Abstract
We report the design and synthesis of the acyclic cucurbit[n]uril–β-cyclodextrin chimeric host H1. The goal of the study is to deepen the cavity of the receptor to allow β-CD complexation of moieties on the guest (especially fentanyl) that protrude from the cavity of the primary acyclic CB[n] binding site to enhance binding affinity and deliver new supramolecular antidotes for fentanyl intoxication. 1H NMR spectroscopy was used to deduce the geometry of the complexes between H1 and H2 and the guest panel (G1 – G8 and fentanyl) whereas isothermal titration calorimetry was used to determine the thermodynamic parameters of complexation. Hosts H1 and H2 retain the essential molecular recognition features of CB[n] receptors, but chimeric host H1 binds slightly stronger toward the guest panel than H2 for reasons that remain unclear. Compared to tetraanionic hosts M1 and M2, the dianionic hosts H1 and H2 are less potent receptors which reflects the importance of electrostatic (ion-ion and ion-dipole) interactions in this series of hosts. The work highlights the challenges inherent in the optimization of binding affinity of hosts as potential supramolecular antidotes.
Keywords: Cucurbit[n]uril, cyclodextrins, molecular recognition, fentanyl, supramolecular antidotes
Table of Contents Graphic
Introduction.
The covalent synthesis and non-covalent self-assembly of molecular container compounds and studies of their molecular recognition properties has occupied a central space in the field of supramolecular chemistry for the past several decades.(1) For example, the design principles for the preparation of metal-organic cages and frameworks by reversible metal•ligand interactions have been delineated, their fundamental host•guest recognition properties studied, and a variety of applications have been demonstrated (e.g. supramolecular nanoreactors, components of sensing arrays, drug delivery vehicles, supramolecular metallo drugs, and materials for separation and sequestration).(2) Within the realm of covalent molecular containers, a variety of classes of compounds (Figure 1) have been studied including cyclodextrins (CD), calixarenes, cyclophanes, cucurbiturils (CB[n]), resorcinarenes and related compounds, and most recently pillararenes.(1b,1f,3) These covalent host systems have been used for a variety of applications including the purification of precious metals, the construction of molecular machines, the preparation of (chiral) stationary phases, as transmembrane ion channels, as household deodorizing products, as supramolecular antidotes, and as critical components of glucose sensors.(4) Most relevant to human health has been the in vivo use of HP-β-CD and Captisol™ as solubilizing excipients for hydrophobic insoluble pharmaceuticals(5) and Sugammadex as a reversal agent for the neuromuscular blockers rocuronium and vecuronium.(6)
Figure 1.
Structures of cyclodextrins and (acyclic) cucurbit[n]urils.
Our research group has been most interested in the synthesis and molecular recognition properties of the CB[n] family of macrocycles (Figure 1).(7) CB[n] are composed of n glycoluril units connected by 2n CH2-groups which define a central hydrophobic cavity flanked by two electrostatically negative ureidyl carbonyl lined portals.(8) Accordingly, CB[n] hosts bind with unusually high affinity and selectivity to hydrophobic diammonium ions with Ka values up to 1017 M−1 in water.(3e,9) CB[n]•guest complexes are, therefore, highly responsive to electrochemical, photochemical, and chemical (e.g. pH or competing guest) stimuli.(10) Accordingly, macrocyclic CB[n] and their derivatives have been used in a variety of applications including as components of sensing arrays, molecular machines, separations materials, supramolecular materials, non-covalent inducers of protein dimerization, as reversal agents, and for (targeted) drug delivery.(4a,10a,11) More recently, we and others,(10d,12) have explored the synthesis and molecular recognition properties of acyclic CB[n]-type receptors (e.g. M1 and M2, Figure 1). Acyclic CB[n]-type receptors are composed of a central glycoluril oligomer, two aromatic sidewalls, and alkoxy chains terminated by sulfonate solubilizing groups. Despite being acyclic, the polycyclic backbone of M1, M2, and analogues preorganizes them into a C-shaped conformation that preserves the essential recognition properties of macrocyclic CB[n] but with more straightforward routes toward synthetic modification.(12e) In a series of papers, we have explored the influence of the glycoluril oligomer length (monomer – pentamer), the aromatic sidewall (e.g. benzene, naphthalene, anthracene, triptycene), the length of the alkylene linking group, and the nature of the ionic group (e.g. carboxylate, sulfonate, ammonium, sulfate) on the molecular recognition properties of acyclic CB[n]-type receptors.(13) By virtue of its high aqueous solubility, acyclic CB[n]-type receptor M1 was shown to be particularly effective as a solubilizing excipient for insoluble drugs for in vivo drug delivery.(13a,14) Conversely, the high affinity displayed by acyclic CB[n]-type receptors toward their guests enables M1 and analogues to function as in vivo reversal agents for the neuromuscular blockers rocuronium and vecuronium which are commonly used by anesthesiologists in the operating room as well as the anesthetics ketamine and etomidate.(15) Most recently, the ability of M1 and M2 to function as in vivo sequestration agents for fentanyl and methamphetamine by complexation of their phenethyl ammonium ion moieties, respectively, have been demonstrated.(16) In this paper, we explore the replacement of the O(CH2)SO3Na groups of acyclic CB[n]-type receptors with β-CD units to deepen the cavity of the receptor and complement regions of guests that protrude from the central acyclic CB[n] cavity as a means to enhance binding affinity toward fentanyl to create an improved supramolecular antidote.
Results and Discussion.
This results and discussion section is organized as follows. First, we describe the design and synthesis of acyclic cucurbituril–cyclodextrin chimeric host H1 along with receptor H2 as comparator. Next, we describe the selection of the guests used in this study along with qualitative investigations of their host•guest binding processes by 1H NMR spectroscopy. Subsequently, we perform direct isothermal titration calorimetry (ITC) titrations to measure the thermodynamic parameters of host•guest binding. Finally, we discuss the results and offer some conclusions.
Design and Synthesis of Host H1.
