Abstract
Genome-wide analyses in the last decade have uncovered the presence of a large number of long non-protein-coding transcripts that show highly tissue- and state-specific expression patterns. High-throughput sequencing analyses in diverse subsets of immune cells have revealed a complex and dynamic expression pattern for these long noncoding RNAs (lncRNAs) that correlate with the functional states of immune cells. Although the vast majority of lncRNAs expressed in immune cells remain unstudied, functional studies performed on a small subset have indicated that their state-specific expressions pattern frequently has a regulatory impact on the function of immune cells. In vivo and in vitro studies have pointed to the involvement of lncRNAs in a wide variety of cellular processes, including both the innate and adaptive immune response through mechanisms ranging from epigenetic and transcriptional regulation to sequestration of functional molecules in subcellular compartments. This review will focus mainly on the role of lncRNAs in CD4+ and CD8+ T cells, which play pivotal roles in adaptive immunity. Recent studies have pointed to key physiological functions for lncRNAs during several developmental and functional stages of the life cycle of lymphocytes. Although lncRNAs play important physiological roles in lymphocytic response to antigenic stimulation, differentiation into effector cells, and secretion of cytokines, their dysregulated expression can promote or sustain pathological states such as autoimmunity, chronic inflammation, cancer, and viremia. This, together with their highly cell type-specific expression patterns, makes lncRNAs ideal therapeutic targets and underscores the need for additional studies into the role of these understudied transcripts in adaptive immune response.
Keywords: CD4+ T cells, CD8+ T cells, immunoregulation, long noncoding RNAs, T cell differentiation
INTRODUCTION
Cells of the immune system have evolved to rapidly respond to various environmental cues, including molecular patterns that indicate the presence of invading pathogens and a diverse array of cytokines. Whereas the initial phases of the response are mediated through signaling cascades that operate largely through posttranslational modification of proteins, the final outcome of the majority of the signaling cascades is the induction of rapid, finely tuned transcriptomic changes (1–3). Long noncoding RNAs (lncRNAs), a diverse and largely unexplored class of cellular regulatory factors, are uniquely suitable to mediate the temporal and specificity requirements of the immune response (4–6). A role for lncRNAs as orchestrators of gene expression in the context of various branches of the immune system has become increasingly evident over the last decade (1–3). A large body of literature reports on the diverse roles played by lncRNAs in processes critical to immune cell function, such as metabolism, survival, proliferation, differentiation, and cytokine secretion in response to external stimuli (1, 2, 7). Indeed, many of these processes seem to be negatively or positively regulated by networks of lncRNAs acting at different levels (8–17). This review will focus on the most recent examples of the regulatory function of lncRNAs in CD4+ and CD8+ T lymphocytes, which play key roles in the adaptive immune response, and the way these regulatory events fit into the emerging picture of lncRNA-mediated immunoregulation.
lncRNAs AS A HETEROGENEOUS AND VERSATILE CLASS OF CELLULAR REGULATORS
lncRNAs are a highly heterogeneous group of transcripts that either completely lack protein-coding capacity or affect cellular processes through a mechanism that does not involve a protein product (4, 5, 18, 19). A large fraction of studied lncRNAs lack significant protein-coding capacity, and many are strictly nuclear, precluding a protein-mediated function (4, 6, 20, 21). Another subset of lncRNAs are translated into functional peptides; however, the RNA transcript itself affects cellular processes in a manner that is not dependent on its protein product (22–24). LncRNAs are highly heterogeneous in their length, ranging from tens of thousands of nucleotides to only a couple of hundred nucleotides or even fewer, and are defined as functional RNA molecules which are generally longer than and functionally distinct from small noncoding RNA classes such as tRNAs, snRNA, miRNAs, and snoRNAs.
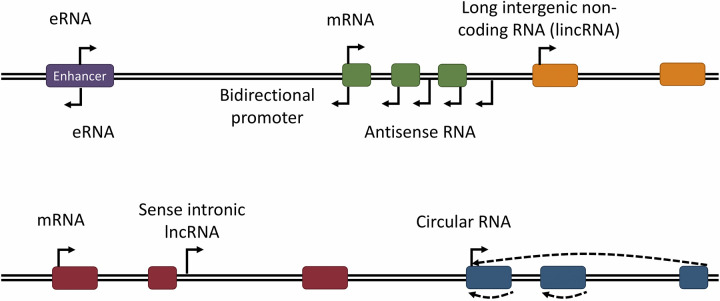
LncRNAs are also very diverse in terms of the way they affect cellular processes. A subset of lncRNAs, referred to as long intergenic noncoding RNAs (lincRNAs), are encoded by genes that do not overlap other known genic regions. However, many lncRNAs are transcribed from genes that overlap another gene in the sense or antisense direction or originate from genomic elements such as enhancers (Fig. 1) (16, 25, 26). In many studied cases, the genomic locus of a lncRNA determines its cellular impact. For example, lncRNAs that overlap another gene in the sense or antisense direction frequently impact the expression of the overlapped gene (5, 27, 28). Based on several studied cases, it has been proposed that lncRNAs transcribed from the locus of an enhancer element are necessary for the function of the enhancer (5, 6, 29). Existing evidence, in aggregate, indicates that lncRNAs play important roles in countless cellular processes through highly diverse molecular mechanisms. For example, through the act of being transcribed, lncRNAs can modify the local chromatin architecture and/or transcriptional activity of nearby genes (14, 16, 30). Similarly to all cellular RNAs, lncRNAs also form extensive interactions with proteins and may also interact with other RNAs or genomic DNA, altering key aspects of the function of these molecules, including their localization, structure, stability, and interactions with other factors (16, 26, 30–32). To date, close to 20,000 lncRNA genes are listed in reference genomic annotations, with a very small fraction functionally studied, but it has been estimated that mammalian genomes may encode as many as 100,000 lncRNAs (25, 31, 33). In general, lncRNAs are transcribed at lower copy numbers per cell than protein-coding RNAs but are more cell type and state specific than mRNAs (12, 26, 31, 32).
Figure 1.
Schematic representation of abundant long noncoding RNA (lncRNA) subtypes based on their transcriptional origin in the genome. Although long intergenic noncoding RNAs (lincRNAs) are transcribed from an independent noncoding gene, many lncRNAs are not. eRNAs are transcribed from enhancer sequences, frequently from bidirectional promoters in these DNA elements. Antisense RNAs overlap 1 or more genes in the opposite orientation and often originate from bidirectional promoters as well. Additional lncRNAs are transcribed from intronic sequences (sense intronic lncRNAs) or 3′-untranslated regions (UTRs). Circular RNAs are typically transcribed from protein-coding genes but undergo backsplicing to generate a circular transcript that may contain intronic, exonic, or both intronic and exonic sequences.
THE lncRNA TRANSCRIPTOME OF CD4+ AND CD8+ T CELLS
A plethora of lncRNAs with likely regulatory functions are expressed in CD4+ and CD8+ T cells. Ranzani et al. (34) performed RNA-seq in 13 different primary human lymphocyte subsets, including naïve, effector, and memory CD4+ and CD8+ T cells, and could detect the expression of 4,201 annotated and 563 unannotated lncRNAs in these cells. Pang et al. (35) generated a microarray expression data set in mouse naïve, memory, and effector CD8+ T cells utilizing 4,329 lncRNA-targeting probes and found that 1,106 lncRNAs were expressed. Ten percent of these lncRNAs were differentially expressed among the CD8+ T cell subsets examined, including 21 that changed in expression during naïve to memory cell differentiation, 81 that changed during effector cell activation, and four that were differentially expressed in both transitions. Many of the expressed lncRNAs were intergenic, but 16–22% overlapped with a protein-coding gene in the antisense direction (Fig. 1). In several instances, expressed lncRNA genes neighbored or overlapped with protein coding genes known to participate in CD8+ T cell function (35).
The lncRNA transcriptome of CD4+ and CD8+ T cells is highly dynamic, exhibiting major changes during development, differentiation, and activation. In the study by Ranzani et al. (34), 172 lncRNAs showed a difference in expression of 2.5-fold or more in one lymphocyte subset relative to the 12 others examined. When genes were classified according to their expression profiles by unsupervised K-means clustering, 73% of lncRNAs and only 34% of protein-coding genes were assigned to a cluster specific to an individual lymphocyte subset, consistent with the known, highly cell type-specific expression pattern of these transcripts. Strikingly, even membrane receptors, which are generally considered accurate markers of lymphocyte differentiation, showed less subtype specificity than lncRNAs when assessed in this way (34). To confirm that the lncRNAs differentially expressed in naïve and memory CD4+ T cells were truly naïve and memory specific, the authors assessed their expression using quantitative PCR in an independent set of naïve and ex vivo polarized T helper 1 (Th1) and T helper 2 (Th2) CD4+ T cell samples. As anticipated, lncRNAs associated with naïve T cells decreased in expression upon activation and polarization, whereas Th1 and Th2 signature lncRNAs were upregulated according to the polarizing conditions used.
lncRNAs AS KEY REGULATORS OF T CELL DIFFERENTIATION AND RESPONSE TO PATHOGENS
One of the first reported roles for lncRNAs in T cells was the fine-tuning of antigen-driven immune responses. lncRNAs have long been associated with regulation of epigenetic modifications (23, 36, 37), a process that is critical to the differentiation of T cells in response to pathogenic stimuli (38–41). T cells undergo major functional and phenotypic changes during the course of response to an infection and require highly context-specific epigenetic and transcriptional regulation, in which lncRNAs play key roles (38, 39, 42, 43).
In order to understand how the lncRNA transcriptome of CD8+ T cells respond to viral infection, Hudson et al. (31) performed RNA-seq in virus-specific CD8+ T cell subsets from mice infected with lymphocytic choriomeningitis virus (LCMV) and human volunteers given live attenuated YFV-17D yellow fever vaccine. In both mouse and human, ∼4,000 annotated and novel lncRNAs were expressed, with ∼800 showing differential expression throughout CD8+ T cell differentiation. Strikingly, principal component analysis indicated that naïve, effector, and memory CD8+ T cell populations could be distinguished based on lncRNA expression profile alone, suggesting a critical role for lncRNAs in defining these subsets. In further support of this idea, the authors reported that lncRNAs with synteny or sequence homology between mouse and human tended to exhibit similar changes in expression during CD8+ T cell differentiation (31). The findings of Hudson et al. (31) are consistent with previous reports of individual lncRNAs regulating T cell differentiation. For example, GATA3-AS1, which was found to be upregulated in CD4+ Th2 cells (44), is expressed from a genomic locus adjacent to GATA3, a master regulator of Th2 lineage commitment (44–46). Knockdown of GATA3-AS1 in human Th2 cells resulted in decreased expression of GATA3 and Th2-related genes under its control, including those encoding IL-5 and IL-13 (Table 1 and Fig. 2) (44). H3K27 acetylation and H3K4 di- and trimethylation at the GATA3 locus were also decreased in GATA3-AS1 knockdown cells. GATA3-AS1 showed binding to WDR5, a component of the MLL H3K4-methyltransferase complex. Thus, it is plausible that GATA3-AS1 contributes to the recruitment of this complex to the GATA3 locus, promoting maintenance of the open chromatin state (44).
