Abstract
Necrotizing enterocolitis (NEC) is a devastating disease affecting premature newborns with no known cure. Up to half of survivors subsequently exhibit cognitive impairment and neurodevelopmental defects. We created a novel probiotics delivery system in which the probiotic Lactobacillus reuteri (Lr) was induced to form a biofilm [Lr (biofilm)] by incubation with dextranomer microspheres loaded with maltose (Lr-DM-maltose). We have previously demonstrated that a single dose of the probiotic Lr administered in its biofilm state significantly reduces the incidence of NEC and decreases inflammatory cytokine production in an animal model of the disease. The aim of our current study was to determine whether a single dose of the probiotic Lr administered in its biofilm state protects the brain after experimental NEC. We found that rat pups exposed to NEC reached developmental milestones significantly slower than breast fed pups, with mild improvement with Lr (biofilm) treatment. Exposure to NEC had a negative effect on cognitive behavior, which was prevented by Lr (biofilm) treatment. Lr administration also reduced anxiety-like behavior in NEC-exposed rats. The behavioral effects of NEC were associated with increased numbers of activated microglia, decreased myelin basic protein (MBP), and decreased neurotrophic gene expression, which were prevented by administration of Lr (biofilm). Our data indicate early enteral treatment with Lr in its biofilm state prevented the deleterious effects of NEC on developmental impairments.
Keywords: Necrotizing enterocolitis (NEC), Cognitive function, Learning, Memory, Probiotics, Lactobacillus reuteri
Highlights
-
•
Early treatment with Lr in its biofilm state improves cognitive function in pups that survive experimental NEC.
-
•
Lr in its biofilm state reduces microglia activation and MBP loss, and maintains memory and learning-related gene expression.
-
•
Administration of Lr in its biofilm state protects the brain, as well as intestines, during experimental NEC.
1. Introduction
Necrotizing enterocolitis (NEC) is one of the most common and devastating diseases in neonates, occurring in approximately 10% of low birthweight, premature infants. 50%–70% of extremely low birth weight babies with NEC will require surgical intervention, with mortality rates as high as 30%. There has been no significant decrease in the incidence or mortality of NEC over the last three decades (Neu and Walker, 2011; Rees et al., 2007; Seeman et al., 2016; Thyoka et al., 2012). Of those infants who survive NEC, about 45% will have poor neurodevelopmental outcomes, such as cerebral palsy or cognitive, psychomotor, visual or auditory impairment at 18–24 months of age (Rees et al., 2007; Robinson et al., 2018). Studies on school aged children between 6 and 13 years of age with a history of NEC have revealed generally lower cognitive abilities, educational achievement, and poor mental health compared to gestational age-matched NICU patients without NEC (Robinson et al., 2018; Roze et al., 2011). The exact etiology of this neurodevelopmental impairment remains unclear but is considered multifactorial (Niemarkt et al., 2019).
Factors leading to poor neurodevelopmental outcomes associated with NEC are complicated. Premature gut, hypoxia-ischemia, formula feeding and microbial dysbiosis can all contribute to the long-term adverse effects (Niemarkt et al., 2019). Several lines of evidence suggest a connection between gut microbiota and brain function in a bidirectional gut-brain axis, which regulates the communication between the enteric nervous system (ENS) and the central nervous system (CNS) (Lu and Claud, 2019; Moschopoulos et al., 2018). Altered gut bacterial composition, or dysbiosis, not only plays an important role in disease development and prognosis, but is also associated with the pathogenesis and long-term adverse neurological outcomes of preterm infants after NEC or sepsis (Niemarkt et al., 2019; Underwood and Sohn, 2017). There are several possible mechanisms related to the role of the microbiota in brain development and function, including the production or modulation of metabolites. For example, the microbiota influence the production of neurotransmitters, such as serotonin, melatonin, gamma-amino butyric acid, and dopamine (Ehrlich et al., 2015; Sarro et al., 2014). In addition, microbial-produced short-chain fatty acids (SCFAs) are well known for their ability to support the intestinal epithelial barrier and have been implicated in several neurological diseases (Lu and Claud, 2019). Altered levels of SCFAs, including butyrate, propionate, and acetate, have been observed during NEC, and likely lead to increased epithelial and blood brain barrier permeability (Niemarkt et al., 2019). The gut microbiota also plays a prominent role in immune activation, which is essential for the development of NEC.
Necrotizing enterocolitis involves excessive intestinal inflammation marked by the release of pro-inflammatory cytokines and mediators, including tumor necrosis factor alpha (TNFα), interleukin-6 (IL6), platelet-derived growth factor (PDGF), high-mobility group protein 1 (HMGB-1), and nitric oxide that can lead to neuroinflammation (Rentea et al., 2013). These proinflammatory cytokines can trigger and activate microglia, which are resident macrophages that comprise 10–15% of all cells in the brain and play an important role in immune defense and brain homeostasis (Gehrmann et al., 1995; Ransohoff and Perry, 2009). Recently, it was demonstrated that HMGB1 released by intestinal epithelial cells during experimental NEC activates brain microglia through a toll-like receptor 4 (TLR4)-dependent mechanism (Nino et al., 2018). Microglial activation, which is marked by morphological (e.g., increased cell body size and shortened/thickened dendritic processes) and functional (e.g., increased phagocytosis, antigen presentation, and cytokine production) changes, leads to a loss of oligodendrocyte precursor cells and subsequent reduction in myelination (Nino et al., 2018). Low myelination is largely responsible for the neurodevelopmental sequelae of NEC. This is consistent with clinical observations of significant neurodevelopmental delays and white matter abnormalities in NEC survivors (Strunk et al., 2014; Volpe, 2008, 2019). Interestingly, these abnormalities are most pronounced in infants with NEC-induced sepsis, who have significantly more white matter abnormalities compared to infants with NEC, but not sepsis (Shah et al., 2008). Taken together, gut microbes are regarded as a potential therapeutic target to relieve the long-term, detrimental effects of NEC.
Probiotics are live microorganisms that when administered in adequate amounts confer a health benefit on the host (Swanson et al., 2020). Multiple studies demonstrate the ability of probiotics to affect intestinal physiology and inflammation, and oral probiotic administration has been successfully used to prevent and treat NEC. Studies in our laboratories (Olson et al., 2016, 2018) and others (Patel and Underwood, 2018) have demonstrated the protective effect of probiotics in NEC by down-regulating pro-inflammatory cytokine gene expression as well as up-regulating anti-inflammatory cytokine gene expression (Olson et al., 2018; Underwood et al., 2014; Wickramasinghe et al., 2015), improving competition with detrimental microbes (Martinez et al., 2013), and inducing maturation and improved function of the gut epithelial barrier (Patel et al., 2012). In addition to these experimental animal studies, randomized trials have been performed using different strains of probiotics to prevent and treat NEC. The overall results support the beneficial effect of probiotics, however, due to the variety of probiotic strains, formulations, timing of administration, and quality control standards used, there are inconsistencies between different trials, and there are limited follow-up studies on long-term outcomes of infants with NEC (Patel and Underwood, 2018). Although probiotics have been shown to reduce anxiety-like behavior, the stress response, and other psychological disorders in both the clinical setting and in animal studies (Bravo et al., 2011; Cryan and Dinan, 2012), it is not yet known whether probiotics can prevent the deleterious effects of NEC on infant neurodevelopment. We have previously shown that a single dose of Lr delivered in its biofilm state, but not in its free-living planktonic state, protects the intestine from NEC (Olson et al., 2018). Thus, we assessed neurodevelopmental milestones and cognitive behaviors to determine whether the probiotic Lr (either in a planktonic or biofilm state) prevents the long-term neurodevelopmental consequences of NEC.
2. Materials and methods
2.1. Generation of L. reuteri in its biofilm state
Preparation of L. reuteri (Lr) in its biofilm state was performed as we have described previously (Olson et al., 2016, 2018). In short, free living planktonic L. reuteri 23272 was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in de Man Rogosa and Sharpe (MRS) broth (Fisher Scientific, Hampton, NH) at 37 °C with 5% CO2. Planktonic Lr (free-living) was pelleted and resuspend in sterile 0.9% saline (1 × 109 CFU/ml). Lr was induced to form a biofilm by incubation with sterile dextranomer microspheres (DM, 20 mg/ml, Sephadex G-25 Superfine, GE Healthcare Bio-Sciences, Piscataway, NJ) pre-equilibrated with 1M maltose for 30 min at room temperature, to form Lr-DM-maltose (biofilm state), resulting in 1 × 109 CFU and 20 mg DM/ml saline. Probiotics in their planktonic and biofilm state, as well as control treatments, were stored in amber-colored Eppendorf tubes to maintain blinding and were administered blindly to rat pups by gastric gavage. Keys to unblinding were only revealed after all experimental results were determined.
