Abstract
Extracellular vesicles (EVs), including exosomes, are emerging as important carriers of signals in normal and pathological physiology. As EVs are a long-range communication or signaling modality—just like hormones are—the field of endocrinology is uniquely poised to offer insight into their functional biology and regulation. EVs are membrane-bound particles secreted by many different cell types and can have local or systemic effects, being transported in body fluids. They express transmembrane proteins, some of which are shared between EVs and some being specific to the tissue of origin, that can interact with target cells directly (much like hormones can). They also contain cargo within them that includes DNA, RNA, miRNA, and various metabolites. They can fuse with target cells to empty their cargo and alter their target cell physiology in this way also. Similar to the endocrine system, the EV system is likely to be under homeostatic control, making the regulation of their biogenesis and secretion important aspects to study. In this review, we briefly highlight select examples of how EVs are implicated in normal physiology and disease states. We also discuss what is known about their biogenesis and regulation of secretion. We hope that this paper inspires the endocrinology field to use our collective expertise to explore these new multimodal “hormones.”
Keywords: extracellular vesicle, exosome, regulation, general endocrinology, physiology, intercellular communication
The field of endocrinology and our understanding of it have evolved through the ages. The pioneering work of Berthold, finding that a chemical in the testes was important for secondary sexual characteristics in roosters, followed by the description of an intestinal factor (secretin) controlling pancreatic secretions, led to our first definition of hormone as put forth by Starling: “the chemical messengers which speeding from cell to cell along the blood stream, may coordinate the activities and growth of different parts of the body” (1). This pioneering work led to the development of the classic age of discovery in endocrinology. That is, remove a tissue (gland) and see what happens, and then add back that tissue and see what happens. The development of the radioimmunoassay in the late 1950s by Rosalyn Yalow and Solomon Berson allowed the field to quantify circulating hormones allowing the field to pursue what regulates hormone release and half-life (2, 3). The notion that some hormones induce secondary messengers, followed by the discovery that steroid hormones regulate transcription, ultimately resulted in the first evidence of a hormone receptor; Jensen and colleagues demonstrated that radiolabeled 17β-estradiol formed a complex with a protein in the nucleus of female reproductive tissues (4, 5). The 1980s through the 1990s brought the so-called molecular era and resulted in the cloning and description of hormone receptors based on homology—many of these first descriptions being of orphan receptors, their ligands not being known (6). Adoption of the orphan receptors in the 2000s, coupled with continued research allowed us to develop a firm grasp on hormones, hormone receptors, and their actions.
Extracellular Vesicles—Biology Mirroring Endocrinology
Up to this point, a hormone had been described as a singular chemical that is secreted and has activity on other cells that can be very distal to the secreting cell. However, during the last 40 years, we were also gaining an appreciation for another circulating factor with signaling properties—extracellular vesicles (EVs; a list of key abbreviations is provided in Table 1), including exosomes. These vesicles have now been well characterized as being able to circulate in the blood and alter the physiology of distal tissues. Is it time we update our definition of hormone to include EVs? Regardless of whether we should or not, many of the principles of endocrinology apply to EVs, making endocrinologists uniquely poised to make serious headway into our understanding of EV biology.
Table 1.
List of key abbreviation definitions
| Abbreviation | Definition |
|---|---|
| EV | Extracellular vesicle |
| ISEV | International Society for Extracellular Vesicles |
| ESCRT | Endosomal sorting complex required for transport |
| MVB | Multivesicular bodies |
| ILV | Intraluminal vesicles |
| Alix | Apoptosis linked gene-2 interacting protein X |
| TSG101 | Tumor susceptibility gene 101 |
| CD | Cluster of differentiation |
| Rab | Ras-related brain/protein |
| Hsc | Heat shock cognate |
| Hsp | Heat shock protein |
| EGFR | Epidermal growth factor receptor |
| PI3K | Phosphoinositide-3-kinase |
| FGFR | Fibroblast growth factor receptor |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| IFN | Interferon |
| HFD | High fat diet |
| PPARγ | Peroxisome proliferator activator gamma |
| miR | microRNA |
| SNAP-23 | Synaptosome associated protein-23 |
| GREM2 | Gremlin-2 |
| TGFβ | Transforming growth factor beta |
| STAM | Signal transducing adapter molecule |
| Eps15 | Epidermal growth factor receptor pathway substrate 15 |
| Hrs | Hepatocyte growth factor–regulated tyrosine kinase substrate |
| ARF6 | ADP ribosylation factor 6 |
| PLD2 | Phospholipase D2 |
| GTPase | Guanosine triphosphatase |
| SNARE | Soluble N-ethylmaleimide-sensitive factor attachment receptor |
| VAMP-7 | Vesicle associated membrane protein-7 |
| SUMO | Small ubiquitin-like modifier |
| ATG | Autophagy-related |
| ISG15 | Interferon-stimulated gene 15 |
| PKM2 | Protein kinase M2 |
| IAA | Indole-3-acetic acid |
| 2,4-DNP | 2,4-Dinitrophenol |
| ATP | Adenosine triphosphate |
| AMP | Adenosine monophosphate |
| cAMP | cyclic adenosine monophosphate |
| KIBRA | Kidney and brain related protein |
| HIF-1α | Hypoxia-inducible factor-1 alpha |
| RAD52 | Radiation sensitive 52 |
| HOXA1 | Homeobox A1 |
| Src | Proto-oncogene tyrosine-protein kinase Src |
| RAS | Rat sarcoma kinase family |
| ERK | Extracellular signal-regulated kinase |
| MAPK | Mitogen associated protein kinase |
| NMDA | N-methyl-D-aspartate |
| iPSC | Induced pluoripotent stem cell |
Extracellular Vesicles—A Brief Synopsis
In 1946, Chargaff and West first postulated the existence of membrane-derived vesicles based on their characterization of centrifuged plasma on blood clotting (7). In 1967, Wolf described small, 20 to 50 nm, platelet-derived vesicles (“platelet dust”) with a density of 1.020 to 1.025 g/mL, which were present in the plasma (8). A decade later in the early 1980s, two groups of researchers described the secretion of small vesicles, from the reticulocytes of rats and sheep, that had transferrin receptors (9-11). Shortly after this discovery, the term exosomes was established and used for the first time (12). Nonetheless, unbeknownst to these early pioneers, their discoveries would contribute to a fundamentally new signaling mechanism, since exosomes reverse the commonly held notion that exosomes/EVs were solely for the disposal of cellular “garbage.” More recently (since ~2006), interest in EVs has gained significant attention. This increase in interest was driven in part by the independent discovery of two research groups that EVs contain RNA (13, 14). Since then, several different types of EVs have been described, based on their properties and biogenesis—apoptotic bodies, microvesicles, ectosomes, and exosomes (Table 2) (15-19). The International Society for Extracellular Vesicles (ISEV) now defines EVs as “particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate” (17). Thanks to their ability to transfer a set of functional molecules over long distances and to integrate with target cells, they can participate in both physiological and pathological processes in the body.
Table 2.
Characteristics of different extracellular vesicles (EVs)
| Extracellular vesicle | Diameter (nm) | Biogenesis pathway |
Common markers |
Described role(s) |
References |
|---|---|---|---|---|---|
| Exosomes | 30-100 | Transported and release via multivesicular bodies (MVBs) ESCRT-dependent and independent ways Endosomal origin |
Alix, TSG101, CD9, CD63, CD81, Rab5a/b, HSP70, HSP90, transferrin receptors | (15-19) | |
| Ectosomes | 100-500 | Created directly from cells’ plasma membrane | β1 integrins, selectins, CD40, MMP, lineage markers | Cell-to-cell communication | (15) |
| Microvesicles | 50-1000 | Created directly from cells’ plasma membrane | No characteristic markers; lack of transferrin receptors | Cell-to-cell communication | (16-18) |
| Apoptotic bodies | 100-5000 | Cell death | Surface markers for the recognition by phagocytic cells markers: Annexin-V | Response to injury and/or stress of cells | (16-18) |
In this review, we will focus on exosomes as they are the most well characterized in the literature. We will reference other EV subtypes when appropriate. According to the latest classification, exosomes are one type of EV, also referred to as “small EVs” (17). It is important to note that the subcategories of EVs and exosomes themselves are still evolving, and there is much discussion in the field as to how exactly we should classify them. Exosomes are the only endosome-origin nanovesicles and are the smallest EV subgroup, with a diameter of 30 to 100 nm (Table 2) (20). The endosomal sorting complex required for transport (ESCRT) machinery was described for the first time in 2008 (21) as well as an alternate (ESCRT-independent) pathway of exosome biogenesis and secretion (22) (discussed below and summarized in Fig. 2). Currently, the ESCRT-dependent pathway is considered the most prevalent pathway used for the biogenesis of exosomes via multivesicular bodies (MVBs) creation and secretion, although this observation could be biased from the limited studies of the ESCRT-independent or alternative pathways (19,20,23).
