Abstract
Objective
To examine the relationship between SARS-CoV-2 infection during pregnancy and the risk for preeclampsia.
Data Sources
MEDLINE, Embase, POPLINE, CINAHL, LILACS, and the World Health Organization COVID-19, Chinese, and preprint databases (all from December 1, 2019, to May 31, 2021). Google Scholar, bibliographies, and conference proceedings were also searched.
Study Eligibility Criteria
Observational studies that assessed the association between SARS-CoV-2 infection during pregnancy and preeclampsia and that reported unadjusted and/or adjusted risk estimates and 95% confidence intervals or data to calculate them.
Study Appraisal and Synthesis Methods
The primary outcome was preeclampsia. Secondary outcomes included preeclampsia with severe features, preeclampsia without severe features, eclampsia, and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. Two reviewers independently reviewed studies for inclusion, assessed their risk of bias, and extracted data. Pooled unadjusted and adjusted odds ratios with 95% confidence intervals, and 95% prediction interval were calculated. Heterogeneity was quantified using the І2 statistic, for which І2≥30% indicated substantial heterogeneity. Subgroup and sensitivity analyses were performed to test the robustness of the overall findings.
Results
A total of 28 studies comprising 790,954 pregnant women, among which 15,524 were diagnosed with SARS-CoV-2 infection, met the inclusion criteria. The meta-analysis of unadjusted odds ratios showed that the odds of developing preeclampsia were significantly higher among pregnant women with SARS-CoV-2 infection than among those without SARS-CoV-2 infection (7.0% vs 4.8%; pooled odds ratio, 1.62; 95% confidence interval, 1.45–1.82; P<.00001; І2=17%; 26 studies; 95% prediction interval of the odds ratio, 1.28–2.05). The meta-analysis of adjusted odds ratios also showed that SARS-CoV-2 infection during pregnancy was associated with a significant increase in the odds of preeclampsia (pooled odds ratio, 1.58; 95% confidence interval, 1.39–1.80; P<.0001; І2=0%; 11 studies). There was a statistically significant increase in the odds of preeclampsia with severe features (odds ratio, 1.76; 95% confidence interval, 1.18–2.63; І2=58%; 7 studies), eclampsia (odds ratio, 1.97; 95% confidence interval, 1.01–3.84; І2=0%, 3 studies), and HELLP syndrome (odds ratio, 2.10; 95% confidence interval, 1.48–2.97; 1 study) among pregnant women with SARS-CoV-2 infection when compared to those without the infection. Overall, the direction and magnitude of the effect of SARS-CoV-2 infection during pregnancy on preeclampsia was consistent across most prespecified subgroup and sensitivity analyses. Both asymptomatic and symptomatic SARS-CoV-2 infections significantly increased the odds of developing preeclampsial; however, it was higher among patients with symptomatic illness (odds ratio, 2.11; 95% confidence interval, 1.59–2.81) than among those with asymptomatic illness (odds ratio, 1.59; 95% confidence interval, 1.21–2.10).
Conclusion
SARS-CoV-2 during pregnancy is associated with higher odds of preeclampsia.
Key words: coronavirus, COVID-19, eclampsia, HELLP syndrome, hepatic damage, hypertension, hypertensive disorders of pregnancy, liver enzymes, maternal morbidity, preeclampsia with severe features, preeclampsia without severe features, proteinuria, thrombocytopenia, viral infection
Introduction
Preeclampsia, a multisystem syndrome that complicates about 5% of pregnancies, is one of the leading causes of maternal mortality worldwide, accounting for approximately 14% of all maternal deaths.1 , 2 In 2018, preeclampsia was responsible for 5.3% of maternal deaths in the United States.3 Preeclampsia is also associated with an increased risk for maternal morbidity and perinatal morbidity and mortality worldwide, mainly in low- and middle-income countries.4, 5, 6, 7, 8, 9, 10, 11, 12 In addition, women with preeclampsia are at a greater risk for developing cardiovascular disease later in life.7 , 11, 12, 13 Although the etiology of preeclampsia remains unclear, it is currently believed that abnormal placentation leading to later placental malperfusion and dysfunction, syncytiotrophoblast stress, oxidative stress, imbalances in circulating placental angiogenic factors, perturbation of the renin-angiotensin system (RAS), placental senescence, inflammation, endothelial dysfunction, and immune abnormalities influenced by maternal genetics, epigenetics, lifestyle, and environmental factors are involved in the pathophysiology of this disorder.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29
AJOG at a Glance.
Why was this study conducted?
Current evidence indicates that urinary tract infections and periodontal disease during pregnancy are associated with an increased risk for preeclampsia. We performed a systematic review and meta-analysis to assess whether SARS-CoV-2 infection during pregnancy also increases the risk for preeclampsia.
Key findings
Pregnant women with a SARS-CoV-2 infection had significantly increased odds of developing preeclampsia when compared to those without the infection (pooled odds ratio, 1.62; 95% confidence interval, 1.45-1.82). Moreover, SARS-CoV-2 infection during pregnancy was associated with increased odds of developing preeclampsia with severe features, eclampsia, and hemolysis, elevated liver enzymes, low platelet count syndrome. Both asymptomatic and symptomatic SARS-CoV-2 infections significantly increased the risk for preeclampsia.
What does this add to what is known?
Pregnant women with a SARS-CoV-2 infection are more likely to develop preeclampsia. Healthcare professionals should be aware of this association and closely monitor pregnant women with SARS-CoV-2 infection for early detection of preeclampsia.
In 2008, our group published a systematic review and meta-analysis that demonstrated that urinary tract infection and periodontal disease during pregnancy were associated with a significantly increased risk of preeclampsia.30 Similar findings were reported in more recent meta-analyses.31, 32, 33, 34, 35, 36, 37 Several studies have assessed the relationship between viral infections during pregnancy, such as those caused by HIV,38 , 39 human papillomavirus,40 , 41 cytomegalovirus,42, 43, 44 hepatitis B virus,45, 46, 47, 48, 49 herpes simplex virus,50 , 51 Epstein-Barr virus,52 and influenza A (H1N1),53 and the risk for preeclampsia. The results of these studies have been conflicting. The mechanisms that have been proposed to explain the association between infection during pregnancy and preeclampsia include (1) direct effects of the infectious agents on trophoblast function and the arterial wall, including endothelial injury or dysfunction; (2) acute atherosis; (3) local inflammation that might cause relative uteroplacental ischemia; and (4) indirect effects through exaggerated maternal systemic inflammatory response.54, 55, 56, 57, 58, 59
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was first reported in China in December 2019.60 Individuals of all ages are at risk for infection and severe disease. Current evidence suggests that pregnancy does not increase susceptibility to SARS-CoV-2 infection, but it appears to worsen the clinical course of COVID-19 when compared to nonpregnant females of the same age.61 , 62 Overall, pregnant women with COVID-19 were at higher risk for intensive care unit (ICU) admission, invasive mechanical ventilation use, need for extra corporeal membrane oxygenation, and maternal death than nonpregnant women with COVID-19.61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74
Three meta-analyses have compared the risk for adverse maternal and perinatal outcomes between pregnant women with and without SARS-CoV-2 infection.62 , 75 , 76 Results from these studies indicate that pregnant women with SARS-CoV-2 infection have a significant increase in the risk for maternal death, admission to the ICU, preterm birth, and stillbirth when compared to those without SARS-CoV-2 infection. Moreover, infants born to mothers with SARS-CoV-2 infection were more likely to be admitted to the neonatal ICU than those born to mothers without the disease. The relationship between SARS-CoV-2 infection during pregnancy and the risk for preeclampsia has received less attention. This topic is relevant for public health and clinical practice. Hence, we performed a systematic review with the primary aim of compiling and critically assessing the existing evidence about the relationship between SARS-CoV-2 infection during pregnancy and the risk for preeclampsia by using formal methods of systematic review and meta-analytic techniques.
Material and Methods
This systematic review was conducted in accordance with a prospectively registered protocol (PROSPERO number CRD42021239092) and reported in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for meta-analyses of observational studies.77 Both authors independently reviewed studies for inclusion, assessed their risk of bias, and extracted data. Disagreements were resolved through consensus.
Literature search
To identify potentially eligible studies, we searched MEDLINE, Embase, CINAHL, LILACS, the World Health Organization COVID-19 database, China National Knowledge Infrastructure, Wanfang, and preprint databases such as medRxiv, bioRxiv, and search.bioPreprint (all from December 1, 2019, to May 31, 2021). Our search terms included a combination of keywords and text words related to SARS-CoV-2 (“SARS-CoV-2,” “COVID-19,” “2019-nCoV,” “nCov-2019,” “SARS-CoV-19,” “coronavirus,” “betacoronavirus,” and “severe acute respiratory syndrome”), preeclampsia (“preeclampsia,” “eclampsia,” “gestosis, EPH,” “pregnancy toxemia,” “pregnancy-induced hypertension,” “hypertensive disorders of pregnancy,” “gestational hypertension,” “pregnancy-associated hypertension,” and “pregnancy hypertension”), and pregnancy (“pregnancy,” “gestation,” and “gravidity”). Google Scholar, proceedings of congresses on obstetrics, maternal-fetal medicine, pediatrics, and neonatology, reference lists of identified studies, previously published systematic reviews, and review articles were also searched. No language restrictions were applied.
Study selection
We included observational studies that assessed the relationship between SARS-CoV-2 infection during pregnancy and preeclampsia and reported unadjusted and/or adjusted odds ratio (OR) or relative risk (RR) estimates and 95% confidence intervals (CIs) or data to calculate these values. The exposed group were pregnant women with a current or previous diagnosis of SARS-CoV-2 infection at any stage of gestation, which was based on a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result or positive antigen test result in a sample collected from the upper respiratory tract, or a positive result for anti–SARS-CoV-2 antibodies in serum. The unexposed group were pregnant women with a negative RT-PCR or antigen test result in a sample collected from the upper respiratory tract or a negative serum antibody test result, or those who were pregnant and delivered before the pandemic. Given that no routine diagnostic testing for SARS-CoV-2 infection was available at the beginning of the pandemic, we included studies in which pregnant women were assigned to exposed or unexposed groups based on the presence or absence of clinical signs or symptoms and/or computed tomography or radiography images of the chest.
Studies were excluded from the review if they (1) were case series or reports, editorials, comments, reviews, or letters without original data; (2) examined only the relationship between SARS-CoV-2 infection and gestational hypertension (new onset hypertension at ≥20 weeks of gestation in the absence of proteinuria or new signs of end-organ dysfunction); or (3) did not report risk estimates or CIs and sufficient information to calculate these values could not be retrieved. Studies published only as abstracts were excluded if information on methodological issues and results were not clearly reported. If a study included women with preeclampsia and gestational hypertension, it was not considered for inclusion in the review unless the data for women with preeclampsia were extractable or obtainable separately. For multiple or duplicate publications of the same dataset, we included only the most recent or complete study and supplemented it if additional information appeared in other publications.
Outcome measures
The primary outcome was preeclampsia, defined as hypertension (at or after 20 weeks of gestation in a previously normotensive woman, or that precedes pregnancy or is present before 20 weeks of gestation in a woman with preexisting chronic hypertension) accompanied by one or more of the following features at or after 20 weeks of gestation: proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, neurologic complications,78 , 79 or fetal growth restriction.79 Secondary outcomes included preeclampsia with severe features, preeclampsia without severe features, eclampsia, and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome.
Risk of bias assessment
The risk of bias in each included study was assessed by the judgement of 6 domains that were deemed by the authors to be important for the quality of observational studies evaluating the association between SARS-CoV-2 infection during pregnancy and preeclampsia. Each domain was judged as a “low,” “high,” or “unclear” risk of bias. The domains that were evaluated and how they were interpreted were as follows:
-
1.
Selection of participants – “low risk of bias”: women, both with and without SARS-CoV-2 infection, were recruited from the same population and during the same time period; “high risk of bias”: women, both with and without SARS-CoV-2 infection, were not recruited from the same population or during the same time period.
-
2.
Inclusion in the exposed cohort – “low risk of bias”: ≥90% of included women had a positive RT-PCR or antigen test result or tested positive for the presence of serum SARS-CoV-2 antibodies; “high risk of bias”: <90% of included women had a positive RT-PCR or antigen test result or tested positive for serum SARS-CoV-2 antibodies.
-
3.
Inclusion in the non-exposed cohort – “low risk of bias”: ≥90% of included women had a negative RT-PCR or antigen test result or tested negative for the presence of serum SARS-CoV-2 antibodies, or women were drawn from a historical control group who delivered before the beginning of the COVID-19 pandemic; “high risk of bias”: <90% of included women had a negative RT-PCR or antigen test result or tested negative for serum SARS-CoV-2 antibodies.
-
4.
