Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has led to a surge in clinical trials evaluating investigational and approved drugs. Retrospective analysis of drugs taken by COVID-19 inpatients provides key information on drugs associated with better or worse outcomes.
Methods
We conducted a retrospective cohort study of 10 741 patients testing positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection within 3 days of admission to compare risk of 30-day all-cause mortality in patients receiving ondansetron using multivariate Cox proportional hazard models. All-cause mortality, length of hospital stay, adverse events such as ischemic cerebral infarction, and subsequent positive COVID-19 tests were measured.
Results
Administration of ≥8 mg of ondansetron within 48 hours of admission was correlated with an adjusted hazard ratio for 30-day all-cause mortality of 0.55 (95% CI, 0.42–0.70; P < .001) and 0.52 (95% CI, 0.31–0.87; P = .012) for all and intensive care unit–admitted patients, respectively. Decreased lengths of stay (9.2 vs 11.6; P < .001), frequencies of subsequent positive SARS-CoV-2 tests (53.6% vs 75.0%; P = .01), and long-term risks of ischemic cerebral ischemia (3.2% vs 6.1%; P < .001) were also noted.
Conclusions
If confirmed by prospective clinical trials, our results suggest that ondansetron, a safe, widely available drug, could be used to decrease morbidity and mortality in at-risk populations.
Keywords: coronavirus, pneumonia, viral, nausea, vomiting
In this retrospective study of 10,741 hospitalized COVID-19 infected veterans, we found a significant reduction in mortality among those who received ondansetron during the first 2 days of admission, including those requiring ICU care or with increased comorbidities.
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has produced significant morbidity and mortality worldwide [1]. Potential therapies are limited in number or are currently under investigation in clinical trials. This has motivated efforts by the biomedical community to perform in vitro and in silico testing of repurposed drugs with potential [2]. These methods are limited by their requirement of either supportive in vitro data from which a hypothesis can be developed and tested in a retrospective population or the need for extensive literature analysis.
Recently, an association between metformin and improved outcomes such as decreased mortality in COVID-19 patients has been recognized and is currently being investigated in multiple prospective trials listed in clinicaltrials.gov [3–6]. Nicotine is also being studied by several groups, which have found, counterintuitively, that smokers and nicotine product users have generally less severe COVID-19 symptoms, with one of the principal hypotheses being that nicotine inhibits cytokine storm development [7–9]. Clinical trials utilizing nicotine patches for prevention and treatment of COVID-19 infection in hospitalized patients are currently ongoing [10, 11].
Ondansetron is a commonly used serotonin 5-HT3 receptor antagonist given to patients experiencing nausea and/or vomiting—experienced in 8%–9% of SARS-CoV-2-infected patients, due mostly to the virus’s propensity to infect ACE2 receptor–expressing cells of the gut [12]. Previous studies using ondansetron have shown that 5-HT3 receptor antagonism is associated with antiviral, anti-inflammatory, and anticoagulant effects [13–15]. In a retrospective cohort study, ondansetron was found to be associated with reduced rates of venous thromboembolisms in hospitalized patients, almost to the same extent as aspirin [16]. Additionally, ondansetron is postulated to have a neuroprotective effect in relation to cerebral ischemic infarctions (strokes) [17, 18].
After ondansetron administration, viral shedding was decreased in rotavirus-infected patients and in mice, although the underlying mechanism remains unknown [19, 20]. Potential antiviral effects of ondansetron against SARS-CoV-2 have recently been described. Ondansetron was found to significantly inhibit the cytopathic effects of SARS-CoV-2 infection in vitro [21]. An in silico study predicted that ondansetron could inhibit the SARS-CoV-2 E protein’s calcium ion trafficking [22]. The importance of calcium signaling in the viral life cycle has been well documented [23], and it has been proposed that decreasing intracellular calcium accumulation could play an important role in reducing COVID-19 severity [24].
The objective of this study was to examine the impact of ondansetron on COVID-19 infection 30-day all-cause mortality and other clinical outcomes.
METHODS
Sources of Data
The US Department of Veterans Affairs (VA) health care system serves >9 million veterans at >1200 Veterans Health Administration sites of care throughout the United States and US territories [25]. All VA facilities were included in this analysis. We collected data from 10 741 US veteran inpatients who were admitted from March 1 through December 3, 2020, and had a positive SARS-CoV-2 qualitative polymerase chain reaction (PCR) or antigen assay result within 3 days of their admission. Inpatient barcode medication administration (BCMA) records and SARS-CoV-2 test sample times permitted us to calculate the time interval between the first positive SARS-CoV-2 test and the administration of specific drug doses. Only admitted patients who survived at least 2 days after their first positive SARS-CoV-2 test were included in this analysis, as they were deemed to have had the opportunity to have had at least 2 days of therapeutic interventions. SARS-CoV-2 test data, BCMA, inpatient and outpatient medication, laboratory data for the hospital stay of interest, intensive care unit (ICU) admission status, and comorbidity, demographic, and self-reported ethnicity data for the prior 3 years of outpatient and inpatient visits were included in our analysis. Relevant data sources from VA sites were maintained, integrated, and normalized using the Bitscopic Praedico platform [26].
