Abstract
Background
Blebs are rupture risk factors in intracranial aneurysms (IAs), but their prevalence, distribution, and associations with clinical factors as well as their causes and effects on aneurysm vulnerability remain unclear.
Methods
A total of 122 blebs in 270 IAs selected for surgery were studied using patient-specific vascular reconstructions from 3D angiographic images. Bleb geometry, location on the aneurysm, and frequency of occurrence in aneurysms at different locations were analyzed. Associations between gender, age, smoking, hypertension, hormone therapy, dental infection, and presence of blebs were investigated.
Results
Of all aneurysms with blebs 77% had a single bleb and 23% had multiple blebs. Only 6% of blebs were at the neck, while 46% were in the body, and 48% in the dome. Aneurysms harboring blebs were larger (p<0.0001), more elongated (p=0.0002), and with wider necks than aneurysms without blebs. Bleb presence was associated with dental infection (p=0.0426), and negatively associated with hormone therapy (p=0.0426) in females. Anterior and posterior communicating arteries had larger percentages of aneurysms harboring blebs than internal carotid arteries. Patients with history of hypertension tended to have larger percent of aneurysms with blebs. However, these trends did not reach significance in this sample
Conclusions
Blebs are common in IAs, and most aneurysms harboring blebs have a single bleb. Blebs in the aneurysm neck are rare, but they are equally common in the body and dome. Presence of blebs in IAs was associated with dental infection, and negatively associated with hormone replacement therapy.
Introduction
Blebs are focal bulges observed on the surface of intracranial aneurysms (IAs) walls. Thin blebs have been observed in association with rupture sites in autopsies, and adjacent to fatty walls during surgery.1 Irregular aneurysm shape including blebs have been considered risk factors for aneurysm growth and rupture.2–8 As such, presence of blebs has been incorporated into aneurysm scoring scales,9,10 and recently they have been proposed as strong risk indicators independent from aneurysm size and location.11
Blebs have been suggested to be a reflection of focal weakening of the wall,11,12 which may develop in response to locally adverse flow conditions,13,14 and may influence aneurysm vulnerability by concentrating wall stresses at the neck of the bleb15 while decreasing tensile stress at the bleb fundus.16
Despite progress in the analysis of blebs, the overall bleb prevalence and distribution in the IA population, the causes and factors associated to their development, and their effect on aneurysm vulnerability are not fully understood. This report provides a description of bleb prevalence and associations with clinical and anatomical characteristics and sets the stage for further studies analyzing the relationship between blebs and flow, biomechanic, and tissue characteristics.
Methods
Data
A total of 199 patients with 270 intracranial aneurysms selected for surgical clipping at 3 hospitals and imaged with 3D rotational angiography (3DRA) or computed tomographic angiography (CTA) before surgery were included in this study.17 As part of a larger project,18 clinical information was gathered through a patient questionnaire before surgery, including: patient sex, age, smoking history, hypertension, hormone treatment, and dental infection (see Supplementary Table I). Protocols for consent, data handling and analysis were approved by the institutional review board (IRB) at the University of Pittsburgh, University of Illinois at Chicago, Allegheny General Hospital, and Helsinki University Hospital. The average patient age was 55.7y (median=57y, range=25–81y), there were 131 (74%) females and 45 (26%) males (questionnaire information was unavailable for a subset of patients from Helsinki University Hospital, missing data is unrelated to any selection bias), and mean aneurysm size was 7.5mm (range=1.3 – 42.6mm).
Bleb Identification and Characterization
Blebs are defined as secondary outpouchings or structures clearly visible and different from the original aneurysm. Well defined blebs are clearly visible because of the change in the surface curvature that occurs at their necks. Our methodology for bleb identification and characterization is illustrated in Figure 1 with an example aneurysm harboring two blebs and described below.
Figure 1.
