Abstract
Asthma is a heterologous disease that is influenced by complex interactions between multiple environmental exposures, metabolism, and host immunoregulatory processes. Specific metabolites are increasingly recognized to influence respiratory inflammation. However, the role of protein‐derived metabolites in regulating inflammatory responses in the lung are poorly described. The aims of the present study were to quantify polyamine levels in bronchoalveolar lavages (BALs) from healthy volunteers and asthma patients, and to evaluate the impact of each polyamine on inflammatory responses using in vitro models and in a house dust mite (HDM)‐induced respiratory allergy model. Spermidine levels were decreased, while cadaverine levels were increased in BALs from asthma patients compared to healthy controls, using Ultra Performance Liquid Chromatography (UPLC). Both spermine and spermidine inhibit lipopolysaccharide (LPS)‐induced cytokine secretion from human peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) in vitro. In addition, oral gavage with spermine or spermidine modulate HDM‐induced cell infiltration, cytokine secretion, and epithelial cell tight junction expression in murine models. Spermidine also reduces airway hyper‐responsiveness. These results suggest that modulation of polyamine metabolism, in particular spermidine, is associated with respiratory inflammation and these molecules and pathways should be further explored as biomarkers of disease and potential targets for novel therapies.
Keywords: asthma, mouse models, polyamines, spermidine, spermine
Spermine and spermidine display anti‐inflammatory effects using in vitro models and protect against house dust mite (HDM) extract‐induced airway inflammation in murine models.

1. INTRODUCTION
Asthma is a chronic inflammatory disorder of the airways, usually associated with airway hyper‐responsiveness and variable airflow obstruction that is often reversible spontaneously or during treatment. Asthma affects more than 300 million people worldwide and is thought to be caused by a combination of genetic, microbiome, dietary and environmental factors, resulting in inflammation of the airways and tissue remodeling. 1 However, the complex interactions between these factors and host immunoregulatory processes are not well‐understood at a mechanistic level in humans. In particular, the role of protein‐derived metabolites in regulating inflammatory responses in the lung is poorly described.
Diamines (e.g. cadaverine and putrescine), tri‐aimes (e.g. spermidine), and tetra‐amines (e.g. spermine), collectively termed polyamines, play multiple important roles in maintaining host physiology, including influencing cell proliferation, cell growth, mitochondrial function, gene translation, and autophagy. 2 , 3 , 4 , 5 These polyamines can be generated by host metabolic processes, can be absorbed from a wide variety of dietary sources, or can be produced by intestinal microbes in the presence of appropriate substrates. 6 , 7 , 8 In addition to their effects on cellular physiology, polyamines are increasingly being recognized as modulators of immune and inflammatory responses. In murine models, serum levels of pro‐inflammatory cytokines and LPS‐induced nitric oxide related damage was inhibited by oral treatment with spermine. 9 Spermidine decreased the severity of colitis in a DSS model, while topical administration of putrescine, spermine, and spermidine decreased skin inflammation following TPA application. 10 , 11 In respiratory allergy murine models, polyamines were found to be increased following challenge with ovalbumin, while allergen challenge in asthma patients was associated with increased putrescine and spermine, but not spermidine, levels in sputum. 12 In addition, putrescine, spermidine, and spermine levels were increased in the peripheral blood of asthma patients with active symptoms. 13 Spermine has been shown to influence cell types relevant to asthma. In vitro experiments suggest that spermine promotes survival, upregulates integrin expression, and stimulates chemotaxis in human eosinophils, while spermine can also induce bronchial epithelial cell injury in a concentration dependent manner. 14 , 15
Given the potential immune modulatory effects of polyamines, the aims of the present study were to quantify polyamine levels in bronchoalveolar lavages from healthy volunteers and asthma patients, and to evaluate the impact of each polyamine on lung inflammatory responses in a house dust mite‐induced respiratory allergy model. Our results show that spermidine levels decrease in BALs from asthma patients, while oral supplementation of mice decreased lung inflammation. Modulation of polyamine metabolism is associated with asthma pathogenesis and therapeutic targeting of these molecules and pathways should be further explored in human clinical studies.
2. MATERIALS AND METHODS
2.1. Human samples
A total of 22 asthma patients and 18 healthy matched volunteers were recruited for this study. Asthma patients had a physician diagnosis of asthma. Patients were recruited under informed consent at ALL‐MED Medical Research Institute, Wroclaw, Poland. Ethical approval was granted from the local ethical committee for all study procedures (KB‐567/2014). BAL was obtained and filtered through a 70 μm filter into sterile tubes. Aliquots of 500 μl were stored at −80℃ for later analysis.
