Abstract
Aims
Guideline-directed medical therapy (GDMT) is underutilized in patients with coronary artery disease (CAD). However, there are no studies evaluating the impact of GDMT adherence on mortality among patients with CAD and heart failure with reduced ejection fraction (HFrEF). We sought to investigate the association of GDMT adherence with long-term mortality in patients with CAD and HFrEF.
Methods and results
Surgical Treatment for Ischaemic Heart Failure (STICH) was a trial of patients with an left ventricular ejection fraction ≤35% and CAD amenable to coronary artery bypass graft surgery (CABG) who were randomized to CABG plus medical therapy (N = 610) or medical therapy alone (N = 602). Median follow-up time was 9.8 years. We defined GDMT for the treatment of CAD and HFrEF as the combination of at least one antiplatelet drug, a statin, a beta-blocker, and an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. The primary outcome was all-cause mortality. Assessment of the independent association between GDMT and mortality was performed using multivariable Cox regression with GDMT as a time-dependent covariate. In the CABG arm, 63.6% of patients were on GDMT throughout the study period compared to 66.5% of patients in the medical therapy arm (P = 0.3). GDMT was independently associated with a significant reduction in mortality (hazard ratio 0.65, 95% confidence interval 0.56–0.76; P < 0.001).
Conclusion
GDMT is associated with reduced mortality in patients with CAD and HFrEF independent of revascularization with CABG. Strategies to improve GDMT adherence in this population are needed to maximize survival.
Keywords: Coronary artery disease, Cardiomyopathy, Heart failure, Outcome
Introduction
Current guidelines for the management of chronic coronary syndromes and heart failure from the European Society of Cardiology (ESC) stress the importance of guideline-directed medical therapy (GDMT) in the treatment of patients with coronary artery disease (CAD) and heart failure with reduced ejection fraction (HFrEF).1,2 Recommendations also suggest implementation of revascularization strategies with either percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) in select patients in whom obstructive CAD is thought to be contributing to symptomatic HFrEF or when angina symptoms persist despite GDMT. However, current studies indicate that use of GDMT for secondary prevention is low following revascularization, particularly after CABG.3–5
A recent systematic review of the use of medical therapy after coronary revascularization found a paucity of high-quality evidence with only three randomized controlled trials (RCTs) reporting the use of medications during the trial period, only one of which was performed during the modern treatment era.6 Notably, these trials either had very low numbers of or excluded patients with left ventricular systolic dysfunction. The Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial was a large contemporary all-comers trial of patients with CAD randomized to either PCI or CABG.7 A recent post hoc analysis of SYNTAX found that <50% of patients were on GDMT over the 5-year study period, but GDMT was an independent predictor of survival with a 36% reduction in the hazard of mortality.6
The Surgical Treatment for Ischaemic Heart Failure (STICH) was a large, contemporary RCT of patients with CAD and HFrEF, defined by the presence of CAD amenable to revascularization and left ventricular ejection fraction (LVEF) ≤35% that evaluated the role of revascularization with CABG and medical therapy vs. medical therapy alone on clinical outcomes. Using data from STICH, we sought to evaluate the association between GDMT adherence and long-term mortality in patients with CAD and HFrEF.
Methods
The STICH trial
STICH was a multicentre randomized clinical trial of 1212 patients with CAD and HFrEF who were randomized to CABG plus medical therapy or medical therapy alone between 2002 and 2007. Patients were eligible for enrolment if they had CAD amenable to CABG and an LVEF ≤35%. Patients were excluded if they had left main stenosis of 50% or more or Canadian Cardiovascular Society Class 3 or 4 angina. Details regarding the design, enrolment characteristics, and outcomes regarding the STICH trial have been published previously.8,9 STICH data were obtained from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repositories Information Coordinating Center (BioLINCC). This study complied with the Declaration of Helsinki. The Washington University Human Research Protection Office granted this study an exemption from IRB oversight due to the deidentified nature of the data.
