Abstract
Background:
Recent studies suggest an intergenerational influence of stress such that maternal exposure even before pregnancy could impact offspring health outcomes later in life. In humans, investigations on the impact of maternal stressors on offspring health outcomes, including stress-sensitive biomarkers, have largely been limited to extreme stressors. Prior studies have not addressed more moderate maternal stressors, such as rotating night shift work, on offspring stress markers in young adulthood.
Methods:
We investigated the association between maternal rotating night shift work before conception and offspring salivary cortisol and alpha amylase (sAA) patterns in young adulthood among mothers enrolled in the Nurses’ Health Study II (NHSII) and their offspring participating in the Growing Up Today Study 2 (GUTS2). Our sample included over 300 mother-child pairs where, between 2011 and 2014, the children provided 5 saliva samples over the course of one day. We used piecewise linear mixed models to compare awakening responses, overall slopes as well as several other diurnal patterns of cortisol and sAA between offspring born to shift working versus non-shift working mothers.
Results:
Offspring born to shift working mothers had a flattened late decline in cortisol (percent differences in slope (%D): 2.1%; 95%CI: 0.3, 3.8) and their sAA awakening response was steeper (%D −37.4%; 95%CI: −59.0, −4.4), whereas sAA increase before bedtime appeared less pronounced (%D −35.9%; 95%CI: −55.3, −8.3), compared to offspring born to mothers without shift work. For cortisol, we observed a significant difference in the Area Under the Curve (AUC) (%D 1.5%; 95%CI: 0.3, 2.7) with higher AUC for offspring of mothers who worked rotating night shifts. In offspring-sex-stratified analyses we found differences primarily among males.
Conclusion:
Our results provide some – albeit modest - evidence that maternal rotating night shift work—a moderate stressor—influences offspring stress markers. Future studies with larger samples sizes, more detailed exposure assessment (particularly during maternal pregnancy), and multiple offspring biomarker assessments at different developmental stages are needed to further investigate these associations.
Keywords: Maternal night shift work, Circadian disruption, Intergenerational, Offspring stress, Cortisol, Salivary alpha amylase
1. Introduction
Stress-related biomarkers can offer insights about the pathophysiology of downstream disease phenotypes. Among other stress response pathways, dysregulation of hypothalamic-pituitary-adrenocortical (HPA) axis production of cortisol and the sympathetic-adrenal-medullary (SAM)-axis release of catecholamines (e.g., epinephrine) have received much attention in recent literature [23].
Several exposures including smoking, physical activity as well as chronic stress have been linked to altered cortisol and sAA levels [26,29]. These, in turn, have been associated with adverse health outcomes in adults [25,28]. In addition, there is also recent evidence for trans- and intergenerational influences on offspring health outcomes, possibly mediated by altered stress responsive biomarkers [7,9,19,38].
Night shift work is an established reality in most Western countries: e.g. in a 2004 survey the Bureau of Labor and Statistics reported that almost 18% of the US work force worked alternative shifts [21]. Rotating night shift work, in particular, is emerging as an occupational risk factor for chronic diseases (obesity, type 2 diabetes, cardiovascular diseases [24,32,33] and mental health [11]), likely because of social and biological stress [16] due to chronic misalignment between the internal circadian rhythm and the behavioral rhythm imposed by external factors.
In addition to health consequences experienced by the exposed individual, recent studies also report epigenetic alterations in nurses working night shifts [5,39]. Keenan et al. [17] recently suggested to extend the developmental origins of disease model to include preconception stress exposure as e.g. results from animal models show that adult mice exposed to social defeat stress before conception bear offspring with increased baseline levels of corticosterone, the main glucocorticoid involved in regulation of stress [10]. Babb et al. [3] studied the transgenerational effects of maternal chronic social stress on offspring behavior and endocrinology in rats. They found that offspring of rats who were exposed to stress across two generations presented with impaired social behavior and decreased basal concentrations of corticosterone compared to offspring of non-exposed rats, independent of offspring sex. While there is a rich literature on sex differences in stress responses to exposures occurring at different developmental stages also including the pre-natal phase [4] - only little is known regarding differential offspring responses by sex to parental stress exposure occurring before conception [7].
Particularly studies in humans are scarce so far, and have primarily focused on associations between extreme and in some cases acute stress exposures before [12] or during pregnancy [37] and offspring mental health and stress responses.
To date, little is known about the relationships between more moderate chronic health stressors, such as circadian disruption (due to rotating night shift work) before pregnancy and offspring stress-sensitive biomarkers. To explore these associations, we utilized information on maternal preconception shift work exposure as well as offspring salivary cortisol and alpha amylase levels in more than 300 mother/child pairs participating in the Nurses’ Health Study II (NHSII) and Growing Up Today Study 2 (GUTS2).
2. Methods
2.1. Study population
Participants are mothers in the Nurses’ Health Study II (NHSII) as well as their children enrolled in the Growing Up Today Study 2 (GUTS2). Both studies are ongoing prospective cohort studies. NHSII was initiated in 1989 with the enrollment of 116,429 female US nurses, aged 25–42 years at that time, who returned a mailed questionnaire reporting on their lifestyle habits and health status. Information on these factors has been updated biennially since. GUTS2 was initiated in 2004, when invitations and questionnaires were sent to 17,280 children of NHSII participants after obtaining maternal consent. 10,918 children aged 9 to 16 years at that time responded and provided information on health, lifestyle factors and growth indicators and were administered follow-up questionnaires in 2006, 2008, 2011 and annually from 2013 to 2016.
The current study is based on a subset of GUTS2 participants who took part in the Saliva Substudy conducted between 2011 and 2014; this sub-study was originally designed to investigate stress response physiology in relation to sexual orientation and recent stressful life events [2]. Therefore, participants reporting their sexual minority status (participants who identified as mostly heterosexual, bisexual, mostly homosexual, gay, lesbian or who reported any history of same-sex sexual partners) were oversampled when recruiting participants via e-mail invitations in 2011. Furthermore, participants were considered eligible if they were not currently pregnant or had not been pregnant in the previous six months, had not reported use of oral or inhaled steroids in the past month, had no history of cancer treatment, and had never been diagnosed with an endocrine disorder. Participants received $25 upon return of saliva samples as an incentive.
All involved studies have approval from the Committees on the Use of Human Subjects in Research at the Brigham and Women’s Hospital (Boston, MA, USA). For both cohorts, if participants returned the baseline self-administered questionnaire, it was assumed to imply informed consent.
2.2. Ascertainment of night shift work before pregnancy
In the baseline questionnaire in 1989 NHSII participants were asked to report the total number of years they had worked rotating night shifts, defined as “at least three nights per month in addition to working days or evenings in the respective month.” In biennial follow-up questionnaires in 1991 and 1993 and a retrospective assessment in 2001 for the time between 1993 and 1995, this information was updated. We calculated cumulative shift work exposure before conception for children born between 1989 and 1995 by adding together the number of years the nurses reported rotating night shift work before conception for each child and also categorized mothers into ever - or never night shift workers. A timeline of exposure and outcome assessments is shown in Supplemental Fig. 1.