2.2. BAL polyamine quantification
475 μl of BAL was mixed with 25 μl of 5 g/L 1,7‐diaminoheptane (Internal standard) 100 µl of 2 M NaOH, 150 µl of saturated sodium bicarbonate solution, and 1 ml of dansyl chloride solution (10 mg/ml in acetone), and was then incubated at 40℃, 200 rpm, for 45 min. Residual dansyl chloride was removed by adding 50 µl of 25% ammonium hydroxide. After 30 min at 25℃, the volume was adjusted to 2.5 ml with acetonitrile, centrifuged at 3500 rpm for 5 min, and supernatants were filtered (0.22 µm) prior to Ultra Performance Liquid Chromatography (UPLC) analysis. Duplicate samples were analysed in parallel. Separation was carried out by UPLC on an ACQUITY UPLC H‐Class Bio System equipped with a quaternary solvent manager, sample manager FTN, column manager, and diode array detector. Data processing was performed using MassLynxV4.1. Based on their different hydrophobicity, the dansylated polyamines were separated on an ACQUITY UPLC BEHC18 column and the samples were eluted with a gradient elution of (A) Acetonitrile (100%), (B) Acetonitrile (50%) as follows: 0–0.72 min, A 40%, B 60%; 0.72–1.07 min, A 40%–80%, B 60%–20%; 1.07–1.42 min, A 80%–90%, B 20%–10%; 1.42–2.11 min, A 90%–95%, B 10%–5%; 2.11–2.46 min, A 95%–40%, B 5%–60%, 2.46–4.20 min, A 40%, B 60%. The flow rate was kept at 0.6 ml/min, column temperature at 25℃, injection 1 µl, and detection wavelength was 217 nm.
2.3. Cell isolation and culture
Peripheral blood mononuclear cells (PBMC`s) were isolated from buffy coats and whole blood using density gradient centrifugation. CD14+ monocytes were isolated from healthy human PBMCs by using AutoMACS and CD14+ beads. IL‐4 (1000 IU/ml) and GM‐CSF (1000 IU/ml) were used for 5 days to differentiate CD14+ monocytes into monocyte‐derived dendritic cells (MDDCs) in vitro. Cells were cultured with polyamines at 37℃ in 5% CO2, in cRPMI 1640 medium supplemented with 1 mM sodium pyruvate, 1% MEM nonessential amino acids and vitamins, 2 mM l‐glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 50 mM 2‐Mercaptoethanol containing 10% of human serum. THP‐1–XBlue cells contain an NF‐kB/activator protein 1 (AP‐1) reporter system. THP‐1 cells have been transfected with a reporter plasmid expressing a secreted embryonic alkaline phosphatase under the control of a promoter that is induced by the transcription factors NF‐κB and AP‐1. For mouse as well human cells culture supernatants, concentration of cytokines and chemokines were quantified with the BioPlex Multiples Array System (Bio‐Rad Laboratories) or with MSD (Meso Scale Discovery). Spermine as well as spermidine used in in vitro and in vivo experiments were freshly prepared in cRPMI containing 10% human serum.
2.4. Animals
Female C57BL/6 mice aged 6–8 weeks were housed for at least for 2 weeks before experiment, at the AO Research Institute in Davos. Mice were housed at 4–6 animals per cage in individually ventilated cages in a 12‐h/12‐h light/dark cycle, with food and water available ad libitum. All experimental procedures were carried out in accordance with Swiss law and approved by the animal experiment commission of the canton Grisons in Switzerland.
2.5. House dust mite model of allergic airway inflammation in mice
Mice were treated at day 0 with 1 µg and on days from 7 to 11 with 10 µg of house dust mite extract or NaCl administered intranasally. Mice were sacrificed and the experiment was analysed on day 12. Mice received 10 mM/200 μl of polyamines by means of gavage starting 5 days before and at the day 0 of first house dust mite or NaCl application. This equates to approximately 15 mg/kg in a 20 g mouse. Subsequently, polyamines were administrated from day 7 to day 11 by means of gavage.
2.6. Murine BAL (bronchoalveolar lavage)
BAL was performed with 1 ml of PBS containing 1 × protease inhibitor cocktail. Red blood cells were removed by resuspending BAL cells in red blood cell lysis buffer (Sigma‐Aldrich) for 2 min at room temperature followed by washing in 1 ml PBS. The total number of leukocytes was counted with a Neubauer counting chamber. Cells were centrifuged and the cell‐free supernatant was stored at −80℃ until cytokines were measured with BioPlex (Bio‐Rad, Hercules).
Single cell suspensions were obtained from lung tissue using GentleMACS. Lung‐derived single‐cell suspensions were plated at a concentration of 1 × 106 cells/ml in complete RPMI (Sigma‐Aldrich) and were re‐stimulated with HDM (Greer) for 48 h at 37℃. Culture supernatants were assayed for cytokine levels by Bio‐Plex Multiplex Immunoassay System (Bio‐Rad). For lung function measurements, mice were intubated under anaesthesia and airway resistance was assessed using the forced oscillation technique (FlexiVent system). Airway resistance was measured in response to increasing concentrations of methacholine from 0, 0.1, 0.3, 1, 3, 10, to 30 mg/ml.
2.7. Lung histology & Immunofluorescence staining of tight junction proteins
Lung tissues were fixed with 4% PFA for 4 h and stored in 70% isopropanol at 4℃. Lung tissues were embedded into paraffin and 3‐µm sections were stained with hematoxylin and eosin (H&E). For immunofluorescence staining of mouse lung, paraffin‐embedded tissue slides (5 mm) were subjected to antigen retrieval in citrate buffer, pH 6 (Fluka). Antibodies used for immunofluorescence were as follows: anti–ZO‐1 anti‐claudin‐4 and secondary antibody goat anti‐rabbit Alexa Fluor 488, and goat anti‐rabbit Alexa Fluor 546. After staining, tissues were mounted with 49–6‐diamidino‐phenylindole dihydrochloride–containing mounting media. Stained slides were stored at 22℃ in the dark. Confocal images were taken and analyzed with an LSM510 Meta Laser Scanning microscope.
