Remdesivir has been recently recognized as a beneficial antiviral therapy for the treatment of hospitalized patients with coronavirus disease 2019 (COVID-19) with lower respiratory tract infection. Cardiac disorders (including atrial fibrillation, supraventricular arrhythmias and other nonspecific arrhythmias) as adverse events occurred in 2.6% of the patients treated with remdesivir.1 Recently, the occurrence of marked sinus bradycardia has been associated with remdesivir administration.2 The aim of our single-center prospective observational study was to evaluate the incidence and clinical impact of arrhythmic events in hospitalized patients receiving remdesivir treatment for COVID-19.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
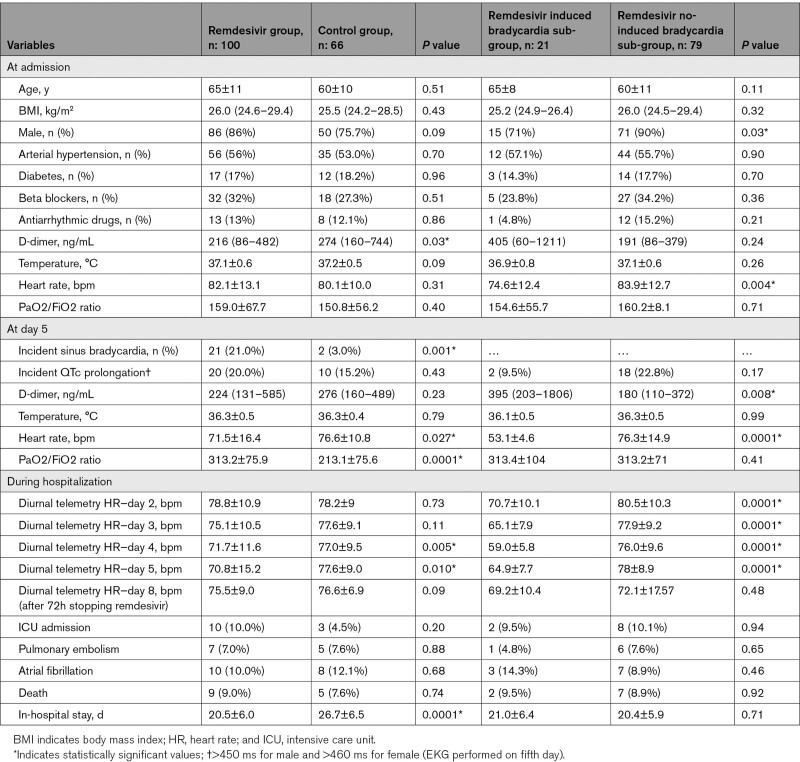
We prospectively included 166 consecutive laboratory-confirmed COVID-19 patients hospitalized at Cotugno Hospital, Naples—Italy, from September 15 to December 1, 2020, 100 patients received remdesivir treatment according to Italian Medicines Agency indications,3 66 patients could not be treated with remdesivir due to the late hospital admission (beyond 10 days from the symptoms onset). All patients were treated with azitromicin 500 mg daily for 6 days, dexamethasone 8 to 16 mg per day depending on the severity of the COVID-19 pneumonia and heparin (therapeutic dose if pulmonary embolism was documented). Clinical evaluation, laboratory examinations, and 12 leads ECG were performed before (at hospital admission) and on the fifth day of therapy (from 8 to 11 am). All data are shown in Table. The distribution of continuous data was tested with the Kolmogorov–Smirnov and the Shapiro-Wilk test. Normally distributed variables were expressed as mean±SD, whereas non-normal distributed ones as median and interquartile range. Categorical variables were reported as numbers and percentages. Continuous normally distributed variables were compared by using the Student t test; differences between non-normally distributed variables were tested with the Mann-Whitney U test. Categorical variables were compared with χ2 test, or Fisher exact test, when appropriate. For all test, a P value <0.05 was considered statistically significant.
Table.
