To the Editor,
Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 outbreak that began in 2019 and spread rapidly across the world has been demonstrated to cause viral pneumonia, acute respiratory distress syndrome (ARDS) and multi-organ system failure [1]. Given the lack of scientific data, efforts are focused on an empirical search for therapeutic strategies to ensure the adequate gas exchange, including methods that can be applied in intensive care unit (ICU) setting. Iloprost is a synthetic analogue of prostacyclin and recent studies investigated its efficacy when applied via infusion in the context of COVID-19 [2, 3]. In addition, inhaled iloprost is a well-known option for the treatment of pulmonary hypertension (PH) [4]. Therefore, in the current study we have analyzed the effects of inhaled iloprost on gas exchange in patients with COVID-19 associated ARDS.
This case–control study was conducted in the Pulmonology Department of university-affiliated hospital (Sechenov University) between April 8, 2020, and May 20, 2020. The study was approved by the local ethics committee of Sechenov University, and written informed consent was obtained from all patients. Eligible patients were subjects aged over 18 years with SARS-CoV-2 infection confirmed by real-time PCR and ARDS according to the Berlin definition [5] and PaO2/FiO2 ≤ 200 mmHg. The exclusion criteria considered need for immediate endotracheal intubation and unstable hemodynamics. The primary objective was to assess the effect of inhaled iloprost on PaO2/FiO2 in patients with ARDS on Day 5. Iloprost was administered with a vibrating mesh nebulizer (Aeroneb Solo; Aerogen) four times per day (20 μg per administration) for 5 days. The control patients were selected based on the same enrollment criteria and we have prospectively recorded the measured parameters on the same data chart. The matching of the controls and patients treated with iloprost was performed based on the following criteria: age (within ± 5 years); National Early Warning Score (NEWS)-2 score on admission (within ± 1 points) and PaO2/FiO2 on admission (within ± 20 mmHg). Computed tomography (CT) scan was performed and CT severity score was calculated as 5-point scale according to the degree of lung involvement: (0) no involvement, (1) less than 25%, (2) 25–50%, (3) 50–75% and (4) more than 75% [6]. All adverse events (AE) and serious AE possibly related to inhaled iloprost were documented.
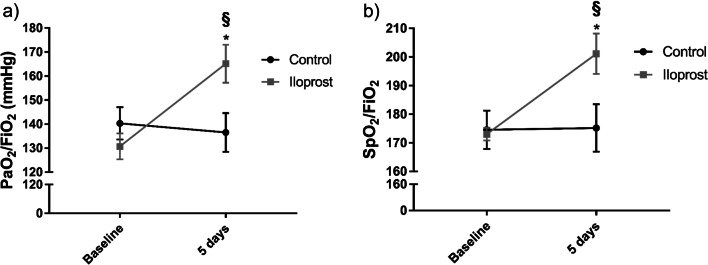
Twenty-three consecutive patients received at least one iloprost inhalation and 22 patients were included into the control group. The baseline demographic, clinical and laboratory characteristics did not differ significantly between the groups (Table 1). Time between the symptom onset and iloprost administration was 8.0 ± 0.5 days. On day 5, iloprost therapy led to the significant improvement in SpO2/FiO2 and PaO2/FiO2 compared to the baseline and controls (Fig. 1). There was also a significant reduction of the Borg dyspnea score (6 vs. 4, p = 0.01). Three patients in iloprost group and 6 patients in control group were transferred to ICU due to rapidly progressive respiratory failure. Remaining patients were free of supplemental oxygen/continuous positive airway pressure at the end of follow-up. The overall iloprost safety profile was similar to that observed in previous studies. The most common AE were flushing (n = 5; 21.7%) and jaw pain (n = 3; 13.0%). There were no cases of AE-related iloprost discontinuation.
Table 1.
