Abstract
The remarkable advances coming about through nanotechnology promise to revolutionize many aspects of modern life, however, these advances come with a responsibility for due diligence to assure that they are not accompanied by adverse consequences for human health or the environment. Many novel nanomaterials (having at least one dimension < 100 nm) could be highly mobile if released into the environment and are also very reactive, which has raised concerns for potential adverse impacts including, among others, the potential for neurotoxicity. Several lines of evidence led to concerns for neurotoxicity, but perhaps none more than observations that inhaled nanoparticles impinging on the mucosal surface of the nasal epithelium could be internalized into olfactory receptor neurons and transported by axoplasmic transport into the olfactory bulbs without crossing the blood brain barrier. From the olfactory bulb there is concern that nanomaterials may be transported deeper into the brain and affect other brain structures. Of course, people will not be exposed to only engineered nanomaterials, but rather such exposures will occur in a complex mixture of environmental materials, some of which are incidentally generated particles of a similar inhalable size range to engineered nanomaterials. To date, most experimental studies of potential neurotoxicity of nanomaterials have not considered the potential exposure sources and pathways that could lead to exposure, and most studies of nanomaterial exposure have not considered potential neurotoxicity. Here, we present a review of potential sources of exposures to nanoparticles, along with a review of the literature on potential neurotoxicity of nanomaterials. We employ the linked concepts of an Aggregate Exposure Pathway (AEP) and an Adverse Outcome Pathway (AOP) in order to organize and present the material. The AEP includes a sequence of Key Events progressing from material sources, release to environmental media, external exposure, internal exposure, and distribution to the target site. The AOP begins with toxicant at the target site causing a Molecular Initiating Event and, like the AEP, progress sequentially to actions at the level of the cell, organ, individual and population. Reports of nanomaterial actions are described at every key event along the AEP and AOP, except for changes in exposed populations that have not yet been observed. At this last stage, however, there is ample evidence of population level effects from exposure to ambient air particles which may act similarly to engineered nanomaterials. The data give an overall impression that current exposure levels may be considerably lower than those reported experimentally to be neurotoxic. This impression, however, is tempered by the absence of long-term exposure studies with realistic routes and levels of exposure to address concerns for chronic accumulation of materials and/or damage. Further, missing across the board are “Key Event Relationships”, which are quantitative expressions linking the Key Events of either the AEP or the AOP, making it impossible to project quantitatively the likelihood of adverse neurotoxic effects from exposure to nanomaterials, or to estimate margins of exposure for such relationships.
Keywords: Nanoparticles, neurotoxicity, biopersistence, metals, nasal uptake, blood-brain-barrier
Graphical Abstract
Introduction
The revolution of nanotechnology has led to a wide variety of innovative products and applications but has also raised concerns for potential exposure of the general population and adverse health effects, including effects on the nervous system. Among the innovative applications are biocompatible nanomaterials that enhance drug delivery across biological barriers and into target cells (e.g. neurons 1 or cancer cells 2). The concerns for nanomaterials arise because, just by their size (< 100 nm), they can interact with biological structures (e.g. cell-surface receptors, proteins, etc. 3) that are not targeted by larger particles or materials 4. Moreover, by these interactions with the biological environment the characteristics of nanomaterials can change dramatically, especially with respect to their biokinetics 5. It appears that although nanomaterial uptake into the body and translocation to the nervous system may be slow, their clearance may be even slower, raising an opportunity for biopersistence 6–8. The possible biopersistence and unwanted bioactivity of nanomaterials or released components (e.g. toxic metal ions) 9 raises concerns for human neurotoxicity. Over the last decade or so, there has been a growing amount and variety of research on the potential risks of nanomaterials to the nervous system 10. The potential for exposure to engineered nanomaterials (ENM) co-exists in a complex environment where other common exposures, such as ambient air ultrafine particles (UFP according to ISO/TC 146/SC 2/WG1 N 320), may also cause adverse neurological effects. In comparison to larger particles, just the size of this material offers the possibility that inhaled nanosized materials deposit in the olfactory region during nasal breathing and might translocate to the olfactory bulb and the brain 11. Thereby, additional routes of exposure need to be considered when airborne ENM are inhaled during nasal breathing. However, computational fluid dynamics (CFD) simulations 11 as well as measures in human nasal replicate casts 12 revealed that very small nanoparticles (1–2 nm) will deposit in the olfactory region of the human nose to a larger extent than larger particles (> 10nm). Such characteristics have to be considered in human health risk assessment when (a) describing the relevant routes of exposure, and (b) identifying the relevant target organs of ENM toxicity.
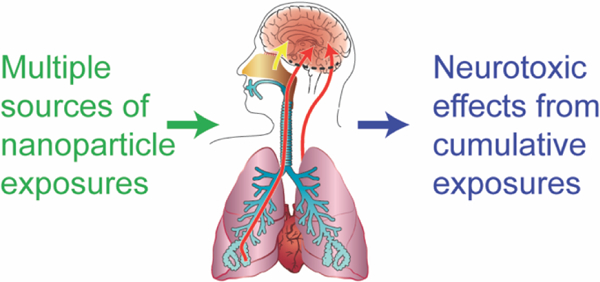
It becomes important to consider the potential for exposure to engineered nanoparticles and neurotoxic effects in the context of total aggregate exposures and cumulative insults (see Figure 1). In this article, we attempt to synthesize the growing research in the context of aggregate exposures and adverse outcome pathways. This effort will reveal areas of research concordance, areas where additional work is required, and help guide development of efficient testing strategies for the potential risks of nanomaterials to the nervous system.
Figure 1.
A schematic overview representing exposure to nanomaterials/ -particles (1), aggregated across sources and over time, their absorption and distribution in the body (2), and accumulated outcomes from exposure accumulated across materials and over time at the cellular, organ (3) and individual or population level (4).
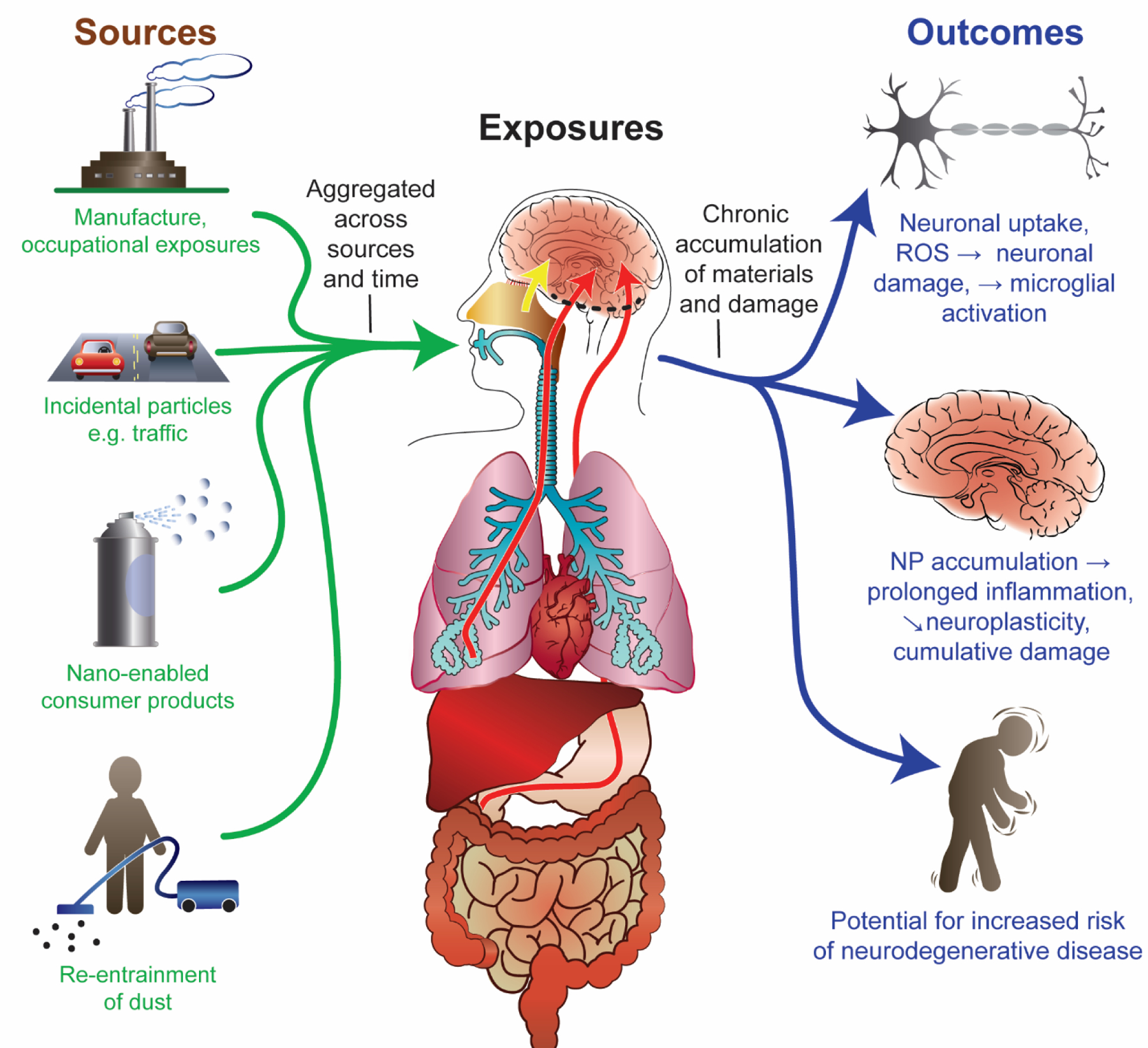
Nanoparticles readily disperse in air, soil or water systems. The properties of being both highly reactive and widely dispersed raised concerns that nanoparticle releases would lead to inadvertent exposures, rapid biouptake and distribution, and potential toxicity. It has become important to consider the potential for nanoparticle release in occupational and environmental contexts, including from nanomaterial-enabled consumer products across their complete life ranging from manufacture, use and disposal at end of life cycle 13. Although many nanomaterial product formulations apparently have little or no potential for substantial release of nanomaterials into the environment, there is a clear potential for occupational exposure during manufacturing 14, and for exposure to members of the public from use of some nanomaterial products marketed in dispersive formulations. There is also the possibility of industrial or transportation accidents which could lead to large volumes of nanomaterials being released.
The complex nature of possible exposures to known environmental sources of nanomaterials that aggregate across time and sources; the uptake and translocation across portals of entry such as the nasal olfactory epithelium, the respiratory tract, and the GI system; the transport to various target organs in the human body (including the brain), possible damage to molecular and cellular structures in the nervous system, and finally adverse health effects related to these perturbations are schematically given in Figure 1.
For human risk assessment it is important to consider all relevant exposure scenarios, the biological intermediate steps including organ distribution, tissue/ cell uptake, clearance/ accumulation, and finally the (neuro)toxic outcome. These four aspects schematically given in Figure 1 are subsequently described in more details.
Sources of nanoparticle exposure include: manufacture of engineered nanomaterials, both for occupational exposure to workers, and for potential releases during the manufacture process to the surrounding communities; generation of incidental nanoparticles such as from common sources such as ultrafine air pollution particles (UFP) from automobiles, as well as nanoparticles associated with traffic such as particles from degradation of tires or brakes; use of nano-enabled consumer products such as nanomaterial containing spray cleaners but also from foodstuff; and re-entrainment of dust from various sources that had settled on indoor or outdoor surfaces, but is disturbed and re-entrained into the airborne breathing zone. The relative contribution of these sources will vary from time to time and place to place, but the overall exposure assessment should consider an aggregated sum across sources and time.
Routes of exposure: The predominant routes of exposure to nanoparticles are inhalation 15 and ingestion 16. Nanomaterials also encounter the skin, such as with use of cosmetic products or sunscreens, but absorption through the dermal route is typically found to be negligible 17. The major route of absorption is inhalation, where particle size and density determine the deposition pattern of particles along the respiratory system. Small and large particles both impinging on the mucosal lining of the head region including the nasal cavity, where absorption and transport along the neurons of the olfactory or trigeminal nerve (axonal transport) and paracellular pathways 18 into the olfactory bulb or other brain structures can occur (yellow arrow). This pathway into the brain does not enter the blood or pass the blood-brain-barrier before entering the central nervous system. Inspired particles may also be engulfed by pulmonary macrophages in the tracheal and bronchial regions to either enter the lymphatic system or be cleared either through the mucociliary escalator to be swallowed and become a source of oral exposure. Nanometer (ultrafine) size particles also penetrate deep into the alveolar region of the lung where they may translocate into the blood in the particle form or as dissolved ionic particle constituents. Finally, particles may be swallowed either following lung clearance or from ingestion of particles present in food or water. A small proportion of these nanoparticles may be absorbed in the gastrointestinal (GI) tract and become present in the blood stream 16. This uptake is also relevant for nanoparticles in foodstuff (e.g. food-grade TiO2 as a whitening agent). Following systemic absorption, particles may be distributed to the other organs, including the brain (red arrow), where they would need to cross the blood-brain barrier to enter the brain tissue.
Biodistribution to the brain: Nanoparticles may enter the brain directly through the nasal olfactory pathway as described above, or to a lesser extent after being absorbed into other cranial nerves such as the trigeminal or facial and transported by axoplasmic transport into the brain (yellow arrow). This pathway might also be exploited for the nose-to-brain delivery of drugs 19–21. In addition, particles may be absorbed into the blood stream either from the lungs or GI tract, where depending on coatings and size, they become associated with serum proteins. Some blood borne particles can enter the brain (red arrows) by transporting across the blood brain barrier (dashed black line), or in a few locations where there is no blood brain barrier (e.g. circumventricular organs). After entering the brain, the limited evidence currently available suggests that particle clearance from the brain may be slow.
Outcomes: Given that clearance of particles may be slow, and damage recovery in the central nervous system (CNS) is limited, it is important to consider the potential for accumulation of materials and damage over time. At the level of the neuron, nanomaterials have been shown to alter neuronal function 22,23, cause generation of reactive oxygen species and oxidative damage 24–30, neuronal apoptosis 24,31–35 and reactive microglia activation 24. At the neuronal systems level, particle exposure and accumulation can lead to persistent inflammation 24, altered function of neuronal networks 30,36, reduced neuroplasticity 37, and potentially cumulative damage 38. At the level of the individual, these cellular and organ-level changes, interacting with factors such as a person’s genetic susceptibility and lifestyle factors, could overtime increase the potential for development of neurodegenerative conditions.
Targeted application of ENM as drug carrier to the brain
While there is concern for inadvertent exposures, at the same time nanomaterials have beneficial biomedical applications. Neuroscientists became fascinated by nanotechnology tools 39 and used them in basic (e.g. functionalized quantum dots 40) and clinical neuroscience (e.g. fullerene-based antioxidants 41). Drug delivery either via the nose-to-brain pathway 18 or by functionalized nanomaterials that are able to cross the blood-brain barrier 42 after systemic administration are thought to be powerful tools for the treatment of various brain diseases 43. Currently, there are three clinical trials available on PubMed (MeSH Terms: nanoparticles; neurodegenerative diseases 44–46) showing for instance the beneficial effects of small interfering RNA encapsulated in lipid nanoparticles in the treatment of transthyretin amyloidosis 45. The TTR siRNA (ALN-TTR01) successfully reduced levels of mutant and non-mutant forms of transthyretin in the patients. Another clinical trial in transthyretin-mediated amyloidosis using lipid nanoparticles as siRNA carriers confirmed the safe use with no dose-limiting side effects 46. Karussis et al. 44 used mesenchymal stem cells (MSCs) that were labeled with superparamagnetic iron oxide ferumoxides in the treatment of multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) patients. Based on magnetic resonance imaging scans, they showed the presence of supraparamagnetic particles (ferumoxides-labeled MSCs) in CNS areas such as the meninges and the spinal cord parenchyma. Neither the intrathecal injection of MSCs via a standard lumbar puncture nor the ferumoxides labeling of stem cells cause major toxic or inflammatory side-effects. Even though nose-to-brain nanodelivery systems have been discussed 47 approaches that use nanocarriers to translocate across the blood-brain-barrier (BBB) after systemic drug application 48 are probably more important. However, the BBB has also been identified as a possible target of ENM such as silver nanoparticles 49 and also UFP found in air pollution 50. Nevertheless, the examples of functionalized ENMs illustrate that not only the size but also other intrinsic properties of such materials determine their behavior or fate when humans are exposed to ENMs. Accordingly, standardized safety or toxicity testing with a strong focus on the brain is necessary for assessing the risks of ENM 51.
Safety Assessment and Neurotoxicity
With respect to the safety assessment of ENM, there is growing emphasis on developing non-animal alternative methods (NAM) for testing potential toxicity. Traditional toxicity testing based on administering substances to laboratory animals is expensive and time consuming. Developmental neurotoxicity testing protocols are one of the most animal-intensive of the testing guidelines required for registering chemical substances under OECD harmonized testing guidelines. Toxicity testing in live animals, based on well standardized protocols published by the OECD, however, has been internationally recognized standards for providing data for risk assessments. Due to both economic and humanitarian goals, and to speed assessment of previously untested substances, there is now a strong incentive to transform traditional toxicity testing to emphasize in vitro and computational approaches to screen compounds for potential toxicity. The US Environmental Protection Agency (EPA) has stated a goal of 30% reduction of funds for animal testing by 2025 and eliminate animal testing by 2030 (https://www.epa.gov/sites/production/files/2019–09/documents/image2019–09-09–231249.pdf).
The nervous system is one of the more complex systems of human health concern 52. The nervous system contains multiple types of cells that operate in a complex network which, despite much sophisticated research, remains incompletely understood. The functions of the nervous system include such important roles as life-supporting autonomic and neuroendocrine systems, sensory perception, coordinated motion, memory and cognition. These operations depend on elaborate network interactions that cannot be fully studied in isolated components, such as simple in vitro systems, other than the formation and function of simple neural connections. The development of the nervous system adds considerations of the timing and sequencing of developmental processes to the already complex study of the nervous system. Similarly, nanotoxicology is arguably one of the more complex challenges of the field of toxicology. The size of nanoparticles makes their movement in the body across cell membranes and normal diffusion barriers possible, and their reactivity due to their large surface area to mass makes them potentially toxic. The kinetic behavior of nanoparticles is dependent on particle physics and complex physical-biochemical interactions occurring on a scale too small for most instrumentation to observe. Whereas standard toxicological assessments focus largely on the chemical composition under considerations, nanotoxicology must consider not only chemical composition (often of mixtures of materials), but also unique properties related to the physicochemical properties of the nanoparticles 53. For these reasons, considerations of the neurotoxicity, including developmental neurotoxicity, of engineered nanomaterials presents a challenging case study for implementing alternative approaches for environmental health and safety assessments.
A strategy has evolved to help organize diverse sets of information relevant to potential environmental health implications of chemical substances, that can be referred to as Aggregate Exposure Pathway / Adverse Outcome Pathway (AEP/AOP) (Figure 2). AOP structures were described first 54–56 and featured a sequence of steps/ events beginning with a molecular initiating event (MIE) in which a toxic substance interacts at the molecular level with a biological target to initiate a toxic response. The MIE is followed by a sequence of Key Events (KE) at the cellular, organ, individual and perhaps population level that characterize the development of a particular type of toxic response. The AOP concept stipulates that the pathway is chemically agnostic so that any material causing the MIE can initiate the sequence of KEs leading to the adverse outcome. This structure has the virtue of helping illustrate the biological significance of molecular events for potential toxicity. While the AOP may simplify complex multi-channel toxicity pathways, the concept is useful for organizing different levels of information. The usefulness of the AOP concept gave rise to a companion exposure concept referred to as the Aggregate Exposure Pathway (AEP) 57. The AEP delineates KEs leading from the original generation of a material through its use, release, exposure of an individual, and eventual delivery to the target tissue. Both AEPs and AOPs are important, and a combination AEP/AOP concept represents a useful structure to consider available information related to potential neurotoxicity of nanomaterials. The depiction of the AEP/AOP concept in Figure 2 is presented with issues specific to nanomaterial exposures and neurotoxic outcomes.
Figure 2.
