med. 2015;89:793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kiyohara T, Miyano K, Kamakura S, Hayase J, Chishiki K, Kohda A, Sumimoto H, Genes Cells. Differential cell surface recruitment of the superoxide-producing NADPH oxidases Nox1, Nox2 and Nox5: The role of the small GTPase Sar1. 2018;23(6):480–493. [DOI] [PubMed] [Google Scholar]
- 112.Chen J, Wang Y, Zhang W, Zhao D, Zhang L, Fan J, Li J, Zhan Q. Membranous NOX5-derived ROS oxidizes and activates local Src to promote malignancy of tumor cells. Signal Transduct Target Ther. 2020;5(1):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Touyz RM, Anagnostopoulou A, Rios F, Montezano AC, Camargo LL. NOX5: Molecular biology and pathophysiology. Exp Physiol. 2019;104(5):605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cortés A, Pejenaute Á, Marqués J, Izal Í, Cenoz S, Ansorena E, Martínez-Irujo JJ, de Miguel C, Zalba G. NADPH Oxidase 5 Induces Changes in the Unfolded Protein Response in Human Aortic Endothelial Cells and in Endothelial-Specific Knock-in Mice. Antioxidants. 2021;10(2):194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marqués J, Cortés A, Pejenaute Á, Ansorena E, Abizanda G, Prósper F, Martínez-Irujo JJ, Miguel C, Zalba G. Induction of Cyclooxygenase-2 by Overexpression of the Human NADPH Oxidase 5 (NOX5) Gene in Aortic Endothelial Cells. Cells. 2020;9(3):637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol. 2012;302(10):H1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pai WY, Lo WY, Hsu T, Peng CT, Wang H. Angiotensin-(1–7) Inhibits Thrombin-Induced Endothelial Phenotypic Changes and Reactive Oxygen Species Production via NADPH Oxidase 5 Downregulation. J.Front Physiol 2017;8:994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keshtgar S, Ebrahimi B, Shid-Moosavi SM, Erfani N. NADPH oxidase 5 activation; a novel approach to human sperm cryoinjury. Cell Tissue Bank. 2020;21(4):675–684. [DOI] [PubMed] [Google Scholar]
- 120.Pi X, Xie L, Portbury AL, Kumar S, Lockyer P, Li X, Patterson C. NADPH oxidase-generated reactive oxygen species are required for stromal cell-derived factor-1alpha-stimulated angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34(9):2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gole HK, Tharp DL, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5). PLoS One. 2014;9(8):e105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rivera J, Sobey CG, Walduck AK, Drummond GR. Nox isoforms in vascular pathophysiology: insights from transgenic and knockout mouse models. Redox Rep. 2010;15(2):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H and Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. [DOI] [PubMed] [Google Scholar]
- 124.Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HHW, Owens GK, Lambeth JD and Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. [DOI] [PubMed] [Google Scholar]
- 125.Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension. 2008;51(2):500–506. [DOI] [PubMed] [Google Scholar]
- 126.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension. 2005;45(4):530–537 [DOI] [PubMed] [Google Scholar]
- 127.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47(2):238–244. [DOI] [PubMed] [Google Scholar]
- 128.Harrison CB, Trevelin SC, Richards DA, Santos CXC, Sawyer G, Markovinovic A, Zhang X, Zhang M, Brewer AC, Yin X, Mayr M, Shah AM. Fibroblast Nox2 Regulates ANG II-Induced Vascular Remodeling and Hypertension via Paracrine Signaling to Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2020; 41(2):ATVBAHA120315322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Michihara A, Oda A, Mido M. High Expression Levels of NADPH Oxidase 3 in the Cerebrum of Ten-Week-Old Stroke-Prone Spontaneously Hypertensive Rats. Biol Pharm Bull. 2016;39(2):252–358. [DOI] [PubMed] [Google Scholar]
- 130.Ye S, Zhong H, Yanamadala S, Campese VM. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension. 2006;48(2):309–315. [DOI] [PubMed] [Google Scholar]
- 131.Radkowski P, Wątor G, Skupien J, Bogdali A, Wołkow P. Analysis of gene expression to predict dynamics of future hypertension incidence in type 2 diabetic patients. BMC Proc. 2016;10:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen G, Adeyemo AA, Zhou J, Chen Y, Doumatey A, Lashley K, Huang H, Amoah A, Agyenim-Boateng K, Eghan BA Jr, Okafor G, Acheampong J, Oli J, Fasanmade O, Johnson T, Rotimi C. A genome-wide search for linkage to renal function phenotypes in West Africans with type 2 diabetes. Am J Kidney Dis. 2007;49(3):394–400. [DOI] [PubMed] [Google Scholar]
- 133.Kumar V, Kurth T, Zheleznova NN, Yang C, Cowley AW Jr. NOX4/H(2)O(2)/mTORC1 Pathway in Salt-Induced Hypertension and Kidney Injury. Hypertension. 2020;76(1):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cowley AW Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension. 2016;67(2):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pavlov TS, Palygin O, Isaeva E, Levchenko V, Khedr S, Blass G, Ilatovskaya DV, Cowley AW Jr, Staruschenko A. NOX4-dependent regulation of ENaC in hypertension and diabetic kidney disease. FASEB J. 2020;34(10):13396–13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li L, Lai EY, Luo Z, Solis G, Mendonca M, Griendling KK, Wellstein A, Welch WJ, Wilcox CS. High Salt Enhances Reactive Oxygen Species and Angiotensin II Contractions of Glomerular Afferent Arterioles From Mice With Reduced Renal Mass. Hypertension. 2018;72(5):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, Kennedy CR. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol. 2014;25:784–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holterman CE, Boisvert NC, Thibodeau JF, Kamto E, Novakovic M, Abd-Elrahman KS, Ferguson SSG, Kennedy CRJ. Podocyte NADPH Oxidase 5 Promotes Renal Inflammation Regulated by the Toll-Like Receptor Pathway. Antioxid Redox Signal. 2019;30(15):1817–1830. [DOI] [PubMed] [Google Scholar]