2.3. Saliva collection
GUTS2 participants were mailed saliva collection tools, along with a brief questionnaire, and were instructed to collect 5 saliva samples during one day. Specifically, they were asked to provide samples at: awakening; 45 min, 4 h and 10 h after awakening; and just before going to bed. Participants were asked not to brush their teeth before the first sample at awakening and further not to engage in vigorous physical activity, drinking, eating or chewing for at least 30 min before each subsequent sample. To assess adherence, participants filled out a log recording collection times and indicating whether they brushed their teeth, exercised, drank or ate in the previous 30 min.
2.4. Sample processing
Filled tubes were stored at the participant’s home refrigerator until completion of sampling and were returned via two-day delivery in a postage-paid mailer together with an ice pack to the BWH/Harvard Cohorts Biorepository where they were stored at −130 Centigrade.
Assays were conducted in the Rohleder Lab at Brandeis University. Cortisol was measured in duplicate using a competitive chemiluminescence immunoassay (CLIA; IBL-International, Toronto, ON, Canada) and sAA was measured using commercially available luminescence immunoassay kits (CLIA; IBL, Hamburg, Germany) along with quality control (QC) samples provided by the BWH/Harvard Cohorts Biorepository. Aliquots of three distinct QC pools were distributed randomly among the participant samples and their identity was blinded from the assay laboratory. Two QC pool samples from at least two of the three available pool were included on each batch of samples and each pool was represented in at least one-third of the analyzed batches [2]. Coefficients of variation for the study met the biorepository standard of 15% or below. Finally, potential batch effects were adjusted for using the average batch method [27].
2.5. Offspring saliva diary measures and additional maternal covariates
In questionnaires sent along with the saliva collection kits, offspring were asked to report on factors that might impact diurnal rhythms. In addition to information on sample collection compliance (including with the “wake-up” sample to confirm it was indeed obtained upon awakening), they reported sleep duration and quality in the previous night as well as on usual nights. Female participants were also asked whether they had used any form of hormonal contraception in the past month. Additionally, participants reported their current mood (“Do you feel worried, anxious, or fearful right now?” and “Do you feel happy, excited, or content right now?” with options ranging from 1-Not at all to 4-Extremely) for each of the five saliva collection. Since the saliva study was originally motivated by an hypothesis related to sexual orientation and stressful life events, we also had information on whether the participants belonged to a sexual minority group or self-reported exposure to traumatic experiences during the past month and past year using a modified version of the Stressful Life Events Screening Questionnaire [13].
In addition, we obtained data on maternal characteristics from the biennial NHSII questionnaires. Maternal age at delivery was calculated as the difference between mother and offspring dates of birth. Maternal diet quality was assessed in 1991 using a validated food frequency questionnaire (FFQ) [35], based on which the Alternative Health Eating Index (AHEI) score was derived [20]. Energy expenditure in metabolic equivalent (MET) hours per week was calculated based on self-reported frequency of various physical activities in 1989 [1]. Information on smoking habits, weight and height was utilized from the time most proximal to and before the conception of the first included child. Information on lifetime history of depression was based on self-reports, first ascertained in 2003, and as proxy for socioeconomic status, we ascertained information on their husband’s level of education in 1999. Further, maternal chronotype information was obtained in 2009 by asking a single question from the Morningness-Eveningness Questionnaire [14], previously validated for classifying individuals as “definitely a morning type,” “rather more a morning than an evening type,” “rather more an evening than a morning type,” “definitely an evening type” or neither.
2.6. Statistical analysis
Both cortisol (nmol/L) and sAA (nmol/L) were log-transformed due their skewed distributions and saliva collection time was centered at awakening. Before log-transforming cortisol and sAA values, we replaced extreme outlier values with values equal to the mean plus 2.5 standard deviations. In exploratory analyses, we fit piecewise cubic spline functions with 3 knots chosen at the preplanned saliva collection times (45 min, 4 h, and 10 h after awakening) to identify time points at which inflections in diurnal rhythms occurred (see Fig. 1). Informed by these exploratory curves, we decided to place a knot at 16 h rather than at 10 h to capture the corresponding rhythm patterns. Similar patterns and analyses approaches were previously reported in Huang et al. [15]. Specifically, we set out to investigate differences in five key metrics: 1) awakening level (baseline level at awakening), 2) awakening response (change between awakening and 45 min after awakening), 3) early decline (change between 45 min and 4 h after awakening), 4) late decline (change between 4 h and 16 h after awakening), and 5) night rise (change between 16 h after awakening and bedtime). We examined differences in these 5 metrics across categories of maternal rotating night shift work exposure using linear mixed models with piecewise linear splines; differences were quantified by including multiplicative interaction terms between the shift work exposure variable and each of the three spline functions and defining respective contrasts through linear combinations of the estimated parameters. Results are presented as percentage differences, calculated by exponentiating the respective coefficients (or linear combinations of these) and subtracting one. Additionally, we computed differences in the area under the curve (AUC) from awakening to 16 h to compare differences in diurnal output. Overall slopes, capturing changes from awakening to bedtime as well as slopes based on only 4 measurement times were also calculated while leaving out the peak values at 45 min after awakening. Geometric means and geometric standard deviation factors of the outlier corrected data on the original scale are also reported for each of the 5 time points.
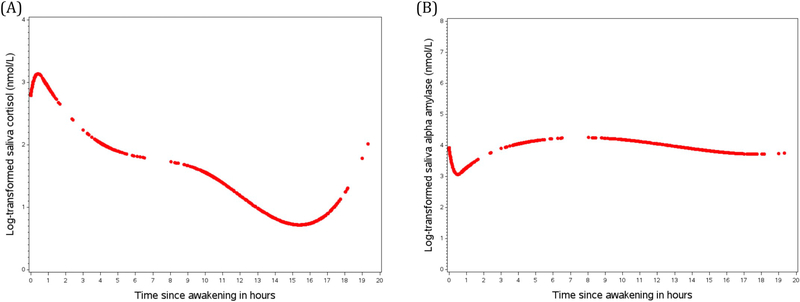
Fig. 1.
(A) Diurnal cortisol rhythms including the entire sample (n = 355 participants). (B) Diurnal alpha amylase (sAA) rhythms including the entire sample (n = 320 participants). Curves were created based on predictive values from mixed models with piecewise cubic spline terms.