Table 1.
List of the lncRNAs covered in this review and a brief description of their function
| lncRNA | Cell Type | Role | Ref. Nos. |
|---|---|---|---|
| ARIEL | CD4+ T cells | Positive regulator of TAL1 and MYC | 16, 26 |
| GATA3-AS1 | CD4+ Th2 | Positive regulator of GATA3 | 44 |
| HEAL | CD4+ T cells | Positive regulator of HIV replication | 25 |
| IFNG-AS1 | CD4+ Th1, CD8+ T effector | Positive regulator IFNγ production | 15, 47–49 |
| LINC01882 | CD4+ T cells | Negative regulator of KLF12 and the MAPK pathway | 10 |
| linc-MAF-4 | CD4+ T cells | Negative regulator of MAF | 17 |
| lnc-EGFR | CD4+ T cells | Positive regulator of FOXP3 | 13 |
| lnc-ITSN1-2 | CD4+ Th17 | Positive regulator of IL-23R | 50 |
| lnc-TIM3 | CD8+ T cells | Positive regulator of MDM2 and BCL2 | 12 |
| MALAT1 | CD4+ T cells | Negative regulator of epigenetic silencing at HIV proviral locus | 51 |
| Morrbid | CD8+ T cells | Negative regulator of PI3K-AKT | 14 |
| Morrbid | CD8+ T cells, CD4+ effector memory, CD4+ central memory | Positive regulator of Bcl2l11 | 14 |
| NEAT1 | CD4+ Th1, CD8+ T effector | Negative regulator of IFN-γ production | 9 |
| NEAT1 | CD4+ T cells | Negative regulator of HIV replication | 52 |
| NKILA | CD8+ T cells, CD4+ Th1 | Negative regulator of NF-κB | 11 |
| NRON | CD4+ T cells | Negative regulator of HIV proviral transcription | 53 |
| PVT1 | CD4+ T cells | Positive regulator of MYC | 8 |
| XIST | CD4+ T cells | Negative regulator of X-linked genes involved in cell cycle control | 54 |
| XLOC_000261 | CD4+ Th17 | Negative regulator of RORγt | 55 |
The T cell subset(s) in which each long noncoding RNA (lncRNA) has been reported to carry out the specified regulatory role(s) are shown. Morrbid and NEAT1 have multiple known functions that are listed as separate entries based on the T cell subset(s) studied in each case. Many of the described lncRNAs have additional functions in non-lymphocytic cells, which have not been included in this table. ARIEL, ARID5B-inducing enhancer-associated long noncoding RNA; Th2, T helper 2; Th1, T helper 1; Th17, T helper 17; PI3K, phosphatidylinositol 3-kinase.
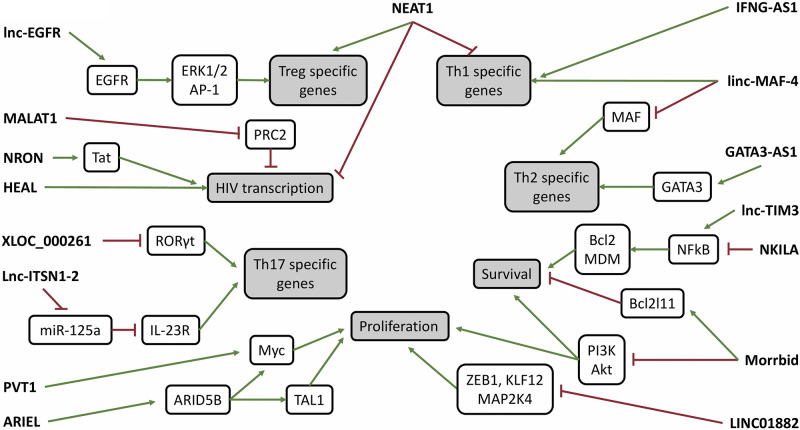
Figure 2.
Recently investigated long noncoding RNAs (lncRNAs) regulate processes critical to T cell function such as survival, proliferation, and lineage-specific gene expression. The lncRNAs act through distinct mechanisms at different levels within known signaling pathways, demonstrating their dynamic and versatile nature. Although many lncRNAs are known to act in a competitive or synergistic manner within a single system, further studies are required to understand the relationship between the lncRNAs shown in this schematic, as the summarized data were collected in different models under different conditions. ARIEL, ARID5B-inducing enhancer-associated long noncoding RNA; PI3K, phosphatidylinositol 3-kinase; Th1, T helper 1; Th2, T helper 2; Th17, T helper 17.
In addition to their role in antigen-stimulated T cell differentiation, lncRNAs have been shown to regulate CD4+ and CD8+ T cell effector functions. Secretion of IFNγ by CD4+ Th1 and CD8+ T effector cells is regulated by the lncRNA IFNG-AS1 (also known as Tmevpg1 and NeST) (Table 1 and Fig. 2) (15, 47–49). In mouse and human, the conserved IFNG-AS1 gene is positioned near to the IFNγ gene (IFNG). According to Gomez et al. (49), transgenic expression of IFNG-AS1 in mice is sufficient to increase CD8+ T cell IFNγ production and influence the outcome of Theiler’s virus and Salmonella enterica infections. Collier et al. (47) report that IFNG-AS1 overexpression contributes to but cannot independently induce upregulation of IFNG in primary mouse Th1 cells. In another study, Th1 cells from mice with a premature polyadenylation signal in the IFNG-AS1 gene showed a decrease in expression of IFNG RNA and a lower survival rate in response to Toxoplasma gondii infection (15). IFNG-AS1 binds to WDR5 and has been linked to epigenetic modifications at the IFNG locus, suggesting a regulatory mechanism that involves chromatin modifying complex recruitment (48, 49).
Another lncRNA, NEAT1, which plays a structural role in paraspeckles, has been implicated in negative regulation of IFNγ production and other processes involved in the immune response. Gast et al. (9) reported that expression of IFNG mRNA in unstimulated splenocytes of NEAT1−/− mice was 14-fold higher than those of wild-type mice. Elevated IFNγ levels could also be detected in the sera of NEAT1−/− mice. Additionally, NEAT1−/− mice exhibited an abnormally high ratio of T helper to T regulatory cells in the spleen and circulating blood (Table 1 and Fig. 2) (9). Further investigation is required to understand the function of this lncRNA in lymphocytes, but the preliminary data are intriguing.
In another study, Kotzin et al. (14) demonstrated an immunosuppressive role for the lncRNA Morrbid, which is induced by LCMV infection or T cell receptor stimulation in mouse CD8+ T cells. To investigate the role of this lncRNA, the authors transferred Morrbid−/− splenocytes to wild-type mice before infection with LCMV. Surprisingly, circulating CD8+ T cell count became abnormally high at the acute phase of the infection and beyond. Morrbid also appeared to suppress secretion of IFNγ, TNFα, and Granzyme B (14). Bcl2l11, an apoptosis-inducing factor required for CD8+ T cell contraction following viral clearance, had been identified previously as a downstream target of Morrbid in myeloid cells (56). Immunofluorescent staining in Morrbid−/− lymphocytes revealed that Morrbid is required for the upregulation of Bcl2l11 in CD8+ T cells, effector memory CD4+ T cells, and central memory CD4+ T cells following in vitro stimulation (14). Additionally, Morrbid was linked to negative regulation of phosphatidylinositol 3-kinase (PI3K)/Akt, a noncanonical type I interferon signaling pathway leading to proliferation, survival, and CD8+ T cell effector function (Table 1 and Fig. 2) (14, 57, 58).
One of the most highly expressed lncRNAs, MALAT1, is found in many cell types, including T cells, and has been linked to the regulation of diverse cellular processes such as metastasis, alternative splicing, epigenetic modification, synapse formation, and myogenesis. Kim et al. (59) showed that in T cell lymphoma tissues and cell lines, MALAT1 binds subunits of PRC2 and may facilitate H3K27 trimethylation by recruiting the complex to target genes. Somewhat surprisingly, although MALAT1 has been implicated in regulation of proliferation and differentiation of T cells (60, 61), it does not seem to play an essential role in the normal formation and function of CD4+ and CD8+ T cells in mice. LCMV-infected MALAT1−/− mice showed no defects in the development and differentiation of effector and memory CD8+ T cells and T follicular helper cells (30). Subtle functional changes due to alteration of T cell epigenetic or transcriptional state induced by MALAT1 ablation cannot be ruled out, as MALAT1−/− T cells were assessed only for surface marker expression and effector molecule production in this study. Taken together, the above studies point to crucial roles for lncRNAs in adaptive immune response, including regulatory functions in differentiation into effector cells and cytokine secretion. As the studied lncRNAs constitute a small fraction of lncRNAs showing changes in expression during T cell activation, it is certain that future studies will reveal many additional lncRNA-mediated functions in T cell and adaptive immune responses.
ROLE OF lncRNAs DURING THE INFECTION OF CD4+ T CELLS BY HIV
In addition to their role in mediating the function of T cells in launching the adaptive immune response, lncRNAs also play a role in the response of CD4+ T cells to being infected by HIV. The Human Immunodeficiency Virus, a lentivirus that primarily targets CD4+ T cells, is the cause of a major global health crisis, with more than 37.9 million people currently infected with the virus worldwide (62–64). Anti-retroviral therapy (ART) suppresses viral replication to undetectable levels and vastly improves the outlook of people living with HIV but fails to eliminate the latent reservoir, which mainly consists of a population of quiescent CD4+ T cells bearing transcriptionally silenced proviruses. The latent reservoir will rebound into transcriptional activation if ART is discontinued and can negatively affect the health and longevity of the patient even while under ART (65–67). The process by which HIV latency is established and maintained is not entirely clear (68–71). However, understanding this process may be key to the development of a cure, as the most promising strategies presented so far involve either permanently blocking the reversal of latency or reactivating HIV transcription in latently infected cells to make them vulnerable to clearance by immune surveillance and ART therapeutic agents. Although a few existing studies have identified potential roles for lncRNAs in HIV pathogenesis, cellular response to infection, and the formation of the silent reservoir, the role of lncRNAs in these processes has remained largely unexplored.
