Abstract
Immune thrombocytopenia (ITP) is an autoimmune condition associated with multiple risk factors including viral infections (hepatitis B virus/hepatitis C virus/cytomegalovirus, HIV, and recently severe acute respiratory syndrome coronavirus 2) and vaccines. Though immune mechanisms have been proposed to explain the pathogenesis of acute ITP, autoimmunity with the coronavirus disease 2019 (COVID-19) vaccine is still unclear and needs further research. We report a case of acute ITP after administration of the Pfizer-BioNTech mRNA COVID-19 vaccine in a patient with previously stable ITP.
Keywords: COVID-19, immune thrombocytopenic purpura, Pfizer, SARS-CoV-2, vaccination
The Food and Drug Administration (FDA) issued Emergency Use Authorization (EUA) for the coronavirus disease 2019 (COVID-19) vaccine on December 11, 2020. In New York, as of February 19, 2021, 2 147 076 first doses and 1 033 267 second doses of the COVID-19 mRNA vaccines have already been administered [1]. The FDA requires that any vaccine-related adverse events be reported to the Vaccine Adverse Events Reporting System (VAERS). The VAERS currently has 15 865 reports of adverse events from the COVID vaccine ranging from mild symptoms to severe anaphylaxis [2].
Immune thrombocytopenia (ITP) is an autoimmune pathology that is defined by isolated platelet count <100×10E9/L, petechiae or purpuric rashes, and episodes of possible hemorrhage caused by antiplatelet antibodies. Typically, the disease follows a stable course, with intermittent and episodic flares leading to relevant thrombocytopenia [3]. ITP has been described following several viral infections including but not limited to hepatitis B/C, cytomegalovirus (CMV), and HIV. More recently, there have been numerous cases of new-onset ITP in COVID-19 patients described in the literature [4]. Following the administration of the COVID-19 vaccine, rare cases of ITP have been reported to the federal government’s Vaccine Adverse Event Reporting System. There have been reports, for example, of thrombocytopenia following the Pfizer vaccine with and without a history of ITP. Here we present the case of a patient with a known history of ITP and subsequent flare-up after the first dose of the Pfizer-BioNTech COVID mRNA vaccine.
CASE REPORT
A 47-year-old female with a medical history of hypothyroidism (secondary to Hashimoto’s thyroiditis), iron deficiency anemia, lymphadenopathy, and ITP received her first dose of the Pfizer-BioNTech mRNA vaccine and experienced mild arm soreness for 2 days postvaccination but did not experience any other adverse effects.
The patient had ITP diagnosed in 2002, during pregnancy, when platelets were decreased to as low as 4000/mcL. After initial treatment with prednisone, she had a response of complete remission (CR) (platelets >100 000, as described by Rodeghiero et al.) [5]. She had another ITP flare-up 6 years later, with platelets decreasing to 3000/mcL, which responded to human immune globulin (IVIG) and dexamethasone in another CR response. Workup during this time frame yielded a direct platelet antibody level of 1962 and a mildly elevated rheumatoid factor of 20.7 IU/mL. Negative tests included antinuclear antigen (ANA), double-stranded DNA (dsDNA), anticardiolipin immunoglobulin M and immunoglobulin G, and lupus anticoagulant. A bone marrow biopsy yielded no morphologic or immunophenotypic evidence of a lymphoproliferative disorder, increased blasts, or presence of clonal blast cells. Karyotyping showed a normal karyotype of 46XX. Her platelet count was recorded on many occasions in the interval years and ranged from 164 000 to 36 000. She was not maintained on any therapy for ITP during this time.
She was found to have an enlarged left axillary lymph node of routine mammogram in 2018 as well as left axillary and retroperitoneal adenopathy on chest computed tomography (CT) in 12/2018 and abdominal pelvis CT in 6/2019. Biopsy of the left axillary lymph node in 2018 showed no malignancy, and an excisional biopsy in 3/2019 of the area showed follicular hyperplasia without malignancy. Flow cytometry was negative for lymphocytic malignancy in 2018, and fine needle aspirate of adenopathy in the breast and 2 excisional biopsies were negative for malignancy in 2019.
