Abstract
Several lines of preclinical and clinical research have confirmed that chronic low-grade inflammation of adipose tissue is mechanistically linked to metabolic disease and organ tissue complications in the overweight and obese organism. Despite this widely confirmed paradigm, numerous open questions and knowledge gaps remain to be investigated. This is mainly due to the intricately intertwined cross-talk of various pro- and anti-inflammatory signaling cascades involved in the immune response of expanding adipose depots, particularly the visceral adipose tissue. Adipose tissue inflammation is initiated and sustained over time by dysfunctional adipocytes that secrete inflammatory adipokines and by infiltration of bone marrow-derived immune cells that signal via production of cytokines and chemokines. Despite its low-grade nature, adipose tissue inflammation negatively impacts remote organ function, a phenomenon that is considered causative of the complications of obesity. The aim of this review is to broadly present an overview of adipose tissue inflammation by highlighting the most recent reports in the scientific literature and summarizing our overall understanding of the field. We also discuss key endogenous anti-inflammatory mediators and analyze their mechanistic role(s) in the pathogenesis and treatment of adipose tissue inflammation. In doing so, we hope to stimulate studies to uncover novel physiological, cellular, and molecular targets for the treatment of obesity.
Keywords: adipocytes, immunity, inflammation, insulin resistance, obesity
INTRODUCTION
Obesity is now considered as a worldwide epidemic (1) and a strong risk factor for insulin resistance, type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), immune disorders, and nonalcoholic fatty liver disease (NAFLD), in addition to several type of cancers. Overall, obesity is associated with a reduction of quality of life, shortened life span, and increased healthcare costs (2–5). Because of a pathophysiology that recapitulates the more complicated picture of multifactorial chronic disease similar to the aging process, obviously obesity cannot be simply considered the result of an energy imbalance between calorie intake and expenditure. Thus, a host of metabolic abnormalities, oxidative stress, mitochondrial dysfunction, immune dysfunction, and chronic low-grade inflammation, have been identified in the overweight obese organism (6, 7). Although it has been almost universally established that adipose tissue (AT) responds to overnutrition by mounting an immune response, the initial inflammatory trigger remains unfortunately unknown. As a result, the clinical efficacy of drugs targeting the presently discovered inflammatory pathways has been disappointing. Perhaps, as discussed later in this review, little attention has been devoted to endogenous anti-inflammatory mediators, other potentially intriguing therapeutic targets in AT inflammation. In this review, we summarize the most recent findings about AT inflammation and obesity, highlighting physiological, cellular, and molecular mechanisms through which AT inflammation contributes to AT dysfunction and related systemic complications.
THE INFLAMMATORY PHENOTYPE OF WHITE ADIPOSE TISSUE
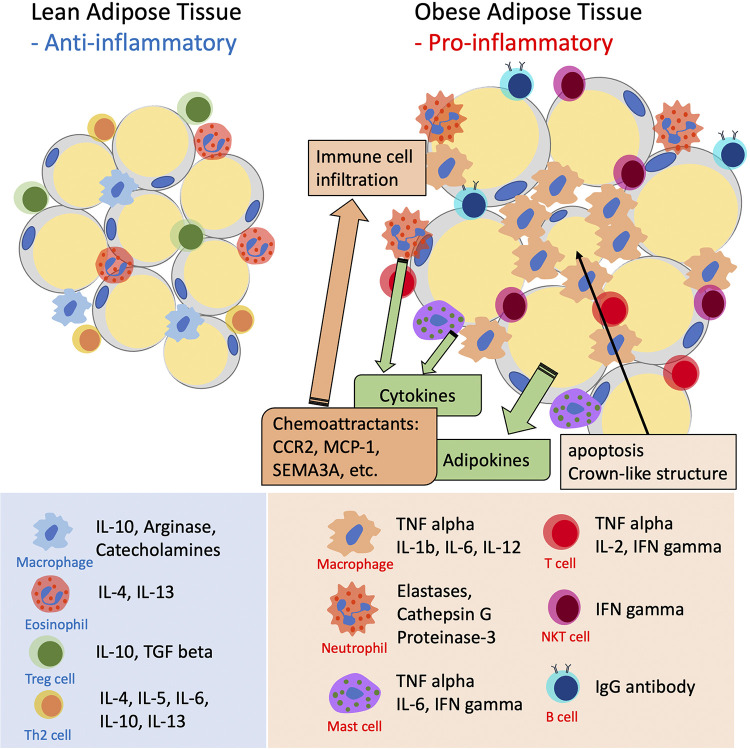
White adipose tissue (WAT) is the major fat-storing depot and also serves as the largest endocrine organ to secrete adipokines and cytokines systemically. Adipokines are involved in various metabolic and physiological signaling cascades and regulate insulin signaling, glucose uptake, fatty acid oxidation, and other energy-producing and metabolic processes (8). Cytokines regulate inflammation and resolution of inflammation along with adaptive and reparative angiogenesis. Weight gain and obesity cause a phenotypic switch of WAT, which is characterized by the appearance of inflamed, dysfunctional adipocytes along with infiltration of immune cells in the stromal vascular fraction (9, 10). Inflamed adipocytes secrete, both locally and systemically, proinflammatory cytokines, which in turn disrupt the normal function of AT itself as well as that of remote organs (11). From this standpoint, AT can be considered an immune and secretory organ, and obesity an inflammatory immune disease (Fig. 1).
Figure 1.
The inflammatory phenotype of expanding adipose tissue. Hypertrophic adipocytes and tissue resident immune cells experience phenotype changes that halt secretion of anti-inflammatory, protective cytokines to begin secretion of inflammatory adipokines and cytokines that act both locally and systemically to induce peripheral insulin resistance. The inflammatory reaction is sustained by adipocyte-derived chemoattractants such as C-C chemokine receptor type 2 (CCR2), monocyte chemoattractant protein (MCP), and semaphorin 3A (SEMA3A).
Both animal and human studies have confirmed the association between increased adiposity and AT inflammation following excessive caloric intake. Using several immunocompromised mouse models, Lee et al. (12) demonstrated the essential role of inflammation in the establishment of insulin resistance following long-term consumption of an obesogenic Western-type diet. A unique feature of the inflammatory responses of expanding WAT is its duration and intensity, i.e., a persistent, low-grade inflammation that fails to resolve. Such obesity-related chronic low-grade inflammation and subsequent altered metabolism have been termed “metaflammation” (9). Inflammation in general is an energy-wasting process that enhances energy expenditure and reduces energy intake in direct and indirect manners. Directly, inflammatory cytokines, such as TNFα, IL-1, and IL-6, induce energy expenditure by binding to signaling receptors located in the central nervous system or in the tissue of metabolic active organs. Indeed, they are provided with leptin-like properties that promote energy expenditure (13–15). Induction of leptin expression represents a molecular mechanism of the inflammatory activity. Leptin expression is increased in adipose tissue by inflammation. Leptin transcription is induced by hypoxia (16) and inflammatory mediators (17), all of which are experienced by expanding adipose depots. Furthermore, leptin receptor expression is induced by TNFα (18), which provides a mechanism by which proinflammatory cytokines enhance leptin activity for energy expenditure. Leptin is an adipokine that inhibits appetite and induces energy expenditure (19), thus indirectly increasing energy expenditure.
Surprisingly, the unique features of AT inflammation induced by overnutrition are not associated with a significant increase in energy expenditure, which permits the coexistence of inflammation and weight gain in obese people (20). Nonetheless, inflammation of AT still shares several commonalities with the traditional inflammatory response, that is, the infiltration of bone marrow-derived immune cells and the secretion of inflammatory mediators including, but not limited to, chemokines and cytokines by adipocytes and resident immune cells. Moreover, because of its conspicuous mass and distribution, even in the apparently healthy normal-weight individual, the inflamed WAT can cause widespread systemic inflammation via release of cytokines (21).
Based on its anatomic location and morphological structure (reviewed in Ref. 22), WAT develops diverse and unique inflammatory phenotypes. Accordingly, it is currently well established that obesity instigates a more complex and intense inflammatory reaction in visceral WAT (VAT) than in subcutaneous WAT (SAT). Indeed, VAT contains more macrophages compared with SAT in obese mice (23–25) and obese humans (26–28). With obesity and insulin resistance, VAT adipocytes experience a much higher degree of hypertrophy than SAT both in humans (29, 30) and in animal models (31). Furthermore, VAT inflammation in obese humans is associated with decreased expression of lipogenic markers (32), probably due to the fact that more cells are switched to an inflammatory rather than a lipid storage phenotype, which leads to the development of metabolic complications, such as ectopic lipid deposition in skeletal muscle and liver (32). Since ectopic lipid deposition dampens peripheral insulin signaling, VAT inflammation is considered to have a major impact on obesity-related metabolic disorders such as systemic insulin resistance and development of T2DM (29, 33, 34). While these lines of research support a higher involvement of VAT inflammation in obesity-related complications, it should be nonetheless noted that many investigators have reported convincing evidence of a role for SAT inflammation in metabolic complications (34–36). The ongoing controversy on the role that different WAT depots play in metaflammation has been partially addressed in mice. To explain the mechanism underlying the different impacts of VAT and SAT on metabolism, Rytka et al. (37) artificially increased fat mass in mice by transplanting epididymal VAT obtained from littermate C57Bl6/J donor mice into the parietal peritoneum that drains in the caval system or the mesenterium that drains in the liver portal system. The procedure induced AT inflammation in both experimental groups of transplanted mice. However, only the mice with mesenterium-transplanted fat experienced elevated IL-6 levels and free fatty acid (FFA) in the portal vein and developed impaired glucose tolerance (37). Human studies of meal FFA uptake further confirm the heterogeneity in the metabolism of VAT and SAT. Expressed relative to the same mass of AT, meal FFA uptake is greater in intra-abdominal than in abdominal subcutaneous fat in both sexes (38, 39). The direct uptake of plasma FFA is also greater in omental than in abdominal subcutaneous fat of women (40). These data suggest that the anatomic location and the unique structural features of VAT enable it to directly feed inflammatory cytokines and metabolites to important organs, such as the liver, that play a role in the regulation of insulin action and systemic metabolism.
In the next sections, we will summarize our current understanding of the cellular and molecular mechanisms implicated in the inflammatory response of the WAT in obesity.
THE ROLE OF BONE MARROW ADIPOSE TISSUE
In healthy adults, the approximatively 10% of adipose tissue located in the bone marrow also regulates whole body energy metabolism, along with local and systemic inflammatory responses. The role of bone marrow adipose tissue (MAT) in energy metabolism and inflammation has been recently reviewed (41, 42); thus, here we will briefly discuss key relevant aspects of MAT’s role in obesity. In terms of metabolic responses, MAT expresses and secretes both adiponectin and leptin. Whereas in vitro studies have reported that primary cultured MAT express a lower level of adiponectin (43, 44), studies in mice and humans have found higher levels of adiponectin expression and secretion in MAT over WAT (45). Furthermore, modulation of MAT mass is correlated to serum adiponectin abundance following caloric restriction (45) or treatment with thiazolidinediones (46). MAT also expresses and secretes leptin (47, 48).
