Abstract
Bioelectric devices can probe fundamental biological dynamics and improve the lives of human beings. However, direct application of traditional rigid electronics onto soft tissues can cause signal transduction and biocompatibility issues. One common mitigation strategy is the use of soft–hard composites to form more biocompatible interfaces with target cells or tissues. Here, we identify several soft–hard composite designs in naturally occurring systems. We use these designs to categorize the existing bioelectric interfaces and to suggest future opportunities. We discuss the utility of soft–hard composites for a variety of interfaces, such as in vitro and in vivo electronic or optoelectronic sensing and genetic and non-genetic modulation. We end the review by proposing new soft–hard composites for future bioelectric studies.
The Need for Soft–Hard Composites
Most human tissues, with the exception of bones and cartilage, display a relatively low Young’s modulus (see Glossary) at the kilopascal scale [1,2]. However, the Young’s modulus of many functional rigid materials (e.g., inorganic semiconductors) can be as high as hundreds of gigapascal. Due to this significant mismatch in modulus, the direct application of traditional hard electronics onto soft tissues presents challenges; such an interface can generate unstable signal transduction and also cause severe long-term tissue damage [3]. One solution to this mismatch is the use of a soft–hard composite as a biomaterial; the hard components are stable, easy to control, and provide the active functionalities, while the soft components reduces inflammatory responses (Figure 1A) and promote compliant mechanical adhesion (Figure 1B). Additionally, as substrates or encapsulation layers, the soft components can protect the high modulus electronic elements (e.g., through impact absorption or by confining the rigid component near the neutral stress planes, Figure 1C), and can facilitate mechanical operations (e.g., stretching, bending, delivery, or removal) of the entire composite. Last but not the least, advanced soft materials can display dynamically responsive or even living behaviors, which are currently lacking in rigid electronic systems (see Outstanding Questions) (Figure 1D).
Figure 1. Soft–Hard Composites May Establish Better Biointerfaces and Are Common in Nature.
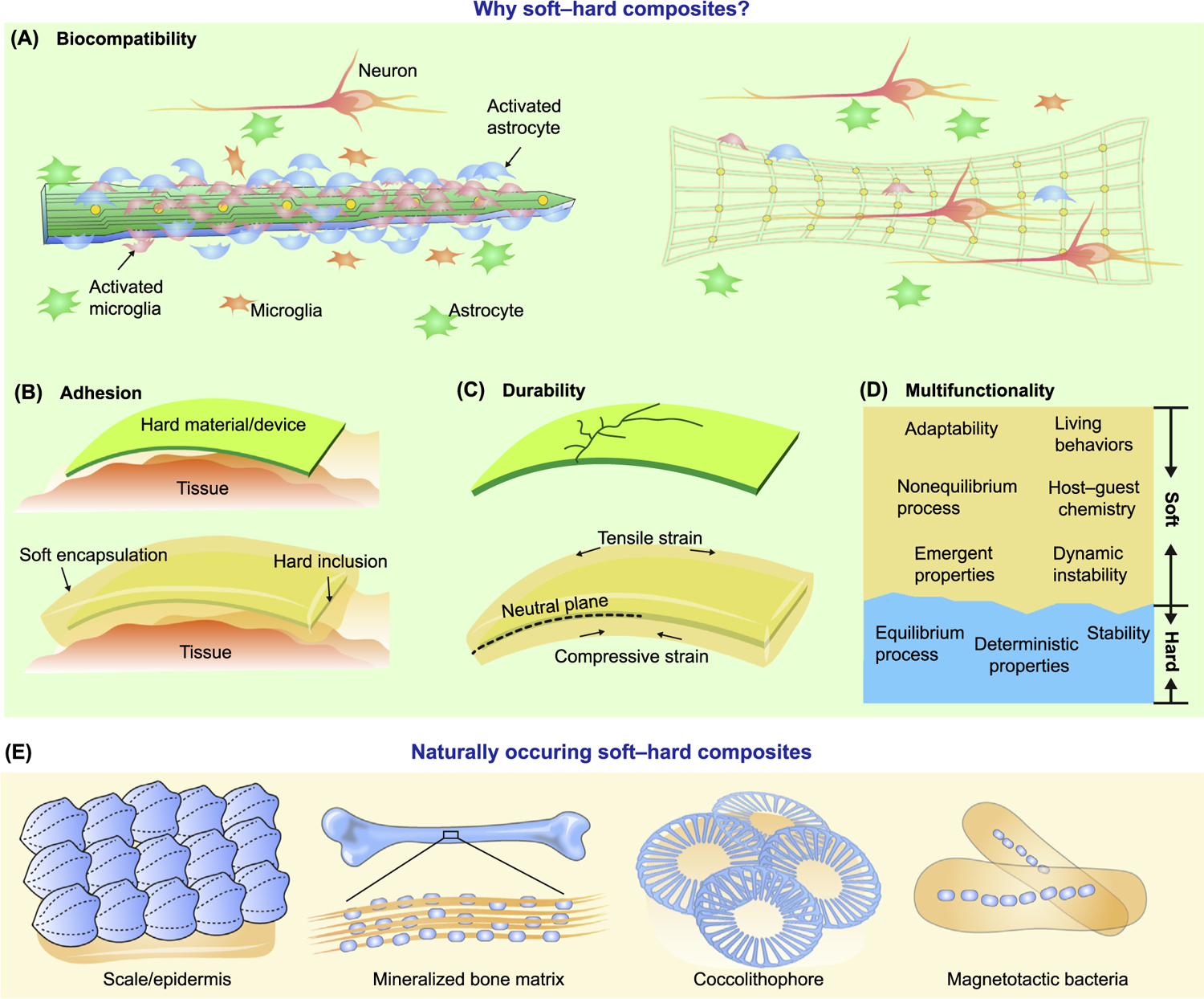
(A) Rigid devices usually trigger significant inflammatory response upon implantation, activating microglia and astrocytes. Soft–hard composites have lower effective modulus and are more biocompatible. (B) Hard materials/devices do not conform well to the curvilinear tissue surfaces. With either partial (see Figure 1 in Box 1, row 1) or complete coverage by the soft materials, the adhesion at the device/tissue interface gets improved. (C) Hard materials are usually brittle, easily fracture under strain, and display poor impact absorption. Incorporation of soft components alleviate such issues by, for example, producing a neutral stress plane for the hard material. (D) Soft and hard materials have different properties. Integration of both in a composite can yield multiple functions. (E) Diverse naturally occurring soft–hard composites. Solid scales (blue) attached to a soft epidermis layer (orange) form a bendable composite. The mineralized matrix of the bone consists of homogenously distributed soft organic components (mainly type I collagen, orange) and hard inorganic components (mainly hydroxyapatite, blue). A coccolithophore is enclosed in a collection of coccoliths which make up its exoskeleton or coccosphere. Magnetotactic bacteria use specialized organelles called magnetosomes to store magnetic material.
Outstanding Questions.
What are the next promising bioelectric composites that can be enabled by biomechanical or biochemical processes? Developmental biology approaches have produced numerous soft–hard interfaces in nature. Recent work on genetically targeted chemical assembly of materials over neuronal membranes suggests one of the possibilities.
How can soft and hard materials contribute synergistically to better bioelectric interface designs? Soft materials usually produce nonlinear behaviors, instabilities, and emergent structures, such as dynamic defect flows and reaction-diffusion processes. Many soft material behaviors are obtained under far-from-equilibrium conditions and are maintained by energy dissipation. In particular, many biological and synthetic soft materials are motile, stimuli-responsive and adaptable, self-healing and regenerative, and symmetry-breaking. Hard materials, in contrast, have well-defined and persistent properties, are insensitive to subtle environmental perturbation, and most of their behaviors are produced at or close to equilibrium. These different properties and behaviors from the soft–hard composites can be leveraged at the same biointerfaces.
The current approaches to probe the biomechanical properties of cells and tissues are distinct from those employed for bioelectric studies. Can future biointerface devices or theoretical models allow the integration of these two types of studies in a seamless and coherent manner? One experimental strategy is to explore piezoelectric materials as either soft or hard components in the bioelectronics composites.
What are the immediate areas where traditional bioelectronics can incorporate tissue-like material components such as granular particles and advanced hydrogels? Electronic skin and cardiac pacing patch may benefit from these new components.
Naturally Occurring Soft–Hard Composites
Many naturally occurring biomaterials contain soft–hard interfaces. While many soft–hard composites used in bioelectric interfaces were not initially inspired by those in nature, we have identified several common features among them. This commonality suggests future bioinspired designs in bioelectronics. The natural composites that are particularly relevant include scales/epidermis, bone, coccolithophore, and magnetotactic bacteria.
In the skin of fish and reptiles, solid scales attach to softer epidermal tissues (Figure 1E) and form a locally rigid but globally bendable layer that protects the tissues [4]. This quasi-layered configuration is reminiscent of the bioelectronic devices that incorporate the sequential patterning of rigid components onto soft matrices, thereby establishing multilayered soft–hard interfaces [5] (Figure 2A) or more complicated multimodal interfaces with movable soft–hard contact points (Figure 2B) [6,7].
Figure 2. Soft–Hard Composites Can Be Categorized for Bioelectric Interfaces.
