Abstract
Lead toxicity is one of the causative agents of male infertility that raised concern from environmental contamination worldwide. L‐carnitine, a biologically active amino acid, present in high concentration in the reproductive organs such as the epididymis, is involved in sperm maturation. The possible protective effect of L‐carnitine in experimentally lead‐induced male reproductive toxicity in rats was evaluated in this study. Thirty adult male Wistar rats were divided into three groups. Group 1: the negative control group was treated with normal saline; Group 2: exposed to 50 mg/kg lead acetate (2% solution in saline); and Group 3: treated with lead acetate 50 mg/kg (2% solution in saline) + L‐carnitine 100 mg/kg. At the end of the experimental period, body and testicular weights were determined, blood samples were withdrawn for hormonal assays of FSH, LH and testosterone. Sperm parameters as sperm count, morphology, viability and motility were measured. Testicular tissue homogenates were prepared for enzymatic assays and for measuring oxidative stress parameters. Lead significantly increased both oxidative stress and the concentration of lactate dehydrogenase‐C in the testicular tissues with a decrease in sperm count, motility and viability. Lead acetate treatment, induced alteration in sperms with normal morphology together with reductions in the serum FSH, LH, testosterone, body and testicular weights. The concentration of 17β‐hydroxysteroid dehydrogenase was significantly reduced. Co‐administration of L‐carnitine significantly reduced testicular oxidative stress, improved sperm parameters, elevated serum FSH, LH and testosterone with an insignificant reduction in the testicular weight. The concentrations of 17β‐hydroxysteroid dehydrogenase and lactate dehydrogenase‐C were significantly improved by L‐carnitine. The overall results indicate that L‐carnitine is expected to improve the lead acetate‐induced male reproductive toxicity.
Keywords: antioxidant, L‐carnitine, lead acetate, oxidative stress, reproductive toxicity, sperm
Chronic lead acetate exposure significantly impairs male reproduction. L‐carnitine supplementation ameliorates the mechanisms of lead acetate‐induced reproductive dysfunction largely by its antioxidant properties. L‐carnitine can be recommended to ameliorate the reproductive toxicity of chronic lead exposure among lead workers.
![]()
1. INTRODUCTION
Heavy metals poisoning is a major public health problem in the world. Lead is one of the metals that causes death and diseases especially in developing countries (Needleman, 2004). Its toxicity can originate from contaminated air, water, food and dust. It is most common in children (Dapul & Laraque, 2014). Lead can cause disease in different organs in the body. The symptoms of toxicity include abdominal pain, headaches, memory problems, constipation, inability to have children, behavioural abnormalities (Dapul & Laraque, 2014). It may be behind unexplained male infertility which is an important problem in humans. Lead can decrease the weights of the body, testes, epididymes, prostate and seminal vesicle (Pinon‐Lataillade et al., 1995). Numerous studies documented that lead exposure has been linked to altered sperm parameters which subsequently leads to male infertility (Kumar, 2018; Martin et al., 2017; Wahab et al., 2019). Searching for protective therapeutic agents against lead‐induced reproductive toxicity has been a great interest in scientific research.
L‐carnitine is a biologically active amino acid, present mainly in high energy‐consuming tissues such as cardiac and skeletal muscles. It also concentrates in reproductive organs such as the epididymis and testis (Kobayashi et al., 2005). It plays a significant role in the mitochondrial function for oxidation and energy production. Moreover, it causes modification of acyl‐CoA and CoA ratio, energy storage as acetylcarnitine and decreases toxic effects of non‐metabolized acyl groups (Vaz & Wanders, 2002). Previous studies reported beneficial effects of L‐carnitine and its acetylated form on sperm parameters and reproduction. Casillas (1973) observed that spermatozoa accumulate L‐carnitine in human epididymis, which is closely related to increased fertility of spermatozoa. In the epididymis, a high concentration of free L‐carnitine is collected from the blood plasma and is transferred into the epididymal fluid. Then, it diffuses into the spermatozoa, where it is present as both free and acetylated forms (Abdelrazik & Agrawal, 2009). An increase in sperm motility is associated with a high concentration of free L‐carnitine in the epididymal fluid (Ng et al., 2004).
Our current study aimed to investigate the possible ameliorative effect of L‐carnitine on the mechanisms of reproductive toxicity induced by chronic lead acetate treatment in male Wistar rats. This was performed by measuring the sperm parameters including sperm count, morphology, motility and viability. The testicular malondialdehyde (MDA) and total antioxidant capacity (TAC) were measured together with the enzymatic concentrations of 17β‐hydroxysteroid dehydrogenase (17β‐HSD) and lactate dehydrogenase‐C (LDH‐C). Serum levels of hormones that play role in male reproduction as follicle‐stimulating hormone (FSH), leuteinizing hormone (LH) and testosterone were measured.
2. MATERIALS AND METHODS
2.1. Chemicals
Lead acetate was supplied by Alpha Chemicals Company, Egypt, and L‐carnitine was purchased from Epico Company, Egypt.
2.2. Experimental animals
Thirty adult male Wistar rats (weight 200 g ± 20 g, age three to four months) were purchased from the animal house of Faculty of Medicine, Assiut University. They were placed in clean cages; left in the animal house under standard living conditions and fed with a standard pellet diet and water. All procedures performed in the study were complying with the ethical standards of Faculty of Medicine, Assiut University for humane animal treatment, and complies with relevant legislation.
2.3. Experimental design
Rats were randomly assigned into three groups, each consisting of 10 animals. Group I served as the negative control group treated with normal saline. Group II the positive control treated with 50 mg/kg lead acetate (2% solution in saline). Group III was treated with lead acetate 50 mg/kg (2% solution in saline) + L‐carnitine 100 mg/kg (El‐Sherbini et al., 2,107). Rats were treated by oral gavage once daily for 40 days. Lead acetate was given to group III on the 5th day after administration of L‐carnitine and continued for 35 days according to the established protocol of Sudjarwo et al., (2017). At the end of the treatment period, body weights were measured and blood samples were withdrawn from the retro‐orbital plexuses while rats were under light ether anaesthesia. Both testes were dissected out and weighted. The relative weight of the testes was calculated according to the following formula:
Relative weight of testes = Weight of testes (g)/weight of the body (g) ×100.
2.4. Sperm collection and testis homogenate preparation
The caudae epididymis of each rat was quickly removed as described by Raji et al., (2005), minced in 1ml phosphate buffer saline (PBS) pH (7.2) (D’Souza, 2004), incised to allow free exit of the sperms out of the epididymes. Approximately 50 µl was pipetted from the sperm concentrate, diluted 20 times with phosphate buffer saline and used for assessment of sperm parameters. One testis was washed by ice‐cold saline and homogenized (10% w/v) in PBS and the supernatant was pipetted after centrifugation for 20 min. The supernatant was used for estimation of the tissue levels of MDA, TAC, 17β‐HSD and LDH‐C using standard kits.