The contribution, though, of bone marrow adipocytes to the metabolic and inflammatory complications of obesity remains somewhat controversial. Differently than visceral fat adipocytes, bone marrow adipocytes are characterized by lower expression levels of adipose-specific genes such as peroxisome proliferator-activated receptor (PPARγ) and fatty acid-binding protein-4 (FABP4), and higher abundance of inflammatory response signaling pathways. In vitro studies have shown that primary cultured bone marrow adipocytes secrete significant levels of IL-6 but only small levels of IL-1 and TNFα (49). Others instead have reported increased expression of inflammatory response genes such as TNFα, IL-6, and IL-1β (43). With high-fat feeding, mice experience increases bone marrow adiposity with adipocytes that surprisingly express decreased inflammatory genes such as TNFα and IL-1β, (50). It should be noted, though, that MAT is unquestionably the prevalent source of immune cells for other adipose depots and metabolic organs in obesity. Thus, it is reasonable to conclude that cytokine production by MAT adipocytes might be mainly important to regulate bone marrow kinetics and that MAT’s contribution to systemic inflammation in obesity is indirectly supported by production and release of immune cells.
MACROPHAGES, MASTER REGULATORS OF WAT INFLAMMATION
Infiltration of bone marrow-derived inflammatory cells is a key feature of the WAT metaflammation. It is well established that infiltration of inflammatory cells in expanding WAT almost invariably causes adipocyte dysfunction and metabolic dysfunction, such as glucose intolerance and insulin resistance. AT macrophages (ATMs) were the first immune cell population to be discovered and studied in this process (51), although more recent reports have emphasized the role of earlier neutrophil infiltration (discussed later). During local and systemic inflammatory responses, tissue resident macrophages present antigens to initiate recruitment of other immune cells and secrete cytokines to regulate inflammatory signaling cascades in the host tissue. This physiological role of macrophages appears to be maintained also in the metaflammation of expanding WAT depots (51, 52). The relevance of this phenomenon is confirmed by several clinical reports demonstrating ATM infiltration in the WAT of obese humans (28, 34, 53–56). Furthermore, clinical studies have also clarified the relationship between ATM infiltration in WAT and insulin resistance. WAT obtained from insulin-resistant and obese patients contains more macrophages with increased expression of proinflammatory mediators (29), and this phenomenon strongly correlates with metabolic dysfunction (33, 57).
Activated ATMs are the main source of proinflammatory mediators such as TNFα, inducible NO synthase (iNOS, monocyte chemoattractant protein-1 (MCP-1), and IL-6 (51, 58). Hotamisligil et al. (59) initially reported in 1993 that the gene and protein expression of TNFα are upregulated in AT, especially in ATMs, obtained from obese animal models. This first observation was followed by many confirmatory studies in laboratory animals and humans showing the upregulation of proinflammatory adipokines including TNFα, IL6, IL-18, and MCP-1 in obesity and their role in the development of insulin resistance and T2DM (51, 58–61). The importance of cytokines secreted by ATMs in whole body metabolism was mechanistically elucidated by Aouadi et al. (62), who showed that ATM-specific silencing of TNFα and osteopontin using siRNA improves insulin sensitivity and glucose tolerance. Osteopontin is a more recently studied inflammatory mediator that appears to favor infiltration, survival, and proliferation of monocytes/macrophages in WAT (63). IL-1β produced by the NOD-, LRR-, and pyrin domain-containing 3 (NLRP3) inflammasome regulates adipose tissue inflammation during obesity (64–66). And inhibition of NLRP3 reduces proinflammatory cytokines and subsequent macrophage infiltration (67). Proteoglycans are also known to regulate inflammation. A recent report revealed that, in obese condition, adipocytes produce versican and macrophages produce biglycan, and the cross-talk between these proteoglycans affects adipose tissue inflammation and insulin resistance (68).
Recent studies have also emphasized the existence of a complex interplay between WAT signaling and the central nervous system (CNS), an organ that contributes to regulation of metabolism at multiple steps (reviewed in Ref. 69). A recent line of research demonstrates that factors involved in axonal growth are also implicated in WAT inflammation. Neuroimmune guidance cue netrin-1 and semaphorin 3E (SEMA3E) with its receptor olexinD1 are regulators of the development of the neuronal system that are upregulated in the obese WAT, where they function as chemoattractants for macrophages (70–72), also by inhibiting macrophage egression (73). Similarly, the neuronal cytokine fractalkine (CX3CL1) and its receptor modulate monocyte adhesion to adipocytes (74).
The classic accepted paradigm is that tissue resident macrophages originate from bone marrow monocytes that infiltrate tissue during physiological immunosurveillance or in response to inflammatory events. Interestingly, diet-induced obesity increases the circulating levels of CD11b(+) monocytes, which express the chemoattractant receptor leukotriene B (75) receptor (BLT-1), and BLT-1 has been shown to sustain monocyte trafficking to WAT (76). More recently, it has been shown that proinflammatory ATMs express genes involved in myelopoiesis and immune cell recruitment, a process that affects circulating levels of monocytes and neutrophils (77, 78). Thus, obesity generates a self-feeding cycle of monocyte/macrophage infiltration to sustain low-grade chronic inflammation of WAT.
While this classic monocyte/macrophage recruitment remains a confirmed mechanism of inflammatory cell infiltration in expanding WAT, a new emerging concept in the field is that of local macrophage proliferation. Cytokines such as IL-4 are reported to stimulate local macrophage proliferation in AT (79). This local proliferation of ATMs occurs mainly at crown-like structures (CLS) that surround necrotic adipocytes, resulting in a preferential increase of M2 macrophage in WAT (80). The consequence and impact of local ATM proliferation, especially of the M2 type, in the pathophysiology of AT dysfunction and metaflammation remains uncharacterized and fertile ground for future research in the field.
The phenotype of obese ATMs is polarized to a proinflammatory state, a phenomenon that has been associated with metabolic complications (57, 81–83). In lean, insulin-sensitive mice, ATMs express anti-inflammatory genes such as IL-10 and arginase-1, whereas ATMs in obese mice highly express proinflammatory genes such as TNFα and iNOS (84). Similarly, the major characteristic of WAT obtained from patients with metabolic abnormalities, such as T2DM patients, is adipocyte hypertrophy with high infiltration of proinflammatory macrophages (35). These “metabolically activated” proinflammatory ATMs function within the context of low-grade inflammation and therefore appear to have important distinctions compared with classically activated M1 macrophages that occur within the context of a full-fledged inflammatory reaction. In fact, whereas M1 macrophages in acute inflammatory reactions highly express CD38, CD274, and CD319 on their cell surface, the ATMs of the obese organism do not (85); they actually show a less inflammatory phenotype with a different gene expression profile compared with classically activated M1 macrophages (86). As result, the expression of proinflammatory cytokines such as TNFα and IL-6 in obese ATMs remains lower than that of classically activated M1 macrophages (85). Although these observations are consistent with the occurrence of low-grade inflammation in obesity, the question remains as to how this lower degree of immune cell activation is achieved and maintained. Current research supports the concept that the concomitant activation of endogenous anti-inflammatory cascades is responsible for dampening macrophage activation and overall WAT inflammation in obesity. Thus, recruitment of anti-inflammatory macrophages via increased IL-10 expression has been observed in WAT after a high-fat diet (HFD) (81), and deletion of IL-10 from the hematopoietic system worsened HFD-induced inflammation and insulin resistance, as one might have predicted (87). Similarly, we have observed increased expression levels of the proangiogenic and anti-inflammatory cytokine IL-19 in stromal vascular fraction and visceral adipocytes of mice given a HFD (our unpublished observation and Fig. 2). Others have implicated a role for IFNγ, another key regulator of macrophage activation and phenotypic switches. Thus, IFNγ knockout mice exhibit less inflammatory ATM in conjunction with decreased adipocyte size and increased insulin sensitivity following HFD (88). Furthermore, interferon-regulatory factor (IRF)-5 expression is upregulated in obesity, and IRF-5 expression is positively correlated with macrophage infiltration and the secretion of proinflammatory adipokines (89).
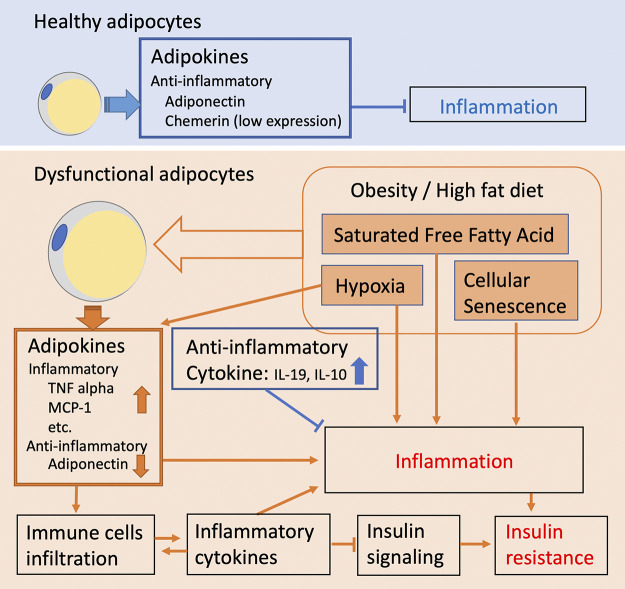
Figure 2.
With increased energy storage and free fatty acid (FFA) cargo, white adipocytes undergo abnormal expansion, which results in hypoxia and remodeling-induced senescence. Hypoxia and senescence initiate and sustain chronic low-grade inflammation. Under these conditions, adipocytes experience endoplasmic reticulum (ER) stress and increased reactive oxygen species (ROS) production. Dysfunctional adipocytes also secrete inflammatory cytokines at the expense of production of protective adipokines, such as adiponectin. Noteworthy, inflamed adipose depots also express anti-inflammatory mediators, such as IL-10 and IL-19, whose role in the overall regulation of the immunometabolic response of adipose tissue remains largely unexplored.