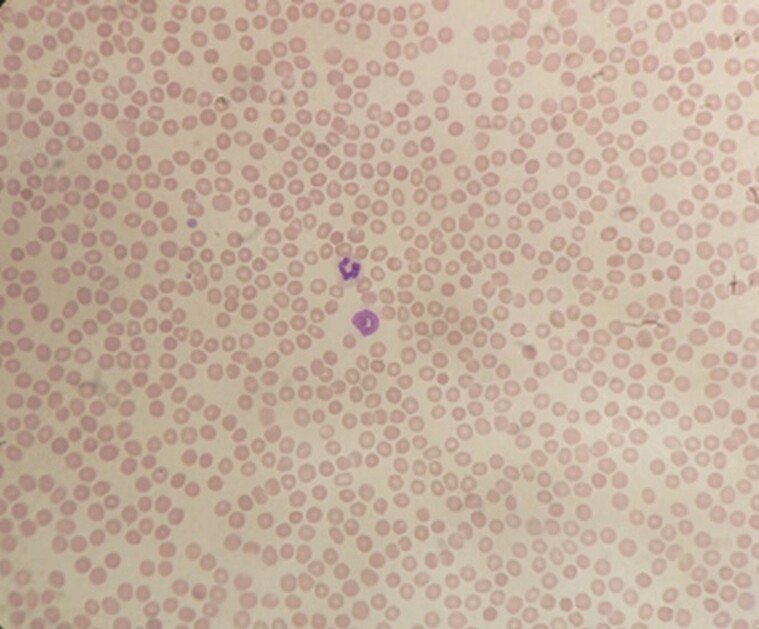
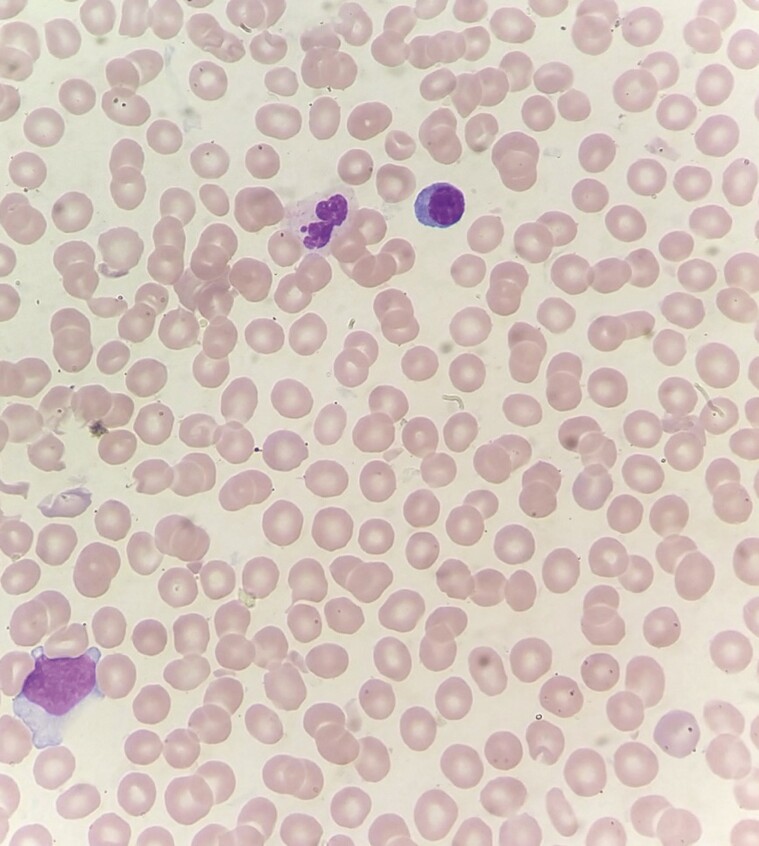
Eighteen days postadministration, the patient presented to the emergency room with complaints of easy bruising, gum bleeding, and an episode of epistaxis. She denied any history of trauma, headache, visual changes, rectal bleeding, or gastrointestinal symptoms. Physical exam was remarkable for ecchymosis and petechiae on her bilateral upper and lower extremities and dried blood within her oropharynx. No splenomegaly or hepatomegaly was noted. A CT of the head was negative for hemorrhage, and a chest radiograph was clear. Her platelet count was noted to be 1000/mcL (her last platelet count 3 months prior was 62 000/mcL). Peripheral smear confirmed the thrombocytopenia and showed normal red blood cell (RBC) morphology (Figures 1 & 2). Her prothrombin time was 16.2 seconds and international normalized ratio 1.5 mg/dL, her reticulocyte count showed mildly elevated reticulocytes of 2.2%, lactate dehydrogenase (LDH) was mildly elevated at 310 U/L, and a manual white blood cell differential showed 13 atypical lymphocytes. She had a mildly elevated alkaline phosphatase of 109 U/L, and antinuclear antibody screen (ANA) was negative. Her other cell lines and labs were within normal limits. The labs within normal limits included white blood cell count and differential, hemoglobin, red cell distribution width (RDW), aPPT, haptoglobin, and basic metabolic panel; a point-of-care test was negative for SARS CoV-2 as well as flu A and B. She had fibrinogen, which was normal at 280, and denied headaches, but no D-dimer or DVT study was done. She received dexamethasone and was admitted to the intensive care unit (ICU).
Figure 1.
Peripheral smear with thrombocytopenia and normal red blood cell (RBC) morphology.
Figure 2.
Peripheral smear with thrombocytopenia normal red blood cell (RBC) morphology.
In the ICU, 1 unit of platelets and 1 unit of IVIG (Privigen) were given, with improvement of platelets to 61 000/mcL. On day 2, the platelet level again fell to 19 000/mcL, and 1 unit of platelet transfusion and IVIG were again given, with platelets subsequently rising to 72 000/mcL. CT scans of the chest, abdomen, and pelvis were