2.5. Sperm parameters
2.5.1. Sperm count
Sperms were counted using the improved two‐chambered Neubauer haematocytometer. Approximately 10 µl of the diluted sperm suspension was added to each chamber and counting was done according to the method described by Raji et al., (2005). The sperm concentration was expressed as sperm number × 106/ml.
2.5.2. Sperm motility
Immediately after sperm isolation and diluting the sperm suspension, about 10 μl was added to the slide chamber. The number of sperms with progressive motility, non‐progressive motility as well as immotile ones was counted as described by Soleimanzadeh and Saberivand (2013).
2.5.3. Sperm viability
Sperm viability was done using the eosin staining technique as described by Sudjarwo et al., (2017). Two drops of eosin stain solution (1% in distilled water) were added to one drop of the freshly collected sperm suspension in a test tube. Sperm viability was determined by counting viable and non‐viable sperms in each chamber. Sperm viability was expressed as the number of viable sperms.
2.5.4. Sperm morphology
Sperm smears were prepared from diluted sperm suspension, allowed to dry in air and examined for alterations in normal sperm morphology (head and tail abnormalities) as close to those described by Wyrobek and Bruce, (1975).
2.5.5. Biochemical assays
The collected blood samples were centrifuged at 10,000 rpm for 15 min to separate serum. The serum was stored at −20°C to measure the levels of pituitary gonadotrophic hormones, FSH and LH using Pishtaz Teb FSH and LH enzyme‐linked immunosorbent assay (ELISA) kits at 450 nm (Pishtaz Teb Diagnostics) according to the enclosed instructions. Rat Testosterone ELISA kit supplied by Biosource, Europe, Belgium, Catalog MBS282195, was used for measuring serum testosterone levels.
The supernatant recovered after homogenization of the testicular tissue was used for the measurement of malondialdehyde level by MDA assay kits for research use only (Biodiagnostic, Giza, Egypt, Catalog Number: MD 25 28) according to the method of Ohkawa et al., (1979) and the enclosed instructions. Total antioxidant capacity was measured by the TAC assay kit for research use only (Biodiagnostic, Giza, Egypt, Catalog Number: TA 25 12) according to the method of Koracevic et al., (2001). The concentrations of 17β‐HSD and LDH‐C were measured using the rat enzyme‐linked immunosorbent assay kit for 17‐beta‐hydroxysteroid dehydrogenase (Catalog MBS2104946) and LDH‐C (Catalog MBS2024970) respectively (Biosource, Europe, Belgium).
2.5.6. Statistical analysis
The software Graphpad Prism version 5 was used for analysing data. Unpaired t test was performed to compare means between two groups. Significance was considered at p‐value < 0.05.
3. RESULTS
3.1. Effect on body and testicular weight
Lead acetate caused a significant (p < 0.05) reduction in rats’ body, absolute and relative testicular weights in comparison with the negative control group. Co‐administration of L‐carnitine improved the relative testicular weight in comparison with the lead acetate‐treated group (Table 1).
TABLE 1.
The effect of lead acetate and lead acetate + L‐carnitine on rats’ body weight, absolute and relative testicular weights
| Groups | Body weight (g) | Absolute testicular weight (g) | Relative testicular weight (%) |
|---|---|---|---|
| Negative control | 206.10 ± 2.656 | 1.35 ± 0.009 | 0.65 ± 0.011 |
| Lead acetate 50 mg/kg (2%) | 185.10 ± 2.597* | 1.12 ± 0.017* | 0.60 ± 0.015* |
| Lead acetate 50 mg/kg (2%) +L‐carnitine 100 mg/kg | 174.80 ± 1.730** | 1.09 ± 0.020 | 0.62 ± 0.020 |
p < 0.05 vs. negative control.
p < 0.05 vs. lead acetate group. Data presented as Mean ± SE.
3.2. Effect on sperm count and morphology
A significant reduction in total sperm counts and elevation in sperms with abnormal morphology were observed in the lead acetate‐treated groups in comparison with the negative control group. The addition of L‐carnitine improved both parameters significantly (p < 0.05) (Figure 1). The detected abnormal sperm heads (headless sperm, amorphous head and head break) are presented in the lead acetate‐treated group (Figure 2).
FIGURE 1.
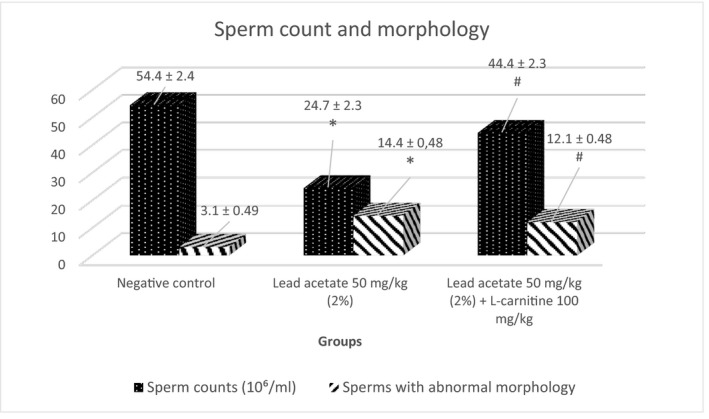
The ameliorative effect of L‐carnitine on lead acetate‐induced alterations in sperm count and morphology (Mean ± SE). *p < 0.05 vs. negative control; # p < 0.05 vs. lead acetate group
FIGURE 2.
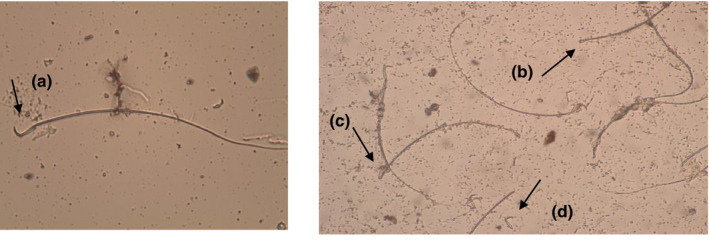
Sperm shape abnormalities detected in male rats treated with lead acetate (2%) solution (50 mg/kg) showing head and tail abnormalities as following: (a) normal sperm, (b) headless sperm, (c) amorphous head, (d) head break (head without tail). Magnification power 400×
3.3. Effect on sperm viability and motility
Lead acetate induced a significant (p < 0.05) reduction in the number of viable and motile sperms in comparison with the negative control group. Sperm viability and motility improved significantly with L‐carnitine (Figure 3). Eosin stain (1%) stained the heads of dead sperms (pink) while heads of viable sperms remained colourless (Figure 4).
