Abstract
Purpose of review
To give an overview on the role of therapeutic drug monitoring (TDM) of biologics in patients with inflammatory bowel disease (IBD).
Recent findings
Numerous prospective exposure-response relationship studies and post-hoc analyses of randomized controlled trials show a positive correlation between biologic drug concentrations and favorable clinical outcomes in IBD. These studies also demonstrate that higher drug concentrations appear to be needed to achieve more stringent objective therapeutic outcomes. Reactive TDM rationalizes the management of primary non-response and secondary loss of response to anti-tumor necrosis factor (anti-TNF) therapy and is more cost-effective when compared to empiric dose optimization. Furthermore, recent data suggest that proactive TDM, with the goal of targeting a threshold drug concentration, is associated with better therapeutic outcomes when compared to empiric dose escalation and/or reactive TDM of infliximab or adalimumab. Finally, proactive TDM can also efficiently guide infliximab de-escalation or discontinuation in patients with IBD in remission.
Summary
Reactive TDM is currently considered as standard of care, while proactive TDM is emerging as a new therapeutic strategy for better optimizing anti-TNF therapy in IBD. However, more data from prospective studies are needed before a wide implementation of TDM-based algorithms in real life clinical practice for newer biologics.
Keywords: inflammatory bowel disease, rheumatoid arthritis, psoriasis, biologics, immunogenicity, therapeutic drug monitoring, anti-TNF therapy, ustekinumab, vedolizumab
Introduction
Biologic therapies are very effective for treating moderate to severe inflammatory bowel diseases (IBD), namely Crohn’s disease (CD) and ulcerative colitis (UC). These agents include the tumor necrosis factor (TNF) inhibitors infliximab, adalimumab, certolizumab pegol and golimumab, the anti-integrin inhibitors vedolizumab and natalizumab, and the IL-12/23 p40 inhibitor ustekinumab [1, 2]. Unfortunately, not all patients respond to induction therapy, and many others lose response over time [3, 4]. Therapeutic drug monitoring (TDM) helps to explain these negative therapeutic outcomes can be attributed to either pharmacokinetic issues, characterized by low drug concentrations with or without the development of anti-drug antibodies (ADA), or a mechanistic failure in patients with adequate drug concentrations [5].
Numerous prospective exposure-response relationship studies and post-hoc analyses of randomized controlled trials show a positive correlation between biologic drug concentrations and favorable clinical outcomes in IBD [6-41*]. These studies in IBD also suggest that higher drug concentrations are required to achieve more stringent objective therapeutic outcomes (from clinical response to histologic remission) [42, 43]. On the other hand, low drug concentrations predispose to ADA formation and treatment failure [44-46].
Reactive TDM is defined as the evaluation of drug concentration and ADA levels in the setting of primary non-response or secondary loss of response (LOR) to a biologic agent. The use of reactive TDM has rationalized the management of these unwanted clinical outcomes [47-49] and is more cost-effective when compared to empiric dose escalation [50-52] (Figure 1). Patients who will benefit from more drug (low drug concentrations) are given it, and those patients who will benefit from another therapy (adequate drug concentrations or high ADA) are switched. Proactive TDM is defined as the evaluation of trough concentration and ADA levels with the goal of optimizing biological therapy to achieve a threshold drug concentration. Recent data suggest that proactive TDM is associated with better therapeutic outcomes when compared to empiric dose optimization and/or reactive TDM of anti-TNF therapy in IBD [53-59]. Proactive TDM can also effectively guide infliximab de-escalation [60, 61] or discontinuation [15, 62-64] in patients with IBD in remission TDM (Figure 2). However, there are perceived knowledge gaps regarding the role of TDM that have hampered the wide implementation of TDM-based algorithms in real-life clinical practice, as reflected also in some of the current guidelines and recommendations (Table 2) [65-70].
Figure 1.
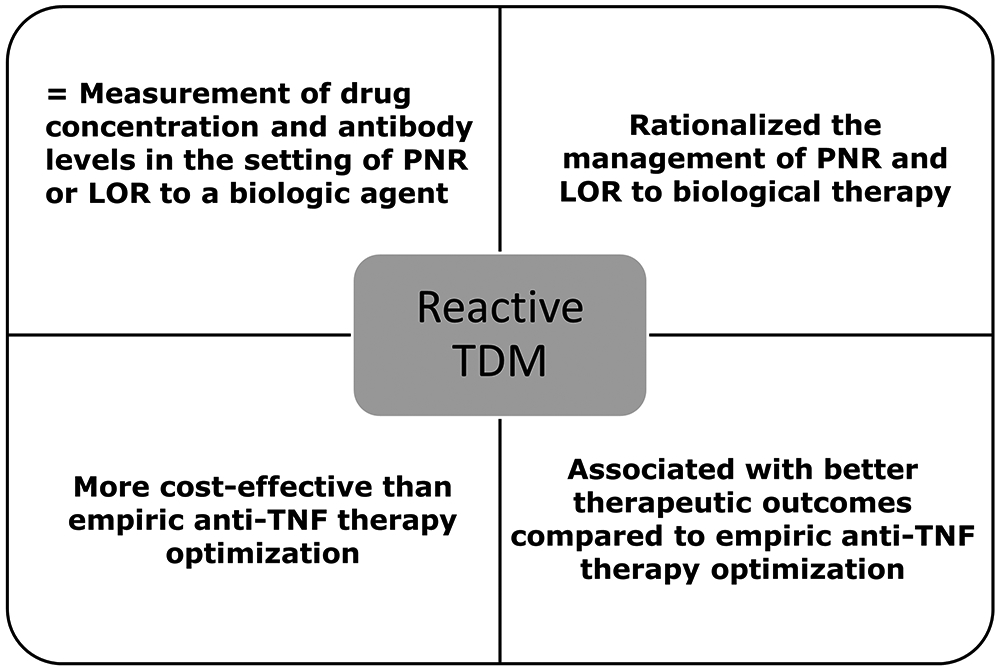
Definition and role of reactive therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease.
