Abstract
Background
Tuberculosis (TB) incidence in Los Angeles County, California, USA (5.7 per 100,000) is significantly higher than the U.S. national average (2.9 per 100,000). Directly observed therapy (DOT) is the preferred strategy for active TB treatment but requires substantial resources. We partnered with the Los Angeles County Department of Public Health (LACDPH) to evaluate the cost-effectiveness of AiCure, an artificial intelligence (AI) platform that allows for automated treatment monitoring.
Methods
We used a Markov model to compare DOT versus AiCure for active TB treatment in LA County. Each cohort transitioned between health states at rates estimated using data from a pilot study for AiCure (N = 43) and comparable historical controls for DOT (N = 71). We estimated total costs (2017, USD) and quality-adjusted life years (QALYs) over a 16-month horizon to calculate the incremental cost-effectiveness ratio (ICER) and net monetary benefits (NMB) of AiCure. To assess robustness, we conducted deterministic (DSA) and probabilistic sensitivity analyses (PSA).
Results
For the average patient, AiCure was dominant over DOT. DOT treatment cost $4,894 and generated 1.03 QALYs over 16-months. AiCure treatment cost $2,668 for 1.05 QALYs. At willingness-to-pay threshold of $150K/QALY, incremental NMB per-patient under AiCure was $4,973. In univariate DSA, NMB were most sensitive to monthly doses and vocational nurse wage; however, AiCure remained dominant. In PSA, AiCure was dominant in 93.5% of 10,000 simulations (cost-effective in 96.4%).
Conclusions
AiCure for treatment of active TB is cost-effective for patients in LA County, California. Increased use of AI platforms in other jurisdictions could facilitate the CDC’s vision of TB elimination.
Background
The United States has one of the lowest tuberculosis (TB) case rates in the world, but there is still considerable progress to be made before TB can be eliminated from the US, with over 9,000 cases reported in 2017 [1]. One of the challenges continues to be considerable geographical heterogeneity; for instance, the TB case rate in Los Angeles County (LAC) (5.7 cases per 100,000 population) is significantly higher than the U.S. national average (2.9 cases per 100,000 population). In 2016, there were 550 confirmed TB cases in LAC [2]. LAC had the highest number of TB cases and the 10th highest TB incidence rate among California’s 61 health jurisdictions. We therefore focus on LAC in the present study on technology to improve TB treatment.
Directly observed therapy (DOT) is the preferred strategy for active tuberculosis treatment. DOT is defined as an in person, direct observation by a health care worker of the patient ingesting each dose of medication. DOT directs partial responsibility of treatment to the provider and helps ensure that patients complete an adequate course of TB treatment [3]. While this is an effective method for ensuring TB treatment adherence– 92.6% of TB patients in LAC completed treatment within a year in 2013 –DOT is resource-intensive and requires a high number of nurse-hours per TB patient [4]. Although some DOT sessions are conducted during patients’ clinic visits, in LAC, DOT is mostly carried out in the field, whereby nurses visit TB patients at home to directly observe ingestion of TB drugs which in turn incurs travel expenses and additional time to coordinate home visits [5].
A common alternative to DOT is video directly observed therapy (VDOT), where healthcare professionals observe patients take their medications through a video conferencing software. Many recent studies find success and/or adherence rates with VDOT to be comparable to in-person DOT [6–14]. Some studies have also investigated VDOT costs to the healthcare system in addition to adherence outcomes. Buchman and Cabello (2017) studied traditional DOT vs Skype observed therapy (SOT) in 24 patients from Nassau County, New York [15]. They found cost savings under SOT of $1,008 per-month and $42 per-case per-month. Holzman, Zenilman, and Shah (2018) conducted a prospective pilot study of a VDOT intervention called miDOT (emocha Mobile Health Inc.) in three tuberculosis clinics in Maryland [7]. They followed 28 adult patients receiving active or latent TB treatment. For a standard 6-month treatment, VDOT saved $1,391 per-patient compared to DOT and adherence rates of 98% and 94% for DOT and VDOT, respectively (P = 0.17). Garfein et al. (2018) studied asynchronous VDOT—videos of medication ingestion are recorded by the patient and watched by a health care worker at a later time—versus traditional DOT in five health districts in California (San Diego, San Francisco, Santa Clara, San Joaquin, and Imperial) [8]. They enrolled a total of 174 adults to VDOT and compared outcomes to 159 historical controls treated with DOT. The 6-month treatment cost with VDOT (range $3,031-$3,911) was 32% (range 6%-46%) less than DOT ($3,212-$5,788) across districts.