Molecular mechanisms operating within macrophages themselves have also been investigated. Thus, it has been reported that suppression of macrophage activation in obesity is partially controlled via adenosine receptors expressed on macrophages and that these receptors could respond to accumulation of local catabolites (90, 91). In a similar line of research, the inositol-requiring enzyme 1α (IRE1α), a key regulator of endoplasmic reticulum (ER) stress in metabolic organs, has been recently investigated. Shan et al. (92) have implicated IRE1α in the regulation of WAT inflammation by showing that myeloid-specific IRE1α knockout mice have a reduced inflammatory phenotype and are protected from HFD-induced obesity, insulin resistance, hyperlipidemia, and hepatic steatosis (92). More clinically relevant, PPARγ has also been implicated in regulation of insulin resistance as well as inflammation. PPARγ is essential for modulation of metabolically activated macrophages, suggesting that PPARγ may increase macrophages with an anti-inflammatory phenotype, and therefore, it improves glucose metabolism via an anti-inflammatory mechanism in addition to its direct metabolic actions (93, 94).
Despite this wealth of knowledge, currently anti-inflammatory therapies have unfortunately failed to correct the metaflammation of obesity, which poses both a puzzling research question and a therapeutic obstacle. To help explain these unexpected negative results, Scherer and colleagues (95) have recently demonstrated that inflammatory cytokines signaling is paradoxically required for appropriate expansion and metabolic flexibility of WAT. They found that congenital silencing of TNFα or IL-1β function in diet induced obese mice further exacerbates insulin resistance and glucose intolerance due to loss of compensatory angiogenesis and insufficient WAT remodeling. Consistent with this study, clinical data have yielded contrasting evidence on the effect of TNFα and IL-1β blocking therapy on glycemic control and insulin sensitivity in obese, diabetic patients. Thus, systemic blockade of TNFα does not improve insulin resistance in humans, and blockade of IL-1β with canakinumab was not associated with any reduction in the rate of incident diabetes in prediabetic insulin resistance patients (96, 97). Overall, how the balance between pro- and anti-inflammatory cytokines ultimately determines whether or not an expanding adipose depot develops a dysfunctional degree of inflammation which impairs its metabolic activity remains insufficiently explored and poorly understood. Further studies are needed to fully understand the link between cytokines and metabolism to identify new therapeutic targets.
INFILTRATION OF OTHER IMMUNE CELLS
Whereas macrophages are the most studied and undoubtedly important immune cell type involved in AT inflammation, other immune cells such as neutrophils and lymphocytes appear to play relevant mechanistic roles (98, 99) (Fig. 1). Animal studies demonstrate that administration of a HFD to lean mice causes a very early infiltration of neutrophils in VAT before the onset of insulin resistance and obesity (100). These infiltrated neutrophils initiate recruitment of macrophages and other antigen-presenting cells. Bidirectional cross-talk between adipocytes and neutrophils has been recently reported to cause WAT inflammation via IL-1β production with the involvement of infiltrating macrophages (101). This neutrophil-dominated early inflammatory cascade was found causal to the development of hepatic insulin resistance and metabolic disorders (102, 103). Prevention of WAT neutrophil infiltration by inhibiting cytosolic phospholipase-A2α (cPLA2a) or the endothelial cell adhesion molecule ICAM-1 reduced TNFα secretion from adipocytes, preserved hepatic insulin signaling, and prevented abnormal gluconeogenesis (102). Neutrophil elastase secreted from infiltrated neutrophils was found to be one of the key components affecting metabolic functions. Neutrophil elastase inhibitors or the deletion of neutrophil elastase in mice preserved insulin sensitivity and attenuated ATMs infiltration in HFD-fed mice (103).
The infiltration of T cells into expanding adipose depots is also considered to be another important, although controversial (12), mechanism for macrophage recruitment and WAT inflammation (104, 105). Similar to ATMs, views on the immunomodulation of T cells in expanding adipose depots remains somewhat controversial, especially for the role of regulatory T cells (Tregs). Some studies reported downregulation of Tregs in obese states, potentially resulting in chronic WAT inflammation and subsequent insulin resistance (106–109). Others have negated a role for Tregs in obesity (110, 111). The natural killer T (NKT) cells have also been implicated, with more recent reports suggesting that HFD increases invariant NKT-1 cells (iNKT1) along with proinflammatory ATMs and decreases invariant NKT 10 cells (iNKT10) along with anti-inflammatory ATMs, indicating a role for iNKT cells in the amount of M1 ATMs that invade WAT depots (112, 113). The reader should be aware also of the fact that T cell infiltration in WAT fluctuates with weight cycling between obese states and weight loss states (114). Interestingly, weight loss induces WAT inflammation, which in turn impairs insulin sensitivity by decreasing PI 3-kinase, phosphorylated protein kinase B (PKB), and glucose transporter (GLUT)4 expression (115). In contrast, a long-term ketogenic diet containing extremely high fat causes the depletion of γ/δ T cells, and mice lacking γ/δ T cells show impaired glucose metabolism (116). These results suggested that γ/δ T cells had protective effects on adipose tissue inflammation.
Obesity has been also associated with upregulation of B cells (117), which, although based on limited studies, induces insulin resistance via the activation of T cells and AMTs in obese conditions (117, 118). B cell cytokine production appears to be regulated by activation of the Toll-like receptors (TLRs) in type 2 diabetes inflammation (119).
Mast cells and dendritic cells also accumulate in obese AT (120, 121). Dendric cells promote macrophage infiltration into WAT and liver (122), or maintain T cell homeostasis in WAT (123).
Last, the role of eosinophils has been also explored by Wu et al. (124), who who reported that eosinophils play unique roles in metabolic homeostasis by regulating alternatively activated macrophages.
THE CONTRIBUTION OF ADIPOKINES
Expanding adipocytes themselves produce various mediators in an autocrine fashion, that are able to promote or attenuate WAT inflammation (Fig. 2). These mediators have both immunomodulating and metabolic functions and are collectively termed adipokines. Although the functions of adipokines and their cross-talk are not fully understood, adipokines have emerged as key regulators of WAT metabolic homeostasis and perhaps systemic inflammation. Adipokines secreted from adipocytes not only contribute to localized WAT inflammation (125), but can also induce systemic elevation of cytokines responsible for peripheral insulin resistance (126, 127). Adipokines also facilitate infiltration of macrophage in WAT via IRF7-mediated upregulation of MCP-1 (128) and its receptor C-C chemokine receptor 2 (CCR2) (129–133). An important example of this paradigm is offered by chemerin, an adipokine associated with obesity, inflammation, angiogenesis, and metabolic syndrome. Chemerin regulates leukocyte recruitment into inflamed organs (134–136); adipose depots expressing low level of chemerin maintain normal insulin signaling and a noninflammatory phenotype (137, 138). Calprotectin has been elucidated as another adipokine upregulated in obesity, implicated in WAT inflammation via enhancing adhesion of circulating monocytes or recruitment of macrophages (139–141).
Along with proinflammatory cytokines, adipocytes also secrete anti-inflammatory cytokines, whose abundance and secretion appear to be reduced with weight gain since obesity definitively skews the balance in favor of inflammatory adipokines. Adiponectin is considered the most important of these mediators, with several seminal findings by our laboratory and others’ elucidating its anti-inflammatory actions (142). Silencing of adiponectin exacerbates insulin sensitivity (143, 144). Of note, the expression of adiponectin in WAT is suppressed in acute inflammatory conditions as well as in chronic obesity (145, 146), whereas its overexpression reduces WAT inflammation and improves insulin sensitivity (31). Several molecules have been involved in the regulation of adiponectin expression, which may represent targets of anti-inflammatory drug therapy. Thus, treatment of adipocytes with glucagon-like peptide-1 (GLP-1) analogs increases adiponectin expression (147). Estrogen also increases adiponectin expression and attenuates WAT inflammation (148, 149), which protects from insulin resistance. Notably, a recent study reported that women with metabolic syndrome showed lower adiponectin and IL-6 levels compared with men with metabolic syndrome (150), suggesting that there were sex-specific pathways regulating obesity-induced inflammation. Fat-specific deletion of Fsp27, a lipid droplet-associated protein expressed in adipocytes in mice causes attenuation of WAT inflammation via upregulation of adiponectin (151). However, the Fsp27-null mice model remains insulin resistant, possibly because of hepatic steatosis (151), which underscores the complexity of multiorgan integrated functions such as the metaflammation of obesity and the challenge of interpretation of genetic mouse models.
Taken together, these data suggested the existence of a vicious cycle between dysfunctional adipocytes and the immune system that helps perpetuate low-grade chronic inflammation in the obese organism. Time course studies aimed at dissecting the temporal relationship between activation of relevant WAT cell types, including those found in the stromal vascular fraction, during feeding studies may provide important mechanistic information to help resolve the pathophysiology conundrum of WAT inflammation by uncovering potential therapeutic targets and intervention timing.
MOLECULAR MECHANISMS REGULATING ADIPOCYTE INFLAMMATION
Many molecular mechanisms operating within adipocytes have been suggested as possible regulators of WAT inflammation, including ER stress, hypoxia, and cellular senescence (Fig. 2). With longstanding knowledge linking ER stress with the insulin resistance of obesity and diabetes (152, 153), several recent studies have explored this field and uncovered a number of important data. In mice, a HFD induces ER stress and chronic inflammation in WAT via a FFA-mediated increase of reactive oxygen species (ROS) (154). A more direct causal role for ER stress was demonstrated with genetic manipulation of the ER chaperone 78-kDa glucose-regulated protein (GRP78), an essential ER chaperone and a master regulator of ER homeostasis that operates as a repressor of the unfolded protein response (UPR) stress sensors. Macrophage-selective ER chaperone GRP78 knockout mice are protected from HFD-induced AT inflammation and insulin resistance (155). In addition, ER stress preconditioning protects against FFA-induced adipocyte inflammation through inhibition of the IKK/NF-κB pathway via X-box binding protein-1 (XBP1), a modulator of the UPR (156). Interestingly, obese, insulin-resistant humans also experience higher levels of circulating saturated fatty acid (FFAs) compared with lean individuals (157–160). Furthermore, in obese patients, adipocyte markers of ER stress significantly correlate with BMI or percent body fat (161), and a recent clinical study reported that the amount and types of FFAs in cell membrane phospholipids are related to the inflammatory phenotype of ATMs; for instance, the proportion of proinflammatory ATMs increased with the proportions of palmitic or palmitoleic acids (162). Conversely, omega-3 fatty acids have been shown to exert protective effects on WAT inflammation via G protein-coupled receptor 120 (GPR120), an omega-3 fatty acid receptor (163).
