Abstract
Inflammation is central to the pathogenesis of pulmonary vascular remodeling and pulmonary hypertension (PH). Inflammation precedes remodeling in preclinical models, thus supporting the concept that changes in immunity drive remodeling in PH. Platelets are recognized as mediators of inflammation, but whether platelets contribute to hypoxia-driven inflammation has not been studied. We utilized a murine hypoxia model to test the hypothesis that platelets drive hypoxia-induced inflammation. We evaluated male and female 9-wk-old normoxic and hypoxic mice and in selected experiments included hypoxic thrombocytopenic mice. Thrombocytopenic mice were generated with an anti-GP1bα rat IgG antibody. We also performed immunostaining of lung sections from failed donor controls and patients with idiopathic pulmonary arterial hypertension. We found that platelets are increased in the lungs of hypoxic mice and hypoxia induces platelet activation. Platelet depletion prevents hypoxia-driven increases in the proinflammatory chemokines CXCL4 and CCL5 and attenuates hypoxia-induced increase in plasma CSF-2. Pulmonary interstitial macrophages are increased in the lungs of hypoxic mice; this increase is prevented in thrombocytopenic mice. To determine the potential relevance to human disease, lung sections from donors and patients with advanced idiopathic pulmonary arterial hypertension (iPAH) were immunostained for the platelet-specific protein CD41. We observed iPAH lungs had a two-fold increase in CD41, compared with controls. Our data provide evidence that the platelet count is increased in the lungs and activated in mice with hypoxia-induced inflammation and provides rationale for the further study of the potential contribution of platelets to inflammatory mediated vascular remodeling and PH.
Keywords: hypertension, hypoxia, inflammation, platelet, pulmonary hypertension
INTRODUCTION
Pulmonary hypertension (PH) is a progressive and lethal disease driven in part by inflammation (1–6). The pulmonary vasculature in patients with PH and animals with experimental PH is characterized by infiltration of innate and adaptive immune cells (4, 5, 7). Proinflammatory chemokines and cytokines are increased within the circulation and lungs of patients with PH (8–10). Inflammation precedes pulmonary vascular remodeling and blockade of inflammatory signaling prevents the development of PH in preclinical models, thus supporting the concept that changes in immunity drive vascular remodeling in PH (3, 7, 11, 12). Immune cells including platelets, are key mediators of vascular inflammation but the role of platelets in hypoxia-induced inflammation has not been established (13, 14).
Platelets are small, anucleated cells derived from megakaryocytes, best known for their role in hemostasis. Platelets also play a role in immune regulation and promote vascular inflammation (14, 15). Aberrant platelet activation mediates several inflammatory conditions including atherosclerosis, sepsis, and acute lung injury and has recently been reported in COVID-19 (15–18). Platelet granules store numerous growth factors, vasoactive mediators, chemokines, cytokines, and angiogenic agents, many of which have been implicated in vascular remodeling and thrombosis in PH (19).
Platelets contribute to vascular inflammation directly via interaction with the extracellular matrix and/or interaction with resident vascular cells (14, 15, 18). Platelets may also indirectly drive vascular inflammation via recruitment and differentiation of circulating immune cells, including monocytes (20). Binding of platelets to monocytes via P-selectin promotes monocyte synthesis of proinflammatory chemokines and increases monocyte expression of adhesion molecules, enhancing monocyte adhesion to the endothelium (20). Monocyte and macrophage recruitment and accumulation within the perivascular/adventitial space is a consistent feature of pulmonary vascular remodeling associated with PH in both humans and all animal models and depletion of monocytes prevents the development of experimental PH (1, 4, 5, 11, 12). Whether platelets contribute to the recruitment of monocytes and macrophages at the time of peak hypoxia-induced inflammation is unknown.
Accumulating data from both human and animal studies demonstrate a role for platelets in the pathogenesis of PH (21–27). The majority of investigation into platelets in the pathogenesis of PH thus far has focused on the hemostatic consequences of platelet activation and pathologic vascular remodeling. However, given the heterogeneity of pulmonary hypertension, there is lack of convincing data supporting the use of antiplatelet therapies which target the platelet hemostatic response, in patients with forms of PH other than chronic thromboembolic PH (CTEPH) (28, 29). Therefore, investigation into novel consequences of platelet activation in PH is warranted. The aim of our work is to identify novel immunoregulatory functions of platelets in the setting of PH. Our study utilized a murine hypoxia model to test the hypothesis that platelets are increased in the lungs of hypoxic mice, circulate in an active state, and modulate hypoxia-induced inflammation. Our data support the premise that platelets modulate inflammation and provide rationale for the study of platelets in the regulation of inflammation in the pathogenesis of PH.
METHODS
Mouse Model
C57BL/6 mice (male and female; 7 wk of age) were purchased from Jackson Laboratories. Animals were acclimatized for 1 wk in a sea-level (SL) chamber (barometric pressure [PB] = 760 mmHg). Control groups remained in the SL chambers, and experimental groups were placed into hypobaric (PB = 380 mmHg) hypoxic chambers (with oxygen levels ∼12%). The Denver Institutional Animal Care and Use Committee (IACUC) at the University of Colorado approved all studies. To deplete platelets, mice were injected intraperitoneally with 4 µg/g of the rat monoclonal antibody directed against GPIbα (Emfret Analytics #3R00, Wurzburg, Germany). Thrombocytopenia was confirmed at 1 h and 3 days after antibody delivery using the hematologic analyzer Heska HT5 (Loveland, CO).
Preparation of Mouse Blood, Platelets, and Plasma
Mice were anesthetized with isoflurane blood, and platelet-poor plasma (PPP) were obtained as previously described (30). Platelet-rich plasma (PRP) was obtained by centrifugation of whole blood at 100 g for 10 min. PRP was supplemented with PGI2 (1 µg/mL) and incubated at room temperature for 3 min before centrifugation at 2000 g × 2 min to obtain platelet-poor plasma (PPP). Complete blood counts were obtained using the hematologic analyzer Heska HT5 (Loveland, CO).