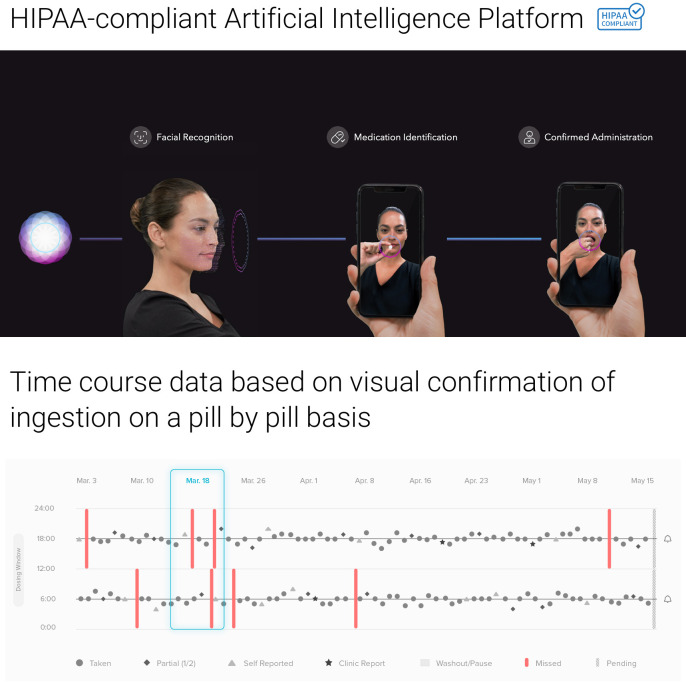
Recent technological advances have introduced further innovations in this area–artificial intelligence (AI)-based software that uses computer vision and machine learning to confirm the patient, the drugs, and ingestion in real time, further reducing health worker burden. However, there is still limited data on the efficacy of AI-based monitoring methods for TB treatment adherence, and no prior studies to our knowledge have evaluated its cost-effectiveness. In this study, we examine both for a cohort of pulmonary TB patients tracked by the Los Angeles County Department of Public Health (LACDPH) using AiCure (New York, NY), an AI platform that uses computer vision and machine learning to automatically confirm the patient, the drugs, and ingestion in real time. Under AiCure, failure in any one of these criteria leads to an email or SMS text message alert sent to a nurse (see Fig 1 for illustration) who then follows up with the patient in real time. Patients can access AiCure using a smart phone application which uses the device camera to record medication consumption; the platform includes the option to have the video sent to nurses to review later. This platform is currently used in drug development trials and population health settings, across multiple therapeutic areas including neuroscience, cardiovascular, and infectious disease [16–18]. Additional details on AiCure are provided in the online S1 File. By focusing on LAC, a large metropolitan area with high TB morbidity and substantial patient heterogeneity, we hope to provide insight into the cost-effectiveness of AI-based treatment monitoring platforms for a critical TB population in the U.S.
Fig 1. AiCure artificial intelligence platform.
Software automatically observes and confirms patient, medication, and ingestion. HIPAA Health Insurance Portability and Accountability Act. Source: AiCure, LLC (New York, NY) with written permission to use and adapt.
Methods
Model overview
We developed a cohort Markov transition state model in Microsoft Excel (Microsoft Corporation, Redmond, WA) to compare cost and treatment completion outcomes of traditional in-person DOT versus AiCure for active TB patients undergoing treatment in LAC. The Markov model tracked monthly TB outcomes for patients over 16 total months, the longest duration on treatment we observed in our data. At each month, patients either continued treatment, defaulted, or successfully completed their treatment; these probabilities were calculated from deidentified LACDPH surveillance data for active TB patients in one of the LACDPH clinics at Pacoima, Los Angeles, CA. Both DOT and AiCure patients were assumed to undergo a standard treatment regimen with an average of 11 observed doses per month. This average reflected the local practice of using both a daily TB treatment regimen as well as a twice a week treatment regimen, according to patient and provider preference. We assumed patients initially unsuccessful on AiCure would be moved to DOT, as is common in clinical practice. We considered patients unsuccessful on DOT as lost to follow-up. Patients must have had a confirmed diagnosis, been without multidrug-resistance (MDR), and been HIV negative to be included in the study population. Our cost-effectiveness study was ruled exempt from Institutional Review Board (IRB) review by the University of Southern California IRB. The pilot research study using AiCure for patients with TB was reviewed and approved by the LACDPH IRB. All experiments were performed in accordance with relevant guidelines and regulations. Informed written consent was obtained from all subjects in the pilot study or, if subjects were under 18, from a parent and/or legal guardian.
Costs by treatment arm, including personnel, technology, and licensing fees, were provided by the LACDPH. We obtained drug costs from the Veterans Affairs Federal Supply Schedule [19]. We estimated total costs on treatment (2017, USD) and total quality-adjusted life years (QALYs) by treatment arm. Our outcomes of interest included incremental cost-effectiveness ratios (ICER) and net monetary benefits (NMB) at various willingness to pay (WTP) values to compare AiCure to DOT. Outcomes were evaluated from the United States societal perspective. To address uncertainty and assess robustness of base results, all parameters were fit to probability distributions and varied in deterministic (DSA) and probabilistic sensitivity analysis (PSA).
Data
Patients and nurses were trained to use the AiCure software in a pilot study of 43 active TB patients in Pacoima, Los Angeles, CA. In this pilot study, eligible patients were provided with an internet and camera-enabled smart phone and trained to record their doses using the software. This data has not been published elsewhere, and we present it here for the first time. Our final analysis sample for the AiCure arm of our model included 43 patients confirmed during 2015 to 2017 who either began and completed or began and moved/defaulted from active TB treatment. Mean age at diagnosis in this sample was 48.4 years, with 21% of patients having multiple comorbidities, 21% with diabetes, and 76% with pulmonary TB disease (Table 1).