FIGURE 3.
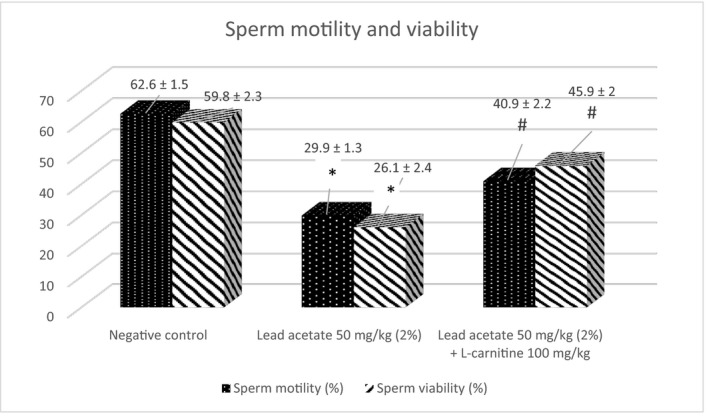
The ameliorative effect of L‐carnitine on lead acetate‐induced alterations in sperm motility and viability (Mean ± SE). *p < 0.05 vs. negative control; # p < 0.05 vs. lead acetate group
FIGURE 4.
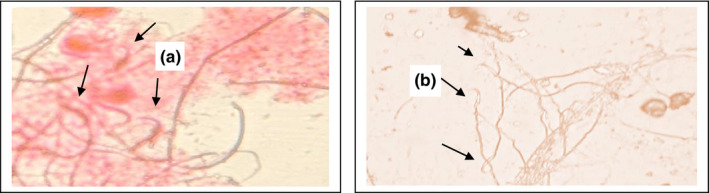
Sperm viability; (a) dead sperm (pink), (b) viable sperm (colourless), (Black arrows); (Eosin 1%, 400×)
3.4. Effect on serum FSH, LH and testosterone
Serum FSH and LH levels were significantly reduced (p < 0.05) in the lead acetate‐treated group and a significant decrease (p < 0.05) was noticed in the serum testosterone in the lead acetate group when compared with the negative control. Co‐administration of L‐carnitine with lead acetate caused a significant increase (p < 0.05) in the serum levels of pituitary gonadotropins and testosterone relative to the lead acetate group (Figure 5).
FIGURE 5.
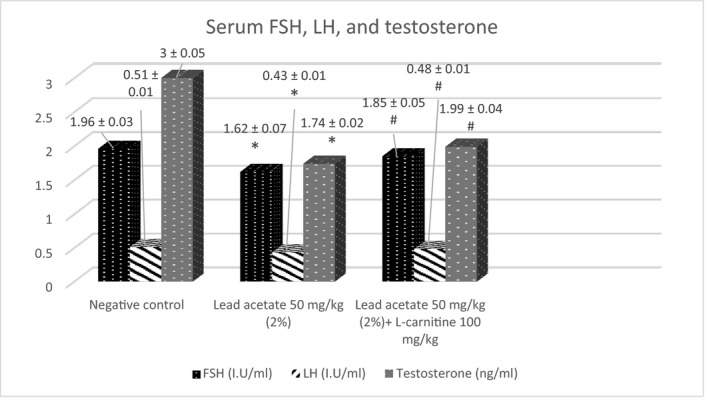
The ameliorative effect of L‐carnitine on lead acetate‐induced alterations in FSH, LH and testosterone serum levels (Mean ± SE). *p < 0.05 vs. negative control; # p < 0.05 vs. lead acetate group
3.5. Effect on testicular MDA and TAC
A significant increase in MDA and reduction in TAC was observed in lead acetate‐treated groups vs. negative control (3.91 ± 0.16 vs. 2.43 ± 0.16 nmol/gm tissue for the MDA) and (1.21 ± 0.10 vs. 2 ± 0.05 mmol/L for the TAC). Co‐administration of L‐carnitine with lead acetate reduced MDA and elevated TAC significantly compared with the lead acetate group (3.28 ± 0.24 vs. 3.91 ± 0.16 nmol/gm tissue for the MDA; and 1.67 ± 0.02 vs. 1.21 ± 0.10 mmol/L for the TAC) (Figure 6).
FIGURE 6.
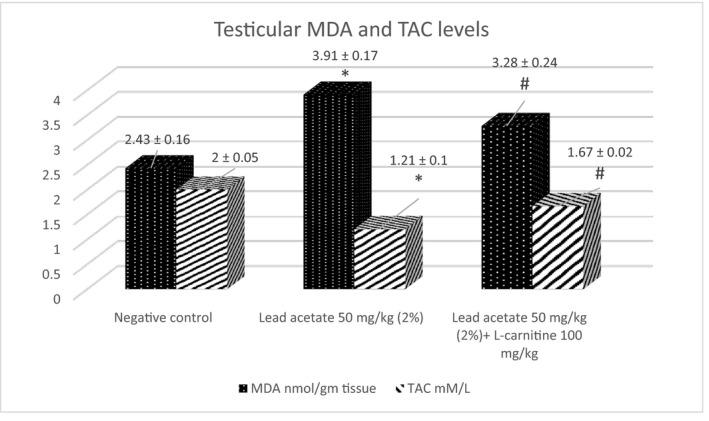
The effect of L‐carnitine on lead acetate‐induced oxidative stress (Mean ± SE). *p < 0.05 vs. negative control; # p < 0.05 vs. lead acetate group
3.6. Effect on testicular 17β‐HSD and LDH
A significant reduction in the concentration of 17β‐HSD was observed in lead acetate‐treated groups vs. negative control (0.027 ± 0.0008 vs. 0.055 ± 0.001 ng/mg tissue). Co‐administration of L‐carnitine with lead acetate increased the tissue concentration of 17β‐HSD significantly compared with the lead acetate group (0.035 ± 0.001 vs. 0.027 ± 0.0008 ng/mg tissue) (Table 2).
TABLE 2.
The effect of lead acetate and lead acetate + L‐carnitine on testicular 17β‐HSD and LDH‐C concentrations
| Groups | 17β‐HSD (ng/mg tissue) | LDH‐C (ng/mg tissue) |
|---|---|---|
| Negative control | 0.055 ± 0.001 | 1.20 ± 0.715 |
| Lead acetate 50 mg/kg (2%) | 0.027 ± 0.001* | 1.60 ± 2.128* |
| Lead acetate 50 mg/kg (2%) +L‐carnitine 100 mg/kg | 0.035 ± 0.001** | 1.46 ± 1.145** |
p < 0.05 vs. negative control.
p < 0.05 vs. lead acetate group. Data presented as Mean ± SE.