Metabolism of FFA is mainly regulated by cell surface-expressed lipases and cytosolic FABP4. In mice, WAT deletion of the hormone-sensitive lipase (HSL) that regulates mobilization of stored fat induces WAT dysfunction and fatty liver (164), which is consistent with clinical observations showing that null mutation in the HSL gene is associated with WAT inflammation or increased risk of T2DM (165). FABP4 is secreted from adipocytes, where it controls lipolysis, and circulating FABP4 is elevated in metabolic disorders. In vitro treatment of adipocytes with FABP4 inhibits adipocyte differentiation and instigates adipocyte inflammation via the p38/NF-κB pathway (166). In a similar line of research, Koliwad et al. (167) reported that overexpression of diacylglycerol O-acyltransferase-1 (DGAT1) in adipocytes, the enzyme that catalyzes the conversion of diacylglycerol and FFAs to triglyceride (TG), attenuates AT inflammation with improved insulin sensitivity and glucose tolerance in HFD-fed mice, despite the exacerbated accumulation of TGs in liver or skeletal muscle. Hematopoietic deletion of the fatty acid translocase and the scavenger receptor CD36 that takes up long-chain fatty acids (FA) also attenuates WAT inflammation (168).
Research has focused on TLR4 in the inflammatory action of FFAs in obesity. Earlier reports suggested that FFAs serve as chemokine inducers to initiate macrophage infiltration via IKKβ and JNK pathways, and this effect of FFAs is partially through TLR4 (169). More recently, it has been reported that FFAs more directly stimulate TLR4 and initiate TLR4-dependent gene expression of lipid metabolic pathways and membrane lipid composition that promote the inflammatory cascade (170, 171). Furthermore, the expression level of tenascin C, an endogenous activator of TLR4, is also increased in obese WAT (172, 173). Accordingly, TLR4 mutant mice exhibit reduced AT inflammation, preserved adiponectin expression, and increased insulin sensitivity despite higher epididymal AT mass and adipocyte hypertrophy (174). Furthermore, global TLR4 silencing protects mice from AT inflammation, probably via reduced ER stress, augmented autophagy activity, attenuated senescence (175), and reduced oxidative stress through metabolic reprogramming of mitochondria (176). On the other hand, TLR4 silencing in myeloid cells alone does not preserve insulin sensitivity, indicating the importance of TLR4 expression in metabolic organs in obesity (177, 178) At the mechanistic level, the high-mobility group box protein-1 (HMGB1) initiates TLR4-dependent inflammation via NF-κB and p38 MAPK signaling with subsequent IL-6 secretion (179). TLR4-mediated macrophage activation (180, 181) has also been associated with alterations of gut microbiota due to changes in gut permeability following intake of fat diets (180–182). Similar to what we have discussed for cytokines, counterregulatory expression of other TLR types appears to take place in obesity. Thus, TLR9 expressed in adipocytes was found to have WAT anti-inflammatory actions, as demonstrated by significantly reduced adiponectin secretion and increased MCP1 secretion in TLR9 knockout mice (183).
ADAPTIVE REACTIONS INDUCED BY AT INFLAMMATION
Chronic low-grade inflammation mediates not only metabolic dysfunctions but also the adaptive reactions necessary to maintain physiological conditions in WAT. Interestingly, many of these adaptive reactions are inflammatory in nature. For instance, cellular senescence is another contributor to WAT inflammation, and adipocyte death is enhanced in obese mice and humans (24, 184). Furthermore, accumulation of senescent T cells and endothelial cells occurs in the obese WAT (185–187), as well as in the insulin-resistant organism (185, 188). Upregulation of senescence markers such as p16, EGFP, and β-galactosidase has been reported in expanding VAT depots (185). Since senescent cells are known to secrete proinflammatory cytokines (189), it is quite likely that cellular senescence instigates WAT inflammation in obesity. In support of this view, mice lacking programmed cell death-4 (PDCD4), a selective protein translation inhibitor, are protected from HFD-induced obesity and WAT inflammation (190).
With expansion, AT also becomes hypoxic due to insufficient vascular supply (reviewed in Ref. 191). The reduced oxygenation of obese fat depots remains even in the face of compensatory angiogenesis (192, 193). The ensuing hypoxia contributes to the increased secretion of inflammatory adipokines, decreased expression of adiponectin, and upregulation of hypoxia-inducible factor 1α (HIF-1α) (194–196). HIF-1α increases macrophage infiltration and WAT inflammation (197). Relevant to what we discussed in the section above, Snodgrass et al. (198) reported that FFAs exacerbated macrophage-mediated inflammation in response to HIF-1α. Indeed, HIF-1α favors the M1 ATM switch by increasing saturated fatty acid stimulation of the adenine nucleotide translocase-2 (ANT2) (199), and activation of the interleukin-1 receptor-associated kinase M (200). In addition to macrophages, infiltration of other myeloid cells is also mediated by HIF-1α (201).
Hypoxia also negatively impacts adipocyte homeostasis. Thus, hypoxia enhances adipocytokine promoter hypomethylation via the HIF-1α and ten-eleven translocation-1 (TET1) pathway in human adipocytes (202). Taken together, hypoxia in expanded adipocytes is one of the major regulatory mechanisms regulating WAT inflammation and insulin resistance (203).
Adaptive changes have also been described in the lymphatic system of WAT. The lymphatic system is involved in adipose metabolism by regulating lipid absorption and lipid transport and the trafficking of immune cells. HFD-fed obese mice have impaired lymphatic vessel function (204, 205). Clinical studies also found evidence of lymphatic vessel dysfunction in humans (206, 207). Obesity-induced lymphatic dysfunction appears to be related to perilymphatic inflammation (208–210) and is causative of WAT and fibrosis (211) via increased lymphatic system permeability (212). Recent publications have linked adipokines such as leptin and adiponectin to lymphatic function. Leptin causes lymphedema in obese patients via morphological change of lymphatic ducts (213). Importantly, adiponectin can improve lymphedema in obese mice by promoting lymphatic formation via AMPK/ALT/eNOS signaling in lymphatic endothelial cells (214).
INFLAMMATION AS A THERAPEUTIC TARGET IN OBESITY
Attenuation of WAT inflammation in obesity has been explored in preclinical and clinical studies as a possible treatment strategy to avert the metabolic and vascular complication of obesity. Unfortunately, the data obtained have yielded controversial results and have also made clear the difficulty of translating results obtained in animal models of obesity to humans.
Clinical reports have investigated anticytokine therapy in the fight against insulin resistance, based on evidence of elevated TNFα in the serum of obese patients. The mechanistic value of this correlative association has been confirmed with studies demonstrating that administration of TNFα to laboratory animals and humans impairs insulin sensitivity (9, 128). In line with these results, some human studies have reported reduced glucose intolerance in overweight individuals and reduced progression to T2D in patients with rheumatoid arthritis following TNFα neutralization (132, 133). To the contrary, others have reported lack of improved insulin sensitivity in people with T2D patients (129, 130) or rheumatoid arthritis (131) following anti-TNFα therapy. Discrepant results have been reported also in healthy volunteers in whom TNFα infusion increased glucose uptake in skeletal muscle (134). Recent evidence in humans have also implicated IL-1β. However, in the recent CANTOS trial, IL-1β inhibition with canakinumab failed to reduce HbA1c, glucose or insulin levels in diabetic patients (150). This dichotomy between WAT inflammation and metabolism is also observed at the cellular level and in response to diet and lifestyle intervention and even bariatric surgery. Although it is well established that obesity causes infiltration of ATMs in WAT, calorie restriction, or rapid weight loss, which improve insulin sensitivity, paradoxically it increases the number of ATMs or proinflammatory cytokines both in animal models (215–217) and human obesity (75, 218–222). Thus, despite improvement of systemic metabolic functions, WAT inflammation or the infiltration of resident macrophages are not changed after weight loss (217, 223). Similarly, the expression of proinflammatory genes in WAT is unchanged in the early phase of a very-low-calorie diet but increases in the later phase (224). Instead, high-intensity exercise improves macrophage polarization toward the anti-inflammatory phenotypes (225). Calorie restriction accompanied by eccentric exercise is also reported to upregulate anti-inflammatory macrophages and downregulate proinflammatory macrophages (226). To complicate matters, short-term overfeeding to healthy normal or overweight individuals did not cause upregulation of proinflammatory gene expression or macrophage infiltration in SAT despite the increased body weight and reduction in insulin sensitivity (227, 228). What appears to be important in reducing ATM infiltration and WAT tissue inflammation is the duration of weight loss more than its total reduction (218, 229–231). Future studies should focus on understanding how chronic adaptation to weight gain and weight cycling links WAT inflammation to insulin resistance. More importantly, integrative physiological studies should be undertaken to fully evaluate the interplay between diet-induced WAT inflammation and the functions of other metabolic organs such as the cardiovascular system, skeletal muscle, and liver.
Many researchers have also investigated the impact of rapid weight loss with bariatric surgery on WAT inflammation, but the results are not consistent. Some studies reported the reduction of WAT inflammation with improvement of insulin sensitivity after bariatric surgery (230, 232, 233). Conversely, other studies show no or only minor changes in AT inflammation after surgery (219, 220, 234). Schmitz et al. (235) reported that improved inflammation and insulin action in liver could improve systemic glucose homeostasis such as insulin sensitivity after bariatric surgeries, even if the WAT inflammation parameters, including the number of ATMs, were not significantly affected. Others have reported increased neutrophil infiltration in WAT after bariatric surgery (219, 220).
Several pharmacological and genetic modification strategies have also been investigated in the fight against obesity and metaflammation. In this context, melatonin, a potent antioxidant that improves inflammatory responses and energy metabolism, has been shown to attenuate obesity-associated complications via decreasing inflammatory adipokines including IL-6, MCP-1, leptin, and TNFα (236, 237). Inhibition of glycogen synthase kinase-3 (GSK3), a protein-serine kinase that lies downstream of multiple cell-signaling pathways, also reduces WAT inflammation, by suppressing migration of monocytes, favoring apoptosis of macrophages, inactivating STAT3, and reducing the levels of FFAs or chemokines secreted by VAT (238).
Genetic modifications have been performed in several mouse models in which the link between WAT inflammation and insulin resistance has been carefully investigated. For example, mice overexpressing calpastatin, the endogenous inhibitor of the calcium-dependent protease calpain, show reduced adipocyte apoptosis and macrophage infiltration in WAT (239). Deletion of C1q/TNF-related protein-7 (CTRP7), a secretory protein of the C1q family, in mice is also improved WAT inflammation and insulin resistance, probably via decreased oxidative and ER stress (240).