2.8. Flow cytometry
MDDCs were stained with anti‐CD80, ‐CD86, ‐PDL1, ‐PDL2, ‐CD11c, and ‐7AAD antibodies. After staining for 25 min in dark at 8℃, cells were washed with PBS and acquired with flow cytometer.
For analysis of intracellular cytokine production by T cells, cells were collected from cell culture and stained with the fixable viability dye eFluor780. For intracellular cytokine staining, cells were stimulated with phorbol 12‐myristate 13‐acetate/ionomycin (50 and 500 ng/ml) for 4 h at 37℃ in 5% CO2 atmosphere in the presence of Brefeldin A solution to inhibit cytokine secretion. Cells were fixed and stained for surface markers CD3 and CD4 for 25 min at 8℃. Next, cells were permeabilized with intracellular staining perm wash buffer and fixation buffer. For intracellular cytokines staining, anti‐IL‐17A, IL‐13, IFN‐gamma, IL‐4, IL‐10, IL‐5 antibodies were used. After staining cells were washed with PBS and acquired by flow cytometry.
Flow cytometric acquisition was performed on the Gallios Flow Cytometer (Beckman Coulter). Flow cytometry data were analysed using FlowJo software.
2.9. Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology. 16 The data were analyzed by an investigator blinded to the experimental conditions. Each in vitro experiment was conducted independently at least three times, and more than five animals per group were required for the animal experiments. The Kolmogorov–Smirnov normality test was used to determine data normality. The data were expressed as mean ± SEM. Statistical significance between the two groups was calculated using the nonparametric Mann–Whitney test. Intergroup differences for multiple groups were assessed by one‐way analysis of variance (ANOVA) followed by post hoc analysis (Dunnett's multiple comparisons test). All data analysis was carried out using GraphPrism software (GraphPad Software, Inc). Differences were considered statistically significant at p < .05.
2.10. Materials
For polyamine quantification in BAL fluid: 1,7‐diaminoheptane, NaOH, saturated sodium bicarbonate solution, dansyl chloride were obtained from Sigma (Sigma‐Aldrich), ammonium hydroxide from (Merck), acetonitrile from Biosolve Chimie, Ultra Performance Liquid Chromatography (UPLC) on an ACQUITY UPLC H‐Class Bio System, and MassLynxV4.1 software from Waters Corp.
For cell culture and isolation: density gradient solution was purchased from Biochrom (L6155), CD14+ isolation kit and Automacs from Miltenyi Biotec, IL‐4 from Novartis, GM‐CSF from Peprotech, RPMI medium from Gibco (21875034), medium supplements from Gibco, Human Serum Type AB from Sigma‐Aldrich (H4522).THP‐1‐XBlue cells were obtained from InvivoGen and BioPlex Multiples Array System from Bio‐Rad Laboratories. Spermine and spermidine used in in vivo experiments were purchased from Sigma‐Aldrich (S4264, S0266, Sigma‐Aldrich).
Mice were obtained from Charles River, house dust mite extract from GREER Laboratories, protease inhibitor cocktail from Roche, red blood cell lysis buffer from Sigma‐Aldrich, FlexiVent system from SCIREQ, and methacholine from Sigma‐Aldrich.
Rabbit polyclonal anti‐ZO‐1 antibody was purchased from Invitrogen, rabbit polyclonal anti‐claudin‐4 antibody from Abcam, goat‐anti‐rabbit AF388, antibody and goat‐antirabbit AF546 from Invitrogen. LSM510 Meta Laser Scanning Microscope from Zeiss.
Flow cytometry antibodies: anti‐CD80 from Biolegend (104706, FITC), anti‐CD86 from Biologend (105106, PE), anti‐CD274 from Biolegend (124314, Pe/Cy7), anti‐CD273 from Biolegend (107210, APC), anti‐CD11c from Biolegend (117322, Pacific Blue), 7AAD from ThermoFisher Scientific (A1310, 7‐aminoactinomycin D), viability dye eFluor780 from Thermofisher Scientific (65‐0865‐18), phorbol 12‐myristate 13‐acetate, ionomycin, brefeldin A from eBioscience, anti‐CD3 from Biolegend (100220, Pe/Cy7), anti CD4 from BD Horizon (560782, V500), perm wash buffer from Biolegend (421002), fixation buffer from Biolegend (420801), anti‐IL‐17A from Biolegend (506910, AlexaFluor), anti‐IL‐13 from eBioscience (50–112–9339, PE‐eFluor 610), anti‐IFN‐gamma from Biolegend (505822 PerCp/Cyanine 5.5), anti‐IL‐4 from Biolegend (504106, APC), anti‐IL‐10 from eBioscience (56–7101–82, AlexaFluor 700), anti‐IL‐5 from Biolegend (504311, Briliant Violet 421), and Gallios Flow Cytometer from Beckman Coulter.
2.11. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 17 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 18
3. RESULTS
3.1. Altered polyamine levels in BALs from asthma patients
BALs were obtained from asthma patients (n = 22, male:female 7:15, mean age = 42.4) and healthy volunteers (n = 18, male:female 7:11, mean age = 42.3). Spermine was the polyamine found at highest concentrations in human BALs, but levels were similar for asthma patients and healthy controls (Figure 1). Spermidine levels were significantly lower in BALs from asthma patients, while cadaverine levels were significantly elevated in BALs from asthma patients. Putrescine levels were similar for both groups.