PNR: primary non-response, LOR: loss of response; TDM: therapeutic drug monitoring; TNF: tumor necrosis factor.
Figure 2.
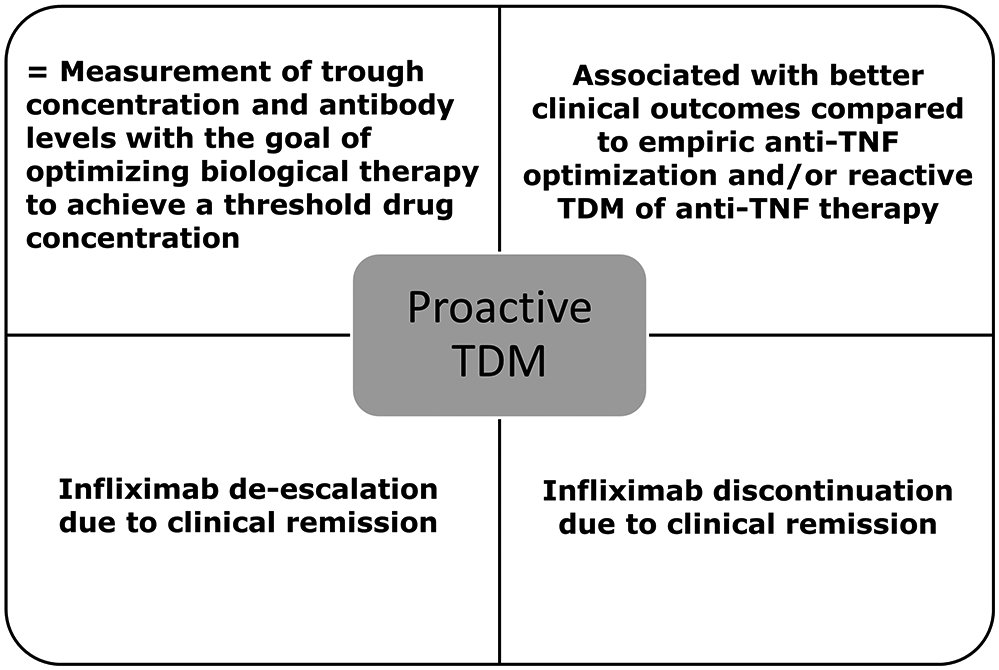
Definition and role of proactive therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease.
TDM: therapeutic drug monitoring; TNF: tumor necrosis factor.
Table 2.
Current recommendations and guidelines from medical societies/organizations as well as expert groups.
| Medical society / organization or expert group |
Method | Reactive TDM | Proactive TDM | Ref. |
|---|---|---|---|---|
| AGA | GRADE | In adults with active IBD treated with anti-TNF agents reactive TDM to guide treatment changes is suggested. (Conditional recommendation, very low quality of evidence) | In adult patients with quiescent IBD treated with anti-TNF agents, no recommendation regarding the use of routine proactive TDM is made. (Knowledge gap) | 65 |
| BSG | GRADE | Treatment options for failure of initial anti-TNF therapy (increase dose, shorten dosage interval, switch to alternative anti-TNF, or switch to different drug class) may be informed by the clinical context and by measurement of serum drug and ADA concentrations. (Weak recommendation, low-quality evidence). Patients with LOR to anti-TNF therapy may have serum drug and ADA concentrations measured to inform appropriate changes in treatment. (Weak recommendation, moderate-quality evidence) | All IBD patients should be reviewed 2-4 weeks after completing loading doses of anti-TNF therapy to assess response and optimize maintenance dosing based on clinical response and measures such as serum drug and ADA concentrations, blood inflammatory markers, fecal biomarkers or endoscopy. (Good practice recommendation) | 66 |
| ECCO | GRADE | In CD patients who have lost response to an anti-TNF agent, there is currently insufficient evidence to recommend for or against the use of reactive therapeutic drug monitoring to improve clinical outcomes. (Weak recommendation, low-quality evidence) | In CD patients in clinical remission under anti-TNF treatment, there is currently insufficient evidence to recommend for or against the use of proactive TDM to improve clinical outcomes as compared to routine care. (Weak recommendation, moderate-quality evidence) | 67 |
| Australian IBD, consensus Working Group | Modified Delphi | TDM should be performed in patients with secondary loss-of-response to guide clinical decision-making | In patients in clinical remission following anti-TNF therapy induction, TDM should be considered to guide management. TDM should be considered periodically in patients in clinical remission if the results are likely to impact management | 68 |
| CAG | GRADE | In patients with CD who have a suboptimal clinical response to anti-TNF induction therapy or LOR to maintenance therapy, we suggest regimen intensification informed by TDM. (Conditional recommendation, very-low-quality evidence) | N/A | 69 |
| BRIDGe | Modified Delphi | It is appropriate to order drug/antibody concentration testing for all anti-TNFs in patients with confirmed LOR. It is appropriate to order drug/antibody concentration testing of anti-TNFs at the end of induction in PNRs. | It is appropriate to order drug/antibody concentration testing at least once during maintenance for patients on all anti-TNFs. It is appropriate to order drug/antibody concentration testing in responders at the end of induction for all anti-TNFs. | 42 |
| ACG | GRADE | In patients with moderately to severely active UC who are responders to anti-TNF therapy and now losing response, we suggest measuring serum drug levels and ADA (if there is not a therapeutic level) to assess the reason for loss of response. (Conditional recommendation, very low quality of evidence) | N/A | 70 |
AGA: American Gastroenterological Association; IBD: inflammatory bowel disease; TDM: therapeutic drug monitoring; TNF: tumor necrosis factor; LOR: loss of response; ADA: anti-drug antibodies; N/A: not applicable; GRADE: Grading of Recommendations Assessment, Development and Evaluation; BSG: British Society of Gastroenterology; PNRs: primary non-responders; CAG: Canadian Association of Gastroenterology; UC: ulcerative colitis; CD: Crohn’s disease; ACG: American College of Gastroenterology; BRIDGe: Building Research n IBD Globally; IBD: inflammatory bowel disease; ECCO: European Crohn’s and colitis organization.