We present unadjusted models, as well was two multivariable (MV) adjusted models. In MV model 1 we adjusted for offspring age at sample collection, saliva collection characteristics (teeth brushed, ate, drank, exercised, happy, worried), usual hours of sleep, last night’s hours of sleep, trauma score in the last month, belonging to a sexual orientation minority group, compliance with the wake-up sample, and oral contraceptive use in females. In MV model 2 we additionally adjusted for maternal characteristics, such as maternal age at birth, body mass index (BMI) before pregnancy, smoking status before pregnancy, diet quality, physical activity, husband’s education, maternal lifetime history of depression and chronotype. Missing indicators were created for missing covariate values.
In addition to analyses in the entire sample, we also present analyses stratified by sex of the offspring to allow adequate consideration of contraceptive hormone use. Since we recently found evidence for an effect modification by chronotype of the association between maternal night shift work exposure before pregnancy and offspring lifetime depression risk [31], we also performed analyses stratified by maternal chronotype. All analyses were performed in SAS 9.4 for UNIX (SAS Institute).
2.7. Final sample for the analyses
In total n = 6980 GUTS participants were invited whereof n = 1966 participated in the saliva collection. Out of these n = 774 GUTS2 participants provided at least one usable saliva sample. Since maternal shift work history was assessed for the first time at the 1989 NHSII baseline questionnaire, we considered only GUTS2 participants (n = 392) who were born to NHSII participants (n = 375) in 1989 or later. We also excluded twins and triplets (11 children, 4 mothers). For both the cortisol and sAA analyses we restricted to participants who provided at least 4 saliva samples and whose reported saliva collections times were within a 24-h window after waking up. In the cortisol analyses, we included 355 participants (345 mothers) and a total of 1758 cortisol samples in the final sample for analyses. In the sAA analyses, 320 participants (310 mothers) and a total of 1543 sAA samples were included after applying the same exclusion criteria.
3. Results
Among the 355 young adults included in our cortisol analysis sample (Table 1), 228 (64%) were born to mothers who had worked rotating night shifts before conception. Their average age at saliva collection was 20.0 (SD 1.1) years and 250 (70%) were female. About 23% of the sample reported belonging to a sexual minority group, with similar distributions across exposure groups and for male versus female offspring. Compliance with obtaining the wake up sample upon awakening was similar in males versus females, while in both groups the compliance was slightly higher for offspring born to mothers with a history of rotating night shifts versus those with no such history. Reports of being worried or happy were comparable across groups of maternal night shift work history and offspring sex, while males reported shorter sleep duration. Maternal age at birth of the included offspring was 33.4 (SD 3.6) years overall and was similar across offspring sex and exposure groups. Distributions of BMI before pregnancy, diet quality and physical activity also did not differ markedly across the different strata. However, mothers who worked rotating night shifts tended to smoke less, were more likely to be definite morning types and were more likely to have a history of depression in our sample.
Table 1.
Mother and offspring characteristics by history of maternal rotating night shift work before pregnancy for 345 mothers and 355 children, born between 1989 and 1995, enrolled in the Growing Up Today Study 2 and participating in the saliva sub-study conducted between 2011 and 2014.
| Female offspring |
Male offspring |
Overall offspring |
||||
|---|---|---|---|---|---|---|
| Maternal history of night shift work |
Maternal history of night shift work |
Maternal history of night shift work |
||||
| Never | Ever | Never | Ever | Never | Ever | |
| Maternal characteristics | ||||||
| Number of mothers, n | 75 | 167 | 42 | 61 | 117 | 228 |
| Age at birth, mean (SD) | 33.1 (3.3) | 33.7 (3.8) | 32.5 (3.0) | 33.5 (3.6) | 32.9 (3.2) | 33.7 (3.7) |
| BMI before pregnancy a, mean (SD) | 22.9 (3.4) | 23.3 (4.6) | 23.4 (4.7) | 22.9 (4.5) | 23.1 (3.9) | 23.2 (4.6) |
| Smoking before pregnancy a, %b | ||||||
| Smoker | 5.3 | 4.8 | 4.8 | 1.6 | 5.1 | 4.0 |
| Past smoker | 22.7 | 19.3 | 11.9 | 13.1 | 18.8 | 17.6 |
| Never smoker | 72.0 | 75.9 | 83.3 | 85.3 | 76.1 | 78.4 |
| Diet quality (AHEI without alcohol)c, mean (SD) | 44.1 (9.5) | 42.9 (9.2) | 44.4 (11.2) | 43.5 (10.8) | 44.2 (10.1) | 43.1 (9.6) |
| Alcohol intake (gm)c, mean (SD) | 2.0 (2.8) | 2.1 (3.1) | 3.2 (5.8) | 2.5 (5.4) | 2.4 (4.1) | 2.2 (3.8) |