Baseline characteristics of the study population
| Iloprost (n = 23) | Control (n = 22) | |
|---|---|---|
| Demographic variables | ||
| Age, years | 62 (53–68) | 60 (54–69) |
| Male, n (%) | 15 (65.2) | 17 (77.3) |
| Caucasian, n (%) | 23 (100) | 22 (100) |
| Anthropometric measures and risk factors | ||
| Smokers, n (%) | 8 (34.8) | 10 (45.5) |
| BMI, kg/m2 | 31.0 (28.0–34.8) | 32.0 (26.5–39.6) |
| Medical history | ||
| Cardiovascular disease, n (%) | 8 (34.8) | 9 (40.9) |
| Chronic lung disease, n (%) | 0 (0) | 1 (4.5) |
| Diabetes mellitus, n (%) | 6 (26.1) | 7 (31.8) |
| Chronic kidney disease, n (%) | 3 (13.0) | 1 (4.5) |
| Clinical variables | ||
| Cough, n (%) | 21 (91.3) | 21 (95.4) |
| Dyspnea, n (%) | 21 (91.3) | 19 (86.4) |
| Fever, n (%) | 19 (82.6) | 18 (81.8) |
| Borg dyspnea scale | 6 (5–8) | 5 (2–8) |
| Laboratory tests | ||
| WBC, 109/L | 5.9 (5.1–8.8) | 6.8 (5.2–8.3) |
| C-reactive protein, mg/L | 131 (102–190) | 128 (89–186) |
| D-dimer, µg/mL | 2.9 (1.9–3.8) | 3.5 (1.9–4.6) |
| Blood gases | ||
| PaO2, mmHg | 65.8 (55.1–78.1) | 62.0 (49.0–77.7) |
| PaCO2, mmHg | 32.0 (29.2–35.0) | 28.8 (23.8–32.7) |
| SpO2, % | 89 (88–90) | 90 (87–93) |
| PaO2/FiO2, mmHg | 131 (120–138) | 130 (114–168) |
| Computed tomography | ||
| CT severity scale, 0/1/2/3/4, n (%) | 0 (0)/0 (0)/7 (30.4)/9 (39.1)/7 (30.4) | 0 (0)/0 (0)/5 (22.7)/14 (63.6)/3 (13.6) |
| Medications | ||
| Vasopressors, n (%) | 0 (0) | 0 (0) |
| Corticosteroids, n (%) | 15 (65.2) | 17 (77.3) |
| Hydroxychloroquine, n (%) | 21 (91.3) | 19 (86.4) |
| Azithromycin, n (%) | 21 (91.3) | 19 (86.4) |
| Respiratory support | ||
| Supplemental oxygen, n (%) | 16 (69.6) | 14 (63.6) |
| CPAP, n (%) | 7 (30.4) | 8 (36.4) |
Data are expressed as absolute values (%) or median (interquartile range)
BMI body mass index, WBC white blood cells, PaO2 arterial oxygen tension, PaCO2 arterial carbon dioxide tension, SpO2 oxygen saturation, FiO2 fraction of inspired oxygen, CT computed tomography, CPAP continuous positive airway pressure
Fig. 1.
Effects of inhaled iloprost on oxygenation parameters a PaO2/FiO2; b SpO2/FiO2). Results are presented as mean ± SEM (n = 16–23). PaO2/FiO2 arterial oxygen tension-to-inspired oxygen fraction ratio, SpO2/FiO2 arterial oxygen saturation-to-inspired oxygen fraction ratio. Variables were compared with two-way ANOVA with Sidak’s multiple comparisons test. *p < 0.05. *Baseline versus 5 days; §Control versus iloprost
In the context of COVID-19, still limited literature sources highlighted the usage of iloprost as potential therapeutic option [2, 3]. In the line with the literature, our findings revealed promising effects of inhaled iloprost with improved oxygenation parameters in patients with COVID-19-associated ARDS. It must be noted that our small pilot study is hypothesis generating rather than confirmatory and its results should be proved in randomized controlled trials.
Acknowledgements
None.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ICU
Intensive care unit
- PaO2
Partial pressure of oxygen
- FiO2
Fraction of inspired oxygen
- SpO2
Oxygen saturation
- NEWS-2
National Early Warning Score
- AE
Adverse events
Authors' contributions
SNA took part in concept and design of the study, and drafting of the manuscript; DK was involved in supervision and drafting of the manuscript; NAT, NVT and GVN took the measurements and collected the data; RTS had contributed to significant intellectual content. All authors were involved in data analysis and interpretation. Finally, all authors were involved in writing, reviewing and editing of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials can be obtained from the corresponding author upon the reasonable request.
Declarations
Ethics approval and consent to participate
The local ethics committee (LEC No. 05-20) approved the study, and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al, China Medical Treatment Expert Group for C. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 2.Johansson PI, Bestle M, Soe-Jensen P, Kristiansen KT, Stensballe J, Clausen NE, Perner A. The effect of prostacyclin (Iloprost) infusion at a dose of 1 ng/kg/min for 72 hours compared to placebo in mechanically ventilated patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:746. doi: 10.1186/s13063-020-04696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moezinia CJ, Ji-Xu A, Azari A, Horlick S, Denton C, Stratton R. Iloprost for COVID-19-related vasculopathy. Lancet Rheumatol. 2020;2:e582–e583. doi: 10.1016/S2665-9913(20)30232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al, Aerosolized Iloprost Randomized Study Group. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002;347:322–9. 10.1056/NEJMoa020204. [DOI] [PubMed]
- 5.Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials can be obtained from the corresponding author upon the reasonable request.