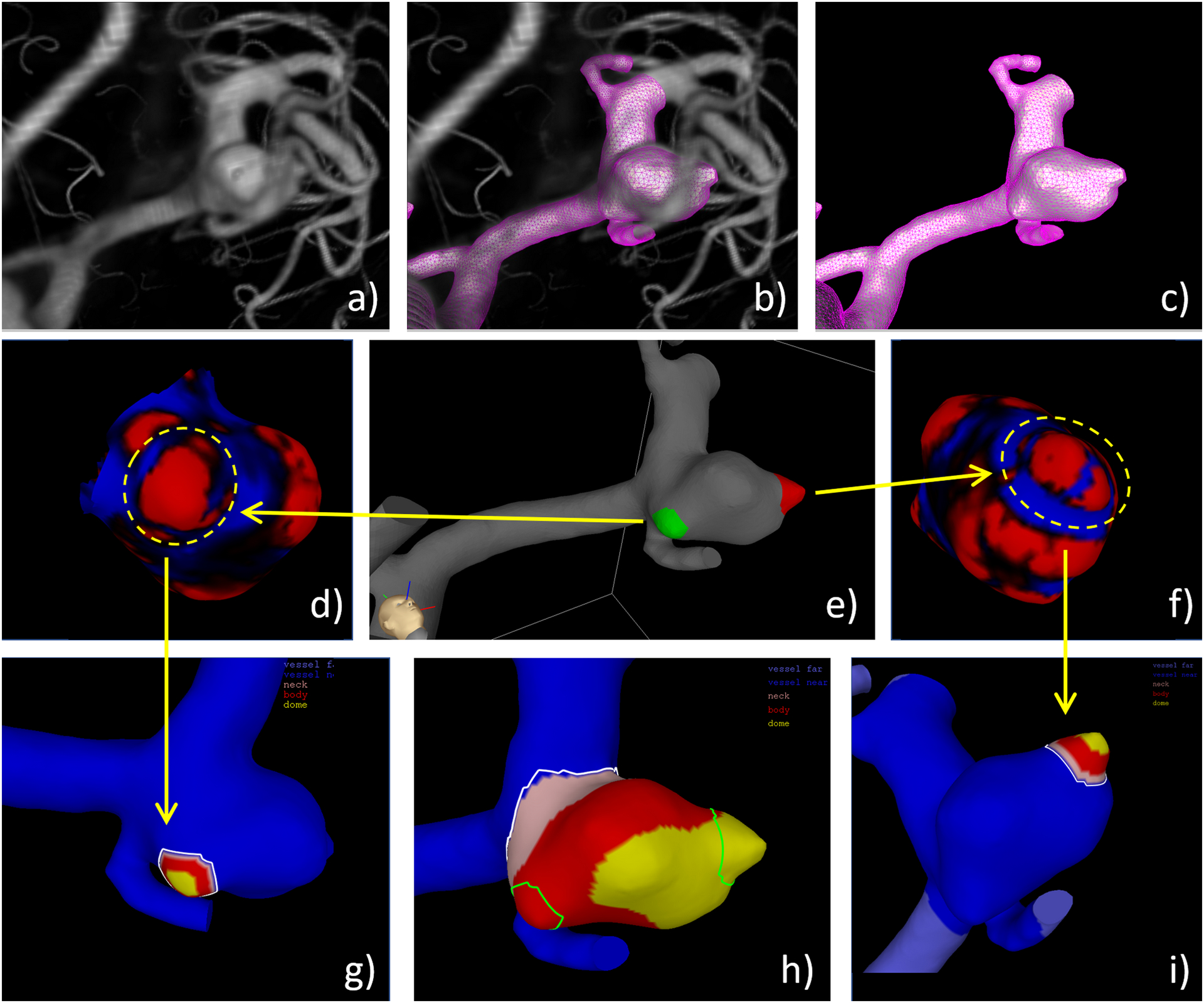
Bleb identification and marking on example aneurysm with two blebs: a) volume rendered 3DRA image, b) superposition of reconstructed vascular model and 3DRA image, c) vascular model, d) curvature map around bleb 1 (red=positive curvature, blue=negative curvature), e) labeling on bleb 1 (green) and 2 (red) by observer using ChePen3D tool as shown in Figure 2, f) curvature map around bleb 2, g) geometric characterization of bleb 1, h) overlapping of blebs and aneurysm regions (pink=neck, red=body, yellow=dome) to determine bleb location on aneurysm sac, and i) geometric characterization of bleb 2. Note the blue band observed in bleb 2 (f) which corresponds to a small surface indentation that was not sufficiently large to consider this bleb as two separate structures.
Patient-specific vascular models were reconstructed from pre-surgical 3D images as previously described.18 Figure 1 shows the original 3DRA image (a,b) and the reconstructed patient-specific vascular model (b,c).