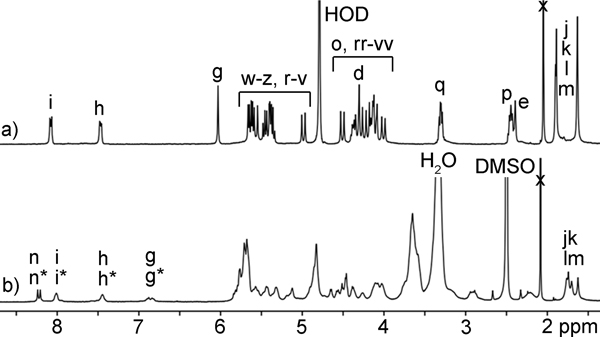
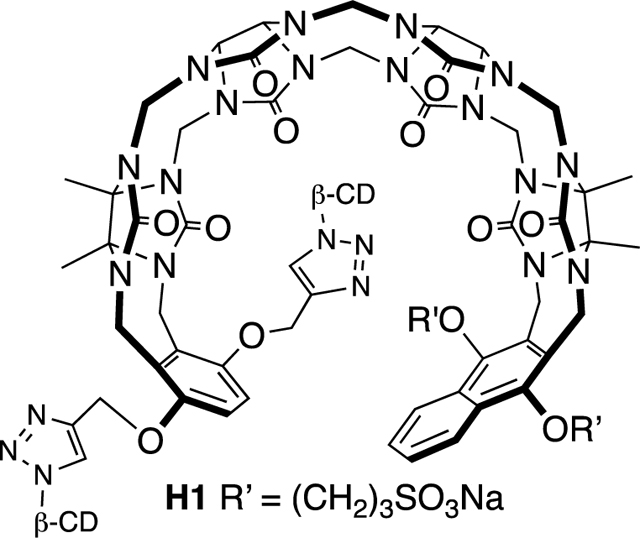
In a previous study we measured the binding of M1 and M2 toward a panel of drugs of abuse and observed tight binding (Ka ≈ 107 M−1) toward fentanyl in 20 mM phosphate buffered water;(16a) follow up in vivo experiments showed that M1 is capable of modulating the physiological effects of fentanyl in Sprague Dawley rats.(16b,17) 1H NMR investigations showed that M1 and M2 bound to the phenethyl ammonium ion binding epitope of fentanyl, whereas the pendant piperidine and amido groups are outside the cavity. Accordingly, as a means to improve binding affinity toward fentanyl and perhaps improve its function as an in vivo sequestration agent we decided to append β-cyclodextrin rings on the arms of the acyclic CB[n] receptor in the form of H1 (Scheme 1) to complement the protruding functional groups of fentanyl. Molecular modelling (Supporting Information, Figure S44) of H1•fentanyl supports the molecular and supramolecular design elements. Synthetically, we allowed glycoluril tetramer (Tet) to react with a mixture of W1 (2 equiv.) and W2 (1 equiv.) in a 1:1 (v:v) mixture of CF3CO2H and Ac2O as solvent at 70 ˚C to promote the envisioned double electrophilic aromatic substitution reactions(13c,18) which delivered H2 in 10% yield. In this reaction we use an excess of W1 with respect to W2 to limit the quantity of M2 formed and enhance the amount of the insoluble byproduct with two W1 walls which allowed H2 to be isolated by recrystallization from H2O / acetone mixtures.(19) Host H2 features a mirror plane passing through its equator but is unsymmetrical from end-to-end due to the different aromatic sidewalls and accordingly is Cs-symmetric. Figure 2a shows the 1H NMR spectrum recorded for H2 in water. As expected based on Cs-symmetry, only 3 aromatic C-H resonances (Hg, Hh, Hi) are observed for H2 at 8.08 (Hi), 7.47 (Hh), and 6.03 (Hg) ppm. The surprisingly upfield shifted resonance for Hg can be explained based on the conformation of H2 in water which features edge-to-face π−π interactions between the tip of the benzene and face of the naphthalene sidewall which places Hg in the anisotropic shielding region of the naphthalene ring. Only two resonances (each integrating to 6H) are observed for the four different Me groups of H2 due to accidental overlap. On the basis of Cs-symmetry, a total of 35 13C NMR resonances would be expected for H2; experimentally, we observe 33 resonances (2 resonances missing due to overlap in the C=O and aromatic region). In the electrospray ionization mass spectrum of H2 we observe an ion at m/z = 1355 which can be assigned to the [H2 – Na]- ion. Having firmly established the structure of H2 we moved on to its transformation into H1. Scheme 1 shows the click reaction between H2 and β-CD-N3 which was carried out in DMSO at 50 ˚C in the presence of CuSO4 and sodium ascorbate as catalyst system. Acyclic cucurbituril–cyclodextrin chimeric host H1 was isolated in 27% yield after purification by silica gel chromatography eluting with acetonitrile–water mixtures. Because the β-CD units of H1 are homochiral and enantiomerically pure, the mirror plane present in H2 is no longer present in H1. Therefore, H1 is C1-symmetric and every proton and every carbon atom in the structure of H1 (C142H198N22Na2O86S2; MW = 3699) is chemically different. The 1H NMR spectrum recorded for H1 in water is broadened, but the spectrum recorded in DMSO-d6 (Figure 2b) is sufficiently sharp to analyze essential features. For example, two singlets are observed at 8.24 and 8.21 ppm which are assigned to the two different triazolyl protons (Hn and Hn*) along with two resonances for Hi / Hi* and Hh / Hh* centered at 8.01 and 7.44 ppm. Protons Hg and Hg* appear as a pair of coupled doublets at 6.88 and 6.84 ppm as expected. The downfield shift of the Hg and Hg* resonances in DMSO-d6 (Figure 2b) relative to that observed for Hg (6.03, Figure 2a) of H1 in D2O is due to differences in cavity solvation where DMSO acts as a guest that changes the orientation of the tips of the aromatic sidewalls. Finally, four methyl resonances are observed (j, k, l, m) at 1.76, 1.74, 1.70, 1.63 ppm as expected which reflects the absence of left-to-right symmetry. The electrospray ionization mass spectrum shows an ion at m/z = 1826 which can be ascribed to the [H1 – 2Na]2- ion. Having established the structures of H1 and H2 we moved on to an examination of their host•guest recognition properties.
Scheme 1.
Synthesis of H1 featuring an acyclic CB[n] core with pendant β-cyclodextrins.
Figure 2.
1H NMR spectra recorded for: a) H2 (400 MHz, D2O, RT), and b) H1 (400 MHz, DMSO-d6, RT).
Qualitative Investigation of the Host Guest properties of H1 and H2.
After completing the synthesis and charaterization of the H1 and H2 hosts, we decided to qualitatively investigate their host•guest recognition properties by 1H NMR spectroscopy. We selected guests G1 – G8 and fentanyl as the members of our guest panel (Figure 3). Guests G1 – G4 were selected because they are commonly investigated with macrocyclic and acyclic CB[n]-type receptors,(9a,20) and differ in the width / length of the hydrophobic moiety between the cationic N-atoms. Compounds G5 – G8 are derivatives of G3 which contain a common central hexanediammonium ion moiety which constitutes an excellent binding domain for acyclic CB[n] hosts and two pendant (CH2)nPh groups (n = 1, 2, 3, 4) that are intended to protrude from the acyclic CB[n] cavity and allow complexation by the pendant β-cyclodextrin rings of H1. Compounds G5 – G7 are known in the literature(21) but the synthetic routes and characterization data were not reported for G6 and G7, whereas G8 is unknown. Compounds G5 – G8 were prepared by the alkylation of N,N,N’,N’-tetramethyl hexanediamine with the appropriate alkyl halide in hot DMF to give G5 – G8 in 50 – 56% yield. Finally, we selected fentanyl as a member of the guest panel because it represents a biologically relevant target that can benefit from the presence of the pendant β-CD cavities of H1.