The goal of this review is to provide the most up to date information regarding the role of TDM for optimizing biologic therapy in IBD.
Exposure-outcomes relationship studies
Numerous exposure-outcomes relationship studies demonstrate that higher biologic drug concentrations, during both induction and maintenance therapy, are associated with better therapeutic outcomes in both CD and UC [6-41*]. Drug thresholds to target may vary depending on the IBD phenotype, investigated therapeutic outcome and type of TDM assay used; and typically, higher concentrations are associated with more stringent outcomes [31, 42, 43, 71, 72]. These studies include adult populations as well as pediatrics (Table 1) [7-17]. Furthermore, though not discussed here, there are several exposure-response studies in other immune-mediated inflammatory diseases, such as rheumatoid arthritis, ankylosing spondylitis and psoriasis [35]. Though most of the data relates to anti-TNF therapies, all therapies have been shown to have positive exposure-outcome relationships. We have chosen to highlight only a few of the more recent studies.
Table 1.
Exposure-outcome relationship data of infliximab in pediatric IBD.
| IBD type |
Treatment time point | Threshold (μg/mL) |
Therapeutic outcome and time point | TDM assay |
Ref. |
|---|---|---|---|---|---|
| CD | Induction (w2) | >9.2 | Clinical remission (w14) | ELISA | 7 |
| CD | Induction (w2) | ≥26.7 | Clinical response (w14) | ELISA | 8 |
| CD | Induction (w6) | >2.2 | Drug retention beyond one year of treatment | ELISA | 7 |
| CD | Induction (w6) | ≥18 | CRP<0.5 mg/dL | ELISA | 8 |
| CD | Induction (w6) | ≥15.9 | Clinical response (w14) | ELISA | 8 |
| CD | Induction (w6) | >8.3 | Clinical remission (w14) | ELISA | 9 |
| CD/UC | Induction (w6) | >9.8 | CRP <0.5 mg/dL | ELISA | 10 |
| CD | Post-induction (w10) | ≥9.1 | Drug retention (w52) | HMSA | 11 |
| CD | Post-induction (w14) | >12.7 | Fistula response (w24) | ELISA | 12 |
| CD/UC | Post-induction (w14) | >5.5 | Clinical remission (w54) | HMSA | 13 |
| CD/UC | Post-induction (w14) | >2 | ESR <18 mm/hr | ELISA | 10 |
| CD/UC | Post-induction (w14) | >3.1 | Sustained clinical remission | ELISA | 14 |
| CD | Maintenance | ≥2.5 | Relapse after drug withdrawal for remission | ELISA | 15 |
| CD | Maintenance | >4.9 | Biochemical remission | ELISA | 16 |
| CD | Maintenance | >5 | Mucosal healing | ELISA | 16 |
| CD/UC | Maintenance | >5.4 | Endoscopic remission | ELISA | 17 |
| CD/UC | Maintenance | >1.6 | ESR <18 mm/hr | ELISA | 10 |
ELISA: enzyme-linked immunosorbent assay; HMSA: homogeneous mobility shift assay; CRP: C-reactive protein, FC: fecal calprotectin; TDM: therapeutic drug monitoring; CD: Crohn’s disease; UC: ulcerative colitis; Ref.: reference; w: week; hr: hour.
A post-hoc analysis of the ACT-1 and 2 (A Safety and Efficacy Study for Infliximab in Patients with Active Ulcerative Colitis) RCTs showed that infliximab concentrations ≥18.6 μg/mL at week 2 and ≥10.6 μg/mL at week 6 were associated with an endoscopic improvement at week 8 [18*]. A post-hoc analysis of the TAILORIX (Drug-concentration versus Symptom-driven Dose Adaptation of Infliximab in patients with active Crohn's disease) RCT identified an infliximab threshold of 23.1 μg/mL at week 2 and 10 μg/mL at week 6 discriminating patients with early endoscopic remission at week 12 [19*]. The prospective PANTS (personalised anti-TNF therapy in Crohn's disease) study showed that the optimal week 14 drug concentrations associated with remission at both week 14 and week 54 were 7 mg/L for infliximab and 12 mg/L for adalimumab [20*]. A recent prospective study showed that a vedolizumab trough concentration cut-off of 16.55 μg/ml at week 14 predicted drug persistence within the first year of therapy [40]. The VISIBLE 1 (Efficacy and Safety of Vedolizumab Subcutaneously (SC) as Maintenance Therapy in Ulcerative Colitis) RCT showed that the proportion of patients receiving vedolizumab SC for maintenance who achieved clinical remission increased with increasing vedolizumab exposure from 50% (quartile 1) to 83% (quartile 4). Similarly, the proportion of patients with endoscopic improvement increased with increasing exposure from 50% (quartile 1) to 89% (quartile 4) [41*]. The prospective multi-center LOVE-CD (LOw countries VEdolizumab in CD) study, including 110 patients with active CD who received open-label vedolizumab (300 mg) infusions at weeks 0, 2, and 6, and every 8 weeks thereafter through week 52, showed that serum concentrations of vedolizumab >10 μg/ml at week 22 were associated with endoscopic remission at week 26 [39]. A recent systemic review and meta-analysis showed that in patients with UC, week 6 vedolizumab trough concentrations ≥18.5-20.8 μg/mL, and maintenance trough concentrations ≥9-12.6 μg/mL were associated with favorable clinical outcomes [37]. In addition, a recent post-hoc analysis of the UNIFI (A Study to Evaluate the Safety and Efficacy of Ustekinumab Induction and Maintenance Therapy in Participants With Moderately to Severely Active Ulcerative Colitis) RCT identified a target concentration threshold ≥3.7μg/mL at week 8 for achievement of clinical response at week 8 and a target concentration threshold ≥1.3μg/mL for clinical remission at week 44 [38*].