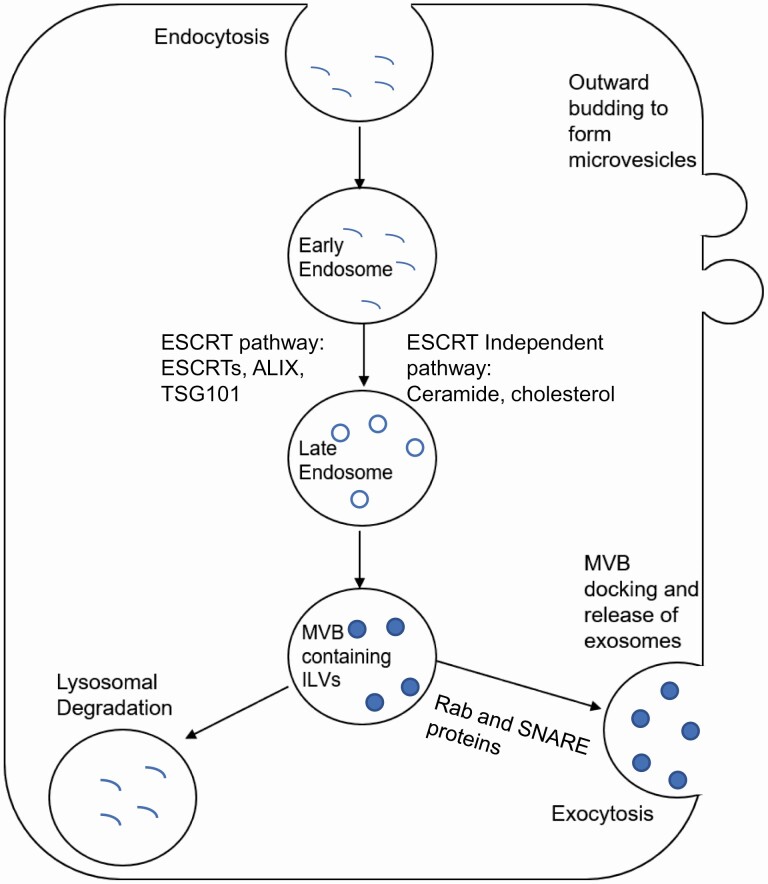
Figure 2.
Biogenesis and secretion of different EV types. Exosomes, the more widely studied EV are formed using the endocytic pathway, which involves formation of endosomes, followed by formation of multivesicular bodies (MVBs), containing intraluminal vesicles (ILVs). These MVBs dock and fuse at the plasma membrane, releasing exosomes. Microvesicles, another type of EV, are formed by outward budding of the plasma membrane.
Based on several studies, EVs (and exosomes) are heterogeneous in their size, even when derived from the same cell. The biological cargo carried by EVs generally reflects the composition of the parent cells. However, the cargo can be different, particularly in the case of exosomes, likely due to a different mechanism of formation than that of other EVs (17). Nevertheless, all EVs are made of a lipid bilayer membrane. The membrane and contents within contain proteins, lipids, sugars, other metabolites, and nucleic acids (DNA and RNA) (24). Within the lipid bilayer, lipid rafts enriched in sphingolipids (including ceramide, which distinguishes these vesicles from lysosomes), as well as cholesterol, have been described and are even considered a characteristic component of the exosome membrane (25).
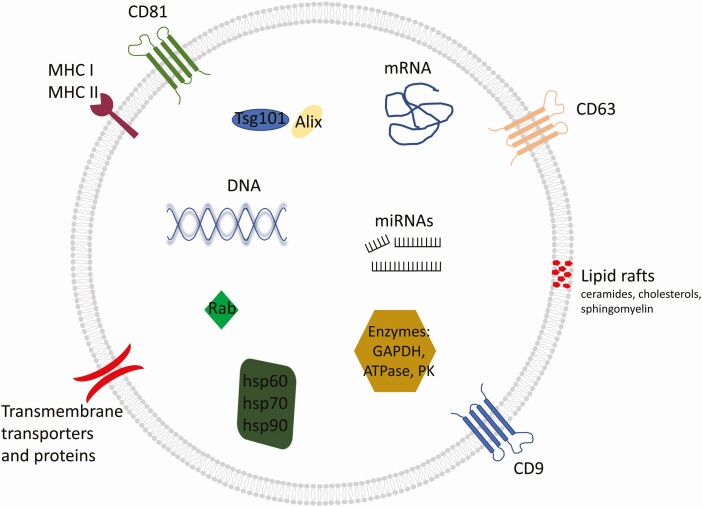
Exosomes have characteristic surface markers which include, among others: (1) tetraspanins (CD9, CD63, CD81); (2) proteins involved in transport and membrane fusion (GTPase, flotillin); (3) proteins of the major histocompatibility complex (MHC) I and II; (4) lipid binding proteins (MFGE8); and (5) integrins, peptidases, and other transmembrane proteins (Fig. 1). Not all exosomes have all of these proteins, which has resulted in a challenge for the field as to how to definitively identify and discriminate exosomes/EVs compared with other cells or cellular debris. On the other hand, exosomes and EVs can express surface markers that are specific to their cell of origin.
Figure 1.
An overview of exosome structure and cargo. Many exosomes have these features, but not all. This diversity has complicated the field as there is not one marker that is specific to exosomes. Adapted from Kalluri and LeBleu, “The biology, function, and biomedical applications of exosomes,” Science 2020;367(6478) (26), with permission from The American Association for the Advancement of Science and Copyright Clearance Center (License Number 5095470918967).
In comparison, the cargo of EVs includes a whole array of different components: heat shock proteins (Hsc70, Hsp90, Hsp60, Hsp70), proteins involved in MVB biogenesis (apoptosis linked gene-2 interacting protein X [Alix], tumor susceptibility gene 101 [TSG101]), proteins from the Rab family, signaling proteins (eg, epidermal growth factor receptor [EGFR], phosphoinositide-3-kinase [PI3K]), and enzymes (eg, glyceraldehyde phosphate dehydrogenase, phosphoglycerate kinase, ATPase, ALG-2), as well as cytoskeleton proteins (eg, actin, fibronectin), and many others. Moreover, RNAs (mRNA, microRNA, non-coding RNA), and DNA can also be found in the cargo of EVs (27-31). There is overlapping cargo between exosomes and other EVs, making it difficult to classify EVs based on cargo alone. However, a protein signature for 5 different categories of small EVs has been reported, at least for dendritic cells (32). For reference, there are resources that catalog the described cargo of EVs (exoRBase (33), ExoCarta (34), and EVmiRNA (35)).
Extracellular Vesicles in Biology and Pathology
Select Examples in Normal Physiology
EVs, including exosomes, have been implicated in several processes in normal physiology and diseases. Similar to how we define hormones, our current understanding of EV function indicates that their main function is intercellular communication from short to long distances using body fluids. Signaling can occur via surface interactions with target cells or uptake and content transfer. The influence of EVs on different processes is visible in almost all tissues of living organisms, which confirms their enormous functional potential. One of the examples is the impact of EVs on human body development processes, even at the embryonic stage. For instance, the involvement of EVs in transmitting Hedgehog and Wnt proteins (36, 37), which regulate growth and patterning (38, 39) during embryonic development. Another example is the involvement of EVs in terms of brain communication. Interestingly, the communication between neural cells is not restricted to synaptic crosstalk but also via nonsynaptic means. EVs have been implicated in mediating communication between neuron-to-neuron (40), neuron-to-glial cells (41), and neuron-to-astrocyte cells (42). Moreover, there is evidence of communication via EVs between blood and the blood-brain barrier (43).
Another example of EVs being implicated in physiological processes is participation in the transmission of immunological signals as well as the transport of antigens, which then activate T lymphocytes either directly, or via transfer to dendritic cells and subsequent presentation (44-46). It was reported that EVs also take part in tissue regeneration by stimulating angiogenesis (47, 48). For example, the protein and microRNA content of mesenchymal stem cell derived EVs can promote cell proliferation and angiogenesis which leads to tissue regeneration (49). However, EVs may have anti-angiogenesis properties too (48). For instance, microRNA (miR-16) found in EV cargo derived from mesenchymal stem cells (50), caused reductions in FGFR1, VEGF, and VEGFR2 (51). Moreover, EVs derived from mesenchymal stem cells have the ability to suppress cell apoptosis (52). These are but a few examples of the involvement of EVs in normal physiological processes. Overall, it appears that the properties and biological functions of EVs result from several factors, the most important of which are the type of cell from which they are derived and the cargo loading system that dictates the content of EVs (53).
