Abstract
Purpose
Cardiovascular comorbidity anticipates severe progression of COVID-19 and becomes evident by coronary artery calcification (CAC) on low-dose chest computed tomography (LDCT). The purpose of this study was to predict a patient’s obligation of intensive care treatment by evaluating the coronary calcium burden on the initial diagnostic LDCT.
Methods
Eighty-nine consecutive patients with parallel LDCT and positive RT-PCR for SARS-CoV-2 were included from three centers. The primary endpoint was admission to ICU, tracheal intubation, or death in the 22-day follow-up period. CAC burden was represented by the Agatston score. Multivariate logistic regression was modeled for prediction of the primary endpoint by the independent variables “Agatston score > 0”, as well as the CT lung involvement score, patient sex, age, clinical predictors of severe COVID-19 progression (history of hypertension, diabetes, prior cardiovascular event, active smoking, or hyperlipidemia), and laboratory parameters (creatinine, C-reactive protein, leucocyte, as well as thrombocyte counts, relative lymphocyte count, d-dimer, and lactate dehydrogenase levels).
Results
After excluding multicollinearity, “Agatston score >0” was an independent regressor within multivariate analysis for prediction of the primary endpoint (p<0.01). Further independent regressors were creatinine (p = 0.02) and leucocyte count (p = 0.04). The Agatston score was significantly higher for COVID-19 cases which completed the primary endpoint (64.2 [interquartile range 1.7–409.4] vs. 0 [interquartile range 0–0]).
Conclusion
CAC scoring on LDCT might help to predict future obligation of intensive care treatment at the day of patient admission to the hospital.
Introduction
Coronavirus Disease 2019 (COVID-19) most commonly manifests by unspecific and mild symptoms [1]. Severe complications include respiratory failure, sepsis and acute cardiac injury, which regularly imply obligation of intensive care treatment and invasive ventilation [2–5]. Such severe progression of COVID-19 is promoted by preexisting comorbidity and, above all, cardiovascular risk factors (CRF) [2, 3, 5–8]. I.e., the most commonly reported risk factors for severe complications of COVID-19 include arterial hypertension, diabetes and history of a cardiovascular event [8].
One aspect of cardiovascular disease is atherosclerotic transformation of the vessel wall. Vessel calcification, such as coronary artery calcification (CAC), represent the lifetime cumulative burden of all risk factors for atherosclerotic artery disease [9–11]. Generally acknowledged risk factors for development of atherosclerotic calcification include high age, active smoking, diabetes mellitus, dyslipidemia and arterial hypertension [12]. These promoters of atherosclerosis are also known to entail a poor prognosis in COVID-19, as explained above.
Low-dose chest CT (LDCT) is regularly performed in the context of COVID-19 to recognize complications, to confirm the diagnosis of laboratory testing, and for staging of the disease [13]. Aside from evaluation of the lungs, LDCT allows for assessment of the heart and coronary vessels to a limited extent. Calcified atherosclerotic plaques stand out by the high intrinsic contrast to the adjacent soft tissue. The most commonly used and best evaluated grading for clinical quantification of CAC is the Agatston score [10]. The Agatston score respects overall volume and maximum attenuation of calcified atherosclerotic plaques on a CT scan. Several studies demonstrated comparable CAC-scoring results of ECG-gated, dedicated calcium scoring CTs and ungated LDCTs, which implies the feasibility to estimate the Agatston score based on a COVID-19 LDCT [14, 15].
Hence, the hypothesis of this study is that the Agatston score obtained from LDCT can serve as a surrogate for cardiovascular comorbidity to identify patients at risk of severe COVID-19 progression.
Methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was waived due to retrospective study characteristics. The study was approved by the local ethics committee of the corresponding author’s institution (ethics committee of the Medical Faculty of the University Cologne, application number 20–1219). All imaging was performed for clinical indications. No scan was conducted explicitly for the purpose of this study.