Assessment of Platelet Activation by Flow Cytometry
Flow cytometry was performed by diluting washed platelets (1 × 106 platelets/mL) in Tyrode’s buffer containing 1 mM CaCl2. Murine platelets were activated with thrombin (0.1 IU/mL) in the presence of anti-mouse CD41-BV421 antibody (Biolegend, Clone No. MWReg30; 1:50) and P-selectin-APC (Biolegend, Clone No. APM-1; 1:25). The activation was quenched at 5 min using ice-cold 1% PFA Tyrodes buffer. Samples were run in the Gallios analyzer (Beckman Coulter, Brea, CA). Flow cytometry data were analyzed using Kaluza flow analysis software (Beckman Coulter, Brea, CA) and Flowjo (Flowjo, LLC) (30).
Measurement of Platelet Monocyte Aggregates
Ten microliters of blood were aliquoted in 1.5 mL Eppendorf tubes that were prepared with platelet and monocyte antibody reagents. Antibodies included CD41-BV421(Biolegend; Clone MWReg30 Cat. No. 133912; 1:8) and Ly6C-FITC (BD; Clone AL-21 Cat. No. 553104; 1:8). Blood samples were covered in foil and stained for 15 min at RT and fixed with 2% PFA. Samples were run on the Gallios 561. Data were analyzed using Kaluza.
ELISA
PRP and PPP samples were obtained. Lung homogenates were prepared in lysis buffer containing protease and phosphatase inhibitors. CXCL4 levels were measured using the mouse CXCL4 ELISA kit (Abcam, Cambridge, MA). Platelet-poor plasma CCL5 and CSF-2 were measured with the Discovery Assay (Eve Technologies, Calgary, AB Canada).
Immunohistochemistry
Mouse lungs were flushed and inflated for paraffin embedding and immunohistochemistry was performed for CD41 as previously described (30). Staining for control and experimental conditions were performed on the same day, and images were collected at the same time under the same conditions. All vessels between 50–200 µm were identified, and the number of platelets adjacent to the endothelium was quantified per vessel. Distal lung platelets were assessed by quantifying CD41+ pixels per high-powered field (×20). Lung fields containing large vessels or airways were excluded, and 10 fields were included per mouse. IHC staining and quantification was performed on human lung sections for CD41 using polyclonal antibody GTX113758 from Gene Tex and Dako Envision anti-rabbit detecting system as described above at 1:250 dilution. CD41-positive pixels were quantified in whole lung sections using a random nonbiased approach as previously described (4).
Quantification of Lung Platelets by FACS
Mice were retro-orbitally injected with 100 µL of a 1:10 dilution of CD41-BV421 (Biolegend, Clone No. MWReg30) in PBS 5 min before collecting lungs to label intravascular platelets. Lungs were homogenized and quantification of lung interstitial platelets was performed as previously described (30). Briefly, the lungs were flushed to remove the majority of circulating platelets. The lungs were then digested using a gentleMACS in 1 mL of HBSS with no calcium or magnesium (Sigma), Liberase TM (Sigma, 0.4 mg/mL), or DNAse I (Sigma, 100 U/mL). The gentleMACS was digested using m_lung_01 and incubated for 20 min and then digested again using m_lung_02 for 20 s. Whole lung digests were stained with CD41-APC (Clone No. MWReg30, BD Biosciences) and CD42b-FITC (Clone No. Xia.G5, Emfret, Eibelstadt, Germany) to label lung platelets. Lung interstitial platelets were defined by positive staining for CD41-APC and CD42b-FITC but negative staining for CD41-BV421 (CD41-APCHi, CD42b-FITCHi, and CD41-BV421Lo). Quantification was performed using 123eCount beads (Thermo Fisher) as previously described (31).
Quantification of Lung Macrophages by FACS
Mice were retro-orbitally injected with 100 μL of a 1:10 dilution of CD45-FITC (Biolegend, Clone No. 30-F11) in PBS 5 min before collecting lungs to label circulating leukocytes. Lungs were homogenized and quantification of lung interstitial macrophages was performed as previously described (3). Briefly, the lungs were flushed to dislodge the majority of circulating leukocytes. The lungs were then digested using a gentleMACS in 1 mL of HBSS with no calcium or magnesium (Sigma), Liberase TM (Sigma, 0.4 mg/mL), or DNAse I (Sigma, 100 U/mL). The gentleMACS was digested using m_lung_01 and incubated for 20 min and then digested again using m_lung_02 for 20 s. Lungs were then stained with CD64-AF647 (Biolegend, Clone No. X54-5/7.1), CD11b-PE/Cy7 (Biolegend, Clone No. M1/70), SigF-PE (BD Biosciences, Clone No. E50-2440), Ly6C-BV421 (BD Biosciences, Clone No. AL-21), Ly6G-BV421 (BD Biosciences, Clone No. 1A8), CD3-BV421 (Biolegend, Clone No. 17A2), B220-BV421 (BD Biosciences, Clone No. RA3-6B2), and DAPI. A dump gate was used which combined DAPI+ cells and all aforementioned BV421 antibodies. Interstitial macrophages were identified by negative expression of RO-CD45−, Dump−, CD64+, CD11b+, and SigF−.
Human Tissues
Human lung specimens from control (rejected lung transplant donors) and patients with idiopathic pulmonary arterial hypertension (iPAH) were provided by the Pulmonary Hypertension Breakthrough Initiative, funded through a National Heart, Lung, and Blood Institute (NHLBI) R24 grant (No. R24HL123767) and the Cardiovascular Medical Research Education Fund. These cases have been described previously in detail (4). The present study was approved by the Colorado Multiple Institutional Review Board. Lung tissue collection was approved by each Institutional Review Board at all lung transplant sites.