FIGURE 1.
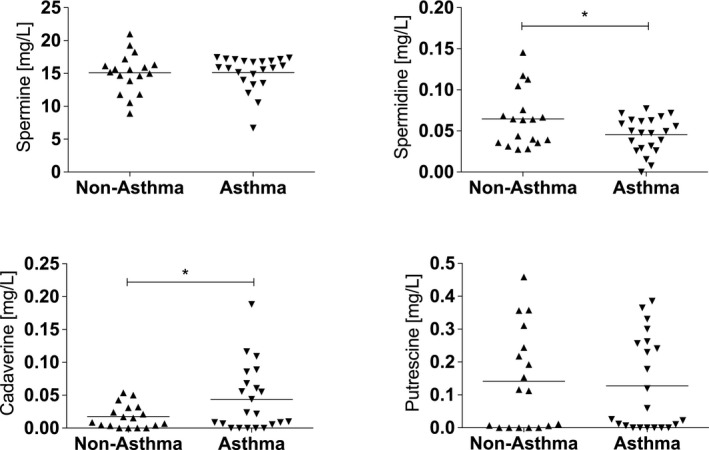
Spermine levels are decreased in BAL from asthma patients. In BAL fluid from patients with asthma (n = 22), spermidine levels are decreased and cadaverine levels are increased as compared to non‐asthmatic controls (n = 18). (*p < .05 between groups [Mann–Whitney test])
3.2. Spermine and spermidine modulate LPS‐induced cell activation
To investigate the effect of polyamines on NF‐κB activation, THP1‐Blue cells were stimulated with LPS (50 ng/ml) for 24 h in the presence or absence of putrescine (PUT), spermine (SPM), spermidine (SPD), or cadaverine (CAD). Activation levels of NF‐κB stimulated with LPS alone were calculated as 100% and served as maximum activation levels. Among the tested polyamines, SPM and SPD reduced LPS‐induced NF‐κB activation at all three tested doses (100 μM, 1000 μM, and 10 000 μM), while CAD had a significant effect only at 10 000 μM (Figure 2A).
FIGURE 2.
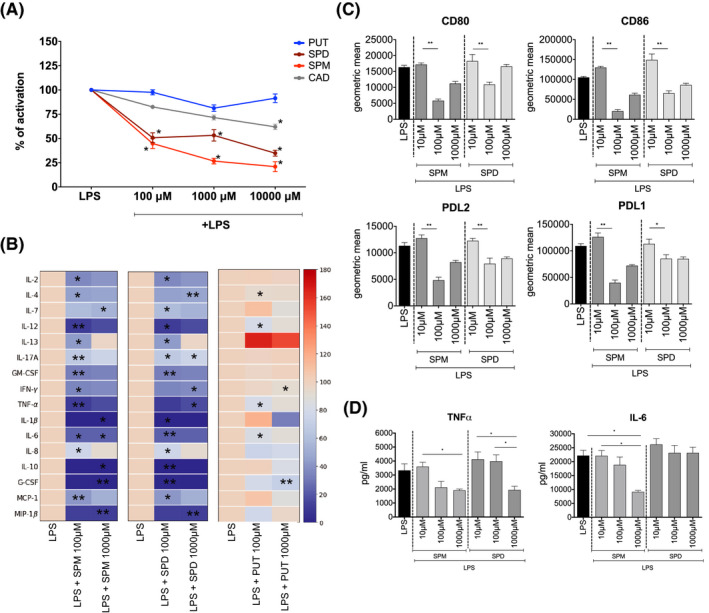
Spermine and spermidine reduce pro‐inflammatory responses in vitro. Spermine (SPM) and spermidine (SPD) decrease LPS induced NF‐κB activation of THP‐1‐Blue cells, in comparison to cells stimulated with LPS only (A). Putrescine (PUT) or cadaverine (CAD) have no effect. 100% activation represents the level of NF‐κB activation following LPS stimulation for 24h, as measured by secretion of NF‐κB‐dependent embryonic alkaline phosphatase (SEAP). The impact of each polyamine on LPS‐induced NF‐κB activation was calculated relative to the LPS alone positive control in each experiment. Human PBMCs stimulated with SPD or SPM in presence of LPS, decrease the production of cytokines during 24 h of culture. Data represented in heatmap as average cytokine concentration with LPS alone condition as 100% of production (B). Expression of co‐stimulatory (CD80, CD86) as well as co‐inhibitory (PDL1, PDL2) molecules (C) and production of proinflammatory cytokines (IL‐6, TNF‐alpha) (D) is decreased in LPS activated monocyte‐derived dendritic cells by SPD and SPM. (*p < .05 between groups, **p < .01 between groups [ANOVA])
As SPM and SPD were the only polyamines tested that impacted THP1 NF‐κB activation at all tested doses, we further explored their immunomodulatory potential using human primary cells. Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers and were LPS (50 ng/ml) stimulated for 24 h with or without SPM and SPD. 100μM Spermine reduced IL‐2, IL‐4, IL‐12, IL‐13, IL‐17A, GM‐CSF, IFN‐ γ, TNF‐ α, IL‐6, IL‐8, MIP‐1 β and 1000 μM reduced IL‐7, IL‐1 β, IL‐6, IL‐10, G‐CSF, MIP‐1β secretion from LPS stimulated PBMCs. Similarly, 100 μM spermidine reduced secretion of IL‐2, IL‐7, IL‐12, IL‐13, IL‐17A, GM‐CSF, IL‐1β, IL‐6, IL‐8, IL‐10, G‐CSF, MCP‐1 and 1000 μM reduced IL‐4, IL‐17A, IFN‐γ, TNF‐α, and MIP‐1β.(Figure 2B and Figure S1). As a polyamine control, we exposed LPS stimulated PBMCs to PUT, but in contrast, PUT reduced secretion of IL‐4, IL‐12, TNF‐α, IL‐6 only at lower doses and IFN‐γ, and G‐CSF only at the higher dose, from LPS‐stimulated PBMCs (Figure 2B and Figure S1).