- 139.Jha JC, Dai A, Holterman CE, Cooper ME, Touyz RM, Kennedy CR, Jandeleit-Dahm KAM. Endothelial or vascular smooth muscle cell-specific expression of human NOX5 exacerbates renal inflammation, fibrosis and albuminuria in the Akita mouse. Diabetologia. 2019;62(9):1712–1726. [DOI] [PubMed] [Google Scholar]
- 140.Deliyanti D, Alrashdi SF, Touyz RM, Kennedy CR, Jha JC, Cooper ME, Jandeleit-Dahm KA, Wilkinson-Berka JL. Nox (NADPH Oxidase) 1, Nox4, and Nox5 Promote Vascular Permeability and Neovascularization in Retinopathy. Hypertension. 2020;75(4):1091–1101. [DOI] [PubMed] [Google Scholar]
- 141.Montezano AC, De Lucca Camargo L, Persson P, Rios FJ, Harvey AP, Anagnostopoulou A, Palacios R, Gandara ACP, Alves-Lopes R, Neves KB, Dulak-Lis M, Holterman CE, de Oliveira PL, Graham D, Kennedy C and Touyz RM. NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function. J Am Heart Assoc. 2018; 15;7(12):e009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elbatreek MH, Sadegh S, Anastasi E, Guney E, Nogales C, Kacprowski T, Hassan AA, Teubner A, Huang PH, Hsu CY, Schiffers PMH, Janssen GM, Kleikers PWM, Wipat A, Baumbach J, De Mey JGR, Schmidt H. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. PLoS Biol. 2020;18:e3000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neves KB, Harvey AP, Moreton F, Montezano AC, Rios FJ, Alves-Lopes R, Nguyen Dinh Cat A, Rocchicciolli P, Delles C, Joutel A, Muir K, Touyz RM. ER stress and Rho kinase activation underlie the vasculopathy of CADASIL. JCI Insight. 2019;4(23):e131344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao GJ, Zhao CL, Ouyang S, Deng KQ, Zhu L, Montezano AC, Zhang C, Hu F, Zhu XY, Tian S, Liu X, Ji YX, Zhang P, Zhang XJ, She ZG, Touyz RM, Li H. Ca(2+)-Dependent NOX5 (NADPH Oxidase 5) Exaggerates Cardiac Hypertrophy Through Reactive Oxygen Species Production. Hypertension. 2020;76(3):827–838. [DOI] [PubMed] [Google Scholar]
- 145.Touyz RM, Anagnostopoulou A, Camargo LL, Rios FJ, Montezano AC. Vascular Biology of Superoxide-Generating NADPH Oxidase 5-Implications in Hypertension and Cardiovascular Disease. Antioxid Redox Signal. 2019;30(7):1027–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Furmanik M, Chatrou M, van Gorp R, Akbulut A, Willems B, Schmidt H, van Eys G, Bochaton-Piallat ML, Proudfoot D, Biessen E, Hedin U, Perisic L, Mees B, Shanahan C, Reutelingsperger C, Schurgers L. Reactive Oxygen Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ Res. 2020;127(7):911–927. [DOI] [PubMed] [Google Scholar]
- 147.Cifuentes-Pagano E, Pagano P. Rational Design and Delivery of NOX-Inhibitory Peptides. J. Methods Mol Biol 2019;1982:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mazumdar S, Marar T, Devarajan S, Patki J. Functional relevance of Gedunin as a bona fide ligand of NADPH oxidase 5 and ROS scavenger: An in silico and in vitro assessment in a hyperglycemic RBC model. Biochem Biophys Rep. 2021;25:100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Casas AI, Nogales C, Mucke HAM, Petraina A, Cuadrado A, Rojo AI, Ghezzi P, Jaquet V, Augsburger F, Dufrasne F, Soubhye J, Deshwal S, Di Sante M, Kaludercic N, Di Lisa F, Schmidt HHHW. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol Rev. 2020;72(4):801–828. [DOI] [PubMed] [Google Scholar]
- 150.Tejero J, Shiva S, Gladwin MT. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev. 2019;99(1):311–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zweier JL, Chen CA, Druhan LJ, Zweier JL, et al. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14(10):1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Benson MA, Batchelor H, Chuaiphichai S, Bailey J, Zhu H, Stuehr DJ, Bhattacharya S, Channon KM, Crabtree MJ. A pivotal role for tryptophan 447 in enzymatic coupling of human endothelial nitric oxide synthase (eNOS): effects on tetrahydrobiopterin-dependent catalysis and eNOS dimerization. J Biol Chem. 2013;288(41):29836–29845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu Y, Ding Y, Ramprasath T, Zou MH. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid Redox Signal. 2021;34(9):750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15(4 Pt 1):326–332. [DOI] [PubMed] [Google Scholar]
- 155.Thida M, Earl J, Zhao Y, Wang H, Tse CS, Vickers JJ, Sutton M, Ong SL, Mori TA, Croft KD, Whitworth JA, Zhang Y. Effects of sepiapterin supplementation and NOS inhibition on glucocorticoid-induced hypertension. Am J Hypertens. 2010;23(5):569–574. [DOI] [PubMed] [Google Scholar]
- 156.Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hashimoto T, Sivakumaran V, Carnicer R, Zhu G, Hahn VS, Bedja D, Recalde A, Duglan D, Channon KM, Casadei B, Kass DA. Tetrahydrobiopterin Protects Against Hypertrophic Heart Disease Independent of Myocardial Nitric Oxide Synthase Coupling. J Am Heart Assoc. 2016;5(3):e003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, Crabtree MJ, Francis JM, Sayeed R, Ratnatunga C, Pillai R, Choudhury RP, Neubauer S, Channon KM. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation. 2012;125:1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Youn JY, Gao L, Cai H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia. 2012;55(7):2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dikalova AE, Góngora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299(3):H673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang K, Wang Y, Siu KL, Zhang Y, Cai H. Targeting feed-forward signaling of TGFbeta/NOX4/DHFR/eNOS uncoupling/TGFbeta axis with anti-TGFbeta and folic acid attenuates formation of aortic aneurysms: Novel mechanisms and therapeutics. Redox Biol. 2021;38:101757–101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol. 2017;174(12):1670–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Young CN. Endoplasmic reticulum stress in the pathogenesis of hypertension. Exp Physiol. 2017;102(8):869–884. [DOI] [PubMed] [Google Scholar]