Select Examples in Disease
It has become increasingly evident that EV communication systems can become hijacked and, in certain contexts, promote pathogenesis and progression of different diseases. For example, EVs play a role in cardiovascular disorders (28), both in promoting the disease (54) and in having a protective effect on heart tissue (55). Furthermore, disorders of the nervous system can be exacerbated by transporting molecular signals to new, healthy cells (43, 56-58). In a longitudinal study, EVs isolated from the plasma of patients who ultimately developed Alzheimer’s disease had elevated concentrations of p-tau181 and p-tau231 (59), indicating that EVs crossing the blood-brain barrier to systemic circulation could represent an early biomarker for this disease (43, 56). Moreover, EVs can play a significant role in other neurological diseases, like Parkinson’s disease, amyotrophic lateral sclerosis, and prion disease (60-62). In 2017, Hartmann et al published results showing that EVs play an important role in the spread of prions that cause neurodegenerative disorder (63). This happens through the cellular prion protein being packaged into EVs, which plays a key role in this disorder.
It has been demonstrated that EVs may improve the spread of retroviruses through the human body. The mechanism by which the HIV components are internalized through the MVB complex and infect T lymphocytes was described (64, 65). This is not the only example of the critical function of EVs in the spread of the viruses. Nakai et al published an article in which it was shown that EVs are commonly secreted from liver cells infected with the hepatitis C virus (HCV) (66). In this case the authors suggested that EVs loaded with viral RNA are delivered to the endosomes of dendritic cells, resulting in the induction of Type III interferon (IFN) through the toll-like receptor 3 (TLR3)/TICAM-1 pathway. Type III IFNs (IFN-λ1, 2, and 3) were first described as interleukin (IL)-29, IL-28a, and IL-28b and share many of the same immune properties as type I IFNs, including blocking viral replication (67-69), with TLR3 and its adaptor protein (TICAM-1) being able to induce the IFN response (70).
Of importance, EVs have also been implicated in obesity, diabetes mellitus, and metabolic syndrome (diagnosed with the presence of any 3 of central obesity, hypertension, high blood triglycerides, hypercholesterolemia (high LDL and/or low HDL), or impaired fasting glucose). These aspects have been nicely reviewed recently (71, 72), so we will only provide a few key findings here. For example, body mass index (BMI) is positively associated with plasma EV concentration, although weight loss due to bariatric surgery or lifestyle changes (exercise and diet) had no impact after a 1-year follow-up (reviewed in (73)). The cargo of EVs in humans with elevated BMI is also altered (74). Interestingly, another group found that the cargo can change following bariatric surgery and weight loss (75). In a preclinical model, EVs have been described to help mediate a high fat diet (HFD) associated decrease in insulin sensitivity; when EVs isolated from feces of HFD-fed obese mice were administered to lean mice, the lean mice developed insulin resistance (76). EVs secreted from adipose tissue–associated macrophages of HFD-fed mice also modulate insulin sensitivity, as they resulted in both glucose intolerance and insulin sensitivity when administered in lean mice; this is likely mediated by miR-155, which is overexpressed in adipose tissue–associated macrophages and targets peroxisome proliferator-activated receptor γ (PPARγ), a well-known insulin sensitizer (77). Many other preclinical studies implicate EVs in the pathophysiology of diabetes, many of which were reviewed recently (73).
Several groups have now reported EVs in diabetes mellitus. Initial studies found EVs isolated from plasma were higher in diabetic individuals compared with controls, and that antiplatelet drugs were able to reduce EVs within these patients (78-80). At the time, EVs were isolated with flow cytometry using cell-specific antibodies, and as such were referred to as microparticles due to the limitations of flow cytometry (73). More recently, a meta-analysis confirmed that circulating EVs of different categories were significantly higher in patients with type 2 diabetes mellitus compared with healthy controls (81). Not only are there more EVs in patients with diabetes mellitus, but the EVs also have altered cargo and function (82).
In rats, aldosterone-related salt-induced hypertension resulted in increased circulating EVs (83). Likewise, angiotensin II or hypertensive conditions increase EV release from several cell sources, including monocytes, macrophages, and endothelial cells, with macrophage-derived EVs under hypertensive conditions being able to induce inflammatory factors in endothelial cells (83-86). As reviewed previously, EVs may represent biomarkers of hypertension and even contribute to the disease, but more work is required in this field (87).
EVs have also been studied in hypercholesterolemia, another aspect of the metabolic syndrome. Patients with familial hypercholesterolemia exhibit increased circulating EVs containing the following surface markers: CD31+/CD42−, CD235+, and tissue factor (TF+) (88). In preclinical animal models with high lipidemia (but normoglycemia), increased circulating EVs with the CD11B surface marker have been observed (89). Regulation of EVs by cholesterol and its metabolites will be discussed in more detail later in this paper. When considered collectively, it appears that EVs are implicated in all aspects of metabolic syndrome and diabetes mellitus, highlighting the relevance of these signaling molecules in endocrine-related diseases.
Although there are many publications describing the involvement of EVs in various pathological conditions in the human body, as mentioned above, the vast majority of research has focused on the contribution of EVs in cancer. It is believed that the initiation for cancerous changes is genetic, but the tumor pathway consists of a number of processes in which the interaction of cells (both short and long distance) is essential for carcinogenesis and progression. It turns out that EVs/exosomes can transport molecules impacting cellular communication, promote angiogenesis, promote progression, are able to induce changes in the tumor microenvironment, promote metastases, and promote drug resistance (18, 90). A wide diversity of cargo has been attributed to these processes, including genetic, protein-based, and metabolic factors. For example, in the case of breast cancer, researchers found microRNAs (eg, miR-10b (91)) and proteins (eg, hsp90α (92), EDIL3 (93)) that promote cancer progression within EVs from cancer cells. Interestingly, EVs can regulate miRNA synthesis independent of their parental cells (94). It has also been observed that tumor-derived EVs can promote tumor angiogenesis through delivery of different molecules, among others, miR-155 (95), oncogenic EGFR (96) or VEGF189 (97) to endothelial cells. Moreover, it has been reported that the surface proteins of EVs are involved in preparing the premetastatic niche, allowing cancer cells to preferentially colonize certain tissues over others; cell lines with distinct tropism for certain metastatic tissues were found to secrete EVs that were critical for this tropism (98). This was illustrated by the ability of EVs to redirect the colonization of different MDA-MB-231 breast cancer sublines to tissues that were not their preferred seeding sites (98), and, as described by another group, breast cancer EVs breaching the blood-brain barrier and “preparing” brain microenvironment for subsequent metastatic cancerous cells (99).
Interestingly, EVs also have a significant effect on drug resistance, with several different mechanisms being implicated. EVs have also been associated with the development of drug resistance through several different mechanisms: (1) anticancer drugs may have a reduced therapeutic effect due to the competition between the drug and the EV cargo (100); (2) cells may directly efflux the drug outside the cells by loading the drug into EVs; (3) EVs can transfer drug efflux pumps to sensitive cancer cells to allow drug efflux; and (4) EVs can also transfer biologically active cargo to recipient cells to promote cancer cell survival by upregulating complementary pathways (101). Direct efflux of drugs via EVs is seen in the case of cisplatin resistance in ovarian cancer. Specifically, O-GlcNAcylation of SNAP-23 is vital for regulating EV secretion in ovarian cancer cells and downregulation of O-GlcNAcylation leads to increased EV secretion, resulting in efflux of cisplatin from the cells (102). EVs derived from cancer-associated fibroblasts (CAFs) induce drug resistance against gemcitabine and improve the survival of lymphoma cells by enhancement of glycolysis; suppression of EV secretion restores their sensitivity (103). This is due to the decrease in expression of the pyrimidine transporter, equilibrative nucleoside transporter 2 (ENT2), which is the transporter for gemcitabine and cytarabine (103). CAF-derived EVs containing miR-423-5p have also been shown to enhance drug resistance to taxane in prostate cancer cells by inhibiting GREM2 through the transforming growth factor β (TGFβ) pathway (104). In gastric cancer, cisplatin and paclitaxel promote CAFs to secrete miR-522–containing EVs, which are taken up by cancer cells and lead to a decrease in lipid-ROS accumulation in cells and decreased chemosensitivity (105).
Overall, researchers have shown that EVs play an important role in the human body. The multitude of their functions applies not only to the state of physiology but also to disease. This provides a unique opportunity to apply the research techniques of endocrinology for the study of not only EV function, but potential intervention strategies. As with hormones, the biogenesis and secretion of EVs are likely regulated in a controlled manner. The next section of this review will summarize our current understanding of this regulation. Although this field is still underdeveloped, it likely represents heretofore untapped therapeutic targets.
