Abstract
We compared 90–90–90 targets in 2020, during the coronavirus disease 2019 (COVID-19) pandemic, with the targets across the period 2017–2019 in people with HIV. We observed a significant loss in the 90–90–90 objectives in 2020 when compared with 2017–2019 that might be attributable to the COVID-19 crisis.
Keywords: COVID-19, care model, HIV target, 90-90-90 goals, UNAIDS
The impact of the coronavirus disease 2019 (COVID-19) pandemic on health systems around the world has the potential to alter the road map toward the end of AIDS. Mathematical models predict that in high-burden settings, deaths due to HIV over 5 years could increase by up to 10%, compared with if there was no COVID-19 pandemic [1]. This might be the consequence of a shifted focus from HIV to the COVID-19 pandemic, which led to less attention given to the needs of people with HIV (PWH) in the era of COVID-19 and less funding for HIV care and prevention. Additionally, in the current HIV–COVID-19 syndemic, health care workers who are primarily involved in HIV care at present are also providing COVID-19 care.
The contemporary HIV care framework is based on the “90–90–90 targets” launched by UNAIDS [2]. They promised the end of AIDS by 2030 through achieving 3 ambitious goals: HIV diagnosis in 90% of all PWH, provision of antiretroviral therapy (ART) for 90% of the diagnosed individuals, and viral suppression for 90% of the treated patients. The fear is that each of these targets may encounter specific obstacles in the COVID-19 era.
The objective of the study was to compare the 90–90–90 targets in 2020, during the COVID-19 pandemic, with the targets across the period 2017–2109 in PWH.
METHODS
This retrospective observational study assessed epidemiological and HIV clinical data in the period January–September across 2017–2020 in the province of Modena, Emilia-Romagna region, Northern Italy, which comprises 700 000 habitants and 7 hospitals.
The network of laboratories within the Modena province and Modena HIV clinic feed the provincial HIV observatory database. The former provided the data regarding the number of performed HIV tests and established HIV diagnosis in the observational period, while the paper and electronic clinical charts of the Modena HIV clinic were used to describe the 90–90–90 targets. Of note, 32.5% of PWH who are followed at Modena HIV clinic have a residence outside Modena province.
The first “90” target was indirectly estimated using the following formula: PWH in care for the current year + PWH who entered care in the subsequent year – PWH who died in the current year.
The second “90” target was calculated as a ratio of PWH receiving ART and PWH who had a confirmed diagnosis of HIV infection. The third “90” target was defined as a ratio of PWH with undetectable HIV RNA (<40 copies/mL) and PWH receiving ART for at least 6 months. Viral blips were defined as HIV RNA between 40 and 200 copies/mL. The chi-square test for linear trend was used to describe differences across the observational period.
Categorical variables were described using frequencies and percentages, while continuous variables were described using mean, median, and interquartile range (IQR) values. Means for continuous variables were compared using the Student t test and the Mann-Whitney test. Proportions for categorical variables were compared using the Cochran-Armitage test for linear trend. Multivariable logistic regression was performed to explore risk factors for loss of care, using as predictors age, sex, migrant status, and being a nonresident of the Emilia-Romagna region.
The study was approved by the local institutional review board in the context of the local HIV cohort description.
RESULTS
In the period January–September 2020, 14 712 HIV tests were performed in the Modena province, with an average reduction of about 20% compared with previous years. Eleven new HIV cases were diagnosed, and 15 PWH died, none of COVID-19. A total of 1512 PWH were in care: The median age (IQR) was 54 (45–59) years, 69.6% were males, and the median CD4 (IQR) was 697 (511–935).
Table 1 compares epidemiological and clinical variables during the study period. Comprehensively, in the period January–September 2017–2020, 116 new HIV diagnoses were observed, with no change in the proportion of AIDS presenters (10, 5, 12, and 4 cases, respectively; P = .07), and 67 deaths were observed. Viral blips and viral failures (>200 copies/mL) were not different across 2017–2020.
Table 1.