The level of LDH‐C was significantly (p < 0.05) increased in the lead acetate‐treated group compared with the negative control (1.602 ± 2.128 vs. 1.203 ± 0.71 ng/mg tissue). L‐carnitine treatment to the intoxicated rats caused a significant (p < 0.05) reduction in LDH‐C level compared with the lead acetate group (1.46 ± 1.145 vs. 1.602 ± 2.128 ng/ml) (Table 2).
4. DISCUSSION
Male infertility is a major health problem, characterized by decreased both sperm production and sperm function. One of the main aetiologies of male infertility is occupational and environmental exposures to toxic heavy metals, such as lead. Lead exposure leads to permanent damage to various organs and it has complex and multiple pathways in infertility. L‐carnitine, widely found in nature, has many effects on human health which have been popularized. This study attempts to investigate the negative effects of lead acetate exposure in experimental animals and the possible protective effect of L‐carnitine on male fertility.
In the present study lead acetate caused significant (p < 0.05) reductions in rats’ body weight, absolute and relative testicular weights in comparison with the negative control group. These results are consistent with Elgawish and Abdelrazek (2014), who reported a significant reduction in testicular weight in lead‐treated rats. Gani et al., (2016) reported a significant reduction in rats’ body weights after treatment with lead acetate. This was explained by the interruption of absorption and metabolism of food and nutrients essential for health. Co‐administration of L‐carnitine to lead acetate‐treated rats caused a further reduction in body and testicular weights compared with the lead‐treated group. Abo‐Ghanema et al., (2012) documented similar results. The decrease in the testicular weight may be attributed to the hypolipidaemic effect of L‐carnitine as it increases the influx of fatty acids into the mitochondria for energy production resulting in loss of fat in tissues.
Lead acetate exposure caused a significant decrease in total sperm counts and elevation in sperms with abnormal morphology in the current study. Moreover, lead acetate produced a significant reduction in the number of motile and viable sperms in comparison with the negative control group. These results are in agreement with other previous studies which reported that lead exposure leads to alterations in sperm parameters as a decrease in sperm count. Chronic lead exposure impairs spermatogenesis which can subsequently decline sperm counts (Pizent et al., 2012). Moreover, lead exposure leads to a high level of abnormal sperms and immature spermatozoa (Chowdhury et al., 1986). Another study observed that high blood and semen lead levels produced a negative relation with sperm count, morphology, viability and motility among battery factory workers. This was associated with high lipid peroxidation and reduction in seminal plasma ascorbic acid as reported by Naha et al., (2005). Furthermore, Li and his co‐worker (2018) showed that high concentrations of lead in drinking water decreased motility of sperms, density, viability and produced significant morphological abnormalities of spermatozoa (Li et al., 2018). Chronic lead exposure caused a significant reduction in sperm vitality, motility, density and morphology in the exposed rats as well as a significant reduction in zinc concentration in testicular homogenates (Martin et al., 2017). The deteriorative effects of lead acetate on sperm parameters were reported by other previous studies (He et al., 2016; Jegede et al., 2015; Sudjarwo et al., 2017).
The deleterious effects of lead acetate on sperm parameters were greatly related to lead‐induced oxidative stress. These observations were evidenced in this study especially that it was accompanied by a significant elevation in lipid peroxide (MDA) level and reduction in TAC in the testicular homogenate. Similar results were reported by El‐Tohamy and El‐Nattat (2010), where lead acetate caused a significant increase in semen MDA and a significant reduction in semen TAC of male rabbits.
The ability of lead acetate to induce oxidative stress has been documented by several previous studies (El‐Sherbini et al., 2017; Haleagrahara et al., 2011; Patri et al., 2017; Samarghandian et al., 2013; Sharma & Thakur, 2017; Wang et al., 2013). Moreover, chronic exposure of rats to lead resulted in an elevation in lipid peroxide concentration in the reproductive organs (Marchlewicz et al., 2007). Oxidative stress ensues when the generation of reactive oxygen species (ROS) overwhelms the antioxidant defence mechanisms of body organs. Dysfunction and damage of several body organs occur under the influence of oxidative stress. The reproductive system is mostly affected as the sperms are highly vulnerable to oxidative stress according to Saleh and Agarwal (2002). Although minute amounts of ROS are produced by sperms to facilitate their functions in fertilization (Faure et al., 2011; Griveau & Le Lannou, 1997), large amounts of ROS appear to negatively impact these sperms. The ROS attack and destroy the cellular membranes of sperms, initiate lipid peroxidation, damage nucleic acids and impair DNA repair (Vigeh et al., 2011). These contribute to a reduction in sperm viability with an increase in abnormal sperm morphologies. In the eosin staining technique, the viable sperms remained colourless while dead sperms were stained in pink as eosin stain can penetrate into dead sperms but not in viable sperms. Destruction of spermatic cell membranes contributes to altered membrane permeability and diffusion of eosin dye into sperms indicating its death.
The effect of lead‐induced oxidative stress cannot be ruled out in its effect on sperm motility. Reactive oxygen species may impair sperm motility through inducing phosphorylation of axonemal proteins or reducing ATP generation within the spermatic cells which are necessary for the movement of sperms (Armstrong et al., 1999; de Lamirande & Gagnon, 1992).
The addition of L‐carnitine to lead‐treated rats improved sperm parameters significantly. The oxidant and antioxidant status in testicular tissue were also significantly improved. L‐carnitine is a bioactive substance, derived from lysine and methionine amino acids, which could accelerate lipid metabolism. It has an important role in mitochondrial β‐oxidation that is involved in cellular energy production. Furthermore, L‐carnitine protects DNA and cell membranes from damage induced by free oxygen radicals (Lenzi et al., 2004; Ye et al., 2010). Free L‐carnitine and acetylated L‐carnitine which are present in high concentrations in the epididymal fluid and spermatozoa than in blood play a significant role in sperm motility (Vicari & Calogero, 2001). Aliabadi et al., (2012) reported a significant increase in sperm motility induced by L‐carnitine and L‐acetyl carnitine in the sperm cells extracted from testes. Moncada et al., (1992) showed a statistically significant increase in the progressive sperm motility after treatment with L‐acetyl carnitine in patients with idiopathic oligoasthenozoospermia. The values of progressive motility returned to pre‐treatment values after cessation of L‐acetyl carnitine which indicated that it was drug related (Moncada et al., 1992). The high concentrations of carnitine are believed to improve sperm motility through the enhancement of mitochondrial fatty acid oxidation and ATP production (Ng et al., 2004). According to Aitken and Baker, the generated reactive oxygen species can disturb sperm motility by interrupting ATP production or flagellar axoneme phosphorylation (Aitken & Baker, 2006). The anti‐oxidant property of L‐carnitine can therefore improve sperm motility as documented by our study and supported by Solarska et al., (2010).
