Key summary points
Aim
The objective of this study is to describe the baseline characteristics of oldest-old patients admitted with COVID-19 to an acute geriatric unit and to determine the factors associated with in-hospital mortality.
Findings
Dementia, incident delirium, and the CURB-65 score ≥ 3 are independent mortality risk factors. The concurrent use of angiotensin-converting enzyme inhibitors is a protective factor.
Message
Recognition of geriatric syndromes may be useful to help clinicians establish the prognosis of oldest-old patients admitted to hospital with COVID-19.
Keywords: Covid-19, SARS-COV-2, Mortality, Older adults, Risk factors
Abstract
Purpose
To determine predictors of in-hospital mortality related to COVID-19 in oldest-old patients.
Design
Single-center observational study.
Setting and participants
Patients ≥ 75 years admitted to an Acute Geriatric Unit with COVID-19.
Methods
Data from hospital admission were retrieved from the electronic medical records: demographics, geriatric syndromes (delirium, falls, polypharmacy, functional and cognitive status) co-morbidities, previous treatments, clinical, laboratory, and radiographic characteristics. Cox proportional hazard models were used to evaluate in-hospital mortality.
Results
Three hundred patients were consecutively included (62.7% females, mean age of 86.3 ± 6.6 years). Barthel Index (BI) was < 60 in 127 patients (42.8%) and 126 (42.0%) had Charlson Index CI ≥ 3. Most patients (216; 72.7%) were frail (Clinical Frailty Scale ≥ 5) and 134 patients (45.1%) had dementia of some degree. The overall in-hospital mortality rate was 37%. The following factors were associated with higher in-hospital mortality in a multi-variant analysis: CURB-65 score = 3–5 (HR 7.99, 95% CI 3.55–19.96, p < 0.001), incident delirium (HR 1.72, 1.10–2.70, p = 0.017) and dementia (HR 3.01, 95% CI 1.37–6.705, p = 0.017). Protective factors were concurrent use of angiotensin-converting enzyme inhibitors (HR 0.42, 95% CI 0.25–0.72, p = 0.002) or prescription of hydroxychloroquine (HC 0.37 95% CI 0.22–0.62, p < 0.001) treatment during admission.
Conclusions and implications
Our findings suggest that recognition of geriatric syndromes together with the CURB-65 score may be useful tools to help clinicians establish the prognosis of oldest-old patients admitted to hospital with COVID-19.
Introduction
Background
Spain was one of the countries most affected by the COVID-19 pandemic during 2020. By February 2021 more than 3,000,000 cases and 64,700 deaths had been reported [1]. In particular, individuals aged more than 74 were affected, with an expected mortality excess (any cause) in 2020 of 78% between March and May 2020 [2].
During recent months, several studies on prognostic factors associated with mortality in older adults have been published, but very few in oldest-old patients. Identifying predictive factors of in-hospital mortality in this vulnerable population is critical to establish appropriate goals of care. Age has been identified as one of the most important factors associated with mortality in older patients admitted to hospital [3, 4]. A meta-analysis showed that co-morbidities such as arterial hypertension, chronic obstructive pulmonary disease (COPD), cardiomyopathy, renal disease, and cerebrovascular disease (CVD) are mortality-related factors in older adults with COVID-19 [5]. Frailty has been positively associated with mortality from COVID-19 in many studies in older people, but not in every case. For this reason, it should be used with caution as a prognostic marker alone [6]. The use of ACEIs or ARBs before COVID-19 illness has not been associated with mortality [7]. However, the effects in older inpatients have not been completely established.
The objective of this study was to describe the baseline characteristics of oldest-old patients admitted with COVID-19 to an acute geriatric unit and to determine the factors associated with in-hospital mortality, including the concurrent use of ACEI or the treatment with hydroxychloroquine. Secondary outcomes were to describe the development of non-cardiac medical complications, mortality, and early hospital readmissions 30 days after discharge from hospital.
Methodology
Study population
This was a single-center longitudinal observational ambispective study that included all patients consecutively admitted to the acute geriatric unit of a secondary-care university hospital between March and May 2020. This hospital at the end of March was transformed into a hospital center dedicated to COVID-19. Over the course of two weeks, it was transformed from having 111 medical beds, 42 surgical beds, and 4 surgical recovery beds to 190 COVID-19 beds, with 5 ICU beds.
Potential study participants were identified on admission by an attending physician (geriatrician), who alerted the investigation team. Inclusion criteria were: (1) men and women ≥ 75 years of age; (2) COVID-19 infection diagnosed by hospital protocol [8]: either confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) for the SARS-CoV2 and/or high clinical and radiological characteristics); and (3) patients (or their next of kin) understood the study and freely agreed to participate in it by giving their written informed consent. Furthermore, we included patients retrospectively once the patient or their families gave their consent.
Exclusion criteria were: (1) patients with palliative needs, diagnosed by the attending team, or (2) patients who declined to participate.
The protocol was approved by the ethics committee of Hospital Universitario La Paz, under the ID: I-4131.
This article was written following the STROBE statement of cohort studies [9].
Data collection
We collected the following data during the first 24 h after admission from the electronic medical records: (1) demographic characteristics: age, gender; (2) functional status (Barthel Index, BI) [10]. BI is a scale to measure performance in daily living activities, with values ranging from 100 (totally independent) to 0 (totally dependent). We considered: independent (BI 100), slight dependence (BI 60–99), moderate dependence (BI 40–59), severe dependence (BI 20–39), and total dependence BI (0–19) 3) Dementia (any type) categorized by The Global Deterioration Scale (GDS) [11]: mild cognitive decline (GDS: 3), mild dementia (GDS: 4), moderate dementia (GDS: 5), moderately severe dementia (GDS: 6) and severe dementia (GDS: 7); (4) delirium, assessed by the Confusion Assessment Method (CAM) [12]. The CAM score is based on four features: acute onset and fluctuating changes in mental status, inattention, disorganized or incoherent thinking, and altered consciousness. Delirium was considered if a patient showed an acute onset and fluctuating discourse and inattention, with either disorganized thinking or an altered level of consciousness. A diagnosis of prevalent delirium was established if it was a clinical sign in the emergency department or within 24 h from admission. Incident delirium was diagnosed if the delirium was present 24 h after hospital admission; (5) The Clinical Frailty Scale (CFS) [13] was used to evaluate frailty (2 weeks before admission). The CFS is a scale that ranks frailty from 1 to 9, with a score of 1 being very fit and 9 terminally ill. We considered fit (CFS 1–4), mild and moderate frailty (CFS 5–6), and severe or very severe frailty (CFS 7–8) as it was stated in previous studies [14]; (6) polypharmacy, that was defined as the use of more than five drugs. The use of antihypertensive treatments (ACEI/ARB), anticoagulants, antipsychotics, and antidepressants as regular medication was recorded too; (7) The Charlson Comorbidity Index (CCI) was used as a co-morbidity scale [15]. This index weights 19 chronic conditions based on a scale of 1–6, with higher scores indicating increased multimorbidity. Our population was dichotomized according to a cutoff point ≥ 3 to determine the influence of the CCI on mortality (8) other co-morbidities recorded included: hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), cerebrovascular (CVD) and liver disease, chronic kidney disease (CKD), atrial fibrillation (AF), heart failure (HF), cancer, and thromboembolic disease.