EV Biogenesis, Cargo Sorting, and Secretion
Different types of EVs have different biogenesis mechanisms. Exosome biogenesis is a complex process involving multiple steps. The process begins by endocytosis or double invagination of the plasma membrane to form early endosomes. These mature to form late endosomes, where the formation of intraluminal vesicles (ILV) begin, and eventually lead to formation of MVBs containing ILVs. These MVBs move to the plasma membrane and fuse to it, leading to the secretion of ILVs as cup-shaped exosomes (26, 106). A schematic overview of the EV biogenesis process is presented in Fig. 2. Key proteins that have been implicated in the EV biogenesis pathways are outlined (summarized) in Table 3.
Table 3.
Proteins implicated in EV biogenesis, cargo selection and secretion
| Protein | Function | Effect of knockdown or inhibition | Biogenesis pathway implicated | Cell type | Reference |
|---|---|---|---|---|---|
| Alix (PDCD6IP) | Sorting of contents into MVBs | Decrease exosome secretion | ESCRT-dependent | Breast cancer cells- MCF7 | (107) |
| TSG101 | Sorting of contents into MVBs | Decrease exosome secretion | ESCRT-dependent | Cervical cancer cells- HeLa-CIITA-OVA | (108) |
| Syntenin | Formation of ILV | Decrease exosome secretion | ESCRT-dependent | Breast cancer cells- MCF7 | (107) |
| Rab 5 | Endosomal trafficking | Decrease exosome secretion | ESCRT-dependent | Cervical cancer cells- HeLa-CIITA | (109) |
| Rab 9a | Endosomal trafficking | Decrease exosome secretion | ESCRT-dependent | Cervical cancer cells- HeLa-CIITA | (109) |
| Rab 7 | Endosomal trafficking | Decrease exosome secretion | ESCRT-dependent | Breast cancer cells- MCF7 | (107) |
| Rab 27a, Rab 27b | Multivesicular endosome docking to plasma membrane | Decrease exosome secretion | ESCRT-dependent | Cervical cancer cells- HeLa-CIITA | (109) |
| nSMase2 | Conversion of sphingomyelin to ceramide | Decrease exosome secretion | ESCRT-independent | Oligodendroglial precursor cells- Oli-neu | (22) |
| ISG15 | Colocalization and degradation of MVBs in lysosomes | Increase exosome secretion | ESCRT-dependent | Jurkat T-cells, HEK293 cells | (110) |
| hnRNPA2B1 | Sorting of miRNA | ESCRT-dependent | Primary T lymphocytes, HEK293 cells | (111) | |
| ATG12-ATG3 | Interacts with Alix, endolysosomal trafficking | Decrease exosome secretion | ESCRT-dependent | Mouse Embryonic Fibroblasts | (112) |
| ATG5 | Decreases acidification of MVB and diverts the MVB away from lysosomal degradation | Decrease exosome secretion | ESCRT-dependent | Breast cancer cells- MDA-MB-231 | (113) |
| ARF6 | Late endosome trafficking Receptor mediated endocytosis |
Decrease exosome and microvesicle secretion | ESCRT-dependent, release of microvesicles | Breast cancer cells- MCF7, CHO cells |
(114, 115) |
| aSMase | Microvesicle shedding | Decrease microvesicle secretion | release of microvesicles | Glial cells | (116) |
| PKM2 | Phosphorylation of SNAP-23- docking of MVBs | Decrease exosome secretion | ESCRT-dependent | Lung cancer cells- A549, Liver cancer cells- HepG2 | (117) |
| KIBRA | Membrane trafficking | Decrease exosome secretion | ESCRT-dependent | Neuronal cells-HT22, Murine podocyte cells- MPC5 | (118) |
| HIF-1α | Regulates multiple proteins | Decrease exosome and microvesicle secretion | ESCRT-dependent, release of microvesicles | Breast cancer cells- MCF7, MDA-MB-231. MDA-MB-435 | (119, 120) |
Abbreviations: EV, extracellular vesicle; ESCRT, endosomal sorting complex required for transport; ILV, intraluminal vesicle; MVB, multivesicular body; SNAP-23, synaptosome associated protein-23.
The Endosomal Sorting Complex Required for Transport (ESCRT) is the predominant mechanism for the sorting of proteins into ILV and recruitment of ubiquitinated proteins for lysosomal degradation (121), but it is also used for exosome secretion. ESCRT-0 is the first complex to be recruited and is involved in cargo clustering. This is required for the ubiquitination of the endocytosed receptors. ESCRT-0 comprises several proteins, including hepatocyte growth factor–regulated tyrosine kinase substrate (Hrs), signal transducing adaptor molecule (STAM), Eps15, and clathrin. Hrs is required for the localization of ESCRT-I to the endosomal membrane (122). ESCRT-I component tumor susceptibility gene 101 (TSG101) is then recruited, which forms a complex with the ubiquitinated cargo proteins. This complex activates ESCRT-II and induces bud formation. ESCRT-II carries out sequestration of MVB proteins and recruits de-ubiquitination enzymes to remove ubiquitin from cargo proteins. Next, ESCRT-III sorts these proteins into ILVs (108, 123). Apoptosis linked gene-2 interacting protein X (Alix), an ESCRT accessory protein, binds to late endosomes via lysobisphosphatidic acid (LBPA), found only in late endosomes, and recruits ESCRT-III (124). Syntenin and syndecan are also ESCRT accessory proteins that are involved in the formation of ILVs within the MVBs. Syntenin is a cytosolic protein that binds to syndecan, forming a complex. Alix also binds to syntenin and recruits this complex to the ESCRT machinery to carry out budding of the late endosomal membrane, forming ILVs (125). ADP ribosylation factor (ARF6) and phospholipase D2 (PLD2) regulate this process by binding syntenin (114). After protein sorting, ESCRT-III is disassembled by VPS4 ATPase (108, 123).
Along with the ESCRT-dependent pathway, there are also other components involved in exosome biogenesis, which are collectively called ESCRT-independent mechanisms. Lipids, such as ceramides, induce inward curvature of the limiting membrane of MVB to form ILV. Cholesterol and phosphatidic acid have also been implicated in exosome biogenesis (123). Mass spectrometry analysis showed that exosomal lipid composition was similar to that of lipid rafts and is enriched in cholesterol, sphingolipids, and ceramide (22). Neutral sphingomyelinase (nSMase) is an enzyme that converts sphingomyelin, a sphingolipid, into ceramide by removal of a phosphocholine moiety (126). Exogenous treatment of cells with nSMase leads to an increase in exosome release, implying that ceramides play a role in the ESCRT-independent biogenesis of exosomes (22).
Once the MVB are formed, they may be directed to the lysosomes for their degradation or fuse with the plasma membrane to release exosomes (22).The process of fusion of the MVBs to the plasma membrane for the release of exosomes is carried out by members of the Rab family of GTPases. The Ras-related GTPases or Rab GTPases are a family of 70 proteins, some of which have been found to be involved in vesicular trafficking, budding, and membrane fusion. Rab 27a and Rab 27b are involved in the docking of MVB at the plasma membrane (109). Rab 9a and Rab 5 are involved in the trafficking of early and late endosomes (109). Rab 5 is also involved in controlling the size of exosomes and its knockdown increases their size (107). Rab 7 is involved in mediating lysosomal degradation of endosomes (127). Once the MVBs are docked at the plasma membrane, they fuse with the plasma membrane, releasing the exosomes. This is mediated by the SNARE (Soluble N-ethylmaleimide-Sensitive Factor Attachment Receptor) complex of proteins, including VAMP-7 and YKT-6 (128). Cortactin and Fascin-1 are 2 actin binding proteins that have also been implicated in the process of endosomal trafficking (129).
Microvesicles (or microparticles or ectosomes) are generated by the outward budding and fission of the plasma membrane (130). These are also a heterogenous population of vesicles and some proteins that have been implicated in exosome biogenesis and have also been implicated in the biogenesis of microvesicles. Acid sphingomyelinase (aSMase) has been implicated in shedding of microparticles from glial cells by its activation and translocation to the plasma membrane (116). Small GTPases such as ARF6, Rab 22a, and RhoA have also been implicated in microvesicle shedding (131). The GTP-binding protein ARF6 aids microvesicle release by activating phospholipase D2, which, through a series of phosphorylation events activates the myosin light chain that is required for microvesicle release (132).