A recent high-throughput RNA sequencing study by Trypsteen et al. (72) in latently infected primary CD4+ T cells identified the global lncRNA transcriptome of latently infected cells for the first time. Using two different primary T cell models of HIV latency, the authors identified a number of differentially expressed lncRNAs, including PVT1 and RP110347C18.3, which were both significantly upregulated, and RP11-539L10.2, which was downregulated. Association analyses linked these lncRNAs to the proteasome, spliceosome, p53 signaling, and mammalian target of rapamycin (mTOR) pathways. Treatment of latently infected T cells with latency reversing agents Romidepsin or SAHA induced downregulation of the three identified lncRNAs, suggesting that the latency reversal agents affect the lncRNA transcriptome and thus may act at least in part via lncRNA modulation (72).
Another recent study identified the lncRNA HEAL to be upregulated in the PBMCs of HIV-infected individuals and in CD4+ T cells infected with HIV ex vivo (25). Importantly, depletion of HEAL was shown to inhibit HIV replication. It was proposed that HEAL, together with the RNA-binding protein FUS, binds to the HIV and CDK2 promoters, recruiting histone acetyltransferase p300 and enhancing transcription at these sites (Table 1 and Fig. 2) (25). Conversely, the lncRNAs NRON and NEAT1 have been reported to negatively impact HIV proviral transcription (52, 53). NRON is reported to be highly expressed in resting CD4+ T cells but decreases in expression upon activation (53). Knockdown of NRON in human embryonic kidney (HEK)-293T cells before transfection with an HIV proviral plasmid significantly increased expression of total HIV mRNA and HIV proteins Gag and Tat (53). Although the impact of NRON knockdown on HIV transcription in primary CD4+ T cells remains to be determined, these results are intriguing, as it has been previously reported that NRON regulates the NFAT signaling pathway (53, 73, 74). The HIV proviral promoter contains two NFAT binding sites (75–77), but they do not appear to be required for NRON-mediated transcriptional repression (53), and thus, the effect of NRON on HIV transcription may be indirect. Alternatively, NRON may promote the degradation of the HIV viral transactivator protein Tat (Table 1 and Fig. 2). As evidence of this, NRON was found to interact directly with Tat and ubiquitin/proteasome components CUL4B and PSMD11 (53).
In a recent study by Liu et al. (52), PBMCs from both healthy donors and HIV patients showed a decrease in expression of the lncRNA NEAT1 upon stimulation with the T cell-activating reagent phytohemagglutinin (PHA). Furthermore, HIV-infected, Jurkat-immortalized CD4+ T cells showed increased viral replication when NEAT1 was knocked out, suggesting that the lncRNA, which impacts the biogenesis of a subset of cellular RNAs (78–80), may exert a direct or indirect anti-viral effect (Table 1 and Fig. 2). It was proposed that the decrease in NEAT1 expression upon T cell stimulation contributes to the enhanced efficiency of HIV infection and replication in the activated state (52). Further investigation is required to elucidate the mechanism of NEAT1 action in this context. The study by Gast et al. (9) mentioned above showed that NEAT1 is an immunosuppressive factor that modulates the balance of T helper to T regulatory cells in the CD4+ T lymphocyte population. It is not uncommon for one lncRNA to play different roles in different systems or multiple roles within the same system (81–83). NEAT1, which is expressed in a wide variety of mammalian cell types and impacts gene expression through several different mechanisms, is known to be multifunctional (9, 84, 85). Interestingly, another group quantified NEAT1 mRNA in 60 HIV patient plasma samples and found that the levels were significantly lower than those in healthy individuals (86). There was no difference in NEAT1 between samples from ART-naïve and ART-suppressed donors, but there was a positive correlation between NEAT1 and CD4+ T cell count. The authors suggested that plasma NEAT1 level may be a useful biomarker for the diagnosis of HIV (86).
Additional evidence for lncRNA-mediated regulation of HIV expression is provided by Qu et al. (51) in a recent study showing a role for lncRNA MALAT1. In a high-throughput sequencing study in the CD4+ T cell line H9, MALAT1 was identified as the most upregulated lncRNA after HIV infection. Following up on this result, which was also confirmed in primary CD4+ T cells, the authors provide data indicating that MALAT1 facilitated the expression of HIV provirus by binding to PRC2 complex and displacing it from the HIV promoter, thus preventing the epigenetic silencing of HIV (Table 1 and Fig. 2) (51). The above results in aggregate paint a complex picture in which lncRNAs directly or indirectly impact the expression of the HIV provirus in a positive or negative manner, underscoring a critical role for lncRNAs in the antiviral response (87–91).
IMPACT OF lncRNAs ON IMMUNE DYSREGULATION
A growing number of studies have linked dysregulation of lncRNAs in immune cells to pathological immune responses, including autoimmunity, chronic inflammation, and aberrant immune cell proliferation, migration, invasion, and apoptosis (2, 92, 93). A variety of strategies have been used in recent years to narrow in on relevant lncRNAs and identify their functions in the context of immune dysregulation (8, 10, 17, 32, 54).
In a study by Zhang et al. (17), PBMCs from patients with multiple sclerosis (MS) showed significant upregulation of linc-MAF-4. Furthermore, expression of this lncRNA correlated with the annual relapse rate of the disease. Linc-MAF-4 was named for the strong negative correlation in its expression with that of MAF, a Th2 transcription factor expressed 56.6 kilobases upstream. The authors knocked down or overexpressed linc-MAF-4 in naïve CD4+ T cells from MS patients and saw a respective increase or decrease in the expression of MAF, suggestive of a regulatory relationship between the two factors. Additionally, linc-MAF-4 overexpression promoted ex vivo differentiation of naïve CD4+ T cells from MS patients into the Th1 effector subset and inhibited ex vivo differentiation of these cells into the Th2 effector subset (Table 1 and Fig. 2) (17). Expansion of Th1 effector cells has been associated with the pathogenesis of MS (94–97), whereas expansion of Th2 effector cells has been shown to decrease MS severity in a mouse model (98–101). The existing evidence in aggregate suggests that linc-MAF-4 promotes MS by inhibiting the expression of MAF, skewing CD4+ T cell differentiation toward the Th1 lineage (17).
Crohn’s disease and ulcerative colitis are chronic inflammatory diseases of the gastrointestinal tract that, like multiple sclerosis, are associated with T cell lineage imbalance. These two diseases, which are both commonly referred to as inflammatory bowel disease (IBD), are characterized by overexpression of T helper 17 (Th17)-specific genes in CD4+ T cells of the gut lamina propria (102–104). A novel lncRNA has recently been implicated in regulation of Th17 differentiation and lineage-specific gene expression in the context of IBD. The lncRNA, referred to as XLOC_000261, was first identified by Braga-Neto et al. (55) in a transcriptomic study of lamina propria CD4+ T cells from Crohn’s disease patients. The authors noted that expression of lncRNAs and adjacent protein-coding genes tended to correlate across the 22 samples in the study. XLOC_000261 and the downstream transcription factor BATF were among the most significantly upregulated gene pairs. The authors measured XLOC_000261 in in vitro polarized T regulatory (Treg), Th1, Th2, and Th17 CD4+ T cells and found that the lncRNA was enriched in the Th17 subset. Knockdown of XLOC_000261 in CD4+ T cells following Th17 polarization increased the RORγt/RORC-expressing fraction of the population detected by flow cytometry. RORγt is a Th17-defining transcription factor that controls Th17 differentiation and expression of proinflammatory cytokines IL-17A and IL-17F (105–107). The lncRNA appears to negatively regulate RORγt/RORC, seemingly at odds with the finding that XLOC_000261 is upregulated in lamina propria CD4+ T cells of Crohn’s disease patients (Table 1 and Fig. 2) (55). XLOC_000261 may be part of a negative feedback mechanism triggered by an excess of proinflammatory cytokines in the T cell environment. Whether the influence of XLOC_000261 on RORγt involves cis-regulation of the downstream transcription factor BATF or another mechanism is unclear.
Lnc-ITSN1-2, a second IBD-associated lncRNA, has been shown to regulate Th17 differentiation on another level (50). Nie and Zhao (50) report that lnc-ITSN1-2 is strongly upregulated in the intestinal mucosa and PBMCs of IBD patients relative to healthy controls and is predictive of disease severity. Knockdown or overexpression of lnc-ITSN1-2 in CD4+ T cells isolated from IDB patients leads to a respective decrease or increase in expression of Th1- and Th17-specific genes, including IFNγ, TNFα, IL-17, and RORC. The IL-23 receptor IL-23R was also downregulated by lnc-ITSN1-2 knockdown and upregulated by lnc-ITSN1-2 overexpression. IL-23R has been implicated in the etiology of IBD, as IL-23 signaling is a key inducer of Th17 differentiation and cytokine production (104, 108, 109). Using the miRanda database, Nie and Zhao (50) identified miR-125a as a potential lnc-ITSN1-2 binding partner and confirmed the binding relationship in a luciferase reporter assay. Expression of Th1- and Th17-specific genes in lnc-ITSN1-2 knockdown cells could be rescued at least partially by knockdown of miR-125a, suggesting that lnc-ITSN1-2 exerts its effect on Th1 and Th17 differentiation via negative regulation of miR-125a. Through additional rescue experiments, the authors were able to show a direct regulatory relationship between miR-125a and IL-23R. These data are consistent with a model in which lnc-ITSN1-2 acts as a “molecular sponge” for miR-125a, preventing it from targeting IL-23R and potentially other genes involved in Th1- and Th17-specific gene expression (Table 1 and Fig. 2) (50).