Antibody Validation
All antibodies have been previously validated in prior publications and in our laboratory using positive and negative controls (30, 32).
Statistical Analysis
Data were analyzed using Prism (GraphPad Software, La Jolla, CA) by unpaired t test or one-way ANOVA. Data are expressed as means ± SD; significance defined as P < 0.05.
RESULTS
Platelets Are Increased in the Lungs of Mice following 3 Days of Hypoxia
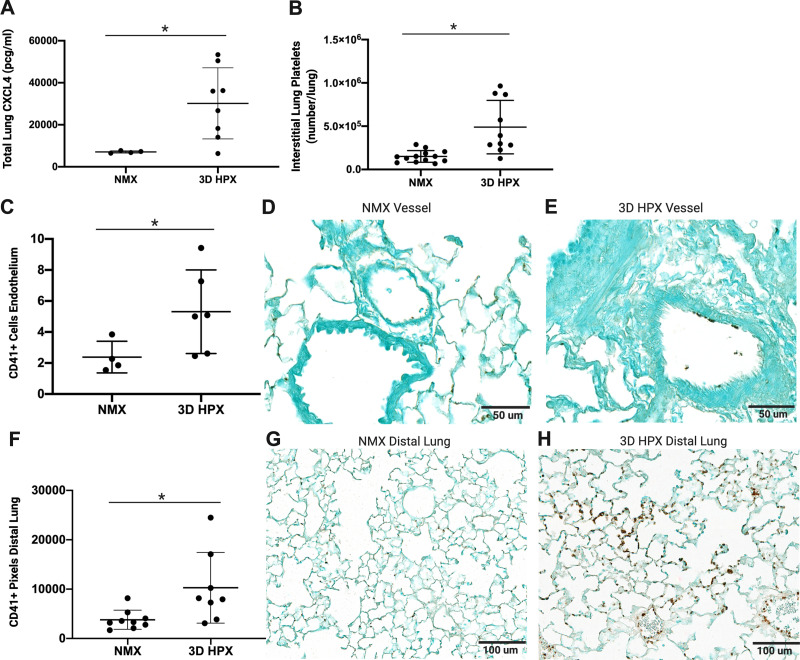
We used several complementary methods to evaluate whether platelets were increased in the lungs of mice at the time of peak hypoxia-induced inflammation. CXCL4 is synthesized by megakaryocytes and stored within platelet alpha granules. It is the most abundant platelet-derived protein. Our data reveal that the platelet-derived protein, CXCL4, is increased in the lungs of mice following hypoxia (Fig. 1A). To determine the levels of platelets in the lung interstitial compartment, we selectively labelled intravascular and interstitial platelets in whole lung digests of control and hypoxic mice and quantified lung interstitial platelets by flow cytometry. Hypoxia significantly increased interstitial lung platelets (Fig. 1B). To determine the location of increased platelets within the lung, we performed IHC for the platelet-specific protein CD41. We counted the number of positive cells adjacent to the pulmonary endothelium (Fig. 1, C–H). Platelets accumulate at the pulmonary endothelium of pulmonary vessels between 50 and 200 µm in mice exposed to hypoxia (Fig. 1, C–E) and are increased 2.5-fold in the distal lung of hypoxic mice (Fig. 1, F–H).
Figure 1.
Platelets are increased in the lungs of mice following hypoxia. A: CXCL4 levels in mouse whole lung homogenates, *P < 0.01 by t test (n = number of rats), normoxia (NMX): n = 4 male; 3D hypoxia (HPX): n = 7 male, 1 female. B: lung interstitial platelets in mouse whole lung homogenates, **P < 0.001 by t test, NMX: n = 8 male, 5 female; 3D HPX: n = 6 male, 4 female. CD41+ cells on the endothelium of pulmonary arterioles between 50 and 200 µm (C) NMX, ×40 magnification (D), and HPX, ×40 magnification (E); scale bars = 50 µm, *P < 0.05 by t test; NMX: n = 3 male, 1 female; HPX: n = 3 male, 3 female. Analysis and representative CD41 staining in mouse distal lung sections (F) NMX, ×20 magnification (G), and HPX, ×20 magnification (H); scale bars = 100 µm, *P < 0.05 by t test; NMX: n = 6 male, 3 female; HPX: n = 5 male, 3 female.
Platelets are Activated following the Exposure of Mice to Hypoxia
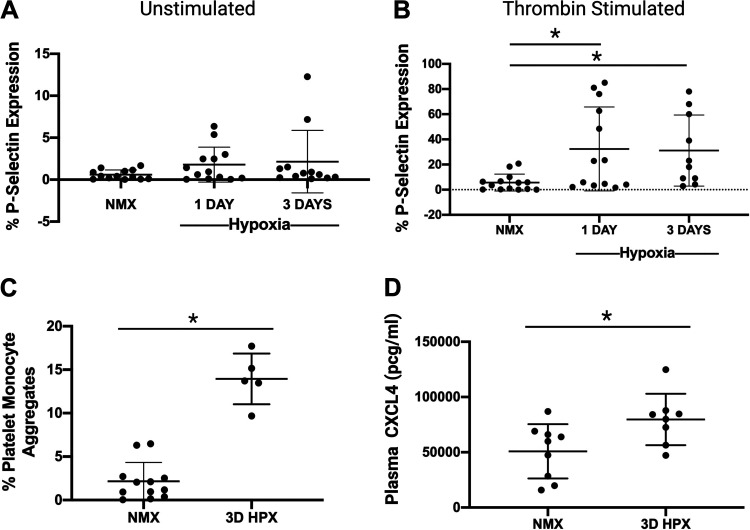
Activated platelets increase membrane expression of the alpha granule protein P-selectin. At baseline, we detect a subtle but nonsignificant trend toward higher exposure of P-selectin in isolated washed platelets from mice exposed to hypoxia. However, upon activation with thrombin, platelets from mice exposed to hypoxia exhibit significantly higher amounts of P-selectin expression compared with platelets from normoxic mice. These results are consistent with hypoxia-induced priming of platelet activation and thrombo-inflammatory potential (Fig. 2, A and B). A sensitive marker of in vivo platelet activation is the generation of platelet-monocyte aggregates (PMA) (16). PMA increased sevenfold following hypoxia over controls (Fig. 2C). Following 3 days of hypoxia, plasma levels of the platelet-specific alpha granule protein CXCL4 were elevated (Fig. 2D). The aggregate of these results supports that hypoxia is associated with platelet alpha granule release and platelets are activated in mice exposed to short-term hypoxia.