Clinical features, laboratory, and radiological characteristics were assessed in the emergency department (ED): temperature, heart rate, blood pressure, oxygen saturation level, and respiratory rate. CURB-65 (Pneumonia Severity Score) [16] and Quick Sequential Organ Failure Assessment (qSOFA) [17] scores were used as measures of severity and sepsis, respectively. CURB-65 (age ≥ 65 years, new-onset confusion; urea > 7 mmol/L; respiratory rate ≥ 30/min, systolic blood pressure < 90 mmHg and/or diastolic blood pressure ≤ 60 mmHg; and; attributing 1 point for each item) has been used to stratify patients with community-acquired pneumonia into low (CURB-65 = 0–1), moderate (CURB-65 = 2) and high (CURB-65 = 3–5) mortality risk [16]. The quickSOFA score (qSOFA) uses three criteria, assigning one point for low blood pressure (SBP ≤ 100 mmHg), high respiratory rate (≥ 22 breaths per min), or altered mental state (Glasgow coma scale < 15). The score ranges from 0 to 3 points. The presence of 2 or more qSOFA points near the onset of sepsis is associated with a greater risk of death [17]. Falls were included as a presentation symptom of COVID-19.
Treatments during admission, including the prescription of medications thought to be helpful in COVID-19 treatment or management, such as hydroxychloroquine, azithromycin, (anticoagulation, prophylactic or therapeutic doses), corticosteroids, lopinavir, ritonavir, darunavir, or colchicine, were retrieved from electronic records.
Non-cardiac complications were evaluated during admission: acute respiratory distress syndrome (ARDS) [18], acute kidney injury (defined by a serum creatinine rise of at least 50% from baseline as per NICE) [19], bacterial co-infection (defined as raised procalcitonin and C-reactive protein), sepsis [17] and sodium disturbance (< 135 or > 145 mEq/L). The length of hospital stay was also collected.
Outcomes
The primary outcome was in-hospital mortality, with the number of days from hospital admission until death also being recorded. Secondary outcomes were non-cardiac medical complications as well as readmission (any cause) or mortality 30 days after discharge from the hospital.
Statistical analysis
Categorical variables were expressed as numbers and percentages (%) and continuous variables as mean and SD or median and interquatile range (IQR) according to their distribution. Normality of the continuous data was checked using the Kolmogorov–Smirnov test. The differences between the discharged and deceased groups for categorical variables were examined using the chi-square test, with the likelihood ratio correction or Fisher's exact test for small samples. The differences for continuous variables were examined using independent samples T-Test or Mann–Whitney U as appropriate.
Curves for the absence of in-hospital mortality were built using the Kaplan–Meier method, and comparison between groups was performed using the log-rank test. Moreover, univariate and multivariate Cox proportional regression analyses were performed, using the stepwise forward method. Variables with p value < 0.15 in univariate analysis were included in multivariate analysis.
Results were considered significant at p values < 0.05. All data were analyzed with SPSS Statistics version 26.0 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Results
Characteristics of the study population
A total of 300 older patients were included (188 women; 62.7%), mean age was 86.3 ± 6.6. Baseline characteristics of the population are shown in Tables 1 and 2. Most patients (200; 66.6%) were diagnosed by positive RT-PCR, 56 patients (18.6%) met clinical and radiological criteria for COVID-19 diagnosis, 37 (12.3%) met clinical and 6 (2%) radiological criteria. Barthel Index (BI) was < 60 in 127 patients (42.8%) and 126 (42.0%) had Charlson Comorbidity Index (CCI) ≥ 3, reflecting the poor functional status and the high co-morbidities burden of the cohort. Most patients (216; 72.7%) were frail (Clinical Frailty Scale ≥ 5) and 134 patients (45.1%) had dementia of some degree.
Table 1.