Autoimmune diseases are often linked to single nucleotide polymorphisms (SNPs) in specific genomic loci. Surprisingly, many of the SNPs that have been identified are located in noncoding regions (110–114). Teimuri et al. (32) recently proposed that autoimmune disease-associated SNPs influence the expression of lncRNAs encoded nearby, which then regulate protein coding genes and miRNAs involved in pathogenesis of the autoimmune conditions. The authors identified eight lncRNAs suspected to play a role in MS based on a set of selection criteria and then measured their expression in PBMCs from MS patients versus healthy controls. One of the candidates with the most significant differential expression was AC009948.5, a lncRNA selected for its genomic proximity to an MS-associated SNP and its potential to base pair with an MS-associated miRNA (32). Although these observations are intriguing, the proposed function of AC009948.5 has not yet been experimentally confirmed. However, similar approaches, combined with existing functional annotations and coexpression databases can be highly fruitful in identification of suitable candidates for in-depth study.
In the same vein, Houtman et al. (10) reported that differential expression of LINC01882, a lncRNA near to an SNP in the PTPN2 locus, may explain the link between the SNP and the associated autoimmune disease. The authors showed a strong correlation between PTPN2 risk variants and decreased expression of LINC01882 in whole blood of both healthy individuals and rheumatoid arthritis patients. Knockdown of LINC01882 in Jurkat-immortalized CD4+ T cells resulted in downregulation of genes with a range of different roles, including KLF12, a transcriptional repressor with unclear impact on lymphocytes (115), ZEB2, a transcription factor that promotes terminal differentiation of CD8+ T cells (116, 117), and MAP2K4, a kinase involved in antigen, costimulatory, and death receptor signaling (118, 119). Further investigation is required to elucidate the relationship between LINC01882, the PTPN2 locus SNP, and autoimmune disease (Table 1 and Fig. 2) (10).
Another lncRNA with a potential role in development of autoimmune conditions is PVT1, which is elevated in the PBMCs of patients with Sjogren’s syndrome (8). Sjogren’s syndrome is an autoimmune disease characterized by hyperproliferation and hyperactivation of CD4+ T cells in salivary gland tissue. Fu et al. (8) reported that knockdown of PVT1 in Jurkat-immortalized CD4+ T cells and mouse primary CD4+ T cells attenuated the expression of cell cycle markers and glycolytic proteins in response to phorbol myristate acetate (PMA) and ionomycin or anti-CD3 and anti-CD28 stimulation. Among the attenuated cell cycle markers was MYC, a key regulator of metabolic reprogramming and cellular proliferation in T cells, which is encoded from a locus adjacent to PVT1 (Table 1 and Fig. 2) (120–122). PVT1 is a well-studied lncRNA and has been reported to control oncogenesis via MYC in a wide variety of cancers (123–125), but Fu et al. (8) were among the first to demonstrate a role for PVT1 and MYC in the pathogenesis of Sjogren’s syndrome.
Perhaps the most studied of all lncRNAs is XIST, which is transcribed from a locus on the X chromosome and plays a key role in X inactivation and dosage compensation in females (126–128). Syrett et al. (54) used fluorescence in situ hybridization (FISH) to show that the lncRNA XIST is aberrantly expressed and localized in T cells from systemic lupus erythematosus (SLE) patients. XIST is known to mediate dosage compensation of X-linked genes in female primates by recruiting chromatin-silencing factors to the inactive X chromosome (Xi) (126–128). Normally, XIST is undetectable via FISH in naïve CD4+ and CD8+ T cells but increases in expression and becomes tightly localized to Xi upon activation. In T cells from SLE patients, however, XIST fails to fully localize to the X chromosome upon activation. Analysis of RNA-seq data from peripheral CD4+ T cells of SLE patients revealed that many X-linked genes were overexpressed, including several involved in metabolism, cell cycle control, proliferation, cell division, and splicing (Table 1). These data point to dysregulation of XIST and resulting errors in dosage compensation as a potential driver of SLE. Consistent with this model, SLE predominantly affects women and others with duplicated X chromosomes (54). The studies discussed above in aggregate point to multiple critical steps at which inappropriate expression of lncRNAs can induce or potentiate pathological immune responses through dysregulation of immune cell activation, cytokine secretion, and effector subset imbalance. Together with the intriguing association of multiple lncRNAs with disease-related SNPs, it is likely that many additional lncRNAs impact the development of autoimmune diseases and thus constitute novel therapeutic targets.
lncRNA INVOLVEMENT IN CANCER IMMUNOSURVEILLANCE
Cancer immunosurveillance, in which cancer cells are identified and destroyed by antigen-specific CD8+ T cells, is one of the main protective mechanisms against tumorigenesis. Factors in the tumor microenvironment often lead to apoptosis or exhaustion of infiltrating T cells, compromising anti-tumor immunity (11, 12, 129). lncRNAs are known to orchestrate many aspects of the innate and adaptive immune response, but the study of their role in cancer immunosurveillance is a relatively new frontier.
Tregs normally suppress various aspects of the immune response to maintain self-tolerance and immune homeostasis, but within the tumor microenvironment they can be co-opted by cancer cells to facilitate immune evasion. Jiang et al. (13) report that in the tumor infiltrating CD4+ T cells of hepatocellular carcinoma patients, an upregulated lncRNA that they name lnc-EGFR promotes Treg differentiation, leading to the suppression of CD8+ effector T cell activity. In a mouse model of liver cancer, lnc-EGFR expression correlated positively with tumor size and expression of the Treg marker Foxp3 but was negatively correlated with expression of the CD8+ T cell marker IFNγ. The authors proposed that lnc-EGFR binds and blocks ubiquitination of the EGFR protein (Table 1 and Fig. 2). Stabilized EGFR activates ERK1/2 and AP-1, triggering AP1-dependent lnc-EGFR and Foxp3 expression, thus promoting Treg differentiation in addition to creating a positive feedback loop (13).
Two groups identified NF-κB-regulating lncRNAs, which impede cancer immunosurveillance, although the mechanism proposed for lncRNA-mediated NF-κB regulation in each case was distinct. Ji et al. (12) reported that lnc-TIM3 promotes exhaustion in tumor-infiltrating CD8+ T cells by inhibiting the binding of immune checkpoint molecule TIM-3/HAVCR2 with ligand BAT3. TIM-3/BAT3 binding represses T cell exhaustion, whereas free BAT3 promotes the expression of NF-κB targets MDM2 and BCL2, allowing exhausted CD8+ T cells to survive (12) (Table 1 and Fig. 2). Another example of lncRNAs involved in immune surveillance was provided by Huang et al. (11), who showed that the lncRNA NKILA promotes apoptosis in tumor-infiltrating CD8+ T cells and CD4+ Th1 cells by inhibiting NF-κB and its anti-apoptotic target genes via interaction with IκBα (Table 1 and Fig. 2). The authors proposed that NKILA-mediated apoptosis of CD8+ T cells and CD4+ Th1 cells in the tumor microenvironment leads to the preferential accumulation of immunosuppressive CD4+ Treg and CD4+ Th2 cells, a phenomenon that is known to contribute to tumor immune evasion (11). Several studies have proposed additional functions for NKILA in cancer and chronic inflammatory conditions (130, 131).
More recently, Wang et al. (129) demonstrated for the first time that exhausted CD8+ T cells may be able to promote an exhausted phenotype in other CD8+ T cells within the tumor microenvironment via exosome-associated lncRNAs. Exosomes isolated from exhausted CD8+ T cells were taken up by nonexhausted CD8+ T cells and resulted in decreased IFNγ and IL-2 production and increased PD-1 and TIM-3 expression, all markers of exhaustion. Furthermore, microarray analysis identified a large number of lncRNAs that were differentially expressed in exhausted and nonexhausted CD8+ T cell exosomes. The differentially expressed lncRNAs were significantly enriched in gene ontology terms “metabolism” and “biosynthetic process,” suggesting that some may play a functional role in the establishment of exhaustion (129). These findings could represent a significant step in our understanding of tumor-immune evasion and provide a foundation for future studies of the role of lncRNAs in the critical process of immune surveillance.
ROLE OF lncRNAs IN T CELL ACUTE LYMPHOBLASTIC LEUKEMIA
Although lncRNAs play a critical role in mediating aspects of the function of CD8+ T cells in identification and clearance of cancer cells of diverse origin, they are also involved in the pathogenesis of cancer within T cells. T cell acute lymphoblastic leukemia (T-ALL) is characterized by both uncontrolled proliferation and arrested differentiation of T cells (132–134). There are several T-ALL subtypes that display dysregulation of different cellular pathways (133–135). In 40–60% of all cases, genes contributing to T-ALL pathogenesis are activated by the oncogenic transcription factor TAL1 (26). In 30% of adult and 5–10% of pediatric T-ALL cases, the overexpression of transcription factor TLX1 drives the malignancy (133). Regardless of subtype, most T-ALL cases display constitutive activation of the NOTCH-1 signaling pathway. Early studies on T-ALL-associated lncRNAs focused on those induced by NOTCH-1, and until recently, subtype-specific changes in the noncoding transcriptome had not been profiled (16, 26, 134).
The study by Ngoc et al. (26) was one of the first to compare lncRNA expression among T-ALL subtypes using high-throughput RNA sequencing. The authors found that elevated expression of a number of lncRNAs, including lnc-FAM160A1-6, was specific to TAL1-positive cases. To investigate the potential role of TAL1 in the dysregulation of lncRNAs, the authors knocked down TAL1 in Jurkat T cells, which are a TAL1-positive T-ALL cell line. Among the significantly downregulated lncRNAs, five were expressed within regions of clustered transcription activator binding sites known as super-enhancers (26). Super-enhancer-associated lncRNAs have been found to play a role in the enhancer-mediated regulation of pivotal downstream genes (136–138). Intriguingly, one of the five super-enhancer-associated lncRNAs was expressed upstream of ARID5B, a gene that has been reported to positively regulate TAL1 and the growth-promoting transcription factor MYC (26). This lncRNA, which was subsequently named ARID5B-inducing, enhancer-associated, long noncoding RNA (ARIEL), was investigated further in a second paper from the same group. The authors reported that ARIEL promotes expression of ARID5B and its regulatory targets by recruiting mediator proteins to the upstream super-enhancer (Table 1 and Fig. 2), thus creating a positive feedback loop. As evidence of ARIEL’s oncogenic function, ARIEL knockdown prevented growth of T-ALL cells in culture and in mouse xenografts (16).