| Physical activity, MET-hrs/wk. d, mean (SD) | 19.1 (17.5) | 18.3 (17.7) | 20.4 (23.9) | 21.0 (23.0) | 19.5 (19.9) | 19.0 (19.2) |
| Chronotype, % | ||||||
| Definite morning type | 29.6 | 32.5 | 30.0 | 50.9 | 29.7 | 37.4 |
| Intermediate type | 63.4 | 57.1 | 57.5 | 40.7 | 61.3 | 52.7 |
| Definite Evening type | 7.0 | 10.4 | 12.5 | 8.5 | 9.0 | 9.9 |
| Lifetime depression history, e % | 10.7 | 19.2 | 19.1 | 23.0 | 13.6 | 20.2 |
| Husbands holding a graduate degree f | 40.9 | 35.0 | 38.5 | 38.6 | 40.0 | 35.9 |
| History of rotating night shift work, % | ||||||
| None | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| < 3 years | 0.0 | 48.5 | 0.0 | 60.7 | 0.0 | 51.8 |
| 3–5 years | 0.0 | 38.9 | 0.0 | 23.0 | 0.0 | 34.6 |
| ≥ 6 years | 0.0 | 12.6 | 0.0 | 16.4 | 0.0 | 13.6 |
| Offspring characteristics | ||||||
| Number of offspring, n | 78 | 172 | 44 | 61 | 122 | 233 |
| Age at saliva collection, mean (SD) | 19.8 (1.1) | 20.0 (1.1) | 20.0 (1.2) | 19.8 (1.2) | 19.9 (1.2) | 20.0 (1.1) |
| Belonging to a sexual orientation minority,g % | 23.1 | 23.3 | 22.7 | 21.3 | 23.0 | 22.8 |
| Hormonal contraception use (female only), % | 32.1 | 45.0 | − | − | − | − |
| Compliant with wakeup sample, % | 94.9 | 97.1 | 93.2 | 96.7 | 94.3 | 97.0 |
| Stressful events in the past month, % | ||||||
| None | 84.6 | 82.0 | 77.3 | 88.5 | 82.0 | 83.7 |
| 1 | 12.8 | 14.0 | 18.2 | 8.2 | 14.8 | 12.5 |
| 2 + | 2.6 | 4.1 | 4.6 | 3.3 | 3.3 | 3.9 |
| Sleep, mean (SD) | ||||||
| Hours of Sleep in previous night | 7.8 (1.3) | 8.0 (1.4) | 7.3 (1.5) | 7.8 (1.5) | 7.6 (1.4) | 8.0 (1.4) |
| Hours of sleep on typical night | 7.8 (1.0) | 8.0 (1.0) | 7.6 (1.3) | 7.7 (1.1) | 7.8 (1.1) | 7.9 (1.0) |
| Time-varying worry score,h mean (SD) | ||||||
| Sample 1 | 1.4 (0.6) | 1.4 (0.6) | 1.4 (0.5) | 1.4 (0.6) | 1.4 (0.6) | 1.4 (0.6) |
| Sample 2 | 1.6 (0.7) | 1.5 (0.6) | 1.4 (0.5) | 1.4 (0.6) | 1.5 (0.6) | 1.5 (0.6) |
| Sample 3 | 1.4 (0.6) | 1.5 (0.6) | 1.4 (0.7) | 1.3 (0.5) | 1.4 (0.6) | 1.4 (0.6) |
| Sample 4 | 1.6 (0.8) | 1.5 (0.6) | 1.5 (0.6) | 1.5 (0.7) | 1.6 (0.7) | 1.5 (0.7) |
| Sample 5 | 1.6 (0.7) | 1.6 (0.7) | 1.4 (0.5) | 1.4 (0.6) | 1.5 (0.6) | 1.5 (0.7) |
| Time-varying happy score h | ||||||
| Sample 1 | 2.0 (0.7) | 1.9 (0.6) | 1.9 (0.6) | 1.9 (0.7) | 1.9 (0.7) | 1.9 (0.6) |
| Sample 2 | 2.2 (0.8) | 2.1 (0.6) | 1.9 (0.7) | 2.1 (0.6) | 2.1 (0.7) | 2.1 (0.6) |
| Sample 3 | 2.4 (0.8) | 2.2 (0.7) | 2.2 (0.7) | 2.3 (0.7) | 2.3 (0.7) | 2.2 (0.7) |
| Sample 4 | 2.3 (0.9) | 2.3 (0.7) | 2.2 (0.6) | 2.3 (0.7) | 2.2 (0.8) | 2.3 (0.7) |
| Sample 5 | 2.2 (0.9) | 2.0 (0.7) | 2.0 (0.9) | 2.0 (0.6) | 2.2 (0.9) | 2.0 (0.6) |
Abbreviations: AHEI, alternative healthy eating index; METS, metabolic-equivalent hours; SD, standard deviation
Recorded on the most recent questionnaire prior to conception of first included offspring.
Percentages of non-missing values.
Recorded in 1991.
One metabolic-equivalent-hour is proportional to the amount of energy spent sitting quietly for one hour.
Self-reported physician/clinician-diagnosed depression; first enquired in 2001 and updated through 2013
Recorded in 1999.
Sexual minority defined based on reporting in 2011 to identify as mostly heterosexual, bisexual, mostly homosexual, gay or lesbian or to have reported any history of same-sex sexual partners.
Do you feel worried, anxious, or fearful right now?’ and ‘Do you feel happy, excited, or content right now?’ with options ranging from 1-Not at all to 4-Extremely.
As shown in Fig. 1A, cortisol level generally increased after awakening, reaching a peak slightly before 1 h after awakening, followed by a sharp decline until roughly 4 h after awakening and less rapid decline until 16 h after awakening. After 16 h, cortisol levels rose until bedtime. Compared to cortisol, sAA profiles followed an almost inverse pattern (Fig. 1B), with a sharp decline within the first hour after awakening, followed by a relatively rapid increase until 5 h after awakening and flattening out afterwards.
Overall, we did not observe any major differences in diurnal rhythms of cortisol or sAA between offspring born to mothers who worked rotating night shifts before pregnancy compared to those whose mothers did not work rotating night shifts. Late decline in cortisol levels (between 4 h and 16 h after awakening) was flattened (percent differences in slope: 2.1%; 95%CI: 0.3, 3.8; Table 2, Fig. 2(A)), sAA awaking response (decline between wake-up sample and 45 min after awakening) was steeper (percentage difference −37.4%; 95%CI: −59.0, −4.4; Table 3, Fig. 3(A)), and sAA showed a decrease before bedtime (−35.9%; 95%CI: −55.3, −8.3; Table 3, Fig. 3(A)) in offspring born to shift working mothers, compared to those born to mothers without shift work.
Table 2.
Percent differences and 95% confidence intervals of diurnal salivary cortisol components comparing GUTS2 saliva sub study participants born between 1989 and 1995 to mothers who worked night shifts before pregnancy to those whose mothers did not work night shifts before pregnancy.