Patient enrollment
Study inclusion was based on screening of the picture archiving and communication system (PACS) and electronic medical records at three level-I hospitals (XX, XX and XX). Diagnostic imaging was indicated by clinical considerations. At each institution LDCT was performed for patients with suspected or RT-PCR positive COVID-19 and
moderate to severe clinical features, or
worsening of respiratory status.
Inclusion criteria to our study comprised
LDCT at initial presentation at hospital according to the hereafter specified imaging protocol,
imaging between 10th of March and 30th of June, 2020, as well as imaging between 30th of June and 15th of October, 2020 at center 1,
mouth swab positive for SARS-CoV-2 as indicated by RT-PCR, and
age ≥18 years.
One patient was excluded due to extensive beam hardening artifacts, impeding the identification of CAC. Twelve patients were excluded due to history of coronary artery disease. This resulted in a total of 89 patients (61, 14, and 14 at centers 1, 2, and 3, respectively).
Clinical follow-up, comorbidity, and laboratory tests
A radiologist reviewed the clinical records for the primary endpoint “admission to ICU, tracheal intubation or death” at each site. Follow-up time was 22 days after the initial LDCT, based on reported data [6]. Clinical outcome was dummy encoded for each patient by completion of the primary endpoint events (0/1). Comorbidities were reviewed from the clinical records and dummy encoded (0/1) for each of the five following items: 1. history of arterial hypertension or current anti-hypertensive medication, 2. history of diabetes or current anti-diabetic medication, 3. prior cardiovascular event (myocardial infarction or revascularization procedure, stroke or transient ischemic attack, or diagnosis of peripheral arterial disease), 4. history of hyperlipidemia or lipid-lowering agents in current medication, 5. active smoking habit. Further, commonly reported laboratory values for high-risk COVID-19 were noted from the clinical records (creatinine, C-reactive protein, leucocyte as well as thrombocyte counts, relative lymphocyte count, d-dimer, and lactate dehydrogenase levels) [16–19]. Laboratory tests were performed on the day of the CT scan (+/- 1 day).
LDCT scanning protocol and image reconstruction
All scans were performed on clinically available CT scanners (iCT 256, Philips Healthcare, SOMATOM Definition AS+, Siemens Healthineers, and Revolution ACT, GE Healthcare). Patients were placed in a head-first supine position. No contrast agent was administered.
Scan parameters for the Philips scanner were: collimation 80.0 × 0.6 mm, pitch 0.76, tube voltage 120 kV, mean CTDIvol 2.1 ± 0.6 mGy, mean DLP 87.4 ± 28.3 mGy*cm (Siemens: collimation 38.4 × 0.6 mm, pitch 1.2, tube voltage 120 kV, CTDIvol 6.8 ± 2.6 mGy, mean DLP 232.4 ± 95.0 mGy*cm; GE: collimation 20.0 × 1.3 mm, pitch 1.38, tube voltage 120 kV, CTDIvol 5.0 ± 3.2 mGy, mean DLP 172.4 ± 97.3 mGy*cm).
For the Philips scanner, images were reconstructed in a 512 x 512 matrix containing pixels of 0.68 x 0.68 mm. Slice thickness was 2.0 mm with an overlap of 1.0 mm (Siemens: 512 x 512 pixels of 0.64 x 0.64 mm, slice thickness 1.0 mm, overlap 0 mm; GE: 512 x 512 pixels of 0.70 x 0.70 mm, slice thickness 1.3 mm, overlap 0.6 mm).
Calcium quantification and lung involvement score
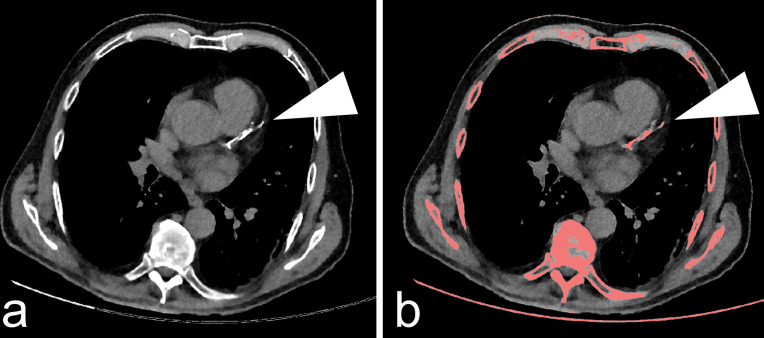
Assessment of the Agatston score was performed using the vendor’s software (IntelliSpace Portal, HeartBeat-CS, Philips Healthcare). The software highlighted all regions with a minimum volume of 0.5 mm3 and attenuation above 130 HU with a colored overlay (Fig 1). A blinded observer manually identified highlighted lesions with a mouse click. CAC clearly associated with aortic valve calcifications were excluded from the measurements. The software automatically performed three-dimensional volumetry of all connected voxels and calculated the Agatston score without specific user interaction.