Another effort at understanding the lncRNA transcriptome of T-ALL was undertaken by Verboom et al. (133), who also used high-throughput transcriptome sequencing to compare T-ALL subsets, focusing on lncRNAs with expression specific to TLX1-positive T-ALL cases. The authors identified 442 known and 158 novel lncRNAs that met this description. Upon knockdown of TLX1 in T-ALL cells displaying TLX1 overexpression, the authors noted that lncRNAs were predominantly downregulated, whereas protein-coding genes were predominantly upregulated. Nearly one-third of the TLX1-induced lncRNAs showed evidence of TLX1 binding to the vicinity of their genomic loci based on ChIP-seq studies. Furthermore, TLX1 binding was significantly enriched among super-enhancer associated, TLX1-induced lncRNAs (133). Taken together, existing evidence suggests that the distinct set of lncRNAs upregulated in the two major subtypes of T-ALL both play potential roles in the pathogenesis of the disease and in some cases may mediate this effect by regulating the activity of super-enhancers. In addition, these studies highlight the critical role played by lncRNAs in cellular networks regulating proliferation and transcriptional regulation in T cells.
CONCLUSIONS
Although the protein-based signaling pathways that regulate the transcriptional cascades during the immune response are well studied, exploration of the role of lncRNAs as regulatory molecules in the immune response is in its infancy. There are many unknowns surrounding this novel class of transcripts and their function. For example, lncRNA sequences are generally not well conserved across species (20, 139, 140). At first, this raised doubts about the ability of lncRNAs to mediate biological function. However, subsequent studies proved that lack of phylogenetic conservation is not an indicator of lack of function (139–142). One possible explanation is that many of the regulatory roles of lncRNAs emerged relatively recently in evolutionary history. Indeed, a large fraction of human lncRNAs is specific to primates and not found in lower organisms (20, 143, 144). Considering the critical roles played by the small number of studied lncRNAs in immune response, the recent evolutionary origin of lncRNAs can be extremely important in understanding the evolution of the immune response. Recent evidence has raised an alternative explanation for the lack of sequence conservation in lncRNAs, as it has been proposed that the three-dimenstional (3D) structure of lncRNAs is more conserved and may be more important to lncRNA function (28, 141, 145). The role of secondary and tertiary structure elements in lncRNA function has been largely overlooked due to the lack of appropriate tools for study. However, with recent progress in the development of appropriate tools (146–149), defining the role of 3D structure in the function of lncRNAs will be a major area of progress in the near future. In either case, study of the role of lncRNAs in immune cell function will provide a novel perspective in regulation and evolution of the immune response.
New molecular and computational tools for the study of cellular RNAs are constantly being introduced, many of which can be adapted for analysis of low-expression level RNAs, a category that encompasses the majority of lncRNAs. These include new methods for assessing lncRNA sequence conservation (150), fluorescence-based CRISPR-Cas13 techniques for tracking the cellular location of lncRNAs in real time (151), and single-cell approaches for defining the impact of loss of function of cellular RNAs (152) and mapping RNA/protein interactions (153). Perhaps one of the most critical remaining obstacles to in-depth study of lncRNAs is the extremely low representation of lncRNAs in single-cell transcriptomic studies, which stems from the low level of expression of most lncRNAs. Overcoming this technical limitation in the coming years is likely to open the door to a much more comprehensive understanding of the function of this highly abundant and understudied class of cellular transcripts.
GRANTS
This work was supported in part by National Institutes of Health Grants CFAR P30-AI036219, 1R01AI120204-01, and 1R21AI127252-01 (to. S.V.), and 5T32AI127201-4 (to L.M.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M.P. prepared figures; L.M.P. drafted manuscript; S.V. edited and revised manuscript; L.M.P. and S.V. approved final version of manuscript.
REFERENCES
- 1.Ahmad I, Valverde A, Ahmad F, Naqvi AR. Long noncoding RNA in myeloid and lymphoid cell differentiation, polzartization, and function. Cells 9: 269, 2020. doi: 10.3390/cells9020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YG, Satpathy AT, Chang HY. Gene regulation in the immune system by long noncoding RNAs. Nat Immunol 18: 962–972, 2017. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Cao X. Long noncoding RNAs in innate immunity. Cell Mol Immunol 13: 138–147, 2016. doi: 10.1038/cmi.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonel P, Guttman M. Approaches for understanding the mechanisms of long noncoding RNA regulation of gene expression. Cold Spring Harb Perspect Biol 11: a032151, 2019. doi: 10.1101/cshperspect.a032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair L, Chung H, Basu U. Regulation of long non-coding RNAs and genome dynamics by the RNA surveillance machinery. Nat Rev Mol Cell Biol 21: 123–136, 2020. doi: 10.1038/s41580-019-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raj A, Rinn JL. Illuminating genomic dark matter with RNA imaging. Cold Spring Harb Perspect Biol 11: a032094, 2019. doi: 10.1101/cshperspect.a032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P. The opening of Pandora’s Box: an emerging role of long noncoding RNA in viral infections. Front Immunol 9: 3138, 2019. doi: 10.3389/fimmu.2018.03138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu J, Shi H, Wang B, Zhan T, Shao Y, Ye L, Wu S, Yu C, Zheng L. LncRNA PVT1 links Myc to glycolytic metabolism upon CD4+ T cell activation and Sjögren’s syndrome-like autoimmune response. J Autoimmun 107: 102358, 2020. doi: 10.1016/j.jaut.2019.102358. [DOI] [PubMed] [Google Scholar]
- 9.Gast M, Rauch BH, Haghikia A, Nakagawa S, Haas J, Stroux A, Schmidt D, Schumann P, Weiss S, Jensen L, Kratzer A, Kraenkel N, Müller C, Börnigen D, Hirose T, Blankenberg S, Escher F, Kühl AA, Kuss AW, Meder B, Landmesser U, Zeller T, Poller W. Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc Res 115: 1886–1906, 2019. doi: 10.1093/cvr/cvz085. [DOI] [PubMed] [Google Scholar]
- 10.Houtman M, Shchetynsky K, Chemin K, Hensvold AH, Ramsköld D, Tandre K, Eloranta ML, Rönnblom L, Uebe S, Catrina AI, Malmström V, Padyukov L. T cells are influenced by a long non-coding RNA in the autoimmune associated PTPN2 locus. J Autoimmun 90: 28–38, 2018. doi: 10.1016/j.jaut.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Chen J, Yang L, Ouyang Q, Li J, Lao L, Zhao J, Liu J, Lu Y, Xing Y, Chen F, Su F, Yao H, Liu Q, Su S, Song E. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat Immunol 19: 1112–1125, 2018. doi: 10.1038/s41590-018-0207-y. [DOI] [PubMed] [Google Scholar]
- 12.Ji J, Yin Y, Ju H, Xu X, Liu W, Fu Q, Hu J, Zhang X, Sun B. Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis 9: 478, 2018. doi: 10.1038/s41419-018-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, Wang K, Jia W, Chu WM, Sun B. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun 8: 15129, 2017. doi: 10.1038/ncomms15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotzin JJ, Iseka F, Wright J, Basavappa MG, Clark ML, Ali MA, Abdel-Hakeem MS, Robertson TF, Mowel WK, Joannas L, Neal VD, Spencer SP, Syrett CM, Anguera MC, Williams A, Wherry EJ, Henao-Mejia J. The long noncoding RNA morrbid regulates CD8 T cells in response to viral infection. Proc Natl Acad Sci U S A 116: 11916–11925, 2019. doi: 10.1073/pnas.1819457116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petermann F, Pękowska A, Johnson CA, Jankovic D, Shih HY, Jiang K, Hudson WH, Brooks SR, Sun HW, Villarino AV, Yao C, Singleton K, Akondy RS, Kanno Y, Sher A, Casellas R, Ahmed R, O’Shea JJ. The magnitude of IFN-γ responses is fine-tuned by DNA Architecture and the non-coding transcript of Ifng-as1. Mol Cell 75: 1229–1242.e5, 2019. doi: 10.1016/j.molcel.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan SH, Leong WZ, Ngoc PCT, Tan TK, Bertulfo FC, Lim MC, An O, Li Z, Yeoh AEJ, Fullwood MJ, Tenen DG, Sanda T. The enhancer RNA ARIEL activates the oncogenic transcriptional program in T-cell acute lymphoblastic leukemia. Blood 134: 239–251, 2019. doi: 10.1182/blood.2018874503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Liu G, Wei C, Gao C, Hao J. Linc-MAF-4 regulates Th1/Th2 differentiation and is associated with the pathogenesis of multiple sclerosis by targeting MAF. FASEB J Off Publ Fed Am Soc Exp Biol 31: 519–525, 2017. doi: 10.1096/fj.201600838R. [DOI] [PubMed] [Google Scholar]