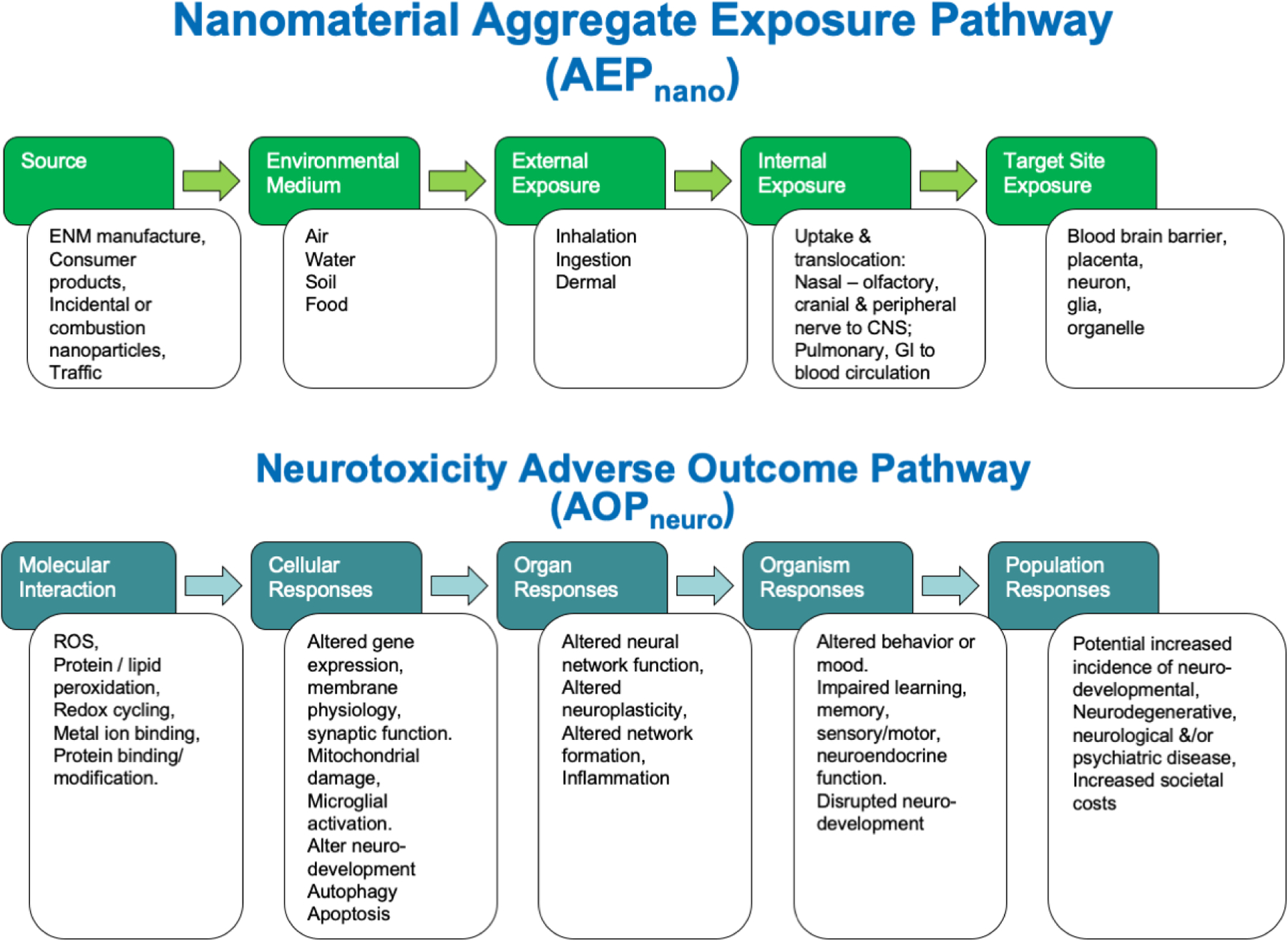
A schematic depicting the combination of an Aggregate Exposure Pathway for nanomaterials (AEPnano) with an Adverse Outcome Pathway for neurotoxicity (AOPneuro).
Aggregate Exposure Pathway: The AEPnano depicts potential pathways of exposure to nanoparticles in a sequence of KEs including: Sources, environmental medium, external exposures internal exposures and delivery to the biological target site. Multiple potential sources and pathways are considered, and the target site exposure reflects the summed aggregate across sources and pathways. It is important also to consider nanoparticles transformations and agglomerations along the AEP since nanomaterials transform rapidly in environmental and biological media, and the ultimate exposure to the target site may be to forms of the material different than that originally released from the source. The arrows in the diagram between the KEs reflect Key Events Relationships (KER) which are quantitative expressions of the transition from one step to the next. There is currently a lack of quantitative information for each of the KERs that would enable prediction of nanoparticle target tissue doses from any given potential exposure scenario (Adapted from the original concept of Teeguarden et al., 2016 57).
Adverse Outcome Pathway: The AOPnano depicts sequential Key Events between the molecular initiating event (MIE) where the nanoparticle causes the initial toxic molecular interaction that leads subsequently to changes at the cellular, organ, organism and population level. In the diagram, examples are listed under each KE of results that have been reported following exposures to nanoparticles. At the population level, the evidence is primarily from epidemiological studies of populations exposed to ultrafine air pollution particles having increased risks of neurodegenerative diseases. As for the AEP, the arrows between KEs of the AOP depict KERs that should be quantitative functions linking the measurable events between adjacent KE. While specific instances of nanoparticle effects can be identified at each KE, there are currently no qualitative data for the KERs. Therefore, it is not currently possible to predict the likelihood or dose-response relationships between generation of the MIE and the progression to an ultimate adverse outcome at the individual or population level (Adapted from the original concept of Ankley et al. 54).
Using these two conceptual frameworks the subsequent sections will provide an overview of the current scientific knowledge addressing the various aspects of exposure and neurotoxicity of ENM.
Aggregate Exposure Pathway (AEP)
The Aggregate Exposure Pathway concept involves release of the material from one or more sources, transport through the environmental media (air, water, food, soil etc.) exposure to individuals, absorption, transport to target tissues, and finally presentation to the molecular target initiating the MIE 57. One of the primary features of nanomaterials relative to conventional chemicals is their unique transport in the environment, biodistribution in the body, time-course and dose delivered to the target site. To understand potential risks from inadvertent exposure to engineered nanoparticles, it is first necessary to understand potential sources of nanomaterial release.
Sources
There are already many nanomaterials available on the commercial market for wholesale purchase and application to industrial processes or to consumer products. One commercial wholesale market lists over 3500 different nanomaterials for sale (https://www.nanowerk.com/). The variety of materials includes single and multi-walled carbon nanotubes, fullerenes, graphene, nanoparticles including metals, metal oxides, binary and higher order combinations of materials, quantum dots, nanowires, nanofibers, and non-carbon nanowires. The group of products labeled simply as “nanoparticles” is by far the largest group on this website. Many of these as “nanoparticles” are core element composition nanoparticles containing metals as their core (e.g. silver or gold). Here, there are commercial products available across a range of particle sizes, coatings or capping agents. The diversity of formulations is especially wide for gold nanoparticles, which can be conjugated with a range of biomolecules or fluorescent tags intended primarily for biomedical research and pharmaceutical development. Nanoparticles made from other core materials might be destined for any variety of applications. Thus, there are a large number of nanomaterials currently used in industry and commerce.
Products Containing Nanomaterials
Many consumer products are being developed using nanotechnology, but the number of such products is almost impossible to know. Currently, most companies are not required to report the nanomaterial content of their products, and the composition of many products is considered as confidential business information. Some companies, however, do advertise products that incorporate nanomaterials, presumably for perceived marketing advantages were nanotechnology is viewed as an asset. Different groups have endeavored to catalogue lists of nanomaterial containing consumer products based on voluntary submissions or from searching the internet for mentions of nano-enabled products. One of these efforts began at the US Woodrow Willson Center, which afterward was transferred to Virginia Tech University 58. Unfortunately, this database is not currently being curated. Another effort in Denmark is being actively curated and contains over 3000 entries 59. Our analysis of two of these datasets revealed several factors that limit understanding of the nanomaterial content of consumer products. The inventories are based on voluntary self-reported nanomaterial content and therefore are not comprehensive since many companies chose to not reveal such information. Among the self-reported nano-enabled products, over half the entries do not report the composition of the nanomaterials used. There is very little information regarding the percentage of nanomaterial content in the products, or the nanomaterial physical-chemical properties (size, shape, functionalization, coating, etc.). With a few exceptions, there is very little information about nanomaterial release from nano-enabled products during their normal use or disposal at the end-of-product life cycle. Finally, very little is known regarding the market penetration of nanomaterial containing products or the proportion of the population using them.
Nano cerium dioxide (CeO2) has been introduced as a fuel borne catalyst for on-road diesel engines in Europe 60. Although no longer registered for on-road vehicles in the US, nano CeO2 may be used in fuel for off-road applications such as railroad and mining equipment. When introduced into diesel fuel, CeO2 reduced the emissions of multiple exhaust components including CO2, CO, total particulate mass, and some volatile organic compounds and polycyclic aromatic hydrocarbons 61. However, there was a corresponding increase in other exhaust components, notably the number of ultrafine particles. The greater the concentration of cerium in the fuel, the smaller the diameter of emitted ultrafine particles. Air monitoring near a busy bus terminal in New Castle England before and after introduction of CeO2 to the bus fleet fuel showed an increase of soluble forms of cerium in PM10 samples 60,62. Thus, because of the large number of on-road diesel vehicles, the use of nano CeO2 as a fuel-borne catalyst may represent one of the most dispersive applications of an engineered nanomaterial to date.
Occupational exposure
Workplaces are typically where humans are first exposed to new materials 14, and the workers are exposed at greater concentrations than occur to the general population. A systematic review summarized the results of 46 studies providing quantitative date on airborne exposures to ENMs 63. Here, data for only 14 types of ENMs could be summarized: (1) six carbonaceous nanomaterials (e.g. fullerene C60, multiwalled carbon nanotubes (MWCNTs), single-walled carbon nanotubes (SWCNTs), (2) seven metallic nanomaterials (aluminum oxide, titanium dioxide, silver, silicon dioxide, iron, cerium oxide, and zinc oxide), and (3) nanoclays. For the most frequently used ENMs, dendrimers and gold nanomaterials, no exposure data were available. In contrast to the enormous production and widespread use of ENMs, empirical evidence about real exposure situations in working environments is far from conclusive.
Another systematic review identified 27 epidemiological investigations of workplace exposures to engineered nanomaterials 14. One or more studies were identified in which workers were occupationally exposed to carbon black, silica nanoparticles, titanium dioxide, multiwalled carbon nanotubes, carbon nanotubes /carbon nanofibers, silver, and various nanomaterials (Ag, iron-oxide, nanogold, CNT, TiO2, SiO2, nanoresins, nanoclay, nanoalumina and metal oxides). The most common route of exposure was by inhalation, and the primary target effects evaluated were respiratory and cardiovascular. For the most part, these studies found limited evidence of adverse effects related to ENM exposures, although in some cases changes were observed in inflammatory markers, eosinophil counts, markers of oxidative stress or damage, increased levels of antioxidant enzymes, altered pulmonary or cardiovascular function, or increased respiratory allergies. In one severe case, female workers without respiratory protection were spray-painting silica nanoparticles in a polyacrylic ester and experienced shortness of breath, pleural and pericardial effusion, pulmonary inflammation and 2 of 7 workers died 64. Most of the studies were cross-sectional, and relatively little time had elapsed since the onset of exposures for the observation of chronic exposure effects. No studies were identified that evaluated potential neurotoxicity in nanomaterial-exposed workers for instance by including standardized neurobehavioral testing.
In addition to manufacturing of ENM or ENM-containing products, occupational exposure to ENM may occur through the workplace usage of ENM enabled products. Examples of this exposure scenario occur in the construction industries, where building materials, paints, glues or other construction products may incorporate nanotechnology. A website dedicated to nano-enabled building materials categorized products as: additives for asphalt, additives for coatings, additives for concrete/cement, adhesives, boiler additives and caulking, with currently more than 60 products listed (http://www.nano.elcosh.org/). Exposure to construction workers is possible during both the construction phase where these materials are first applied, and during demolition where dust containing nanomaterials could be generated and workers may have little or no awareness of the composition of the materials being removed.
Consumer products, foodstuff, indoor sources
Potential sources of non-occupational human exposure to engineered nanomaterials include from nanomaterial-enabled consumer products or foodstuff that might release nanomaterials during usage or at other stages along the product life cycle, such as end-of-life disposal. Consumer products in which the nanomaterials are constituents of solid articles, such as sports equipment with carbon nanofibers, have little chance of releasing ENM during their in-use phase. In contrast, nanoparticles in liquid sprays or formulas such as silver nanoparticles in spray surface cleaners and personal care products, or TiO2 or ZnO as UV blockers in skin creams are more likely to cause direct consumer exposures. While research suggests that little TiO2 from sunscreens is systemically absorbed through intact skin, absorption may be increased after sunburn or in otherwise damaged skin 65. The prospect of inhalation exposure to nano-sized silver particles in spray cleaners is feasible. Commercially available spray disinfectants or dietary supplements advertised to contain colloidal silver, consisted of wide ranges of silver concentrations, with only approximately 20% of the products being within their nominally claimed concentrations 66. When analyzed by TEM silver containing products showed particle size distributions that were, for the most part, either <5 nm or between 20–40 nm 66. Both particle size distributions are highly respirable. The US FDA declared that all over the counter drug products containing colloidal silver ingredients or silver salts are not generally recognized as safe and effective https://www.govinfo.gov/content/pkg/FR-1999–08-17/pdf/99–21253.pdf). This warning was echoed by the US National Institutes of Health (https://nccih.nih.gov/health/colloidalsilver). TiO2 is not only used in sunscreens, it is also a white pigment used as food additive. Thus, external exposure to nanoparticles from foodstuffs might be possible. Food-grade TiO2 containing a fraction of nanosized TiO2 is approved as a pigment (E171 in Europe) in common foodstuffs 67. However, due to a recent study in rats showing adverse effects of food-grade TiO2 on intestinal and systemic immune homeostasis 68 French authorities (ANSES; French Agency for Food, Environmental and Occupational Health & Safety) asked for a better characterization of the hazard and the risks of E171 and recommended the promotion of products that do not contain nanomaterials (https://www.anses.fr/en/search/site/food-grade?iso1=fr&iso2=en). More recently, the generation of NPs emitted by appliances operated by brush electric motors has been discussed 69 and especially in indoor environments NPs containing metals (e.g. Cu) might be a relevant source. However, these non-combustion related nanoparticles are not engineered nanomaterials in the narrower sense.
Environmental Medium (Air, water, soil, food)
Should engineered nanomaterials be released into the environment, they will enter air, water or soil media and contact and interact with elements of the natural environment 13. Nanomaterials are subject by multiple forces affecting interactions and attractions with other particles and surfaces. In natural systems there will usually be an abundance of natural particles and surfaces relative to the expected low concentrations of released engineered nanomaterials, meaning that released ENM will rapidly become hetero-aggregated with prevailing environmental substrates, such as airborne particles or waterborne natural organic matter. Some nanoparticle compositions are relatively stable, such as TiO2. Others, such as silver, may react quickly so, for example, silver nanoparticles entering a sewer system will rapidly convert to silver sulphide 70,71. While graphene entering river system will complex with natural organic matter and eventually sink to the sediment layer, graphene oxide is more polar, less hydrophobic, and able to stay suspended in water columns for a longer period of time enabling transport over greater distances from the source of contamination 72. The extent of ENM attachments and transformations in natural environments can be influenced by multiple factors including the ENM composition or coating, and the environment it enters. These factors make prediction of environmental fate transport and transformations complex 73. Similarly, the consequences of environmental exposures are also difficult to project. Accordingly, for the risk assessment of ENM it is important to address these interactions with environmental media. The OECD provides guidelines (e.g. OECD TG 29) that are especially relevant for metals and metal compounds such as silver nanoparticles 74.
There have been efforts to model the quantities of nanomaterials along the phases of their product life cycles extending from manufacture and use to final disposal. The first attempt to quantify global life cycle releases of nanomaterials evaluated market information for the top ten ENMs by production volume, and estimated material flows into the environment and their final disposal site 75. The ten materials modeled included silica (SiO2), titania (TiO2), alumina (aluminum oxides), iron and iron oxides, zinc oxides, ceria, nano-silver, nano-copper, carbon nanotubes and nanoclays. Limitations of the analysis included that for many materials, the production and emission data did not distinguish nanoparticle from bulk chemical forms, and several of the release parameters had to be bounded by high and low estimates. The assumptions for the percentages released into air varied according to usages but ranged from a low of zero % (filtration, packaging, paper and boards, sensors) to a high of 5 % (academia and research, aerospace, automotive, catalysts, composites, electronics and optics, medical and textiles). Overall, it appeared that the ultimate destination of 63–91% of ENM would be landfills. Only about 0.1–0.5 % of ENM were expected to be released into air and 0.4–7 % into bodies of water. These estimates suggest that human exposure to ENM from environmental releases is overall a low probability, and that inadvertent human exposures were more likely to occur occupationally or through nearfield use of nano-enabled consumer products.
External exposure
After their release to any environmental medium external exposure of humans may take place via various routes of exposures. Like for environmental or unintentionally produced nanoparticles 76,77 inhalation, ingestion, and dermal uptake are the potential routes for external exposure to engineered nanomaterials (ENMs). According to Figure 1 and the epidemiological studies among workers, the respiratory tract is the most important portal of entry for ENM as well as for UFP from air pollution. However, for some external exposures the gastrointestinal tract is also relevant when discussing internal exposures and subsequent uptake and translocation. In both cases, different compartments of these portals of entry need to be considered because of (a) physiological differences (e.g. cellular composition) of these sections, and (b) deposition differences due to intrinsic characteristics of the nanomaterial (e.g. particle size). The respiratory tract is usually divided into the upper and lower respiratory tract (URT and LRT). The URT consists of the nasal cavity, the pharynx, and the larynx. The LRT can be divided into trachea, primary bronchi, and lungs (including the alveoli). In the GI tract especially the intestines (gut) are relevant for the uptake of ENM into the bloodstream and possible translocation to other organs including the brain. External exposure of the skin is likely when applying consumer products containing ENM or in the indoor environment (see previous section). In consumer products, such as sunscreens, dermal uptake through intact skin is negligible 17 and local toxicity is usually low for most ENMs 78. However, controlled and targeted functionalization (e.g. lipid nanocarriers for topical drug-delivery) seem to be necessary to increase transdermal uptake and the subsequent internal exposure of specific targets in the organism 79. Here, targeting the brain is less relevant and the next section about internal exposure will focus on the respiratory and gastrointestinal tract.
Internal and target site exposure
The route of exposure is relevant for determining the organ or cell types (e.g. airway or skin epithelial cells) that will be affected from the toxic interactions with the agent, or in this case ENM 80. One needs to distinguish local effects (e.g. eye or skin irritation), occurring at the site where the toxin/ ENM comes in contact with the organism from systemic effects (e.g. liver fibrosis, neurodegeneration) that are related to toxicity in other organs after the translocation of the toxin/ ENM from the initial target site. Translocation of ENMs to the nervous system is thought to be a prerequisite to cause any neurotoxicity associated with neuropathological changes. However, the innate immune system plays an important role in defense mechanisms related to the protection of the organism against xenobiotics including particles and ENM 81. As a consequence of peripheral inflammation the functionality of the brain can be altered as shown by increased hippocampal slice excitability in a model of inflammatory bowel disease using 2,4,6-trinitrobenzene sulfonic acid (TNBS) treatment in rats 82. Such an “indirect” pathway of neurotoxicity has been shown in the context of air pollution 83 and might be a possible mechanism for the neurotoxicity of ENM.
Due to size, shape, and various biophysicochemical properties, ENM interact with biological components in the body (e.g. proteins, membranes, phospholipids, endocytic vesicles, organelles, DNA and biological fluids) 53. Therefore, almost all cells in the human body are capable of taking up ENMs via various physiological pathways 3. Here, the three most relevant uptake mechanisms are macropinocytosis, clathrin- and caveolae-mediated endocytosis. With respect to internal exposure and neurotoxicity the nasal cavity is of utmost importance as nanoparticles can deposit especially in this region (see Figure 1). Here, the olfactory epithelium provides some unique features as nanoparticles are in direct contact with CNS structures. The absence of a blood-brain barrier on this pathway prompted the olfactory receptor neurons (ORN) located in this structure to be called a “window to the brain” 84. In the context of nanomaterials the first demonstration of the relevance of this pathway dated back to the 1970 when De Lorenzo 85 showed via electron microscopy that 50 nm colloidal gold traveled from ORNs to mitral cells located in the olfactory bulb.