The positive impact of L‐carnitine on sperm parameters have been studied in previous studies. It was directly related to improvements in sperm parameters as sperm counts, viability and morphology (Ahmed et al., 2011; Al‐Daraji & Tahir, 2014; Khademi et al., 2004). The improvements in sperm parameters can be attributed to the antioxidant properties of L‐carnitine as it prevents the production of free radicals in the semen as reported by Agarwal and Said (2004). According to Surai (2015), several antioxidant mechanisms for L‐carnitine were proposed, including its ability to directly scavenge free radicals; chelate catalytic metals‐promoters of ROS, such as Fe and Cu; maintain mitochondrial integrity and prevent ROS formation and inhibit ROS‐generating enzymes, such as xanthine oxidase and NADPH oxidases with an additional synthesis of antioxidant enzymes.
Chronic administration of lead acetate resulted in a significant reduction in the serum levels of pituitary gonadotropins—FSH and LH as well as testosterone in the current study. These results are coinciding with Wahab et al., (2019). Low testosterone level has also been reported in lead exposure (Doumouchtsis et al., 2009; Yu et al., 2010). Impairment of spermatogenesis in chronic lead exposure is attributed to hormonal imbalance induced by disruption of the hypothalamic–pituitary axis causing a reduction in follicle‐stimulating hormone/luteinizing hormone and gonadotropin‐releasing hormone (Doumouchtsis et al., 2009). Disruption in gonadotropin‐releasing hormone by lead could be a possible mechanism for the reduced levels of FSH and LH (Gore, 2001). Compromised pituitary gonadotropin levels impair spermatogenesis and negatively impact sperm counts (Ahmed & Abdel‐Emam, 2019). Follicle‐stimulating hormone directly enhances spermatogenesis, increases the testicular size and Sertoli cell differentiation (O'Shaughnessy et al., 2010). Luteinizing hormone is the primary hormone necessary for maturation of and steroidogenesis in Leydig cells. Reduction in serum testosterone levels of lead acetate‐exposed rats could be attributed to the reduction in serum LH. This can impair spermatogenesis and reduced sperm counts as reported by Almansour, (2009). Treatment of lead‐intoxicated rats with L‐carnitine in our study showed a significant elevation in serum levels of FSH, LH and testosterone. L‐carnitine treatment showed similar results in previous studies (Rahimi & Shariati, 2017; Rezaei et al., 2018). Moreover, Al‐Daraji and Tahir (2014) reported that L‐carnitine can increase serum levels of FSH and LH which subsequently improves spermatogenesis and testosterone level. As an antioxidant, L‐carnitine can inhibit free radicals and increase the expression of the antioxidant enzymes leading to reduced oxidative stress (Zambrano et al., 2014). Oxidative stress can decrease FSH, LH and testosterone levels (Rezaei et al., 2018). Additionally, L‐carnitine can increase the release of luteinizing hormone‐releasing hormone, which activates the release of LH which activates the release of testosterone (Abo‐Ghanema et al., 2012).
17β‐hydroxysteroid dehydrogenase is the main enzyme expressed in the testis to convert androstenedione into testosterone. The level of testicular 17β‐HSD was reduced in the lead acetate‐treated rats which coincides with the reduced serum levels of testosterone hormone. Wahab et al., (2019) reported reduced testicular 17β‐HSD activity in the lead acetate‐treated rats for 35 days. The reduced serum levels of LH can explain the reduced activity and concentration of 17β‐HSD as LH signals the initiation of steroidogenesis in the testes and hence the observed decrease in serum testosterone levels. Down‐regulation of 17β‐HSD expression leads to the inhibition of LH‐stimulated testosterone production (Al‐Rubiey, 2012). Co‐administration of L‐carnitine with lead acetate resulted in an elevation in the testicular levels of 17β‐HSD. As L‐carnitine can increase the serum levels of LH, the stimulating effects of luteinizing hormone in the initiation of steroidogenesis may also promote the activity and the concentration of 17β‐HSD (Ribeiro & Abucham, 2009).
Lactate dehydrogenase‐C (formerly known as lactate dehydrogenase‐X) is the first testis‐specific isozyme discovered in male germ cells. It is involved in the conversion of glucose to lactate to be utilized in energy production by the spermatozoa. Lead intoxication caused a significant elevation in testicular LDH‐C level which coincides with the results of Bello and Idris (2018), who reported a significant increase in LDH in the serum of lead acetate intoxicated rats. The oxidative damage of the testicular tissue and increased leakage of the enzyme from the damaged tissues could be a possible explanation for the elevated LDH‐C level. The addition of L‐carnitine to lead acetate intoxicated rats resulted in a significant reduction in LDH‐C concentrations. The antioxidant effect of L‐carnitine can minimize the oxidative‐induced damage within the testicular tissue and subsequently reduce the leakage of LDH‐C. Abd‐Allah et al., (2009) and Aliabadi et al., (2013) reported an increase in LDH‐X and LDH‐C4 activity in the testicular tissue after treatment with L‐carnitine. A recent study showed that antioxidant agents can reduce the LDH activity in lead toxicity (Udefa et al., 2020). It seems that chronic lead intoxication increases the leakage of LDH‐C from the damaged testicular tissues and increases its activity (as a compensatory mechanism) due its inhibitory effect on spermatogenesis.
5. CONCLUSION
From the abovementioned results, chronic lead acetate exposure significantly impairs sperm parameters. This is associated with an increase in testicular oxidative stress markers, alterations in enzymatic levels of lactate dehydrogenase and 17beta‐hydroxysteroid dehydrogenase and reduction in pituitary gonadotropins and testosterone which may negatively impact male reproduction. L‐carnitine supplementation improved the deteriorated sperm parameters, improved testicular oxidative stress, hormonal and enzymatic levels by its antioxidant properties. Therefore, L‐carnitine can be recommended to ameliorate the reproductive toxicity of chronic lead exposure among lead workers.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
Rania Abdel‐Emam: Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Validation; Visualization; Writing‐review & editing. Esraa Ahmed: Conceptualization; Methodology; Validation; Visualization; Writing‐original draft.
ACKNOWLEDGEMENTS
We would like to acknowledge Dr. Nahed A. Elossily and Dr. Esam O. Kamel for their kind assistance to achieve this work. Every kind hand participated in accomplishment of this study is deeply acknowledged.
Abdel‐Emam RA, Ahmed EA. Ameliorative effect of L‐carnitine on chronic lead-induced reproductive toxicity in male rats. Vet Med Sci. 2021;7:1426–1435. 10.1002/vms3.473
Funding information
This study did not receive any funding source.