Statistical Analysis
To test for correlations between patient outcomes and each of >200 medications, we focused on the 84 medications given to at least 400 patients. Outcomes were assessed with adjusted and multivariate models. Factors associated with 30-day and 90-day mortality were investigated using Cox proportional hazards models. Charlson Comorbidity Index (CCI) scores were determined using comorbidity-associated International Classification of Diseases, Tenth Revision (ICD-10), codes from the prior 3 years of health visits. No imputation was performed. For subsequent analysis, patients were divided into 3 ondansetron groups: patients receiving no ondansetron post-SARS-CoV-2 test (group 0), those receiving up to 8 mg in the first 48 hours post-SARS-CoV-2 test (group 1), and those receiving ≥8 mg in the same time period (group 2). The proportional hazards assumption for the Cox models was investigated and confirmed graphically through Kaplan-Meier survival analysis. No censoring was required due to the broad availability of patient follow-up data postadmission. All results are presented with 95% confidence intervals. Statistical tests were 2-tailed, with P < .05 considered significant.
For the analysis of subsequent development of venous thromboembolism, pulmonary thromboembolism, ischemic cerebral infarction, and myocardial infarction, presented as 100-day incidence, we included patients who survived at least 2 weeks postadmission and whose admission date was at least 60 days before the date of the analysis (January 24, 2021). For this Student t test analysis, we combined ondansetron groups 1 and 2. All analyses were conducted with R [27] and Excel (Microsoft) [28].
RESULTS
Characteristics of SARS-CoV-2-Positive Inpatients
A total of 10 741 hospitalized inpatients testing positive for SARS-CoV-2 and surviving for at least 48 hours in the hospital were included. The median age was 71 years, and 94.9% were male. Demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics of 10741 COVID-19-Positive Inpatients
| Ondansetron Group 0 (No Ondansetron; n = 9540) | Ondansetron Group 1 (<8 mg in First 24 h; n = 750) | Ondansetron Group 2 (≥8 mg in First 24 h; n = 451) | All Patients (n = 10 741) | |
|---|---|---|---|---|
| Male sex, No. (%) | 9125 (95.6) | 675 (90.0) | 388 (86.0) | 10 188 (94.9) |
| Median age (IQR), y | 72 (63–78) | 66 (55–73) | 63 (49–72) | 71 (62–77) |
| Age group 1 (≤49), No. (%) | 708 (7.4) | 127 (16.9) | 117 (25.9) | 952 (8.9) |
| Age group 2 (50–59), No. (%) | 963 (10.1) | 121 (16.1) | 78 (17.3) | 1162 (10.8) |
| Age group 3 (60–69), No. (%) | 2193 (23.0) | 204 (27.2) | 102 (22.6) | 2499 (23.3) |
| Age group 4 (≥70), No. (%) | 5676 (59.5) | 298 (39.7) | 154 (34.1) | 6128 (57.1) |
| White, non-Hispanic (%) | 4694 (49.2) | 385 (51.3) | 231 (51.2) | 5310 (49.4) |
| Black or African American (%) | 3179 (33.3) | 222 (29.6) | 132 (29.3) | 3533 (32.9) |
| Hispanic or Latino (%) | 773 (8.1) | 71 (9.5) | 40 (8.9) | 884 (8.2) |
| Other race (%) | 167 (1.6) | 21 (2.8) | 15 (2.7) | 203 (1.8) |
| Unknown race (%) | 664 (7.0) | 41 (5.5) | 30 (6.7) | 735 (6.8) |
| Previously healthy, No. (%) | 1223 (12.8) | 126 (16.8) | 94 (20.8) | 1443 (13.4) |
| At least 1 underlying condition, including obesity, No. (%) | 8317 (87.2) | 624 (83.2) | 357 (78.8) | 9298 (86.6) |
| Respiratory, No. (%) | 3325 (34.9) | 237 (31.6) | 105 (23.2) | 3667 (34.1) |
| Hypertension, No. (%) | 7476 (78.4) | 557 (74.3) | 308 (68.0) | 8341 (77.7) |
| Diabetes, No. (%) | 4882 (48.8) | 341 (54.5) | 211 (53.0) | 5210 (48.5) |
| Obesity, No. (%) | 3300 (34.6) | 304 (40.5) | 163 (36.1) | 3167 (29.5) |
| Average length of stay excluding patients who died at discharge ± SD, d | 11.60 ± 12.90 | 10.01 ± 11.10 | 9.22 ± 9.28 | 11.38 ± 12.65 |
| Median length of stay excluding patients who died at discharge ± SD, d (P value relative to group 0) | 6.86 ± 13.66 | 6.01 ± 11.76 (P = .0034**) | 6.50 ± 9.55 (P < .001***) | 6.81 ± 13.39 |
Numbers of patients and percentages from each of the above demographics and clinical comorbidities for each of the ondansetron groups are shown. In addition, the average and median lengths of stay are shown.
