Abstract
EFSA received a mandate from the European Commission to assess the effectiveness of some of the control measures against diseases included in the Category A list according to Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’). This opinion belongs to a series of opinions where these control measures will be assessed, with this opinion covering the assessment of control measures for Classical swine fever (CSF). In this opinion, EFSA and the AHAW Panel of experts review the effectiveness of: (i) clinical and laboratory sampling procedures, (ii) monitoring period and (iii) the minimum radii of the protection and surveillance zones, and the minimum length of time the measures should be applied in these zones. The general methodology used for this series of opinions has been published elsewhere; nonetheless, details of the model used for answering these questions are presented in this opinion as well as the transmission kernels used for the assessment of the minimum radius of the protection and surveillance zones. Several scenarios for which these control measures had to be assessed were designed and agreed prior to the start of the assessment. Here, several recommendations are given on how to increase the effectiveness of some of the sampling procedures. Based on the average length of the period between virus introduction and the reporting of a CSF suspicion, the monitoring period was assessed as non‐effective. In a similar way, it was recommended that the length of the measures in the protection and surveillance zones were increased from 15 to 25 days in the protection zone and from 30 to 40 days in the surveillance zone. Finally, the analysis of existing Kernels for CSF suggested that the radius of the protection and the surveillance zones comprise 99% of the infections from an affected establishment if transmission occurred. Recommendations provided for each of the scenarios assessed aim to support the European Commission in the drafting of further pieces of legislation, as well as for plausible ad hoc requests in relation to CSF.
Keywords: disease control, CSF, sampling procedures, monitoring period, protection zone, surveillance zone, intervention
Summary
This opinion is part of a series of opinions, in which the three‐first terms of reference (ToR) of a mandate received from the European Commission have been considered. The background and specific details of this mandate can be found in the opinion. The ToRs in this mandate request an assessment of the effectiveness of:
the clinical and laboratory examination in their capacity to detect disease (or estimate the disease prevalence within an establishment), either in suspect or confirmed animals in a single establishment or in establishments within restriction zones (ToR 1);
the effectiveness of the duration of the monitoring period (for different scenarios) in the control of suspected and confirmed outbreaks (ToR 2);
the size and duration of the restriction zones, in their capacity for mitigating disease spread (ToR 3).
In order to harmonise the approach to these assessments, the methodology used in this series of opinions, covering all Category A diseases, was agreed on and published in a separate technical report (EFSA, 2020).
A qualitative assessment of the existing clinical examination (ToR 1) procedures for Classical swine fever virus (CSFV) was carried out. For assessing the effectiveness of the laboratory examination, the within‐herd dynamics of CSFV were modelled for six different scenarios (considering low, medium and high transmission rates, combined with long or short infectious periods), using a stochastic SEIR epidemic model. More specifically, the number of dead pigs and prevalence of pyrexic and seropositive pigs, respectively, at different time points post introduction of the virus to the herd (as predicted by the model) was used for the assessment. The median time (days) to reach a 10% and 20% seroprevalence, and 10% prevalence of pigs with fever, respectively, were calculated. The time to detection, when five dead pigs (or five pigs with clinical signs) were virologically tested, was also estimated. Aside, the time to detection of a potential CSF outbreak in a herd given a surveillance scheme based on weekly sampling of at least two dead post weaning pigs for virus detection was also assessed. The assessment confirmed the effectiveness for early detection in the event of a suspicion due to clinical signs (13 days post infection in the event of a high transmission rate strain, and within 16 days in the event of low transmission strains) of the collection and the virological sampling of at least five pigs (dead or with clinical signs if a sufficient number of dead pigs is not found). In contrast, serological testing of randomly selected pigs in herds, where clinical signs have not been observed, can be considered effective only in specific situations (e.g. in support to the epidemiological investigation) and should not be recommended as a general procedure. For surveillance purposes aiming at early detection in the absence of a suspicion, a weekly sampling of at least two dead post weaning pigs (or dead pigs older than 2 months in each epidemiological unit) was assessed as effective and would lead to virus detection at median times of between 14 and 16 days post infection in the event of infection with a virus of high transmission rate and between 24 and 30 days in the event of a low transmission rate virus, with 95% confidence, assuming a 3% baseline mortality.
To answer ToR 2, and to assess the minimum length of time measures should be implemented in the protection and surveillance zones (ToR 3.2), an extensive literature search (ELS) was carried out. This ELS aimed to assess the average, shortest and longest period between the earliest point of infection of a pig herd with an CSFV virus, and the time of reporting of a suspicion by the competent authority. The average time to the reporting of a suspicion report was used then to assess the effectiveness of the length of the monitoring period. For the majority of the scenarios, the existing length of the monitoring period for CSF (15 days) was considered insufficient. In those cases, extending the length of the monitoring period to 25 days was recommended (this number should be extended further to 40 days for primary outbreaks). To assess the effectiveness of the minimum length of time the measures should be applied in the protection and surveillance zones, the average time assessed via the ELS was also used. Based on this, the minimum duration of the protection zone (15 days) and the surveillance zone (30 days), according to existing legislation, were recommended to be increased to 25 and 40 days, respectively.
To assess the effectiveness of the minimum radii to be implemented in the protection and surveillance zones (ToR 3.1), transmission kernels were used. These kernels were built using data from previous outbreaks and represent the relative risk of transmission to each individual establishment from an affected establishment. Assuming transmission occurs from an affected establishment, the probability of CSF transmission beyond the protection zone and surveillance zone was 2% and 0.2%, respectively. The minimum radius was thus considered highly effective when focusing on the control of the spread of the disease among and between domestic pig herds. It is important to note, however, that the transmission kernels presented cover only some of the risk pathways associated with spread from the index case and that these probabilities do not take into account the risk of transmission due to wild boar, or movements of live animals and products off the establishment prior to confirmation.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’), hereinafter referred to as AHL, requires the Commission to lay down detailed rules on the disease control measures against listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). The Commission is empowered to adopt delegated acts supplementing the rules laid down in Part III of Regulation (EU) 2016/429 on transmissible animal diseases (Animal Health Law) on disease control measures for listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). Therefore, the Commission has developed and adopted a Delegated Regulation laying down rules for the prevention and control of certain diseases (‘the Delegated Regulation’). The rules laid down in the Delegated Regulation are in respect of terrestrial animals largely replicating the rules currently in force concerning the disease control measures in the event of animal diseases with serious effects on the livestock as they have proven to be effective in preventing the spread of those diseases within the Union. Consequently, many animal disease control measures laid down in existing Directives will be, to the extent that not already done by the Animal Health Law, replaced by the rules provided in the Delegated Regulation. At the same time, these rules have been aligned with the international standards from the World Organisation for Animal Health (OIE), wherever these existed. However, certain disease control measures proposed in the Delegated Regulation, in particular in its Annexes, were considered as outdated i.e. possibly not based on most recent scientific evidence at the time of development. Their review is considered as necessary. Moreover, for those category A diseases for which rules were not established before or were not detailed enough, certain disease control and risk mitigating measures are, due to the lack of scientific basis, extrapolated from other diseases, for which rules existed in the past. Finally, for some other diseases the evidence and scientific knowledge, was not available to the Commission and to the Member States at the time of developing the Delegated Regulation due to the time constraints. The following diseases are examples of the later: infection with Rift Valley fever (RVF), infection with Mycoplasma mycoides subsp. Mycoides SC (Contagious bovine pleuropneumonia) (CBPP), Contagious caprine pleuropneumonia (CCPP), Sheep pox and goat pox, infection with peste des petits ruminants virus (PPR), African horse sickness (AHS), Glanders. In this regard, the existing rules will cease to apply as from the date of application of the Animal Health Law and its complementing legislation including the Delegated Regulation, i.e. from 21 April 2021. Certain of the proposed measures for the prevention and control of category A diseases of terrestrial animals should therefore be assessed in order to ensure that they are effective and updated based on the latest scientific knowledge in this new set of legislation. This is particularly important in the case of those diseases that are less common or have been never reported in the Union.
1.1.1. ToR 1: Sampling of animals and establishments for the detection of category A diseases in terrestrial animals
Based on available scientific information, assess the effectiveness of existing sampling procedures to detect or rule out the presence of each category A disease of terrestrial animals and, in case of absence of effective procedures, develop them, in order to complete the rules provided for in Annex I to the Delegated Regulation. In particular, provide for disease‐specific procedures for the sampling of:
ToR 1.1 Animals for clinical examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 13(3)(c), 14(1) and 26(2) of the Delegated Regulation.
ToR 1.2 Animals for laboratory examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 12(3), 13(3)(c), 14(1), 26(2) of the Delegated Regulation.
ToR 1.3 Establishments to ensure the detection of the relevant category A disease for the performance of visits in establishments located in protection zones larger than 3 km and establishments located in the surveillance zone in accordance with Articles 26(5) and 41 of the Delegated Regulation.
ToR 1.4 Animals for clinical and laboratory examinations to ensure the detection of the relevant category A disease for the movement of animals from restricted zones in accordance with Articles 28(5), 43(5), 56(1)(c) of the Delegated Regulation.
ToR 1.5 Animals for laboratory examinations to ensure the detection of the relevant category A disease before and after being introduced in the affected establishment for repopulation, in accordance with Article 59(2), (3) and (9) of the Delegated Regulation.
1.1.2. ToR 2: Monitoring period
ToR 2.1 Assess the effectiveness of the length of the monitoring periods set out in Annex II of the Delegated Regulation for each category A disease of terrestrial animals. In this regard, it is important to take into consideration that the monitoring period was introduced as a management tool, which represents a time frame of reference assigned to each category A disease for the competent authority to apply certain control measures and to carry out investigations in the event of suspicion and confirmation of category A diseases in terrestrial animals.
This assessment should be carried out with respect to the following situations:
-
a)
the records analysis carried out by the competent authority in the framework of the epidemiological enquiry referred to in Article 57 of Regulation (EU) 2016/429, in the event of suspicion of a category A disease (Article 8(4) of the Delegated Regulation);
-
b)
the derogation from killing in the event of an outbreak of a category A disease in establishments keeping animals of listed species in two or more epidemiological units (Article 13(1) of the Delegated Regulation);
-
c)
the tracing carried out by the competent authority to identify establishments and other locations epidemiologically linked to an establishment affected by a category A disease (Article 17(2) of the Delegated Regulation);
-
d)
the exemption applied to certain products from the prohibitions laid down in Annex VI taking into account the date they were produced (Article 27(3)(c) of the Delegated Regulation);
-
e)
the specific conditions for authorising movements of semen from approved germinal product establishments in the protection and surveillance zones (Article 32(c) and 48(c) of the Delegated Regulation);
-
f)
the repopulation of establishments affected by a category A disease (Article 57(1)(b) and 59(4)(b) of the Delegated Regulation).
ToR 2.2 Propose the length of what should be the monitoring period in those diseases for which the time is assessed as not effective.
1.1.3. ToR 3: Minimum radius of restricted zones and duration of the disease control measures in restricted zones
ToR 3.1 Assess the effectiveness to control the spread of the disease of the minimum radius of the protection and surveillance zones set out in Annex V of the Delegated Regulation for each category A disease of terrestrial animals.
ToR 3.2 Assess the effectiveness to control the spread of the disease of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones as set out in Annex X and XI for each category A disease of terrestrial animals.
1.1.4. ToR 4: Prohibitions in restricted zones and risk‐mitigating treatments for products of animal origin and other materials
ToR 4.1 Assess the effectiveness to control the spread of disease of prohibitions set out in Annex VI of the Delegated Regulation with respect to the risk associated for each category A disease, to the listed activities and commodities.
ToR 4.2 Review the available scientific information on risk‐mitigating treatments that are effective to control the presence of category A disease agents in products of animal origin and other relevant materials. Based on this:
-
a)
provide an opinion on the effectiveness of the risk‐mitigating treatments for products of animal origin and other materials produced or processed in the restricted zone set out in Annex VII and VIII, and
-
b)
if relevant, suggest new treatments or procedures that can be effective to mitigate or to eliminate such risk.
1.2. Interpretation of the Terms of Reference
To address the ToRs of the mandate, EFSA proposed and agreed with the European Commission the following:
The publication of 14 individual opinions, one per each of the diseases included in the list of category A diseases for terrestrial animals, with each of these opinions providing the answer to ToRs 1, 2 and 3. The current manuscript is one of the 14 opinions covering ToRs 1, 2 and 3 for Classical swine fever (CSF).
The publication of a unique opinion covering ToR 4 for all diseases listed (i.e. ToR 4 is not covered in this opinion).
To address ToR 1 (effectiveness of sampling procedures), EFSA agreed with the European Commission on 21 scenarios based on different articles of the Delegated Regulation (EC) 2020/687 (hereinafter referred to as Delegated Regulation), for which the effectiveness of the sampling procedures will be assessed (Annex B). Although these scenarios will be assessed independently, some of these scenarios may be merged if the assessment processes and/or results are the same.
To address ToR 2 (effectiveness of the monitoring period), seven scenarios previously agreed with the contractor were defined (Annex D). The assessment of the effectiveness of the monitoring period will be done by assessing its ability to ensure that specific actions can be carried out without posing a risk of disease spread, if the monitoring period is calculated backwards or forwards from a specific date. If the length of the monitoring period estimated by EFSA is longer than the existing monitoring periods, the existing monitoring period will be considered non‐effective. If the length of the monitoring period estimated by EFSA is shorter than the existing monitoring period, this existing monitoring period will be considered effective from a disease control point of view. No assessment of the plausible unnecessary economic burden that may be placed on the stakeholders as a result of an excessive length of the monitoring periods will be done by EFSA.
The assessment of the minimum duration and the length of the radii of the protection and surveillance zones (ToR 3) will be done independently. The setting of these two zones (protection and surveillance zones) surrounding an affected establishment and the control measures implemented in each one of the zones are based on the general principle that the probability of disease spread is larger the closer the establishment is to an affected establishment. The validity of this statement will not be assessed in this manuscript; nonetheless, the limitations that this assumption may have in the control of certain diseases will, when relevant, be discussed.
-
The following scenarios in ToR 1 (Annex B) were not relevant for CSF, and therefore were not included in the assessment:
scenario 4 because there are no non‐listed species for which CSFV sampling is recommended,
scenario 7 because the minimum radius of the protection zone for CSF is 3 km,
scenarios 10, 11, 16 and 17 because they refer to poultry, and
scenario 14 as it refers to ungulates.
The duration of the monitoring period for CSF as described in Annex II of the Delegated Regulation is 15 days.
The minimum length of the radii of the protection (PZ) and surveillance zones (SZ) for CSF as described in Annex V of the Delegated regulation are 3 and 10 km, respectively.
The minimum duration of the measures in the PZ and SZ for CSF as described in Annex X and XI of the Delegated Regulation are 15 and 30 days, respectively.
2. Epidemiology and geographical distribution of CSF
2.1. Epidemiology
Classical swine fever (CSF) or Hog cholera is a highly contagious viral disease affecting pigs and Eurasian wild boar (Sus scrofa). The causative agent is the CSF virus (CSFV), an RNA virus, of the family Flaviviridae, genus Pestivirus which includes ruminant viruses such as Bovine viral diarrhoea virus (BVDV) and Border disease virus (BDV). A single serotype of CSFV is present, but there are three major genotypes and numerous subgenotypes (OIE, 2020).
CSFV typically causes a severe clinical disease in domestic pigs and sometimes in wild boar. It can infect peccaries and warthogs, which usually develop an asymptomatic or subacute form of disease; experimental infection without clinical signs is possible in ruminants (Spickler, 2015).
CSFV is transmitted mainly by the oronasal route, between animals in close contact through secretions, faeces, urine, semen and blood. Indirect transmission can occur through fomites (vehicles, clothes), needles (iatrogenic infection) and insects acting as mechanical vectors. The virus survives for months in uncooked meat including frozen, smoked or salt cured meat so that infection through swill feed containing contaminated pork meat can occur (Pastoret, 2003; Spickler, 2015).
The disease has been eradicated from Northern America, Argentina, Australia, New Zealand and the EU, and is endemic in other parts of the world. In some countries, it is only present in the wild boar population and biosecurity measures are necessary to avoid transmission of CSFV to domestic pigs.
Countries may obtain official CSFV‐free status from OIE if no outbreak nor evidence of CSFV infection has been observed during the last 12 months in domestic and captive wild pigs based on clinical, virological and serological surveillance programme. In such countries, strict import and quarantine rules are in place. In case of an outbreak, all pigs in affected farms must be slaughtered and biosecurity measures must be taken, including pig movement restrictions. Live‐attenuated vaccines and marker vaccines are available for the control of the disease in endemic countries or as emergency ring vaccination in case of an outbreak in a free country, provided it is allowed by the country regulation. Oral live‐attenuated vaccines are used to control the disease in wild boar in Europe (OIE, 2019b, 2020).
The incubation period is usually 3–7 days (range 2–14 days). In a review of experimental infections, the median and the minimum incubation period in pigs were 3 days and 1 day, respectively (Dórea et al., 2021). The severity of the disease depends on the virulence of the strain, the age and the immune status of the animal, being more pronounced in young and naïve animals (Spickler, 2015; OIE, 2020). The morbidity and mortality in a susceptible population infected with a highly virulent strain can reach 100%; however, most of the outbreaks worldwide are currently caused by moderately virulent strains, although some less virulent strains have been also reported (Pastoret, 2003; OIE, 2020). In experimental infections with these less virulent strains, a case fatality rate (CFR) of 20% was found, with lower mortality in adults compared to piglets (Spickler, 2015).
In the acute form, infected animals develop high fever (40–41°C), anorexia, depression and conjunctivitis. Cutaneous symptoms may include hyperaemia or haemorrhagic lesions on the abdomen, inner thighs and ears and cyanosis of the snout, ears and tail. Severe digestive disorders such as vomiting and episodes alternating between constipation and watery diarrhoea may appear. Dyspnoea and coughing can occur. Nervous signs such as staggering, paresis and convulsions are common in the final stage, with death occurring within 1–3 weeks.
A chronic form can develop with less virulent strains or in partially immune herds. Clinical signs are similar to the acute form, but less severe and pigs appear to recover after a few weeks before a relapse of the disease with death that often occurs, sometimes after several months. Wasting, stunted growth, dermatitis and reproductive disorders, including abortion, stillbirths and congenital tremor or malformations are also observed (Pastoret, 2003; Kramer et al., 2009; Spickler, 2015; OIE, 2020). Seroconversion occurs 1–3 weeks after infection, and in the event of infection with high virulent strains, the few survival pigs develop antibodies that persist for years (Kramer et al., 2009; Spickler, 2015, OIE, 2019a, 2020). When pigs are infected with the less virulent strains, a larger number of animals (up to a one‐third of the experimentally infected animals) may survive for some time, although they are unable to clear the infection, succumbing to the disease at a later stage (Weesendorp et al., 2009b). These chronically infected pigs remain carriers until death, excreting large amounts of virus and they may have fluctuating or undetectable level of antibodies (Pastoret, 2003; Kramer et al., 2009; Weesendorp et al., 2009b; OIE, 2019b).
Congenital infections of the fetuses can occur at all stages of pregnancy, including through artificial insemination with infected semen (de Smit et al., 1999), leading to abortions, stillbirths, mummifications, congenital defects, congenital tremors or weak piglets dying shortly within days or weeks after birth. Sows infected at a late stage of pregnancy can give birth to clinically healthy but persistently infected (PI) piglets that remain seronegative but with lifelong shedding of the virus; PI animals later develop clinical signs such as poor growth, chronic diarrhoea, depression, ataxia and finally die, usually within 6–12 months (Pastoret, 2003; Kramer et al., 2009; Spickler, 2015; OIE, 2020).
Detection of CSFV is routinely done by reverse transcription polymerase chain reaction (RT‐PCR) on tonsil swabs or on unclotted blood or serum samples from live animals obtained during the acute phase, or on tissue samples taken at necropsy (tonsils, lymph nodes, spleen, kidneys). RT‐PCR can also be used to differentiate a wild strain from some vaccine strains. Antigen capture enzyme‐linked immunosorbent assays (ELISAs) are available but are used for herd‐level screening rather than individual test due to their low sensitivity and specificity. Virus isolation can be performed for genetic characterisation of the CSFV strain (Kramer et al., 2009; Spickler, 2015; OIE, 2019b, 2020).
Serological tests include antibody ELISA and viral neutralisation test (VNT), which is the reference test. Infection of pigs with ruminant pestivirus (BVDV or BDV) is responsible for cross reactions with CSF serological tests so that any positive ELISA test should be confirmed by comparative VNT or by ELISAs using monoclonal antibodies. There are specific ELISA tests that can differentiate between antibodies to wild strains and antibodies due to vaccination with a marker vaccine.
2.2. Geographical distribution of CSF
Classical swine fever is endemic in large parts of the world including parts of Asia, Latin America and Eastern Europe. In the European Union, the last outbreak in domestic pigs was reported in 2014, and the last case in wild boar in 2015.
Figure 1 below depicts countries reporting CSF outbreaks in domestic swine and wild boar between 2015 and 2021, and Figure 2 countries with the OIE official free status for CSF in 2021.
Figure 1.
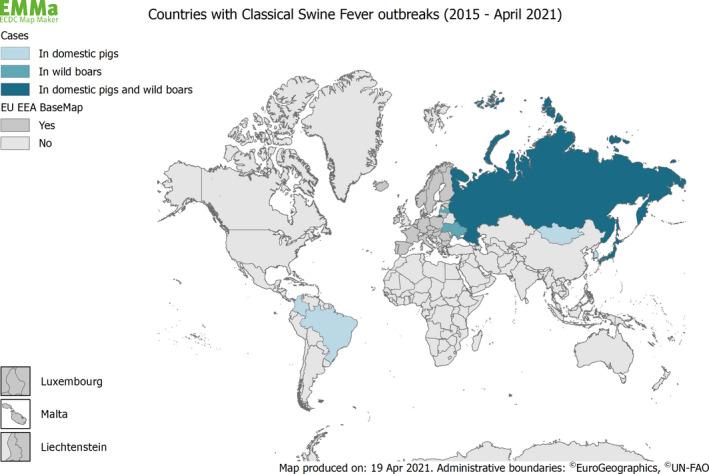
Map of countries with reported outbreaks of CSF in domestic swine and wild boar between 2015 and 2021 (Data sources: ADNS and OIE)
Figure 2.