Blebs were then identified by visual inspection of the vascular reconstructions, volume rendered 3D images, and surface curvature maps (measure of the local bending of the aneurysm surface). In these curvature maps, in general blebs appear as red regions of positive curvature surrounded by a blue ring of negative curvature. Examples are shown in Figure 1(d,f) to illustrate this point.
Blebs were then interactively marked (painted) on the vascular reconstructions (surface triangulations) using a previously developed tool (ChePen3D)18. Different labels (colors) were assigned to different blebs occurring on the same aneurysm. The markings of the two blebs on the example model are shown in Figure 1(e).
Blebs were identified and delineated independently by three observers. A bleb was considered “true” if it was simultaneously identified by the majority of observers. Similarly, the bleb domain was defined as the (overlap) region delineated (painted) by the majority of observers. The agreement in the number of blebs of each aneurysm as well as the extent of their region on the aneurysm sac between each observer and the majority was then quantified. The curvature maps were used to visually confirm that the final bleb region was acceptable.
The same methods and tools used to characterize aneurysm geometries19 were used to characterize the bleb geometry, as illustrated in Figure 1(g,i). In particular, the automated calculation of the following geometric features for the blebs was carried out: bleb size (maximum distance between points on the bleb surface), bleb neck size (maximum distance between points on the bleb neck), and bleb aspect ratio (bleb height divided by bleb neck size).
The bleb location on the aneurysm sac was defined as the neck, body, or dome based on the maximum overlap between the area of the bleb and aneurysm regions between 0–20%, 20–60%, 60%−100% of the maximum geodesic distance to the aneurysm neck, respectively.19 Figure 1(h) shows the overlap of the blebs and the aneurysm regions labeled as neck (pink), body (red), and dome (yellow) in our example. Finally, the multiplicity of blebs was quantified by counting the number of blebs present in each aneurysm.
Figure 2 shows the tool used to mark blebs on 3D vascular reconstructions as well as example aneurysms with blebs. Note the different size, shape and location of the blebs on the aneurysm sac.
Figure 2.
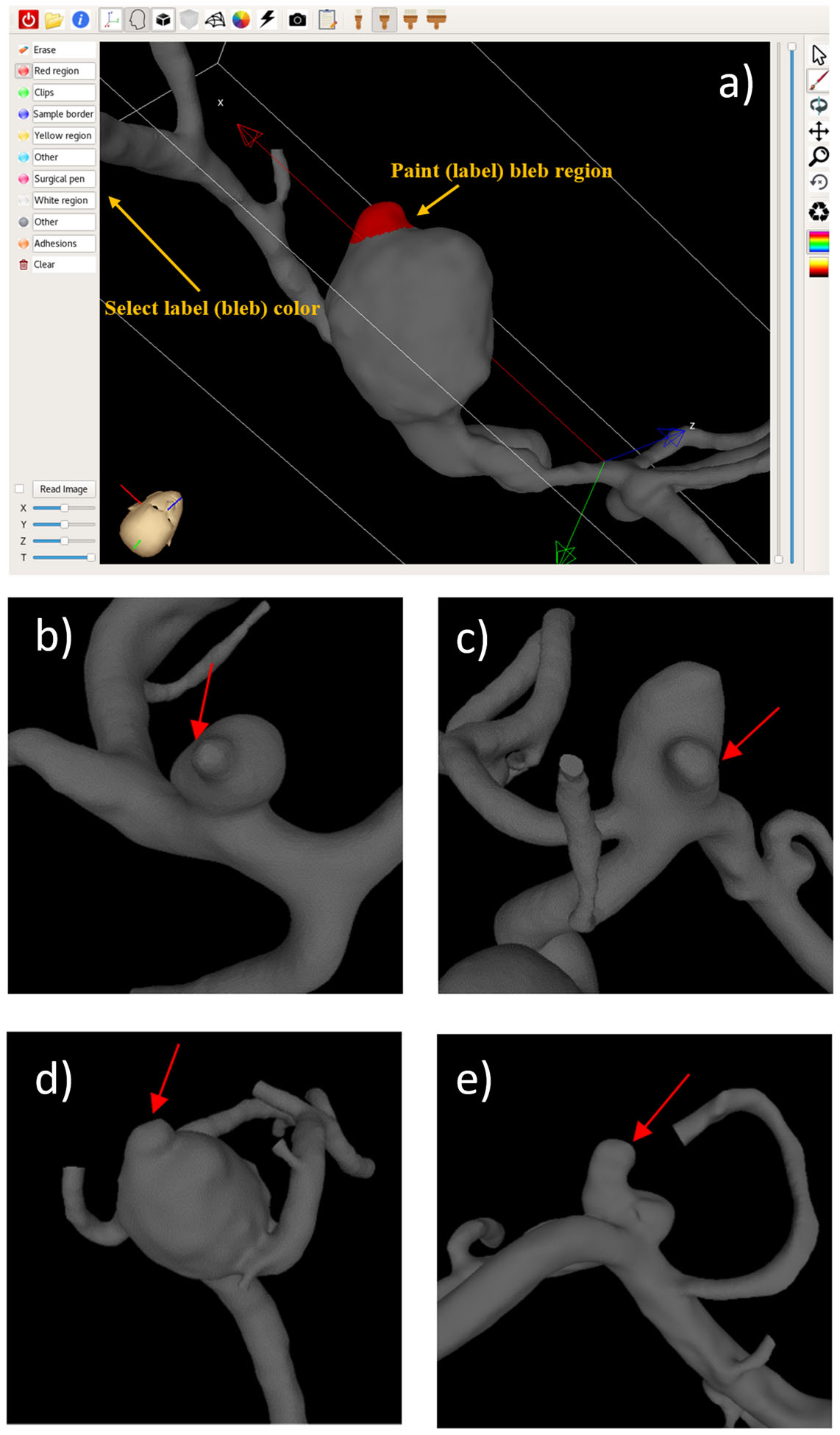
Tool for marking the blebs on the 3D vascular models (a), and example aneurysms with blebs (b–e). Arrows point to the identified blebs.
Data Analysis