Finally, human monocyte‐derived dendritic cells (MDDCs) were differentiated from peripheral blood derived monocytes in the presence of IL‐4 and GM‐CSF. Addition of 100 μM or 1000 μM SPM or SPD to MDDCs stimulated with LPS for 24 h reduced cell surface expression of CD80, CD86, PDL1, and PDL2 (Figure 2C). Additionally, both polyamines reduced production of LPS‐induced TNF‐α by MDDCs but only SPM was able to reduce MDDC`s production of IL‐6 (Figure 2D).
3.3. Airway inflammation severity is reduced by oral administration of SPM and SPD
To investigate the effect of oral administration of polyamines on respiratory allergic inflammation, we gavaged mice with polyamines every day, for 6 days before HDM sensitization and every day, for 5 days, during challenge with HDM (Figure 3A). Preliminary dose ranging experiments suggested that 10 mM was the highest dose that was well‐tolerated by all animals and this dose was used in further experiments (approximately 15 mg/kg). A significant increase in BAL inflammatory cells was observed in animals that received intranasal HDM and were gavaged with the medium control (Figure 3B). In contrast, oral gavage of either SPM or SPD (10 mM) but not of CAD (10mM), significantly reduced the number of total cells in BAL in HDM exposed animals (Figure 3B, Figure S2A). In addition to BAL cell numbers, cytokine levels in BALs were also reduced by SPD and SPM (Figure 3C). Compared to animals exposed to HDM alone, KC, MCP‐1, and TNF‐α were reduced by SPM and Eotaxin, MCP‐1 and TNF‐α were reduced by SPD in BAL. (Figure 3C, Figure S2B). In addition, single cell suspensions isolated from lung tissue were re‐stimulated in vitro for 48 h with HDM. Lung cells isolated from mice treated with SPM (IL‐1 α, IL‐1β, IL‐2, IL‐3, IL‐5, IL‐6, IL‐9, IL‐10, IL‐12(p70), IL‐13, IL‐17, Eotaxin, G‐CSF, GM‐CSF, IFN‐γ, KC, MCP‐1, MIP‐1β, RANTES, TNF‐α) and SPD (IL‐1α, IL‐12(p70), IL‐13, IL‐17, Eotaxin, IFN‐γ, RANTES, TNF‐α) produced significantly less cytokines when restimulated with HDM in vitro (Figure 3D, Figure S3). Flow cytometric analyses of lung tissue derived CD3+CD4+ T cells revealed that SPM reduced the percentage of IFN‐γ producing T cells and SPD reduces the percentage of IL‐5, IL‐13, and IFN‐γ producing T cells (Figure 3E).
FIGURE 3.
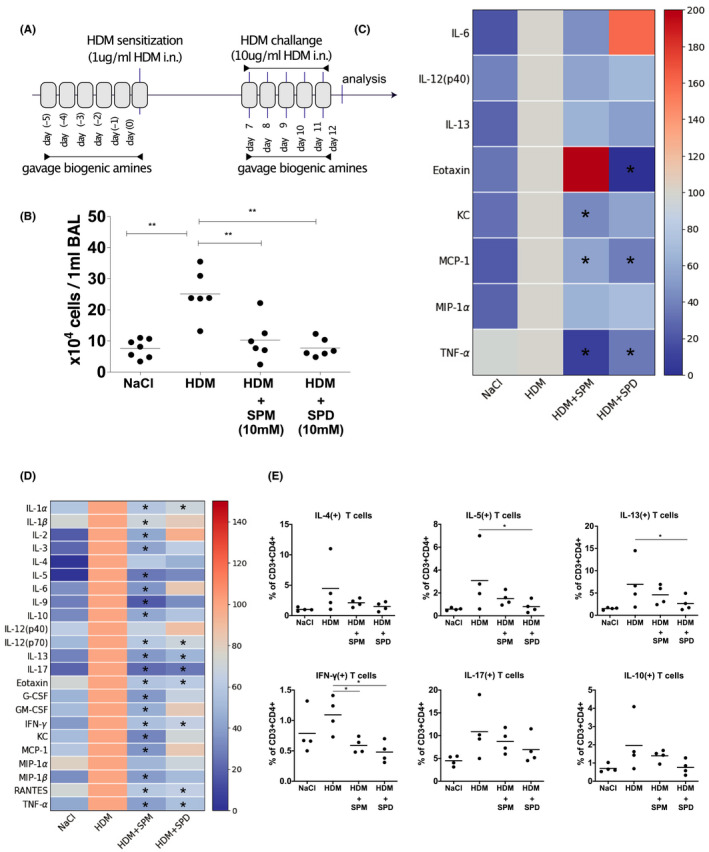
Oral gavage with spermine and spermidine decreases HDM‐induced airway inflammation. SPM and SPD gavage 5 days before and during HDM‐sensitization on day 0, as well as during HDM‐challenge from day 7 to 11 (A), decrease total number of immune cells (B), and cytokine levels (C) in bronchial alveolar lavage fluid in mouse model of HDM‐induced allergic lung inflammation. For heatmaps (C, D), the condition where mice received only intranasally HDM served as control and was set to 100% of cytokine concentration. Cells isolated from lungs were restimulated with HDM for 48h in vitro and decrease of cytokine production was observed in culture of cells isolated from mice gavaged with either SPM or SPD (D). Isolated lung T cells were intracellularly stained for IL‐10, IL‐17, IL‐13, IL‐5, IL‐4, and IFN‐gamma and results expressed as % cytokine positive of all CD4 lymphocytes (E). (*p < .05 between groups, **p < .01 between groups [ANOVA])
Furthermore, inflammatory cell infiltration (Figure 4A) in tissue sections from the lungs of mice administered SPD were substantially reduced, while SPM reduced these changes to a lesser extent. Additionally, to assess disruption of epithelial tight junctions integrity that leads to loss of barrier function, immunofluorescence staining for two key tight junction molecules ZO‐1 and cld‐4 was performed on tissue sections. Confocal microscopy analysis revealed that orally administration of SPD, but not SPM, reconstituted lung expression of claudin‐4 and ZO‐1 that were reduced in HDM‐exposed mice (Figure 4B).