Regulation of EV Biogenesis, Secretion, and Cargo
Role of Posttranslational Modifications
Ubiquitination is a posttranslational modification which involves tagging proteins with ubiquitin and targeting them for degradation. Posttranslational modifications are also involved in sorting of proteins into MVB and then targeting them either for lysosomal degradation or secretion as EVs. Ubiquitin-like modifications have been implicated in regulating EV secretion and EV cargo. Some of the ubiquitin-like proteins include the product of interferon-stimulated gene 15 (ISG15), small ubiquitin-related modifier (SUMO), NEDD8, autophagy-related gene 8 (ATG8), and ATG12 (133). ISGylation has also been implicated in modulating release of EVs. ISGylation of TSG101 decreases the number of MVBs and reduces secretion of EVs by increasing their colocalization with lysosomes and degradation of the proteins (110). SUMOylation is involved in the modulation of EV cargo, specifically in the sorting of miRNA. The protein, heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1), binds specific motifs on miRNA and controls their loading on MVBs. This binding is controlled by SUMOylation of hnRNPA2B1 (111). Autophagy-related gene ATG12-ATG3 interaction promotes late endosome to lysosome trafficking by interacting with Alix and also mediates other Alix-related ESCRT functions (112). ATG 5 and ATG 16L1 increase release of EVs by de-acidification of MVBs via V1V0 ATPase control (113).
Role of Glycolysis and Oxidative Phosphorylation
Tumor cells carry out higher amounts of glycolysis than normal cells. Higher levels of aerobic glycolysis have been shown to increase EV formation in A549 (lung cancer) cells and HepG2 (liver cancer) cells. This effect was shown to be mediated by pyruvate kinase M2 (PKM2), whose expression is increased in tumor cells. PKM2 increases EV release by phosphorylating Ser95 of SNAP-23, a component of the synaptosome/SNARE complex, involved in docking and release of MVB contents (117). In another study, however, the simultaneous inhibition of glycolysis and oxidative phosphorylation by indole-3-acetic acid (IAA) and 2,4-DNP has been shown to stimulate the secretion of EVs, both in cell lines and in vivo. Inhibition of these pathways leads to a decrease in overall cellular energy. The study showed that addition of these compounds decreased the levels of ATP and increased AMP levels, which indicates that there is some involvement of cAMP in this process (134).
Role of KIBRA
Kidney and brain expressed protein (KIBRA) is a scaffolding protein which is involved in processes such as cell migration and membrane trafficking. Knockdown of KIBRA was shown to decrease secretion of EVs and increase the size and number of MVBs in HT22 cells and MPC5 cells. The expression of Rab27a was downregulated at the protein level but not at the mRNA level due to ubiquitination and degradation (118).
Role of Hypoxia
Hypoxia acts as a critical factor in the progression of cancer. Hypoxia leads to an increase in EV secretion in granulocytic myeloid derived suppressor cells (G-MDSCs) in a hypoxia-inducible factor (HIF)-1α dependent manner, in a colon cancer model (135). A HIF-1α dependent increase in EV secretion was also observed in the noncancerous renal proximal tubular cells of the kidney. Interestingly, these EVs had a protective effect on the cells, by inhibiting apoptosis (136). Activation of signal transducer and activator of transcription factor 3 (STAT3) by HIF-1α leads to an increase in EV secretion in ovarian cancer cells by the upregulation of Rab27a and downregulation of Rab7 (127). Hypoxia increased EV secretion in breast cancer cell lines MCF7 and MDA-MB231with an enrichment of miR-210 in the cells and in EVs (119). The increased miR-210 was also observed in murine 4T1 mammary cancer cells and EVs in another study (137). The hypoxia-inducible miR-210 has been previously implicated in repressing DNA repair pathways by repressing RAD52 expression (138) and in inhibiting cell proliferation by targeting homeobox A1 (HOXA1) and fibroblast growth factor receptor like 1 (FGFRL1) (139). Elevated cellular miR-210 has been correlated with poor survival in cancers such as acute myeloid leukemia (140) and in breast cancer (141). In human U87 glioblastoma cells, hypoxia induced EV secretion and the hypoxia induced EVs had a significantly different miRNA profile compared to normoxia EVs (142). Hypoxia has also been shown to stimulate microvesicle secretion by HIF-1α–induced Rab22a expression in MDA-MB231 and MDA-MB435 cells (120).
Role of Drugs and Lifestyle Modifications Related to the Treatment of Diabetes Mellitus
There is emerging evidence from humans and preclinical models indicating that EV abundance and cargo can be altered by the same regimens commonly used to treat diabetes mellitus (73). The PPARγ agonist pioglitazone hydrochloride resulted in decreased EV levels compared with metformin in newly diagnosed patients (143). As mentioned previously, treatment of diabetic patients with antiplatelet therapies also decreased plasma EV concentrations (79, 80). Dietary factors can also influence EV concentration; a diet rich in oats decreased EVs from myeloid cells in humans, while skeletal muscle isolated from mice fed a high-palm-oil diet had higher EV concentrations (144, 145). In this case, the EVs isolated from skeletal muscle of mice fed a high-palm-oil diet were able to reduce markers of muscle differentiation and promoted proliferation when in vitro muscle was exposed to them (145). In fact, this study indicated that lipids could be transferred from cells via EVs (145). Exercise induces EV secretion and alters their cargo. Exercise-induced EVs have been suggested to mediate some of the beneficial effects of exercise (146, 147). Intriguingly, EVs from skeletal muscle can affect β-cell function of the pancreas in islet explants (148). Collectively, it appears that those strategies to influence physiology related to diabetes mellitus and energy homeostasis impact EV secretion and cargo.
Role of Proto-Oncogenes and Oncogenes
Src
Activation of c-Src, a proto-oncogene, is common in the case of cancers. Activated c-Src is known to be localized in the endosomal membrane, and it increases EV secretion by associating with and activating Alix, which promotes ILV formation in MVB (149). Src is also required for the internalization of syndecan, which promotes recruitment of syntenin to the endosomes and then increases ILV formation (125).
Ras
Ras genes, namely HRAS, KRAS, and NRAS are mutated and activated in many cancers and are involved in activation of the ERK/MAPK pathway. Based on data from ExoCarta (34), there are multiple proteins, lipid mRNA, and miRNA that are regulated or influenced by the Ras family of proteins (150). Loading of specific miRNA and proteins have been shown to be regulated by the mutation status of KRAS. Mutation of KRAS in colon cancer decreases the loading of Argonaute2 (Ago2; a regulatory protein for miRNA secretion) in EVs due to phosphorylation of Ago2 at Ser 378 (151). Mutation of KRAS also leads to increased exosomal loading and secretion of miR-100 and miR-10b (152). In another study, proteomic analysis of EVs from mutant KRAS colon cancer cells showed that the EVs contained elevated levels of SRC, KRAS, EGFR, and integrins (153).
Role of Sirtuins
Sirtuins are NAD+ dependent deacetylases that are involved in various physiological processes and in maintaining cellular homeostasis. Sirtuin 1 (SIRT 1) is the most studied sirtuin, mainly due to its role in aging and in cancer. SIRT 1 expression is decreased in aggressive types of breast cancer, and this increases EV secretion (154). It does this by reduction of V-ATPase, which is involved in acidification of lysosomes for protein degradation. The impairment of lysosomal function leads to formation of larger MVBs and directs them toward the plasma membrane for exosome release in MDA-MB-231 cells (154). Another study in using HEK-293 cells also showed that loss of SIRT 1 increases release of EVs by formation of larger late endosomes (155). They also studied SIRT 2 in the context of EV release and found that loss of SIRT 2 also led to increased release of EVs, but in a manner distinct from that of SIRT 1. SIRT 2 exerts its effects by interacting with L-type lectin (LMAN2) and does not increase the size of endosomes (155). LMAN2 is involved in limiting the trans-Golgi to endosome trafficking of exosomal cargo protein GPRC5B, and loss of SIRT 2 increases EV release of GPRC5B, implying that it is involved in modulating the cargo of EV (155).
Effect of Genetic Alterations in Neurodegenerative Diseases
EV signaling in the brain is involved in transcriptional regulation, neurogenesis, and neuro-immune modulation. Neurons and glial cells secrete exosomes containing the same markers, such as Alix, TSG101, and tetraspanins, but their cargos are characterized by specific molecules which are not common to other types of cells (156).
In oligodendroglial cells, glutamate, a neurotransmitter, increases EV secretion. The cells responded to glutamate in a calcium-dependent manner via the activation of NMDA receptors (157). In the context of Alzheimer disease, it was shown that expression of the apolipoprotein E4 (APOE4) allele led to reduced secretion of EVs, potentially implicating cholesterol in EV secretion. This was due to the downregulation of Rab35, which is involved in the docking of MVB to plasma membrane. The released EVs were also enriched in lipids such as cholesterol, ceramide, and gangliosides, compared with regular concentrations in the brain (158). Cholesterol accumulation in the cell has been shown to impair MVB secretion by impairing inactivation of Rab7 (159). The authors suggest that APOE4 causes some disruption of cholesterol metabolism within the cell, which leads to modifications in the endosomal pathway. Excess cholesterol coupled with the associated increases in sphingolipids can disrupt the endosomal pathway, impacting both the morphology and motility of endosomal compartments (159, 160). Specifically, accumulation of cholesterol within the endosome can impair the inactivation of Rab7 and thus immobilize MVBs (159, 161). More on cholesterol and EVs is discussed in the next subsection “Cholesterol and its Metabolites.”