Reactive therapeutic drug monitoring
Reactive TDM has rationalized the management of LOR to anti-TNF therapy in IBD. It can stratify patients with subtherapeutic drug concentrations who will respond to dose escalation from those patients who already have enough drug exposure and would benefit from an alternative mechanism of medication from those patients with high ADA that cannot be overcome with dose optimization [47-49]. Yanai et al. showed that at the time of LOR, infliximab concentrations >3.8 μg/mL and adalimumab concentrations and >4.5 μg/mL identify patients who probably have a mechanistic failure and benefit more from changing out-of-class than dose escalation or switching within drug class [47]. Furthermore, Roblin et al. showed that adalimumab concentrations >4.9 μg/mL are associated with failure to a second anti-TNF, thus helping to identify patients likely failing adalimumab due to pharmacodynamic issues who would benefit from a non-anti-TNF agent [49]. Additionally, several studies have demonstrated that reactive TDM is more cost-effective [50-52] and is associated with higher rates of endoscopic remission when compared to empiric infliximab dose optimization [74]. Thus, we recommend reactive TDM in patients who develop LOR to anti-TNF therapy. A suggested reactive TDM-based algorithm for optimizing infliximab therapy in IBD is depicted in Figure 3. As adequate drug concentrations suggest a loss mechanistic effect, in practice we do not abandon infliximab or adalimumab unless drug concentrations are greater than 10-15 μg/ml.
Figure 3.
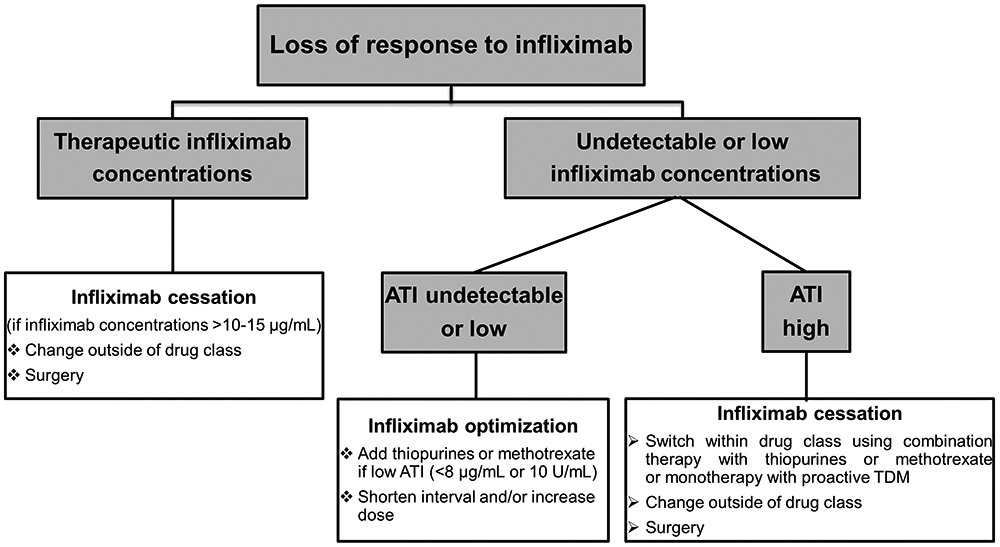
Reactive therapeutic drug monitoring-based algorithm for optimizing infliximab therapy in inflammatory bowel disease.
ATI: antibodies to infliximab; TDM: therapeutic drug monitoring.
A recent RCT, showed that patients with LOR and antibodies to a first anti-TNF benefit from the use of azathioprine in combination with the second anti-TNF. In these patients the addition of azathioprine was associated with a significant reduction in the risk of developing ADA, low drug concentrations and LOR to a second anti-TNF [73**]. Thus, if patients develop ADA to one anti-TNF, the addition on an immunomodulator (IMM) (or proactive TDM) should be recommended with the use of a second anti-TNF. This becomes even more clinically relevant in patients with a genetic predisposition for developing ADA [75]. Though the data for reactive TDM for new biologics is only theoretical at this time, though based on exposure-response studies, makes sense.
Proactive therapeutic drug monitoring
Proactive TDM for optimizing medications is not a new concept. It has been used for cyclosporine and tacrolimus in treating UC and in solid organ transplantation as well as with various antibiotics (gentamycin and vancomycin). The goal of proactive TDM is to improve response rates and prevent secondary LOR by targeting drug concentrations which are considered to be in the optimal therapeutic range. Proactive TDM of anti-TNF therapy has been associated with better therapeutic outcomes when compared to empiric dose escalation and/or reactive TDM in IBD including a lower risk of relapse, improved clinical remission rates, higher rates of mucosal healing as well as less treatment failure, need for IBD-related surgery or hospitalization, risk of ADA and serious infusion reactions [53-59]. Most recently, the PAILOT (Paediatric Crohn’s disease Adalimumab-Level-based Optimisation Treatment) RCT randomized 80 biological-naïve children with luminal CD who responded to adalimumab induction therapy to proactive TDM or reactive TDM. This study met its primary endpoint and showed that the steroid-free clinical remission rate at week 72 was higher in children undergoing proactive compared to those undergoing reactive TDM [32 (82%) vs. 19 (46%), p<0.001), respectively] [57**]. Furthermore the proactive TDM group had a higher rate of the stringent composite remission (defined as corticosteroid-free clinical remission, C-reactive protein ≤0.5 mg/dL and fecal calprotectin ≤150 mg/g) throughout week 8 to 72 when compared to those undergoing reactive TDM [16/38 (42%) vs. 5/40 (12%), p=0.003), respectively] [57**]. Interestingly, in this study 90% of the proactive group required dose-optimization compared to almost 60% of the reactive group. Furthermore, a recent 3-year prospective observational study showed that proactive TDM compared to empirical dosing is associated with a significant reduction in the risk of treatment failure (hazard ratio [HR]: 0.51; 95% confidence interval [CI]: 0.27-0.92; p=0.037), IBD-related surgery (HR: 0.14; 95%CI: 0.03-0.65; p=0.012) and hospitalization (HR: 0.38; 95% CI: 0.17-0.87; p=0.022) [58].