Table 1. Summary statistics by treatment arm.
| DOT | AiCure | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-value | |
| Demographics | |||||
| Age (years) | 50.9 | (17.07) | 48.4 | (19.84) | 0.530 |
| Year confirmed | 2013.5 | (0.50) | 2016.7 | (0.47) | 0.001 |
| Demographics, cont. | No. | Proportion | No. | Proportion | P-value |
| Male | 45 | (0.63) | 22 | (0.65) | 0.895 |
| Previous treatment for TB | 0 | (0.00) | 2 | (0.06) | 0.039 |
| PZA drug resistance | 0 | (0.00) | 1 | (0.03) | 0.147 |
| Race/Ethnicity | |||||
| White | 36 | (0.51) | 19 | (0.56) | 0.619 |
| Asian | 33 | (0.46) | 14 | (0.41) | 0.609 |
| Black | 1 | (0.01) | 0 | (0.00) | 0.487 |
| Other | 1 | (0.01) | 1 | (0.03) | 0.591 |
| Hispanic | 32 | (0.45) | 16 | (0.47) | 0.848 |
| Country of birth | |||||
| United States | 2 | (0.03) | 3 | (0.09) | 0.176 |
| Mexico | 23 | (0.32) | 7 | (0.21) | 0.210 |
| Philippines | 25 | (0.35) | 7 | (0.21) | 0.128 |
| Other | 21 | (0.30) | 17 | (0.50) | 0.042 |
| Years since arrival in USA | 15.0 | (11.82) | 20.2 | (13.78) | 0.073 |
| Comorbidities | |||||
| Pulmonary complications | 56 | (0.79) | 26 | (0.76) | 0.781 |
| HIV positive | 0 | (0.00) | 0 | (0.00) | . |
| Diabetic | 20 | (0.28) | 7 | (0.21) | 0.406 |
| Renal impairment | 2 | (0.03) | 0 | (0.00) | 0.323 |
| Immunocompromised | 4 | (0.06) | 0 | (0.00) | 0.158 |
| Pleural complications | 3 | (0.04) | 3 | (0.09) | 0.342 |
| Bone joint complications | 1 | (0.01) | 1 | (0.03) | 0.591 |
| Meningitis | 6 | (0.08) | 1 | (0.03) | 0.290 |
| Comorbid | 25 | (0.35) | 7 | (0.21) | 0.128 |
| Risk factors | |||||
| Homeless | 0 | (0.00) | 0 | (0.00) | . |
| Injecting drug user | 0 | (0.00) | 0 | (0.00) | . |
| Non-injecting drug user | 0 | (0.00) | 1 | (0.03) | 0.149 |
| Risk for alcohol abuse | 0 | (0.00) | 5 | (0.15) | 0.001 |
| Smoker | 10 | (0.14) | 0 | (0.00) | 0.021 |
| No risk factors | 38 | (0.54) | 25 | (0.74) | 0.050 |
| Observations | 71 | 43 | 114 | ||
Category totals may not sum to N due to missing values. P-values are calculated using two-sided, two-sample t-tests for proportions or means with unequal variances.
We compared these patients with a cohort of patients with active TB treated with DOT in the same clinic, confirmed between 2013 and 2014 (n = 172), the years immediately preceding the AiCure pilot. To best approximate the population that would have been eligible for AiCure, we included only patients who clinicians determined to be eligible for AiCure treatment. Our control final sample included 71 patients previously treated for active TB under DOT. Average age at diagnosis in this sample was 50.9, with 35% having multiple comorbidities, including 28% diabetic and 79% with pulmonary disease (Table 1). We calculated P-values using two-sided, two-sample t-tests for proportions or means with unequal variances. No demographic factors including age, gender, or race were statistically significantly different across treatment arms. Although not selected for, the demographic characteristics and proportion of patients with comorbidities in each treatment arm were similar. In both arms most patients had no behavioral risk factors and no major morbidities other than pulmonary complications.
Costs
For both treatment arms, we calculated drug acquisition costs to be $174.16 per-patient per-month, assuming treatment with the standard regimen of isoniazid (INH), rifampin (RIF), pyrazinamide (PZF), and ethambutol (EMB) [3, 19]. AiCure-specific fees include licensing for $750 per clinic per month up to 50 patients, $1,500 per clinic per month for technical support, a fixed cost of $52 per patient for a phone, and $47.50 per patient per month for phone service. We estimated the remaining costs in the model using Licensed Vocational Nurse (LVN) or Registered Nurse (RN) hourly compensation rates provided by LACDPH (Table 2). Additional details on costs are available in the online S1 File.
Table 2. Baseline model parameters.