| Maternal history of night shift work before pregnancy | |||||||
|---|---|---|---|---|---|---|---|
| Never | Ever | Ever | Ever | ||||
| Participants | N = 122 | N = 233 Unadjusted | N = 233 MV model 1* | N = 233 MV model 2** | |||
| %D | 95% CI | %D | 95% CI | %D | 95% CI | ||
| Overall | |||||||
| Awakening level a | 0 (ref.) | 10.0 | (−3.0, 24.7) | 12.1 | (−1.1, 27.1) | 11.8 | (−1.4, 26.9) |
| Awakening response b | 0 (ref.) | −4.8 | (−24.7, 20.4) | −4.8 | (−24.7, 20.5) | −5.2 | (−25.1, 19.9) |
| Early decline c | 0 (ref.) | −2.6 | (−7.4, 2.4) | −2.6 | (−7.4, 2.5) | −2.6 | (−7.4, 2.5) |
| Late decline d | 0 (ref.) | 2.0 | (0.3, 3.8) | 2.0 | (0.3, 3.8) | 2.1 | (0.3, 3.8) |
| Night rise e | 0 (ref.) | 0.5 | (−21.7, 28.8) | −0.3 | (−22.2, 27.8) | −0.9 | (−22.6, 27.0) |
| AUC f | 0 (ref.) | 1.2 | (0.0, 2.5) | 1.6 | (0.3, 2.8) | 1.5 | (0.3, 2.7) |
| Overall slope g | 0 (ref.) | 0.8 | (−0.3, 1.9) | 0.7 | (−0.4, 1.9) | 0.7 | (−0.4, 1.9) |
| Overall slope without peak h | 0 (ref.) | 0.9 | (−0.4, 2.1) | 0.9 | (−0.4, 2.1) | 0.8 | (−0.4, 2.1) |
| Females | N = 78 | N = 172 | N = 172 | N = 172 | |||
| Awakening level a | 0 (ref.) | −0.3 | (−13.7, 15.2) | 0.5 | (−13.0, 16.1) | −0.1 | (−13.8, 15.8) |
| Awakening response b | 0 (ref.) | −6.0 | (−27.9, 22.4) | −4.6 | (−27.0, 24.6) | −4.6 | (−27.0, 24.7) |
| Early decline c | 0 (ref.) | 3.1 | (−2.6, 9.1) | 2.4 | (−3.3, 8.5) | 2.4 | (−3.3, 8.5) |
| Late decline d | 0 (ref.) | 0.9 | (−1.0, 2.9) | 1.2 | (−0.8, 3.2) | 1.2 | (−0.8, 3.2) |
| Night rise e | 0 (ref.) | 16.7 | (−14.8, 59.9) | 14.8 | (−16.0, 56.9) | 15.0 | (−15.9, 57.2) |
| AUC f | 0 (ref.) | 1.2 | (−0.2, 2.7) | 1.4 | (0.0, 2.8) | 1.4 | (−0.1, 2.8) |
| Overall slope g | 0 (ref.) | 1.4 | (0.1, 2.8) | 1.5 | (0.1, 2.9) | 1.5 | (0.1, 2.9) |
| Overall slope without peak h | 0 (ref.) | 1.3 | (−0.2, 2.8) | 1.4 | (0.0, 2.9) | 1.4 | (0.0, 2.9) |
| Males | N = 44 | N = 61 | N = 61 | N = 61 | |||
| Awakening level a | 0 (ref.) | 35.5 | (5.5, 74.0) | 38.6 | (7.8, 78.3) | 34.6 | (4.0, 74.2) |
| Awakening response b | 0 (ref.) | −8.7 | (−43.1, 46.7) | −11.6 | (−45.1, 42.3) | −12.9 | (−45.8, 39.8) |
| Early decline c | 0 (ref.) | −13.0 | (−21.5, −3.7) | −12.5 | (−21.1–3.2) | −12.5 | (−21.0, −3.1) |
| Late decline d | 0 (ref.) | 4.5 | (1.1, 8.1) | 4.6 | (1.2, 8.2) | 4.6 | (1.2, 8.2) |
| Night rise e | 0 (ref.) | −28.3 | (−54.6, 13.1) | −29.3 | (−55.3, 11.7) | −29.4 | (−55.3, 11.7) |
| AUC f | 0 (ref.) | 0.8 | (−1.5, 3.2) | 1.0 | (−1.4, 3.5) | 0.5 | (−2.0, 2.9) |
| Overall slope g | 0 (ref.) | −0.7 | (−2.6, 1.4) | −0.6 | (−2.6, 1.4) | −0.6 | (−2.6, 1.4) |
| Overall slope without peak h | 0 (ref.) | −0.3 | (−2.5, 2.1) | −0.3 | (−2.6, 2.0) | −0.4 | (−2.7, 2.0) |
Abbreviations: %D, Percent Differences; CI, confidence interval; AUC, area under curve
Adjusted for age at sample collection in years, wake-up sample within 15 min of waking up (yes/no), saliva collection characteristics (brushed, ate, drank, exercised, happy, worried), usual hours of sleep, last night’s hours of sleep, trauma score in last month (0,1, 2+ events), belonging to sexual orientation minority (yes/no); birth control (yes/no, in females only).
Additionally adjusted for maternal characteristics: maternal age at birth, BMI before pregnancy, smoking status before pregnancy (smoker, past smoker, never smoker), alternative healthy eating score (quintiles), physical activity (MET-hours/week; quintiles), husband’s education (< 2 yr college, 4 yr college, grad school), maternal lifetime history of depression diagnosis (yes/no), chronotype (definite morning type, intermediate, definite evening type).
Cortisol level at awakening.
Change from awakening to 45 min after awakening.
Change from 45 min after awakening to 4 h after awakening.
Change from 4 h after awakening to 16 h after awakening.
Change from 16 h after awakening to bedtime.
Differences in area under the curve, levels from wake up time to 16 h after awakening.
Change from awakening to bedtime.
Change from awakening to bedtime without morning peak (at 45 min).
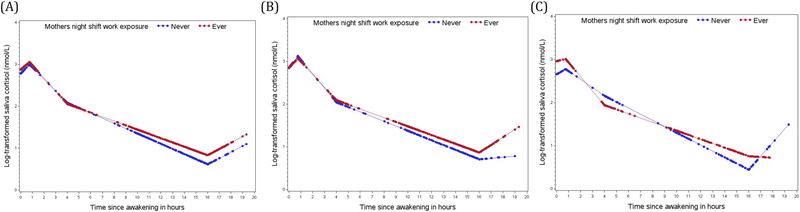
Fig. 2.
Log- transformed diurnal salivary cortisol rhythms of the total sample (A), the female sample (B) and the male sample (C) by maternal shift work exposure before pregnancy comparing offspring whose mothers worked night shift work before pregnancy (red curve) to offspring whose mothers never worked night shifts before pregnancy (blue curve). The curves are based on predictive values obtained from a linear mixed effect models with piecewise linear terms of the time variable.
Table 3.
Percent differences and 95% confidence intervals of diurnal salivary alpha amylase components comparing GUTS2 saliva sub study participants born between 1989 and 1995 to mothers who worked night shifts before pregnancy to those whose mothers did not work night shifts before pregnancy.