Upper Respiratory Tract/Nasal-Olfactory Route
Airborne ENMs as well as ambient ultrafine particles have been studied with respect to their ability to translocate to the brain via olfactory receptor neurons, the olfactory bulb and finally to neuroanatomically connected central brain regions. To our knowledge, magnetite, a strong magnetic (ferrimagnetic) mixed Fe2+/Fe3+ iron oxide is the only nanosized air pollution particle that could be found in human brain tissue (frontal cortex) 86. The authors concluded that iron or other transition metal nanoparticles have the ability to enter the brain via the olfactory bulb. However, the neuropathological findings were limited to brain samples taken from the frontal lobes and the translocation from the olfactory epithelium along olfactory pathways has not been studied. In contrast, an animal study that exposed rats to radiolabeled aerosols of water-soluble 59Fe(II)SO4 (mass median aerodynamic diameter: 2,990 nm) for approximately 90 minutes showed that over a time course of 21 days 59Fe was not transported via the olfactory route to the brain 87. As Fe(II)SO4 is water-soluble ferrous sulfate was dissolved in the nasal lining fluid of the olfactory and respiratory mucosa of the nose as the radioactivity measured in these compartments of the nasal cavity was elevated in the treated rats/ nostrils. Iron nanoparticles might behave differently and after intranasal instillation of radiolabeled Fe3O4 nanoparticles (mean size: 30 nm, hydrodynamic diameter of agglomerates in physiological saline: 462 nm). In rats the instillation of radiolabeled Fe3O4 led to increased radioactivity measured in the olfactory bulb, the striatum and other brain areas 88. In the histopathology of the rat brain tissue the presence of the particles was not confirmed by Electron microscopy (EM) as suggested by Yokel 7. Accordingly, the translocation of the ENM was not verified. Moreover, in this study 20 µg in 10 µl physiological saline were instilled into the nostrils of rats, leading to concentrations of 2 g/l. These exposure units are not comparable to airborne exposure concentrations in the working environment. For example, respirable Fe in welding fumes in the breathing zone of active welders were measured as 370 µg/m3 that would be 0.00000037 g/l 89. Moreover, the size of the particles when applied to the animals was above 100 nm, and thus, Wu et al. 88 investigated the possible translocation of larger particles. While Maher et al. 86 provided transmission electron microscopy images of brain sections showing the size and morphology of the magnetite nanoparticles in the tissue this information is not provide by Wu et al. 88. This difference as well as the different exposure scenarios of these studies hamper the direct comparison of these iron oxide (magnetite) nanoparticle studies.
As mentioned in a previous section, metals are often the core element of ENM with respect to the olfactory transport and various rodent studies have investigated the translocation of metal oxides, including TiO2 90. In addition, many “translocation” studies have systematically investigated the olfactory uptake of manganese (Mn), a neurotoxic metal 91 that can be found in welding fumes 92. In several studies using rats, the results showed that inhalation or nasal instillation of MnO NP increased Mn in olfactory bulb and brain regions 93. Moreover, the nasal olfactory uptake of MnO NP is clearly size dependent, whereas 1.3 µm particles were taken up but 18 µm particles were not 94. Both studies showed that Mn accumulation in the olfactory bulb was dramatically higher than in any, more remote brain areas like the cortex or the basal ganglia. Here, it must be mentioned that the outcome measures of such studies (e.g. inductively coupled plasma mass spectrometry (ICP-MS)) are only capable of measuring the concentration of the elemental metal, and cannot distinguish whether to metal is in particulate or dissolved ionic form.
A widely used nanomaterial TiO2, when repeatedly administered intranasally to mice, led to increased Ti concentrations in olfactory bulb, cortex, hippocampus and cerebellum. The increase in Ti content was time-dependent, showing the highest concentrations of Ti, as measureed by ICP-MS, after 15 installations over 30 days. t Moreover, the concentrations of Ti depended on both particle size and brain region. After 30 days of instillations, the smaller NPs (80 nm) were associated with higher Ti tissue concentration in the hippocampus. During the other durations tested (2, 10, and 20 days), the concentration in the olfactory bulb was higher than in the other brain areas, namely the cerebellum and cortex 95. In another 90-day inhalation study 96 the translocation to the olfactory bulb was not reported.
Nanoscale aluminum oxides of different sizes (13, 20, and 40–50 nm), as well as aluminum salts, were used to determine the olfactory uptake of this metal into the brain 97. The authors observed a dose- and time-dependent increase of the Al concentration in the olfactory bulb only for the water-soluble Al salts. For the different aluminum oxides, no translocation to the olfactory bulb could be observed after intranasal installation.
Silica nanoparticles (SiO2-NPs) were investigated with respect to their ability to reach more remote brain areas after intranasal installation 98. After treatment with radiolabeled SiO2-NPs, radioactivity was detected in the striatum which is closely connected to the olfactory bulb 99.
With respect to carbon UFP that are relevant in the context of air pollution the translocation into the olfactory bulb has been shown by using 36nm 13C-labelled UFP 100. This example shows that the olfactory pathway might be relevant for nanomaterials without a metal core.
In addition to the olfactory epithelium, the respiratory epithelium in the nasal cavity is richly innervated by peripheral nerve fibers, especially the maxillary branch of the trigeminal nerve (fifth paired cranial nerve). The trigeminal sensory system is involved in nociception, and mechano, thermo, and polymodal nociceptors are located in specialized nerve endings in the nasal mucosa 101. The relevance of this cranial nerve as a way of directing uptake into the brain was investigated for manganese chloride (MnCl2) aerosols, as this material translocates to the olfactory bulb and into the brain. After 10-days of controlled inhalation, increased Mn concentrations could be measured in the trigeminal ganglion of rats and mice. Even 14 days after the exposure, the Mn levels were still significantly elevated. The authors also observed weak elevation of Mn in the spinal trigeminal nucleus in the medulla, indicating translocation to neuroanatomically-connected but more remote brain areas 102.
For some ENM the trigeminal pathway might be even more important than the olfactory pathway, as described for curcumin (Cur)-loaded polycaprolactone nanoparticles (PCL NPs) in rats after nasal instillation 103. Thus, for intact polymeric nanoparticles the uptake from the respiratory epithelium might be more relevant.
The relevance of these animal studies for humans is limited due to the large neuroanatomical and -physiological differences between the upper respiratory tract of rodents and humans 104. As direct comparisons are often not possible due to the invasiveness of the methods that are needed to estimate transport and deposition on metals/ nanomaterials into the human brain, computational fluid dynamics (CFD) studies are one attempt to estimate the deposition of particles in the various compartments of the upper respiratory tract. A CFD study simulated the particle deposition pattern in the nasal cavity of humans exposed to particulate matter such as welding fumes 105. The authors showed that especially small particles (< 20 nm) and compact agglomerate morphology are associated with a deposition in the olfactory region of the human nose. In general, they estimated that 0.1 to 1% of the inhaled welding fume agglomerates were deposited on the olfactory mucosa. To our knowledge such estimates are not available for exposures of ENM to humans, so the results obtained in rodent studies have to be interpreted with caution if they are to be extrapolated to humans.
Nevertheless, the exact pathway of the uptake of ENM along the olfactory or trigeminal nerve needs to be addressed in future research to shed more light on this particular pathway of “internal brain exposure”. Neurobiologically, neurons are phagocytic as shown for apoptotic and necrotic cell debris or 2.8 µm microspheres 106 and neurophysiologically this is important during embryonic development as well as during postnatal life. Therefore, it appears to be possible that intranasal nerve endings as well as ORNs are able to internalize nano-sized materials using a endocytosis. Ion channels like the transient receptor potential channels (TRP channels) located on the free nerve endings of the trigeminal nerve are Ca2+-permeable, nonselective cation channels 107. Other metal ions (e.g. Mn2+) might be transported into the nerve fibers via this pathway. In case of the olfactory pathway, the opening of cyclic nucleotide–gated ion channels after the activation of ORN might provide an entrance for metal ions dissolved from ENM or UFPs. The subsequent and dynein-driven transport along axons and dendrites has been described and modelled by Kuznetsov 108. These mechanistic considerations, demonstrated by the use of nanocarriers for intranasal drug delivery to the brain 18, need to be considered when estimating the internal exposures to ENM in the context of the AEP. Here, the stability of the ENM in the biological environment is crucial and alternative in vitro assays have been suggested to provide such information for the multitude of possible formulations of nanomaterials 109.
Lower Respiratory Tract/ Lung
During the production of ENMs, inhalation is the most relevant route of exposure and toxicological risk assessments typically emphasized the lungs and the respiratory tract as primary target organs 76. Sufficient in vivo data were available to perform a recent meta-analysis of transcriptomic responses to seven ENMs (i.e. carbon nanotubes, carbon black (CB), TiO2 nanoparticles, mixtures of metallic nanoparticles in welding fumes) in comparison to a pathogen-induced mouse models of lung diseases (e.g. bleomycin) 110. The response of other organs, including the brain were not investigated in this meta-analysis. Clear signatures for two groups of ENMs (metal-based/ carbon black vs. multiwalled carbon nanotubes (MWCNT)) could be derived and at least the m MWCNTs response showed some similarity to the disease models. For the MWCNTs, time-dependent expression profiles could be investigated that also fit with the progression of the bacteria-driven and Th2-response-mediated allergy disease models that are associated with lung fibrosis. In contrast, nanoTiO2 and CB induced inflammation was predominantly neutrophilic. Welding fume related transcriptomic responses were comparable to nanoTiO2 response patterns, showing that this particular metal nanoparticle might act via similar pathways. In addition, the metal response profiles were unrelated to lung fibrosis. The nanoTiO2 effects depended on the route of exposure, and also the way the NPs were administered 110. Intratracheal instillation of TiO2, but not cerium dioxide (CeO2) 111, induced inflammatory responses in bronchoalveolar lavage fluid (BALF) 112. TiO2 given to rats in a single intratracheal dose showed slow pulmonary clearance to thoracic lymph nodes, but no translocation to brain 108. CeO2 and ZnO administered to Calu-3 lung epithelial cells in an in vitro epithelial translation system showed low particle translocation (<0.01%) at 24 hours 109. In humans occupationally exposed to ENMs, inhalation is the more relevant and realistic exposure scenario and quantitative data about the exposure is a prerequisite before epidemiological studies could estimate adverse health effects at the respiratory tract.
TiO2 given to rats in a single intratracheal dose showed slow pulmonary clearance to thoracic lymph nodes, but no translocation to brain 113. The translocation of ENM across the epithelial layer of the lungs can also be studied in vitro by using transwell systems like the In Vitro Epithelial Translocation system (INVET) 114. Here, CeO2 and ZnO administered to Calu-3 lung epithelial cells in this in vitro epithelial translation system showed low particle translocation (<0.01%) at 24 hours 114.
The translocation of ENM across the air-blood barrier seems to be age dependent 115. Neonatal animals showed a higher translocation of AuNPs (100 nm) from the air to the blood than 21-day old rats, who were comparable to adult rats with respect to the translocation. Accordingly, the internal blood concentration of ENM might be higher in infants and, in combination with the less mature BBB in children 116, age-dependent effects might be relevant for the uptake of ENMs from the lungs into the blood and finally into the brain.
The primary target organ of airborne organic and inorganic particles is the respiratory tract, including both cancer and non-cancer lesions 117. With the advent of nanotechnology and increase in research on the multiorgan effects of ultrafine particles (UFP) 118 extrapulmonary target organs such as liver, kidney, gastrointestinal tract and brain have been included into the toxicological risk assessment of nanoparticles (NPs) 119. Particle size is an important predictor of deposition in the different compartments of the respiratory tract 11. Size also appears to be crucial for the subsequent translocation of NPs from the nose into the brain 100. Environmental nanoparticles with at least one dimension smaller than 100 nm are mainly combustion-derived nanoparticles (CDNP) and represent a diverse group of materials 120. In this size range a large proportion of the inhaled particles will deposit in the nose 11 and at least in animal studies carbon NPs 121 as well as manganese oxide can translocate to the olfactory bulb and more remote parts of the brain 93. Size also alters toxicity as shown for TiO2 particles. TiO2 nanoparticles in the size of 20 nm induced a stronger inflammatory response (percentage of neutrophils in lung lavage of rats and mice) than larger NPs (250 nm) 76.
The gastrointestinal tract
Humans given oral TiO2 NP showed increased particles in blood by dark-field microscopy, increased titanium in blood by ICP-MS, and systemic absorption of TiO2 122. Engineered nanomaterials are also used in various food products 16 and metal oxides and silicon dioxide are the most frequently used ENMs in food (e.g. food-grade TiO2 (E171) as a whitening agent). The food industries use ENMs as food additives, in food packaging, as antimicrobials for improving food preservation, for nutrient encapsulation and enhancing bioavailability, as well as in sensing applications for microorganism detection and identification (see 123,124 for review). The amount of daily ingested microparticles, including ultrafine (< 0.1 µm) particles, has been estimated to be around 1012 particles/ person 125 mainly consisting of TiO2 and mixed silicates. Especially for TiO2 the widespread use of some food products (e.g. salad dressing, sugar coating) can lead to high uptake levels of TiO2 via ingestion 125. Lomer et al. 125 showed that in patients suffering from Crohn’s disease, the reduction of dietary intake of particles reduced the disease severity. In addition to this clinical relevance, the review by Sohal et al. 16 provides an extensive overview about the safety of ENM in food products. Various in vitro and in vivo studies, including gut microbiome models, were summarized and several toxicological endpoints as well as aspects of dissolution in cell culture media, body fluids or cellular compartments (e.g. dissociation of Zn ions from ZnO in lysosomes and mitochondria 126). In their conclusion, the authors stated that in most of the available studies relevant test material (e.g. use of food-grade nanomaterials, most susceptible cell type), dose ranges (e.g. considering daily intake), dosimetry and dissolution kinetics (e.g. acidity in lysosomes) were not always considered carefully. Illustrating the importance of these factors, silver nanoparticles, when exposed to simulated stomach fluid (water, HCl and glycine at pH 1.5), rapidly agglomerated and fused, and the particle surfaces were converted largely to AgCl 127. Accordingly, the toxicological risk assessment of ingested ENM in humans addressing local effects in the gut is difficult. However, EFSA provides guidance for the safe intake of ENMs as food additives on their website (https://www.efsa.europa.eu/en/data/chemical-hazards-data). In nutritional epidemiology and controlled human ingestion studies the intake of ENM has not been addressed yet. If this aspect would be incorporated into recent cohort studies the local and systemic health effects of chronic consumption of ENM containing food could be estimated more precisely. Effects on the gut microbiome in the context of Parkinson’s disease (PD) has recently gained attention, 128 and ENM effects on the microbiota-gut-brain axis might be relevant for neuropathological changes caused by ingested ENMs.
Translocation into the brain/ nervous system
An in vitro model of the blood-brain barrier (BBB) showed increased permeability, disruption of tight junctions, reduced antioxidant defenses, inflammation, and apoptosis following treatment with AgNP 32. Similarly, an in vitro BBB model showed disruption of epithelial cell monolayer, implying BBB breakdown, from TiO2 129.
Rats were exposed by inhalation for 90 days to 14–15 nm silver nanoparticles, and elevated tissue concentrations of silver were measured at the end of the exposure period, and while they declined after 4 or 12 weeks of recovery 8. Although there was some variability across groups, in general silver was retained during the recovery period to a greater extent in the eyes, brains and, for females, ovaries, than in the other tissues measured. This slow clearance suggests a greater potential for bioaccumulation in the eye, brain and ovaries than other tissues.
Yokel et al. 130 published a review about the interaction of metal-based nanoparticles with the nervous system. They focused particularly on the flux across the blood-brain barrier after systemic injection of ENM and summarized that for nanoceria, nanogold, nanosilica, nanotitania, and nanoiron less than 0.1% of the applied dose can be found in the brain of the treated animals. However, coating of NPs with polyethylene glycol (PEG), also called “PEGylation”, markedly increased the brain concentration of metal-based nanoparticles. Other groups showed that only high dose intratracheal instillation of PbO NP or MnO NP increased brain Pb 131 or brain Mn 132, respectively. Another study injecting silver nanoparticles (average size: 36 nm) at doses of 10, 25, and 50 mg/kg BW ip to male mice for seven days showed a dose-dependent increase of Ag in the hippocampus 133. Up to 0.5 µg/g wet weight of Ag could be found in this brain area but, as outlined later in this review, the learning and memory abilities of the animals were not compromised. As a dose comparison, a dose of 2.5 µg/kg BW/d (with a safety factor of 100) was recommended as a Tolerable Daily Intake (TDI) value for elemental silver 134,135, meaning that the dose levels of 10, 25, or 50 mg/kg are 4,000, 10,000 or 20,000 times the TDI, or 400, 1000 or 2000 times the Low Observable Adverse Effect Level (LOAEL) for oral silver, respectively. The relevance of such high dose exposures for typically exposed humans is unclear.
The ip or iv injections of nanomaterials, however, outside of some medical procedures, are worst-case exposure scenarios that are not comparable with the typical non-medical routes of exposure for humans. Many biological barriers are bypassed with these injection routes, and such uptake and translocation data must be interpreted with caution when extrapolated to humans and their exposure to ENM in everyday life.
At the cellular level, metallic nanoparticles can be observed to be taken up into cells using a combination of dark-field and fluorescent microscopy 136. Using retinal pigment epithelial cells, silver particles were observed to grow in brightness after being internalized into the cytoplasm, presumably reflecting agglomeration of particles to form larger brighter reflective surfaces. The particles were translocated intracellularly to the vicinity of the endoplasmic reticulum. When stained also for lysosomes, collections of silver nanoparticles were co-located with the lysosomes, apparently as a cellular defensive process.
Adverse Outcome Pathway
The AOP begins with a toxic substance reaching the molecular target in the target tissue and the generation of a molecular initiating event (MIE). Subsequent “Key Events” along the AOP reflect measurable consequences of the MIE at successively more complex levels of the organism including the cellular, organ, and individual. In some cases, such as ecosystem studies or when exposures can be linked to demographic groups, it may be of interest to project outcomes to a population level.
Molecular Initiating Event (MIE)
The molecular mechanisms of action through which nanoparticles exert toxicity is an active research area. Much of the molecular and cellular toxicity work has been addressed in non-neuronal cell types. Buchman et al. 137 proposed three general categories of cytotoxic mechanisms of nanomaterials including (1) direct interactions at the cell surface, (2) dissolution of material releasing toxic ions, and (3) generation of reactive oxygen species leading to oxidative stress and damage. In the first category, cytotoxic mechanisms are related to highly reactive nanoparticles that exert toxicity through direct interactions with the cell surface either damaging the membrane or initiating signaling pathways that damage the cell 137. These highly reactive materials will likely cause toxic interactions at the point of entry to the body and, because of multiple intervening tissues, never reach the brain. The second and third categories of action, however, are actively hypothesized as molecular initiating events for the neurotoxicity of nanomaterials. In addition, other proposed mechanisms of nanoparticle neurotoxicity include inflammation, altered function of nerve membrane ion channels or receptors, and actions disrupting key stages of neuronal development.
With respect to the detection of MIEs in the context of nanotoxicity the use of alternative species such as zebrafish or caenorhabditis elegans can provide some relevant information that has been recently summarized in a meta-analysis 138. The authors processed transcriptomic data of various experiments investigating different ENMs within alternative species in a highly standardized procedure. Biostatistics showed that, regardless of the applied nanomaterial, genes that are related to energy generation are among those most frequently deregulated. When generating toxicity profiles for the Ti-, Ce-, Zn-, and Au-based ENMs across the different species the gene ontology (GO) term “energy generation” was also overrepresented. Thus, after interaction with the different cells of these species a general mechanism of ENM toxicity seemed to be related to the depletion of various cellular energy sources such as adenosine triphosphate (ATP). However, in this meta-analysis the individual studies differed markedly with respect to the exposure duration, the size of the ENM, etc.
Dissolution of metal particles to toxic metal ions (Ag, Zn, Cu)
One of the principal theories for the mechanism of toxicity for some nanoparticles is that they dissolve to release toxic metal ions. This is relevant for materials that may be relatively soluble in biologically fluids, such as silver, copper, or zinc but unlikely for more stable particles such as TiO2 or CeO2. For example, similar effects on brain neurochemistry were reported following 28 days of oral administration to 14 nm silver particles and the same dose level (9 mg/kg/d) of silver acetate 31. Singh et al. 139 observed murine macrophages took up silver particles, where the particles rapidly dissolved releasing silver ions, and the cytotoxicity was remediated in the presence of Ag ion-reactive, thiol containing compounds. This suggested that the proximal toxic entity was the Ag+ ions delivered into the cell via the nanoparticles.
