Investigating the evolutionary history of widespread higher taxa, subjected to multiple tectonic events, can provide evidence for or against various palaeogeographical models of early Earth history [1,2]. Contemporary biotic distributions have been strongly influenced by events associated with the breakup of Gondwana into present-day Africa, Antarctica, Australia, South America, Madagascar and India, during the Late Mesozoic and Early Palaeogene [2]. The fragmentation of Gondwana and subsequent tectonic drift ultimately allowed biotic exchanges between Laurasia and Gondwana [2,3], influencing the global distributions of many taxa.
The relative positions of the post-breakup Gondwanan landmasses during the Late Cretaceous, especially of the Indian and Australian plates around the Indian Ocean, are highly debated [1]. The plate reshuffling was probably accompanied by the formation of multiple temporary land bridges and involved biotic exchange among the plates. Although most models agree that the Indian Plate carried a biotic ‘ferry’ of taxa (both plants and animals) to Asia after it broke away from other Gondwanan landmasses from about 88 to 55Ma [2,4], both geological and paleontological data also support land bridges or minor marine barriers that permitted biotic exchanges with other Gondwanan landmasses (e.g. Africa, Madagascar; Fig.1)
Figure 1.
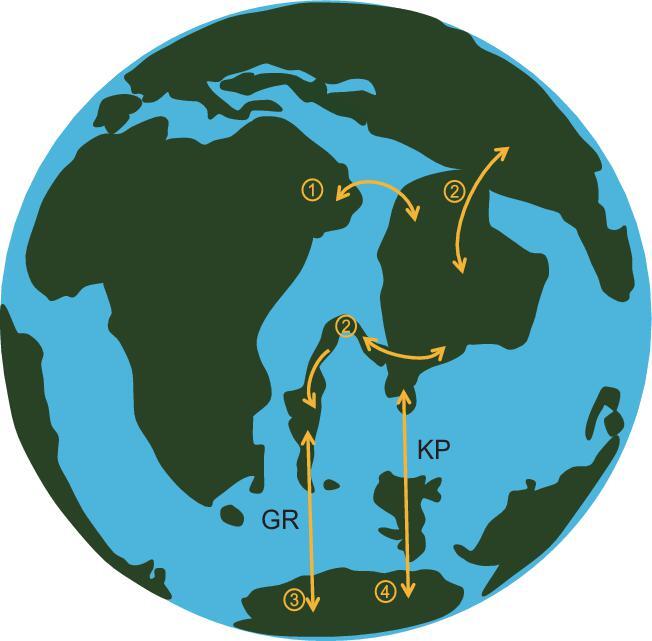
Schematic representation of different hypotheses regarding land connections and corridors for dispersal among the landmasses around the Indian Ocean from 88 to 55Ma. (1) Africa and India were reconnected to each other directly [3,5]; (2) Asia and Madagascar were linked by India, with possible dispersal between Asia and Madagascar over India and the Seychelles plateau [5]; (3) Antarctic–Australia–New Guinea and Madagascar were connected by the Gunnerus Ridge (GR) [6,7]; (4) Antarctic–Australia–New Guinea and India were connected by the Kerguelen Plateau (KP) [13]. Paleo-reconstructions are modified from Briggs [5] and Bossuyt etal. [12].
[3,5]. There is also debate about whether Antarctica–Australia–New Guinea was connected to: (i) the Indian Plate via the Kerguelen Plateau (KP) land bridge and (ii) Madagascar via the Gunnerus Ridge (GR) land bridge in the Late Cretaceous [6–9]. Previous studies addressed some of these issues from palaeobiogeographical and evolutionary perspectives, but were inconclusive due to the selection of taxa that did not include all landmasses or the limited recovery of evolutionary relationships due to the use of sequence data from only a few genes [10–13].
The neobatrachian clade Natatanura are an ideal group to infer Gondwanan geological and environmental history due to their ancient origins (divergence from Afrobatrachia at around 100Ma), high species diversity (>1500 extant species), almost cosmopolitan distribution (absent only from Antarctica), general low terrestrial vagility and poor overwater dispersal capabilities [14]. Previous studies suggested the divergence of Natatanura was characterized by a historical association with the breakup of Gondwanan plates [12]. These frogs are thus an appropriate group of organisms to test hypotheses of Cretaceous–Palaeogene biotic exchanges between Laurasia and Gondwana around the Indian Ocean. However, prior studies that have included Natatanura failed to resolve the major nodes in its phylogeny or suffered from incomplete lineage
sampling, which, until now, hampered conclusive testing of these hypotheses [12,15].