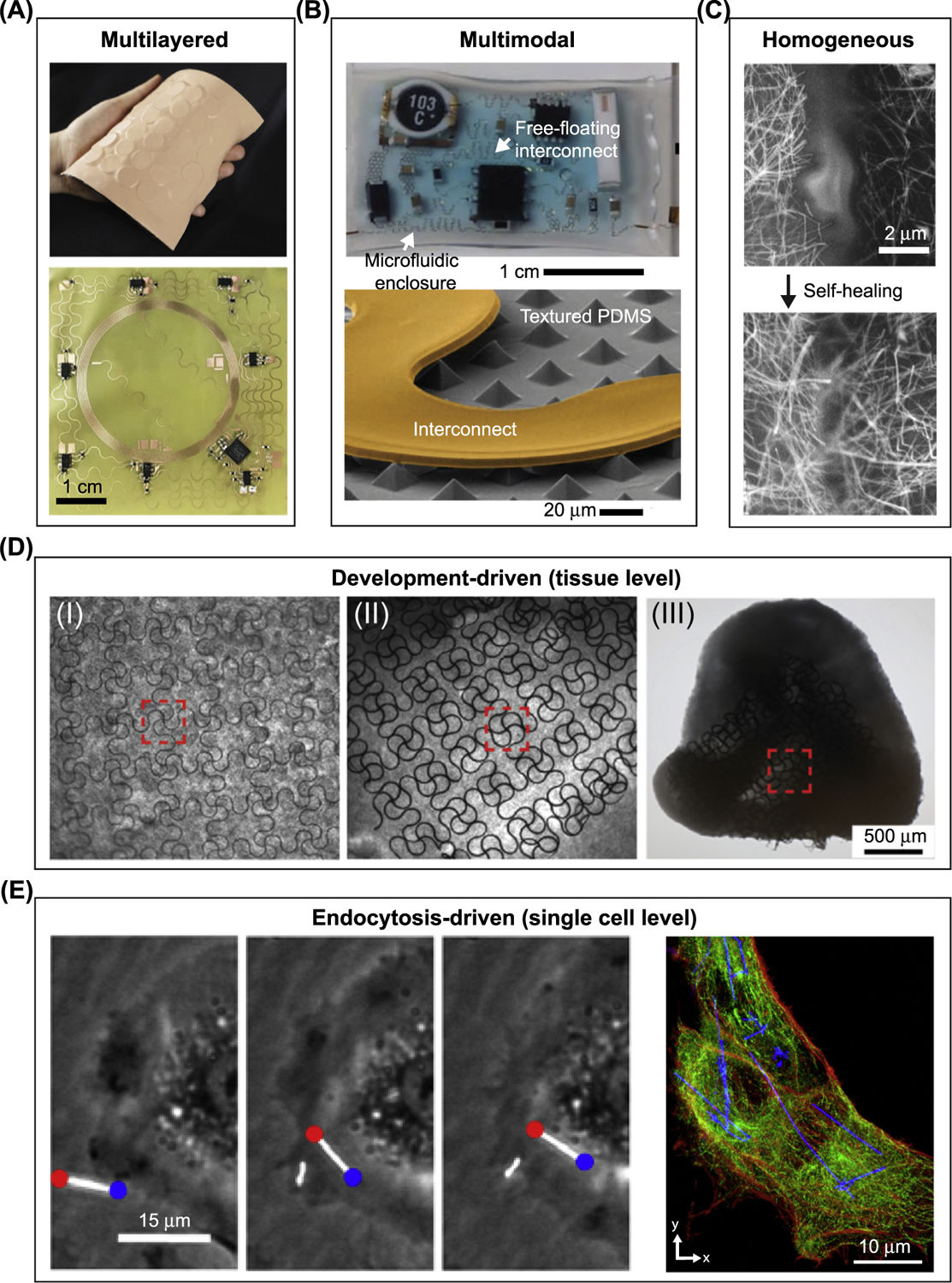
(A–C) Soft–hard composites enabled by synthetic materials. (A) Multilayered interface: (top) photograph of an epidermal virtual reality device, which integrates silicon, actuators, electronics, a Cu-based near-field communication coil (NFC), and polyimide. (Bottom) Optical image shows the NFC coil after integrating the electronic components. Adapted, with permission, from [5]. (B) Multimodal interface: (top) photograph of a soft microfluidic assembly of sensors, circuits, and radios used as epidermal electronics. (Bottom) Scanning electron microscopy (SEM) image of part of the internal device shows a textured polydimethylsiloxane (PDMS) surface and the free-standing interconnect. Liquid can be injected to help impact absorption. Adapted, with permission, from [6]. (C) Homogeneous interface: SEM images of a broken Ag nanowire/PDMS–MPU0.4–IU0.6 composite conductor before (top) and after (bottom) self-healing. Adapted, with permission, from [9]. (D,E) Biology-driven formation of the soft–hard composites. (D) Mechanobiology-driven interface: optical microscopy images of different stages in ‘cyborg’ cardiac organoid formation show the organogenesis force-induced deformation and 3D incorporation of mesh-like nanoelectronics. Adapted, with permission, from [11]. (E) Endocytosis-driven interface: (left) scatter-enhanced phase-contrast images show the internalization of Si nanowires into the human umbilical vein endothelial cell (HUVEC). (Right) confocal fluorescence microscopy images of a silicon nanowire/HUVEC composite shows intracellular distribution of Si nanowires. Actin, red; tubulin, green. Adapted, with permission, from [14]. Abbreviations: IU, Isophorone bisurea unit; MPU, 4,4′ -methylenebis(phenyl urea) unit.
Bones contain 3D mineralized matrices [4,8], in which hydroxyapatite nanocrystals are distributed in soft type I collagen (Figure 1E). These mineralized matrices can inform future design of a 3D-distributed bioelectronics composite, especially given some current devices already display homogeneously incorporated soft (e.g., elastomers) and hard phases (e.g., inorganic conductors) (Figure 2C) [9]. The surrounding soft matrix can induce unique soft material functions to the entire bioelectronics composite, such as self-healing of the electronic network (Figure 2C) [9].
Coccoliths are produced through a developmental process in which a unicellular plankton (coccolithophore) drives collection of mineralized plates to form an exoskeleton or coccosphere (Figure 1E) [10]. This biology-driven organization highlights the recent development of 3D bioelectronics composites, such as nanoelectronics-innervated cardiac organoids [11] in which mesh-like nanoelectronics are folded into cardiac tissue via tissue growth and cellular traction forces (Figure 2D). This process is also relevant to the latest discovery of genetically targeted neuronal synthesis of extracellular conducting polymer networks [12]. Such a developmental biology-driven 3D bioelectronics formation is an emerging area (see Outstanding Questions), where biochemical and biomechanical processes (e.g., focal adhesions and cytoskeletal dynamics) can be explored to enhance the electronics–cell interactions at the molecular and organelle levels.
Magnetotactic bacteria build specialized organelles called magnetosomes to store intracellular magnetic materials (Figure 1E) [13]. The structure of magnetotactic bacteria evokes potential designs for intracellular soft–hard interfaces, in which synthetic hard materials (e.g., silicon nanowires) can be internalized by mammalian cells to form intracellular biointerfaces with vesicles, cytoskeletal filaments, and motor proteins (Figure 2E) [14]. As the composite is living, proliferative, motile, and biocompatible, this approach may be adopted for building living bioelectric interfaces in vivo where the cellular composites are implanted for therapeutic purpose.
In these examples of soft–hard composites, soft components, either a synthetic material with a relatively low Young’s modulus (Figure 2A–C) or biological tissues or cells (Figure 2D,E), are integrated with a hard synthetic counterpart to establish a soft–hard composite (Table 1). One advantage of the soft–hard composite strategy is that it leverages mature technology (i.e., semiconductor fabrication) for immediate applications such as tissue-interfacing bioelectronics, without developing new synthetic solutions. In this short review, we highlight recent progress (primarily over the past 5 years) in the design and utilization of soft–hard composites for biointerfaces, where connections are made between these composites and the naturally occurring ones (Figure 1). We focus our discussion on bioelectronics or bioelectric systems. Traditional drug-delivery systems [15–17] have been reviewed extensively elsewhere and are not included here. We end the review with our perspective on the utilization of advanced hydrogels and granule-based tissue-like materials as next-generation soft materials for soft–hard composites in biomedical applications.
Table 1.