Figure 3.
Chemical structures of guests G1 – G8 and fentanyl that we investigated in this study.
To qualitatively assess the host-guest recognition properties of H1 and H2 toward the guest panel, we initially collected 1H NMR spectra of H1 or H2 in the presence of 1 equiv. and 2 equiv. of guests G1 – G8. Figure 4 shows the 1H NMR spectra recorded for mixtures of host H1 and guest G2. The 1H NMR spectrum of H1 in D2O is heavily broadened and spin-spin splitting cannot be observed; nonetheless some assignments can be made based on chemical shift and intensity. Interestingly, at a 1:1 H1:G2 ratio (Figure 4c) the spectrum sharpens dramatically indicating a well defined H1•G2 complex. Upon formation of the H1•G2 complex, host resonances Hg and Hg* shift downfield as the edge-to-face π−π interactions between the benzene and naphthalene sidewalls are disrupted upon host•guest complexation.(19) Conversely, the four different host Me groups (j, k, l, m) become well resolved upon formation of the H1•G2 complex. Interestingly, upon formation of the H1•G2 complex, guest protons Hc shift upfield to 6.18 ppm and appear as a pair of coupled doublets which is due to the fact that the top and the bottom of H1 are different. The upfield shifting is consistent with the binding of the p-phenylene unit of G2 inside the anisotropic shielding region of H1. Similarly, two upfield shifted NMe3 resonances (a’ and a’’) are observed upon formation of H1•G2. When an excess of guest G2 is present (Figure 4d), separate sharp resonances are observed for H1•G2 and uncomplexed G2 which indicates that the guest exchange process is slow on the chemical shift timescale. Slow kinetics of guest exchange are generally observed for tighter host•guest complexes which encouraged us to measure the thermodynamics of host•guest complexation by isothermal titration calorimetry as described below. Related 1H NMR spectra were acquired for the remainder of the H1•guest and H2•guest complexes (Supporting Information). In these complexes, we generally observe upfield shifting of the resonances corresponding to the central hydrophobic domain of guests G1 – G8 as they bind inside the anisotropic shielding region of the acyclic CB[n] cavity. Precipitation is observed when preparing mixtures of H1 with G5 – G8 at 2.5 mM concentrations; sufficient concentrations of H1•G5 – H1•G8 remain in solution and 1H NMR spectra were obtained (Supporting Information). This result could mean that the pendant aryl rings do not bind inside the intended β-CD cavity and instead cause intermolecular aggregation. However, we also observed precipitation when preparing host•guest complexes of H2 with G5 – G8 which instead suggests that the complexation of dianionic hosts H1 or H2 with dicationic guests G5 – G8 results in the overall neutral zwitterionic complexes which might be expected to display lower aqueous solubility. Slow to intermediate kinetics of guest exchange on the chemical shift timescale are typically observed for H1 and H2.
Figure 4.
1H NMR spectra recorded (400 MHz, D2O, RT) for: a) H1 (2.5 mM), b) G2 (2.5 mM), c) a mixture of H1 (2.5 mM) and G2 (2.5 mM), and d) a mixture of H1 (2.5 mM) and G2 (5.0 mM).
Determination of the Thermodynamic Parameters for Host•Guest Complexes by Isothermal Titration Microcalorimetry.
After having demonstrated binding of guests G1– G8 into the cavity of H1 and H2 by analysis of the complexation induced changes in chemical shift, we decided to measure the thermodynamic parameters for formation of the complexes. Host•guest complexes of macrocyclic and acyclic CB[n] are often too strong to measure by 1H NMR or even UV/Vis titrations, so we turned to ITC which can be conducted at low μM concentrations and deliver Ka values up to ≈ 107 M−1 reliably by direct ITC titrations. Figure 5a shows the thermogram recorded during the titration of a solution of H1 (50 μM) in the ITC cell with a solution of fentanyl (500 μM) in the ITC injection syringe. In this experiment we used [H1] = 50 μM so as to lower the c-value (c = Ka × [Host]) into the range required for reliable measurements.(22) Figure 5b shows the a plot of ΔH versus the H1:fentanyl molar ratio with was fitted with the PEAQ ITC analysis software to a single set of sites binding model with Ka = (5.30 ± 0.23) × 106 M−1 and ΔH = −10.8 ± 0.04 kcal mol-1. Related direct ITC titrations were performed for the remainder of the host•guest complexes of H1 and H2 with guests G1 – G8 and fentanyl and the values of Ka and ΔH are presented in Table 1. Table 1 also presents the thermodynamic parameters for the complexes between hosts M1 and M2 with guests G1 – G3 and fentanyl drawn from the literature.(13d,16a,20b,23) The Ka values for H1 and H2 fall in the relatively narrow range of 1.72 × 105 M−1 – 1.78 × 107 M-1. Interestingly, H1 is generally (8 out of 9 cases) a more potent than H2 toward a specific guest ranging from a low of 0.78-fold (for G6) to a high of 40-fold (for G4). Clearly the presence of the pendant β-CD rings increase the binding affinity of H1 relative to H2. However, the reasons for this increase remain unclear because one would expect higher Ka values only for guests G5 – G8 and fentanyl which contain pendant aryl rings that protrude from the acyclic CB[n] cavity but not for guests G1 – G4 which does not agree with the experimental results. The potential influence of the inclusion of the hydrophilic sulfonate groups of H1 in the hydrophobic cavities of the adjacent β-CD units on binding thermodynamics is considered unlikely. As expected based on precedent from macrocyclic and acyclic CB[n]-type receptors, the complexation enthalpies (ΔH) are uniformly negative values which reflect the fact that the uncomplexed receptors encapsulate H2O molecules that do not have a full complement of H-bonds which leads to enthalpic gains upon host•guest complexation.(24) The ΔH values for the complexes of H1 with a specific guest are uniformly larger negative values than the corresponding complex of H2. One can also perform a comparison between the complexation abilities of H1 and H2 with the previously studied M1 and M2.(13d,20b,23) Of course, H1 and H2 possess one phenyl wall and one naphthalene wall whereas M1 possesses two phenyl walls and M2 possesses two naphthalene walls. As such, the comparisons should be made with caution. Table 1 shows that M1 and M2 bind slightly more weakly (≈ 0.6-fold) than H1 toward cyclohexanediammonium ion G1 which is not surprising given that G1 is never a tight binder to acyclic CB[n] because the 4 C-atom spacing between N-atoms is not optimal to complement the distance between ureidyl carbonyl portals of the host. For guests G2 and G3, however, host H1 is inferior to hosts M1 and M2 (G2: M1 10-fold and M2 142-fold; G3: M1 29-fold and M2 151-fold). This is not surprising given that M1 and M2 are two of our tightest binding hosts(13a,15a,16) and that H1 and H2 are dianionic hosts whereas M1 and M2 are tetraanionic hosts. The electrostatic component of the binding of macrocyclic and acyclic CB[n]-type receptors is known to make a significant contribution toward the overall binding free energy of the host•guest complexes.