Fig 1. Quantification of coronary artery calcification.
Exemplary axial computed tomography slice of a patient with evidence of coronary artery calcification (a). Connected voxels with an attenuation above 130 HU and minimum volume of 0.5 mm3 were highlighted by the vendor’s software (b). Coronary artery plaques were manually selected, excluding aortic valve calcifications, e.g., calcification of the Ramus interventricularis anterior (white arrowheads). The Agatston score was noted for each patient.
Lung involvement was assessed semi-quantitatively for each patient, using the COVID-19 chest CT score established by Francone et al. [20]. Based on the extent of lobar involvement in COVID-19 pneumonia, each lobe is addressed by a particular score from 0–5 (0: 0%; 1: < 5%; 2: 5–25%; 3: 26–50%; 4: 51–75%; 5: > 75%). The total score is obtained by the sum of lobar scores (global lung involvement score with range 0–25).
Statistics and data analysis
Data analysis was carried out in R language for statistical computing (R Foundation, Vienna, Austria, version 4.0.0). Wilcoxon test was performed for comparison of ordinal data and non-parametric data, chi-squared test for nominal data, and student’s t-test for parametric data. Shapiro-Wilk’s test was performed to test data for normality, using the R library dplyr [21]. Multivariate regression was implemented by the glm() function in “binomial” mode. As a measure for the accuracy of fit of the logistic regression, pseudo-R2 was calculated by the Nagelkerke method (> 0.2 acceptable, > 0.4 good, > 0.5 very good) [22]. The variance inflation factor (VIF) was calculated for each independent variable using the cars package for R to preclude multicollinearity (< 2.5 no significant multicollinearity) [23, 24]. Statistical power was calculated post-hoc using G*Power [25]. Intra- and inter-observer variability were reported on a randomly selected subset of 18% of our data by the intraclass correlation coefficient (ICC), using the R library psych [26, 27].
All mentioned values are stated as average ± standard deviation or median [first quartile, third quartile]. A p-value ≤0.05 was considered to indicate statistical significance.
Results
Eighty-nine patients were evaluated (49 male, mean age 58.1 ± 15.9 years). The primary endpoint was observed in 36 patients (40.4%), seven patients deceased during the follow-up period (mortality rate 7.9%). All deceased patients died during hospitalization (three patients deceased in the ICU, four in an isolation unit). The most commonly reported cause of death was severe respiratory distress (five patients), followed by acute decompensated heart failure (two patients). Patient details are summarized in Table 1.
Table 1. Patient details.