- 164.Zito E ERO1: A protein disulfide oxidase and H2O2 producer. Free Radic Biol Med. 2015;83:299–304. [DOI] [PubMed] [Google Scholar]
- 165.Rainbolt TK, Saunders JM, Wiseman RL. Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends Endocrinol Metab. 2014;25(10):528–537. [DOI] [PubMed] [Google Scholar]
- 166.Gao P, Yan Z, Zhu Z. Mitochondria-Associated Endoplasmic Reticulum Membranes in Cardiovascular Diseases. Front Cell Dev Biol. 2020;8:604240. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic Reticulum Stress and Associated ROS. Int J Mol Sci. 2016;17(3):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hetz C The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. [DOI] [PubMed] [Google Scholar]
- 168.Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. 2021. February 22. [DOI] [PubMed] [Google Scholar]
- 169.Santos CX, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV, Lopes LR. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid Redox Signal. 2014;20(1):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Laurindo FR, Araujo TL, Abrahão TB. Nox NADPH oxidases and the endoplasmic reticulum. Antioxid Redox Signal. 2014;20(17):2755–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11(10):2409–2427. [DOI] [PubMed] [Google Scholar]
- 172.Ma S, Wang Q, Zhang Y, Yang D, Li D, Tang B, Yang Y. Transgenic overexpression of uncoupling protein 2 attenuates salt-induced vascular dysfunction by inhibition of oxidative stress. Am J Hypertens. 2014;27(3):345–350. [DOI] [PubMed] [Google Scholar]
- 173.Cunard R, Cunard R. Endoplasmic Reticulum Stress, a Driver or an Innocent Bystander in Endothelial Dysfunction Associated with Hypertension? Curr Hypertens Rep. 2017;19(8):64–69. [DOI] [PubMed] [Google Scholar]
- 174.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122(11):3960–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Choi SK, Lim M, Byeon SH, Lee YH. Inhibition of endoplasmic reticulum stress improves coronary artery function in the spontaneously hypertensive rats. Sci Rep. 2016;6:31925–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Han S, Bal NB, Sadi G, Usanmaz SE, Tuglu MM, Uludag MO, Demirel-Yilmaz E. Inhibition of endoplasmic reticulum stress protected DOCA-salt hypertension-induced vascular dysfunction. Vascul Pharmacol. 2019;113:38–46. [DOI] [PubMed] [Google Scholar]
- 177.Liu G, Wu F, Jiang X, Que Y, Qin Z, Hu P, Lee KSS, Yang J, Zeng C, Hammock BD, Tong X. Inactivation of Cys(674) in SERCA2 increases BP by inducing endoplasmic reticulum stress and soluble epoxide hydrolase. Br J Pharmacol. 2020;177(8):1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Z, do Carmo JM, da Silva AA, Fu Y, Hall JE. Mechanisms of Synergistic Interactions of Diabetes and Hypertension in Chronic Kidney Disease: Role of Mitochondrial Dysfunction and ER Stress. Curr Hypertens Rep. 2020;22(2):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mostafa RG, El-Aleem Hassan Abd El-Aleem A, Mahmoud Fouda EA, Ahmed Taha FR, Amin Elzorkany KM. A pilot study on gene expression of endoplasmic reticulum unfolded protein response in chronic kidney disease. Biochem Biophys Rep. 2020;24:100829–100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Paggio A, Checchetto V, Campo A, Menabò R, Di Marco G, Di Lisa F, Szabo I, Rizzuto R, De Stefani D. Identification of an ATP-sensitive potassium channel in mitochondria. Nature. 2019;572(7771):609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension. 2016;67(6):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Castro SD, Mennuni S, Volpe M. Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci. 2014;16(1):823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu J, Zeng Z, Zhang W, Deng Z, Wan Y, Zhang Y, An S, Huang Q, Chen Z. Emerging role of SIRT3 in mitochondrial dysfunction and cardiovascular diseases. Free Radic Res. 2019;53(2):139–149. [DOI] [PubMed] [Google Scholar]
- 184.Vaka VR, McMaster KM, Cunningham MW Jr, Ibrahim T, Hazlewood R, Usry N, Cornelius DC, Amaral LM, LaMarca B, Vaka VR, et al. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension. 2018;72(3):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Daiber A Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797(6–7):897–906. [DOI] [PubMed] [Google Scholar]
- 186.Müllebner A, Dorighello GG, Kozlov AV, Duvigneau JC. Interaction between Mitochondrial Reactive Oxygen Species, Heme Oxygenase, and Nitric Oxide Synthase Stimulates Phagocytosis in Macrophages. Front Med. 2018;4:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brandt M, Garlapati V, Oelze M, Sotiriou E, Knorr M, Kröller-Schön S, Kossmann S, Schönfelder T, Morawietz H, Schulz E, Schultheiss HP, Daiber A, Münzel T, Wenzel P. NOX2 amplifies acetaldehyde-mediated cardiomyocyte mitochondrial dysfunction in alcoholic cardiomyopathy. Sci Rep. 2016;6:32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang GS, Wang RJ, Zhang HN, Zhang GP, Luo MS, Luo JD. Effects of chronic treatment with honokiol in spontaneously hypertensive rats. Biol Pharm Bull. 2010;33(3):427–431. [DOI] [PubMed] [Google Scholar]
- 189.Zeng H, He X, Chen JX. A Sex-Specific Role of Endothelial Sirtuin 3 on Blood Pressure and Diastolic Dysfunction in Female Mice. Int J Mol Sci. 2020;21(24):9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.He P, Li Z, Yue Z, Gao H, Feng G, Wang P, Huang Y, Luo W, Hong H, Liang L, Chen S, Liu P. SIRT3 prevents angiotensin II-induced renal tubular epithelial-mesenchymal transition by ameliorating oxidative stress and mitochondrial dysfunction. Mol Cell Endocrinol. 2018;460:1–13. [DOI] [PubMed] [Google Scholar]