performed to evaluate lymphadenopathy and showed resolution of the previously noted adenopathy. Further workup was significant for Sjogren’s SS-A antibody (RO) at 2.8 AI, a repeat ANA was positive at 1:80 with a speckled pattern, an Epstein-Barr virus (EBV) DNA by polymerase chain reaction (PCR) test was positive at 429 IU/mL, and hepatitis C, hepatitis B, HIV, and thyroid-stimulating hormone were negative. She was discharged with clinic follow-up. Up to the time of this report, she has received only the first dose of the vaccine.
DISCUSSION
Immune thrombocytopenic purpura is a rare autoimmune disease described by platelet count <100×109/L, which is associated with an increased risk of bleeding [6]. Risk factors include environmental factors (eg, infections, drugs, and malignancy), genetic predisposition, and viral infections. Moreover, some vaccines against infectious agents have also been associated with acute ITP including measles, mumps, and rubella (MMR), pneumococcus, Haemophilus influenzae B, hepatitis B virus, and varicella-zoster virus (VZV) [6, 7].
Thrombocytopenia is being reported in association with SARS-CoV-2 infection and is a risk factor for increased morbidity and mortality. Thrombocytopenia in these patients may be caused by disseminated intravascular coagulation (DIC) and sepsis; drug-induced and acute ITP have been reported [6]. Multiple mechanisms have been reported, including infection of the bone marrow cells leading to inhibition of platelet production or direct effect on platelets by the virus (conceivably via CD-13 receptors), virus-induced liver damage resulting in thrombopoietin synthesis, pulmonary endothelial injury with formation of platelet clumps in the lungs resulting in formation of microthrombi and thrombocytopenia, and degradation of platelets by the immune system [7]. There has not been adequate discussion on these immune mechanisms to date. Certain phenomena that can explain this autoimmunity by viruses include molecular mimicry, cryptic antigen expression, and epitome spreading. Molecular mimicry has been well explained in viruses including HIV, HCV, VZV, and H. Pylori. However, the sequence homology of SARS-CoV-2 and platelets has yet to be identified [4].