For silver, however, the case may be somewhat unclear 140,141. Several authors have included silver ion control conditions to experiments of silver nanoparticle toxicity, and observed that the effects of ionic silver differed from those of silver particles, leading to a conclusion that silver nanoparticles have a different molecular initiating event than release of toxic ions, or in addition to the release of toxic ions. Differences between treatment with ionic silver and silver nanoparticles include the biodistribution, and locally delivered dose and time course. Nanoparticles can be taken up into cellular cytoplasm via endocytosis, where they traverse to the perinuclear region, perhaps interacting with the endoplasmic reticulum, and eventually being concentrated in lysosomes. The low pH of the lysosomal environment promotes the dissolution of AgNP and the locally concentrated release of Ag ions over a prolonged time. By contrast, silver ions taken up into the cell are available to cause lipid peroxidation or protein adduction more widely and rapidly across the cell. The nanoparticles therefore may have a different cellular distribution than silver ions and have a more localized and prolonged time course of dissolution and release silver ions. The spatial distribution, local concentration, and time course of action could differ between treatment with silver nanoparticles and silver ions. The dose-response relationship and time course of exposure are important determinants of toxicity. In addition, it is very difficult to match the dose of ionic silver to that of silver nanoparticles, other than by matching total mass of treatment under the assumption of total silver particle dissolution. For these reasons, it is reasonable to expect different toxicity manifestations following treatment with ionic silver and silver nanoparticles, even if the ultimate molecular toxicity of silver nanoparticles is the release of toxic silver ions.
Generation of reactive oxygen species (ROS)
The generation of free radicals followed by formation of reactive oxygen species, oxidative damage to the cellular components, and expression of antioxidant response pathways, is a common theme following cellular treatment with engineered nanomaterials. The formation of ROS in neuronal or glial cells treated with nanomaterials has been reported often (e.g. 24–30). The molecular responses of cells to treatment with metal based nanomaterials include reduced cellular glutathione from exposure to gold and silica NP in murine microglia cells 142; altered gene expression profiles in N27 rat dopaminergic neurons following AgNP treatment, including activation of NRF2 pathway which controls the expression of an array of antioxidant response genes 143,144.
One refinement of the theory of free radical generation as the initiating event for nanomaterial toxicity was proposed for the case of metal oxide nanoparticles causing pulmonary inflammation by Zhang et al. 145. Metal oxide nanoparticles have semi-conducting properties by which the outer shell electron can be elevated from the valence band to the conduction band, with the energy difference between the bands referred to as the band gap. Some metal oxide nanoparticles in an aqueous environment such as the interior of cells have band gap energies in the range of −4.12 to 4.84 eV, a magnitude that may interact with biomolecular redox reactions, interfering with cellular redox cycling and in the process generate reactive oxygen species. Zhang et al. 145 tested 24 metal oxide nanoparticles and observed that those within this range of bandgap energies were more cytotoxic than those with higher or lower values. The Zhang et al analysis was based on actions in pulmonary cells, but similar reactions are conceivable in the nervous system should the nanomaterials reach those tissues.
The theory that semiconductor metal oxides of an optimal bandgap participate in cellular redox cycling and generate free radicals which disrupt cellular metabolism has a related feature for photoactive semiconductor nanomaterials. Titanium dioxide, in particular, is both a semiconductor and a photocatalyst, in which absorption of a photon of light elevates valence band electrons to the conduction band. This excitation generates both loose electrons in the conduction band and holes where the electrons left the valence band. In an aqueous environment, the generated free conduction-band electrons and the valence holes catalyze the degradation of water molecules, creating both oxygen and hydroxy free radicals that then precipitate oxidation or hydroxylation reactions with nearby biomolecules. Phototoxic mechanisms are relevant in tissues exposed to the sun, including the skin and eye. In the eye, the retina is the only part of the central nervous system exposed to light. In retinal pigment epithelial cells, exposure TiO2 is much more toxic following co-exposure to UVA wavelengths of light 146. UVA irradiation reaches the retina only for young animals prior to the maturation of the UV filtering aspects of the lens. Some materials such as fullerene (hydroxy-fullerene), however, have a photoactivation spectrum extending into the visible wavelengths of light, and can be a phototoxic risk for any age 147,148.
Inflammation
Perhaps in reaction to the generation of ROS, or perhaps related to other injuries, treatment with nanoparticles causes cells to increase expression of proinflammatory gene expression pathways, and the release of proinflammatory chemicals. Microglia, immune cells of the central nervous system, take up larger amounts of carboxylated polystyrene nanoparticles than do neurons, showing higher local doses for immunological cells 149. AgNP increased the expression pro-inflammatory gene pathways including NRf2 and NFkB 143,144,150. Release of pro-inflammatory markers leads to activation of microglia and other inflammatory responses that, if prolonged, can themselves become neurotoxic. Silver NP caused mouse microglia to secrete cytokines that were toxic to hippocampal neurons 151. TiO2 nanoparticles applied to mouse primary striatal cultures containing microglia and dopaminergic neurons caused the release of inflammatory markers, ROS formation, and eventually lead to neuronal apoptosis. TiO2 was more toxic in mixed neuron-glia cultures than pure neurons, possibly due to microglia activation and ROS release 24. The cytokines and other factors isolated from a mouse microglia cell line (BV2) after treatment with AgNP, were toxic to mouse hypothalamic cells 151.
In response to nanoparticles of silver or gold, primary rat brain microvessel endothelial cells showed size dependent release of proinflammatory mediators and increases in permeability 49,152. TiO2 also caused inflammation and disruption of an in vitro model of the BBB 129. In an in vitro co-culture BBB model system containing rat brain microvascular endothelial cells, pericytes and astrocytes, treatment with AgNP triggered inflammatory responses that were associated with increased BBB permeability, disruption of tight junctions, reduced antioxidant defenses and apoptosis 32.
Cellular Events
Altered function of nerve membrane ion channels and/or receptors
Silver nanoparticles (50–100 nm) at 10−5 g/ml (10 ug/ml) inhibited hippocampal CA1 neuron membrane voltage-gated sodium currents in rat hippocampal slice preparations 23. Exposure of primary cultures of rat cerebellar granule cells to AgNP in vitro showed initial activation of NMDA receptors, leading to intracellular calcium imbalance, altered mitochondrial function and ROS production, culminating in excitotoxic cytotoxicity 22. In this scenario, ROS generation was a consequence of prior molecular interactions of the nanoparticles, rather than the original initiating event.
Altered neurodevelopment at the cellular level
The potential effects of chemical substances on neurodevelopment is currently an active research area. In particular, there is an ongoing search for alternative approaches to evaluate developmental neurotoxicity to replace traditional developmental neurotoxicity testing guidelines involving whole animals (e.g. 153,154). There are several alternative approaches being considered and many include, either explicitly or implicitly, an adverse outcome pathway-like approach 155,156. Among the Key Events of neurodevelopment identified in AOP approaches are: proliferation, migration, differentiation, neurite outgrowth, synaptogenesis, network formation and network function, synaptic pruning and apoptosis 157,158. These frameworks for evaluating developmental neurotoxicity of chemicals are also relevant for assessing nanoparticles.
Research on neurodevelopmental effects of nanomaterials has included some of the Key Events being targeted for developmental neurotoxicity (DNT) assays including proliferation, differentiation, and neurite outgrowth. In primary rat cortical cultures, AgNP inhibited of neurite outgrowth, reduced cell viability, and also caused degeneration of mature neurons 159. In PC12 cell cultures, silver nanoparticles impaired cell replication, and differentiation into a cholinergic cell type in a manner that was dependent on particle coating and size 160,161. Cultures of human embryonic stem cells treated with silver nanoparticles showed an increased astrocyte/neuron ratio, altered astrocyte morphology, reduced neurite outgrowth, decreased expression of synaptic proteins, and neurodegeneration 162. Other studies have shown neurodevelopmental effects of nanomaterials at the network and organ level and will be discussed in those sections below.
Apoptosis/cytotoxicity
Many nanomaterials have been reported to cause autophagy, apoptosis and/or cytotoxicity in neuronal or glial cell culture systems, usually in a dose and time-dependent fashion. These include: copper oxide 163; manganese 27, silver 31,49; gold 152; graphene and graphene oxide 28,33,164; single-walled carbon nanotubes 28,29; silica-coated iron oxide 165; superparamagnetic iron 34, and TiO2 26. Clearly, most materials when added to cell culture will kill cells if the concentration added is sufficiently high, so the relevance of these reports depends on the dose and the sensitivity relevant to other outcome measures. In an AOP framework, the critical considerations are quantitating Key Event Relationships between molecular level effects, changes in cellular functions and cytotoxicity or apoptosis. This quantification of Key Event Relationships has yet to be done for the neurotoxicity of nanomaterials.
In one possible exception, TiO2 toxicity to human astrocyte and neuronal cells in vitro was compared to the exposure levels causing brain damage in vivo 35. In this study, 69 nm anatase TiO2 was given to human glial (D384) and neuronal (SH-SY5Y) cell lines either acutely or for 7–10 days of prolonged exposure. Acute exposure caused cytotoxicity based on the MMT assay or calcein-AM/PI staining. After 7 d exposure colony formation was reduced by dose levels as low as 0.2 μg/ml, which the authors conclude were comparable to brain Ti concentrations in lab animal intranasally administered TiO2 and demonstrating neurotoxic effects as reported by 95 and 37. Note however, that the TiO2 dose levels of Ze et al. 37 were very high, as previously discussed.
Organ Responses
Altered neural network functioning
In primary mammalian (mouse or rat) cortical neurons grown on a microelectrode array (MEA), carbon black, iron oxide (Fe2O3), TiO2, and CeO2 nanoparticles were all shown to alter patterns of spontaneous neuronal network activity 30,166. The potency of carbon black, TiO2 and Fe2O3 nanoparticles for generating ROS was opposite to that for altering neuronal network activity, indicating that ROS formation was unrelated to changing neuronal firing patterns 30. Subtle changes in the firing rates of neuronal networks at dose levels below those producing cytotoxicity were caused by CeO2 and TiO2 nanoparticles 166. The effects of silver nanoparticles in an MEA system following a bicuculline challenge varied depending on particle size and coating, with increased network activity caused by 10 nm Ag-citrate, but decreased network activity following 75 nm Ag-PVP 36. In this case, the patterns of effects caused by silver nitrate as an ion control differed from those of silver nanoparticles, arguing for mechanistic actions of particulate nanosilver on neuronal network firing that were independent from the release of dissolved silver ions. Thus, in reports of the effects of ENM on neuronal network firing patterns, there is evidence that ENM actions are unrelated to either the generation of ROS or the dissolution of metal ions; two of the most prominent theories for molecular mechanisms of the neurotoxicity of ENM.
Generation of reactive oxygen species and inflammation at the organ level in vivo
The generation of reactive oxygen species and subsequent oxidative damage is commonly cited as a potential MIE for the effects of nanoparticles, including the neurotoxic effects. Much of this data has come from in vitro experiments, as discussed previously. The generation of ROS and oxidative damage to the brain has also been reported from in vivo animal experiments. Much of the data, however, involves relatively high dose levels or unrealistic routes of exposure. For example, in a study comparing three nanomaterials, adult male mice given suspensions of either TiO2, ZnO, or Al2O3 by oral gavage at a dose of 500 mg/kg/day for 21 consecutive days, showed increased reactive oxygen species in brain and other tissues, as well as decreases in dopamine and norepinephrine concentrations, and evidence of nanoparticles in brain tissue observed by TEM 167. Mice given 25 nm silver nanoparticles at doses of 0, 100, 500, or 1000 mg/kg, (ip) and tested 24 h afterward showed gene expression profiles at the two higher doses indicative of oxidative stress, apoptosis and neurotoxicity in caudate, frontal cortex, and hippocampus 168. Antioxidant gene expression in the brain differed between TiO2 or AgNP given by a single iv injection to adult rats and measured 28 days later 169. In these studies, paradoxically, AgNP decreased brain oxidative stress parameters, where TiO2 did not. TiO2 (80 or 155 nm) was administered to adult mice via intranasal instillation (500 μg/mouse), every other day for 1, 5, 10 or 15 times 95. Titanium concentration was increased in brain regions in a rank order were hippocampus > olfactory bulb > cerebellum > cortex. Increased antioxidative markers (GSH-Px, GST, SOD) were observed for 80 nm at 10 days, but not at 30 days. MDA, a marker of oxidative damage, was greater for 155 nm than 80 nm TiO2. Increased tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) in were found in brain following 155, but not 80, nm TiO2. Across these studies, it is unrealistic to imagine human exposure scenarios involving intravenous injection of metal or metal oxide nanoparticles, or exposure by any route where exposure levels approach 500 or 1000 mg/kg/d for multiple consecutive days.
Mice treated intranasally with TiO2 (2.5, 5, or 10 mg/kg/d for 90 days) showed proliferation of spongiocytes and hemorrhage in the brain, increased RNA and protein expression, including increased heme oxygenase 1 (HO-1) levels, indicative of p38-Nrf-2 signaling 170. The changes were associated with production of ROS and oxidative damage to the brain. The same dosing paradigm also reportedly produced a decrease in brain weight, an increase in whole brain titanium concentration, glial proliferation, apoptosis of hippocampal cells, and a host of gene expression changes 171, however these studies were criticized for the unreasonably extended period of daily intranasal instillation, and the high dose levels 172.
Male mice were given intranasal FITC-labeled SiO2 (115 nm, 8 mg/kg/day for 81 days) 173. When assessed 1 or 2 months later, nanoparticle deposition was observed in prefrontal cortex and in CA1 and CA3 regions of the hippocampus, but not in the dentate gyrus. Activated microglia and inflammatory cytokines were increased in the hippocampus. Mice also showed altered behavior in several test paradigms, neurodegeneration, altered synaptic physiology, and neuro-inflammation evident by increased microglia.
In addition to metal and metal oxide nanoparticles, reports investigating oxidative stress in the brain also include olfactory instillation of carbon black 174. In this case, mice treated with 95 μg/kg carbon black by nasal instillation on GD 5 and 9 showed differential gene expression profiles when offspring were tested at 6 or 12 weeks. These changes, however, were not reduced by 500 mg/kg/d ascorbic acid (ip GD5 and 9), suggesting a lack of protection from antioxidant co-treatment.
Alterations of brain neurochemistry in vivo
Female mice, given either 14 nm silver nanoparticles ip (4.5 or 9 mg Ag/kg/d) or ionic silver (9 mg/kg/d) for 28 d caused an increase of brain dopamine (DA) concentration 31. A lower dose for a shorter time (2.25 or 4.5 mg/kg/d for 14 d), in contrast, reduced brain DA. Brain weight was not affected, nor were brain concentrations of norepinephrine (NE) or serotonin (5-HT). The similarities of AgNP and Ag ion treatment led the authors to conclude that the effects of AgNP were likely related to dissolution and release of toxic silver ions, however, the reversal of effects on brain DA with extended dosing is concerning.
The effects of particle size and surface properties were examined in adult female mice given repeated intranasal instillations of TiO2 in either nano (10 nm) or bulk (1 µm) size particle and rendered either hydrophobic (no coating) or hydrophilic (3 – 4.8 % Si) composition 175. The titanium concentration in cerebral cortex was greater following hydrophilic than hydrophobic nanoparticles, and greater for nano- than bulk-sized particles. Similarly, the nano-sized hydrophilic nanoparticles caused cortical neuropathology, and had greater effects on dopamine (DA) and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) and 5-hydroxytryptamine (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA).
Mice given daily ip injections of TiO2 (5, 10, 50, 100, and 150 mg/kg BW (nano); 150 mg/kg (bulk) / day) for 14 days showed a decrease in the brain / body weight ratio, increase in brain ROS, lipid peroxidation, reduced brain antioxidants, reduced acetylcholinesterase (AChE) activity, reduced brain glutamate content/g protein and increased nitric oxide synthases (NOS) and nitric oxide (NO) concentrations in brain 176. Repeated intragastric administration of anatase TiO2 (5–10 nm) to adult rats at dose levels of 0, 50, 100, or 200 mg/kg/d for 60 d caused dose-related reduction of brain weight, reduction of brain AChE activity, increased cerebellar interleukin 6 (IL-6), and increased cortical GFAP 177. Thus, there are two reports that repeated ip injections of high doses of TiO2 reduce brain AChE activity, along with changes in multiple other brain parameters.
In summary, the effects of repeated treatments nanomaterials on brain neurochemistry have been reported, although the evidence is slim. Silver nanoparticles were reported to first reduce but then increase brain DA concentration, while high dose levels of TiO2 were reported to reduce brain AChE activity.
Alterations in behavior, including learning and memory
There are multiple reports that systemic exposure to engineered nanoparticles altered the behavior of rats or mice. Nanoparticles of TiO2 (5–6 nm) given to adult mice by daily nasal administration for 90 days 37 altered behavior in the Morris Water Maze. The later study, however, was criticized for repeated daily intranasal administrations, which may have produced local nasal irritation or worse 172. Silver nanoparticles (20–30 nm) given to mice by iv injections (1 injection/week for 1, 2, or 3 weeks) increased latencies in the Morris Water Maze, as well as altering tests of social and motor behavior 178. TiO2 given to rats (intratracheal, 5d/w, 6 w) in a high dose increased lung weight, reduced brain/weight to body weight ratios, and reduced grip strength. Both low and high doses, increased latencies of somatosensory, auditory and visual evoked potentials. Somatosensory latencies correlated with the concentration of titanium in cortex 179.
Silver nanoparticles (20–30 nm) given to mice by iv injections (1 injection/w for 1, 2, or 3 weeks) increased latencies in the Morris Water Maze, as well as altering tests of social and motor behavior 178. Adult mice treated with 36 nm silver nanoparticles (10, 25, 50 mg/kg/d for 7 d) did not show any deficit in Morris Water Maze or in neuro-progenitor cells 133. Following much higher acute doses, adult mice given 0, 100, 500 or 1000 mg/kg in a single ip injection showed changes in gene expression profiles in caudate, frontal cortex indicative of oxidative stress 168. Adult mice given 10 nm AgNP orally for 14 d (0.2 mg/kg/d) showed ultrastructural alterations of brain synapses, blurred synaptic structure, enhanced density of synaptic vessels, disturbed synaptic membranes, synaptic degeneration, myelin and membrane-like fragments, neurodegenerative processes, decreased levels of synapsin I, synaptophysin and PSD-95 protein 180. Effects were more pronounced in hippocampus than cortex. Cerebellar glial fibrillary acidic protein (GFAP), a marker of reactive gliosis in response to neuronal damage, was increased relative to controls following intranasal instillation of up to 1 mg/kg/d for 14 weeks 180. The effects were attenuated by vitamin E. Thus, repeated exposure to silver nanoparticles was able to alter behavior in the Morris Water Maze and other behavioral paradigms and also show changes in silver content of the brain, changes in brain structure and biochemistry. The dose ranges applied in these studies varied widely, as did the routes of exposure, making it difficult to compare across studies. The report of silver nanoparticle effects being attenuated by vitamin E suggests the involvement of oxidative damage in the pathogenesis.
Zinc and zinc oxide nanomaterials are among the more soluble of the metal/metal oxide nanomaterials, and therefore more likely to be transformed to soluble zinc ions after in vivo treatment. Rats given 25 mg/kg/d of 20–30 nm ZnO by ip injection for 10 days showed altered concentrations of the trace elements Fe and Ca, but not Zn, in the brain, and minimal changes in behavior on the plus maze 181. In another study, very different dose levels of ZnO (68.96 nm) were given to mice for 5 days, including “environmental” dose levels of 5.625 × 10−5 mg/kg (ip), and “toxic” dose levels of 300 mg/kg (ip) 182. Mice in the “toxic” dose group lost body weight and showed an increased brain-to-body weight ratio. No changes were observed in the elevated plus maze or the forced swim tests, but in an open field test ZnO exposed animals in both dose groups spent significantly less time in the center segments of the open field. Zinc concentrations were elevated above controls in both groups, even the low “environmental” dose level. Note that the concentrations of Zn reported by Amara et al. 181 in rat brain tissue of control animals was about 258 μg/g tissue, where that reported by de Souza et al. 182 in mice was only about 15 μg/g tissue raising concerns about the validity of the analytical chemistry.