Loss to follow-up or exclusions – “low risk of bias”: losses to follow-up or nonvalid exclusions <10%; “high risk of bias”: losses to follow-up or nonvalid exclusions ≥10%.
-
5.
Control for confounding factors – “low risk of bias”: the study controlled for potential confounding factors related to both SARS-CoV-2 infection and preeclampsia; “high risk of bias”: the study did not control for potential confounding factors related to both SARS-CoV-2 infection and preeclampsia.
-
6.
Temporality between the exposure and outcome – “low risk of bias”: the study reported the time elapsed between SARS-CoV-2 infection diagnosis and preeclampsia diagnosis; “high risk of bias”: the study did not report the time elapsed between SARS-CoV-2 infection diagnosis and preeclampsia diagnosis.
If there was insufficient information available to make a judgment about the bias of a domain, the domain was scored as having an “unclear risk of bias.”
Data extraction
Data were extracted by using a standardized data collection form. The following information was extracted from each study: first author’s name, date of publication, geographic location of the study, study design, inclusion and exclusion criteria, characteristics of the study population, sample size, case definition, tests used for diagnosing SARS-CoV-2 infection, gestational age at diagnosis of SARS-CoV-2 infection, severity of SARS-CoV-2 infection, criteria used to include women in the unexposed group, mean or median time elapsed between SARS-CoV-2 infection diagnosis and preeclampsia diagnosis, definition and severity of preeclampsia, confounding factors controlled for by matching or adjustment, report of dose-response gradient, and unadjusted and/or adjusted RRs or ORs with 95% CIs. The corresponding authors of primary studies were contacted to obtain additional information on methods used and/or unpublished relevant data.
Statistical analysis
The exposure (independent) variable was the presence or absence of a current or previous SARS-CoV-2 infection, and the outcome (dependent) variable was the presence or absence of preeclampsia. We estimated pooled unadjusted and adjusted ORs with 95% CIs as the measure of the association between SARS-CoV-2 infection during pregnancy and preeclampsia. The basic data used in the unadjusted analyses consisted of a series of 2×2 tables defined by the dichotomous SARS-CoV-2 infection/non–SARS-CoV-2 infection and preeclampsia/nonpreeclampsia for each study. The results from individual studies were then combined to produce the pooled unadjusted OR with 95% CI by using a random-effects model. This analysis model was chosen because of the high likelihood of between-study variance in observational studies. We also calculated the pooled adjusted OR with 95% CI by only taking into consideration studies that provided an adjusted estimate, either using appropriate methods of analysis or through matching of variables in the study design. The data needed from each study were the estimated adjusted effect (either the adjusted RR or the adjusted OR, the latter being a good approximation of the adjusted RR if the prevalence of the disease is low) and its estimated standard error (often obtained indirectly from the CI reported in the study).
The heterogeneity of the results among studies was evaluated by visually inspecting forest plots and by estimating the quantity І 2.80 A significant level of heterogeneity was defined as І 2≥30%.80 Subgroup analyses were performed to test the robustness of the overall findings and to explore potential sources of heterogeneity. We also addressed heterogeneity by calculating the 95% prediction interval for the pooled unadjusted OR, which gives an estimate of the point at which the true effects are to be expected for 95% of similar studies that might be conducted in the future.81, 82, 83 Prespecified subgroup analyses were carried out according to the severity of SARS-CoV-2 infection (asymptomatic illness vs symptomatic illness), study design (retrospective cohort vs prospective cohort vs cross-sectional), study of the association (as primary aim vs as secondary aim), control for confounding factors (yes vs no), geographic location (North America vs Europe vs Asia vs Latin America vs Multiregion), sample size (<200 vs 200–999 vs 1000–5000 vs >5000), test used for diagnosing SARS-CoV-2 infection (RT-PCR vs RT-PCR or antigens vs antibodies in serum vs mixed or unclear), and timing of the diagnosis of SARS-CoV-2 infection (at any time during pregnancy vs at admission for delivery).
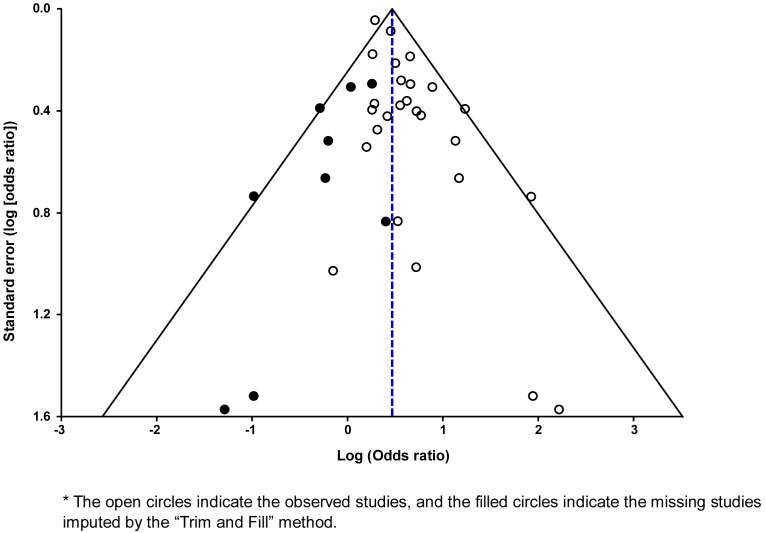
The impact of the risk of bias on the results was examined by performing a sensitivity analysis that included only studies with a low risk of bias in at least 5 of the 6 domains evaluated. We assessed publication and related biases visually by examining the symmetry of the funnel plots and statistically by measuring the degree of asymmetry with the Egger84 and Begg-Mazumdar85 tests, with P<.10 indicating significant asymmetry. In the presence of funnel plot asymmetry, we assessed the potential impact of publication bias on the overall effect size obtained in the meta-analysis by using the “Trim and Fill” method developed by Duval and Tweedie.86 , 87
Statistical analyses were performed by using Review Manager (RevMan, version 5.4.1, The Cochrane Collaboration, London, United Kingdom) and StatsDirect (version 3.3.5; StatsDirect Ltd, Merseyside, United Kingdom).
Results
Selection, characteristics, and risk of bias of studies
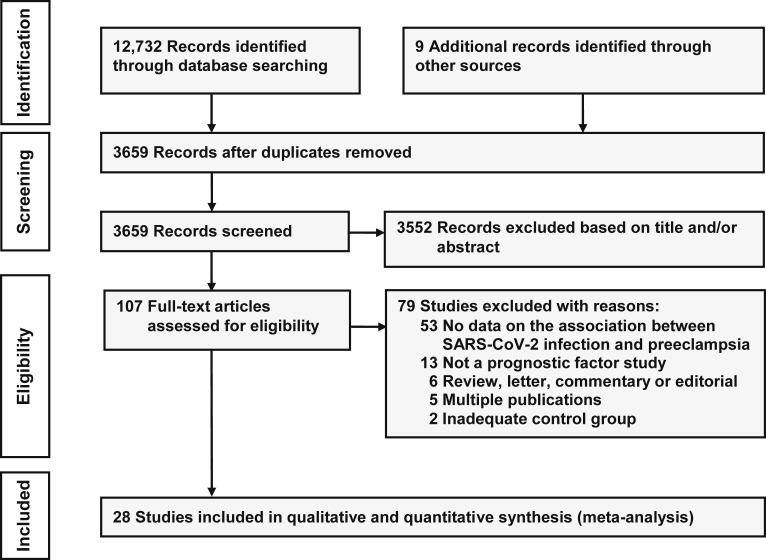
Figure 1 shows the process of the literature search and selection of studies. The searches produced 3659 records of which 107 were considered relevant. Of these, 79 were excluded, the main reason being a lack of data on the relationship between SARS-CoV-2 infection and preeclampsia. A total of 28 studies (14 prospective cohort, 12 retrospective cohort, and 2 cross-sectional) including 790,954 pregnant women, among which 15,524 were diagnosed with SARS-CoV-2 infection, met the inclusion criteria.88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115 Four studies were specifically designed to evaluate the association between SARS-CoV-2 infection during pregnancy and preeclampsia.98 , 108 , 112 , 115 The remaining 24 studies compared the maternal and perinatal outcomes for pregnant women with and those without SARS-CoV-2 infection and reported on the risk of preeclampsia. The corresponding authors of 8 studies supplied additional data.90 , 93 , 96 , 107, 108, 109 , 111 , 113
Figure 1.
Flow diagram of the study selection process
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
The main characteristics of the studies included in the systematic review are presented in Table 1 . A total of 14 studies were conducted in North American countries (13 in the United States and 1 in Canada), 6 in European countries, 5 in Asian countries, and 2 in Latin America. The remaining study was performed across 18 countries. The sample size ranged from 2499 to 406,446104 (median, 907). Two cross-sectional studies, 1 from the United States and 1 from the United Kingdom, included a total of 748,526 pregnant women.104 , 114 An RT-PCR test was used to diagnose SARS-CoV-2 infection in 18 studies, an RT-PCR or antigen test was used in 3 studies, and a serum antibody test was used in 3 studies. In the remaining 4 studies, SARS-CoV-2 infection was diagnosed based on laboratory tests and/or clinical signs and symptoms of COVID-19 and/or chest imaging suggestive of the disease. Among the 22 studies that reported on the severity of SARS-CoV-2 infection, 16 had a higher proportion of patients with asymptomatic illness, 5 had a higher proportion of patients with symptomatic illness, and 1 had the same proportion of patients with asymptomatic and symptomatic illness. Fifteen studies included women among whom SARS-CoV-2 infection was diagnosed at any time during pregnancy and 13 studies included women among whom SARS-CoV-2 infection was diagnosed at admission for delivery. Most patients were diagnosed with SARS-CoV-2 infection during the third trimester. In 25 studies, women with and without SARS-CoV-2 infection were recruited from the same population and during the same time period. In the remaining 3 studies, the comparison group without SARS-CoV-2 infection were pregnant women who delivered before the beginning of the COVID-19 pandemic.
Table 1.