Vaccines are now required for prevention of diseases such as SARS-CoV-2 in the general population and for those who are at high risk of complications [8]. As of December 2020, 212 vaccines had been developed across the globe including 4 nonreplicating viral vector vaccines, 3 inactivated vaccines, 2 protein subunit vaccines, and 2 RNA vaccines that had entered in phase III clinical trials. Among these companies, the leading companies, Moderna, BioNTech/Pfizer, and Inovio, generated nucleic acid–based vaccines. Early studies from BioNTech/Pfizer and Moderna have shown a strong antibody response [9, 10]. Pfizer generated a BNT162b2 COVID-19 vaccine, which is a lipid nanoparticle–formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein [11]. Reported side effects include local reactions like redness, swelling, pain at the injection site, and systemic-like fever, chills, fatigue, headache, new or worsening muscle pain, and vomiting [11, 12]. Lymphadenopathy in the arm and neck region particularly was also reported but was not common. Rarely reported side effects include Bell’s palsy, appendicitis, acute MI, and cerebrovascular accident [12]. There are very few reports of ITP exacerbation following COVID-19 vaccine administration. There was 1 reported case of acute ITP in a patient with stable chronic ITP within 2 weeks of administration of the Moderna vaccine [13].
Our patient with a history of ITP received the first dose of the Pfizer-BioNTech mRNA vaccine. Her last ITP flare was 13 years ago, and she has had a stable platelet count above 30 000/mcL since then. After 18 days of vaccine administration, she presented with acute on chronic ITP. Workup ruled out any other etiology of thrombocytopenia, including active COVID-19 infection. The patient did not complain of any headaches, and thus the likelihood of thrombosis was very low. Although the positive EBV DNA PCR may have been a factor contributing to the acute on chronic ITP, there is no way to tell if the vaccine may have caused a reactivation of EBV, which triggered the acute ITP. However, given her infrequency of ITP flares and the fact that the event occurred after receiving the vaccine, an association of thrombocytopenia with the COVID-19 vaccine should be considered. The underlying mechanism requires further research.
CONCLUSIONS
The temporal relation of ITP exacerbation after receiving the Pfizer-BioNTech mRNA COVID-19 vaccine in this patient, especially considering the infrequency of her flare-ups and her stable platelet count for many years, is of considerable significance. The best available evidence from all clinical trials and reported side effects from vaccinated individuals suggest that ITP secondary to the COVID vaccine is an exceedingly rare reportable adverse effect, but the benefits of vaccination outweigh the risks of COVID infection in these patients. Considering the few similar cases recently reported, patients with chronic ITP should be monitored closely for any suspicious symptoms following vaccination. The underlying mechanism of this autoimmunity is still unclear and needs further research.
Acknowledgments
Financial support. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Potential conflicts of interest. No conflicts of interests were declared by any of the authors listed. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Written consent was obtained from the patient for the case report.
References
- 1. COVID-19 vaccine tracker. New York State. Available at:https://covid19vaccine.health.ny.gov/covid-19-vaccine-tracker. Accessed 02/20/21.
- 2. The Vaccine Adverse Event Reporting System (VAERS) request. Centers for Disease Control and Prevention (CDC). Available at: https://wonder.cdc.gov/vaers.html. Accessed 02/20/21.
- 3. Justiz Vaillant AA, Gupta N.. ITP-Immune Thrombocytopenic Purpura. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 4. Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr Clin Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009; 113:2386–93. [DOI] [PubMed] [Google Scholar]
- 6. Bomhof G, Mutsaers PGNJ, Leebeek FWG, et al. COVID-19-associated immune thrombocytopenia. Br J Haematol 2020; 190:e61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood 2009; 113:6511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano 2020; 14:12522–37. [DOI] [PubMed] [Google Scholar]
- 10. Zhao J, Zhao S, Ou J, et al. COVID-19: coronavirus vaccine development updates. Front Immunol 2020; 11:602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1922–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toom S, Wolf B, Avula A, et al. Familial thrombocytopenia flare-up following the first dose of mRNA-1273 COVID-19 vaccine. Am J Hematol. In press. [DOI] [PMC free article] [PubMed]