Soft and Hard Components in Bioelectric Interfacesa
| Representative work | Interface type | Sensing/modulation | Hard components | Soft components | Refs |
|---|---|---|---|---|---|
| Skin-integrated wireless haptic interfaces | Multilayered | Tactile sensing | Circuit components, copper | Polyimide, silicone, cloth | [5] |
| Soft microfluidic bioelectronics assembly | Multimodal | EMG, EEG, ECG | Circuit components | Elastomer, fluid | [6] |
| 3D integrated stretchable electronics | Multilayered | EMG, EEG, ECG, tactile sensing | Circuit components, Copper | Polyimide, silicone | [7] |
| Self-healable electronic skin | Homogeneous | ECG | CNT, silver nanowire, LED | PDMS, SEBS | [9] |
| Cyborg organoids | Development-driven | Extracellular cardiac recording | Platinum, gold | SU-8, cultured cells | [11] |
| 3D intracellular FET probes for cardiomyocytes | Multilayered | Intra- and extracellular cardiac recording | Silicon nanowire, metallic interconnects | SU-8, lipid bilayer | [25] |
| 3D intracellular FET probes for neurons | Multilayered | Intra- and extracellular neural recording | Silicon/silicide nanowire, metallic interconnects | SU-8, lipid bilayer | [30] |
| Electronic skin for prosthetic tactile sensation | Multilayered | Tactile sensing | Circuit components, gold | PDMS, liquid metal | [36] |
| Electronic skin capable of differentiating mechanical stimuli | Multilayered | Tactile sensing | CNT | PDMS | [37] |
| Silk protein-supported on-skin electronics | Multilayered | Tactile sensing | Gold | Plasticized silk | [39] |
| Bending-insensitive pressure sensor | Multilayered | Tactile sensing | Gold, ITO, CNT, graphene | PET, parylene | [41] |
| Direction-sensitive electronic skin | Multilayered | Tactile sensing | CNT | Polyurethane, PHB-PHV | [43] |
| Epidermal electronic systems for neonatal intensive care | Multilayered | ECG, photoplethysmograms | Circuit components, copper | Silicone, ionic liquid | [47] |
| MRI-compatible epidermal electronic interfaces | Multilayered | EMG, EEG, ECG, tactile sensing | Gold | Silicone, polyimide, PET | [48] |
| 3D macroporous brain probes | Multilayered | Extracellular neural recording | Gold, palladium | SU-8 | [51] |
| Injectable mesh electronics for brain | Multilayered | Extracellular neural recording | Gold | SU-8 | [52] |
| Injectable mesh electronics for retina | Multilayered | Extracellular neural recording | Gold, platinum | SU-8 | [53] |
| Capacitively coupled silicon transistor arrays | Multilayered | Extracellular cardiac recording | Silicon, silicon oxide, gold | Polyimide | [58] |
| Bioresorbable pressure sensors | Multilayered | Intracranial temperature and pressure sensing | Silicon, silicon oxide | PDMS | [60] |
| One-step optogenetics with multifunctional flexible polymer fibers | Multimodal | Optogenetics, extracellular neural recording | Graphite | Polycarbonate, cyclic olefin copolymer | [64] |
| Multifunctional fibers for multimodal interrogation of neural circuits in vivo | Multimodal | Optogenetics, extracellular neural recording | Graphite, tin | Polycarbonate, cyclic olefin copolymer | [65] |
| Closed-loop system for peripheral neuromodulation | Multilayered | Optogenetics | Circuit components, copper | Polyimide, PDMS | [74] |
| Thin-film microelectrodes for auditory brainstem implants | Multilayered | Electrical stimulation | Platinum | Polyimide, PDMS | [76] |
| SU-8-supported silicon nanowire arrays for cardiac modulation | Multilayered | Nongenetic optical modulation | Silicon nanowires | SU-8 | [79] |
| PDMS-supported silicon membranes for neuromodulation | Multilayered | Nongenetic optical modulation | Silicon | PDMS | [80] |
| Myofibroblast-silicon composites for cardiac modulation | Endocytosis-driven | Nongenetic optical modulation | Silicon nanowires | Cultured cells | [81] |
Abbreviations: CNT, Carbon nanotube; ITO, indium tin oxide; PDMS, polydimethylsiloxane; PET, polyethylene terephthalate; PHB, polyhydroxybutyrate; PHV, polyhydroxyvalerate; SEBS, styrene ethylene butylene styrene; SU-8, SU-8 photoresist (a commonly used epoxy-based negative photoresist).
Figure 1.
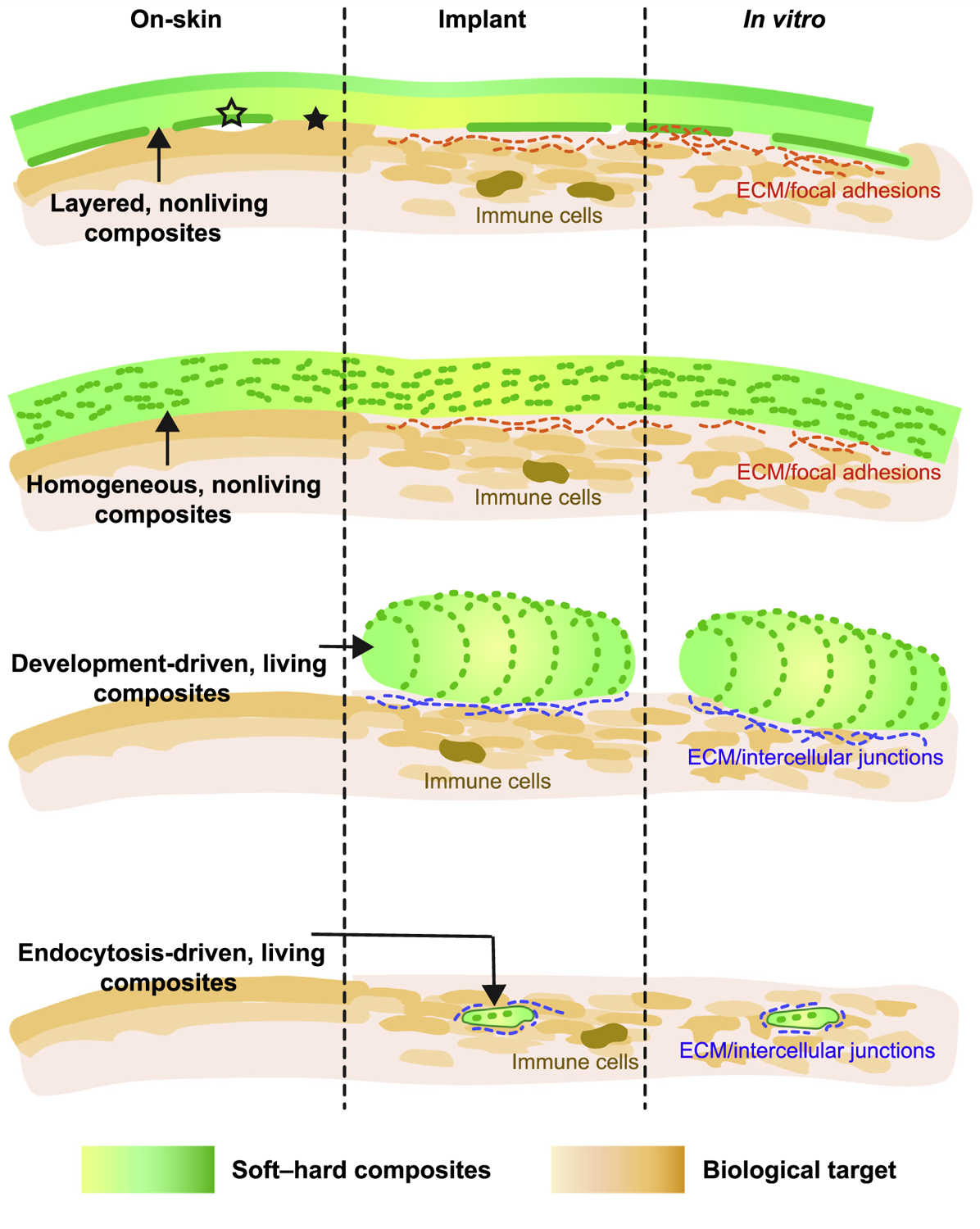
Classifying Types of Soft–Hard Composites (Rows) and the Intended Locations of the Biointerfaces (Columns).
Soft–Hard Composites for Sensing/Recording
Sensing and recording not only play essential roles in medical diagnostics, improved patient care, and reduced healthcare spending, but are also important in disease prevention [18–20]. Many clinical sensing/recording devices cannot provide customized, long-term monitoring without interfering in daily activity. Sensing/recording covers a wide range of applications, including but not limited to electrocardiogram (ECG), electroencephalogram (EEG), and electromyogram (EMG) [21,22]. Utilization of soft–hard composites in sensing/recording devices (Table 1) can significantly improve patient experience, as well as device performance and longevity; soft–hard composites can even endow the devices with unique properties such as self-healing [9]. We highlight selected sensing/recording device applications below, which take the form of supported or layered structures (Figures 1E and 2A,B) and uniformly distributed structures (Figures 1E and 2C).
Intracellular Sensing
Over the past decade, designs for intracellular recording devices have advanced from traditional rigid glass micropipettes [23,24] to layered soft–hard configurations. One such design incorporates a flexible intracellular probe based on a kinked Si nanowire field-effect transistor (FET) that was used to record action potentials from isolated cardiomyocytes [25]. The rigid components, such as the metallic interconnects and part of the kinked Si nanowire, were fabricated over a flexible SU-8 backbone. This composite structure yields bendable device where the FET height and orientation can both be adjusted [26]. Such kinked protrusions have been utilized as rigid components for cellular penetration in a number of reports [27–29]. Recently, a highly sensitive and flexible U-shaped nanowire probe was developed through transfer printing and short-channel formation, the curved tip of which was rigid enough to enter single neurons. Notably, the recorded intracellular action potentials can reach the amplitude over 100 mV from primary neurons and show device tip-curvature dependence (Figure 3A) [30]. In these intercellular sensing devices, a phospholipid bilayer (another soft component) coating over the FET promoted intracellular entry of the device, likely through a membrane fusion process. In addition to a phospholipid coating, Si nanowires may be modified with a cell-penetrating peptide for active intracellular entry [31]. The use of soft components, such as an SU-8 substrate and a phospholipid bilayer, yielded minimally invasive intracellular recording device whose cell penetration did not perturb the natural firing patterns of the targeted cardiomyocytes or neurons [32].