Characteristics of the Study Population; PaO2/FiO2 Ratio of Arterial Oxygen Partial Pressure (PaO2 in mm Hg) to Fractional Inspired Oxygen (FiO2 in %)
The statistical analyses were performed with IBM SPSS software (SPSS, version 20; IBM, NY). Informed consent was obtained under the institutional review board policies of hospital administration. There were no significant differences in baseline clinical characteristics between remdesivir and control group, expect for D-dimer value (216 [86–482] versus 274 [160–744] ng/mL; P=0.03). On the fifth day, remdesivir group showed higher incidence of sinus bradycardia compared with control group (21% versus 3.0%; P=0.001), despite the higher PaO2/FiO2 ratio values (313.2±75.9 versus 213.1±75.6; P=0.0001). In the remdesivir sub group, 4 of 21 patients (19 %) experienced extreme sinus bradycardia, defined as heart rate <50 bpm. No significant differences in electrocardiographic parameters, incident arrhythmias, thromboembolic events and in-hospital mortality were reported between the 2 groups. The remdesivir group has been dichotomized into 2 sub-groups according to the incident sinus bradycardia. Patients with sinus bradycardia were more frequently female (29% versus 10%; P=0.03); they showed lower resting heart rate (75.2±12.9 versus 83.9±12.7 bpm; P=0.006) and higher D-dimer value (395 [203–1806] versus 180 [110–372] ng/mL; P=0.008) at admission; however, at univariable regression model only male sex (relative risk, 0.28 [95% CI, 0.85–0.93]; P=0.038) was associated to reduced risk of incident sinus bradycardia. The multivariable model could not be performed due to low rate of adverse events. The heart rate reduction (considered as delta heart rate before and after remdesivir administration) was 22 bpm (11.5–31.0, interquartile range) and 9 bpm (0–18.0, interquartile range), in the bradycardia group and in nonbradycardia group, respectively (P=0.001). We performed a sub-analysis in the whole population considering only patients with body temperature <37.2 °C at admission and we still found a significant heart rate reduction after remdesivir was started (from 81 to 70 bpm; P<0.0001). In all cases, sinus bradycardia was reversible at remdesivir discontinuation. No patients were sedated or required blood pressure support during remdesivir treatment. No patients required temporary pacemaker, neither presented hemodynamic instability or ventricular arrhythmias. Patients with sinus bradycardia did not show significant differences in intensive care unit admission rate (9.5% versus 10.1%; P=0.94) and overall mortality (9.5% versus 8.9%; P=0.92) compared with those without remdesivir induced bradycardia. The in-hospital stays were similar between the 2 sub-groups (21.4±6.5 versus 20.3±5.9 days; P=0.48). Remdesivir related incident sinus bradycardia might be due to its status of nucleoside analogue that resembles ATP, justifying its negative reversible chronotropic effect on sinus node cells.4 According to our findings the incident sinus bradycardia following remdesivir administration did not seem to impact on patients’ prognosis in terms of intensive care unit admission and in-hospital mortality. Our results, despite the limitation of potential confounding factors, support a safe use of remdesivir among patients with COVID-19 in a real-world setting. Further studies with larger sample sizes are needed to elucidate the safety profile of remdesivir with regard to cardiovascular events, especially in patients with preexisting arrhythmic disorders.
Sources of Funding
None.
Disclosures
None.
Nonstandard Abbreviation and Acronym
- COVID-19
- Coronavirus Disease 2019
For Sources of Funding and Disclosures, see page 673.
Contributor Information
Emilio Attena, Email: emilioattena@hotmail.it.
Alberto Enrico Maraolo, Email: albertomaraolo@gmail.com.
Mariano Mollica, Email: mollicamariano@gmail.com.
Annunziata De Rosa, Email: annunziataderosa@yahoo.it.
Raffaella Pisapia, Email: raffaella.pisapia@ospedalideicolli.it.
Giuseppe Fiorentino, Email: giuseppe.fiorentino@ospedalideicolli.it.
Roberto Parrella, Email: roberto.parrella@ospedalideicolli.it.
Sergio Severino, Email: sergio.severino@ospedalideicolli.it.
Vincenzo Russo, Email: v.p.russo@libero.it.
References
- 1.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19 - Final Report. N Engl J Med. 2020; 383:1813–1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubitosa JC, Kakar P, Gerula C, Nossa H, Finkel D, Wong K, Khatri M, Ali H. Marked sinus bradycardia associated with remdesivir in COVID-19: a case and literature review. JACC Case Rep. 2020; 2:2260–2264. doi: 10.1016/j.jaccas.2020.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Italian Medicines Agency. Remdesivir nella terapia dei pazienti adulti con COVID-19. 24/11. 2020:1–6. Accessed March 3, 2021. https://www.aifa.gov.it/documents/20142/1123276/remdesivir_update01_24.11.2020.pdf/f600b68a-7aea-6781-3dbf-934db269087c
- 4.Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020; 40:659–671. doi: 10.1002/phar.2429 [DOI] [PMC free article] [PubMed] [Google Scholar]