Figure 2.
Platelets are activated in mice following hypoxia exposure. A: platelets from mice exposed to hypoxia have similar levels of P-selectin expression at baseline (unstimulated), ns, not significant; n = 12–13 rats (sex not recorded). B: platelets from mice exposed to hypoxia for both 1 day and 3 days have increased expression of P-selectin after thrombin stimulation compared with controls, *P < 0.05 by one-way ANOVA, n = 10–14 rats (sex not recorded). C: increased platelet-monocyte aggregates following exposure to hypoxia, *P < 0.0001 by t test, n = number of rats; NMX: n = 7 male 5 female; HPX: n = 2 male 3 female. D: plasma CXCL4 levels from mice following hypoxia are increased compared with controls, *P < 0.05 by t test; NMX: n = 5 male 4 female; 3D HPX: n = 6 male 2 female. HPX, hypoxia; NMX, normoxia.
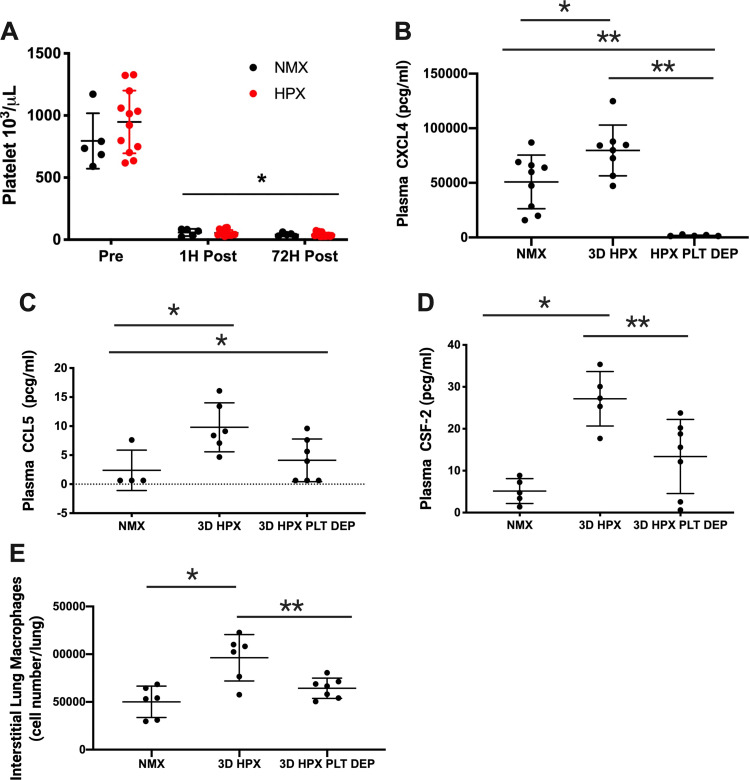
Figure 3.
Platelet depletion prevents inflammation in mice exposed to hypoxia. A: antibody-mediated platelet depletion results in profound thrombocytopenia that persists throughout the study period, *P < 0.001, n = number of rats, NMX: n = 5 male; HPX, n = 10 male, 2 female. Platelet depletion prevents hypoxia-induced increase in plasma CXCL4 *P < 0.05, NMX vs. 3D HPX, **P < 0.005 (B). 3D HPX + platelet depletion vs. NMX and 3D HPX by one-way ANOVA; NMX: n = 5 male, 4 female; 3D HPX: n = 6 male, 2 female; 3D HPX + platelet depletion: n = 5 male, and plasma CCL5, *P < 0.05, 3D HPX + platelet depletion vs. NMX and 3D HPX by one-way ANOVA; NMX: n = 4 male; 3D HPX: n = 6 male; 3D HPX + platelet depletion: n = 7 male (C). Platelet depletion attenuates hypoxia-associated increase in Plasma CSF-2, *P < 0.005, NMX vs. 3D HPX and **P < 0.05, 3D HPX vs. 3D HPX + platelet depletion by one-way ANOVA, NMX: n = 5 M, 3 D HPX: n = 5 M, 3 D HPX + platelet depletion: n = 7 M (D). Platelet depletion prevents hypoxia-induced expansion of lung interstitial macrophages, *P < 0.001, NMX vs. 3D HPX and **P < 0.05 3D HPX vs. 3D HPX + platelet depletion by one-way ANOVA; NMX: n = 6 male; HPX: n = 6 male, 3D HPX + platelet depletion: n = 7 male (E). HPX, hypoxia; NMX, normoxia.