Study medications, outcomes, and definitions
Following trial enrolment, patients underwent follow-up evaluations at the time of discharge from the CABG hospitalization or 30 days after randomization for medical therapy patients, then every 4 months for the first year and every 6 months thereafter. A full medication history was obtained at study enrolment, hospital discharge, or 30 days after enrolment, 1 year, 5 years, and 10 years. GDMT for the treatment of chronic coronary disease and HFrEF was defined as the combination of at least one antiplatelet drug, a statin, a beta-blocker, and an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. Mineralocorticoid receptor antagonists (MRAs) were not included in the definition of GDMT due to inconsistency in guideline recommendations during study enrolment and follow-up. The primary endpoint was all-cause mortality.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and compared using Student’s t-test. Categorical variables are presented as counts and percentages of the total and compared with the χ2 test. Assessment of the independent association between GDMT and mortality was performed by constructing a Cox regression model with GDMT as a time-dependent covariate. Other variables included in the model were those that differed with a P ≤ 0.1 in bivariate comparisons between the GDMT and non-GDMT groups at time of hospital discharge or 30 days after enrolment, as well as those supported by clinical experience and prior literature. Variables included in the model were age, treatment assignment, minority status, New York Heart Association (NYHA) class, atrial fibrillation/flutter, current smoking, depression, hyperlipidaemia, prior myocardial infarction, prior CABG, prior implantable cardiac defibrillator, creatinine, peripheral vascular disease, prior stroke, and haemoglobin. Hazard ratios (HRs) with 95% confidence intervals (CIs) are shown for treatment effect.
Inverse probability of treatment weighting
To reduce the impact of potential selection bias, an inverse probability of treatment weighting method was used. First, a non-parsimonious, multivariable logistic regression model was created to obtain the probability of receiving GDMT. The inverse of the probability of GDMT assignment was then used to create a weight for each patient.10 Independent variables for the logistic regression model were age, treatment assignment, minority status, NYHA class, atrial fibrillation/flutter, current smoking, depression, hyperlipidaemia, prior myocardial infarction, prior CABG, prior implantable cardiac defibrillator, creatinine, peripheral vascular disease, prior stroke, and haemoglobin.
The χ2 test was used to assess the interaction between treatment effect and patient characteristics. A value of P < 0.05 was considered statistically significant. All analyses were performed with SPSS 23 (IBM Corp., Armonk, NY, USA) and SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
GDMT use in the STICH trial
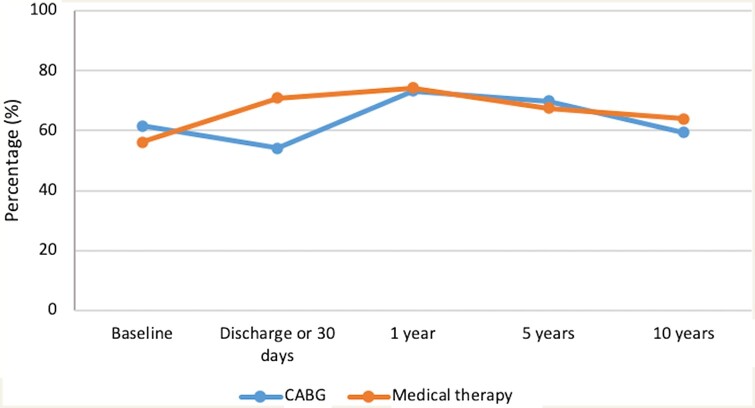
Of the 1212 patients enrolled in STICH, 610 were randomized to CABG plus medical therapy and 602 to medical therapy alone. The median follow-up was 9.8 years. Twenty-five participants withdrew consent or were lost to follow-up (13 in the CABG arm and 12 in the medical therapy arm). There was no significant difference in rates of GDMT adherence between the CABG and medical therapy arms across the entire study trial time period, with 63.6% in the CABG arm and 66.5% in the medical therapy arm on GDMT (P = 0.3). Figure 1 demonstrates the percentage of patients taking GDMT at different time points stratified by treatment assignment. There was a statistically significant difference in GDMT compliance at hospital discharge or 30 days after enrolment between the two arms, with 54.2% patients in the CABG arm on GDMT compared to 70.9% in the medical therapy alone arm (P < 0.001). However, at subsequent follow-up times, GDMT rates were similar in both arms. Individuals on GDMT at hospital discharge or 30 days after trial enrolment were younger, had lower NYHA class, and lower rates of atrial fibrillation, current smoking, and depression. Patients on GDMT were less likely to be members of a minority group and had higher rates of hyperlipidaemia and prior myocardial infarction. There was no difference in sex, diabetes, and hypertension between those who were or were not treated with GDMT (Table 1).