- 191.Zhou L, Zhang S, Bolor-Erdene E, Wang L, Tian D, Mei Y. NAMPT/SIRT1 Attenuate Ang II-Induced Vascular Remodeling and Vulnerability to Hypertension by Inhibiting the ROS/MAPK Pathway. Oxid Med Cell Longev. 2020;2020:1974265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sosnowska B, Mazidi M, Penson P, Gluba-Brzózka A, Rysz J, Banach M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis. 2017;265:275–282. [DOI] [PubMed] [Google Scholar]
- 193.Gao J, Zhang K, Wang Y, Guo R, Liu H, Jia C, Sun X, Wu C, Wang W, Du J, Chen J. A machine learning-driven study indicates emodin improves cardiac hypertrophy by modulation of mitochondrial SIRT3 signaling. Pharmacol Res. 2020;155:104739. [DOI] [PubMed] [Google Scholar]
- 194.Yang Y, Li N, Chen T, Zhang C, Li J, Liu L, Qi Y, Zheng X, Zhang C, Bu P. Sirt3 promotes sensitivity to sunitinib-induced cardiotoxicity via inhibition of GTSP1/JNK/autophagy pathway in vivo and in vitro. Arch Toxicol. 2019;93(11):3249–3260. [DOI] [PubMed] [Google Scholar]
- 195.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Park JB, Touyz RM, Chen X, Schiffrin EL. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2002;15:78–84. [DOI] [PubMed] [Google Scholar]
- 198.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–611. [DOI] [PubMed] [Google Scholar]
- 199.Ahmad KA, Yuan Yuan D, Nawaz W, Ze H, Zhuo CX, Talal B, Taleb A, Mais E, Qilong D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic Res. 2017;51(4):428–438 [DOI] [PubMed] [Google Scholar]
- 200.Costa CA, Amaral TA, Carvalho LC, Ognibene DT, da Silva AF, Moss MB, Valença SS, de Moura RS, Resende AC. Antioxidant treatment with tempol and apocynin prevents endothelial dysfunction and development of renovascular hypertension. Am J Hypertens. 2009;22(12):1242–1249. [DOI] [PubMed] [Google Scholar]
- 201.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol. 2004;15(2):306–15. [DOI] [PubMed] [Google Scholar]
- 202.Peixoto EB, Pessoa BS, Biswas SK, Lopes de Faria JB. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am J Nephrol. 2009;29(4):309–318. [DOI] [PubMed] [Google Scholar]
- 203.Carlström M, Brown RD, Sällström J, Larsson E, Zilmer M, Zabihi S, Eriksson UJ, Persson AE. SOD1 deficiency causes salt sensitivity and aggravates hypertension in hydronephrosis. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R82–92. [DOI] [PubMed] [Google Scholar]
- 204.Archer SL. Acquired Mitochondrial Abnormalities, Including Epigenetic Inhibition of Superoxide Dismutase 2, in Pulmonary Hypertension and Cancer: Therapeutic Implications. Adv Exp Med Biol. 2016;903:29–53. [DOI] [PubMed] [Google Scholar]
- 205.Godin N, Liu F, Lau GJ, Brezniceanu ML, Chénier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int. 2010;77(12):1086–1097. [DOI] [PubMed] [Google Scholar]
- 206.Farooqui Z, Mohammad RS, Lokhandwala MF, Banday AA. Nrf2 inhibition induces oxidative stress, renal inflammation and hypertension in mice. Clin Exp Hypertens. 2020;43(2):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao S, Ghosh A, Lo CS, Chenier I, Scholey JW, Filep JG, Ingelfinger JR, Zhang SL, Chan JSD. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1–7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology. 2018;159(2):836–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Myint PK, Luben RN, Wareham NJ, Khaw KT. Association between plasma vitamin C concentrations and blood pressure in the European prospective investigation into cancer-Norfolk population-based study. Hypertension. 2011;58(3):372–379. [DOI] [PubMed] [Google Scholar]
- 209.Huang J, Weinstein SJ, Yu K, Männistö S, Albanes D. Relationship Between Serum Alpha-Tocopherol and Overall and Cause-Specific Mortality. Circ Res. 2019;125(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li Z, Chen J, Zhang D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007–2014. J Hypertens. 2019;37(12):2371–2379. [DOI] [PubMed] [Google Scholar]
- 211.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. [DOI] [PubMed] [Google Scholar]
- 212.Castrejón-Téllez V, Villegas-Romero M, Rubio-Ruiz ME, Pérez-Torres I, Carreón-Torres E, Díaz-Díaz E, Guarner-Lans V. Effect of a Resveratrol/Quercetin Mixture on the Reversion of Hypertension Induced by a Short-Term Exposure to High Sucrose Levels Near Weaning and a Long-Term Exposure That Leads to Metabolic Syndrome in Rats. Int J Mol Sci. 2020;21(6):2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Prysyazhna O, Wolhuter K, Switzer C, Santos C, Yang X, Lynham S, Shah AM, Eaton P, Burgoyne JR. Blood Pressure-Lowering by the Antioxidant Resveratrol Is Counterintuitively Mediated by Oxidation of cGMP-Dependent Protein Kinase. Circulation. 2019;140(2):126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dornas WC, Silva M, Tavares R, de Lima WG, dos Santos RC, Pedrosa ML, Silva ME, Dornas WC, et al. Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: a meta-analysis. J Hypertens. 2015;33(1):14–23. [DOI] [PubMed] [Google Scholar]
- 215.Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Mol Interv. 2010;10(6):354–362. [DOI] [PubMed] [Google Scholar]
- 216.Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J Am Coll Cardiol. 2018;71(22):2570–2584. [DOI] [PubMed] [Google Scholar]
- 217.Zinovkin RA, Zamyatnin AA. Mitochondria-Targeted Drugs. Curr Mol Pharmacol. 2019;12(3):202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Javkhedkar AA, Quiroz Y, Rodriguez-Iturbe B, Vaziri ND, Lokhandwala MF and Banday AA. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid Med Cell Longev. 2019;2019:9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marques BCAA, Trindade M, Aquino JCF, Cunha AR, Gismondi RO, Neves MF, Oigman W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin Exp Hypertens. 2018;40(3):218–223. [DOI] [PubMed] [Google Scholar]