Baseline characteristics [1]
| Total | Survivors | Non-survivors | p value | |
|---|---|---|---|---|
| n | 300 | 189 | 111 | |
| Woman (%) | 188 (62.7) | 130 (68.8) | 58 (52.3) | 0.004 |
| Age (years, SD) | 86.3 ± 6.6 | 85.7 ± 6.7 | 87.5 ± 6.3 | 0.022 |
| Length of hospital stay (days) [median (IQR)] | 11 (7/18) | 14 (10/21) | 7 (4/11) | < 0.001 |
| Number of days from onset of symptoms [median (IQR)] | 5 (2/7) | 5 (2/8) | 5 (3/7) | 0.509 |
| Community-dwelling | 97 (32.3) | 64 (33.9) | 33 (29.7) | 0.460 |
| Nursing home | 203 (67.7) | 125 (66.1) | 78 (70.3) | |
| Co-morbidities | ||||
| Hypertension | 201 (67.0) | 130 (68.8) | 71 (64.0) | 0.391 |
| Diabetes mellitus (DM) | 85 (28.3) | 43 (22.8) | 42 (37.8) | 0.005 |
| COPD | 48 (16.0) | 31 (16.4) | 17 (15.3) | 0.804 |
| Chronic cerebrovascular disease | 73 (24.3) | 44 (23.3) | 29 (26.1) | 0.579 |
| Chronic liver disease | 9 (3.0) | 4 (2.1) | 5 (4.5) | 0.242 |
| Chronic kidney disease (CKD) | 52 (17.3) | 34 (18.0) | 18 (16.2) | 0.695 |
| Atrial Fibrillation | 83 (27.7) | 46 (24.3) | 37 (33.3) | 0.093 |
| Heart failure | 73 (24.3) | 47 (24.9) | 26 (23.4) | 0.778 |
| Cancer | 38 (12.7) | 26 (13.8) | 12 (10.8) | 0.459 |
| VTE/DTV | 33 (11.0) | 17 (9.0) | 16 (14.4) | 0.147 |
| Comprehensive Geriatric Assessment (CGA) | ||||
| Charlson Comorbidity Index (CCI) | ||||
| CCI (mean) | 2.5 ± 2.1 | 2.3 ± 2.2 | 2.8 ± 1.8 | 0.049 |
| CCI ≥ 3 | 126 (42.0) | 69 (36.5) | 57 (51.4) | 0.012 |
| Barthel Index (BI) | ||||
| Independent (BI 100) | 48 (16.2) | 36 (19.4) | 12 (10.9) | 0.013 |
| Slight dependence (60–99) | 121 (40.9) | 82 (44.1) | 39 (35.5) | |
| Moderate dependence (40–59) | 40 (13.5) | 17 (9.1) | 23 (20.9) | |
| Severe dependence (20–39) | 33 (11.1) | 21 (11.3) | 12 (10.9) | |
| Total dependence (0–19) | 54 (18.2) | 30 (16.1) | 24 (21.8) | |
| Dependence (BI < 100) | 248 (83.8) | 150 (80.6) | 98 (89.1) | 0.057 |
| Frailty | ||||
| Fit or very mild frailty (CFS 1–4) | 81 (27.3) | 63 (33.7) | 18 (16.4) | 0.003 |
| Mild or moderate frailty (CFS: 5–6) | 131 (44.1) | 79 (42.2) | 52 (47.3) | |
| Severe or very severe frailty (CFS: 7–8) | 85 (28.6) | 45 (24.1) | 40 (36.4) | |
| Frail (CFS ≥ 5) | 216 (72.7) | 124 (66.3) | 92 (83.6) | 0.001 |
| Dementia: Global Deterioration Scale, GDS | ||||
| GDS: 0–3 | 164 (55.2) | 113 (60.1) | 51 (46.8) | 0.050 |
| GDS: 4 | 35 (11.8) | 20 (10.6) | 15 (13.8) | |
| GDS: 5 | 38 (12.8) | 20 (10.6) | 18 (16.5) | |
| GDS: 6 | 42 (14.1) | 28 (14.9) | 14 (12.8) | |
| GDS: 7 | 18 (6.1) | 7 (3.7) | 11 (10.1) | |
| Dementia (GDS ≥ 4) | 134 (45.1) | 76 (40.4) | 58 (53.2) | 0.033 |
| Polypharmacy | 213 (71.0) | 133 (70.4) | 80 (72.1) | 0.754 |
| Delirium (prevalent) | 117 (39.0) | 59 (31.2) | 58 (52.3) | < 0.001 |
| Delirium (incident) | 84 (28.0) | 37 (19.6) | 47 (42.3) | < 0.001 |
| Fall | 32 (10.7) | 19 (10.1) | 13 (11.7) | 0.653 |
Bold value indicates statistical significance
Table 2.
Baseline characteristics [2]
| Total | Survivors | Non-survivors | p value | |
|---|---|---|---|---|
| Clinical variables | ||||
| Blood pressure | 129.6 ± 25.3 | 132.2 ± 25.0 | 124.9 ± 25.2 | 0.017 |
| Heart rate | 85.9 ± 19.3 | 83.6 ± 16.7 | 89.7 ± 22.7 | 0.015 |
| Respiratory rate, breath/min | 23.2 ± 7.8 | 21.0 ± 6.9 | 26.3 ± 8.0 | < 0.001 |
| Fever | 154 (51.3) | 91 (48.1) | 63 (56.8) | 0.150 |
| Blood oxygen level (pulse oximeter) | 91.1 ± 6.0 | 92.0 ± 5.3 | 89.7 ± 6.8 | 0.001 |
| CURB-65, mean | 2.1 ± 0.9 | 1.8 ± 0.9 | 2.7 ± 0.8 | < 0.001 |
| Severity of pneumonia | ||||
| CURB-65: 0–1 (low risk) | 83 (28.9) | 75 (42.1) | 8 (7.3) | < 0.001 |
| CURB-65: 2 (moderate risk) | 105 (36.6) | 69 (38.8) | 36 (33.0) | |
| CURB-65: 3–5 (high risk) | 99 (34.5) | 34 (19.1) | 65 (59.6) | |
| qSOFA, mean | 0.9 ± 0.8 | 0.6 ± 0.7 | 1.3 ± 0.8 | < 0.001 |
| Laboratory data | ||||
| Lymphocytes, cells/mm3 (median (IQR)) | 0.89 (0.60/1.26) | 0.93 (0.62/1.35) | 0.77 (0.49/1.00) | 0.007 |
| Albumin, g/dL [median (IQR)] | 3.3 (3.0/3.7) | 3.4 (3.1/3.7) | 3.2 (2.9/3.7) | 0.068 |
| Ferritin, ng/mL [median (IQR)] | 266 (155/463.5) | 246 (147/392) | 401 (203/755) | 0.002 |
| d-Dimer, ng/mL [median (IQR)] | 1.5 (0.8/2.8) | 1.4 (0.8/2.6) | 1.6 (0.9/3.0) | 0.281 |
| C-reactive protein, mg/L [median (IQR)) | 62.0 (26.3/143.0) | 53.7 (21.6/114.0) | 86.0 (46.8/181.5) | < 0.001 |
| Thrombocytes, 106 (median (IQR)) | 206 (1254/289) | 221 (158/317) | 190 (141/252) | 0.004 |
| Creatinine, mg/dL [median (IQR)] | 0.97 (0.70/1.40) | 0.80 (0.60/1.20) | 1.14 (0.87/1.63) | < 0.001 |
| Treatment | ||||
| ACEI | 88 (29.3) | 63 (33.3) | 25 (22.5) | 0.047 |
| Anticoagulants (any dose) | 83 (27.7) | 48 (25.4) | 35 (31.5) | 0.251 |
| Benzodiazepines | 96 (32.0) | 63 (33.3) | 33 (29.7) | 0.518 |