| no primary endpoint in 22 days follow-up (n = 53) | ICU, tracheal intubation or death (n = 36) | Data available | |
|---|---|---|---|
| Most severe clinical outcome in 22 days follow-up | No hospitalization (47.2%), hospitalization (52.8%) | Admission to ICU (36.2%), tracheal intubation (44.4%), death (19.4%) | 100.0% |
| Agatston score*** | 0 [Q1 = 0, Q3 = 0] | 64.2 [Q1 = 1.7, Q3 = 409.4] | 100.0% |
| CT lung involvement score* | 10.6 ± 5.1 | 12.8 ± 5.0 | 100.0% |
| Patient age*** | 52.0 ± 14.2 | 67.2 ± 14.0 | 100.0% |
| Patient sex | 52.8% male | 58.3% male | 100.0% |
| Arterial hypertension | 45.3% | 61.1% | 100.0% |
| Diabetes* | 7.5% | 30.1% | 100.0% |
| Prior cardiovasc. event | 11.3% | 19.4% | 100.0% |
| Hyperlipidemia | 22.6% | 27.8% | 100.0% |
| Active smoker | 9.4% | 22.2% | 100.0% |
| Creatinine** (norm: 0.5–1.1 mg/dl) | 0.9 [Q1 = 0.7, Q3 = 1.1] | 1.1 [Q1 = 0.8, Q3 = 1.7] | 97.8% |
| C-reactive protein* (norm: <5.0 mg/l) | 32.9 [Q1 = 9.0, Q3 = 71.8] | 54.2 [Q1 = 25.9, Q3 = 97.5] | 97.8% |
| Leucocyte count** (norm 4.4–11.3 x1E9/l) | 6.6 [Q1 = 4.6, Q3 = 9.9] | 9.6 [Q1 = 5.9. Q3 = 13.0] | 97.8% |
| Thrombocyte count (norm: 150–400 x1E9/l) | 186 [Q1 = 147, Q3 = 250] | 201 [Q1 = 127, Q3 = 268] | 97.8% |
| Rel. lymphocyte count* (norm: 19.1–47.9%) | 23.5 [Q1 = 15.0, Q3 = 38.4] | 14.5 [Q1 = 8.8, Q3 = 32.8] | 94.4% |
| D-dimer** (norm: <0.5 μg/l) | 0.7 [Q1 = 0.4, Q3 = 1.2] | 1.7 [Q1 = 0.9, Q3 = 2.8] | 73.0% |
| Lactate dehydrogenase (norm: < 245 U/l) | 276.0 [Q1 = 223.0, Q3 = 373.5] | 350.5 [Q1 = 249.5, Q3 = 478.3] | 97.8% |
*/**/*** differ significantly for COVID-19 cases with observed primary endpoint vs. no primary endpoint (Wilcoxon test, two-sided T-test, chi-squared test
*p<0.05
**p<0.01
***p<0.001)
A multivariate logistic regression model was fit to predict the primary endpoint by the main independent variable “Agatston score >0”, as well as the control variables patient age, sex, CT lung involvement score, and the five considered clinical anamnestic risk factors. To identify the most relevant laboratory parameters from the abundancy of available tests, only those parameters were respected in the regression model, which differed significantly between the two study groups. To acknowledge possible multicollinearity issues, the VIFs were calculated for each independent variable of our preliminary model. Independent variables with VIF >2.5 were dismissed from the model from top to bottom, ultimately removing the two clinical parameters hyperlipidemia (VIF = 6.6) and arterial hypertension (VIF = 2.8). The final regression model is summarized in Table 2.
Table 2. Multivariate logistic regression model for completion of the primary endpoint “admission to ICU, tracheal intubation or death” in COVID-19 during follow-up of 22 days.