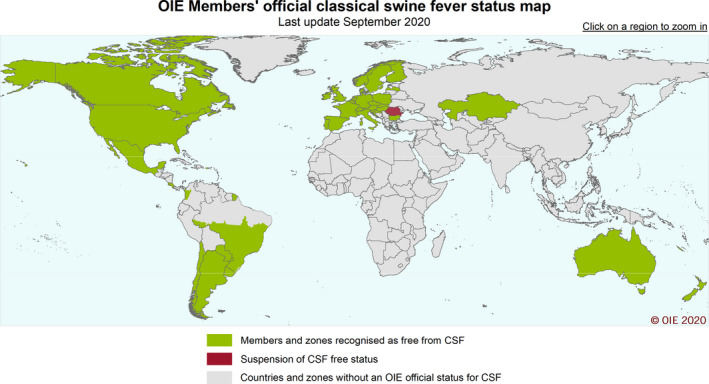
Map of countries with the OIE official free status for Classical swine fever, 2021 (Source: OIE; © OIE)
3. Data and methodologies
3.1. Methodology used in ToR 1
Although the general methodology applied to all opinions covering the assessment of control measures for the Category A diseases produced under this mandate has been published elsewhere, specific details of the methodology related to the CSF opinion are presented below.
Mathematical model and transmission scenarios considered
The within‐herd dynamics of classical swine fever virus (CSFV) were modelled using a stochastic SEIR epidemic model (Keeling and Rohani, 2008). The pig population was divided into four classes: susceptible (i.e. uninfected), S; exposed (i.e. infected, but not yet infectious), E; infectious, I; and recovered, R. Disease associated mortality was assumed to occur at a constant rate during the infectious period.
The force of infection is given by,
where β is the transmission rate, I(t) is the number of infectious pigs at time t and N(t) is the total number of pigs at time t. This formulation assumes homogeneous mixing (i.e. individuals uniformly and randomly contact each other) and frequency‐dependent transmission (i.e. the number of contacts is independent of the population size) (Keeling and Rohani, 2008). The durations of the latent and infectious periods were assumed to follow gamma distributions with means μE and μI and shape parameters kE and kI, respectively (i.e. with variances μE 2/kE and μI 2/kI). This was incorporated in the model by subdividing the latent and infectious classes into kE and kI stages each of mean duration μE/kE and μI/kI, respectively (Anderson and Watson, 1980).
The number of pigs in each class takes an integer value, while transitions between classes are stochastic processes. The number of transitions of each type during a small‐time interval δt was drawn from a binomial distribution with number of pigs in the class, n, and transition probability, q (the appropriate per capita rate multiplied by δt) as parameters.
The initial herd size was assumed to be 50, 100, 200 or 1,000 pigs. Transmission parameters were extracted from published analyses of transmission experiments (Klinkenberg et al., 2002; Backer et al., 2009; Durand et al., 2009; Weesendorp et al., 2009a, 2011, 2014), from which three scenarios were identified for the transmission rate (low, medium and high) and two for the infectious period (short and long) (i.e. a total of six scenarios in all; Table 1). The CFR was assumed to be 20% representing the lower range of CFR estimated based on experimental infections using recent CSFV strains originating from different parts of the world (Floegel‐Niesmann et al., 2009; Spickler, 2015).
Table 1.
Parameters used for modelling the transmission of Classical swine fever virus; six different scenarios were considered based on the length of the infectious period (short, long) and the transmission rate category (low, medium and high)
| Disease scenario | β | μE | kE | μI | kI | |
|---|---|---|---|---|---|---|
| Short infectious period | Low transmission rate | 0.3 | 4 | 10 | 15 | 7 |
| Medium transmission rate | 1.5 | 15 | ||||
| High transmission rate | 6.0 | 15 | ||||
| Long infectious period | Low transmission rate | 0.3 | 25 | |||
| Medium transmission rate | 1.5 | 25 | ||||
| High transmission rate | 6.0 | 25 | ||||
β – transmission rate
μE – mean latent period (days)
kE – shape parameter for gamma‐distributed latent period
μI – mean infectious period (days)
kI – shape parameter for gamma‐distributed infectious period
Within‐herd dynamics of CSFV
The within‐herd dynamics of CSFV is shown in Figure 3. Here, the median (solid line) and 95% prediction interval (shading) for the number of (from left to right): exposed, infectious and recovered pigs, and for the cumulative number of dead pigs, are shown for the six scenarios considered in Table 1 (rows); these scenarios differ in terms of the R0 and infectious period considered (see details in Table 1).
Figure 3.
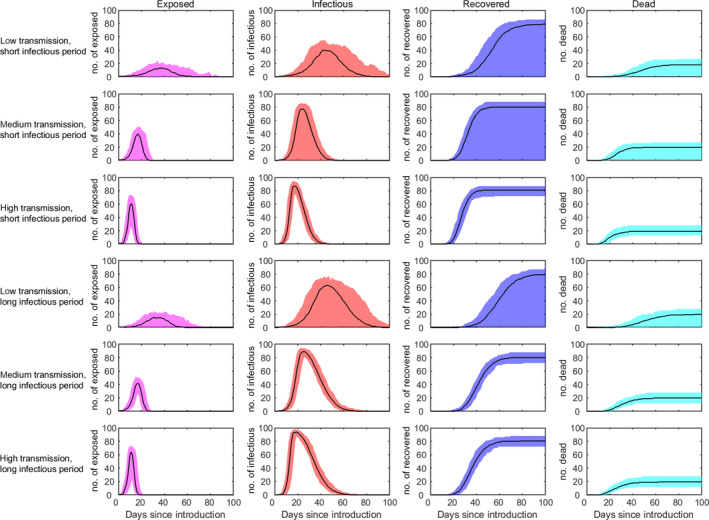
Within‐herd dynamics of CSFV in pigs. The plots show the median (solid line) and 95% prediction interval (shading) for the number of exposed pigs (magenta), infectious pigs (red), recovered pigs (blue) and cumulative number of dead pigs (cyan) for the six scenarios considered in Table 1; these scenarios differ in terms of R 0 and infectious period (rows; see Table 1 for details)
Detection of Classical swine fever virus
Sampling live pigs
The prevalence of virus‐positive pigs was assumed to correspond to the prevalence of infectious pigs, while pigs in the recovered class were assumed to be seropositive.
The prevalence is the proportion of pigs either infected or seropositive, so the denominator in the calculations is the initial herd size minus the cumulative number of pigs that have died of CSF.
The sensitivity and specificity of the diagnostic test used to confirm infection were assumed to be 100% (even if specificity for some tests might not be 100% due to cross reactions with other pestiviruses, in the event of a positive case being found, further diagnostic tests will be used for confirmation).
Sampling pigs with fever
The number of infectious pigs with fever was given by F(t)˜Binomial (I(t), pF), where pF is the proportion of infected animals with fever (assumed to be 87% (Elbers et al., 2002)). The prevalence of fever is the number of pigs with fever divided by the initial herd size minus the cumulative number of pigs that have died of CSFV.
When sampling five pigs with fever, the probability of detection, pfever, was computed using the hypergeometric distribution (i.e. sampling without replacement), so that
where M is the total number of pigs with fever (uninfected and infected) at the time of sampling, K is the number of infected pigs with fever and SS is the number of pigs with fever sampled (i.e. 5 or all if < 5). Baseline morbidity (i.e. proportion of pigs with fever) was assumed to be 1%.
Sampling dead pigs
The number of pigs dying each day of non‐CSF‐related causes in each class (x = S, E, I or R) was given by Dx(t) ˜ Binomial (x(t), mB), where mB is the baseline mortality (i.e. proportion of pigs dying as a result of non‐CSFV‐related causes each day). This was assumed to be 0.033% based on 3% post‐weaning mortality during a 90‐day production cycle. The number of uninfected dead pigs each day was given by DS(t) + DE(t) + DR(t) and the number of infected dead pigs each day was given by D(t) + DI(t) where D(t) is the number of pigs dying of CSF.
The probability of detection, pdead, was computed using the hypergeometric distribution (i.e. sampling without replacement), so that
where M is the cumulative number of dead pigs (uninfected and infected) in the preceding week, K is the cumulative number of dead infected pigs in the preceding week and SS is the number of dead pigs sampled (i.e. 5 or all dead pigs if < 5). If there were fewer than five dead pigs, the remaining sample (i.e. so the total number of animals sampled was 5) was made up of pigs with fever (which are euthanised). In this case, the probability of detection is given by pdead + pfever – pdead × pfever where pdead and pfever are as given above. The sensitivity and specificity of the diagnostic test used to confirm infection in sampled pigs were assumed to be 100%.
For weekly testing of two dead pigs, the time of the initial sample was drawn uniformly from day 1 to 7 post introduction and testing was subsequently carried out every 7 days. The probability of detection at each sampling time, pD, was computed as described above (but with SS = 2) and sampling was carried out until CSFV was detected (i.e. there was at least one dead infected pig in the sample).
3.2. Methodology used in ToR 2
To estimate the time lag between infection and reporting of a CSF suspicion (ToR 2), an extensive literature search (ELS) was outsourced by EFSA (OC/EFSA/ALPHA/2020/02 – LOT 2). The aim of this ELS was to answer the epidemiological question of: ‘what is the average, shortest and longest period of time for an outbreak of CSF to be reported (measured as the number of days from the earliest point of infection with CSF virus, to the time of declaration of a suspicion by the competent authority after the clinical investigation by an official veterinarian)?’. To answer this question, an ELS on case reports, papers describing outbreaks or epidemics of CSF and any other relevant grey literature or data was carried out. For the inclusion criteria in the ELS, the earliest point of infection had to have been estimated by carrying out an epidemiological investigation. Papers and other sources of data where the earliest point of infection was determined purely by subtracting a known incubation period from the date of the suspicion of the outbreak, were excluded. The ELS was restricted to studies conducted in Europe or describing results obtained in Europe. If none or very few articles were retrieved (less or equal to 5) in the first search, the search was extended to the rest of the world. An ELS protocol similar to that shown in Annex 5 of the Methodology report was followed (EFSA, 2020).
3.3. Methodology used in ToR 3
The assessment of radius size of restricted zones (ToR 3), to prevent further disease spread at a given probability, was performed by using disease transmission kernels.
To estimate the duration of measures in the protection and surveillance zones, the outputs obtained from the ELS described in Section 3.2 were used. Further details can be found in the Methodology report (EFSA, 2020).
3.4. Uncertainty
A description of the methodology followed to deal with uncertainty is provided in a Methodology report published by EFSA (EFSA, 2020). All sources of uncertainty identified during the assessment were recorded. In addition, for this opinion, the impact of the uncertainties identified in the assessment of ToRs 2 and 3 were assessed collectively after transforming the objective of these ToRs into well‐defined quantities of interest.
For ToR 2, aiming at the assessment of the effectiveness of the length of the monitoring period under different scenarios, it was agreed that a given length would be considered effective if it would serve its scenario‐specific purpose in at least 95% of the cases in which it was implemented. Three different quantities of interest were defined, each referring to different scenarios among those listed in Annex D:
Scenarios 1, 2 and 4: probability that, in 95% or more of all pig establishments suspected and eventually confirmed, the initial infection would have occurred within 15 days before the date of notification of the suspicion.
Scenario 3: probability that 95% or more of the independent epidemiological units within CSF‐affected establishments, that eventually become infected, would have been infected within 15 days before the date of confirmation of the disease in the establishment.
Scenario 6: probability that, in 95% or more of CSF‐affected establishments that are depopulated and are in the vicinity of an unknowingly infected establishment, the disease in the surrounding establishment is detected in the 15 days following the cleaning and disinfection of the CSF‐affected establishment.
For ToR3, aiming at the assessment of the effectiveness of the minimum radii established in the protection and surveillance zones, it was agreed that a given radius would be considered effective if it would prevent transmission to outside the zone in the 15 days (protection zone) or 30 days (surveillance zones) following its implementation, in 95% or more of the establishments in which it was implemented. Therefore, in this case two quantities of interest were considered:
Protection zone: probability that in 95% or more of all protection zones, there is no transmission to outside the zone in the 15 days following their establishment.
Surveillance zone: probability that in 95% or more of all surveillance zones, there is no transmission to outside the zone in the 30 days following their establishment.
Members of the WG provided their judgements individually, along with the rationale supporting them, for each of the five quantities of interest defined using the probability scale (Table 2) proposed in the EFSA uncertainty guidance (EFSA Scientific Committee, 2018).
Table 2.
Approximate probability scale used for quantification of the uncertainty in the assessment
| Probability term | Subjective probability range | Additional options | |
|---|---|---|---|
| Almost certain | 99–100% | More likely than not: > 50% | Unable to give any probability: range is 0–100% Report as ‘inconclusive’, ‘cannot conclude’ or ‘unknown’ |
| Extremely likely | 95–99% | ||
| Very likely | 90–95% | ||
| Likely | 66–90% | ||
| About as likely as not | 33–66% | ||
| Unlikely | 10–33% | ||
| Very unlikely | 5–10% | ||
| Extremely unlikely | 1–5% | ||
| Almost impossible | 0–1% | ||
Individual judgements and rationales were then discussed during an online meeting in order to elicit a consensus group judgement for each quantity of interest. The outputs of this assessment are placed in their respective sections.
4. Assessment
4.1. Assessment of sampling procedures (ToR 1)
4.1.1. Assessment of sampling procedures in the event of suspicion or confirmation of CSF
4.1.1.1. In the event of a suspicion of CSF in an establishment where animals of the listed species are kept
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect CSFV in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). For further details, see Annexes B and C.
1.
1st Scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 6(2) of the Delegated Regulation (EU) 2020/687
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario were taken into consideration for the assessment:
It refers to an event of suspicion of CSF in an establishment with kept animals of the listed species;
The listed species for CSF as provided in Commission Implemented Regulation 2018/1882 are those belonging to the Suidae family;
Subsequent to the suspicion, the competent authority shall immediately conduct an investigation to confirm or rule out the presence of CSF;
The official veterinarian must perform a clinical examination and collect samples for further laboratory examination.
Summary of sampling procedures
Several sources of literature, where guidelines on the sampling procedures to be followed in the event of a suspect CSF outbreak were described, were examined and are presented in Scenario 1 of the table presented in Annex C. Out of them, the guidelines enacted by Council Directive 2001/89/EC and described in Commission Decision 2002/106/EC (hereinafter Diagnostic Manual) were the most detailed, and are those discussed and analysed further here (as no new procedures were described in the rest of the literature found).
In summary, inspection of animals in each subunit of the holding must be carried out to select the pigs to be clinically examined.
The clinical examination must include a body temperature check and must primarily concern, sick or anorexic pigs, pigs recently recovered from disease, pigs recently introduced from confirmed outbreaks or from other suspected sources, pigs kept in subunits recently visited by external visitors, which had a recent close contact with CSV‐suspected or infected pigs or for which other particularly risky contacts with a potential source of CSFV have been identified, pigs already sampled and serologically tested for CSF, in case the results of these tests do not allow to rule out CSF, and in‐contact pigs.
If the inspection in the suspected holding has not indicated the presence of the pigs listed above, the competent authority should carry out further examinations on pigs selected at random in the subunits, for which a risk of introduction of CSFV has been identified or is suspected. The minimum number of pigs to be examined must allow for the detection of fever if it occurs at a prevalence of 10% with 95% confidence in these subunits of fattening pigs. In case of breeding sows, the prevalence of pigs with fever should be assumed to be 5%, and at semen collection centres, all boars must be examined.
If dead or moribund pigs are detected in a suspected holding, post‐mortem examinations must be carried out, preferably on at least five of these pigs and in particular on pigs that have shown before death or are showing very evident signs of disease, pigs with high fever and pigs that have died recently.
Irrespective of the presence or absence of lesions suggesting CSF, samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests.
If the post‐mortem examinations have not shown lesions suggesting CSF but, due to the epidemiological situation, further investigations are deemed necessary, clinical examination of randomly selected pigs as described above should be carried out.
Additionally, blood samples should be collected for serological testing from the suspected pigs and from pigs randomly selected during clinical examination. Post‐mortem examinations may be carried on three to four in‐contact pigs.
The number of samples to be taken for virological tests is left upon the decision of the competent authority.
If the suspicion of CSF in the holding is the result of serological positive findings, but the diagnostic test used does not allow differentiation between CSF infection and other pestiviruses, the following procedures are foreseen:
-
a)
if the seropositive pigs are pregnant sows, some of them, preferably not less than three, shall be euthanised and subjected to a post‐mortem examination. Prior to killing, a blood sample must be taken for further serological tests. The fetuses shall be subjected to examination for classical swine fever virus, virus antigen or virus genome.
-
b)
if the seropositive pigs are sows with suckling piglets, blood samples must be taken from all piglets and shall be subjected to examination for classical swine fever virus, virus antigen or virus genome. Blood samples must also be taken from the sows for further serological tests.
If, after the examination carried out in a suspected holding, clinical signs or lesions suggestive of classical swine fever are not detected, but further laboratory tests are deemed necessary by the competent authority to rule out classical swine fever, the random sampling as described above should be carried out.
Figure 4.
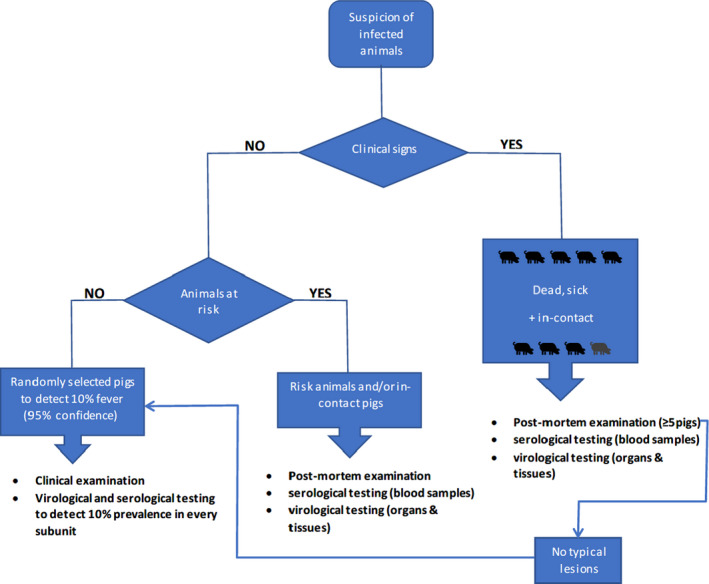
Decision tree of the diagnostic procedure for CSF confirmation
Assessment
In case the suspicion has been raised due to the presence of clinical signs and mortality, the pigs with clinical signs must be clinically examined and sampled for virological and serological testing.
The median length of time between introduction of the virus and report of the CSF suspicion as obtained from the ELS carried out to answer ToR 2, and shown in Section 4.2.2, is 25 days for secondary outbreaks, and 40 days for primary outbreaks.
From the model output, it can be observed that the number of dead pigs in the herd at a time of suspicion is dependent of the transmission rate of the virus and herd size.
In case of highly transmissible CSFV strains, the number of pigs dying from CSF will range from 1 to 10 (median) depending on herd size during the week preceding day 15 post introduction (Table 3).
Table 3.
Median (M), lower (L) and upper (U) 95% prediction intervals for the number of pigs dying of classical swine fever in the week preceding day 15 post introduction to the herd
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 3 | 3 | 0 | 7 |
| Medium transmission rate | 1 | 0 | 3 | 1 | 0 | 4 | 1 | 0 | 4 | 3 | 0 | 7 | |
| High transmission rate | 2 | 0 | 6 | 4 | 0 | 9 | 6 | 0 | 12 | 10 | 3 | 22 | |
| Long infectious period | Low transmission rate | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 3 | 2 | 0 | 6 |
| Medium transmission rate | 0 | 0 | 2 | 1 | 0 | 3 | 1 | 0 | 4 | 3 | 0 | 8 | |
| High transmission rate | 1 | 0 | 6 | 2 | 0 | 5 | 3 | 0 | 8 | 8 | 3 | 16 | |
This indicates that at least one (median) dead pig would be available in affected herds for necropsy and sampling for virological testing at this stage.
In Table 4, the time to reach 10% fever prevalence is also shown. In this table, it can be seen that a 10% fever prevalence would be reached before day 15 post introduction (median ranging between 9 and 14 days) in the event of infection with a virus strain with high transmission rates.
Table 4.
Median (M), lower (L) and upper (U) 95% prediction intervals for the time (days post introduction) to 10% fever prevalence of classical swine fever virus in a pig herd of a unit size of 50, 100, 200 and 1,000 pigs
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 23 | 13 | 41 | 27 | 18 | 49 | 32 | 24 | 52 | 45 | 35 | 59 |
| Medium transmission rate | 12 | 9 | 16 | 14 | 11 | 19 | 16 | 13 | 21 | 20 | 17 | 24 | |
| High transmission rate | 10 | 7 | 13 | 10 | 7 | 13 | 11 | 9 | 14 | 14 | 12 | 17 | |
| Long infectious period | Low transmission rate | 21 | 13 | 36 | 25 | 16 | 40 | 30 | 22 | 43 | 41 | 33 | 57 |
| Medium transmission rate | 12 | 9 | 17 | 14 | 11 | 17 | 16 | 12 | 21 | 20 | 17 | 24 | |
| High transmission rate | 9 | 7 | 12 | 10 | 8 | 14 | 11 | 9 | 15 | 14 | 12 | 17 | |
Further, the results of the model analyses presented in Table 5 confirm that in case of such a scenario, and assuming a 100% sensitivity of the virological test employed, the virus would be detected, with 95% confidence, after 11–13 days (median) post introduction if five pigs are tested, prioritising the dead pigs and complementing with a sufficient number of pyrexic pigs to make up this total.