Figure 3. Soft–Hard Composites Can Sense in Neuronal Signals.
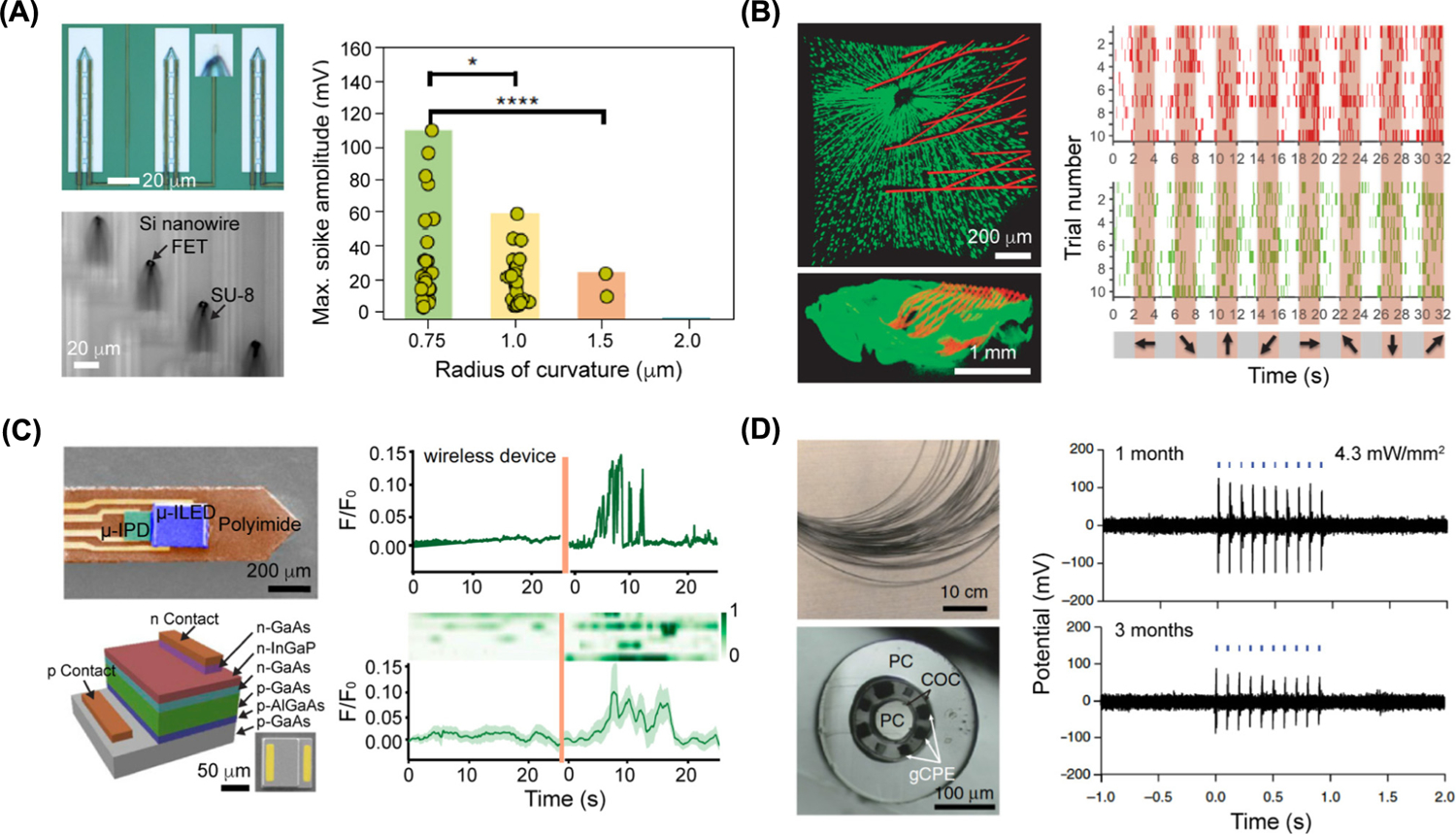
(A) Ultra-small 3D transistor probes for intracellular recording. (Top left) Optical image of 3D transistor probes with magnified view (inset) showing that the U-shaped nanowire is transferred onto the device tip. (Bottom left) Optical image of the bend-up device array in water. (Right) Plot of maximum spike amplitude obtained from action potential recordings in dorsal root ganglion neurons using the ultra-small 3D transistor probes. The P values of the statistical studies were obtained using Student’s t-test. *P < 0.1, ****P < 0.0001. Adapted, with permission, from [30]. (B) Mesh electronics for chronic recording in retina from awake mice at the single-neuron level. (Top left) Ex vivo imaging of the interface between the retinal ganglion cells (green) and injected mesh electronics (red) on day 7 after injection (top view). (Bottom left) Side view image of the interface. (Right) Plots showing the firing events of two neurons (red and green) in response to grating stimulations on day 7 after injection. Shaded regions (orange) correspond to times when gratings stimulations were performed. Adapted, with permission, from [53]. (C) Wireless optoelectronic photometer neuron recording in deep brain. (Top left) Colorized scanning-electron-microscopy (SEM) image of the probe. (Bottom left) Schematic of a GaAs μ-IPD with a representative SEM image of μ-IPD in the right corner. (Top right) Plot of fluorescence changes before/after animal was shocked with wireless optoelectronic photometers. (Bottom right) Heatmap of signals (eight trails) recorded before/after animal was shocked. Heatmap is aligned with plotted trace. Adapted, with permission, from [62]. (D) Flexible polymer fibers capable of collocated neural recordings. (Top left) Photograph of a bundle of multimodal fibers (before etching of the sacrificial polycarbonate cladding). (Bottom left) Cross-sectional image of the multimodal fiber. (Right) Electrophysiological recording plots of optically evoked potentials in the medial prefrontal cortex of wild type mice performed at 1 month and 3 months after the one-step implantation and transfection surgery. Adapted, with permission, from [64]. Abbreviations: COC, Cyclic olefin copolymer; FET, field-effect transistor; gCPE, conductive polyethylene (CPE) and 5% graphite; PC, polycarbonate.
Tactile Sensing
Flexible bioelectronics for tactile sensing can adopt multilayered, multimodal, or homogeneous soft–hard interfaces (Figures 1E and 2A–C) [33–35]. For instance, a wearable elastomer-based electronic skin, composed of a silicone dielectric layer (1st soft component), a cracked gold electrode, and liquid metal wires (2nd soft component), was successfully used to monitor finger articulation, demonstrating sensitivity over a wide range of pressure values [36]. Uniformly mixing polydimethylsiloxane (PDMS) and single-walled carbon nanotubes into films for electronic skin has enabled tactile sensing as well as energy harvesting [37]. Integration of such tactile sensors with electrochromic polymers results in devices that can indicate different colors at different pressures [38]. Beyond traditional elastomers or polymer substrates, silk protein can also be used as the soft component in biointerfaces to provide conformable contact with skin [39].
Additional structure or composition designs in either the soft or the hard components can enhance the sensing performance of on-skin bioelectronics. Examples include a microhairy sensor capable of pulse signal amplification [40], a bending-insensitive pressure sensor [41] made from a composite of carbon nanotubes and graphene nanofibers, and a highly biocompatible on-skin gold nanomesh sensor with sacrificial polyvinyl alcohol nanofiber-based soft substrate [42]. Recent work also reported on an electronic skin that can measure and discriminate both normal and shear forces in real time and detect the direction of applied pressures for robotics [43]. Notably, an on-skin, wireless bioelectronics system was recently applied as a touch-based interface for virtual reality and augmented reality technology (Figure 2A). This composite was capable of softly laminating onto curvilinear skin surfaces to communicate information through localized mechanical vibrations [5].
Epidermal Electrophysiology Recording
One of the main applications for on-skin bioelectronics is ECG/EEG/EMG recording for medical diagnostics and disease prevention. Most recording systems use a layer-by-layer design and include multimaterial integration and heterogeneous structures (Figures 1E and 2A,B). Flexible plastic substrates, such as Kapton, are frequently used in devices for monitoring temperature, ECG signals, and heart rate in humans [44]. Many other recording systems employ softer substrates or even composites with movable contact points between the soft and hard components. For example, a microfluidic physiology recording platform was developed, where high-modulus functional electronic elements achieved high stretchability through a free-floating configuration and controlled buckling over a structured PDMS surface (Figure 2B) [6]. The soft components in this example include the PDMS supporting substrate and encapsulation layer (or superstrate), as well as the silicon oligomer fluid filled inside the enclosure. This overall device has ultra-low modulus and can softly laminate onto the skin surface to enable wireless monitoring of ECG, EEG, and EMG signals in humans [6].
ECG/EEG/EMG recordings can also be adapted for diverse biological environments or to tackle multiple medical problems. For example, a soft, curved electrode system was integrated with the auricle of the ear and functioned as a persistent brain–computer interface for high-quality EEG recording over 2 weeks [45]. An on-skin device capable of mechano-acoustic recording has been used in electrophysiological sensing, seismocardiography recording, and heart murmur detection [46]. A wireless epidermal electronic system developed for a neonatal intensive care unit was used to monitor and analyze data in-sensor from neonates in real time without a battery [47]. A large-area epidermal electronic system, which covered the entire scalp and the full circumference of the forearm, has been used for multifunctional control of a transhumeral prosthesis [48].