CONCLUSION AND FUTURE DIRECTION
In this this review article, we have attempted to summarize our current understanding of the role that WAT inflammation plays in the metabolic complications of obesity by highlighting the most recent preclinical and clinical studies. The past decade has clearly demonstrated the existence of a strong relationship between WAT inflammation and insulin resistance, glucose intolerance, type 2 diabetes, and obesity. However, numerous knowledge gaps continue to limit our understanding of the complex interactions among adipokines and systemic pathophysiology. Investigations in WAT inflammation have several difficulties to overcome. First, other organs, such as brain, liver, and skeletal muscles, also contribute to metabolic dysfunction, and an improvement of WAT inflammation is usually associated with an improvement of other organ function that also impact on insulin sensitivity. These integrative metabolic responses make difficult, if not almost impossible, to isolate the mechanistic contribution of WAT inflammation to obesity complications in intact physiological systems. Second, a current limitation of the research in the field is that the fragility of adipocytes makes it difficult to preserve the integrity of the tissue under study. Thus, our current models of the basic mechanisms governing WAT remodeling in obesity are mostly based on extrapolations from in vitro or ex vivo biochemical approaches or conventional histological analysis. Although these approaches remain useful, they yield limited translational value, as they are inadequate to study integrated physiological responses. Obviously, our understanding of these phenomena would greatly benefit from the availability of tools enabling in vivo analysis. Third, there are substantial differences in characteristics and cell surface markers of immune cells between rodents and humans, and therefore careful interpretation of data obtained in animal models of obesity are warranted. More importantly, genetically modified mice should be validated with the use of wild-type mice in which changes in the target gene byproducts should be observed following induction of obesity. Finally, clinical studies have a limited view of WAT tissue inflammation due to the fact that they are largely based on omental fat pad because of accessibility. Since fat depots in different regions of the body assume diverse inflammatory phenotypes, inclusion of additional adipose depots in future studies may provide a more comprehensive view in the field. We hope the current review will stimulate new interest in this field and will promote obesity research.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK096521 to R. Scalia and by National Heart, Lung, and Blood Institute Grants HL141108 and HL117724 to M. V. Autieri.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.K. prepared figures; T.K. and R.S. drafted manuscript; M.V.A. and R.S. edited and revised manuscript; M.V.A. and R.S. approved final version of manuscript.
REFERENCES
- 1.Roberto CA, Swinburn B, Hawkes C, Huang TT, Costa SA, Ashe M, Zwicker L, Cawley JH, Brownell KD. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet 385: 2400–2409, 2015. doi: 10.1016/S0140-6736(14)61744-X. [DOI] [PubMed] [Google Scholar]
- 2.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, , et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363: 2211–2219, 2010 [Erratum in N Engl J Med 365: 869, 2011]. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 377: 1085–1095, 2011. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer–viewpoint of the IARC Working Group. N Engl J Med 375: 794–798, 2016. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn S, Lawes CM, Ali MK, Mozaffarian D, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating G, Asia-Pacific Cohort Studies C, Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in E, Emerging Risk Factor C, and Prospective Studies C. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One 8: e65174, 2013. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pararasa C, Bailey CJ, Griffiths HR. Ageing, adipose tissue, fatty acids and inflammation. Biogerontology 16: 235–248, 2015. doi: 10.1007/s10522-014-9536-x. [DOI] [PubMed] [Google Scholar]
- 7.Perez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Galvez BG. “Adipaging”: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol 594: 3187–3207, 2016. doi: 10.1113/JP271691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 22: 1023–1031, 2008. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 10.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 542: 177–185, 2017. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity 47: 406–420, 2017. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483, 2011. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anforth HR, Bluthe RM, Bristow A, Hopkins S, Lenczowski MJ, Luheshi G, Lundkvist J, Michaud B, Mistry Y, Van Dam AM, Zhen C, Dantzer R, Poole S, Rothwell NJ, Tilders FJ, Wollman EE. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur Cytokine Netw 9: 279–288, 1998. [PubMed] [Google Scholar]
- 14.Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC. Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology 150: 4124–4134, 2009. doi: 10.1210/en.2009-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 16.Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem 277: 42953–42957, 2002. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 17.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest 97: 2152–2157, 1996. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan L, Guo K, Cremona ML, McGraw TE, Leibel RL, Zhang Y. TNF-α up-regulates protein level and cell surface expression of the leptin receptor by stimulating its export via a PKC-dependent mechanism. Endocrinology 153: 5821–5833, 2012. doi: 10.1210/en.2012-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers MG Jr, Olson DP. Central nervous system control of metabolism. Nature 491: 357–363, 2012. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- 20.Carneiro IP, Elliott SA, Siervo M, Padwal R, Bertoli S, Battezzati A, Prado CM. Is obesity associated with altered energy expenditure? Adv Nutr 7: 476–487, 2016. doi: 10.3945/an.115.008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis 56: 426–433, 2014. doi: 10.1016/j.pcad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Scalia R. The microcirculation in adipose tissue inflammation. Rev Endocr Metab Disord 14: 69–76, 2013. doi: 10.1007/s11154-013-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res 52: 480–488, 2011. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res 49: 1562–1568, 2008. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto Y, Higashiyama H, Rong JX, McVey MJ, Kinoshita M, Asano S, Hansen MK. Comparison of mitochondrial and macrophage content between subcutaneous and visceral fat in db/db mice. Exp Mol Pathol 83: 73–83, 2007. doi: 10.1016/j.yexmp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clement K. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 55: 1554–1561, 2006. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 27.Harlev A, Aricha-Tamir B, Shaco-Levy R, Tarnovscki T, Bashan N, Rudich A, Sheiner E, Press F, Wiznitzer A. Macrophage infiltration and stress-signaling in omental and subcutaneous adipose tissue in diabetic pregnancies. J Matern Fetal Neonatal Med 27: 1189–1194, 2014. doi: 10.3109/14767058.2013.853734. [DOI] [PubMed] [Google Scholar]
- 28.Harman-Boehm I, BlüHer M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, KlöTing N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92: 2240–2247, 2007. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 29.Hardy OT, Perugini RA, Nicoloro SM, Gallagher-Dorval K, Puri V, Straubhaar J, Czech MP. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Relat Dis 7: 60–67, 2011. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connell J, Lynch L, Cawood TJ, Kwasnik A, Nolan N, Geoghegan J, McCormick A, O'Farrelly C, O'Shea D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One 5: e9997, 2010. doi: 10.1371/journal.pone.0009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulain-Godefroy O, Lecoeur C, Pattou F, Frühbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol 295: R1–R7, 2008. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]
- 33.Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 299: E506–E515, 2010. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 34.Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, FerranteAW, Jr, Krakoff J. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes 58: 385–393, 2009. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acosta JR, Douagi I, Andersson DP, Bäckdahl J, Rydén M, Arner P, Laurencikiene J. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 59: 560–570, 2016. doi: 10.1007/s00125-015-3810-6. [DOI] [PubMed] [Google Scholar]
- 36.van der Kolk BW, Kalafati M, Adriaens M, van Greevenbroek MMJ, Vogelzangs N, Saris WHM, Astrup A, Valsesia A, Langin D, van der Kallen CJH, Eussen SJPM, Schalkwijk CG, Stehouwer CDA, Goossens GH, Arts ICW, Jocken JWE, Evelo CT, Blaak EE. Subcutaneous adipose tissue and systemic inflammation are associated with peripheral but not hepatic insulin resistance in humans. Diabetes 68: 2247–2258, 2019. doi: 10.2337/db19-0560. [DOI] [PubMed] [Google Scholar]
- 37.Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes 60: 56–63, 2011. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 285: E1282–E1288, 2003. doi: 10.1152/ajpendo.00220.2003. [DOI] [PubMed] [Google Scholar]
- 39.Marin P, Lonn L, Andersson B, Oden B, Olbe L, Bengtsson BA, Bjorntorp P. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 81: 1018–1022, 1996. doi: 10.1210/jcem.81.3.8772568. [DOI] [PubMed] [Google Scholar]
- 40.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 60: 2032–2040, 2011. doi: 10.2337/db11-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benova A, Tencerova M. Obesity-induced changes in bone marrow homeostasis. Front Endocrinol (Lausanne) 11: 294, 2020. doi: 10.3389/fendo.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boroumand P, Klip A. Bone marrow adipose cells—cellular interactions and changes with obesity. J Cell Sci 133: jcs238394, 2020. doi: 10.1242/jcs.238394. [DOI] [PubMed] [Google Scholar]
- 43.Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics 12: 212, 2011. doi: 10.1186/1471-2164-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, Cinti S, Olivieri A, Leoni P. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol 41: 558–566, 2013. doi: 10.1016/j.exphem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 20: 368–375, 2014. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulston RJ, Learman BS, Zhang B, Scheller EL, Parlee SD, Simon BR, Mori H, Bree AJ, Wallace RJ, Krishnan V, MacDougald OA, Cawthorn WP. Increased circulating adiponectin in response to thiazolidinediones: investigating the role of bone marrow adipose tissue. Front Endocrinol (Lausanne) 7: 128, 2016. doi: 10.3389/fendo.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laharrague P, Truel N, Fontanilles AM, Corberand JX, Penicaud L, Casteilla L. Regulation by cytokines of leptin expression in human bone marrow adipocytes. Horm Metab Res 32: 381–385, 2000. doi: 10.1055/s-2007-978658. [DOI] [PubMed] [Google Scholar]
- 48.Uchihashi K, Aoki S, Shigematsu M, Kamochi N, Sonoda E, Soejima H, Fukudome K, Sugihara H, Hotokebuchi T, Toda S. Organotypic culture of human bone marrow adipose tissue. Pathol Int 60: 259–267, 2010. doi: 10.1111/j.1440-1827.2010.02511.x. [DOI] [PubMed] [Google Scholar]
- 49.Laharrague P, Fontanilles AM, Tkaczuk J, Corberand JX, Pénicaud L, Casteilla L. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur Cytokine Netw 11: 634–639, 2000. [PubMed] [Google Scholar]
- 50.Tencerova M, Figeac F, Ditzel N, Taipaleenmäki H, Nielsen TK, Kassem M. High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res 33: 1154–1165, 2018. doi: 10.1002/jbmr.3408. [DOI] [PubMed] [Google Scholar]