Here, we integrate phylogenetic, biogeographic and molecular dating methods to reconstruct the spatiotemporal diversification of Natatanura (see Supplementary Data). Results resolve the evolutionary history of Natatanuran frogs (Supplementary Figs 1–3), based on molecular data from 376 nuclear loci, representing by far the largest molecular data set assembled for this group. Samples include all major lineages, 85 Natatanura species and 20 outgroup taxa (Supplementary Data). The novel evidence reveals how Natatanuran frogs interchanged between Laurasia and Gondwana around the Indian Ocean during the Cretaceous–Palaeogene, challenging recent biogeographical assumptions and providing new insights into Indian Ocean biotic exchanges.
STEPPING-STONE ROLE OF THE INDIAN PLATE FOR BIOTIC EXCHANGE BETWEEN AFRICA, ASIA AND MADAGASCAR
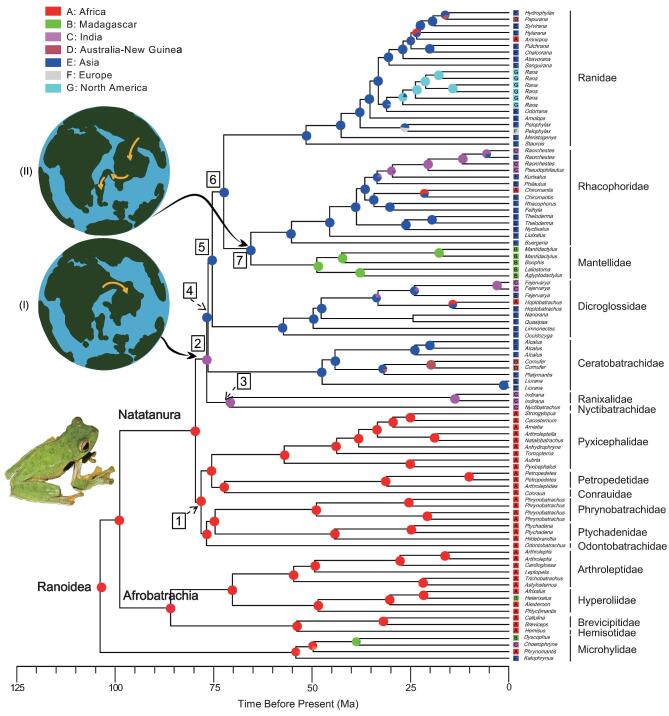
Using the traditional Gondwana and Laurasia model, it has been commonly assumed that the Indian Plate was an isolated island between ∼88–55Ma, and the Indian landmass served as an ‘ark’ to transport lineages from various biotic groups ‘Out-of-India’ into Asia, after India broke away and drifted northward from Gondwana in the Late Cretaceous [4]. According to this model, there was no biotic exchange (which would have required crossing marine barriers) between India and its nearby landmass after it separated from Madagascar ∼88Ma, and dispersal events only resumed after India's collision with Asia in the early Eocene (∼65–55Ma, [16]). ‘Rafting’ of the flora and fauna on the Indian Plate enabled unidirectional migration of Gondwanan taxa into Asia. However, our phylogenomic results reject this model. We do not find any periods between 88 and 55Ma, when there was no biotic exchange occurring between Africa and India, India and Asia, or India and Madagascar. In contrast, our ancestral reconstruction suggests Natatanura originated in Africa and then dispersed to Asia through India ∼75.6–72.8Ma (Fig.2 and Supplementary Fig. 3). It is unlikely that frogs could cross a large saltwater barrier, although a few extant species may have made more modest oceanic dispersals (e.g. Ptychadena mascareniensis, [17]). Briggs [5] and Chaterjee et al. [3] suggested a geographical model in which there were ‘corridors’ or ‘landspans’ that reconnected Africa and India from ∼75 to ∼60Ma. Our topologies and divergence time estimates are consistent with this scenario. A terrestrial route possibly existed from Africa to Asia via India, which would have allowed frogs to disperse among these landmasses. Moreover, Malagasy mantellid frogs are phylogenetically deeply nested within the larger Asian clade and the ancestral reconstruction supports this clade originating from Asia and dispersing to Madagascar (Fig.2). Taking into account that the oldest known rhacophorid fossils (Indorana prasadi) are from the Indian landmass [18] and that the Indian Plate would have been well placed to minimize oceanic dispersal distances between Asia and Madagascar, the Indian Plate could have served as a stepping stone for long-distance dispersal, as suggested previously [13,15].