Main characteristics of studies included in the systematic review
| First author, y (country) | Design (sample size) | Group with SARS-CoV-2 infection | Timing of the diagnosis of SARS-CoV-2 infection | Group without SARS-CoV-2 infection | Adjustment for confounders or matching of variables | Outcome |
|---|---|---|---|---|---|---|
| Ahlberg,88 2020 (Sweden) | Retrospective cohort (759) | n=155; women with a positive RT-PCR test result (98%) or positive for antibodies against SARS-CoV-2 (2%); 65% asymptomatic and 35% symptomatic | Admission for delivery (91%) and antepartum period (9%); ∼90% during the third trimester | n=604; women in labor with a negative RT-PCR test result (100%) | Maternal age, parity, body mass index, country of birth, living with partner, and prepregnancy comorbidity | Preeclampsia |
| Yang,89 2020 (China) | Retrospective cohort (11,078) | n=65; women with a positive RT-PCR test result (100%) | “During late pregnancy” | n=11,013; women with a negative RT-PCR test result (57%) or without signs or symptoms of COVID-19 (43%) | Maternal age, occupation, education, gravidity, parity, gestational hypertension, preeclampsia, gestational diabetes mellitus, and premature rupture of membranes | Preeclampsia |
| Prabhu,90 2020 (United States) | Prospective cohort (675) | n=70; women with a positive RT-PCR test result (100%); 79% asymptomatic and 21% symptomatic | Admission for delivery (100%); median gestational age, 39.0 wk | n=605; women admitted for delivery with a negative RT-PCR test result (100%) | No | Preeclampsia |
| Grechukhina,91 2020 (United States) | Retrospective cohort (8768) | n=77; women with a positive RT-PCR test result (100%); 53% asymptomatic and 47% symptomatic | Admission for delivery (67%), antepartum period (24%), and postpartum period (9%); most during the third trimester | n=8691; prepandemic (2018–2019) control group of pregnant women | No | Preeclampsia |
| Adhikari,92 2020 (United States) | Retrospective cohort (3280) | n=245; women with a positive RT-PCR test result (100%); 39% asymptomatic, 56% with mild or moderate illness and 5% with severe or critical illness | Admission for delivery (68%), antepartum period (30%), and unclear (2%); 93% during the third trimester and 7% during the second trimester | n=3035; women with a negative RT-PCR test result (100%) | No | Preeclampsia with severe features |
| Pirjani,93 2020 (Iran) | Prospective cohort (199) | n=66; women with a positive RT-PCR test result, or signs or symptoms of COVID-19 plus a chest CT scan suggestive of the disease; 100% symptomatic | During the second (24%) and third (74%) trimester; mean gestational age, 32.6 wk | n=133; healthy women without signs or symptoms of COVID-19 (100%) | Maternal age, body mass index, previous delivery type, gestational age, previous pregnancy problems, and preexisting medical problems | Preeclampsia |
| Wang,94 2020 (United States) | Retrospective cohort (813) | n=53; women with a positive RT-PCR or antigen test result (100%); 85% with asymptomatic or mild illness and 15% with moderate, severe, or critical illness | Admission to the hospital (100%); mean gestational age, 38.1 wk | n=760; women with a negative RT-PCR or antigen test result, or without signs or symptoms of COVID-19 | No | Preeclampsia with severe features |
| Egerup,95 2021 (Denmark) | Prospective cohort (1313) | n=28; women with a positive result for anti–SARS-CoV-2 IgM or IgG antibodies in serum (100%); no woman had a positive RT-PCR test result; 50% asymptomatic and 50% symptomatic | Admission for delivery (100%); median gestational age, 40.1 wk | n=1285; women with a negative result for anti–SARS-CoV-2 IgM and IgG antibodies in serum (100%); one woman had a positive RT-PCR test result | No | Preeclampsia |
| Hcini,96 2021 (French Guiana) | Prospective cohort (507) | n=137; women with a positive RT-PCR test result (100%); 63% asymptomatic, 33% with mild illness, and 4% with severe illness | Admission for delivery (100%); most during the third trimester | n=370; women admitted for delivery with a negative RT-PCR test result (100%) | “Unbalanced maternal characteristics” | Preeclampsia |
| Mahajan,97 2021 (India) | Retrospective cohort (73) | n=10; women with multiple gestation and a positive RT-PCR test result (100%); 80% asymptomatic and 20% symptomatic | During the second (25%) and third (75%) trimesters; median gestational age, 34.5 wk | n=63; prepandemic (2019–2020) control group of pregnant women with multiple gestation | No | Preeclampsia and eclampsia |
| Madden,98 2021 (United States) | Retrospective cohort (1715) | n=167; women with a positive RT-PCR test result (100%) | Admission to the hospital (100%) | n=1548; women with a negative RT-PCR test result (100%) | No | Preeclampsia, preeclampsia with severe features, and preeclampsia without severe features |
| Colon-Aponte,99 2021 (United States) | Prospective cohort (24) | n=12; women with a positive RT-PCR test result (100%) | Admission for delivery (100%); mean gestational age, 39.0 wk | n=12; women with a negative RT-PCR test result (100%) | No | Preeclampsia |
| Yazihan,100 2021 (Turkey) | Prospective cohort (187) | n=95; women with a positive RT-PCR test result (100%); 74% with mild illness, 24% with moderate illness, and 2% with severe illness | During the first (34%), second (34%) and third (33%) trimesters | n=92; healthy women without signs or symptoms of COVID-19 | No | Preeclampsia |
| Brandt,101 2021 (United States) | Prospective cohort (183) | n=61; women with a positive RT-PCR test result (100%); 89% with asymptomatic or mild illness, and 11% with severe or critical illness | Mean gestational age, 38.8 wk for women with asymptomatic or mild illness and 33.6 wk for those with severe or critical illness | n=122; women with a negative RT-PCR test result or those without signs or symptoms of COVID-19 | Maternal age, obesity, maternal race, and comorbid medical problems (chronic hypertension, diabetes mellitus, renal disease, asthma, immunocompromised state, and anemia) | Preeclampsia |
| Cardona-Pérez,102 2021 (Mexico) | Retrospective cohort (231) | n=67; women with a positive RT-PCR test result (100%); 86% asymptomatic and 14% symptomatic | Admission for delivery (100%); <28 wk, 10%; 28–36 wk, 24%, ≥37 wk, 66% | n=164; women with a negative RT-PCR test result (100%) | Maternal age, body mass index, preexisting comorbidities, and gestational age at admission | Preeclampsia |
| Steffen,103 2021 (United States) | Prospective cohort (1000) | n=61; women with a positive result for anti–SARS-CoV-2 IgG antibodies in serum (84%) or RT-PCR test (5%) or both tests (11%); 51% asymptomatic and 49% symptomatic | Admission for delivery (100%); median gestational age, 39.0 wk | n=939; women with a negative result for anti–SARS-CoV-2 IgG antibodies in serum or RT-PCR test (100%) | No | Preeclampsia, preeclampsia with severe features, preeclampsia without severe features, and eclampsia |
| Jering,104 2021 (United States) | Cross-sectional (406,446) | n=6380; women giving birth with a diagnosis of COVID-19 at discharge (ICD-10 code U07.1). Diagnostic criteria for SARS-CoV-2 infection were not reported | At birth; 98% in the third trimester | n=400,066; women giving birth without a diagnosis of COVID-19 at discharge (ICD-10 code U07.1) | Adjusted for propensity score, which included the following covariates: maternal age, race and ethnicity, geographic region, urban population, teaching hospital, discharge month, trimester of pregnancy, obesity, smoking, hypertension, gestational hypertension, diabetes, gestational diabetes, kidney disease, pulmonary disease | Preeclampsia, eclampsia, and HELLP syndrome |
| Vousden,105 2021 (United Kingdom) | Prospective cohort (1842) | n=1148; women with a positive RT-PCR test result within 7 days of admission to hospital (99%) or chest imaging suggestive of COVID-19 (1%); 37% asymptomatic and 63% symptomatic | <22 wk, 7%; 22–27 wk, 7%; ≥28 wk, 86% | n=694; prepandemic (2017–2018) control group of pregnant women | Maternal age, ethnicity, body mass index, any relevant previous medical problem, cigarette smoking | Preeclampsia |
| Abedzadeh-Kalahroudi,106 2021 (Iran) | Prospective cohort (149) | n=55; women with a positive RT-PCR test result, signs or symptoms of COVID-19, or laboratory tests and a chest CT scan suggestive of the disease; >90% symptomatic | During the first (7%), second (14%), and third (79%) trimesters; mean gestational age, 31.9 wk | n=94; healthy women without clinical signs or symptoms of COVID-19 (100%) | No | Preeclampsia |
| Crovetto,107 2021 (Spain) | Prospective cohort (1304) | n=176; women with a positive result for anti–SARS-CoV-2 IgG or IgM or IgA antibodies in serum (∼99%) and/or RT-PCR test; 60% asymptomatic and 40% symptomatic | Admission for delivery (100%); 24–42 wk | n=1128; women with a negative result for anti–SARS-CoV-2 IgG and IgM or IgA antibodies in serum or negative RT-PCR test (100%) | No | Preeclampsia |
| Rosenbloom,108 2021 (United States) | Retrospective cohort (249) | n=83; women with a positive RT-PCR or antigen test result (100%); 58% asymptomatic and 42% symptomatic | Any time during pregnancy | n=166; women with a negative RT-PCR test result (100%) | Race, parity | Preeclampsia, preeclampsia with severe features, and preeclampsia without severe features |
| Trahan,109 2021 (Canada) | Retrospective cohort (270) | n=45; women with a positive RT-PCR test result (100%); 27% asymptomatic and 73% symptomatic | Any time during pregnancy; 98% in the third trimester | n=225; women with a negative RT-PCR test result (100%) | No | Preeclampsia |
| Soto-Torres,110 2021 (United States) | Retrospective cohort (209) | n=106; women with a positive RT-PCR or antigen test result (100%); 54% asymptomatic and 46% symptomatic (28% with mild illness and 18% with severe illness) | Any time during pregnancy; median gestational age, 32.9 wk (range, 10.9–40.4 wk); | n=103; women with a negative RT-PCR or antigen test result (100%) | Maternal age, body mass index, parity, gestational age | Preeclampsia |
| Katz,111 2021 (United States) | Prospective cohort (1454) | n=490; women with a positive RT-PCR test result within 14 d of delivery (100%); 64% asymptomatic and 36% symptomatic | Within 14 d of delivery; most in the third trimester | n=964; women with a negative RT-PCR test result (84%) or without signs or symptoms of COVID-19 (16%) | Maternal age, race, ethnicity, body mass index, and maternal comorbidities (including diabetes, preexisting hypertension, cardiac, pulmonary, or autoimmune disease) | Preeclampsia |
| Chornock,112 2021 (United States) | Retrospective cohort (1008) | n=73; women with a positive RT-PCR test result (100%); 84% asymptomatic and 16% symptomatic | Admission for delivery (99.2%) and antepartum period (0.8%); mean gestational age, 40.1 wk | n=935; women with a negative RT-PCR test result at admission for delivery (100%) | Race, body mass index, aspirin use, and chronic hypertension | Preeclampsia, preeclampsia with severe features, and preeclampsia without severe features |
| Cruz Melguizo,113 2021 (Spain) | Prospective cohort (2954) | n=1347; women with a positive RT-PCR test result (100%); 51% asymptomatic and 49% symptomatic (35% with mild or moderate illness and 14% with severe or critical illness) | Any time during pregnancy; most in the third trimester | n=1607; women with a negative RT-PCR test result at admission for delivery (100%) | No | Preeclampsia, preeclampsia with severe features, and preeclampsia without severe features |
| Gurol-Urganci,114 2021 (United Kingdom) | Cross-sectional (342,080) | n=3527; women with a positive RT-PCR test result (100%) | At the time of birth (100%) | n=338,553; women without laboratory-confirmed SARS-CoV-2 infection (ICD-10 code U07.1) | Maternal age, ethnicity, parity, preexisting diabetes, preexisting hypertension, and socioeconomic deprivation | Preeclampsia |
| Papageorghiou,115 2021 (Multicountry)a | Prospective cohort (2184) | n=725; women with a positive RT-PCR test result (92.7%), clinical signs or symptoms of COVID-19 (6.8%), or chest imaging suggestive of COVID-19 (0.6%); 40% asymptomatic and 60% symptomatic | ≤26 wk, 5%; >26 wk, 95%; median gestational age, 37.6 wk (IQR 34.3–39.1); 71% of women were diagnosed <10 d before giving birth | n=1459; women with a negative RT-PCR or antigen test result (50%) or women without signs or symptoms of COVID-19 (50%) | Maternal age, parity, cigarette smoking, overweight or obesity, history of diabetes, cardiac disease, hypertension, or renal disease, and history of adverse pregnancy outcomes | Preeclampsia |
CT, computed tomography; HELLP, hemolysis, elevated liver enzymes, low platelet count; ICD, International Classification of Diseases; IgM, immunoglobulin M; IQR, interquartile range; RT-PCR, reverse transcription polymerase chain reaction.
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
Includes cases from Argentina, Brazil, Egypt, France, Ghana, India, Indonesia, Italy, Japan, Mexico, Nigeria, North Macedonia, Pakistan, Russia, Spain, Switzerland, the United Kingdom, and the United States.
A total of 14 studies controlled for potential confounding factors related to both SARS-CoV-2 infection and preeclampsia. Most of them adjusted their results for maternal age, body mass index, preexisting comorbidities, and race or ethnicity. Twenty-six studies provided data on the relationship between SARS-CoV-2 infection and preeclampsia (with and without severe features), 7 on the relationship with preeclampsia with severe features, 5 on the relationship with preeclampsia without severe features, 3 on the relationship with eclampsia, and 1 on the relationship with HELLP syndrome.
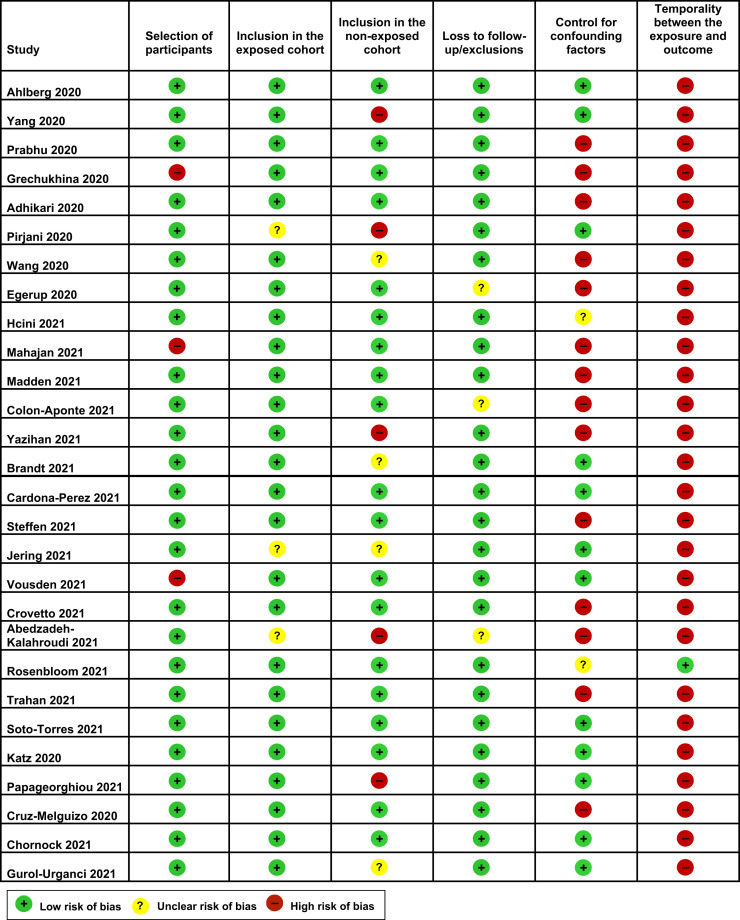
The risk of bias in each included study is shown in Supplemental Figure 1. No study was judged to be at low risk of bias for all 6 domains. Six studies were deemed to be at low risk of bias for 5 domains and 13 were judged to be at low risk of bias for 4 domains. The remaining 9 studies were judged to be at low risk of bias for ≤3 domains. The most common shortcomings were the failure to report temporality of the association between SARS-CoV-2 infection and preeclampsia and the lack of adjustment for confounding factors.