- 51.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr.. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72: 219–246, 2010. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 53.Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumié A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 117: 806–815, 2008. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 54.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumié A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 53: 1285–1292, 2004. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 55.Curat CA, Wegner V, Sengenès C, Miranville A, Tonus C, Busse R, Bouloumié A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49: 744–747, 2006. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 56.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive proinflammatory mediator production. Int J Obes (Lond) 31: 1420–1428, 2007. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 57.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Proinflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 59: 1648–1656, 2010. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 60.Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab 93: 3215–3221, 2008. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- 61.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 12: 593–605, 2010. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aouadi M, Tencerova M, Vangala P, Yawe JC, Nicoloro SM, Amano SU, Cohen JL, Czech MP. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci U S A 110: 8278–8283, 2013. doi: 10.1073/pnas.1300492110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tardelli M, Zeyda K, Moreno-Viedma V, Wanko B, Grun NG, Staffler G, Zeyda M, Stulnig TM. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Mol Metab 5: 1131–1137, 2016. doi: 10.1016/j.molmet.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee H-M, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62: 194–204, 2013. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vandanmagsar B, Youm Y-H, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17: 179–188, 2011. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin Z, Deng T, Peterson LE, Yu R, Lin J, Hamilton DJ, Reardon PR, Sherman V, Winnier GE, Zhan M, Lyon CJ, Wong ST, Hsueh WA. Transcriptome analysis of human adipocytes implicates the NOD-like receptor pathway in obesity-induced adipose inflammation. Mol Cell Endocrinol 394: 80–87, 2014. doi: 10.1016/j.mce.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang T, Fang Z, Linghu KG, Liu J, Gan L, Lin L. Small molecule-driven SIRT3-autophagy-mediated NLRP3 inflammasome inhibition ameliorates inflammatory crosstalk between macrophages and adipocytes. Br J Pharmacol 177: 4645–4665, 2020. doi: 10.1111/bph.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. CY, Kang I, Harten IA, Gebe JA, Chan CK, Omer M, Alonge KM, den Hartigh LJ, Gomes Kjerulf D, Goodspeed L, Subramanian S, Wang S, Kim F, Birk DE, Wight TN, Chait A. Adipocyte-derived versican and macrophage-derived biglycan control adipose tissue inflammation in obesity. Cell Rep 31: 107818–107818, 2020. doi: 10.1016/j.celrep.2020.107818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jais A, Brüning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest 127: 24–32, 2017. doi: 10.1172/JCI88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramkhelawon B, Hennessy EJ, Ménager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner KJ, Moore KJ. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med 20: 377–384, 2014. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma M, Schlegel M, Brown EJ, Sansbury BE, Weinstock A, Afonso MS, Corr EM, van Solingen C, Shanley LC, Peled D, Ramasamy R, Schmidt AM, Spite M, Fisher EA, Moore, KJ. Netrin-1 alters adipose tissue macrophage fate and function in obesity. Immunometabolism 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu I, Yoshida Y, Moriya J, Nojima A, Uemura A, Kobayashi Y, Minamino T. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab 18: 491–504, 2013. doi: 10.1016/j.cmet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 73.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol 13: 136–143, 2012. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah R, Hinkle CC, Ferguson JF, Mehta NN, Li M, Qu L, Lu Y, Putt ME, Ahima RS, Reilly MP. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 60: 1512–1518, 2011. doi: 10.2337/db10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aleman JO, Iyengar NM, Walker JM, Milne GL, Da Rosa JC, Liang Y, Giri DD, Zhou XK, Pollak MN, Hudis CA, Breslow JL, Holt PR, Dannenberg AJ. Effects of rapid weight loss on systemic and adipose tissue inflammation and metabolism in obese postmenopausal women. J Endocr Soc 1: 625–637, 2017. doi: 10.1210/js.2017-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol 187: 1942–1949, 2011. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bijnen M, Josefs T, Cuijpers I, Maalsen CJ, van de Gaar J, Vroomen M, Wijnands E, Rensen SS, Greve JWM, Hofker MH, Biessen EAL, Stehouwer CDA, Schalkwijk CG, Wouters K. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut 67: 1317–1327, 2018. doi: 10.1136/gutjnl-2016-313654. [DOI] [PubMed] [Google Scholar]
- 78.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 19: 821–835, 2014. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332: 1284–1288, 2011. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haase J, Weyer U, Immig K, Klöting N, Blüher M, Eilers J, Bechmann I, Gericke M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia 57: 562–571, 2014. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 81.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58: 2574–2582, 2009. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56: 16–23, 2007. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 83.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes 59: 1171–1181, 2010. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 20: 614–625, 2014. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante, AW Jr.. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab 18: 816–830, 2013. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kowalski GM, Nicholls HT, Risis S, Watson NK, Kanellakis P, Bruce CR, Bobik A, Lancaster GI, Febbraio MA. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia 54: 888–899, 2011. doi: 10.1007/s00125-010-2020-5. [DOI] [PubMed] [Google Scholar]
- 88.O'Rourke RW, White AE, Metcalf MD, Winters BR, Diggs BS, Zhu X, Marks DL. Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism 61: 1152–1161, 2012. doi: 10.1016/j.metabol.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sindhu S, Thomas R, Kochumon S, Wilson A, Abu-Farha M, Bennakhi A, Al-Mulla F, Ahmad R. Increased adipose tissue expression of interferon regulatory factor (IRF)-5 in obesity: association with metabolic inflammation. Cells 8: 1418, 2019. doi: 10.3390/cells8111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeOliveira CC, Paiva Caria CR, Ferreira Gotardo EM, Ribeiro ML, Gambero A. Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. Eur J Pharmacol 799: 154–159, 2017. doi: 10.1016/j.ejphar.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 91.Pei Y, Li H, Cai Y, Zhou J, Luo X, Ma L, McDaniel K, Zeng T, Chen Y, Qian X, Huo Y, Glaser S, Meng F, Alpini G, Chen L, Wu C. Regulation of adipose tissue inflammation by adenosine 2A receptor in obese mice. J Endocrinol 239: 365–376, 2018. doi: 10.1530/JOE-18-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S, Yang L, Zhang M, Nan F, Li J, Liu J, Liu J, Jia W, Qiu Y, Song B, Han JJ, Rui L, Duan SZ, Liu Y. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol 18: 519–529, 2017. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 93.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447: 1116–1120, 2007. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prieur X, Mok CY, Velagapudi VR, Nuñez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O'Rahilly S, Sethi JK, Dopazo J, Oresic M, Ricote M, Vidal-Puig A. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 60: 797–809, 2011. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 20: 103–118, 2014. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dhorepatil A, Ball S, Ghosh RK, Kondapaneni M, Lavie CJ. Canakinumab: promises and future in cardiometabolic diseases and malignancy. Am J Med 132: 312–324, 2019. doi: 10.1016/j.amjmed.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 97.Ferraz-Amaro I, Arce-Franco M, Muñiz J, López-Fernández J, Hernández-Hernández V, Franco A, Quevedo J, Martínez-Martín J, Díaz-González F. Systemic blockade of TNF-α does not improve insulin resistance in humans. Horm Metab Res 43: 801–808, 2011. doi: 10.1055/s-0031-1287783. [DOI] [PubMed] [Google Scholar]
- 98.McDonnell ME, Ganley-Leal LM, Mehta A, Bigornia SJ, Mott M, Rehman Q, Farb MG, Hess DT, Joseph L, Gokce N, Apovian CM. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity (Silver Spring) 20: 1372–1378, 2012. doi: 10.1038/oby.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 100.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 49: 1894–1903, 2008. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Watanabe Y, Nagai Y, Honda H, Okamoto N, Yanagibashi T, Ogasawara M, Yamamoto S, Imamura R, Takasaki I, Hara H, Sasahara M, Arita M, Hida S, Taniguchi S, Suda T, Takatsu K. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J 33: 11821–11835, 2019. doi: 10.1096/fj.201900477RR. [DOI] [PubMed] [Google Scholar]
- 102.Hadad N, Burgazliev O, Elgazar-Carmon V, Solomonov Y, Wueest S, Item F, Konrad D, Rudich A, Levy R. Induction of cytosolic phospholipase a2alpha is required for adipose neutrophil infiltration and hepatic insulin resistance early in the course of high-fat feeding. Diabetes 62: 3053–3063, 2013. doi: 10.2337/db12-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 18: 1407–1412, 2012. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Blüher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28: 1304–1310, 2008. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 105.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 106.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, Sun Q, Satoskar AR, Lumeng C, Moffatt-Bruce S, Rajagopalan S. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One 6: e16376, 2011. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939, 2009. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pandolfi JB, Ferraro AA, Sananez I, Gancedo MC, Baz P, Billordo LA, Fainboim L, Arruvito LA. Induced inflammation drives tissue-resident Th17 Cells in metabolically unhealthy obesity. J Immunol 196: 3287–3296, 2016. doi: 10.4049/jimmunol.1502506. [DOI] [PubMed] [Google Scholar]
- 109.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15: 921–929, 2009. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Travers RL, Motta AC, Betts JA, Bouloumié A, Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes 39: 762–769, 2015. doi: 10.1038/ijo.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeyda M, Huber J, Prager G, Stulnig TM. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring) 19: 743–748, 2011. doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- 112.Chen D, Zhao H, Gao X, Chen S, Liu H, Zhang J, Zhang J, Meng M. Subcutaneous administration of alpha-GalCer activates iNKT10 cells to promote M2 macrophage polarization and ameliorates chronic inflammation of obese adipose tissue. Int Immunopharmacol 77: 105948, 2019. doi: 10.1016/j.intimp.2019.105948. [DOI] [PubMed] [Google Scholar]