2.2. Neonatal rat model of experimental NEC
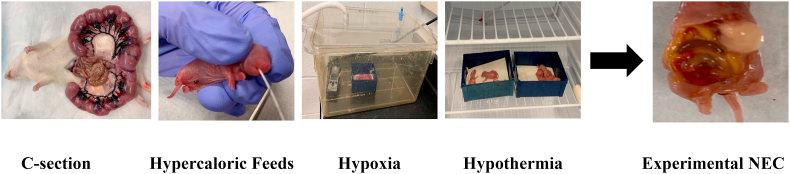
All animal studies were carried out in compliance with the guide for Care and Use of Laboratory Animals of the National Institute of Health. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of The Research Institute at Nationwide Children’s Hospital (protocol #AR15–00012). The well-established neonatal rat model of experimental NEC from our lab has been described previously (Olson et al., 2018). Briefly, neonatal rat pups from timed-pregnant Sprague-Dawley dams (Envigo, Indianapolis, IN) were delivered prematurely via cesarean section at E20.5. Pups were randomized to receive either PBS control (NEC), 0.1 ml of planktonic Lr (1 × 108 CFU/pup) or 0.1 ml of Lr-DM-maltose ((Lr (biofilm), 1 × 108 CFU and 2 mg DM/pup) via orogastric gavage using a 1.9 French PICC-Nate silicone catheter (Utah medical products Inc, Midvale, Utah) into the stomach. To induce NEC, rat pups were exposed to repeated episodes of hypoxia, hypothermia, and hypercaloric feeds over a 96-h time period (Fig. 1). Hypercaloric feeds consisted of 5 daily gavage feedings of increasing volumes of formula containing 15 g Similac 60/40 (Abbott Nutrition, Columbus, OH) in 75 mL of Esbilac (Pet-Ag, New Hampshire, IL) which provided a combined calorie of 836.8 kJ kg−1·day−1. Daily hypercaloric feedings were combined with 3 episodes of hypoxia (<1.5% of oxygen for 90 s) and hypothermia (4 °C environment for 10 min). We (Olson et al., 2016; Pisano et al., 2020; Rager et al., 2016) and others (Good et al., 2014; Miyake et al., 2020; Nino et al., 2018) have found that administration of lipopolysaccharide is essential to achieving an acceptable incidence of NEC in this model. Therefore, pups received gastric gavage of 2 mg/kg lipopolysaccharide (Sigma-Aldrich, St. Louis, MO) on the first day of life. Pups were housed in a temperature (35 °C) and humidity controlled Bistos baby incubator (BT-500, Medical Device Depot, Ellicott City, MD). Pups that survived the 96-h period of stress were placed with a foster dam on day of life 5. Additional control animals were breast-fed (BF) uninjured pups that were not exposed to experimental NEC.
Fig. 1.
Neonatal rat model of experimental NEC. Neonatal rats were delivered prematurely via cesarean section at E20.5. To induce NEC, pups were subjected to repeated episodes of hypercaloric feeds and hypoxia/hypothermia for 96 h.
2.3. Assessment of developmental milestones in rat pups
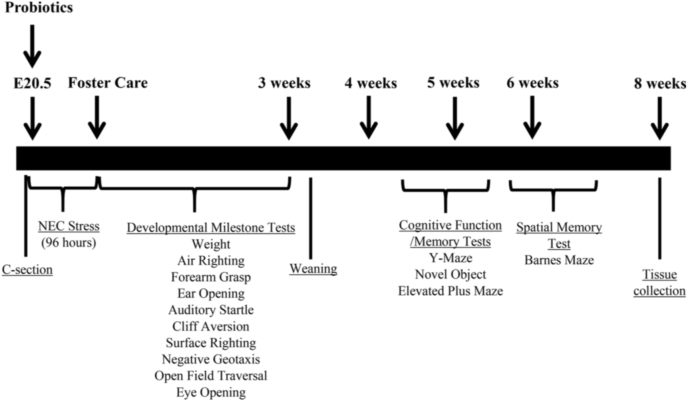
Pups that survived the stress of experimental NEC were placed with a foster dam along with age-matched BF control pups on day 5 of life. Pups were weighed daily and developmental milestones (eye and ear opening, surface and air righting reflexes, strength of forelimb grasp, auditory startle, negative geotaxis, cliff aversion, and open field traversal) assessed for 23 days. Postnatal development after NEC was examined following the timeline shown in Fig. 2. Developmental milestones were measured using modified protocols from previously published methods (Heyser, 2004). All tests were performed in a cell culture hood by investigators who were blinded to the treatments that the pups had received.
Fig. 2.
Study timeline. Neonatal rats were delivered prematurely via cesarean section at E20.5. To induce NEC, pups were subjected to repeated episodes of hypercaloric feeds and hypoxia/hypothermia x 96 h. Surviving pups were placed with foster dams and subjected to developmental milestone testing daily for 23 days. Cognitive function and memory tests were performed between 4 and 8 weeks of age. Rats were sacrificed and tissues collected at 2 months of age.
2.3.1. Air righting
The air righting test measures labyrinthine, body righting and coordination. For this test, a folded 4-layer towel was placed on a heating pad. The rat pup was gently held upside down at approximately 25 cm in height and released onto the towel below (Fig. 3A). The task was considered successfully completed when the pup turned right side up and landed on all four paws on the pad. The test was repeated daily until the pup landed on all four paws for two consecutive days. The day of life at the second consecutive day the test was completed was recorded and used in statistical analyses.
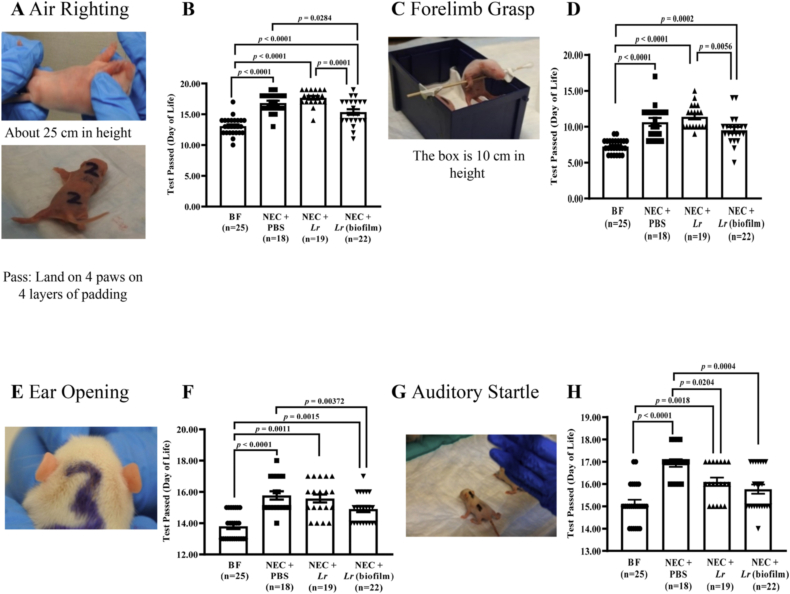
Fig. 3.
Developmental milestones after experimental NEC. Rat pups were exposed to experimental NEC, surviving pups were placed with foster dams, and developmental milestones were measured daily for 23 days. (A, B) air righting test; (C,D) forelimb grasp test; (E,F) ear opening; (G,H) auditory startle. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA).
2.3.2. Forelimb grasp
The forelimb grasp test is a measure of strength. For this test, a Q-tip stick was suspended on top of a box of 10 cm in height containing a folded 4-layer towel and a heating pad underneath. A pup was held with its forepaws resting on the stick and then released (Fig. 3C). The length of time the pup gripped the stick was measured. If the pup fell off immediately, the pup was retested once. Successful completion occurred when the pup held on to the stick for at least 2 s. The test was repeated daily until the pup completed the task on 2 consecutive days. The day of life at the second consecutive day of completion was recorded.
2.3.3. Ear opening and eye opening
Rat pups were examined daily to determine the first day that both ears (Fig. 3E) and eyes were open. For eye and ear opening, once they were open, they stayed open, but for the other developmental milestone tests, the pup may pass accidently, therefore we defined pass as when they pass the tests on 2 consecutive days.
2.3.4. Auditory startle
The auditory startle test assesses the auditory reflex. In this test, rat pups were placed on a pad with a heating pad underneath, in a cell culture hood with the hood blower off. The investigator clapped hands 5 times at a distance of 10 cm from the pup (Fig. 3G). A passing mark was given if the pup responded to the hand clapping with a startle. Auditory startle was tested once daily until the rat pup passed the test on two consecutive days. The day of life at the second consecutive day passed was recorded.
2.3.5. Surface righting
Surface righting tests the rat pup’s labyrinthine and body righting mechanisms, strength, and coordination. Pups were placed in the supine position on a towel with a heating pad underneath. The time to reach a prone position was recorded for each pup (Supplemental Fig. 2A). If the pup was able to flip within 30 s, they were considered successful at the task. Surface righting was measured once daily until the rat pup completed the task on two consecutive days. The day of life at the second consecutive day passed was recorded.
2.3.6. Negative geotaxis
The negative geotaxis test also evaluates labyrinthine and body righting mechanisms, strength, and coordination. To test negative geotaxis, a mouse cage lid was placed at an angle of 35°–45°on top of bedding. Rat pups were placed head down and the time was recorded for the pup to turn 180° to the “head up” position (Supplemental Fig. 2C). If the pup lost footing and slipped to the bottom of the lid, the test was repeated once. Pups were considered successful at the task when they could turn themselves 180° and start to climb within 30 s. Negative geotaxis was measured once daily until the rat pups could right themselves in less than 30 s for two consecutive days. The day of life at the second consecutive day passed was recorded.
2.3.7. Open field traversal
This test measures animal locomotion and the extinguishing of pivoting behavior. Pat pups were placed on the center of a pad and the time required to crawl out of a 12.7 cm radius circle was recorded (Supplemental Fig. 2E). A passing mark was achieved if the rat pup exited the circle within 30 s. Open field traversal was tested once daily until the rat pups could exit the circle in less than 30 s for two consecutive days. The day of life at the second consecutive day passed was recorded.
2.3.8. Cliff aversion
The cliff aversion test evaluates labyrinthine reflex, strength, and coordination in the rat pup. Rat pups were positioned on a flipped pipette box of 10 cm in height with the forepaws and the snout hanging over the edge of the box with a pad underneath (Supplemental Fig. 32G). If the pup fell off the box, the test was not repeated. Pups passed the test when they turned around or crawled away from the edge within 30 s. The cliff aversion test was measured once daily until the rat pup passed the test on two consecutive days. The day of life at the second consecutive day passed was recorded and marked as a passed test.