Supplemental Figure 1.
Risk of bias for each included study
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
Association between SARS-CoV-2 infection during pregnancy and preeclampsia
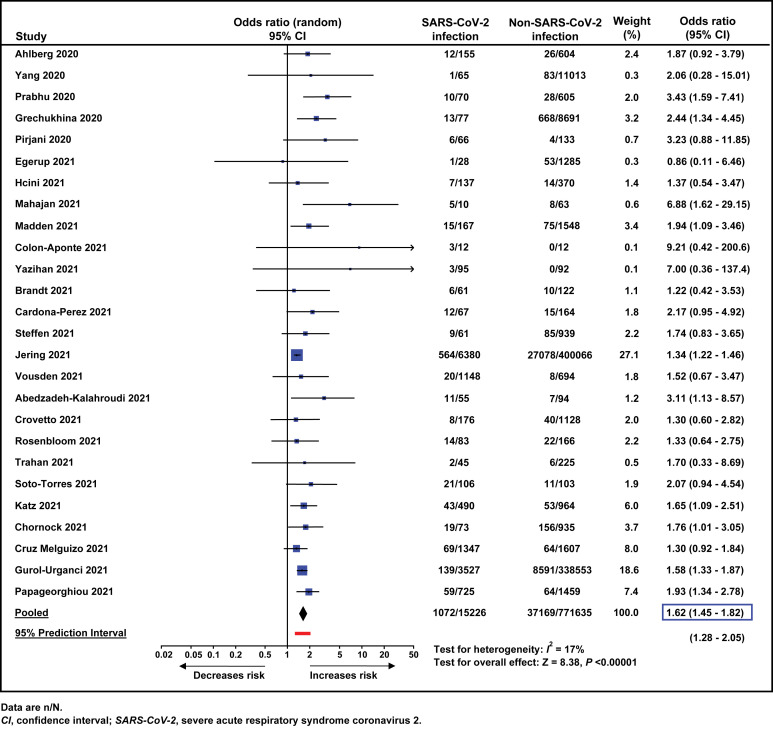
A total of 26 studies,88, 89, 90, 91 , 93 , 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115 comprising 786,861 women, reported on the association between SARS-CoV-2 infection during pregnancy and the risk of preeclampsia (with and without severe features). All but 1 study95 found that the frequency of preeclampsia was higher among pregnant women with a diagnosis of SARS-CoV-2 infection than among those without a diagnosis of SARS-CoV-2 infection. The meta-analysis of unadjusted ORs showed that the odds of developing preeclampsia were significantly higher among pregnant women with SARS-CoV-2 infection than among those without SARS-CoV-2 infection (7.0% vs 4.8%; pooled unadjusted OR, 1.62; 95% CI, 1.45–1.82; P<.00001; 95% prediction interval of the OR, 1.28–2.05) (Figure 2 ).
Figure 2.
Meta-analysis of unadjusted odds ratios for the association between SARS-CoV-2 infection during pregnancy and preeclampsia
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
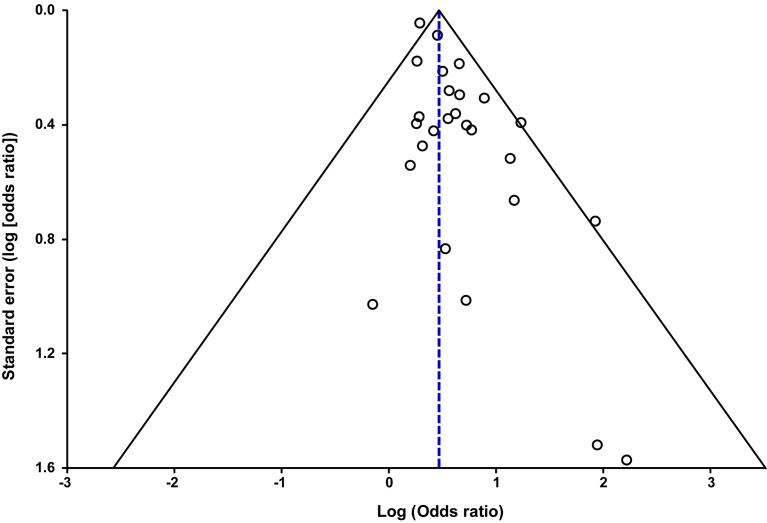
There was evidence of low statistical heterogeneity (I 2=17%), which indicates little variability among studies. This degree of heterogeneity was primarily due to the study by Jering et al104 since the exclusion of this study from the meta-analysis produced a pooled OR of 1.70 (95% CI, 1.52–1.89) without statistical heterogeneity (I 2=0%). Visual examination of the funnel plot suggested the presence of asymmetry (Supplemental Figure 2), which was statistically significant according to the Egger’s test (P<.002) but not according to the Begg and Mazumdar’s test (P=.63). After applying the “Trim and Fill” method (Supplemental Figure 3), the pooled unadjusted estimate reduced slightly but remained statistically significant (OR, 1.53; 95% CI, 1.30–1.81).
Supplemental Figure 2.
Funnel plot of the meta-analysis on the association between SARS-CoV-2 infection during pregnancy and preeclampsia
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
Supplemental Figure 3.
Funnel plot of the meta-analysis on the association between SARS-CoV-2 infection during pregnancy and preeclampsia after applying the “Trim and Fill” method∗
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
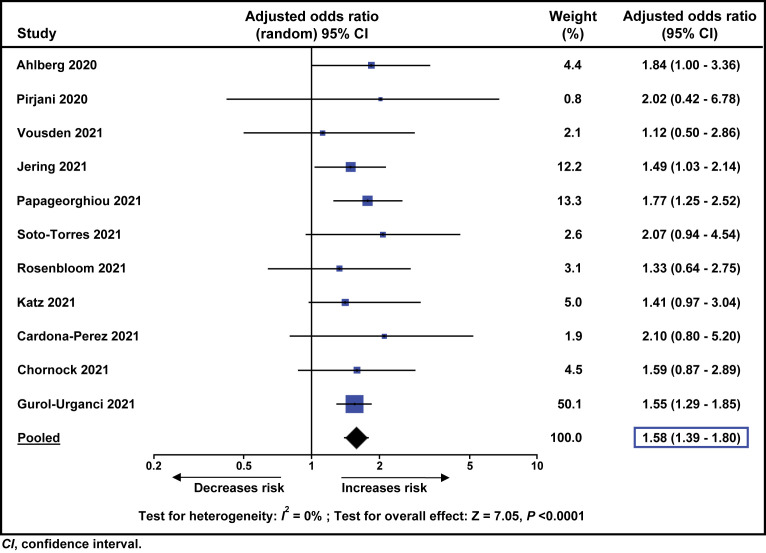
The meta-analysis of adjusted ORs also showed that, compared to pregnant women without SARS-CoV-2 infection, those with the disease had increased odds of developing preeclampsia (pooled adjusted OR, 1.58; 95% CI, 1.39–1.80; P<.0001; I 2=0%; 11 studies, 756,661 women) (Figure 3 ).
Figure 3.
Meta-analysis of adjusted odds ratios for the association between SARS-CoV-2 infection during pregnancy and preeclampsia
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
The results of the pooled unadjusted ORs for the association between SARS-CoV-2 infection and preeclampsia-related disorders are depicted in Table 2 . There was a statistically significant increase in the odds of preeclampsia with severe features (OR, 1.76; 95% CI, 1.18–2.63; I 2=58%; 7 studies, 11,019 women), eclampsia (OR, 1.97; 95% CI, 1.01–3.84; I 2=0%; 3 studies, 407,519 women), and HELLP syndrome (OR, 2.10; 95% CI, 1.48–2.97; 1 study, 406,446 women) among pregnant women with SARS-CoV-2 infection, as compared to those without the infection. There was no significant difference in the odds of preeclampsia without severe features between pregnant women with and those without SARS-CoV-2 infection (OR, 1.25; 95% CI, 0.81–1.93; I 2=29%; 5 studies, 6926 women).
Table 2.
Pooled unadjusted odds ratios for the association between SARS-CoV-2 infection and preeclampsia-related disorders
| Outcome | Number of studies | SARS-CoV-2 infection | No SARS-CoV-2 infection | Pooled odds ratio (95% CI) | P value | I2, % |
|---|---|---|---|---|---|---|
| Preeclampsia (with and without severe features) | 2688, 89, 90, 91,93,95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115 | 1072/15,226 | 37,169/771,635 | 1.62 (1.45–1.82) | <.00001 | 17 |
| Preeclampsia with severe features | 792,94,98,103,108,112,113 | 101/2029 | 629/8990 | 1.76 (1.18–2.63) | .006 | 58 |
| Preeclampsia without severe features | 598,103,108,112,113 | 61/1731 | 190/5195 | 1.25 (0.81–1.93) | .31 | 29 |
| Eclampsia | 397,103,104 | 9/6451 | 290/401,068 | 1.97 (1.01–3.84) | .048 | 0 |
| HELLP syndrome | 1104 | 33/6380 | 989/400,066 | 2.10 (1.48–2.97) | <.0001 | NA |
Data are presented as n/N.
CI, confidence interval; HELLP, hemolysis, elevated liver enzymes, low platelet count; NA, not applicable.
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
Subgroup and sensitivity analyses
The results of the subgroup analyses for the association between SARS-CoV-2 infection and preeclampsia are shown in Table 3 . Overall, the direction and magnitude of the effect of SARS-CoV-2 infection during pregnancy on preeclampsia were consistent across most prespecified subgroup analyses. However, studies with a retrospective cohort design, those that did not adjust for confounding factors, those that were conducted in Asia, or those that had a sample size <200 were associated with higher pooled ORs. On the other hand, studies with a cross-sectional design, those that controlled for confounding factors, or those that used serum antibody tests for diagnosing SARS-CoV-2 infection were associated with a slight reduction in the pooled ORs. Both asymptomatic and symptomatic SARS-CoV-2 infections significantly increased the odds of preeclampsia; however, the odds were higher among patients with symptomatic illness (OR, 2.11; 95% CI, 1.59–2.81) than among those with asymptomatic illness (OR, 1.59; 95% CI, 1.21–2.10).
Table 3.