Figure 5.
a) Thermogram obtained during the titration of a solution of H1 (50 μM) in the cell with a solution of fentanyl (500 μM) in the syringe at 298 K in 20 mM sodium phosphate buffered water at pH 7.4. b) Plot of ΔH (kcal mol−1) versus host:guest molar ratio which was fitted to a single set of sites model to extract Ka = (5.30 ± 0.23) × 106 M−1 and ΔH = −10.8 ± 0.04 kcal mol-1.
Table 1.
Thermodynamic parameters (Ka (M−1); ΔH (kcal mol−1)) measured for the host•guest complexes by isothermal titration calorimetry. Conditions: 20 mM sodium phosphate buffered water, pH 7.40, 298 K.
| Guest | Host H1 | Host H2 | Host M1 (16a,23) | Host M2 (13d,16a,20b) | ||||
|---|---|---|---|---|---|---|---|---|
| Ka | ΔH | Ka | ΔH | Ka | ΔH | Ka | ΔH | |
| G1 | (3.02 ± 0.30) × 106 | −7.25 ± 0.070 | (5.26 ± 0.36) × 105 | −4.35 ± 0.059 | (1.95 ± 0.09) × 106 | −5.70 ± 0.027 | (1.77 ± 0.09) × 106 | −5.54 ± 0.03 |
| G2 | (1.78 ± 0.18) × 107 | −9.74 ± 0.073 | (9.20 ± 0.54) × 106 | −7.88 ± 0.035 | (1.78 ± 0.07) × 108 | −11.4 ± 0.022 | (2.53 ± 0.12) × 109 | −14.3 ± 0.04 |
| G3 | (3.02 ± 0.30) × 106 | −7.71 ± 0.050 | (1.46 ± 0.07) × 106 | −5.74 ± 0.036 | (8.93 ± 0.33) × 107 | −9.35 ± 0.021 | (4.59 ± 0.09) × 108 | −10.6 ± 0.15 |
| G4 | (6.99 ± 0.42) × 106 | −7.06 ± 0.034 | (1.72 ± 0.28) × 105 | −3.80 ± 0.031 | n.d. | n.d. | n.d. | n.d. |
| G5 | (4.15 ± 0.25) × 106 | −20.00 ± 0.260 | (1.67 ± 0.29) × 105 | −6.87 ± 0.340 | n.d. | n.d. | n.d. | n.d. |
| G6 | (2.36 ± 0.25) × 105 | −9.30 ± 0.270 | (3.01 ± 0.52) × 105 | −5.62 ± 0.240 | n.d. | n.d. | n.d. | n.d. |
| G7 | (2.84 ± 0.38) × 106 | −8.90 ± 0.120 | (3.14 ± 0.25) × 105 | −3.88 ± 0.077 | n.d. | n.d. | n.d. | n.d. |
| G8 | (3.84 ± 0.82) × 106 | −10.10 ± 0.200 | (2.92 ± 0.18) × 106 | −4.21 ± 0.027 | n.d. | n.d. | n.d. | n.d. |
| Fent- anyl |
(5.30 ± 0.23) × 106 | −10.80 ± 0.040 | (6.81 ± 0.28) × 105 | −5.16 ± 0.038 | 1.1 ± 0.04 × 107 | −20.9 ± 0.06 | (7.6 ± 0.5) × 106 | −20.2 ± 0.07 |
n.d. = not determined
Conclusions.
In summary, we have reported the design and synthesis of acyclic CB[n]-type receptor H1 which contains two pendant β-cyclodextrin rings via click reaction of β-CD-N3 with H2 as a potential route to deepen the cavity of the host and complement pendant groups on the guest (especially fentanyl) that protrude from the cavity of the acyclic CB[n] receptors. The molecular recognition properties of H1 and H2 toward a panel of diammonium ion guests (G1 – G8) and fentanyl were investigated by means of 1H NMR spectroscopy and isothermal titration calorimetry. We find that H1 and H2 retain the essential molecular recognition features of macrocyclic and acyclic CB[n]-type receptors (e.g. the ability to bind to diammonium ions in water with single digit μM to 100 nM dissociation constants). Acyclic CB[n]-cyclodextrin chimeric host H1 binds slightly stronger toward the guest panel than H2 but the origin of the tighter binding cannot be ascribed to the occupation of the β-CD cavities by the pendant functional groups on the guest. Dianionic hosts H1 and H2 are less potent receptors than tetraanionic hosts M1 and M2 which reflects the importance of electrostatic (ion-ion and ion-dipole) interactions on the strength of acyclic CB[n]•guest complexes. In conclusion, we find that the incorporation of β-CD rings into H1 slightly enhances guest binding relative to propargylated H2. However, the removal of the two anionic sulfonate groups that synthetically enabled β-CD attachment by click reaction more than offset the expected gains in binding affinity relative to M1 and M2. The work highlights the challenges inherent in the optimization of binding affinity of hosts as potential supramolecular antidotes.(4a,4j)
Experimental Details.
Compounds Tet, W1, W2, M1, M2 and β-CD-N3 were prepared according to literature procedures.(14,25) NMR spectra were measured on 400 MHz, 500 MHz, and 600 MHz spectrometers (400, 500, 600 MHz for 1H NMR; 100, 126 MHz for 13C NMR) at room temperature in the stated deuterated solvents unless otherwise stated. Low resolution mass spectrometry was performed using a JEOL AccuTOF electrospray instrument.
Host H2.