Table 5.
Median (M), lower (L) and upper (U) 95% prediction intervals for the time (days post introduction) to 95% confidence in detecting classical swine fever virus in a pig herd when testing five dead or sick pigs†
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 5 | 2 | 8 | 4 | 2 | 7 | 5 | 3 | 9 | 20 | 5 | 34 |
| Medium transmission rate | 5 | 3 | 8 | 4 | 3 | 8 | 5 | 2 | 9 | 13 | 6 | 20 | |
| High transmission rate | 5 | 3 | 8 | 4 | 2 | 7 | 5 | 2 | 8 | 10 | 4 | 15 | |
| Long infectious period | Low transmission rate | 5 | 3 | 8 | 5 | 2 | 7 | 4 | 3 | 8 | 21 | 6 | 37 |
| Medium transmission rate | 5 | 2 | 7 | 5 | 2 | 7 | 5 | 3 | 9 | 13 | 6 | 21 | |
| High transmission rate | 5 | 3 | 8 | 4 | 3 | 7 | 5 | 2 | 8 | 11 | 7 | 15 | |
If fewer than five dead pigs were available at the time of sampling, additional pigs with fever were assumed to be (euthanised and) tested, so that a total of five pigs were tested.
In case of the spread of CSF virus strains with lower transmission rates, the number of dead pigs would be lower than in outbreaks caused by high transmission rate viruses and limited to single individuals per week (or even none) by day 25 post introduction (see Table 6) and between 1 and 7 by day 40 post introduction (see Table 7).
Table 6.
Median (M), lower (L) and upper (U) 95% prediction intervals for the number of pigs dying of classical swine fever in the preceding week at 25 days post introduction to the herd
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 0 | 0 | 3 | 1 | 0 | 3 | 1 | 0 | 4 | 3 | 0 | 8 |
| Medium transmission rate | 4 | 1 | 8 | 6 | 1 | 13 | 12 | 3 | 21 | 28 | 8 | 53 | |
| High transmission rate | 3 | 0 | 8 | 7 | 3 | 15 | 17 | 10 | 23 | 89 | 66 | 105 | |
| Long infectious period | Low transmission rate | 1 | 0 | 3 | 0 | 0 | 3 | 1 | 0 | 4 | 3 | 0 | 8 |
| Medium transmission rate | 3 | 0 | 7 | 5 | 2 | 11 | 8 | 1 | 15 | 16 | 3 | 37 | |
| High transmission rate | 3 | 0 | 6 | 6 | 2 | 10 | 12 | 5 | 18 | 59 | 45 | 71 | |
Table 7.
Median (M), lower (L) and upper (U) 95% prediction intervals for the number of pigs dying of classical swine fever in the preceding week at 40 days post introduction to the herd
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 1 | 0 | 6 | 3 | 0 | 8 | 4 | 0 | 10 | 7 | 1 | 19 |
| Medium transmission rate | 1 | 0 | 3 | 3 | 0 | 7 | 7 | 2 | 17 | 62 | 40 | 86 | |
| High transmission rate | 0 | 0 | 2 | 1 | 0 | 4 | 2 | 0 | 6 | 18 | 8 | 38 | |
| Long infectious period | Low transmission rate | 2 | 0 | 5 | 3 | 0 | 7 | 4 | 0 | 10 | 6 | 1 | 17 |
| Medium transmission rate | 2 | 0 | 4 | 4 | 1 | 7 | 9 | 2 | 16 | 53 | 4 | 65 | |
| High transmission rate | 1 | 0 | 4 | 3 | 0 | 6 | 6 | 2 | 11 | 39 | 26 | 47 | |
Nonetheless, it may be assumed that in such a situation the notification of the suspicion would be delayed as well (being the number of days to detection larger than the average). In any case, in Table 8 below it is shown that even if no dead pigs are found at the inspection on day 25 or 40 post introduction, the disease would be detected in a herd with 95% confidence by testing 5 pyrexic animals.
Table 8.
Median (M), lower (L) and upper (U) 95% prediction intervals for the time (days post introduction) to 95% confidence in detecting classical swine fever virus in a pig herd when testing 5 pigs with fever or fewer if < 5 pigs with fever were present at the time of sampling
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 5 | 2 | 9 | 5 | 2 | 8 | 5 | 2 | 8 | 15 | 5 | 32 |
| Medium transmission rate | 5 | 3 | 8 | 4 | 3 | 7 | 4 | 2 | 7 | 11 | 5 | 15 | |
| High transmission rate | 5 | 3 | 7 | 5 | 2 | 7 | 5 | 2 | 8 | 9 | 5 | 11 | |
| Long infectious period | Low transmission rate | 5 | 3 | 7 | 4 | 2 | 7 | 5 | 3 | 7 | 16 | 4 | 28 |
| Medium transmission rate | 5 | 3 | 8 | 5 | 2 | 7 | 5 | 3 | 9 | 11 | 5 | 15 | |
| High transmission rate | 5 | 3 | 8 | 5 | 3 | 8 | 4 | 2 | 8 | 9 | 5 | 12 | |
In relation to the effectiveness of the clinical examination in randomly selected animals, the model analysis shows (see Table 4 above), that in case of spread of CSFV with high transmission rate, the prevalence of infectious (virus positive) pigs with fever will reach 10% in 9–14 days (median) post introduction, with the number of days increasing with herd size. As sows may show less clinical signs, i.e. a smaller proportion of infected sows could develop fever as compared to infected finishing pigs, a lower prevalence of animals with fever (5%) should be assumed in sample size calculation for sows.
In case of the spread of CSFV strains with lower transmission rates, the time period to reach 10% prevalence of animals with fever may be considerably delayed (21–45 days as median depending on herd size, see Table 4 above).
In summary, for all strains, regardless of the transmission rate, considering the median time of reporting of the suspicion of CSF to the authorities (25 days for secondary outbreaks and 40 days for primary outbreaks, based on the results obtained in Section 4.2.1), it may be concluded that it is highly likely that the disease will be detected in the herd where clinical suspicion has been raised, if the sampling procedures described in the diagnostic manual regarding the sampling of dead pigs, and pigs with clinical signs or fever, are followed (i.e. by virological testing of at least five dead pigs or pigs with clinical signs or fever).
In case the suspicion is raised due to the suspect establishment being epidemiologically linked to an affected establishment, and no pigs are presenting clinical signs when arriving to the establishment (and unless a group of animals with high risk has been identified, e.g. pigs introduced from an affected establishment, that could be targeted for testing), clinical examination and sampling of randomly selected animals for the detection of fever and antibodies (serological testing) have been foreseen in the diagnostic manual. The model analysis shows that the 10% prevalence of animals with fever, caused by CSFV will be reached by 9–14 days (median) post introduction (see Table 4) whereas the same seroprevalence will be reached only 20–30 days (median) post introduction in case of the high transmission rate of the virus (Table 9).
Table 9.
Median (M), lower (L) and upper (U) 95% prediction intervals for the time (days post introduction) to 10% seroprevalence of classical swine fever virus in a pig herd
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 34 | 26 | 51 | 39 | 31 | 56 | 44 | 36 | 65 | 55 | 46 | 71 |
| Medium transmission rate | 24 | 20 | 30 | 25 | 22 | 31 | 27 | 24 | 33 | 31 | 28 | 35 | |
| High transmission rate | 20 | 16 | 24 | 21 | 18 | 24 | 22 | 19 | 25 | 24 | 22 | 27 | |
| Long infectious period | Low transmission rate | 41 | 30 | 56 | 44 | 36 | 57 | 49 | 41 | 63 | 62 | 52 | 77 |
| Medium transmission rate | 30 | 25 | 36 | 31 | 28 | 36 | 33 | 30 | 38 | 37 | 34 | 41 | |
| High transmission rate | 26 | 22 | 31 | 27 | 23 | 31 | 28 | 25 | 31 | 30 | 28 | 33 | |
In case of the lower transmission rate of the virus the 10% seroprevalence would be reached much later; in larger herds it could be as late as day 62 post introduction (as the largest median observed for herds of 1,000 pigs). This indicates that with serological testing of pigs in suspect herds, according to the diagnostic manual procedures, the detection of the disease would be delayed. Serological testing would nonetheless be effective, if the spread of a low virulent strain could be suspected that has been present in the herd for a prolonged period without causing remarkable disease signs in pigs.
Table 10 below shows how the median seroprevalence in large herds could still be below 10% 40 days after virus introduction if the virus has a low transmission rate.
Table 10.
Median (M), lower (L) and upper (U) 95% prediction intervals for the seroprevalence of classical swine fever virus in a pig herd at 40 days post introduction
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 20 | 0 | 61 | 12 | 0 | 33 | 6 | 0 | 19 | 1 | 0 | 4 |
| Medium transmission rate | 92 | 72 | 100 | 85 | 0 | 97 | 79 | 26 | 91 | 58 | 37 | 76 | |
| High transmission rate | 98 | 90 | 100 | 98 | 93 | 100 | 97 | 90 | 99 | 92 | 85 | 96 | |
| Long infectious period | Low transmission rate | 9 | 0 | 26 | 5 | 0 | 18 | 3 | 0 | 9 | 0 | 0 | 2 |
| Medium transmission rate | 51 | 29 | 72 | 44 | 26 | 57 | 36 | 13 | 46 | 19 | 0 | 30 | |
| High transmission rate | 69 | 51 | 88 | 65 | 49 | 81 | 61 | 47 | 71 | 51 | 39 | 60 | |
Nonetheless the median number of days to observe 10% of pigs with fever in the event of infection with a virus with low transmission rates is below 40 (between 15 and 39), even for herds of 1,000 pigs (see Table 4).
It can be concluded that on average, in the event of a low transmission rate virus, random sampling of pigs to detect fever assuming a 10% prevalence would help to identify infected pigs if 40 days or more have elapsed since the virus introduction. However, if after taking temperatures, no pigs are identified with fever, the random sampling of pigs for serological testing in herds would not be effective for detecting the infection in the herd.
If the suspicion of CSF in the holding is related to a previous positive or suspicious serological test result, the diagnostic manual foresees additional sampling of pigs with an aim to isolate the virus from fetuses of seropositive sows or perform serological tests in contact pigs. These procedures could be considered adequate as detection of the virus in infected fetuses and molecular characterisation of the detected virus allow with highest certainty to rule out or confirm the CSFV infection in these animals. If virus detection is not successful, discriminatory serological tests are available to identify the type of the Pestivirus inducing antibodies in these animals.
Development of new procedures
Random sampling of pigs for serological testing could be omitted in herds that are suspected to be infected due to an epidemiological link with an affected establishment, but where clinical signs in pigs have not been detected (as clinical signs would be apparent ahead of a positive serological result), unless, there are specific epidemiological considerations such as: suspicion of delayed reporting, spread of a low virulent strain or in the event that only‐sows farms, or a boar station are involved. The serological sampling could be replaced by enhanced passive surveillance during the monitoring period (the assessment of the length of the recommended monitoring period is described in Section 4.2.2), with animals dying or showing clinical signs being sampled and tested for CSFV as described in the assessment section above and in Section 4.1.1.5.
Two groups of animals at risk of being infected not mentioned in the diagnostic manual are pigs with stunted growth, and sows that had aborted; these two groups could be added in any future guidelines.
4.1.1.2. For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in an establishment affected and officially confirmed with CSF
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing and in their ability to support the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. For further details, see Annexes B and C.
1.
2nd Scenario of sampling procedures
ToR 1.2 in accordance with Mandate
Article 12(3) and the Art. 7 (4) (Preventive killing) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
The following elements of the scenario were taken into consideration for the assessment:
It refers to an officially confirmed establishment;
Kept animals of listed species found dead or before/when they are killed are sampled;
Competent authority collects samples for laboratory examination;
The purposes of the sampling are:
-
a)
supporting the epidemiological enquiry to:
-
i
identify the likely origin of the disease;
-
ii
calculate the likely length of time that the disease is present;
-
iii
identify establishments where the animals could have contracted the disease and movements from the affected establishment that could have led to the spread of the disease; and
-
iv
obtain information on the likely spread of the listed disease in the surrounding environment, including the presence and distribution of disease vectors
-
b)
confirming/ruling out disease in the event of preventive killing.
Summary of sampling procedures
According to the diagnostic manual (Annex, CHAPTER IV.B.) to support the epidemiological investigation in an affected holding, blood samples for serological tests must be taken at random from the pigs when they are killed. The minimum number of pigs to be sampled must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit of the holding. Samples for virological tests may also be taken in accordance with the instructions of the competent authority.
In case of secondary outbreaks, the competent authority may establish ad hoc sampling procedures, taking into account the epidemiological information already available on the source and means of virus introduction into the holding and the potential spread of disease from the holding.
When pigs are killed as a preventive measure on a suspected holding, blood samples for serological tests, as well as blood or tonsils samples for virological tests must be taken in accordance with the procedure laid down in the Diagnostic Manual (Annex, CHAPTER IV.C.).
Sampling must primarily concern pigs showing clinical signs or post‐mortem lesions suggesting CSF and their in‐contact pigs, along with other pigs which might have had risky contacts with infected or suspected pigs or which are suspected to have been contaminated with CSFV.
Furthermore, pigs from each of the subunits of the holding must be sampled at random. In this case, the minimum number of samples to be taken for serological tests must allow for the detection of 10% seroprevalence with 95% confidence in the subunit in question. In the case of breeding sows: the minimum number of sows to be sampled must allow for the detection of 5% seroprevalence with 95% confidence. In certain cases, when lower seroprevalence could be assumed it is recommended to increase the sample size. At semen collection centres blood samples from all boars should be taken.
The type of samples to be taken for virological tests and the test to be used are to be decided by the competent authority, which should take into account the range of tests that can be performed, the sensitivity of these tests and the epidemiological situation.
Assessment
For the epidemiological investigation, it is important to examine the distribution of disease and infection across an infected farm because it may provide information regarding the potential site of virus entry on the farm and the potential for onward spread to other farms. For that reason, a clinical inspection on all subunits of the farm is useful. In addition, a serological survey is useful as it may help estimate the time of virus introduction. Because sows in the EU can no longer be denied social interaction, with individual isolation only occurring in some periods of their life cycle, larger opportunities for disease transmission are facilitated as compared to crate housing. Based on this, there is no reason to aim for the detection of a lower seroprevalence in sows compared to that in other pigs (Stegeman et al., 1999).
In case of preventive culling, in order to confirm CSFV infection, the sampling strategy described in section 4.1.1.1 related to the sampling in a suspect herd with an epidemiological link with an affected establishment, should be followed.
Development of new procedures
To assess the length of time the disease has been in the farm: clinical inspection of all subunits of the farm to explore how widespread the infection is across the farm must be carried out. Based on the clinical inspection, animals may be selected for clinical examination in various parts of the farm and tested for the presence of virus. This sampling aims to detect virus presence on different sites of the farm, not the prevalence, so the exact number of pigs to be tested is best decided by the local authorities based on the situation at hand, using as guidance the results presented in section 4.1.1.1. In addition, serum samples should be collected from a random sample of pigs across the farm aiming to detect a 10% seroprevalence. In case these prove negative, it is highly likely that the virus was introduced into the farm less than 20–30 days before, if referring to a high transmission strain (these are the minimum number of days needed to reach that seroprevalence in the event of a high transmission rate strain; this minimum number would be much larger if the virus has low transmissibility, see Table 9). This information retrieved form serology would be valuable to determine the time window for contact tracing, moreover, it may help identify in which part of the farm the infection started.
In case of preventive killing the clinical inspection, examination and sample collection should be according to that described in Section 4.1.1.1 for farms suspected based on epidemiological links. As these farms cannot be followed further, it is useful to also collect blood samples for serology as described above, to confirm or rule out unnoticed infections.
4.1.1.3. For granting a specific derogation from killing animals of the categories described in article 13.2 of the Delegated Regulation in an CSF‐affected establishment
1.
3rd Scenario of sampling procedure
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 13(3)c of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration during for the assessment:
-
1
It refers to an officially confirmed establishment;
-
2
In an establishment where there are kept animals of listed species of the following specific categories animal categories based on article 13(2):
-
a)
animals kept in a confined establishment;
-
b)
animals kept for scientific purposes or purposes related to conservation of protected or endangered species;
-
c)
animals officially registered in advance as rare breeds;
-
d)
animals with a duly justified high genetic, cultural or educational value.
-
a)
-
3
the competent authority may grant a specific derogation from killing all the animals of listed species belonging to any of the above categories in an affected establishment, provided that specific conditions are fulfilled;
-
4
The animals should be subjected to clinical surveillance, including laboratory examinations;
-
5
Sampling procedures should ensure that the animals do not pose a risk of transmission of CSF if left alive.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. For further details, see Annexes B and C.
Summary of sampling procedures
There are no sampling procedures to grant a derogation from killing of animals in an affected establishment.
Assessment
The course of infection in CSF may vary considerably between virus strains and age of the pigs. Highly virulent strains are usually associated with (per) acute disease, high mortality and a very small number of survival pigs that would develop antibodies for life. In case of moderately virulent strains, primary infection can result in death, recovery or chronical infection. In case of recovery, pigs will have long lasting antibodies. In case of chronical infection, however, the animals remain infected, and die of the disease at a later stage, with the length of time to death being variable. These chronically infected pigs continue to shed virus (intermittently) and are infectious to in‐contact pigs (Weesendorp et al., 2009b). Antibody levels in these animals may be variable including levels that are undetectable. In addition, when sows are infected during gestation, they can give birth to immunotolerant carrier pigs due to transplacental transmission of the virus in the period when the fetuses are not yet immunocompetent (before 70 days of gestation). These piglets will acquire antibodies from the colostrum of their dam after birth and will develop disease and die (at a variable time) after birth that is influenced by the acquired maternal immunity from their dam. The possibilities of finding chronically infected pigs (particularly when a moderately virulent strain is in circulation), and immunotolerant carriers, must be taken into account before a derogation is granted. Due to the potential presence of three different categories of animals that have been infected: (i) antibodies/fully recovered, (ii) antibodies/infected (chronically infected pigs/carrier piglet with maternally derive antibodies) and (iii) no antibodies/infected (immunotolerant carriers pigs), the granting of the derogation should not be based on serological sampling only.
Development of new procedures
The aim should be to detect any virus present on the farm before the restrictions in the area are/can be lifted.
Three options (and a combination of those) can be considered:
-
a)
to test all pigs for virus and antibodies twice a month (interval related to length of an infectious period during primary infection) removing chronically infected pigs from the farm, because they will not recover
-
b)
to test piglets born to seropositive sows for virus and antibodies to detect immunotolerant piglets, with the latter being removed from the farm.
For a) and b) testing should continue until three consecutive samplings with negative results in polymerase chain reaction (PCR) (no single virus positive pigs or piglets has been observed) are attained over a period of a month.
-
c)
sentinel animals comingling with surviving animals could also be used to exclude any virus transmission. Sentinel animals should be tested as described in Section 4.1.3.3 (section related to sampling of repopulated animals).
4.1.1.4. For wild animals of the listed species in a CSF‐affected establishment and its surroundings
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species. For further details, see Annex B.
1.
5th scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Article 14(1) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario were taken into consideration for the assessment:
It concerns a CSF‐affected establishment (officially confirmed).
It refers to wild animals of listed species within the establishment and in the surroundings of the establishment.
As listed in Commission Implementing Regulation (EU) 2018/1882 for CSF; the wild animals of listed species animals are those of wild boar species.
The competent authority may establish these sampling procedures in addition to other measures.
The purpose of the sampling procedures in wild animals of listed species is to ensure the detection of the virus, if the virus is present in these wild animals.
Summary of sampling procedures
No guidelines for the sampling of wild animals of listed species are described for confirmation of CSFV infection in animals within an establishment and its surroundings.
Assessment
In case wild boars have entered the territory of the affected establishment (e.g. pastures), there is a risk of dispersal of the virus into the wild boar population in the surroundings of the affected establishment. Contrarily, infection may have originated in the wild boar population, with wild boar being the source of infection for pigs in the establishment. The sampling procedures should ensure the detection of the infection in wild boar caught within the establishment and found dead or hunted in its surroundings to support the management of the related risks.
Development of new procedures
If incursion of wild boar into the territory of the establishment has occurred and those animals have been caught and culled, blood and tissue samples should be collected for laboratory examination and virus and antibody detection with relevant diagnostic tests performed.
Enhanced passive surveillance (wild boar carcass search) in the area surrounding the establishment should be implemented. All wild boars found dead should be tested for virus and antibodies. If hunting is ongoing in the surrounding, the shot animals should also be tested.
Effective surveillance for CSF, especially when due to low virulence strains, will require the use of PCR‐based assays for the detection of early infections (< 14 days) and antibody‐based assays thereafter (Panyasing et al., 2018). Modelling has shown that current active surveillance for CSF in wild boar as carried out in Germany (59 randomly chosen samples per year, tested by virological and serological methods; Schulz et al., 2017a) fulfils the requirements of detecting an outbreak with 95% confidence within one year after the introduction of CSF into the population. By contrast, passive surveillance alone is not sufficient to meet the requirements for detecting CSF. Nevertheless, there is room for improved performance and efficiency by more homogeneous (active and passive) sampling of wild boar over the year. In times of disease freedom, evaluating active surveillance samples by serology is sufficient and can thus save resources. Adaptive, situation‐based surveillance designs can be meaningful regarding recurrent wildlife diseases such as CSF (Thulke et al., 2009). Risk‐based approaches, for instance, sampling only among sub‐adults, might result in better acceptability and timeliness (Schulz et al., 2017b).
4.1.1.5. For animals of listed species in non‐affected establishments located in a protection zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. For further details, see Annexes B and C.
1.
6th Scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 26(2) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario should be taken into consideration during for the assessment:
It concerns the protection zone with radius up to 3 km;
Official veterinarians must visit at least once all the non‐affected establishments with kept animals of listed species located in the protection zone;
Among others, they must perform a clinical examination of kept animals of listed species and if necessary, collection of samples for laboratory examination;
The purpose of sampling procedures is to confirm or rule out the presence of CSF.