- 18.Deveson IW, Hardwick SA, Mercer TR, Mattick JS. The Dimensions, Dynamics, and Relevance of the Mammalian Noncoding Transcriptome. Trends Genet TIG 33: 464–478, 2017. doi: 10.1016/j.tig.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17: 47–62, 2016. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 20.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22: 1775–1789, 2012. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, , et al. Landscape of transcription in human cells. Nature 489: 101–108, 2012. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubé F, Francastel C. Coding and non-coding RNAs, the frontier has never been so blurred. Front Genet 9: 140, 2018. doi: 10.3389/fgene.2018.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumari P, Sampath K. cncRNAs: Bi-functional RNAs with protein coding and non-coding functions. Semin Cell Dev Biol 47-48: 40–51, 2015[Erratum in Semin Cell Dev Biol 53: 168, 2016]. doi: 10.1016/j.semcdb.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulveling D, Francastel C, Hubé F. When one is better than two: RNA with dual functions. Biochimie 93: 633–644, 2011. doi: 10.1016/j.biochi.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Chao T-C, Zhang Q, Li Z, Tiwari SK, Qin Y, Yau E, Sanchez A, Singh G, Chang K, Kaul M, Karris MAY, Rana TM. The long noncoding RNA HEAL regulates HIV-1 replication through epigenetic regulation of the HIV-1 promoter. mBio 10, 2019. doi: 10.1128/mBio.02016-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngoc PCT, Tan SH, Tan TK, Chan MM, Li Z, Yeoh AEJ, Tenen DG, Sanda T. Identification of novel lncRNAs regulated by the TAL1 complex in T-cell acute lymphoblastic leukemia. Leukemia 32: 2138–2151, 2018. doi: 10.1038/s41375-018-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol 10: 637–643, 2009. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Froberg JE, Lee JT. Long noncoding RNAs: fresh perspectives into the RNA world. Trends Biochem Sci 39: 35–43, 2014. doi: 10.1016/j.tibs.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam MT, Li W, Rosenfeld MG, Glass CK. Enhancer RNAs and regulated transcriptional programs. Trends Biochem Sci 39: 170–182, 2014. doi: 10.1016/j.tibs.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Y, Guo W, Chen J, Guo P, Yu G, Liu J, Wang F, Liu J, You M, Zhao T, Kang Y, Ma X, Yu S. Long noncoding RNA Malat1 is not essential for T cell development and response to LCMV infection. RNA Biol 15: 1477–1486, 2018. doi: 10.1080/15476286.2018.1551705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson WH, Prokhnevska N, Gensheimer J, Akondy R, McGuire DJ, Ahmed R, Kissick HT. Expression of novel long noncoding RNAs defines virus-specific effector and memory CD8+ T cells. Nat Commun 10: 196, 2019. doi: 10.1038/s41467-018-07956-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teimuri S, Hosseini A, Rezaenasab A, Ghaedi K, Ghoveud E, Etemadifar M, Nasr-Esfahani MH, Megraw TL. Integrative analysis of lncRNAs in Th17 cell lineage to discover new potential biomarkers and therapeutic targets in autoimmune diseases. Mol Ther Nucleic Acids 12: 393–404, 2018. doi: 10.1016/j.omtn.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, , et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res 47: D766–D773, 2019. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranzani V, Rossetti G, Panzeri I, Arrigoni A, Bonnal RJ, Curti S, Gruarin P, Provasi E, Sugliano E, Marconi M, De Francesco R, Geginat J, Bodega B, Abrignani S, Pagani M. The long intergenic noncoding RNA landscape of human lymphocytes highlights the regulation of T cell differentiation by linc-MAF-4. Nat Immunol 16: 318–325, 2015. doi: 10.1038/ni.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang KC, Dinger ME, Mercer TR, Malquori L, Grimmond SM, Chen W, Mattick JS. Genome-wide identification of long noncoding RNAs in CD8+ T cells. J Immunol 182: 7738–7748, 2009. doi: 10.4049/jimmunol.0900603. [DOI] [PubMed] [Google Scholar]
- 36.Boon RA, Jaé N, Holdt L, Dimmeler S. Long noncoding RNAs: from clinical genetics to therapeutic targets? J Am Coll Cardiol 67: 1214–1226, 2016. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 37.Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet 16: 71–84, 2015. doi: 10.1038/nrg3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durek P, Nordström K, Gasparoni G, Salhab A, Kressler C, de Almeida M, DEEP Consortium, , et al. Epigenomic profiling of human CD4+ T cells supports a linear differentiation model and highlights molecular regulators of memory development. Immunity 45: 1148–1161, 2016. doi: 10.1016/j.immuni.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Gaballa JB, Neto MB, Ramos GP, Bamidele AO, Gonzalez MM, Sagstetter MR, Sarmento OF, Faubion WA Jr.. The Role of Histone Methyltransferases and Long Non-coding RNAs in the Regulation of T Cell Fate Decisions. Front Immunol 9: 2955, 2018. doi: 10.3389/fimmu.2018.02955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henning AN, Roychoudhuri R, Restifo., NP. Epigenetic control of CD8+ T cell differentiation. Nat Rev Immunol 18: 340–356, 2018. doi: 10.1038/nri.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yerinde C, Siegmund B, Glauben R, Weidinger C. Metabolic Control of Epigenetics and Its Role in CD8+ T Cell Differentiation and Function. Front Immunol 10: 2718, 2019. doi: 10.3389/fimmu.2019.02718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panzeri I, Rossetti G, Abrignani S, Pagani M. Long intergenic non-coding RNAs: novel drivers of human lymphocyte differentiation. Front Immunol 6: 175, 2015. doi: 10.3389/fimmu.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valadkhan S, Plasek LM. Long non-coding RNA-mediated regulation of the interferon response: a new perspective on a familiar theme. PAI 3: 126–148, 2018. doi: 10.20411/pai.v3i1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibbons HR, Shaginurova G, Kim LC, Chapman N, Spurlock CF 3rd, Aune TM. Divergent lncRNA GATA3-AS1 regulates GATA3 transcription in T-helper 2 cells. Front Immunol 9: 2512, 2018. doi: 10.3389/fimmu.2018.02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Nestor CE, Zhao S, Lentini A, Bohle B, Benson M, Wang H. Profiling of human CD4+ T-cell subsets identifies the TH2-specific noncoding RNA GATA3-AS1. J Allergy Clin Immunol 132: 1005–1008, 2013. doi: 10.1016/j.jaci.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res 16: 3–10, 2006. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 47.Collier SP, Collins PL, Williams CL, Boothby MR, Aune TM. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J Immunol Baltim Md 1950 189: 2084–2088, 2012. [Erratum in J Immunol 192: 533, 2014]. doi: 10.4049/jimmunol.1200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collier SP, Henderson MA, Tossberg JT, Aune TM. Regulation of the Th1 genomic locus from Ifng through Tmevpg1 by T-bet. J Immunol Baltim Md 1950 193: 3959–3965, 2014. doi: 10.4049/jimmunol.1401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez JA, Wapinski OL, Yang YW, Bureau JF, Gopinath S, Monack DM, Chang HY, Brahic M, Kirkegaard K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 152: 743–754, 2013. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nie J, Zhao Q. Lnc-ITSN1-2, derived from rna sequencing, correlates with increased disease risk, activity and promotes CD4+ T cell activation, proliferation and Th1/Th17 cell differentiation by serving as a ceRNA for IL-23R via sponging miR-125a in inflammatory bowel disease. Front Immunol 11, 2020. doi: 10.3389/fimmu.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qu D, Sun WW, Li L, Ma L, Sun L, Jin X, Li T, Hou W, Wang JH. Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Res 47: 3013–3027, 2019. doi: 10.1093/nar/gkz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Hu PW, Couturier J, Lewis DE, Rice AP. HIV-1 replication in CD4+ T cells exploits the down-regulation of antiviral NEAT1 long non-coding RNAs following T cell activation. Virology 522: 193–198, 2018. doi: 10.1016/j.virol.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Chen C, Ma X, Geng G, Liu B, Zhang Y, Zhang S, Zhong F, Liu C, Yin Y, Cai W, Zhang H. Long noncoding RNA NRON contributes to HIV-1 latency by specifically inducing tat protein degradation. Nat Commun 7: 11730, 2016. doi: 10.1038/ncomms11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syrett CM, Paneru B, Sandoval-Heglund D, Wang J, Banerjee S, Sindhava V, Behrens EM, Atchison M, Anguera MC. Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight 4, 2019. doi: 10.1172/jci.insight.126751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braga-Neto MB, Gaballa JM, Bamidele AO, Sarmento OF, Svingen P, Gonzalez M, Ramos GP, Sagstetter MR, Aseem SO, Sun Z, Faubion WA. Deregulation of long intergenic non-coding RNAs in CD4+ T cells of lamina propria in Crohn’s Disease through transcriptome profiling. J Crohns Colitis 14: 96–109, 2020. doi: 10.1093/ecco-jcc/jjz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotzin JJ, Spencer SP, McCright SJ, Kumar DBU, Collet MA, Mowel WK, Elliott EN, Uyar A, Makiya MA, Dunagin MC, Harman CCD, Virtue AT, Zhu S, Bailis W, Stein J, Hughes C, Raj A, Wherry EJ, Goff LA, Klion AD, Rinn JL, Williams A, Flavell RA, Henao-Mejia J. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537: 239–243, 2016. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaur S, Sassano A, Dolniak B, Joshi S, Majchrzak-Kita B, Baker DP, Hay N, Fish EN, Platanias LC. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci U S A 105: 4808–4813, 2008. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saleiro D, Mehrotra S, Kroczynska B, Beauchamp EM, Lisowski P, Majchrzak-Kita B, Bhagat TD, Stein BL, McMahon B, Altman JK, Kosciuczuk EM, Baker DP, Jie C, Jafari N, Thompson CB, Levine RL, Fish EN, Verma AK, Platanias LC. Central role of ULK1 in type I interferon signaling. Cell Rep 11: 605–617, 2015. doi: 10.1016/j.celrep.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SH, Kim SH, Yang WI, Kim SJ, Yoon SO. Association of the long non-coding RNA MALAT1 with the polycomb repressive complex pathway in T and NK cell lymphoma. Oncotarget 8: 31305–31317, 2017. doi: 10.18632/oncotarget.15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masoumi F, Ghorbani S, Talebi F, Branton WG, Rajaei S, Power C, Noorbakhsh F. Malat1 long noncoding RNA regulates inflammation and leukocyte differentiation in experimental autoimmune encephalomyelitis. J Neuroimmunol 328: 50–59, 2019. doi: 10.1016/j.jneuroim.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 61.Wu J, Zhang H, Zheng Y, Jin X, Liu M, Li S, Zhao Q, Liu X, Wang Y, Shi M, Zhang S, Tian J, Sun Y, Zhang M, Yu B. The long noncoding RNA MALAT1 induces tolerogenic dendritic cells and regulatory T cells via miR155/dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin/IL10 Axis. Front Immunol 9: 1847, 2018. doi: 10.3389/fimmu.2018.01847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barbier F, Mer M, Szychowiak P, Miller RF, Mariotte É, Galicier L, Bouadma L, Tattevin P, Azoulay É. Management of HIV-infected patients in the intensive care unit. Intensive Care Med 46: 329–342, 2020. doi: 10.1007/s00134-020-05945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primer 1: 15035, 2015. doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 64.Ghosn J, Taiwo B, Seedat S, Autran B, Katlama, C. HIV. Lancet Lond Engl 392: 685–697, 2018. doi: 10.1016/S0140-6736(18)31311-4. [DOI] [PubMed] [Google Scholar]