Abbreviation: COVID-19, coronavirus disease 2019.
*P < .05; **P < .01; ***P < .001.
Medications and Outcomes of Hospitalized Patients
We first examined the associated 30-day all-cause mortalities for the 84 drugs or drug classes received by at least 400 patients. The 3 drugs associated with the lowest 30-day all-cause mortalities were metformin, nicotine, and ondansetron (Supplementary Table 1). Given that ondansetron has not been studied in hospitalized COVID-19 patients and there are no clinical trials underway in COVID-19 patients, and unlike metformin and nicotine, which are both essentially continuation of care for comorbidities of diabetes and nicotine addiction, respectively, and thus may have known and unknown confounders, we decided to focus on ondansetron for the remainder of this study.
The median ondansetron dose in the first 48 hours of admission of patients in group 1 and group 2 was 4 mg (n = 750) and 8 mg (n = 451), respectively. Patients generally received the majority of the total doses they would receive during their hospitalization in the first 48 hours after admission (Supplementary Figure 1), with 75% of doses given intravenously and 25% as oral tablets.
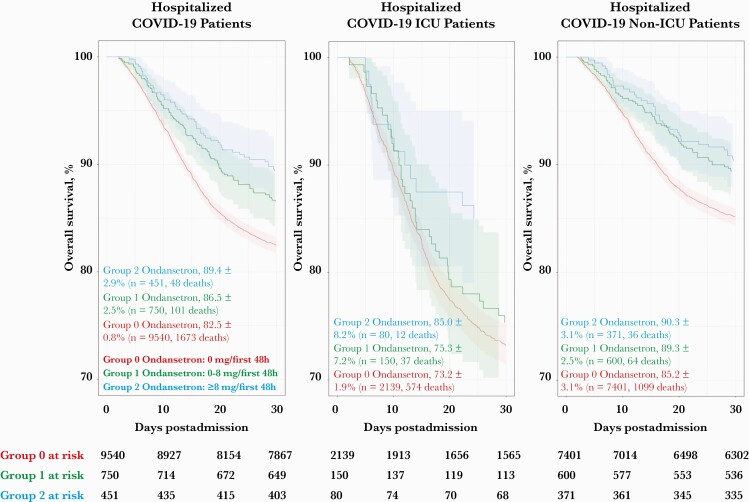
Thirty-day all-cause mortality estimates are listed in Table 2 and shown in Figure 1. These measurements showed significant mortality reduction in ondansetron groups 1 and 2 vs no ondansetron (13.5% and 10.6% vs 17.5%; P = .005 and <.001, respectively). Mortality reduction was significant for both ICU and non-ICU patients (15.0% vs 26.8% for ICU patients in ondansetron group 2 vs group 0; P = .026; and 9.7% vs 14.9% for non-ICU patients in ondansetron group 2 vs group 0; P = .008), as well as for those with hypertension (in ondansetron group 2 vs group 0, 12.3% vs 18.5%; P = .007). The following cohorts also suggested improved survival with ondansetron, but the cohort sizes were insufficient to achieve statistical significance: females (4.8% vs 10.1%; P = .176), diabetes (15.2% vs 18.2%; P = .298), COPD/emphysema (13.3% vs 19.8%; P = .128), moderate to severe kidney disease (16.7% vs 24.1%; P = .213), and cancer history (13.8% vs 20.3%; P = .101).
Table 2.