| Maternal history of night shift work before pregnancy | |||||||
|---|---|---|---|---|---|---|---|
| Never | Ever | Ever | Ever | ||||
| Participants | N = 122 | N = 208 Unadjusted | N = 208 MV model 1* | N = 208 MV model 2** | |||
| %D | 95% CI | %D | 95% CI | %D | 95% CI | ||
| Overall | |||||||
| Awakening level a | 0 (ref.) | 27.0 | (−3.5, 67.3) | 28.9 | (−2.0, 69.4) | 25.4 | (−4.9, 65.3) |
| Awakening response b | 0 (ref.) | −37.8 | (−59.4, −4.8) | −37.7 | (−59.2, −4.9) | −37.4 | (−59.0, −4.4) |
| Early decline c | 0 (ref.) | 2.6 | (−6.1, 12.1) | 2.8 | (−5.9, 12.3) | 2.7 | (−6.0, 12.2) |
| Late decline d | 0 (ref.) | 0.0 | (−2.8, 2.8) | −0.1 | (−2.8, 2.7) | 0.0 | (−2.7, 2.8) |
| Night rise ef | 0 (ref.) | −33.2 | (−53.1, −4.8) | −34.3 | (−54.1, −6.0) | −35.9 | (−55.3, −8.3) |
| AUC f | 0 (ref.) | −0.6 | (−3.2, 2.0) | −0.3 | (−2.9, 2.3) | −0.7 | (−3.3, 1.9) |
| Overall slope g | 0 (ref.) | −1.3 | (−2.9, 0.4) | −1.3 | (−2.9, 0.4) | −1.3 | (−2.9, 0.4) |
| Overall slope without peak h | 0 (ref.) | −1.9 | (−3.8, 0.1) | −1.9 | (−3.9, 0.1) | −1.9 | (−3.9, 0.1) |
| Females | N = 73 | N = 161 | N = 161 | N = 161 | |||
| Awakening level a | 0 (ref.) | 8.0 | (−22.4, 50.2) | 10.8 | (−20.3, 54.1) | 1.2 | (−28.0, 42.4) |
| Awakening response b | 0 (ref.) | −16.4 | (−49.8, 39.2) | −17.6 | (−50.5, 37.2) | −4.2 | (−42.9, 60.8) |
| Early decline c | 0 (ref.) | −0.9 | (−10.9, 10.3) | −0.2 | (−10.3, 11.0) | −0.4 | (−10.6, 11.0) |
| Late decline d | 0 (ref.) | 0.2 | (−3.1, 3.6) | −0.1 | (−3.4, 3.4) | 0.0 | (−3.4, 3.5) |
| Night rise e | 0 (ref.) | −30.7 | (−57.5, 12.9) | −32.3 | (−58.9, 11.6) | −31.6 | (−58.0, 11.4) |
| AUC f | 0 (ref.) | −1.1 | (−4.3, 2.0) | −0.8 | (−3.9, 2.3) | −0.5 | (−3.7, 2.7) |
| Overall slope g | 0 (ref.) | −1.2 | (−3.2, 0.9) | −1.3 | (−3.3, 0.8) | −1.2 | (−3.2, 0.8) |
| Overall slope without peak h | 0 (ref.) | −1.2 | (−3.6, 1.4) | −1.3 | (−3.8, 1.2) | −1.3 | (−3.8, 1.2) |
| Males | N = 39 | N = 47 | N = 47 | N = 47 | |||
| Awakening level a | 0 (ref.) | 67.5 | (−0.6, 182.3) | 75.4 | (3.7196.6) | 67.9 | (−3.9, 193.2) |
| Awakening response b | 0 (ref.) | −64.4 | (−83.9, −21.3) | −65.6 | (−84–5, −23.7) | −65.1 | (−84.5, −21.4) |
| Early decline c | 0 (ref.) | 12.9 | (−4.3, 33.1) | 12.7 | (−4.5, 32.9) | 13.1 | (−4.5, 34.0) |
| Late decline d | 0 (ref.) | −1.5 | (−6.5, 3.7) | −1.1 | (−6.1, 4.2) | −1.7 | (−6.8, 3.6) |
| Night rise e | 0 (ref.) | −30.0 | (−63.4, 34.0) | −32.1 | (−64.6, 30.1) | −12.8 | (−56.0, 73.0) |
| AUC f | 0 (ref.) | 0.4 | (−4.4, 5.3) | 1.0 | (−3.9, 5.8) | 0.1 | (−5.3, 5.6) |
| Overall slope g | 0 (ref.) | −1.7 | (−4.7, 1.4) | −1.7 | (−4.6, 1.4) | −1.6 | (−4.5, 1.5) |
| Overall slope without peak h | 0 (ref.) | −3.7 | (−7.1, −0.2) | −3.7 | (−7.1, −0.2) | −3.6 | (−7.1, −0.1) |
Abbreviations: %D, Percent Differences; CI, confidence interval; AUC, area under curve
Adjusted for age at sample collection, wake-up sample within 15 min of waking up (yes/no), saliva collection characteristics (brushed, ate, drank, exercised, happy, worried), usual hours of sleep, last night’s hours of sleep, trauma score in last month (0,1, 2+ events), belonging to sexual orientation minority (yes/no); birth control (yes/no, in females only).
Additionally adjusted for maternal characteristics: maternal age at birth, BMI before pregnancy, smoking status before pregnancy (smoker, past smoker, never smoker), alternative healthy eating score (quintiles), physical activity (MET-hours/week; quintiles), husband’s education (<2yr college, 4yr college, grad school), maternal lifetime history of depression diagnosis (yes/no), chronotype (definite morning type, intermediate, definite evening type).
Amylase level at awakening.
Change from awakening to 45 min after awakening.
Change from 45 min after awakening to 4 h after awakening.
Change from 4 h after awakening to 16 h after awakening.
Change from 16 h after awakening to bedtime.
Area under the curve, levels from wake up time to 16 h after awakening.
Change from awakening to bedtime.
Change from awakening to bedtime without morning peak (at 45 min).
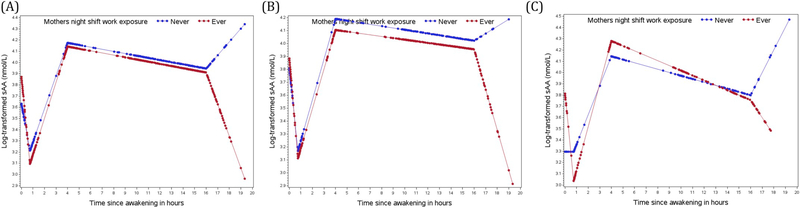
Fig. 3.
Log- transformed diurnal salivary alpha amylase (sAA) rhythms of the total sample (A), the female sample (B) and the male sample (C) by maternal shift work exposure before pregnancy comparing offspring whose mothers worked night shift work before pregnancy (red curve) to offspring whose mothers never worked night shifts before pregnancy (blue curve). The curves are based on predictive values obtained from a linear mixed effect models with piecewise linear terms of the time variable.
In sex stratified analyses, cortisol slopes based on all collection times were slightly flatter in female offspring born to night shift working mothers (percent difference in overall slope: 1.5%; 95%CI: 0.1, 2.9 Table 2, Fig. 2(B)), compared to female offspring whose mothers did not work night shifts. Male offspring born to shift working mothers had higher awakening cortisol levels (34.6%; 95%CI: 4.0, 74.2; Table 2, Fig. 2(C)), followed by a steeper early decline (between 45 min and 4 h after awakening. −12.5%; 95%CI: −21.0, −3.1) and a flatter late decline (between 4 h and 16 h after awakening, 4.6%; 95%CI: 1.2, 8.2) compared to male offspring whose mothers did not work night shifts. None of the sAA components were significantly different in female offspring (Table 3, Fig. 3(B)), while male offspring born to night shift working mothers showed a more pronounce awakening response (change between wake up and 45 min after awakening, −65.1%; 95%CI: −84.5, −21.4, Fig. 3(C)).
Stratified results by maternal chronotype are shown in Supplemental Tables 1 and 2. Overall, we did not observe any consistent differences in cortisol or sAA patterns by chronotype, although these analyses were limited by small numbers in each stratum.
In Supplemental Tables 3 and 4 geometric means and geometric standard deviation factors of the outlier corrected cortisol and sAA data on the original scale for the 5 measurement times.