The agreement between the three independent observers identifying and marking blebs was quantified using Fleiss’ Kappa statistics. Associations between categorical variables (e.g. patient sex) and presence of blebs were analyzed using contingency tables and the Pearson’s Chi-squared test, unless the assumptions for Chi-square test were not met, in which case, the Fisher’s Exact test was used. The mean values of continuous variables (e.g. aneurysm size) were statistically compared between the group of aneurysms with and without blebs using the two-sample unpaired Wilcoxon (Mann-Whitney) test. All statistical analyses were carried out using R scripts. Associations were considered significant if the corresponding p-value<0.05. The p-values were then adjusted for multiple testing using the Benjamini & Hochberg (BH) method implemented in R.
Results
Bleb Identification: Inter-Observer Agreement
Out of 270 aneurysms, all three observers unanimously agreed on the number of blebs present in 223 aneurysms (83%). After reaching consensus about the number of blebs in each aneurysm by a simple majority vote, the agreement between each observer and the majority was 98% for observer 1, 95% for observer 2, and 88% for observer 3, for a mean observer agreement with the majority of 94%. The Fleiss’ Kappa statistics to quantify observers’ agreement gave a kappa value of k=0.755, which indicates excellent agreement beyond chance,20 further confirmed by a p-value<0.0001, demonstrating that kappa is significantly different from zero.
Similarly, the percent of the area of the blebs marked by each observer overlapping with the area marked by the majority reached a mean of 88.3% with a minimum of 71% and a maximum of 98.4%, and only 5 bleb markings had an agreement with the majority below 75%.
Bleb Distribution and Frequency
Blebs were identified in 97 (36%) of the 270 aneurysms studied, the remaining 173 (64%) were classified as having no blebs. The mean bleb size was 3.0 ± 1.4 mm, the mean size of the blebs neck was 3.0 ± 1.8 mm, and the mean aspect ratio of the blebs was 0.49 ± 0.25. Of the 97 aneurysms with blebs 75 (77%) had a single bleb, while 22 (23%) had multiple blebs (two or more). Only 7 blebs (6%) were found in the neck region of the aneurysm, while 56 blebs (46%) were located in the body of the aneurysm, and 59 blebs (48%) were located in the dome of the aneurysm. This data is summarized in Supplementary Table II.
Associations with Presence of Blebs
Statistical comparisons between patient and aneurysm characteristics between aneurysms with blebs and aneurysms without blebs are presented in Table 1. Statistically significant associations remained significant after adjusting for multiple comparisons, except aneurysm neck size (see below).
Table 1.