Summary of sampling procedures
According to Articles 4 and 5 of the old Directive 92/102/EEC, all establishments in the protection zone had to be visited by an official veterinarian (within 7 days after establishment of the protection zone) for a clinical examination of the pigs, and for a check of the register and of the pig identification marks. Further, all dead or diseased pigs on a holding had to be immediately notified to the competent authority, which had to carry out appropriate investigations in accordance with the procedures laid down in the diagnostic manual.
The measures in the protection zone had to be applied at least until: a) cleaning and disinfection in the infected holdings had been carried out; b) pigs on all holdings had undergone clinical and laboratory examinations with negative results, in accordance with the diagnostic manual in order to detect the possible presence of CSFV. The examinations referred to in point b) could not take place before 30 days had elapsed since the completion of the preliminary cleaning and disinfection of the infected holding that originated the protection zone.
Assessment
For the purpose of early detection of the virus in these establishments, the guidelines provided in Section 4.1.1.1 can be followed at the first visit and depending on whether clinical signs are observed or not at the clinical examination. For points already discussed in section 4.1.1.1, the assessment remains as per section 4.1.1.1.
If no clinical signs are observed, and there are no other reasons to suspect CSFV at the first visit, random sampling of the pigs to detect a 10% fever prevalence would lead to detection of viruses with low transmission rates by day 40 (between 15 and 39) post introduction, even for herds of subunits of 1,000 pigs (see Table 4). Detecting 10% seroprevalence would take longer, (a median of up to 62 days post introduction, see Table 9). Random sampling to detect fever is therefore recommended.
Development of new procedures
Even if no pyrexic pigs are found at the first visit, this would not be sufficient to rule out the disease in herds of the protection zone. Surveillance based on continuous sampling of at least two dead post‐weaning pigs, or pigs older than 2 months per week in each epidemiological unit for virus detection for the duration of the restricted zone as prescribed for ASF in accordance with the Strategic approach to the management of African Swine Fever for the EU – Rev. February 2020, SANTE/7113/2015 – Rev 12, could be considered also for CSF.
In relation to such continuous sampling, the predicted time (median and 95% confidence) to detect CSFV when testing two dead pigs weekly, is shown in Table 11. The simulated time to detection is also shown in Figure 5.
Table 11.
Median (M), lower (L) and upper (U) 95% prediction intervals for the time to detection (days post introduction) of classical swine fever virus when PCR testing two dead pigs per week
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 24 | 11 | 46 | 25 | 7 | 40 | 26 | 10 | 40 | 27 | 9 | 45 |
| Medium transmission rate | 17 | 8 | 27 | 17 | 8 | 25 | 18 | 9 | 25 | 19 | 10 | 27 | |
| High transmission rate | 15 | 9 | 22 | 14 | 7 | 21 | 14 | 8 | 21 | 16 | 9 | 21 | |
| Long infectious period | Low transmission rate | 27 | 10 | 46 | 28 | 10 | 44 | 28 | 10 | 43 | 30 | 9 | 49 |
| Medium transmission rate | 19 | 9 | 28 | 19 | 11 | 26 | 19 | 9 | 27 | 20 | 9 | 29 | |
| High transmission rate | 16 | 8 | 25 | 16 | 10 | 22 | 15 | 9 | 21 | 16 | 9 | 22 | |
Figure 5.
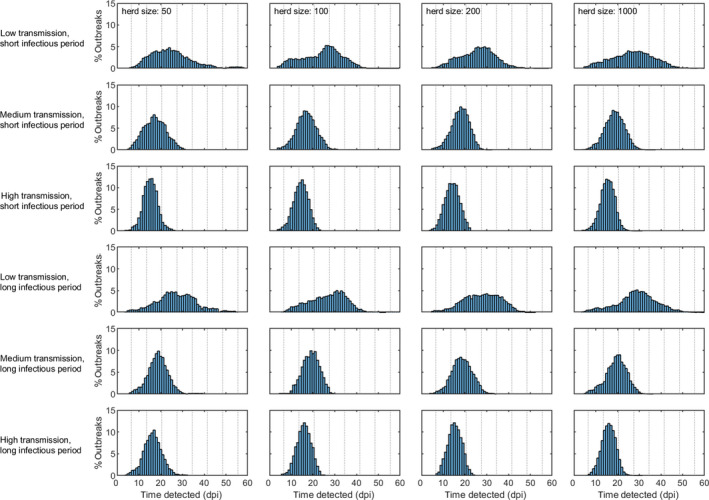
Simulated time (days post introduction) in order to detect CSFV in a pig herd when testing of two dead pigs each week is implemented. The plots show the time to detection in a herd of 50 100, 200 or 1,000 pigs (indicated at the top of each column) for six scenarios which differ in transmission parameters and infectious period. The vertical dotted lines demarcate the weekly sampling periods for a period up to 8 weeks
This sampling scheme will detect the virus in an affected herd at median times of between 14 and 16 days post infection in the event of infection with a virus of high transmission rate and between 24 and 30 days in the event of a low transmission rate virus, with 95% confidence, assuming a 3% baseline mortality over the whole production cycle in the target age groups (see Table 11).
Serological testing of randomly selected animals should only be conducted in the event of an only sows farm or a boar station.
4.1.1.6. For non‐affected establishments located in a surveillance zone
1.
8th scenario of sampling procedures:
ToR 1.3 in accordance with Article 41 of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
Ιt refers to the surveillance zone;
Sample of the establishments of kept animals of listed species in the surveillance zone;
Official veterinarians carry out visits to a sample of the establishments among others perform clinical examination of kept animals of listed species and if necessary, collection of samples for laboratory examination;
The purpose of sampling procedure is to ensure the detection of the disease if the disease is present in any of the establishments.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure early detection if the virus is present in establishments within the surveillance zone. For further details, see Annex B.
Summary of sampling procedures
According to the old diagnostic manual, in farms of the surveillance zone, all dead or diseased pigs on a holding had to be immediately notified to the competent authority (passive surveillance), which had to carry out appropriate investigations in accordance with the procedures laid down in this diagnostic manual.
Further, and before the restrictions were lifted in the surveillance zone, all farms had to be visited not earlier than 20 days after initial cleaning and disinfection of the index case. Clinical inspection had to take place, and in case of clinical signs, it had to be followed by the sampling procedures described in this diagnostic manual.
In addition, serological sampling (aiming at 10% seroprevalence) had to take place on those farms, where no pigs between 2 and 6 months of age were present, whenever the competent authority deemed that CSFV could have spread unnoticed amongst breeding sows; in any other holding where sampling was deemed necessary by the competent authority; and in all semen collection centres.
Assessment
No sampling procedures for the early detection of the virus in establishments located in the surveillance zone have been found in the literature/past legislation. The sampling described in the summary above referred to ensuring freedom of the disease before the restrictions were lifted, but they were not designed to achieve an early detection. To ensure early detection in these establishments, the enhanced passive surveillance described for the establishments in the protection zone (Section 4.1.1.5), could be put in place. This enhanced surveillance would require the continuous sampling of at least two dead post‐weaning pigs or pigs older than 2 months in each epidemiological unit for virus detection during the period the surveillance zone is in place. If this enhanced surveillance was to be put in place, the final visit to ensure freedom of CSF could be limited to those establishments that did not follow it. Random sampling of a number of establishments within the surveillance zone was not considered the best alternative as the sample would require a large number of establishments to be sampled (maybe all) in order to have sufficient power to detect the disease.
Development of new procedures
Enhanced passive surveillance is recommended as described above.
4.1.2. Assessment of sampling procedures to grant derogations for animal movements
4.1.2.1. From non‐affected establishments located in the protection zone to slaughterhouses located within the protection zone or in the surveillance zone or outside the restricted zone
1.
9th Scenario of sampling procedures
ToR 1.4 in accordance with Article 28(5) of the Delegated Regulation (EU) 2020/687
Article 29 of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the protection zone;
Grant derogation for movement of kept animals of listed species from a non‐affected establishment in the protection zone;
Animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29). For further details, see Annex B.
Summary of sampling procedures
Based on the old directive assessed in this opinion, a clinical examination of pigs had to be carried out in each subunit in which the pigs to be moved were kept. In case of pigs older than 3–4 months, this examination had to include taking the temperature of a proportion of pigs allowing for the detection of fever if it occurred at a prevalence of 20% with 95% confidence in the subunits in question. In the case of breeding sows or boars, this number of animals had to be increased to detect a 5% prevalence with 95% confidence.
Blood samples for serological tests or blood or tonsil for virological tests had to be taken at slaughter from pigs proceeding from each of the subunits from which pigs had been moved. The minimum number of samples to be taken had to allow for the detection of 10% seroprevalence or virus prevalence with 95% confidence in each subunit, in case of breeding pigs this design (sero)prevalence had to be 5%.
Assessment
In order to detect 20% prevalence with a 95% confidence, rectal temperature would need to be taken from up to 14 animals (minimum sample size) within 24 h before the shipment. In case animals with a higher temperature than normal are observed, it is recommended that sampling is performed according to Section 4.1.1.1 and animals are not shipped until a negative test result is available and the animals are healthy. Higher than normal temperatures in otherwise healthy pigs may happen due to stress reactions. Elbers et al. (2002) reported fever as a clinical sign in 87% of CSF infected pigs submitted to post‐mortem. While assuming that viraemia in pigs is associated with fever, taking temperature to detect a 20% prevalence of pigs with fever, would result in detection of the CSFV in a shipment of 100 pigs at a minimum of 12 and a maximum of 33 days (median) post introduction (Table 12). The latter number being a situation with a slowly transmitting virus inducing a short infectious period.
Table 12.
Median (M), lower (L) and upper (U) 95% prediction intervals for the time (days post introduction) to 20% fever prevalence of classical swine fever virus in a pig herd
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 29 | 17 | 50 | 33 | 24 | 61 | 39 | 30 | 59 | 51 | 42 | 65 |
| Medium transmission rate | 15 | 10 | 19 | 16 | 13 | 21 | 18 | 15 | 23 | 22 | 19 | 26 | |
| High transmission rate | 11 | 8 | 14 | 12 | 9 | 14 | 13 | 11 | 16 | 15 | 13 | 18 | |
| Long infectious period | Low transmission rate | 25 | 18 | 41 | 30 | 21 | 47 | 35 | 28 | 49 | 47 | 38 | 63 |
| Medium transmission rate | 14 | 11 | 19 | 16 | 13 | 19 | 18 | 15 | 23 | 22 | 19 | 26 | |
| High transmission rate | 11 | 9 | 14 | 12 | 9 | 15 | 13 | 10 | 16 | 15 | 13 | 18 | |
Age dependent susceptibility to the development of clinical signs may result in fewer sows with fever than in younger pigs, depending on the virulence of the virus strain. For that reason, using a higher sample size (sample size to detect 5% fever prevalence in sows versus 10% in finishing pigs) for temperature collection in sows could be justified.
Serological testing of pigs at slaughter to detect 10% seroprevalence will detect infections that have been present in the group for at least 20–62 days (range of medians for the different groups considered and shown in Table 9). Given that farms in the protection zone must be visited for clinical inspection, it is highly unlikely to find seropositive pigs in the absence of any previous clinical suspicion. Virological testing at slaughter detects the earlier stages of infection. In case of a virulent or moderately virulent strain, this will contribute little to detection, when the temperature is being taken on the farm, because virus positivity will be associated with fever. In case of a strain with low virulence additional confidence of infection freedom of the batch could be obtained by virological testing. Nevertheless, using different sample sizes for serologically testing breeding pigs (to detect a 5% seroprevalence) compared to finishing pigs (to detect a 10% seroprevalence) does not seem scientifically justified nowadays, given that both types of pigs are kept in groups (at least for some periods of their life cycle), enabling a similar contact structure to allow infection transmission (as opposed to sows in crates before 2013).
A clinical inspection of the entire farm, before the group to be shipped is clinically examined, is recommended. This could show suspicion of infection in other parts of the farm. In that case, movement of the pigs should not take place even if the group to be moved is clinically healthy and no animal with fever has been detected among them.
Development of new procedures
Clinical inspection of the entire farm before clinical examination of the group to be shipped in accordance with Section 4.1.1.1.
The temperature should be taken in the group of pigs to be shipped according to the described protocol. Additional sampling at the abattoir could be omitted.
4.1.2.2. From non‐affected establishments located in the protection zone to a plant approved for processing or disposal of animal by‐products in which the animals are immediately killed
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37). For further details, see Annexes B and C.
1.
12th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 28(5) and article 37 of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the protection zone;
To grant derogation for movement of kept animals of listed species from a non‐affected establishment in the protection zone;
The animals to be moved to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed;
Clinical examinations and laboratory examinations of animals kept in the establishment, including those animals to be moved.
The following elements of the scenario were taken into consideration for the assessment:
It concerns the protection zone;
To grant derogation for movement of kept animals of listed species from a non‐affected establishment in the protection zone;
The animals to be moved to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed;
Clinical examinations and laboratory examinations of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
As per Section 4.1.2.1.
Assessment
As per Section 4.1.2.1.
Development of new procedures
As per Section 4.1.2.1.
4.1.2.3. From an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone and from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b) from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone. For further details, see Annexes B and C.
1.
13th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 43(5) and article 44 of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the:
It concerns kept animals of listed species of the establishments in the surveillance zone;
To grant derogation for movement from an establishment in the surveillance zone to be moved to a slaughterhouse within the restricted zone or outside the restricted zone;
To grant derogation for movement from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
As per Section 4.1.2.1.
Assessment
Pigs from outside the surveillance zone moved into the zone to a slaughterhouse do not increase the risk, because they come from a CSF‐free zone. No extra checks are needed on the farm of origin in those cases and no virological or serological testing is useful after ante mortem checks, because the duration of transport is much shorter that the period needed to get positive test results.
Development of new procedures
As per Section 4.1.2.1.
No additional testing is needed for farms located outside the surveillance zone.
4.1.2.4. From an establishment in a surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone
1.
15th scenario of sampling procedures
ToR 1.4 in accordance with article 43(5) and article 45(2) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the surveillance zone;
Grant derogation for movement of kept animals of listed species;
From the surveillance zone;
To be moved to an establishment belonging to the same supply chain, located in or outside the surveillance zone, to complete the production cycle before slaughter;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter. For further details, see Annex B.
Summary of sampling procedures
In the sampling procedures described in Council Directive 2001/89/EC it was foreseen a clinical examination of the pigs in the holding and in particular those to be moved, including taking the body temperature of a proportion thereof. According to the Diagnostic Manual (Annex Chapter D) the clinical examination of pigs had to be carried out by an official veterinarian within the 24‐h period before moving the pigs. In addition, a clinical examination of pigs had to be carried out in each subunit of the holding in which the pigs to be moved were kept. In case of pigs older than three to four months, this examination had to include the taking of temperature of a proportion of pigs. The minimum number of pigs to be checked had to allow for the detection of fever if it occurred at a prevalence of 10% with 95% confidence in these subunits. In case the breeding sows were moved, the minimum number of sows to be examined had to allow for the detection of fever if it occurred at a prevalence of 5% with 95% confidence in the subunit where the sows to be moved were kept and in case of boars, all boars to be moved had to be examined.
Assessment
The enhanced passive surveillance suggested for establishments in the surveillance zone as described in Section 4.1.1.6 would ensure early detection of the disease in the establishments within the zone.
Clinical and laboratory examination of animals to be moved would provide additional confidence in disease freedom in these animals.
According to the model analysis in case of the spread of highly virulent CSFV strains the prevalence of animals with fever reaches the level of 10% depending on herd size after 9–14 days post infection as a median (Table 4). However, in such a situation, animals with clinical signs are assumed to emerge in the herd and such animals would undergo sampling for laboratory testing. Thus, the clinical examination of pigs according to the old diagnostic manual could be considered effective in preventing the spread of the infection. In case of detection of any disease signs in animals, the sampling procedures according to Section 4.1.1.1 (sampling procedures in the event of a suspicion) should be applied.
In case the outbreaks are caused by CSFV strains of lower virulence the manifestation of clinical disease (including fever) may be variable. In these circumstances onset of clinical signs may be delayed. According to model analysis, the 5% prevalence of animals with fever would be reached by day 33 or later post introduction in larger herds. In these circumstances the additional laboratory testing for presence of virus in the animals to be moved would be necessary to assure disease freedom of moved pigs.
Development of new procedures
If a CSFV strain of lower virulence has been circulating, additionally to the clinical examination foreseen in present procedures, testing for virus of all animals being dispatched should be considered necessary to prevent transmission of the virus.
4.1.2.5. From an establishment located in the restricted zone to move within and from the restricted zone when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation
1.
18th scenario of sampling procedures
ToR 1.4 in accordance with article 56(1) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the restricted zone when restriction measures are maintained beyond the period set out in Annex XI;
To grant derogation for movement of kept animals of listed species from an establishment within the restricted zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within and from the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation. For further details, see Annex B.
Summary of sampling procedures as described in the diagnostic manual
According to Council Directive 2001/89/EC a clinical examination of the pigs in the holding and in particular those to be moved, including the taking of the body temperature of a proportion thereof, had to be carried out by an official veterinarian. See section 4.1.2.4.
Assessment
As per Section 4.1.2.4.
Development of new procedures
As per Section 4.1.2.4.
4.1.3. Assessment of sampling procedures for repopulation purposes
4.1.3.1. For the animals that are kept for the repopulation prior to their introduction
1.
19th scenario of sampling procedures
ToR 1.5 in accordance with article 59(2) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It refers to the repopulation of a previously affected establishment;
Animals intended for repopulation shall be sampled prior to their introduction into the establishment of destination;
The samples shall be collected from a representative number of animals in each consignment from each establishment or from a representative number of animals of each consignment (if animals are all to be introduced at different times or from different establishments of origin);
Laboratory examinations;
The purpose sampling procedures is to rule out the presence of the disease.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. For further details, see Annex B.
Summary of sampling procedures as described in the diagnostic manual
No specific procedures for sampling of animals intended for repopulation were found in the literature review.
Assessment
If the animals intended for repopulation originate from a holding located in a surveillance zone, the procedures in place for movement of pigs to other establishments from such holdings will apply. See Section 4.1.2.4.
If the pigs intended for repopulation come from a CSF‐free area, there are no requirements for prior testing of pigs before being moved and general regulations in place for moving live pigs will apply.
Development of new procedures
As per Section 4.1.2.4.
No testing for animals that come from outside the restricted zones is needed.
4.1.3.2. In the event of unusual mortalities or clinical signs being notified during the repopulation
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. For further details, see Annex B.
1.
20th scenario of sampling procedures
ToR 1.5 in accordance with article 59(9) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It refers to a repopulated establishment;
Unusual mortalities or clinical signs during the repopulation;
The official veterinarians shall without delay collect samples for laboratory examination;
The purpose of sampling procedures is to rule out the presence of the disease.
Summary of sampling procedures as described in the diagnostic manual
As per Section 4.1.1.1.
Assessment
As per Section 4.1.1.1.
Development of new procedures
As per Section 4.1.1.1.
4.1.3.3. For animals that have been repopulated
1.
21st scenario of sampling procedures
ToR 1.5 in accordance with article 59(5) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It refers to a repopulated establishment;
Animals that have been used for repopulation;
Laboratory examinations;
Sampling procedures to rule out the presence of the disease.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. For further details, see Annex B.
Summary of sampling procedures as described in the diagnostic manual
No guidelines for the clinical examination of repopulated animals were found, only for laboratory testing. Based on the old diagnostic manual, in case of an open‐air pig holding, repopulation had to start with sentinel pigs that were placed throughout the infected holding, with the exact details being decided by the competent authority. In other farms, repopulation could be based on sentinel pigs or on total repopulation. All pigs had to come from unrestricted holdings and they had to arrive within a period of 20 days. In case of sentinel pigs, a sample allowing detection of a 10% seroprevalence had to be collected per subunit 40 days after introduction of the pigs; in case of total restocking a sample allowing detection of a 20% (10% breeding pigs) seroprevalence had to be collected per subunit not earlier than 40 days after introduction of the last pigs.
If more than six months have passed since cleaning and disinfection, the competent authority was able to provide a derogation from the testing.
Assessment
Using sentinel pigs for the repopulation of an open air‐pig holding (versus complete repopulation) is recommended, because cleaning and disinfection may not be possible in all parts of these holdings (e.g. pastures). In the event of a highly virulent strain being circulating, serology will add little to the passive surveillance in the farm, as some mortality, and pigs with clinical signs will be observed before day 40 (see Tables 4 and 7). However, serology will provide added value to passive surveillance in case of a virus with limited virulence and in the event that under reporting by passive surveillance is suspected. Unless the strain has low transmissibility the seroprevalence in the farm will exceed 20% 40 days after introduction of the pigs. In the event of a low transmissibility virus, and particularly in large herds, detection of 20% seroprevalence might take up to 83 days (see Table 13 below); in that event, it would be recommended to increase the sample size by reducing the aimed seroprevalence to 10%.
Table 13.
Median (M), lower (L) and upper (U) 95% prediction intervals for the time (days post introduction) to 20% seroprevalence of classical swine fever virus in a pig herd
| Scenario | Herd size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 1,000 | ||||||||||
| M | L | U | M | L | U | M | L | U | M | L | U | ||
| Short infectious period | Low transmission rate | 40 | 30 | 59 | 44 | 36 | 65 | 50 | 41 | 70 | 61 | 52 | 76 |
| Medium transmission rate | 26 | 23 | 31 | 28 | 24 | 33 | 30 | 27 | 35 | 34 | 31 | 37 | |
| High transmission rate | 22 | 19 | 26 | 23 | 19 | 26 | 24 | 22 | 27 | 26 | 24 | 29 | |
| Long infectious period | Low transmission rate | 46 | 38 | 60 | 49 | 42 | 66 | 55 | 48 | 68 | 67 | 58 | 83 |
| Medium transmission rate | 33 | 28 | 39 | 35 | 31 | 39 | 36 | 34 | 41 | 41 | 38 | 45 | |
| High transmission rate | 29 | 25 | 33 | 30 | 27 | 34 | 31 | 28 | 35 | 33 | 31 | 36 | |
Given that all pigs are kept in groups nowadays, the design prevalence in breeding pigs and finishing pigs should be the same. In all of the scenarios considered in this assessment, clinical signs (i.e. pigs with fever) will arise within 6 months from the repopulation date, in this regard, the lack of need for sampling procedures when more than 6 months have elapsed since the cleaning and disinfection can be justified.