DATA AVAILABILITY STATEMENT
The original research articles that support the results of this study are publicly available.
REFERENCES
- Abd‐Allah, A. R. , Helal, G. K. , Al‐Yahya, A. A. , Aleisa, A. M. , Al‐Rejaie, S. S. , & Al‐Bakheet, S. A. (2009). Pro‐inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L‐carnitine. Oxidative Medicine and Cellular Longevity, 2(2), 73–81. 10.4161/oxim.2.2.8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelrazik, H. , & Agrawal, A. (2009). L‐carnitine and assisted reproduction. Archives of Medical Science, 5, 43–47. [Google Scholar]
- Abo‐Ghanema, I. I. , El‐Nasharty, M. A. , El‐Far, A. H. , & Ghonium, H. A. (2012). Effect of ginger and L‐carnitine on the reproductive performance of male rats. World Acad Sci Eng Technol, 64, 980–986. [Google Scholar]
- Agarwal, A. , & Said, T. M. (2004). Carnitine and male infertility. Reproductive Biomedicine Online, 8(4), 376–384. [DOI] [PubMed] [Google Scholar]
- Ahmed, E. A. , & Abdel‐Emam, R. A. (2019). The potential impact of 1st and 2nd generation antihistamines on male fertility. Comparative Clinical Pathology, 28, 1465–1470. 10.1007/s00580-019-02993-0 [DOI] [Google Scholar]
- Ahmed, S. D. H. , Karira, K. A. , & Jagdesh, A. S. (2011). Role of L‐carnitine in male infertility. The Journal of the Pakistan Medical Association, 61(8), 732–736. [PubMed] [Google Scholar]
- Aitken, R. J. , & Baker, M. A. (2006). Oxidative stress, sperm survival and fertility control. Molecular and Cellular Endocrinology, 250, 66–69. 10.1016/j.mce.2005.12.026 [DOI] [PubMed] [Google Scholar]
- Al‐Daraji, H. J. , & Tahir, A. O. (2014). Effect of L‐carnitine supplementation on drake semen quality. South African Journal of Animal Science, 44(1), 18–25. 10.4314/sajas.v44i1.3 [DOI] [Google Scholar]
- Aliabadi, E. , Karimi, F. , Rasti, M. , Akmali, M. , & Esmaeilpour, T. (2013). Effects of L‐carnitine and pentoxifylline on the activity of lactate dehydrogenase C4 isozyme and motility of testicular spermatozoa in mice. Journal of Reproduction & Infertility, 14(2), 56–61. [PMC free article] [PubMed] [Google Scholar]
- Aliabadi, E. , Soleimani Mehranjani, M. , Borzoei, Z. , Talaei‐Khozani, T. , Mirkhani, H. , & Tabesh, H. (2012). Effects of L‐carnitine and L‐acetyl‐carnitine on testicular sperm motility and chromatin quality. Iranian Journal of Reproductive Medicine, 10(2), 77–82.PMID: 25242977; PMCID: PMC4163266. [PMC free article] [PubMed] [Google Scholar]
- Almansour, M. I. (2009). Histological alterations induced by lead in the testes of the Quail Coturnix coturnix . Research Journal of Environmental Toxicology, 3, 24–30. [Google Scholar]
- Al‐Rubiey, F. K. (2012). Effect of L‐carnitine and meloxicam treatment on testicular Leydig cell numbers of varicocelized rats. Middle East Fertility Society Journal, 17, 47–53. 10.1016/j.mefs.2011.08.009 [DOI] [Google Scholar]
- Armstrong, J. S. , Rajasekaran, M. , Chamulitrat, W. , Gatti, P. , Hellstrom, W. J. , & Sikka, S. C. (1999). Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radical Biology and Medicine, 26(7–8), 869–880. 10.1016/S0891-5849(98)00275-5 [DOI] [PubMed] [Google Scholar]
- Bello, T. H. , & Idris, O. A. (2018). The Effect of antioxidant (Gallic acid) on the testes of lead acetate induced Wistar rat. Toxicology and Environmental Health Sciences, 10(5), 261–267. 10.1007/s13530-018-0374-0 [DOI] [Google Scholar]
- Casillas, E. R. (1973). Accumulation of carnitine by bovine spermatozoa during maturation in the epididymis. Journal of Biological Chemistry, 248, 8227–8232. 10.1016/S0021-9258(19)43218-3 [DOI] [PubMed] [Google Scholar]
- Chowdhury, A. R. , Chinoy, N. J. , Gautam, A. K. , Rao, R. V. , Parikh, D. J. , Shah, G. M. et al (1986). Effect of lead on human semen. Advances in Contraceptive Delivery Systems: CDS, 2, 208–210. [PubMed] [Google Scholar]
- Dapul, H. , & Laraque, D. (2014). Lead poisoning in children. Advances in Pediatrics, 61(1), 313–333. 10.1016/j.yapd.2014.04.004 [DOI] [PubMed] [Google Scholar]
- de Lamirande, E. , & Gagnon, C. (1992). Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. Journal of Andrology, 13(5), 368–378. [PubMed] [Google Scholar]
- Doumouchtsis, K. K. , Doumouchtsis, S. K. , Doumouchtsis, E. K. , & Perrea, D. N. (2009). The effect of lead intoxication on endocrine functions. Journal of Endocrinological Investigation, 32, 175–183. 10.1007/BF03345710 [DOI] [PubMed] [Google Scholar]
- D'Souza, U. J. (2004). Effect of tamoxifen on spermatogenesis and tubular morphology in rats. Asian Journal of Andrology, 6(3), 223–226. [PubMed] [Google Scholar]
- Elgawish, R. A. R. , & Abdelrazek, H. M. A. (2014). Effects of lead acetate on testicular function and caspase‐3 expression with respect to the protective effect of cinnamon in albino rats. Toxicology Reports, 1, 795–801. 10.1016/j.toxrep.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sherbini, E. , El‐Sayed, G. , El Shotory, R. , Gheith, N. , Abou‐Alsoud, M. , Harakeh, S. M. , & Karrouf, G. I. (2017). Ameliorative effects of L‐carnitine on rats raised on a diet supplemented with lead acetate. Saudi Journal of Biological Sciences, 24, 1410–1417. 10.1016/j.sjbs.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Tohamy, M. M. , & El‐Nattat, W. S. (2010). Effect of antioxidant on lead‐induced oxidative damage and reproductive dysfunction in male rabbits. J Am Sci, 6(11), 613–622. [Google Scholar]
- Faure, C. , Dupont, C. , Sermondade, N. , & Lévy, R. (2011). Antioxydants et infertilité masculine.mt Médecine de la Reproduction. Gynécologie Endocrinologie, 13(4), 275–283. [Google Scholar]