2.4. Assessment of neurodevelopment, learning, and memory in juvenile rats
After weaning, rats were left undisturbed except for a series of tests to assess developmental milestones, as well as cognitive and anxiety-like behaviors between 4 and 8 weeks of life (Fig. 2). These tests were performed by investigators who were blinded to the treatments that the rats had received. Behavior equipment was cleaned with Process NPD (Steris Corporation, Mentor, OH) between tests to eliminate residual odorant cues.
2.4.1. Y-maze test
The Y-maze assesses spatial learning and reference memory in the animal (Kraeuter et al., 2019b). The apparatus consists of 3 open arms of equal length in the shape of a “Y", with each arm labeled A, B, or C (Fig. 4A). A or B arm is 15 cm long and 5 cm wide while C arm is 20 cm long and x 5 cm wide. 4.5–5.5-week-old rats were placed in the center of the Y maze and allowed to freely explore the maze for 8 min. The location of each rat was recorded for the entire 8 min with a Sony Handycam HDR-CX405 camera (Sony, Japan) and scored for order of arm entries by two independent researchers blinded to treatment groups. An arm entry was recorded when all four paws of the rat entered the arm. Behavior was scored in triads with a unique triad consisting of three different arm choices in succession (e.g. ABC, BCA, ACB, etc.). Unique and non-unique triads were reported as percentages of the number of totals triads recorded. The number of total triads and the % of those triads that were unique were recorded.
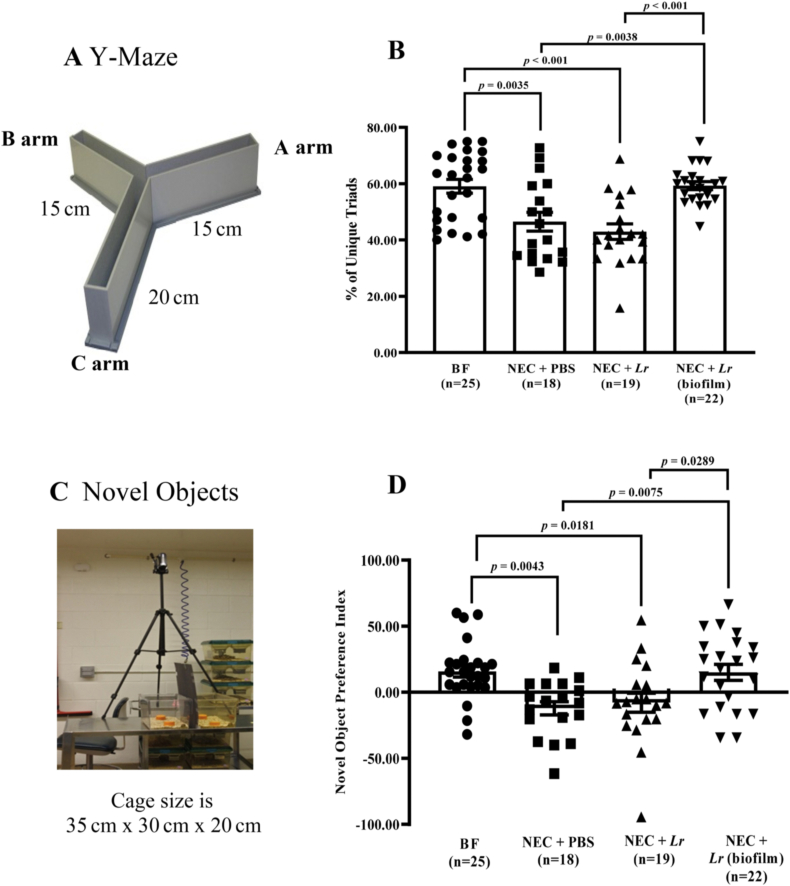
Fig. 4.
Short-term spatial and non-spatial working memory after experimental NEC. Rat pups were subjected to experimental NEC and surviving pups were placed with foster dams. Behavioral activities were measured after weaning. (A) Y-Maze setting; (B) % of unique triads; (C) Novel Objects setting; and (D) novel object preference index. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA).
2.4.2. Novel object recognition test
The novel object recognition test is used to measure non-spatial working memory and recognition memory (Lueptow, 2017). In this test, each rat was placed in a test cage (35 cm × 30 cm x 20 cm in size) containing 2 identical objects (autoclaved caps from a 1L wide mouth glass bottle) on the opposite end of the cage for 10 min, and then returned to its home cage. Rats were left in their home cages for 3 h before returning to the test cage with one original object replaced by a novel object of similar size but different shape (25 ml cell culture flask). The behavior of the rats was recorded for 10 min (Fig. 4C). Videos were scored by two independent researchers in a blinded fashion. The time the rat spent exploring the novel object vs. the original object was recorded, and scores were averaged. If there was a significant difference between the two observers, videos were watched and scored again. The novel object preference was calculated as [(time spent with novel object – time spent with original object)/(time spent with novel object + time spent with original object) x 100].
2.4.3. Barnes maze test
The Barnes maze is used to evaluate spatial learning and memory. The Barnes maze platform was obtained from Harvard Apparatus (Halliston, MA) and consists of a circular (122 cm in diameter) platform that is raised 120 cm off the ground. The platform contains eighteen holes of 10 cm diameter around the edge. The maze is brightly lit and there are three different shaped distal visual cues approximately 20cm2 in size in the area surrounding the platform. One of the holes leads to an enclosed, hidden escape compartment under the maze (Fig. 5A). Since rodents are nocturnal and naturally fearful of brightly lit open spaces and heights, they will seek out and prefer the enclosed compartment of the maze. Rats received 4 training trials per day for 3 consecutive days. During the training trial, the rats were first placed under a plastic cup in the center of the maze for 15 s. After removing the cup, the rats were allowed to explore the maze, and a number of parameters recorded to assess their ability to learn the task, including the latency to locate the escape hole and the number of incorrect holes checked prior to locating the correct hole. If the rats did not find the escape hole within 90 s, they were guided to the escape hole. Two days after the final session of training, the rats underwent three probe trials with a different investigator. To assess the ability of the rats to remember where the previous escape hole was, the latency to find the previous escape hole and the distance traveled prior finding the hole was recorded, along with the amount of time the rats spent in proximity to the correct hole. To avoid bias, an independent researcher timed and recorded results for all three trials performed on the testing day.
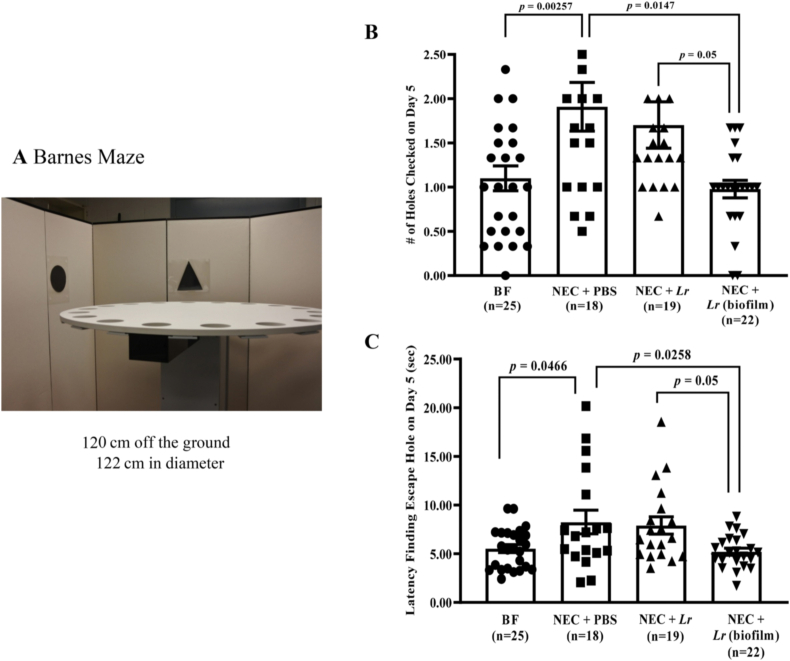
Fig. 5.
Barnes maze test after experimental NEC. Rat pups were subjected to experimental NEC, and surviving pups were placed with surrogate dams. Behavioral activities were measured after weaning. (A) Barnes Maze setting; (B) number of holes checked prior to finding the escape hole; and (C) latency to finding escape hole. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA).
2.4.4. Elevated plus maze test
The elevated plus maze is a validated test for anxiety-like behavior that couples the willingness of rodents to explore novel areas with the aversiveness of brightly lit open areas. The maze consists of two open arms and two closed arms 70 cm off the ground. The closed arm area is 35 cm × 7.5 cm x 20 cm (Fig. 6A). The elevated plus maze was connected to an automatic tracking device and software Med-PC IV (Med Associations, Inc, St Albans, VT). Each rat was put in the middle of the elevated plus maze and its movement was recorded by the tracking software for a total of 10 min.
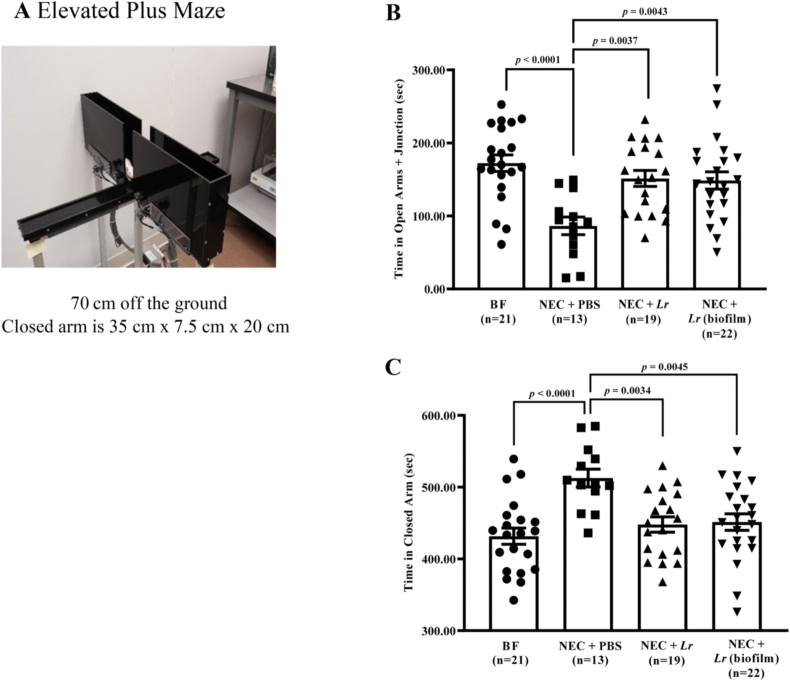
Fig. 6.