Platelet Depletion Prevents Hypoxia-Induced Increase in Plasma ProInflammatory Chemokines and Lung Interstitial Macrophages in Mice
Antibody-mediated platelet depletion results in profound thrombocytopenia that persists throughout the 3-day study period (Fig. 3A). Chemokines CXCL4 and CCL5 increase in patients with PH and have been implicated in the pathogenesis of PH (8, 10). We show that plasma CXCL4 and CCL5 levels are increased following hypoxia and that hypoxia-induced increase in CXCL4 and CCL5 is prevented in thrombocytopenic mice (Fig. 3, B and C). CSF-2, a proinflammatory cytokine involved in the activation of monocytes and macrophages, is increased in the lungs of patients with PH and pulmonary arteries of hypoxic mice (2, 33). Plasma CSF-2 is increased in hypoxic mice and hypoxia-induced increase in CSF-2 is attenuated in thrombocytopenic hypoxic mice (Fig. 3D). Macrophage recruitment and accumulation within the perivascular/adventitial space is a consistent feature of pulmonary vascular remodeling associated with PH in both humans and animal models (1, 3, 5, 12). Hypoxia increases lung interstitial macrophages in platelet-replete mice, whereas platelet depletion prevents hypoxia-induced increase in lung interstitial macrophages (Fig. 3E).
Platelets Are Increased in the Lungs of Patients with End-Stage PAH
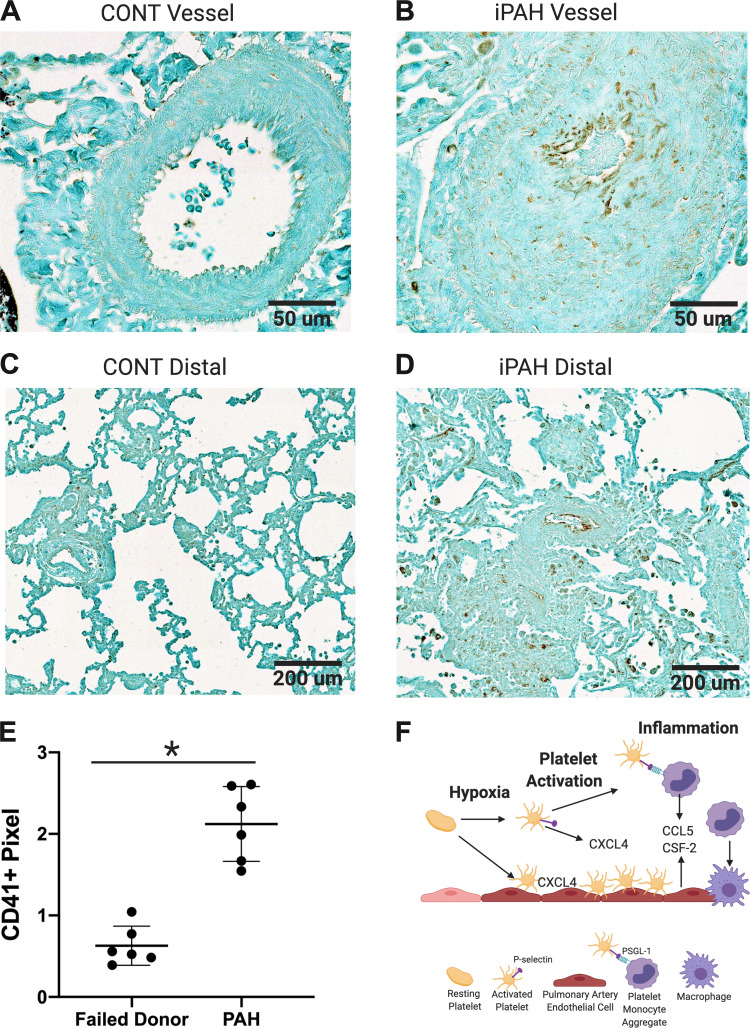
To extend the study in rodent models to humans, we evaluated whether platelets are increased in the lungs of patients with advanced iPAH (24, 30). We performed IHC for the platelet integrin CD41 and quantified the pixel counts of CD41-positive staining in whole lung sections using a random nonbiased approach (4). We found that CD41 staining was increased twofold in the lungs of patients with advanced iPAH compared with control lungs from lung transplant donors (Fig. 4, A–E).
Figure 4.
Platelets are increased in the lungs of patients with advanced iPAH compared with that of failed donor controls. Representative ×40 (A and B) and ×10 (C and D) magnification of CD41 staining in human lung sections from 2 control (A and C) and 2 patients with iPAH (B and D); scale bar = 50 µm (A and B), scale bar = 200 µm (C and D), CD41+ pixel count normalized to total lung surface area, *P < 0.0001 by unpaired t test, Failed donor controls: n = 4 male, 2 female; PAH: n = 2 male, 4 female (E). iPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension. F: graphical abstract created with BioRender.com.
DISCUSSION
Inflammation is central to the pathogenesis of PH. Although monocytes and macrophages have received the most attention and support for driving inflammation in PH, other immune cells, including platelets, are key mediators of vascular inflammation (3, 12, 15). Platelets may modulate inflammation and immunity directly through release of platelet-stored chemokines or indirectly by driving monocyte proinflammatory activation (14, 20, 34, 35). We hypothesized that platelets are increased in the lungs of hypoxic mice, circulate in an active state, and modulate hypoxia-induced inflammation. Our data demonstrate that platelets are increased in the lungs of hypoxia exposed mice at the timepoint associated with peak hypoxia-induced inflammation. We also show that mouse platelet activation is induced by hypoxia and platelet depletion prevents hypoxia-driven increases in plasma proinflammatory chemokines, CXCL4 and CCL5, and attenuates hypoxia-induced increase in plasma CSF-2. Furthermore, platelet depletion prevents hypoxia-induced pulmonary interstitial macrophage expansion.