| Risperidone | 13 (4.3) | 5 (2.6) | 8 (7.2) | 0.079 |
| Quetiapine | 34 (11.3) | 17 (9.0) | 17 (15.3) | 0.095 |
| Antidepressants | 109 (36.3) | 71 (37.6) | 38 (34.2) | 0.562 |
| Haloperidol | 24 (8.0) | 15 (7.9) | 9 (8.1) | 0.958 |
| Trazodone | 34 (11.3) | 23 (12.2) | 11 (9.9) | 0.551 |
| Treatment during admission | ||||
| Anticoagulant prophylaxis | 270 (90.6) | 176 (93.6) | 94 (85.5) | 0.020 |
| None | 29 (9.7) | 12 (6.4) | 17 (15.5) | 0.032 |
| Prophylactic dose | 186 (62.4) | 124 (66.0) | 62 (56.4) | |
| Therapeutic dose | 83 (27.9) | 52 (27.7) | 31 (28.2) | |
| Hydroxychloroquine | 261 (87.0) | 175 (92.6) | 86 (77.5) | < 0.001 |
| Azithromycin | 188 (63.1) | 121 (64.4) | 67 (60.9) | 0.551 |
| Corticosteroids | 109 (37.1) | 61 (33.0) | 48 (44.0) | 0.058 |
| Lopinavir plus ritonavir | 22 (7.3) | 13 (6.9) | 9 (8.1) | 0.693 |
| Colchicine | 3 (1.0) | 2 (1.1) | 1 (0.9) | 0.999 |
| Darunavir | 5 (1.7) | 4 (2.1) | 1 (0.9) | 0.655 |
| Radiology | ||||
| Pneumonia | 252 (84.0) | 149 (78.8) | 103 (92.8) | 0.001 |
| Respiratory tract infection | 48 (16.0) | 450 (21.2) | 8 (7.2) | 0.002 |
| Unilobar pneumonia | 25 (8.3) | 15 (7.9) | 10 (9.0) | 0.746 |
| Unilateral multilobar pneumonia | 15 (5.0) | 8 (4.2) | 7 (6.3) | 0.426 |
| Bilateral multilobar pneumonia | 212 (70.7) | 126 (66.7) | 86 (77.5) | 0.047 |
| Ground-glass opacification | 52 (17.9) | 35 (18.6) | 17 (16.5) | 0.653 |
| Unilobar pneumonia, unilateral multilobar pneumonia, bilateral multilobar pneumonia, ground-glass opacification | 252 (84.0) | 149 (78.8) | 103 (92.8) | 0.001 |
| Non-cardiac complication | ||||
| Respiratory distress syndrome | 15 (5.2) | 1 (0.6) | 14 (13.2) | < 0.001 |
| Acute renal failure | 38 (13.2) | 22 (12.2) | 16 (15.1) | 0.578 |
| Co-infection | 65 (22.6) | 42 (23.2) | 23 (21.7) | 0.022 |
| Sepsis | 31 (10.8) | 4 (2.2) | 27 (25.5) | < 0.001 |
| Sodium disturbance | 17 (5.9) | 10 (5.5) | 7 (6.6) | 0.714 |
Bold value indicates statistical significance
Fever was present in 154 (51%) of patients in the ED 117 (39%) of patients were diagnosed with delirium in ED, while 84 (28%) of them developed delirium during admission.
Pneumonia was present in 252 (84%) of patients, 212 (70%) had multilobar bilaterally, 204 patients (more than 70%) were moderate or high-risk CURB-65 ≥ 2. qSOFA mean was 0.9 ± 0.8.
Bacterial co-infection was present in 65 (22.6%) of patients, whereas acute kidney injury and sepsis were diagnosed in 13% and 11% of them, respectively.
Treatments during admission were as follows: 270 patients (90.6%) received anticoagulant therapy: 186 (62%) of them received prophylactic dose anticoagulation and 83 (27.9%) therapeutic doses of anticoagulant therapy. Other treatments were hydroxychloroquine, azithromycin and parenteral glucocorticosteroids that were prescribed for 261 patients (87%), 188 (63%) and 109 (37%), respectively.
The overall in-hospital mortality rate was 37% (n: 111). Median time to in-hospital death was 7 days (interquartile range, IQR 4–11). Survivors’ median length of hospital stay was 14 days.
Basic comparison between survivors and non-survivors
Baseline differences between in-hospital survivors and non-survivors are shown in Tables 1 and 2. 62.7% of patients in the study were women, but women only represented 52.3% of those who died. Components identified during Comprehensive Geriatric Assessment were significantly different between those surviving and not: those dying during admission had a higher co-morbidity burden as assessed by the Charlson Comorbidity Index (CCI ≥ 3, p = 0.012), worse functional status (Barthel Index, BI, < 60, p = 0.013), were more frail (CFS ≥ 5 p = 0.001), had an increased prevalence of delirium (incident or prevalent, p < 0.001) and pre-existing dementia (p = 0.033). Length of hospital stay was shorter in the group of non-survivors (p < 0.001). Diabetes mellitus and the presence of pneumonia were more prevalent in the group of non-survivors (p = 0.005 and p = 0.001 respectively), but no difference was seen in those with a diagnosis of COPD.
Regarding laboratory examinations: lower median values of lymphocytes (p = 0.007), and higher ferritin (p = 0.002), CRP (p < 0.001), and creatinine (p < 0.001) were associated with an increased risk of in-hospital mortality.
Concurrent use of ACEI at hospital admission was associated with lower in-hospital mortality (p = 0.047). Prescription of hydroxychloroquine and anticoagulants during hospital admission were also associated with lower in-hospital mortality (p < 0.001 and p = 0.020, respectively). Although it was not statistically significant (p = 0.058), there was a trend showing potential benefits from the use of glucocorticoids during admission for in-hospital mortality.
