Abstract
Introduction
Protective pulmonary mechanical ventilation, higher positive end-expiratory pressure, neuromuscular blockade, prone positioning, and pulmonary recruitment procedures are all strategies in severe COVID-19 cases. Extracorporeal Membrane Oxygenation (ECMO) can be seen as an alternative to traditional treatment in certain patients when conventional therapy fails. We present a study that intends to systematically review and meta-analysis ECMO use in COVID-19 patients.
Methods
We search major medical databases (Cochrane Library, PubMed, EMBASE, MedRxiv) for clinical trials that were released between January 2020 until February 2021, had full-text availability, were written in English, and humans studies.
We used National Heart, Lung, and Blood Institute (NHLBI) quality evaluation methods for retrospective cohort and cross-sectional studies to evaluate the quality of the articles. In addition, we used the Mantel–Haenszel random-effects meta-analysis of using RevMan 5.4.
Results
A total of 33 studies involving 3090 patients were included in the systematic review and six studies in the meta-analysis. There were 828 patients admitted to the ICU, of which 779 patients had ARDS (94%). Of the total study, 527 patients received ECMO therapy (17%). ARDS incidence was associated with complications during ICU care compared to non-ICU care (OR 107.98; 95% CI 55.51–210.03; p < 0.00001). Indirect comparisons, the incidence of mortality was associated with ECMO compared with non-ECMO (OR 15.79; 95% CI 4.21–59.28; p < 0.0001).
Conclusion
The incidence of ARDS was associated with complications during ICU stay, and the incidence of mortality was associated with ECMO. Further study about mechanisms involving illness and death of patients from COVID-19 is needed.
Keywords: Acute respiratory distress syndrome, Extracorporeal membrane oxygenation, ECMO, Coronavirus disease, COVID-19
1. Introduction
Coronavirus disease 2019 (COVID-19) has spread rapidly in China and many other countries since the outbreak began person-to-person transmission and is highly contagious [1]. Until February 2021, there are 110 million people infected with COVID-19 worldwide [2]. The majority of patients of COVID-19 suffer from mild symptoms and recover completely. However, about 14% of cases fell in severe and critical conditions with an estimated 2.3–3.83% mortality. Much is unknown about this virus, including natural history, the incidence of advanced complications, virus persistence, or prognosis in different patients’ subsets [1].
SARS-CoV-2 infection can develop into acute respiratory distress syndrome (ARDS) [3]. In some cases, the treatment has been pulmonary mechanical ventilation, neuromuscular blockade, higher pressure positive end-expiration, pulmonary recruitment technique, and pronation position. If conventional therapy fails, extracorporeal membrane oxygenation (ECMO) can be considered an alternative therapy. ECMO can be considered in patients who experience severe heart and lung failure due to COVID-19, refractory to mechanical ventilation, and other optimal medical therapies [4].
Two basic methods can be used in ECMO therapy: veno-venous (VV-ECMO) or veno-arterial (VA-ECMO). Regarding respiratory complications of COVID-19, VV-ECMO is the recommended type. In principle, ECMO functions as a cardiopulmonary bypass, exchanging oxygen with carbon dioxide through the artificial membrane into deoxygenated veins and then returned to the patient through the venous or arterial system [5]. In previous pandemics, the role of ECMO has been proven to support recovery from severe respiratory distress and cardiovascular disorders due to ARDS [6]. However, the role of ECMO in COVID-19 and its implications is not yet known and understood.
2. Method
Article search, quality assessment of each article, data extraction, and analysis, as well as summary and interpretation of results, were all done following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) guidelines. We searched all articles assessing ECMO as a therapy in COVID-19. The search was carried out on various major medical databases (PubMed, Cochrane Library, EMBASE, and medRxiv) systematically using keywords: “extracorporeal membrane oxygenation”, AND/OR “ECMO”, AND/OR “Sars-Cov-2”, AND/ OR “COVID-19”, AND/OR “Coronavirus Disease”, in the title, abstract, and medical subject heading (MeSH). Search parameters were limited to English, human studies, clinical trials, and fully published studies or studies in progress if preliminary results were published. Reference lists from the literature that met the inclusion criteria were also manually screened to identify additional relevant studies.