Thirty-Day All-Cause Mortality for Ondansetron Groups for Specific Subgroups
| Group 0 (n = 9540) | Group 1 (n = 750) | Group 2 (n = 451) | P Value Comparing Groups 0 and 1 | P Value Comparing Groups 0 and 2 | P Value Comparing Groups 0 and 1/2 | |
|---|---|---|---|---|---|---|
| Overall, % | 17.5 | 13.5 | 10.6 | .005** | <.001*** | <.001*** |
| Non-ICU, % | 14.9 | 10.7 | 9.7 | .006** | .008** | <.001** |
| ICU, % | 26.8 | 24.7 | 15.0 | .628 | .026* | .070 |
| Obesity, % | 12.3 | 13.3 | 8.6 | .863 | .524 | .232 |
| Ventilation, % | 39.4 | 26.2 | 20.8 | .126 | .107 | <.001*** |
| Intubation, % | 58.8 | 62.0 | 52.2 | .765 | .676 | .984 |
| Hypertension, % | 18.5 | 14.9 | 12.3 | .038* | .007** | <.001*** |
| Diabetes, % | 18.2 | 16.7 | 15.2 | .529 | .298 | .222 |
| Moderate or severe kidney disease, % | 24.1 | 20.4 | 16.7 | .362 | .213 | .114 |
| COPD/emphysema, % | 19.8 | 18.1 | 13.3 | .588 | .128 | .161 |
| Cancer history, % | 20.3 | 18.3 | 13.8 | .500 | .101 | .117 |
| Female, % | 10.1 | 6.7 | 4.8 | .47 | .260 | .124 |
| African American or Black, % | 15.1 | 10.8 | 11.4 | .103 | .296 | .041* |
| Hispanic or Latino, % | 15.9 | 16.9 | 10.0 | .961 | .435 | .686 |
| Age group 1: ≤49, % | 2.4 | 3.1 | 1.7 | .851 | .897 | .960 |
| Age group 2: 50–59, % | 5.0 | 5.8 | 6.4 | .874 | .777 | .544 |
| Age group 3: 60–69, % | 12.4 | 13.2 | 9.8 | .816 | .530 | .877 |
| Age group 4: ≥70, % | 23.6 | 21.1 | 20.1 | .374 | .372 | .182 |
Groups were defined as follows: ICU = patients who were in the ICU at any time during their COVID-19 admission; non-ICU = patients who were never in the ICU during their COVID-19 admission; hypertension = patients with an ICD-10 code of hypertension in the prior 3 years; obesity = patients with either a BMI ≥30 kg/m2 in the past year or an ICD-10 code of obesity in the prior 3 years; diabetes = patients with an ICD-10 code of diabetes in the prior 3 years; Hispanic = patients who self-identified in 2019–2020 as Hispanic or Latino; African American = patients who self-identified in 2020 as Black or African American.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ICD-10, International Classification of Diseases, Tenth Revision; ICU, intensive care unit.
*P < .05; **P < .01; ***P < .001.
Figure 1.
Kaplan-Meier curve showing survival rates of hospitalized patients who received or did not receive ondansetron in the 30 days postadmission for their first positive COVID-19 admission. Patients in groups 1 and 2 ondansetron (0–8 mg/first 48 hours admission [green] and ≥8 mg/first 48 hours admission [blue], respectively) had improved survival compared with group 0 (no ondansetron [red]) on Kaplan-Meier analysis at 30 days. Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Cox proportional hazard analysis was used to test for potential confounding variables such as other demographic and clinical variables like age group, gender, cancer history, diabetes, and CCI, as well as treatment with remdesivir or dexamethasone (Table 3). We noted that groups 1 and 2 had fewer comorbidities and were on average younger (Table 1). After controlling for CCI, the 30-day mortality hazard ratio for group 2 was 0.65 (95% CI, 0.49–0.87; P = .003), and for group 1 it was 0.79 (95% CI, 0.65–0.97; P = .023). After controlling for age group, the 30-day mortality hazard ratio for group 2 was 0.84 (95% CI, 0.63–1.12; P = .23), and for group 1 it was 0.95 (95% CI, 0.77–1.16; P = .59). In summary, comorbidities as determined by the Charlson Comorbidity Index score are not a confounder, but age is a likely confounder for 30-day mortality. We also examined 90-day all-cause mortality for age as a confounder and presented it in Supplementary Table 2, with results for all of the same variables reported in Table 2. Controlling for age group, the 90-day mortality hazard ratio for group 2 was 0.78 (95% CI, 0.61–1.01; P = .06), and for group 1 it was 0.89 (95% CI, 0.74–1.06; P = .20). None of the other variables were found to have a significant interaction term in the Cox analysis for 30- and 90-day mortality, so we focused on results for the univariate models. We found that a total dose of ≥8 mg of ondansetron given in the first 48 hours of hospital admission (group 2) was associated with a decrease in all-cause 30-day mortality of 45% for all patients and 48% for ICU patients (Table 3) vs those given no ondansetron. Group 1 (>0 and <8 mg) was also associated with better outcomes, but to a lesser degree, indicating that the improved survival associated with ondansetron is likely dose-sensitive.
Table 3.