Figure 1.
Use of GDMT in the CABG plus medical therapy versus medical therapy alone arms of the Surgical Treatment for Ischemic Heart Failure trial. There was a significant difference in rates of GDMT at hospital discharge or 30 days after enrolment between the two arms. However, at subsequent follow-up, only about two-thirds of patients in both treatment arms were treated with GDMT. CABG, coronary artery bypass grafting.
Table 1.
Patient characteristics according to GDMT status at hospital discharge or 30 days
| GDMT (N = 757) | No GDMT (N = 454) | P-value | |
|---|---|---|---|
| Age (years), mean ± SD | 59.6 ± 9.2 | 61.4 ± 9.4 | 0.001 |
| Treatment assignment, n (%) | <0.001 | ||
| CABG + medical therapy | 331 (43.7) | 279 (61.5) | |
| Medical therapy alone | 426 (56.3) | 175 (38.5) | |
| Male, n (%) | 662 (87.5) | 401 (88.3) | 0.65 |
| BMI (kg/m2) | 27.4 ± 4.4 | 27.1 ± 5.1 | 0.41 |
| Minority, n (%) | 235 (31) | 185 (40.7) | 0.001 |
| Current NYHA class, n (%) | <0.001 | ||
| I | 111 (14.7) | 28 (6.2) | |
| II | 399 (52.7) | 226 (49.8) | |
| III | 228 (30.1) | 184 (40.5) | |
| IV | 19 (2.5) | 16 (3.5) | |
| Atrial fibrillation/flutter, n (%) | 71 (9.4) | 82 (18.1) | <0.001 |
| Current smoker, n (%) | 141 (18.6) | 111 (24.5) | 0.02 |
| Cancer, n (%) | 8 (1.1) | 6 (1.3) | 0.68 |
| Depression, n (%) | 36 (4.8) | 40 (8.8) | 0.005 |
| Diabetes mellitus, n (%) | 292 (38.6) | 185 (40.7) | 0.45 |
| Hyperlipidaemia, n (%) | 482 (63.8) | 247 (54.4) | 0.001 |
| Hypertension, n (%) | 465 (61.4) | 263 (57.9) | 0.23 |
| PVD, n (%) | 111 (14.7) | 73 (16.1) | 0.51 |
| Prior myocardial infarction, n (%) | 601 (79.4) | 332 (73.1) | 0.01 |
| Prior stroke, n (%) | 54 (7.1) | 38 (8.4) | 0.43 |
| Prior CABG, n (%) | 16 (2.1) | 20 (4.4) | 0.02 |
| Prior PCI, n (%) | 105 (13.9) | 51 (11.2) | 0.19 |
| Prior ICD, n (%) | 13 (1.7) | 16 (3.5) | 0.05 |
| Creatinine (mg/dL), mean ± SD | 1.15 ± 0.5 | 1.21 ± 0.8 | 0.08 |
| Duke coronary artery disease index (0–100), mean ± SD | 58.2 ± 21.2 | 59.9 ± 21.8 | 0.16 |
BMI, body mass index; CABG, coronary artery bypass grafting; GDMT, guideline-directed medical therapy; ICD, implantable cardiac defibrillator; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease.
Survival
On multivariable Cox regression analysis with GDMT as a time dependent variable, GDMT was independently associated with a significant reduction in mortality [adjusted HR (aHR) 0.65, 95% CI 0.56–0.76; P < 0.001]. The beneficial effect of GDMT was observed after 1 year of follow-up and was sustained over the next 9 years (Figure 2). The propensity-weighted analysis also demonstrated a mortality benefit associated with GDMT treatment after 1 year (aHR 0.72, 95% CI0.62–0.84; P < 0.001) that increased at 5 (aHR 0.61, 95% CI 0.53–0.71; P < 0.001) and 10 years (aHR 0.58, 95% CI 0.50–0.67; P < 0.001).
Figure 2.