We included all studies regarding ECMO as a therapy in COVID-19. The inclusion criteria are; (i) studies in humans (i) adults (>18 years) with an indication of ECMO insertion, namely, hypoxic respiratory failure despite adequate ventilation therapy (Extracorporeal Life Support Organization/ELSO Guidelines on ARDS), severe hypercapnia (pH < 7,2 and PaCO2> 80 over 6 h), prolonged ventilation <7 days, cardiogenic shock refractory to conventional therapy, Murray score> 3, or 1 organ failure with or without comorbidity in COVID-19 patients; (iii) obtained output data in the form of ICU care or death with the use of ECMO; (iv) obtained data on COVID-19 patients and complications in the form of ARDS and non-ARDS complications; or (v) data on the number of patients treated in ICU and non-ICU with COVID-19 will be obtained. The exclusion criteria were; (i) the population is not COVID 19; (ii) is a review study, editorial, or conference paper; and (iii) have incomplete data.
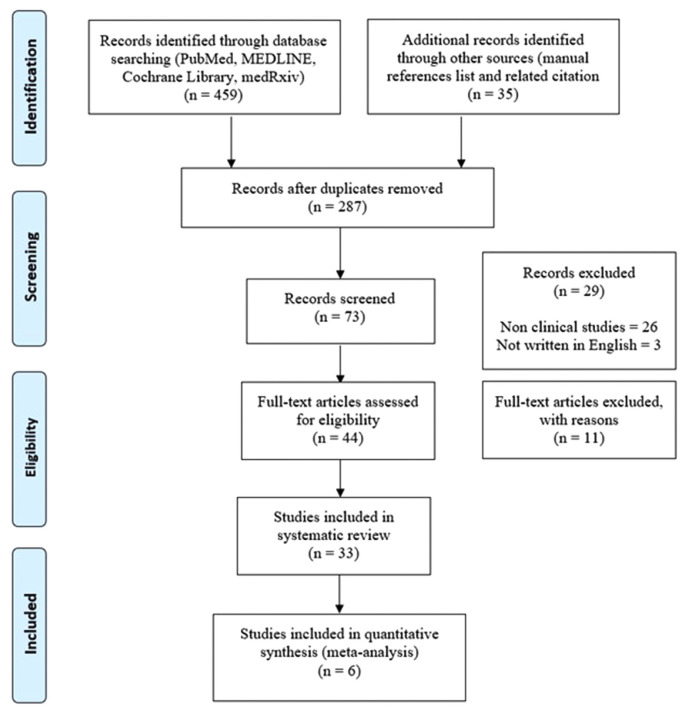
A total of 494 articles were selected after a comprehensive search, yielded 459 articles and 35 related articles were added to the main journal reference list. We found 287 full-text studies and, after removing duplicates leaving with 73 publications. Furthermore, reviewing the title and abstracts against the inclusion and exclusion criterion eliminated 29 papers and left 44 articles. A thorough reading of the entire articles resulted in the exclusion of 11 articles due to a lack of complete data on outcomes and incomplete text, so 33 studies were conducted for the systematic review report. The final meta-analysis contained a total of six articles (Fig. 1).
Fig. 1.
Flowchart of literature search based on PRISMA.
Researchers assessed each article’s methodological quality for the meta-analysis using quality assessment tools from the National Heart, Lung, and Blood Institute (NHLBI) for observational cohorts and cross-sectional studies with poor, fair, and good quality results.
Meta-analysis was conducted using the Mantel-Haenszel fixed-effects model and the Review Manager (RevMan v5.4.1 2020). The outcome assessment was measured using the odds ratio (OR). The sensitivity analysis was carried out by excluding studies judged to have a high risk of bias. The chi-square test and I2 were used to evaluate heterogeneity between and within the sample. If the I2 statistic revealed more significant than 50% variability, a random-effects model analysis was performed. Continuous data were shown as mean ± SD, and dichotomous variables were shown as percentages (%). If the p-value was less than 0.05, the statistical significance was considered significant.
3. Results
Thirty-three studies with a total of 3090 patients (66% male and 34% female) were included in the systematic review. The baseline characteristics of article inclusion presented in Table 1. Of the 33 studies, there were 11 case report studies, seven case series studies, five cross-sectional studies, and ten retrospective cohort studies (Table 1). Study locations vary from Europe, America, and Asia. Only 23 studies have data on the number of patients treated in the ICU, 828 patients and 779 patients experienced ARDS (94%). Five hundred twenty-seven patients received ECMO therapy (17%). The studies that listed the type of ECMO contained 16 VV ECMO and 12 VA ECMO. There was an average of 20.1% of total deaths from the total population of 17 studies.
Table 1.