| Description | DOT | AiCure | Source |
|---|---|---|---|
| General Parameters | |||
| Patients | 100 | 100 | Assumed |
| Clinic capacity | 100 | 100 | Assumed |
| Doses, monthly | 11 | 11 | LACDPH |
| FDOT proportion | 0.69 | - | LACDPH |
| FDOT appointment time, hours | 0.83 | - | LACDPH |
| FDOT miles, per appointment | 7.60 | - | LACDPH |
| FDOT missed appointment time, hours | 0.42 | - | LACDPH |
| FDOT missed appointment frequency, PMPM | 2.50 | - | LACDPH |
| CDOT appointment time | 0.25 | - | LACDPH |
| CDOT missed appointment time | 0.17 | - | LACDPH |
| CDOT missed appointment frequency, monthly | 2.60 | - | LACDPH |
| AI dose time | - | 0 | LACDPH |
| AI missed dose time | - | 0.208 | LACDPH |
| AI missed dose frequency, monthly | - | 1.3 | LACDPH |
| AI appointment frequency, monthly | - | 1.5 | LACDPH |
| Baseline Completion Probabilitiesa | |||
| P(Complete | t ≤ 120 days) | 0.000 | 0.000 | LACDPH |
| P(Complete | 120 < t ≤ 150 days) | 0.000 | 0.000 | LACDPH |
| P(Complete | 150 < t ≤ 180 days) | 0.000 | 0.051 | LACDPH |
| P(Complete | 180 < t ≤ 210 days) | 0.217 | 0.306 | LACDPH |
| P(Complete | 210 < t ≤ 240 days) | 0.149 | 0.120 | LACDPH |
| P(Complete | 240 < t ≤ 270 days) | 0.050 | 0.045 | LACDPH |
| P(Complete | 270 < t ≤ 300 days) | 0.684 | 0.524 | LACDPH |
| P(Complete | 300 < t ≤ 330 days) | 0.083 | 0.600 | LACDPH |
| P(Complete | 330 < t ≤ 360 days) | 0.091 | 0.250 | LACDPH |
| P(Complete | 360 < t ≤ 390 days) | 0.600 | 0.333 | LACDPH |
| P(Complete | 390 < t ≤ 420 days) | 0.500 | 0.500 | LACDPH |
| P(Complete | 420 < t ≤ 450 days) | 0.500 | 1.000 | LACDPH |
| P(Complete | 450 < t ≤ 480 days) | 1.000 | 1.000 | LACDPH |
| Utilities (QALY weights) | |||
| Active tuberculosis | 0.663 | 0.663 | (20, 21) |
| Healthy, post treatment | 0.942 | 0.942 | (20, 21) |
| Costs (2017 USD) | |||
| Licensed vocational nurse, hourly | $35.45 | $35.45 | LACDPH |
| Registered nurse, hourly | $79.26 | $79.26 | LACDPH |
| Mileage, per mile | $0.54 | $0.54 | IRS |
| Licensing per 50 patients, monthly | - | $750.00 | LACDPH |
| Phone purchase | - | $52.00 | LACDPH |
| Phone service, monthly | - | $47.50 | LACDPH |
| Technical support, monthly | - | $1,500.00 | LACDPH |
| Pharmaceutical acquisition, monthly | $174.16 | $174.16 | (19) |
a See S1 Table in S1 File for complete table of continuation, completion, and default probabilities. LACDPH, Los Angeles County Department of Public Health; FDOT, field directly observed therapy; CDOT, clinic directly observed therapy; PMPM, per-member per-month; AI, AiCure; QALY, quality-adjusted life year.
Health states and treatment progression
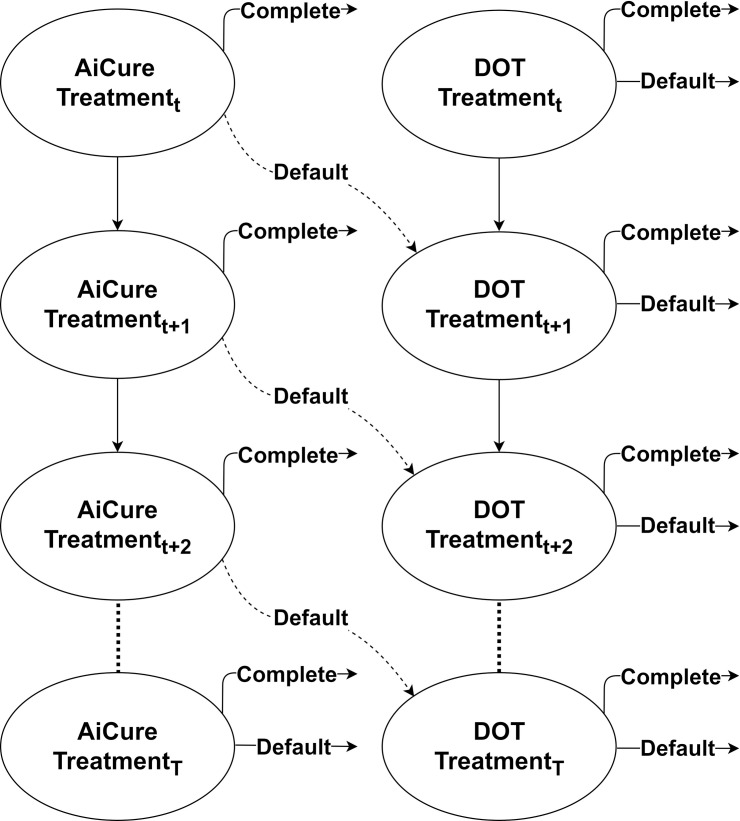
We modeled patient progression from treatment initiation to completion using a Markov model. In the model, individuals were on-treatment, had completed treatment, or had defaulted (Fig 2). Consistent with LACDPH active TB treatment guidelines, we assumed a minimum successful treatment length of five months for all patients [3]. At each 30-day increment from 4–16 months of treatment, patients had some probability of completing or defaulting from treatment on each treatment arm (values found using the LACDPH dataset). Consistent with clinical practice, non-adherent patients on AiCure would be moved to DOT for the remainder of treatment. See S1 Table in S1 File for detailed transition probabilities by arm.
Fig 2. Markov state transition model for AiCure and DOT arms.
AiCure patients (left) could default to DOT treatment (right). DOT directly observed therapy.
Health utilities
We identified health-related quality of life (HR-QoL) utilities for each health state from the medical literature. The utility for living with active drug-susceptible TB and taking TB treatment was 0.663 and healthy with past TB treatment was 0.942 [20, 21]. A summary of utility values and sensitivity analysis ranges are provided in Table 2 and S2 Table in S1 File, respectively. We assumed that the health utility for being on AiCure was the same as for DOT, as the only difference is the method of treatment monitoring. We counted defaulting patients as living with active TB for the remainder of the observation period.