Compound Tet (1.00 g, 1.28 mmol) was first dissolved in a mixture of CF3CO2H and Ac2O (v:v = 1:1, 30 mL) and heated at 70 ˚C for 5 min. Compounds W1 (0.47 g, 2.56 mmol) and W2 (0.57 g, 1.28 mmol) were added and the reaction mixture was stirred and heated at 70 ˚C for another 12 h. The reaction mixture was poured into acetone (40 mL) to give a yellow precipitate. The precipitate was collected by centrifugation and then dried under high vacuum overnight. The crude solid was recrystallized from water (15 mL) and acetone (30 mL) to yield H2 as a white solid (0.18 g, 0.13 mmol, 10%). M.p. > 310 ˚C. IR (ATR, cm−1): 1722s, 1457s, 1223s, 1179s, 1081m, 795m. ESI-MS: m/z 1355.59, calcd. for [H2-Na]-: 1355.36. 1H NMR (400 MHz, D2O): δ 8.09 (ABq, 2H), 7.47 (ABq, 2H), 6.03 (s, 2H), 5.64 (d, J = 15.7 Hz, 2H), 5.62 (d, J = 15.3 Hz, 2H), 5.56 (d, J = 16.4 Hz, 2H), 5.47 (d, J = 9.0 Hz, 1H), 5.42 (d, J = 15.5 Hz, 2H), 5.39 (d, J = 9.0 Hz, 1H), 5.35 (d, J = 9.0 Hz, 1H), 4.99 (d, J = 16.1 Hz, 2H), 4.51 (d, J = 16.4, 2H), 4.45–4.35 (m, 2H), 4.30 (s, 2H), 4.28 (d, J = 16.0, 2H), 4.20 (d, J = 15.8, 2H), 4.20–4.10 (m, 2H), 4.14 (d, J = 15.4, 2H), 4.01 (d, J = 16.0, 2H), 3.31 (m, 4H), 2.45 (m, 4H), 2.39 (s, 2H), 1.89 (s, 6H), 1.63 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 155.4, 154.3, 154.1, 149.4, 148.3, 128.3, 127.9, 126.6, 122.8, 114.6, 79.9, 77.8, 77.6, 76.4, 76.3, 74.3, 70.5, 67.1, 67.0, 57.6, 52.7, 48.7, 48.5, 48.0, 36.0, 34.4, 30.7, 30.7, 26.5, 25.2, 25.1, 16.9, 16.0, 15.9, 15.7.
Host H1.
Compound H2 (0.020 g, 0.015 mmol) and β-CD-N3 (0.036 g, 0.032 mmol) were dissolved in DMSO (3 mL) and stirred for 5 min. Subsequently, CuSO4•5H2O (9.0 mg, 0.036 mmol) and sodium ascorbate (0.014 g, 0.072 mmol) were added to the solution and the reaction mixture was stirred and heated at 50 ˚C for 24 h. The reaction mixture was poured into acetone (20 mL) to give a yellow precipitate which was isolated by filtration and then dried under high vacuum. The crude solid was purified by column chromatography (SiO2, CH3CN/H2O, 2:3) to give H1 (15.0 mg, 27%) as a white solid. M.p. > 310 ˚C. IR (ATR, cm−1): 3358m, 1727m, 1658m, 1462m, 1026s, 797w. ESI-MS: m/z 1826, calcd. for [H1-2Na]2- 1826. 1H NMR (400 MHz, DMSO-d6): δ 8.24 (s, 1H), 8.20 (s, 1H), 8.01 (m, 2H), 7.44 (m, 2H), 6.88 (d, J = 7.7 Hz, 1H), 6.84 (d, J = 6.7 Hz, 1H), 5.90–5.10 (m, 43H), 5.00–4.75 (br m, 17H), 4.75–3.90 (m, 30H), 3.90–3.40 (m, 40H), 3.05–2.80 (m, 6H), 2.30–2.10 (br, 4H), 1.76 (s, 3H), 1.74 (s, 3H), 1.70 (s, 3H), 1.63 (s, 3H). 13C NMR (150 MHz, DMSO-d6): δ 156.1, 155.0, 154.8, 154.7, 150.7, 150.4, 148.8, 148.6, 143.4, 143.3, 128.7, 128.7, 128.3, 128.0, 127.2 (br), 126.9, 126.4, 123.2 (br), 114.8 (br), 102.7, 102.5 (br), 102.1, 101.9, 83.6, 82.4, 82.1, 81.9, 81.6, 78.1, 77.9, 76.9, 76.8, 74.5 (br), 73.5 (br), 72.9, 72.7, 72.5, 70.9, 70.6, 70.1, 66.8, 63.7, 63.4, 60.4 (br), 59.8, 53.0 (br), 50.5, 48.9, 48.4, 40.9, 36.4 (br), 34.9 (br), 31.1, 26.8, 17.5, 16.5, 16.1.
Supplementary Material
title
Funding. We thank the National Institutes of Health (GM132345) for financial support.
Footnotes
Disclosure Statement. L.I. is an inventor on patents held by the University of Maryland on the biomedical application of acyclic cucurbiturils.
References.
- 1) a).Cram DJ, “The design of molecular hosts, guests, and their complexes (nobel lecture).” Angew. Chem. Int. Ed 1988, 27, 1009–1020; [PubMed] [Google Scholar]; b) Diederich F, “Complexation of neutral molecules by cyclophane hosts.” Angew. Chem., Intl. Ed. Engl 1988, 27, 362–386; [Google Scholar]; c) Lehn J-M, “Supramolecular chemistry – scope and perspectives: Molecules, supermolecules, and molecular devices (nobel lecture).” Angew. Chem., Int. Ed. Engl 1988, 27, 89–112; [Google Scholar]; d) Rebek J, “Molecular behavior in small spaces.” Acc. Chem. Res 2009, 42, 1660–1668; [DOI] [PubMed] [Google Scholar]; e) Yoshizawa M; Klosterman JK; Fujita M, “Functional molecular flasks: New properties and reactions within discrete, self-assembled hosts.” Angew. Chem. Int. Ed 2009, 48, 3418–3438; [DOI] [PubMed] [Google Scholar]; f) Jordan JH; Gibb BC, “Molecular containers assembled through the hydrophobic effect.” Chem. Soc. Rev 2015, 44, 547–585. [DOI] [PubMed] [Google Scholar]
- 2) a).Samanta SK; Isaacs L, “Biomedical applications of metal organic polygons and polyhedra (mops).” Coord. Chem. Rev 2020, 410, 213181; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zarra S; Wood DM; Roberts DA; Nitschke JR, “Molecular containers in complex chemical systems.” Chem. Soc. Rev 2015, 44, 419–432; [DOI] [PubMed] [Google Scholar]; c) Pluth MD; Raymond KN, “Reversible guest exchange mechanisms in supramolecular host-guest assemblies.” Chem. Soc. Rev 2007, 36, 161–171; [DOI] [PubMed] [Google Scholar]; d) Chakrabarty R; Mukherjee PS; Stang PJ, “Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles.” Chem. Rev 2011, 111, 6810–6918; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Furukawa H; Cordova KE; O’Keeffe M; Yaghi OM, “The chemistry and applications of metal-organic frameworks.” Science 2013, 341, 1230444; [DOI] [PubMed] [Google Scholar]; f) Klemes MJ; Ling Y; Ching C; Wu C; Xiao L; Helbling DE; Dichtel WR, “Reduction of a tetrafluoroterephthalonitrile-β-cyclodextrin polymer to remove anionic micropollutants and perfluorinated alkyl substances from water.” Angew. Chem., Int. Ed 2019, 58, 12049–12053. [DOI] [PubMed] [Google Scholar]