- 65.Abner E, Jordan A. HIV “shock and kill” therapy: In need of revision. Antiviral Res 166: 19–34, 2019. doi: 10.1016/j.antiviral.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Datta PK, Kaminski R, Hu W, Pirrone V, Sullivan NT, Nonnemacher MR, Dampier W, Wigdahl B, Khalili K. HIV-1 latency and eradication: past, present and future. CHR 14: 431–441, 2016. doi: 10.2174/1570162x14666160324125536. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz C, Bouchat S, Marban C, Gautier V, Van Lint C, Rohr O, Le Douce V. On the way to find a cure: Purging latent HIV-1 reservoirs. Biochem Pharmacol 146: 10–22, 2017. doi: 10.1016/j.bcp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, Boucher G, Haddad EK, Sekaly RP, Harman AN, Anderson JL, Jones KL, Mak J, Cunningham AL, Jaworowski A, Lewin SR. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci U S A 107: 16934–16939, 2010. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rezaei SD, Lu HK, Chang JJ, Rhodes A, Lewin SR, Cameron PU. The pathway to establishing HIV latency is critical to how latency is maintained and reversed. J Virol 92: e02225-17, 2018. doi: 10.1128/JVI.02225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 110: 4161–4164, 2007. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 71.Shan L, Deng K, Gao H, Xing S, Capoferri AA, Durand CM, Rabi SA, Laird GM, Kim M, Hosmane NN, Yang HC, Zhang H, Margolick JB, Li L, Cai W, Ke R, Flavell RA, Siliciano JD, Siliciano RF. Transcriptional reprogramming during effector-to-memory transition renders CD4+ T cells permissive for latent HIV-1 infection. Immunity 47: 766–775.e3, 2017. doi: 10.1016/j.immuni.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trypsteen W, White CH, Mukim A, Spina CA, De Spiegelaere W, Lefever S, Planelles V, Bosque A, Woelk CH, Vandekerckhove L, Beliakova-Bethell N. Long non-coding RNAs and latent HIV - A search for novel targets for latency reversal. PloS One 14: e0224879, 2019. doi: 10.1371/journal.pone.0224879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol 12: 1063–1070, 2011. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willingham AT, Orth AP, Batalov S, Peters EC, Wen BG, Aza-Blanc P, Hogenesch JB, Schultz PG. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309: 1570–1573, 2005. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 75.Cron RQ, Bartz SR, Clausell A, Bort SJ, Klebanoff SJ, Lewis DB. NFAT1 enhances HIV-1 gene expression in primary human CD4 T cells. Clin Immunol Orlando Fla 94: 179–191, 2000. doi: 10.1006/clim.1999.4831. [DOI] [PubMed] [Google Scholar]
- 76.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity 6: 235–244, 1997. doi: 10.1016/s1074-7613(00)80326-x. doi:. [DOI] [PubMed] [Google Scholar]
- 77.Romanchikova N, Ivanova V, Scheller C, Jankevics E, Jassoy C, Serfling E. NFAT transcription factors control HIV-1 expression through a binding site downstream of TAR region. Immunobiology 208: 361–365, 2003. doi: 10.1078/0171-2985-00283. [DOI] [PubMed] [Google Scholar]
- 78.Bond CS, Fox AH. Paraspeckles: nuclear bodies built on long noncoding RNA. J Cell Biol 186: 637–644, 2009. doi: 10.1083/jcb.200906113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fox AH, Nakagawa S, Hirose T, Bond CS. Paraspeckles: where long noncoding RNA meets phase separation. Trends Biochem Sci 43: 124–135, 2018. doi: 10.1016/j.tibs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Nakagawa S, Hirose T. Paraspeckle nuclear bodies–useful uselessness? Cell Mol Life Sci CMLS 69: 3027–3036, 2012. doi: 10.1007/s00018-012-0973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 43: 904–914, 2011. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu B, Gerin I, Miao H, Vu-Phan D, Johnson CN, Xu R, Chen X-W, Cawthorn WP, MacDougald OA, Koenig RJ. Multiple roles for the non-coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PloS One 5: e14199, 2010. doi: 10.1371/journal.pone.0014199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, Hamblin MH, Yin KJ. The long noncoding RNA Malat1: Its physiological and pathophysiological functions. RNA Biol 14: 1705–1714, 2017. doi: 10.1080/15476286.2017.1358347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klec C, Prinz F, Pichler M. Involvement of the long noncoding RNA NEAT1 in carcinogenesis. Mol Oncol 13: 46–60, 2019. doi: 10.1002/1878-0261.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z, Li K, Huang W. Long non-coding RNA NEAT1-centric gene regulation. Cell Mol Life Sci 77: 3769–3779, 2020. doi: 10.1007/s00018-020-03503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin C, Peng X, Xie T, Lu X, Liu F, Wu H, Yang Z, Wang J, Cheng L, Wu N. Detection of the long noncoding RNAs nuclear-enriched autosomal transcript 1 (NEAT1) and metastasis associated lung adenocarcinoma transcript 1 in the peripheral blood of HIV-1-infected patients. HIV Med 17: 68–72, 2016. doi: 10.1111/hiv.12276. [DOI] [PubMed] [Google Scholar]
- 87.Fortes P, Morris KV. Long noncoding RNAs in viral infections. Virus Res 212: 1–11, 2016. doi: 10.1016/j.virusres.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kambara H, Niazi F, Kostadinova L, Moonka DK, Siegel CT, Post AB, Carnero E, Barriocanal M, Fortes P, Anthony DD, Valadkhan S. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res 42: 10668–10680, 2014. doi: 10.1093/nar/gku713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lemler DJ, Brochu HN, Yang F, Harrell EA, Peng X. Elucidating the role of host long non-coding rna during viral infection: challenges and paths forward. Vaccines 5: 37, 2017. doi: 10.3390/vaccines5040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valadkhan S, Fortes P. Regulation of the interferon response by lncRNAs in HCV infection. Front Microbiol 9: 181, 2018. doi: 10.3389/fmicb.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valadkhan S, Gunawardane LS. lncRNA-mediated regulation of the interferon response. Virus Res 212: 127–136, 2016. doi: 10.1016/j.virusres.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martínez JA, Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J Off Publ Fed Am Soc Exp Biol 29: 3595–3611, 2015. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- 93.Wang J, Yan S, Yang J, Lu H, Xu D, Wang Z. Non-coding RNAs in Rheumatoid Arthritis: From Bench to Bedside. Front Immunol 10: 3129, 2019. doi: 10.3389/fimmu.2019.03129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao Y, Goods BA, Raddassi K, Nepom GT, Kwok WW, Love JC, Hafler DA. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med 7: 287ra74, 2015. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, Mari ER, Safavi F, Leist TP, Zhang G-X, Rostami A. Expression of GM-CSF in T cells Is increased in multiple sclerosis and suppressed by IFN-β therapy. J Immunol Baltim Md 1950 194: 5085–5093, 2015. doi: 10.4049/jimmunol.1403243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmitt N. Role of T Follicular Helper cells in Multiple Sclerosis. J Nat Sci 1: e139, 2015. [PMC free article] [PubMed] [Google Scholar]
- 97.Tao Y, Zhang X, Chopra M, Kim M-J, Buch KR, Kong D, Jin J, Tang Y, Zhu H, Jewells V, Markovic-Plese S. The role of endogenous IFN-β in the regulation of Th17 responses in patients with relapsing-remitting multiple sclerosis. J Immunol Baltim Md 1950 192: 5610–5617, 2014. doi: 10.4049/jimmunol.1302580. [DOI] [PubMed] [Google Scholar]
- 98.Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci USA 97: 11472–11477, 2000. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature 453: 1051–1057, 2008. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol Baltim Md 1950 183: 7169–7177, 2009. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Connor RA, Prendergast CT, Sabatos CA, Lau CWZ, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol Baltim Md 1950 181: 3750–3754, 2008. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, Gevers D, McGovern DPB, Singh N, Braun J, Jacobs JP, Clemente JC, Grinspan A, Sands BE, Colombel JF, Dubinsky MC, Faith JJ. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50: 212–224.e4, 2019. doi: 10.1016/j.immuni.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JY, Hall JA, Kroehling L, Wu L, Najar T, Nguyen HH, Lin WY, Yeung ST, Silva HM, Li D, Hine A, Loke P, Hudesman D, Martin JC, Kenigsberg E, Merad M, Khanna KM, Littman DR. Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell 180: 79–91.e16, 2020. doi: 10.1016/j.cell.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H. Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun 87: 38–49, 2018. doi: 10.1016/j.jaut.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 105.Eberl G. RORγt, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol 10: 27–34, 2017. doi: 10.1038/mi.2016.86. [DOI] [PubMed] [Google Scholar]
- 106.Korn T. Disentangling the manifold functions of RORγt. Nat Immunol 18: 1059–1060, 2017. doi: 10.1038/ni.3831. [DOI] [PubMed] [Google Scholar]
- 107.Rutz S, Eidenschenk C, Kiefer JR, Ouyang W. Post-translational regulation of RORγt-A therapeutic target for the modulation of interleukin-17-mediated responses in autoimmune diseases. Cytokine Growth Factor Rev 30: 1–17, 2016. doi: 10.1016/j.cytogfr.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 108.Davies JM, Abreu MT. The innate immune system and inflammatory bowel disease. Scand J Gastroenterol 50: 24–33, 2015. doi: 10.3109/00365521.2014.966321. [DOI] [PubMed] [Google Scholar]
- 109.Eken A, Singh AK, Treuting PM, Oukka M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol 7: 143–154, 2014. doi: 10.1038/mi.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, Wellcome Trust Case Control Consortium, , et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 44: 1336–1340, 2012. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, Schuldt D, Nikolaus S, Rosenstiel P, Krawczak M, Schreiber S. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet 40: 713–715, 2008. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 112.Hinks A, Cobb J, Marion MC, Prahalad S, Sudman M, Bowes J, Boston Children's JIA Registry, , et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet 45: 664–669, 2013. doi: 10.1038/ng.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, , et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 337: 1190–1195, 2012. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Genetics of Type 1 Diabetes in Finland, , et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39: 857–864, 2007. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roth C, Schuierer M, Günther K, Buettner R. Genomic structure and DNA binding properties of the human zinc finger transcriptional repressor AP-2rep (KLF12). Genomics 63: 384–390, 2000. doi: 10.1006/geno.1999.6084. [DOI] [PubMed] [Google Scholar]