Development of new procedures
The sampling procedures described in the old diagnostic manual are effective. The detection threshold for serology could be 20% for breeding and other pigs, although it will need to be reduced to 10% in the event of a virus with low transmissibility.
4.2. Assessment of the length of the monitoring period
The concept of the monitoring period was introduced as a management tool for the investigation and control of suspected and confirmed outbreaks of Category A diseases in terrestrial animals. This tool aimed to standardise the methodology by which relevant authorities responded to suspected and confirmed cases of these diseases. In this regard, a disease‐specific monitoring period was set for each of the 14 diseases included in the Category A list. Throughout the EU legislation, the monitoring period is used as an aid in the control of these diseases, although the specific purpose in which the monitoring period is used varies depending on the articles of the legislation.
The length of the monitoring period for each disease is set out in Annex II of the Commission Delegated Regulation (EU) 2020/687 supplementing the rules laid down in Part III of Regulation (EU) 2016/429 (Animal Health Law).
The table in Annex C in this Scientific Opinion describes the seven scenarios, for which an assessment of the length of the monitoring period for CSF had been requested.
4.2.1. Results
A search was carried out identifying 832 references published after 01/01/1995. Among these references, 9 were selected to be included in the qualitative review. The full selection process is displayed in Figure 6.
Figure 6.
PRISMA diagram CSF Monitoring period ELS
The majority of the references reported dates instead of periods (7 references out of 9); these dates were used to calculate the periods of interest. Information on the main outcome of interest, the period between the earliest point of infection and the suspicion report, was retrieved in six references and is summarised in Table 14.
Table 14.
Summary of CSF literature extraction for the outcome ‘period between earliest point of infection and suspicion report’
| Reference | Country | Year | Species/farm type | Period (days) |
|---|---|---|---|---|
| Elbers et al. (1999) | Netherlands | 1992 | Pig/NA | 421 |
| Laevens et al. (1998) | Belgium | 1993 | Pig/fattening | 182 |
| Elbers et al. (1999) | Netherlands | 1997 | Pig/mixed Insemination center | 423 304 |
| Elbers et al. (1999) | Germany | 1997 | Pig/NA | 561 |
| Elbers et al. (1999) | Spain | 1997 | Pig/NA | 631 |
| Mintiens et al. (2001) | Belgium | 1997 | Pig/fattening | 195 |
| Moennig et al. (2013) | Germany | 2006 | Pig/NA | 706 |
| David et al. (2011) OIE (2009) | Israel | 2009 | Pig/closed | 217 |
NA: Not available.
Primary outbreak. Based on date of arrival of infected pig from Germany.
Primary outbreak. Based on the serological findings at depopulation (See method described in Stegeman et al. (1999)).
Secondary outbreak. Based on the serological findings at depopulation (See method described in Stegeman et al. (1999)). As the most likely route of infection, it is presumed that CSFV was introduced into the AI centre during or after the removal and transport of four boars by an external transporter.
Secondary outbreak. Based on the date when weaned pigs originating from the primary infected herd were introduced and probably served as a source of infection.
Primary outbreak. No information on estimation method; From Table in Moennig et al. (2013).
Primary outbreak. Based on the date when the index case was artificially inseminated with imported semen from Germany.
Based on the results from Table 14, the shortest period found was 18 days. This was found in the context of the first farm infected during the 1993–1994 epidemic in Belgium (Laevens et al., 1998). It was found that the infection originated from pigs that were imported from Germany.
The longest period between the earliest point of infection and the suspicion report found in the selected references, 70 days, was mentioned in reference to the primary outbreak of the 2006 epidemic in Germany (Elbers et al., 2012; Moennig et al., 2013). The average period was calculated to 40 days.
Seroconversion in animals
According to the available scientific literature reviewed, when detection of serum CSFV‐specific antibodies was performed with Erns ELISA, the range of days for seroconversion for pigs that were directly challenged intranasally, orally or oronasally, was 10–22 days post infection (dpi) (Depner et al., 2001; Uttenthal et al., 2001; Reimann et al., 2004; Koenig et al., 2007; Dortmans et al., 2008; Kaden et al., 2008; Leifer et al., 2009; Gabriel et al., 2012; Renson et al., 2013; Blome et al., 2014; Eblé et al., 2014; Madera et al., 2016).
When detection of serum CSFV specific antibodies was performed with the use of virus neutralisation tests (VNT: FAVN = fluorescent antibody virus neutralisation or NPLA = neutralising peroxidase‐linked assay), the range of days until seroconversion for pigs that were directly challenged intranasally, orally or oronasally, was 6–15 dpi (Depner et al., 2001; Rasmussen et al., 2007; Dortmans et al., 2008; Kaden et al., 2008; Tignon et al., 2008; Huang et al., 2011; Gabriel et al., 2012; Graham et al., 2012; Renson et al., 2013; Blome et al., 2014; Eblé et al., 2014).
A contrasting result compared to previously mentioned studies in the range of days for seroconversion was observed by Uttenthal et al. (2001): pigs in Denmark that were orally challenged with 104.4 TCID50 of CSF 277/Pader strain (genotype 2.1) seroconverted between 21 and 42 dpi. The researchers concluded that differences in the inoculation procedure (nasal, oral), the number of cell culture passages of the challenge CSFV stock and the management of the pigs among studies performed in different countries, might have influenced the course of infection.
4.2.2. Assessment
Considering the results presented above, an assessment of the effectiveness of the current monitoring period for CSF, depending on the purpose of that period in the different scenarios shown in Annex C, was carried out. For CSF, the length of the monitoring period as defined in Annex II of the Delegated Regulation is 15 days.
1.
1st scenario of monitoring period
ToR 2 in accordance with Article 8 and Annex II of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion of a CSF outbreak
2nd scenario of monitoring period
ToR 2 in accordance with article 17(2) and Annex II of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a Category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of a CSF outbreak
3rd scenario of monitoring period
ToR 2 in accordance with article 13(b) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a CSF outbreak in an epidemiological unit in which the disease has not been confirmed, in order to provide derogations from killing the animals in this unit, if this unit has been completely separated, and handled by different personnel during this monitoring period
Scenarios 1, 2 and 3
For the first 3 scenarios, the main purpose of the use of the monitoring period is to be able to carry a full epidemiological investigation (Scenarios 1 and 2 being at the time of the suspicion and confirmation, respectively), or part of the epidemiological investigation (i.e. Scenario 3 where the aim is to identify any possible epidemiological links between the affected establishment and any separated non‐affected epidemiological units). The length of the monitoring period should then dictate how far back or forward the activities related to tracing (and other activities needed during an epidemiological investigation) should go (checks for production records, animal movement records, etc.). This monitoring period is the time/duration where the infection could have been present unknowingly/undetected in an establishment, and due to the regular activities carried out in this establishment, could have spread to other epidemiological units. In the case of scenario 3, if no epidemiological links between the establishment that has been confirmed positive and the other epidemiological units are found during the investigation (and only if other conditions described in the legislation are met), a derogation from killing the animals in the separated non‐affected epidemiological units could be granted.
The period of time for which the disease could have been present, unknowingly/undetected, in an establishment, equates then to the time period between the entry of the CSFV into the establishment, and the reporting of the suspicion. Once the suspicion has been officially reported, control measures are implemented, and further spread should in this way be prevented.
Based on the ELS carried out and presented above, the length of the time between the earliest point of infection and the suspicion report was estimated at between 18 and 70 days, with an average of 40 days. The monitoring period as defined in Annex II of the Delegated Regulation of 15 days does not fall within this range and is much lower than the average of 40 days. It should be noted, however, that the majority of the papers included in the ELS describe investigations of primary outbreaks. In general, it can be expected that the period between the introduction of the infection and the suspicion report will be shorter for secondary outbreaks compared to primary outbreaks (given a likely increase in awareness among the relevant stakeholders that will occur after the primary outbreak). This is also supported by the results from the ELS and more specifically the two included papers describing secondary outbreaks, with periods between the earliest point of infection and suspicion report of 19 and 30 days, respectively, i.e. an average of 25 days.
In addition, the current situation as regards ASF in several of the EU MS can be expected to have a positive effect on the capacity to detect an incursion also of CSF given the high awareness of ASF (the primary differential diagnosis to CSF) combined with the common practice of testing suspected cases of ASF/CSF for both diseases.
Taken together the monitoring period as defined in Annex II of the Delegated Regulation of 15 days, however, cannot be considered effective. Based on the considerations above, and given the current level of awareness of ASF, a monitoring period of 25 days is recommended (the average period reported in the ELS for secondary outbreaks), except for the first affected establishments detected in an area, where a monitoring period of 40 days (the average period reported in ELS, including primary outbreaks) is recommended. A revision of these proposed recommendations should be carried out if the level of awareness due to ASF in the EU were to decrease.
Once the impact of the sources of uncertainty described above regarding the time periods available for the assessment (coming mostly from investigations carried out on primary outbreaks that occurred over 20 years ago and often based on different assumptions for their calculation), it was concluded with a 70–100% certainty (based on expert opinion), that 95% or more of all pig establishments suspected and eventually confirmed in a region, where CSF has been already described, will have become initially infected within 25 days before the date of notification of the suspicion. For index cases in a region (in which thus lower awareness is expected), there is a 70–100% certainty that 95% or more of all pig establishments will have become initially infected within 40 days before the date of notification of the suspicion. The same certainty existed regarding the same time periods for independent units in a CSF‐affected establishment that become infected.
1.
4th scenario of monitoring period
ToR 2 in accordance with article 27(3)c and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the CSF outbreak in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements
Scenario 4
The main purpose of the monitoring period in Scenario 4 is to ensure that certain products or materials, likely to spread the disease, that have been produced in a non‐affected establishment located in the protection zone of an affected establishment, can be moved safely and without posing a risk of disease spread. In this scenario, and in contrast with the previous three scenarios, the establishment of concern is neither a suspect establishment nor an affected establishment. For the assessment of this scenario, we assume that the earliest plausible point of infection of these products or materials in the establishment of concern would be the earliest plausible point of infection of the establishment that originated the protection zone. If these products have been obtained or produced before the earliest point of infection of the affected establishment, then they could be exempted from prohibitions to be moved, as long as other conditions specified in the legislation are met (e.g. the products must have been clearly separated during the production process, storage and transport, from products not eligible for dispatch outside the restricted zone).
As above, the monitoring period as defined in Annex II of the Delegated Regulation of 15 days cannot be considered effective for this scenario. Given the considerations for scenarios 1,2 and 3 above and the fact that the disease has already been detected in the area, and that high awareness is thus expected, a monitoring period of 25 days, i.e. the average length estimated for secondary outbreaks in the ELS, could instead be suggested for this scenario. A revision of this proposed recommendations should be carried out if the level of awareness due to ASF in the EU were to decrease.
Because the assessment of the effectiveness of the proposed alternative monitoring period is subjected to the same uncertainties described for Scenarios 1, 2 and 3, the same conclusion was reached for Scenario 4 (i.e. it was concluded with a 70–100% certainty that 95% or more of all affected establishments would have become initially infected within 25 days before the date of notification of the suspicion and thus products obtained or produced before then would not represent a risk of infection).
1.
5th scenario of monitoring period
ToR 2 in accordance with article 32 (c), article 48(c) and Annex II of the Delegated Regulation (EU) 2020/687
The purpose of this section is to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period
Scenario 5
The aim of the monitoring period is to ensure that semen from animals in a non‐affected establishment (located in a protection or surveillance zone) that has been collected and frozen after the earliest time of infection of the affected establishment that originated the protection zone, is safe to be moved without posing a risk of disease spread. In this scenario, EFSA is requested to assess the length of time, after the semen was taken, when the animal should be tested in order to allow that semen to be moved. Here, it is assumed that the earliest point of infection of the animal would be on, or after the earliest point of infection of the affected establishment that originated the protection zone, and the latest date the semen could have become contaminated would be the date the semen was collected.
In the case of a CSF outbreak, based on the existing legislation, the pigs would have to be tested not earlier than the time in days of the monitoring period plus 7 days (15 + 7 = 22 days) counted after the semen was taken.
Experimental studies have shown that adult boars infected with CSF virus can excrete virus with semen and can, subsequently, transmit the virus to sows and their fetuses via artificial insemination (de Smit et al., 1999).
In the scenario, where the semen might have been contaminated, the latest at the date of collection from an infected donor without clinical signs or with mild clinical signs that remained unnoticed, a serological test would indicate if the donor has ever been exposed to CSFV and therefore if the semen could be contaminated.
Based on the results from the ELS presented in Section 4.2.1 in relation to the seroconversion in non‐vaccinated naive animals, a wide range of studies have reported that seroconversion occur at between 10 and 22 days post infection as detected by Erns ELISA, and between 6 and 15 days by VNT. Only one study report contrasting results with delayed seroconversion at between 21 and 42 days post infection after oral challenge.
Based on the ELS, sampling the animals at least 22 (15 + 7) days after semen collection as foreseen in the Delegated Regulation is considered effective to detect antibodies with several laboratory methods, given that the infection may have occurred at the latest at the day of semen collection.
1.
6th scenario of monitoring period
ToR 2 in accordance with article 57 (1) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date of the final cleaning and disinfection in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority (assuming relevant control of insects and rodents was carried out).
7th scenario of monitoring period
ToR 2 in accordance with article 59 (4) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date the first animal was introduced for the purpose of repopulation, during this monitoring period, all animals of the listed species intended for repopulation should be introduced.
Scenarios 6 and 7
In Scenarios 6 and 7, the monitoring period is used in the context of repopulation. In Scenario 6, the monitoring period is used to ensure that the repopulation process is not put at risk due to the disease still being present unknowingly in establishments within the surrounding area of the establishment to be repopulated (if an establishment tested positive to CSF virus within a distance equal or lower to the radius of the surveillance zone, the repopulation process could not take place). Repopulation can only take place after a number of days equal to the monitoring period has elapsed since the final cleaning and disinfection of the affected establishment.
In this regard the number of days of the monitoring period for CSF, counted from the day of the final cleaning and disinfection must ensure enough time for any potentially infected surrounding establishment to be reported as a suspicion. Considering the results presented above.
As above, the monitoring period as defined in Annex II of the Delegated Regulation of 15 days cannot be considered effective for this scenario. Given the considerations for Scenarios 1, 2 and 3 above, and the fact that the disease has already been detected in the area, and that high awareness is thus expected, a monitoring period of 25 days, i.e. the average length estimated for secondary outbreaks in the ELS, could instead be suggested for this scenario.
Also as above, and given the uncertainties identified, it was concluded with a 70–100% certainty that in 95% or more of all affected establishments depopulated and subjected to cleaning and disinfection, if surrounded by unknowingly infected establishments, the infection in these surrounded establishments would be detected within 25 days from the date of the cleaning and disinfection, and therefore, before animals are brought in for repopulation.
In Scenario 7, the monitoring period must be counted forwards from the date in which the first animal is introduced into the establishment to be repopulated, with all the animals intended for repopulation of this establishment being introduced within the length of time of this monitoring period.
The aim of the monitoring period in this scenario is to ensure the early detection of any potentially recently infected animal intended for repopulation once they have been moved into the repopulated establishment. Although the preferred option is that all animals are introduced into the establishment to be repopulated at the same time, this is not always feasible. The first clinical and laboratory sampling of the repopulated animals takes place once all the animals are in situ. By restricting the period of time during which animals may be introduced into the establishment, the period of time during which the disease could be unknowingly spreading within the establishment is reduced. Assuming that the latest point of infection of the first pig or batch of pigs introduced into the repopulated establishment is the day when the animals are moved, clinically ill pigs would be observed at the first visit, if this visit is carried out a number of days equal to the incubation period. For CSF the incubation period is usually 3–7 days, with a reported range of 2–14 days. The EFSA AHAW Panel thus considers the existing length of the monitoring period as defined in Annex II of the Delegated Regulation (15 days) effective as it would allow for early detection of potentially infected pigs at the first visit following re‐stocking.
4.3. Assessment of the minimum radius and time periods of the protection and surveillance zones set in place subsequent to a disease outbreak
4.3.1. Assessment of the minimum radius
The purpose of this section is to assess the effectiveness to control the spread of CSF by implementing a protection and surveillance zones of a minimum radius, as set out in Annex V of the Delegated Regulation, surrounding the establishment where the disease has been confirmed. Based on this regulation, the minimum radius of the protection and surveillance zones for CSF should be of 3 and 10 km, respectively (see Annex E).
Results
To answer this ToR, transmission kernels have been estimated for two epidemics of CFV: the 1997–1998 epidemic in the Netherlands (Backer et al., 2009; Boender et al., 2014); and the 2000 epidemic in the UK (Gamado et al., 2017). All three studies used the same functional form for the kernel, namely,
where d0 is the distance at which the probability of transmission is reduced by half and α controls how rapidly the kernel decays with distance. The estimated parameters are shown in Table 15.
Table 15.
Kernels for the transmission of classical swine fever virus
| Epidemic | Parameters* | Reference | |
|---|---|---|---|
| d0 (km) | α | ||
| The Netherlands 1997–1998 | 1.0 | 2.2 | Backer et al. (2009) |
| The Netherlands 1997–1998 | 0.55 (0.42, 0.73) | 2.27 (2.15, 2.40) | Boender et al. (2014) |
| UK 2000 | 0.28 (0.04, 5.53) | 1.71 (0.94, 3.80) | Gamado et al. (2017) |
95% confidence intervals are shown in brackets if they were reported in the original reference.
Figure 7.
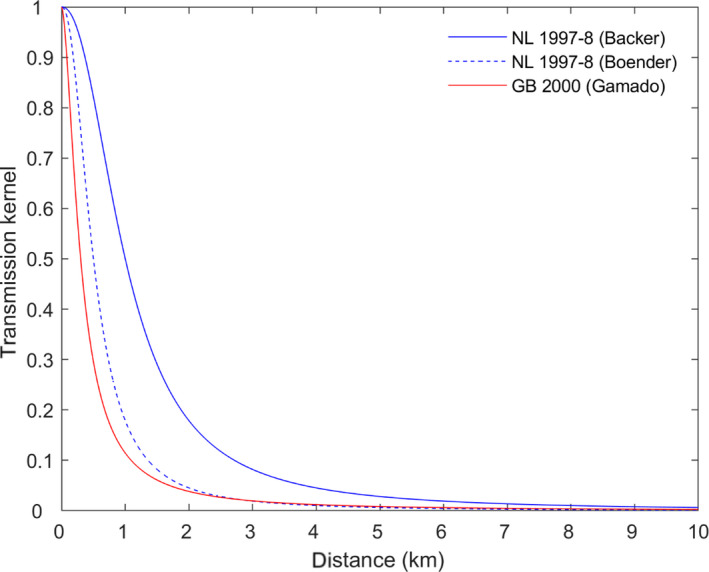
Kernels for the transmission of classical swine fever virus
For the three kernels in Table 15, the probability of transmission beyond given distances (if transmission were to occur from an infected establishment) was computed using the estimates, lower 95% confidence limits and upper 95% confidence limits, including beyond the proposed radius for the protection and surveillance zones (3 km and 10 km, respectively) (Figure 8). In addition, the distances at which a threshold probability of transmission beyond that distance is reached were also calculated for each kernel using the estimates, lower 95% confidence limits and upper 95% confidence limits (Figure 8). The corresponding values computed using the estimates are summarised in Tables 16 and 17.
Figure 8.
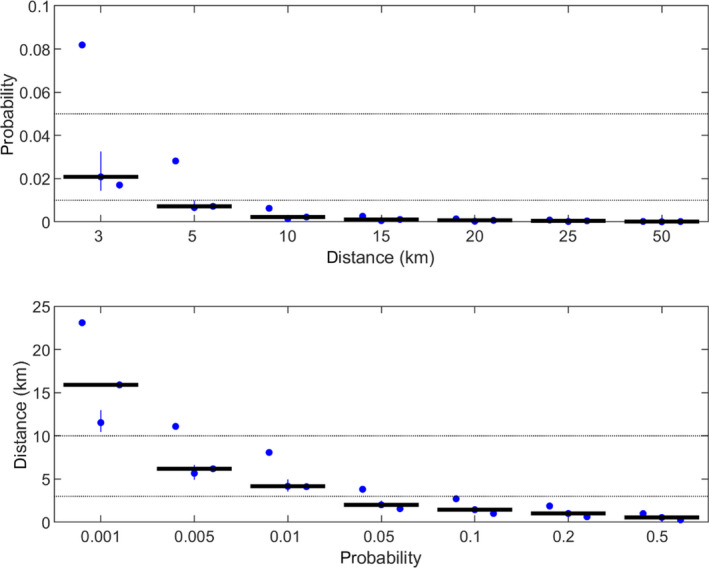
Assessment of the radius of the protection and surveillance zone for classical swine fever virus. The top panel shows the probability of transmission beyond a given distance (if transmission were to occur from an infected establishment) computed using the estimates (blue circles) and the lower and upper 95% confidence limits (error bars) for each kernel (and in the same order as) in Table 15. The thick black line indicates the median probability for all kernels. The black dotted lines indicate threshold probabilities of 0.05 and 0.01. The bottom panel shows the distances at which a threshold probability of transmission beyond that distance is reached calculated using the estimates (circles) and lower and upper 95% confidence limits (error bars) for each kernel. The thick black line indicates the median distance for all kernels. The black dotted lines indicate distances of 3 km and 10 km (i.e. the proposed radius of the protection and surveillance zones, respectively)
Table 16.