Results of Cox Proportional Hazards Models Examining the Relation of Ondansetron-Taking in the First 48 Hours of Hospital Admission to 30-Day Mortality in Hospitalized Patients
| Total Population (n = 10 741) | ICU (n = 2369) | Non-ICU (n = 8372) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| All COVID-19 inpatients | 1 | 1.83 (1.68–2.00) | <.001*** | |||
| Ondansetron therapy | ||||||
| <8 mg within 48 h (group 1) | 0.75 (0.61–0.91) | .004** | 0.9 (0.64–1.25) | .527 | 0.7 (0.54–0.9) | .005** |
| ≥8 mg within 48 h (group 2) | 0.58 (0.44–0.77) | <.001*** | 0.53 (0.3–0.94) | .029* | 0.63 (0.45–0.88) | .006** |
| Remdesivir therapy | 1.12 (1.02–1.23) | .015* | 1.06 (0.91–1.25) | .441 | 1.08 (0.96–1.22) | 0.18 |
| Dexamethasone therapy | 1.45 (1.33–1.59) | <.001*** | 1.28 (1.1–1.5) | .002** | 1.47 (1.31–1.65) | <.001*** |
| Risk factors | ||||||
| Charlson Comorbidity Index ≥7 | 1.88 (1.72–2.07) | <.001*** | 1.74 (1.49–2.04) | <.001*** | 1.96 (1.74–2.19) | <.001*** |
| Obesity | 0.72 (0.65–0.79) | <.001*** | 0.8 (0.68–0.95) | .011* | 0.65 (0.57–0.74) | <.001*** |
| Hypertension | 1.4 (1.24–1.58) | <.001*** | 1.13 (0.92–1.38) | .236 | 1.52 (1.3–1.77) | <.001*** |
| Diabetes | 1.14 (1.04–1.25) | .004** | 1.16 (0.99–1.36) | .058 | 1.11 (0.99–1.25) | .062 |
| Cancer | 1.4 (1.24–1.57) | <.001*** | 1.28 (1.05–1.57) | .016* | 1.45 (1.25–1.67) | <.001*** |
| Age | ||||||
| 50–59 y | 2.16 (1.34–3.49) | .002** | 2.3 (1.04–5.09) | .041* | 1.99 (1.09–3.64) | .026* |
| 60–69 y | 5.37 (3.52–8.21) | <.001*** | 5.67 (2.78–11.59) | <.001*** | 4.94 (2.92–8.37) | <.001*** |
| 70+ y | 10.85 (7.19–16.38) | <.001*** | 9.06 (4.5–18.23) | <.001*** | 11.58 (6.95–19.29) | <.001*** |
| Female | 0.49 (0.37–0.65) | <.001*** | 0.72 (0.48–1.09) | .125 | 0.4 (0.27–0.58) | <.001*** |
| Race | ||||||
| African American or Black | 0.79 (0.71–0.88) | <.001*** | 0.89 (0.75–1.07) | .225 | 0.73 (0.64–0.84) | <.001*** |
| American Indian or Alaska Native | 1.46 (0.96–2.2) | .075 | 2.03 (1.04–3.93) | .037* | 1.26 (0.74–2.14) | .393 |
| Asian | 1.09 (0.66–1.82) | .728 | 0.75 (0.24–2.34) | .619 | 1.26 (0.71–2.22) | .435 |
| Hispanic or Latino | 0.85 (0.71–1.02) | .081 | 1.39 (1.06–1.83) | .018* | 0.67 (0.53–0.85) | <.001*** |
| Native Hawaiian or Pacific Islander | 1.05 (0.68–1.61) | .838 | 0.83 (0.31–2.23) | .715 | 1.16 (0.72–1.88) | .536 |
| Unknown | 1.11 (0.93–1.32) | .259 | 1.23 (0.9–1.68) | .201 | 1.08 (0.88–1.34) | .453 |
| Ondansetron controlling for age | ||||||
| Ondansetron group 1 | 0.95 (0.77–1.16) | .595 | 1.11 (0.8–1.56) | .523 | 0.9 (0.7–1.16) | .413 |
| Ondansetron group 2 | 0.84 (0.63–1.12) | .228 | 0.69 (0.39–1.23) | .206 | 0.94 (0.67–1.31) | .698 |
| Age 50–59 y | 2.14 (1.32–3.46) | .002** | 2.29 (1.03–5.08) | .041* | 1.98 (1.08–3.62) | .027* |
| Age 60–69 y | 5.29 (3.46–8.08) | <.001*** | 5.64 (2.76–11.53) | <.001*** | 4.89 (2.88–8.29) | <.001*** |
| Age 70+ y | 10.63 (7.03–16.07) | <.001*** | 8.99 (4.46–18.11) | <.001*** | 11.41 (6.84–19.03) | <.001*** |
| Ondansetron controlling for CCI | ||||||
| Ondansetron group 1 | 0.79 (0.65–0.97) | .023* | 0.91 (0.65–1.27) | .592 | 0.75 (0.58–0.97) | .026* |
| Ondansetron group 2 | 0.65 (0.49–0.87) | .003** | 0.56 (0.32–0.99) | .047* | 0.72 (0.51–1) | .05 |
| CCI score | 1.1 (1.09–1.11) | <.001*** | 1.09 (1.07–1.11) | <.001*** | 1.11 (1.09–1.12) | <.001*** |
Patients receiving ≥8 mg of ondansetron in the first 48 hours (group 2) had better mortality outcomes than those receiving none across all patients, whether ICU or non-ICU. Patients receiving 0–8 mg of ondansetron (group 1) also had better outcomes, although the small sample size (n = 150) prevented statistical significance from being attained for ICU patients. The reference group for the hazard ratio for each risk factor is the group of all patients in the study without that risk factor. Results for ondansetron, remdesivir, and dexamethasone therapies are from univariate analysis; adding the Charlson Comorbidity Index or other therapies as covariates did not significantly alter the results.