Sensivity analyses
To check for robustness of our results, we conducted deterministic and probabilistic sensitivity analyses. In the scenario in which the base case resulted in a dominant treatment option, the outcome of interest was changed from ICERs to incremental net monetary benefits (INMB) of AiCure with willingness to pay (WTP) of $150,000 per QALY [22]. For one-way deterministic sensitivity analysis, we varied model inputs within their 95% confidence intervals, predicted ranges, or +/- 50% when neither were available. Probabilistic sensitivity analysis involved fitting likely distributions to input parameters and simulating outcomes in 10,000 Monte Carlo repetitions [23–25].
Scenario analyses
As the number of observed patients on AiCure was relatively small, there exists uncertainty around the monthly treatment completion rate. We therefore examined several scenario analyses to identify thresholds for which AiCure would still be cost-effective even if monthly treatment rates are lower. In the first scenario, we examined what would happen if monthly AiCure completion rates are lower by 5 percentage points per month. We repeated this analysis where AiCure completion rates are lower than observed in our data by 10 percentage points and 15 percentage points.
As a secondary scenario analysis, we also examined the “best case” and “worst case” scenario where the non-transition probability parameters from one-way DSA are at their 95% confidence interval bounds to either make AiCure/DOT perform well or poorly. Outcomes of interest in all scenario analyses were the variation in ICER and cost-effectiveness outcomes for AiCure.
We additionally include a third scenario analysis, a comparison of AiCure to VDOT, where we examine the cost-effectiveness outcomes if additional resources were needed in adopting AiCure in a new location. Any jurisdiction seeking to adopt an AI treatment monitoring system may have nurses monitor patient video records (similar to VDOT) to ensure the AI system is operating correctly. We therefore examine the scenario where nurses must review patient video on the AiCure platform, spending 7 minutes per video.
Results
Among AiCure patients, average total days on treatment was 252.5 (SD = 69.4, range 94 to 401), slightly higher but not statistically significantly different from the DOT average of 247.1 (SD = 89.1, range 8 to 456) (p = 0.729) (Table 3). Among those who successfully completed treatment, mean days were 258.9 (SD = 64.4) and 270.5 (SD = 67.4) for AiCure and DOT, respectively (p = 0.395).
Table 3. Result statistics by treatment arm.
| DOT | AiCure | ||||
|---|---|---|---|---|---|
| No. | Prop. | No. | Prop. | P-value | |
| Treatment status counts | |||||
| Completed | 62 | (0.87) | 38 | (0.88) | 0.869 |
| Moved | 6 | (0.08) | 3 | (0.07) | 0.777 |
| Defaulteda | 3 | (0.04) | 2 | (0.05) | 0.914 |
| Days on treatment for TB | Mean | SD | Mean | SD | P-value |
| Total | 247.1 | (89.07) | 252.5 | (69.36) | 0.729 |
| Completers | 270.5 | (67.39) | 258.9 | (64.44) | 0.395 |
| Defaultersa | 124.0 | (3.00) | 130.5 | (51.62) | 0.888 |
| Observations | 71 | 43 | 114 | ||
a Default is defined as patients who refused continued treatment, were lost to follow-up, or went off protocol for medical reasons during our observation period. Category totals may not sum to N due to missing values. Movers were excluded from transition probability calculation. P-values are calculated using two-sided, two-sample t-tests for proportions or means with unequal variances.
Using the model, we found that AiCure results in greater benefits at a lower cost over DOT. In our base case analysis, AiCure cost an average of $2,668 per-patient, while DOT cost 83% more, at $4,894 per-patient over the 16-month horizon (Table 4). Our model results indicated AiCure produced 1.05 QALYs per patient over the 16-month time horizon and DOT produced 1.03 QALYs.
Table 4. Model results under base case and scenario analyses.
| Scenario | AiCure | DOT | AiCure vs. DOT | ||||
|---|---|---|---|---|---|---|---|
| Cost (2017 USD) | QALYs | Cost (2017 USD) | QALYs | Cost (2017 USD) | QALYs | ICER (2017 USD) | |
| Base case | $2,668 | 1.05 | $4,894 | 1.03 | -$2,226 | 0.02 | AI Dominant |
| AI 5% worsea | $2,860 | 1.03 | $4,894 | 1.03 | -$2,034 | 0.00 | AI Dominant |
| AI 10% worsea | $3,011 | 1.02 | $4,894 | 1.03 | -$1,882 | -0.01 | $244,651 (DOT) |
| AI 15% worsea | $3,164 | 1.01 | $4,894 | 1.03 | -$1,730 | -0.02 | $89,889 (DOT) |
| AI WC/DOT BC | $5,278 | 1.08 | $1,607 | 1.08 | $3,672 | 0.01 | $433,646 (AI) |
| AI BC/DOT WC | $2,883 | 1.01 | $10,485 | 0.98 | -$7,603 | 0.03 | AI Dominant |
a x% worse implies an x percentage-point decrease in all monthly completion probabilities up to 15 months, with a floor of zero. The worst/best scenario involves model one-way sensitivity parameters tested at their 95% confidence interval lower or upper bounds. All costs and QALYs are reported per-patient. AI, AiCure; DOT, directly observed therapy; ICER, incremental cost-effectiveness ratio, QALY, quality-adjusted life year; BC, best case; WC, worst case.
Incrementally, AiCure patients gain an additional 0.02 QALYs at cost savings of $2,226 over DOT. At WTP of $50K, $100K, and $150K per QALY, resulting in incremental NMB per patient under AiCure versus DOT that were all above zero ($3,142, $4,057, and $4,973 respectively).