Probability of transmission of classical swine fever virus beyond different distances
| Distance (km) | |||||||
|---|---|---|---|---|---|---|---|
| 3 | 5 | 10 | 15 | 20 | 25 | 50 | |
| Median | 0.02 | 0.007 | 0.002 | 0.001 | 0.001 | 0.001 | < 0.001 |
| Minimum | 0.02 | 0.006 | 0.001 | 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Maximum | 0.08 | 0.03 | 0.006 | 0.003 | 0.001 | 0.001 | < 0.001 |
Table 17.
Distances (km) at which the probability of transmission of classical swine fever virus beyond that distance reaches a threshold level
| Threshold probability of transmission | |||||||
|---|---|---|---|---|---|---|---|
| 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.2 | 0.5 | |
| Median | 15.9 | 6.2 | 4.2 | 2.0 | 1.4 | 1.0 | 0.6 |
| Minimum | 11.5 | 5.7 | 4.1 | 1.6 | 1.0 | 0.6 | 0.3 |
| Maximum | 23.1 | 11.1 | 8.1 | 3.8 | 2.7 | 1.9 | 1.0 |
Assessment
Based on the kernel results above, if transmission occurs from an infected farm, the median relative probability of transmission beyond the protection (3 km) and surveillance zones (10 km) is 2% and 0.2%, respectively. In several articles of the AHL, a threshold of 95% is used for different purposes; if we use this threshold as to determine whether or not the minimum radius is efficient, the assessment based on the data presented will lead to the conclusion that the minimum radius is effective if/when focusing on the control of the spread of the disease among and between domestic pig herds. Using the same threshold, hypothetical protection and surveillance zones with radii of 2 and 4 km, respectively, would also be considered effective. Nonetheless, it is crucial to note that these probabilities do not take into account the risk of transmission due to wild boar.
Several sources of uncertainty that could impact the assessment made based on the evidence available were identified:
Data used in the models found in the scientific literature originated from two epidemics that occurred in only two countries in 1998–1999 (Netherlands) and 2000 (UK). Swine populations and management practices are known to vary both between regions (i.e. across different EU countries) and over time (increase in farm sizes and decrease in farm densities in several countries), and thus, the results from the kernels may not necessarily be representative of transmission dynamics now or in the future in future locations.
The models used were based on a single functional form for the kernel (NL 1998–1999 outbreak) and on four functional forms with only the best‐fitting kernel being used in the zone size assessment (UK 2000 outbreak). Kernels are a simple form of spatial model and incorporate all routes of transmission in a single description. This could have consequences for the probability of spread if different routes are of greater or lesser importance in different locations/epidemics.
Finally, there are additional uncertainties derived from the process of extrapolating from the models outputs to the parameter of interest: since the quantity of interest is the probability of transmission beyond the set thresholds occurs, and the output of the kernels is the probability of such transmission beyond the set thresholds occurs assuming there is in fact some transmission, the output of the kernel can be interpreted as an upper limit of the quantity of interest. How similar these two numbers will be, depends on how likely it is that transmission from an affected establishment occurs, i.e. how likely it is that there will be spread of viable CSF virus from an affected establishment by any possible mean (aerosolisation, indirect contacts between farms, etc.), something that vary depending on the biosecurity of the farms among other factors.
When the impact of all these uncertainties on the evaluation of the effectiveness of the proposed minimum radius for the protection (3 km) and surveillance (10 km) was assessed collectively, it was concluded with a 90–100% certainty (from very likely to almost certain) that the minimum radius of 3 km would prevent transmission outside the protection zone in the 15 days following their setting up in 95% or more of all the zones that are established. Similarly, it was also concluded with a 95–100% certainty (from extremely likely to almost certain) that the minimum radius of 10 km would prevent transmission outside the surveillance zone in the 30 days following their setting up in 95% or more of the zones that are established. The certainty on the effectiveness of both radii was very high yet more uncertainty existed regarding the effectiveness of the protection zone (90–100%) than the surveillance zone (95–100% certainty). The lower certainty related to effectiveness of the protection zone is because the kernels were not considered perfect and may not represent all possible ways of transmission of CSF that could take place during an outbreak (and that could be different from those that had been modelled before). Certainty was higher regarding the effectiveness of the surveillance zone because it was considered very unlikely that transmission occurred to outside without being detected first in the protection zone.
4.3.2. Assessment of the minimum period
The purpose of this section is to assess the effectiveness to control CSF spread of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones as set out in Annex X and XI.
The length of the minimum period of the protection zone and surveillance zone are 15 and 30 days, respectively (see Annex E). In the protection zone, all farms are visited for a clinical inspection. This visit aims to quickly identify infected farms, where infection has started before control measures were implemented. The movement control applies for 30 days, ensuring that possibly infected pigs in both protection and surveillance zones are not moved to uninfected farms.
From Table 14 in Section 4.2.1 it follows that the median time between introduction and suspicion for secondary outbreaks is 25 days and 40 for primary outbreaks, with a maximum time between introduction and suspicion of 70 days. In the methodology developed for answering this question (EFSA, 2020), the average and maximum time between introduction and the report of a suspicion (as estimated via the ELS) was considered as the recommended minimum length of time the protection and surveillance zones had to be in place, respectively. Nonetheless, the EFSA AHAW Panel considers that the maximum time reported in Table 14 (70 days) would be excessive considering the low relative probability of farms in the surveillance zone to become infected (see Table 17), and the uncertainty associated to this number (see footnote on Table 14). As a result, the EFSA AHAW panel recommends that the minimum time for maintenance of the measures in the protection zone should be increased to 25 days, and to 40 days in the surveillance zone.
Based on the same reasoning explained for ToR2, it was concluded with a 70–100% certainty that the minimum alternative periods proposed (25 days for the protection zone and 40 days for the surveillance zone) would allow the detection of additional affected establishments where infection started before control measures were implemented in 95% or more of the implemented zones.
4.3.3. Uncertainty analysis
Additional sources of uncertainty that were not considered in the assessment (i.e. those affecting ToR 1) were also identified (see Annex F), but their impact on the outputs of the assessment could not be quantified.
5. Conclusions and recommendations
| Sampling procedure | Laboratory guidelines based on Commission Decision 2002/106/EC if not stated otherwise | Conclusions | Recommendations | |
|---|---|---|---|---|
| ToR 1: In the event of suspicion or confirmation | ||||
| 1st scenario 4.1.1.1 In the event of a suspicion of CSF in an establishment where animals of the listed species are kept |
|
|
||
| 2nd scenario 4.1.1.2. For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in an CSF officially confirmed establishment | For epidemiological investigation it is important to examine the distribution of infections and seroconversions across infected farms. Given the adoption of group housing in sows there is no reason to aim for a lower seroprevalence in sows than in younger pigs. To identify possible infections thorough clinical and laboratory examination is needed on farms pre‐emptively culled. | It is recommended that a clinical inspection and, if deemed indicated from clinical examination, sample collection for virus detection to establish the distribution of infection across infected farms, is carried out. The serological survey should aim for detection of a seroprevalence of 10% in all age groups of pigs. | ||
| 3rd scenario 4.1.1.3. For granting a specific derogation from killing animals of the categories of article 13.2 of the Delegated Regulation in an CSF‐affected establishment | While testing to grant a derogation the possible presence of chronically infected pigs and immunotolerant carriers should be considered. | It is recommended that all pigs are tested twice a month for virus detection and derogation is not granted before all pigs have been negative for virus for at least three consecutive times. | ||
| 5th scenario 4.1.1.4. For wild animals of the listed species within the CSF‐affected establishment and its surroundings. | If wild boars have entered the territory of the affected establishment (e.g. pastures), there is a risk of spread of the virus to the wild boar population in the surroundings of the affected establishment. Contrarily, infection may have originated in the wild boar population, being wild boars the source of infection for pigs in the establishment. | If incursion of wild boars to the territory of the establishment has occurred and those animals have been caught and culled, blood and tissue samples should be collected for laboratory examination and virus and antibody detection with relevant diagnostic tests performed. Enhanced passive surveillance (wild boar carcass search) in the area surrounding the establishment should be implemented. All wild boars found dead should be tested for virus and antibodies. If hunting is ongoing in the surroundings, all hunted animals should also be tested. | ||
| 6th scenario 4.1.1.5. For animals of listed species in the non‐affected establishments located in a protection zone | Visit and examination as per 1st scenario is effective as first screening. | Weekly testing of 2 dead pigs > 2 months of age after visit. | ||
| 8th scenario 4.1.1.6. For non‐affected establishments located in a surveillance zone | Weekly testing of 2 dead pigs > 2 months of age to replace visit before lifting restrictions. | |||
| ToR 1: To grant derogations for animal movements | ||||
| 9th scenario 4.1.2.1. From non‐affected establishments located in the protection zone to slaughterhouses located within the protection zone or in the surveillance zone or outside the restricted zone | Infection in a farm can be present even if the group to be shipped looks healthy. Collecting samples for laboratory testing at the slaughterhouse has limited added value if clinical inspection at the farm and group to be shipped has not shown any indication for CSF. | Clinical inspection of the entire farm before shipment is recommended. Sample collection for laboratory testing at the slaughterhouse may be omitted. | ||
| 12th scenario 4.1.2.2 From non‐affected establishments located in the protection zone to a plant approved for processing or disposal of animal by‐products in which the animals are immediately killed | As per Section 4.1.2.1. | As per Section 4.1.2.1. | ||
| 13th scenario 4.1.2.3. From an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone and from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone | As per Section 4.1.2.1. | As per Section 4.1.2.1 No additional testing is needed for farms located outside the surveillance zone. | ||
| 15th scenario 4.1.2.4 From an establishment in a surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone | If enhanced surveillance is carried out in the establishments of the surveillance zone (ongoing weekly sampling of two dead pigs as described in Section 4.1.1.6), the assessed measures are considered sufficient, except in the event of infection with a strain off low virulence. | If infection with a CSFV strain of lower virulence is suspected, additionally to the clinical examination foreseen in present procedures, testing for virus of all animals being dispatched should be considered necessary to prevent transmission of the virus. | ||
| 18th scenario 4.1.2.5 From an establishment located in the restricted zone to move within the restricted zone when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation | As per Section 4.1.2.4 | As per Section 4.1.2.4 | ||
| ToR 1: For repopulation purposes | ||||
| 19th scenario 4.1.3.1 For the animals that are kept for the repopulation prior to their introduction | As per Section 4.1.2.4 | As per Section 4.1.2.4 No need for prior testing of animals introduced from outside the restricted zones. | ||
| 20th scenario 4.1.3.2 In the event of unusual mortalities or clinical signs being notified during the repopulation | As per Section 4.1.1.1 | As per Section 4.1.1.1 | ||
| 21st scenario 4.1.3.3 For animals that have been repopulated | Current procedures for repopulation are deemed effective. | It is recommended to use the same detection threshold for serology in all age groups of pigs. | ||
| ToR 2 | ||||
| Description | Conclusions | Recommendations | ||
| 4.2 Assessment of the length of the monitoring period of CSF | Scenarios 1–3, 4 and 6 The monitoring period as defined in Annex II of the Delegated Regulation of 15 days cannot be considered effective. Scenario 5 Based on the results of the ELS, sampling the animals at least 22 (15 + 7) days after semen collection as foreseen in the Delegated Regulation is considered effective to detect antibodies with several laboratory methods, given that the infection may have occurred at the latest at the day of semen collection. Scenario 7 For the purpose of this scenario the existing length of the monitoring period as defined in Annex II of the Delegated Regulation (15 days) effective as it would allow for early detection of potentially infected pigs at the first visit following re‐stocking. | Scenarios 1–3, 4 and 6 Given the current level of awareness of ASF, a monitoring period of 25 days is recommended (the average period reported in the ELS for secondary outbreaks), except for the first affected establishments detected in an area (index cases), where a monitoring period of 40 days (the average period reported in ELS, including primary outbreaks) is recommended. A revision of these proposed recommendations should be carried out if the level of awareness due to ASF in the EU were to decrease. Based on available estimates of the time between likely entry of infection and notification of suspicion, the panel is 70–100% certain that infection in 95% or more of establishments suspected and eventually confirmed will have become initially infected within 25 days before the date of notification of suspicion except for index cases (within 40 days in that case). Scenario 5 The existing length of the monitoring period is considered effective. Scenario 7 The existing length of the monitoring period is considered effective. | ||
| ToR 3 | ||||
| Description | Conclusions | Recommendations | ||
| 4.3.1 Assessment of the minimum radius | Based on available estimates on spatial spread of CSFV in previous outbreaks occurring in Europe, the panel is 90–100% certain the minimum radius of 3 km will prevent transmission outside the protection zone in 95% or more of the established zones. Regarding surveillance zones, the panel is 95–100% certain the minimum radius of 10 km will prevent transmission outside the surveillance zone in 95% or more of the established zones. | It is recommended that the current radii of the protection (3 km) and surveillance zones (10 km) are maintained. | ||
| 4.3.2 Assessment of the minimum period | Considering the time between introduction and the reporting of a suspicion of CSF, as assessed in this opinion, the existing minimum times for maintenance of the measures in the protection (15 days) and surveillance zones (30 days) are not considered efficient. | It is recommended that the minimum time for maintenance of the measures in the protection zone should be increased to 25 days, and to 40 days in the surveillance zone. It is concluded with a 70–100% certainty that maintenance of the measures in the protection zone for 25 days and for 40 days in the surveillance zone will allow the detection of additional affected establishments where infection started before control measures were implemented in 95% or more of the implemented zones. | ||
Abbreviations
- AHS
African horse sickness
- ASF
African swine fever
- CBPP
Contagious bovine pleuropneumonia
- CCPP
Contagious caprine pleuropneumonia
- CSF
Classical swine fever
- Dpi
days post inoculation
- ELISA
enzyme‐linked immunosorbent assay
- ELS
extensive literature search
- FMD
Foot and mouth disease
- HPAI
Highly Pathogenic Avian Influenza
- LSD
Lumpy skin disease virus
- NCD
Newcastle disease virus
- OIE
World Organization for Animal Health
- PCR
polymerase chain reaction
- PZ
protection zone
- RP
rinderpest virus
- RT‐PCR
reverse transcription polymerase chain reaction
- RVFV
Rift Valley fever virus
- SPGP
Sheep pox and goat pox
- SZ
surveillance zone
- ToR
Terms of Reference
Annex A – Definitions in EU legislation
1.
| Terms | Definitions |
|---|---|
| Clinical examination | The clinical examination comprises: (i) an initial general evaluation of the animal health status of the establishment which comprises all the animals of listed species kept in the establishment; and (ii) an individual examination of the animals included in the sample referred to in point (a). The sampling of animals for clinical examination is carried out in accordance with point A.1 of Annex I for terrestrial animals (Delegated Regulation article 3) |
| Confined establishment | Means any permanent, geographically limited establishment, created on a voluntary basis and approved for the purpose of movements, where the animals are: a) kept or bred for the purposes of exhibitions, education, the conservation of species or research; b) confined and separated from the surrounding environment; and c) subject to animal health surveillance and biosecurity measures; (AHL: Regulation 2016/429 article 4(48)) |
| Epidemiological unit | Means a group of animals with the same likelihood of exposure to a disease agent; (AHL: Regulation 2016/429 article 4(39)) |
| Establishment | Means any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: a) households where pet animals are kept; b) veterinary practices or clinics; (AHL: Regulation 2016/429 article 4(27)) |
| Health status | Means the disease status as regards the listed diseases relevant for a particular listed species with respect to: a) an animal; b) animals within: (i) an epidemiological unit; (ii) an establishment; (iii) a zone; (iv) a compartment; (v) a Member State; (vi) a third country or territory; (AHL: Regulation 2016/429 article 4(34)) |
| Infected zone | Means a zone in which restrictions on the movements of kept and wild animals or products and other disease control and biosecurity measures may be applied with the view to preventing the spread of a category A disease in the event of official confirmation of the disease in wild animals. (Delegated Regulation article 2(15)) |
| Kept animals | Means animals which are kept by humans, including, in the case of aquatic animals, aquaculture animals; (AHL: Regulation 2016/429 article 4(5)) |
| Outbreak | Means the officially confirmed occurrence of a listed disease or an emerging disease in one or more animals in an establishment or other place where animals are kept or located; (AHL: Regulation 2016/429 article 4 (40) |
| Protection zone | Means a zone around and including the location of an outbreak, where disease control measures are applied in order to prevent the spread of the disease from that zone; (AHL: Regulation 2016/429 article 4(42)) |
| Listed diseases | Means diseases listed in accordance with Article 5(1); (AHL: Regulation 2016/429 article 4 (18)) List of the diseases (AHL: Regulation 2016/429, Annex II) |
| Listed species | Means an animal species or group of animal species listed in accordance with Article 8(2), or, in the case of emerging diseases, an animal species or group of animal species which meets the criteria for listed species laid down in Article 8(2); (AHL: Regulation 2016/429 article 4(20)) List of species and groups of species (Commission Implemented Regulation 2018/1882) |
| Monitoring periods | It is appropriate to follow a single approach for the measures to apply in the event of a category A disease. However, the epidemiology of diseases should be taken into account to establish the appropriate moment for the competent authority to apply control measures and to carry out investigations if there is suspicion or confirmation of those diseases. Therefore ‘monitoring periods’ should be provided, as reference time frames for each category A disease affecting terrestrial animals based on incubation periods and other relevant elements that may affect the spread of the disease. (Delegated Regulation whereas 10). |
| Restricted zone | Means a zone in which restrictions on the movements of certain animals or products and other disease control measures are applied, with a view to preventing the spread of a particular disease into areas where no restrictions are applied; a restricted zone may, when relevant, include protection and surveillance zones; (AHL: Regulation 2016/429 article 4(41)) |
| Surveillance zone | Means a zone which is established around the protection zone, and where disease control measures are applied in order to prevent the spread of the disease from the protection zone; (AHL: Regulation 2016/429 article 4(43)) |
| Wild animals | Means animals which are not kept animals; (AHL: Regulation 2016/429 article 4(8)) |
| Zone | Means: a) for terrestrial animals, an area of a Member State, third country or territory with a precise geographical delimitation, containing an animal subpopulation with a distinct health status with respect to a specific disease or specific diseases subject to appropriate surveillance, disease control and biosecurity measures; (AHL: Regulation 2016/429 article 4 (35)) |
Annex B – Scenarios of ToR 1
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenario |
|---|---|---|---|---|
| In the event of suspicion or confirmation | ||||
| ToR 1.1 ToR 1.2 | 6(2) of the Delegated Regulation | 1st scenario | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). |
|
| ToR 1.2 | Art. 12(3) Art. 7 (4) (Preventive killing) of the Delegated Regulation, and Art. 57 Reg 2016/429 | 2nd scenario | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. |
|
| ToR 1.1 ToR 1.2 | Article 13(3)c of the Delegated Regulation | 3rd scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. |
|
| ToR 1.1 ToR 1.2 | Article 14(1) of the Delegated Regulation Art. 57 Reg.2016/429 | 4th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. |
|
| ToR 1.1 ToR 1.2 | Article 14(1) of the Delegated Regulation Art. 57 Reg. 2016/429 | 5th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species |
|
| ToR 1.1 ToR 1.2 | Article 26(2) of the Delegated Regulation | 6th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. |
|
| ToR 1.3 | Article 26(5) of the Delegated Regulation point A.3 of Annex I | 7th scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of establishments located in a protection zone when the radius is larger than 3 km. The purpose of the sampling procedure is to ensure disease detection of the virus if the virus is present in establishments within the protection zone |
|
| ToR 1.3 | Article 41 of the Delegated Regulation | 8th scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone |
|
| Derogations to allow animal movements | ||||
| ToR 1.4 | Article 28(5) of the Delegated Regulation Article 29 of the Delegated Regulation | 9th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29) |
|
| ToR 1.4 | Article 28(5) and Article 30(1) of the Delegated Regulation | 10th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of day‐old‐chicks located in the protection zone and hatched from eggs originating in the restricted zone or outside the restricted zone. The sampling procedures should ensure that the movement of these day‐old‐chicks to an establishment located in the same Member State but if possible, outside the restricted zone |
|
| ToR 1.4 | Article 28(5) and Article 30(2) of the Delegated Regulation | 11th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the protection zone to establishments located in the same MS and if possible within the restricted zone. |
|
| ToR 1.4 | Article 28(5) and Article 37 of the Delegated Regulation | 12th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37) |
|
| ToR 1.4 | Article 43(5) and Article 44 of the Delegated Regulation | 13th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b)from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone |
|
| ToR 1.4 | Article 43(5) and Article 45(1) of the Delegated Regulation | 14th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant a derogation and allow for the animals to be moved from an establishment in the surveillance zone to pastures situated within the surveillance zone |
|
| ToR 1.4 | Article 43(5) and Article 45(2) of the Delegated Regulation | 15th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter |
|
| ToR 1.4 | Article 43(5) and Article 46(1) of the Delegated Regulation | 16th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations to grant derogation of movements of day‐old‐chicks hatched from establishment located in the surveillance zone, from eggs originating within the surveillance zone and eggs originating outside the restricted zone, to an establishment located in the same Member State where they were hatched |
|
| ToR 1.4 | Article 43(5) and Article 46(2) of the Delegated Regulation | 17th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the surveillance zone to establishments located in the same MS. |
|
| ToR 1.4 | Article 56(1)c of the Delegated Regulation | 18th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI |
|
| Repopulation | ||||
| ToR 1.5 | Article 59(2), (3) of the Delegated Regulation | 19th scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(9) of the Delegated Regulation | 20th scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(5) of the Delegated Regulation | 21st scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. |
|
Annex C – Existing sampling procedures for CSF
1.