| Independent variable | p-value | Variance inflation factor | Odds ratio [95% confidence interval] |
|---|---|---|---|
| Agatston score >0 | <0.01 | 2.4 | 33.6 [4.2–456.8] |
| CT lung involvement score | 0.72 | 1.5 | 1.0 [0.8–1.3] |
| Patient age | 0.56 | 2.2 | 1.0 [0.9–1.1] |
| Patient sex | 0.18 | 1.4 | 0.3 [0.04–1.6] |
| Diabetes | 0.52 | 1.6 | 0.4 [0.03–4.9] |
| Prior cardiovasc. event | 0.06 | 1.9 | 0.1 [0.06–0.9] |
| Active smoker | 0.06 | 2.2 | 19.7 [1.1–826.9] |
| Creatinine | 0.02 | 1.8 | 10.2 [1.7–95.6] |
| C-reactive protein | 0.56 | 1.3 | 1.0 [1.0–1.0] |
| Leucocyte count | 0.04 | 1.9 | 1.3 [1.0–1.8] |
| Rel. lymphocyte count | 0.27 | 1.8 | 1.0 [0.9–1.0] |
| D-dimer | 0.83 | 1.4 | 1.0 [0.8–1.4] |
After adjusting for the included control variables, the main independent variable “Agatston score >0” was significantly associated with the primary outcome within the multivariate logistic regression (p<0.01, odds ratio (OR) 33.6 [4.2–456.8]). Further, the creatinine level (p = 0.02, OR 10.2 [1.7–95.6]) and leucocyte count (p = 0.04, OR 1.3 [1.0–1.8]) were significant regressors of the primary outcome. Assuming average values for the control variables, patients with Agatston score 0 had a 0.16 risk (standard error 0.09) to complete the primary endpoint within 22 days. An Agatston score of >0 imposed to a risk of 0.86 (standard error 0.10) to complete the primary endpoint. Pseudo-R2 by Nagelkerke as an indicator for the accuracy of fit of the regression model was 0.64. That is, 64% of the variance of the dependent variable “primary endpoint observed” is accounted for by our regression, which is rated a “very good” model. As a comparison, a multivariate logistic regression model comprising only patient age, sex, the three considered clinical risk factors, and laboratory parameters achieved a pseudo-R2 of 0.50. Sensitivity and specificity to recognize completion of the primary endpoint by the dichotomous variable “Agatston score >0” within our study population was 0.75 (27/36) and 0.83 (44/53), respectively. The positive predictive value was 0.75 (27/36).
Statistical power for regression of the primary endpoint by the main independent variable in our multivariate model was >0.99, which exceeds the typically desired power level of 0.80 (S1 File). Intra- and inter-observer reliability for evaluation of Agatston scores and lung involvement were excellent (S2 File).
Discussion
From the beginning of the COVID-19 pandemic Chinese physicians observed a coincidence of cardiovascular comorbidity and severe progression of the disease, which was consistently confirmed by international authors [2–6, 8]. However, a comprehensive clinical assessment of cardiovascular comorbidity might not be achievable for every patient during the pandemic, since it consumes time and medical resources. Instead, we suggest assessment of CAC on LDCT as a surrogate of individual cardiovascular comorbidity to anticipate severe progression of COVID-19. Coronary calcium scoring on LDCT has been proven viable in several studies [14, 15]. An experienced radiologist can obtain the Agatston score from a COVID-19 LDCT in a few seconds and automated software using deep learning is readily available [28]. In our study, the detection of CAC resulted in an increased risk to require intensive care treatment from 0.16 (standard error 0.09) to 0.86 (standard error 0.10). Our data suggest that the detection of CAC is an independent risk factor for severe progression of COVID-19, which remained significant after inclusion of demographic and clinical risk factors to the regression model (p<0.01, VIF = 2.4). The most accurate prediction of the primary endpoint was achieved by a model combining CAC, demographic and clinical risk factors (pseudo-R2 by Nagelkerke 0.64, “very good” model). This implies that a combination of calcium scoring on LDCT, basic laboratory tests, assessment of CT lung involvement, and a quick anamnesis for comorbidities explain 64% of the variance of the risk of ICU admission in the context of COVID-19.
Several recent studies explored the influence of CAC on the prognosis of COVID-19. An early single-center study from Italy identified a cutoff at Agatston score >400 as a risk factor for admission to an ICU or patient death on a population of 53 patients with subclinical coronary artery disease [29]. In a further single-center study by Dillinger et al., detection of CAC was independently associated with the primary outcome (mechanical noninvasive or invasive ventilation, extracorporeal membrane oxygenation, patient death) after inclusion of demographic and clinical risk factors (age, sex, hypertension, smoking, diabetes) [30]. The largest study up-to-date reports >1000 patients from Italy and approved CAC as an independent risk factor for in-hospital mortality [31]. However, to the best of our knowledge, this work is the first research to confirm CAC as an independent biomarker for severe COVID-19 after adjusting for demographic, anamnestic, and laboratory factors, as well as lung involvement in LDCT.