Studies are available in which rats were given either Mn or MnO particles by inhalation, intranasal or intratracheal instillation. Intranasal instillation or inhalation of 3–8 nm Mn particles led to increased concentrations of Mn in the olfactory bulb, striatum, frontal cortex and cerebellum 93. Following nose- only inhalation, particles of 1.3 μm MnO were taken up into the olfactory bulb, but larger particles (18 μm) were not 94. Repeated intratracheal instillation of MnO (23–30 nm) to adult rats reduced body weight, increased brain Mn concentration, and impaired locomotion 183.
Lead oxide nanoparticles (2 or 4 mg/kg) given via intra- tracheal instillation to adult rats (5 d/w, 3 or 6 w), increased lead concentration in the brain and other organs, while altering neurophysiological function of peripheral nerves and the ascending somatosensory system 131. Silica particles (115 nm) given to adult mice by intranasal instillation (8 mg/kg/d for 81 d) impaired performance on Morris Water Maze and other behavioral tasks, and also caused nanoparticle deposition in prefrontal cortex and hippocampus, neurodegeneration and increased microglia as a sign of inflammation 173. Copper oxide nanoparticles given to adult rats by daily ip injections (0.5 mg/kg/d) impaired performance in the Morris Water Maze and altered electrophysiological measurements including the slope of excitatory post-synaptic potentials (EPSP) in long term potentiation (LTP) testing, this was associated with reduced NR2A expression and impaired pre- and post-synaptic glutamate neurotransmission 184.
Autonomic function
A 5-hour daily inhalation exposure for 7 days to MWCNT-7 (5 mg/m3) increased heart rate variability of rats, indicating a change in sympathetic/parasympathetic balance 185. Heart rate variability has been a marker for the effects of exposure to airborne particulate matter on the autonomic control of cardiovascular function, and a proposed causal event for increased risk of cardiovascular death from exposure to ambient air pollution. The proposed mechanism for particulate matter involves activation of irritant receptors on pulmonary C-fiber sensory afferent neurons, activation of medullary cardiovascular regulatory centers, and autonomic efferent neuronal activity modulating heart rate.
Neurodegeneration/neuropathology
Neuropathology has been reported in the brains of rodents treated systemically with engineered nanomaterials. Rats were treated with citrate-capped 10 nm AgNP ip at a dose of 0.2 mg/kg/d for 14 d 186. Ultrastructural evaluation of brain tissue revealed alterations of brain synapses, blurred synaptic structure, enhanced density of synaptic vesicles, disturbed synaptic membranes, synaptic degeneration, presence of myelin and membrane-like fragments, and neuro-degenerative processes. In addition, there were decreased levels of synapsin I, synaptophysin and PSD-95 protein. The effects of AgNP treatment were more pronounced in hippocampus than cortex. Following long term (90 d) intra-nasal administration of TiO2, studies that were criticized as noted above 172, mice showed reduced body and brain weight, hippocampal neuropathology, and other alterations including deficits in Morris Water Maze, as described previously 37. Also following long-term (81 d) intranasal deposition, this time with SiO2 (115nm, FITC-tagged), the prefrontal cortex and hippocampus of mice showed nanoparticle deposition, neurodegeneration, and increased microglia indicative of neuroinflammation 173.
Developmental Neurotoxicity in vivo
Developmental neurotoxicity is an important consideration and has been studied in several in vivo experiments in which rats or mice were treated pre- or postnatally after which the effects were studied in resultant offspring. The neurodevelopmental effects of TiO2 have been reported in multiple studies 187. Pregnant rats were given oral doses of 100 mg/kg/d from GD2-GD21 188. The offspring had increased titanium concentrations, reduced cell proliferation in the hippocampus, and impaired performance on Morris Water Maze and Passive Avoidance tasks at postnatal day 60. Mice were given much lower doses of 6.5 nm TiO2, either 1, 2, or 3 mg/kg/d, but over a time period encompassing both prenatal and postnatal lactational periods (GD0 – PND21) 187. After PND 21, the offspring mice showed a thinning of cerebral and cerebellar cortex, decrease in neuron density in cerebrum, damage in hippocampal pyramidal cells, and a decrease in learning and memory. These deficits were related to alterations in the expression of Rho protein family, which are involved in several key neurodevelopmental processes 187.
In a study evaluating potential developmental neurotoxicity of (20–50 nm) AgNP, pregnant rats were given ip injections of uncoated AgNP, PVP-coated AgNP silver nitrate or sodium nitrate every other day from GD10-GD18 189. Silver NP dose levels were 20 mg/kg. Escape latency relative to the other groups was prolonged over successive days of testing in the offspring treated with PVP-coated AgNP, however the silver content of the hippocampus was much higher in pups receiving uncoated AgNP than PVP-coated AgNP or the other groups. The authors interpreted this result as showing that PVP-coating stabilized the nanoparticles, allowing fewer toxic silver ions to be released into brain tissue than from the uncoated AgNP. Neonatal rats were treated with intranasal drops of 0.1, 0.2, 0.5, or 1 mg/kg/d AgNP (20–25 nm) daily for 14 weeks 180. Animals in all dose groups lost weight and showed increased glial fibrillary acidic protein (GFAP) in cerebellum 180. In this report, vitamin E (100 mg/kg/d) co-treatment reduced the effects of AgNP.
In an in vivo study paired to an in vitro study, Hawkins et al. 190 studied cobalt chromium (CoCr) nanoparticles, which can disrupt autophagy in the placenta, thereby altering placental function in pregnant mice. The impaired placenta did not adequately provide signaling factors critical for neurodevelopment to the fetus, and neurodevelopment of the fetal brain was impaired. DNA damage was observed in neonatal blood and liver without increased levels of Co or Cr, and neonatal brains had reactive gliosis indicated by increased GFAP mRNA and GFAP immunohistochemistry in the neonatal hippocampus. Therefore, exposure to CoCr nanoparticles during pregnancy altered fetal neurodevelopment without penetrating the placenta or directly contacting the fetus or the fetal brain.
Maternal carbon black inhalation (45 m/d to 0, 4.6 or 37 mg/m3 GD 4–18) increased perivascular GFAP expression in cerebral cortex and hippocampus, enlarged lysosomes in brain perivascular macrophages, and decreased parvalbumin positive interneurons in offspring, with deficits observed as long as 120 days post exposure. Behavior in an open field was altered in a dose-dependent manner at 90 days of age 191. Carbon black was given by bilateral intranasal instillation to pregnant mice GD5–9 and gene expression by microarray was tested in the frontal cortex of offspring 174. Differential gene expression at 6 and 12 weeks of age showed 1353 total genes up or down regulated. Effected gene ontology families were epithelial cell development, cytokine activity, transforming growth factor beta receptor signaling, hemostasis (6 weeks), and cysteine-like endopeptidase inhibition involved in apoptosis, and muscle organ development (12 weeks) 174.
Pregnant mice were exposed to alumina (13 or 50 nm, or 10 μm (“bulk”); 50 mg/kg) by nasal drip three times/day throughout pregnancy 192. The body weight of pups at birth was reduced by both nanoparticle treatments but not by the bulk material. All three conditions raised the AL content in pup brains, with the smaller nanoparticle causing the greatest increase and the bulk material the least. Developmental physical markers (ear, teeth and eye appearance/opening) were delayed by the nanoparticles. Nanoparticles also increased the distance traveled, and reduced the time spent in the central region of an open field, which the authors interpreted as anxiety-like behavior 192.
Population Level
Ultrafine Air Pollution Particles
To date, there have been few studies of humans exposed to engineered nanomaterials, and all have either related to measuring exposure or potential health effects other than neurotoxicity. There have been no systematic studies related to the potential effects of inadvertent exposure to ENM on the human nervous system. There are clinical applications of nano-sized superparamagnetic iron oxide particles that can be used as MR contrast agents, such as Ferumoxtran 193 but clinical studies 194 as well as toxicological risk assessment 195 did not reveal any elevated risk for neurotoxicity. However, these ENM are specifically designed to support the diagnosis and treatment of severe diseases, are applied under controlled conditions, and their side-effects might be tolerated due to their clinical benefits 194.
There is, however, a growing body of evidence related to potential neurotoxicity of inhalation of ambient air particle matter (PM) of the same size range as engineered nanoparticles 50,196. There is concern particularly that, in addition to the cardiovascular and pulmonary systems, the nervous system may be a susceptible target of exposure to PM, especially to particles less than 2.5 µm in size (PM2.5). Epidemiological studies now correlate exposure to air pollution, including PM, to increased rates of stroke or neurodegenerative diseases including Alzheimer’s disease, and dementia or cognitive impairments that together comprise an Alzheimer’s-like phenotype 118. In a review article Jayaraj et al. 38 identified 30 epidemiological studies investigating a measure of air pollution compared to the risk of an “Alzheimer’s Disease (AD)-like” phenotype, which included dementia, a diagnosis of AD, cognitive dysfunction, neuroimaging changes, or a diagnosis of mild cognitive impairment. Of those 30 studies, 25 showed a statistically positive relationship between air pollution and an AD-like phenotype. For studies specifically looking at PM2.5 as the measure of air pollution exposure, there were 12 positive and 1 negative studies. Similarly, there is also growing concern that air pollution particles may be related to neurodevelopmental deficits, such as autism 197. The US Environmental Protection Agency recently published a final revision after review of the Integrated Science Assessment of Particulate Matter (EPA/600/R-19/188, December 2019, www.epa.gov/isa). This is an extensive review and evaluation of the available scientific literature that is required periodically by the U.S. Clean Air Act. The current document is almost 2000 pages long. In that document the EPA concluded that the linkage between long-term exposure to PM2.5 and nervous system effects was likely to be causal. Short term exposure to PM2.5, or short or long-term exposure to PM10–2.5 and ultrafine particulate matter was considered to be suggestive, but the current evidence was not sufficient to infer causality. Since ultrafine particles (< 100 nm) and PM2.5 share a common size range with engineered nanoparticles, may be taken up into the brain through similar pathways, and may serve as a source of ROS generation as do engineered nanoparticles, evidence from exposure to ultrafine particles or PM2.5 adds to the concern that there may be neurotoxic risks for exposure to engineered nanoparticles, particularly by the inhalation route. To date, the population exposure to air pollution particles far exceeds that of engineered nanoparticles, but nevertheless there is concern that ENM exposures may add to the burden of PM exposure or, for some individuals, become a sufficient source of exposure by themselves to cause adverse effects.
General conclusions regarding potential neurotoxicity of engineered nanomaterials
As a general conclusion from this and other reviews 10,130,198,199 there is scientific evidence that ENM can cause neurotoxic effects. By using general and related concepts in toxicology to structure the existing knowledge more systematically, namely the aggregate exposure pathway (AEP) and adverse outcome pathway (AOP), we showed that relevant exposure to ENM exists, internal exposure of the nervous system to ENM is possible, general toxic mechanisms (e.g. oxidative stress) or neuron-specific mechanisms (e.g. synaptic degeneration) can cause perturbations of neurotransmission, and finally the possible impairment of neuroplasticity and the risk of subsequent neurodegeneration. However, we also identified knowledge gaps that are described in the subsequent statements:
In comparing the large number and variety of engineered nanomaterials being developed and incorporated into products with the relatively small number and narrow range of materials that have been studied for neurotoxicity, there is a large gap, with many currently used materials having little or no published data regarding environmental health and safety. In particular, there is a dearth of data for most materials regarding potential neurotoxicity.
The gap between the number of nanomaterials being produced and the capacity to test them argues for development of efficient higher throughput screening approaches. There is an active effort to develop screening tests for chemical-induced neurodevelopmental disorders that is conceptually linked to an AOP designation through selecting outcomes for screening that reflect Key Events of neurodevelopment. This approach may also have merit for developing screening tests for potential developmental toxicity of nanomaterials. The primary difference being the need to consider dosimetric issues of nanomaterials, which may differ from traditional chemicals.
Given a sufficiently high dose to adult animals, nanoparticles can enter the brain in quantities large enough to cause neurotoxic effects, including: neuropathology, neuroinflammation and changes in behavior. The development of screening approaches for nanomaterial neurotoxicity in adults, however, is receiving less attention than neurodevelopment.
Given a sufficiently high dose to pregnant animals, nanoparticles can enter the fetus and the fetal brain to damage neurodevelopment, effecting the brain and behavior of the offspring.
Very little attempt has been made to match expected exposure patterns in terms of dose levels, routes of exposure and exposure durations between expected exposures to the general public and those used in experimental studies. In fact, the dose levels employed in many experimental studies appear to be very high, and the durations of exposure unreasonably long, given anticipated usage patterns of non-occupationally exposed members of the general public. In order to detect effects of infrequent exposures to low dose levels expected for the general public, subtle effects on neurological functioning should be considered.
Very little attempt has been made to establish Key Event Relationships quantitatively between any of the Key Events of either AEP or AOP constructs, which would be necessary to construct quantitative exposure-dose-response relationships of existing materials or of predictive risk estimates of novel materials.
It appears that the rate of uptake and distribution of nanomaterials to the brain is slow by all systemic routes of exposure, with nasal olfactory uptake perhaps being relative higher than others, but still small in mass transport volume. It also appears the clearance of ENM from the brain is also slow, and likely, slower than uptake. This opens the possibility of long-term low-level exposure leading to bioaccumulation of ENM in brain tissue. This may be a particular concern for nanomaterials composed of relatively stable and insoluble materials such as TiO2 or CeO2. The types of studies required to address long-term mass balance and quantifying rates of nanomaterials uptake and clearance from the brain have yet to be performed.
Insufficient research has been conducted in which the physical/chemical properties of engineered nanomaterials are systematically manipulated (such as comparative studies of nanoparticles differing in size, composition or surface properties) and their effects directly compared on the nervous system. This would be required for each core-nanomaterial. Those studies to date indicate greater biodistribution and toxicity for smaller nanoparticles.
Across the AEP and AOP schematic, examples of nanomaterial exposure or nervous system effects can be found at virtually every stage, leading to the impression that neurotoxic effects from exposure to nanomaterials may pose possible risks. However, the examples are scattered across exposures to nanomaterials of different composition, size and surface conditions, and the effects scattered across different cell types, species, exposure routes, dose levels and durations, and outcomes measured. Therefore, there is no example of a single nanomaterial that has been studied across the linkages spanning manufacture, exposure, effects and adverse outcomes.
Some experimenters have measured metal content of whole non-perfused brain tissues using chemical detection methods such as ICP-MS or AA, and concluded that nanoparticles entered the brain, without considering that the technology employed does not discriminate between particle form and dissolved ionic form of the metal (especially a concern for moderately or highly soluble compounds such as silver or zinc), and also considering that the measurement of whole non-perfused brain tissue may contain nanomaterials in the blood or embedded in the vascular endothelium which not have actually crossed the blood-brain barrier and entered the neuronal parenchyma of the central nervous system.
Many studies have demonstrated that treatment with nanomaterials has increased reactive oxygen species and/or caused oxidative damage in brain tissues and concluded or implied that the generation of ROS is the primary mechanism leading to neurotoxicity. However, it is also possible that the generation of ROS is a secondary response to some other action of the nanoparticles. For example, intracellular nanoparticles are trafficked to the lysosomes 136, where generation of ROS is a cellular defensive reaction designed to breakdown foreign materials or pathogens. Similarly, microglial responses may also generate ROS as a component of reactive inflammation. In such cases the generation of ROS is a secondary reaction to the ENM or the damage caused by the ENM, and unless neuroinflammation is the key toxic mechanism, may not be the primary mechanism initiating neurotoxicity.
Two primary theories of nanotoxicity explain the molecular generation of oxygen or hydroxy free radicals. One involves semiconductor metal oxides of an optimal bandgap participating in the cellular redox cycling and generating free radicals that disrupt cellular metabolism. The other theory has similar elements involving elevation of valence band electrons to the conduction band following absorption of a photon of light and subsequent excitation of photoreactive materials such as TiO2 146 leading to generation of oxygen and hydroxy free radicals that then precipitate oxidation or hydroxylation reactions with nearby biomolecules. Of course, phototoxic mechanisms are relevant for neurotoxicity solely in the retina, the only part of the central nervous system exposed to light 147,148.
Although it might be assumed that a prerequisite for nanomaterials to cause neurotoxicity is the penetration of nanoparticles into the nervous system, two recent lines of evidence argue otherwise. First, it is hypothesized that inhalation of particles into the respiratory tract initiates pulmonary inflammation and the systemic release of proinflammatory chemical markers, which then enter the CNS and initiate an inflammatory response in the brain including persistent activation on microglia that, if prolonged, has adverse neurotoxic implications 38. Second, an analogous process has been proposed for developmental exposures in which the nanoparticles initiate inflammatory reactions in the placenta, preventing the placenta from providing adequate support for rapidly developing fetal nervous system with persistent adverse consequences for the developing offspring 190.
Despite of the uncertainties addressed in these thirteen statements, the economic and societal benefits that can be achieved by the safe use of nanotechnology are enormous, including the combination of nanocarriers for drug-delivery with stem cell therapy for the treatment of neurodegenerative diseases 200. Nevertheless, the whole repertoire of experimental and epidemiological research in neuroscience/ toxicology needs to be exploited to help cure and not harm the brain.
Acknowledgments
Funding Sources
none.
ABBREVIATIONS
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin/ 5-hydroxytryptamine
- AA
atomic absorption
- AChE
acetylcholinesterase
- AD
Alzheimer’s Disease
- AEP
aggregate exposure pathway
- ALS
amyotrophic lateral sclerosis
- ANSES
French Agency for Food, Environmental and Occupational Health & Safety
- AOP
averse outcome pathway
- BALF
bronchoalveolar lavage fluid
- BBB
blood brain barrier
- BW
bodyweight
- CB
carbon black
- CDNP
combustion-derived nanoparticles
- CFD
computational fluid dynamics
- CNS
central nervous system
- DA
dopamine
- DNA
deoxyribonucleic acid
- DNT
developmental neurotoxicity
- DOPAC
3,4-dihydroxyphenylacetic acid
- ENM
engineered nanomaterial
- FDA
Food and Drug Administration
- FITC
fluorescein isothiocyanate
- GD
gestation day
- GFAP
glial fibrillary acidic protein
- GI
gastrointestinal tract
- HVA
homovanillic acid
- ICP-MS
inductively coupled plasma mass spectrometry
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- KE
key event
- KER
key event relationship
- LRT
lower respiratory tract
- MIE
molecular initiating event
- MR
Magnetic resonance
- MS
multiple sclerosis
- MWCNT
multiwalled carbon nanotube
- NAM
non-animal alternative methods
- NE
norepinephrine
- NM
nanomaterial
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NOS
nitric oxide synthases
- NP
nanoparticles
- OECD
Organization for Economic Co-operation and Development
- ORN
olfactory receptor neuron
- PD
Parkinson’s disease
- PEG
polyethylene glycol
- PM
particle matter
- PND
postnatal day
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- SWCNT
single-walled carbon nanotube
- TDI
Tolerable Daily Intake
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- TNF-α
tumor necrosis factor alpha
- TRP
transient receptor potential
- UFP
ultrafine particles
- URT
upper respiratory tract
- UV
ultraviolet
Biography

William K. Boyes, PhD, U.S. Environmental Protection Agency
Dr. Boyes is a neurotoxicologist with over 39 years of experience at the U.S. Environmental Protection Agency. Dr. Boyes has a PhD in Environmental Health from the University of Cincinnati and postdoctoral training in neurotoxicology. He is the author/coauthor of numerous publications including peer reviewed manuscripts, book chapters, neurotoxicity testing and risk assessment guidelines, national research strategies for hazardous air pollutants and nanomaterials, and reports to Congress on topics such as fuel additives and biofuels. His current research focuses on potential effects of environmental exposure to engineered nanomaterials.

Christoph van Thriel, PhD, Leibniz Research Centre for Working Environment and Human Factors at the TU Dortmund
Dr. van Thriel is a neurotoxicologist with over 20 years of experience at the Leibniz Research Centre for Working Environment and Human Factors. Dr. van Thriel received the venia legendi in biological psychology from the Ruhr-University Bochum. His research is related to the interaction of chemical in the working environment with the central and peripheral nervous system investigating the adverse health effects neurotoxicity and sensory irritation. He is the author/coauthor of numerous publications including peer reviewed manuscripts, book chapters, and scientific justifications of various Occupational Exposure Limits.