Basic characteristic of research.
| No | Author/year | Study Type | Country | N (3090) | Age | Sex M/F (n) |
Admitted to ICU (n = 829) | ARDS (n = 779) | On ECMO (n = 527) | Type of ECMO | Overall mortality (%) | ECMO Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abou-Arab et al./2020 [7] | Case report | France | 2 | 59 67 |
F M |
2 | 2 | 2 | N/A VV |
N/A | N/A |
| 2 | Barrasa et al./2020 [8] | Retrospective | Spain | 48 | 63 (median) | M (27) F (21) |
48 | 48 | 1 | VV | 6 (15) | N/A |
| 3 | Bemtgen et al./2020 [9] | Case report | Germany | 1 | 52 | M | 1 | 1 | 1 | VA to VV | N/A | on ECMO |
| 4 | Chen et al./2020 [10] | Retrospective | China | 99 | 55.5 (mean) | M (67) F (32) |
23 | 17 | 3 | N/A | 11 (11) | 1 death |
| 5 | Firstenberg et al./2020 [11] | Case report | USA | 1 | 51 | M | 1 | 1 | 1 | VV | N/A | discharged day 28 |
| 6 | Giani et al./2020 [12] | Case report | Italy | 1 | 66 | M | 1 | 1 | 1 | VV | N/A | N/A |
| 7 | Guan et al./2020 [13] | Cross sectional | China | 1099 | 47 (median) | M (640) F (459) |
55 | 37 | 5 | N/A | 14 (1.4) | 5 on ECMO meet primary endpoints |
| 8 | Hartman et al./2020 [14] | Case report | USA | 1 | 44 | M | N/A | N/A | 1 | VV | N/A | taken off ECMO day 7 |
| 9 | Huang et al./2020 [15] | Cross sectional | China | 41 | 49 (median) | M (30) F (11) |
13 | 12 | 2 | N/A | 6 (14.6) | N/A |
| 10 | Jacobs et al./2020 [16] | Cross sectional | USA | 32 | 52.4 (mean) | M (22) F (10) |
N/A | N/A | 32 | VA VAV VV |
10 (31.3) | 10 deaths, 5 weaned off, 17 on ECMO |
| 11 | Japan ECMsOne/ 2020 [17] | Cross sectional | Japan | 26 | 71 (mean) | N/A | N/A | N/A | 26 | N/A | N/A | 16 weaned off, 6 extubated and referred for rehabilitation, 10 on ECMO. |
| 12 | Kato et al./ 2020 [18] | Case series | Japan | 70 | 67 (mean) | M (47) F (23) |
N/A | N/A | 2 | VV | 2 (14.8) | 2 weaned off |
| 13 | Li et al./2020 [1] | Case series | China | 8 | 65 (mean) | M (6) F (2) |
N/A | N/A | 8 | VV VA |
N/A | 4 deaths, 3 weaned off, 1 on ECMO |
| 14 | Loforte et al./ 2020 [19] | Observational | Italy | 59 | 49 (mean) | M (4) F (55) |
59 | 59 | 4 | VV | 1 (25) | 1 death, 3 weaned off |
| 15 | Marullo et al./ 2020 [3] | Retrospective | Europe | 333 | 51.8 (mean) | M (285) F (48) |
N/A | N/A | 333 | VA VAV VV VVV |
57 (17.1) | 57 deaths, 54 weaned off |
| 16 | Nakamura et al./ 2020 [20] | Case report | Japan | 1 | 45 | M | 1 | 1 | 1 | VV | N/A | discharged |
| 17 | Ruan et al./ 2020 [21] | Retrospective | China | 150 | 67 (median) | M (102) F (48) |
41 | 62 | 7 | N/A | 68 (48.3) | 7 deaths |
| 18 | Schmiady et al./ 2020 [22] | Case report | Swiss | 1 | 54 | F | 1 | 1 | 1 | VV | N/A | N/A |
| 19 | Shen et al./ 2020 [23] | Case series | China | 5 | 60 (mean) | M (3) F (2) |
5 | 5 | 1 | N/A | N/A | weaned off |
| 20 | Sultan et al./ 2020 [24] | Case series | USA | 10 | 31–62 | M (7) F (3) |
N/A | 10 | 10 | VV | 1 (10) | 1 death, 3 weaned off |
| 21 | Takeda et al./ 2020 [25] | LTE | Japan | 26 | 71 | N/A | N/A | N/A | 26 | N/A | N/A | 16 weaned off, 10 on ECMO |
| 22 | Tang et al./ 2020 [26] | Retrospective case control | China | 179 | 62 (median) | M (105) F (74) |
73 | 73 | 10 | N/A | 21 (28.3) | N/A |
| 23 | Taniguchi et al./ 2020 [27] | Case report | Japan | 1 | 72 | F | 1 | 1 | 1 | VV | N/A | Weaned off ECMO day 12 |
| 24 | Tavazzi et al./ 2020 [28] | Case report | Italy | 1 | 69 | N/A | N/A | 1 | 1 | VA | N/A | Patient died |