- Gani, M. U. , Siddiqui, M. S. I. , Rashid, M. H. O. , Islam, K. , Moonmoon, S. , Ahmed, S. , & Mostofa, M. (2016). Effect of lead acetate alone and in combination with whole milk (Star ship®) on body growth and liver functions in an experimentally induced lead toxicity in rat. Asian Journal of Medical and Biological Research, 2(2), 183–189. 10.3329/ajmbr.v2i2.29009 [DOI] [Google Scholar]
- Gore, A. C. (2001). Environmental toxicant effects on neuroendocrine function. Endocrine, 14, 235–246. 10.1385/ENDO:14:2:235 [DOI] [PubMed] [Google Scholar]
- Griveau, J. F. , & Le Lannou, D. (1997). Reactive oxygen species and human spermatozoa: Physiology and pathology. International Journal of Andrology, 20, 61–69. 10.1046/j.1365-2605.1997.00044.x [DOI] [PubMed] [Google Scholar]
- Haleagrahara, N. , Jachie, T. , Chakavarthi, S. , & Kulur, A. B. (2011). Protective effect of alpha‐lipoic acid against lead‐acetate induced oxidative stress in the bone marrow of rats. International Journal of Pharmacology, 7(2), 217–227. 10.3923/ijp.2011.217.227 [DOI] [Google Scholar]
- He, Y. , Zou, Q. , Chen, H. , Weng, S. , Luo, T. , & Zeng, X. (2016). Lead inhibits human sperm functions by reducing the levels of intracellular calcium, cAMP, and tyrosine phosphorylation. Tohoku Journal of Experimental Medicine, 238(4), 295–303. 10.1620/tjem.238.295 [DOI] [PubMed] [Google Scholar]
- Jegede, A. I. , Offor, U. , Azu, O. O. , & Akinloye, O. (2015). Red palm oil attenuates lead acetate induced testicular damage in adult male Sprague‐Dawley rats. Evidence‐Based Complementary and Alternative Medicine, 2015, 1–7. 10.1155/2015/130261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi, A. , Safdarian, L. , Alleyassin, A. , Agha‐Hosseini, M. , Hamed, E. A. , Saeidabadi, H. S. , & Pooyan, O. (2004). The effect of L‐carnitine on sperm parameters in patients candidate for intracytoplasmic sperm injection. Iran J Reprod Med, 2(2), 65–69. [Google Scholar]
- Kobayashi, D. , Goto, A. , Maeda, T. , Nezu, J. I. , Tsuji, A. , & Tamai, I. (2005). OCTN2‐mediated transport of carnitine in isolated Sertoli cells. Reproduction, 129, 729–736. 10.1530/rep.1.00507 [DOI] [PubMed] [Google Scholar]
- Koracevic, D. , Koracevic, G. , Djordjevic, V. , Andrejevic, S. , & Cosic, V. (2001). Colorimetric method for determination of total antioxidant capacity. Journal of Clinical Pathology, 54, 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. (2018). Occupational and environmental exposure to lead and reproductive health impairment: An overview. Indian Journal of Occupational and Environmental Medicine, 22(3), 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi, A. , Sgrò, P. , Salacone, P. , Paoli, D. , Gilio, B. , Lombardo, F. , Santulli, M. , Agarwal, A. , & Gandini, L. (2004). A placebo‐controlled double‐blind randomized trial of the use of combined L‐Carnitine and l‐acetyL‐Carnitine treatment in men with asthenozoospermia. Fertility and Sterility, 81, 1578–1584. 10.1016/j.fertnstert.2003.10.034 [DOI] [PubMed] [Google Scholar]
- Li, C. , Zhao, K. , Zhang, H. , Liu, L. , Xiong, F. , Wang, K. , & Chen, B. (2018). Lead exposure reduces sperm quality and DNA integrity in mice. Environmental Toxicology, 33(5), 594–602. 10.1002/tox.22545 [DOI] [PubMed] [Google Scholar]
- Marchlewicz, M. , Wiszniewska, B. , Gonet, B. , Baranowska‐Bosiacka, I. , Safranow, K. , Kolasa, A. , Głąbowski, W. , Kurzawa, R. , Jakubowska, K. , & Rać, M. E. (2007). Increased lipid peroxidation and ascorbic acid utilization in testis and epididymis of rats chronically exposed to lead. BioMetals, 20, 13–19. 10.1007/s10534-006-9009-z [DOI] [PubMed] [Google Scholar]
- Martin, K. K. , Arsene, A. M. , Melaine, M. G. , Ernest, Z. N. , Joseph, D. A. , Mireille, D. , & David, N. J. (2017). Effects of chronic lead exposure on zinc concentration and spermatic parameters in Wistar rats. AMBS, 3(2), 51–58. [Google Scholar]
- Moncada, M. L. , Vicari, E. , Cimino, C. et al (1992). Effect of acetylcarnitine treatment in oligoasthenospermic patients. Acta Europaea Fertilitatis, 23, 221–224. [PubMed] [Google Scholar]
- Naha, N. , Bhar, R. B. , Mukherjee, A. , & Chowdhury, A. R. (2005). Structural alteration of spermatozoa in the persons employed in lead acid battery factory. Indian Journal of Physiology and Pharmacology, 49, 153–162. [PubMed] [Google Scholar]
- Needleman, H. (2004). Lead poisoning. Annual Review of Medicine, 55, 209–222. 10.1146/annurev.med.55.091902.103653 [DOI] [PubMed] [Google Scholar]
- Ng, C. M. , Blackman, M. R. , Wang, C. , & Swerdloff, S. (2004). The role of carnitine in the male reproductive system. Annals of the New York Academy of Sciences, 1033, 177–188. 10.1196/annals.1320.017 [DOI] [PubMed] [Google Scholar]
- Ohkawa, H. , Ohishi, N. , & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95(2), 351–358. 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy, P. J. , Monteiro, A. , Verhoeven, G. , De Gendt, K. , & Abel, M. H. (2010). Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin‐deficient hypogonadal mice lacking androgen receptors. Reproduction, 139(1), 177–184. 10.1530/REP-09-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patri, S. , Sahu, S. , Parida, B. , Baral, B. , Prusty, A. , Samanta, L. , & Jena, S. (2017). Effect of lead acetate on oxidative stress and antioxidant defence system of Bacillus subtilis and plasmid (pBSIISK) isolated from DH5α. Canadian Journal of Biotechnology, 1(Special Issue), 154. 10.24870/cjb.2017-a140 [DOI] [Google Scholar]
- Pinon‐Lataillade, G. , Thoreux‐Manlay, A. , Coffigny, H. , Masse, R. , & Soufir, J. (1995). Reproductive toxicity of chronic lead exposure in male and female mice. Human and Experimental Toxicology, 14(11), 872–878. 10.1177/096032719501401103 [DOI] [PubMed] [Google Scholar]