4. Discussion
Utilizing night shift work history information from mothers participating in NHSII and saliva samples of their offspring enrolled in GUTS2, we found no major overall differences in cortisol or alpha amylase patterns between offspring born to mothers who had worked rotating night shifts before conception versus offspring whose mothers did not. However, we observed some differences in cortisol and sAA patterns suggesting a slightly different stress marker profile in offspring of women with chronic exposure to night shift work prior to pregnancy, compared to offspring of women who never worked night shifts. The observed differences were primarily found in male offspring, suggesting possible sex-specific differences in response to maternal pre-conceptional stress exposure.
Rotating night shift work is a recognized social and biological stressor [30], and studies have previously shown higher risk of several chronic disease outcomes [33,34] as well as altered cortisol [8] and sAA [22] levels among night workers, compared to non-night workers. However, to the best of our knowledge no prior study has investigated the effect of rotating night shift work exposure before pregnancy on offspring stress markers in adulthood.
Previous research in animals suggests a transgenerational effect of stress, as reviewed by Klengel et al. [18]. More particularly, studies by Dietz et al. [10] in mice and Babb et al., (2014) in rats include reports on altered corticosterone levels in offspring born to mice exposed to chronic social defeat stress during adulthood and rats exposed to a chronic social stress protocol during early life [6]. Recently Bale and Epperson [4] reviewed data findings and mechanisms driving sex differences in stress responses through all stages of life, starting with exposure during gestation. However, literature on responses to parental stress exposure before conception is still scare [7] and potential sex difference and underlying mechanisms remain to be elucidated.
To date, few studies on inter- and transgenerational effects of stress exposure have been conducted in humans. Most of the research has focused on severe stressors, such as PTSD (post-traumatic stress disorder) following the World Trade Center attack or the Holocaust [12,36,37]. Relevant in this context, Yehuda et al. investigated cortisol levels of babies born to mothers who were exposed to the World Trade Center attack during pregnancy and found that babies born to mothers who developed PTSD had lower awakening and bedtime cortisol levels [37]. However, literature regarding the effects of prenatal stress exposures on stress marker profiles assessed later in the life of the offspring are scarce. Our study provides evidence to suggest changes in the stress marker profiles of offspring born to mothers with chronic night work exposure before pregnancy; thus, further study on potential transand intergenerational effects of chronic moderate and low-level stress in mothers is warranted.
Strengths of this study include its consideration of numerous potential confounding variables, including maternal lifestyle and socioeconomic status as well as detailed information about behaviors and mood on the day of sample collection. Further, the repeated collection of saliva at five well-defined time points throughout one day allowed us to characterize diurnal cortisol and alpha amylase patterns in great detail.
Several limitations need to be mentioned. The self-reported nature of the rotating night shift work exposure could have led to exposure misclassification. However, potential misclassifications are most likely non-differential and hence would have made it less likely to detect differences in the outcome (i.e., would tend to be biased toward, rather than away, from the null). Unfortunately, we did not have information on night shift work exposure during pregnancy. Therefore, we could not disentangle the total effect of maternal pre-conceptional shift work exposure on offspring stress markers into components solely driven by pre-conceptional shift work, solely shift work during pregnancy or the combination of pre-conceptional and during pregnancy exposure. Compliance to sampling time (especially regarding the first morning sample) was simply assessed by comparing self-reported wake-up and sample collection times. These assessments could be improved by electronic sampling protocols in future studies. While it is possible to measure cortisol levels in saliva, most research involving the SAM-axis still involves collection of urine or blood. However, Nater and colleagues established methods to determine SAM-axis activity in samples of saliva, which is easier to collect; salivary alpha-amylase (sAA) is a stress-sensitive biomarker of SAM-axis activities [26]. Despite the overall large sample size of the saliva sub-study, the sample size for the current analysis was smaller, resulting in power limitations, particularly in the stratified analyses. Finally, our sample consisted predominantly of non-Latino white U.S. nurses and their children raised in largely middle-income households. Hence, our findings might not be directly generalizable to other populations.
In conclusion, we found no major differences in salivary cortisol and alpha amylase patterns in early adulthood comparing offspring of mothers who worked rotating night shifts before conception to those whose mothers did not. Future studies with larger samples sizes, more detailed exposure assessment, particularly also in utero information, and multiple offspring biomarker assessments at different developmental stages are needed to further investigate these associations, also investigating differences by offspring sex.
Supplementary Material
Acknowledgement
We thank the thousands of participants in the Growing Up Today Study as well as their mothers.
Funding
This study was supported by The National Institute for Occupational Safety and Health grant 5R01OH009803 (PI: Schernhammer E), as well as grant U01 CA176726 National Cancer Institute, and grants HD057368 and HD066963 from the National Institutes of Child Health and Human Development.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Financial disclosures
Dr. Devore has received consulting fees from Epi Excellence and Bohn Epidemiology.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.physbeh.2019.05.007.