Proactive TDM can also be applied to better guide biologic withdrawal or de-escalation in patients in remission [60-63]. A recent observational study showed that in IBD patients in clinical remission infliximab de-escalation based on TDM (when infliximab concentrations at the time of de-escalation were higher than 7 μg/mL) was associated with less relapse compared to only clinically-based infliximab de-escalation [60]. In our clinical practice, dose de-escalation is typically performed patients in stable clinical remission with an infliximab concentration > 15 μg/mL. Following dose de-escalation, patients should continue to be followed with proactive TDM to maintain adequate infliximab concentrations and avoid relapse [61].
Proactive TDM can also be used to support the concept of ‘optimized monotherapy’ instead of using combination anti-TNF therapy with an IMM (thiopurines or methotrexate) which poses a risk for serious and opportunistic infections and lymphoma [76]. Two recent observational studies showed that proactive TDM-based infliximab monotherapy is as effective as infliximab combination therapy with an IMM [77, 78]. This concept is further reinforced by a recent post-hoc analysis of the SONIC (Study of Biologic and Immunomodulator Naive Patients in Crohn Disease) RCT which demonstrated that patients stratified by infliximab concentration quartiles have comparable outcomes regardless of concomitant azathioprine [79**]. In our clinical practice, we perform proactive TDM, typically optimized monotherapy, with infliximab and adalimumab. For infliximab our goal threshold is typically 5-10 μg/mL, but in certain scenarios may be as high as 15 for infliximab. For adalimumab, our goal threshold is typically >10 μg/mL. If not performing optimized monotherapy with anti-TNF, patients with IBD should be on a concomitant IMM to decrease ADA and improve outcomes.
However, before a wide implementation of TDM-based algorithms in real life clinical practice, several knowledge gaps need to be addressed, including when to measure biologic drug concentrations (peak vs. intermediate vs. trough; induction vs. post-induction concentrations) and what are the optimal drug concentrations to target (depending on the therapeutic outcome, IBD phenotype and type of TDM assay used). Moreover, the detection, quantification and interpretation of ADA can be challenging depending largely on the analytical properties of the assay used [80]. For example, the previously established cutoff of 8 μg/ml with the first-generation enzyme-linked immunosorbent assay (ELISA) seems to correspond to the cutoff of 374 ng/ml with the second-generation ELISA and a cutoff of 119 ng/ml in the ready-to-use ELISA kit [81]. Additionally, more data from well-designed prospective studies and RCTs are also needed. For example, the NOR-DRUM (NORwegian DRUg Monitoring) randomised, open, controlled, parallel-group, comparative, multi-centre, national, superiority, phase IV study will aim to assess the effectiveness of TDM in patients with rheumatoid arthritis, psoriatic arthritis, spondyloarthritis, UC, CD and psoriasis. Participants will be randomised 1:1 to either TDM of infliximab (intervention group) or to standard treatment with infliximab without TDM (control group) [82]. Finally, future perspectives to better optimize TDM include the incorporation of pharmacokinetic dashboard models and the use of rapid point of care assays for an early drug optimization [83, 84].
Conclusion
Many studies show the positive correlation of drug concentrations and outcomes. Currently, reactive TDM is considered the standard of care, while proactive TDM is emerging as a new therapeutic strategy for better optimizing anti-TNF therapy in IBD. However, more data from prospective studies are needed before a wide implementation of TDM-based algorithms in real life clinical practice for newer biologics.
Key points.
There is a positive correlation between biologic drug concentrations and favorable therapeutic outcomes in inflammatory bowel disease (IBD).
Reactive therapeutic drug monitoring (TDM) can efficiently guide the management of patients with primary non-response and secondary loss of response to anti-tumor necrosis factor (anti-TNF) therapy in IBD and has been proven more cost-effective when compared to empiric dose escalation.
Preliminary data suggest that proactive TDM of anti-TNF therapy is associated with better therapeutic outcomes when compared to empiric dose optimization and/or reactive TDM in IBD.
Proactive TDM can efficiently guide infliximab de-escalation or discontinuation in patients with IBD in remission.
Acknowledgments
Financial support and sponsorship: K.P. is supported by Ruth L. Kirschstein NRSA Institutional Research Training Grant 5T32DK007760-18 from National Institutes of Health (NIH).
Abbreviations:
- TDM
therapeutic drug monitoring
- IBD
inflammatory bowel disease
- ADA
anti-drug antibody
- CD
Crohn’s disease
- UC
ulcerative colitis
- ELISA
enzyme-linked immunosorbent assay
- TNF
tumor necrosis factor
- ADA
anti-drug antibodies
- SAE
serious adverse events
- HR
hazard ratio
- CI
confidence interval
- LOR
loss of response
- IMM
immunomodulator.
Footnotes
Declaration of interest: K.P. received a lecture fee from Mitsubishi Tanabe Pharma. A.S.C.: received a consultancy fee from Janssen, Abbvie, Takeda, Pfizer, Samsung, Arena, Bacainn, EMD Serono, Arsanis, Grifols, Prometheus; and research support from Inform Diagnostics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as: *of special interest **of outstanding interest.