Subgroup analyses for the association between SARS-CoV-2 infection and preeclampsia
| Subgroup | Number of studies | SARS-CoV-2 infection | No SARS-CoV-2 infection | Pooled odds ratio (95% CI) | I2, % |
|---|---|---|---|---|---|
| Severity of SARS-CoV-2 infection | |||||
| Asymptomatic | 690,95,105,107,111,115 | 63/1206 | 246/6135 | 1.59 (1.21–2.10) | 0 |
| Symptomatic | 790,93,95,105,107,111,115 | 84/1497 | 250/6268 | 2.11 (1.59–2.81) | 0 |
| Study design | |||||
| Prospective cohort | 1490,93,95,96,99, 101,103,105, 106, 107,111,113,115 | 255/4471 | 430/9504 | 1.69 (1.42–2.01) | 0 |
| Retrospective cohort | 1088,89,91,97,98,102,108, 109, 110,112 | 114/848 | 1070/23,512 | 1.98 (1.56–2.52) | 0 |
| Cross- sectional | 2104,114 | 703/9907 | 35,669/738,619 | 1.43 (1.22–1.67) | 65 |
| Study of the association | |||||
| As primary aim | 498,108,112,115 | 107/1048 | 317/4108 | 1.81 (1.41–2.33) | 0 |
| As secondary aim | 2288, 89, 90, 91,93,95, 96, 97,99, 107,109, 110, 111,113,114 | 965/14,178 | 36,852/767,527 | 1.61 (1.41–1.83) | 20 |
| Control for confounding factors | |||||
| Yes | 1488,89,93,96,101,102,104,105,108,110, 111, 112,114,115 | 923/13,083 | 36,135/755,346 | 1.43 (1.33–1.54) | 0 |
| No | 1290,91,95,97, 100,103,106,107,109,113 | 149/2143 | 1034/16,289 | 1.98 (1.49–2.64) | 25 |
| Geographic location | |||||
| North America |
1290,91,98,99,101,103,104,108, 109, 110, 111, 112 | 719/7625 | 28,192/414,376 | 1.67 (1.38–2.02) | 26 |
| Europe | 688,95,105,107,113,114 | 249/6381 | 8782/343,871 | 1.52 (1.31–1.75) | 0 |
| Asia | 589,93,97,100,106 | 26/291 | 102/11,395 | 3.65 (1.92–6.96) | 0 |
| Latin America |
296,102 | 19/204 | 29/534 | 1.77 (0.96–3.28) | 0 |
| Multiple regions | 1115 | 59/25 | 64/1459 | 1.93 (1.34–2.78) | NA |
| Sample size | |||||
| <200 | 693,97,99, 101,106 | 34/299 | 29/516 | 2.90 (1.65–5.09) | 0 |
| 200–999 | 788,90,96,102,108, 109, 110 | 78/663 | 122/2237 | 1.94 (1.42–2.65) | 0 |
| 1000–5000 | 995,98,103,105,107,111, 112, 113,115 | 243/4215 | 598/10,559 | 1.62 (1.36–1.92) | 20 |
| >5000 | 489,91,104,114 | 717/10,049 | 36,420/758,323 | 1.50 (1.25–1.80) | 53 |
| Test used for diagnosing SARS-CoV-2 infection | |||||
| RT-PCR | 1788, 89, 90, 91,96, 102,105,109,111, 112, 113,115 | 299/4744 | 1278/29,168 | 1.79 (1.53–2.10) | 0 |
| RT-PCR or antigens | 2108,110 | 35/189 | 33/269 | 1.63 (0.95–2.78) | 0 |
| Antibodies in serum | 395,103,107 | 18/265 | 178/3352 | 1.46 (0.87–2.44) | 0 |
| Mixed or unclear | 493,104,106,114 | 720/10,028 | 35,680/738,846 | 1.50 (1.23–1.84) | 56 |
| Timing of the diagnosis of SARS-CoV-2 infection | |||||
| At any time during pregnancy | 1289,91,93,97,100,105,106,108, 109, 110,113,115 | 224/3822 | 945/24,340 | 1.80 (1.47–2.21) | 4 |
| At admission for delivery | 1488,90,95,96,98,99,101, 102, 103, 104,107,111,112,114 | 848/11,404 | 36,224/747,295 | 1.49 (1.35–1.66) | 9 |
Data are presented as n/N.
CI, confidence interval; NA, not applicable; RT-PCR, Reverse transcription polymerase chain reaction.
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
The effect of SARS-CoV-2 infection during pregnancy on the risk of preeclampsia did not change after a sensitivity analysis limited to the 6 studies88 , 102 , 108 , 110, 111, 112 with a low risk of bias in at least 5 of the 6 domains evaluated (pooled OR, 1.74; 95% CI, 1.35–2.23; I 2=0%). The pooled OR for the subgroup of 20 studies89, 90, 91 , 93 , 95, 96, 97, 98, 99, 100, 101 , 103, 104, 105, 106, 107 , 109 , 113, 114, 115 with a low risk of bias in less than 5 of the 6 domains evaluated was 1.65 (95% CI, 1.43–1.91) with an І 2=30%.
Temporality of the association between SARS-CoV-2 infection and preeclampsia
Only the study by Rosenbloom et al108 provided data on the time elapsed between SARS-CoV-2 infection diagnosis and preeclampsia diagnosis. In this study, the median interval from the diagnosis of SARS-CoV-2 infection to the diagnosis of preeclampsia was 3.79 weeks (interquartile range, 0.43–13.0). The hazard ratios for the association between SARS-CoV-2 infection and the development of preeclampsia were 2.88 (95% CI, 1.20–6.93) for the infection diagnosed before 32 weeks of gestation and 2.74 (95% CI, 0.98–7.71) for the infection diagnosed at or after 32 weeks of gestation.
Comment
Principal findings
This systematic review shows that women with SARS-CoV-2 infection during pregnancy had a significantly higher odds (62%) of developing preeclampsia than those without SARS-CoV-2 infection during pregnancy. This association was remarkably consistent across all predefined subgroups. Moreover, SARS-CoV-2 infection during pregnancy was associated with a significant increase in the odds of preeclampsia with severe features, eclampsia, and HELLP syndrome. Both asymptomatic and symptomatic SARS-CoV-2 infections significantly increased the risk for preeclampsia. Nevertheless, the odds of developing preeclampsia were higher among patients with symptomatic illness than among those with asymptomatic illness, which suggests a dose-response gradient effect.
Assessing the causal relationship between SARS-CoV-2 infection during pregnancy and preeclampsia
The results of this systematic review provide convincing evidence for an association between SARS-CoV-2 infection during pregnancy and the risk for preeclampsia for the following reasons: (1) there was a significant effect based on the random-effects model at P<.00001 and P<.0001 for the pooled unadjusted and adjusted effect size estimates, respectively; (2) the magnitude of the effect estimate was relatively large; (3) the CIs observed were relatively narrow, implying that the estimates of effect size are more precise; (4) the relationship was consistently present in the majority of the studies, across diverse populations worldwide, and in virtually every way the data were interrogated; (5) the large number of pregnant women with and without SARS-CoV-2 infection included in the analyses; (6) in most studies, an association between SARS-CoV-2 infection and a higher risk for preeclampsia was neither anticipated nor hypothesized when the study was carried out; (7) the low statistical heterogeneity; (8) evidence of clinical homogeneity across the included studies; (9) a statistically significant effect was reported in the largest studies; (10) the 95% prediction interval that excluded a null effect and that was in the same direction as the 95% CI; and (11) the sensitivity analysis that was restricted to studies at low risk of bias (and thus supporting the overall findings).
Additional information could support the hypothesis that SARS-CoV-2 infection during pregnancy plays a causal role in the development of preeclampsia. When assessing causality, evidence of a dose-response relationship suggests that the association is less likely to be due to confounding.116 , 117 We found evidence for a dose-response gradient effect on the basis that patients with symptomatic illness were more likely to develop preeclampsia (OR=2.11) than those with asymptomatic illness (OR=1.59). Recently, a multicenter cohort study by Metz et al,118 which did not include women without SARS-CoV-2 infection, reported that the frequency of hypertensive disorders of pregnancy among women with asymptomatic SARS-CoV-2 infection, mild or moderate disease, and severe or critical disease was 18.8%, 23.8%, and 40.4%, respectively. Compared to asymptomatic patients, those with mild or moderate and severe or critical disease were at an increased risk for hypertensive disorders of pregnancy (adjusted RR, 1.24; 95% CI, 0.98–1.58 for mild or moderate disease; and adjusted RR, 1.61; 95% CI, 1.18–2.20 for severe or critical disease). These data strongly suggest a dose-response effect.
Temporality is considered fundamental when assessing the likelihood of a causal relationship between an exposure and an outcome.116 , 117 Evidence that the participants were exposed before the occurrence of the outcome strengthens a causal relationship argument. The study by Rosenbloom et al108 provided clear evidence to support a meaningful temporal relationship between SARS-CoV-2 infection and preeclampsia. The median time interval between SARS-CoV-2 infection diagnosis and preeclampsia diagnosis was 3.79 weeks. Both cases of SARS-CoV-2 infection diagnosed before 32 weeks of gestation and cases diagnosed at or after 32 weeks of gestation were associated with a higher risk for developing preeclampsia. However, the association was only statistically significant for SARS-CoV-2 infection diagnosed before 32 weeks of gestation. Rosenbloom et al108 hypothesized that SARS-CoV-2 infection that was diagnosed closer to term was not associated with a significant increase in the risk for preeclampsia because the time remaining to develop the clinical disorder was limited. Nevertheless, a subgroup analysis in our meta-analysis showed that SARS-CoV-2 infection diagnosed either at delivery or at any time during pregnancy was significantly associated with an increased risk for preeclampsia.
Several mechanisms could explain how SARS-CoV-2 infection during pregnancy might be involved in the pathogenesis of preeclampsia. SARS-CoV-2 enters the cell after the N-terminal portion of the viral spike protein binds to the cell membrane angiotensin-converting enzyme 2 (ACE2) receptor.119, 120, 121 The ACE2 receptor is an important component of the RAS, which converts angiotensin II into angiotensin 1 to 7.122 The RAS is an important regulator of placental function, because it plays a role in the control of trophoblast proliferation, angiogenesis, and blood flow. The RAS significantly modulates uteroplacental blood flow through the balance of its vasoconstrictive and vasodilatory pathways.122 The binding of SARS-CoV-2 to ACE2 receptors causes down-regulation of the RAS system with reduced levels of vasodilatory angiotensin 1 to 7, thereby leaving the vasoconstrictive and proinflammatory effects of angiotensin II unopposed. These alterations in the RAS could have a role in the pathophysiology of preeclampsia.123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138
There is evidence that SARS-CoV-2 can infect the syncytiotrophoblast and activate inflammatory responses in placentas of women with a positive RT-PCR test result.139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151 Recently, Verma et al152 showed that SARS-CoV-2 colonizes ACE2 receptor-expressing maternal and fetal cells in the placenta, which leads to alterations in the local RAS. The infected placentas had a significant reduction in the expression of ACE2 receptors, with a concomitant increase in soluble fms-like tyrosine kinase-1 (sFlt-1) production and a decrease in proangiogenic factors. In addition, the serum levels of sFlt-1 and angiotensin II type 1-receptor autoantibodies, both markers of preeclampsia, were significantly higher among women with SARS-CoV-2 infection before delivery than among uninfected women. The findings of this study provide a plausible mechanism for explaining the association between SARS-CoV-2 infection and preeclampsia.
Seethy et al153 explored in silico potential interactions between SARS-CoV-2 proteins and proteins involved in the key functions of the placenta. Potential SARS-CoV-2 interactions with placental proteins such as MFGE8, PLAT, and PAR2, which may play key roles in trophoblast invasion, migration, proliferation, and differentiation processes, were identified. The authors concluded that these interactions could be involved in the development of preeclampsia. Beys-da-Silva et al154 assessed differentially expressed genes from clinical and experimental datasets of SARS-CoV-2 infection and their potential roles in preeclampsia. It was found that SARS-CoV-2 infection upregulates sFlt-1 and endoglin, vasoconstrictive peptides, nitric oxide modulators, and prothrombotic-related molecules. Therefore, SARS-CoV-2 infection can affect different molecular pathways related to the pathogenesis of preeclampsia such as angiogenesis, hypoxia, inflammatory signaling, thrombin or platelet activation, and imbalance of vasoactive peptides. In summary, multiple mechanisms link SARS-CoV-2 infection to the subsequent development of vascular disease and preeclampsia.
Strengths and limitations
The main strength of our study is the use of the most rigorous methodology for performing a systematic review and meta-analysis of observational studies, which included the use of a prospective protocol designed to address a specific research question, the extensive literature searches without language restrictions, the risk of bias assessment in the included studies, the quantitative summarization of the evidence, the performance of multiple subgroup and sensitivity analyses, the exploration of sources of heterogeneity, the calculation of the 95% prediction interval, and the use of methods for addressing potential publication bias. Other strengths of our review are the inclusion of a relatively large number of studies and of women from different populations throughout the world and the inclusion of additional unpublished data from 29% of the included studies.
Some potential limitations of our study should be considered. First, only 1 study reported on the temporality of the association between SARS-CoV-2 infection during pregnancy and preeclampsia. Second, only one-half of the included studies controlled for potential confounding factors. However, there were no significant differences in the pooled unadjusted and adjusted ORs obtained from the subgroup analysis of studies that controlled for confounding factors and the overall estimate obtained for all included studies. Even if adjustments for potential confounding factors are made, residual confounding remains a potentially serious problem in observational research. Third, there was evidence of funnel plot asymmetry, which raises the possibility of publication bias. Nevertheless, funnel plot asymmetry may also be caused by other reporting biases (eg, selective outcome or analysis reporting), poor methodological quality of smaller studies, true heterogeneity, statistical artifact, or chance.155, 156, 157 We assessed the potential impact of publication bias in our meta-analysis by using the “Trim and Fill” approach and the “adjusted” estimate obtained (OR, 1.53; 95% CI, 1.30–1.79) was slightly lower than that obtained in the original analysis (OR, 1.62; 95% CI, 1.45–1.82). Therefore, we concluded that the potential impact of publication bias is probably trivial. Finally, the number of studies that reported on the relationship between SARS-CoV-2 infection and preeclampsia according to the severity of the infection was limited.