In univariate DSA, no variation in the model inputs within reasonable ranges resulted in a negative incremental NMB for AiCure. The model was most sensitive to the number of doses taken per-member per-month (11 in the base case). Varying this parameter between 5.5 and 16.5 doses per-month resulted in an NMB range of $3,633 to $6,313 WTP of $150K/QALY. The model was also highly sensitive to hourly wage for licensed vocational nurses (LVN) of $35.45; varying this value (+/- 50%) resulted in an NMB range of $3,885 to $6,061 (see tornado diagram in S1 Fig in S1 File). These results suggest much of the cost savings from AiCure can be attributed to the significant decrease in personnel costs required per dose. As DOT treatment requires LVNs or other skilled personnel to engage in significant travel, the trip frequency/distance and hourly rate of these individuals drives large cost differences.
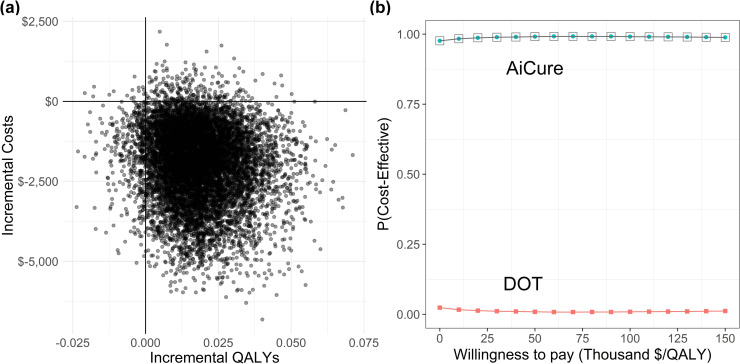
In PSA, AiCure produced more QALYs per patient at a lower cost than DOT in 93.5% of 10,000 simulations (Fig 3). At WTP/QALY of $50K, $100K, and $150K, the probability of AiCure being cost-effective is 95.3%, 95.9%, and 96.4%, respectively. These results suggest the base scenario is robust to univariate and probabilistic variations in the input parameters. However, we find there is some probability that DOT is the more cost-effective choice as the WTP for a QALY decreases (see cost-effectiveness acceptability curve [CEAC] in Fig 3B).
Fig 3. Probabilistic sensitivity analysis results.
Panel a: Incremental values per-patient of AiCure relative to DOT. Panel b: Cost-effectiveness acceptability curve (CEAC) by treatment arm. DOT directly observed therapy, QALY quality-adjusted life year.
Our scenario analyses also suggest that the most a clinic could lose by adopting AiCure would be relatively small (Table 4). In the “worst case” scenario, we find that AiCure would not be cost-effective, generating 0.01 more QALYs per patient for $3,672 more dollars than DOT (AiCure ICER of $433,646). On the other hand, clinics could stand to gain much more by adopting AiCure: in the “best case” scenario, we find that AiCure could generate 0.03 more QALYs per patient for $7,603 less costs than DOT. The difference in health outcomes are quite small in both the “best” and “worst” cases, indicating that patient health would not be substantially affected with or without AiCure, although the financial impact could be large. See the online S1 File for additional details on our primary and secondary scenario analyses.
In the third scenario analysis, we compared AiCure to VDOT, where nurses monitor patient video records to ensure the AI system is operating correctly. We find that even with this additional monitoring the system would still be cost saving. If 7 minutes were spent reviewing each dose (as observed in LA County’s system after AiCure’s initial adoption during implementation of the parent research study), AiCure still saved $1,517 per patient relative to DOT. This 7-minute figure per-dose is included in the “worst case” scenario analysis previously described and is comparable to figures found for VDOT in previous studies. AI monitoring systems could potentially save a few hundred dollars per patient compared to VDOT systems. Consistent with prior studies, we estimate that there would be a cost savings of roughly $1,500 per patient if nurses reviewed video of each dose, which is about $700 less savings than using the AI monitoring system in our base case [7, 8, 15]. This could amount to substantial savings for large health systems if AI monitoring was used at scale.
Discussion
The data from LAC patients suggest that AiCure would be an effective method of reducing nurse and patient burden and achieving similar levels of DOT success. Patients on AiCure completed treatment in a statistically indistinguishable amount of time as DOT (p = 0.729) while costing $2,226 less, on average. Sensitivity analysis results showed that despite the uncertainty around completion times due to the limited number of patients in the LAC data, AiCure would be the cost-effective strategy over 95% of the time with willingness-to-pay levels over $50,000 per QALY. Even in cases where DOT was cost-effective over AiCure, the largest amount DOT could save over AiCure over all possible input values was modest ($3,672 saved per-patient) relative to the largest savings for AiCure over all possible input values ($7,603 saved per-patient).
However, these results may not be generalizable to all TB clinics and all patients. The cohorts used for comparison in this study were not perfectly statistically similar across all characteristics, constitute a small sample of all patients, and indeed may not be fully representative of the LAC TB patient population. We limited our analyses to non-drug resistant pulmonary TB cases only, as most TB cases in LAC are not complicated by extra-pulmonary TB or drug resistance–in 2015, 71.5% of TB patients had exclusively pulmonary disease. These would also be the most likely patients to be first shifted to an AI Platform, as their treatment regimens are relatively simpler. The cost savings and treatment outcomes in this analysis may not apply to a patient population with more complicated case histories or comorbidities, nor to health programs less geographically sprawling than those in LAC–the costs included those of LVN travel (an average of $35.45 per hour and 54 cents per mile in the base analysis). However, while cost-savings may be lower in smaller counties, variation in travel costs or nurse wages in the sensitivity analyses did not lead to DOT becoming cost-effective over AiCure in any scenario.