Endothelial cells from patients and animals with PH demonstrate increased expression of proteins, ICAM-1, VCAM, and E-selectin, which promote platelet-endothelial adhesion (36, 37). Platelet endothelial adhesion promotes inflammatory cell influx to the vasculature, leading to vascular remodeling in several inflammatory conditions (38, 39). Accumulation of inflammatory cells within the lungs is a consistent early and potentially therapeutically targetable feature of experimental PH. We were therefore interested to determine whether platelets were increased in the lung in early inflammation. We show through several complementary approaches that platelets accumulate both along the endothelium of medium-sized vessels and within the distal lungs of mice at the timepoint associated with peak hypoxia-induced inflammation. Given our finding that platelets are increased in the lungs of mice exposed to short-term hypoxia and prior observations that platelets are increased in the lungs of rodents with experimental PH, we evaluated whether platelets are increased in the lungs of patients with advanced iPAH compared with that of failed donor controls (24–26, 30). We show that platelets accumulate within the lungs of patients with advanced iPAH.
In addition to defining whether platelet numbers were increased within the lung, we were also interested in establishing the activation state of platelets in hypoxia-induced inflammation. Activation of platelets alters platelet function and is well-described in patients with systemic inflammation (15). Rats exposed to 6 h of acute hypoxia and mice exposed to 3 wk of chronic hypoxia demonstrate increased platelet reactivity (40, 41). In accordance with these data, we show through several methods, which measure different aspects of activation, that platelets from mice exposed to 3 days of hypoxia are activated. We first measured the expression of P-selectin on the platelet surface, as the expression of P-selectin is one marker of platelet activation. The results demonstrate that P-selectin expression was not increased at baseline in platelets from hypoxic mice but platelets from hypoxic mice are more activatable by thrombin, supporting that platelets from hypoxic mice are primed for further agonist-induced activation. Thrombin activation of platelets is relevant to our model, as it is one of the most potent platelet agonists and can be locally or systemically generated by acute and chronic inflammation (42). We next collected whole blood and performed whole-blood flow cytometry to measure the percentage of circulating platelet monocyte aggregates as the generation of platelet monocyte aggregates is a sensitive marker of in vivo platelet activation and found that hypoxia increases platelet monocyte aggregates. Finally, we measured platelet-poor plasma levels of the platelet-derived alpha granule protein, CXCL4 and found increased plasma CXCL4 under hypoxic conditions. All together these data support our conclusion that hypoxia leads to platelet activation.
When platelets are activated, they aggregate and release granule stored vasoactive mediators, angiogenic agents, growth factors, chemokines, and cytokines, many of which have been implicated in the pathogenesis of pathologic pulmonary vascular remodeling and PH (13, 36).
Platelet activation may result in not only thrombosis but also vascular injury and inflammation (35, 39). Activated platelets may modulate inflammation through the secretion of cplatelet stored chemokines or by inducing the release of chemokines stored within other cells such as endothelial cells or leukocytes (14, 18). We found that platelet activation was associated with an increase in circulating proinflammatory chemokines CXCL4, CCL5, and CSF-2. CXCL4 is the most abundant platelet chemokine and has numerous immunomodulatory functions including preventing monocyte apoptosis and promoting a proinflammatory macrophage phenotype (43). CCL5 also has several immunoregulatory functions including the recruitment of monocytes, and promotion of leukocyte adhesion to the vascular wall and is increased in the remodeled vessels of patients with PH (8, 44). CSF-2 is increased in the lungs of patients with iPAH and the pulmonary arteries of hypoxic mice and regulates monocyte and macrophage recruitment in experimental PH (2, 33). Our data demonstrate that plasma CXCL4, CCL5, and CSF-2 are increased in hypoxic mice and that platelet depletion prevents hypoxia-induced increase in CXCL4 and CCL5 and attenuates hypoxia-induced increase in plasma CSF-2. The mechanism by which platelet depletion attenuates hypoxia-induced increase in these chemokines will require subsequent investigation. As our data demonstrate both increased platelet-endothelial adhesion and increased platelet-monocyte adhesion with hypoxia, it is possible that platelets may modulate the increased expression of these chemokines by endothelial cells and/or monocytes.
The chemokines CXCL4, CCL5, and CSF-2 modulate monocyte activation and recruitment (33, 43, 44). As these chemokines were decreased in platelet-depleted hypoxic mice compared with platelet-replete hypoxic mice, we tested whether the expansion of pulmonary interstitial macrophages was prevented in hypoxic platelet-depleted mice. A key finding of our study is that platelet depletion prevents hypoxia-induced expansion of pulmonary interstitial macrophages. As the prevention of pulmonary interstitial macrophage expansion is protective in experimental models of PH, the impact of early platelet depletion on additional endpoints such as vascular remodeling and PH warrants further investigation (11, 12).
There are a few potential limitations to consider. Although we demonstrate that platelets are activated in mice exposed to hypoxia, the mechanism of hypoxia-mediated platelet activation remains unclear. Elucidation of the mechanisms by which hypoxia induces platelet activation has the potential to impact the development of treatments targeted at novel platelet activation pathways and warrants further investigation. Our data demonstrate significantly higher numbers of platelets within the lungs of mice exposed to hypoxia. However, at the time point studied, the number of circulating platelets were similar to controls (data not shown). As the lung is a known site for extramedullary platelet biogenesis, it is conceivable that increased platelets within the lungs of these mice is due to increased local production from resident lung megakaryocytes (45). It is also possible that circulating platelets are sequestered in the lung, but our assays are not sensitive enough to detect these subtle differences in circulating platelet number. In this study we examined whether hypoxia-induced platelet activation is associated with early inflammation. As platelet granules store both pro- and anti-inflammatory mediators and the immune modulatory functions of platelets are known to depend both on type and length of injury, it will be important in future studies to test whether prolonged platelet depletion is protective or promotes further injury.