Epidemiological and Clinical Variables During the Study Period
| 2017 | 2018 | 2019 | 2020 | P | |
|---|---|---|---|---|---|
| 90–90–90 targets | |||||
| No. of HIV tests | 18 324 | 18 529 | 18 726 | 14 712 | |
| Incident HIV diagnosis, No. | 47 | 30 | 28 | 11 | |
| AIDS presentation at diagnosis, No. (%) | 10 (21.7) | 5 (19.2) | 12 (44.4) | 4 (44.4) | .07 |
| Estimated PWH, No. (%) | 1708 (100) | 1721 (100) | 1741 (100) | 1761 (100) | |
| In care, No. (%) | 1604 (93.9) | 1631 (94.8) | 1673 (96.9) | 1512 (85.9) | .003 |
| New patients linked to care, No. | 144 | 143 | 73 | ||
| On treatment, No. (%) | 1574 (98.1) | 1608 (98.6) | 1659 (99.2) | 1510 (99.9) | <.01 |
| Undetectable HIV RNA (≤40 c/mL), No. (%) | 1388 (93.8) | 1466 (95.3) | 1500 (95.1) | 1382 (94.3) | <.01 |
| Demographic and HIV characteristics | |||||
| Age, median (IQR), y | 51 (43–56) | 52 (43–57) | 53 (44–58) | 54 (45–59) | |
| Sex, males, No. (%) | 1111 (69.3) | 1132 (69.4) | 1163 (69.5) | 1052 (69.6) | .84 |
| Italians, No. (%) | 1287 (80.2) | 1310 (80.3) | 1318 (78.8) | 1196 (79.1) | .27 |
| Migrants, No. (%) | 317 (19.8) | 321 (19.7) | 355 (21.2) | 316 (20.9) | .27 |
| Men who have sex with men, No. (%) | 467 (29.1) | 484 (29.7) | 499 (29.8) | 460 (30.4) | .43 |
| Intravenous drug users, No. (%) | 376 (23.4) | 368 (22.6) | 357 (21.3) | 319 (21.1) | .08 |
| Heterosexual intercourse, No. (%) | 663 (41.3) | 675 (41.4) | 711 (42.5) | 640 (42.3) | .46 |
| Others, No. (%) | 98 (6.1) | 104 (6.4) | 106 (6.3) | 93 (6.2) | .97 |
| CD4+ T-cell count, median (IQR), c/mL | 664 (476–884) |
689 (480–892) |
684 (481–899) |
697 (511–935) |
.01 |
| Deaths, No. | 13 | 23 | 16 | 15 | |
| Detectable HIV RNA (>40–≤200 c/mL), No. (%) | 42 (2.8) | 46 (3.0) | 42 (2.7) | 47 (3.2) | .70 |
| Detectable HIV RNA (>200 c/mL), No. (%) | 50 (3.4) | 26 (1.7) | 36 (2.3) | 36 (2.5) | .23 |
Abbreviations: IQR, interquartile range; PWH, people with HIV.
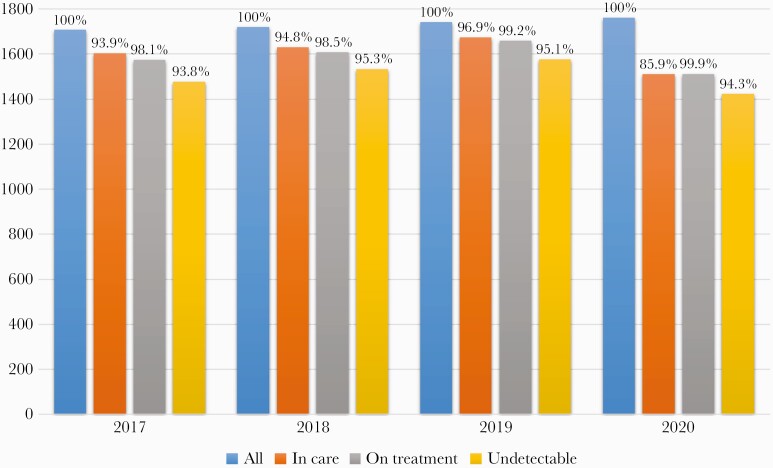
The cascade of care across 2017–2020 is shown in Figure 1. The test for linear trend implied a significant loss in the target objectives in 2020 (P < .001); in particular, the first “90” was the most affected (Figure 1). In comparison with 2019, 249 PWH who were in care in 2019 did not have access to the HIV clinic, depicting an alarming decrease of 14% of PWH in care (Table 1). In multivariable analyses, predictors of loss of care in 2020 were migrant status (odds ratio [OR]; 95% CI, 1.04–2.14) and being a nonresident of the Emilia Romagna region (OR, 3.1; 95% CI, 3.1–2.2), after correction for sex (OR, 0.99; 95% CI, 0.71–1.38) and age (OR, 0.98; 95% CI, 0.97–1.0).
Figure 1.
The cascade of care across 2017–2020.
DISCUSSION
During the study period, the response to the pandemic reduced the availability of routine outpatient HIV care worldwide. Specifically, many infectious disease physicians were redeployed to COVID-19 care, and many patients have not been able to reach hospitals and clinics due either to fear of contracting severe acute respiratory syndrome coronavirus 2 or to confinement measures [3].