We also wish to acknowledge the assumptions in our model. We do not consider in the model possible side effects or delays in appropriate care due to a reduction in frequency of patient-nurse contacts, as we assume that patients’ monthly clinic visits are sufficient to address any treatment complications. We note that our base case may underestimate the true cost savings of AI treatment monitoring platforms for patients with more than 2–3 DOT field or clinic appointments per week; an increased frequency of clinic or field visits would only widen cost differences through necessary LVN and RN personnel hours. Recent ATS/CDC TB treatment guidelines recommend the use of daily rather than intermittent (such as twice or thrice weekly) TB therapy [26, 27]. This recommendation has been difficult to implement fully due to staff capacity. As LACDPH increasingly meets this standard, we would expect the true cost savings of AiCure to increase. We do not consider transmission in this analysis, with the assumption that both intervention arms effectively limit spread of TB soon after the patient starts treatment. We also assume that all patients who complete care would have roughly equivalent quality of life post-treatment whether on DOT or AiCure, and we therefore only consider the 16-month treatment for the time horizon.
Despite these limitations, our analysis suggests that AiCure would be a cost-saving alternative to DOT with no sacrifice in quality of care, provided it is offered to uncomplicated non-drug resistant pulmonary TB cases. Increased access to and implementation of AI treatment monitoring systems could potentially reduce the costs of already resource-constrained public health systems and free nurses to perform skilled tasks instead of traveling to patients or reviewing videos of patient to ensure adherence. These results suggest that the AiCure platform may be a reasonable option to consider for patients beyond those in the pilot study.
Conclusions
The evidence from this pilot study suggests that AI treatment monitoring platforms for TB may be worth investing in as cost-savings under typical operation should be substantial compared to DOT without degradation of patient outcomes. Our findings contribute to a growing literature on the value and application of artificial intelligence in medicine to improve clinical decision-making, implement efficiencies, and drive behavioral modification across large patient populations, especially with regards to remote treatment options for conditions which disproportionately affect disadvantaged populations in the US. Future work may look at the heterogeneity in outcomes by patient population and the value of conducting additional, larger-scale multi-arm studies to reduce uncertainty around efficacy and cost estimates.
Supporting information
(DOCX)
Acknowledgments
We thank Stuart McMullen, of the Centers for Disease Control and Prevention and Ana Delia Hernandez, of the Los Angeles County Tuberculosis Control Program for managing all aspects of the clinical project using AiCure. We thank Wendy Gilman, Angela Lozano, Youghaper Bornazyan, Joselito Florendo, of the Pacoima Health Center for their work in the clinical study using AiCure. We also thank Adam Hanina and Laura Shafner of AiCure (New York, NY) for helpful comments on an earlier version of our cost-effectiveness study.
Abbreviations
- AI
artificial intelligence
- ATS
American Thoracic Society
- BC
best case
- CA
California
- CDC
Centers for Disease Control and Prevention
- CEAC
cost-effectiveness acceptability curve
- DOT
directly observed therapy
- DSA
deterministic sensitivity analyses
- EMB
ethambutol
- HIV
human immunodeficiency virus
- HR-QoL
health-related quality of life
- ICER
incremental cost-effectiveness ratio
- INH
isoniazid
- INMB
incremental net monetary benefits
- IRB
institutional review board
- LA
Los Angeles
- LAC
Los Angeles County
- LACDPH
Los Angeles County Department of Public Health
- LVN
licensed vocational nurse
- MDR
multidrug-resistance
- NMB
net monetary benefits
- NY
New York
- PSA
probabilistic sensitivity analyses
- PZF
pyrazinamide
- QALY
quality-adjusted life year
- RIF
rifampin
- RN
registered nurse
- SD
standard deviation
- SI
supplementary information
- SMS
short message service
- SOT
Skype observed therapy
- TB
tuberculosis
- US
United States
- USA
United States of America
- USD
United States dollar
- VDOT
video directly observed therapy
- WA
Washington
- WC
worst case
- WTP
willingness to pay
Data Availability
All data generated or analyzed during our cost-effectiveness study are included in this published article and its supplementary information files. Patient-level data from the pilot study and historical controls are not publicly available as the data include information which could be used to identify individuals. Patient-level data is housed and collected by the LA County Department of Public Health tuberculosis control program (email: tbc@ph.lacounty.gov).
Funding Statement
This work (our clinical study using AiCure) was supported by the Los Angeles County Quality and Productivity Commission (http://qpc.lacounty.gov/). The funding source had no involvement in the design, analysis, interpretation, writing, or decision to submit for publication our cost effectiveness study.
References
- 1.Centers for Disease Control and Prevention. Burden of TB in the United States 2018 [updated November 15; cited 2019 March 27]. Available from: https://www.cdc.gov/features/burden-tb-us/index.html.
- 2.Los Angeles County Department of Public Health. Tuberculosis in Los Angeles County: Surveillance Report (2016) 2018 [Available from: http://publichealth.lacounty.gov/tb/docs/SurveillanceReport2016.pdf.
- 3.Los Angeles County Department of Public Health. Tuberculosis Control Program Manual 2003 [Available from: http://publichealth.lacounty.gov/tb/TBManual/TBmanual.pdf.