Abbreviations: CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; ICU, intensive care unit.
*P < .05; **P < .01; ***P < .001.
Medical Chart Review of High Ondansetron-Administered Patients
To investigate whether nausea/vomiting was associated with reduced disease severity, which would introduce a possible confounding variable in our analysis, we examined mortality associated with the anti-emetic drug metoclopramide, a dopamine D2 and 5-HT-3 receptor antagonist used for the same indication. Cox analysis found that COVID-19 patients receiving metoclopramide had an elevated 30-day mortality hazard ratio of 1.74 (95% CI, 1.45–2.08; P < .001). The inclusion of ICU status and a CCI score >7 as possible confounding variables in the analysis resulted in no evidence of interaction effects.
We further reviewed records of 100 random patients in ondansetron group 2 to determine indication for ondansetron use. We found 94% exhibiting nausea, 63% vomiting, 44% diarrhea, 25% mild respiratory symptoms (not requiring supplemental oxygen), and 56% moderate to severe respiratory symptoms (Supplementary Figure 2). In this random sample of patients, ondansetron was therefore taken by patients for its labeled use (nausea/vomiting).
Duration of Hospitalization, Subsequent PCR Positivity, and Venous Thromboembolism Risk
Excluding patients who died or the small number not yet discharged (n = 33), patients in ondansetron groups 1 and 2 had shorter hospital stays compared with group 0 (group 0 mean, 11.60 days; group 1 mean, 10.01 days; P = .0034; group 2 mean, 9.22 days; P < .001) (Table 1). Patients in ondansetron group 2 were also less likely to have a positive SARS-CoV-2 test over the subsequent 4 weeks after their initial positive test (group 2, 15/28 [53.6%]; vs group 0, 393/524 [75.0%]; P = .012) (Table 4).
Table 4.
Results of Subsequent SARS-CoV-2 PCR or Antigen Tests Within 28 Days After Admission Date
| 7–14-Day SARS-CoV-2-Positive Test Patients, No. (%) | 14–21-Day SARS-CoV-2-Positive Test Patients, No. (%) | 21–28-Day SARS-CoV-2-Positive Test Patients, No. (%) | 7–28-Day SARS-CoV-2-Positive Test Patients, No. (%) | |
|---|---|---|---|---|
| Group 0 (no ondansetron) | 250/307 (81.4) | 123/167 (73.7) | 54/92 (58.8) | 427/566 (75.4) |
| Group 1 (0–8 mg ondansetron/first 48 h) | 18/21 (86) | 9/14 (64) | 3/4 (75) | 30/39 (76) |
| Group 2 (≥8 mg ondansetron/first 48 h) | 11/15 (73) | 4/8 (50) | 0/5 (0) | 15/28 (54) |
Patients who received ondansetron in the first 48 hours postadmission were less likely to have positive SARS-CoV-2 test results in the subsequent 4 weeks.
Abbreviations: PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Rates of ischemic cerebral ischemia and myocardial infarction events for groups 1 and 2 combined were significantly decreased among those who received ondansetron (3.2% vs 6.1%; P < .001; and 0.0% vs 0.3%; P = .045, respectively), while venous thromboembolism and pulmonary embolism for groups 1 and 2 combined did not differ significantly from group 0 (3.5% vs 3.7%; P = .79; and 4.9% vs 5.0%; P = .84, respectively) (Supplementary Table 3).
Remdesivir and Dexamethasone Analysis
We next determined whether improved survival with ondansetron use was related to remdesivir and dexamethasone usage in our Cox proportional hazards model. Remdesivir and dexamethasone did not significantly affect survival in the presence of ondansetron (Table 3). Regardless of whether patients received either of these 2 medications for COVID-19 treatment, administration of ondansetron resulted in higher survival.