Limitations of our study include a small patient population, which leads to relatively wide 95% confidence intervals for our independent variables. Even though the association of our main independent variable to completion of the primary endpoint is highly significant (p<0.01), the wide OR confidence interval precludes accurate specification of the strength of the relationship. Further, endpoints of our statistical analysis were simplified to predict the clinical outcome that we recognized as most essential, considering the major issue of scarce intensive care capacities in the ongoing pandemic [32]. The reconstructed slice thickness of the CT scans in our study varied between 1 mm and 2 mm for the three centers, which should not affect measurements of CAC on non ECG-gated CT [33]. Similarly, modification of the tube current and consequently the radiation dose of a CT scan affects the standard deviation of measurements (noise), but not the average CT density [34]. An inter-vendor analysis to exclude a further scanner-specific bias was precluded by our sample size. Further, our work investigates only those individuals with COVID-19, who qualified for medical imaging due to clinical considerations (worsening of respiratory status, moderate to severe clinical features); any clinical application based on this study should be limited to such patient population. Future studies should be realized in a prospective setting and include a larger patient population to elaborate on the predictive benefit of CAC. A uniform study population concerning the days after symptom onset might help to investigate the predictive power of lung involvement, compared to the more stable anamnestic risk factors and the CAC burden.
During the ongoing COVID-19 pandemic, opportunistic quantification of CAC might allow for early recognition of patients at risk to require intensive care treatment. Our data indicate that evaluation of the CAC burden on LDCT can help to delimitate the number of required intensive care beds on the day of patient admission to the hospital. Since ICU capacity is a major bottleneck in the ongoing pandemic, this might facilitate resource management and collaboration on a national and international scale, ultimately promoting containment of COVID-19.
Supporting information
(CSV)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files (S1 Data).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2020;14: 247–250. doi: 10.1016/j.dsx.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driggin E, Madhavan M V., Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. Journal of the American College of Cardiology. Elsevier USA; 2020. pp. 2352–2371. doi: 10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020. doi: 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan: A Retrospective Observational Study. SSRN Electron J. 2020. doi: 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368: m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clinical Research in Cardiology. Springer; 2020. pp. 531–538. doi: 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nature Reviews Cardiology. 2020. doi: 10.1038/s41569-020-0413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman DS, Arnson Y, Rozanski A. Coronary Artery Calcium Scanning: The Agatston Score and Beyond. JACC: Cardiovascular Imaging. Elsevier Inc.; 2016. pp. 1417–1419. doi: 10.1016/j.jcmg.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 10.Rusnak J, Behnes M, Henzler T, Reckord N, Vogler N, Meyer M, et al. Comparative analysis of high-sensitivity cardiac troponin i and T for their association with coronary computed tomography-assessed calcium scoring represented by the Agatston score. Eur J Med Res. 2017;22. doi: 10.1186/s40001-017-0263-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: A report from the Committee on Vascular Lesions of the council on arteriosclerosis, American heart association. Circulation. Lippincott Williams and Wilkins; 1995. pp. 1355–1374. doi: 10.1161/01.cir.92.5.1355 [DOI] [PubMed] [Google Scholar]
- 12.Djekic D, Angerås O, Lappas G, Fagman E, Fagerberg B, Bergström G, et al. Impact of socioeconomic status on coronary artery calcification. Eur J Prev Cardiol. 2018;25: 1756–1764. doi: 10.1177/2047487318792103 [DOI] [PubMed] [Google Scholar]