Footnotes
Publisher's Disclaimer: Disclaimer:
The information in this document was funded wholly (or in part) by the U.S. Environmental Protection Agency. It was subjected to review by the Center for Public Health and Environmental Assessment and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- (1).Re F; Gregori M; Masserini M. Nanotechnology for Neurodegenerative Disorders. Maturitas 2012, 73 (1), 45–51. [DOI] [PubMed] [Google Scholar]
- (2).Hrkach J; Von Hoff D; Ali MM; Andrianova E; Auer J; Campbell T; De Witt D; Figa M; Figueiredo M; Horhota A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med 2012, 4 (128), 128ra39–128ra39. [DOI] [PubMed] [Google Scholar]
- (3).Mao Z; Zhou X; Gao C. Influence of Structure and Properties of Colloidal Biomaterials on Cellular Uptake and Cell Functions. Biomaterials Science. Royal Society of Chemistry July 30, 2013, pp 896–911. [DOI] [PubMed]
- (4).Nel A; Xia T; Madler L; Li N. Toxic Potential of Materials at the Nanolevel. Science (80-. ). 2006, 311 (5761), 622–627. [DOI] [PubMed] [Google Scholar]
- (5).Konduru NV; Molina RM; Swami A; Damiani F; Pyrgiotakis G; Lin P; Andreozzi P; Donaghey TC; Demokritou P; Krol S.; et al. Protein Corona: Implications for Nanoparticle Interactions with Pulmonary Cells. Part. Fibre Toxicol. 2017, 14 (1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lee JH; Kim YS; Song KS; Ryu HR; Sung JH; Park JD; Park HM; Song NW; Shin BS; Marshak D.; et al. Biopersistence of Silver Nanoparticles in Tissues from Sprague-Dawley Rats. Part. Fibre Toxicol. 2013, 10 (1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Yokel RA Nanoparticle Brain Delivery: A Guide to Verification Methods. Nanomedicine 2020, 15 (4), 409–432. [DOI] [PubMed] [Google Scholar]
- (8).Song KS; Sung JH; Ji JH; Lee JH; Lee JS; Ryu HR; Lee JK; Chung YH; Park HM; Shin BS; et al. Recovery from Silver-Nanoparticle-Exposure-Induced Lung Inflammation and Lung Function Changes in Sprague Dawley Rats. Nanotoxicology 2013, 7 (2), 169–180. [DOI] [PubMed] [Google Scholar]
- (9).Gebel T; Foth H; Damm G; Freyberger A; Kramer PJ; Lilienblum W; Rohl C; Schupp T; Weiss C; Wollin KM; et al. Manufactured Nanomaterials: Categorization and Approaches to Hazard Assessment. Arch Toxicol 2014, 88 (12), 2191–2211. [DOI] [PubMed] [Google Scholar]
- (10).Bencsik A; Lestaevel P; Guseva Canu I. Nano- and Neurotoxicology: An Emerging Discipline. Prog. Neurobiol 2018, 160, 45–63. [DOI] [PubMed] [Google Scholar]
- (11).Garcia GJM; Schroeter JD; Kimbell JS Olfactory Deposition of Inhaled Nanoparticles in Humans. Inhal. Toxicol 2015, 27 (8), 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Swift DL; Montassier N; Hopke PK; Karpen-Hayes K; Cheng YS; Yin Fong Su; Hsu Chi Yeh; Strong JC Inspiratory Deposition of Ultrafine Particles in Human Nasal Replicate Cast. J. Aerosol Sci 1992, 23 (1), 65–72. [Google Scholar]
- (13).Boyes WK; Thornton BLM; Al-Abed SR; Andersen CP; Bouchard DC; Burgess RM; Hubal EAC; Ho KT; Hughes MF; Kitchin K.; et al. A Comprehensive Framework for Evaluating the Environmental Health and Safety Implications of Engineered Nanomaterials. Crit. Rev. Toxicol 2017, 1–44. [DOI] [PubMed]
- (14).Schulte PA; Leso V; Niang M; Iavicoli I. Current State of Knowledge on the Health Effects of Engineered Nanomaterials in Workers: A Systematic Review of Human Studies and Epidemiological Investigations. Scand. J. Work. Environ. Health 2019, 45 (3), 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Oberdorster G. Safety Assessment for Nanotechnology and Nanomedicine: Concepts of Nanotoxicology. J Intern Med 2010, 267 (1), 89–105. [DOI] [PubMed] [Google Scholar]
- (16).Sohal IS; O’Fallon KS; Gaines P; Demokritou P; Bello D. Ingested Engineered Nanomaterials: State of Science in Nanotoxicity Testing and Future Research Needs. Part. Fibre Toxicol 2018, 15 (1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Larese Filon F; Mauro M; Adami G; Bovenzi M; Crosera M. Nanoparticles Skin Absorption: New Aspects for a Safety Profile Evaluation. Regul. Toxicol. Pharmacol 2015, 72 (2), 310–322. [DOI] [PubMed] [Google Scholar]
- (18).Bourganis V; Kammona O; Alexopoulos A; Kiparissides C. Recent Advances in Carrier Mediated Nose-to-Brain Delivery of Pharmaceutics. Eur. J. Pharm. Biopharm 2018, 128, 337–362. [DOI] [PubMed] [Google Scholar]
- (19).Musumeci T; Serapide MF; Pellitteri R; Dalpiaz A; Ferraro L; Dal Magro R; Bonaccorso A; Carbone C; Veiga F; Sancini G.; et al. Oxcarbazepine Free or Loaded PLGA Nanoparticles as Effective Intranasal Approach to Control Epileptic Seizures in Rodents. Eur. J. Pharm. Biopharm 2018, 133, 309–320. [DOI] [PubMed] [Google Scholar]
- (20).Bonaccorso A; Musumeci T; Serapide MF; Pellitteri R; Uchegbu IF; Puglisi G. Nose to Brain Delivery in Rats: Effect of Surface Charge of Rhodamine B Labeled Nanocarriers on Brain Subregion Localization. Colloids Surfaces B Biointerfaces 2017, 154, 297–306. [DOI] [PubMed] [Google Scholar]
- (21).Musumeci T; Pellitteri R; Spatuzza M; Puglisi G. Nose-to-Brain Delivery: Evaluation of Polymeric Nanoparticles on Olfactory Ensheathing Cells Uptake. J. Pharm. Sci 2014, 103 (2), 628–635. [DOI] [PubMed] [Google Scholar]
- (22).Ziemińska E; Stafiej A; Struzyńska L. The Role of the Glutamatergic NMDA Receptor in Nanosilver-Evoked Neurotoxicity in Primary Cultures of Cerebellar Granule Cells. Toxicology 2014, 315 (1), 38–48. [DOI] [PubMed] [Google Scholar]
- (23).Liu Z; Ren G; Zhang T; Yang Z. Action Potential Changes Associated with the Inhibitory Effects on Voltage-Gated Sodium Current of Hippocampal CA1 Neurons by Silver Nanoparticles. Toxicology 2009, 264 (3), 179–184. [DOI] [PubMed] [Google Scholar]
- (24).Long TC; Tajuba J; Sama P; Saleh N; Swartz C; Parker J; Hester S; Lowry GV; Veronesi B. Nanosize Titanium Dioxide Stimulates Reactive Oxygen Species in Brain Microglia and Damages Neurons in Vitro. Environ. Health Perspect 2007, 115 (11), 1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Long TC; Saleh N; Tilton RD; Lowry GV; Veronesi B. Titanium Dioxide (P25) Produces Reactive Oxygen Species in Immortalized Brain Microglia (BV2): Implications for Nanoparticle Neurotoxicity. Environ. Sci. Technol 2006, 40 (14), 4346–4352. [DOI] [PubMed] [Google Scholar]
- (26).He Q; Zhou X; Liu Y; Gou W; Cui J; Li Z; Wu Y; Zuo D. Titanium Dioxide Nanoparticles Induce Mouse Hippocampal Neuron Apoptosis via Oxidative Stress- and Calcium Imbalance-Mediated Endoplasmic Reticulum Stress. Environ. Toxicol. Pharmacol 2018, 63, 6–15. [DOI] [PubMed] [Google Scholar]
- (27).Afeseh Ngwa H; Kanthasamy A; Gu Y; Fang N; Anantharam V; Kanthasamy AG Manganese Nanoparticle Activates Mitochondrial Dependent Apoptotic Signaling and Autophagy in Dopaminergic Neuronal Cells. Toxicol. Appl. Pharmacol 2011, 256 (3), 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhang Y; Ali SF; Dervishi E; Xu Y; Li Z; Casciano D; Biris AS Cytotoxicity Effects of Graphene and Single-Wall Carbon Nanotubes in Neural Phaeochromocytoma-Derived Pc12 Cells. ACS Nano 2010, 4 (6), 3181–3186. [DOI] [PubMed] [Google Scholar]
- (29).Zhang Y; Xu Y; Li Z; Chen T; Lantz SM; Howard PC; Paule MG; Slikker W; Watanabe F; Mustafa T.; et al. Mechanistic Toxicity Evaluation of Uncoated and PEGylated Single-Walled Carbon Nanotubes in Neuronal PC12 Cells. ACS Nano 2011, 5 (9), 7020–7033. [DOI] [PubMed] [Google Scholar]
- (30).Gramowski A; Flossdorf J; Bhattacharya K; Jonas L; Lantow M; Rahman Q; Schiffmann D; Weiss DG; Dopp E. Nanoparticles Induce Changes of the Electrical Activity of Neuronal Networks on Microelectrode Array Neurochips. Environ. Health Perspect 2010, 118 (10), 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hadrup N; Loeschner K; Mortensen A; Sharma AK; Qvortrup K; Larsen EH; Lam HR The Similar Neurotoxic Effects of Nanoparticulate and Ionic Silver in Vivo and in Vitro. Neurotoxicology 2012, 33 (3), 416–423. [DOI] [PubMed] [Google Scholar]
- (32).Xu L; Dan M; Shao A; Cheng X; Zhang C; Ayokel RA; Takemura T; Hanagata N; Niwa M; Watanabe D. Silver Nanoparticles Induce Tight Junction Disruption and Astrocyte Neurotoxicity in a Rat Blood-Brain Barrier Primary Triple Coculture Model. Int. J. Nanomedicine 2015, 10, 6105–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Feng X; Chen L; Guo W; Zhang Y; Lai X; Shao L; Li Y. Graphene Oxide Induces P62/SQSTM-Dependent Apoptosis through the Impairment of Autophagic Flux and Lysosomal Dysfunction in PC12 Cells. Acta Biomater 2018, 81, 278–292. [DOI] [PubMed] [Google Scholar]
- (34).Liu Y; Li J; Xu K; Gu J; Huang L; Zhang L; Liu N; Kong J; Xing M; Zhang L.; et al. Characterization of Superparamagnetic Iron Oxide Nanoparticle-Induced Apoptosis in PC12 Cells and Mouse Hippocampus and Striatum. Toxicol. Lett 2018, 292, 151–161. [DOI] [PubMed] [Google Scholar]
- (35).Coccini T; Grandi S; Lonati D; Locatelli C; De Simone U. Comparative Cellular Toxicity of Titanium Dioxide Nanoparticles on Human Astrocyte and Neuronal Cells after Acute and Prolonged Exposure. Neurotoxicology 2015, 48, 77–89. [DOI] [PubMed] [Google Scholar]
- (36).Strickland JD; LeFew WR; Crooks J; Hall D; Ortenzio JN; Dreher K; Shafer TJ In Vitro Screening of Silver Nanoparticles and Ionic Silver Using Neural Networks Yields Differential Effects on Spontaneous Activity and Pharmacological Responses. Toxicology 2016, 355–356, 1–8. [DOI] [PubMed] [Google Scholar]
- (37).Ze Y; Hu R; Wang X; Sang X; Ze X; Li B; Su J; Wang Y; Guan N; Zhao X.; et al. Neurotoxicity and Gene-Expressed Profile in Brain-Injured Mice Caused by Exposure to Titanium Dioxide Nanoparticles. J. Biomed. Mater. Res. - Part A 2014, 102 (2), 470–478. [DOI] [PubMed] [Google Scholar]
- (38).Jayaraj RL; Rodriguez EA; Wang Y; Block ML Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Heal. Reports 2017, 4 (2), 166–179. [DOI] [PubMed] [Google Scholar]
- (39).Silva GA Neuroscience Nanotechnology: Progress, Opportunities and Challenges. Nature Reviews Neuroscience Nature Publishing Group; January 1, 2006, pp 65–74. [DOI] [PubMed] [Google Scholar]
- (40).Dahan M; Lévi S; Luccardini C; Rostaing P; Riveau B; Triller A. Diffusion Dynamics of Glycine Receptors Revealed by Single-Quantum Dot Tracking. Science (80-. ). 2003, 302 (5644), 442–445. [DOI] [PubMed] [Google Scholar]
- (41).Dugan LL; Lovett EG; Quick KL; Lotharius J; Lin TT; O’Malley KL Fullerene-Based Antioxidants and Neurodegenerative Disorders. Park. Relat. Disord 2001, 7 (3), 243–246. [DOI] [PubMed] [Google Scholar]
- (42).Cox A; Vinciguerra D; Re F; Magro RD; Mura S; Masserini M; Couvreur P; Nicolas J. Protein-Functionalized Nanoparticles Derived from End-Functional Polymers and Polymer Prodrugs for Crossing the Blood-Brain Barrier. Eur. J. Pharm. Biopharm 2019, 142, 70–82. [DOI] [PubMed] [Google Scholar]
- (43).Reynolds JL; Mahato RI Nanomedicines for the Treatment of CNS Diseases. J. Neuroimmune Pharmacol 2017, 12 (1), 1–5. [DOI] [PubMed] [Google Scholar]
- (44).Karussis D; Karageorgiou C; Vaknin-Dembinsky A; Gowda-Kurkalli B; Gomori JM; Kassis I; Bulte JWM; Petrou P; Ben-Hur T; Abramsky O.; et al. Safety and Immunological Effects of Mesenchymal Stem Cell Transplantation in Patients with Multiple Sclerosis and Amyotrophic Lateral Sclerosis. Arch. Neurol 2010, 67 (10), 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Coelho T; Adams D; Silva A; Lozeron P; Hawkins PN; Mant T; Perez J; Chiesa J; Warrington S; Tranter E.; et al. Safety and Efficacy of RNAi Therapy for Transthyretin Amyloidosis. N. Engl. J. Med 2013, 369 (9), 819–829. [DOI] [PubMed] [Google Scholar]
- (46).Suhr OB; Coelho T; Buades J; Pouget J; Conceicao I; Berk J; Schmidt H; Waddington-Cruz M; Campistol JM; Bettencourt BR; et al. Efficacy and Safety of Patisiran for Familial Amyloidotic Polyneuropathy: A Phase II Multi-Dose Study. Orphanet J. Rare Dis 2015, 10 (1), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Bourganis V; Kammona O; Alexopoulos A; Kiparissides C. Recent Advances in Carrier Mediated Nose-to-Brain Delivery of Pharmaceutics. Eur. J. Pharm. Biopharm 2018. [DOI] [PubMed]
- (48).Kim K-T; Lee HS; Lee J-J; Park EK; Lee B-S; Lee J-Y; Bae J-S Nanodelivery Systems for Overcoming Limited Transportation of Therapeutic Molecules through the Blood–Brain Barrier. Future Med. Chem 2018, 10 (22), 2659–2674. [DOI] [PubMed] [Google Scholar]
- (49).Trickler WJ; Lantz SM; Murdock RC; Schrand AM; Robinson BL; Newport GD; Schlager JJ; Oldenburg SJ; Paule MG; Slikker W.; et al. Silver Nanoparticle Induced Blood-Brain Barrier Inflammation and Increased Permeability in Primary Rat Brain Microvessel Endothelial Cells. Toxicol. Sci 2010, 118 (1), 160–170. [DOI] [PubMed] [Google Scholar]
- (50).Heusinkveld HJ; Wahle T; Campbell A; Westerink RH; Tran L; Johnston H; Stone V; Cassee FR; Schins RP Neurodegenerative and Neurological Disorders by Small Inhaled Particles. Neurotoxicology 2016, 56, 94–106. [DOI] [PubMed] [Google Scholar]
- (51).Hardy A; Benford D; Halldorsson T; Jeger MJ; Knutsen HK; More S; Naegeli H; Noteborn H; Ockleford C; Ricci A.; et al. Guidance on Risk Assessment of the Application of Nanoscience and Nanotechnologies in the Food and Feed Chain: Part 1, Human and Animal Health. EFSA J 2018, 16 (7), 2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).van Thriel C; Westerink RH; Beste C; Bale AS; Lein PJ; Leist M. Translating Neurobehavioural Endpoints of Developmental Neurotoxicity Tests into in Vitro Assays and Readouts. Neurotoxicology 2012, 33 (4), 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Nel AE; Mädler L; Velegol D; Xia T; Hoek EMV; Somasundaran P; Klaessig F; Castranova V; Thompson M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat. Mater 2009, 8 (7), 543–557. [DOI] [PubMed] [Google Scholar]
- (54).Ankley GT; Bennett RS; Erickson RJ; Hoff DJ; Hornung MW; Johnson RD; Mount DR; Nichols JW; Russom CL; Schmieder PK; et al. Adverse Outcome Pathways: A Conceptual Framework to Support Ecotoxicology Research and Risk Assessment. Env. Toxicol Chem 2010, 29 (3), 730–741. [DOI] [PubMed] [Google Scholar]
- (55).Villeneuve DL; Crump D; Garcia-Reyero N; Hecker M; Hutchinson TH; LaLone CA; Landesmann B; Lettieri T; Munn S; Nepelska M.; et al. Adverse Outcome Pathway (AOP) Development I: Strategies and Principles. Toxicol. Sci 2014, 142 (2), 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Villeneuve DL; Crump D; Garcia-Reyero N; Hecker M; Hutchinson TH; LaLone CA; Landesmann B; Lettieri T; Munn S; Nepelska M.; et al. Adverse Outcome Pathway Development II: Best Practices. Toxicol. Sci 2014, 142 (2), 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Teeguarden JG; Tan YM; Edwards SW; Leonard JA; Anderson KA; Corley RA; Kile ML; Simonich SM; Stone D; Tanguay RL; et al. Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework. Environ. Sci. Technol 2016, 50 (9), 4579–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Vance ME; Kuiken T; Vejerano EP; McGinnis SP; Hochella MF; Hull DR Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory. Beilstein J. Nanotechnol 2015, 6 (1), 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Foss Hansen S; Heggelund LR; Revilla Besora P; Mackevica A; Boldrin A; Baun A. Nanoproducts - What Is Actually Available to European Consumers? Environ. Sci. Nano 2016, 3 (1), 169–180. [Google Scholar]
- (60).Cassee FR; Van Balen EC; Singh C; Green D; Muijser H; Weinstein J; Dreher K. Exposure, Health and Ecological Effects Review of Engineered Nanoscale Cerium and Cerium Oxide Associated with Its Use as a Fuel Additive. Critical Reviews in Toxicology. March 2011, pp 213–229. [DOI] [PubMed]
- (61).Zhang J; Nazarenko Y; Zhang L; Calderon L; Lee KB; Garfunkel E; Schwander S; Tetley TD; Chung KF; Porter AE; et al. Impacts of a Nanosized Ceria Additive on Diesel Engine Emissions of Particulate and Gaseous Pollutants. Environ. Sci. Technol 2013, 47 (22), 13077–13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Gantt B; Hoque S; Willis RD; Fahey KM; Delgado-Saborit JM; Harrison RM; Erdakos GB; Bhave PV; Zhang KM; Kovalcik K.; et al. Near-Road Modeling and Measurement of Cerium-Containing Particles Generated by Nanoparticle Diesel Fuel Additive Use. Environ. Sci. Technol 2014, 48 (18), 10607–10613. [DOI] [PubMed] [Google Scholar]
- (63).Debia M; Bakhiyi B; Ostiguy C; Verbeek JH; Brouwer DH; Murashov V A Systematic Review of Reported Exposure to Engineered Nanomaterials. Annals of Occupational Hygiene. Oxford University Press; October 1, 2016, pp 916–935. [DOI] [PubMed] [Google Scholar]
- (64).Song Y; Li X; Du X. Exposure to Nanoparticles Is Related to Pleural Effusion, Pulmonary Fibrosis and Granuloma. Eur. Respir. J 2009, 34 (3), 559–567. [DOI] [PubMed] [Google Scholar]
- (65).Monteiro-Riviere NA; Wiench K; Landsiedel R; Schulte S; Inman AO; Riviere JE Safety Evaluation of Sunscreen Formulations Containing Titanium Dioxide and Zinc Oxide Nanoparticles in UVB Sunburned Skin: An In Vitro and in Vivo Study. Toxicol. Sci 2011, 123 (1), 264–280. [DOI] [PubMed] [Google Scholar]
- (66).Rogers KR; Navratilova J; Stefaniak A; Bowers L; Knepp AK; Al-Abed SR; Potter P; Gitipour A; Radwan I; Nelson C.; et al. Characterization of Engineered Nanoparticles in Commercially Available Spray Disinfectant Products Advertised to Contain Colloidal Silver. Sci. Total Environ 2018, 619–620, 1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).European Food Safety (EFSA). EFSA Statement on the Review of the Risks Related to the Exposure to the Food Additive Titanium Dioxide (E 171) Performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J 2019, 17 (6), e05714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Bettini S; Boutet-Robinet E; Cartier C; Coméra C; Gaultier E; Dupuy J; Naud N; Taché S; Grysan P; Reguer S.; et al. Food-Grade TiO 2 Impairs Intestinal and Systemic Immune Homeostasis, Initiates Preneoplastic Lesions and Promotes Aberrant Crypt Development in the Rat Colon. Sci. Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Manigrasso M; Protano C; Astolfi ML; Massimi L; Avino P; Vitali M; Canepari S. Evidences of Copper Nanoparticle Exposure in Indoor Environments: Long-Term Assessment, High-Resolution Field Emission Scanning Electron Microscopy Evaluation, in Silico Respiratory Dosimetry Study and Possible Health Implications. Sci. Total Environ. 2019, 653, 1192–1203. [DOI] [PubMed] [Google Scholar]
- (70).Impellitteri CA; Harmon S; Silva RG; Miller BW; Scheckel KG; Luxton TP; Schupp D; Panguluri S. Transformation of Silver Nanoparticles in Fresh, Aged, and Incinerated Biosolids. Water Res. 2013, 47 (12), 3878–3886. [DOI] [PubMed] [Google Scholar]
- (71).Lombi E; Donner E; Taheri S; Tavakkoli E; Jämting ÅK; McClure S; Naidu R; Miller BW; Scheckel KG; Vasilev K. Transformation of Four Silver/Silver Chloride Nanoparticles during Anaerobic Treatment of Wastewater and Post-Processing of Sewage Sludge. Environ. Pollut 2013, 176, 193–197. [DOI] [PubMed] [Google Scholar]
- (72).Chowdhury I; Duch MC; Mansukhani ND; Hersam MC; Bouchard D. Colloidal Properties and Stability of Graphene Oxide Nanomaterials in the Aquatic Environment. Environ. Sci. Technol 2013, 47 (12), 6288–6296. [DOI] [PubMed] [Google Scholar]
- (73).Hendren CO; Lowry GV; Unrine JM; Wiesner MR A Functional Assay-Based Strategy for Nanomaterial Risk Forecasting. Sci. Total Environ. 2015, 536, 1029–1037. [DOI] [PubMed] [Google Scholar]
- (74).Wasmuth C; Rüdel H; Düring RA; Klawonn T. Assessing the Suitability of the OECD 29 Guidance Document to Investigate the Transformation and Dissolution of Silver Nanoparticles in Aqueous Media. Chemosphere 2016, 144, 2018–2023. [DOI] [PubMed] [Google Scholar]
- (75).Keller AA; McFerran S; Lazareva A; Suh S. Global Life Cycle Releases of Engineered Nanomaterials. J. Nanoparticle Res. 2013, 15 (6), 1692. [Google Scholar]
- (76).Oberdörster G; Oberdörster E; Oberdörster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113 (7), 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Buzea C; Pacheco II; Robbie K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2 (4), MR17–MR71. [DOI] [PubMed] [Google Scholar]
- (78).Patzelt A; Mak WC; Jung S; Knorr F; Meinke MC; Richter H; Rühl E; Cheung KY; Tran NBNN; Lademann J. Do Nanoparticles Have a Future in Dermal Drug Delivery? J. Control. Release 2017, 246, 174–182. [DOI] [PubMed] [Google Scholar]
- (79).Dey S; Datta S; Dasgupta S; Mazumder B; Pathak YV Lipid Nanoparticles for Topical Application of Drugs for Skin Diseases. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier, 2016; Vol. 59, pp 327–361. [Google Scholar]
- (80).Klaassen CD Casarett and Doull’s Toxicology - The Basic Science of Poisons (9th Edition); McGraw-Hill: New York, 2018. [Google Scholar]
- (81).Labib S; Williams A; Yauk CL; Nikota JK; Wallin H; Vogel U; Halappanavar S. Nano-Risk Science: Application of Toxicogenomics in an Adverse Outcome Pathway Framework for Risk Assessment of Multi-Walled Carbon Nanotubes. Part. Fibre Toxicol. 2016, 13 (1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Riazi K; Galic MA; Kuzmiski JB; Ho W; Sharkey KA; Pittman QJ Microglial Activation and TNF Production Mediate Altered CNS Excitability Following Peripheral Inflammation. Proc. Natl. Acad. Sci 2008, 105 (44), 17151–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Mumaw CL; Levesque S; McGraw C; Robertson S; Lucas S; Stafflinger JE; Campen MJ; Hall P; Norenberg JP; Anderson T.; et al. Microglial Priming through the Lung-Brain Axis: The Role of Air Pollution-Induced Circulating Factors. FASEB J. 2016, 30 (5), 1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Perry C; Mackay-Sim A; Feron F; McGrath J. Olfactory Neural Cells: An Untapped Diagnostic and Therapeutic Resource. Laryngoscope 2002, 112 (4), 603–607. [DOI] [PubMed] [Google Scholar]
- (85).De Lorenzo AJD The Olfactory Neuron and the Blood-Brain Barrier. Ciba Foundation Symposium - Internal Secretions of the Pancreas (Colloquia on Endocrinology). January 1, 1970, pp 151–176.