We assume that in Modena province in Italy, in which the 90–90–90 targets of the cascade of care have already been reached since 2017, a significant decrease in each target was due to the COVID-19 crisis. In detail, despite an increase of HIV testing in the period June–September, the first “90” goal was lost in 2020.
The first 90% target has been also linked to sexually transmitted disease screening and access to pre-exposure prophylaxis (PrEP). As a result of the COVID-19 pandemic, an 85% reduction in HIV/gonorrhea/chlamydia tests was reported in the first quarter of the year in the Boston area [4]. However, a report from Lombardy, Italy, showed a rise in acute bacterial STIs during the period of lockdown [5]. A report from Melbourne showed that 25% of PrEP users stopped taking PrEP during the COVID-19 outbreak, and 5% switched from daily to on-demand PrEP [6].
Although the long-term consequences of interruption in the scale-up of PrEP in people at risk are still unknown, the potential negative consequences may be mitigated by a reduction in condomless sex and number of partners during the COVID-19 pandemic [6]. Data from a sexual health clinic in London have shown that there was an 80% reduction in postexposure prophylaxis (PEP) use within 4 weeks of the lockdown [7]. Unfortunately, we did not examine PrEP access and uptake, so we could not address the contribution of this issue in our population.
Reduction in HIV testing may further worsen the still alarming proportion of PWH diagnosed with advanced HIV disease. Moreover, the risk of stigma related to COVID-19 may delay presentation of patients with HIV-related opportunistic infections to health care services [8], but at the same time, COVID-19 offers an opportunity to increase HIV testing in acute medical settings [9]. In detail, some respiratory manifestations of HIV disease may be similar to COVID-19, and increased hospitalizations due to COVID-19 may be considered an opportunity to conduct more broad screening for HIV.
On the other hand, the COVID-19 crisis could represent an opportunity to increase the availability of self-testing and rapid test screening in nonhospital settings including community check points, given that “traditional” testing clinics are deployed in providing care for COVID-19. However, it is noteworthy that even if the diagnosis is made in nonhospital settings, it is important to optimize counseling and linkage to care.
This interaction represents a crucial step in the beginning of a long-lasting patient–physician relationship based on mutual trust and compassion and represents an essential resource for the continuum of care.
The second 90% target refers to “retention in care” and is critical to the management of HIV, as it is associated with improved survival, decreased HIV-related complications, and reduced HIV transmission. Although small case series have described a positive impact of COVID-19 in terms of service re-engagement, whether this is maintained remains to be seen.
In our clinic, almost 200 PWH did not have any contact with the hospital during the COVID-19 crisis, and some of them could be at risk of being lost to care for a long period of time. Indeed, being lost to care represents an important risk factor for clinical progression, and these patients may account for up to 62% of all AIDS cases [10]. In particular, risk factors for progression to AIDS in patients who are lost to care include psychiatric comorbidities, migration status, and alcohol and substance abuse [11].
Loss to care was more often observed in nonresidents of the Emilia-Romagna region. This is easily interpretable as being caused by lockdown measures that did not allow mobility between regions. Some PWH, who are nonresidents of Emilia-Romagna, asked to transfer their ART prescription to hospital pharmacies in their region of residence. Conversely, PWH who are residents of Emilia-Romagna (to which Modena province belongs) had easier and less limited access to provincial pharmacies that supply ART medications.
More crucial is the risk of loss to care in migrants. We defined migrants as people born outside of Italy who came to live in our province searching for work to improve their life condition. These individuals may represent a socially and economically vulnerable group that needs dedicated strategies for retention in care. Anecdotally, a Ghanaian lady was admitted to our unit in June 2020 with HIV-associated encephalopathy secondary to ART interruption. She admitted that, due to a language barrier, she had not understood where to get ART when the infectious disease clinic pharmacy was moved to a different place, in order to avoid PWH entering the restricted COVID area.
These vulnerable populations may be the most difficult to reach using the telemedicine approach, and therefore dedicated programs including same-day ART and personalized interventions are needed. This is also true for dedicated programs for re-engagement, particularly for PWH lost to care at the time of HIV service interruption caused by COVID-19.
Concerning the third 90%, difficult access to the hospital clinic and drug delivery services could have a deleterious impact on this target. Actually, our data show that the percentage of PWH with an undetectable viral load was similar to previous years. It is notable that during the COVID-19 crisis alternative drug delivery methods were organized. A particular problem was represented by blood testing for monitoring HIV infection. As current ART regimens substantially reduce the risk of emergence of drug resistance, HIV societies, in particular the British HIV Association, recommended the postponement of routine monitoring of viral load in the current era beyond the usual 6 months. Nevertheless, it will be important to monitor these approaches over a longer period of time as it is not known whether the postponement of routine monitoring of viral load is safe or how to interpret the viral blips. An annual interaction between patients and physicians may be perceived to be insufficient on both sides, and patient–physician virtual encounters should be promoted even beyond the communication of blood test results. In the near future, accessibility of long-lasting HIV drugs, including injectables, will also change HIV care, though this is dependent on the setting of drug delivery.