In the context of dementia, frontotemporal dementia-associated N279K tau mutant led to the impairment of endocytic trafficking in iPSC-derived neural stem cells isolated from patients. The cells showed signs of reduced lysosomes and accumulation of endosomes (162). Null mutation of the gene progranulin in frontotemporal dementia also reduced EV secretion and cargo by altering the secretion pathway in cells (163).
Cholesterol and its Metabolites
Cholesterol and its metabolites such as 27- hydroxycholesterol (27HC) have been found within EVs, likely within the membrane (164, 165). Interestingly though, these may also be involved in the regulation of EV biogenesis and/or secretion. An inhibitor of cholesterol synthesis (simvastatin) was found to inhibit EV secretion in various cell types (166). In a recent study, it was found that 27HC promoted the secretion of EVs in neutrophils, RAW 264.7 monocytic cells, and 4T1 mammary cancer cells (167). EVs from neutrophils treated with 27HC had an altered metabolic signature and promoted mammary tumor growth and metastasis compared to EVs from vehicle treated neutrophils. This would indicate that 27HC was not only altering secretion, but also cargo loading into EVs. Given that 27HC is a known ligand for the estrogen receptors and the liver X receptors, it is of interest to see if these endocrine receptors are involved in regulating EV biogenesis and secretion. On the other hand, 25-hydroxycholesterol has been found to prevent EV uptake in certain cell types (168).
Drugs Implicated in the Modulation of EV Biogenesis and Cargo
Drug repurposing is becoming an increasingly used approach for treatment of diseases. Several groups have attempted drug repurposing for the inhibition of EVs in the context of cancer. Table 4 is a summary of compounds that have shown evidence of inhibiting EVs, their mechanism of inhibition and the type of cells in which they have been studied.
Table 4.
Drugs that inhibit EV biogenesis
| Compound | Drug target / disease indication | Mechanism of inhibition | Cell type | Reference |
|---|---|---|---|---|
| Simvastatin | HMGCoA-reductase/ hypercholesterolemia | Alters MVB trafficking and leads to their accumulation near the plasma membrane; reduced Rab27b levels | Macrophage-like cells- THP-1 | (166) |
| Manumycin A | Ras farensyltransferase/ antibiotic and antitumor | Acts via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1; reduced Hrs, Alix, Rab27a levels | Prostate cancer (CRPC) cells- C4-2B | (169) |
| Sulfisoxasole | Endothelin receptor A/ antibacterial compound | Triggers colocalization of MVB with lysosomes for degradation | Breast cancer cells- MCF7 and MDA-MB231 and melanoma cells-SK-MEL28 | (170) |
| Imipramine | aSMase/ antidepressant | Inhibits microvesicle shedding by reducing translocation of aSMase to outer leaflet of plasma membrane | Prostate cancer cells -PC3, breast cancer cells -MCF7, glial cells | (116, 171) |
| GW4869 | nSMase2 | Reduces ceramide levels and ESCRT-independent ILV formation | HEK293 cells | (22) |
| Chloramidine/ bisindolylmaleimide-I | Protein kinase C inhibitor/ anticancer | Affects posttranslational protein deamination, prevents externalization of phosphatidyl serine | Prostate cancer cells -PC3, breast cancer cells -MCF7 | (171) |
| Cannabidol | STAT3/ anti-inflammatory, antiproliferative | Decreases STAT3 and CD63 levels | Prostate cancer cells- PC3, liver cancer cells- HepG2, breast cancer cells MDA-MB231 | (172) |
| Y27632 | Rho A | Inhibits Rho-associated protein kinases which inhibits microvesicle release | Platelets | (19) |
| Tipifarnib | Farensyltransferase; targeted inhibition of Ras/Raf/ERK1/2 signaling/anticancer | Reduces Alix, nSMase2, Rab27a | Prostate cancer cells- C4-2B, PC3 | (173) |
| Shikonin | Pyruvate kinase M2-Suppresses aerobic glycolysis/anticancer | Inhibits PKM2 mediated phosphorylation of SNAP-23, which decreases exocytosis | Lung cancer cells-A549, liver cancer cells- HepG2 | (117) |
Conclusion
Similar to hormones, EVs and exosomes are increasingly being recognized as mediators of cellular communication, involved in the homeostatic regulation of many physiological processes. On the other hand, they have also been implicated in the pathophysiology of several diseases. Much work has been devoted to their role in cancer, where they have been shown to contribute to growth, invasiveness, and even where metastatic cells home to. The exact pathways and mechanisms that regulate EV biogenesis and secretion are still poorly understood. Some conflicting results being reported suggests that these processes may be cell-type or context dependent. Of course, just like hormones, EVs appear to have distinct target cells. At present, very little is known about why certain EVs are taken up by certain cell types, but this should be an important aspect of future research. Likewise, how the surface proteins and cargo of EVs are regulated are critical questions in the field. One confounding factor to the field has been the diversity of isolation and characterization techniques used. Regardless, it is likely that defining how EVs are loaded with cargo, what regulates this, and what regulates their ultimate secretion will provide new avenues for drug development. The field of endocrinology is uniquely poised to provide insight, as many of the questions revolving around EVs are questions the endocrinology field has been addressing for over a century.
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health (R01CA234025) and Department of Defense Breast Cancer Research Program Era of Hope Scholar Award (W81XWH-20-BCRP-EOHS / BC200206).
Glossary
Abbreviations
- 27HC
27-hydroxycholesterol
- ARF
ADP ribosylation factor
- ATG
autophagy-related gene
- CAF
cancer-associated fibroblast
- EV
extracellular vesicle
- ESCRT
endosomal sorting complex required for transport
- HIF
hypoxia-inducible factor
- IFN
interferon
- ILV
intraluminal vesicle
- KIBRA
kidney and brain expressed protein
- MVB
multivesicular body
- PPARγ
peroxisome proliferator-activated receptor γ
- SIRT
sirtuin
Additional Information
Disclosures: The authors have nothing to declare.
Conflict of Interest Statement : The authors have no conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability
Not applicable.