In Vivo Electronic or Optoelectronic Sensing
Designs for in vivo sensing devices are increasingly incorporating supported or layered soft–hard composites. In a number of emerging designs, such as mesh electronics [49], an open-framework polymeric backbone supports the rigid electronic elements such as Si FETs or metal electrodes [49,50]. The soft–hard composites can be syringe-injected into biological tissues or cavities for electrical recording of, for example, local field potentials and single-neuron action potentials from the somatosensory cortex [51], and stable chronic brain mapping for least 8 months at the single-neuron level [52], both of which have been demonstrated in rodents. In one notable example, mesh electronics were injected into mice retinas for chronic retinal recording. The injected mesh electronics expanded inside the eye to cover the retina, without compromising normal eye function, and enabled recording of retinal ganglion cell responses to visual stimuli at single-neuron level for at least 2 weeks in awake mice (Figure 3B) [53].
Polymer-supported Si nanomembrane-based sensors have been developed for various in vivo applications [54–57]. Si nanomembrane-based sensors, composed of thermally grown oxide and capacitively coupled arrays of Si transistors over a polymeric substrate, are capable of cardiac electrophysiology recordings with a low current-leakage level and a long operation lifetime [58]. Furthermore, doped Si nanomembranes can be bound to layers of thermally grown SiO2 to form a stable conductively coupled interface for chronic neural electrophysiology recording [59]. Conversely, the degradation speed of an Si-based system can be controlled for transient bioelectronics and this has been utilized in a bioresorbable pressure sensor that enabled 25-day monitoring of intracranial pressure in rats [60].
Optoelectronic sensors can also be configured into soft–hard composites for biointerfaces. For example, layered structures of gallium/indium compound semiconductors over a plastic substrate have been developed for multimodal neural interfaces, as well as position- and angle-independent wireless power harvesting [61]. The similar principle was utilized in a wireless, injectable fluorescence photometer capable of stable and chronic calcium recording in the deep brain (Figure 3C). Such device is able to record the neuron activities in deep brain at regions of interest in freely moving animals, with a performance comparable with traditional photometry systems based on fiber-optics [62].
Polymeric components, in addition to serving as the soft substrate, can enable scalable device fabrication through printing or drawing [63], such as the preparation of carbon-based composite electrodes for in vivo sensing [64]. Therein, thermal drawing techniques were used to fabricate polymeric probes that composed of carbon-black doped conductive polyethylene, cyclic olefin co-polymer, and polycarbonate (Figure 3D). Such fibers could be utilized for optical stimulation of spinal cord in live mice [64]. The electrical performance of neural recording could be further improved by integrating the polymeric probes with low-melting metals such as tin, through increasing the electrical conductivity [65]. Recent work demonstrated that both high probe strength and toughness could be achieved by controlling the orientation of the polymeric nanofibrils; such a design principle suggests a route to increasing the longevity of neural probes [66].
Soft–Hard Composites for Modulation
In addition to sensing/monitoring, bioelectronic devices play important roles in biological modulation. Implantable microelectrode arrays can deliver neural stimulation, but are traditionally made of metallic microwire or silicon-based rigid materials, which have significantly higher Young’s modulus than the interfacing tissues. This often causes shear-induced inflammation and foreign body reactions [67,68]. Utilization of soft–hard composites allows the hard stimulating materials to be packaged within, or over, soft synthetic components (Figure 2A–C) or biological cells or tissues (Figure 2D,E).
Optogenetic Approach
Optogenetics is a neuromodulation method that combines optics and genetics techniques to control the activity of individual neurons in living tissue, even in free-moving animals [69]. Soft–hard composites for optogenetics can provide the light source or delivery system, such as a light-emitting diode (LED)/laser or optical waveguide. Optogenetic modulation with flexible optoelectronics has enabled numerous advances [61,70–73], such as a closed-loop system for optogenetic peripheral neuromodulation to control bladder functions upon recognizing abnormal bladder voiding patterns (Figure 4A) [74]. The optogenetic components can also be integrated with other functional devices in a single composite. For example, it is possible to integrate a microfluidic drug delivery system with micro-LED arrays to fabricate a wireless optofluidic neural probe [75]. In another example, an optical waveguide used for optogenetics was integrated with six electrodes and two microfluidic channels to achieve long-term, electrophysiological, and optical neural interrogation in a mouse brain [64].
Figure 4. Soft–Hard Composites Can Modulate Biological Activities.
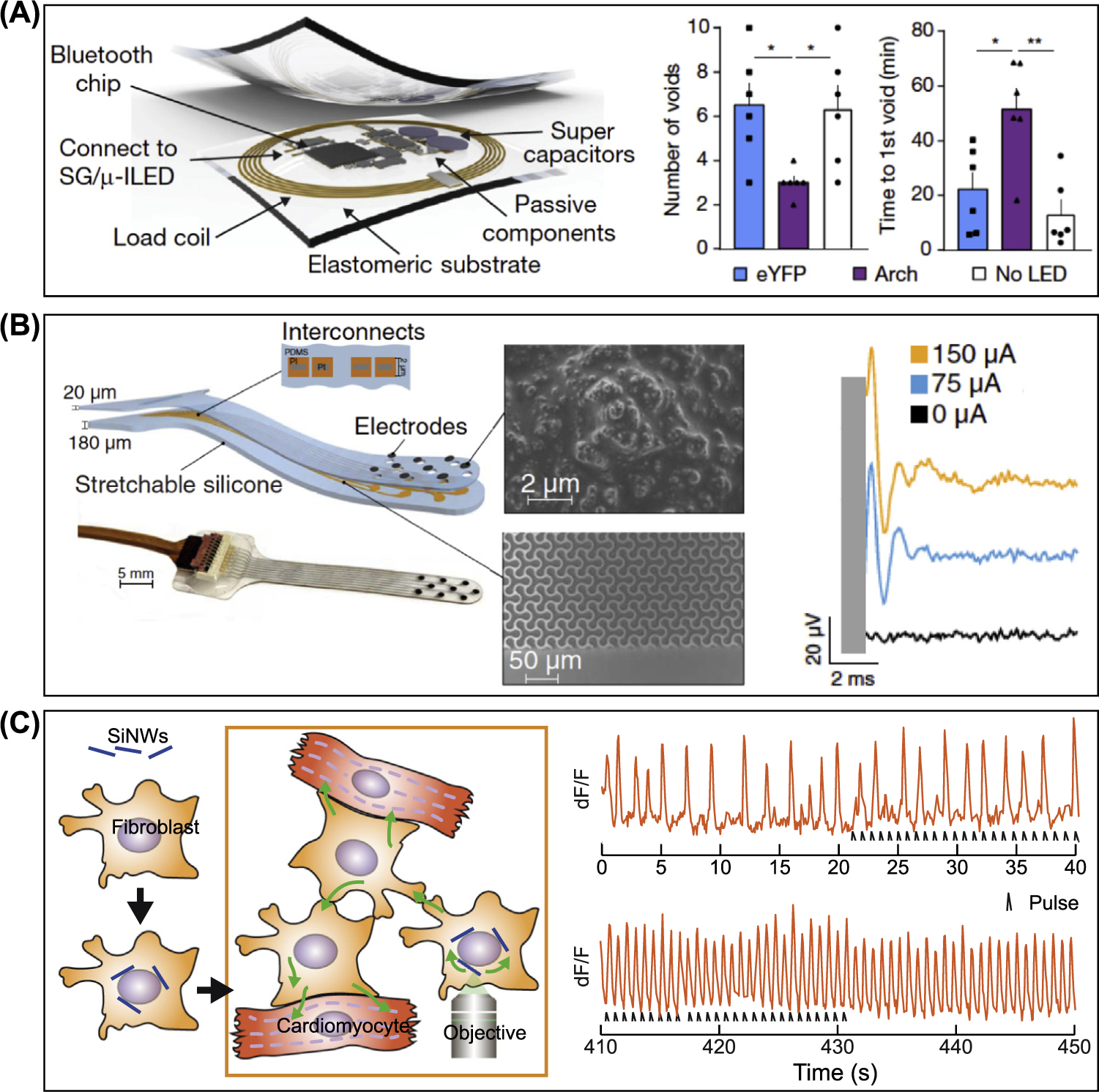
(A) Wireless bio-optoelectronic implant for optogenetic peripheral neuromodulation. (Left) Schematic of the implantable wireless control and power module. (Right) Plot of number of voids after cyclophosphamide injection (3 h later) and plot of the time to formation of the first void following injection in experimental and control groups. Plots demonstrate that the system was capable of recognizing abnormal voiding patterns and conditionally activating the μ-LED to attenuate the increase in voiding induced by cyclophosphamide. The filled squares, triangles, and circles represent the data points; n = 6 rats/group. *P < 0.05, **P < 0.01; two-way analysis of variance with Tukey’s multiple comparison test. Adapted, with permission, from [74]. (B) Soft auditory brainstem implants (ABIs) for evoking auditory neural activity. (Left) Schematics and a photograph of the soft ABI. ABIs are composed of polyimide layers, platinum, and silicone layers. Interconnects formed by polyimide and the platinum are embedded between the silicon layers. (Middle) Scanning electron microscopy images of the Pt-polydimethylsiloxane (PDMS) composite that coats the electrode to decrease the impedance and the microstructured multilayer (polyimide) of the interconnects. (Right) Plot of auditory brainstem response (ABR) waveforms evoked via monopolar electrical stimulation of a single mouse (week 4). Adapted, with permission, from [76]. (C) Myofibroblast-silicon composites for cardiac stimulation. (Left) Schematic of the myofibroblast-silicon nanowire hybrid methodology with Si nanowires (SiNWs) seeded on myofibroblasts. Hybrids can be cocultured with cardiomyocytes or injected into the heart tissue to provide high-resolution photo-modulation. (Right) Plot of dF/F versus time of electrical activity at the spots that were not in direct contact with the hybrid. The initial slow rate of electrical activity gradually increases and synchronizes with the laser pulses, demonstrating that optical modulation of myofibroblast-silicon composites could induce override pacing in cocultured cardiomyocytes. Adapted, with permission, from [81].