| 25 | Yousefzai et al./ 2020 [29] | Case report | USA | 1 | 56 | M | 1 | 1 | 1 | VA | N/A | Weaned off ECMO |
| 26 | Wang et al./ 2020 [30] | Retrospective | China | 138 | 56 (median) | M (75) F (63) |
36 | 22 | 4 | N/A | 6 (4.3) | N/A |
| 27 | Wu et al./ 2020 [31] | Retrospective | China | 201 | N/A | N/A | 53 | 84 | 1 | N/A | 44 (22.9) | Patient died |
| 28 | Yang et al./ 2020 [32] | Retrospective | China | 52 | 59.7 (median) | M (35) F (17) |
52 | 35 | 6 | N/A | 32 (4.5) | 5 deaths |
| 29 | Yu et al./ 2020 [33] | Cross sectional | China | 226 | 64 (mean) | M (139) F (87) |
226 | 161 | 14 | N/A | N/A | 9 death |
| 30 | Zangrillo et al./ 2020 [34] | Case series | Italy | 73 | 61 (median) | M (61) F (12) |
73 | 73 | 5 | N/A | 17 (23.3) | N/A |
| 31 | Zeng et al./ 2020 [35] | Case series | China | 12 | 50.9 (mean) | M (11) F (1) |
12 | 12 | 12 | N/A | 5 (41.6) | 5 deaths, 3 weaned off, 4 on ECMO, 2 coma |
| 32 | Zhan et al./ 2020 [36] | Case report | China | 1 | 54 | M | N/A | N/A | 1 | VV | N/A | discharged |
| 33 | Zhou et al./ 2020 [37] | Retrospective | China | 191 | 56 (median) | M (119) F (72) |
50 | 59 | 3 | N/A | 54 (28.3) | 3 deaths |
Abbreviations: VA veno-arterial; VV veno-venous;, N/A: not available.
Six studies were included in the inclusion criteria for the meta-analysis (two cross-sectional studies and four studies were retrospective cohort studies) (Table 2). All studies published in 2020 and located in China. The comorbid factors attached are hypertension, cardiovascular disease, chronic obstructive pulmonary disease, and diabetes mellitus. Although all articles published in peer-reviewed journals, we assessed the six studies’ methodological quality that was mapped using the NHLBI score. Overall, all studies in this study were of good methodological quality.
Table 2.
Basic characteristic of meta-analysis study.
| No | Author/ Years | Country | Period | n | Age (median) | Male (n) | HT (n) | DM (n) | CVD (n) | COPD (n) | ICU (n) | non ICU (n) | ARDS (n) | ARDS in ICU (n) | ARDS non ICU (n) | ECMO (n) | Non ECMO (n) | Overall mortality (n) | ECMO mortality (n) | Non ECMO mortality (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Huang et al./ 2020 [15] | China | 16 Dec 2019–2 Jan 2020 | 41 | 49 | 30 | 6 | 8 | 6 | 1 | 13 | 28 | 12 | 11 | 1 | 2 | 39 | 6 | N/A | N/A |
| 2 | Wang et al./ 2020 [30] | China | 1 Jan 2020–28 Jan 2020 | 138 | 56 | 75 | 64 | 14 | 20 | 4 | 36 | 102 | 27 | 22 | 5 | 4 | 134 | 6 | N/A | N/A |
| 3 | Yang et al./ 2020 [32] | China | 24 Dec 2019–26 Jan 2020 | 52 | 59,7 | 35 | N/A | 2 | 2 | 2 | 52 | 146 | 36 | 35 | 1 | 6 | 46 | 32 | 6 | 26 |
| 4 | Guan et al./ 2020 [13] | China | 11 Dec 2019–31 Jan 2020 | 1099 | 47 | 640 | 165 | 81 | 27 | 12 | 55 | 1044 | 37 | 27 | 10 | 5 | 1094 | 67 | 5 | 62 |
| 5 | Zhou et al./ 2020 [37] | China | discharged by 31 Jan 2020 | 191 | 56 | 119 | 58 | 36 | 15 | 6 | 50 | 141 | 59 | N/A | N/A | 3 | 188 | 54 | 3 | 51 |
| 6 | Ruan et al./ 2020 [21] | China | N/A | 150 | 67 | 102 | N/A | N/A | N/A | N/A | 41 | 109 | 62 | N/A | N/A | 7 | 143 | 68 | 7 | 61 |
| TOTAL | 1671 | 1001 | 293 | 141 | 70 | 25 | 247 | 1570 | 233 | 95 | 17 | 27 | 1644 | 233 | 21 | 20 | ||||
Abbreviation: HT: hypertension, DM: Diabetes Mellitus, CVD: Cardiovascular Disease, COPD: Chronic Obstructive Pulmonary Disease, ICU: Intensive Care Unit, ARDS: Acute Respiratory Distress Syndrome, ECMO: Extracorporeal Membrane Oxygenation, N/A: not available.