- 221.Theodotou M, Fokianos K, Mouzouridou A, Konstantinou C, Aristotelous A, Prodromou D, Chrysikou A. The effect of resveratrol on hypertension: A clinical trial. Exp Ther Med. 2017;13(1):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Movahed A, Ostovar A, Iranpour D, Thandapilly SJ, Raj P, Louis XL, Smoliga JM, Netticadan T. The efficacy of resveratrol in controlling hypertension: study protocol for a randomized, crossover, double-blinded, placebo-controlled trial. Trials. 2016;17(1):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Patel B, Mann GE, Chapple SJ. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radic Biol Med. 2018;122:150–160. [DOI] [PubMed] [Google Scholar]
- 224.Gupte SA, Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal. 2008;10(6):1137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10(6):1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51(7):1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oliveira PVS, Laurindo FRM. Implications of plasma thiol redox in disease. Clin Sci. 2018;132(12):1257–128 [DOI] [PubMed] [Google Scholar]
- 228.Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. [DOI] [PubMed] [Google Scholar]
- 230.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda). 2006;21:269–280. [DOI] [PubMed] [Google Scholar]
- 231.Garcia-Redondo AB, Briones AM, Martinez-Revelles S, Palao T, Vila L, Alonso MJ, Salaices M. c-Src, ERK1/2 and Rho kinase mediate hydrogen peroxide-induced vascular contraction in hypertension: role of TXA2, NAD(P)H oxidase and mitochondria. J Hypertens. 2015;33:77–87. [DOI] [PubMed] [Google Scholar]
- 232.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. [DOI] [PubMed] [Google Scholar]
- 233.Dustin CM, Heppner DE, Lin MJ, van der Vliet A. Redox regulation of tyrosine kinase signalling: more than meets the eye. J Biochem. 2020;167(2):151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Datla SR, McGrail DJ, Vukelic S, Huff LP, Lyle AN, Pounkova L, Lee M, Seidel-Rogol B, Khalil MK, Hilenski LL, Terada LS, Dawson MR, Lassegue B, Griendling KK. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am J Physiol Heart Circ Physiol. 2014;307:H945–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vukelic S, Xu Q, Seidel-Rogol B, Faidley EA, Dikalova AE, Hilenski LL, Jorde U, Poole LB, Lassegue B, Zhang G, Griendling KK. NOX4 and Poldip2 Induce Filamentous Actin Oxidation and Promote Its Interaction With Vinculin During Integrin-Mediated Cell Adhesion. Arterioscler Thromb Vasc Biol. 2018;38:2423–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Acevedo A, González-Billault C. Crosstalk between Rac1-mediated actin regulation and ROS production. Free Radic Biol Med. 2018;116:101–113. [DOI] [PubMed] [Google Scholar]
- 237.Cuello F, Wittig I, Lorenz K, Eaton P. Oxidation of cardiac myofilament proteins: Priming for dysfunction? Mol Aspects Med. 2018;63:47–58. [DOI] [PubMed] [Google Scholar]
- 238.Lakshminarayanan V, Drab-Weiss EA and Roebuck KA. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the IL-8 promoter in endothelial and epithelial cells. J Biol Chem. 1998;273:32670–32678. [DOI] [PubMed] [Google Scholar]
- 239.Barančík M, Grešová L, Barteková M and Dovinová I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol Res. 2016;65:S1–s10. [DOI] [PubMed] [Google Scholar]
- 240.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. [DOI] [PubMed] [Google Scholar]
- 241.Alves-Lopes R, Neves KB, Anagnostopoulou A, Rios FJ, Lacchini S, Montezano AC, Touyz RM. Crosstalk Between Vascular Redox and Calcium Signaling in Hypertension Involves TRPM2 Cation Channel. Hypertension. 2020;75:139–149. [DOI] [PubMed] [Google Scholar]
- 242.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. [DOI] [PubMed] [Google Scholar]
- 243.Gibhardt CS, Cappello S, Bhardwaj R, Schober R, Kirsch SA, Bonilla del Rio Z, et al. Oxidative Stress-Induced STIM2 Cysteine Modifications Suppress Store-Operated Calcium Entry. Cell Reports. 2020;33. [DOI] [PubMed] [Google Scholar]
- 244.Martín-Garrido A, Boyano-Adánez MC, Alique M, Calleros L, Serrano I, Griera M, Rodríguez-Puyol D, Griendling KK, Rodríguez-Puyol M. Hydrogen peroxide downregulates inositol 1,4,5-trisphosphate receptor content through proteasome activation. Free Radic Biol Med. 2009;47:1362–1370. [DOI] [PubMed] [Google Scholar]
- 245.Camargo LL, Harvey AP, Rios FJ, Tsiropoulou S, Da Silva RNO, Cao Z, Graham D, McMaster C, Burchmore RJ, Hartley RC, Bulleid N, Montezano AC, Touyz RM. Vascular Nox Compartmentalization, Protein Hyperoxidation, and Endoplasmic Reticulum Stress Response in Hypertension. Hypertension. 2018;72:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ushio-Fukai M Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. [DOI] [PubMed] [Google Scholar]
- 247.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Patel D, Alhawaj R, Kelly MR, Accarino JJ, Lakhkar A, Gupte SA, Sun D, Wolin MS. Potential role of mitochondrial superoxide decreasing ferrochelatase and heme in coronary artery soluble guanylate cyclase depletion by angiotensin II. Am J Physiol Heart Circ Physiol. 2016;310:H1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ushio-Fukai M Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11(6):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pasqua T, Pagliaro P, Rocca C, Angelone T, Penna C. Role of NLRP 3 inflammasome in hypertension: A potential therapeutic target. Curr Pharm Biotechnol. 2018;19(9):708–714. [DOI] [PubMed] [Google Scholar]