- (86).Maher BA; Ahmed IAM; Karloukovski V; MacLaren DA; Foulds PG; Allsop D; Mann DMA; Torres-Jardón R; Calderon-Garciduenas L. Magnetite Pollution Nanoparticles in the Human Brain. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (39), 10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Rao DB; Wong BA; McManus BE; McElveen AM; James AR; Dorman DC Inhaled Iron, Unlike Manganese, Is Not Transported to the Rat Brain via the Olfactory Pathway. Toxicol. Appl. Pharmacol 2003, 193 (1), 116–126. [DOI] [PubMed] [Google Scholar]
- (88).Wu J; Ding T; Sun J. Neurotoxic Potential of Iron Oxide Nanoparticles in the Rat Brain Striatum and Hippocampus. Neurotoxicology 2013, 34 (1), 243–253. [DOI] [PubMed] [Google Scholar]
- (89).Pesch B; Dydak U; Lotz A; Casjens S; Quetscher C; Lehnert M; Abramowski J; Stewig C; Yeh CL; Weiss T.; et al. Association of Exposure to Manganese and Iron with Relaxation Rates R1 and R2*- Magnetic Resonance Imaging Results from the WELDOX II Study. Neurotoxicology 2018, 64, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Pujalte I; Dieme D; Haddad S; Serventi AM; Bouchard M. Toxicokinetics of Titanium Dioxide (TiO2) Nanoparticles after Inhalation in Rats. Toxicol Lett 2017, 265, 77–85. [DOI] [PubMed] [Google Scholar]
- (91).Chen P; Chakraborty S; Peres TV; Bowman AB; Aschner M. Manganese-Induced Neurotoxicity: From C. Elegans to Humans. Toxicol. Res. (Camb) 2015, 4 (2), 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Antonini JM; Santamaria AB; Jenkins NT; Albini E; Lucchini R. Fate of Manganese Associated with the Inhalation of Welding Fumes: Potential Neurological Effects. Neurotoxicology 2006, 27 (3), 304–310. [DOI] [PubMed] [Google Scholar]
- (93).Elder A; Gelein R; Silva V; Feikert T; Opanashuk L; Carter J; Potter R; Maynard A; Ito Y; Finkelstein J.; et al. Translocation of Inhaled Ultrafine Manganese Oxide Particles to the Central Nervous System. Env. Heal. Perspect 2006, 114 (8), 1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Fechter LD; Johnson DL; Lynch RA The Relationship of Particle Size to Olfactory Nerve Uptake of a Non-Soluble Form of Manganese into Brain. Neurotoxicology 2002, 23 (2), 177–183. [DOI] [PubMed] [Google Scholar]
- (95).Wang J; Liu Y; Jiao F; Lao F; Li W; Gu Y; Li Y; Ge C; Zhou G; Li B.; et al. Time-Dependent Translocation and Potential Impairment on Central Nervous System by Intranasally Instilled TiO(2) Nanoparticles. Toxicology 2008, 254 (1–2), 82–90. [DOI] [PubMed] [Google Scholar]
- (96).Hext PM; Tomenson JA; Thompson P. Titanium Dioxide: Inhalation Toxicology and Epidemiology. Ann. Occup. Hyg 2005, 49 (6), 461–472. [DOI] [PubMed] [Google Scholar]
- (97).Chalansonnet M; Carabin N; Boucard S; Merlen L; Melczer M; Antoine G; Devoy J; Remy A; Gagnaire F. Study of Potential Transfer of Aluminum to the Brain via the Olfactory Pathway. Toxicol. Lett 2018, 283, 77–85. [DOI] [PubMed] [Google Scholar]
- (98).Wu J; Wang C; Sun J; Xue Y. Neurotoxicity of Silica Nanoparticles: Brain Localization and Dopaminergic Neurons Damage Pathways. ACS Nano 2011, 5 (6), 4476–4489. [DOI] [PubMed] [Google Scholar]
- (99).Yamaguchi M. Functional Sub-Circuits of the Olfactory System Viewed from the Olfactory Bulb and the Olfactory Tubercle. Front. Neuroanat 2017, 11. [DOI] [PMC free article] [PubMed]
- (100).Oberdörster G; Sharp Z; Atudorei V; Elder A; Gelein R; Kreyling W; Cox C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol 2004, 16 (6–7), 437–445. [DOI] [PubMed] [Google Scholar]
- (101).Van der Cruyssen F; Verhelst P-J; Politis C. Channels and Transmitters Involved in Trigeminal Nociception. Open Access J Surg OAJS.MS.ID 2018, 7 (5), 5–7. [Google Scholar]
- (102).Lewis J; Bench G; Myers O; Tinner B; Staines W; Barr E; Divine KK; Barrington W; Karlsson J. Trigeminal Uptake and Clearance of Inhaled Manganese Chloride in Rats and Mice. Neurotoxicology 2005, 26 (1), 113–123. [DOI] [PubMed] [Google Scholar]
- (103).Li Y; Wang C; Zong S; Qi J; Dong X; Zhao W; Wu W; Fu Q; Lu Y; Chen Z. The Trigeminal Pathway Dominates the Nose-to-Brain Transportation of Intact Polymeric Nanoparticles: Evidence from Aggregation-Caused Quenching Probes. J. Biomed. Nanotechnol 2019, 15 (4), 686–702. [DOI] [PubMed] [Google Scholar]
- (104).Brüning T; Bartsch R; Bolt HM; Desel H; Drexler H; Gundert-Remy U; Hartwig A; Jackh R; Leibold E; Pallapies D.; et al. Sensory Irritation as a Basis for Setting Occupational Exposure Limits. Arch Toxicol 2014 , 88 (10), 1855–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Tian L; Inthavong K; Liden G; Shang Y; Tu J. Transport and Deposition of Welding Fume Agglomerates in a Realistic Human Nasal Airway. Ann Occup Hyg 2016, 60 (6), 731–747. [DOI] [PubMed] [Google Scholar]
- (106).Bowen S; Ateh DD; Deinhardt K; Bird MM; Price KM; Baker CS; Robson JC; Swash M; Shamsuddin W; Kawar S.; et al. The Phagocytic Capacity of Neurones. Eur. J. Neurosci 2007, 25 (10), 2947–2955. [DOI] [PubMed] [Google Scholar]
- (107).Mickle AD; Shepherd AJ; Mohapatra DP Sensory TRP Channels: The Key Transducers of Nociception and Pain. Prog Mol Biol Transl Sci 2015, 131, 73–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Kuznetsov AV Modelling Transport of Layered Double Hydroxide Nanoparticles in Axons and Dendrites of Cortical Neurons. Comput. Methods Biomech. Biomed. Engin 2012, 15 (12), 1263–1271. [DOI] [PubMed] [Google Scholar]
- (109).Hartung T; Sabbioni E. Alternative in Vitro Assays in Nanomaterial Toxicology. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2011, 3 (6), 545–573. [DOI] [PubMed] [Google Scholar]
- (110).Nikota J; Williams A; Yauk CL; Wallin H; Vogel U; Halappanavar S. Meta-Analysis of Transcriptomic Responses as a Means to Identify Pulmonary Disease Outcomes for Engineered Nanomaterials. Part. Fibre Toxicol 2016, 13 (1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Morimoto Y; Izumi H; Yoshiura Y; Tomonaga T; Oyabu T; Myojo T; Kawai K; Yatera K; Shimada M; Kubo M.; et al. Pulmonary Toxicity of Well-Dispersed Cerium Oxide Nanoparticles Following Intratracheal Instillation and Inhalation. J. Nanoparticle Res. 2015, 17 (11), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Morimoto Y; Izumi H; Yoshiura Y; Tomonaga T; Lee BW; Okada T; Oyabu T; Myojo T; Kawai K; Yatera K.; et al. Comparison of Pulmonary Inflammatory Responses Following Intratracheal Instillation and Inhalation of Nanoparticles. Nanotoxicology 2016, 10 (5), 607–618. [DOI] [PubMed] [Google Scholar]
- (113).Shinohara N; Oshima Y; Kobayashi T; Imatanaka N; Nakai M; Ichinose T; Sasaki T; Kawaguchi K; Zhang G; Gamo M. Pulmonary Clearance Kinetics and Extrapulmonary Translocation of Seven Titanium Dioxide Nano- and Submicron Materials Following Intratracheal Administration in Rats. Nanotoxicology 2015, 9 (8), 1050–1058. [DOI] [PubMed] [Google Scholar]
- (114).Cohen JM; Derk R; Wang L; Godleski J; Kobzik L; Brain J; Demokritou P. Tracking Translocation of Industrially Relevant Engineered Nanomaterials (ENMs) across Alveolar Epithelial Monolayers in Vitro. Nanotoxicology 2014, 8 (sup1), 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Tsuda A; Donaghey TC; Konduru NV; Pyrgiotakis G; Van Winkle LS; Zhang Z; Edwards P; Bustamante J-M; Brain JD; Demokritou P. Age-Dependent Translocation of Gold Nanoparticles across the Air–Blood Barrier. ACS Nano 2019, 13 (9), 10095–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Saunders NR; Liddelow SA; Dziegielewska KM Barrier Mechanisms in the Developing Brain. Front. Pharmacol 2012, 3, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Borm PJA; Driscoll K Particles, Inflammation and Respiratory Tract Carcinogenesis. In Toxicology Letters; Elsevier, 1996; Vol. 88, pp 109–113. [DOI] [PubMed] [Google Scholar]
- (118).Block ML; Calderon-Garciduenas L. Air Pollution: Mechanisms of Neuroinflammation and CNS Disease. Trends Neurosci 2009, 32 (9), 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Boyes WK; Chen R; Chen C; Yokel RA The Neurotoxic Potential of Engineered Nanomaterials. Neurotoxicology 2012, 33 (4), 902–910. [DOI] [PubMed] [Google Scholar]
- (120).Donaldson K; Tran L; Jimenez LA; Duffin R; Newby DE; Mills N; MacNee W; Stone V. Combustion-Derived Nanoparticles: A Review of Their Toxicology Following Inhalation Exposure. Part. Fibre Toxicol. 2005, 2 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Oberdorster G; Sharp Z; Atudorei V; Elder A; Gelein R; Lunts A; Kreyling W; Cox C. Extrapulmonary Translocation of Ultrafine Carbon Particles Following Whole-Body Inhalation Exposure of Rats. J Toxicol Env. Heal. A 2002, 65 (20), 1531–1543. [DOI] [PubMed] [Google Scholar]
- (122).Pele LC; Thoree V; Bruggraber SF; Koller D; Thompson RP; Lomer MC; Powell JJ Pharmaceutical/Food Grade Titanium Dioxide Particles Are Absorbed into the Bloodstream of Human Volunteers. Part. Fibre Toxicol 2015, 12 (1), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Cushen M; Kerry J; Morris M; Cruz-Romero M; Cummins E. Nanotechnologies in the Food Industry - Recent Developments, Risks and Regulation. Trends Food Sci. Technol 2012, 24 (1), 30–46. [Google Scholar]
- (124).Morris VJ Emerging Roles of Engineered Nanomaterials in the Food Industry. Trends Biotechnol. 2011, 29 (10), 509–516. [DOI] [PubMed] [Google Scholar]
- (125).Lomer MCE; Thompson RPH; Powell JJ Fine and Ultrafine Particles of the Diet: Influence on the Mucosal Immune Response and Association with Crohn’s Disease. Proc. Nutr. Soc 2002, 61 (01), 123–130. [DOI] [PubMed] [Google Scholar]
- (126).Wang Y; Yuan L; Yao C; Ding L; Li C; Fang J; Sui K; Liu Y; Wu M. A Combined Toxicity Study of Zinc Oxide Nanoparticles and Vitamin C in Food Additives. Nanoscale 2014, 6 (24), 15333–15342. [DOI] [PubMed] [Google Scholar]
- (127).Rogers KR; Bradham K; Tolaymat T; Thomas DJ; Hartmann T; Ma L; Williams A. Alterations in Physical State of Silver Nanoparticles Exposed to Synthetic Human Stomach Fluid. Sci. Total Environ. 2012, 420, 334–339. [DOI] [PubMed] [Google Scholar]
- (128).Felice VD; Quigley EM; Sullivan AM; O’Keeffe GW; O’Mahony SM Microbiota-Gut-Brain Signalling in Parkinson’s Disease: Implications for Non-Motor Symptoms. Park. Relat. Disord 2016, 27, 1–8. [DOI] [PubMed] [Google Scholar]
- (129).Brun E; Carrière M; Mabondzo A. In Vitro Evidence of Dysregulation of Blood-Brain Barrier Function after Acute and Repeated/Long-Term Exposure to TiO2 Nanoparticles. Biomaterials 2012, 33 (3), 886–896. [DOI] [PubMed] [Google Scholar]
- (130).Yokel R; Grulke E; Macphail R. Metal-Based Nanoparticle Interactions with the Nervous System: The Challenge of Brain Entry and the Risk of Retention in the Organism. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2013, 5 (4), 346–373. [DOI] [PubMed] [Google Scholar]
- (131).Oszlánczi G; Papp A; Szabó A; Nagymajtényi L; Sápi A; Kónya Z; Paulik E; Vezér T. Nervous System Effects in Rats on Subacute Exposure by Lead-Containing Nanoparticles via the Airways. Inhal. Toxicol 2011, 23 (4), 173–181. [DOI] [PubMed] [Google Scholar]
- (132).Oszlánczi G; Vezér T; Sárközi L; Horváth E; Kónya Z; Papp A. Functional Neurotoxicity of Mn-Containing Nanoparticles in Rats. Ecotoxicol. Environ. Saf 2010, 73 (8), 2004–2009. [DOI] [PubMed] [Google Scholar]
- (133).Liu P; Huang Z; Gu N. Exposure to Silver Nanoparticles Does Not Affect Cognitive Outcome or Hippocampal Neurogenesis in Adult Mice. Ecotoxicol. Environ. Saf 2013, 87, 124–130. [DOI] [PubMed] [Google Scholar]
- (134).Nielsen E; Ostergaard G; Larsen JC Toxicological Risk Assessment of Chemicals. A Practical Guide, 1st Editio.; CRC Press: Boca Raton, USA, 2008. [Google Scholar]
- (135).Hadrup N; Lam HR Oral Toxicity of Silver Ions, Silver Nanoparticles and Colloidal Silver – A Review. Regul. Toxicol. Pharmacol 2014, 68 (1), 1–7. [DOI] [PubMed] [Google Scholar]
- (136).Zucker RM; Ortenzio J; Degn LL; Lerner JM; Boyes WK Biophysical Comparison of Four Silver Nanoparticles Coatings Using Microscopy, Hyperspectral Imaging and Flow Cytometry. PLoS One 2019, 14 (7), e0219078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Buchman JT; Hudson-Smith NV; Landy KM; Haynes CL Understanding Nanoparticle Toxicity Mechanisms to Inform Redesign Strategies to Reduce Environmental Impact. Acc. Chem. Res 2019, 52 (6), 1632–1642. [DOI] [PubMed] [Google Scholar]
- (138).Burkard M; Betz A; Schirmer K; Zupanic A. Common Gene Expression Patterns in Environmental Model Organisms Exposed to Engineered Nanomaterials: A Meta-Analysis. Environ. Sci. Technol 2020, 54, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Singh RP; Ramarao P. Cellular Uptake, Intracellular Trafficking and Cytotoxicity of Silver Nanoparticles. Toxicol. Lett 2012, 213 (2), 249–259. [DOI] [PubMed] [Google Scholar]
- (140).Johnston HJ; Hutchison G; Christensen FM; Peters S; Hankin S; Stone V. A Review of the in Vivo and in Vitro Toxicity of Silver and Gold Particulates: Particle Attributes and Biological Mechanisms Responsible for the Observed Toxicity. Crit. Rev. Toxicol 2010, 40 (4), 328–346. [DOI] [PubMed] [Google Scholar]
- (141).Lubick N. Nanosilver Toxicity: Ions, Nanoparticles - Or Both? Environ. Sci. Technol 2008, 42 (23), 8617. [DOI] [PubMed] [Google Scholar]
- (142).Koch F; Möller AM; Frenz M; Pieles U; Kuehni-Boghenbor K; Mevissen M. An in Vitro Toxicity Evaluation of Gold-, PLLA- and PCL-Coated Silica Nanoparticles in Neuronal Cells for Nanoparticle-Assisted Laser-Tissue Soldering. Toxicol. Vitr 2014, 28 (5), 990–998. [DOI] [PubMed] [Google Scholar]
- (143).Veronesi B; Chorley B; Ward W; Simmons SO; Tennant A; Vallanat B The Physicochemistry of Capped Nanosilver Predicts Its Biological Activity in Rat Brain Endothelial Cells (RBEC4). In ACS Sustainable Chemistry and Engineering; 2014; Vol. 2, pp 1566–1573. [Google Scholar]
- (144).Chorley B; Ward W; Simmons SO; Vallanat B; Veronesi B. The Cellular and Genomic Response of Rat Dopaminergic Neurons (N27) to Coated Nanosilver. Neurotoxicology 2014, 45, 12–21. [DOI] [PubMed] [Google Scholar]
- (145).Zhang H; Ji Z; Xia T; Meng H; Low-Kam C; Liu R; Pokhrel S; Lin S; Wang X; Liao YP; et al. Use of Metal Oxide Nanoparticle Band Gap to Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation. ACS Nano 2012, 6 (5), 4349–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Sanders K; Degn LL; Mundy WR; Zucker RM; Dreher K; Zhao B; Roberts JE; Boyes WK In Vitro Phototoxicity and Hazard Identification of Nano-Scale Titanium Dioxide. Toxicol. Appl. Pharmacol 2012, 258 (2), 226–236. [DOI] [PubMed] [Google Scholar]