Elevated plus maze test after experimental NEC. Rat pups were subjected to experimental NEC, and surviving pups were placed with surrogate dams. Behavioral activities were measured after weaning. (A) elevated plus maze setting; (B) time in open arm and junction; (C) time in closed arm. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA).
2.5. Immunofluorescent staining of the rat brain
In a different cohort of animals, 2-month-old rats were euthanized, and brains were harvested. Where indicated, the hippocampus was separately dissected for analysis with the aid of the rat brain matrix tissue dissection instrument (ASI Instruments, Houston, Texas). Whole brain or hippocampal sections were fixed in freshly made 4% paraformaldehyde for 8–20 h at room temperature followed by cryoprotection in 15% sucrose for 12–18 h and then 30% sucrose for 12–18 h. Tissues were then embedded in OCT cryostat sectioning medium (Fisher Scientific, Hampton, NH) and frozen on dry ice. Frozen tissues were sectioned (5 μm thickness) and tissue sections rehydrated in PBS wash buffer and blocked in buffer containing 2% donkey serum, 0.1% Triton, 0.05% tween 20 for 1 h at room temperature. Samples were incubated with Iba-1 (Wako Chemical, Richmond, VA 1:500) or Myelin Basic Protein (Abcam, Cambridge, MA 1:1000) primary antibody at 4 °C overnight followed by incubation with Fluor488-conjugated donkey anti-rabbit IgG polyclonal secondary antibody (Thermo Fisher 1:1000), and mounted with Vectashield mounting media with DAPI. A minimum of 8 random confocal images were obtained randomly across the whole brain using a Zeiss 800 confocal microscope equipped with Zen 3.0 software (Carl Zeiss Microscopy GmbH, Jena Germany). Microglia from each image were counted manually, and in addition, % positive staining per high powered field (HPF) was measured with Image J software (1.47v NIH, USA). MBP staining densitometry was assessed using Zen 3.0 software, and in addition, % positive staining per high power field was measured with Image J software. Images were evaluated by four different researchers in a blinded fashion, and independent scorings were averaged.
2.6. RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
In a different cohort of animals, 2-month-old rats were euthanized, and brains were harvested. The prefrontal cortex and hippocampus were dissected with the aid of the rat brain matrix tissue dissection instrument (ASI Instruments), and separately stored in RNAlater (Thermo Fisher, Waltham, MA). Tissues (100 mg) were disrupted with 1.0 mm Zirconia beads (Biospec Products, Bartlesville, OK) in a TissueLyser II (Qiagen, Germantown, MD) at 30 Hz for 30 s, repeated three times. Total RNA was isolated using the Purelink RNA mini kit (Thermo Fisher) according to the manufacturer’s instructions. cDNA was produced from 1 μg RNA using the Superscript IV VILO cDNA synthesis kit with ezDNase Enzyme to remove genomic DNA (Thermo Fisher, Waltham, MA). Quantitative real-time PCR (qRT-PCR) was performed in duplicate using primers for rat BDNF and Grin2A (Qiagen), and Power up SYBR Green PCR Master mix (Thermo Fisher) on a Master cycler realplex2 (Eppendorf). Target gene expression was normalized to the average of GAPDH and β-actin endogenous controls and expressed as relative copy number (RCN) using 2(−ΔΔCt). These tests were performed by investigators who were blinded to the treatments that the rats had received.
2.7. Statistical analyses
Data were collected in a blinded fashion. After normality of the dataset and equal variance between groups were confirmed, we analyzed data using a one-way ANOVA with GraphPad Prism 8.2.0 (GraphPad Software, Inc. La Jolla, CA). Error bars represent SEM with statistical significance defined as p ≤ 0.05.
3. Results
3.1. Lr (biofilm) treatment attenuates NEC-induced delays in rat pup developmental milestones
3.1.1. Air righting test
Rat pups exposed to experimental NEC showed reduced labyrinthine, body righting and coordination in the air righting test (p < 0.001 compared with BF pups) (Fig. 3B). Treatment of NEC-exposed pups with planktonic Lr resulted in no improvement in pups turning right side up and landing on all four paws (p<0.001 vs. BF pups). However, treatment with Lr (biofilm) attenuated this NEC-induced delay in development (p = 0.0284 vs. NEC + PBS pups; p = 0.001 vs. planktonic Lr pups).
3.1.2. Forelimb grasp test
Exposure to experimental NEC reduced overall strength in pups gripping the stick for at least 2 s (p<0.0001 vs. BF pups) (Fig. 3D). Although Lr (biofilm) treatment did not recover strength to the BF level, it did significantly improve strength compared to the planktonic Lr group (p = 0.0056).
3.1.3. Ear opening test
Pups exposed to experimental NEC had delayed ear opening (p < 0.0001 vs. BF pups) (Fig. 3F). Lr (biofilm) treatment attenuated the NEC effect (p = 0.00372 vs. NEC + PBS pups), but ear opening was still delayed compared to BF (p = 0.0015).
3.1.4. Auditory reflex test
Exposure to experimental NEC resulted in a delay in the auditory reflex in pups responding to the hand clapping with a startle (p < 0.0001 vs. BF pups). Both planktonic Lr and Lr (biofilm) treatment prevented the delayed auditory reflex (p = 0.0204 and p = 0.0004 vs. NEC + PBS, respectively) (Fig. 3H).
3.1.5. Body weight and additional developmental tests
Probiotic treatment did not improve all aspects of developmental milestones tested. Pups exposed to experimental NEC all had reduced body weight compared to BF pups up to 21 days of life, and this was not improved with administration of planktonic Lr or Lr (biofilm) (Supplemental Fig. 1). Surface righting for pups to flip (labyrinthine and body righting mechanisms, strength, and coordination), negative geotaxis for pups turning 180° to a “head up” position (labyrinthine and body righting mechanisms, strength, and coordination), open field traversal for pups to crawl out 12.7 cm radius circle (locomotion and the extinguishing of pivoting behavior), cliff aversion for pups crawling away from the edge (labyrinthine reflex, strength, and coordination) and eye opening tests did not reveal significant improvement with probiotic administration (Supplemental Figs. 2A–I). Overall, we observed an attenuation of the negative effects of NEC on some, but not all developmental milestone tests.
3.2. Lr (biofilm) treatment promotes short-term working memory in rats exposed to NEC
Learning, memory, risk taking, and exploratory behaviors increase in rat pups as the brain matures. Thus, we next tested neurocognitive function of rats after exposure to experimental NEC using validated tests of learning and memory.
3.2.1. Y-maze test
After weaning from surrogate dams, all pups were left undisturbed for 1.5 weeks. We then assessed learning and spatial memory behavior with the Y-Maze test. Rodents typically seek novel environments, and thus prefer to investigate new arms in the Y-Maze rather than returning to the arm that was previously visited. Rats exposed to NEC had a significantly lower % of unique triads explored (p = 0.0035 vs. BF rats) (Fig. 4B). Planktonic Lr treatment did not enhance the % of unique triads explored, whereas treatment with Lr (biofilm) prevented the effects of NEC on the % of unique triads explored (p = 0.0038 vs. NEC + PBS rats and p < 0.001 vs. planktonic Lr).
3.2.2. Novel objects recognition test
This test was performed after the Y-maze test and was used to assess rodent non-spatial working memory and recognition memory. The novel object preference index revealed that rats exposed to NEC spent less time exploring the novel object compared to BF rats (p = 0.0043 vs. BF rats) (Fig. 4D). Planktonic Lr had no effect, whereas Lr (biofilm) significantly increased the amount of time exploring the novel object (p = 0.0075 vs. NEC + PBS and p = 0.0289 vs. planktonic Lr).
3.2.3. Barnes Maze test
This test was performed to further examine spatial learning and memory ability (Fig. 5B and C). Exposure to experimental NEC did not significantly affect the ability of rats to learn the location of the escape hole during training trials (data not shown). However, exposure to NEC significantly reduced the ability of the rats to remember the location of the hole. NEC rats checked more holes (p = 0.00257 vs. BF rats) and required a longer time to find the escape hole compared with BF rats (p = 0.0466 vs. BF rats) (Fig. 5B and C). Whereas planktonic Lr had no significant effects, Lr (biofilm) prevented the effects of NEC on the number of holes checked (p = 0.0147 vs. NEC + PBS and p = 0.05 vs. planktonic Lr) (Fig. 5B) and latency in finding the escape hole (p = 0.0258 vs. NEC + PBS and p = 0.05 vs. planktonic Lr) (Fig. 5C). Taken together, these results indicate that Lr in its biofilm state enhances spatial and non-spatial short-term working memory after exposure to experimental NEC.
3.3. Lr treatment reduces anxiety in rats exposed to NEC
Since Lr (biofilm) prevented NEC-induced reduction in memory, we next examined anxiety-like behavior after probiotic treatment.