Multivariant analysis of risk factors associated with in-hospital mortality
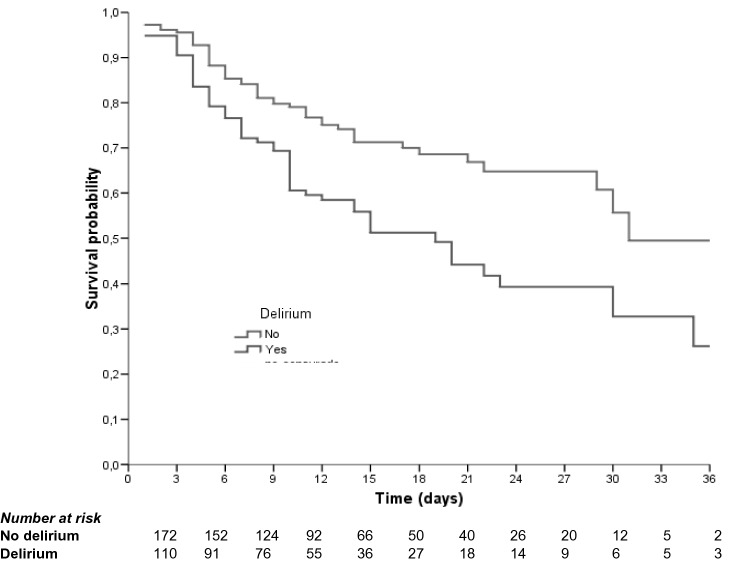
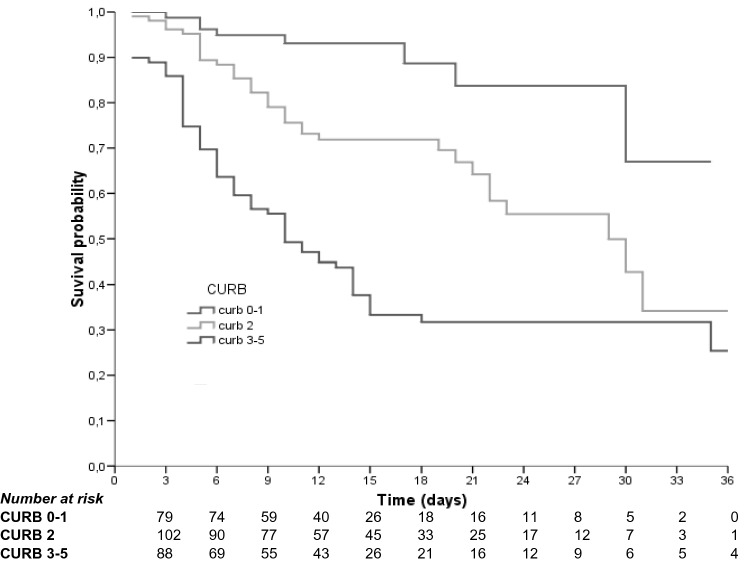
In multi-variant analysis (Table 3), CURB-65 score = 3–5 (HR 7.99, 95% CI 3.55–19.96, p < 0.001), incident delirium (HR 1.72, 1.10–2.70, p = 0.017) and dementia (HR 3.01, 95% CI 1.37–6.705, p = 0.017) were associated with higher in-hospital mortality. Kaplan–Meier survival rate analyses for those patients with and without delirium are shown in Fig. 1, and according to CURB-65 score in Fig. 2. Pre-admission treatment with ACEI (HR 0.42 95% CI 0.25–0.72, p = 0.002) and hydroxychloroquine treatment during admission (HR 0.37 95% CI 0.22–0.62, p < 0.001) appeared to be protective factors. Anticoagulation treatment (any kind of anticoagulant treatment), whether at prophylactic or therapeutic doses, also seemed to be protective (HR 0.38 95% CI 0.19–0.73, p = 0.004 and HR 0.33 95% CI 0.16–0.67, p = 0.002).
Table 3.
Univariable and multivariable Cox regression analysis of mortality
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Dementia (GDS ≥ 4) | 1.567 (0.859/2.857) | 0.143 | 3.012 (1.373/6.705) | 0.017 |
| Delirium (prevalent) | 2.372 (1.628/3.454) | < 0.001 | – | 0.777 |
| Delirium (incident) | 1.857 (1.276/2.702) | 0.001 | 1.728 (1.104/2.705) | 0.017 |
| CURB-65: 0–1 (low risk) | 1 | – | 1 | |
| CURB-65: 2 (moderate risk) | 3.350 (1.557/7.208) | 0.002 | 3.802 (1.639/8.816) | 0.002 |
| CURB-65: 3–5 (high risk) | 7.764 (3.723/16.191) | < 0.001 | 7.991 (3.555/17.965) | < 0.001 |
| ACEI | 0.578 (0.368/0.910) | 0.018 | 0.428 (0.253/0.725) | 0.002 |
| Hydroxychloroquine | 0.415 (0.263/0.654) | < 0.001 | 0.372 (0.220/0.627) | < 0.001 |
| No anticoagulant treatment | 1 | 1 | ||
| Prophylactic dose | 0.347 (0.202/0.598) | < 0.001 | 0.380 (0.196/0.736) | 0.004 |
| Therapeutic dose | 0.395 (0.217/0.720) | 0.002 | 0.331 (0.163/0.671) | 0.002 |
Bold value indicates statistical significance
Fig. 1.
Kaplan–Meier survival curves Delirium (Long-Rank: p = 0.001)
Fig. 2.
Kaplan–Meier survival curves CURB-65 (Long-Rank: p < 0.001)
Mortality and 30-day hospital readmission
The total number of readmissions 30 days after discharge was 28 (15.0%): 24 patients (8.0%) had one hospital admission and four patients (1.3%) two hospital admissions during that month. The most frequent causes of readmission were pneumonia n = 5 (1.7%/) followed by falls, acute urinary tract infection, and acute heart failure n = 4 (1.3%). The overall causes of hospital readmission are defined in Table 4. The global mortality rate 30 days after hospital discharge was 6.1% (11 patients).
Table 4.
Causes of readmission (30 days after hospital discharge)
| Cause | N | % |
|---|---|---|
| Pneumonia | 5 | 1.7 |
| Fall | 4 | 1.3 |
| Acute urinary tract infection | 4 | 1.3 |
| Acute heart failure | 4 | 1.3 |
| Fall with fracture | 3 | 1.0 |
| Vomiting and diarrhea | 2 | 0.7 |
| Syncope | 1 | 0.3 |
| Pressure ulcer and osteomyelitis | 1 | 0.3 |
| Rectorrhagia | 1 | 0.3 |
Discussion
To the best of our knowledge, this is the first study to assess the importance of geriatric syndromes, such as dementia and incident delirium, as risk factors for mortality in a cohort of Spanish oldest-old inpatients with high co-morbidity burden and functional decline. Moreover, it provides information on the process of care for older patients with COVID-19 in a secondary-care hospital where most of the patients were considered to be non-ICU candidates.