In summary, we report that platelets of hypoxic mice are activated, and platelets are increased in the lungs of hypoxic mice and patients with advanced iPAH. In addition, our data demonstrate that hypoxia-induced increase in the chemokines CXCL4, CCL5, and CSF-2 is decreased in thrombocytopenic hypoxic mice. Finally, we demonstrate that expansion of pulmonary interstitial macrophages is prevented in thrombocytopenic mice. Although our findings do not elucidate how platelets may modulate inflammation, they lend support to a potential role of platelets in this process and warrant further investigation. We speculate that pharmacologic strategies targeting immune activation of platelets may provide novel treatment strategies for patients with PH.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants P01 HL152961 (to C.D., K.R.S., and R.M.T.); P01 HL014985 (to K.R.S.); K08 HL132041-01 (to C.D.); 1R35HL139726-01 (to E.S.N.); R33HL141794, R01HL151984, and R01HL120728 (to K.N.); R01HL120728 (to J.D.P.), R01HL141794 (to J.D.P.), and P01 HL144457 (to J.D.P).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.D. conceived and designed research; C.D., P.D.-C., and A.G. performed experiments; C.D. and P.D.-C. analyzed data; C.D., P.D.-C., and E.S.N. interpreted results of experiments; C.D. and J.P. prepared figures; C.D. drafted manuscript; C.D., J.P., A.G., K.N., A.A., R.M.T., K.R.S., and E.S.N. edited and revised manuscript; C.D., J.P., A.G., K.N., K.R.S., and E.S.N. approved final version of manuscript.
REFERENCES
- 1.Floren tin J, Coppin E, Vasamsetti SB, Zhao J, Tai YY, Tang Y, Zhang Y, Watson A, Sembrat J, Rojas M, Vargas SO, Chan SY, Dutta P. Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J Immunol 200: 3612–3625, 2018. doi: 10.4049/jimmunol.1701287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frid MG, McKeon BA, Thurman JM, Maron BA, Li M, Zhang H, Kumar S, Sullivan T, Laskowsky J, Fini MA, Hu S, Tuder RM, Gandjeva A, Wilkins MR, Rhodes CJ, Ghataorhe P, Leopold JA, Wang RS, Holers VM, Stenmark KR. Immunoglobulin-driven complement activation regulates proinflammatory remodeling in pulmonary hypertension. Am J Respir Crit Care Med 201: 224–239, 2020. doi: 10.1164/rccm.201903-0591OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugliese SC, Kumar S, Janssen WJ, Graham BB, Frid MG, Riddle SR, El Kasmi KC, Stenmark KR. A time- and compartment-specific activation of lung macrophages in hypoxic pulmonary hypertension. J Immunol 198: 4802–4812, 2017. doi: 10.4049/jimmunol.1601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994. [PMC free article] [PubMed] [Google Scholar]
- 6.Yu YRA, Malakhau Y, Yu CA, Phelan SJ, Cumming RI, Kan MJ, Mao L, Rajagopal S, Piantadosi CA, Gunn MD. Nonclassical monocytes sense hypoxia, regulate pulmonary vascular remodeling, and promote pulmonary hypertension. J Immunol 204: 1474–1485, 2020. doi: 10.4049/jimmunol.1900239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 897–908, 2012. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 8.Dorfmuller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb-Lhermine A, Marfaing-Koka A, Simonneau G, Emilie D, Humbert M. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 165: 534–539, 2002. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 151: 1628–1631, 1995. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol 298: H1235–H1248, 2010. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alterntive activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 123: 1986–1995, 2011. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubes P. The versatile platelet contributes to inflammation, infection, hemostasis, coagulation and cancer. Semin Immunol 28: 535, 2016. doi: 10.1016/j.smim.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Mezger M, Nording H, Sauter R, Graf T, Heim C, von Bubnoff N, Ensminger SM, Langer HF. Platelets and immune responses during thromboinflammation. Front Immunol 10: 1731, 2019. doi: 10.3389/fimmu.2019.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost 106: 827–838, 2011. doi: 10.1160/TH11-08-0592. [DOI] [PubMed] [Google Scholar]
- 16.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pao CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 136: 1330–1341, 2020. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest 119: 3450–3461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middleton EA, Weyrich AS, Zimmerman GA. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev 96: 1211–1259, 2016. doi: 10.1152/physrev.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hundelshausen P, Petersen F, Brandt E. Platelet-derived chemokines in vascular biology. Thromb Haemost 97: 704–713, 2007. doi: 10.1160/TH07-01-0066. [DOI] [PubMed] [Google Scholar]
- 20.Lam FW, Vijayan KV, Re R. Platelets and their interactions with other immune cells. Compr Physiol 5: 1265–1280, 2015. doi: 10.1002/cphy.c140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aytekin M, Aulak KS, Haserodt S, Chakravarti R, Cody J, Minai OA, Dweik RA. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: role of nitric oxide. Am J Physiol Lung Cell Mol Physiol 302: L512–L520, 2012. doi: 10.1152/ajplung.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer EM, Chanthaphavong RS, Sodhi CP, Hackam DJ, Billiar TR, Bauer PM. Genetic deletion of toll-like receptor 4 on platelets attenuates experimental pulmonary hypertension. Circ Res 114: 1596–1600, 2014. doi: 10.1161/CIRCRESAHA.114.303662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diehl P, Aleker M, Helbing T, Sossong V, Germann M, Sorichter S, Bode C, Moser M. Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. J Thromb Thrombolysis 31: 173–179, 2011. doi: 10.1007/s11239-010-0507-z. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Ohnuma N, Ohno K, Takasaki K, Okamoto S, Asai T, Okuda M, Nakamoto T, Iizuka M. Changes in sequestered leukocytes and platelets in the pulmonary microvasculature of rats with monocrotaline-induced pulmonary hypertension. Int J Microcirc 17: 290–297, 1997. doi: 10.1159/000179243. [DOI] [PubMed] [Google Scholar]