Nongenetic Approach
Optogenetics provides a powerful method for remote control of neural activities, but there is a parallel need for non-genetic modulation of biological activities. Our discussion of non-genetic modulation methods focuses on those that utilize advanced materials to deliver local stimulations, such as electrochemical, photothermal, and photoelectrochemical effects.
Direct electrical stimulation has been practiced for decades although progress has been rather slow. Recent efforts include the design of new stretchable electrodes or implantation into specific brain regions for chronic control. One example is the recently demonstrated auditory brainstem implant with stretchable composite electrodes (Figure 4B) [76]. The electrodes are composed of polyimide, platinum, and silicone layers, and they can evoke auditory neural activities. The geometries of the layered soft matrices and the rigid electrodes are usually critical for long-term device performance.
Recently, several studies showed that Si nanostructures and their composites with polymers or cells could be utilized for photothermal [77] or photoelectrochemical [78] modulation of neural or cardiac activities. For instance, coaxial Si nanowires with dopant modulated structures possess photovoltaic properties and photoelectrochemical effect under physiological condition [78]. To form a soft–hard composite, these optically active Si nanowires were assembled over flexible SU-8 mesh substrate. The composite can conform to an isolated heart surface and optically stimulate the heart to beat at a higher target frequency [79]. In a related example, dopant modulated Si membranes, when integrated with holey PDMS, can be implanted over the motor and somatosensory cortex regions and are capable of inducing limb motion in animals upon light stimulation [80].
Besides synthetic polymeric support, Si nanostructures can also be integrated with living cells (Figures 1E and 2E). For example, hybrid Si nanowire/myofibroblasts were recently injected into heart tissues to seamlessly integrate with contractile tissue in vivo for bioelectric studies (Figure 4C) [81]. This study demonstrated that the use of biological cells to ‘encapsulate’ the rigid nanostructures could minimize the inflammatory tissue response [81]. Similar principles can be used for the recently developed cyborg organoids in future implantation and in vivo sensing and recording applications [11]. These biohybrids hold great promise as minimally invasive interfaces to naturally occurring cells and tissues for cell-based therapeutics and diagnosis.
Emerging Soft Materials for Future Bioelectric Interfaces
The synthetic soft components in the current soft–hard composites-based bioelectric interfaces are usually passive, serving mostly as substrate or encapsulation materials, with rare cases showing tissue-like behaviors such as self-healing. When biological components such as cells or tissues are leveraged for building the composites, the intended studies or applications need to consider biocompatibility (e.g., only highly biocompatible semiconductors such as Si nanostructures can form intracellular composites) and the intrinsic biological lifetime. Given these limits, we expect an opportunity of a rapid deployment of advanced soft components in future bioelectronics (see Outstanding Questions). The new soft components can either introduce additional tissue-like functions into the composites, such as adaptability, hemostasis, and motility, or serve as a reservoir for biologics.
Hydrogels
Hydrogels have been used for bioelectric interfaces [82] in the form of hydrogel coatings/encapsulations [83,84], ionically defined circuitries [85,86], conductive nanocomposites [87,88], and conductive polymeric networks [89,90]. Recently, hydrogels that can adhere to diverse substrates have been developed [91], some of which are conductive and hold promise in bioelectronics in vivo [92]. Nevertheless, many advanced properties of hydrogels are yet to be explored for the bioelectric interfaces.
Hydrogels can be tuned in composition and surface chemistry to modulate biological activities. For example, functional proteins can be reversibly patterned within the hydrogels to achieve 4D control over cellular activities [93]. Molecularly engineered hydrogels have also been widely used to support the development and maturation of organoid cultures [94]. Stress relaxation properties were discovered in an alginate-based hydrogel, which significantly influenced stem cell activity; stress relaxation is a key parameter of cell–extracellular matrix (ECM) interaction [95]. These chemical, biological, and mechanical properties in advanced hydrogels are appealing in future bioelectronics, as they can enhance biointegration, trigger tissue regeneration and development, and even modulate biomechanical activities through modulus control or biochemical moiety patterning in the hydrogels (Figure 1D; also see Outstanding Questions).
Hydrogels can be engineered to sense or adapt to biochemical and biomechanical signals. For example, hydrogels hybridized with bacterial cells can be patterned via 3D printing as a living tattoo to sense chemicals on human skin [96]. It is further possible to utilize a stimulus-sensitive, polymer-based microcapsule to encapsulate engineered bacteria for partial lysis at high local density, as programmed for desired application scenarios [97]. For use in gastric devices, a pH-responsive supramolecular gel was developed through chemical modification. While stable in an acidic environment, the gel will dissolve in the small and large intestines, which are in a neutral pH environment [98]. To treat myocardial infarction, a viscoelastic adhesive epicardial patch based on starch hydrogel was recently developed. Such adhesive patch possesses a self-adaptive dynamic stiffness and it can balance the solid and fluidic properties in response to the deformation of heart contraction–relaxation cycles [99]. If integrated as components in bioelectronics, these hydrogels can produce bioresponsive changes and self-adaptability in the devices, which are significantly lacking in the current bioelectric interfaces (Figure 1D; also see Outstanding Questions).
Granular Materials
Tissues, such as human skin, are multicomponent and hierarchical, mechanically heterogeneous and anisotropic, self-healing, impact-absorbing, and dynamically responsive. Despite numerous efforts to develop ECM-like materials using polymeric systems, such as hydrogels, one challenge for tissue-like materials lies in developing a dynamically responsive cell-like building block that can be combined with ECM-like polymer platforms. Recently, it was discovered that dispersion of granular materials in hydrogels produces several dynamic responses to external stress, which is due to strong intergranular interactions and granules-enhanced mechanochemistry [100,101]. Furthermore, soft dendritic microparticle granules have been shown to yield unusual adhesion properties at the interfaces [102]. When integrated with synthetic hydrogel networks, these granule-based composites may be considered better analogs of biological tissues in terms of both the structure (hierarchical assembly of cells and ECM) and the static and dynamic mechanical properties. These granules can enhance the tissue-like behaviors in the soft substrate, matrix, or superstrate that supports the hard electronics elements. The integrated bioelectronics composite may serve as either an adaptable implant that modulates the biomechanical and bioelectric environment (Figure 1D; also see Outstanding Questions), or a humanoid skin element for enhanced human–robot interaction.
Concluding Remarks and Future Perspectives
In this review, we have identified several naturally occurring soft–hard interface designs, which can be used to categorize the existing bioelectric interfaces where soft–hard composites are involved (see Outstanding Questions). Given this commonality, we believe that future bioelectric interfaces can be explored with intentional bioinspired designs (Figure 1). We expect that advanced microfabrication [103–106] could serve as a powerful toolset to produce complex bioinspired soft–hard composites for bioelectronics. Indeed, 3D printing has already produced many bioinspired constructs, such as a flexible armor [107] and voxelated materials with stiffness ranges over several orders of magnitude [108].
Incorporation of tissue-like soft materials into the composites may yield bioelectric devices with new functions, such as treating chronic cutaneous wounds, gastric ulcerative lesions, traumatized skeletal muscle, and peripheral neuronal injuries. Furthermore, new soft–hard bioelectric composites may incorporate various types of chemical, physical (light, electricity, heat, magnetic, tension), and biological stimuli along specific pathways at biointerfaces to complement biophenotypes and/or modulate biosystems locally or systematically. For example, topically implantable tissue-like bioelectric devices may accomplish secretory, rhythmic, or electrophysiological modulation of the functions of multiple tissues/organs, including the endocrine and exocrine glands, and parasympathetic control of the heart, lungs, and digestive tract. New bioelectric composite designs should also consider supramolecular chemistry, biosafety and biocompatibility, environmental concerns and recycling, sterilization, product management and clinical practice, and cost-effectiveness. With these future designs, bioinspired tissue-like soft–hard composites may revolutionize bioelectronic therapy, medical product design, and manufacturing.
Highlights.
The direct application of traditional hard electronics onto soft tissues can cause unstable signal transduction and long-term tissue damage. One solution to this issue is the use of a soft–hard composite to establish the biointerface.
There are several naturally occurring configurations for soft–hard composites, which can be used to categorize the existing bioelectric sensing or modulation devices.
Multilayered configurations have been used frequently in biointerface devices. Biology-driven processes may yield more living soft–hard composites for future bioelectric studies.