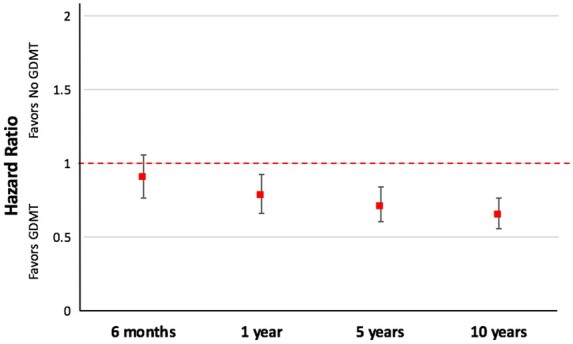
Effect of GDMT on all-cause mortality in the Surgical Treatment for Ischemic Heart Failure trial. GDMT was associated with significantly reduced mortality at 1 year that was sustained for 10 years. Adjusted hazard ratios with 95% confidence intervals are shown.
In addition to GDMT, other covariates independently associated with mortality included randomization to CABG (aHR 0.78, 95% CI 0.67–0.91; P = 0.001), age (aHR 1.17, 95% CI 1.07–1.28; P < 0.001), creatinine (aHR 1.18, 95% CI 1.06–1.16; P < 0.001), NYHA Class 3 (aHR 1.51, 95% CI 1.15–2.01; P = 0.004) and 4 (aHR 2.17, 95% CI 1.35–3.51; P = 0.002), and prior stroke (aHR 1.47, 95% CI 1.13–1.91; P = 0.004) (Table 2). There was no interaction between GDMT and revascularization with CABG (P-interaction = 0.39), with each variable independently associated with a reduction in mortality.
Table 2.
Independent predictors of mortality
| Hazard ratio | 95% confidence interval | P-value | |
|---|---|---|---|
| GDMT | 0.65 | 0.56–0.76 | <0.001 |
| Randomization to CABG | 0.78 | 0.67–0.91 | 0.001 |
| Age (per 10-year increase) | 1.17 | 1.07–1.28 | <0.001 |
| Atrial fibrillation | 1.24 | 0.99–1.55 | 0.05 |
| NYHA class | |||
| III | 1.52 | 1.15–2.01 | 0.004 |
| IV | 2.17 | 1.35–3.51 | 0.002 |
| PVD | 1.20 | 0.98–1.47 | 0.07 |
| Prior MI | 1.19 | 0.99–1.43 | 0.06 |
| Prior Stroke | 1.47 | 1.13–1.91 | 0.004 |
| Current smoker | 1.17 | 0.97–1.41 | 0.10 |
| Creatinine | 1.18 | 1.06–1.16 | <0.001 |
CABG, coronary artery bypass grafting; GDMT, guideline-directed medical therapy; MI, myocardial infarction; NYHA, New York Heart Association; PVD, peripheral vascular disease.
Discussion
There are three significant findings of this post hoc analysis of the STICH trial. First, use of GDMT, defined as the combination of at least one antiplatelet drug, a statin, a beta-blocker, and an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, in patients with CAD and HFrEF, is underutilized with approximately one-third of patients not receiving GDMT. Second, GDMT was associated with reduced mortality independent of the effect of revascularization with CABG, suggesting incremental mortality reduction when adding GDMT to revascularization. Of note, the treatment effect with GDMT (35% reduction in HR of mortality) was numerically greater than the treatment effect of CABG (22% reduction in HR of mortality). Third, the reduction in mortality associated with GDMT was observed as early as 1 year after randomization and was maintained throughout the 10-year study. These findings persisted after propensity adjustment for GDMT.
The objective of the STICH trial was to compare treatment with medical therapy plus CABG with medical therapy alone in patients with CAD and HFrEF. At 5 years of follow-up, the intention-to-treat analysis demonstrated no significant difference between the two arms with respect to the primary outcome of death from any cause.8 After the follow-up period was extended to 10 years, a significant reduction in mortality was found for CABG plus medical therapy compared to medical therapy alone (HR 0.84, 95% CI 0.73–0.97; P = 0.02).9 However, in both the medical therapy and CABG arms, only about two-thirds of patients received GDMT as defined by practice guidelines at the time of the study. The beneficial effect of GDMT, independent of revascularization, was observed as early as 1 year (aHR 0.79, 95% CI 0.66–0.93; P = 0.004) and persisted throughout the 10-year follow-up. In contrast, the benefit of CABG was not apparent at 5 years and became significant only after 10 years of follow-up. It should be emphasized that 10-year follow-up of the STICH trial and the current analysis demonstrate the survival benefit from CABG. Therefore, treatment with GDMT should not preclude CABG in appropriate patients. Conversely, performance of CABG does not justify less robust adherence to GDMT.