Figure 2.
Ancestral-area estimations for the species of Ranoidea, using the DEC+J model in BioGeoBEARS. Circles at nodes represent the set of possible ancestral areas and the colours reflect biogeographic designations (see area code key). Clades of interest are numbered in boxes. Models show the stepping-stone role of the Indian Plate to biotic exchange between India and Africa (I) and among India, Asia and Madagascar (II). Paleo reconstructions are modified from Chatterjee et al. [3] and Briggs [5].
Geological and paleontological evidence has previously challenged the ‘Indian-biotic Ark’ model. For example, Briggs [5] proposed that India was in close proximity to other landmasses during its journey northwards because a significant endemic biota did not evolve on the Indian Plate during this period. Ali and Aitchison [8] proposed the existence of a palaeogeographic connection between Madagascar and India in the Late Cretaceous, which may have been formed by the Seychelles–Mascarene Plateau. More recently, Chatterjee et al. [3] argued that biotic links were possibly re-established between India and Africa during the Late Cretaceous, during India's collision with the Kohistan–Ladakh Arc along the Indus Suture in the Late Cretaceous. Sister relationships and divergence dates for some vertebrate fossil groups (Supplementary Data) also support stepping-stone biotic exchange via the Indian Plate, consistently with our results. However, we did not find any studies of nonvolant extant groups that provide substantial evidence for using the Indian Plate as a stepping-stone route among these three plates from 88 to 55Ma. Although several plant and animal groups exhibit sister relationships between India (or Asia) and Madagascar (or Africa) (e.g. [19,20]), their deep divergences (>88Ma) attribute this relationship to ancient vicariance coinciding with the breakup of Gondwana. Nevertheless, other taxa with younger divergences, and distributions that thus cannot be attributed to ancient Gondwanan vicariance, will represent good candidates for testing ‘Indian stepping-stone hypotheses’ in the future (e.g. microhylid frogs, [21]). We provide the first case of extant taxa that appears to have taken advantage of the Indian Plate as a stepping-stone route between Africa, Asia and Madagascar, although the exact positions of land bridges or traversable ocean channels still remain unclear. And we predict that this geographic scenario will also be recovered for other nonvolant organisms with Indian Ocean distributions.
DISPERSALS WITH AUSTRALIA–NEW GUINEA AND ASIA
The Antarctica–Australia–New Guinea Plate has been proposed to have been connected to the Indian Plate by the KP land bridge, or connected to Madagascar by the GR land bridge (Fig.1) in the Late Cretaceous [6,7], although these land bridges have been disputed due to a lack of evidence that they were sub-aerial during this period [8,9]. Concerning Natatanura diversification, we find no sister relationships between frogs from Antarctica–Australia–New Guinea and either India or Madagascar (Fig.2), and thus find no support for biota exchanges among these landmasses via a KP and GR land bridge. In addition, we date Natatanura dispersal into Australia–New Guinea to be much later than these hypothetical Late Cretaceous land bridges.
Bossuyt et al. [12] suggested that Australia–New Guinea acted as a raft, enabling Gondwanan Natatanura frogs to colonize Southeast Asia, although most of their basal relationships were not well resolved. Our genomic-based estimates of phylogeny, divergence times and biogeographic reconstruction cast doubt on this dispersal route. We found
strong support that the two Australia–New Guinea clades, Cornufer and Papurana, were embedded within Asian clades of Ceratobatrachidae and Ranidae, respectively. Ancestral state analysis suggests Asian origins, followed by migration into Australia–New Guinea for Cornufer
(30.2Ma, 95% highest posterior density (HPD): 21.3–40.0Ma) and Papurana (14.9Ma, 95% HPD: 10.4–19.5Ma) independently (Fig.2 and Supplementary Fig. 3). These dispersals could have occurred after the Australia–New Guinea Plate first collided with
Sundaland in the Early Miocene [22]. Tectonic collision and extensive island formations in Wallacea provided a direct colonization route between Asia and Australasia, and are suggested to have triggered much of the biotic interchange between the regions. For example, Miocene dispersals between these two regions are known in plants [23], birds [24] and mammals [25]. However, few dated phylogenies of herpetofauna exist that directly shed light on this issue, although those that do show similar patterns with evidence of immigrations [26]. Additional studies with increased taxon sampling should help to identify the common time periods and directions of dispersals taken by these species.