- 253.Wang J, Wen Y, Lv LL, Liu H, Tang RN, Ma KL, Liu BC. Involvement of endoplasmic reticulum stress in Ang II-induced NLRP3 iinflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin. 2015;36(7):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zewinger S, Reiser J, Jankowski V, Alansary D, Hahm E, Triem S, Klug M, Schunk SJ, Schmit D, Kramann R, Körbel C, Ampofo E, Laschke MW, Selejan SR, Paschen A, Herter T, Schuster S, Silbernagel G, Sester M, Sester U, Aßmann G, Bals R, Kostner G, Jahnen-Dechent W, Menger MD, Rohrer L, März W, Böhm M, Jankowski J, Kopf M, Latz E, Niemeyer BA, Fliser D, Laufs U, Speer T. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol. 2020;21(1):30–41. [DOI] [PubMed] [Google Scholar]
- 255.Numata T, Takahashi K, Inoue R. “TRP inflammation” relationship in cardiovascular system. Semin Immunopathol. 2016;38(3):339–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun. 2013;4:1611–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang X, Hong S, Qi S, Liu W, Zhang X, Shi Z, Chen W, Zhao M, Yin X. NLRP3 inflammasome is involved in calcium-sensing receptor-induced aortic remodeling in SHRs. Mediators Inflamm. 2019;2019:6847087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Villegas LR, Kluck D, Field C, Oberley-Deegan RE, Woods C, Yeager ME, El Kasmi KC, Savani RC, Bowler RP, Nozik-Grayck E. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal. 2013;18(14):1753–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ferreira NS, Bruder-Nascimento T, Pereira CA, Zanotto CZ, Prado DS, Silva JF, Rassi DM, Foss-Freitas MC, Alves-Filho JC, Carlos D, Tostes RC. NLRP3 Inflammasome and Mineralocorticoid Receptors Are Associated with Vascular Dysfunction in Type 2 Diabetes Mellitus. Cells 2019;8(12). pii: E1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao MX, Wang JJ, Zhou YB, Han Y, Chen Q, Li YH, Kang YM, Zhu GQ. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8(10):e3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lian D, Lai J, Wu Y, Wang L, Chen Y, Zhang Y, Boini KM, Huang Y, Chen Y. Cathepsin B-Mediated NLRP3 Inflammasome Formation and Activation in Angiotensin II -Induced Hypertensive Mice: Role of Macrophage Digestion Dysfunction. Cell Physiol Biochem. 2018;50(4):1585–1600. [DOI] [PubMed] [Google Scholar]
- 262.Zhao M, Bai M, Ding G, Zhang Y, Huang S, Jia Z, Zhang A. Angiotensin II Stimulates the NLRP3 Inflammasome to Induce Podocyte Injury and Mitochondrial Dysfunction. Kidney Dis (Basel). 2018;4(2):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gan W, Ren J, Li T, Lv S, Li C, Liu Z, Yang M. The SGK1 inhibitor EMD638683, prevents Angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim Biophys Acta Mol Basis Dis. 2018;1864(1):1–10. [DOI] [PubMed] [Google Scholar]
- 264.Valinsky WC, Touyz RM, Shrier A. Aldosterone, SGK1, and ion channels in the kidney. Clin Sci (Lond). 2018;132(2):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maron BA, Tang SS, Loscalzo J. S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antioxid Redox Signal. 2013;18(3):270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Méndez L, Pazos M, Giralt M, Nogués MR, Pérez-Jiménez J, Torres JL, Gallardo JM, Medina I. Targets of protein carbonylation in spontaneously hypertensive obese Koletsky rats and healthy Wistar counterparts: a potential role on metabolic disorders. J Proteomics. 2014;106:246–59. [DOI] [PubMed] [Google Scholar]
- 267.Tyther R, Ahmeda A, Johns E, McDonagh B, Sheehan D. Proteomic profiling of perturbed protein sulfenation in renal medulla of the spontaneously hypertensive rat. J Proteome Res. 2010;9(5):2678–2687. [DOI] [PubMed] [Google Scholar]
- 268.Chen X, Mori T, Guo Q, Hu C, Ohsaki Y, Yoneki Y, Zhu W, Jiang Y, Endo S, Nakayama K, Ogawa S, Nakayama M, Miyata T, Ito S. Carbonyl stress induces hypertension and cardio-renal vascular injury in Dahl salt-sensitive rats. Hypertens Res. 2013;36(4):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yıldırım E, İpek E, Bavunoğlu I, Yıldırım N, Cengiz M, Yavuzer S, Yavuzer H, Erman H, Uzun H. The impact of protein oxidation on sustained and white coat hypertension. Anatol J Cardiol. 2017;17(3):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xu Q, Huff LP, Fujii M, Griendling KK. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic Biol Med. 2017;109:84–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Varland S, Vandekerckhove J, Drazic A. Actin Post-translational Modifications: The Cinderella of Cytoskeletal Control. Trends Biochem Sci. 2019;44(6):502–516. [DOI] [PubMed] [Google Scholar]
- 272.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107(1):117–125. [DOI] [PubMed] [Google Scholar]
- 273.Atar S, Ye Y, Lin Y, Freeberg SY, Nishi SP, Rosanio S, Huang MH, Uretsky BF, Perez-Polo JR, Birnbaum Y. Atorvastatin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cyclooxygenase-2. Am J Physiol Heart Circ Physiol. 2006;290(5):H1960–1968. [DOI] [PubMed] [Google Scholar]
- 274.Wang SB, Murray CI, Chung HS, Van Eyk JE. Redox regulation of mitochondrial ATP synthase. Trends Cardiovasc Med. 2013;23(1):14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bubb KJ, Birgisdottir AB, Tang O, Hansen T, Figtree GA. Redox modification of caveolar proteins in the cardiovascular system- role in cellular signalling and disease. Free Radic Biol Med. 2017;109:61–74. [DOI] [PubMed] [Google Scholar]
- 276.Radi R Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc Natl Acad Sci U S A. 2018;115(23):5839–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Stomberski CT, Hess DT, Stamler JS. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid Redox Signal. 2019;30(10):1331–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tsiropoulou S, Montezano AC, Scott A, Burchmore RJ, Touyz RM. Vascular protein oxidation and redox proteomics in human hypertension. Heart. 2015;101;A13–A14. [Google Scholar]