Although still suboptimal, GDMT use in STICH was greater than in previous trials of patients with CAD. This is likely a byproduct of the STICH trial design which mandated frequent follow-up and guidance that physicians pay close attention to use of GDMT during the trial. The SYNTAX trial, which was designed to compare complex CAD revascularization with PCI vs. CABG found that only about one-third of patients were on GDMT, which represented a clear missed opportunity since GDMT use was an independent predictor of survival with a 36% reduction in the hazard of mortality.7 The different rates of GDMT adherence between STICH and SYNTAX were likely due to the fact that the SYNTAX trial design did not require providers to actively monitor and prescribe GDMT during the trial follow-up period. The Reduction of Atherothrombosis for Continued Health (REACH) trial, an international observational registry, found that only about half of patients with CAD were on GDMT.11 Two other contemporary RCTs evaluating revascularization vs. medical therapy, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) and Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D) trials, also placed a strong emphasis on medical therapy and reported rates of individual medication use (antiplatelet agents, statins, beta-blockers, angiotensin-converting enzyme inhibitor inhibitors or angiotensin receptor blockers) ranging from 75% to 95%. However, neither trial reported the percentage of patients on the combination of all therapies.12,13 The Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial compared PCI with CABG in patients with multivessel CAD and Type 2 diabetes mellitus. No information on medication use and successful risk factor modification was reported, although it recommended providers follow guideline-driven goals for risk factor reduction including target low-density lipoprotein cholesterol level of <70 mg per decilitre, blood pressure <130/80 mmHg, and glycated haemoglobin <7%.14
The underutilization of GDMT in patients with CAD is not fully understood, is likely multifactorial, and may reflect patient difficulty in medication compliance, healthcare system limitations, or provider misconception of the long-term benefit of medical therapy in patients with or without revascularization. It is possible that providers continue to see GDMT and revascularization as competing rather than synergistic treatment approaches, thus contributing to GDMT underutilization, particularly in patients who have undergone revascularization. Nevertheless, our findings confirm that GDMT should be instituted in all patients with complex CAD and HFrEF. These findings extend those of the SYNTAX trial that demonstrated that lack of GDMT was associated with an increase in adverse events, including death.7 In fact, the benefit of GDMT treatment was greater than the incremental benefit of CABG compared to PCI in the SYNTAX trial. A post hoc analysis of the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) IV trial of over 2000 patients undergoing CABG15 found that the individual components of GDMT (except antiplatelet medications) did not improve clinical outcomes, but that patients on GDMT experienced a reduction in mortality and recurrent myocardial infarction at 2 years.16
The recently published International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial, failed to find incremental benefit of early revascularization (PCI or CABG) over medical therapy in patients with stable ischaemic coronary disease after 3.2 years of follow-up.17 Notably, the ISCHEMIA trial excluded patients with left ventricular systolic dysfunction but nevertheless confirms the clinical importance of medical therapy in reduction of cardiovascular events, including mortality.
Study limitations
This is a post hoc analysis of the STICH trial and thus has limitations inherent to any such analysis. First, patients in the GDMT and non-GDMT groups had differences in baseline characteristics. Although a multivariable Cox regression analysis was used to adjust for these differences, unmeasured or unrecognized differences could exist between groups. Second, medication use was ascertained at regular intervals by patient self-report which may be inaccurate.18 Third, knowledge of specific drug doses was not available and, thus, it is unknown if patients in the GDMT arm were treated with optimal doses. Fourth, the reasons for non-adherence with components of GDMT were not available and we were therefore unable to assess if non-adherence was due to patient or physician behaviour or contraindications to medications. Fifth, as participants in a clinical trial, patients enrolled in STICH had frequent, regular contact with medical providers during the trial, which may not be achievable by usual care in the community, thus limiting the generalizability of our results. Sixth, we did not include MRAs in the definition of GDMT as the guideline-based indications for these agents underwent multiple changes during the design and conduct of the STICH trial. Finally, as in other adherence studies, we cannot rule out a healthy adherer bias, whereby a patient adherent to one chronic preventive therapy is more likely to engage in other healthy behaviours such as exercise and a healthy diet.19
Clinical implications and future perspectives
This study has important implications for clinical practice. First, by demonstrating an early mortality reduction after initiation of GDMT, these findings should encourage physicians to quickly initiate GDMT in patients with CAD and HFrEF. Since conduct of the STICH trial, there have been many advances in pharmacotherapy for CAD and HFrEF such as PCSK9 inhibitors, more effective antiplatelet and anticoagulant therapies, sacubitril/valsartan, and novel diabetes agents (GLP1 agonists, SGLT-2 inhibitors). Our findings of an independent 35% reduction in mortality with GDMT may, in fact, represent a conservative estimate of the potential mortality benefits of currently available medical therapy agents. Thus, efforts to assess and improve patient adherence to medical therapy should be intensified, including educating patients on their disease and the important role of medications in their treatment. The frequency of medication dosing, insurance coverage, and out-of-pocket cost to the patient should be considered when prescribing medications.20,21 The development of a single tablet polypill may also improve patient adherence.22 Studies to understand the reason for GDMT underutilization and to develop interventions to improve use and adherence are urgently needed.