We found, in line with previous studies, that the mortality rate in our population was high (37%). Blomaard et al. [20] in a cohort study in the Netherlands with more than 1300 patients included and a mean age of 78 years found a mortality rate of 38%. De Smet et al. [21] described a lower mortality rate (23%) in a Belgian cohort of 81 patients with similar characteristics regarding age, dementia, and polypharmacy as in our study, with a mean CFS score of 7, although the majority of the patients in this study population were considered potential candidates for ICU treatment. It is not known whether the rates of co-infection, CURB-65, or co-morbidities such as diabetes mellitus were similar to the ones in our study.
In a multi-variant analysis, factors associated with mortality were delirium, dementia, and CURB-65. Our results demonstrated that patients with dementia had three times higher risk of dying than patients without dementia. These results are in line with those assessed by Bianchetti et al. who stated that dementia is associated with higher mortality OR 1.84 (95% CI 1.09–3.13, p < 0.05) in older adults admitted to acute hospital wards with COVID-19 in Northern Italy [22]. A recent meta-analysis of nine studies showed that the mortality rate in patients with dementia and COVID-19 was higher than those without dementia OR: 5.17 (95% CI 2.31–11.59) [23]. This may be explained by several reasons. Firstly dementia is often associated with other co-morbidities such as high blood pressure and diabetes mellitus, which worsen the prognosis of patients with COVID-19. In addition, the clinical presentation of older patients with dementia is often atypical, which makes early diagnosis more difficult [24]. The ApoE4 genotype has also been associated with a probable increase in the development of the cytokine storm whose effects can be more deleterious in patients with dementia who already have a higher baseline inflammatory state [23].
Although those who were more frail (CFS > 5) were more likely to have died, this factor did not come out as a significant predictor for mortality in the multivariate analysis. This differs from other studies [14]. This may be because, at the beginning of the pandemic, the most frail institutionalized patients were treated in their nursing homes where possible rather than being referred to hospitals. Furthermore, given the characteristics of our hospital, such as the limited availability of ICU beds, the robust patients who are candidates for invasive therapies are less likely to be admitted to our center, which could have been a source of bias. Most studies have reported a positive association between frailty and mortality, but not all of them, as has been stated in a recent systematic review [6]. This may be because frailty has been associated with a lower degree of inflammation on admission which could lead to better health outcomes [25]. Most of the studies that assessed the relationship between frailty and mortality were relatively small sample sizes, single center with different patient selection criteria which explains the heterogeneity of the results [6]. Comprehensive geriatric assessment (CGA) is important, as per Table 1, which highlights the significant differences between survivors and non-survivors. However, BI did not come out as significant in the multivariate analysis. Ramos-Rincón et al. found that a severe degree of dependence (BI ≤ 60) was an independent predictor of death in a cohort of 2772 Spanish patients ≥ 80 diagnosed with COVID-19 included in the SEMI-COVID-19 Registry [26]. Our sample had more than twice as many patients with severe dependence (BI ‹ 60), which may explain this different result. There was not a significant difference in CCI of those who died or survived in the current study or in the study of Ramos-Rincón et al. probably due to the high prevalence of co-morbidity in both series or the fact that the CCI is not sufficiently discriminatory in these population.
Other major geriatric syndromes affecting our population were the prevalent and incident delirium (39% and 28%, respectively). This can be explained by the fact that as described, the study population was frail, with a high rate of pre-morbid dementia, cardiovascular and cerebrovascular disease. In addition, there was a reasonably high rate of use of drugs such as benzodiazepines (32%), known to exacerbate delirium, to add to the risks of delirium conferred by factors such as the presence of infection, hypoxia, and isolation in a strange environment.
The presence of delirium was associated with a survival rate of only 10% after 30 days in the COVIDAge Study [27]. Rebora et al. [28] in an Italian cohort of 516 patients established that delirium during admission was significantly associated with in-hospital mortality (HR) = 1.88, 95% CI 1.25–2.83). We assessed both, delirium present during the emergency room admission and the delirium developed during hospitalization (prevalent and incident). In a multi-variant analysis, only incident delirium was a powerful predictor of in-hospital mortality. The occurrence of delirium in COVID-19 patients has been significantly associated with threefold higher mortality, compared to those without delirium in a recent meta-analysis [29]. This is due to multiple factors, the presence of delirium has been associated with greater severity of COVID-19 infection, and delirium can aggravate pre-existing co-morbidities [29]. Prevention, early diagnosis, and management (including non-pharmacological approaches) of delirium should be a standard of care in older patients diagnosed with COVID-19.
On hospital admission, scores of between three and five on the CURB-65 scale were associated with a substantial increase in mortality in this study. Although those who died in the current study had significantly higher qSOFA scores, this did not come out as significant in the multivariate analysis. In a much larger study of 10.238 Spanish patients (mean age of 66.6 years, 57% with an age-adjusted Charlson Comorbidity Index ≥ 3), CURB-65 ≥ 3 was also found to be better than qSOFA in predicting mortality (AUROC = 0.825) [30]. We can conclude that the CURB-65 scale could be used to assess the prognosis of older inpatients with pneumonia due to COVID-19.
Bacterial co-infection was present in 22.6% of the global sample both during hospital admission and hospital stay. Lansbury et al. [31] in a recent meta-analysis stated that overall, 7% of hospitalized COVID-19 patients had a bacterial co-infection (95% CI 3–12%, n = 2183, I2 = 92.2%). However, the heterogeneity of the studies included was high and the information of the methods used to assess co-infection is scarce. Nevertheless, we know that high levels of procalcitonin and C-reactive protein may appear in patients with COVID-19 without a bacterial co-infection, which might have resulted in an overestimation of our results.
Also interesting is that there was no significant difference in the mortality of those with or without COPD. Perhaps, the use of steroids during admission could have contributed to a beneficial survival in a presumed high-risk group.
Nearly 30% of our patients were receiving ACE inhibitors/ARBs for an underlying condition at admission, and these were not routinely discontinued. This was less than those reported in other studies published in older Spanish adults, where nearly 50% of patients were using these treatments. Whereas that study just included people with heart failure, the current study included all patients over the age of 75, including those with any type of cardiovascular disease [32]. The use of ACE inhibitors/ARBs was associated significantly with less risk of dying in our study. These treatments were stopped during admission on an individual basis by the attending physician, e.g., due to acute kidney injury or arterial hypotension. It is known that the angiotensin-converting enzyme 2 (ACE2) serves as a gateway for the virus to enter the cell [33]. In the early stages of the pandemic, it was hypothesized that the use of ACE inhibitors and ARBs could increase ACE2 expression which would facilitate infection with COVID-19 [34]. Nowadays, the role of ACE inhibitors in the development of the disease is not fully established [33]. Lee et al. in a recent meta-analysis of 11 studies with more than 12,600 patients concluded that they are not associated with an increase in mortality [35], so their discontinuation is not indicated on admission if there are no other clinical reasons to support this [36].