Of the total patients, 247 patients (14.7%) admitted to the ICU, and 233 patients (13.9%) were diagnosed with ARDS. Four studies presented ARDS complications in ICU and non-ICU care, with a total of 95 patients experiencing ARDS in ICU (84%) and 17 patients experiencing ARDS during follow-up in non-ICU care (16%). The number of deaths due to all complications was 233 patients (13.9%). The use of ECMO in the total population is insufficient compared to support without ECMO (nasal cannula, non-invasive ventilation/high flow nasal cannula, or invasive ventilation), which was only 27 patients (1.6%). During follow-up, of the 27 patients who were given ECMO support, 21 patients experienced death (77.8%).
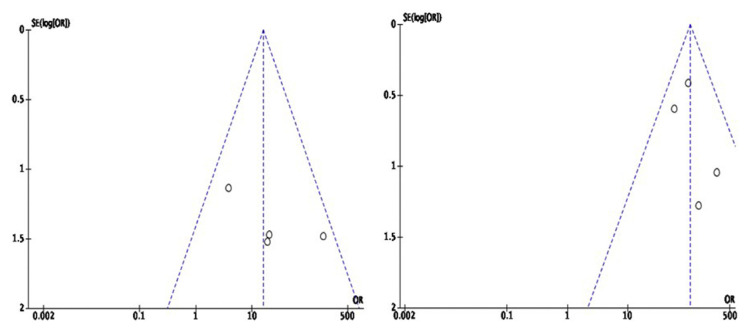
Funnel plot analysis shown in Fig. 2. Visually on the funnel plot, we obtain a symmetrical model, which indicates no visible publication bias from the four studies analyzing mortality on ECMO use (Fig. 2a). The other four studies that analyzed the outcome of ARDS incidence in patients admitted to the ICU also presented an asymmetrical model indicating no apparent publication bias (Fig. 2b).
Fig. 2.
Funnel plot (a) Mortality in ECMO use in COVID-19 patients (b) COVID-19 patients with ARDS in ICU care.
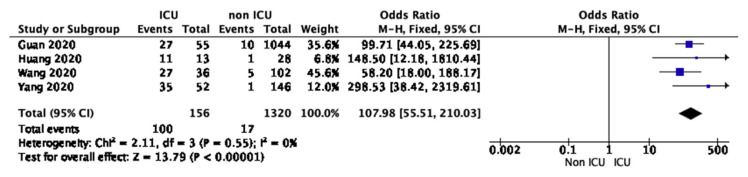
The outcome of this meta-analysis was to seek an association between the incidence of ARDS in ICU care and mortality from ECMO use in ARDS patients. Four studies involving 1476 patients reported 156 patients admitted to the ICU (10.5%). In direct comparison, ARDS incidence was associated with complications during ICU stay compared with ARDS incidence in non-ICU care (OR 107.98; 95% CI 55.51–210.03; p < 0.00001). There was no significant heterogeneity (I2 = 0%; P < 0.00001) for the entire population (Fig. 3).
Fig. 3.
Forest plot of COVID-19 patients who become diagnosed with ARDS during ICU care and non-ICU care.
Four studies involving 1491 patients reported 21 patients who received ECMO support (1.4%). On a direct comparison, the incidence of mortality associated with ECMO compared with non-ECMOs (OR 15.79; 95% CI 4.21–59.28; p < 0.0001). However, there was no significant substantial heterogeneity (I2 30%; p < 0.0001) for the entire population (Fig. 4).
Fig. 4.
Forest plot of mortality of ECMO use in COVID-19 patients.