Conclusion
GDMT, defined as the combination of at least one antiplatelet drug, a statin, a beta-blocker, and an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, in patients with CAD and HFrEF, is associated with a significant reduction in mortality independent of CABG with a 35% reduction in the hazard of mortality at 10 years. The benefit of GDMT is apparent at 1 year and sustained over 10 years of follow-up. Given the early benefits, GDMT should be initiated as soon as possible after diagnosis regardless of revascularization strategy.
Data availability statement
The data underlying this article are available from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repositories Information Coordinating Center (BioLINCC) at https://biolincc.nhlbi.nih.gov.
Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002345.
Conflict of interest: none declared.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016;69:1167. [DOI] [PubMed] [Google Scholar]
- 2. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 3. Hiratzka LF, Eagle KA, Liang L, Fonarow GC, LaBresh KA, Peterson ED; Get with the Guidelines Steering Committee. Atherosclerosis secondary prevention performance measures after coronary bypass graft surgery compared with percutaneous catheter intervention and nonintervention patients in the Get With the Guidelines database. Circulation 2007;116:I207–I212. [DOI] [PubMed] [Google Scholar]
- 4. Newby LK, Allen LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER. et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation 2006;113:203–212. [DOI] [PubMed] [Google Scholar]
- 5. Borden WV, Redbery RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA.. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA 2011;305:1882–1889. [DOI] [PubMed] [Google Scholar]
- 6. Mahfoud F, Bohm M, Baumhakel M.. Inadequate reporting of concomitant drug treatment in cardiovascular interventional head to head trials. Clin Cardiol 2012;35:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iqbal J, Zhang YJ, Holmes DR, Morice MC, Mack MJ, Kappetein AP. et al. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial at the 5-year follow-up. Circulation 2015;131:1269–1277. [DOI] [PubMed] [Google Scholar]
- 8. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A. et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE. et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robins JM, Hernan MA, Brumback B.. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 11. Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Hoffman E. et al.; REduction of Atherothrombosis for Continued Health Registry Investigators. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med 2013;126:693–700. [DOI] [PubMed] [Google Scholar]
- 12.The BARI-2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ. et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 14. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ. et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 15. Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson JT, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM; PREVENT IV Investigators. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005;294:2446–2454. [DOI] [PubMed] [Google Scholar]
- 16. Goyal A, Alexander JH, Hafley GE, Graham SH, Mehta RH, Mack MJ. et al. Outcomes associated with the use of secondary prevention medications after coronary artery bypass graft surgery. Ann Thorac Surg 2007;83:993–1001. [DOI] [PubMed] [Google Scholar]
- 17. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE. et al.; Ischemia Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME. et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Behav Med Pract Policy Res 2015;5:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shrank WH, Patrick AR, Brookhart AM.. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011;26:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toy EL, Beaulieu NU, McHale JM, Welland TR, Plauschinat CA, Swensen A. et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med 2011;105:435–441. [DOI] [PubMed] [Google Scholar]
- 21. Choudhry NK, Patrick AR, Antman EM, Avorn J, Shrank WH.. Cost-effectiveness of providing full drug coverage to increase medication adherence in post-myocardial infarction Medicare beneficiaries. Circulation 2008;117:1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castellano JM, Sanz G, Peñalvo JL, Bansilal S, Fernández-Ortiz A, Alvarez L. et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol 2014;64:2071–2082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repositories Information Coordinating Center (BioLINCC) at https://biolincc.nhlbi.nih.gov.