3.3.1. Elevated plus maze test
In this test, rats explore both the open and closed arms of the plus maze, but prefer the relative security of the closed arms. An increased preference for the closed arms is reflective of anxiety-like behavior (Kraeuter et al., 2019a). After exposure to NEC, rats spent less time in the open arms/junction and more time in the closed arms (p<0.0001 vs. BF rats) (Fig. 6bB, 6C). Both planktonic Lr and Lr (biofilm) prevented the effects of NEC on these anxiety-like behaviors with more time spent in open arms/junction (p = 0.0037 for NEC + PBS vs. Lr and p = 0.0043 for NEC + PBS vs. Lr (biofilm).
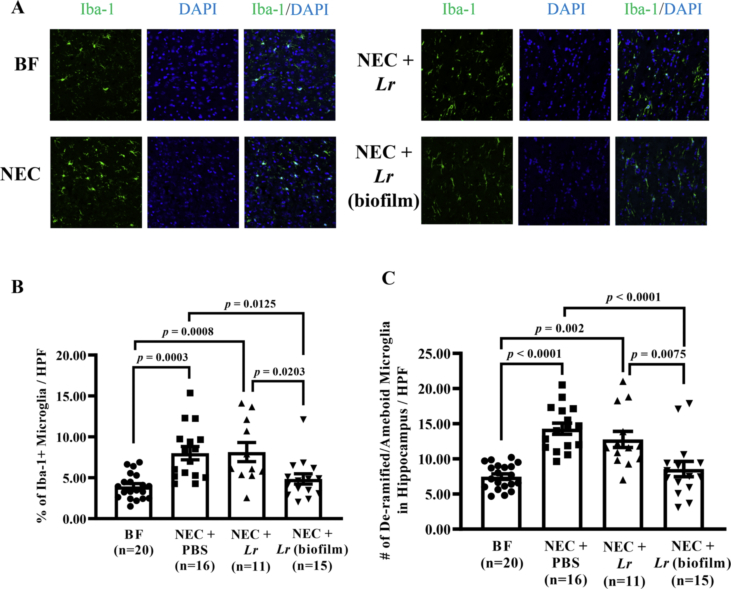
3.4. Lr (biofilm) prevents NEC-induced increases in Iba-1+ microglia activation
To begin to elucidate the pathways leading to NEC-induced impairment in the brain, we first examined microglia activation. Sagittal sections of whole brain were immunostained with an antibody to the calcium-binding adaptor molecule 1 (Iba-1) as a microglia marker, and random images were obtained at 20X magnification across the whole brain (Fig. 7A). Iba-1 protein is specifically and constitutively expressed in all microglia and is widely accepted as a marker for both surveillant (ramified) and activated (de-ramified or ameboid) microglia (Verdonk et al., 2016). Two months after exposure to experimental NEC, there were more Iba-1+ microglia per field of view (p = 0.0003 vs. BF rats) (Fig. 7B). Lr (biofilm) but not planktonic Lr prevented the increased % of Iba-1+ microglia (p = 0.0125 vs. NEC + PBS and p = 0.0203 vs. planktonic Lr). Next, we identified whether there were more microglia with an activated phenotype by counting the number of microglia with increased cell body thickness, reduced/thickened dendritic processes, and/or ameboid shape (collectively referred to as de-ramified/ameboid). Two-month-old rats exposed to experimental NEC at birth had higher numbers of de-ramified/ameboid microglia (p < 0.0001 vs. BF rats). Whereas planktonic Lr had no effect, Lr (biofilm) treatment prevented the NEC-induced increase in the number of de-ramified/ameboid microglia in the brain (p < 0.0001 vs. NEC and p = 0.0075 vs. planktonic Lr) (Fig. 7C).
Fig. 7.
Microglia in whole brain 2 months after experimental NEC. Rat pups were subjected to experimental NEC and surviving pups were placed with surrogate dams. Rats were sacrificed at two months of age, and brains harvested and fixed in 4% PFA. Frozen sections were stained with Iba-1 antibody and Alexa 488 conjugated secondary antibody. At least 8 confocal pictures per section were imaged using 20X objectives. (A) Representative immunohistochemical images of the whole brain; (B) percent of Iba-1+ cells/HPF quantified using Image J software; (C) numbers of de-ramified/ameboid microglia manually counted/HPF. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA).
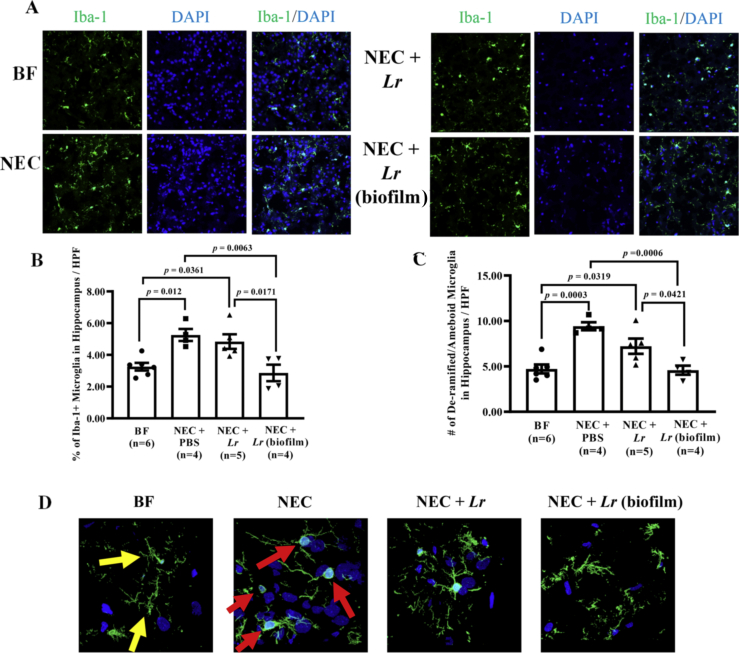
In light of the observed differences in hippocampal-dependent memory, we next focused on microglia in the isolated hippocampus of 2-month-old rats. Sagittal sections of the hippocampus were immunostained for Iba-1, and random images obtained at 20X magnification (Fig. 8A). Similar to the observations in whole brain, % of Iba-1+ microglia and the number of de-ramified/ameboid microglia were significantly increased in rats exposed to experimental NEC as pups (p = 0.012 and p = 0.0003 vs. BF respectively). Administration of Lr in its biofilm state reduced % Iba-1+ microglia and the number of de-ramified/ameboid microglia in the hippocampus (p = 0.0063 and p = 0.0006 vs. NEC + PBS; p = 0.0171 and p = 0.0421 vs. planktonic Lr) (Fig. 8B and C). Microglia morphology was confirmed with higher magnification using 63× objectives (Fig. 8D). These data demonstrate that Lr in its biofilm state reduces the number of activated microglia, which may contribute to the prevention of the detrimental neurological effects caused by NEC.
Fig. 8.
Microglia in hippocampus 2 months after experimental NEC. Rat pups were subjected to experimental NEC and surviving pups were placed with surrogate dams. Rats were sacrificed at two months of age, brains were harvested, and hippocampus sections were fixed in 4% PFA. Frozen sections were stained with Iba-1 antibody and Alexa 488 conjugated secondary antibody. At least 8 confocal pictures per section were imaged using 20X objectives. (A) Representative immunohistochemical images of the hippocampus; (B) percent of Iba-1+ cells/HPF quantified using Image J software; (C) numbers of de-ramified/ameboid microglia manually counted/HPF. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA). (D) Representative images using 63X objectives. Yellow arrows, elongated dendritic cell processes of ramified (non-activated) microglia; red arrows, de-ramified and ameboid cell bodies of activated microglia. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
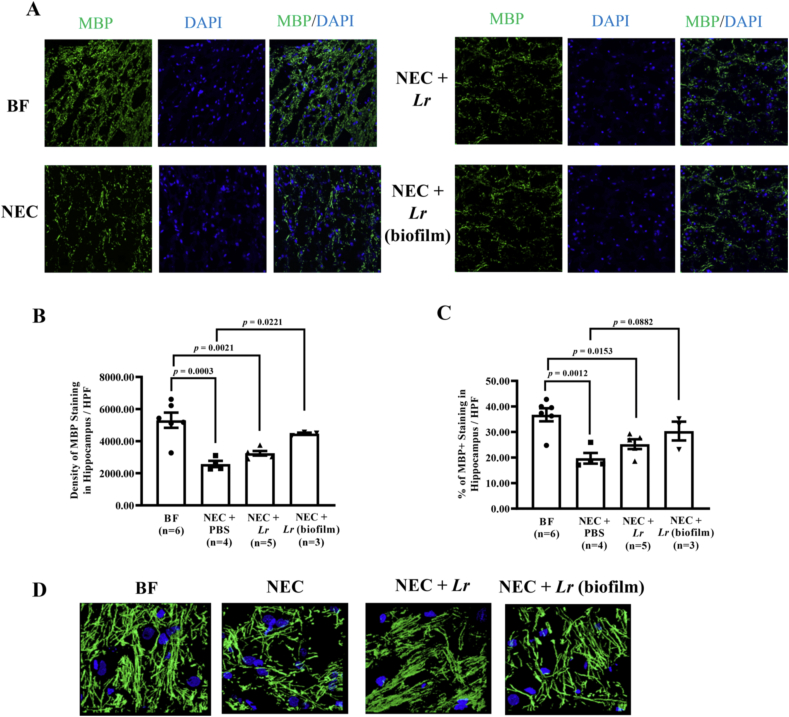
3.5. Lr (biofilm) attenuates NEC-induced reduction in myelin basic protein (MBP)
To begin to determine how microglia may influence cognitive behavior and memory, we examined myelination in the hippocampus by staining with MBP antibody. Sagittal sections of the hippocampus were immunostained for MBP, and random images obtained at 20X magnification (Fig. 9A). We observed that exposure to NEC reduced overall MBP staining in the hippocampus as assessed by densitometry and by determining the % of MBP + pixels (p = 0.0003 and p = 0.0012 vs. BF respectively) (Fig. 9B and C). Treatment with planktonic Lr did not lead to any statistically significant differences. However, rats treated with Lr (biofilm) prior to experimental NEC displayed an increase in MBP densitometry and in the % of MBP + cells compared to untreated rats (p = 0.0221 and p = 0.0882 respectively) (Fig. 9B and C). There were no significant differences between the BF group and rats treated with Lr (biofilm) prior to exposure to experimental NEC (Fig. 9B and C). MBP staining was confirmed with higher magnification using 63× objectives (Fig. 9D). Taken together, these data show that Lr in its biofilm state prevents NEC-induced decreases in MBP, which may help to promote cognitive development.