MULTIPLE DISPERSALS FROM ASIA TO AFRICA
Reconnections between Gondwana and Laurasia-origin landmasses in the Neogene allowed extensive biotic interchanges between Africa and Eurasia [3]. These biotas could have migrated across the western margin of the Mediterranean Sea or through the Afro-Arabian to Eurasian land bridge [27]. Our results suggest three groups of ranoid frogs dispersed independently from Asia to Africa. The dispersals of Ranidae (Amnirana) and Rhacophoridae (Chiromantis) appear to have occurred in a similar time period: ∼21.6 and ∼20.6Ma, respectively (Fig.2 and Supplementary Fig. 3). This period is consistent with collision of the Afro-Arabian Plate with Eurasia during the mid-Burdigalian (∼19–21Ma) causing the emergence of a terrestrial corridor, called ‘the Gomphotherium Land Bridge’ [28]. This land bridge later became disconnected intermittently, but it appears to have been continuously present since ∼15Ma ago, triggering mammals [29], reptiles [30], invertebrate [31] and possibly also frogs (our results) to exchange between Africa and Eurasia. Interestingly, our results also support another colonization from Asia to Africa by a lineage of Dicroglossidae (Hoplobatrachus), which occurred much later (∼12.7Ma). This implies that habitats in the North Africa and Afro-Arabian plates were suitable for amphibian dispersal during the middle Miocene. As a consequence of shrinkage of the Tethys Sea, desert conditions expanded across North Africa in the late Miocene (∼7Ma), marking the origin of the Sahara Desert, and also the deserts of the Middle East and the Arabian Peninsula [32], which subsequently hindered the migration of most mesic-adapted species between Africa and Eurasia.
Supplementary Material
Acknowledgements
We extend our sincere gratitude to Robert W. Murphy, Amy Lathrop, Abigail Cramer, Alan Resetar, Jimmy A. McGuire, Carol Spencer, Robert Drewes, Jens Vindum, Bryan L. Stuart, Zoe Davids, Aaron Bauer, Raj Patel and John Smith for the use of tissues held in their collections. We are grateful to Alyssa Bigelow Hassinger, Hannah Ralicki, Kirby Birch and Ameer Jalal at the Florida State University Center for Anchored Phylogenomics for assistance with molecular data collection and bioinformatics analysis.
FUNDING
This work was supported by the programs of the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDA20050201), the National Natural Science Foundation of China (NSFC 31672268, 31622052), the International Partnership Program of Chinese Academy of Sciences (152453KYSB20170033) and the Animal Branch of the Germplasm Bank of Wild Species, CAS (Large Research Infrastructure Funding) to J.C.; the National Science Foundation (USA) BIO-DEB 1021247 to E.S.P. and C.J.R.; BIO-DEB 135500 to D.W.W.; BIO-GRFP 3048109801 to P.M.H.; BIO-DEB 1021299 to K.M. Kjer; BIO-DEB 1120516 to E.M.L.; and NSF-IIP to A.R.L. and E.M.L.; the Strategic Priority Research Program, CAS (XDPB020406), the National Key Research and Development Program of China (2017YFC0505202), Southeast Asia Biodiversity Research Institute, CAS (Y4ZK111B01: 2017CASSEABRIQG002) to W.-W.Z.; the Sino-Africa Joint Research Center, CAS (SAJC201611) to M.-S.P.; the funding from the State Key Laboratory of Genetic Resources and Evolution (GREKF18-15) and NSFC (31501843, 31702008) to Z.-Y.Y.; J.C and M.-S.P are supported by the Youth Innovation Promotion Association CAS.