Incorporation of tissue-like materials, together with advanced manufacturing, may yield bioelectric devices with new functions and revolutionize bioelectronic therapy, medical product design, and manufacturing.
Box 1. How Do Soft–Hard Composites Establish Bioelectric Interfaces?
Depending on the types of soft–hard composites (rows, Figure 1) and the intended locations of the biointerfaces (columns, Figure 1), mechanisms of the interface formation are different. Tight biointerfaces are desirable for all cases, as the distances between the recording/modulation devices and the biological targets usually affect the amplitude and specificity of the transduced signals. Poor device biointegration can cause fluctuating and unpredictable biointerface geometry or detachment of the devices, yielding unstable signal transduction or even device failure.
On-Skin Devices
Multilayered and homogenous soft–hard composites establish on-skin biointerfaces through physical adhesion (first and second rows, left column, Figure 1). To form a conformal contact between the devices and the skin, it should satisfy [109]:
| [1] |
where γ, , , hrough, and λrough are the effective work of adhesion, effective bending stiffness of device, the plane-strain modulus of the skin, skin roughness amplitude, and wavelength, respectively. Therefore, low bending stiffness device, soft and smooth skin, and strong interfacial adhesion promote conformal contact. In particular, soft materials usually have higher work of adhesion (e.g., 0.2 N/m for silicone/skin interface, versus ~0 N/m for Au/skin interface), thus improving the overall device attachment; the filled and open stars in Figure 1 highlight the local adhesion heterogeneity.
Implants
All soft–hard composites discussed in this review can potentially work as implants, although implantable development-driven living composites (third row, Figure 1) are yet to be demonstrated. For nonliving composites (first and second rows, Figure 1), cellular focal adhesions and extracellular matrix (ECM) can contribute to the device biointegration (in addition to the physical adhesion). For example, when a cell binds to an ECM protein (e.g., fibronectin) that is deposited over the implant surface, integrins aggregate into nascent focal adhesions over the substrate. This leads to the unfolding of cytoskeletal proteins, such as talin and vinculin, located within the adhesion. The protein conformational changes then induce cytoskeletal contractility and traction forces over the focal adhesion, ‘tighten’ the biointerfaces.
For living composites (third and fourth rows, Figure 1), they can also form a special biointerface (i.e., intercellular junctions between cells in the composites and the interfacing cells). These intercellular junctions (e.g., gap junctions, electrical synapses, plasmodesmata) can mediate the communications of chemical or electrical signals in either excitable or nonexcitable tissues. For example, an electrical coupling through gap junctions synchronizes cardiomyocyte contraction.
Devices for In Vitro Cultures
The scenarios are similar to those of implants, except that no inflammable responses are expected.
Acknowledgments
This work is supported by the Air Force Office of Scientific Research (AFOSR FA9550-18-1-0503), the National Science Foundation (NSF 1848613), and the National Institutes of Health (NIH NS101488).
Glossary
- Electrocardiogram (ECG)
with electrodes placed over the skin, ECG yields a noninvasive electrophysiological recording of the electrical activity of the heart. It is typically a graph showing a voltage change over time
- Electroencephalogram (EEG)
with electrodes placed over the scalp, EEG produces a noninvasive recording of the electrical activity of the brain. The recorded voltage fluctuates as a result of the dynamic changes of the ionic currents of the neurons
- Electromyogram (EMG)
measures the electrical activities of skeletal muscle tissues. It can be either noninvasive or invasive, depending on whether the electrodes are placed on the skin surface or insert into the muscle tissues
- Extracellular matrix (ECM)
a dynamic network of hydrated protein-and sugar-based macromolecules. They provide chemical and mechanical cues to cells and tissues. They establish the acellular tissue microenvironment
- Field-effect transistors (FET)
a three-terminal (source, drain, and gate) semiconductor device. The source and drain electrodes are used to inject and collect currents, respectively. The conductivity of the semiconductor channel can be modulated by voltage inputs applied to the gate electrode
- Young’s modulus
Young’s modulus can also be called elastic modulus or modulus of elasticity in tension. It is the ratio of stress-to-strain in the linear elasticity regime, which is usually calculated from the initial slope of a stress-strain curve in a tensile measurement
References
- 1.Feiner R and Dvir T (2017) Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater 3, 1–16 [Google Scholar]
- 2.Cowin SC and Doty SB (2007) Tissue Mechanics, Springer Science & Business Media [Google Scholar]
- 3.Chen R et al. (2017) Neural recording and modulation technologies. Nat. Rev. Mater 2, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegst UGK et al. (2015) Bioinspired structural materials. Nat. Mater 14, 23–36 [DOI] [PubMed] [Google Scholar]
- 5.Yu X et al. (2019) Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 575, 473–479 [DOI] [PubMed] [Google Scholar]
- 6.Xu S et al. (2014) Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 [DOI] [PubMed] [Google Scholar]
- 7.Huang Z et al. (2018) Three-dimensional integrated stretchable electronics. Nat. Electron 1, 473–480 [Google Scholar]
- 8.Reznikov N et al. (2016) A materials science vision of extracellular matrix mineralization. Nat. Rev. Mater 1, 1–14 [Google Scholar]
- 9.Son D et al. (2018) An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol 13, 1057–1065 [DOI] [PubMed] [Google Scholar]
- 10.Gal A et al. (2016) Macromolecular recognition directs calcium ions to coccolith mineralization sites. Science 353, 590–593 [DOI] [PubMed] [Google Scholar]
- 11.Li Q et al. (2019) Cyborg organoids: implantation of nanoelectronics via organogenesis for tissue-wide electrophysiology. Nano Lett. 19, 5781–5789 [DOI] [PubMed] [Google Scholar]
- 12.Liu J et al. (2020) Genetically targeted chemical assembly of functional materials in living cells, tissues, and animals. Science 367, 1372–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faivre D and Schüler D (2008) Magnetotactic bacteria and magnetosomes. Chem. Rev 108, 4875–4898 [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman JF et al. (2016) Cellular uptake and dynamics of unlabeled freestanding silicon nanowires. Sci. Adv 2, e1601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y et al. (2016) Bioresponsive materials. Nat. Rev. Mater 2, 1–17 [Google Scholar]
- 16.Webber MJ and Langer R (2017) Drug delivery by supramolecular design. Chem. Soc. Rev 46, 6600–6620 [DOI] [PubMed] [Google Scholar]
- 17.Kakkar A et al. (2017) Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem 1, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang A and Lieber CM (2016) Nano-bioelectronics. Chem. Rev 116, 215–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Z et al. (2019) Electrically-transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev 119, 478–598 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y et al. (2017) Nature-inspired structural materials for flexible electronic devices. Chem. Rev 117, 12893–12941 [DOI] [PubMed] [Google Scholar]
- 21.Kumari P et al. (2017) Increasing trend of wearables and multimodal interface for human activity monitoring: a review. Biosens. Bioelectron 90, 298–307 [DOI] [PubMed] [Google Scholar]
- 22.Faust O et al. (2018) Deep learning for healthcare applications based on physiological signals: a review. Comput. Methods Prog. Biomed 161, 1–13 [DOI] [PubMed] [Google Scholar]
- 23.Spira ME and Hai A (2013) Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol 8, 83–94 [DOI] [PubMed] [Google Scholar]
- 24.Frank JA et al. (2019) Next-generation interfaces for studying neural function. Nat. Biotechnol 37, 1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian B et al. (2010) Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian B and Lieber CM (2019) Nanowired bioelectric interfaces. Chem. Rev 119, 9136–9152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qing Q et al. (2014) Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat. Nanotechnol 9, 142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Z et al. (2012) Kinked p–n junction nanowire probes for high spatial resolution sensing and intracellular recording. Nano Lett. 12, 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L et al. (2013) Design and synthesis of diverse functional kinked nanowire structures for nanoelectronic bioprobes. Nano Lett. 13, 746–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y et al. (2019) Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotechnol 14, 783–790 [DOI] [PubMed] [Google Scholar]
- 31.Lee J-H et al. (2016) Spontaneous internalization of cell penetrating peptide-modified nanowires into primary neurons. Nano Lett. 16, 1509–1513 [DOI] [PubMed] [Google Scholar]
- 32.Acarón Ledesma H et al. (2019) An atlas of nano-enabled neural interfaces. Nat. Nanotechnol 14, 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi S et al. (2019) High-performance stretchable conductive nanocomposites: materials, processes, and device applications. Chem. Soc. Rev 48, 1566–1595 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y et al. (2019) Organic crystalline materials in flexible electronics. Chem. Soc. Rev 48, 1492–1530 [DOI] [PubMed] [Google Scholar]
- 35.Zhu B et al. (2019) Softening gold for elastronics. Chem. Soc. Rev 48, 1668–1711 [DOI] [PubMed] [Google Scholar]
- 36.Gerratt AP et al. (2015) Elastomeric electronic skin for prosthetic tactile sensation. Adv. Funct. Mater 25, 2287–2295 [Google Scholar]
- 37.Park S et al. (2014) Stretchable energy-harvesting tactile electronic skin capable of differentiating multiple mechanical stimuli modes. Adv. Mater 26, 7324–7332 [DOI] [PubMed] [Google Scholar]
- 38.Chou H-H et al. (2015) A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat. Commun 6, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G et al. (2018) Plasticizing silk protein for on-skin stretchable electrodes. Adv. Mater 30, 1800129. [DOI] [PubMed] [Google Scholar]