References
- 1. The Croonian Lectures on the chemical correlation of the functions of the body. Lancet. 1905;166(4275):339-341. [Google Scholar]
- 2. Berson SA, Yalow RS, Bauman A, Rothschild MA, Newerly K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J Clin Invest. 1956;35(2):170-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berson SA, Yalow RS. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest. 1959;38:1996-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jensen EV. On the mechanism of estrogen action. Perspect Biol Med. 1962;6:47-59. [DOI] [PubMed] [Google Scholar]
- 5. Jensen EV, Suzuki T, Kawashima T, Stumpf WE, Jungblut PW, DeSombre ER. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968;59(2): 632-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tata JR. One hundred years of hormones. EMBO Rep. 2005;6(6):490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189-197. [PubMed] [Google Scholar]
- 8. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269-288. [DOI] [PubMed] [Google Scholar]
- 9. Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983;113(2):650-658. [DOI] [PubMed] [Google Scholar]
- 10. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967-978. [DOI] [PubMed] [Google Scholar]
- 11. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412-9420. [PubMed] [Google Scholar]
- 13. Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847-856. [DOI] [PubMed] [Google Scholar]
- 14. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659. [DOI] [PubMed] [Google Scholar]
- 15. Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr Biol. 2018;28(8):R435-R444. [DOI] [PubMed] [Google Scholar]
- 16. Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125-R134. [DOI] [PubMed] [Google Scholar]
- 17. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto T, Kosaka N, Ochiya T. Latest advances in extracellular vesicles: from bench to bedside. Sci Technol Adv Mater. 2019;20(1):746-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2020;9(1):1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213-228. [DOI] [PubMed] [Google Scholar]
- 21. Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20(1):4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244-1247. [DOI] [PubMed] [Google Scholar]
- 23. Tschuschke M, Kocherova I, Bryja A, et al. Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. J Clin Med. 2020;9(2):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Javeed N, Mukhopadhyay D. Exosomes and their role in the micro-/macro-environment: a comprehensive review. J Biomed Res. 2017;31(5):386-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalluri R, LeBleu VS. Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harb Symp Quant Biol. 2016;81:275-280. [DOI] [PubMed] [Google Scholar]
- 30. Meng Y, Sun J, Wang X, et al. Exosomes: a promising avenue for the diagnosis of breast cancer. Technol Cancer Res Treat. 2019;18:1533033818821421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126(4):1152-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968-E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. exoRBase. Accessed April 2, 2021.http://www.exorbase.org
- 34. ExoCarta. Accessed April 2, 2021. http://exocarta.org/index.html
- 35. ExosomeRNA. Accessed April 2, 2021. https://exosome-rna.com
- 36. Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036-1045. [DOI] [PubMed] [Google Scholar]
- 37. Vyas N, Walvekar A, Tate D, et al. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep. 2014;4:7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb Perspect Biol. 2012;4(8):a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205-3214. [DOI] [PubMed] [Google Scholar]
- 40. Polanco JC, Li C, Durisic N, Sullivan R, Götz J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol Commun. 2018;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morel L, Regan M, Higashimori H, et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288(10):7105-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019;9(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36(7):1772-1781. [DOI] [PubMed] [Google Scholar]
- 45. Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156-1162. [DOI] [PubMed] [Google Scholar]
- 46. Wang J, Sun X, Zhao J, et al. Exosomes: a novel strategy for treatment and prevention of diseases. Front Pharmacol. 2017;8:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes. 2013;2(1):54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zimta AA, Baru O, Badea M, Buduru SD, Berindan-Neagoe I. The role of angiogenesis and pro-angiogenic exosomes in regenerative dentistry. Int J Mol Sci. 2019;20(2):406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Katsuda T, Ochiya T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res Ther. 2015;6:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fafián-Labora J, Lesende-Rodriguez I, Fernández-Pernas P, et al. Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci Rep. 2017;7:43923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernández-Hernando C, Suárez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31(11):2595-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li SP, Lin ZX, Jiang XY, Yu XY. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin. 2018;39(4):542-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bang C, Batkai S, Dangwal S, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301-312. [DOI] [PubMed] [Google Scholar]
- 56. Jan AT, Malik MA, Rahman S, et al. Perspective insights of exosomes in neurodegenerative diseases: a critical appraisal. Front Aging Neurosci. 2017;9:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu W, Bai X, Zhang A, Huang J, Xu S, Zhang J. Role of exosomes in central nervous system diseases. Front Mol Neurosci. 2019;12:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B. Exosomes, an unmasked culprit in neurodegenerative diseases. Front Neurosci. 2017;11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kapogiannis D, Mustapic M, Shardell MD, et al. Association of extracellular vesicle biomarkers with alzheimer disease in the baltimore longitudinal study of aging. JAMA Neurol. 2019;76(11):1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Longoni B, Fasciani I, Kolachalam S, et al. Neurotoxic and neuroprotective role of exosomes in Parkinson’s disease. Curr Pharm Des. 2019;25(42):4510-4522. [DOI] [PubMed] [Google Scholar]
- 61. Gagliardi D, Bresolin N, Comi GP, Corti S. Extracellular vesicles and amyotrophic lateral sclerosis: from misfolded protein vehicles to promising clinical biomarkers. Cell Mol Life Sci. 2021;78(2):561-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheng L, Zhao W, Hill AF. Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Mol Aspects Med. 2018;60:62-68. [DOI] [PubMed] [Google Scholar]
- 63. Hartmann A, Muth C, Dabrowski O, Krasemann S, Glatzel M. Exosomes and the Prion Protein: More than One Truth. Front Neurosci. 2017;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Izquierdo-Useros N, Naranjo-Gómez M, Archer J, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113(12):2732-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103(3):738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakai M, Oshiumi H, Funami K, et al. Interferon (IFN) and cellular immune response evoked in RNA-pattern sensing during infection with hepatitis C virus (HCV). Sensors (Basel). 2015;15(10):27160-27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80(9):4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kotenko SV, Gallagher G, Baurin VV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4(1):69-77. [DOI] [PubMed] [Google Scholar]
- 69. Sheppard P, Kindsvogel W, Xu W, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4(1):63-68. [DOI] [PubMed] [Google Scholar]
- 70. Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4(2):161-167. [DOI] [PubMed] [Google Scholar]
- 71. Martínez MC, Andriantsitohaina R. Extracellular vesicles in metabolic syndrome. Circ Res. 2017;120(10):1674-1686. [DOI] [PubMed] [Google Scholar]
- 72. Javeed N. Shedding perspective on extracellular vesicle biology in diabetes and associated metabolic syndromes. Endocrinology. 2019;160(2):399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Noren Hooten N, Evans MK. Extracellular vesicles as signaling mediators in type 2 diabetes mellitus. Am J Physiol Cell Physiol. 2020;318(6):C1189-C1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ferrante SC, Nadler EP, Pillai DK, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77(3):447-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hubal MJ, Nadler EP, Ferrante SC, et al. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25(1):102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kumar A, Sundaram K, Mu J, et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun. 2021;12(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ying W, Riopel M, Bandyopadhyay G, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372-384.e12. [DOI] [PubMed] [Google Scholar]
- 78. Nomura S, Shouzu A, Omoto S, et al. Effect of cilostazol on soluble adhesion molecules and platelet-derived microparticles in patients with diabetes. Thromb Haemost. 1998;80(3):388-392. [PubMed] [Google Scholar]
- 79. Omoto S, Nomura S, Shouzu A, et al. Significance of platelet-derived microparticles and activated platelets in diabetic nephropathy. Nephron. 1999;81(3):271-277. [DOI] [PubMed] [Google Scholar]
- 80. Shouzu A, Nomura S, Hayakawa T, et al. Effect of sarpogrelate hydrochloride on platelet-derived microparticles and various soluble adhesion molecules in diabetes mellitus. Clin Appl Thromb Hemost. 2000;6(3):139-143. [DOI] [PubMed] [Google Scholar]
- 81. Li S, Wei J, Zhang C, et al. Cell-Derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem. 2016;39(6):2439-2450. [DOI] [PubMed] [Google Scholar]
- 82. Freeman DW, Noren Hooten N, Eitan E, et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018;67(11):2377-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. López Andrés N, Tesse A, Regnault V, et al. Increased microparticle production and impaired microvascular endothelial function in aldosterone-salt-treated rats: protective effects of polyphenols. Plos One. 2012;7(7):e39235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31(8):1898-1907. [DOI] [PubMed] [Google Scholar]
- 85. Osada-Oka M, Shiota M, Izumi Y, et al. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res. 2017;40(4):353-360. [DOI] [PubMed] [Google Scholar]
- 86. Cordazzo C, Neri T, Petrini S, et al. Angiotensin II induces the generation of procoagulant microparticles by human mononuclear cells via an angiotensin type 2 receptor-mediated pathway. Thromb Res. 2013;131(4):e168-e174. [DOI] [PubMed] [Google Scholar]
- 87. Bei Y, Chen T, Banciu DD, Cretoiu D, Xiao J. Circulating exosomes in cardiovascular diseases. Adv Exp Med Biol. 2017;998:255-269. [DOI] [PubMed] [Google Scholar]
- 88. Nielsen MH, Irvine H, Vedel S, Raungaard B, Beck-Nielsen H, Handberg A. The impact of lipoprotein-associated oxidative stress on cell-specific microvesicle release in patients with familial hypercholesterolemia. Oxid Med Cell Longev. 2016;2016:2492858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ousmaal Mel F, Martínez MC, Andriantsitohaina R, et al. Increased monocyte/neutrophil and pro-coagulant microparticle levels and overexpression of aortic endothelial caveolin-1β in dyslipidemic sand rat, Psammomys obesus. J Diabetes Complications. 2016;30(1):21-29. [DOI] [PubMed] [Google Scholar]
- 90. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee JE, Moon PG, Cho YE, et al. Identification of EDIL3 on extracellular vesicles involved in breast cancer cell invasion. J Proteomics. 2016;131:17-28. [DOI] [PubMed] [Google Scholar]