FIGURE 4.
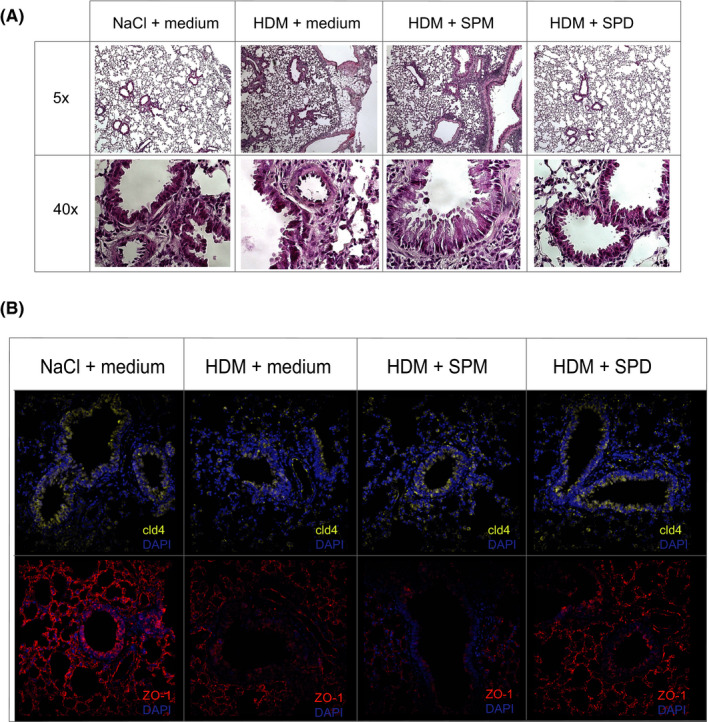
Oral gavage with spermidine decrease lung tissue inflammation. Oral gavage with SPD decreases number of lung infiltrating cell as show in hematoxylin and eosin staining (A) and reconstitute impaired epithelial barrier (B) with increased expression of tight junction proteins (ZO‐1, cld‐4) as observed in immunofluorescence staining of lung tissue. SPM gavage did not exert the same effects on the lung tissue
Lastly, as SPD seemed to be the most potent polyamine with effects on the lung tissue, we examined if SPD could influence airway hyper‐responsiveness in response to methacholine challenge. With increasing doses of methacholine, animals that were gavaged with SPD, displayed improved tissue damping properties as compared to control animals (Figure 5). Tissue dampening is closely related to tissue resistance but unlike tissue resistance it is frequency independent and reflects the energy dissipation in alveoli.
FIGURE 5.
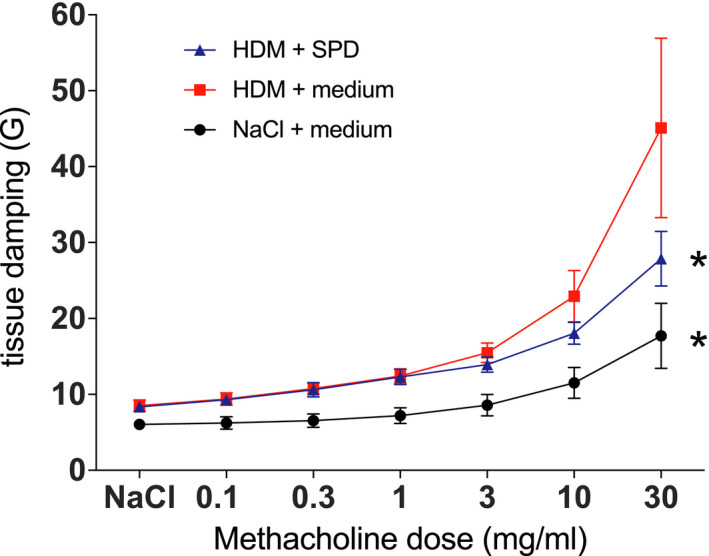
Mice gavaged with SPD improve lung function. Lung function was measured using low frequency forded oscillations and represented as tissue damping (G). Mice which were gavaged with SPD show improved tissue resistance with increasing doses of methacholine as compared to control mice. (*p < .05 between groups [two‐way ANOVA])
4. DISCUSSION
Our results demonstrate that polyamines are detectable in bronchoalveolar lavages from asthma patients, with significant differences in spermidine and cadaverine levels compared to healthy volunteers. In vitro experiments confirmed that spermidine and spermine exert anti‐inflammatory effects on THP1 monocytes and primary immune cells stimulated with LPS. Oral gavage with spermidine and spermine also were associated with anti‐inflammatory effects in a murine model of HDM‐induced lung inflammation, with spermidine exerting a greater protective effect compared to spermine.