- 116.Dominguez CX, Amezquita RA, Guan T, Marshall HD, Joshi NS, Kleinstein SH, Kaech SM. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J Exp Med 212: 2041–2056, 2015. doi: 10.1084/jem.20150186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Omilusik KD, Best JA, Yu B, Goossens S, Weidemann A, Nguyen JV, Seuntjens E, Stryjewska A, Zweier C, Roychoudhuri R, Gattinoni L, Bird LM, Higashi Y, Kondoh H, Huylebroeck D, Haigh J, Goldrath AW. Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J Exp Med 212: 2027–2039, 2015. doi: 10.1084/jem.20150194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swat W, Fujikawa K, Ganiatsas S, Yang D, Xavier RJ, Harris NL, Davidson L, Ferrini R, Davis RJ, Labow MA, Flavell RA, Zon LI, Alt FW. SEK1/MKK4 is required for maintenance of a normal peripheral lymphoid compartment but not for lymphocyte development. Immunity 8: 625–634, 1998. doi: 10.1016/s1074-7613(00)80567-1. doi:. [DOI] [PubMed] [Google Scholar]
- 119.Tran SE, Meinander A, Holmström TH, Rivero-Müller A, Heiskanen KM, Linnau EK, Courtney MJ, Mosser DD, Sistonen L, Eriksson JE. Heat stress downregulates FLIP and sensitizes cells to Fas receptor-mediated apoptosis. Cell Death Differ 10: 1137–1147, 2003. doi: 10.1038/sj.cdd.4401278. [DOI] [PubMed] [Google Scholar]
- 120.Marchingo JM, Sinclair LV, Howden AJ, Cantrell DA. Quantitative analysis of how Myc controls T cell proteomes and metabolic pathways during T cell activation. eLife 9, 2020. doi: 10.7554/eLife.53725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saravia J, Zeng H, Dhungana Y, Bastardo Blanco D, Nguyen TM, Chapman NM, Wang Y, Kanneganti A, Liu S, Raynor JL, Vogel P, Neale G, Carmeliet P, Chi H. Homeostasis and transitional activation of regulatory T cells require c-Myc. Sci Adv 6: eaaw6443, 2020. doi: 10.1126/sciadv.aaw6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35: 871–882, 2011. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cho SW, Xu J, Sun R, Mumbach MR, Carter AC, Chen YG, Yost KE, Kim J, He J, Nevins SA, Chin SF, Caldas C, Liu SJ, Horlbeck MA, Lim DA, Weissman JS, Curtis C, Chang HY. Promoter of lncRNA Gene PVT1 Is a tumor-suppressor DNA boundary element. Cell 173: 1398–1412.e22, 2018. doi: 10.1016/j.cell.2018.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olivero CE, Martínez-Terroba E, Zimmer J, Liao C, Tesfaye E, Hooshdaran N, Schofield JA, Bendor J, Fang D, Simon MD, Zamudio JR, Dimitrova N. p53 Activates the long noncoding RNA Pvt1b to inhibit Myc and suppress tumorigenesis. Molecular Cell, 77: 761–774, 2020. doi: 10.1016/j.molcel.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Porter JR, Fisher BE, Baranello L, Liu JC, Kambach DM, Nie Z, Koh WS, Luo J, Stommel JM, Levens D, Batchelor E. Global inhibition with specific activation: how p53 and MYC redistribute the transcriptome in the DNA double-strand break response. Mol Cell 67: 1013–1025.e9, 2017. doi: 10.1016/j.molcel.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Froberg JE, Yang L, Lee JT. Guided by RNAs: X-inactivation as a model for lncRNA function. J Mol Biol 425: 3698–3706, 2013. doi: 10.1016/j.jmb.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Galupa R, Heard E. X-chromosome inactivation: a crossroads between chromosome architecture and gene regulation. Annu Rev Genet 52: 535–566, 2018. doi: 10.1146/annurev-genet-120116-024611. [DOI] [PubMed] [Google Scholar]
- 128.Sahakyan A, Yang Y, Plath K. The role of Xist in X-chromosome dosage compensation. Trends Cell Biol 28: 999–1013, 2018. doi: 10.1016/j.tcb.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X, Shen H, He Q, Tian W, Xia A, Lu XJ. Exosomes derived from exhausted CD8+ T cells impaired the anticancer function of normal CD8+ T cells. J Med Genet 56: 29–31, 2019. doi: 10.1136/jmedgenet-2018-105439. [DOI] [PubMed] [Google Scholar]
- 130.Bird L. lncRNA NKILA: a killer regulator. Nat Rev Immunol 18: 666–667, 2018. doi: 10.1038/s41577-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 131.Gupta SC, Awasthee N, Rai V, Chava S, Gunda V, Challagundla KB. Long non-coding RNAs and nuclear factor-κB crosstalk in cancer and other human diseases. Biochim Biophys Acta Rev Cancer 1873: 188316, 2020. doi: 10.1016/j.bbcan.2019.188316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soulier J, Clappier E, Cayuela JM, Regnault A, García-Peydró M, Dombret H, Baruchel A, Toribio ML, Sigaux F, HOXA genes are included in genetic and biologic networks defining human acute T-cell leukemia (T-ALL). Blood 106: 274–286, 2005. doi: 10.1182/blood-2004-10-3900. [DOI] [PubMed] [Google Scholar]
- 133.Verboom K, Van Loocke W, Volders PJ, Decaesteker B, Cobos FA, Bornschein S, de Bock CE, Atak ZK, Clappier E, Aerts S, Cools J, Soulier J, Taghon T, Van Vlierberghe P, Vandesompele J, Speleman F, Durinck K. A comprehensive inventory of TLX1 controlled long non-coding RNAs in T-cell acute lymphoblastic leukemia through polyA+ and total RNA sequencing. Haematologica 103: e585–e589, 2018. doi: 10.3324/haematol.2018.190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wallaert A, Durinck K, Taghon T, Van Vlierberghe P, Speleman F. T-ALL and thymocytes: a message of noncoding RNAs. J Hematol OncolJ Hematol Oncol 10: 66, 2017. doi: 10.1186/s13045-017-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spinella JF, Cassart P, Richer C, Saillour V, Ouimet M, Langlois S, St-Onge P, Sontag T, Healy J, Minden MD, Sinnett D. Genomic characterization of pediatric T-cell acute lymphoblastic leukemia reveals novel recurrent driver mutations. Oncotarget 7: 65485–65503, 2016. doi: 10.18632/oncotarget.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anderson KM, Anderson DM, McAnally JR, Shelton JM, Bassel-Duby R, Olson EN. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539: 433–436, 2016. doi: 10.1038/nature20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ounzain S, Micheletti R, Arnan C, Plaisance I, Cecchi D, Schroen B, Reverter F, Alexanian M, Gonzales C, Ng SY, Bussotti G, Pezzuto I, Notredame C, Heymans S, Guigó R, Johnson R, Pedrazzini T. CARMEN, a human super enhancer-associated long noncoding RNA controlling cardiac specification, differentiation and homeostasis. J Mol Cell Cardiol 89: 98–112, 2015. doi: 10.1016/j.yjmcc.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 138.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, Zhang S, Wang HB, Ge J, Lu X, Yang L, Chen LL. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res 24: 513–531, 2014[Erratum in Cell Res 24: 1150, 2014]. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Amaral PP, Dinger ME, Mattick JS. Non-coding RNAs in homeostasis, disease and stress responses: an evolutionary perspective. Brief Funct Genomics 12: 254–278, 2013. doi: 10.1093/bfgp/elt016. [DOI] [PubMed] [Google Scholar]
- 140.Ulitsky I, Bartel DP. lincRNAs: Genomics, evolution, and mechanisms. Cell 154: 26–46, 2013. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pang KC, Frith MC, Mattick JS. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet TIG 22: 1–5, 2006. doi: 10.1016/j.tig.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 142.Robinson EK, Covarrubias S, Carpenter S. The how and why of lncRNA function: an innate immune perspective. Biochimica Biophys Acta Gene Regul Mech 1863: 194419, 2020. doi: 10.1016/j.bbagrm.2019.194419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Awan HM, Shah A, Rashid F, Shan G. Primate-specific Long Non-coding RNAs and MicroRNAs. Genomics Proteomics Bioinformatics 15: 187–195, 2017. doi: 10.1016/j.gpb.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tay SK, Blythe J, Lipovich L. Global discovery of primate-specific genes in the human genome. Proc Natl Acad Sci 106: 12019–12024, 2009. doi: 10.1073/pnas.0904569106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel DP. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 147: 1537–1550, 2011[Erratum in Cell 151: 684-686, 2012]. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Delli Ponti R, Marti S, Armaos A, Tartaglia GG. A high-throughput approach to profile RNA structure. Nucleic Acids Res 45: e35, 2017. doi: 10.1093/nar/gkw1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hawkes EJ, Hennelly SP, Novikova IV, Irwin JA, Dean C, Sanbonmatsu KY. COOLAIR antisense RNAs form evolutionarily conserved elaborate secondary structures. Cell Rep 16: 3087–3096, 2016. doi: 10.1016/j.celrep.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim DN, Thiel BC, Mrozowich T, Hennelly SP, Hofacker IL, Patel TR, Sanbonmatsu KY. Zinc-finger protein CNBP alters the 3-D structure of lncRNA Braveheart in solution. Nat Commun 11: 148, 2020. doi: 10.1038/s41467-019-13942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smola MJ, Christy TW, Inoue K, Nicholson CO, Friedersdorf M, Keene JD, Lee DM, Calabrese JM, Weeks KM. SHAPE reveals transcript-wide interactions, complex structural domains, and protein interactions across the Xist lncRNA in living cells. Proc Natl Acad Sci U S A 113: 10322–10327, 2016. doi: 10.1073/pnas.1600008113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kirk JM, Kim SO, Inoue K, Smola MJ, Lee DM, Schertzer MD, Wooten JS, Baker AR, Sprague D, Collins DW, Horning CR, Wang S, Chen Q, Weeks KM, Mucha PJ, Calabrese JM. Functional classification of long non-coding RNAs by k-mer content. Nat Genet 50: 1474–1482, 2018. doi: 10.1038/s41588-018-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davis BJ, O'Connell MR. Put on your para-spectacles: the development of optimized CRISPR-Cas13-based approaches to image RNA dynamics in real time. Mol Cell 77: 207–209, 2020. doi: 10.1016/j.molcel.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 152.Dixit A, Parnas O, Li B, Chen J, Fulco CP, Jerby-Arnon L, Marjanovic ND, Dionne D, Burks T, Raychowdhury R, Adamson B, Norman TM, Lander ES, Weissman JS, Friedman N, Regev A. Perturb-Seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167: 1853–1866.e17, 2016. doi: 10.1016/j.cell.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McMahon AC, Rahman R, Jin H, Shen JL, Fieldsend A, Luo W, Rosbash M. TRIBE: Hijacking an RNA-editing enzyme to identify cell-specific targets of RNA-binding proteins. Cell 165: 742–753, 2016. doi: 10.1016/j.cell.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]