DISCUSSION
In this large retrospective study of patients hospitalized for SARS-CoV-2 infection, we found that a total dose of ≥8 mg of ondansetron given in the first 48 hours of hospital admission (group 2) was associated with decreases in all-cause 30-day mortality of 39% for all patients and 46% for ICU patients (Table 3). Group 1 (>0 and <8 mg) was also associated with better outcomes, but to a lesser degree, indicating that the improved survival associated with ondansetron was likely dose-sensitive. Next we tested the hypothesis that these patients were somehow different, perhaps with respect to nausea and vomiting. In a meta-study of 55 studies comprising 10 014 COVID-19 patients, 8.3% of patients exhibited nausea and 6.5% vomiting. Neither was found to be statistically significantly associated with more severe illness [29]. These numbers are compatible with our study, where 1435/13 612 (10.5%) were given ondansetron within the first 48 hours of hospital admission. Patients who took ondansetron in the first 48 hours were more likely to be admitted to the ICU than those who were not (26.0% vs 20.9%), another indication that they are not patients with milder illness. Consistent with this, another meta-analysis of COVID-19 patients revealed that symptoms such as nausea and vomiting, associated with gastrointestinal tract infection, were more likely to be associated with multiorgan involvement, to develop acute respiratory distress syndrome, and to be admitted to the ICU [30]. This discounts the hypothesis that nausea and vomiting are symptoms associated with milder disease.
Limitations
A limitation of our analysis was that this was not a randomized clinical trial in which patients receiving these drugs were carefully matched for COVID-19 disease severity, age, and comorbidities. We noted that the ondansetron groups were younger and had fewer comorbidities for unknown reasons, and there was an interaction with age but not comorbidities. The retrospective design of this study also limited our ability to fully address variability in ondansetron dosing regimens. Although we observed that ≥8 mg administered within the first 48 hours of hospitalization was associated with improved survival, the exact optimal dosing regimen and duration remain unestablished. In contrast with other existing intravenously administered treatment options, however, the availability of ondansetron as an oral dosage form offers significant advantages in being more readily accessible to patients with milder disease, making it a potential candidate for adjunctive use in SARS-CoV-2 infections upon (or before) hospitalization. Lastly, although the numbers in this study were large, the identification of a significant impact of ondansetron use in subgroups such as those patients with a diabetes diagnosis was limited by subgroup size.
CONCLUSIONS
In one of the largest cohorts of hospitalized COVID-19 patients to date, we retrospectively identified that administration of the anti-emetic drug ondansetron was associated with an adjusted 45% reduction in 30-day all-cause mortality in hospitalized patients and a 48% reduction in ICU patients. Our data also suggest that a reduction in ischemic cerebral infarctions and myocardial infarctions may play a significant role in this, through possible anti-inflammatory or antiviral effects of ondansetron [21, 22]. Ondansetron’s widespread availability, affordability, and limited side effect profile make it an excellent candidate for future prospective clinical trials. Further analysis of the other drugs that we identified preliminarily as being associated with positive outcomes may also be warranted.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Renle Chu and Michael Luo, who assisted with literature review, and Chong Lee, Hemal Parekh, and Joel Mewton for assistance with data extraction and troubleshooting. We thank Drs. Prashant Loyalka and Paul Khavari for critical reading of the manuscript.
Financial support. This work was supported by Bitscopic’s R&D budget and intramural funding from the Department of Veterans Affairs.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Potential conflicts of interest. All of the authors, with the exception of Dr. Mark Holodniy, are all employees of Bitscopic, Inc. Otherwise they declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. V.B., R.R., S.P., E.G., F.S., P.E., and M.H. contributed to study conception, design, data analysis, and writing of the manuscript. All authors have read, edited, and approved the final manuscript.
Patient consent. This project was approved by the Stanford University Institutional Review Board under a protocol entitled “Public Health Surveillance in the Department of Veterans Affairs.” As the project was considered minimal risk, consent to participate was not required. Bitscopic is operating under a 10-year Research and Development agreement with the VA, signed in 2019.