Characteristics of aneurysms with and without blebs.
| Characteristics | Values | Aneurysms with blebs | Aneurysms without blebs | p-value | Adjusted p-value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age | Age | 56.8 ± 10 y | 54.9 ± 10.6 y | 0.2506 | 0.3062 |
| Sex | Female | 52 (40%) | 79 (60%) | 0.8202 | 0.8202 |
| Male | 17 (38%) | 28 (62%) | |||
| Unknown | 9 | 14 | |||
| Smoking | Yes | 34 (37%) | 59 (63%) | 0.5831 | 0.6414 |
| No | 23 (41%) | 33 (59%) | |||
| Unknown | 21 | 29 | |||
| Hypertension | Yes | 36 (45%) | 44 (55%) | 0.0905 | 0.1321 |
| No | 21 (31%) | 46 (69%) | |||
| Unknown | 21 | 31 | |||
| Dental Infection | Yes | 8 (80%) | 2 (20%) | † 0.0154* | 0.0426* |
| No | 31 (37%) | 52 (63%) | |||
| Unknown | 39 | 67 | |||
| Hormone Therapy (females only) | Yes | 2 (13%) | 13 (87%) | 0.0194* | 0.0426* |
| No | 43 (45%) | 52 (55%) | |||
| Unknown | 7 | 14 | |||
| Aneurysm characteristics | |||||
| Rupture Status | Ruptured | 29 (48%) | 31 (52%) | 0.0168* | 0.0426* |
| Unruptured | 59 (31%) | 129 (69%) | |||
| Unknown | 9 | 13 | |||
| Geometry | Aneurysm Size | 9.1 ± 3.3 mm | 6.7 ± 4.5 mm | <0.0001* | <0.0001* |
| Neck Size | 5.5 ± 1.9 mm | 4.9 ± 2.5 mm | 0.0318* | 0.05831 | |
| Aspect Ratio | 1.24 ± 0.52 | 0.93 ± 0.63 | <0.0001* | 0.0002* | |
| Location | ACA | 3 (23%) | 10 (77%) | 0.0961 | 0.1321 |
| ACOM | 22 (46%) | 26 (54%) | |||
| ICA | 8 (18%) | 37 (82%) | |||
| MCA | 49 (40%) | 75 (60%) | |||
| PCOM | 13 (41%) | 19 (59%) | |||
| PICA | 2 (40%) | 3 (60%) | |||
| Other | 0 (0%) | 3 (100%) | |||
Statistically significant p-values are indicated with a “*”.
ACA=anterior cerebral artery; ACOM=anterior communicating artery; ICA=internal carotid artery; MCA=middle cerebral artery; PCOM=posterior communicating artery; and PICA=posterior inferior cerebellar artery.
Fisher’s Exact test was used for dental infection.
In general, aneurysm with blebs tended to be larger (p<0.0001), more elongated as measured by larger aspect ratio (p=0.0002), and with wider necks (p=0.0318 before adjusting, p=0.05831 after adjustment) than aneurysms without blebs.
Interestingly, prevalence of dental infection was associated with the presence of blebs (p=0.0426). In contrast, treatment with hormone replacement therapy was negatively associated with presence of blebs in female patients (p=0.0426). Specifically, the proportion of aneurysms without blebs went from 55% to 87% for female patients under hormone therapy.
No significant associations were observed between bleb presence and patient sex (p=0.8202), age (p=0.3062), or smoking history (p=0.6414). Curiously, the percentage of aneurysms with blebs increased for patients with history of hypertension (45%) compared with patients without history of hypertension (36%). Additionally, certain anatomical locations tended to have larger percentage of aneurysms with blebs. ACOM was the location with the largest proportion of aneurysms with blebs (46%), followed by PCOM (41%), and MCA (40%), while ICA had the smallest proportion of aneurysms with blebs (18%). However, these trends observed for hypertension and aneurysm location did not reach significance in this sample (only 90% confidence before adjustment), but may be worth studying further with larger datasets.