Fig. 9.
Myelin Basic Protein (MBP) in hippocampus 2 months after experimental NEC. Rat pups were subjected to experimental NEC and surviving pups were placed with surrogate dams. Rats were sacrificed at two months of age, brains were harvested, and hippocampus sections were fixed in 4% PFA. Frozen sections were stained with MBP antibody and Alexa 488 conjugated secondary antibody. At least 5 confocal pictures per section were imaged using 20X objectives. (A) Representative immunohistochemical images from hippocampus sections; (B) densitometry analysis using Zen software; (C) % positive MBP staining/HPF analyzed using Image J software. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA). (D) Selective images were shown in 63X objectives.
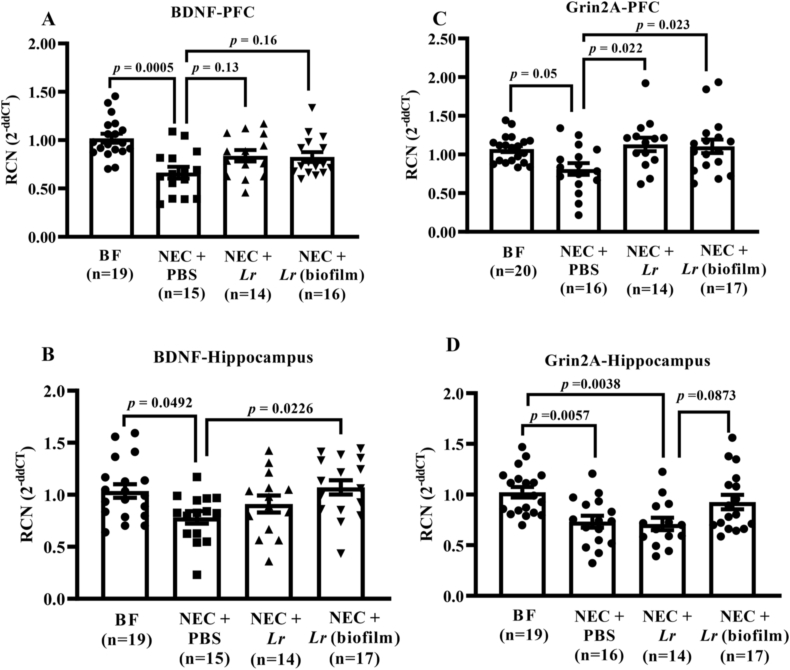
3.6. Lr (biofilm) prevents NEC-induced reduction in BDNF and Grin2A gene expression
It has been shown that early life adversity induces changes in gene transcription that influence physiological and behavioral activities (Meaney, 2001). Therefore, we next assessed changes in gene expression in the brain of rats after exposure to experimental NEC. The prefrontal cortex (PFC) plays an important role in attention and short-term working memory, and the hippocampus is actively involved in memory acquisition, formation, and maintenance (Dupret et al., 2008). Based on the observed effects of NEC on these behaviors, we isolated RNA from the PFC and hippocampus and measured the expression of several memory- and learning-related genes.
3.6.1. Brain-derived neurotrophic factor (BDNF)
BDNF plays an important role in brain growth, neurodevelopment, synapse remodeling, and responses to stress and injury (Kowianski et al., 2018; Rao et al., 2009), in addition to the acquisition of both spatial and non-spatial memories (Miranda et al., 2018). Two months after exposure to experimental NEC, there was reduced BDNF gene expression in both the prefrontal cortex (p = 0.0005 vs. BF rats) and hippocampus (p = 0.0492 vs. BF rats) (Fig. 10A and B). There was a trend for treatment with probiotics to attenuate the NEC-induced decrease in BDNF expression (p = 0.16 NEC + PBS vs. Lr (biofilm)) in the prefrontal cortex (Fig. 10A). This effect was more pronounced in the hippocampus, were Lr (biofilm) prevented the NEC-induced decrease in BDNF (p = 0.0226 vs. NEC + PBS) (Fig. 10B).
Fig. 10.
Neurotrophic gene expression after experimental NEC. Rat pups were subjected to experimental NEC and surviving pups were placed with surrogate dams. Rats were sacrificed at 2 months of age, brains harvested, the prefrontal cortex (PFC) and hippocampus collected, and gene expression measured by qRT-PCR. (A) BDNF expression in prefrontal cortex; (B) BDNF expression in hippocampus; (C) Grin2A expression in prefrontal cortex; and (D) Grin2 expression in hippocampus. Shown are relative copy numbers from independent rats. Error bars represent SEM with statistical significance defined as p ≤ 0.05 (one-way ANOVA).
3.6.2. NMDA receptor subunit 2A (Grin2A)
We next tested the effects of experimental NEC on brain NMDA receptor subunit 2A (Grin2A) gene expression in rats. Grin2A is a glutamate receptor and ion channel protein found in nerve cells. Ca2+ flux through NMDA receptors is thought to be critical in synaptic plasticity, a cellular mechanism for learning and memory (Baez et al., 2018). Two months after exposure to experimental NEC, there was reduced Grin2A gene expression in both the prefrontal cortex (p = 0.05 vs. BF rats) and hippocampus (p = 0.0057 vs. BF rats) (Fig. 10C and D). Treatment with planktonic Lr or Lr (biofilm) equivalently prevented the effects of NEC on Grin2A expression in the prefrontal cortex (p = 0.022 Lr vs. NEC; p = 0.023 Lr (biofilm) vs. NEC + PBS) (Fig. 10C). However, in the hippocampus, planktonic Lr had no significant effect whereas Lr (biofilm) administration resulted in Grin2A levels that were similar to BF rats (p = 0.6499) (Fig. 10D).
4. Discussion
Severe neurocognitive injury is a common and debilitating long-term complication that affects approximately 40% of children who survive NEC (Matei et al., 2020). Although it is recognized that intestinal injury in premature infants contributes to neuroinflammation and subsequent aberrant neurodevelopment, there is currently a lack of therapeutic options to prevent these long-term consequences of NEC. We have shown previously that administration of a single dose of Lr in its biofilm state, but not its planktonic state, reduces the incidence and severity of NEC (Olson et al., 2018). Here we demonstrated that multiple components of neurodevelopment are significantly delayed in rats that survive experimental NEC. Importantly, enteral administration of the probiotic Lr administered in its biofilm state prevented many of the deleterious effects of NEC on neurodevelopmental milestones, as well as cognitive and anxiety-like behaviors. The beneficial effects of Lr (biofilm) on neurodevelopment and behavior were evident early in pup development (i.e., 1–2 weeks of age), persisted into pup adolescence (i.e., 1–2 months of age), and were associated with differences in microglia and key factors for neurodevelopment in brain regions related to affected behaviors. Thus, administration of Lr in its biofilm state protects the brain, as well as the intestines, from the detrimental consequences of NEC.
Cognitive impairment in NEC survivors is related to disease severity. Infants with Bell’s stage 2 or 3 NEC are significantly more likely to suffer neurodevelopmental impairment at 20 months of age (Hintz et al., 2005; Rees et al., 2007), which persists into childhood (Shah et al., 2008). In addition, infants requiring surgery have a significantly increased chance of developing cerebral palsy and psychomotor impairment compared to infants whose NEC was treated medically (Matei et al., 2020). In our experimental NEC model, ~60–70% of rats develop NEC and the majority of those rats die if left untreated (Besner, 2015; Radulescu et al., 2009). In the current study, surviving pups were placed with foster dams and left undisturbed until behavioral testing was performed. Although these surviving pups may not have experienced advanced stages of NEC, and are likely to bear disease hallmarks more comparable to human babies suffering from medical NEC as opposed to the more severe surgical NEC, they still showed significant neurodevelopmental and behavioral differences compared to BF rats. These effects were prevented by one single administration of Lr (biofilm). Although we were not able to compare the effectiveness of Lr (biofilm) at different NEC severities (e.g., stage 2 vs. stage 4 NEC), it is possible that the benefits of Lr (biofilm) are even more pronounced with more severe NEC, but this will require further study. Of note, in our experimental rodent NEC model, premature rat pups are delivered via C-section. It is not possible to determine the sex of these premature pups at birth, so we randomly assigned them to the treatment groups. Thus, both male and female rats were used in these studies. After 96 h of exposure to NEC stress, surviving pups were placed with foster dams. We could determine the sex when pups were ~14 days old. We did look for any behavior difference between male and female rats but did not find any statistically significant differences between males and females (data not shown). Nonetheless, we demonstrate an effectiveness of Lr (biofilm) in preventing NEC-induced reductions in cognitive behaviors in rats that are similar to the learning and memory disabilities reported in human infants who survive NEC (Shah et al., 2008).