Some limitations of our study must be acknowledged. First, we considered a relatively small number of variables to address the HIV cascade of care, which comprises a huge variety of aspects. Second, our formula to calculate the first “90” target is not standardized and relied on the previous proposals regarding the estimation of the first “90” [12]. Third, as already mentioned, we did not explore PrEP use during the observational period.
Despite these limitations, our findings contribute to a better understanding of the current HIV cascade of care. We were able to estimate all 3 “90” targets, which provided an important insight into the management of HIV care during the COVID-19 pandemic. However, we are still missing a national and a European “snapshot” that might help us to provide tailored intervention in the COVID-19 era.
It is important to consider the impact of COVID-19 on health challenges faced by PWH, including HIV itself, chronic noncommunicable diseases, mental health burden, substance abuse, and other infections, all of which are catalyzed by biological, behavioral, psychosocial, and structural drivers. The COVID-19 pandemic imposed many challenges on the HIV cascade of care, as most outpatient and nonurgent services were interrupted during the lockdown. This also compromised careful and comprehensive assessment of adherence, ART toxicity, and efficacy. Without these aspects of the HIV cascade, it is difficult to guarantee the second and third “90” targets.
Given the lockdown restrictions that were in place in Modena province from March 9 to May 18, 2020, that limited access to HIV outpatient services, we argue that the reverse trend in the HIV cascade might be attributable to the COVID-19 crisis. Understanding the impact of the COVID-19 crisis in PWH in a syndemic framework provides a meaningful and robust paradigm to understand how to re-design health services for PWH in light of the COVID-19 era.
Acknowledgments
Financial support. This study was not funded.
Potential conflicts of interest. G.G. and C.M. received research grants and speaker honoraria from Gilead, ViiV, MERCK, and Jansen. G.G. and C.M. attended advisory boards of Gilead, ViiV, and MERCK. The other authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study does not include factors necessitating patient consent.
References
- 1. Sands P. HIV, tuberculosis, and malaria: how can the impact of COVID-19 be minimised? Lancet Glob Health 2020; 8:e1102–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2020. https://www.unaids.org/en/resources/909090
- 3. Guaraldi G, Milic J, Martinez E, et al. HIV care models during the COVID-19 era. Clin Infect Dis 2020; ciaa1864.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krakower DS, Solleveld P, Levine K, Mayer KH. Impact of COVID-19 on HIV preexposure prophylaxis care at a Boston community health center. Paper presented at: IAC 2020 Virtual; July 6-10, 2020.
- 5. Latini A, Magri F, Donà MG, et al. Is COVID-19 affecting the epidemiology of STIs? The experience of syphilis in Rome. Sex Transm Infect. 2021; 97:78.. [DOI] [PubMed] [Google Scholar]
- 6. Chow EPF, Hocking JS, Ong JJ, et al. Changing the use of HIV pre-exposure prophylaxis among men who have sex with men during the COVID-19 pandemic in Melbourne, Australia. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Junejo M, Girometti N, McOwan A, et al. HIV postexposure prophylaxis during COVID-19. Lancet HIV 2020; 7:e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sotgiu G, Dobler CC. Social stigma in the time of coronavirus. Eur Respir 2020; 56:2002461.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly S, Waters L, Cevik M, et al.2020. p. ; 20:590–2.. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin Med (Lond) [DOI] [PMC free article] [PubMed]
- 10. Scourfield A, Jackson A, Nelson M. Will earlier diagnosis of HIV infection in late presenters reduce the frequency of serious opportunistic infections? HIV Med 2011; 12:449–50. [DOI] [PubMed] [Google Scholar]
- 11. Lee MJ, Rayment M, Scourfield A, Gazzard B. Comparison of two cohorts of patients presenting with AIDS: patients with previously known HIV diagnoses and true late presenters. Sex Transm Infect 2013; 89:553–6. [DOI] [PubMed] [Google Scholar]
- 12. Sohail M, Levitan EB, Rana AI, et al. Estimating the first 90 of the UNAIDS 90–90–90 goal: a review. J Int Assoc Provid AIDS Care 2020; 19:2325958220919290. [DOI] [PMC free article] [PubMed] [Google Scholar]