- Pizent, A. , Tariba, B. , & Živković, T. (2012). Reproductive toxicity of metals in men. Archives of Industrial Hygiene and Toxicology, 63(Suppl 1), 35–46. 10.2478/10004-1254-63-2012-2151 [DOI] [PubMed] [Google Scholar]
- Rahimi, S. , & Shariati, M. (2017). Investigation of the improvement effect of L‐carnitine on cyclophosphamide‐induced resticular damage in adult male rats. Indo American Journal of Pharmaceutical Sciences, 4(08), 2255–2263. 10.5281/zenodo.839841 [DOI] [Google Scholar]
- Raji, Y. , Salman, T. M. , & Akinsomisoye, O. S. (2005). Reproductive functions in male rats treated with methanolic extract of Alstoniabooneistem bark. African J Biomed Res, 8, 105–111. [Google Scholar]
- Rezaei, N. , Mardanshahi, T. , Shafaroudi, M. M. , Abedian, S. , Mohammadi, H. , & Zare, Z. (2018). Effects of L‐carnitine on the follicle‐stimulating hormone, luteinizing hormone, testosterone, and testicular tissue oxidative stress levels in streptozotocin‐induced diabetic rats. Journal of Evidence‐Based Integrative Medicine, 23, 1–10. 10.1177/2515690X18796053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, R. S. , & Abucham, J. (2009). Recovery of persistent hypogonadism by clomiphene in males with prolactinomas under dopamine agonist treatment. European Journal of Endocrinology, 161, 163–169. 10.1530/EJE-09-0084 [DOI] [PubMed] [Google Scholar]
- Saleh, R. A. , & Agarwal, A. (2002). Oxidative stress and male infertility: From research bench to clinical practice. Journal of Andrology, 23, 737–752. [PubMed] [Google Scholar]
- Samarghandian, S. , Borji, A. , Afshari, R. , Delkhosh, M. B. , & Gholami, A. (2013). The effect of lead acetate on oxidative stress and antioxidant status in rat bronchoalveolar lavage fluid and lung tissue. Toxicology Mechanisms and Methods, 23(6), 432–436. 10.3109/15376516.2013.777136 [DOI] [PubMed] [Google Scholar]
- Sharma, S. , & Thakur, A. (2017). Biochemical studies on the mice heart regarding lead acetate induced oxidative stress. International Journal of Pharmaceutical Sciences and Research, 8(3), 1388–1392. [Google Scholar]
- Solarska, K. , Lewińska, A. , Karowicz‐Bilińska, A. , & Bartosz, G. (2010). The antioxidant properties of carnitine in vitro. Cellular & Molecular Biology Letters, 15, 90–97. 10.2478/s11658-009-0036-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimanzadeh, A. , & Saberivand, A. (2013). Effect of curcumin on rat sperm morphology after the freeze‐thawing process. Vet Res Forum, 4(3), 185–189. [PMC free article] [PubMed] [Google Scholar]
- Sudjarwo, S. A. , Sudjarwo, G. W. , & Koerniasari. (2017). Protective effect of curcumin on lead acetate‐induced testicular toxicity in Wistar rats. Research in Pharmaceutical Sciences, 12(5), 381–390. 10.4103/1735-5362.213983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai, P. F. (2015). Antioxidant action of carnitine: Molecular mechanisms and practical applications. ECVE, 2(1), 66–84. [Google Scholar]
- Udefa, A. L. , Amamaa, E. A. , Archibonga, E. A. , Nwangwaa, J. N. , Adamaa, S. , Inyanga, V. U. , Inyakaa, G. U. , Ajua, G. J. , Okpaa, S. , & Inahb, I. O. (2020). Antioxidant, anti‐inflammatory and anti‐apoptotic effects of hydro‐ethanolic extract of Cyperus esculentus L. (tigernut) on lead acetate‐induced testicular dysfunction in Wistar rats. Biomedicine & Pharmacotherapy, 129, 110491. [DOI] [PubMed] [Google Scholar]
- Vaz, F. M. , & Wanders, R. J. A. (2002). Carnitine biosynthesis in mammals. The Biochemical Journal, 361, 417–429. 10.1042/bj3610417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari, E. , & Calogero, A. (2001). Effects of treatment with carnitines in infertile patients with prostate‐vesiculo‐epididymitis. Human Reproduction, 16, 2338–2342. [DOI] [PubMed] [Google Scholar]
- Vigeh, M. , Smith, D. R. , & Hsu, P. (2011). How does lead induce male infertility? Iran J Reprod Med, 9(1), 1–8. [PMC free article] [PubMed] [Google Scholar]
- Wahab, O. A. , Princely, A. C. , Oluwadamilare, A. A. , Oore‐oluwapo, D. O. , Blessing, A. O. , & Alfred, E. F. (2019). Clomiphene citrate ameliorated lead acetate‐induced reproductive toxicity in male Wistar rats. JBRA Assisted Reproduction, 23(4), 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zhu, H. , Yang, Z. , & Liu, Z. (2013). Antioxidative effects of hesperetin against lead acetate‐induced oxidative stress in rats. Indian Journal of Pharmacology, 45(4), 395–398. 10.4103/0253-7613.115015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrobek, A. J. , & Bruce, W. R. (1975). Chemical induction of sperm abnormalities in mice. Proceedings of the National Academy of Sciences, 72, 4425–4429. 10.1073/pnas.72.11.4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, J. , Li, J. , Yu, Y. , Wei, Q. , Deng, W. , & Yu, L. (2010). L‐carnitine attenuates oxidant injury in HK‐2 cells via ROS‐mitochondria pathway. Regulatory Peptides, 161(1–3), 58–66. 10.1016/j.regpep.2009.12.024. Epub 2010 Jan 20 [DOI] [PubMed] [Google Scholar]
- Yu, T. , Li, Z. , Wang, X. , Niu, K. , Xiao, J. , Li, B. et al (2010). Effect of lead exposure on male sexual hormone. Wei Sheng Yan Jiu, Journal of Hygiene Research, 39, 413–415. [PubMed] [Google Scholar]
- Zambrano, S. , Blanca, A. J. , Ruiz‐Armenta, M. V. , Miguel‐Carrasco, J. L. , Arevalo, M. , Mate, A. , & Vazquez, C. M. (2014). L‐Carnitine attenuates the development of kidney fibrosis in hypertensive rats by upregulating PPAR‐γ. American Journal of Hypertension, 27, 460–470. 10.1093/ajh/hpt268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original research articles that support the results of this study are publicly available.