- 1.Katsanos KH, Papamichael K, Feuerstein JD, et al. Biological therapies in inflammatory bowel disease: Beyond anti-TNF therapies. Clin Immunol 2019;206:9–14 [DOI] [PubMed] [Google Scholar]
- 2.Papamichael K, Lin S, Moore M, et al. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019. March 26;10:2040622319838443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparrow MP, Papamichael K, Ward MG, et al. Therapeutic drug monitoring of biologics during induction to prevent primary non-response. J Crohns Colitis. 2019. September 24. pii: jjz162. doi: 10.1093/ecco-jcc/jjz162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine S, Papamichael K, Cheifetz AS. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2019;15:656–665. [PMC free article] [PubMed] [Google Scholar]
- 5.Papamichael K, Cheifetz AS. Therapeutic drug monitoring in inflammatory bowel disease: for every patient and every drug? Curr Opin Gastroenterol. 2019. April 9. doi: 10.1097/MOG.0000000000000536. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeire S, Dreesen E, Papamichael K, et al. How, when, and for whom should we perform therapeutic drug monitoring? Clin Gastroenterol Hepatol. 2019. October 4. pii: S1542-3565(19)31092-4. doi: 10.1016/j.cgh.2019.09.041. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Ungar B, Glidai Y, Yavzori M, et al. Association Between Infliximab Drug and Antibody Levels and Therapy Outcome in Pediatric Inflammatory Bowel Diseases. J Pediatr Gastroenterol Nutr 2018;67:507–512 [DOI] [PubMed] [Google Scholar]
- 8.Clarkston K, Tsai YT, Jackson K, et al. Development of infliximab target concentrations during induction in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr 2019;69:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courbette O, Aupiais C, Viala J, et al. Trough levels of infliximab at w6 are predictive of remission at w14 in pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2020;70:310–317 [DOI] [PubMed] [Google Scholar]
- 10.Choi SY, Kang B, Choe YH. Serum infliximab cutoff trough level values for maintaining hematological remission in pediatric inflammatory bowel disease. Gut Liver 2019;13:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein R, Lee D, Leonard MB, et al. Serum infliximab, antidrug antibodies, and tumor necrosis factor predict sustained response in pediatric Crohn's disease. Inflamm Bowel Dis 2016;22:1370–1377. [DOI] [PubMed] [Google Scholar]
- 12.El-Matary W, Walters TD, Huynh HQ, et al. Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal Crohn's disease in children. Inflamm Bowel Dis 2019;25:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh N, Rosenthal CJ, Melmed GY, et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014. October;20(10):1708–1713. [DOI] [PubMed] [Google Scholar]
- 14.Naviglio S, Lacorte D, Lucafò M, et al. Causes of treatment failure in children with inflammatory bowel disease treated with infliximab: a pharmacokinetic Study. J Pediatr Gastroenterol Nutr 2019;68:37–44. [DOI] [PubMed] [Google Scholar]
- 15.Kang B, Choi SY, Choi YO, et al. Subtherapeutic infliximab trough levels and complete mucosal healing are associated with sustained clinical remission after infliximab cessation in paediatric-onset Crohn's disease patients treated with combined immunosuppressive therapy. J Crohns Colitis 2018;12:644–652. [DOI] [PubMed] [Google Scholar]
- 16.Kang B, Choi SY, Choi YO, et al. Infliximab trough levels are associated with mucosal healing during maintenance treatment with infliximab in paediatric Crohn's disease. J Crohns Colitis 2019;13:189–197. [DOI] [PubMed] [Google Scholar]
- 17.Van Hoeve K, Dreesen E, Hoffman I, et al. Higher infliximab trough levels are associated with better outcome in paediatric patients with inflammatory bowel disease. J Crohns Colitis 2019;12:1316–1325 [DOI] [PubMed] [Google Scholar]
- 18*.Vande Casteele N, Jeyarajah J, Jairath V, et al. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:1814–1821.This is a post-hoc analysis of the ACT-1 and 2 (A Safety and Efficacy Study for Infliximab in Patients with Active Ulcerative Colitis) RCTs showing that infliximab concentrations ≥18.6 μg/mL at week 2 and ≥10.6 μg/mL at week 6 are associated with a Mayo endoscopic score <2 at week 8.
- 19*.Dreesen E, Baert F, Laharie D, et al. Monitoring a combination of calprotectin and infliximab identifies patients with mucosal healing of Crohn's disease. Clin Gastroenterol Hepatol. 2020;18:637–646.This is a post-hoc of the TAILORIX (Drug-concentration versus Symptom-driven Dose Adaptation of Infliximab in patients with active Crohn’s disease) RCT identifying an infliximab threshold of 23.1 μg/mL at week and 10 μg/mL at week discriminating patients with early endoscopic remission at week 12 or not.
- 20*.Kennedy NA, Heap GA, Harry D, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study Lancet Gastroenterol Hepatol 2019;4:341–353.The PANTS (personalised anti-TNF therapy in Crohn's disease) is a prospective observational study showing that the optimal week 14 drug concentrations associated with remission at both week 14 and week 54 were 7 mg/L for infliximab and 12 mg/L for adalimumab.