- 40.Pang C et al. (2015) Highly skin-conformal microhairy sensor for pulse signal amplification. Adv. Mater 27, 634–640 [DOI] [PubMed] [Google Scholar]
- 41.Lee S et al. (2016) A transparent bending-insensitive pressure sensor. Nat. Nanotechnol 11, 472–478 [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto A et al. (2017) Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol 12, 907–913 [DOI] [PubMed] [Google Scholar]
- 43.Boutry CM et al. (2018) A hierarchically patterned, bioinspired e-skin able to detect the direction of applied pressure for robotics. Sci. Robot 3, eaau6914. [DOI] [PubMed] [Google Scholar]
- 44.Khan Y et al. (2016) Flexible hybrid electronics: direct interfacing of soft and hard electronics for wearable health monitoring. Adv. Funct. Mater 26, 8764–8775 [Google Scholar]
- 45.Norton JJS et al. (2015) Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proc. Natl. Acad. Sci. U. S. A 112, 3920–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y et al. (2016) Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv 2, e1601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung HU et al. (2019) Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian L et al. (2019) Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng 3, 194–205 [DOI] [PubMed] [Google Scholar]
- 49.Dai X et al. (2018) Mesh nanoelectronics: seamless integration of electronics with tissues. Acc. Chem. Res 51, 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong G and Lieber CM (2019) Novel electrode technologies for neural recordings. Nat. Rev. Neurosci 20, 330–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie C et al. (2015) Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater 14, 1286–1292 [DOI] [PubMed] [Google Scholar]
- 52.Fu T-M et al. (2016) Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 [DOI] [PubMed] [Google Scholar]
- 53.Hong G et al. (2018) A method for single-neuron chronic recording from the retina in awake mice. Science 360, 1447–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ning X et al. (2018) Assembly of advanced materials into 3D functional structures by methods inspired by origami and kirigami: a review. Adv. Mater. Interfaces 5, 1800284 [Google Scholar]
- 55.Yoder MA et al. (2018) Semiconductor nanomembrane materials for high-performance soft electronic devices. J. Am. Chem. Soc 140, 9001–9019 [DOI] [PubMed] [Google Scholar]
- 56.Rogers JA et al. (2011) Synthesis, assembly and applications of semiconductor nanomembranes. Nature 477, 45–53 [DOI] [PubMed] [Google Scholar]
- 57.Rogers JA et al. (2010) Materials and mechanics for stretchable electronics. Science 327, 1603–1607 [DOI] [PubMed] [Google Scholar]
- 58.Fang H et al. (2017) Capacitively coupled arrays of multiplexed flexible silicon transistors for long-term cardiac electrophysiology. Nat. Biomed. Eng 1, 0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J et al. (2018) Conductively coupled flexible silicon electronic systems for chronic neural electrophysiology. Proc. Natl. Acad. Sci. U. S. A 115, E9542–E9549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin J et al. (2019) Bioresorbable pressure sensors protected with thermally grown silicon dioxide for the monitoring of chronic diseases and healing processes. Nat. Biomed. Eng 3, 37–46 [DOI] [PubMed] [Google Scholar]
- 61.Gutruf P et al. (2018) Fully implantable optoelectronic systems for battery-free, multimodal operation in neuroscience research. Nat. Electron 1, 652–660 [Google Scholar]
- 62.Lu L et al. (2018) Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proc. Natl. Acad. Sci. U. S. A 115, E1374–E1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loke G et al. (2020) Recent progress and perspectives of thermally drawn multimaterial fiber electronics. Adv. Mater 32, 1904911. [DOI] [PubMed] [Google Scholar]
- 64.Park S et al. (2017) One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci 20, 612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Canales A et al. (2015) Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol 33, 277–284 [DOI] [PubMed] [Google Scholar]
- 66.Liao X et al. (2019) High strength in combination with high toughness in robust and sustainable polymeric materials. Science 366, 1376–1379 [DOI] [PubMed] [Google Scholar]
- 67.Moshayedi P et al. (2014) The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 35, 3919–3925 [DOI] [PubMed] [Google Scholar]
- 68.Nguyen JK et al. (2014) Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng 11, 056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deisseroth K et al. (2006) Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci 26, 10380–10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin G et al. (2017) Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samineni V et al. (2017) Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain 158, 2108–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutruf P and Rogers JA (2018) Implantable, wireless device platforms for neuroscience research. Curr. Opin. Neurobiol 50, 42–49 [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y et al. (2019) Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl. Acad. Sci. U. S. A 116, 21427–21437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mickle AD et al. (2019) A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong J-W et al. (2015) Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vachicouras N et al. (2019) Microstructured thin-film electrode technology enables proof of concept of scalable, soft auditory brainstem implants. Sci. Transl. Med 11, eaax9487. [DOI] [PubMed] [Google Scholar]
- 77.Jiang Y et al. (2016) Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater 15, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parameswaran R et al. (2018) Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol 13, 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parameswaran R et al. (2019) Optical stimulation of cardiac cells with a polymer-supported silicon nanowire matrix. Proc. Natl. Acad. Sci. U. S. A 116, 413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang Y et al. (2018) Rational design of silicon structures for optically controlled multiscale biointerfaces. Nat. Biomed. Eng 2, 508–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rotenberg MY et al. (2019) Living myofibroblast–silicon composites for probing electrical coupling in cardiac systems. Proc. Natl. Acad. Sci. U. S. A 116, 22531–22539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuk H et al. (2019) Hydrogel bioelectronics. Chem. Soc. Rev 48, 1642–1667 [DOI] [PubMed] [Google Scholar]
- 83.Lin S et al. (2016) Stretchable hydrogel electronics and devices. Adv. Mater 28, 4497–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wirthl D et al. (2017) Instant tough bonding of hydrogels for soft machines and electronics. Sci. Adv 3, e1700053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keplinger C et al. (2013) Stretchable, transparent, ionic conductors. Science 341, 984–987 [DOI] [PubMed] [Google Scholar]
- 86.Yang C and Suo Z (2018) Hydrogel ionotronics. Nat. Rev. Mater 3, 125–142 [Google Scholar]
- 87.Balazs AC et al. (2006) Nanoparticle polymer composites: where two small worlds meet. Science 314, 1107–1110 [DOI] [PubMed] [Google Scholar]
- 88.Xu Y et al. (2010) Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4, 4324–4330 [DOI] [PubMed] [Google Scholar]
- 89.Lu B et al. (2019) Pure PEDOT:PSS hydrogels. Nat. Commun 10, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan L et al. (2012) Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. U. S. A 109, 9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yuk H et al. (2019) Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 [DOI] [PubMed] [Google Scholar]
- 92.Inoue A et al. (2020) Strong adhesion of wet conducting polymers on diverse substrates. Sci. Adv 6, eaay5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shadish JA et al. (2019) Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater 18, 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kratochvil MJ et al. (2019) Engineered materials for organoid systems. Nat. Rev. Mater 4, 606–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaudhuri O et al. (2016) Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater 15, 326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu J et al. (2019) High stretchability, strength, and toughness of living cells enabled by hyperelastic vimentin intermediate filaments. Proc. Natl. Acad. Sci. U. S. A 116, 17175–17180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dai Z et al. (2019) Versatile biomanufacturing through stimulus-responsive cell–material feedback. Nat. Chem. Biol 15, 1017–1024 [DOI] [PubMed] [Google Scholar]
- 98.Zhang S et al. (2015) A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater 14, 1065–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin X et al. (2019) A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat. Biomed. Eng 3, 632–643 [DOI] [PubMed] [Google Scholar]
- 100.Peters IR et al. (2016) Direct observation of dynamic shear jamming in dense suspensions. Nature 532, 214–217 [DOI] [PubMed] [Google Scholar]
- 101.James NM et al. (2018) Interparticle hydrogen bonding can elicit shear jamming in dense suspensions. Nat. Mater 17, 965–970 [DOI] [PubMed] [Google Scholar]
- 102.Roh S et al. (2019) Soft dendritic microparticles with unusual adhesion and structuring properties. Nat. Mater 18, 1315–1320 [DOI] [PubMed] [Google Scholar]
- 103.Hu G et al. (2018) Functional inks and printing of two-dimensional materials. Chem. Soc. Rev 47, 3265–3300 [DOI] [PubMed] [Google Scholar]
- 104.Ligon SC et al. (2017) Polymers for 3D printing and customized additive manufacturing. Chem. Rev 117, 10212–10290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parra-Cabrera C et al. (2018) 3D printing in chemical engineering and catalytic technology: structured catalysts, mixers and reactors. Chem. Soc. Rev 47, 209–230 [DOI] [PubMed] [Google Scholar]
- 106.Studart AR (2016) Additive manufacturing of biologically-inspired materials. Chem. Soc. Rev 45, 359–376 [DOI] [PubMed] [Google Scholar]
- 107.Connors M et al. (2019) Bioinspired design of flexible armor based on chiton scales. Nat. Commun 10, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skylar-Scott MA et al. (2019) Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 575, 330–335 [DOI] [PubMed] [Google Scholar]
- 109.Wang S et al. (2012) Mechanics of epidermal electronics. J. Appl. Mech 79, 1022 [Google Scholar]