- 279.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Touyz RM, Montezano AC, Rios F, Widlansky ME, Liang M. Redox Stress Defines the Small Artery Vasculopathy of Hypertension: How Do We Bridge the Bench-to-Bedside Gap? Circ Res. 2017;120:1721–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Virdis A, Gesi M, Taddei S. Impact of apocynin on vascular disease in hypertension. Vascul Pharmacol. 2016;87:1–5. [DOI] [PubMed] [Google Scholar]
- 282.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. [DOI] [PubMed] [Google Scholar]
- 283.Martínez-Revelles S, García-Redondo AB, Avendaño MS, Varona S, Palao T, Orriols M, Roque FR, Fortuño A, Touyz RM, Martínez-González J, Salaices M, Rodríguez C, Briones AM. Lysyl Oxidase Induces Vascular Oxidative Stress and Contributes to Arterial Stiffness and Abnormal Elastin Structure in Hypertension: Role of p38MAPK. Antioxid Redox Signal. 2017;27(7):379–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens. 2001;19:1245–1254. [DOI] [PubMed] [Google Scholar]
- 285.Lemarié CA, Simeone SM, Nikonova A, Ebrahimian T, Deschênes ME, Coffman TM, Paradis P and Schiffrin EL. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res. 2009;105:852–859. [DOI] [PubMed] [Google Scholar]
- 286.Ren J, Crowley SD. Role of T-cell activation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2019;316(6):H1345–H1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Coelho SC, Berillo O, Caillon A, Ouerd S, Fraulob-Aquino JC, Barhoumi T, Offermanns S, Paradis P, Schiffrin EL. Three-Month Endothelial Human Endothelin-1 Overexpression Causes Blood Pressure Elevation and Vascular and Kidney Injury. Hypertension. 2018;71:208–216. [DOI] [PubMed] [Google Scholar]
- 288.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21(4):489–495. [DOI] [PubMed] [Google Scholar]
- 289.Friederich-Persson M, Nguyen Dinh Cat A, Persson P, Montezano AC, Touyz RM. Brown Adipose Tissue Regulates Small Artery Function Through NADPH Oxidase 4-Derived Hydrogen Peroxide and Redox-Sensitive Protein Kinase G-1alpha. Arterioscler Thromb Vasc Biol. 2017;37(3):455–465. [DOI] [PubMed] [Google Scholar]
- 290.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal. 2014;20(1):74–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox Signal. 2016;25(3):119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Qaddumi WN, Jose PA. The Role of the Renal Dopaminergic System and Oxidative Stress in the Pathogenesis of Hypertension. Biomedicines. 2021;9(2):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hsu CN, Tain YL. Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants (Basel). 2020;10(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hong YA, Park CW. Catalytic Antioxidants in the Kidney. Antioxidants (Basel). 2021;10(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chan JYH, Chan SHH. Differential impacts of brain stem oxidative stress and nitrosative stress on sympathetic vasomotor tone. Pharmacol Ther. 2019;201:120–136. [DOI] [PubMed] [Google Scholar]
- 296.Chan SH, Chan JY. Brain stem NOS and ROS in neural mechanisms of hypertension. Antioxid Redox Signal. 2014;20:146–163. [DOI] [PubMed] [Google Scholar]
- 297.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–9. [DOI] [PubMed] [Google Scholar]
- 298.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–9. [DOI] [PubMed] [Google Scholar]
- 299.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. [DOI] [PubMed] [Google Scholar]
- 300.Chan SH, Wu KL, Chang AY, Tai MH, Chan JY. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension. 2009;53:217–227. [DOI] [PubMed] [Google Scholar]
- 301.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. [DOI] [PubMed] [Google Scholar]
- 302.Yin JX, Yang RF, Li S, Renshaw AO, Li YL, Schultz HD, Zimmerman MC. Mitochondria-produced superoxide mediates angiotensin II-induced inhibition of neuronal potassium current. Am J Physiol Cell Physiol. 2010;298:C857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Basu U, Case AJ, Liu J, Tian J, Li YL and Zimmerman MC. Redox-sensitive calcium/calmodulin-dependent protein kinase IIα in angiotensin II intra-neuronal signaling and hypertension. Redox Biol. 2019;27:101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yang Q, Yu XJ, Su Q, Yi QY, Song XA, Shi XL, Li HB, Qi J, Zhu GQ, Kang YM. Blockade of c-Src Within the Paraventricular Nucleus Attenuates Inflammatory Cytokines and Oxidative Stress in the Mechanism of the TLR4 Signal Pathway in Salt-Induced Hypertension. Neurosci Bull. 2020;36:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Jiang L, Zhou X, Yang H, Guan R, Xin Y, Wang J, Shen L, Zhu D, Ma S, Wang J. Upregulation of AT(1) Receptor Mediates a Pressor Effect Through ROS-SAPK/JNK Signaling in Glutamatergic Neurons of Rostral Ventrolateral Medulla in Rats With Stress-Induced Hypertension. Front Physiol. 2018;9:1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ma A, Gao L, Wafi AM, Yu L, Rudebush T, Zhou W, Zucker IH. Overexpression of Central ACE2 (Angiotensin-Converting Enzyme 2) Attenuates the Pressor Response to Chronic Central Infusion of Ang II (Angiotensin II): A Potential Role for Nrf2 (Nuclear Factor [Erythroid-Derived 2]-Like 2). Hypertension. 2020;76:1514–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. [DOI] [PubMed] [Google Scholar]
- 311.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory Role for B Cells in the Development of Angiotensin II-Dependent Hypertension. Hypertension. 2015;66:1023–1033. [DOI] [PubMed] [Google Scholar]
- 313.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. [DOI] [PubMed] [Google Scholar]