- 21.Papamichael K, Rakowsky S, Rivera C, et al. Association between serum infliximab trough concentrations during maintenance therapy and biochemical, endoscopic and histologic remission in Crohn’s disease. Inflamm Bowel Dis 2018;24:2266–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papamichael K, Rakowsky S, Rivera C, et al. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment Pharmacol Ther 2018;47:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juncadella A, Papamichael K, Vaughn B, et al. Maintenance adalimumab concentrations are associated with biochemical, endoscopic and histologic remission in inflammatory bowel disease. Dig Dis Sci 2018;63:3067–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vande Casteele N, Feagan BG, Vermeire S, et al. Exposure–response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn’s disease. Aliment Pharmacol Ther 2018;47:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther 2019;49:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–1946. [DOI] [PubMed] [Google Scholar]
- 27.Pouillon L, Rousseau H, Busby-Venner et al. Vedolizumab trough levels and histological healing during maintenance therapy in ulcerative Colitis. J Crohns Colitis 2019;13:970–975. [DOI] [PubMed] [Google Scholar]
- 28.Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn's disease. Gastroenterology 2018;154:1660–1671. [DOI] [PubMed] [Google Scholar]
- 29.Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Crohn's disease only in part explains limited endoscopic remission rates. J Crohns Colitis 2019;13:864–872 [DOI] [PubMed] [Google Scholar]
- 30.Soufflet N, Boschetti G, Roblin X, et al. Concentrations of ustekinumab during induction therapy associate with remission in patients with Crohn's Disease. Clin Gastroenterol Hepatol 2019;17:2610–2612 [DOI] [PubMed] [Google Scholar]
- 31.Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther 2017;45:933–940. [DOI] [PubMed] [Google Scholar]
- 32.Ungaro RC, Yarur A, Jossen J, et al. Higher trough vedolizumab concentrations during maintenance therapy are associated with corticosteroid-free remission in inflammatory bowel disease. J Crohns Colitis 2019;13:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarur AJ, Bruss A, Naik S, et al. Vedolizumab concentrations are associated with long-term endoscopic remission in patients with inflammatory bowel diseases. Dig Dis Sci 2019;64:1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungar B, Kopylov U, Yavzori M, et al. Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:697–705. [DOI] [PubMed] [Google Scholar]
- 35.Papamichael K, Vogelzang EH, Lambert J, et al. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev Clin Immunol 2019;15:837–848. [DOI] [PubMed] [Google Scholar]
- 36.Feng T, Chen B, Ungar B, et al. Association of infliximab levels with mucosal healing is time-dependent in Crohn's disease: higher drug exposure is required postinduction than during maintenance treatment. Inflamm Bowel Dis 2019;25:1813–1821. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Dulai PS, Vande Casteele N, et al. Systematic review with meta-analysis: association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2019;50:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Adedokun OJ, Xu Z, Marano C, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis: ustekinumab PK and exposure-response in UC. Clin Gastroenterol Hepatol. 2019. December 6. pii: S1542-3565(19)31403-X. doi: 10.1016/j.cgh.2019.11.059. [Epub ahead of print]This is a post-hoc analysis of the UNIFI (A Study to Evaluate the Safety and Efficacy of Ustekinumab Induction and Maintenance Therapy in Participants With Moderately to Severely Active Ulcerative Colitis) RCT identifying a target concentration threshold ≥3.7μg/mL at week 8 for achievement of clinical response at week 8 and a target concentration threshold ≥1.3μg/mL for clinical remission at week 44.
- 39.Löwenberg M, Vermeire S, Mostafavi N, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn's disease. Gastroenterology 2019;157:997–1006. [DOI] [PubMed] [Google Scholar]
- 40.Guidi L, Pugliese D, Tonucci TP, et al. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United European Gastroenterol J 2019;7:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Sandborn WJ, Baert F, Danese S, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2019. August 28. pii: S0016-5085(19)41247-X. doi: 10.1053/j.gastro.2019.08.027. [Epub ahead of print]The VISIBLE 1 [Efficacy and Safety of Vedolizumab Subcutaneously (SC) as Maintenance Therapy in Ulcerative Colitis] is a RCT showing that the proportion of patients receiving vedolizumab SC for maintenance who achieved clinical remission, defined as a total Mayo score of ≤2 and no subscore >1 at week 52, increased with increasing vedolizumab exposure from 50% (quartile 1) to 83% (quartile 4).
- 42.Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2019;17:1655–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Berghe N, Gils A, Thomas D. Achieving mucosal healing in inflammatory bowel diseases: which drug concentrations need to be targeted? Clin Pharmacol Ther 2019;106:945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ungar B, Engel T, Yablecovitch D, et al. Prospective observational evaluation of time-dependency of adalimumab immunogenicity and drug concentrations: The POETIC Study. Am J Gastroenterol 2018;113:890–898. [DOI] [PubMed] [Google Scholar]
- 45.Bar-Yoseph H, Levhar N, Selinger L, et al. Early drug and anti-infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment Pharmacol Ther 2018;47:212–218. [DOI] [PubMed] [Google Scholar]
- 46.Dreesen E, Van Stappen T, Ballet V, et al. Anti-infliximab antibody concentrations can guide treatment intensification in patients with Crohn's disease who lose clinical response. Aliment Pharmacol Ther 2018;47:346–355. [DOI] [PubMed] [Google Scholar]
- 47.Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13:522–530. [DOI] [PubMed] [Google Scholar]
- 48.Restellini S, Chao CY, Lakatos PL, et al. Therapeutic drug monitoring guides the management of Crohn's patients with secondary loss of response to adalimumab. Inflamm Bowel Dis 2018;24:1531–1538. [DOI] [PubMed] [Google Scholar]
- 49.Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014;109:1250–1256. [DOI] [PubMed] [Google Scholar]
- 50.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–927. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y, Lin B, Thilakanathan C, et al. Therapeutic drug monitoring in inflammatory bowel disease reduces unnecessary use of infliximab with substantial associated cost-savings. Intern Med J 2019. October 7. doi: 10.1111/imj.14644. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Guidi L, Pugliese D, Panici Tonucci T, et al. Therapeutic drug monitoring is more cost-effective than a clinically-based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multi-centre study. J Crohns Colitis 2018;12:1079–1088 [DOI] [PubMed] [Google Scholar]
- 53.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–1329. [DOI] [PubMed] [Google Scholar]
- 54.Papamichael K, Vajravelu RK, Vaughn BP, et al. Proactive infliximab monitoring following reactive testing is associated with better clinical outcomes than reactive testing alone in patients with inflammatory bowel disease. J Crohns Colitis 2018;12:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared to standard of care in patients with inflammatory bowel disease. J Crohns Colitis 2019;13:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn’s disease compared with reactive monitoring. Gastroenterology. 2019. October;157(4):985–996.e2.The PAILOT (Paediatric Crohn’s disease Adalimumab-Level-based Optimisation Treatment) is a RCT showing that steroid-free clinical remission rate at week 72 was higher in the proactive compared to the reactive TDM group.