- (147).Roberts JE; Wielgus AR; Boyes WK; Andley U; Chignell CF Phototoxicity and Cytotoxicity of Fullerol in Human Lens Epithelial Cells. Toxicol. Appl. Pharmacol 2008, 228 (1), 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Wielgus AR; Zhao B; Chignell CF; Hu DN; Roberts JE Phototoxicity and Cytotoxicity of Fullerol in Human Retinal Pigment Epithelial Cells. Toxicol. Appl. Pharmacol 2010, 242 (1), 79–90. [DOI] [PubMed] [Google Scholar]
- (149).Murali K; Kenesei K; Li Y; Demeter K; Környei Z; Madarász E. Uptake and Bio-Reactivity of Polystyrene Nanoparticles Is Affected by Surface Modifications, Ageing and LPS Adsorption: In Vitro Studies on Neural Tissue Cells. Nanoscale 2015, 7 (9), 4199–4210. [DOI] [PubMed] [Google Scholar]
- (150).Baruwati B; Simmons SO; Varma RS; Veronesi B. “Green” Synthesized and Coated Nanosilver Alters the Membrane Permeability of Barrier (Intestinal, Brain Endothelial) Cells and Stimulates Oxidative Stress Pathways in Neurons. ACS Sustain. Chem. Eng 2013, 1 (7), 753–759. [Google Scholar]
- (151).Duffy CM; Ahmed S; Yuan C; Mavanji V; Nixon JP; Butterick T. Microglia as a Surrogate Biosensor to Determine Nanoparticle Neurotoxicity. J. Vis. Exp 2016, 2016 (116). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Trickler WJ; Lantz SM; Murdock RC; Schrand AM; Robinson BL; Newport GD; Schlager JJ; Oldenburg SJ; Paule MG; Slikker W Jr; et al. Brain Microvessel Endothelial Cells Responses to Gold Nanoparticles: In Vitro pro-Inflammatory Mediators and Permeability. Nanotoxicology 2011, 5 (4), 479–492. [DOI] [PubMed] [Google Scholar]
- (153).Bal-Price A; Pistollato F; Sachana M; Bopp SK; Munn S; Worth A. Strategies to Improve the Regulatory Assessment of Developmental Neurotoxicity (DNT) Using in Vitro Methods. Toxicol. Appl. Pharmacol 2018, 354, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Crofton KM; Mundy WR; Shafer TJ Developmental Neurotoxicity Testing: A Path Forward. Congenital Anomalies. September 2012, pp 140–146. [DOI] [PubMed]
- (155).Bal-Price A; Crofton KM; Sachana M; Shafer TJ; Behl M; Forsby A; Hargreaves A; Landesmann B; Lein PJ; Louisse J.; et al. Putative Adverse Outcome Pathways Relevant to Neurotoxicity. Crit Rev Toxicol 2015, 45 (1), 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Spinu N; Bal-Price A; Cronin MTD; Enoch SJ; Madden JC; Worth AP Development and Analysis of an Adverse Outcome Pathway Network for Human Neurotoxicity. Arch. Toxicol 2019, 93 (10), 2759–2772. [DOI] [PubMed] [Google Scholar]
- (157).Aschner M; Ceccatelli S; Daneshian M; Fritsche E; Hasiwa N; Hartung T; Hogberg HT; Leist M; Li A; Mundi WR; et al. Reference Compounds for Alternative Test Methods to Indicate Developmental Neurotoxicity (DNT) Potential of Chemicals: Example Lists and Criteria for Their Selection and Use. ALTEX 2017, 34 (1), 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Harrill JA; Freudenrich T; Wallace K; Ball K; Shafer TJ; Mundy WR Testing for Developmental Neurotoxicity Using a Battery of in Vitro Assays for Key Cellular Events in Neurodevelopment. Toxicol. Appl. Pharmacol 2018. [DOI] [PubMed]
- (159).Xu F; Piett C; Farkas S; Qazzaz M; Syed NI Silver Nanoparticles (AgNPs) Cause Degeneration of Cytoskeleton and Disrupt Synaptic Machinery of Cultured Cortical Neurons. Mol. Brain 2013, 6 (1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Powers CM; Wrench N; Ryde IT; Smith AM; Seidler FJ; Slotkin TA Silver Impairs Neurodevelopment: Studies in PC12 Cells. Environ. Health Perspect 2010, 118 (1), 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Powers CM; Badireddy AR; Ryde IT; Seidler FJ; Slotkin TA Silver Nanoparticles Compromise Neurodevelopment in PC12 Cells: Critical Contributions of Silver Ion, Particle Size, Coating, and Composition. Environ. Health Perspect 2011, 119 (1), 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Repar N; Li H; Aguilar JS; Li QQ; Drobne D; Hong Y. Silver Nanoparticles Induce Neurotoxicity in a Human Embryonic Stem Cell-Derived Neuron and Astrocyte Network. Nanotoxicology 2018, 12 (2), 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Niska K; Santos-Martinez MJ; Radomski MW; Inkielewicz-Stepniak I. CuO Nanoparticles Induce Apoptosis by Impairing the Antioxidant Defense and Detoxification Systems in the Mouse Hippocampal HT22 Cell Line: Protective Effect of Crocetin. Toxicol. Vitr 2015, 29 (4), 663–671. [DOI] [PubMed] [Google Scholar]
- (164).Majeed W; Bourdo S; Petibone DM; Saini V; Vang KB; Nima ZA; Alghazali KM; Darrigues E; Ghosh A; Watanabe F.; et al. The Role of Surface Chemistry in the Cytotoxicity Profile of Graphene. J. Appl. Toxicol 2017, 37 (4), 462–470. [DOI] [PubMed] [Google Scholar]
- (165).Fernández-Bertólez N; Costa C; Brandão F; Kiliç G; Duarte JA; Teixeira JP; Pásaro E; Valdiglesias V; Laffon B. Toxicological Assessment of Silica-Coated Iron Oxide Nanoparticles in Human Astrocytes. Food Chem. Toxicol 2018, 118, 13–23. [DOI] [PubMed] [Google Scholar]
- (166).Strickland JD; Lefew WR; Crooks J; Hall D; Ortenzio JN; Dreher K; Shafer TJ In Vitro Screening of Metal Oxide Nanoparticles for Effects on Neural Function Using Cortical Networks on Microelectrode Arrays. Nanotoxicology 2016, 10 (5), 619–628. [DOI] [PubMed] [Google Scholar]
- (167).Shrivastava R; Raza S; Yadav A; Kushwaha P; Flora SJS Effects of Sub-Acute Exposure to TiO2, ZnO and Al2O3 Nanoparticles on Oxidative Stress and Histological Changes in Mouse Liver and Brain. Drug Chem. Toxicol 2014, 37 (3), 336–347. [DOI] [PubMed] [Google Scholar]
- (168).Rahman MF; Wang J; Patterson TA; Saini UT; Robinson BL; Newport GD; Murdock RC; Schlager JJ; Hussain SM; Ali SF Expression of Genes Related to Oxidative Stress in the Mouse Brain after Exposure to Silver-25 Nanoparticles. Toxicol. Lett 2009, 187 (1), 15–21. [DOI] [PubMed] [Google Scholar]
- (169).Krawczyńska A; Dziendzikowska K; Gromadzka-Ostrowska J; Lankoff A; Herman AP; Oczkowski M; Królikowski T; Wilczak J; Wojewódzka M; Kruszewski M. Silver and Titanium Dioxide Nanoparticles Alter Oxidative/Inflammatory Response and Renin-Angiotensin System in Brain. Food Chem. Toxicol 2015, 85, 96–105. [DOI] [PubMed] [Google Scholar]
- (170).Ze Y; Zheng L; Zhao X; Gui S; Sang X; Su J; Guan N; Zhu L; Sheng L; Hu R.; et al. Molecular Mechanism of Titanium Dioxide Nanoparticles-Induced Oxidative Injury in the Brain of Mice. Chemosphere 2013, 92 (9), 1183–1189. [DOI] [PubMed] [Google Scholar]
- (171).Ze Y; Sheng L; Zhao X; Hong J; Ze X; Yu X; Pan X; Lin A; Zhao Y; Zhang C.; et al. TiO2 Nanoparticles Induced Hippocampal Neuroinflammation in Mice. PLoS One 2014, 9 (3), e92230. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (172).Bencsik A; Lestaevel P. Concerns Regarding Nanosized Titanium Dioxide Nasal Exposure and Neurotoxicity Study by Ze et Al. Journal of Biomedical Materials Research - Part A. June 2015, pp 2198–2200. [DOI] [PubMed]
- (173).You R; Ho YS; Hung CHL; Liu Y; Huang CX; Chan HN; Ho SL; Lui SY; Li HW; Chang RCC Silica Nanoparticles Induce Neurodegeneration-like Changes in Behavior, Neuropathology, and Affect Synapse through MAPK Activation. Part. Fibre Toxicol. 2018, 15 (1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Onoda A; Takeda K; Umezawa M. Dysregulation of Major Functional Genes in Frontal Cortex by Maternal Exposure to Carbon Black Nanoparticle Is Not Ameliorated by Ascorbic Acid Pretreatment. Sci. Total Environ. 2018, 634, 1126–1135. [DOI] [PubMed] [Google Scholar]
- (175).Zhang L; Bai R; Li B; Ge C; Du J; Liu Y; Le Guyader L; Zhao Y; Wu Y; He S.; et al. Rutile TiO2 Particles Exert Size and Surface Coating Dependent Retention and Lesions on the Murine Brain. Toxicol. Lett 2011, 207 (1), 73–81. [DOI] [PubMed] [Google Scholar]
- (176).Ma L; Liu J; Li N; Wang J; Duan Y; Yan J; Liu H; Wang H; Hong F. Oxidative Stress in the Brain of Mice Caused by Translocated Nanoparticulate TiO2 Delivered to the Abdominal Cavity. Biomaterials 2010, 31 (1), 99–105. [DOI] [PubMed] [Google Scholar]
- (177).Grissa I; Guezguez S; Ezzi L; Chakroun S; Sallem A; Kerkeni E; Elghoul J; El Mir L; Mehdi M; Cheikh H ben; et al. The Effect of Titanium Dioxide Nanoparticles on Neuroinflammation Response in Rat Brain. Environ. Sci. Pollut. Res 2016, 23 (20), 20205–20213. [DOI] [PubMed] [Google Scholar]
- (178).Greish K; Alqahtani AA; Alotaibi AF; Abdulla AM; Bukelly AT; Alsobyani FM; Alharbi GH; Alkiyumi IS; Aldawish MM; Alshahrani TF; et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in Balb/c Mice. Int. J. Environ. Res. Public Health 2019, 16 (1), 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Horváth T; Vezér T; Kozma G; Papp A. Functional Neurotoxicity and Tissue Metal Levels in Rats Exposed Subacutely to Titanium Dioxide Nanoparticles via the Airways. Ideggyogy. Sz 2018, 71 (1–2), 35–42. [DOI] [PubMed] [Google Scholar]
- (180).Yin N; Yao X; Zhou Q; Faiola F; Jiang G. Vitamin E Attenuates Silver Nanoparticle-Induced Effects on Body Weight and Neurotoxicity in Rats. Biochem. Biophys. Res. Commun 2015, 458 (2), 405–410. [DOI] [PubMed] [Google Scholar]
- (181).Amara S; Slama I. Ben; Omri K; Ghoul J. El; Mir L. El; Rhouma K. Ben; Abdelmelek H; Sakly M. Effects of Nanoparticle Zinc Oxide on Emotional Behavior and Trace Elements Homeostasis in Rat Brain. Toxicol. Ind. Health 2015, 31 (12), 1202–1209. [DOI] [PubMed] [Google Scholar]
- (182).de Souza JM; Mendes B. de O.; Guimarães ATB; Rodrigues A. S. de L.; Chagas TQ; Rocha TL; Malafaia G. Zinc Oxide Nanoparticles in Predicted Environmentally Relevant Concentrations Leading to Behavioral Impairments in Male Swiss Mice. Sci. Total Environ. 2018, 613–614, 653–662. [DOI] [PubMed] [Google Scholar]
- (183).Takács S; Szabó A; Oszlánczi G; Pusztai P; Sápi A; Kónya Z; Papp A. Repeated Simultaneous Cortical Electrophysiological and Behavioral Recording in Rats Exposed to Manganese-Containing Nanoparticles. Acta Biol. Hung 2012, 63 (4), 426–440. [DOI] [PubMed] [Google Scholar]
- (184).Li X; Sun W; An L. Nano-CuO Impairs Spatial Cognition Associated with Inhibiting Hippocampal Long-Term Potentiation via Affecting Glutamatergic Neurotransmission in Rats. Toxicol. Ind. Health 2018, 34 (6), 409–421. [DOI] [PubMed] [Google Scholar]
- (185).Zheng W; McKinney W; Kashon M; Salmen R; Castranova V; Kan H. The Influence of Inhaled Multi-Walled Carbon Nanotubes on the Autonomic Nervous System. Part. Fibre Toxicol 2016, 13 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Skalska J; Frontczak-Baniewicz M; Struzyńska L. Synaptic Degeneration in Rat Brain after Prolonged Oral Exposure to Silver Nanoparticles. Neurotoxicology 2015, 46, 145–154. [DOI] [PubMed] [Google Scholar]
- (187).Hong F; Zhou Y; Ji J; Zhuang J; Sheng L; Wang L. Nano-TiO2 Inhibits Development of the Central Nervous System and Its Mechanism in Offspring Mice. J. Agric. Food Chem 2018, 66 (44), 11767–11774. [DOI] [PubMed] [Google Scholar]
- (188).Mohammadipour A; Fazel A; Haghir H; Motejaded F; Rafatpanah H; Zabihi H; Hosseini M; Bideskan AE Maternal Exposure to Titanium Dioxide Nanoparticles during Pregnancy; Impaired Memory and Decreased Hippocampal Cell Proliferation in Rat Offspring. Environ. Toxicol. Pharmacol 2014, 37 (2), 617–625. [DOI] [PubMed] [Google Scholar]
- (189).Wu J; Yu C; Tan Y; Hou Z; Li M; Shao F; Lu X. Effects of Prenatal Exposure to Silver Nanoparticles on Spatial Cognition and Hippocampal Neurodevelopment in Rats. Environ. Res 2015, 138, 67–73. [DOI] [PubMed] [Google Scholar]
- (190).Hawkins SJ; Crompton LA; Sood A; Saunders M; Boyle NT; Buckley A; Minogue AM; McComish SF; Jiménez-Moreno N; Cordero-Llana O.; et al. Nanoparticle-Induced Neuronal Toxicity across Placental Barriers Is Mediated by Autophagy and Dependent on Astrocytes. Nat. Nanotechnol 2018, 13 (5), 427–433. [DOI] [PubMed] [Google Scholar]
- (191).Umezawa M; Onoda A; Korshunova I; Jensen ACØ; Koponen IK; Jensen KA; Khodosevich K; Vogel U; Hougaard KS Maternal Inhalation of Carbon Black Nanoparticles Induces Neurodevelopmental Changes in Mouse Offspring. Part. Fibre Toxicol 2018, 15 (1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Zhang Q; Ding Y; He K; Li H; Gao F; Moehling TJ; Wu X; Duncan J; Niu Q. Exposure to Alumina Nanoparticles in Female Mice during Pregnancy Induces Neurodevelopmental Toxicity in the Offspring. Front. Pharmacol 2018, 9 (MAR). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Varallyay P; Nesbit G; Muldoon LL; Nixon RR; Delashaw J; Cohen JI; Petrillo A; Rink D; Neuwelt EA Comparison of Two Superparamagnetic Viral-Sized Iron Oxide Particles Ferumoxides and Ferumoxtran-10 with a Gadolinium Chelate in Imaging Intracranial Tumors. AJNR. Am. J. Neuroradiol 2002, 23 (4), 510–519. [PMC free article] [PubMed] [Google Scholar]
- (194).Bernd H; De Kerviler E; Gaillard S; Bonnemain B. Safety and Tolerability of Ultrasmall Superparamagnetic Iron Oxide Contrast Agent: Comprehensive Analysis of a Clinical Development Program. Invest. Radiol 2009, 44 (6), 336–342. [DOI] [PubMed] [Google Scholar]
- (195).Winer JL; Liu CY; Apuzzo MLJ The Use of Nanoparticles as Contrast Media in Neuroimaging: A Statement on Toxicity. World Neurosurg. 2012, 78 (6), 709–711. [DOI] [PubMed] [Google Scholar]
- (196).Costa LG; Cole TB; Coburn J; Chang YC; Dao K; Roqué PJ Neurotoxicity of Traffic-Related Air Pollution. Neurotoxicology 2017, 59, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Costa LG; Chang YC; Cole TB Developmental Neurotoxicity of Traffic-Related Air Pollution: Focus on Autism. Current environmental health reports. June 1, 2017, pp 156–165. [DOI] [PMC free article] [PubMed]
- (198).Yokel RA; MacPhail RC Engineered Nanomaterials: Exposures, Hazards, and Risk Prevention. J. Occup. Med. Toxicol 2011, 6 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Teleanu DM; Chircov C; Grumezescu AM; Teleanu RI Neurotoxicity of Nanomaterials: An up-to-Date Overview. Nanomaterials 2019, 9 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Vissers C; Ming G; Song H. Nanoparticle Technology and Stem Cell Therapy Team up against Neurodegenerative Disorders. Adv. Drug Deliv. Rev 2019, 148, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]