References
- 1. World Health Organization. COVID-19 Weekly Epidemiological Update. Geneva: World Health Organization; 2021. [Google Scholar]
- 2. Vijayvargiya P, Esquer Garrigos Z, Castillo Almeida NE, et al. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof). Mayo Clin Proc 2020; 95:1454–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scheen AJ. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab 2020; 46:423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malhotra A, Hepokoski M, McCowen KC, Y-J Shyy J. ACE2, metformin, and COVID-19. iScience 2020; 23:101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo P, Qiu L, Liu Y, et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg 2020; 103:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ClinicalTrials.gov. Metformin glycinate, treatment of patients with COVID-19 and severe acute respiratory syndrome secondary to SARS-CoV-2 (DMMETCOV19-2). https://clinicaltrials.gov/ct2/show/NCT04625985. Accessed 2 January 2021. [Google Scholar]
- 7. Kloc M, Ghobrial RM, Kubiak JZ. How nicotine can inhibit cytokine storm in the lungs and prevent or lessen the severity of COVID-19 infection? Immunol Lett 2020; 224:28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polosa R, Caci G. COVID-19: counter-intuitive data on smoking prevalence and therapeutic implications for nicotine. Intern Emerg Med 2020; 15:853–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farsalinos K, Barbouni A, Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med 2020; 15:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ClinicalTrials.gov. Efficacy of nicotine in preventing COVID-19 infection (NICOVID-PREV). https://clinicaltrials.gov/ct2/show/NCT04583410. Accessed 2 January 2021. [Google Scholar]
- 11. ClinicalTrials.gov. Evaluation of the efficacy of nicotine patches in SARS-CoV2 (COVID-19) infection in hospitalized patients (NICOVID). https://clinicaltrials.gov/ct2/show/NCT04608201. Accessed 2 January 2021. [Google Scholar]
- 12. Andrews PLR, Cai W, Rudd JA, Sanger GJ. COVID-19, nausea, and vomiting. J Gastroenterol Hepatol 2021; 36:646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Motavallian A, Minaiyan M, Rabbani M, et al. Anti-inflammatory effects of alosetron mediated through 5-HT3 receptors on experimental colitis. Res Pharm Sci 2019; 14:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mainou BA, Ashbrook AW, Smith EC, et al. Serotonin receptor agonist 5-nonyloxytryptamine alters the kinetics of reovirus cell entry. J Virol 2015; 89:8701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fakhfouri G, Rahimian R, Ghia JE, et al. Impact of 5-HT3 receptor antagonists on peripheral and central diseases. Drug Discov Today 2012; 17:741–7. [DOI] [PubMed] [Google Scholar]
- 16. Datta A, Matlock MK, Dang NL, et al. “Black box” to “conversational” machine learning: ondansetron reduces risk of hospital-acquired venous thromboembolism. IEEE J Biomed Health Inform. In press. [DOI] [PubMed] [Google Scholar]
- 17. Murozono M, Miyashita R, Takeda A, et al. Co-administration of cyclosporin A and ondansetron decreases transient local cerebral ischemic injury in the mouse. Neuro Endocrinol Lett 2017; 38:163–8. [PubMed] [Google Scholar]
- 18. Sharma A, Patnaik R, Sharma HS. Neuroprotective effects of 5-HT3 receptor antagonist ondansetron on morphine withdrawal induced brain edema formation, blood-brain barrier dysfunction, neuronal injuries, glial activation and heat shock protein upregulation in the brain. Int Rev Neurobiol 2019; 146:209–28. [DOI] [PubMed] [Google Scholar]
- 19. Bialowas S, Hagbom M, Nordgren J, et al. Rotavirus and serotonin cross-talk in diarrhoea. PLoS One 2016; 11:e0159660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagbom M, Novak D, Ekström M, et al. Ondansetron treatment reduces rotavirus symptoms—a randomized double-blinded placebo-controlled trial. PLoS One 2017; 12:e0186824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Touret F, Gilles M, Barral K, et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep 2020; 10:13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dey D, Borkotoky S, Banerjee M. In silico identification of tretinoin as a SARS-CoV-2 envelope (E) protein ion channel inhibitor. Comput Biol Med 2020; 127:104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clark KB, Eisenstein EM. Targeting host store-operated Ca(2+) release to attenuate viral infections. Curr Top Med Chem 2013; 13:1916–32. [DOI] [PubMed] [Google Scholar]
- 24. Jayaseelan VP, Paramasivam A. Repurposing calcium channel blockers as antiviral drugs. J Cell Commun Signal 2020; 14:467–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. RAND Health. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. 2015. https://www.rand.org/pubs/research_reports/RR1165z2.html. Accessed 2 January 2021. [PMC free article] [PubMed] [Google Scholar]
- 26. Holodniy M, Winston C, Lucero-Obusan C, et al. Evaluation of Praedico™, a next generation big data biosurveillance application. Online J Public Health Inform 2015; 7:e133. [Google Scholar]
- 27. Chambers J. Software for Data Analysis: Programming With R. New York Springer; 2008. [Google Scholar]
- 28. Divisi D, Di Leonardo G, Zaccagna G, Crisci R. Basic statistics with Microsoft Excel: a review. J Thorac Dis 2017; 9:1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon 2020; 6:e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gul F, Lo KB, Peterson J, et al. Meta-analysis of outcomes of patients with COVID-19 infection with versus without gastrointestinal symptoms. Proc (Bayl Univ Med Cent) 2020; 33:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.