- 113.O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, Jobe BA, Roberts CT Jr, Slifka MK, Marks DL. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 33: 978–990, 2009. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes 62: 3180–3188, 2013. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X, Jiang L, Yang M, Wu YW, Sun JZ. Impact of weight cycling on CTRP3 expression, adipose tissue inflammation and insulin sensitivity in C57BL/6J mice. Exp Ther Med 16: 2052–2059, 2018. doi: 10.3892/etm.2018.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goldberg EL, Shchukina I, Asher JL, Sidorov S, Artyomov MN, Dixit VD. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat Metab 2: 50–61, 2020. doi: 10.1038/s42255-019-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 17: 610–617, 2011. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duffaut C, Galitzky J, Lafontan M, Bouloumié A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun 384: 482–485, 2009. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 119.Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, Kantarci A, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia 53: 1461–1471, 2010. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumié A, Gual P, Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61: 2238–2247, 2012. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 15: 940–945, 2009. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stefanovic-Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL, Sipula IJ, Dedousis N, Scott DK, Morel PA, Thomson AW, O'Doherty RM. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes 61: 2330–2339, 2012. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Porsche CE, Delproposto JB, Patrick E, Zamarron BF, Lumeng CN. Adipose tissue dendritic cell signals are required to maintain T cell homeostasis and obesity-induced expansion. Mol Cell Endocrinol 505: 110740, 2020. doi: 10.1016/j.mce.2020.110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332: 243–247, 2011. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 3: 716–724, 2007. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 126.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389: 610–614, 1997. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 127.Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller DE. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 46: 1526–1531, 1997. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- 128.Kuroda M, Nishiguchi M, Ugawa N, Ishikawa E, Kawabata Y, Okamoto S, Sasaki W, Miyatake Y, Sebe M, Masumoto S, Tsutsumi R, Harada N, Sakaue H. Interferon regulatory factor 7 mediates obesity-associated MCP-1 transcription. PloS one 15: e0233390, 2020. doi: 10.1371/journal.pone.0233390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab 90: 2282–2289, 2005. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 130.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mulder P, van den Hoek AM, Kleemann R. The CCR2 inhibitor propagermanium attenuates diet-induced insulin resistance, adipose tissue inflammation and non-alcoholic steatohepatitis. PLoS One 12: e0169740, 2017. doi: 10.1371/journal.pone.0169740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tamura Y, Sugimoto M, Murayama T, Ueda Y, Kanamori H, Ono K, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol 28: 2195–2201, 2008. doi: 10.1161/ATVBAHA.108.168633. [DOI] [PubMed] [Google Scholar]
- 133.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr.. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006[Erratum inJ Clin Invest 116: 1457, 2006]. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148: 4687–4694, 2007. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 135.Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, Morrison S, Carless M, Dyer TD, Cole SA, Goring HH, Moses EK, Walder K, Cawthorne MA, Blangero J, Jowett JB. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab 95: 2476–2485, 2010. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Frühbeck G. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-alpha stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg Obes Relat Dis 9: 306–314, 2013. doi: 10.1016/j.soard.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 137.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med 205: 767–775, 2008. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett 582: 573–578, 2008. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 139.Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes 53: 1979–1986, 2004. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- 140.Catalan V, Gomez-Ambrosi J, Rodríguez A, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Fernández-Real JM, Salvador J, Frühbeck G. Increased levels of calprotectin in obesity are related to macrophage content: impact on inflammation and effect of weight loss. Mol Med 17: 1157–1167, 2011. doi: 10.2119/molmed.2011.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mortensen OH, Nielsen AR, Erikstrup C, Plomgaard P, Fischer CP, Krogh-Madsen R, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK. Calprotectin–a novel marker of obesity. PLoS One 4: e7419, 2009. doi: 10.1371/journal.pone.0007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest 117: 1718–1726, 2007. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145: 367–383, 2004. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 144.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277: 25863–25866, 2002. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 145.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Mohlig M, Pfeiffer AF, Luft FC, Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes 52: 942–947, 2003. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 146.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 147.Pastel E, Joshi S, Knight B, Liversedge N, Ward R, Kos K. Effects of Exendin-4 on human adipose tissue inflammation and ECM remodelling. Nutr Diabetes 6: e235, 2016. doi: 10.1038/nutd.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohlsson C, Hammarstedt A, Vandenput L, Saarinen N, Ryberg H, Windahl SH, Farman HH, Jansson JO, Moverare-Skrtic S, Smith U, Zhang FP, Poutanen M, Hedjazifar S, Sjögren K. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am J Physiol Endocrinol Metab 313: E450–E462, 2017. doi: 10.1152/ajpendo.00093.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qi X, Zhang B, Zhao Y, Li R, Chang HM, Pang Y, Qiao J. Hyperhomocysteinemia promotes insulin resistance and adipose tissue inflammation in PCOS mice through modulating M2 macrophage polarization via estrogen suppression. Endocrinology 158: 1181–1193, 2017. doi: 10.1210/en.2017-00039. [DOI] [PubMed] [Google Scholar]
- 150.Ter Horst R, van den Munckhof ICL, Schraa K, Aguirre-Gamboa R, Jaeger M, Smeekens SP, Brand T, Lemmers H, Dijkstra H, Galesloot TE, de Graaf J, Xavier RJ, Li Y, Joosten LAB, Rutten JHW, Netea MG, Riksen NP. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol 40: 1787–1800, 2020. doi: 10.1161/ATVBAHA.120.314508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou L, Park SY, Xu L, Xia X, Ye J, Su L, Jeong KH, Hur JH, Oh H, Tamori Y, Zingaretti CM, Cinti S, Argente J, Yu M, Wu L, Ju S, Guan F, Yang H, Choi CS, Savage DB, Li P. Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nat Commun 6: 5949, 2015. doi: 10.1038/ncomms6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280: 847–851, 2005[Erratum inJ Biol Chem 280: 30648, 2005]. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 153.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 154.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep 2: 799, 2012. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim JH, Lee E, Friedline RH, Suk S, Jung DY, Dagdeviren S, Hu X, Inashima K, Noh HL, Kwon JY, Nambu A, Huh JR, Han MS, Davis RJ, Lee AS, Lee KW, Kim JK. Endoplasmic reticulum chaperone GRP78 regulates macrophage function and insulin resistance in diet-induced obesity. FASEB J 32: 2292–2304, 2018. doi: 10.1096/fj.201701017R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang M, Chen X, Zheng Z, Yu S, Zhou B, Liu Y, Liu D, Chen Y, Qian X. Beneficial effect of ER stress preconditioning in protection against FFA-induced adipocyte inflammation via XBP1 in 3T3-L1 adipocytes. Mol Cell Biochem 463: 45–55, 2020. doi: 10.1007/s11010-019-03627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 16: 1248–1255, 2008. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 158.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121: 1858–1870, 2011. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 160.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab 93: 4532–4541, 2008. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Poledne R, Malinska H, Kubatova H, Fronek J, Thieme F, Kauerova S, Lesna IK. Polarization of macrophages in human adipose tissue is related to the fatty acid spectrum in membrane phospholipids. Nutrients 12: 8, 2019. doi: 10.3390/nu12010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, 2010. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xia B, Cai GH, Yang H, Wang SP, Mitchell GA, Wu JW. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet 13: e1007110, 2017. doi: 10.1371/journal.pgen.1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S, Blaner WS, Snitker S, O'Connell JR, Gong DW, Breyer RJ 3rd, Ryan AS, McLenithan JC, Shuldiner AR, Sztalryd C, Damcott CM. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N Engl J Med 370: 2307–2315, 2014. doi: 10.1056/NEJMoa1315496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dou HX, Wang T, Su HX, Gao DD, Xu YC, Li YX, Wang HY. Exogenous FABP4 interferes with differentiation, promotes lipolysis and inflammation in adipocytes. Endocrine 67: 587–596, 2020. doi: 10.1007/s12020-019-02157-8. [DOI] [PubMed] [Google Scholar]
- 167.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, Farese, RV Jr.. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest 120: 756–767, 2010[Erratum inJ Clin Invest 121: 1667, 2011]. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nicholls HT, Kowalski G, Kennedy DJ, Risis S, Zaffino LA, Watson N, Kanellakis P, Watt MJ, Bobik A, Bonen A, Febbraio M, Lancaster GI, Febbraio MA. Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes 60: 1100–1110, 2011. doi: 10.2337/db10-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 58: 104–115, 2009. doi: 10.2337/db07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol 29: 1944–1949, 2009. doi: 10.1161/atvbaha.109.194050. [DOI] [PubMed] [Google Scholar]
- 171.Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E, Weir J, Mellett NA, Pernes G, Conway JRW, Lee MKS, Timpson P, Murphy AJ, Masters SL, Gerondakis S, Bartonicek N, Kaczorowski DC, Dinger ME, Meikle PJ, Bond PJ, Febbraio MA. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab 27: 1096–1110, 2018. doi: 10.1016/j.cmet.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 172.Becerril S, Rodriguez A, Catalan V, Mendez-Gimenez L, Ramirez B, Sainz N, Llorente M, Unamuno X, Gomez-Ambrosi J, Frühbeck G. Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: role of tenascin C. Int J Obes 42: 1458–1470, 2018. doi: 10.1038/s41366-018-0005-5. [DOI] [PubMed] [Google Scholar]
- 173.Catalan V, Gomez-Ambrósi J, Rodríguez A, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Salvador J, Frühbeck G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J Clin Endocrinol Metab 97: E1880–1889, 2012. doi: 10.1210/jc.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50: 1267–1276, 2007. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 175.Ghosh AK, O'Brien M, Mau T, Yung R. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging (Albany NY) 9: 1971–1982, 2017. doi: 10.18632/aging.101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin HY, Weng SW, Shen FC, Chang YH, Lian WS, Hsieh CH, Chuang JH, Lin TK, Liou CW, Chang CS, Lin CY, Su YJ, Wang PW. Abrogation of Toll-like receptor 4 mitigates obesity-induced oxidative stress, proinflammation, and insulin resistance through metabolic reprogramming of mitochondria in adipose tissue. Antioxid Redox Signal 33: 66–86, 2020. doi: 10.1089/ars.2019.7737. [DOI] [PubMed] [Google Scholar]
- 177.Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, Lee S, Scherer PE, Elmquist JK. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun 5: 3878, 2014. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab 10: 419–429, 2009. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gunasekaran MK, Virama-Latchoumy AL, Girard AC, Planesse C, Guérin-Dubourg A, Ottosson L, Andersson U, Césari M, Roche R, Hoareau L. TLR4-dependant proinflammatory effects of HMGB1 on human adipocyte. Adipocyte 5: 384–388, 2016. doi: 10.1080/21623945.2016.1245818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3: 559–572, 2011. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 182.Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, Almendros I, Gileles-Hillel A, Qiao Z, Hubert N, Farre R, Chang EB, Gozal D. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep 6: 35405, 2016. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thomalla M, Schmid A, Neumann E, Pfefferle PI, Müller-Ladner U, Schäffler A, Karrasch T. Evidence of an anti-inflammatory toll-like receptor 9 (TLR 9) pathway in adipocytes. J Endocrinol 240: 325–343, 2019. doi: 10.1530/JOE-18-0326. [DOI] [PubMed] [Google Scholar]