Clinical implications
SARS-CoV-2 infection during pregnancy and preeclampsia are independently associated with an increased risk for adverse maternal and perinatal outcomes.4, 5, 6, 7, 8, 9, 10, 11, 12 , 74 , 76 One of the studies included in our systematic review reported that SARS-CoV-2 infection during pregnancy and preeclampsia are associated in an additive fashion with an increased risk for adverse pregnancy outcomes.115 In fact, patients diagnosed with both SARS-CoV-2 infection and preeclampsia had a higher risk for preterm birth, a small-for-gestational-age neonate, and adverse maternal and perinatal outcomes than those diagnosed with only SARS-CoV-2 infection or only preeclampsia. Healthcare professionals need to be aware of the increased risk for preeclampsia among pregnant women diagnosed with SARS-CoV-2 infection, even among those with asymptomatic illness, and the negative additive effect of SARS-CoV-2 infection and preeclampsia to plan close monitoring of the affected pregnancy and to adopt early effective interventions that can reduce risks to mothers and their fetuses or neonates.
The association between low-dose aspirin administration to prevent preeclampsia and SARS-CoV-2 infection remains unclear. Two studies included in our review reported on such an association. Papageorghiou et al115 reported that the significant association between SARS-CoV-2 infection and preeclampsia was not modified by aspirin use during pregnancy (P value for interaction=.42); Chornock et al112 reported that the frequency of aspirin use during pregnancy was higher among women with SARS-CoV-2 infection than among those without the disease; however, this difference was not statistically significant (19.2% vs 11.9%; P=.07). Currently, most professional organizations recommend that low-dose aspirin administration should still be offered to pregnant women during the COVID-19 pandemic for the prevention of preeclampsia.158, 159, 160, 161, 162 Some authors who believe that aspirin could increase the risk for progression of SARS-CoV-2 infection have suggested that the prescription of aspirin for the prevention of preeclampsia should be ceased immediately following a diagnosis of SARS-CoV-2 infection, that aspirin use should be avoided for the duration of the disease, and that medication use can be resumed after full recovery from the infection.163
Public health implications
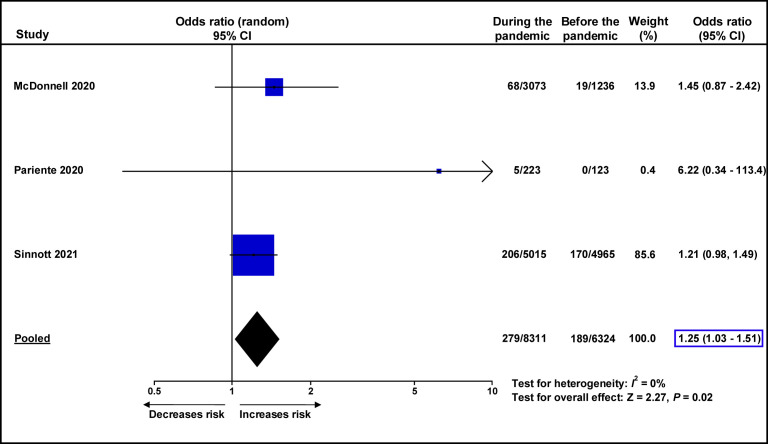
A recent meta-analysis assessed the impact of the COVID-19 pandemic on maternal and perinatal outcomes by comparing the rates of adverse outcomes during the pandemic to those before the pandemic.164 This study reported a significant increase in maternal mortality, stillbirth, maternal stress, and ruptured ectopic pregnancies during the pandemic, as compared to before the pandemic. Unfortunately, this review did not assess the effect of the COVID-19 pandemic on the rates of preeclampsia. We identified 3 studies conducted in high-income countries165, 166, 167 that compared the rates of preeclampsia before and during the COVID-19 pandemic. We performed a meta-analysis of these studies and found that the rate of preeclampsia during the COVID-19 pandemic was significantly higher than before the pandemic (3.4% vs 3.0%; OR, 1.25; 95% CI, 1.03–1.51; P=.02) (Supplemental Figure 4). Another study, which provided insufficient data to be included in this meta-analysis, reported that the risk of preeclampsia was significantly higher during the COVID-19 pandemic than before the pandemic (RR, 1.53; 95% CI, 1.24–1.90 when compared to the 2018 preeclampsia rate; RR, 1.25; 95% CI, 1.02–1.52 when compared to the 2019 preeclampsia rate).168 These data support the findings of our study.
Supplemental Figure 4.
Meta-analysis of unadjusted odds ratios for the risk of preeclampsia during the COVID-19 pandemic in comparison to before the pandemic
CI, confidence interval.
Conde-Agudelo. Association between SARS-CoV-2 infection during pregnancy and preeclampsia. Am J Obstet Gynecol 2022.
The observations from this meta-analysis can be indirectly tested in clinical trials that evaluate the effects of administering COVID-19 vaccines to pregnant women or antiviral therapies to pregnant women with SARS-CoV-2 infection or in studies assessing the impact of COVID-19 mitigation measures on the incidence of preeclampsia. We located a nonrandomized study in the medRxiv database that compared the outcomes between pregnant women who received the COVID-19 vaccine during pregnancy (n=140) and those who did not (n=1862).169 Women vaccinated during pregnancy were less likely than unvaccinated women to be diagnosed with SARS-CoV-2 infection before delivery (1.4% vs 11.3%; RR, 0.13; 95% CI, 0.03–0.50; P=.003). Moreover, pregnant women who received COVID-19 vaccines during pregnancy had a nonsignificant decrease in the risk for preeclampsia when compared to unvaccinated pregnant women (0.7% vs 1.2%; RR, 0.58; 95% CI, 0.08–4.25; P=.59). Evidence from ongoing randomized controlled trials comparing COVID-19 vaccines to placebo in pregnant women will help to determine whether vaccines prevent SARS-CoV-2 infection in this population and reduce the risk of preeclampsia and/or other pregnancy complications. If administration of COVID-19 vaccines to pregnant women or the use of antiviral therapies among pregnant women with SARS-CoV-2 infection, or if the implementation of COVID-19 mitigation measures decreases the risk of preeclampsia, it would strongly support the causal relationship between SARS-CoV-2 infection during pregnancy and preeclampsia.
Research implications
Further research is required to elucidate (1) the mechanisms underlying the association between SARS-CoV-2 infection and preeclampsia; (2) the effects of SARS-CoV-2 infection during the first and second trimesters of pregnancy on the risk of preeclampsia; (3) the interrelationships between low-dose aspirin use, SARS-CoV-2 infection, and preeclampsia; (4) the effects of administering COVID-19 vaccines to pregnant women and antiviral therapies to pregnant women with SARS-CoV-2 infection on the risk of preeclampsia; (5) the impact of implementing COVID-19 mitigation measures on the incidence of preeclampsia; (6) effective interventions to prevent preeclampsia in pregnant women with SARS-CoV-2 infection; and (7) effective interventions in pregnant women with both SARS-CoV-2 infection and preeclampsia to prevent adverse maternal and perinatal outcomes.
Conclusion
This systematic review and meta-analysis indicates that there is an association between SARS-CoV-2 infection during pregnancy and preeclampsia and suggests that this relationship may be causal.
Acknowledgments
We are very grateful to Drs Malavika Prabhu (Department of Obstetrics & Gynecology, Weill Cornell Medicine, New York, NY), Mahdi Sepidarkish (Infertility and Reproductive Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran), Najeh Hcini (Department of Obstetrics and Gynaecology, West French Guiana Hospital Center, Saint-Laurent-du-Maroni, French Guiana), Francesca Crovetto (Department of Maternal-Fetal Medicine, BCNatal, Barcelona Center for Maternal-Fetal and Neonatal Medicine, Hospital Sant Joan de Déu and Hospital Clínic, Universitat de Barcelona, Barcelona, Spain), Joshua I. Rosenbloom (Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, MO), Marie-Julie Trahan (Department of Obstetrics and Gynecology, McGill University, Montreal, Quebec, Canada), Daniel Katz (Department of Anesthesiology, Perioperative and Pain Medicine, Icahn School of Medicine at Mount Sinai, New York, NY), and Oscar Martinez-Perez (Department of Obstetrics and Gynecology, Hospital Universitario Puerta de Hierro, Majadahonda, Madrid, Spain) for providing unpublished data from their studies. None of them have a conflict of interest with respect to our systematic review and meta-analysis.
Footnotes
The authors report no conflict of interest.
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy ShriverNational Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, by federal funds from the NICHD/NIH/DHHS under contract number HHSN275201300006C.
R.R. has contributed to this work as part of his official duties as an employee of the United States Federal Government.
The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Reprints will not be available.
Appendix
References
- 1.Abalos E., Cuesta C., Grosso A.L., Chou D., Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Say L., Chou D., Gemmill A., et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 3.Hoyert D.L., Miniño A.M. Maternal mortality in the United States: changes in coding, Publication, and Data Release, 2018. Natl Vital Stat Rep. 2020;69:1–18. [PubMed] [Google Scholar]
- 4.Abalos E., Cuesta C., Carroli G., et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(Suppl1):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 5.Harmon Q.E., Huang L., Umbach D.M., et al. Risk of fetal death with preeclampsia. Obstet Gynecol. 2015;125:628–635. doi: 10.1097/AOG.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettit F., Mangos G., Davis G., Henry A., Brown M.A. Pre-eclampsia causes adverse maternal outcomes across the gestational spectrum. Pregnancy Hypertens. 2015;5:198–204. doi: 10.1016/j.preghy.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Burton G.J., Redman C.W., Roberts J.M., Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 8.Wadhwani P., Saha P.K., Kalra J.K., Gainder S., Sundaram V. A study to compare maternal and perinatal outcome in early vs. late onset preeclampsia. Obstet Gynecol Sci. 2020;63:270–277. doi: 10.5468/ogs.2020.63.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casagrande L., Rezende G.P., Guida J.P., et al. Maternal and perinatal outcomes related to superimposed pre-eclampsia in a Brazilian cohort of women with chronic hypertension. Int J Gynaecol Obstet. 2020;149:148–153. doi: 10.1002/ijgo.13114. [DOI] [PubMed] [Google Scholar]
- 10.Lai J., Syngelaki A., Nicolaides K.H., von Dadelszen P., Magee L.A. Impact of new definitions of preeclampsia at term on identification of adverse maternal and perinatal outcomes. Am J Obstet Gynecol. 2021;224:518.e1–518.e11. doi: 10.1016/j.ajog.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Society for Maternal-Fetal Medicine (SMFM) Executive Summary: workshop on preeclampsia, January 25-26, 2021, cosponsored by the Society for Maternal-Fetal Medicine and the Preeclampsia Foundation. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.05.043. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Chappell L.C., Cluver C.A., Kingdom J., Tong S. Pre-eclampsia. Lancet. 2021 doi: 10.1016/S0140-6736(20)32335-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Pittara T., Vyrides A., Lamnisos D., Giannakou K. Pre-eclampsia and long-term health outcomes for mother and infant: an umbrella review. BJOG. 2021;128:1421–1430. doi: 10.1111/1471-0528.16683. [DOI] [PubMed] [Google Scholar]
- 14.Falco M.L., Sivanathan J., Laoreti A., Thilaganathan B., Khalil A. Placental histopathology associated with pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:295–301. doi: 10.1002/uog.17494. [DOI] [PubMed] [Google Scholar]
- 15.Sultana Z., Maiti K., Dedman L., Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2018;218:S762–S773. doi: 10.1016/j.ajog.2017.11.567. [DOI] [PubMed] [Google Scholar]
- 16.Than N.G., Romero R., Tarca A.L., et al. Integrated systems biology approach identifies novel maternal and placental pathways of preeclampsia. Front Immunol. 2018;9:1661. doi: 10.3389/fimmu.2018.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brosens I., Brosens J.J., Muter J., Puttemans P., Benagiano G. Preeclampsia: the role of persistent endothelial cells in uteroplacental arteries. Am J Obstet Gynecol. 2019;221:219–226. doi: 10.1016/j.ajog.2019.01.239. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Lopez N., Motomura K., Miller D., Garcia-Flores V., Galaz J., Romero R. Inflammasomes: their role in normal and complicated pregnancies. J Immunol. 2019;203:2757–2769. doi: 10.4049/jimmunol.1900901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brosens I., Puttemans P., Benagiano G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol. 2019;221:437–456. doi: 10.1016/j.ajog.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Garrido-Gomez T., Quiñonero A., Dominguez F., et al. Preeclampsia: a defect in decidualization is associated with deficiency of annexin A2. Am J Obstet Gynecol. 2020;222:376.e1–376.e17. doi: 10.1016/j.ajog.2019.11.1250. [DOI] [PubMed] [Google Scholar]
- 21.Lip S.V., Boekschoten M.V., Hooiveld G.J., et al. Early-onset preeclampsia, plasma microRNAs, and endothelial cell function. Am J Obstet Gynecol. 2020;222:497.e1–497.e12. doi: 10.1016/j.ajog.2019.11.1286. [DOI] [PubMed] [Google Scholar]
- 22.Garrido-Gómez T., Castillo-Marco N., Cordero T., Simón C. Decidualization resistance in the origin of preeclampsia. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.09.039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Staff A.C., Fjeldstad H.E., Fosheim I.K., et al. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.09.026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Rana S., Burke S.D., Karumanchi S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.10.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redman C.W.G., Staff A.C., Roberts J.M. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.09.047. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Collier A.Y., Smith L.A., Karumanchi S.A. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum Immunol. 2021;82:362–370. doi: 10.1016/j.humimm.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagel S., Cohen S.M., Goldman-Wohl D. An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2020.10.023. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Melchiorre K., Giorgione V., Thilaganathan B. The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2020.10.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Miller D., Motomura K., Galaz J., et al. Cellular immune responses in the pathophysiology of preeclampsia. J Leukoc Biol. 2021 doi: 10.1002/JLB.5RU1120-787RR. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conde-Agudelo A., Villar J., Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2008;198:7–22. doi: 10.1016/j.ajog.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Konopka T., Zakrzewska A. Periodontitis and risk for preeclampsia - a systematic review. Ginekol Pol. 2020;91:158–164. doi: 10.5603/GP.2020.0024. [DOI] [PubMed] [Google Scholar]