During homeostasis, the production of polyamines is tightly regulated. The decarboxylation of ornithine by ornithine decarboxylase (ODC1) generates putrescine, which is converted to spermidine by spermidine synthase (SPDS), which is further metabolized to spermine by spermine synthase (SPMS). 19 Spermine can also be back converted to spermidine. ODC1 is considered the rate‐limiting enzyme of this pathway as it's subject to a unique mechanism of ubiquitin‐independent proteasomal degradation. 20 Putrescine and spermine levels were similar between asthma patients and control BALs, while only spermidine levels were decreased in asthma patients. This suggests that there is not a global dysregulation of this pathway in the asthmatic lung, but reduced spermidine levels may be related to altered utilization or metabolism of this specific polyamine by inflammatory cells. In addition, non‐mammalian cellular sources may also influence spermidine levels. We have previously shown that microbes from the human gut can secrete and utilize polyamines, and there are significant differences in the microbial populations present in the asthmatic lung. 7 , 21 Unfortunately, it's not currently possible to discriminate between microbe‐derived polyamines versus mammalian cell‐derived polyamines in vivo, but both cellular compartments are likely to contribute to the overall polyamine pool.
In contrast with spermidine, cadaverine levels were increased in the asthmatic lung. Cadaverine is the product of lysine decarboxylation, rather than ornithine decarboxylation. However, ODC1 is thought to also play a role in cadaverine generation. 2 While accumulation of cadaverine can have adverse metabolic consequences on host cells, cadaverine might not play a significant direct role in driving pro‐inflammatory responses within the lung. Our in vitro data and murine data show that cadaverine did not decrease, but also did not increase inflammatory responses. As with the other polyamines, cadaverine can be generated by both microbial and mammalian cells and increased cadaverine levels might be the result of altered microbial populations as well as changes in host cell metabolism.
Spermidine is being increasingly recognized as a potent regulator of inflammatory responses. Spermidine has been shown to have a protective role in multiple different mouse experimental models of inflammatory diseases including multiple sclerosis, psoriasis, and colitis. 10 , 22 , 23 Multiple mechanisms may underpin this activity. Epigenetic modifications are well‐described whereby spermidine‐induced acetylation of histone 3 led to upregulation of autophagy‐related gene expression, which contributed to autophagy‐mediated removal of dysfunctional organelles, cells or proteins, and subsequently reduces cell damage and increased longevity in murine models. 3 Recently spermidine has been shown to modulate CD4+ T‐cell differentiation in vitro, preferentially committing naive T cells to a regulatory phenotype, which is partially dependent on the autophagy gene Atg5. 24 In addition, spermidine via transformation into hypusine, promotes hypusination and, thus, activation of the critical cellular protein eukaryotic translation factor 5A (eIF5A). 25 Spermidine can also promote dendritic cell immunoregulatory properties via the indoleamine 2,3‐dioxygenase 1 (IDO1) enzyme‐mediated conversion of tryptophan into kynurenines, suggesting an important immunoregulatory circuit involving the aryl hydrocarbon receptor (AhR). 19 , 26 Thus, spermidine may exert immunomodulatory effects via both biochemical pathways and epigenetic modifications, which will need to be explored in future experiments focused on the inflamed lung and asthma.
One interesting aspect of our murine experiments is that we administered the polyamines orally, but not topically to the lung. The protective effect of orally administered spermidine is in keeping with the concept of the “gut‐lung axis” that highlights the dependence of lung health on factors present within the gastrointestinal tract. Previous work by us and others have shown that a variety of dietary and microbial metabolites, including short chain fatty acids and histamine, within the gut can impact lung health. 27 , 28 Our hypothesis is that these gut associated metabolites do not have direct effects on the lungs, but influence the activation and polarization of immune cell subsets that get recruited to the damaged lung mucosa. However, future experiments are required to confirm this hypothesis.
In summary, polyamines are present within the lung and may be relevant to the lung inflammatory processes associated with asthma. However, it's not clear if polyamine levels change as a consequence of these disease processes, or if polyamines are directly involved in the development of the pathology. Studies, such as those ongoing in our laboratory, are required to determine if polyamines are simply asthma biomarkers or if they represent clinically relevant therapeutic targets. Further exploration of the immunometabolic mechanisms by which spermidine impacts host inflammatory responses may also reveal if host or microbial‐derived polyamines are more important within the lung.