4. Discussion
Many COVID-19 patients have only minor symptoms and eventually recover. On the other hand, some advance to severe disease conditions, including spasms and hypoxemia, around a week after onset. Such patients develop ARDS rapidly, leading to multiple organ failure or death [31]. The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) have published recommendations indicating the likelihood of ECMO care in patients with severe/critical respiratory failure and heart involvement who have failed to respond to traditional treatment [17].
ECMO is indicated in patients who have a high risk of mortality. Patients who have refractory hypoxemia with oxygen partial pressure (PaO2)/ inspired oxygen fraction (FiO2) 50 mmHg for 3 h or PaO2/FiO2 80 mmHg for >6 h are candidates for ECMO [22]. In more than 95% of cases, severe and refractory hypoxemia results in death. In this scenario, conventional mechanical ventilation cannot increase the minimal blood oxygenation required by the body. ECMO techniques can be used until the lungs recover and regain their basic function [23].
From our systematic review, 11 studies showed high mortality rates in patients with COVID-19. The main risk factors associated with high mortality include age ≥60 years, various comorbidities (such as cardiovascular disease and diabetes), low lymphocyte count <0.8 ( × 10*9/L), and D-dimer> 1 μg/L. In contrast, 12 studies showed a positive outcome for ECMO (weaning/discharge) administration. These results could be related to earlier ECMO administration. For example, Taniguchi et al. and Zhan et al. reported that earlier ECMO impacted patient outcomes. The oxygen supply remained sufficient to avoid lung injury due to mechanical ventilator damage.
In the meta-analysis, less than half of the patients analyzed used ECMO. The results showed that mortality when using ECMO was very high. There are several ways to measure mortality risk in ARDS. All include PaO2/FiO2 below 100, despite and after optimal care. The recently published EOLIA trial contains three indications that define severe ARDS where ECMO may be helpful for adult respiratory failure. Many standardized algorithms for ARDS therapies. When patients meet indications, ECMO should be initiated immediately in an experienced centre, not days later. In addition, ECMO should be prioritized given to particular patient groups, namely younger patients with a relatively low prevalence of comorbidities and an acceptable probability of reversing pulmonary failure [2].
Several factors must be considered, such as old age, comorbidities that indicate a poor prognosis (diabetes, heart disease, hypertension, and COPD). If the patient experiences intracranial bleeding or multiorgan failure will increase the risk of death when given ECMO therapy [38]. Other factors like disruption in coagulation pathways can further raise the risk of thrombotic and hemorrhagic consequences. This might be due to the use of anticoagulants during ECMO delivery, as well as systemic inflammation. As a result, coagulability levels in patients receiving ECMO need to be monitored more closely [39,40].
Certain patients may be given antiviral, antibacterial, steroids, immunoglobulins, chloroquine, vasoconstrictor agents, or medications as alternative medicines or concurrent therapies such as kidney replacement. It would all depend on the patient’s concerns, which is also an alternative. Among COVID-19 patients with ECMO, several study reports found a strong positive correlation between mortality and high levels of cytokines, especially IL-6 [41]. So that in the future, studies are needed on cytokine analysis that can explain the high mortality of patients undergoing ECMO and other treatments that would be ideal for assisting inpatient recovery [9].
It is important to note that the role of ECMO in treating diseases caused by this new virus is still uncertain because of the lack of a concurrent control group. It is difficult to draw any confirmed conclusion regarding effectiveness as it is difficult to ascertain any observed effect is a “true” intervention effect, as we cannot rule out the contribution of the natural course of the condition, placebo/Hawthorne effect, or the effect of other concurrent treatment. Meanwhile, new studies by a variety of authors are constantly proposed. It should also be noted that extracorporeal ventilation as a treatment modality is not yet widely used. This scenario may be because ECMO is an expensive technology that uses many resources, making it difficult for certain countries affected by COVID-19 to pay. Another essential argument is that it can be conducted in the health care centre with experienced staff, qualified professionals, and a multidisciplinary approach [1]. Based on the description above, it is evident that much more research is needed to be done with a larger population concerning the use of ECMO in COVID-19 patients, especially since this is an alternative to conventional treatment that failed of some critical patients, so it needs to be re-analyzed at ECMO timing initiation and patient selection criteria to reduce mortality.
5. Conclusions
The incidence of ARDS in COVID-19 patients was higher during ICU care than non-ICU care, and the incidence of mortality was higher with ECMO use. Thus, it can be seen that with follow-up studies, the mechanisms involving illness and death of patients due to COVID-19 can be better demonstrated, and the use of ECMO in patients can be considered earlier.