References
- [1].Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, Paffenbarger RS, Compendium of physical activities: classification of energy costs of human physical activities, Med. Sci. Sports Exerc. 25 (1993) 71–80. [DOI] [PubMed] [Google Scholar]
- [2].Austin SB, Rosario M, McLaughlin KA, Roberts AL, Gordon AR, Sarda V, Missmer S, Anatale-Tardiff L, Scherer EA, Sexual orientation and diurnal cortisol patterns in a cohort of U.S. young adults, Psychoneuroendocrinology 69 (2016) 197–208, 10.1016/j.psyneuen.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Babb JA, Carini LM, Spears SL, Nephew BC, Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats, Horm. Behav. 65 (2014) 386–393, 10.1016/J.YHBEH.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bale TL, Epperson CN, Sex differences and stress across the lifespan, Nat. Neurosci. (2015), 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhatti P, Zhang Y, Song X, Makar KW, Sather CL, Kelsey KT, Houseman EA, Wang P, Nightshift work and genome-wide DNA methylation, Chronobiol. Int. 32 (2015) 103–112, 10.3109/07420528.2014.956362. [DOI] [PubMed] [Google Scholar]
- [6].Carini LM, Murgatroyd CA, Nephew BC, Using chronic social stress to model postpartum depression in lactating rodents, J. Vis. Exp. (2013) e50324,, 10.3791/50324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chan JC, Nugent BM, Bale TL, Review Parental Advisory: Maternal and Paternal Stress Can Impact Offspring Neurodevelopment, (2018), 10.1016/j.biopsych.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Charles LE, Fekedulegn D, Burchfiel CM, Hartley TA, Andrew ME, Violanti JM, Miller DB, Shiftwork and diurnal salivary cortisol patterns among police officers, J. Occup. Environ. Med. 58 (2016) 542–549, 10.1097/JOM.0000000000000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, Hultman CM, Långström N, Lichtenstein P, D’Onofrio BM, Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress, Psychol. Med. 44 (2014) 71–84, 10.1017/S0033291713000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ, Paternal transmission of stress-induced pathologies, Biol. Psychiatry 70 (2011) 408–414, 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferri P, Guadi M, Marcheselli L, Balduzzi S, Magnani D, Di Lorenzo R, The impact of shift work on the psychological and physical health of nurses in a general hospital: a comparison between rotating night shifts and day shifts, Risk Manag. Healthc. Policy 9 (2016) 203–211, 10.2147/RMHP.S115326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Flory JD, Bierer LM, Yehuda R, Maternal exposure to the holocaust and health complaints in offspring, Dis. Markers 30 (2011) 133–139, 10.3233/DMA-2011-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goodman LA, Corcoran C, Turner K, Yuan N, Green BL, Assessing traumatic event exposure: general issues and preliminary findings for the stressful life events screening questionnaire, J. Trauma. Stress. 11 (1998) 521–542, 10.1023/A:1024456713321. [DOI] [PubMed] [Google Scholar]
- [14].Horne JA, Ostberg O, A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms, Int. J. Chronobiol. 4 (1976) 97–110. [PubMed] [Google Scholar]
- [15].Huang T, Poole EM, Vetter C, Rexrode KM, Kubzansky LD, Schernhammer E, Rohleder N, Hu FB, Redline S, Tworoger SS, Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women, Psychoneuroendocrinology 84 (2017) 172–180, 10.1016/j.psyneuen.2017.07.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Juda M, Vetter C, Roenneberg T, Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers, J. Biol. Rhythm. 28 (2013) 141–151, 10.1177/0748730412475042. [DOI] [PubMed] [Google Scholar]
- [17].Keenan K, Hipwell AE, Class QA, Mbayiwa K, Extending the developmental origins of disease model: impact of preconception stress exposure on offspring neurodevelopment, Dev. Psychobiol. (2018), 10.1002/dev.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Klengel T, Dias BG, Ressler KJ, Models of intergenerational and transgenerational transmission of risk for psychopathology in mice, Neuropsychopharmacology 41 (2016) 219–231, 10.1038/npp.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matthews SG, Phillips DIW, Minireview: transgenerational inheritance of the stress response: a new frontier in stress research, Endocrinology 151 (2010) 7–13, 10.1210/en.2009-0916. [DOI] [PubMed] [Google Scholar]
- [20].McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC, Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance, Am. J. Clin. Nutr. 76 (2002) 1261–1271. [DOI] [PubMed] [Google Scholar]
- [21].McMenamin TM, A time to work: recent trends in shift work and flexible schedules, Bur. Labor Stat. (2007). [Google Scholar]
- [22].Morita Y, Aida H, Yamaguchi T, Azuma M, Suzuki S, Suetake N, Yukishita T, Lee K, Kobayashi H, Morita Y, Effects of prolonged night shifts on salivary α-amylase, secretory immunoglobulin, cortisol, and chromogranin a levels in nurses, Health (Irvine. Calif.) 6 (2014) 2014–2025, 10.4236/health.2014.615236. [DOI] [Google Scholar]
- [23].Nater UM, Skoluda N, Strahler J, Biomarkers of stress in behavioural medicine, \ 26 (2013) 440–445, 10.1097/YCO.0b013e328363b4ed. [DOI] [PubMed] [Google Scholar]
- [24].Pan A, Schernhammer ES, Sun Q, Hu FB, Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women, PLoS Med. 8 (2011), 10.1371/journal.pmed.1001141e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Piazza JR, Almeida DM, Dmitrieva NO, Klein LC, Frontiers in the use of biomarkers of health in research on stress and aging, J. Gerontol. B. Psychol. Sci. Soc. Sci. 65 (2010) 513–525, 10.1093/geronb/gbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rohleder N, Nater UM, Determinants of salivary α-amylase in humans and methodological considerations, Psychoneuroendocrinology 34 (2009) 469–485, 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- [27].Rosner B, Cook N, Portman R, Daniels S, Falkner B, Determination of blood pressure percentiles in Normal-weight children: some methodological issues, Am. J. Epidemiol. 167 (2008) 653–666, 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- [28].Siddiqui A, Madhu SV, Sharma SB, Desai NG, Endocrine stress responses and risk of type 2 diabetes mellitus, Stress 18 (2015) 498–506, 10.3109/10253890.2015.1067677. [DOI] [PubMed] [Google Scholar]
- [29].Steptoe A, Ussher M, Smoking, cortisol and nicotine, Int. J. Psychophysiol. 59 (2006) 228–235, 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- [30].Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V, WHO International Agency For Research on Cancer Monograph Working Group, Carcinogenicity of shift-work, painting, and fire-fighting, Lancet Oncol. 8 (2007) 1065–1066, 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- [31].Strohmaier S, Devore E, Vetter C, Eliassen AH, Rosner B, Okereke OI, Schernhammer ES, n.d. Night shift work before and during pregnancy in relation to depression and anxiety in adolescent and young adult offspring submitted 10.1007/s10654-019-00525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun M, Feng W, Wang F, Li P, Li Z, Li M, Tse G, Vlaanderen J, Vermeulen R, Tse LA, Meta-analysis on shift work and risks of specific obesity types, Obes. Rev. 19 (2018) 28–40, 10.1111/obr.12621. [DOI] [PubMed] [Google Scholar]
- [33].Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES, Association between rotating night shift work and risk of coronary heart disease among women, JAMA 315 (2016) 1726, 10.1001/jama.2016.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, Laden F, Willett WC, Hankinson SE, Schernhammer ES, Rotating night-shift work and the risk of breast Cancer in the Nurses’ health studies, Am. J. Epidemiol. 186 (2017) 532–540, 10.1093/aje/kwx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE, Reproducibility and validity of a semiquantitative food frequency questionnaire, Am. J. Epidemiol. 122 (1985) 51–65. [DOI] [PubMed] [Google Scholar]
- [36].Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Müller-Myhsok B, Holsboer F, Buxbaum JD, Gene expression patterns associated with posttraumatic stress disorder following exposure to the world trade center attacks, Biol. Psychiatry 66 (2009) 708–711, 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- [37].Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS, Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the world trade center attacks during pregnancy, J. Clin. Endocrinol. Metab. 90 (2005) 4115–4118, 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- [38].Yehuda R, Lehrner A, Bierer LM, Gerlinde M, The public reception of putative epigenetic mechanisms in the transgenerational effects of trauma, Environ. Epigen. 4 (2018), 10.1093/eep/dvy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhu Y, Stevens RG, Hoffman AE, Tjonneland A, Vogel UB, Zheng T, Hansen J, Epigenetic impact of long-term shiftwork: pilot evidence from circadian genes and whole-genome methylation analysis, Chronobiol. Int. 28 (2011) 852–861, 10.3109/07420528.2011.618896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.