DISCLOSURES
CA reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission's Horizon's 2020 Framework Programme, Cure, Novartis Research Institutes, Astra Zeneca, Scibase and Sanofi/Regeneron that are unrelated to the submitted work. LO’M reports grants and/or personal fees from PrecisionBiotics and GSK that are unrelated to the submitted work. The remaining authors declare no competing interests.
AUTHOR CONTRIBUTIONS
MW—experiment design, data acquisition, analysis and interpretation, manuscript drafting; DG, Remo F., Ruth F., PW, KK, BP, WB, PW, AD—data acquisition and analysis; MS, MJ, CA—manuscript revising; LOM—conception and experiment design, manuscript drafting, manuscript revising for important intellectual content.
Supporting information
Figure S1–S3
Wawrzyniak M, Groeger D, Frei R, et al. Spermidine and spermine exert protective effects within the lung. Pharmacol Res Perspect. 2021;9:e00837. 10.1002/prp2.837
REFERENCES
- 1. Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42:5‐15. [DOI] [PubMed] [Google Scholar]
- 2. Bekebrede AF, Keijer J, Gerrits WJJ, Boer VCJ. The molecular and physiological effects of protein‐derived polyamines in the intestine. Nutrients. 2020;12:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305‐1314. [DOI] [PubMed] [Google Scholar]
- 4. Minois N, Carmona‐Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY). 2011;3:716‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pegg AE. Functions of polyamines in mammals. J Biol Chem. 2016;291:14904‐14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atiya Ali M, Poortvliet E, Stromberg R, Yngve A. Polyamines in foods: development of a food database. Food Nutr Res. 2011;55(1):5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pugin B, Barcik W, Westermann P, et al. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb Ecol Health Dis. 2017;28:1353881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanchez‐Jimenez F, Medina MA, Villalobos‐Rueda L, Urdiales JL. Polyamines in mammalian pathophysiology. Cell Mol Life Sci. 2019;76:3987‐4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ter Steege JC, Forget PP, Buurman WA. Oral spermine administration inhibits nitric oxide‐mediated intestinal damage and levels of systemic inflammatory mediators in a mouse endotoxin model. Shock. 1999;11:115‐119. [DOI] [PubMed] [Google Scholar]
- 10. Moron B, Spalinger M, Kasper S, et al. Activation of protein tyrosine phosphatase non‐receptor type 2 by spermidine exerts anti‐inflammatory effects in human THP‐1 monocytes and in a mouse model of acute colitis. PLoS One. 2013;8:e73703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013;62:681‐688. [DOI] [PubMed] [Google Scholar]
- 12. North ML, Grasemann H, Khanna N, Inman MD, Gauvreau GM, Scott JA. Increased ornithine‐derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am J Respir Cell Mol Biol. 2013;48:694‐702. [DOI] [PubMed] [Google Scholar]
- 13. Kurosawa M, Shimizu Y, Tsukagoshi H, Ueki M. Elevated levels of peripheral‐blood, naturally occurring aliphatic polyamines in bronchial asthmatic patients with active symptoms. Allergy. 1992;47:638‐643. [DOI] [PubMed] [Google Scholar]
- 14. Ilmarinen P, Moilanen E, Erjefalt JS, Kankaanranta H. The polyamine spermine promotes survival and activation of human eosinophils. J Allergy Clin Immunol. 2015;136:482‐484 e411. [DOI] [PubMed] [Google Scholar]
- 15. Jain V, Raina S, Gheware AP, et al. Reduction in polyamine catabolism leads to spermine‐mediated airway epithelial injury and induces asthma features. Allergy. 2018;73:2033‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curtis MJ, Alexander S, Cirino G, et al. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol. 2018;175:987‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander SPH, Fabbro D, Kelly E, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: enzymes. Br J Pharmacol. 2019;176(Suppl 1):S297‐S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Proietti E, Rossini S, Grohmann U, Mondanelli G. Polyamines and kynurenines at the intersection of immune modulation. Trends Immunol. 2020;41:1037‐1050. [DOI] [PubMed] [Google Scholar]
- 20. Kahana C. The antizyme family for regulating polyamines. J Biol Chem. 2018;293:18730‐18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michalovich D, Rodriguez‐Perez N, Smolinska S, et al. Obesity and disease severity magnify disturbed microbiome‐immune interactions in asthma patients. Nat Commun. 2019;10:5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li G, Ding H, Yu X, et al. Spermidine suppresses inflammatory DC function by activating the FOXO3 pathway and counteracts Autoimmunity. iScience. 2020;23:100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Q, Zheng C, Cao J, et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016;23:1850‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carriche GM, Almeida L, Stuve P, et al. Regulating T‐cell differentiation through the polyamine spermidine. J Allergy Clin Immunol. 2021;147:335‐348 e311. [DOI] [PubMed] [Google Scholar]
- 25. Mandal A, Mandal S, Park MH. Genome‐wide analyses and functional classification of proline repeat‐rich proteins: potential role of eIF5A in eukaryotic evolution. PLoS One. 2014;9:e111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Casero RA Jr. Targeting the aryl hydrocarbon receptor/polyamine biosynthesis axis of evil for cancer therapy. J Clin Invest. 2018;128:4254‐4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barcik W, Pugin B, Bresco MS, et al. Bacterial secretion of histamine within the gut influences immune responses within the lung. Allergy. 2019;74:899‐909. [DOI] [PubMed] [Google Scholar]
- 28. Roduit C, Frei R, Ferstl R, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74:799‐809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1–S3