- 94. Melo SA, Sugimoto H, O’Connell JT, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Deng T, Zhang H, Yang H, et al. Exosome miR-155 derived from gastric carcinoma promotes angiogenesis by targeting the c-MYB/VEGF axis of endothelial cells. Mol Ther Nucleic Acids. 2020;19:1449-1459. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96. Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106(10):3794-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ko SY, Lee W, Kenny HA, et al. Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake. Commun Biol. 2019;2:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Choy C, Jandial R. Breast cancer exosomes breach the blood-brain barrier. Neurosurgery. 2016;78(6):N10-N11. [DOI] [PubMed] [Google Scholar]
- 100. Ciravolo V, Huber V, Ghedini GC, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227(2):658-667. [DOI] [PubMed] [Google Scholar]
- 101. Dong X, Bai X, Ni J, et al. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020;11(11):987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Qian L, Yang X, Li S, et al. Reduced O-GlcNAcylation of SNAP-23 promotes cisplatin resistance by inducing exosome secretion in ovarian cancer. Cell Death Discov. 2021;7(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kunou S, Shimada K, Takai M, et al. Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in malignant lymphoma. Oncogene. 2021;40(23):3989-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shan G, Gu J, Zhou D, et al. Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-β signaling pathway. Exp Mol Med. 2020;52(11): 1809-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang H, Deng T, Liu R, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677-685. [DOI] [PubMed] [Google Scholar]
- 108. Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553-5565. [DOI] [PubMed] [Google Scholar]
- 109. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19-30. [DOI] [PubMed] [Google Scholar]
- 110. Villarroya-Beltri C, Baixauli F, Mittelbrunn M, et al. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun. 2016;7:13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Murrow L, Malhotra R, Debnath J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17(3):300-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Guo H, Chitiprolu M, Roncevic L, et al. Atg5 disassociates the V1V0-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell. 2017;43(6):716-730.e7. [DOI] [PubMed] [Google Scholar]
- 114. Ghossoub R, Lembo F, Rubio A, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. [DOI] [PubMed] [Google Scholar]
- 115. D’Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267(5201):1175-1178. [DOI] [PubMed] [Google Scholar]
- 116. Bianco F, Perrotta C, Novellino L, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. Embo J. 2009;28(8):1043-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wei Y, Wang D, Jin F, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Song L, Tang S, Han X, et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10(1):1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi:10.1186/1471-2407-12-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang T, Gilkes DM, Takano N, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111(31):E3234-E3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002;3(2):283-289. [DOI] [PubMed] [Google Scholar]
- 122. Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003;162(3):435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219(3):e201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Imjeti NS, Menck K, Egea-Jimenez AL, et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc Natl Acad Sci U S A. 2017;114(47):12495-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu BX, Clarke CJ, Hannun YA. Mammalian neutral sphingomyelinases: regulation and roles in cell signaling responses. Neuromolecular Med. 2010;12(4):320-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dorayappan KDP, Wanner R, Wallbillich JJ, et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37(28):3806-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ruiz-Martinez M, Navarro A, Marrades RM, et al. YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget. 2016;7(32):51515-51524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Beghein E, Devriese D, Van Hoey E, Gettemans J. Cortactin and fascin-1 regulate extracellular vesicle release by controlling endosomal trafficking or invadopodia formation and function. Sci Rep. 2018;8(1):15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Clancy JW, Zhang Y, Sheehan C, D’Souza-Schorey C. An ARF6-Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nat Cell Biol. 2019;21(7):856-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Muralidharan-Chari V, Clancy J, Plou C, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Han HG, Moon HW, Jeon YJ. ISG15 in cancer: beyond ubiquitin-like protein. Cancer Lett. 2018;438:52-62. [DOI] [PubMed] [Google Scholar]
- 134. Ludwig N, Yerneni SS, Menshikova EV, Gillespie DG, Jackson EK, Whiteside TL. Simultaneous inhibition of glycolysis and oxidative phosphorylation triggers a multi-fold increase in secretion of exosomes: possible role of 2’3’-cAMP. Sci Rep. 2020;10(1):6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wang Y, Yin K, Tian J, et al. Granulocytic myeloid-derived suppressor cells promote the stemness of colorectal cancer cells through exosomal S100A9. Adv Sci (Weinh). 2019;6(18):1901278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhang W, Zhou X, Yao Q, Liu Y, Zhang H, Dong Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017;313(4):F906-F913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jung KO, Youn H, Lee CH, Kang KW, Chung JK. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget. 2017;8(6):9899-9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69(3):1221-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Huang X, Ding L, Bennewith KL, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35(6):856-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tang X, Chen L, Yan X, Li Y, Xiong Y, Zhou X. Overexpression of miR-210 is Associated with Poor Prognosis of Acute Myeloid Leukemia. Med Sci Monit. 2015;21:3427-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Camps C, Buffa FM, Colella S, et al. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14(5):1340-1348. [DOI] [PubMed] [Google Scholar]
- 142. Guo X, Qiu W, Liu Q, et al. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene. 2018;37(31):4239-4259. [DOI] [PubMed] [Google Scholar]
- 143. Esposito K, Maiorino MI, Di Palo C, et al. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes–a randomized controlled trial. Diabetes Obes Metab. 2011;13(5):439-445. [DOI] [PubMed] [Google Scholar]
- 144. Zhang X, McGeoch SC, Megson IL, et al. Oat-enriched diet reduces inflammatory status assessed by circulating cell-derived microparticle concentrations in type 2 diabetes. Mol Nutr Food Res. 2014;58(6):1322-1332. [DOI] [PubMed] [Google Scholar]
- 145. Aswad H, Forterre A, Wiklander OP, et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57(10):2155-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Whitham M, Parker BL, Friedrichsen M, et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27(1):237-251.e4. [DOI] [PubMed] [Google Scholar]
- 148. Jalabert A, Vial G, Guay C, et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59(5):1049-1058. [DOI] [PubMed] [Google Scholar]
- 149. Hikita T, Kuwahara A, Watanabe R, Miyata M, Oneyama C. Src in endosomal membranes promotes exosome secretion and tumor progression. Sci Rep. 2019;9(1):3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sexton RE, Mpilla G, Kim S, Philip PA, Azmi AS. Ras and exosome signaling. Semin Cancer Biol. 2019;54:131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. McKenzie AJ, Hoshino D, Hong NH, et al. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 2016;15(5):978-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cha DJ, Franklin JL, Dou Y, et al. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4:e07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Demory Beckler M, Higginbotham JN, Franklin JL, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12(2):343-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Latifkar A, Ling L, Hingorani A, et al. Loss of Sirtuin 1 alters the secretome of breast cancer cells by impairing lysosomal integrity. Dev Cell. 2019;49(3):393-408.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lee BR, Sanstrum BJ, Liu Y, Kwon SH. Distinct role of Sirtuin 1 (SIRT1) and Sirtuin 2 (SIRT2) in inhibiting cargo-loading and release of extracellular vesicles. Sci Rep. 2019;9(1):20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Riva P, Battaglia C, Venturin M. Emerging role of genetic alterations affecting exosome biology in neurodegenerative diseases. Int J Mol Sci. 2019;20(17):4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Frühbeis C, Fröhlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. Plos Biol. 2013;11(7):e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Peng KY, Pérez-González R, Alldred MJ, et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain. 2019;142(1):163-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lebrand C, Corti M, Goodson H, et al. Late endosome motility depends on lipids via the small GTPase Rab7. Embo J. 2002;21(6):1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Marquer C, Laine J, Dauphinot L, et al. Increasing membrane cholesterol of neurons in culture recapitulates Alzheimer’s disease early phenotypes. Mol Neurodegener. 2014;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Chen H, Yang J, Low PS, Cheng JX. Cholesterol level regulates endosome motility via Rab proteins. Biophys J. 2008;94(4):1508-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wren MC, Zhao J, Liu CC, et al. Frontotemporal dementia-associated N279K tau mutant disrupts subcellular vesicle trafficking and induces cellular stress in iPSC-derived neural stem cells. Mol Neurodegener. 2015;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Benussi L, Ciani M, Tonoli E, et al. Loss of exosomes in progranulin-associated frontotemporal dementia. Neurobiol Aging. 2016;40:41-49. [DOI] [PubMed] [Google Scholar]
- 164. Strauss K, Goebel C, Runz H, et al. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem. 2010;285(34):26279-26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Roberg-Larsen H, Lund K, Seterdal KE, et al. Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes. J Steroid Biochem Mol Biol. 2017;169:22-28. [DOI] [PubMed] [Google Scholar]
- 166. Kulshreshtha A, Singh S, Ahmad M, et al. Simvastatin mediates inhibition of exosome synthesis, localization and secretion via multicomponent interventions. Sci Rep. 2019;9(1):16373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Baek AE, Krawczynska N, Das Gupta A, et al. The cholesterol metabolite 27-hydroxycholesterol increases the secretion of extracellular vesicles which promote breast cancer progression. Endocrinology. 2021;162(7):bqab095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ortiz A, Gui J, Zahedi F, et al. An interferon-driven oxysterol-based defense against tumor-derived extracellular vesicles. Cancer Cell. 2019;35(1):33-45 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Datta A, Kim H, Lal M, et al. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 2017;408:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Im EJ, Lee CH, Moon PG, et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat Commun. 2019;10(1):1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kosgodage US, Trindade RP, Thompson PR, Inal JM, Lange S. Chloramidine/Bisindolylmaleimide-I-mediated inhibition of exosome and microvesicle release and enhanced efficacy of cancer chemotherapy. Int J Mol Sci. 2017;18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kosgodage US, Mould R, Henley AB, et al. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018;9:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Datta A, Kim H, McGee L, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep. 2018;8(1):8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.