REFERENCES
- 1. Upchurch P. Trends Ecol Evol 2008; 23: 229–36. [DOI] [PubMed] [Google Scholar]
- 2. Li J, Li Y, Klaus S et al. Proc Natl Acad Sci USA 2013; 110: 441–3446. [Google Scholar]
- 3. Chatterjee S, Goswami A, Scotese CR. Gondwana Research 2013; 23: 238–67. [Google Scholar]
- 4. Scotese CR. Atlas of Earth History. Arlington: University of Texas, 2001. [Google Scholar]
- 5. Briggs JC. J Biogeogr 2003; 30: 381–8. [Google Scholar]
- 6. Case JA. J Vert Paleont 2002; 22: 42A. [Google Scholar]
- 7. Hay WW, DeConto RM, Wold CN et al. Alternative global Cretaceous paleogeography. In: Barrera E, Johnson CC (eds). Evolution of the Cretaceous Ocean Climate System. Boulder CO: Geological Society of America Special Paper, 1999, 1–48. [Google Scholar]
- 8. Ali JR, Aitchison JC. Earth-Sci Rev 2008; 88: 145–66. [Google Scholar]
- 9. Ali JR, Aitchison JC. J Biogeogr 2009; 36: 1778–84. [Google Scholar]
- 10. Samonds KE, Godfrey LR, Ali JR et al. PLoS ONE 2013; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verma O, Khosla A, Goin FJ et al. New Mexico Mus Nat Hist Sci Bull 2016; 71: 317–30. [Google Scholar]
- 12. Bossuyt F, Brown RM, Hillis DM et al. Syst Biol 2006; 55: 579–94. [DOI] [PubMed] [Google Scholar]
- 13. Crottini A, Madsen O, Poux C et al. Proc Natl Acad Sci USA 2012; 109: 5358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. University of California, Berkeley . AmphibiaWeb: Information on Amphibian Biology and Conservation. https://amphibiaweb.org/taxonomy/index.html(15 February 2018, date last accessed). ). [Google Scholar]
- 15. Feng YJ, Blackburn DC, Liang D et al. Proc Natl Acad Sci USA 2017; 29: E5864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aitchison JC, Ali JR, Davis AM. J Geophys Res 2007; 112: B05423. [Google Scholar]
- 17. Vences M, Kosuch J, Rodel M et al. J Biogeogr 2004; 31: 593–601. [Google Scholar]
- 18. Folie A, Rana RS, Rose KD et al. Acta Palaeontol Pol 2013; 58: 511–24. [Google Scholar]
- 19. Kamei RG, San Mauro D, Gower DJ et al. Proc Biol Sci 2012; 279: 2396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas N, Bruhl JJ, Ford A et al. J Biogeogr 2014; 41: 894–904. [Google Scholar]
- 21. van der Meijden A, Vences M, Hoegg S et al. Mol Phylogen Evol 2007; 44: 1017–30. [DOI] [PubMed] [Google Scholar]
- 22. Hall R. J Asian Earth Sci 2002; 20: 353–431. [Google Scholar]
- 23. Sniderman JMK, Jordan GJ. J Biogeogr 2011; 38: 1445–55. [Google Scholar]
- 24. Moyle RG, Oliveros CH, Andersen MJ et al. Nat Comms 2016; 7:12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lecompte E, Aplin K, Denys C et al. BMC Evol Biol 2008; 8: 199–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Byrne M, Steane DA, Joseph L et al. J Biogeogr 2011; 38: 1635–56. [Google Scholar]
- 27. Duggen S, Hoernle K, van den Bogaard P et al. Nature 2003; 422: 602–6. [DOI] [PubMed] [Google Scholar]
- 28. Bosworth W, Huchon P, McClay K. J Afr Earth Sci 2005; 43: 334–78. [Google Scholar]
- 29. Antoine PO, Welcomme J, Marivaux L et al. J Vertebr Paleontol 2003; 23: 977–80. [Google Scholar]
- 30. Tamar K, Carranza S, Sindaco R et al. Mol Phylogen Evol 2016; 103: 6–18. [DOI] [PubMed] [Google Scholar]
- 31. Harzhauser M, Kroh A, Mandic O et al. Zool Anz 2007; 24: 241–56. [Google Scholar]
- 32. Zhang Z, Ramstein G, Schuster M et al. Nature 2014; 513: 401–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.