There is mounting evidence to support a role for gut microbes in neurodevelopment and cognitive function across the lifespan. Gareau et al. demonstrated that working memory is reduced when C57BL/6 mice are challenged with Citrobacter rodentium and exposed to acute stress. The effects on memory were prevented by daily treatment with a combination of the probiotics Lactobacillus rhamnosus (R0011) + Lactobacillus helveticus (R0052) (i.e., Lacidofil) (Gareau et al., 2011). Similarly, the compromised emotional learning from early life stress was reversed in rats when they were provided with L. rhamnosus R0011 and L. helveticus R0052 in the dam’s drinking water during stress (Cowan et al., 2019). Bravo et al. demonstrated that administration of L. rhamnosus reduced stress-induced corticosterone, anxiety and depression, and altered central GABA receptor expression in the brain of mice (Bravo et al., 2011), indicating that probiotics are capable of inducing changes in the CNS. Studies in humans have not been as promising, and in a randomized controlled trial evaluating the impact of oral probiotics on neurodevelopmental outcomes in preterm infants, the probiotics Lactobacillus sporogenes did not affect neuromotor, neurosensory and cognitive outcomes at 18–24 months corrected age (Akar et al., 2017; Sari et al., 2012). Although our study also involved Lr, we used a different strain of the probiotic (i.e., ATCC23272). This is important, because it is well recognized that there are substantial functional differences between Lr strains (Spinler et al., 2014). Moreover, in our experimental rat NEC model, Lr are attached to the surface of biocompatible microspheres loaded with maltose to increase glucosyltransferase (GTFW)-induced biofilm formation (Olson et al., 2016). Indeed, most of the beneficial effects of Lr only occurred when the bacteria were in the biofilm state. The reasons for this are not yet known, but we have previously found that Lr (biofilm) persists in the neonatal rat gut for longer periods of time than planktonic Lr, and helps to normalize neonatal intestinal bacteriome development (Olson et al., 2018). Further research is ongoing to elucidate the mechanisms involved in the neuroprotective effects of Lr (biofilm) in NEC-exposed animals.
An important observation in our rat model of experimental NEC was prolonged microglia activation that persisted for at least 2 months. This is consistent with the findings of Nino et al. (2018) who used a mouse model of NEC to show that TLR4-deficient mice are protected from NEC-associated microglial cell activation, ROS accumulation in neonates, and neurodevelopment impairment into adulthood. Interestingly, we found that administration of Lr (biofilm) in the neonatal period prevented the effects of NEC on the number of microglia in the brain that had a reactive/active morphology at 2 months of age, which is equivalent to middle/high school aged children. Our results demonstrate that the effects of NEC on brain microglia persist far beyond the initial intestinal insult and that this novel probiotic delivery system can prevent long-term, detrimental effects of NEC on microglia and cognitive outcomes. Although it was beyond the scope of this study to confirm microglial activity in NEC animals, cell morphology is strongly associated with microglial activation state (Verdonk et al., 2016). In our study, we specifically characterized cells with de-ramified, unramified, or ameboid morphology as a surrogate marker for activation (Verdonk et al., 2016). Additional studies are needed to assess more subtle morphological variations and intermediate forms of microglia activation. Nonetheless, our study demonstrates the beneficial effects of Lr (biofilm) on microglia in NEC-exposed rats.
Microglial activation leads to reductions in oligodendrocyte progenitor cells in mice exposed to NEC. Because oligodendrocytes are responsible for neuronal myelination, microglial-induced reductions in oligodendrocyte progenitor cells are thought to be responsible for myelination impairment and cognitive impairment after NEC (Baburamani et al., 2014; Back, 2014; Nino et al., 2018; Salmaso et al., 2014). Consistent with our observation of an increased number of de-ramified/ameboid microglia, we observed lower amounts of MBP staining in the brains of rats exposed to NEC. These effects were partially attenuated by Lr (biofilm), but planktonic Lr had no effect on MBP staining in the brain, again supporting the beneficial effects of Lr (biofilm) on neurodevelopment in NEC-exposed rats.
Early life adversity, such as prolonged maternal separation or shock, induces changes in gene transcription in the brain that lead to changes in physiological and behavioral measures throughout life (Meaney, 2001). One such effect of early life adversity is reduction in BDNF, which is a neurotrophic factor related to neurodevelopment, neuroprotection, synapse regulation, learning and memory (Kowianski et al., 2018). Abnormal BDNF expression in the hippocampus and prefrontal cortex has been implicated in several neuropsychiatric disorders (Rosas-Vidal et al., 2018). Increased expression of BDNF can have a positive effect on the generation of long-term potentiation and memory, while frontal cortex restricted BDNF knockout mice show learning deficits and hippocampal-dependent altered pattern discrimination (Gorski et al., 2003). Previous studies have shown that the probiotic Bacillus longum but not L. rhamnosus reduced bacterial infection-induced reductions in brain BDNF, which were found to be related to anxiety-like behavior (Bercik et al., 2010). In our experiments, exposure to NEC led to decreased BDNF expression in both the prefrontal cortex and hippocampus. Treatment with Lr in its biofilm state, but not planktonic Lr, prevented NEC-induced decreases in BDNF in the hippocampus. BDNF and the NMDA receptor are part of the same cellular network for spatial memory in the hippocampus; BDNF promotes phosphorylation of NMDA receptor subunit 2A (Grin2A in rats) and enhances NMDA receptor activity (Miranda et al., 2018). We therefore assessed Grin2A expression and found that NEC reduced Grin2A expression in both the prefrontal cortex and hippocampus. Interestingly, both planktonic Lr and Lr (biofilm) prevented the effects of NEC on the expression of Grin2A in the prefrontal cortex, but only Lr (biofilm) significantly improved Grin 2A expression in the hippocampus. This is consistent with our finding that both planktonic Lr and Lr (biofilm) can attenuate anxiety-like behavior that involves the prefrontal cortex. In contrast, only Lr (biofilm) affected the hippocampus, including BDNF and Grin 2A gene expression. Consistent with this finding of beneficial effects in the hippocampus, Lr (biofilm), but not planktonic Lr, improved hippocampal-dependent cognitive tasks. Additional studies will determine whether the unique, beneficial effects of Lr (biofilm) are limited to the hippocampus.
We have previously shown that a single dose of Lr delivered in its biofilm state protects the intestine from NEC (Olson et al., 2018). Intestinal inflammation can have effects throughout the body, including the brain, and our study is in line with previous studies demonstrating that animals that survive experimental NEC have significant delays in neurodevelopment and disruption of cognitive and anxiety-like behaviors that persist into maturity. We now demonstrate that Lr (biofilm) prevents the detrimental neurodevelopmental sequalae of experimental NEC. Although the mechanisms by which this occurs are not yet fully understood, prevention of NEC-induced increases in microglial activation, which is known to contribute to the neurobehavioral consequences of NEC, as well as effects on key neurodevelopmental growth factors and neurotransmitter receptors, have been demonstrated here. These findings have significant implications as we move towards clinical utilization of this probiotic delivery system to reduce the harmful effects of clinical NEC.
Funding sources
This work was funded by NIH R01 GM123482 (GEB, SDG, MTB) and the Research Institute at Nationwide Children’s Hospital (GEB).
Declaration of competing interest
Drs. Besner, Bailey, and Goodman have stock holdings and an investment interest in Scioto Biosciences, Inc.
Acknowledgements
The authors are grateful to Dr. Miriam Conces in the NCH department of pathology for guidance on microglia classification. We thank Cindy McAllister and the Morphology Core at NCH for technical support in cutting frozen tissue sections for immunofluorescence analysis. We thank Dr. Jacob Allen for providing insightful discussion.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100256.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Akar M., Eras Z., Oncel M.Y., Arayici S., Guzoglu N., Canpolat F.E., Uras N., Oguz S.S. Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. J. Matern. Fetal Neonatal Med. 2017;30:411–415. doi: 10.1080/14767058.2016.1174683. [DOI] [PubMed] [Google Scholar]
- Baburamani A.A., Supramaniam V.G., Hagberg H., Mallard C. Microglia toxicity in preterm brain injury. Reprod. Toxicol. 2014;48:106–112. doi: 10.1016/j.reprotox.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A. Cerebral white and gray matter injury in newborns: new insights into pathophysiology and management. Clin. Perinatol. 2014;41:1–24. doi: 10.1016/j.clp.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez M.V., Cercato M.C., Jerusalinsky D.A. NMDA receptor subunits change after synaptic plasticity induction and learning and memory acquisition. Neural Plast. 2018;2018:5093048. doi: 10.1155/2018/5093048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P., Verdu E.F., Foster J.A., Macri J., Potter M., Huang X., Malinowski P., Jackson W., Blennerhassett P., Neufeld K.A., Lu J., Khan W.I., Corthesy-Theulaz I., Cherbut C., Bergonzelli G.E., Collins S.M. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. e2101. [DOI] [PubMed] [Google Scholar]
- Besner G.E. A pain in the NEC: research challenges and opportunities. J. Pediatr. Surg. 2015;50:23–29. doi: 10.1016/j.jpedsurg.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.S.M., Stylianakis A.A., Richardson R. Early-life stress, microbiota, and brain development: probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev Cogn Neurosci. 2019;37:100627. doi: 10.1016/j.dcn.2019.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Dupret D., Revest J.M., Koehl M., Ichas F., De Giorgi F., Costet P., Abrous D.N., Piazza P.V. Spatial relational memory requires hippocampal adult neurogenesis. PloS One. 2008;3 doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich D.E., Neigh G.N., Bourke C.H., Nemeth C.L., Hazra R., Ryan S.J., Rowson S., Jairam N., Sholar C.A., Rainnie D.G., Stowe Z.N., Owens M.J. Prenatal stress, regardless of concurrent escitalopram treatment, alters behavior and amygdala gene expression of adolescent female rats. Neuropharmacology. 2015;97:251–258. doi: 10.1016/j.neuropharm.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., Macqueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Gehrmann J., Matsumoto Y., Kreutzberg G.W. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Good M., Sodhi C.P., Ozolek J.A., Buck R.H., Goehring K.C., Thomas D.L., Vikram A., Bibby K., Morowitz M.J., Firek B., Lu P., Hackam D.J. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G1021–G1032. doi: 10.1152/ajpgi.00452.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J.A., Balogh S.A., Wehner J.M., Jones K.R. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Heyser C.J. Assessment of developmental milestones in rodents. Curr Protoc Neurosci Chapter. 2004;8 doi: 10.1002/0471142301.ns0818s25. Unit 8 18. [DOI] [PubMed] [Google Scholar]