- 314.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017;21:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Emmerson A, Trevelin SC, Mongue-Din H, Becker PD, Ortiz C, Smyth LA, Peng Q, Elgueta R, Sawyer G, Ivetic A, Lechler RI, Lombardi G, Shah AM. Nox2 in regulatory T cells promotes angiotensin II-induced cardiovascular remodeling. J Clin Invest. 2018;128:3088–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Abais-Battad JM, Lund H, Dasinger JH, Fehrenbach DJ, Cowley AW Jr., Mattson DL. NOX2-derived reactive oxygen species in immune cells exacerbates salt-sensitive hypertension. Free Radic Biol Med. 2020;146:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23(6):981–987. [DOI] [PubMed] [Google Scholar]
- 319.Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, Channon KM. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol. 2004;24(9):1614–1620. [DOI] [PubMed] [Google Scholar]
- 320.Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, Carnevale R, Soresina A, Finocchi A, Cirillo E, Catasca E, Angelico F, Loffredo L. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol 2013;33:406–412 [DOI] [PubMed] [Google Scholar]
- 321.Li H, Han X, Hu Z, Huang J, Chen J, Hixson JE, Rao DC, He J, Gu D, Chen S. Associations of NADPH oxidase-related genes with blood pressure changes and incident hypertension: The GenSalt Study. J Hum Hypertens. 2018;32(4):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Han X, Hu Z, Chen J, Huang J, Huang C, Liu F, Gu C, Yang X, Hixson JE, Lu X, Wang L, Liu DP, He J, Chen S, Gu D. Associations Between Genetic Variants of NADPH Oxidase-Related Genes and Blood Pressure Responses to Dietary Sodium Intervention: The GenSalt Study. Am J Hypertens. 2017;30(4):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Qin YW, Peng J, Liang BY, Su L, Chen Q, Xie JJ, Gu L. The A930G polymorphism ofP22phox (CYBA) gene but not C242T variation is associated with hypertension: a meta-analysis. PLoS One. 2013;8(12):e82465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.San José G, Fortuño A, Beloqui O, Díez J, Zalba G. NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin Sci (Lond). 2008;114(3):173–182. [DOI] [PubMed] [Google Scholar]
- 325.Kraja AT, Cook JP, Warren HR, et al. New blood pressure-associated loci identified in meta-analyses of 475 000 individuals. Circ Cardiovasc Genet. 2017;10;e001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Guarner-Lans V, Ramírez-Higuera A, Rubio-Ruiz ME, Castrejón-Téllez V, Soto ME, Pérez-Torres I. Early Programming of Adult Systemic Essential Hypertension. Int J Mol Sci. 2020;21(4):1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Friso S, Carvajal CA, Fardella CE, Olivieri O Epigenetics and arterial hypertension: The challenge of emerging evidence. Transl. Res 2015;165:154–165 [DOI] [PubMed] [Google Scholar]
- 328.Carlomosti F, D’Agostino M, Beji S, Torcinaro A, Rizzi R, Zaccagnini G, Maimone B, Di Stefano V, De Santa F, Cordisco S, et al. Oxidative Stress-Induced miR-200c Disrupts the Regulatory Loop Among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal 2017;27:328–344. [DOI] [PubMed] [Google Scholar]
- 329.Caimi G, Mulè G, Hopps E, Carollo C, Lo Presti R. Nitric oxide metabolites and oxidative stress in mild essential hypertension. Clin Hemorheol Microcirc. 2010;46(4):321–325. [DOI] [PubMed] [Google Scholar]
- 330.Melton CD, Luo R, Wong BJ, Spasojevic I, Wagenknecht LE, D’Agostino RB Jr, Il’yasova D. Urinary F(2)-isoprostanes and the risk of hypertension. Ann Epidemiol. 2017;27(6):391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Petramala L, Pignatelli P, Carnevale R, Zinnamosca L, Marinelli C, Settevendemmie A, Concistrè A, Tonnarini G, De Toma G, Violi F, Letizia C. Oxidative stress in patients affected by primary aldosteronism. J Hypertens. 2014;32(10):2022–2029 [DOI] [PubMed] [Google Scholar]
- 332.Cuffe JS, Xu ZC, Perkins AV. Biomarkers of oxidative stress in pregnancy complications. Biomark Med. 2017;11(3):295–306 [DOI] [PubMed] [Google Scholar]
- 333.Simic DV, Mimic-Oka J, Pljesa-Ercegovac M, Savic-Radojevic A, Opacic M, Matic D, Ivanovic B, Simic T. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J Hum Hypertens. 2006;20(2):149–155. [DOI] [PubMed] [Google Scholar]
- 334.Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid Med Cell Longev. 2019;2019:8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kashyap MK, Yadav V, Sherawat BS, Jain S, Kumari S, Khullar M, Sharma PC, Nath R. Different antioxidants status, total antioxidant power and free radicals in essential hypertension. Mol Cell Biochem. 2005;277(1–2):89–99 [DOI] [PubMed] [Google Scholar]
- 336.Verma MK, Jaiswal A, Sharma P, Kumar P, Singh AN. Oxidative stress and biomarker of TNF-alpha, MDA and FRAP in hypertension. J Med Life. 2019;12(3):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Chaves FJ, Mansego ML, Blesa S, Gonzalez-Albert V, Jiménez J, Tormos MC, Espinosa O, Giner V, Iradi A, Saez G, Redon J, Chaves FJ. Inadequate cytoplasmic antioxidant enzymes response contributes to the oxidative stress in human hypertension. Am J Hypertens. 2007;20(1):62–69. [DOI] [PubMed] [Google Scholar]
- 338.Rybka J, Kupczyk D, Kędziora-Kornatowska K, Pawluk H, Czuczejko J, Szewczyk-Golec K, Kozakiewicz M, Antonioli M, Carvalho LA, Kędziora J, Rybka J, et al. Age-related changes in an antioxidant defense system in elderly patients with essential hypertension compared with healthy controls. Redox Rep. 2011;16(2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ran L, Zhao W, Tan X, Wang H, Mizuno K, Takagi K, Zhao Y, Bu H. Cardiovasc Ther. Association between Serum Vitamin C and the Blood Pressure: A Systematic Review and Meta-Analysis of Observational Studies. 2020:2020:4940673. [DOI] [PMC free article] [PubMed] [Google Scholar]