In our study, hydroxychloroquine treatment seemed protective from mortality. The main actions of hydroxychloroquine are due to a decrease of the pH in endosomes, which makes it difficult for the virus to enter the cells, as well as a reduction in the production of the pro-inflammatory cytokines (IL6) [37]; so, in vitro studies have suggested that it could inhibit SARS-CoV-2 infection [38]. Based on these findings, at this stage of the pandemic, hydroxychloroquine was administered alone or in combination with azithromycin to all patients during the first five days of admission to our hospital, regardless of age or functional status unless the clinical severity prevented from taking oral medication or there was some contraindication such as QT prolongation. Although other small studies in older patients also suggested the effectiveness of these treatments in the early stages of disease, [39] this has not been supported by large randomized controlled trials [40] which concluded that hydroxychloroquine is ineffective at reducing mortality due to COVID-19, and also highlighted its potential adverse effects such as QT prolongation and elevation of liver enzyme levels. Moreover, its interactions with drugs widely used by older patients (digoxin, insulin, metformin, sertraline, antipsychotics, etc.) have to lead to the conclusion that hydroxychloroquine is not considered beneficial to treat COVID-19, and therefore currently it is not recommended [41].
The present study was done with treatments which differ from current guidelines, and as discussed, suggests some benefit from treatments that are not in current COVID-19 treatment guidelines. This was an observational study and, thus, cannot control for confounders. For example, 37% of patients also received glucocorticoids, which the RECOVERY trial [42] subsequently demonstrated can increase survival. Therefore, we do not consider that our results should modify the current recommendations.
Interestingly, we did not actually find that the use of glucocorticoids in our study increased survival. This could be explained by the fact that the use of steroids was not yet generalized by this point in the pandemic, as steroids had previously been found to increase mortality in a cohort of MERS-CoV patients [43] and, thus, were only prescribed for severe disease. Subsequent studies [42] suggest that earlier use of steroids could have reduced in-hospital mortality in our population. It is theoretically possible that steroids did confer a survival advantage on those with COPD in the current study, helping to explain the similar rates of COPD in those who survived or who died.
In this study, patients received prophylactic dose anticoagulation (40 mg of enoxaparin or equivalent) until discharge unless contraindicated, e.g., an active bleeding, severe thrombocytopenia, or end-of-life care. The therapeutic doses (1 mg/kg/12 h or equivalent) were maintained in patients who required it for underlying conditions. Those patients with suspected or confirmed thromboembolic disease were assessed and treated according to the guidelines [44]. It was demonstrated that anticoagulant treatment during admission both in prophylactic (HR 0.38 95% CI 0.19–0.73, p = 0.004) and therapeutic doses (HR 0.33 95% CI 0.16–0.67, p = 0.002) had a positive effect on mortality. This is in line with another study [45] which demonstrated that early prophylactic anticoagulation was associated with a decreased risk of 30-day mortality (HR 0.73, 95% CI 0.66–0.81) in a cohort of 4297 patients admitted to hospital in the United States with a median age of 68 years (interquartile range 58–75 years). The percentage of bleeding in our population was 9% and there were no cases of severe bleeding. Currently, the guidelines recommend the use of prophylactic dose anticoagulation in older inpatients with COVID-19, the risks versus the benefits of the use of higher doses to prevent VTE are under review, and further research is needed [41].
Lopinavir and ritonavir were used to inhibit SARS-CoV-2 proteases and prevent viral replication but were soon abandoned due to gastrointestinal side effects, so they were only prescribed in 7% of our population. Currently, its use is not recommended to treat COVID-19 [41].
Our study may have some limitations. This was an ambispective single-center observational study, and although only one hospital was involved, the population came from different areas of Madrid. Most of the Spanish oldest-old adults who participated were considered to be non-ICU candidates due to age, co-morbidities, and functional status. Therefore, the results may be different in more robust populations. The population included was entirely of Caucasian origin, thus, the results cannot be extrapolated to other populations. The hospitalized patients included were probably individuals with more severe symptoms than those treated at home. The age of the included patients was very high, with a narrow standard deviation, which led to the assumption that age was not a strong discriminator for prognosis in this population. Clinical management of COVID-19 in nursing homes was not determined in this study, although 67% of the patients included in this study were institutionalized individuals. Another limitation is that due to the rapidly accruing international evidence in the management of COVID-19, routine management of patients within our study changed during the study period. For example, as time went on, guidelines/protocols changed to recommend early use of steroids, a stronger recommendation for thromboprophylaxis, as well as the cessation of the use of hydroxychloroquine. These changes may, therefore, affect the interpretation of the results. The CFS was used to assess frailty, however, it is not known whether the results could have been different with other frailty assessment methods (e.g., Fried). Moreover, these are data from a real-life cohort of a geriatric ward, where the treatment was based on hospital protocols according to the guidelines of the Community of Madrid. Importantly, the scales used to evaluate functional and mental status, co-morbidity burden, and frailty were validated in older populations and used in previous COVID-19 studies, so the results could be compared. Moreover, only short-term follow-up data were provided. Finally, the treatment of older inpatients should consider not only prognostic factors but also goals of care including patient’s values and preferences according to a comprehensive geriatric assessment.
Conclusion
Dementia, incident delirium, and the CURB-65 score were independent risk factors and the strongest mortality predictors in a Spanish cohort of oldest-old patients admitted to an acute geriatric unit with COVID-19. Anticoagulation, ACE inhibitors, and hydroxychloroquine all seemed to be protective factors, but this should be interpreted with some caution given changing guidelines and patient selection during the study. Recognition of geriatric syndromes may be useful in helping clinicians establish the prognosis of oldest-old patients admitted to hospital with COVID-19.
Funding
The OCTA-COVID authors have not declared a specific grant for this research.
Declarations
Conflict of interest
The authors have declared no conflict of interest for this article and no financial conflicts.
Ethical approval
The protocol was approved by the ethics committee of Hospital Universitario La Paz, under the ID: I-4131.