- 3) a).Rekharsky MV; Inoue Y, “Complexation thermodynamics of cyclodextrins.” Chem. Rev 1998, 98, 1875–1917; [DOI] [PubMed] [Google Scholar]; b) Gutsche CD, “Calixarenes.” Acc. Chem. Res 1983, 16, 161–170; [Google Scholar]; c) Dale EJ; Vermeulen NA; Juricek M; Barnes JC; Young RM; Wasielewski MR; Stoddart JF, “Supramolecular explorations: Exhibiting the extent of extended cationic cyclophanes.” Acc. Chem. Res 2016, 49, 262–273; [DOI] [PubMed] [Google Scholar]; d) Gassensmith JJ; Baumes JM; Smith BD, “Discovery and early development of squaraine rotaxanes.” Chem. Commun 2009, 6329–6338; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Shetty D; Khedkar JK; Park KM; Kim K, “Can we beat the biotin–avidin pair?: Cucurbit[7]uril-based ultrahigh affinity host–guest complexes and their applications.” Chem. Soc. Rev 2015, 44, 8747–8761; [DOI] [PubMed] [Google Scholar]; f) Assaf KI; Nau WM, “Cucurbiturils: From synthesis to high-affinity binding and catalysis.” Chem. Soc. Rev 2015, 44, 394–418; [DOI] [PubMed] [Google Scholar]; g) Barrow SJ; Kasera S; Rowland MJ; del Barrio J; Scherman OA, “Cucurbituril-based molecular recognition.” Chem. Rev 2015, 115, 12320–12406; [DOI] [PubMed] [Google Scholar]; h) Hof F; Craig SL; Nuckolls C; Rebek J, “Molecular encapsulation.” Angew. Chem., Int. Ed 2002, 41, 1488–1508; [DOI] [PubMed] [Google Scholar]; i) Johnson AM; Wiley CA; Young MC; Zhang X; Lyon Y; Julian RR; Hooley RJ, “Narcissistic self-sorting in self-assembled cages of rare earth metals and rigid ligands.” Angew. Chem. Int. Ed 2015, 54, 5641–5645; [DOI] [PubMed] [Google Scholar]; j) Xue M; Yang Y; Chi X; Zhang Z; Huang F, “Pillararenes, a new class of macrocycles for supramolecular chemistry.” Acc. Chem. Res 2012, 45, 1294–1308; [DOI] [PubMed] [Google Scholar]; k) Wu J-R; Yang Y-W, “New opportunities in synthetic macrocyclic arenes.” Chem. Commun 2019, 55, 1533–1543. [DOI] [PubMed] [Google Scholar]
- 4) a).Yin H; Zhang X; Wei J; Lu S; Bardelang D; Wang R, “Recent advances in supramolecular antidotes.” Theranostics 2021, 11, 1513–1526; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wu H; Jones LO; Wang Y; Shen D; Liu Z; Zhang L; Cai K; Jiao Y; Stern CL; Schatz GC; Stoddart JF, “High-efficiency gold recovery using cucurbit[6]uril.” ACS Appl. Mater. Interfaces 2020, 12, 38768–38777; [DOI] [PubMed] [Google Scholar]; c) Roy I; Limketkai B; Botros YY; Stoddart JF, “Cyclodextrin metal-organic frameworks: From the research laboratory to the marketplace.” Acc. Chem. Res 2020, 53, 2762; [DOI] [PubMed] [Google Scholar]; d) Hong Y; Thirion D; Subramanian S; Yoo M; Choi H; Kim HY; Stoddart JF; Yavuz CT, “Precious metal recovery from electronic waste by a porous porphyrin polymer.” Proc. Natl. Acad. Sci. U. S. A 2020, 117, 16174–16180; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Kassem S; van Leeuwen T; Lubbe AS; Wilson MR; Feringa BL; Leigh DA, “Artificial molecular motors.” Chem. Soc. Rev 2017, 46, 2592–2621; [DOI] [PubMed] [Google Scholar]; f) Si W; Xin P; Li Z-T; Hou J-L, “Tubular unimolecular transmembrane channels: Construction strategy and transport activities.” Acc. Chem. Res 2015, 48, 1612–1619; [DOI] [PubMed] [Google Scholar]; g) Moyer BA; Custelcean R; Hay BP; Sessler JL; Bowman-James K; Day VW; Kang S-O, “A case for molecular recognition in nuclear separations: Sulfate separation from nuclear wastes.” Inorg. Chem 2013, 52, 3473–3490; [DOI] [PubMed] [Google Scholar]; h) Issaq HJ, “The multimodal cyclodextrin bonded stationary phases for high performance liquid chromatography.” J. Liq. Chromatogr 1988, 11, 2131–2146; [Google Scholar]; i) Febreze home page. https://www.febreze.com/en-us (accessed May 5, 2020);; j) Deng C-L; Murkli SL; Isaacs LD, “Supramolecular hosts as in vivo sequestration agents for pharmaceuticals and toxins.” Chem. Soc. Rev 2020, 49, 7516–7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5) a).Stella VJ; Rajewski RA, “Cyclodextrins: Their future in drug formulation and delivery.” Pharm. Res 1997, 14, 556–567; [DOI] [PubMed] [Google Scholar]; b) Stella VJ; Rao VM; Zannou EA; Zia V, “Mechanisms of drug release from cyclodextrin complexes.” Adv. Drug Delivery Rev 1999, 36, 3–16. [DOI] [PubMed] [Google Scholar]
- 6).Bom A; Bradley M; Cameron K; Clark JK; Van Egmond J; Feilden H; MacLean EJ; Muir AW; Palin R; Rees DC; Zhang M-Q, “A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host.” Angew. Chem., Int. Ed 2002, 41, 265–270. [DOI] [PubMed] [Google Scholar]
- 7) a).Freeman WA; Mock WL; Shih N-Y, “Cucurbituril.” J. Am. Chem. Soc 1981, 103, 7367–7368; [Google Scholar]; b) Kim J; Jung I-S; Kim S-Y; Lee E; Kang J-K; Sakamoto S; Yamaguchi K; Kim K, “New cucurbituril homologues: Syntheses, isolation, characterization, and x-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8).” J. Am. Chem. Soc 2000, 122, 540–541; [Google Scholar]; c) Day AI; Arnold AP; Blanch RJ; Snushall B, “Controlling factors in the synthesis of cucurbituril and its homologues.” J. Org. Chem 2001, 66, 8094–8100. [DOI] [PubMed] [Google Scholar]
- 8.) Lee JW; Samal S; Selvapalam N; Kim H-J; Kim K, “Cucurbituril homologues and derivatives: New opportunities in supramolecular chemistry.” Acc. Chem. Res 2003, 36, 621–630. [DOI] [PubMed] [Google Scholar]