Discussion
The presence of blebs has been proposed as a risk factor for intracranial aneurysm rupture in several previous studies.2–8 However, very limited attention has been given to the prevalence, distribution, and general characteristics of blebs in the cerebral aneurysm population.21
Because there is no standard and objective definition of a bleb, in this study three observers identified and marked blebs in patient-specific vascular reconstructions created from 3DRA or CTA images, aided by curvature maps that highlight bleb structures characterized by focal curvature changes. Changes in curvature are easily observed by the naked eye because they cause a change in the shadowing of 3D surfaces (due to changes in the normal direction) under directional illumination. Inter-observer analysis showed good agreement between observers when identifying and marking blebs, indicating that most blebs were well defined and easily identifiable. However, 17% of the blebs were more difficult to identify, typically this happens when the neck of the bleb is not very well defined (e.g. the ring of negative curvature surrounding the bleb is not closed, but has a “C” shape that confuses the definition of the bleb). Furthermore, the detection and definition of blebs in IAs could be affected by the imaging modality used and could potentially result in a higher detection with newer techniques such as cone-beam CTA.22 However, a simple contingency table analysis presented in Supplementary Table III shows that mixing 3DRA and CTA images in our study seems reasonable since they yield similar bleb prevalence. Additionally, aneurysms with two (or more) lobules of roughly similar sizes were classified as having no blebs if it was not possible to convincingly decide if a lobule was the bleb and the other the aneurysm. Thus, it is important to note that aneurysms without blebs could still have irregular, elongated, and deformed shapes. Although our dataset is not very large, it could be a starting point as a training set for machine learning algorithms to automatically identify blebs.
The presence of blebs was significantly associated with aneurysm rupture status (p=0.0426), in agreement with several previous studies.3,12,9,11
The main findings of our study include:
1-. Bleb Prevalence and Distribution and Mechanistic Implications
Consistently with a previous study,23 it was found that blebs are relatively common, they occur in over 30% of aneurysms, usually as single blebs and more rarely as multiple blebs in the same aneurysm. It has been argued that blebs are an indication of a focalized weakening of the aneurysm wall,11,12 even weaker than the rest of the aneurysm. Thus, this prevalence of blebs suggests that a large number of aneurysms enlarge in a disorganized manner leading to asymmetrical shapes and localized bulging. Since this study included data of patients selected for surgery, we compared the prevalence of blebs against a second database of patients referred to cerebral angiography (Supplementary Table IV). This comparison shows that the presence of blebs in the two populations is quite similar and therefore it suggests our results should apply to the general aneurysm population.
Blebs at the neck are rare (only about 6% occur in this region), with the majority of blebs occurring roughly equally distributed in the aneurysm body or dome. Perhaps this is an indication that vessel remodeling mechanisms are more effective in the neck region where vascular cells can more easily migrate from the parent artery than in the more distal body or dome regions where remodeling mechanisms may be impaired or more limited. A prior study on a smaller dataset (50 blebs) similarly found the smallest number of blebs near the aneurysm neck.21
Blebs are more common in large and elongated aneurysms, and in aneurysms at certain locations such as the ACOM and PCOM. These observations suggest two possible mechanisms involved in the local weakening of the aneurysm wall and development of blebs: a) contacts with extra vascular structures that are more likely to occur in large aneurysms and at certain locations may affect the wall structure locally, and b) flow conditions that are thought to be associated with local weakening of the wall, are more common in aneurysms at certain locations. The ACOM and PCOM locations are known to have increased rupture risk compared to MCA and ICA.24 The increased prevalence of blebs in aneurysms at the ACOM and PCOM could be the reason why they are more likely to rupture. However, similar prevalence of blebs but lower rupture risk in aneurysms at the MCA compared to ACOM and PCOM could be a sign of different types of blebs and associated failure mechanisms.
2-. Associations between Clinical Factors and Bleb Prevalence
Patients with dental infections are more likely to have aneurysms harboring blebs than patients without dental infections. In this study, “dental infection” was considered positive when the patient reported dental issues related to: a) bleeding from gums/teeth when biting, b) presence of loose teeth, c) teeth loss due to periodontitis or caries, d) diagnosed periodontitis by a dentist or other dental clinician. Periodontitis has been previously proposed as a risk factor for aneurysm development and rupture.25 The connection between dental infection and intracranial aneurysms is thought to be through an inflammatory process, where oral bacteria circulating in the blood stream may preferentially migrate into the aneurysm wall because of the locally damaged endothelium, which becomes more permeable as well as local flow stagnation conditions that may increase the chances of bacteria infiltrating the wall, and initiating an inflammatory response that culminates in a focal weakening of the wall.