Sampling scenarios for CSF – Based on Commission Decision 2002/106/EC if not stated otherwise
| Scenario | Description of the Scenario | Clinical guidelines | Laboratory guidelines |
|---|---|---|---|
| 1st | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR1.1) and laboratory examination (TOR1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). | Annex, CHAPTER IV.A.: 2. When an official veterinarian visits a suspected holding to confirm or rule out classical swine fever: – a check of the production and health records of the holding must be carried out, if these records are available; – an inspection in each subunit of the holding must be carried out to select the pigs to be clinically examined. The clinical examination must include the taking of body temperature and must primarily concern the following pigs or group of pigs: – sick or anorexic pigs; – pigs recently recovered from disease; – pigs recently introduced from confirmed outbreaks or from other suspected sources; – pigs kept in subunits recently visited by external visitors which had a recent close contact with classical swine fever suspected or infected pigs or for which other particularly risky contacts with a potential source of classical swine fever virus have been identified; – pigs already sampled and serologically tested for classical swine fever, in case the results of these tests do not allow to rule out classical swine fever, and in‐contact pigs. If the inspection in the suspected holding has not indicated the presence of the pigs or group of pigs referred to in the above subparagraph, the competent authority, without prejudice to other measures that may be applied in the holding in question in accordance with Directive 2001/89/EC and taking into account the epidemiological situation, shall: – carry out further examinations in the holding in question in accordance with subparagraph 3 below; 3. When reference is made to this paragraph, the clinical examination in the holding in question must be carried out on pigs selected at random in the subunits for which a risk of introduction of classical swine fever virus has been identified or is suspected. The minimum number of pigs to be examined must allow for the detection of fever if it occurs at a prevalence of 10% with 95% confidence in these subunits. However, in case of: – breeding sows: the minimum number of sows to be examined must allow for the detection of fever if it occurs at a prevalence of 5% with 95% confidence; – at semen collection centres: all boars must be examined. 4. If dead or moribund pigs are detected in a suspected holding, post‐mortem examinations must be carried out, preferably on at least five of these pigs and in particular on pigs: – that before death have shown or are showing very evident signs of disease; – with high fever; – recently dead. If these examinations have not shown lesions suggesting classical swine fever but, due to the epidemiological situation, further investigations are deemed necessary: – a clinical examination, as laid down in subparagraph 3 and blood sampling as laid down in subparagraph 5 must be carried out in the subunit where the dead or moribund pigs were kept; and – post‐mortem examinations may be carried on 3–4 in‐contact pigs. Emergency animal diseases: A field guide for Australian veterinarians (Department of Agriculture and CSIRO, 2019 ): CSF is an extremely variable disease and you cannot diagnose it based on clinical signs and gross pathology alone. Submit at least five pigs for post‐mortem examination, as individual animals may present with great variability in lesions. Record a composite picture of all lesions seen. Specific Domestic Animal Infectious Disease Quarantine Guidelines on Classical Swine Fever: Classical Swine Fever Diagnostics Manual (Ministry of Agriculture, Forestry and Fisheries of Japan, 2013 ): In case of suspicion, the prefectural animal health inspector shall thoroughly conduct clinical tests on abnormal pigs and pigs, etc. living together including measuring body temperatures. On this occasion, he/she shall record the status of a group of pigs, etc. including abnormal pigs. | Annex, CHAPTER IV.A.: 4. If dead or moribund pigs are detected in a suspected holding, post‐mortem examinations must be carried out, preferably on at least five of these pigs and in particular on pigs: — that before death have shown or are showing very evident signs of disease; — with high fever; — recently dead. If these examinations have not shown lesions suggesting classical swine fever but, due to the epidemiological situation, further investigations are deemed necessary: — a clinical examination, as laid down in subparagraph 3 and blood sampling as laid down in subparagraph 5 must be carried out in the subunit where the dead or moribund pigs were kept; and — post‐mortem examinations may be carried on 3–4 in‐contact pigs. Irrespective of the presence or absence of lesions suggesting classical swine fever, samples of the organs or tissues from pigs that have been subjected to post‐mortem examination must be collected for virological tests in accordance with Chapter V B. 1 These samples must be preferably collected from recently dead pigs. 5. If further clinical signs or lesions that may suggest classical swine fever are detected in a suspected holding, but the competent authority deems that these findings are not sufficient to confirm an outbreak of classical swine fever and that laboratory tests are therefore necessary, blood samples for laboratory tests must be taken from the suspected pigs and from other pigs in each subunit in which the suspected pigs are kept, in accordance with the procedures laid down below. The minimum number of samples to be taken for serological tests must allow for the detection of 10% seroprevalence with 95% confidence in the subunit in question. However, in the case of: – breeding sows: the minimum number of sows to be sampled must allow for the detection of 5% seroprevalence with 95% confidence (1); – a semen collection centre: blood samples must be taken from all boars. The number of samples to be taken for virological tests will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of the laboratory tests that will be used and the epidemiological situation. 6. If the suspicion of classical swine fever in the holding in question is related to the results of previous serological tests, in addition to the blood samples to be taken from the pigs referred to in 2, second subparagraph, fifth indent (i.e. pigs already sampled and serologically tested for classical swine fever, in case the results of these tests do not allow to rule out classical swine fever, and in‐contact pigs), the following procedures shall be applied: a) if the seropositive pigs are pregnant sows, some of them, preferably not less than three, shall be euthanised and subjected to a post‐mortem examination. Prior to killing a blood sample must be taken for further serological tests. The fetuses shall be subjected to examination for classical swine fever virus, virus antigen or virus genome in accordance with Chapter VI to detect intrauterine infection; b) if the seropositive pigs are sows with suckling piglets, blood samples must be taken from all piglets and shall be subjected to examination for classical swine fever virus, virus antigen or virus genome as referred to in Chapter VI. Blood samples must also be taken from the sows for further serological tests. 7. If, after the examination carried out in a suspected holding, clinical signs or lesions suggestive of classical swine fever are not detected, but further laboratory tests are deemed necessary by the competent authority to rule out classical swine fever, the sampling procedures laid down in subparagraph 5 shall be used for guidance purposes. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, heading ‘B. Diagnostic techniques’ (OIE, 2019a ): The variability of the clinical signs and post‐mortem lesions does not provide firm evidence for unequivocal diagnosis. tentative diagnosis based on clinical signs and post‐mortem lesions must therefore be confirmed by laboratory investigations. As pyrexia is one of the first signs of CSF and is accompanied by viraemia (Depner et al., 1994),detection of virus or viral nucleic acid in whole blood, collected in heparin or ethylene diamine tetra‐acetic acid (EDTA), or in tissues, collected from febrile animals, is the method of choice for detecting infected herds at an early stage. Targeted and risk‐based sampling should be performed, random sampling only being applied in cases where no clinical signs of disease are present. To increase the sensitivity of detection of virus, viral antigen or nucleic acid, clinically diseased animals and febrile animals should primarily be sampled. The Foreign Animal Disease Preparedness and Response Plan CLASSICAL SWINE FEVER STANDARD OPERATING PROCEDURES: 3. SURVEILLANCE. Appendix B : Classical Swine Fever (CSF) Surveillance Plan (USDA, 2013 ).: When the Foreign Animal Disease Diagnostician (FADD) concurs that the herd meets the clinical case definition for CSF, the FADD will collect specimens for shipment to FADDL. At a minimum, specimens to be collected from live affected swine are serum, whole blood (EDTA or heparin), tonsil scrapings and nasal swabs. When possible, at least one pig, and ideally five pigs, should be posted and the following tissues collected: tonsil, lymph nodes, spleen, kidney and distal ileum. Emergency animal diseases: A field guide for Australian veterinarians (Department of Agriculture and CSIRO, 2019 ): CSF is an extremely variable disease and you cannot diagnose it based on clinical signs and gross pathology alone. Collect samples from affected, especially pyrexic, pigs killed immediately before a post‐mortem examination and from pigs that have recently died (including stillborn piglets and aborted fetuses). Collect: – serum, 30 samples from suspected chronically infected animals EDTA blood (7–10 ml/animal) from live, clinically affected animals – fresh tissue from the spleen, gastro‐hepatic lymph node, mesenteric lymph node, tonsils, lung, kidney and ileum (2 g of each tissue) – fixed tissue, a full range of tissues (including the brain) in neutral‐buffered formalin. The most rapid, sensitive and specific diagnostic procedure is the detection of viral nucleic acid in blood or in lymphoid tissue by qRT‐PCR. Specific Domestic Animal Infectious Disease Quarantine Guidelines on Classical Swine Fever: Classical Swine Fever Diagnostics Manual (Ministry of Agriculture, Forestry and Fisheries of Japan, 2013): Blood shall be collected from pigs, etc., which show symptoms and pigs, etc. living together (blood serum and blood with anticoagulant added) and be carried with carcasses of pigs, etc. or pigs, etc., which are raising suspicions of infection of classical swine fever or African swine fever, to a livestock hygiene service centre. A livestock hygiene service centre shall collect samples (including amygdalae, kidney and spleen without fail) necessary for the pathological appraisal (classical swine fever and differential diagnosis) from the carcasses of pigs, etc. or pigs, etc., which are raising suspicions of infection of classical swine fever or African swine fever. |
| 2nd | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. | NA | Annex, CHAPTER IV.C.: 1. In order that classical swine fever may be confirmed or ruled out and additional epidemiological information is gained, when pigs are killed as a preventive measure on a suspected holding in accordance with the provisions of Article 4(3)(a) or Article 7(2) of Directive 2001/89/EC, blood samples for serological tests as well as blood or tonsils samples for virological tests must be taken in accordance with the procedure laid down in subparagraph 2. 2. Sampling must primarily concern: – pigs showing signs or post‐mortem lesions suggesting classical swine fever and their in‐contact pigs; – other pigs which might have had risky contacts with infected or suspected pigs or which are suspected to have been contaminated with classical swine fever virus. These pigs must be sampled in accordance with the instructions of the competent authority, which will take into account the epidemiological situation. In this case, the sampling procedures laid down in the second, third and fourth subparagraphs below shall be used for guidance purposes. Furthermore, pigs proceeding from each of the subunits of the holding must be sampled at random. In this case, the minimum number of samples to be taken for serological tests must allow for the detection of 10% seroprevalence with 95% confidence in the subunit in question. However, in the case of: ‐breeding sows: the minimum number of sows to be sampled must allow for the detection of 5% seroprevalence with 95% confidence; In certain cases, e.g. when classical swine fever is suspected in a holding with a limited number of young pigs, the proportion of infected sows may be very small. In these cases a higher number of sows must be sampled. – a semen collection centre: blood samples must be taken from all boars. The type of samples to be taken for virological tests and the test to be used will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of these tests and the epidemiological situation. Annex, CHAPTER IV.B.: 1. In order that the manner of introduction of classical swine fever virus into an infected holding and the length of time elapsed since its introduction may be established, when pigs are killed on a holding following confirmation of an outbreak in accordance with Article 5(1)(a) of Directive 2001/89/EC, blood samples for serological tests must be taken at random from the pigs when they are killed. 2. The minimum number of pigs to be sampled must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit of the holding. Samples for virological tests may also be taken in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of the laboratory tests that will be used and the epidemiological situation. 3. However, in case of secondary outbreaks, the competent authority may decide to derogate from subparagraphs 1 and 2 and establish ad hoc sampling procedures, taking into account the epidemiological information already available on the source and means of virus introduction into the holding and the potential spread of disease from the holding. |
| 3rd | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. | No specific guidelines were found | No specific guidelines were found |
| 5th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species. | No specific guidelines were found | No specific guidelines were found |
| 6th | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. | Article 10 of Directive 2001/89/EC: 1. Member States shall ensure that the following measures are applied in the protection zone: a) a census of all the holdings shall be made as soon as possible; after the establishment of the protection zone these holdings shall be visited by an official veterinarian within not more than seven days for a clinical examination of the pigs and for a check of the register and of the pig identification marks referred to in Articles 4 and 5 of Directive 92/102/EEC; e) all dead or diseased pigs on a holding shall be immediately notified to the competent authority, which shall carry out appropriate investigations in accordance with the procedures laid down in the diagnostic manual → 4. The measures in the protection zone shall continue to be applied at least until: a)… b) pigs on all holdings have undergone clinical and laboratory examinations carried out in accordance with the diagnostic manual in order to detect the possible presence of classical swine fever virus. The examinations referred to in point b) shall not take place before 30 days have elapsed after the completion of preliminary cleaning and disinfection measures on the infected holdings. Annex, CHAPTER IV.F.: 1. In order that the measures referred to in Article 10 of Directive 2001/89/EC may be lifted in a protection zone, in all holdings in the zone: — a clinical examination must be carried out in accordance with the procedures laid down in A.2 and 3; (2. When an official veterinarian visits a suspected holding to confirm or rule out classical swine fever: — a check of the production and health records of the holding must be carried out, if these records are available; — an inspection in each subunit of the holding must be carried out to select the pigs to be clinically examined. The clinical examination must include the taking of body temperature and must primarily concern the following pigs or group of pigs: — sick or anorexic pigs; — pigs recently recovered from disease; — pigs recently introduced from confirmed outbreaks or from other suspected sources; — pigs kept in subunits recently visited by external visitors which had a recent close contact with classical swine fever suspected or infected pigs or for which other particularly risky contacts with a potential source of classical swine fever virus have been identified; — pigs already sampled and serologically tested for classical swine fever, in case the results of these tests do notallow to rule out classical swine fever, and in‐contact pigs. 3. When reference is made to this paragraph, the clinical examination in the holding in question must be carried out on pigs selected at random in the subunits for which a risk of introduction of classical swine fever virus has been identified or is suspected. The minimum number of pigs to be examined must allow for the detection of fever if it occurs at a prevalence of 10% with 95% confidence in these subunits. However, in case of: — breeding sows, the minimum number of sows to be examined must allow for the detection of fever if it occurs at a prevalence of 5% with 95% confidence; — at semen collection centres, all boars must be examined.) Specific Domestic Animal Infectious Disease Quarantine Guidelines on Classical Swine Fever: Classical Swine Fever Diagnostics Manual (Ministry of Agriculture, Forestry and Fisheries of Japan, 2013): Test of surrounding farms in restriction areas: (1) Test to confirm the outbreak status When the outbreak of classical swine fever is confirmed, a prefecture shall enter farms (limited to those that rear six or more pigs, etc.) in areas of restricted movement and conduct the following tests within 24 h as a general rule: (i) Clinical test The prefecture shall enter farms (limited to those that rear six or more pigs, etc.) in areas of restricted movement to confirm the existence or absence of clinical signs set forth in Subsection 4, Item 1. | Annex, CHAPTER IV.F.: 2. The minimum number of blood samples to be taken must allow for the detection of 10% seroprevalence with 95% confidence in pigs in each subunit in the holding. However, in the case of: — breeding sows, the minimum number of samples to be taken must allow for the detection of 5% seroprevalence with 95% confidence; — a semen collection centre, blood samples must be taken from all boars. G. Specific Domestic Animal Infectious Disease Quarantine Guidelines on Classical Swine Fever: Classical Swine Fever Diagnostics Manual (Ministry of Agriculture, Forestry and Fisheries of Japan, 2013): Test of surrounding farms in restriction areas: (1) Test to confirm the outbreak status When the outbreak of classical swine fever is confirmed, a prefecture shall enter farms (limited to those that rear six or more pigs, etc.) in areas of restricted movement and conduct the following tests within 24 hours as a general rule: (ii) Blood test, antigen test and serum antibody test At the time of (i), blood test (leucocyte counting and confirming shift to the left of neutrophilic leukocyte nucleus), antigen test (PCR test, however, dead pigs, etc. shall be tested by the fluorescent antibody method, using amygdalae) and serum antibody test (ELISA method) shall be implemented to a given number of pigs. (2) Free Status Confirmation Test A similar test as (1) shall be conducted when 17 days have elapsed since the completion of quarantine measures at all infected farms in areas of restricted movement to confirm if the area is free from the disease. The number of collected samples for various tests in a test to confirm the outbreak status and a test to confirm disease‐free status is at least 30 (at least five randomly selected from each pig sty) as number enough to expose 10% infection at 95% reliability after consultation with the Animal Health Division and if there is more than one pigsty, samples shall be collected from all pigsties. In addition, Exhibit 1 ‘Classical Swine Fever Diagnostics Manual’ shall be referred to when conducting tests. Besides, samples shall be collected from abnormal pigs and if such pigs, etc. are not recognised, samples should be randomly collected from healthy pigs, etc. |
| 8th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR1.1) and laboratory (ToR1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone. | Article 11 of Directive 2001/89/EC: 1. Member States shall ensure that the following measures are applied in the surveillance zone: (a) a census shall be taken of all pig holdings; (e) all dead or diseased pigs on a holding shall be immediately notified to the competent authority, which shall carry out appropriate investigations in accordance with the procedures laid down in the diagnostic manual. → 3. The measures in the surveillance zone shall continue to be applied at least until: a)/…/ b) pigs on all holdings have undergone clinical and, where necessary, laboratory examinations as laid down in the diagnostic manual in order to detect the possible presence of classical swine fever virus. The examinations referred to in point b) shall not take place before 20 days have elapsed after the completion of preliminary cleaning and disinfection measures on the infected holdings.Annex, CHAPTER IV.G: 1. In order that the restrictions referred to in Article 11 of Directive 2001/89/EC may be lifted in a surveillance zone, a clinical examination must be carried out in all holdings in the zone in accordance with the procedures laid down in A.2. | Annex, CHAPTER IV.G 1./…/ In addition, blood samples for serological tests must be taken from pigs: — in all the holdings where no pigs of between two and eight months of age are kept; — whenever the competent authority deems that classical swine fever might have spread unnoticed amongst breeding sows; — in any other holding where sampling is deemed necessary by the competent authority; — in all semen collection centres 2. Whenever blood sampling for serological tests is carried out in holdings located in the surveillance zone, the number of blood samples to be taken in these holdings must be in accordance with F.2. However, if the competent authority deems that classical swine fever might have spread unnoticed amongst breeding sows, sampling may only be carried out in the subunits where these animals are kept. |
| Derogations to allow animal movements | |||
| 9th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29). | Annex, CHAPTER IV.D.: 1. Without prejudice to the provisions of Article 11(1)(f), second subparagraph of Directive 2001/89/EC, in order that authorisation may be given to move pigs from holdings located in protection or surveillance zones in accordance with Article 10(3) of the said Directive, the clinical examination to be carried out by an official veterinarian must: — be carried out within the 24‐h period before moving the pigs; — be in accordance with the provisions laid down in A.2. 3. In case of pigs to be moved to a slaughterhouse, to a processing plant or to other places to be then killed or slaughtered, in addition to the investigations to be carried out in accordance with subparagraph 1, a clinical examination of pigs must be carried out in each subunit in which the pigs to be moved are kept. In case of pigs older than three to four months, this examination must include the taking of temperature of a proportion of pigs. The minimum number of the pigs to be checked must allow for the detection of fever if it occurs at a prevalence of 20% with 95% confidence in the subunits in question. However, in the case of breeding sows or boars, the minimum number of pigs to be examined must allow for the detection of fever if it occurs at a prevalence of 5% with 95% confidence in the subunit where the pigs to be moved are kept. | Annex, CHAPTER IV.D.: 4. When the pigs referred to in subparagraph 3 are slaughtered or killed, blood samples for serological tests or blood or tonsils samples for virological tests must be taken from pigs proceeding from each of the subunits from which pigs have been moved. The minimum number of samples to be taken must allow for the detection of 10% seroprevalence or virus prevalence with 95% confidence in each subunit. However, in the case of breeding sows or boars the minimum number of pigs to be sampled must allow for the detection of 5% of seroprevalence or virus prevalence with 95% confidence in the subunit where these pigs were kept. The type of samples to be taken and the test to be used will be in accordance with the instructions of the competent authority, which will take into account the range of tests that can be performed, the sensitivity of these tests and the epidemiological situation. |
| 12th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37). | See scenario 9 | See scenario 9 |
| 13th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved : a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b)from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone. | See scenario 9 | See scenario 9 |
| 15th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow for them to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter. | Annex, CHAPTER IV.D.: 1. Without prejudice to the provisions of Article 11(1)(f), second subparagraph of Directive 2001/89/EC, in order that authorisation may be given to move pigs from holdings located in protection or surveillance zones in accordance with Article 10(3) of the said Directive, the clinical examination to be carried out by an official veterinarian must: – be carried out within the 24‐h period before moving the pigs; – be in accordance with the provisions laid down in A.2. 2. In the case of pigs to be moved to another holding, in addition to the investigations to be carried out in accordance with subparagraph 1, a clinical examination of pigs must be carried out in each subunit of the holding in which the pigs to be moved are kept. In case of pigs older than three to four months, this examination must include the taking of temperature of a proportion of pigs. The minimum number of pigs to be checked must allow for the detection of fever if it occurs at a prevalence of 10% with 95% confidence in these subunits. However, in the case of: – breeding sows, the minimum number of sows to be examined must allow for the detection of fever if it occurs at a prevalence of 5% with 95% confidence in the subunit where the sows to be moved are kept; – boars, all boars to be moved must be examined. | No specific guidelines were found |
| 18th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI. | Scenario 9 to slaughter and killing Article 10 of Directive 2001/89/EC: 2. Where the prohibitions provided for in paragraph 1 are maintained beyond 30 days because of further outbreaks of the disease and as a result animal welfare or other problems arise in keeping the pigs, subject to the conditions set out in paragraph 3, the competent authority may, following a reasoned application by the owner, authorise removal of pigs from a holding within the protection zone, to be directly transported to: a) a slaughterhouse designated by the competent authority, preferably within the protection or surveillance zone for the purpose of immediate slaughter; b) a processing plant or a suitable place where the pigs are immediately killed and their carcases are processed under official supervision; or c) under exceptional circumstances, to other premises located within the protection zone. Member States availing themselves of this provision shall immediately inform the Commission thereof in the Standing Veterinary Committee. 3. When reference is made to this paragraph, the competent authority may authorise removal of pigs from the holding concerned, on condition that: a) a clinical examination of the pigs in the holding and in particular those to be moved, including the taking of the body temperature of a proportion thereof, and a check of the register and the pig identification marks referred to in Articles 4 and 5 of Directive 92/102/EEC have been carried out by an official veterinarian; Article 11 of Directive 2001/89/EC: 2. Where the prohibitions provided for in paragraph 1 are maintained beyond 30 days because of further outbreaks of the disease and where as a result animal welfare or other problems arise in keeping the pigs, subject to the conditions set out in Article 10(3), the competent authority may, following a reasoned application by the owner, authorise removal of pigs from a holding within the surveillance zone to be directly transported to: a) a slaughterhouse designated by the competent authority, preferably within the protection or surveillance zone for the purpose of immediate slaughter; b) a processing plant or a suitable place where the pigs are immediately killed and their carcases are processed under official supervision; or c) under exceptional circumstances, to other premises located within the protection or surveillance zone. Member States availing themselves of this provision shall immediately inform the Commission thereof in the Standing Veterinary Committee. | Article 10 of Directive 2001/89/EC: 3. When reference is made to this paragraph, the competent authority may authorise removal of pigs from the holding concerned, on condition that: (e) if the pigs are to be slaughtered or killed, a sufficient number of samples shall be taken from the pigs in accordance with the diagnostic manual in order that the presence of classical swine fever virus in these holdings can be confirmed or ruled out; See scenario 9 |
| Repopulation | |||
| 19th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. | No specific guidelines were found | Article 13 of Directive 2001/89/EC: 2. The reintroduction of pigs shall take account of the type of farming practised on the holding concerned and must conform to the following procedures: a) as regards open‐air pig holdings, the reintroduction of pigs shall start with the introduction of sentinel pigs which have been checked and found negative for the presence of antibodies against classical swine fever virus/…/ |
| 20th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. | No specific guidelines were found | Annex, CHAPTER IV.E: 2. After re‐introduction of pigs, the competent authority shall ensure that in case of any disease or death of the pigs in the holding due to unknown reasons, the pigs in question are immediately tested for classical swine fever. These provisions shall apply until the restrictions referred to in Article 13(2)(a), second subparagraph and Article 19(8), second subparagraph (b), second sentence of Directive 2001/89/EC are lifted in the holding in question. |
| 21st | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. | No specific guidelines were found | Article 13 of Directive 2001/89/EC: 2. The reintroduction of pigs shall take account of the type of farming practised on the holding concerned and must conform to the following procedures: a) as regards open‐air pig holdings, the reintroduction of pigs shall start with the introduction of sentinel pigs which have been checked and found negative for the presence of antibodies against classical swine fever virus or come from holdings not subjected to any restrictions related to classical swine fever. The sentinel pigs shall be placed, in accordance with the requirements of the competent authority, throughout the infected holding and be sampled 40 days after having been placed on the holding, and tested for the presence of antibodies, in accordance with the diagnostic manual. b) as regards all other forms of rearing, the reintroduction of pigs shall either take place in accordance with the measures provided for – in point a) or shall be based on total repopulation, provided that: – all the pigs arrive within a period of 20 days and come from holdings not subjected to any restrictions related to classical swine fever, – pigs in the repopulated herd are subjected to a serological examination in accordance with the diagnostic manual. Sampling for that examination shall be carried out at the earliest 40 days after the arrival of the last pigs, Annex, CHAPTER IV.E 1. When pigs are re‐introduced into a holding in accordance with Article 13(2)(a) or (2)(b) or Article 19(8), second subparagraph (b) of Directive 2001/89/EC, the following sampling procedures must be applied: — in case sentinel pigs are reintroduced, blood samples for serological tests must be taken at random from a number of pigs that allow for the detection of 10% seroprevalence with 95% confidence in each subunit of the holding; — in case of total re‐population, blood samples for serological tests must be taken at random from a number of pigs that allow for the detection of 20% seroprevalence with 95% confidence in each subunit of the holding. However, in the case of breeding sows or boars the number of samples to be taken must be such as to detect 10% seroprevalence with 95% confidence. |
Annex D – Scenarios of ToR 2
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenarios |
|---|---|---|---|---|
| ToR 2 | Article 8 of the Delegated Regulation Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation | 1st scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion. |
|
| ToR 2 | Article 17(2) and Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation | 2nd scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of the disease. |
|
| ToR 2 | Article 13(b) of the Delegated Regulation Annex II of the Delegated Regulation | 3rd scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a category A disease in an establishment with kept animals of listed species, during which the epidemiological units in which the disease has not been confirmed were kept completely separated and handled by different personnel, in order to provide derogations from killing. |
|
| ToR 2 | Article 27(3)c of the Delegated Regulation Annex II of the Delegated Regulation | 4th scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the latest outbreak of a category A disease in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements. |
|
| ToR 2 | Article 32(c) of the Delegated Regulation Article 48(c) of the Delegated Regulation Annex II of the Delegated Regulation | 5th scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period. |
|
| ToR 2 | Article 57(1)b of the Delegated Regulation Annex II of the Delegated Regulation | 6th scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards from the date after the final cleaning and disinfection and when relevant control of insects and rodents was carried out in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority. |
|
| ToR 2 | Article 59(4)b of the Delegated Regulation Annex II of the Delegated Regulation | 7th scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards the date when the first animal was introduced, during which all the animals of listed species intended for repopulation should be introduced. |
|
Annex E – Minimum radius and minimum period of duration of protection and surveillance zones
1.