- 13.Kwee TC, Kwee RM. Chest ct in covid-19: What the radiologist needs to know. Radiographics. 2020;40: 1848–1865. doi: 10.1148/rg.2020200159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: Comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5: 113–118. doi: 10.1016/j.jcct.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe B-K. Coronary Calcium Screening Using Low-Dose Lung Cancer Screening: Effectiveness of MDCT with Retrospective Reconstruction C a r d i a c I m ag i ng • O r ig i n a l R e s e a rc h. 2008. [cited 19 Apr 2020]. doi: 10.2214/AJR.07.2979 [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Cheng X, Dong Q, Zhou C, Wang Y, Song B, et al. The characteristics of laboratory tests at admission and the risk factors for adverse clinical outcomes of severe and critical COVID-19 patients. BMC Infect Dis. 2021;21: 1–8. doi: 10.1186/s12879-020-05706-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leulseged TW, Hassen IS, Ayele BT, Tsegay YG, Abebe DS, Edo MG, et al. Laboratory biomarkers of covid-19 disease severity and outcome: Findings from a developing country. PLoS One. 2021;16: e0246087. doi: 10.1371/journal.pone.0246087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keddie S, Ziff O, Chou MKL, Taylor RL, Heslegrave A, Garr E, et al. Laboratory biomarkers associated with COVID-19 severity and management. Clin Immunol. 2020;221. doi: 10.1016/j.clim.2020.108614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jose Antunez Muiños P, López Otero D, Amat-Santos IJ, López País J, Aparisi A, Cacho Antonio CE, et al. The COVID-19 lab score: an accurate dynamic tool to predict in-hospital outcomes in COVID-19 patients. Sci Reports |. 123AD;11: 9361. doi: 10.1038/s41598-021-88679-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30: 6808–6817. doi: 10.1007/s00330-020-07033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A Grammar of Data Manipulation [R package dplyr version 1.0.6]. 2021 [cited 16 Jun 2021]. Available: https://cran.r-project.org/package=dplyr
- 22.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991. doi: 10.1093/biomet/78.3.691 [DOI] [Google Scholar]
- 23.John F, Sanford W. An R Companion to Applied Regression Third Edition. SAGE Publications, Inc. 2019. [Google Scholar]
- 24.Midi H, Sarkar SK, Rana S. Collinearity diagnostics of binary logistic regression model. J Interdiscip Math. 2010;13: 253–267. doi: 10.1080/09720502.2010.10700699 [DOI] [Google Scholar]
- 25.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. Psychonomic Society Inc.; 2007. pp. 175–191. doi: 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- 26.Revelle W. psych: Procedures for Personality and Psychological Research. In: https://CRAN.R-project.org/package=psych. 2020.
- 27.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15: 155–163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lessmann N, Van Ginneken B, Zreik M, De Jong PA, De Vos BD, Viergever MA, et al. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks with Dilated Convolutions. IEEE Trans Med Imaging. 2018;37: 615–625. doi: 10.1109/TMI.2017.2769839 [DOI] [PubMed] [Google Scholar]
- 29.Nai Fovino L, Cademartiri F, Tarantini G. Subclinical coronary artery disease in COVID-19 patients. Eur Hear J—Cardiovasc Imaging. 2020. [cited 10 Aug 2020]. doi: 10.1093/ehjci/jeaa202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dillinger JG, Benmessaoud FA, Pezel T, Voicu S, Sideris G, Chergui N, et al. Coronary Artery Calcification and Complications in Patients With COVID-19. JACC Cardiovasc Imaging. 2020;13: 2468–2470. doi: 10.1016/j.jcmg.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannini F, Toselli M, Palmisano A, Cereda A, Vignale D, Leone R, et al. Coronary and total thoracic calcium scores predict mortality and provides pathophysiologic insights in COVID-19 patients. J Cardiovasc Comput Tomogr. 2021. [cited 17 Jun 2021]. doi: 10.1016/j.jcct.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritter M, Ott DVM, Paul F, Haynes JD, Ritter K. COVID-19: a simple statistical model for predicting intensive care unit load in exponential phases of the disease. Sci Rep. 2021;11: 5018. doi: 10.1038/s41598-021-83853-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen JL, Sharma E, Gorvitovskaia AY, Watts JP, Assali M, Neverson J, et al. Impact of slice thickness on the predictive value of lung cancer screening computed tomography in the evaluation of coronary artery calcification. J Am Heart Assoc. 2019;8. doi: 10.1161/JAHA.118.010110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deprez FC, Vlassenbroek A, Ghaye B, Raaijmakers R, Coche E. Controversies about effects of low-kilovoltage MDCT acquisition on Agatston calcium scoring. J Cardiovasc Comput Tomogr. 2013;7: 58–61. doi: 10.1016/j.jcct.2012.11.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files (S1 Data).