Aneurysms in female patients under hormone replacement therapy were less likely to harbor blebs than those in female patients under no hormone therapy. It has been speculated that the higher prevalence of cerebral aneurysms in female patients together with the higher prevalence in patients older than 50 years of age may be related to hormonal changes during menopause in women in their fifth decade, and experimental animal models showed that estrogen deficiency causes endothelial dysfunction and inflammation, which may predispose for aneurysm development and rupture.26 The results presented in this study seem to be in agreement with this line of thought and suggest that hormone replacement therapy may be protective against bleb formation (and perhaps of further weakening of the wall and aneurysm progression) in female patients.
In our opinion these conjectures and hypotheses should be further investigated with larger datasets, including refined clinical information (dental infection, hormone therapy, hypertension, anatomical location), hemodynamic, biomechanical, and biological data as they may yield valuable information about the mechanisms responsible for bleb development and aneurysm evolution and rupture. In particular, it would be important to better understand the relationship between aneurysm rupture and blebs. It has been shown that aneurysms with a previous history of rupture are more likely to harbor blebs,3 and it has also been proposed that blebs form after or as a consequence of aneurysm rupture.27 However, other studies based on longitudinal data have suggested that bleb formation is a slow process.28 Furthermore, many unruptured aneurysms exhibit blebs (61% of aneurysms with blebs were unruptured in our sample), and recent analyses of ex-vivo aneurysm samples have found evidence of extensive collagen fiber remodeling at and around blebs,17 supporting the idea that blebs are indeed a sub-structure of the aneurysm wall that develop over extended periods of time. Furthermore, not all blebs have ultra-thin walls, blebs with thick atherosclerotic and hyperplastic walls have also been observed,18 which suggests that there may be more than one mechanism involved in the formation of blebs, and perhaps more than one failure mode associated with blebs.
Conclusions
Blebs are common in IAs, and most aneurysms harboring blebs have a single bleb. Blebs are rare at the neck, but they are equally common in the aneurysm body and dome. Blebs are more common in large and elongated aneurysms, and in aneurysms at ACOM and PCOM locations compared to ICA locations. Bleb presence was associated with dental infection, and negatively associated with hormone replacement therapy. Blebs should be further investigated to better understand how they form and how they affect the rupture risk of IAs.
Supplementary Material
Funding
This work was supported by NIH grant R01NS097457.
Footnotes
Competing Interests
None declared.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon request.
Research Ethics Approval
The protocols for patient consent, handling of patient data and analysis were approved by the institutional review board (IRB) at the University of Pittsburgh (Protocol # STUDY20020015), University of Illinois at Chicago (Protocol # 2015-0322), Allegheny General Hospital (Protocol # RC-5141), and Helsinki University Hospital. The whole study’s IRB is overseen by the University of Pittsburgh’s IRB.
References
- 1.Nyström SH. On factors related to growth and rupture of intracranial aneurysms. Acta Neuropathol (Berl) 1970;16:64–72. [DOI] [PubMed] [Google Scholar]
- 2.Phan TG, Huston J, Brown RD, et al. Intracranial saccular aneurysm enlargement determined using serial magnetic resonance angiography. J Neurosurg 2002;97:1023–8. [DOI] [PubMed] [Google Scholar]
- 3.Beck J, Rohde S, el Beltagy M, et al. Difference in configuration of ruptured and unruptured intracranial aneurysms determined by biplanar digital subtraction angiography. ACTA Neurochir 2003;145:861–5. [DOI] [PubMed] [Google Scholar]
- 4.Tsukahara T, Murakami N, Sakurai Y, et al. Treatment of unruptured cerebral aneurysms; a multi-center study at Japanese national hospitals. Acta Neurochir Suppl 2005;94:77–85. [DOI] [PubMed] [Google Scholar]
- 5.Sonobe M, Yamazaki T, Yonekura M, et al. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke 2010;41:1969–77. [DOI] [PubMed] [Google Scholar]
- 6.Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012;366:2474–82. [DOI] [PubMed] [Google Scholar]