- 184.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46: 2347–2355, 2005. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 185.Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, Mazula DL, Palmer AK, Baker DJ, Jensen MD, Torbenson MS, Miller JD, Ikeda Y, Tchkonia T, van Deursen JM, Kirkland JL, LeBrasseur NK. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65: 1606–1615, 2016. doi: 10.2337/db15-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N, Sano M. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest 126: 4626–4639, 2016. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Villaret A, Galitzky J, Decaunes P, Estève D, Marques MA, Sengenès C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, Bouloumié A. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 59: 2755–2763, 2010. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15: 1082–1087, 2009. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 189.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Q, Dong Z, Liu X, Song X, Song Q, Shang Q, Jiang Y, Guo C, Zhang L. Programmed cell death-4 deficiency prevents diet-induced obesity, adipose tissue inflammation, and insulin resistance. Diabetes 62: 4132–4143, 2013. doi: 10.2337/db13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93: 1–21, 2013. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 192.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124: 67–76, 2011. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 193.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 195.O'Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, Cheang EC, Varlamov O, Corless CL, Roberts CT Jr, Marks DL. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia 54: 1480–1490, 2011. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 197.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58: 718–725, 2009. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Snodgrass RG, Boß M, Zezina E, Weigert A, Dehne N, Fleming I, Brüne B, Namgaladze D. Hypoxia potentiates palmitate-induced proinflammatory activation of primary human macrophages. J Biol Chem 291: 413–424, 2016. doi: 10.1074/jbc.M115.686709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, Quehenberger O, Johnson RS, Olefsky JM. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 157: 1339–1352, 2014. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poblete JMS, Ballinger MN, Bao S, Alghothani M, Nevado JB Jr, Eubank TD, Christman JW, Magalang UJ. Macrophage HIF-1α mediates obesity-related adipose tissue dysfunction via interleukin-1 receptor-associated kinase M. Am J Physiol Endocrinol Metab 318: E689–E700, 2020. doi: 10.1152/ajpendo.00174.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657, 2003[Erratum inCell113: 419, 2003]. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ali MM, Phillips SA, Mahmoud AM. HIF1alpha/TET1 pathway mediates hypoxia-induced adipocytokine promoter hypomethylation in human adipocytes. Cells 9: 134, 2020. doi: 10.3390/cells9010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, Tobe K. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia 56: 1403–1412, 2013. doi: 10.1007/s00125-013-2885-1. [DOI] [PubMed] [Google Scholar]
- 204.Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9: e94713, 2014. doi: 10.1371/journal.pone.0094713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8: e70703, 2013. doi: 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arngrim N, Simonsen L, Holst JJ, Bülow J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond) 37: 748–750, 2013. doi: 10.1038/ijo.2012.98. [DOI] [PubMed] [Google Scholar]
- 207.Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med 366: 2136–2137, 2012. doi: 10.1056/NEJMc1201684. [DOI] [PubMed] [Google Scholar]
- 208.García Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, Mehrara BJ. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 40: 1582–1590, 2016. doi: 10.1038/ijo.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nitti MD, Hespe GE, Kataru RP, Garcia Nores GD, Savetsky IL, Torrisi JS, Gardenier JC, Dannenberg AJ, Mehrara BJ. Obesity-induced lymphatic dysfunction is reversible with weight loss. J Physiol 594: 7073–7087, 2016. doi: 10.1113/JP273061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Torrisi JS, Hespe GE, Cuzzone DA, Savetsky IL, Nitti MD, Gardenier JC, García Nores GD, Jowhar D, Kataru RP, Mehrara BJ. Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci Rep 6: 19817, 2016. doi: 10.1038/srep19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, Mehrara BJ. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 307: H165–H172, 2014. doi: 10.1152/ajpheart.00244.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol 194: 5200–5210, 2015. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sato A, Kamekura R, Kawata K, Kawada M, Jitsukawa S, Yamashita K, Sato N, Himi T, Ichimiya S. Novel mechanisms of compromised lymphatic endothelial cell homeostasis in obesity: the role of leptin in lymphatic endothelial cell tube formation and proliferation. PLoS One 11: e0158408, 2016. doi: 10.1371/journal.pone.0158408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shimizu Y, Shibata R, Ishii M, Ohashi K, Kambara T, Uemura Y, Yuasa D, Kataoka Y, Kihara S, Murohara T, Ouchi N. Adiponectin-mediated modulation of lymphatic vessel formation and lymphedema. J Am Heart Assoc 2: e000438, 2013. doi: 10.1161/JAHA.113.000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW Jr.. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120: 3466–3479, 2010. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lacerda DR, Costa KA, Silveira ALM, Rodrigues DF, Silva AN, Sabino JL, Pinho V, Menezes GB, Soares DD, Teixeira MM, Ferreira AVM. Role of adipose tissue inflammation in fat pad loss induced by fasting in lean and mildly obese mice. J Nutr Biochem 72: 108208, 2019. doi: 10.1016/j.jnutbio.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 217.Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, DelProposto JL, Geletka LM, Muir LA, Lumeng CN. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes 66: 392–406, 2017. doi: 10.2337/db16-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Capel F, Klimcáková E, Viguerie N, Roussel B, Vítková M, Kováciková M, Polák J, Kovácová Z, Galitzky J, Maoret JJ, Hanácek J, Pers TH, Bouloumié A, Stich V, Langin D. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 58: 1558–1567, 2009. doi: 10.2337/db09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hagman DK, Larson I, Kuzma JN, Cromer G, Makar K, Rubinow KB, Foster-Schubert KE, van Yserloo B, Billing PS, Landerholm RW, Crouthamel M, Flum DR, Cummings DE, Kratz M. The short-term and long-term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism 70: 12–22, 2017. doi: 10.1016/j.metabol.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kratz M, Hagman DK, Kuzma JN, Foster-Schubert KE, Chan CP, Stewart S, van Yserloo B, Westbrook EO, Arterburn DE, Flum DR, Cummings DE. Improvements in glycemic control after gastric bypass occur despite persistent adipose tissue inflammation. Obesity (Silver Spring) 24: 1438–1445, 2016. doi: 10.1002/oby.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tam CS, Covington JD, Ravussin E, Redman LM; Pennington CALERIE Team. Little evidence of systemic and adipose tissue inflammation in overweight individuals(dagger). Front Genet 3: 58, 2012. doi: 10.3389/fgene.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Viguerie N, Vidal H, Arner P, Holst C, Verdich C, Avizou S, Astrup A, Saris WH, Macdonald IA, Klimcakova E, Clément K, Martinez A, Hoffstedt J, Sørensen TI, Langin D. Nutrient-Gene Interactions in Human Obesity–Implications for Dietary Guideline p. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia 48: 123–131, 2005. doi: 10.1007/s00125-004-1618-x. [DOI] [PubMed] [Google Scholar]
- 223.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las Fuentes L, He S, Okunade AL, Patterson BW, Klein S. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 23: 591–601, 2016. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Šrámková V, Rossmeislová L, Krauzová E, Kračmerová J, Koc M, Langin D, Štich V, Šiklová M. Comparison of early (2 days) and later (28 days) response of adipose tissue to very low-calorie diet in obese women. J Clin Endocrinol Metab 101: 5021–5029, 2016. doi: 10.1210/jc.2016-2161. [DOI] [PubMed] [Google Scholar]
- 225.Baek KW, Lee DI, Kang SA, Yu HS. Differences in macrophage polarization in the adipose tissue of obese mice under various levels of exercise intensity. J Physiol Biochem 76: 159–168, 2020. doi: 10.1007/s13105-020-00731-7. [DOI] [PubMed] [Google Scholar]
- 226.Luo W, Ai L, Wang B, Wang L, Gan Y, Liu C, Jensen J, Zhou Y. Eccentric exercise and dietary restriction inhibits M1 macrophage polarization activated by high-fat diet-induced obesity. Life Sci 243: 117246, 2020. doi: 10.1016/j.lfs.2019.117246. [DOI] [PubMed] [Google Scholar]
- 227.Johannsen DL, Tchoukalova Y, Tam CS, Covington JD, Xie W, Schwarz JM, Bajpeyi S, Ravussin E. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the "adipose tissue expandability" hypothesis. Diabetes Care 37: 2789–2797, 2014. doi: 10.2337/dc14-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tam CS, Viardot A, Clement K, Tordjman J, Tonks K, Greenfield JR, Campbell LV, Samocha-Bonet D, Heilbronn LK. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes 59: 2164–2170, 2010. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Auerbach P, Nordby P, Bendtsen LQ, Mehlsen JL, Basnet SK, Vestergaard H, Ploug T, Stallknecht B. Differential effects of endurance training and weight loss on plasma adiponectin multimers and adipose tissue macrophages in younger, moderately overweight men. Am J Physiol Regul Integr Comp Physiol 305: R490–R498, 2013. doi: 10.1152/ajpregu.00575.2012. [DOI] [PubMed] [Google Scholar]
- 230.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54: 2277–2286, 2005. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 231.Kováčiková M, Sengenès C, Kováčová Z, Síklová-Vítková M, Klimčaková E, Polak J, Rossmeislová L, Bajzová M, Hejnová J, Hněvkovská Z, Bouloumié A, Langin D, Stich V. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int J Obes (Lond) 35: 91–98, 2011. doi: 10.1038/ijo.2010.112. [DOI] [PubMed] [Google Scholar]
- 232.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 94: 4619–4623, 2009. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 233.Viardot A, Lord RV, Samaras K. The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab 95: 2845–2850, 2010. doi: 10.1210/jc.2009-2371. [DOI] [PubMed] [Google Scholar]
- 234.Pardina E, Ferrer R, Baena-Fustegueras JA, Rivero J, Lecube A, Fort JM, Vargas V, Catalán R, Peinado-Onsurbe J. Only C-reactive protein, but not TNF-alpha or IL6, reflects the improvement in inflammation after bariatric surgery. Obes Surg 22: 131–139, 2012. doi: 10.1007/s11695-011-0546-3. [DOI] [PubMed] [Google Scholar]
- 235.Schmitz J, Evers N, Awazawa M, Nicholls HT, Brönneke HS, Dietrich A, Mauer J, Blüher M, Brüning JC. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol Metab 5: 328–339, 2016. doi: 10.1016/j.molmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.de Farias T, Cruz MM, de Sa R, Severi I, Perugini J, Senzacqua M, Cerutti SM, Giordano A, Cinti S, Alonso-Vale MIC. Melatonin supplementation decreases hypertrophic obesity and inflammation induced by high-fat diet in mice. Front Endocrinol (Lausanne) 10: 750, 2019. doi: 10.3389/fendo.2019.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Farias T, Paixao RID, Cruz MM, de Sa R, Simão JJ, Antraco VJ, Alonso-Vale MIC. Melatonin supplementation attenuates the proinflammatory adipokines expression in visceral fat from obese mice induced by a high-fat diet. Cells 8: 1041, 2019. doi: 10.3390/cells8091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang L, Wang Y, Zhang C, Li J, Meng Y, Dou M, Noguchi CT, Di L. Inhibiting glycogen synthase kinase 3 reverses obesity-induced white adipose tissue inflammation by regulating apoptosis inhibitor of macrophage/CD5L-mediated macrophage migration. Arterioscler Thromb Vasc Biol 38: 2103–2116, 2018. doi: 10.1161/ATVBAHA.118.311363. [DOI] [PubMed] [Google Scholar]
- 239.Muniappan L, Javidan A, Jiang W, Mohammadmoradi S, Moorleghen JJ, Katz WS, Balakrishnan A, Howatt DA, Subramanian V. Calpain inhibition attenuates adipose tissue inflammation and fibrosis in diet-induced obese mice. Sci Rep 7: 14398, 2017. doi: 10.1038/s41598-017-14719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Petersen PS, Lei X, Wolf RM, Rodriguez S, Tan SY, Little HC, Schweitzer MA, Magnuson TH, Steele KE, Wong GW. CTRP7 deletion attenuates obesity-linked glucose intolerance, adipose tissue inflammation, and hepatic stress. Am J Physiol Endocrinol Metab 312: E309–E325, 2017. doi: 10.1152/ajpendo.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]