- 32.Yan L., Jin Y., Hang H., Yan B. The association between urinary tract infection during pregnancy and preeclampsia: a meta-analysis. Medicine (Baltimore) 2018;97:e12192. doi: 10.1097/MD.0000000000012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nourollahpour Shiadeh M., Behboodi Moghadam Z., Adam I., Saber V., Bagheri M., Rostami A. Human infectious diseases and risk of preeclampsia: an updated review of the literature. Infection. 2017;45:589–600. doi: 10.1007/s15010-017-1031-2. [DOI] [PubMed] [Google Scholar]
- 34.Huang X., Wang J., Liu J., et al. Maternal periodontal disease and risk of preeclampsia: a meta-analysis. J Huazhong Univ Sci Technolog Med Sci. 2014;34:729–735. doi: 10.1007/s11596-014-1343-8. [DOI] [PubMed] [Google Scholar]
- 35.Ide M., Papapanou P.N. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes—systematic review. J Clin Periodontol. 2013;84:S181–S194. doi: 10.1902/jop.2013.134009. [DOI] [PubMed] [Google Scholar]
- 36.Sgolastra F., Petrucci A., Severino M., Gatto R., Monaco A. Relationship between periodontitis and pre-eclampsia: a meta-analysis. PLoS One. 2013;8:e71387. doi: 10.1371/journal.pone.0071387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei B.J., Chen Y.J., Yu L., Wu B. Periodontal disease and risk of preeclampsia: a meta-analysis of observational studies. PLoS One. 2013;8:e70901. doi: 10.1371/journal.pone.0070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nourollahpour Shiadeh M., Riahi S.M., Khani S., et al. Human immunodeficiency virus and risk of pre-eclampsia and eclampsia in pregnant women: a meta-analysis on cohort studies. Pregnancy Hypertens. 2019;17:269–275. doi: 10.1016/j.preghy.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Sansone M., Sarno L., Saccone G., et al. Risk of preeclampsia in human immunodeficiency virus-infected pregnant women. Obstet Gynecol. 2016;127:1027–1032. doi: 10.1097/AOG.0000000000001424. [DOI] [PubMed] [Google Scholar]
- 40.Subramaniam A., Lees B.F., Becker D.A., Tang Y., Khan M.J., Edwards R.K. Evaluation of human papillomavirus as a risk factor for preterm birth or pregnancy-related hypertension. Obstet Gynecol. 2016;127:233–240. doi: 10.1097/AOG.0000000000001247. [DOI] [PubMed] [Google Scholar]
- 41.McDonnold M., Dunn H., Hester A., et al. High risk human papillomavirus at entry to prenatal care and risk of preeclampsia. Am J Obstet Gynecol. 2014;210:138.e1–138.e5. doi: 10.1016/j.ajog.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 42.Xie F., von Dadelszen P., Nadeau J. CMV infection, TLR-2 and -4 expression, and cytokine profiles in early-onset preeclampsia with HELLP syndrome. Am J Reprod Immunol. 2014;71:379–386. doi: 10.1111/aji.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strand K.M., Odland M.L., Iversen A.C., Nordbø S.A., Vik T., Austgulen R. Cytomegalovirus antibody status at 17-18 weeks of gestation and pre-eclampsia: a case-control study of pregnant women in Norway. BJOG. 2012;119:1316–1323. doi: 10.1111/j.1471-0528.2012.03420.x. [DOI] [PubMed] [Google Scholar]
- 44.Xie F., Hu Y., Magee L.A., et al. An association between cytomegalovirus infection and pre-eclampsia: a case-control study and data synthesis. Acta Obstet Gynecol Scand. 2010;89:1162–1167. doi: 10.3109/00016349.2010.499449. [DOI] [PubMed] [Google Scholar]
- 45.Bajema K.L., Stankiewicz Karita H.C., Tenforde M.W., Hawes S.E., Heffron R. Maternal hepatitis B infection and pregnancy outcomes in the United States: a population-based cohort study. Open Forum Infect Dis. 2018;5:ofy134. doi: 10.1093/ofid/ofy134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed M.A., Sharif M.E., Rayis D.A., Nasr A.M., Adam I. Hepatitis B infection and preeclampsia among pregnant Sudanese women. Virol J. 2018;15:20. doi: 10.1186/s12985-018-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan J., Liu X., Mao X., et al. HBsAg positivity during pregnancy and adverse maternal outcomes: a retrospective cohort analysis. J Viral Hepat. 2016;23:812–819. doi: 10.1111/jvh.12545. [DOI] [PubMed] [Google Scholar]
- 48.Huang X., Tan H., Li X., Zhou S., Wen S.W., Luo M. Maternal chronic HBV infection would not increase the risk of pregnancy-induced hypertension—results from pregnancy cohort in Liuyang rural China. PLoS One. 2014;9:e114248. doi: 10.1371/journal.pone.0114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lao T.T., Sahota D.S., Cheng Y.K., Law L.W., Leung T.Y. Maternal hepatitis B surface antigen status and incidence of pre-eclampsia. J Viral Hepat. 2013;20:343–349. doi: 10.1111/jvh.12037. [DOI] [PubMed] [Google Scholar]
- 50.Shi L., Wu Y. HSV infection is associated with gestational hypertension: results from the US National inpatient sample. J Investig Med. 2018;66:1–5. doi: 10.1136/jim-2017-000687. [DOI] [PubMed] [Google Scholar]
- 51.Alshareef S.A., Eltom A.M., Nasr A.M., Hamdan H.Z., Adam I. Rubella, herpes simplex virus type 2 and preeclampsia. Virol J. 2017;14:142. doi: 10.1186/s12985-017-0813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott S.E., Parchim N.F., Kellems R.E., Xia Y., Soffici A.R., Daugherty P.S. A pre-eclampsia-associated Epstein-Barr virus antibody cross-reacts with placental GPR50. Clin Immunol. 2016;168:64–71. doi: 10.1016/j.clim.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Laake I., Tunheim G., Robertson A.H., et al. Risk of pregnancy complications and adverse birth outcomes after maternal A(H1N1)pdm09 influenza: a Norwegian population-based cohort study. BMC Infect Dis. 2018;18:525. doi: 10.1186/s12879-018-3435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Dadelszen P., Magee L.A. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;81:642–648. [PubMed] [Google Scholar]
- 55.Dorman E.K., Shulman C.E., Kingdom J., et al. Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound Obstet Gynecol. 2002;19:165–170. doi: 10.1046/j.0960-7692.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- 56.Arechavaleta-Velasco F., Ma Y., Zhang J., McGrath C.M., Parry S. Adeno-associated virus-2 (AAV-2) causes trophoblast dysfunction, and placental AAV-2 infection is associated with preeclampsia. Am J Pathol. 2006;168:1951–1959. doi: 10.2353/ajpath.2006.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardenas I., Means R.E., Aldo P., et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaiworapongsa T., Romero R., Gotsch F., et al. Acute pyelonephritis during pregnancy changes the balance of angiogenic and anti-angiogenic factors in maternal plasma. J Matern Fetal Neonatal Med. 2010;23:167–178. doi: 10.3109/14767050903067378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon J.Y., Romero R., Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387–390. doi: 10.1111/aji.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciapponi A., Bardach A., Comandé D., et al. COVID-19 and pregnancy: an umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badr D.A., Mattern J., Carlin A., et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764–768. doi: 10.1016/j.ajog.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collin J., Byström E., Carnahan A., Ahrne M. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99:819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westgren M., Acharya G. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol. 2020;223:779–780. doi: 10.1016/j.ajog.2020.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qeadan F., Mensah N.A., Tingey B., Stanford J.B. The risk of clinical complications and death among pregnant women with COVID-19 in the Cerner COVID-19 cohort: a retrospective analysis. BMC Pregnancy Childbirth. 2021;21:305. doi: 10.1186/s12884-021-03772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeBolt C.A., Bianco A., Limaye M.A., et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224:510.e1–510.e12. doi: 10.1016/j.ajog.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez-Portilla R.J., Sotiriadis A., Chatzakis C., et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx) Ultrasound Obstet Gynecol. 2021;57:224–231. doi: 10.1002/uog.23575. [DOI] [PubMed] [Google Scholar]
- 71.Lokken E.M., Huebner E.M., Taylor G.G., et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225:77.e1–77.e14. doi: 10.1016/j.ajog.2020.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lokken E.M., Taylor G.G., Huebner E.M., et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am J Obstet Gynecol. 2021;225:75.e1–75.e16. doi: 10.1016/j.ajog.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan D.S.A., Pirzada A.N., Ali A., Salam R.A., Das J.K., Lassi Z.S. The differences in clinical presentation, management, and prognosis of laboratory-confirmed COVID-19 between pregnant and non-pregnant women: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:5613. doi: 10.3390/ijerph18115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez-Portilla R.J., Smith E.R., He S., et al. Young pregnant women are also at an increased risk of mortality and severe illness due to coronavirus disease 2019: analysis of the Mexican National Surveillance Program. Am J Obstet Gynecol. 2021;224:404–407. doi: 10.1016/j.ajog.2020.12.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huntley B.J.F., Mulder I.A., Di Mascio D., et al. Adverse pregnancy outcomes among individuals with and without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a systematic review and meta-analysis. Obstet Gynecol. 2021;137:585–596. doi: 10.1097/AOG.0000000000004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 78.Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237–e260. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 79.Brown M.A., Magee L.A., Kenny L.C., et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Higgins J.P., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riley R.D., Higgins J.P., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 83.IntHout J., Ioannidis J.P., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 86.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 87.Duval S., Tweedie R. A nonparametric ”trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 88.Ahlberg M., Neovius M., Saltvedt S., et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324:1782–1785. doi: 10.1001/jama.2020.19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang R., Mei H., Zheng T., et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18:330. doi: 10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prabhu M., Cagino K., Matthews K.C., et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grechukhina O., Greenberg V., Lundsberg L.S., et al. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am J Obstet Gynecol MFM. 2020;2:100246. doi: 10.1016/j.ajogmf.2020.100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adhikari E.H., Moreno W., Zofkie A.C., et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pirjani R., Hosseini R., Soori T., et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med. 2020;27:taaa158. doi: 10.1093/jtm/taaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang M.J., Schapero M., Iverson R., Yarrington C.D. Obstetric hemorrhage risk associated with novel COVID-19 diagnosis from a single-institution cohort in the United States. Am J Perinatol. 2020;37:1411–1416. doi: 10.1055/s-0040-1718403. [DOI] [PubMed] [Google Scholar]
- 95.Egerup P., Fich Olsen L., Christiansen A.H., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies at delivery in women, partners, and newborns. Obstet Gynecol. 2021;137:49–55. doi: 10.1097/AOG.0000000000004199. [DOI] [PubMed] [Google Scholar]
- 96.Hcini N., Maamri F., Picone O., et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2021;257:11–18. doi: 10.1016/j.ejogrb.2020.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahajan N.N., Ansari M., Gaikwad C., et al. Impact of SARS-CoV-2 on multiple gestation pregnancy. Int J Gynaecol Obstet. 2021;152:220–225. doi: 10.1002/ijgo.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madden N., Emeruwa U., Polin M., Bejerano S., Gyamfi-Bannerman C., Booker W.A. 32 COVID-19 and new hypertensive disease in pregnancy. Am J Obstet Gynecol. 2021;224:S23–S24. doi: 10.1016/j.ajogmf.2021.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colon-Aponte C., Ndubizu C., Jayakumar A., et al. 962 Miami mother-baby COVID collaborative: a prospective study linking circulating factors, placental findings and pregnancy complications. Am J Obstet Gynecol. 2021;224:S597–S598. [Google Scholar]
- 100.Yazihan N., Tanacan A., Erol S.A., et al. Comparison of VEGF-A values between pregnant women with COVID-19 and healthy pregnancies and its association with composite adverse outcomes. J Med Virol. 2021;93:2204–2209. doi: 10.1002/jmv.26631. [DOI] [PubMed] [Google Scholar]