- 25.Shen T, Shi J, Wang N, Yu X, Zhang C, Li J, Wei L, Ma C, Zhao X, Lian M, Jiang C, Zhu D. 15-Lipoxygenase and 15-hydroxyeicosatetraenoic acid regulate intravascular thrombosis in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 309: L449–462, 2015. doi: 10.1152/ajplung.00004.2015. [DOI] [PubMed] [Google Scholar]
- 26.White SM, Roth RA. Pulmonary platelet sequestration is increased following monocrotaline pyrrole treatment of rats. Toxicol Appl Pharmacol 96: 465–475, 1988. doi: 10.1016/0041-008X(88)90006-3. [DOI] [PubMed] [Google Scholar]
- 27.White SM, Wagner JG, Roth RA. Effects of altered platelet number on pulmonary hypertension and platelet sequestration in monocrotaline pyrrole-treated rats. Toxicol Appl Pharmacol 99: 302–313, 1989. doi: 10.1016/0041-008X(89)90012-4. [DOI] [PubMed] [Google Scholar]
- 28.Berger G, Azzam ZS, Hoffman R, Yigla M. Coagulation and anticoagulation in pulmonary arterial hypertension. Isr Med Assoc J 11: 376–379, 2009. [PubMed] [Google Scholar]
- 29.Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, Ogo T, Tapson VF, Ghofrani HA, Jenkins DP. Chronic thromboembolic pulmonary hypertension. Eur Respir J 53: 1801915, 2019. doi: 10.1183/13993003.01915-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davizon-Castillo P, Allawzi A, Sorrells M, Fisher S, Baltrunaite K, Neeves K, Nozik-Grayck E, DiPaola J, Delaney C. Platelet activation in experimental murine neonatal pulmonary hypertension. Physiol Rep 8: e14386, 2020. doi: 10.14814/phy2.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Good RJ, Hernandez-Lagunas L, Allawzi A, Maltzahn JK, Vohwinkel CU, Upadhyay AK, Kompella UB, Birukov KG, Carpenter TC, Sucharov CC, Nozik-Grayck E. MicroRNA dysregulation in lung injury: the role of the miR-26a/EphA2 axis in regulation of endothelial permeability. Am J Physiol Lung Cell Mol Physiol 315: L584–L594, 2018. doi: 10.1152/ajplung.00073.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsujino T, Masuki H, Nakamura M, Isobe K, Kawabata H, Aizawa H, Watanabe T, Kitamura Y, Okudera H, Okuda K, Nakata K, Kawase T. Striking differences in platelet distribution between advanced-platelet-rich fibrin and concentrated growth factors: effects of silica-containing plastic tubes. J Funct Biomater 10: 43, 2019. doi: 10.3390/jfb10030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawada H, Saito T, Nickel NP, Alastalo TP, Glotzbach JP, Chan R, Haghighat L, Fuchs G, Januszyk M, Cao A, Lai YJ, Perez Vde J, Kim YM, Wang L, Chen PI, Spiekerkoetter E, Mitani Y, Gurtner GC, Sarnow P, Rabinovitch M. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J Exp Med 211: 263–280, 2014. doi: 10.1084/jem.20111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilt ZT, Pariser DN, Ture SK, Mohan A, Quijada P, Asante AA, Cameron SJ, Sterling JA, Merkel AR, Johanson AL, Jenkins JL, Small EM, McGrath KE, Palis J, Elliott MR, Morrell CN. Platelet-derived beta2M regulates monocyte inflammatory responses. JCI Insight 4: e122943, 2019. doi: 10.1172/jci.insight.122943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapur R, Zufferey A, Boilard E, Semple JW. Nouvelle cuisine: platelets served with inflammation. J Immunol 194: 5579–5587, 2015. doi: 10.4049/jimmunol.1500259. [DOI] [PubMed] [Google Scholar]
- 36.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmuller P, Guignabert C, Humbert M. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation 129: 1332–1340, 2014. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 37.Kuebler WM, Bonnet S, Tabuchi A. Inflammation and autoimmunity in pulmonary hypertension: is there a role for endothelial adhesion molecules? Pulm Circ 8: 2045893218757596, 2018. doi: 10.1177/2045893218757596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Lopez JA. Interactions of platelets with subendothelium and endothelium. Microcirculation 12: 235–246, 2005. doi: 10.1080/10739680590925484. [DOI] [PubMed] [Google Scholar]
- 39.King SM, McNamee RA, Houng AK, Patel R, Brands M, Reed GL. Platelet dense-granule secretion plays a critical role in thrombosis and subsequent vascular remodeling in atherosclerotic mice. Circulation 120: 785–791, 2009. doi: 10.1161/CIRCULATIONAHA.108.845461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng EM H, Comhair S, Erzurum S, Billiar TR, Bauer PM. Complement C3 deficiency attenuates chronic hypoxia-induced pulmonary hypertension in mice. PLoS One 6: e28578, 2011. doi: 10.1371/journal.pone.0028578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, Chatterjee T, Bajaj N, Sengupta S, Ganju L, Singh SB, Ashraf MZ. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood 123: 1250–1260, 2014. doi: 10.1182/blood-2013-05-501924. [DOI] [PubMed] [Google Scholar]
- 42.Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res 118: 1392–1408, 2016. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 43.Kasper B, Petersen F. Molecular pathways of platelet factor 4/CXCL4 signaling. Eur J Cell Biol 90: 521–526, 2011. doi: 10.1016/j.ejcb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 106: 1523–1529, 2002. doi: 10.1161/01.CIR.0000028590.02477.6F. [DOI] [PubMed] [Google Scholar]
- 45.Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegue E, Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544: 105–109, 2017. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]