| Category A diseases | Minimum radius of Protection zone Annex V | Minimum radius of Surveillance zone Annex V | Minimum period of duration of measures in the protection zone (Article 39(1)) Annex X | Additional period of duration of surveillance measures in the protection zone (Article 39(3)) Annex X | Minimum period of duration of measures in the surveillance zone (as referred to in Articles 55 and 56 of this Regulation) Annex XI |
|---|---|---|---|---|---|
| Foot and mouth disease (FMD) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Infection with rinderpest virus (RP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with Rift Valley fever virus (RVFV) | 20 km | 50 km | 30 days | 15 days | 45 days |
| Infection with lumpy skin disease virus (LSD) | 20 km | 50 km | 28 days | 17 days | 45 days |
| Infection with Mycoplasma mycoides subsp. mycoides SC (Contagious bovine pleuropneumonia) (CBPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| Sheep pox and goat pox (SPGP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with peste des petits ruminant virus (PPR) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Contagious caprine pleuropneumonia (CCPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| African horse sickness (AHS) | 100 km | 150 km | 12 months | Not applicable | 12 months |
| Infection with Burkholderia mallei (Glanders) | Establishment | Establishment | 6 months | Not applicable | Not applicable |
| Classical swine fever (CSF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| African swine fever (ASF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Highly pathogenic avian influenza (HPAI) | 3 km | 10 km | 21 day | 9 days | 30 days |
| Infection with Newcastle disease virus (NCD) | 3 km | 10 km | 21 days | 9 days | 30 days |
Annex F – Non quantified sources of uncertainty
1.
| Source or location of the uncertainty | # | Nature or cause of uncertainty as described by the experts | Impact of the uncertainty on the assessment |
|---|---|---|---|
| ToR 1 | 1 | The model used to answer ToR 1 is based on the assumption of homogeneous mixing, that may not hold for certain production systems | The effectiveness of the sampling strategies could be over or underestimated. |
| 2 | Case fatality rate is based on a study performed under experimental conditions that may not be reflective of possible values occurring under field conditions | The effectiveness of the sampling strategies could be over or underestimated |
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Stegeman JA, Antoniou SE, Aznar I, Broglia A, Lima E, Van der Stede Y, Zancanaro G and Roberts HC, 2021. Assessment of the control measures of the category A diseases of Animal Health Law: Classical Swine Fever. EFSA Journal 2021;19(7):6707, 83 pp. 10.2903/j.efsa.2021.6707
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00797
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to thank the following for the support provided to this scientific output: P95, COVETLAB, Evangelos Tsiamadis, and Verena Oswaldi.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 2: © OIE.
Adopted: 23 June 2021
References
- Anderson D and Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191–198. [Google Scholar]
- Backer JA, Hagenaars TJ, van Roermund HJ and de Jong MC, 2009. Modelling the effectiveness and risks of vaccination strategies to control classical swine fever epidemics. Journal of the Royal Society, Interface, 6, 849–861. 10.1098/rsif.2008.0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blome S, Gabriel C, Schmeiser S, Meyer D, Meindl‐Böhmer A, Koenen F and Beer M, 2014. Efficacy of marker vaccine candidate CP7_E2alf against challenge with classical swine fever virus isolates of different genotypes. Veterinary Microbiology, 169, 8–17. [DOI] [PubMed] [Google Scholar]
- Boender GJ, van den Hengel R, van Roermund HJ and Hagenaars TJ, 2014. The influence of between‐farm distance and farm size on the spread of classical swine fever during the 1997–1998 epidemic in The Netherlands. PLoS ONE, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D, Edri N, Yakobson B, Bombarov V, King R, Davidson I, Pozzi P, Hadani Y, Bellaiche M and Schmeiser S, 2011. Emergence of classical swine fever virus in Israel in 2009. The Veterinary Journal, 190, e146–e149. [DOI] [PubMed] [Google Scholar]
- Department of Agriculture and CSIRO , 2019. Emergency animal diseases: A field guide for Australian veterinarians, Canberra, August. CC BY 4.0. Available online at: https://www.outbreak.gov.au/for-vets-and-scientists/emergency-animal-diseases-guide
- Depner K, Gruber A and Liess B, 1994. Experimental‐infection of weaner pigs with a field isolate of hog‐cholera classical‐swine‐fever virus derived from a recent outbreak in Lower Saxony. 1. Clinical, virological and serological findings. Wiener Tierarztliche Monatsschrift, 81, 370–373. [Google Scholar]
- Depner KR, Bouma A, Koenen F, Klinkenberg D, Lange E, de Smit H and Vanderhallen H, 2001. Classical swine fever (CSF) marker vaccine: trial II. Challenge study in pregnant sows. Veterinary Microbiology, 83, 107–120. [DOI] [PubMed] [Google Scholar]
- Dórea FC, Swanenburg M, Horigan V, Han S, Young B, de Freitas Costa E, de Souza Santos MA, Evans D, Royall E, Aznar I and Dhollander S, 2021. Data collection for risk assessments on animal health: review protocol 2021. EFSA supporting publication 2021.
- Dortmans J, Loeffen W, Weerdmeester K, van der Poel W and de Bruin M, 2008. Efficacy of intradermally administrated E2 subunit vaccines in reducing horizontal transmission of classical swine fever virus. Vaccine, 26, 1235–1242. [DOI] [PubMed] [Google Scholar]
- Durand B, Davila S, Cariolet R, Mesplède A and Le Potier MF, 2009. Comparison of viraemia‐ and clinical‐based estimates of within‐ and between‐pen transmission of classical swine fever virus from three transmission experiments. Veterinary Microbiology, 135, 196–204. 10.1016/j.vetmic.2008.09.056 [DOI] [PubMed] [Google Scholar]
- Eblé P, Quak S, Geurts Y, Moonen‐Leusen H and Loeffen W, 2014. Efficacy of CSF vaccine CP7_E2alf in piglets with maternally derived antibodies. Veterinary Microbiology, 174, 27–38. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Alvarez J, Roberts HC, Stahl K, Viltrop A, De Clercq K, Klement E, Stegeman JA, Gubbins S, Antoniou SE, Zancanaro G and Aznar I, 2020. Technical report on the methodological approach used for the assessment of the control measures for Category A diseases in the context of the new Animal Health Law. EFSA supporting publication 2020;EN‐1988, 60 pp. 10.2903/sp.efsa.2020.EN-1988 [DOI]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/sp.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers AR, Stegeman A, Moser H, Ekker HM, Smak JA and Pluimers FH, 1999. The classical swine fever epidemic 1997–1998 in the Netherlands: descriptive epidemiology. Preventive Veterinary Medicine, 42, 157–184. [DOI] [PubMed] [Google Scholar]
- Elbers AR, Bouma A and Stegeman JA, 2002. Quantitative assessment of clinical signs for the detection of classical swine fever outbreaks during an epidemic. Veterinary Microbiology, 85, 323–332. 10.1016/s0378-1135(01)00519-3 [DOI] [PubMed] [Google Scholar]
- Elbers A, Loeffen W and Koch G, 2012. Classical swine fever and avian influenza epidemics: lessons learned. Berliner und Munchener Tierarztliche Wochenschrift, 125, 21–26. [PubMed] [Google Scholar]
- Floegel‐Niesmann G, Blome S, Gerss‐Dülmer H, Bunzenthal C and Moennig V, 2009. Virulence of classical swine fever virus isolates from Europe and other areas during 1996 until 2007. Veterinary Microbiology, 139, 165–169. [DOI] [PubMed] [Google Scholar]
- Gabriel C, Blome S, Urniza A, Juanola S, Koenen F and Beer M, 2012. Towards licensing of CP7_E2alf as marker vaccine against classical swine fever—duration of immunity. Vaccine, 30, 2928–2936. [DOI] [PubMed] [Google Scholar]
- Gamado K, Marion G and Porphyre T, 2017. Data‐driven risk assessment from small scale epidemics: estimation and model choice for spatio‐temporal data with application to a classical swine fever outbreak. Frontiers Veterinary Science, 4, 16. 10.3389/fvets.2017.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SP, Everett HE, Haines FJ, Johns HL, Sosan OA, Salguero FJ, Clifford DJ, Steinbach F, Drew TW and Crooke HR, 2012. Challenge of pigs with classical swine fever viruses after C‐strain vaccination reveals remarkably rapid protection and insights into early immunity. PLoS ONE, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y‐L, Pang VF, Lin C‐M, Tsai Y‐C, Chia M‐Y, Deng M‐C, Chang C‐Y and Jeng C‐R, 2011. Porcine circovirus type 2 (PCV2) infection decreases the efficacy of an attenuated classical swine fever virus (CSFV) vaccine. Veterinary Research, 42, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden V, Lange E and Faust A, 2008. Oral vaccination against classical swine fever with a chimeric Pestivirus: comparative investigations of liquid and lyophilized virus. European Journal of Wildlife Research, 54, 237–244. [Google Scholar]
- Keeling MJ and Rohani P, 2008. Modeling Infectious Diseases in Humans and Animals. Princeton University Press, Princeton, NJ, USA. [Google Scholar]
- Klinkenberg D, de Bree J, Laevens H and de Jong MC, 2002. Within‐ and between‐pen transmission of Classical Swine Fever Virus: a new method to estimate the basic reproduction ratio from transmission experiments. Epidemiology and Infection, 128, 293–299. 10.1017/s0950268801006537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig P, Lange E, Reimann I and Beer M, 2007. CP7_E2alf: a safe and efficient marker vaccine strain for oral immunisation of wild boar against Classical swine fever virus (CSFV). Vaccine, 25, 3391–3399. [DOI] [PubMed] [Google Scholar]
- Kramer M, Staubach C, Koenen F, Haegeman A, Pol F, Le Potier MF and Greiser‐Wilke I, 2009. Scientific review on Classical Swine Fever. EFSA Supporting Publications, 6, 6E. [Google Scholar]
- Laevens H, Deluyker H, Koenen F, Van Caenegem G, Vermeersch J and de Kruif A, 1998. An experimental infection with a classical swine fever virus in weaner pigs: II. The use of serological data to estimate the day of virus introduction in natural outbreaks. Veterinary Quarterly, 20, 46–49. [DOI] [PubMed] [Google Scholar]
- Leifer I, Lange E, Reimann I, Blome S, Juanola S, Duran JP and Beer M, 2009. Modified live marker vaccine candidate CP7_E2alf provides early onset of protection against lethal challenge infection with classical swine fever virus after both intramuscular and oral immunization. Vaccine, 27, 6522–6529. [DOI] [PubMed] [Google Scholar]
- Madera R, Gong W, Wang L, Burakova Y, Lleellish K, Galliher‐Beckley A, Nietfeld J, Henningson J, Jia K and Li P, 2016. Pigs immunized with a novel E2 subunit vaccine are protected from subgenotype heterologous classical swine fever virus challenge. BMC Veterinary Research, 12, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries of Japan , 2013. Specific Domestic Animal Infectious Disease Quarantine Guidelines on Classical Swine Fever: Classical Swine Fever Diagnostics Manual.
- Mintiens K, Deluyker H, Laevens H, Koenen F, Dewulf J and De Kruif A, 2001. Descriptive epidemiology of a classical swine fever outbreak in the Limburg Province of Belgium in 1997. Journal of Veterinary Medicine, Series B, 48, 143–149. [DOI] [PubMed] [Google Scholar]
- Moennig V, Becher P and Beer M (Karger Publishers), 2013. Classical swine fever. pp. 167–174. Available online: https://pdfs.semanticscholar.org/4d1c/df7f7ec21d2779d17eec8d6c89d1483bb948.pdf [DOI] [PubMed]
- OIE , 2009, online. WAHIS Interface: Immediate notification Classical Swine Fever Israel 2009.
- OIE , 2019a. Classical swine fever (Infection with Classical swine fever). Paris, France. Available online: https://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/3.08.03_CSF.pdf
- OIE , 2019b. Infection with Classical swine fever. Paris, France. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_csf.pdf
- OIE , 2020. Classical swine fever. Technical disease card. Paris, France Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/CLASSICAL_SWINE_FEVER.pdf
- Panyasing Y, Kedkovid R, Thanawongnuwech R, Kittawornrat A, Ji J, Giménez‐Lirola L and Zimmerman J, 2018. Effective surveillance for early classical swine fever virus detection will utilize both virus and antibody detection capabilities. Veterinary Microbiology, 216, 72–78. [DOI] [PubMed] [Google Scholar]
- Pastoret P‐P, 2003. Peste porcine clasique. In: Lefevre P‐C, Blancou J, Chermette R (eds.). Principales maladies infectieuses et parasitaires du bétail ‐ Europe et régions chaudes. Paris, Éditions Tec & Doc : Éditions médicales internationales, Lavoisier. pp. 565–576. [Google Scholar]
- Rasmussen TB, Uttenthal Å, Reimann I, Nielsen J, Depner K and Beer M, 2007. Virulence, immunogenicity and vaccine properties of a novel chimeric pestivirus. Journal of General Virology, 88, 481–486. [DOI] [PubMed] [Google Scholar]
- Reimann I, Depner K, Trapp S and Beer M, 2004. An avirulent chimeric Pestivirus with altered cell tropism protects pigs against lethal infection with classical swine fever virus. Virology, 322, 143–157. [DOI] [PubMed] [Google Scholar]
- Renson P, Le Dimna M, Keranflech A, Cariolet R, Koenen F and Le Potier M‐F, 2013. CP7_E2alf oral vaccination confers partial protection against early classical swine fever virus challenge and interferes with pathogeny‐related cytokine responses. Veterinary Research, 44, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Staubach C, Conraths F and Schulz K, 2017a. A simulation model to determine sensitivity and timeliness of surveillance strategies. Transboundary and Emerging Diseases, 64, 1709–1719. [DOI] [PubMed] [Google Scholar]
- Schulz K, Peyre M, Staubach C, Schauer B, Schulz J, Calba C, Häsler B and Conraths FJ, 2017b. Surveillance strategies for Classical Swine Fever in wild boar–a comprehensive evaluation study to ensure powerful surveillance. Scientific Reports, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smit AJ, Bouma A, Terpstra C and van Oirschot JT, 1999. Transmission of classical swine fever virus by artificial insemination. Veterinary Microbiology, 67, 239–249. 10.1016/s0378-1135(99)00045-0 [DOI] [PubMed] [Google Scholar]
- Spickler AR (Center for food security and public health (CFSPH)), 2015. Classical Swine Fever. Iowa State University. Available online: http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php
- Stegeman A, Elbers AR, Bouma A, de Smit H and de Jong MC, 1999. Transmission of classical swine fever virus within herds during the 1997‐1998 epidemic in The Netherlands. Preventive Veterinary Medicine, 42, 201–218. 10.1016/s0167-5877(99)00076-8 [DOI] [PubMed] [Google Scholar]
- Thulke H‐H, Eisinger D, Freuling C, Fröhlich A, Globig A, Grimm V, Müller T, Selhorst T, Staubach C and Zips S, 2009. Situation‐based surveillance: adapting investigations to actual epidemic situations. Journal of Wildlife Diseases, 45, 1089–1103. [DOI] [PubMed] [Google Scholar]
- Tignon M, Kulcsár G, Belák K, Haegeman A, Barna T, Fábián K, Lévai R, Farsang A, Stede Y and Vrancken R, 2008. Application of a commercial real‐time RT‐PCR assay for surveillance of classical swine fever: evaluation by testing sequential tissue and blood samples. The Open Veterinary Science Journal, 2, 104–110. 10.2174/1874318808002010104 [DOI] [Google Scholar]
- USDA , 2013. The Foreign Animal Disease Preparedness and Response Plan Classical Swine Fever Standard Operating Procedures, National Center for Animal Health Emergency Management.
- Uttenthal Å, Le Potier M‐F, Romero L, De Mia GM and Floegel‐Niesmann G, 2001. Classical swine fever (CSF) marker vaccine: trial I. Challenge studies in weaner pigs. Veterinary Microbiology, 83, 85–106. [DOI] [PubMed] [Google Scholar]
- Weesendorp E, Backer J, Stegeman A and Loeffen W, 2009a. Effect of strain and inoculation dose of classical swine fever virus on within‐pen transmission. Veterinary Research, 40, 59. 10.1051/vetres/2009041 [DOI] [PubMed] [Google Scholar]
- Weesendorp E, Stegeman A and Loeffen W, 2009b. Dynamics of virus excretion via different routes in pigs experimentally infected with classical swine fever virus strains of high, moderate or low virulence. Veterinary Microbiology, 133, 9–22. 10.1016/j.vetmic.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Weesendorp E, Backer J, Stegeman A and Loeffen W, 2011. Transmission of classical swine fever virus depends on the clinical course of infection which is associated with high and low levels of virus excretion. Veterinary Microbiology, 147, 262–273. 10.1016/j.vetmic.2010.06.032 [DOI] [PubMed] [Google Scholar]
- Weesendorp E, Backer J and Loeffen W, 2014. Quantification of different classical swine fever virus transmission routes within a single compartment. Veterinary Microbiology, 174, 353–361. 10.1016/j.vetmic.2014.10.022 [DOI] [PubMed] [Google Scholar]