- 101.Brandt J.S., Hill J., Reddy A., et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol. 2021;224:389.e1–389.e9. doi: 10.1016/j.ajog.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cardona-Pérez J.A., Villegas-Mota I., Helguera-Repetto A.C., et al. Prevalence, clinical features, and outcomes of SARS-CoV-2 infection in pregnant women with or without mild/moderate symptoms: results from universal screening in a tertiary care center in Mexico City, Mexico. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steffen H.A., Swartz S.R., Jackson J.B., et al. SARS-CoV-2 infection during pregnancy in a rural midwest all-delivery cohort and associated maternal and neonatal outcomes. Am J Perinatol. 2021;38:614–621. doi: 10.1055/s-0041-1723938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jering K.S., Claggett B.L., Cunningham J.W., et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181:714–717. doi: 10.1001/jamainternmed.2020.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vousden N., Bunch K., Morris E., et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS One. 2021;16 doi: 10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abedzadeh-Kalahroudi M., Sehat M., Vahedpour Z., Talebian P. Maternal and neonatal outcomes of pregnant patients with COVID-19: a prospective cohort study. Int J Gynaecol Obstet. 2021;153:449–456. doi: 10.1002/ijgo.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crovetto F., Crispi F., Llurba E., et al. Impact of SARS-CoV-2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab104. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosenbloom J.I., Raghuraman N., Carter E.B., Kelly J.C. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2021;224:623–624. doi: 10.1016/j.ajog.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trahan M.J., Malhamé I., O’Farrell P., et al. Obstetrical and newborn outcomes among patients with SARS-CoV-2 during pregnancy. J Obstet Gynaecol Can. 2021;43:888–892.e1. doi: 10.1016/j.jogc.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soto-Torres E., Hernandez-Andrade E., Huntley E., Mendez-Figueroa H., Blackwell S.C. Ultrasound and Doppler findings in pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol. 2021;58:111–120. doi: 10.1002/uog.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katz D., Bateman B.T., Kjaer K., et al. The Society for Obstetric Anesthesia and Perinatology coronavirus disease 2019 Registry: an analysis of outcomes among pregnant women delivering during the initial severe acute respiratory syndrome coronavirus-2 outbreak in the United States. Anesth Analg. 2021;133:462–473. doi: 10.1213/ANE.0000000000005592. [DOI] [PubMed] [Google Scholar]
- 112.Chornock R., Iqbal S.N., Wang T., Kodama S., Kawakita T., Fries M. Incidence of hypertensive disorders of pregnancy in women with COVID-19. Am J Perinatol. 2021;38:766–772. doi: 10.1055/s-0041-1727167. [DOI] [PubMed] [Google Scholar]
- 113.Cruz Melguizo S., de la Cruz Conty M.L., Carmona Payán P., et al. Pregnancy outcomes and SARS-CoV-2 infection: the Spanish Obstetric Emergency Group Study. Viruses. 2021;13:853. doi: 10.3390/v13050853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gurol-Urganci I., Jardine J.E., Carroll F., et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.05.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Papageorghiou A.T., Deruelle P., Gunier R.B., et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal Study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.05.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hill A.B. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 117.Shimonovich M., Pearce A., Thomson H., Keyes K., Katikireddi S.V. Assessing causality in epidemiology: revisiting Bradford Hill to incorporate developments in causal thinking. Eur J Epidemiol. 2020 doi: 10.1007/s10654-020-00703-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Q., Zhang Y., Wu L., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lumbers E.R., Delforce S.J., Arthurs A.L., Pringle K.G. Causes and consequences of the dysregulated maternal renin-angiotensin system in preeclampsia. Front Endocrinol (Lausanne) 2019;10:563. doi: 10.3389/fendo.2019.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abbas A.M., Ahmed O.A., Shaltout A.S. COVID-19 and maternal pre-eclampsia: a synopsis. Scand J Immunol. 2020;92:e12918. doi: 10.1111/sji.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tamanna S., Clifton V.L., Rae K., van Helden D.F., Lumbers E.R., Pringle K.G. Angiotensin converting enzyme 2 (ACE2) in pregnancy: preeclampsia and small for gestational age. Front Physiol. 2020;11:590787. doi: 10.3389/fphys.2020.590787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Todros T., Masturzo B., De Francia S. COVID-19 infection: ACE2, pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2020;253:330. doi: 10.1016/j.ejogrb.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dang D., Wang L., Zhang C., Li Z., Wu H. Potential effects of SARS-CoV-2 infection during pregnancy on fetuses and newborns are worthy of attention. J Obstet Gynaecol Res. 2020;46:1951–1957. doi: 10.1111/jog.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bloise E., Zhang J., Nakpu J., et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol. 2021;224:298.e1–298.e8. doi: 10.1016/j.ajog.2020.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abel T., Moodley J., Naicker T. The involvement of microRNAs in SARS-CoV-2 infection comorbid with HIV-associated preeclampsia. Curr Hypertens Rep. 2021;23:20. doi: 10.1007/s11906-021-01138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Lami R.A., Alrammahi A.M., Algburi A.M.A. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2021;224:550–551. doi: 10.1016/j.ajog.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferrer-Oliveras R., Mendoza M., Capote S., et al. Immunological and physiopathological approach of COVID-19 in pregnancy. Arch Gynecol Obstet. 2021;304:39–57. doi: 10.1007/s00404-021-06061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ouyang Y., Bagalkot T., Fitzgerald W., et al. Term human placental trophoblasts express SARS-CoV-2 entry factors ACE2, TMPRSS2, and Furin. mSphere. 2021;6 doi: 10.1128/mSphere.00250-21. e00250-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jaiswal N., Puri M., Agarwal K., et al. COVID-19 as an independent risk factor for subclinical placental dysfunction. Eur J Obstet Gynecol Reprod Biol. 2021;259:7–11. doi: 10.1016/j.ejogrb.2021.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palanisamy A., Giri T. Reduced severe acute respiratory syndrome coronavirus 2 entry factors and enhanced innate immune gene expression in the nasal epithelium of pregnant rats. Am J Obstet Gynecol. 2021;224:118–120. doi: 10.1016/j.ajog.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nizyaeva N.V., Lomova N.A., Dolgopolova E.L., et al. The impact of the novel coronavirus infection COVID-19 on the mother-placenta-fetus system. Bull RSMU. 2021;2:25–31. [Google Scholar]
- 135.Patberg E.T., Adams T., Rekawek P., et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224:382.e1–382.e18. doi: 10.1016/j.ajog.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smithgall M.C., Liu-Jarin X., Hamele-Bena D., et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020;77:994–999. doi: 10.1111/his.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sherer M.L., Lei J., Creisher P.S., et al. Pregnancy alters interleukin-1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Facchetti F., Bugatti M., Drera E., et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBiomedicine. 2020;59:102951. doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hecht J.L., Quade B., Deshpande V., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vivanti A.J., Vauloup-Fellous C., Prevot S., et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Algarroba G.N., Rekawek P., Vahanian S.A., et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hosier H., Farhadian S.F., Morotti R.A., et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Penfield C.A., Brubaker S.G., Limaye M.A., et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020;2:100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Taglauer E., Benarroch Y., Rop K., et al. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. doi: 10.1016/j.placenta.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alamar I., Abu-Arja M.H., Heyman T., et al. A possible case of vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a newborn with positive placental in situ hybridization of SARS-CoV-2 RNA. J Pediatric Infect Dis Soc. 2020;9:636–639. doi: 10.1093/jpids/piaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Algarroba G.N., Hanna N.N., Rekawek P., et al. Confirmatory evidence of the visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:953–954. doi: 10.1016/j.ajog.2020.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Debelenko L., Katsyv I., Chong A.M., Peruyero L., Szabolcs M., Uhlemann A.C. Trophoblast damage with acute and chronic intervillositis: disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum Pathol. 2021;109:69–79. doi: 10.1016/j.humpath.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu-Culligan A., Chavan A.R., Vijayakumar P., et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y) 2021;2:591–610.e10. doi: 10.1016/j.medj.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schwartz D.A., Baldewijns M., Benachi A., et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med. 2021;145:517–528. doi: 10.5858/arpa.2020-0771-SA. [DOI] [PubMed] [Google Scholar]
- 151.Argueta LB, Lacko LA, Bram Y, Tada T, Carrau L, Zhang T, et al. SARS-CoV-2 infects syncytiotrophoblast and activates inflammatory responses in the placenta. bioRxiv [preprint]. 10.1101/2021.06.01.446676. [DOI]
- 152.Verma S., Joshi C.S., Silverstein R.B., He M., Carter E.B., Mysorekar I.U. SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system. Med (N Y) 2021;2:575–590.e5. doi: 10.1016/j.medj.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seethy A.A., Singh S., Mukherjee I., et al. Potential SARS-CoV-2 interactions with proteins involved in trophoblast functions - an in-silico study. Placenta. 2021;103:141–151. doi: 10.1016/j.placenta.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beys-da-Silva W.O., da Rosa R.L., Santi L., et al. The risk of COVID-19 for pregnant women: evidences of molecular alterations associated with preeclampsia in SARS-CoV-2 infection. Biochim Biophys Acta Mol Basis Dis. 2021;1867:165999. doi: 10.1016/j.bbadis.2020.165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lau J., Ioannidis J.P., Terrin N., Schmid C.H., Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sterne J.A., Sutton A.J., Ioannidis J.P., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 157.Doleman B., Freeman S.C., Lund J.N., Williams J.P., Sutton A.J. Funnel plots may show asymmetry in the absence of publication bias with continuous outcomes dependent on baseline risk: presentation of a new publication bias test. Res Synth Methods. 2020;11:522–534. doi: 10.1002/jrsm.1414. [DOI] [PubMed] [Google Scholar]
- 158.The American College of Obstetricians and Gynecologists COVID-19 FAQs for obstetrician-gynecologists, obstetrics. 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics Available at:
- 159.Society for Maternal-Fetal Medicine Management considerations for pregnant patients with COVID-19. 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf Available at:
- 160.Royal College of obstetricians & Gynaecologysts Guidance for maternal medicine services in the coronavirus (COVID-19) pandemic. 2020. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-12-09-guidance-for-maternal-medicine-services-in-the-coronavirus-covid-19-pandemic.pdf Available at:
- 161.Elwood C., Raeside A., Watson H., et al. Committee Opinion No. 400: COVID-19 and pregnancy. 2020. https://sogc.org/common/Uploaded%20files/Latest%20News/Committee%20Opinion%20No.%20400%20COVID-19%20and%20Pregnancy.pdf Available at:
- 162.Poon L.C., Yang H., Dumont S., et al. ISUOG interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium: information for healthcare professionals—an update. Ultrasound Obstet Gynecol. 2020;55:848–862. doi: 10.1002/uog.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gavillet M., Rolnik D.L., Hoffman M.K., Panchaud A., Baud D. Should we stop aspirin prophylaxis in pregnant women diagnosed with COVID-19? Ultrasound Obstet Gynecol. 2020;55:843–844. doi: 10.1002/uog.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chmielewska B., Barratt I., Townsend R., et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McDonnell S., McNamee E., Lindow S.W., O’Connell M.P. The impact of the Covid-19 pandemic on maternity services: a review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;255:172–176. doi: 10.1016/j.ejogrb.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pariente G., Wissotzky Broder O., Sheiner E., et al. Risk for probable post-partum depression among women during the COVID-19 pandemic. Arch Womens Ment Health. 2020;23:767–773. doi: 10.1007/s00737-020-01075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sinnott C., Freret T.S., Clapp M.A., Little S.E. Increased rates of hypertensive disorders of pregnancy during the COVID-19 pandemic. Am J Obstet Gynecol. 2021;224:S685. doi: 10.1055/a-2295-3543. [DOI] [PubMed] [Google Scholar]
- 168.Patel S., Nguyen L.M., Zhao Z., Newton J. Impact of COVID-19 on obstetrical outcomes at a single academic medical center. Am J Obstet Gynecol. 2021;224:S617. [Google Scholar]
- 169.Theiler RN, Wick M, Mehta R, Weaver A, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. medRxiv 2021.05.17.21257337; 10.1101/2021.05.17.21257337. Accessed May 31, 2021. [DOI] [PMC free article] [PubMed]