- 4.Los Angeles County Department of Public Health. Tuberculosis in Los Angeles County: Surveillance Report (2013) 2015 [Available from: http://publichealth.lacounty.gov/tb/docs/SurveillanceReport2013.pdf.
- 5.Garfein RS. Best Practices Guidelines for Using Mobile Technology to Monitor Tuberculosis Treatment Adherence in California California TB Controllers Meeting2015 [updated April 20, 2015. Available from: http://www.ctca.org/fileLibrary/file_751.pdf.
- 6.Ngwatu BK, Nsengiyumva NP, Oxlade O, Mappin-Kasirer B, Nguyen NL, Jaramillo E, et al. The impact of digital health technologies on tuberculosis treatment: a systematic review. Eur Respir J. 2018;51(1). doi: 10.1183/13993003.01596-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzman SB, Zenilman A, Shah M. Advancing Patient-Centered Care in Tuberculosis Management: A Mixed-Methods Appraisal of Video Directly Observed Therapy. Open Forum Infect Dis. 2018;5(4):ofy046. doi: 10.1093/ofid/ofy046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garfein RS, Liu L, Cuevas-Mota J, Collins K, Munoz F, Catanzaro DG, et al. Tuberculosis Treatment Monitoring by Video Directly Observed Therapy in 5 Health Districts, California, USA. Emerg Infect Dis. 2018;24(10):1806–15. doi: 10.3201/eid2410.180459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D, Jaramillo E, et al. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15(7):e1002595. doi: 10.1371/journal.pmed.1002595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macaraig M, Lobato MN, McGinnis Pilote K, Wegener D. A National Survey on the Use of Electronic Directly Observed Therapy for Treatment of Tuberculosis. J Public Health Manag Pract. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Holzschuh EL, Province S, Johnson K, Walls C, Shemwell C, Martin G, et al. Use of Video Directly Observed Therapy for Treatment of Latent Tuberculosis Infection—Johnson County, Kansas, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(14):387–9. doi: 10.15585/mmwr.mm6614a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Story A, Garfein RS, Hayward A, Rusovich V, Dadu A, Soltan V, et al. Monitoring Therapy Compliance of Tuberculosis Patients by using Video-Enabled Electronic Devices. Emerg Infect Dis. 2016;22(3):538–40. doi: 10.3201/eid2203.151620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuck C, Robinson E, Macaraig M, Alexander M, Burzynski J. Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int J Tuberc Lung Dis. 2016;20(5):588–93. doi: 10.5588/ijtld.15.0738 [DOI] [PubMed] [Google Scholar]
- 14.Garfein RS, Collins K, Munoz F, Moser K, Cerecer-Callu P, Raab F, et al. Feasibility of tuberculosis treatment monitoring by video directly observed therapy: a binational pilot study. Int J Tuberc Lung Dis. 2015;19(9):1057–64. doi: 10.5588/ijtld.14.0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchman T, Cabello C. A New Method to Directly Observe Tuberculosis Treatment: Skype Observed Therapy, a Patient-Centered Approach. J Public Health Manag Pract. 2017;23(2):175–7. doi: 10.1097/PHH.0000000000000339 [DOI] [PubMed] [Google Scholar]
- 16.Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using Artificial Intelligence to Reduce the Risk of Nonadherence in Patients on Anticoagulation Therapy. Stroke. 2017;48(5):1416–9. doi: 10.1161/STROKEAHA.116.016281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bain EE, Shafner L, Walling DP, Othman AA, Chuang-Stein C, Hinkle J, et al. Use of a Novel Artificial Intelligence Platform on Mobile Devices to Assess Dosing Compliance in a Phase 2 Clinical Trial in Subjects With Schizophrenia. JMIR Mhealth Uhealth. 2017;5(2):e18. doi: 10.2196/mhealth.7030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang AH, Shafner L, Hernandez AD, Hanina A. Evaluating The Use Of An Artificial Intelligence (AI) Platform On Mobile Devices To Measure And Support Tuberculosis Medication Adherence. 21st Annual Conference of The Union. 2017. Vancouver, BC, Canada.
- 19.VA Federal Supply Schedule Service. Federal Supply Schedule (FSS)—Pharmaceutical Pricing 2017 [Available from: https://www.va.gov/oal/business/fss/pharmPrices.asp.
- 20.Suen SC, Bendavid E, Goldhaber-Fiebert JD. Cost-effectiveness of improvements in diagnosis and treatment accessibility for tuberculosis control in India. Int J Tuberc Lung Dis. 2015;19(9):1115–24, i-xv. doi: 10.5588/ijtld.15.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43. doi: 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 23.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—6. Value Health. 2012;15(6):835–42. doi: 10.1016/j.jval.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 24.Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8(1):1–2. doi: 10.1111/j.1524-4733.2005.08101.x [DOI] [PubMed] [Google Scholar]
- 25.Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making. 2003;23(4):341–50. doi: 10.1177/0272989X03255922 [DOI] [PubMed] [Google Scholar]
- 26.Control and Prevention/Infectious Diseases Society of America: Treatment of Tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603 [DOI] [PubMed] [Google Scholar]
- 27.Nahid P, Dorman SE, Alipanah N, et al. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis. 2016;63(7):853–867. doi: 10.1093/cid/ciw566 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All data generated or analyzed during our cost-effectiveness study are included in this published article and its supplementary information files. Patient-level data from the pilot study and historical controls are not publicly available as the data include information which could be used to identify individuals. Patient-level data is housed and collected by the LA County Department of Public Health tuberculosis control program (email: tbc@ph.lacounty.gov).