Informed consent
The patients or their families gave consent to participate in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Inf Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European monitoring of excess mortality for public health action. http://www.euromomo.eu/. Accessed 13 Feb 2021
- 3.Rodriguez-Nava G, Yanez-Bello MA, Trelles-Garcia DP, Chung CW, Chaudry S, Khan AS, et al. Clinical characteristics and risk factors for death of hospitalized patients with COVID-19 in a community hospital: a retrospective cohort study. Mayo Clin Proc Innov Qual Outcomes. 2020;5(1):1–10. doi: 10.1016/j.mayocpiqo.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martín-Sánchez FJ, Del Toro E, Cardassay E, Valls Carbó A, Cuesta F, Vigara M, et al. Clinical presentation and outcome across age categories among patients with COVID-19 admitted to a Spanish Emergency Department. Eur Geriatr Med. 2020;11(5):829–841. doi: 10.1007/s41999-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosco TD, Best J, Davis D, Bryden D, Arkill S, van Oppen J, et al. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing. 2021 doi: 10.1093/ageing/afab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bavishi C, Whelton PK, Mancia G, Corrao G, Messerli FH. Renin-angiotensin-system inhibitors and all-cause mortality in patients with COVID-19: a systematic review and meta-analysis of observational studies. J Hypertens. 2021;39(4):784–794. doi: 10.1097/HJH.0000000000002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministerio de Sanidad-Instituto de Salud Carlos III (2020) Estrategia de detección precoz, vigilancia y control de COVID-19. Versión 18 Dic. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf. Accessed 13 Feb 2021
- 9.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidmiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 11.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 12.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence (2020) COVID-19 rapid guideline: acute kidney injury in hospital (NICE Guideline 175). https://www.nice.org.uk/guidance/ng175. Accessed 13 Feb 2021 [PubMed]
- 20.Blomaard LC, van der Linden CMJ, van der Bol JM, Jansen SWM, Polinder-Bos HA, Willems HC, et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing. 2021;50(3):631–640. doi: 10.1093/ageing/afab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Smet R, Mellaerts B, Vandewinckele H, Lybeert P, Frans E, Ombelet S, et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21(7):928–932. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. 2020;24(6):560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu N, Sun J, Wang X, Zhao M, Huang Q, Li H. The impact of dementia on the clinical outcome of COVID-19: a systematic review and meta-analysis. J Alzheimers Dis. 2020;78(4):1775–1782. doi: 10.3233/JAD-201016. [DOI] [PubMed] [Google Scholar]
- 24.Lozano-Montoya I, Quezada-Feijoo M, Jaramillo-Hidalgo J, Gómez-Pavón FJ. Atypical symptoms of COVID-19 in hospitalised oldest old adults. Rev Esp Geriatr Gerontol. 2020;56:120–121. doi: 10.1016/j.regg.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopp P, Miles A, Webb TE, Mcloughlin BC, Mannan I, Raja N, et al. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. Eur Geriatr Med. 2020;11(6):1089–1094. doi: 10.1007/s41999-020-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Rincon J-M, Buonaiuto V, Ricci M, Martín-Carmona J, Paredes-Ruíz D, Calderón-Moreno M, et al. Clinical characteristics and risk factors for mortality in very old patients hospitalized with COVID-19 in Spain. J Gerontol A Biol Sci Med Sci. 2021;76(3):e28–e37. doi: 10.1093/gerona/glaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendes A, Serratrice C, Herrmann FR, Genton L, Périvier S, Scheffler M, et al. Predictors of in-hospital mortality in older patients with COVID-19: The COVIDAge Study. J Am Med Dir Assoc. 2020;21(11):1546–1554.e3. doi: 10.1016/j.jamda.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebora P, Rozzini R, Bianchetti A, Blangiardo P, Marchegiani A, Piazzoli A, et al. Delirium in patients with SARS-CoV-2 infection: a multicenter study. J Am Geriatr Soc. 2020;69(2):293–299. doi: 10.1111/jgs.16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao S-C, Lai C-C, Chen Y-HY-C, Chen Y-HY-C, Hung M-J, Liao S-C. Prevalence, incidence and mortality of delirium in patients with COVID-19: a systematic review and meta-analysis. Age Ageing. 2021 doi: 10.1093/ageing/afab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artero A, Madrazo M, Fernández-Garcés M, Muiño Miguez A, González García A, Crestelo Vieitez A, et al. Severity Scores in COVID-19 pneumonia: a multicenter, retrospective. Cohort Study. J Gen Intern Med. 2021;36(5):1338–1345. doi: 10.1007/s11606-021-06626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becerra-Muñoz VM, Núñez-Gil IJ, Eid CM, Aguado MG, Romero R, Huang J, et al. Clinical profile and predictors of in-hospital mortality among older patients admitted for COVID-19. Age Ageing. 2020;50(2):326–334. doi: 10.1093/ageing/afaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adu-Amankwaah J, Mprah R, Adekunle AO, Noah MLN, Adzika GK, Machuki JO, et al. The cardiovascular aspect of COVID-19. Ann Med. 2021;53(1):227–236. doi: 10.1080/07853890.2020.1861644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HW, Yoon CH, Jang EJ, Lee CH. Renin-angiotensin system blocker and outcomes of COVID-19: a systematic review and meta-analysis. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-215322. [DOI] [PubMed] [Google Scholar]
- 36.Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail. 2020;26(5):370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar R, Sharma A, Srivastava JK, Siddiqui MH, Uddin MS, Aleya L. Hydroxychloroquine in COVID-19: therapeutic promises, current status, and environmental implications. Environ Sci Pollut Res Int. 2021 doi: 10.1007/s11356-020-12200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heras E, Garibaldi P, Boix M, Valero O, Castillo J, Curbelo Y, et al. COVID-19 mortality risk factors in older people in a long-term care center. Eur Geriatr Med. 2020;12(3):601–607. doi: 10.1007/s41999-020-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed Jun 2021 [PubMed]
- 42.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfaraj SH, Al-Tawfiq JA, Assiri AY, Alzahrani NA, Alanazi AA, Memish ZA. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: a cohort study. Travel Med Infect Dis. 2019;29:48–50. doi: 10.1016/j.tmaid.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rentsch CT, Beckman JA, Tomlinson L, Gellad WF, Alcorn C, Kidwai-Khan F, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]