- 7.Burkhardt J-K, Fierstra J, Esposito G, et al. Rapid Documented Growth of Aneurysm Bleb Led to Rupture of an Incidental Intracranial Anterior Communicating Artery Aneurysm. J Neurol Surg Part Cent Eur Neurosurg 2017;78:521–4. [DOI] [PubMed] [Google Scholar]
- 8.Yamano A, Yanaka K, Uemura K, et al. Bleb formation in small unruptured intracranial aneurysm as a predictor of early rupture. J Surg Case Rep 2018;2018:rjy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greving JP, Wermer MJ, Brown RD, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014;13:59–66. [DOI] [PubMed] [Google Scholar]
- 10.Etminan N, Brown RD, Beseoglu K, et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology 2015;85:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindgren AE, Koivisto T, Björkman J, et al. Irregular Shape of Intracranial Aneurysm Indicates Rupture Risk Irrespective of Size in a Population-Based Cohort. Stroke 2016;47:1219–26. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa M, Katada K, Anno H, et al. CT Angiography with electrocardiographically gated reconstruction for visualizing pulsation of intracranial aneurysms: identification of aneurysmal protuberance presumably associated with wall thinning. AJNR Am J Neuroradiol 2005;26:1366–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Tateshima S, Murayama Y, Villablanca JP, et al. In vitro measurement of fluid-induced wall shear stress in unruptured cerebral aneurysms harboring blebs. Stroke 2003;34:187–92. [DOI] [PubMed] [Google Scholar]
- 14.Cebral JR, Sheridan MJ, Putman CM. Hemodynamics and Bleb Formation in Intracranial Aneurysms. AJNR Am J Neuroradiol 2010;31:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Challa V, Han H-C. Spatial variations in wall thickness, material stiffness and initial shape affect wall stress and shape of intracranial aneurysms. Neurol Res 2007;29:569–77. [DOI] [PubMed] [Google Scholar]
- 16.Meng H, Feng Y, Woodward SH, et al. Mathematical model of the rupture mechanism of intracranial saccular aneurysms through daughter aneurysm formation and growth. Neurol Res 2005;27:459–65. [DOI] [PubMed] [Google Scholar]
- 17.Robertson AM, Duan X, Hill MR, et al. Diversity in the strength and structure of unruptured cerebral aneurysms. Ann Biomed Eng 2014;43:1502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cebral JR, Mut F, Gade P, et al. Combining data from multiple sources to study mechanisms of aneurysm disease: Tools and techniques. Int J Numer Methods Biomed Eng 2018;34:e3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mut F, Löhner R, Chien A, et al. Computational hemodynamics framework for the analysis of cerebral aneurysms. Int J Num Meth Biomed Eng 2011;27:822–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleiss J, Levin B, Paik M. Statistical methods for rates and proportions. In: 3rd ed. Hoboken, NJ: John Wiley & Sons; 2003:86–94. [Google Scholar]
- 21.Zhou J, Cebral JR, Maiti S, et al. Distribution of blebs on the walls of intracranial aneurysms. In: BMES 2019 Annual Meeting, Philadelphia, PA; 2019. [Google Scholar]
- 22.Lauric A, Heller RS, Schimansky S, et al. Benefit of cone-beam CT angiography in visualizing aneurysm shape and identification of exact rupture site. J Neuroimaging Off J Am Soc Neuroimaging 2015;25:56–61. [DOI] [PubMed] [Google Scholar]
- 23.Mansour A, Alemam S, Haitham H, et al. Aneurysm geometrics impact on microsurgical modality of ruptured and unruptured middle cerebral artery aneurysms. Menoufia Med J 2018;31:1402–9. [Google Scholar]
- 24.Cebral JR, Raschi M. Suggested connections between risk factors of intracranial aneurysms: a review. Ann Biomed Eng 2013;41:1366–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallikainen J, Lindgren A, Savolainen J, et al. Periodontitis and gingival bleeding associate with intracranial aneurysms and risk of aneurysmal subarachnoid hemorrhage. Neurosurg Rev 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai M, Wali AR, Birk HS, et al. Role of pregnancy and female sex steroids on aneurysm formation, growth, and rupture: a systematic review of the literature. Neurosurg Focus 2019;47:E8. [DOI] [PubMed] [Google Scholar]
- 27.Nomura M, Kida S, Uchiyama N, et al. Ruptured irregularly shaped aneurysms: pseudoaneurysm formation in a thrombus located at the rupture site. J Neurosurg 2000;93:998–1002. [DOI] [PubMed] [Google Scholar]
- 28.O’shaughnessy BA, Getch CC, Bendok BR, et al. Late morphological progression of a dissecting basilar artery aneurysm after staged bilateral vertebral artery occlusion:case report. Surg Neurol 2005;63:236–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