Abbreviations
- ARDS
acute respiratory distress syndrome
- CDC
centers for disease control and prevention
- COVID-19
coronavirus disease 2019
- ECMO
extracorporeal membrane oxygenation
- ELSO
extracorporeal life support organization
- ICU
intensive care unit
- NHBLI
national heart, lung, and blood institute
- VV-ECMO
veno-venous extracorporeal membrane oxygenation
- VA-ECMO
veno-arterial extracorporeal membrane oxygenation
- WHO
world health organization (WHO)
Footnotes
Funding
The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Author contribution
Conception and design of Study: Novia Kusumawardhani, Ivana Purnama Dewi. Literature review: Novia Kusumawardhani, Ivana Purnama Dewi. Acquisition of data: Novia Kusumawardhani, Budi Baktijasa Dharmadjati. Drafting of manuscript: Novia Kusumawardhani, Ivana Purnama Dewi. Revising and editing the manuscript critically for important intellectual contents: Novia Kusumawardhani, Ivana Purnama Dewi. Revising and editing the manuscript critically for important intellectual contents: Novia Kusumawardhani, Ivana Purnama Dewi, Budi Baktijasa Dharmadjati. Supervision of the research: Budi Baktijasa Dharmadjati.
References
- 1.Li X, Guo Z, Li B, Zhang X, Tian R, Wu W, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. Am Soc Artif Intern Organs J. 2020;66:475–81. doi: 10.1097/MAT.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Clinical management of severe acute respiratory infection when COVID-19 is suspected. Geneva: WHO; 2020. [Google Scholar]
- 3.Marullo AG, Cavarretta E, Biondi-Zoccai G, Mancone M, Peruzzi M, Piscioneri F, et al. Extra-corporeal membrane oxygenation for critically ill patients with coronavirus-associated disease 2019: an updated perspective of the European experience. Minerva Cardioangiol. 2020;68:368–72. doi: 10.23736/S0026-4725.20.05328-1. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira TF, Rocha CAO, Santos AGGD, Silva-Junior LCF, Aquino SHS, Cunha EJOD, et al. Extracorporeal membrane oxygenation in COVID-19 treatment: a systematic literature review. Braz J Cardiovasc Surg. 2020 doi: 10.21470/1678-9741-2020-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haiduc AA, Alom S, Melamed N, Harky A. Role of extra-corporeal membrane oxygenation in COVID-19: a systematic review. J Card Surg. 2020;35:2679–87. doi: 10.1111/jocs.14879. [DOI] [PubMed] [Google Scholar]
- 6.Arabi YM, Al-Omari A, Mandourah Y, Al-Hameed F, Sindi AA, Alraddadi B, et al. Critically ill patients with the middle east respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45:1683–95. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 7.Abou-arab O, Huette P, Berna P, Mahjoub Y. Tracheal trauma after difficult airway management in morbidly obese patients with COVID-19. Br J Anaesth. 2020;125:e168–70. doi: 10.1016/j.bja.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrasa H, Rello J, Tejada S, Martín A, Balziskueta G, Vinuesa C, et al. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39:553–61. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bemtgen X, Kruger K, Supady A, Durschmied D, Schibilsky D, Bamberg F, et al. First successful treatment of COVID-19 induced refractory cardiogenic plus vasoplegic shock by combination of pVAD and ECMO - a case report. Am Soc Artif Intern Organs J. 2020;66:607–9. doi: 10.1097/MAT.0000000000001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firstenberg MS, Stahel PF, Hanna J, Kotaru C, Crossno J, Forrester J. Successful COVID-19 rescue therapy by extra-corporeal membrane oxygenation (ECMO) for respiratory failure: a case report. Patient Saf Surg. 2020;14:1–7. doi: 10.1186/s13037-020-00245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giani M, Seminati D, Lucchini A, Foti G, Pagni F. Exuberant plasmocytosis in bronchoalveolar lavage specimen of the first patient requiring extracorporeal membrane oxygenation for SARS-CoV-2 in Europe. J Thorac Oncol. 2020;15:e65–6. doi: 10.1016/j.jtho.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, Wen-hua Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartman ME, Hernandez RA, PateL K, Wagner TE, Trinh T, Lipke AB, et al. COVID-19 respiratory failure: targeting inflammation on VV-ECMO support. Am Soc Artif Intern Organs J. 2020;66:603–6. doi: 10.1097/MAT.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs JP, Stammers AH, St Louis J, Hayanga JWA, Firstenberg MS, Mongero LB, et al. Extra-corporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in Coronavirus disease 2019: experience with 32 patients. Am Soc Artif Intern Organs J. 2020;66:722–30. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japan ECMOnet for COVID-19. Nationwide system to centralize decisions around ECMO use for severe COVID-19 pneumonia in Japan (special correspondence) J Intensive Care. 2020;8:29. doi: 10.1186/s40560-020-00445-4. Erratum in: J Intensive Care 2020;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato H, Shimizu H, Shibue Y, Hosoda T, Iwabuchi K, Nagamine K, et al. Clinical course of 2019 novel coronavirus disease (COVID-19) in individuals present during the outbreak on the Diamond Princess cruise ship. J Infect Chemother. 2020;26:865–9. doi: 10.1016/j.jiac.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loforte A, Dal Checco E, Gliozzi G, Benedetto M, Cavalli GG, Mariani C, et al. Veno-venous extracorporeal membrane oxygenation support in COVID-19 respiratory distress syndrome. Am Soc Artif Intern Organs J. 2020;66:734–8. doi: 10.1097/MAT.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura K, Hikone M, Shimizu H, Kuwahara Y, Tanabe M, Kobayashi M, et al. A sporadic COVID-19 pneumonia treated with extracorporeal membrane oxygenation in Tokyo, Japan: a case report. J Infect Chemother. 2020;26:756–61. doi: 10.1016/j.jiac.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmiady MO, Sromicki J, Kucher N, Ouda A. Successful percutaneous thrombectomy in a patient with COVID-19 pneumonia and acute pulmonary embolism supported by extracorporeal membrane oxygenation. Eur Heart J. 2020;41:3107. doi: 10.1093/eurheartj/ehaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J Am Med Assoc. 2020;323:1582–9. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultan I, Habertheuer A, Usman AA, Kilic A, Gnall E, Friscia ME, et al. The role of extracorporeal life support for patients with COVID-19: preliminary results from a state-wide experience. J Card Surg. 2020;35:1410–3. doi: 10.1111/jocs.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda S. Nationwide system to centralize decisions around extracorporeal membranous oxygenation use for severe COVID-19 pneumonia in Japan. Acute Med Surg. 2020;7:e510. doi: 10.1002/ams2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi H, Ogawa F, Honzawa H, Yamaguchi K, Niida S, Shinohara M, et al. Veno-venous extracorporeal membrane oxygenation for severe pneumonia: COVID-19 case in Japan. Acute Med Surg. 2020;7:e509. doi: 10.1002/ams2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–5. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yousefzai R, Bhimaraj A. Misdiagnosis in the COVID Era: when zebras are everywhere, don’t forget the horses. JACC Case Rep. 2020;2:1614–9. doi: 10.1016/j.jaccas.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y, Xu D, Fu S, Zhang J, Yang X, Xu L, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zangrillo A, Beretta L, Scandroglio AM, Monti G, Forminskiy E, Colombo S, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–11. doi: 10.1016/S1441-2772(23)00387-3. PMID: 32353223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng Y, Cai Z, XianYu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care. 2020;24:148. doi: 10.1186/s13054-020-2840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan WQ, Li MD, Xu M, Lu YB. Successful treatment of COVID-19 using extracorporeal membrane oxygenation, a case report. Eur Rev Med Pharmacol Sci. 2020;24:3385–9. doi: 10.26355/eurrev_202003_20705. [DOI] [PubMed] [Google Scholar]
- 37.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu BS, Hu MZ, Jiang LX, Yu J, Chang Y, Cao Y, et al. Extra-corporeal membrane oxygenation (ECMO) in patients with COVID-19: a rapid systematic review of case studies. Eur Rev Med Pharmacol Sci. 2020;24:11945–52. doi: 10.26355/eurrev_202011_23855. PMID: 33275268. [DOI] [PubMed] [Google Scholar]
- 39.Alom S, Haiduc AA, Melamed N, Axiaq A, Harky A. Use of ECMO in patients with coronavirus disease 2019: does the evidence suffice? JCVA. 2021;35(4):1256–62. doi: 10.1053/j.jvca.2020.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savarimuthu S, BinSaeid J, Harky A. The role of ECMO in COVID-19: can it provide rescue therapy in those who are critically ill? J Card Surg. 2020;35(6):1298–301. doi: 10.1111/jocs.14635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta P, Mcauley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]