- 9) a).Liu S; Ruspic C; Mukhopadhyay P; Chakrabarti S; Zavalij PY; Isaacs L, “The cucurbit[n]uril family: Prime components for self-sorting systems.” J. Am. Chem. Soc 2005, 127, 15959–15967; [DOI] [PubMed] [Google Scholar]; b) Rekharsky MV; Mori T; Yang C; Ko YH; Selvapalam N; Kim H; Sobransingh D; Kaifer AE; Liu S; Isaacs L; Chen W; Moghaddam S; Gilson MK; Kim K; Inoue Y, “A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy-entropy compensation.” Proc. Natl. Acad. Sci. U. S. A 2007, 104, 20737–20742; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cao L; Sekutor M; Zavalij PY; Mlinaric-Majerski K; Glaser R; Isaacs L, “Cucurbit[7]uril.Guest pair with an attomolar dissociation constant.” Angew. Chem. Int. Ed 2014, 53, 988–993. [DOI] [PubMed] [Google Scholar]
- 10) a).Ko YH; Kim E; Hwang I; Kim K, “Supramolecular assemblies built with host-stabilized charge-transfer interactions.” Chem. Commun 2007, 1305–1315; [DOI] [PubMed] [Google Scholar]; b) Sindelar V; Silvi S; Parker SE; Sobransingh D; Kaifer AE, “Proton and electron transfer control of the position of cucurbit[n]uril wheels in pseudorotaxanes.” Adv. Funct. Mat 2007, 17, 694–701; [Google Scholar]; c) Isaacs L, “Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers.” Acc. Chem. Res 2014, 47, 2052–2062; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Del Barrio J; Horton P; Lairez D; Lloyd G; Toprakcioglu C; Scherman O, “Photocontrol over cucurbit[8]uril complexes: Stoichiometry and supramolecular polymers.” J. Am. Chem. Soc 2013, 135, 11760–11763. [DOI] [PubMed] [Google Scholar]
- 11) a).Dsouza R; Hennig A; Nau W, “Supramolecular tandem enzyme assays.” Chem. Eur. J 2012, 18, 3444–3459; [DOI] [PubMed] [Google Scholar]; b) Lee D-W; Park K; Banerjee M; Ha S; Lee T; Suh K; Paul S; Jung H; Kim J; Selvapalam N; Ryu S; Kim K, “Supramolecular fishing for plasma membrane proteins using an ultrastable synthetic host-guest binding pair.” Nat. Chem 2011, 3, 154–159; [DOI] [PubMed] [Google Scholar]; c) Li W; Bockus AT; Vinciguerra B; Isaacs L; Urbach AR, “Predictive recognition of native proteins by cucurbit[7]uril in a complex mixture.” Chem. Commun 2016, 52, 8537–8540; [DOI] [PubMed] [Google Scholar]; d) Liu J; Lan Y; Yu Z; Tan CSY; Parker RM; Abell C; Scherman OA, “Cucurbit[n]uril-based microcapsules self-assembled within microfluidic droplets: A versatile approach for supramolecular architectures and materials.” Acc. Chem. Res 2017, 50, 208–217; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) McCune JA; Mommer S; Parkins CC; Scherman OA, “Design principles for aqueous interactive materials: Lessons from small molecules and stimuli-responsive systems.” Adv. Mater 2020, 32, 1906890; [DOI] [PubMed] [Google Scholar]; f) Tian J; Chen L; Zhang D-W; Liu Y; Li Z-T, “Supramolecular organic frameworks: Engineering periodicity in water through host-guest chemistry.” Chem. Commun 2016, 52, 6351–6362; [DOI] [PubMed] [Google Scholar]; g) Dang DT; Nguyen HD; Merkx M; Brunsveld L, “Supramolecular control of enzyme activity through cucurbit[8]uril-mediated dimerization.” Angew. Chem., Int. Ed 2013, 52, 2915–2919; [DOI] [PubMed] [Google Scholar]; h) Chen Y; Zhang Y-M; Liu Y, “Molecular selective binding and nano-fabrication of cucurbituril/cyclodextrin pairs.” Isr. J. Chem 2011, 51, 515–524; [Google Scholar]; i) Liu Y-H; Zhang Y-M; Yu H-J; Liu Y, “Cucurbituril-based biomacromolecular assemblies.” Angew. Chem. Int. Ed 2021, 60, 3870–3880. [DOI] [PubMed] [Google Scholar]
- 12) a).Stancl M; Hodan M; Sindelar V, “Glycoluril trimers: Selective synthesis and supramolecular properties.” Org. Lett 2009, 11, 4184–4187; [DOI] [PubMed] [Google Scholar]; b) Stancl M; Gilberg L; Ustrnul L; Necas M; Sindelar V, “Synthesis and supramolecular properties of glycoluril tetramer.” Supramol. Chem 2014, 26, 168–172; [Google Scholar]; c) Mao D; Liang Y; Liu Y; Zhou X; Ma J; Jiang B; Liu J; Ma D, “Acid-labile acyclic cucurbit[n]uril molecular containers for controlled release.” Angew. Chem. Int. Ed 2017, 41, 12614–12618; [DOI] [PubMed] [Google Scholar]; d) Prabodh A; Bauer D; Kubik S; Rebmann P; Klaerner FG; Schrader T; Delarue Bizzini L; Mayor M; Biedermann F, “Chirality sensing of terpenes, steroids, amino acids, peptides and drugs with acyclic cucurbit[n]urils and molecular tweezers.” Chem. Commun 2020, 56, 4652–4655; [DOI] [PubMed] [Google Scholar]; e) Ganapati S; Isaacs L, “Acyclic cucurbit[n]uril-type receptors: Preparation, molecular recognition properties and biological applications.” Isr. J. Chem 2018, 58, 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13) a).Zhang B; Isaacs L, “Acyclic cucurbit[n]uril-type molecular containers: Influence of aromatic walls on their function as solubilizing excipients for insoluble drugs.” J. Med. Chem 2014, 57, 9554–9563; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sigwalt D; Moncelet D; Falcinelli S; Mandadapu V; Zavalij PY; Day A; Briken V; Isaacs L, “Acyclic cucurbit[n]uril-type molecular containers: Influence of linker length on their function as solubilizing agents.” ChemMedChem 2016, 11, 980–989; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gilberg L; Zhang B; Zavalij PY; Sindelar V; Isaacs L, “Acyclic cucurbit[n]uril-type molecular containers: Influence of glycoluril oligomer length on their function as solubilizing agents.” Org. Biomol. Chem 2015, 13, 4041–4050; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Brady KG; Gilberg L; Sigwalt D; Bistany-Riebman J; Murkli S; Klemm J; Kulhanek P; Sindelar V; Isaacs L, “Conformationally mobile acyclic cucurbit[n]uril-type receptors derived from an s-shaped methylene bridged glycoluril pentamer.” Supramol. Chem 2020, 32, 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Ma D; Hettiarachchi G; Nguyen D; Zhang B; Wittenberg JB; Zavalij PY; Briken V; Isaacs L, “Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals.” Nat. Chem 2012, 4, 503–510. [DOI] [PubMed] [Google Scholar]