- 58.Fernandes SR, Bernardo S, Simões C, et al. Proactive Infliximab Drug Monitoring Is Superior to Conventional Management in Inflammatory Bowel Disease. Inflamm Bowel Dis 2020;26:263–270 [DOI] [PubMed] [Google Scholar]
- 59.Sanchez-Hernandez JG, Rebollo N, Martin-Suarez A, et al. A three-year prospective study of a multidisciplinary early proactive therapeutic drug monitoring program of infliximab treatments in inflammatory bowel disease. Br J Clin Pharmacol. 2020. February 5. doi: 10.1111/bcp.14229. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucidarme C, Petitcollin A, Brochard C, et al. Predictors of relapse following infliximab de-escalation in patients with inflammatory bowel disease: the value of a strategy based on therapeutic drug monitoring. Aliment Pharmacol Ther 2019;49:147–154. [DOI] [PubMed] [Google Scholar]
- 61.Petitcollin A, Brochard C, Siproudhis L, et al. Pharmacokinetic parameters of infliximab influence the rate of relapse after de-escalation in adults with inflammatory bowel diseases. Clin Pharmacol Ther 2019;106:605–615 [DOI] [PubMed] [Google Scholar]
- 62.Papamichael K, Vande Casteele N, Gils A, et al. Long-term outcome of patients with Crohn’s disease who discontinued infliximab therapy upon clinical remission. Clin Gastroenterol Hepatol 2015;13:1103–1110. [DOI] [PubMed] [Google Scholar]
- 63.Bots SJ, Kuin S, Ponsioen CY, et al. Relapse rates and predictors for relapse in a real-life cohort of IBD patients after discontinuation of anti-TNF therapy. Scand J Gastroenterol 2019;54:281–288. [DOI] [PubMed] [Google Scholar]
- 64.Chapman TP, Gomes CF, Louis E, et al. De-escalation of immunomodulator and biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2020;5:63–79. [DOI] [PubMed] [Google Scholar]
- 65.Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 66.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres J, Bonovas S, Doherty G, et al. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis 2020;14:4–22. [DOI] [PubMed] [Google Scholar]
- 68.Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;46:1037–1053. [DOI] [PubMed] [Google Scholar]
- 69.Mack DR, Benchimol EI, Critch J, et al. Canadian association of gastroenterology clinical practice guideline for the medical management of pediatric luminal Crohn’s disease. Gastroenterology 2019;157:320–348 [DOI] [PubMed] [Google Scholar]
- 70.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 71.Clarke WT, Papamichael K, Casteele NV, et al. Infliximab and adalimumab concentrations may vary between the enzyme-linked immunosorbent assay and the homogeneous mobility shift assay in patients with inflammatory bowel disease: a prospective cross-sectional observational study. Inflamm Bowel Dis 2019;25:e143–e145. [DOI] [PubMed] [Google Scholar]
- 72.Gibson DJ, Ward MG, Rentsch C, et al. Review article: determination of the therapeutic range for therapeutic drug monitoring of adalimumab and infliximab in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020. January 21. doi: 10.1111/apt.15643. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73**.Roblin X, Williet N, Boschetti G, et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut. 2020. January 24. pii: gutjnl-2019–319758. doi: 10.1136/gutjnl-2019-319758. [Epub ahead of print]This RCT demonstrated that in patients with loss of response to a first anti-TNF due to immunogenicity the use of azathioprine in combination with the second anti-TNF was associated with a significant reduction in the risk of developing anti-drug antibodies and low drug concentrations.
- 74.Kelly OB, Donnell SO, Stempak JM, et al. Therapeutic drug monitoring to guide infliximab dose adjustment is associated with better endoscopic outcomes than clinical decision making alone in active inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1202–1209. [DOI] [PubMed] [Google Scholar]
- 75.Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology 2020;158:189–199 [DOI] [PubMed] [Google Scholar]
- 76.Papamichael K, Mantzaris GJ, Peyrin-Biroulet L. A safety assessment of anti-tumor necrosis factor alpha therapy for treatment of Crohn's disease. Expert Opin Drug Saf 2016;15:493–501. [DOI] [PubMed] [Google Scholar]
- 77.Lega S, Phan BL, Rosenthal CJ, et al. Proactively optimized infliximab monotherapy is as effective as combination therapy in IBD. Inflamm Bowel Dis 2019;25:134–141. [DOI] [PubMed] [Google Scholar]
- 78.Drobne D, Kurent T, Golob S, et al. Optimised infliximab monotherapy is as effective as optimised combination therapy, but is associated with higher drug consumption in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:880–889. [DOI] [PubMed] [Google Scholar]
- 79**.Colombel JF, Adedokun OJ, Gasink C, et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin Gastroenterol Hepatol 2019;17:1525–1532.This is a post-hoc analysis of the SONIC (Study of Biologic and Immunomodulator Naive Patients in Crohn Disease) RCT which demonstrated that patients stratified by infliximab concentration quartiles had similar outcomes irrespective of concomitant azathioprine.
- 80.Vande Casteele N Assays for measurement of TNF antagonists in practice. Frontline Gastroenterol. 2017;8:236–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imbrechts M, Van Stappen T, Compernolle G, et al. Anti-infliximab antibodies: How to compare old and new data? J Pharm Biomed Anal. 2020;177:112842. [DOI] [PubMed] [Google Scholar]
- 82.Syversen SW, Goll GL, Jørgensen KK, et al. Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study). Trials 2020;21:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strik A, Berends S, Mould D, et al. Dashboard driven vs. conventional dosing of infliximab in inflammatory bowel disease patients: the PRECISION trial. J Crohn's Colitis 2019;13:S063 [Google Scholar]
- 84.Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther 2018;48:731–739. [DOI] [PubMed] [Google Scholar]