- Hintz S.R., Kendrick D.E., Stoll B.J., Vohr B.R., Fanaroff A.A., Donovan E.F., Poole W.K., Blakely M.L., Wright L., Higgins R., Network N.N.R. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- Kowianski P., Lietzau G., Czuba E., Waskow M., Steliga A., Morys J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A.K., Guest P.C., Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol. Biol. 2019;1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- Kraeuter A.K., Guest P.C., Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 2019;1916:105–111. doi: 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- Lu J., Claud E.C. Connection between gut microbiome and brain development in preterm infants. Dev. Psychobiol. 2019;61:739–751. doi: 10.1002/dev.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow L.M. Novel object recognition test for the investigation of learning and memory in mice. JoVE. 2017 doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.A., Balciunas E.M., Converti A., Cotter P.D., de Souza Oliveira R.P. Bacteriocin production by Bifidobacterium spp. A review. Biotechnol. Adv. 2013;31:482–488. doi: 10.1016/j.biotechadv.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Matei A., Montalva L., Goodbaum A., Lauriti G., Zani A. Neurodevelopmental impairment in necrotising enterocolitis survivors: systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2020;105:432–439. doi: 10.1136/archdischild-2019-317830. [DOI] [PubMed] [Google Scholar]
- Meaney M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miranda M., Kent B.A., Morici J.F., Gallo F., Saksida L.M., Bussey T.J., Weisstaub N., Bekinschtein P. NMDA receptors and BDNF are necessary for discrimination of overlapping spatial and non-spatial memories in perirhinal cortex and hippocampus. Neurobiol. Learn. Mem. 2018;155:337–343. doi: 10.1016/j.nlm.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Miyake H., Lee C., Chusilp S., Bhalla M., Li B., Pitino M., Seo S., O’Connor D.L., Pierro A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2020;36:155–163. doi: 10.1007/s00383-019-04599-7. [DOI] [PubMed] [Google Scholar]
- Moschopoulos C., Kratimenos P., Koutroulis I., Shah B.V., Mowes A., Bhandari V. The neurodevelopmental perspective of surgical necrotizing enterocolitis: the role of the gut-brain Axis. Mediat. Inflamm. 2018;2018:7456857. doi: 10.1155/2018/7456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J., Walker W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemarkt H.J., De Meij T.G., van Ganzewinkel C.J., de Boer N.K.H., Andriessen P., Hutten M.C., Kramer B.W. Necrotizing enterocolitis, gut microbiota, and brain development: role of the brain-gut Axis. Neonatology. 2019;115:423–431. doi: 10.1159/000497420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nino D.F., Zhou Q., Yamaguchi Y., Martin L.Y., Wang S., Fulton W.B., Jia H., Lu P., Prindle T., Jr., Zhang F., Crawford J., Hou Z., Mori S., Chen L.L., Guajardo A., Fatemi A., Pletnikov M., Kannan R.M., Kannan S., Sodhi C.P., Hackam D.J. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 2018;10(471):eaan0237. doi: 10.1126/scitranslmed.aan0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J.K., Navarro J.B., Allen J.M., McCulloh C.J., Mashburn-Warren L., Wang Y., Varaljay V.A., Bailey M.T., Goodman S.D., Besner G.E. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G408–G419. doi: 10.1152/ajpgi.00078.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J.K., Rager T.M., Navarro J.B., Mashburn-Warren L., Goodman S.D., Besner G.E. Harvesting the benefits of biofilms: a novel probiotic delivery system for the prevention of necrotizing enterocolitis. J. Pediatr. Surg. 2016;51:936–941. doi: 10.1016/j.jpedsurg.2016.02.062. [DOI] [PubMed] [Google Scholar]
- Patel R.M., Myers L.S., Kurundkar A.R., Maheshwari A., Nusrat A., Lin P.W. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 2012;180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.M., Underwood M.A. Probiotics and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018;27:39–46. doi: 10.1053/j.sempedsurg.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano C., Galley J., Elbahrawy M., Wang Y., Farrell A., Brigstock D., Besner G.E. Human breast milk-derived extracellular vesicles in the protection against experimental necrotizing enterocolitis. J. Pediatr. Surg. 2020;55:54–58. doi: 10.1016/j.jpedsurg.2019.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulescu A., Zorko N.A., Yu X., Besner G.E. Preclinical neonatal rat studies of heparin-binding EGF-like growth factor in protection of the intestines from necrotizing enterocolitis. Pediatr. Res. 2009;65:437–442. doi: 10.1203/PDR.0b013e3181994fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager T.M., Olson J.K., Zhou Y., Wang Y., Besner G.E. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J. Pediatr. Surg. 2016;51:942–947. doi: 10.1016/j.jpedsurg.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rao R., Mashburn C.B., Mao J., Wadhwa N., Smith G.M., Desai N.S. Brain-derived neurotrophic factor in infants <32 weeks gestational age: correlation with antenatal factors and postnatal outcomes. Pediatr. Res. 2009;65:548–552. doi: 10.1203/PDR.0b013e31819d9ea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees C.M., Pierro A., Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2007;92:F193–F198. doi: 10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentea R.M., Welak S.R., Fredrich K., Donohoe D., Pritchard K.A., Oldham K.T., Gourlay D.M., Liedel J.L. Early enteral stressors in newborns increase inflammatory cytokine expression in a neonatal necrotizing enterocolitis rat model. Eur. J. Pediatr. Surg. 2013;23:39–47. doi: 10.1055/s-0032-1329704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.R., Kennedy C., van Arendonk K.J., Green A., Martin C.R., Blakely M.L. Neurodevelopmental considerations in surgical necrotizing enterocolitis. Semin. Pediatr. Surg. 2018;27:52–56. doi: 10.1053/j.sempedsurg.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Rosas-Vidal L.E., Lozada-Miranda V., Cantres-Rosario Y., Vega-Medina A., Melendez L., Quirk G.J. Alteration of BDNF in the medial prefrontal cortex and the ventral hippocampus impairs extinction of avoidance. Neuropsychopharmacology. 2018;43:2636–2644. doi: 10.1038/s41386-018-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E., Ta B.D., van der Ree M.H., Tanis J.C., van Braeckel K.N., Hulscher J.B., Bos A.F. Functional impairments at school age of children with necrotizing enterocolitis or spontaneous intestinal perforation. Pediatr. Res. 2011;70:619–625. doi: 10.1203/PDR.0b013e31823279b1. [DOI] [PubMed] [Google Scholar]
- Salmaso N., Jablonska B., Scafidi J., Vaccarino F.M., Gallo V. Neurobiology of premature brain injury. Nat. Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari F.N., Eras Z., Dizdar E.A., Erdeve O., Oguz S.S., Uras N., Dilmen U. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am. J. Perinatol. 2012;29:579–586. doi: 10.1055/s-0032-1311981. [DOI] [PubMed] [Google Scholar]
- Sarro E.C., Sullivan R.M., Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258:147–161. doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman S.M., Mehal J.M., Haberling D.L., Holman R.C., Stoll B.J. Infant and maternal risk factors related to necrotising enterocolitis-associated infant death in the United States. Acta Paediatr. 2016;105:e240–246. doi: 10.1111/apa.13390. [DOI] [PubMed] [Google Scholar]
- Shah D.K., Doyle L.W., Anderson P.J., Bear M., Daley A.J., Hunt R.W., Inder T.E. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J. Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. 175 e171. [DOI] [PubMed] [Google Scholar]
- Spinler J.K., Sontakke A., Hollister E.B., Venable S.F., Oh P.L., Balderas M.A., Saulnier D.M., Mistretta T.A., Devaraj S., Walter J., Versalovic J., Highlander S.K. From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol. 2014;6:1772–1789. doi: 10.1093/gbe/evu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk T., Inder T., Wang X., Burgner D., Mallard C., Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect. Dis. 2014;14:751–762. doi: 10.1016/S1473-3099(14)70710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K.S., Gibson G.R., Hutkins R., Reimer R.A., Reid G., Verbeke K., Scott K.P., Holscher H.D., Azad M.B., Delzenne N.M., Sanders M.E. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020 doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyoka M., de Coppi P., Eaton S., Khoo K., Hall N.J., Curry J., Kiely E., Drake D., Cross K., Pierro A. Advanced necrotizing enterocolitis part 1: mortality. Eur. J. Pediatr. Surg. 2012;22:8–12. doi: 10.1055/s-0032-1306263. [DOI] [PubMed] [Google Scholar]
- Underwood M.A., Arriola J., Gerber C.W., Kaveti A., Kalanetra K.M., Kananurak A., Bevins C.L., Mills D.A., Dvorak B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 2014;76:326–333. doi: 10.1038/pr.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood M.A., Sohn K. The microbiota of the extremely preterm infant. Clin. Perinatol. 2017;44:407–427. doi: 10.1016/j.clp.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk F., Roux P., Flamant P., Fiette L., Bozza F.A., Simard S., Lemaire M., Plaud B., Shorte S.L., Sharshar T., Chretien F., Danckaert A. Phenotypic clustering: a novel method for microglial morphology analysis. J. Neuroinflammation. 2016;13:153. doi: 10.1186/s12974-016-0614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J. Pediatr. 2008;153:160–163. doi: 10.1016/j.jpeds.2008.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Microglia: newly discovered complexity could lead to targeted therapy for neonatal white matter injury and dysmaturation. J. Neonatal Perinat. Med. 2019;12:239–242. doi: 10.3233/NPM-190303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe S., Pacheco A.R., Lemay D.G., Mills D.A. Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiol. 2015;15:172. doi: 10.1186/s12866-015-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.