- 15) a).Ma D; Zhang B; Hoffmann U; Sundrup MG; Eikermann M; Isaacs L, “Acyclic cucurbit[n]uril-type molecular containers bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo.” Angew. Chem. Int. Ed 2012, 51, 11358–11362; [DOI] [PubMed] [Google Scholar]; b) Haerter F; Simons JCP; Foerster U; Moreno Duarte I; Diaz-Gil D; Ganapati S; Eikermann-Haerter K; Ayata C; Zhang B; Blobner M; Isaacs L; Eikermann M, “Comparative effectiveness of calabadion and sugammadex to reverse non-depolarizing neuromuscular-blocking agents.” Anesthesiology 2015, 123, 1337–1349; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Diaz-Gil D; Haerter F; Falcinelli S; Ganapati S; Hettiarachchi GK; Simons JCP; Zhang B; Grabitz SD; Duarte IM; Cotten JF; Eikermann-Haerter K; Deng H; Chamberlin NL; Isaacs L; Briken V; Eikermann M, “A novel strategy to reverse general anesthesia by scavenging with the acyclic cucurbit[n]uril-type molecular container calabadion 2.” Anesthesiology 2016, 125, 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16) a).Ganapati S; Grabitz SD; Murkli S; Scheffenbichler F; Rudolph MI; Zavalij PY; Eikermann M; Isaacs L, “Molecular containers bind drugs of abuse in vitro and reverse the hyperlocomotive effect of methamphetamine in rats.” ChemBioChem 2017, 18, 1583–1588; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Thevathasan T; Grabitz SD; Santer P; Rostin P; Akeju O; Boghosian JD; Gill M; Isaacs L; Cotton JF; Eikermann M, “Calabadion 1 selectively reverses respiratory and central nervous system effects of fentanyl in a rat model.” Br. J. Anaesth 2020, 125, e140–e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Leonard MZ; Rostin P; Hill KP; Grabitz SD; Eikermann M; Miczek KA, “The molecular-container calabadion-2 prevents methamphetamine-induced reinstatement in rats: A potential approach to relapse prevention?” International J. Neuropsychopharmacology 2020, 23, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Sijbesma RP; Kentgens APM; Lutz ETG; van der Maas JH; Nolte RJM, “Binding features of molecular clips derived from diphenylglycoluril.” J. Am. Chem. Soc 1993, 115, 8999–9005. [Google Scholar]
- 19).Zhang M; Sigwalt D; Isaacs L, “Differentially functionalized acyclic cucurbiturils: Synthesis, self-assembly and cb[6]-induced allosteric guest binding.” Chem. Commun 2015, 51, 14620–14623. [DOI] [PubMed] [Google Scholar]
- 20) a).Xue W; Zavalij PY; Isaacs L, “Acyclic cucurbit[n]uril type receptors: Secondary versus tertiary amide arms.” Supramol. Chem 2019, 31, 685–694; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Murkli S; Klemm J; King D; Zavalij PY; Isaacs L, “Acyclic cucurbit[n]uril‐type receptors: Aromatic wall extension enhances binding affinity, delivers helical chirality, and enables fluorescence sensing.” Chem. Eur. J 2020, 26, 15249–15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21) a).Cavallito CJ; Gray AP; Spinner EE, “Bis-amamonium salts. Derivatives of fluorene, carbazole, and phenothiazine.” J. Am. Chem. Soc 1954, 76, 1862–1866; [Google Scholar]; b) Gerlach FD; Luellmann H; Ohnesorge FK; Wassermann O, “Anticholinergic effect and structure relations in alkane-α-ω-bisammonium compounds.” Arzneim.-Forsch 1970, 20, 751–754; [PubMed] [Google Scholar]; c) Luellmann H; Ohnesorge FK; Tonner HD; Wassermann O; Ziegler A, “Influence of alkane-bis-onium compounds upon the activity of the acetylcholinesterase and upon its inhibition by diisopropylphosphorofluoridate.” Biochem. Pharmacol 1971, 20, 2579–2586. [DOI] [PubMed] [Google Scholar]
- 22) a).Wiseman T; Williston S; Brandts JF; Lin L-N, “Rapid measurement of binding constants and heats of binding using a new titration calorimeter.” Anal. Biochem 1989, 179, 131–137; [DOI] [PubMed] [Google Scholar]; b) Broecker J; Vargas C; Keller S, “Revisiting the optimal c value for isothermal titration calorimetry.” Anal. Biochem 2011, 418, 307–309. [DOI] [PubMed] [Google Scholar]
- 23).Xue W; Zavalij PY; Isaacs L, “Triazole functionalized acyclic cucurbit[n]uril-type receptors: Host·guest recognition properties.” Org. Biomol. Chem 2019, 17, 5561–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24) a).Biedermann F; Uzunova VD; Scherman OA; Nau WM; De Simone A, “Release of high-energy water as an essential driving force for the high-affinity binding of cucurbit[n]urils.” J. Am. Chem. Soc 2012, 134, 15318–15323; [DOI] [PubMed] [Google Scholar]; b) Biedermann F; Nau WM; Schneider H-J, “The hydrophobic effect revisited-studies with supramolecular complexes imply high-energy water as a noncovalent driving force.” Angew. Chem. Int. Ed 2014, 53, 11158–11171. [DOI] [PubMed] [Google Scholar]
- 25) a).Bi J; Zeng X; Tian D; Li H, “Temperature-responsive switch constructed from an anthracene-functionalized pillar[5]arene-based host−guest system.” Org. Lett 2016, 18, 1092–1095; [DOI] [PubMed] [Google Scholar]; b) Jiang W; Liu Y; Yu C; Li S; Li Y; Zhou Y, “Light-triggered reversible “one-to-two” morphological transition in a “latent double-amphiphilic” linear-hyperbranched supramolecular block copolymer.” Chem. Commun 2016, 52, 8223–8226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.